Abstract
Acute anterior uveitis (AAU) and related spondyloarthropathies (SpAs) such as ankylosing spondylitis, reactive arthritis and psoriatic arthritis have joined the expanding family of inflammatory disease associated with the biology of the intestinal microbiome. This review discusses why AAU and related SpAs have been incorporated into this paradigm shift for understanding disease pathogenesis. We focus in particular on the major risk gene HLA-B27 and how it may be associated with the loss of intestinal tolerance and ocular immune privilege that may accompany AAU. We discuss how perturbed microbiota composition, intestinal immunity and/or barrier function may contribute to the development of inflammatory responses that ultimately target either self or microbial antigen in the eye. These inflammatory processes may be augmented by translocation of intestinally-derived innate immune stimuli, such as microbe associated molecular patterns (MAMPs). Moreover, gut-derived host immune cells or damage-associated molecular patterns (DAMPs) may contribute to the ocular inflammatory cascade. Finally we discuss the novel therapeutic avenues offered by the microbiota for future clinical management of AAU and spondyloarthropathies.
Introduction
If you like puzzles, the pathogenesis of HLA B27-associated uveitis should intrigue you. The clues are abundant. There are multiple genetic factors, none of which is as influential as HLA B27 itself. There are associated diseases: ankylosing spondylitis, reactive arthritis, inflammatory bowel disease, and psoriatic arthritis. There are known environmental triggers for B27-related disease such as Gram-negative dysenteries. The puzzle was first described in 1973 when a group from London noted that half of those developing acute anterior uveitis were B27 positive 1. And yet more than four decades after the discovery of this potent immuno-genetic effect, the puzzle remains unsolved. Could the solution be buried somewhere within the microbiome?
Because uveitis is unusual as a primary symptom in most practices of rheumatology, we begin with some background on uveitis and its relationship to rheumatic diseases. Then we discuss the evidence that gut disease is linked to spondyloarthropathy which in turn is linked to uveitis. Next we review the evidence that HLA molecules in general and HLA B27 in particular shape the microbiome. Finally we offer 4 mechanisms that could account for the link between the microbiome and gut inflammation and conclude with some of the implications that this association has for research and therapy.
References included in this review have been chosen from searches of the National Library of Medicine (www.pubmed.gov). Abstracts have been excluded.
Uveitis
Uveitis is the term to describe inflammation of the iris, ciliary body, or choroid and adjacent ocular structures. It is the fourth leading cause of acquired blindness 2–4. It has a point prevalence of about one person per thousand 5, but because it affects many patients for decades, it is comparable to diabetes or macular degeneration in terms of years of visual loss 4. Uveitis can be subdivided based on the portion of the uveal tract which is predominantly inflamed. Anterior uveitis is much more common than intermediate, posterior or panuveitis and accounts for 85% of all instances of uveitis 5. In one referral series describing patients with anterior uveitis, nearly 3 out of 4 had an acute or sudden onset as opposed to an insidious onset 6. Sudden onset anterior uveitis is the phenotype of uveitis typically associated with HLA B27 7. HLA B27-associated uveitis is the most common uveitis diagnosis in many referral centers throughout the world 8 including Europe 9, North America 8, Australia 10, and parts of southeast Asia 11,12. In addition, HLA B27-associated uveitis frequently goes unrecognized 13,14. In addition to HLA B27, other genetic, environmental, and stochastic factors undoubtedly contribute to the development of AAU. The fact that 50% of patients with AAU are HLA B27 negative indicates that these factors are well worth pursuing, but they lie outside the main focus of this review.
Uveitis has a broad differential diagnosis which includes immune-mediated systemic diseases, immune-mediated syndromes confined to the eye, infections including Lyme disease and Whipple’s disease, masquerade syndromes such as lymphoma, and reactions to medications15. Although rheumatologists generally receive very little training to evaluate uveitis, many forms of arthritis can be associated with uveitis. In addition to the infections mentioned above, uveitis in combination with arthritis could be any form of spondyloarthritis, juvenile idiopathic arthritis, Behçet’s disease, relapsing polychondritis, Sweet’s syndrome, sarcoidosis, lupus, several forms of vasculitis such as Kawasaki’s disease, reactions to medications such as check point inhibitors or TNF inhibitors, and several forms of autoinflammatory disease such as Blau syndrome or NOMID. The frequency of uveitis varies with different forms of spondyloarthritis (see Figure 1). Uveitis will affect up to 50% of patients with ankylosing spondylitis in a lifetime 16. Uveitis is the most common clinically important co-morbidity recognized among patients with ankylosing spondylitis 17. The likelihood of uveitis in association with psoriatic arthritis is more in the range of 7 to 19% 18,19. And the likelihood is lower still in association with either Crohn’s disease or ulcerative colitis 20. The range of tissues affected in spondyloarthritis is illustrated in Figure 2.
Figure 1. Uveitis in association with Spondyloarthritis.
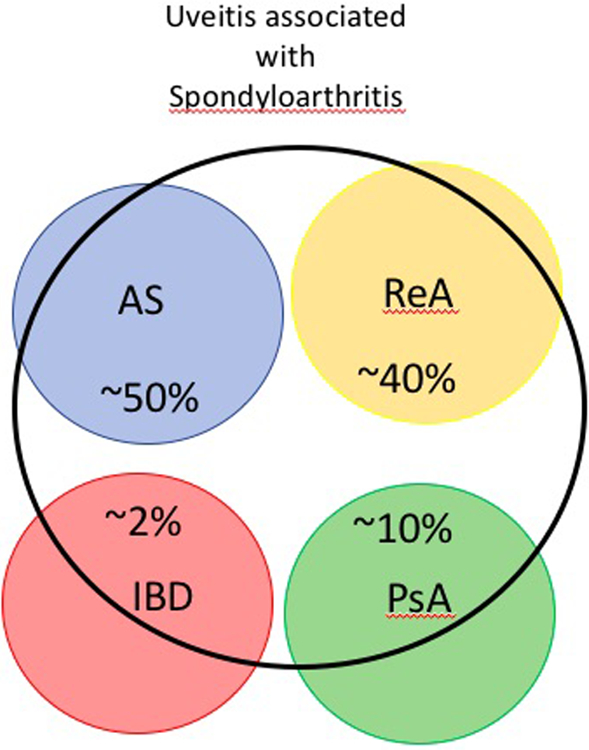
Uveitis here encompasses both B27+ve and B27-ve associated eye inflammation; AS, ankylosing spondylitis; PsA, psoriatic arthritis; ReA, reactive arthritis; SpA-IBD; Spondyloarthropathy-associated IBD. Uveitis associated with AS or ReA almost always fits with AAU 7, while the uveitis associated with PsA 19 or IBD 20 has a greater likelihood to be chronic or posterior to the lens of the eye.
Figure 2. Disease manifestations of the SpA disease family.
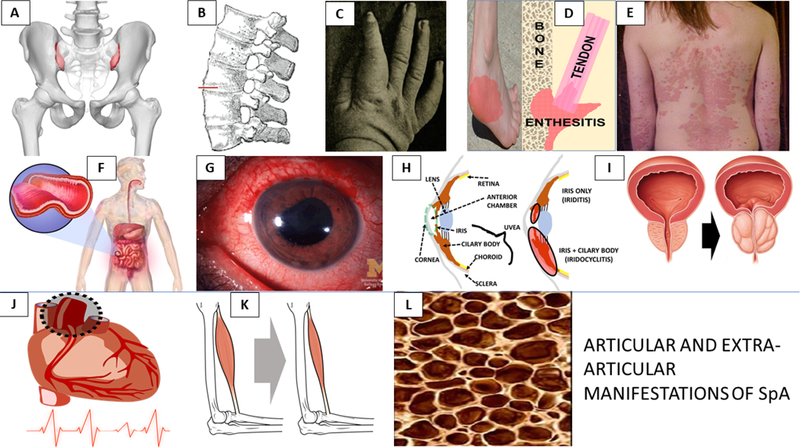
Top row (A-E): A) Sacroiliitis B) Spinal syndesmophytes C) Dactylitis D) Enthesitis E) Psoriasis; Middle row (F-I): F) Enteropathy G,H) Anterior Uveitis with iritis or iridocyclitis I) Prostate inflammation; Bottom row (J-L): J) Aortic root disease and conductive disorder K) Sarcopenia, L) Osteoporosis.
The mechanisms that account for a link between arthritis and uveitis are unknown, although uveitis and arthritis do co-exist in several animal models which include adjuvant arthritis 21, aggrecan-induced arthritis in BALB/C mice 22, and the SKG mouse model of arthritis which is dependent on a mutation in ZAP-70 and then challenge with fungal cell wall 23. The animal model which is arguably most popular for use in studying HLA B27-associated disease, the HLA B27 transgenic rat, develops spondylitis, peripheral arthritis, colitis, and dermatitis, but not uveitis 24.
Spondyloarthropathy and Bowel Inflammation
The spondyloarthropathies including ankylosing spondylitis, reactive arthritis, enthesitis related arthritis in children, psoriatic arthritis, and arthritis associated with inflammatory bowel disease are clearly linked to bowel inflammation, which is often asymptomatic in a disease such as ankylosing spondylitis. Mielants, De Vos, Elewaut, Veys and colleagues firmly established this relationship by histology obtained during routine colonoscopy of patients with spondyloarthritis and no overt bowel symptoms 25,26. Increased bowel permeability has been demonstrated in patients with ankylosing spondylitis 27. A variety of enteric infections including Salmonella, Yersinia, Shigella, and Campylobacter are known to trigger reactive arthritis 28,29. The efficacy of sulfasalazine for the peripheral joint disease associated with spondyloarthritis might derive from its effects on the microbiota of the gut and on bowel permeability 30,31.
A long list of diseases has been linked to the microbiome. Among these diseases, it is now widely believed that both Crohn’s disease and ulcerative colitis 32–35 are caused by changes in the microbiome. Changes in the gut microbiome have been demonstrated as well in ankylosing spondylitis 36–38, psoriatic arthritis 39, and juvenile onset enthesitis related arthritis 40–42. Studies on the fecal microbiome comparing bacteria from patients with disease to bacteria in fecal samples from healthy controls have many limitations. The fecal sample might not reflect the bacterial ecosystem at a critical site such as the intestinal mucosa of the cecum. The fecal sample is certain to be affected by variables such as age, medications, gender, diet, disease duration, and geographic location. Finding differences in the microbiome does not elucidate a mechanism for disease causation and the changes might result from the disease rather than play a causal role. Despite these limitations, comparing the fecal microbiome between patients with or without disease is usually among the first studies to implicate the microbiome in pathogenesis of a specific disease.
HLA molecules and the gut microbiome
The term microbiome was coined by Joshua Lederberg to describe the microbial population that co-exists with another organism such as a human body 43. The Human Microbiome Project was funded in 2007 by the National Institutes of Health to characterize the bacteria that are the main constituents of the microbiome 44. In Europe a similar project called Meta Hit was similarly begun 45. Bacterial cells outnumber mammalian cells within our body. Bacterial transcripts outnumber human transcripts in the body by a ratio greater than 100 to 1 46. As noted above, many factors affect the microbiome including diet, use of antibiotics, and geographic location. The microbiome has now been implicated in the pathogenesis of a vast array of immune-mediated diseases 47 as well as diseases not typically considered to be immune-mediated such as autism 48 and depression 49. In addition, the microbiome metabolizes many medications that range from acetaminophen to cancer therapeutics 50.
HLA (human leukocyte antigen) molecules affect susceptibility to more than 100 diseases 51. Although HLA molecules regulate the immune response, in the majority of diseases, the mechanism by which HLA molecules predispose to disease is not known. The association between HLA B27 and spondyloarthritis is among the strongest. Major histocompatibility complex (MHC) genes like HLA are the most polymorphic genes known 52. A teleologic argument is that the immune response is primarily a defense against infections and the polymorphism minimizes the likelihood that one specific infection could eradicate a species. A corollary to this proposition is that HLA molecules should affect the bacterial composition of the gut.
To test this hypothesis, we and our colleagues studied HLA B27 transgenic rats 53. The rats express multiple copies of HLA B27 along with the invariant MHC light chain, beta 2 microglobulin. The spondyloarthropathy that affects these rats was described by Taurog and colleagues more than two decades ago 53. On the Fischer background, the rats develop diarrhea around two to four months of age. Subsequently many of the animals will develop psoriasiform rashes, peripheral arthritis, and spondylitis. The microbiome plays a major role in this disease because if the rats are raised in a germ free environment, the bowel and joint disease is greatly reduced 54. Some bacteria such as Lactobacillus Rhamnosus GG can be introduced such that the remission is maintained, while other bacteria cause the disease to recur 55. The gut microbiome is clearly affected by HLA B27 in these rodents. Interestingly, one of the earliest immunological changes in these animals is an upregulation of antimicrobial peptides such as RegIIIγ and S100A8 (calprotectin) in the colon of these animals 56. These changes may precede the development of dysbiosis in these animals and we therefore propose changes to the intestinal microbiome may be a primary event in SpA pathogenesis rather than merely an epiphenomenon. Interestingly, calprotectin in the serum has been reported to be a biomarker for posterior uveitis 57, juvenile idiopathic arthritis with uveitis 58, and Behcet’s disease 59.
Mounting evidence indicates the MHC can modify the microbiota. Indeed, the MHC molecules that predispose to celiac disease alter the gut microbiome as demonstrated in a study on infants 60. The MHC molecule that predisposes to rheumatoid arthritis, HLA DR*0401 alters the microbiome in transgenic mice in comparison to those that are transgenic for DR*0402 61. Transgenic expression of human MHC class II molecules can protect mice from the development of diabetes 62 and the fecal microbiome of these animals protects NOD mice from developing hyperglycemia. These studies complement those that more broadly show that MHC haplotype modifies the composition of the microbiota 35.
How could the microbiome contribute to uveitis?
A number of mechanisms have been proposed for how HLA-B27 may contribute to the loss of ocular tolerance and the manifestation of uveitis (Figure 3). Many may also be relevant for the loss of intestinal tolerance that accompanies several forms of SpA. These mechanisms are not mutually exclusive. Many have been discussed in a recent review 63 and summarized in Figure 3A. These B27-dependent mechanisms may impact the gut and/or the eye itself, where loss of tolerance could contribute to uveitogenesis.
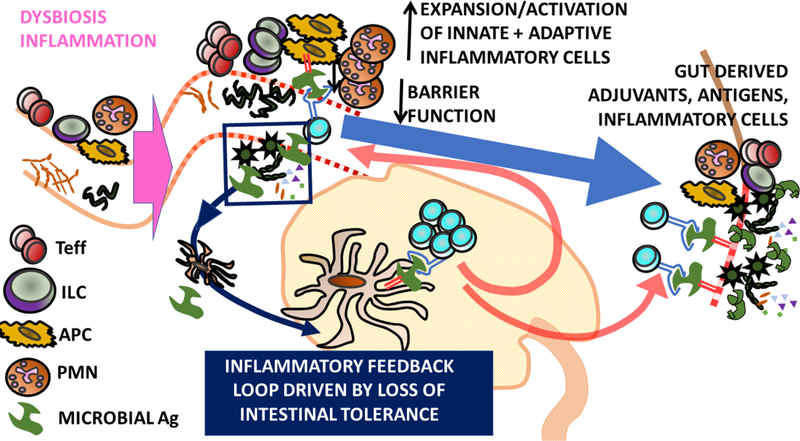
Figure 3. B27-and gut-associated mechanisms that may contribute to AAU pathogenesis.
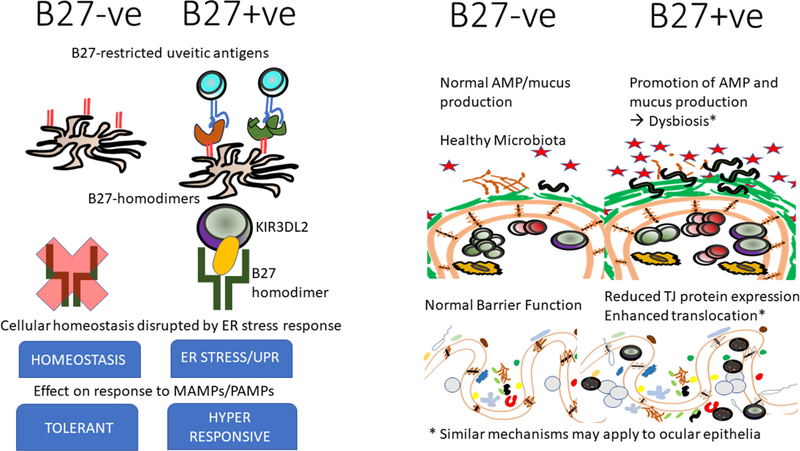
a) HLA B27-associated mechanisms. Figure shows HLA B27-associated changes that may accompany HLA B27 expression either at the cellular (left side) or tissue (right side) level that may predispose HLA-B27 +ve individuals to a loss of gut and/or ocular tolerance.
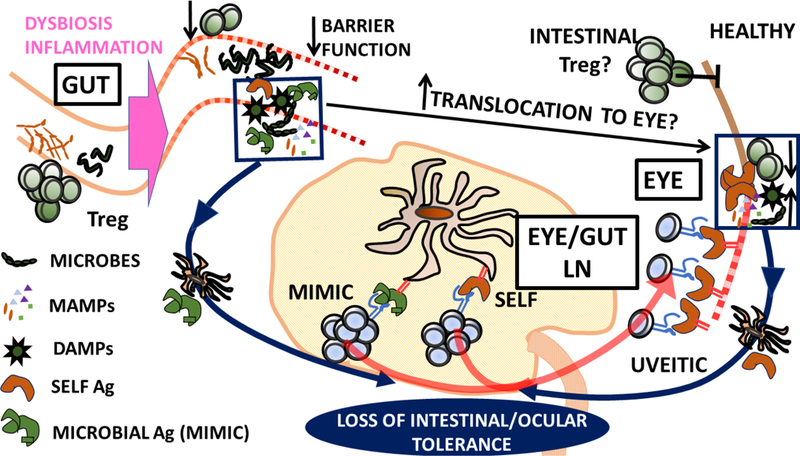
b) Self-Ag dependent model of uveitis. Dysbiosis leads to gut inflammation. Reduction of intestinal Treg that may normally contribute to ocular homeostasis or dissemination of intestinal microbes/MAMPs/DAMPs (arising from tissue damage and increased permeability) that act as adjuvants in the eye may result. Loss of ocular immune privilege leads to activation of uveitic self-reactive T cells in ocular draining LN. Loss of gut tolerance may also drive activation of T cells reactive to microbial antigens with mimicry to self in gut draining LN. These T cells subsequently target self-Ag in the eye.
c) Self-Ag independent model of uveitis. Dysbiosis and/or intestinal inflammation leads to the expansion of intestinal effector immune cells, e.g. conventional and non-conventional effector T cells (Teff), polymorphonuclear cells (PMN), innate lymphoid cells (ILC) and antigen presenting cells (APC). This developing inflammatory environment may be exacerbated by the loss of intestinal tolerance and expansion of microbially-reactive T cells which drive an inflammatory feedback loop. Diminished barrier function may promote dissemination of microbial products from gut to eye. Activation/Expansion of inflammatory immune cells in the intestine may promote their recirculation and potential migration to the eye. Antigen presentation of microbial antigens in the eye may further drive inflammation.
Note by no means are the two models proposed mutually exclusive.
Abbreviations: Ag, antigen; DAMP, damage-associated molecular pattern; LN; lymph node; MAMP, microbe-associated molecular pattern; Treg. regulatory T cell.
Several models can be proposed where the intestinal microbiome is a key component of AAU pathogenesis. First, the microbiome could induce a non-specific loss of gut tolerance that ultimately leads to inflammatory responses to ocular self-antigen (included in Figure 3B). A few general examples of broad mechanisms that may promote loss of tolerance include that gut bacteria regulate the number of FoxP3 + cells in peripheral blood 64, the number of T cells which produce interleukin (IL)-10 65, and the number of Th17 cells 66. Klebsiella cultured from the microbiome of a patient with Crohn’s disease can induce helper T cells 67. A reduction in regulatory T cells that are either FoxP3 positive or which synthesize IL-10 could predispose to immune-mediated inflammation. Similarly, an increase in Th17 T cells could easily predispose to immune-mediated disease. The microbiome also affects multiple populations of non-conventional lymphoid cells which reside in the gut including NK cells and other innate lymphoid cells, intraepithelial lymphocytes, gamma delta T cells and mucosal associated invariant T cells (MAIT cells) 63. Several of these cells types have been implicated in spondyloarthritis 68,69 and some have been implicated in models of uveitis 70,71. Our own group has used the EAU (experimental autoimmune uveitis) model to show that the disease can be markedly ameliorated by reducing gut bacteria with broad spectrum oral antibiotics 72. The antibiotic treatment results in an increase in Fox P3+ T cells in the cervical lymph node, mesenteric lymph node, spleen and retina at selected time points. Similarly, there is a transient increase in TH17 T cells in the cervical lymph node. Since this is a model of posterior and not anterior uveitis, extrapolations to acute anterior uveitis should be made cautiously.
Second, a bacterial antigen could mimic an autoantigen (included in Figure 3B). Rheumatic fever 73 and Guillain Barre syndrome 74 are thought to be immune-mediated diseases triggered by bacterial or viral mimicry. Endogenous peptides presented by the shared epitope that predisposes to rheumatoid arthritis have marked homology to amino acids in bacteria which include Prevotella 75. A single T cell receptor has some plasticity, i.e. an ability to recognize diverse peptides, some of which could cross-react with self-peptides 76. Although mimicry between HLA B27 and a bacterial antigen has been reported 77,78, these reports are more than 20 years old and have not progressed to explain the tissue distribution of B27-related disease. Caspi and colleagues have described a model of posterior uveitis mediated by transgenic T cells that recognize the retinal antigen, IRBP (inter-photoreceptor binding protein). In this model, her studies indicate that a bacterial antigen from the intestine activates these T cells presumably through antigenic mimicry 79. This paradigm, however, might not apply to HLA B27-associated anterior uveitis for which autoantibodies and autoreactive T cells (common markers of autoimmunity) are not generally considered to be markers of disease.
Third, a change in gut bacteria could alter intestinal permeability (see Figure 3C). This would allow bacterial products to disseminate. If these products lodge in the synovium or the uvea, they could trigger either an innate and/or adaptive immune response. Observations that support this hypothesis include: intestinal permeability is increased in ankylosing spondylitis 80 and bacterial products have been detected in synovial fluid from the joint in ankylosing spondylitis 81 and the other B27-related arthritis, reactive arthritis 29,82. A small prior study from Tunisia published in 2008 has also previously reported bacterial products in synovium from patients with reactive arthritis 83. This study did not include ankylosing spondylitis patients nor did it use technology as precise as the current state of the art. Bacterial translocation to the blood has been documented in Crohn’s disease 84 and the subset of patients who demonstrate this translocation are more likely to have flares of disease activity 84. Gonococcal arthritis is an example of the synovium acting as a targeted site for bacterial dissemination 85. Increased bacterial cell wall has been detected by immunostaining in the joints of patients with rheumatoid arthritis 86. Bacterial translocation to the joint is consistent with our own unpublished observations in the HLA B27 positive transgenic rat. It is important to note, however, that translocation of bacterial products has been noted in healthy individuals such as marathon runners 87 or as a potential result from brushing one’s teeth 88
Interestingly, an intriguing recent study by Atarashi and colleagues demonstrated that oral bacteria isolated from IBD patients potentiate inflammatory responses if they colonize the intestine 67. Moreover, colonization of the liver by the intestinal pathobiont Enterococcus gallinarum may contribute to the pathogenesis of systemic lupus erythematosus 89. This serves to reinforce the idea that should bacteria find themselves outside their normal habitat (examples include the eye or the joint) they may be relevant suspects in the search for etiological agents in uveitis. As mentioned, administration of microbial products such as LPS or β-glucan may potentiate uveitic inflammation. Tolerance or tachyphylaxis to these microbe associated molecular patterns might develop in the intestine. Indeed, given the formidable barriers that act to contain bacteria within the gut, and avoid translocation, such as the recently described gut-vascular barrier 90, it may indeed be microbial products rather than live or dead bacteria that reach distal sites such as the eye or the joint.
It is likely, however, that bacterial translocation is necessary but not sufficient to create arthritis. Jabri’s laboratory studied a mouse model with features that predispose to celiac disease including the appropriate human HLA molecules and a diet that included gluten 91. The animals do not develop features of autoimmune disease. However, if the animals are infected with a reovirus that by itself induces no symptoms in the mouse, autoantibodies to transglutaminase become detectable. This paradigm suggests that a “second hit” such as a viral infection might be necessary for bacteria to induce arthritis after translocation to a joint.
In contrast to the joint, we are not aware of any direct evidence that bacterial products are translocated to the anterior uveal tract during non-infectious, acute anterior uveitis. However, the adage that absence of evidence does not equate to evidence of absence may be particularly applicable. The eye is not biopsied except in rare instances during acute inflammation and the amount of fluid available for study from an anterior chamber is limited. This makes it challenging to study or detect bacterial products in the eye of these patients. We have demonstrated that even a footpad injection of bacterial endotoxin induces an acute anterior uveitis in rats 92. The rodent eye expresses a variety of TLR receptors and will become inflamed after direct injection of bacterial products 93. TLR2 receptors are down-regulated on leukocytes from patients with acute anterior uveitis, but these cells produce more interleukin 1 beta than controls in response to TLR2 activation 94. In patients with Crohn’s disease (as noted above) such translocation of bacterial products to blood has been demonstrated; thus, it seems highly plausible that some bacterial product(s) could also disseminate to the anterior uveal tract. In addition, host derived inflammatory products such as damage-associated molecular patterns (DAMPSs) could also be hypothesized to be translocated from the damaged intestine to the eye where they could also contribute to the local inflammatory cascade (Figure 3C).
If translocation is a critical component in the pathogenesis of uveitis, it is appropriate to ask why uveitis is not more common in bowel disease such as Crohn’s disease in comparison to ankylosing spondylitis? Although the answer to this question is not known, we offer two hypotheses. First, as discussed above, the marked increase in permeability in IBD could lead to a tachyphylaxis such that bacterial products were constantly exposed to the immune system and thus induced less inflammation. Second, HLA B27 is the major known genetic factor that predisposes to AAU. As HLA B27 is not associated with IBD, it makes sense that AAU is less common in IBD.
Finally, a fourth mechanism to explain the role of the gut microbiome in anterior uveitis is translocation of lymphocytes or perhaps other inflammatory cells from the gut to the eye (see Figures 3B and 3C). Using the EAU model and lymphocytes that express the pigment, kaede, which can be photoactivated, we and our colleagues have been able to demonstrate that there is indeed migration of lymphocytes from the bowel to the eye 95. Moreover, elegant recent studies have demonstrated that circulating lymphocytes with presumed intestinal origin (that are commensal reactive and express gut homing chemokine receptors) are a part of the normal circulating T cell pool, even in healthy individuals 96.
Clinical and Future Implications
Assuming that the microbiome does contribute to HLA B27-related uveitis and arthritis, its contribution is likely to be considerable if one can extrapolate from the effect of the germ-free state in the B27+ rats. This can lead one to envisage several future treatment modalities based upon the microbiome (see Figure 4). Such a goal might be simplified if a single bacterium is eventually implicated as the cause of HLA B27-related inflammation just as Helicobacter pylori is the culprit in peptic acid disease. But assuming that no single organism is the cause of B27-related inflammation, are the observations likely to be clinically applicable?
Figure 4. Strategies for therapeutic targeting of the intestinal microbiota.
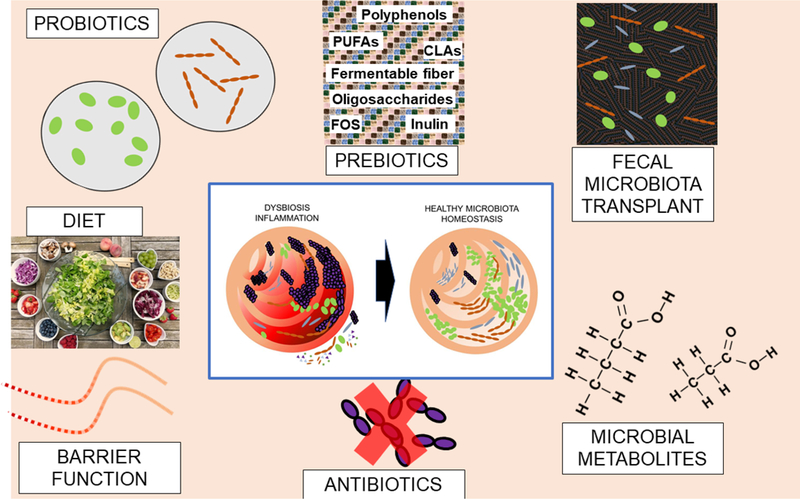
Selective depletion of harmful bacteria (purple) or recolonization with benign bacteria (green or brown) derived from probiotics or fecal microbiota transplant (FMT). Prebiotics may promote the growth of beneficial bacteria. Anti-inflammatory microbial metabolites (e.g. Short Chain Fatty Acids) might also be used to treat B27-associated gut inflammation. Diet is another method to modify the microbiota, and like FMT is likely to contain both probiotics and prebiotics. Finally targeting of intestinal permeability may be another way of attenuating HLA B27-associated inflammatory sequelae.
One option is fecal transplantation. This approach has had dramatic success to treat recalcitrant Clostridia difficile infection 97 and success as well in the treatment of ulcerative colitis 98,99. Obstacles to fecal transplantation include the aesthetics, the safety (since the approach could readily transmit viruses like cytomegalovirus 100 or conceivably prions), and the likely transient nature of the effect such that the transplant would presumably need to be repeated frequently.
An alternative approach could be to target microbial metabolites which are altered in HLA-B27 + rats and SpA patients or their downstream signaling pathways32,83. For instance, we have reported that short chain fatty acids (SCFAs) are reduced in the intestine from these rats 101 and we have shown that oral supplementation with the SCFA propionate, reduces the inflammation in both the cecum and the colon in the B27 + rats. This is accompanied by a reduction of critical inflammatory cytokines in intestinal tissue including IL-1 beta, IL-17 and interferon gamma 101. More recently we have also demonstrated that SCFAs can attenuate disease in an inducible model of experimental autoimmune uveitis 95.
Medications which reduce intestinal permeability could also be effective in preventing the dissemination of bacterial products. As noted above, sulfasalazine’s efficacy might relate in part to this effect 30. Mongersen, which antagonizes downstream effects of transforming growth factor beta, has also been proposed to ameliorate inflammatory bowel disease while reducing the permeability in the gut wall 102.
Most antibiotics are not likely to have sustained benefit to treat or prevent acute anterior uveitis since resistance should rapidly develop. Probiotics are a popular option, but little is objectively known about how successfully probiotics evade the effect of low gastric pH and successfully colonize the intestine. Diet can profoundly affect the microbiome and offers what is arguably the best hope to prevent attacks of acute anterior uveitis. The challenge is the complexity of diet which makes it very difficult to identify the components that need to be added or subtracted for sustained benefit.
Perinatal exposure to bacteria can have lifelong effects. For example, in mice, animals born by Caesarian section have immune system differences that persist into adulthood when compared to animals born trans-vaginally 103. In this regard, it is intriguing that patients with ankylosing spondylitis are reportedly less likely to be breast fed compared to controls 104. If the genetic approach can identify individuals at high risk for developing spondyloarthritis, it is possible that neonatal exposure to protective bacteria could have sustained benefit in the prevention of disease.
In addition to genetic approaches (e.g. HLA-B27 genotyping) that can be used to help establish disease risk, it is also tempting to speculate that the composition of the microbiota itself could prove clinically useful even if it cannot be successfully targeted therapeutically. To this end, if dysbiosis is a significant component of AAU pathogenesis it might be used to predict disease risk, time of onset, progression or treatment response.
Conclusions:
Just a decade ago, the intestinal microbiome could be considered a black box. Most of the organisms that constitute the intestinal microbiome are anaerobic and extremely difficult to culture. As the cost of DNA sequencing has fallen dramatically, it has become feasible to begin to characterize the bacteria with which we have a symbiotic relationship. But this advance is recent such that the microbiome remains in its infancy, or at best adolescence. We believe, however, that the microbiome will ultimately become an essential target in the treatment and prevention of immune-mediated diseases including uveitis.
The puzzle as to why HLA B27 predisposes to disease has resisted solution for more than four decades. The observation that HLA B27 predisposed to a disease came as a surprise. Ankylosing spondylitis was not the main target of the initial HLA and disease study; it was included as a control group for the main object of the study, rheumatoid arthritis. An even greater surprise, arguably, is the possibility that fecal bacteria cause ocular inflammation.
BOX 1. Why might the eye/joint be prone to inflammation but not other sites?
| Higher B27 expression than other tissue sites? |
| Unique mechanical stressors/connective tissue (e.g. entheses, lens/ciliary body)? |
| More prone to dysregulation of homeostatic pathways (ER stress, autophagy, Treg, etc..)? |
| More susceptible to deposition of MAMP/microbes? |
| More permissive to infection due to immune privileged location? |
| Tissue tropism of infectious agents? |
| Mucosal-like addressins make them more susceptible to infiltration by gut-derived immune cells? |
| Increased expression of vascular adhesion molecules that promote infiltration of inflammatory immune cells (these may or may not be gut derived). |
| NOTE these properties may be acting in concert with other factors (e.g. viral infection or other stress of target tissue) |
Key points.
Inflammation of the iris (iritis) and ciliary body (cyclitis) of the front portion (anterior) of the eye represents 85% of all uveitis cases.
Sudden onset or acute anterior uveitis (AAU) is strongly associated with HLA-B27 and a member of the wider SpA family. SpAs have been associated with an altered/dysbiotic intestinal microbiota.
Molecular mimicry of HLA-B27 restricted microbial antigens with self antigen expressed in ocular, synovial or other inflamed tissues in SpA is one proposed contributory pathogenic mechanism.
Intestinal inflammation and/or diminished barrier function have also been proposed to contribute to extra-intestinal disease sequelae by dissemination of microbes, microbial products or host immune cells to affected tissues.
HLA-B27 may contribute to loss of tolerance in affected tissue(s) through a number of mechanisms such as HLA-B27 homodimer formation or disruption of cellular homeostatic pathways through HLA-B27 misfolding/aggregation.
Therapeutic strategies that target the microbiota include pre-,pro- or anti-biotics, microbial metabolites, dietary modification or fecal microbiota transplantation (FMT).
Acknowledgments
Funding: This project was supported by NIH Grant EY 026572, EY029266, the Spondylitis Association of America, the William and Mary Bauman Foundation, the Stan and Madelle Family Trust, the Rheumatology Research Foundation, and Research to Prevent Blindness.
The authors wish to acknowledge many valuable contributions made by the research community that were omitted due to space to constraints.
Footnotes
Conflicts of Interest: Both Drs. Asquith and Rosenbaum have a collaboration with Open Biome
References
- 1.Brewerton DA, Caffrey M, Nicholls A, Walters D & James DC Acute anterior uveitis and HL-A 27. Lancet 302, 994–996 (1973). [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB The natural history of uveitis. Int Ophthalmol 14, 303–308 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Rothova A, Suttorp-van Schulten MS, Frits Treffers W & Kijlstra A Causes and frequency of blindness in patients with intraocular inflammatory disease. Br. J. Ophthalmol 80, 332–336 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suttorp-Schulten MS & Rothova A The possible impact of uveitis in blindness: a literature survey. Br. J. Ophthalmol 80, 844–848 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gritz DC & Wong IG Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 111, 491–500; discussion 500 (2004). [DOI] [PubMed] [Google Scholar]
- 6.D’Alessandro LP, Forster DJ & Rao NA Anterior uveitis and hypopyon. Am J Ophthalmol 112, 317–321 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum JT Characterization of uveitis associated with spondyloarthritis. J Rheumatol 16, 792–796 (1989). [PubMed] [Google Scholar]
- 8.Rosenbaum JT Uveitis. An internist’s view. Arch. Intern. Med 149, 1173–1176 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Fanlo P et al. Profile of patients with uveitis referred to a multidisciplinary unit in northern Spain. Arch Soc Esp Oftalmol 92, 202–209 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Zagora SL et al. Etiology and Clinical Features of Ocular Inflammatory Diseases in a Tertiary Referral Centre in Sydney, Australia. Ocul Immunol Inflamm 25, S107–S114 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Chung YM, Yeh TS & Liu JH Endogenous uveitis in Chinese--an analysis of 240 cases in a uveitis clinic. Jpn J Ophthalmol 32, 64–69 (1988). [PubMed] [Google Scholar]
- 12.Yang P et al. Clinical features of HLA-B27-positive acute anterior uveitis with or without ankylosing spondylitis in a Chinese cohort. Br. J. Ophthalmol 102, 215–219 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Juanola X, Loza Santamaria E, Cordero-Coma M & Group SW Description and Prevalence of Spondyloarthritis in Patients with Anterior Uveitis: The SENTINEL Interdisciplinary Collaborative Project. Ophthalmology 123, 1632–1636 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Haroon M, O’Rourke M, Ramasamy P, Murphy CC & FitzGerald O A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: the DUET (Dublin Uveitis Evaluation Tool). Ann Rheum Dis 74, 1990–1995 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum JT Uveitis: etiology, diagnosis, and treatment www.uptodate.com (2017).
- 16.Robinson PC et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol 67, 140–151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum J & Chandran V Management of comorbidities in ankylosing spondylitis. Am J Med Sci 343, 364–366 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Murray PI & Rauz S The eye and inflammatory rheumatic diseases: The eye and rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis. Best Pract Res Clin Rheumatol 30, 802–825 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Paiva ES, Macaluso DC, Edwards A & Rosenbaum JT Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis 59, 67–70 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons JL & Rosenbaum JT Uveitis associated with inflammatory bowel disease compared with uveitis associated with spondyloarthropathy. Arch Ophthalmol 115, 61–64 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Petty RE et al. Uveitis and arthritis induced by adjuvant: Clinical, immunologic and histologic characteristics. J. Rheumatol 16, 499–505 (1989). [PubMed] [Google Scholar]
- 22.Kezic JM, Davey MP, Glant TT, Rosenbaum JT & Rosenzweig HL Interferon-gamma regulates discordant mechanisms of uveitis versus joint and axial disease in a murine model resembling spondylarthritis. Arthritis Rheum 64, 762–771 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehaume LM et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol 66, 2780–2792 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Baggia S et al. A novel model of bacterially-induced acute anterior uveitis in rats and the lack of effect from HLA B27 expression. J. Invest. Med 45, 295–301 (1997). [PubMed] [Google Scholar]
- 25.De Vos M, Mielants H, Cuvelier C, Elewaut A & Veys E Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastro 110, 1696–1703 (1996). [DOI] [PubMed] [Google Scholar]
- 26.Mielants H, Veys EM, Joos R, Cuvelier C & De Vos M Repeat ileocolonoscopy in reactive arthritis. J Rheumatol 14, 456–458 (1987). [PubMed] [Google Scholar]
- 27.Vaile JH, Meddings JB, Yacyshyn BR, Russell AS & Maksymowych WP Bowel permeability and CD45RO expression on circulating CD20+ B cells in patients with ankylosing spondylitis and their relatives. J Rheumatol 26, 128–135 (1999). [PubMed] [Google Scholar]
- 28.Granfors K et al. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet 335, 685–688 (1990). [DOI] [PubMed] [Google Scholar]
- 29.Granfors K et al. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med 320, 216–221 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Wang F et al. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol 166, 409–419 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Box SA & Pullar T Sulphasalazine in the treatment of rheumatoid arthritis. Br J Rheumatol 36, 382–386 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Halfvarson J et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2, 17004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostic AD, Xavier RJ & Gevers D The microbiome in inflammatory bowel disease: current status and the future ahead. Gastro 146, 1489–1499 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pascal V et al. A microbial signature for Crohn’s disease. Gut 66, 813–822 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubinak JL et al. MHC variation sculpts individualized microbial communities that control susceptibility to enteric infection. Nat Commun 6, 8642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breban M et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis 76, 1614–1622 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Tito RY et al. Dialister as microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol (2016). [DOI] [PubMed]
- 38.Costello ME et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol (2014). [DOI] [PubMed]
- 39.Scher JU et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 67, 128–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal A, Sarangi AN, Gaur P, Shukla A & Aggarwal R Gut microbiome in children with enthesitis-related arthritis in a developing country and the effect of probiotic administration. Clin Exp Immunol 187, 480–489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Paola M et al. Alteration of Fecal Microbiota Profiles in Juvenile Idiopathic Arthritis. Associations with HLA-B27 Allele and Disease Status. Front Microbiol 7, 1703 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoll ML et al. Fecal metabolomics in pediatric spondyloarthritis implicate decreased metabolic diversity and altered tryptophan metabolism as pathogenic factors. Genes Immun 17, 400–405 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lederberg J ‘Ome Sweet’ Omics: A geneological treasury of words. The Scientist (2001).
- 44.Turnbaugh PJ et al. The human microbiome project. Nature 449, 804–810 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin J et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Xie L, Li Y & Wei C More than 9,000,000 unique genes in human gut bacterial community: estimating gene numbers inside a human body. PLoS One 4, e6074 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shreiner AB, Kao JY & Young VB The gut microbiome in health and in disease. Curr Opin Gastroenterol 31, 69–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osokine I & Erlebacher A Inflammation and Autism: From Maternal Gut to Fetal Brain. Trends Mol Med (2017). [DOI] [PMC free article] [PubMed]
- 49.Stevens BR et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut (2017). [DOI] [PMC free article] [PubMed]
- 50.Clayton TA, Baker D, Lindon JC, Everett JR & Nicholson JK Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci U S A 106, 14728–14733 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiina T, Inoko H & Kulski JK An update of the HLA genomic region, locus information and disease associations: 2004. Tissue Antigens 64, 631–649 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Jin P & Wang E Polymorphism in clinical immunology - From HLA typing to immunogenetic profiling. J Transl Med 1, 8 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammer RE, Maika SD, Richardson JA, Tang JP & Taurog JD Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell 63, 1099–1112 (1990). [DOI] [PubMed] [Google Scholar]
- 54.Taurog JD et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 180, 2359–2364 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dieleman LA et al. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut 52, 370–376 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asquith M et al. Perturbed mucosal immunity and dysbiosis accompany clinical disease in a rat model of spondyloarthritis. Arthritis Rheumatol (2016). [DOI] [PMC free article] [PubMed]
- 57.Olson JA et al. Calprotectin is raised in endogenous posterior uveitis. Ocul Immunol Inflamm 4, 91–98 (1996). [DOI] [PubMed] [Google Scholar]
- 58.Walscheid K et al. Elevated S100A8/A9 and S100A12 Serum Levels Reflect Intraocular Inflammation in Juvenile Idiopathic Arthritis-Associated Uveitis: Results From a Pilot Study. Invest. Ophthalmol. Vis. Sci 56, 7653–7660 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Kim DH et al. Fecal calprotectin as a non-invasive biomarker for intestinal involvement of Behcet’s disease. J Gastroenterol Hepatol 32, 595–601 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Olivares M et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 64, 406–417 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Gomez A et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One 7, e36095 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silverman M et al. Protective major histocompatibility complex allele prevents type 1 diabetes by shaping the intestinal microbiota early in ontogeny. Proc Natl Acad Sci U S A 114, 9671–9676 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belkaid Y & Harrison OJ Homeostatic Immunity and the Microbiota. Immunity 46, 562–576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atarashi K et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu X, Zhang M, Yang X, Hong N & Yu C Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J Crohns Colitis 7, e558–568 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Wu HJ et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atarashi K et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciccia F et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis 74, 1739–1747 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Gracey E et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis 75, 2124–2132 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Grajewski RS et al. Activation of invariant NKT cells ameliorates experimental ocular autoimmunity by a mechanism involving innate IFN-gamma production and dampening of the adaptive Th1 and Th17 responses. J Immunol 181, 4791–4797 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Y et al. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol 183, 560–567 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura YK et al. Gut Microbial Alterations Associated With Protection From Autoimmune Uveitis. Invest. Ophthalmol. Vis. Sci 57, 3747–3758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cunningham MW Streptococcus and rheumatic fever. Curr Opin Rheumatol 24, 408–416 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahrizaila N & Yuki N Guillain-barre syndrome animal model: the first proof of molecular mimicry in human autoimmune disorder. J Biomed Biotechnol 2011, 829129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pianta A et al. Two rheumatoid arthritis-specific autoantigens correlate microbial immunity with autoimmune responses in joints. J Clin Invest 127, 2946–2956 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin Y & Mariuzza RA The multiple mechanisms of T cell receptor cross-reactivity. Immunity 31, 849–851 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Schwimmbeck PL & Oldstone MB Molecular mimicry between human leukocyte antigen B27 and Klebsiella. Consequences for spondyloarthropathies. Am J Med 85, 51–53 (1988). [DOI] [PubMed] [Google Scholar]
- 78.van Bohemen CG, Grumet FC & Zanen HC Identification of HLA-B27M1 and -M2 cross-reactive antigens in Klebsiella, Shigella and Yersinia. Immunology 52, 607–610 (1984). [PMC free article] [PubMed] [Google Scholar]
- 79.Horai R et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 43, 343–353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mielants H, Veys EM, De Vos M & Cuvelier C Increased intestinal permeability in ankylosing spondylitis. Gut 33, 1150 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pacheco-Tena C et al. Bacterial DNA in synovial fluid cells of patients with juvenile onset spondyloarthropathies. Rheumatology (Oxford, England) 40, 920–927 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Nikkari S et al. Salmonella-triggered reactive arthritis: use of polymerase chain reaction, immunocytochemical staining, and gas chromatography-mass spectrometry in the detection of bacterial components from synovial fluid. Arthritis Rheum 42, 84–89 (1999). [DOI] [PubMed] [Google Scholar]
- 83.Siala M et al. Analysis of bacterial DNA in synovial tissue of Tunisian patients with reactive and undifferentiated arthritis by broad-range PCR, cloning and sequencing. Arthritis Res Ther 10, R40 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gutierrez A et al. Gut Bacterial DNA Translocation is an Independent Risk Factor of Flare at Short Term in Patients With Crohn’s Disease. Am J Gastroenterol 111, 529–540 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Muralidhar B, Rumore PM & Steinman CR Use of the polymerase chain reaction to study arthritis due to Neisseria gonorrhoeae. Arthritis Rheum 37, 710–717 (1994). [DOI] [PubMed] [Google Scholar]
- 86.Schrijver IA, Melief MJ, Tak PP, Hazenberg MP & Laman JD Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum 43, 2160–2168 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Camus G et al. Mild endotoxaemia and the inflammatory response induced by a marathon race. Clin Sci (Lond) 92, 415–422 (1997). [DOI] [PubMed] [Google Scholar]
- 88.Bhanji S, Williams B, Sheller B, Elwood T & Mancl L Transient bacteremia induced by toothbrushing a comparison of the Sonicare toothbrush with a conventional toothbrush. Pediatr Dent 24, 295–299 (2002). [PubMed] [Google Scholar]
- 89.Manfredo Vieira S et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spadoni I et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Bouziat R et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356, 44–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenbaum JT, McDevitt HO, Guss RB & Egbert PR Endotoxin-induced uveitis in rats as a model for human disease. Nature 286, 611–613 (1980). [DOI] [PubMed] [Google Scholar]
- 93.Allensworth JJ, Planck SR, Rosenbaum JT & Rosenzweig HL Investigation of the differential potentials of TLR agonists to elicit uveitis in mice. J. Leukoc. Biol 90, 1159–1166 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang JH et al. Changes in Toll-like receptor (TLR)-2 and TLR4 expression and function but not polymorphisms are associated with acute anterior uveitis. Invest. Ophthalmol. Vis. Sci 48, 1711–1717 (2007). [DOI] [PubMed] [Google Scholar]
- 95.Nakamura YK et al. Short chain fatty acids ameliorate immune-mediated uveitis potentially by altering migration of lymphocytes from the intestine. Sci Rep accepted (2017). [DOI] [PMC free article] [PubMed]
- 96.Hegazy AN et al. Circulating and Tissue-Resident CD4+ T Cells With Reactivity to Intestinal Microbiota Are Abundant in Healthy Individuals and Function Is Altered During Inflammation. Gastro 153, 1320–1337 e1316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Nood E et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368, 407–415 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Paramsothy S et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Moayyedi P et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 149, 102–109 e106 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Hohmann EL, Ananthakrishnan AN & Deshpande V Case Records of the Massachusetts General Hospital. Case 25–2014. A 37-year-old man with ulcerative colitis and bloody diarrhea. N Engl J Med 371, 668–675 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Asquith M et al. Intestinal Metabolites Are Profoundly Altered in the Context of HLA-B27 Expression and Functionally Modulate Disease in a Rat Model of Spondyloarthritis. Arthritis Rheumatol June 16. doi: 10.1002/art.40183 [Epub ahead of print] (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monteleone G et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med 372, 1104–1113 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Hansen CH et al. Mode of delivery shapes gut colonization pattern and modulates regulatory immunity in mice. J Immunol 193, 1213–1222 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Montoya J et al. Patients with ankylosing spondylitis have been breast fed less often than healthy controls: a case-control retrospective study. Ann Rheum Dis 75, 879–882 (2016). [DOI] [PubMed] [Google Scholar]


