Abstract
Background
In Africa, peanuts are frequently consumed, but severe allergic reactions are rare. We investigated immunological patterns of clinical tolerance to peanut in peanut-sensitized but asymptomatic patients from central Africa compared to peanut-allergic and peanut-sensitized but asymptomatic patients from Sweden.
Methods
Sera from allergic patients (n = 54) from Zimbabwe sensitized to peanut but without allergic symptoms to peanut, and sera from peanut-allergic (n = 25) and peanut-sensitized but asymptomatic (n = 25) patients from Sweden were analyzed toward peanut allergen components (Ara h 1–3, 6, 8–9) and other allergen molecules from important allergen sources using microarray. IgE to Ara h 2 peptide epitopes was analyzed, and allergenic activity was assessed by basophil activation assay.
Results
Forty-six percent of the African and all peanut-allergic Swedish patients showed IgE toward one of the highly allergenic peanut allergens (Ara h 1–3, 6, 9). However, 48% of the African patients had IgE to cross-reactive carbohydrate determinants (CCDs) with low allergenic activity and 60% of the Swedish asymptomatic patients had IgE against the PR protein Ara h 8. IgG and IgG4 specificities and levels could not discriminate between the African asymptomatic and Swedish peanut-allergic patients. Asymptomatic patients almost lacked IgE to Ara h 2 peptides, which were recognized by peanut-allergic patients. Peanut IgE from peanut asymptomatic patients showed poor allergenic activity compared with IgE from peanut-allergic patients.
Conclusions
Natural clinical tolerance to peanut in the African patients can be caused by IgE to low allergenic peanut components and by poor allergenic activity of peanut-specific IgE.
Keywords: Africa, allergen component, allergy, peanut, tolerance
Peanut, Arachis hypogea, is one of the most allergenic vegetable foods in the Western world that may cause mild to life-threatening allergic reactions. However, peanut allergy has rarely been reported from Africa, the Middle East, and South-East Asia, where peanut is consumed in high amounts (1–3). Particularly in West Africa, peanut constitutes a significant cash crop (4).
The major peanut allergens, Ara h 1 (5), Ara h 2 (6), and Ara h 3 (7), are members of the seed storage protein families and are associated with clinical reactivity. The 2S albumin Ara h 2 has been considered the clinically most important allergen in this context (8). The 2S albumin Ara h 6 (9) is very similar to Ara h 2. Ara h 8 (10) belongs to the pathogenesis-related protein family (PR)-10 and is a major allergen in patients with combined sensitisation to birch pollen and peanut. Occurrence of IgE to Ara h 8 is usually associated with very mild or no symptoms at all (11, 12). Ara h 9 (13) belongs to the nonspecific lipid transfer proteins (nsLTPs) and seems to be of clinical importance particularly in the Mediterranean area (14). All these peanut components have improved the diagnosis of peanut allergy (15, 16).
Conflicting results have been reported in studies investigating the role of specific IgG in clinical food tolerance. High IgG4 levels to cow's milk have been found to be associated with maintenance of clinical tolerance to cow's milk in atopic children and adults (17). On the other hand, in peanut-allergic children, increased peanut-specific IgG4 levels have been associated with clinical reactivity (18). Recently, it was reported that peanut-specific IgG levels are elevated in peanut-sensitized children especially in those avoiding peanuts (19). Using a chip containing a variety of microarrayed allergens, among them a comprehensive panel of peanut allergens, plant allergens involved in IgE cross-reactivity, and allergen markers for sensitization to carbohydrates (20), we analyzed a group of patients from Zimbabwe in central Africa who despite IgE sensitization to peanut allergen extracts regularly ate peanuts without ever showing clinical symptoms and compared them with doctor-diagnosed peanut-allergic and peanut-sensitized but asymptomatic patients from Sweden. We also determined allergen-specific IgG and IgG4 reactivity to the microarrayed allergens. In addition, we analyzed the IgE recognition to Ara h 2 peptide epitopes. The allergenic activity of peanut IgE was investigated to elucidate possible mechanisms of natural clinical tolerance in African patients compared with peanut-allergic and peanut-sensitized but asymptomatic patients from Europe.
Methods
Demographic and clinical characterization of the study populations
Sera from 54 allergic patients from Harare, Zimbabwe, who had peanut-specific IgE reactivity (IQR 0.64–10.20 kUA/l; Table 1) but regularly ate peanuts without ever having suffered from any peanut-related allergic symptoms were studied. For comparison, a group of peanut-allergic patients (n = 25) and a group of peanut-sensitized but asymptomatic patients (n = 25) from Sachs' Children's Hospital, Stockholm, Sweden, were enrolled as controls (Table 1). For details, see online supporting information.
Table 1. Demographic and clinical characterization of peanut-sensitized patients from Africa and Sweden, as well as Swedish peanut tolerant patients.
| Zimbabwe tolerant n = 54 | Sweden tolerant n = 25 | Sweden allergic n = 25 | |
|---|---|---|---|
| Age, years: median (range) | 11 (0.9–59) | 11 (3–18) | 9 (3–15) |
| Sex (m/f) | (31/23) | (13/12) | (13/12) |
| Skin prick test or IgE positive | |||
| HDM (n) | 34 | 3 | 1 |
| Pollens (n) | 35 | 25 | 18 |
| Animals (n) | 16 | 15 | 13 |
| Food (n) | 32 | 24 | 12 |
| Peanut IgE (kUA/l) median (range) | 2.5 (0.1–200) | 2.8 (0.23–17) | 280 (28–1300) |
| Total IgE (kU/l) median (range) | 520.5 (23–20000) | 290 (33.9–2398) | 647 (105–4096) |
| Asthma (n /%) | 15 (28) | 18 (72) | 18 (72) |
| Rhinitis/Conjunctivitis (n /%) | 28 (52) | 22 (88) | 12 (48) |
| Skin symptoms*(n /%) | 39 (72) | 9 (36) | 12 (48) |
| GI symptoms†(n /%) | 5 (9) | 2 (8) | 2 (8) |
Skin prick test (SPT) positive ≥3 mm. Peanut IgE ≥ 0.1 kUA/l. Total IgE ≥ 2 kU/l. (n) number of patients, (n /%) number and percentage of patients.
Skin symptoms: dermatitis, eczema, urticaria, angioedema.
GI symptoms: vomiting, bloating, abdominal discomfort.
Measurement of total IgE and allergen-specific IgE, IgG, and IgG4
ImmunoCAP
Total IgE and allergen-specific IgE against peanut extract (f13) were measured with the ImmunoCAP System (Thermo Fisher/Phadia AB, Uppsala, Sweden). The cutoffs were 2 kU/l for total IgE and ≥0.10 kUA/l for allergen-specific IgE.
Microarray
Serum samples were analyzed for IgE and IgG reactivity to microarrayed allergen components using a customized allergen chip based on ISAC technology (Thermo Fisher/Phadia AB) developed in the MeDALL FP7-funded research program.19 For details, see online supporting information.
Ara h 2 peptide synthesis
Eight overlapping synthetic peptides with a length of 22–25 aa (Table S1) covering Ara h 2 were synthesized. For details, see online supporting information.
Dot blot assay of Ara h 2 peptides
Information is provided in the online supporting information.
Basophil activation assays and IgG depletion
Rat basophil leukemia (RBL) assays were performed as previously described (21). For details, see online supporting information.
Ethical aspects
The analysis of patient sera was approved by the local ethics committee of the University of Zimbabwe Medical School, Harare, Zimbabwe, and the Ethical Review Board at Karolinska Institutet, Stockholm, Sweden. Residual serum samples from the routine allergy diagnosis were analyzed in an anonymized manner with permission of the ethical committee of the Medical University of Vienna.
Statistical analysis
The Mann–Whitney U-test was used to evaluate differences between two groups, and P-values <0.05 were considered significant. The Spearman rank correlation coefficient r was used to assess correlations between parameters, significance level (P < 0.01). Data were analyzed using IBM SPSS statistics software version 20 (Chicago, IL, USA) and Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Several of the African peanut-sensitized but asymptomatic patients show high peanut allergen-specific IgE levels as well as reactivity to peanut allergen components associated with severe clinical symptoms
Although the median IgE levels to peanut extract were modest (2.5 kUA/l; IQR 0.64–10.20 kUA/l), ten patients showed peanut allergen-specific IgE levels above 15 kUA/l, a threshold level which is commonly associated with generalized symptomatic reactions (Table 2) (22). Forty-six percent of the asymptomatic patients reacted with peanut allergen components usually associated with systemic reactions to peanut, for example, rAra h 1–3, nAra h 6, and rAra h 9 (Table 2, Fig. 1A,B). The most frequently recognized peanut allergen component was rAra h 1 (16/54). Twenty-four percent of the patients had IgE antibodies against rAra h 2 followed by the peanut LTP rAra h 9 (9/54), nAra h 6 (5/54), and rAra h 3 (3/54). Only one patient showed IgE reactivity to the Bet v 1-homologous peanut allergen rAra h 8 from the PR-10 allergen family. IgE sensitizations to the highly cross-reactive family of plant profilins, which are also present in peanut, were observed in 37% of the patients (Table 2). IgE sensitization to highly glycosylated plant allergens (e.g., MUXF3 (23), nCyn d 1 (24), nPhl p 4 (25), nCry j 1 (26), and nCup a 1 (27), Table 2) was very common. However, it was not correlated with IgE to peanut extract (data not shown). All but seven of the 54 patients mounted IgE reactivity against at least one of these carbohydrate marker allergens.
Table 2. Heat map of IgE reactivities to peanut extract, peanut allergen components, plant profilins, and carbohydrate markers. Ranges of allergen-specific IgE levels are indicated.
| Asymptomatic Africa |
peanut | Profilins | CCD | ß-hexosaminidase release in RBL assay (allergen concentration 0.01 μg/ml) (%) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ImmunoCAP Peanut IgE |
rAra h 1 | rAra h 2 | rAra h 3 | nAra h 6 | rAra h 8 | rAra h 9 | rBet v 2 | rHev b 8 | rMer a 1 | rPhl p 12 | rPru du 4 | rTri a 12 | nCry j 1 | nCyn d 1 | nCup a 1 | MUXF3 | nPhl p 4 | ||
| 1 | 200.00 | 8.36 | 13.38 | ≤0.35 | 7.87 | ≤0.35 | 4.81 | 27.00 | 118.81 | 81.14 | 5.96 | 32.87 | 17.99 | 18.54 | 131.39 | 44.68 | 4.43 | 25.38 | 6.64 |
| 2 | 190.00 | 0.40 | 0.49 | ≤0.35 | ≤0.35 | ≤0.35 | 12.38 | 23.02 | 106.53 | 114.28 | 40.42 | 110.21 | 108.89 | 30.02 | 134.79 | 88.01 | 4.65 | 38.98 | 3.64 |
| 3 | 79.00 | ≤0.35 | 48.38 | 2.95 | 51.62 | ≤0.35 | ≤0.35 | 72.33 | 117.48 | 120.09 | 14.63 | 71.72 | 50.47 | 12.29 | 137.52 | 50.52 | 9.45 | 40.71 | 0.00 |
| 4 | 30.00 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.47 | ≤0.35 | 2.10 | 0.78 | ≤0.35 | 0.43 | ≤0.35 | 0.65 | 130.98 | 34.30 | 3.05 | 13.06 | 0.00 |
| 5 | 28.00 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 6.75 | 36.56 | 18.21 | ≤0.35 | 12.04 | 1.49 | 2.90 | 129.03 | 12.71 | 1.48 | 12.66 | 1.47 |
| 6 | 28.00 | ≤0.35 | 19.84 | 2.70 | 13.30 | ≤0.35 | 5.47 | 18.83 | 39.62 | 35.34 | 1.55 | 14.61 | 4.92 | 0.67 | 90.76 | 3.47 | ≤0.35 | 1.88 | 1.84 |
| 7 | 18.60 | 0.43 | 3.08 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 19.38 | 70.24 | 51.07 | 3.55 | 39.27 | 14.55 | 22.32 | 91.35 | 43.04 | ≤0.35 | 6.39 | 1.94 |
| 8 | 18.40 | 0.36 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 7.09 | ≤0.35 | 1.47 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 9.31 | 133.26 | 31.59 | 0.89 | 17.16 | 3.11 |
| 9 | 17.00 | 0.45 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.25 | ≤0.35 | 0.45 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 6.96 | 55.69 | 23.61 | 0.80 | 13.67 | 1.44 |
| 10 | 15.80 | 0.48 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.56 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 104.71 | 6.53 | ≤0.35 | 12.38 | 2.15 |
| 11 | 13.80 | 13.00 | 0.82 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 4.01 | 23.85 | 10.78 | ≤0.35 | 10.18 | 1.97 |
| 12 | 12.60 | 0.40 | 0.42 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.78 |
| 13 | 12.00 | 0.53 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.38 | 41.30 | 4.38 | 1.56 | 24.81 | 4.36 |
| 14 | 9.60 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.56 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.11 | 4.52 | 3.92 | 0.62 | 2.68 | 0.00 |
| 15 | 8.40 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 6.34 | 42.05 | 19.64 | ≤0.35 | 20.36 | 10.34 | ≤0.35 | 134.63 | 11.44 | ≤0.35 | 1.30 | 3.28 |
| 16 | 7.00 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.47 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 8.74 | 21.17 | 36.88 | 1.51 | 9.55 | 1.43 |
| 17 | 5.80 | ≤0.35 | 0.76 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 142.30 | 14.31 | ≤0.35 | ≤0.35 | 2.63 |
| 18 | 5.30 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.13 | ≤0.35 | ≤0.35 | 1.44 | 0.00 |
| 19 | 4.70 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.53 | 2.03 | 1.39 | ≤0.35 | 0.51 | ≤0.35 | 2.66 | 33.20 | 20.02 | 1.74 | 5.63 | 1.53 |
| 20 | 4.60 | 3.21 | 0.61 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.50 | 6.01 | 3.31 | ≤0.35 | 1.03 | 2.25 | 0.52 | 99.98 | 1.82 | ≤0.35 | 3.93 | 1.20 |
| 21 | 4.10 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.70 | 3.44 | 2.12 | 0.67 | 2.96 | 0.00 |
| 22 | 4.00 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.17 | 6.43 | 5.59 | ≤0.35 | 1.96 | ≤0.35 | ≤0.35 | 63.69 | 15.57 | 0.63 | 2.42 | 0.00 |
| 23 | 4.00 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.78 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.28 | 93.82 | 16.88 | 1.55 | 3.88 | 1.24 |
| 24 | 3.80 | 1.24 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.84 | ≤0.35 | ≤0.35 | 1.57 | 2.14 |
| 25 | 3.70 | 1.02 | 0.48 | ≤0.35 | 0.37 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 7.03 | 0.89 | ≤0.35 | 3.62 | 0.00 |
| 26 | 3.40 | 0.53 | ≤0.35 | 0.63 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.40 | 13.35 | 1.42 | 2.38 | 8.25 | 0.00 |
| 27 | 2.70 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 4.77 | 29.80 | 26.35 | 1.50 | 6.07 | 0.00 |
| 28 | 2.30 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 7.61 | 7.05 | 23.40 | 1.04 | 3.59 | 1.57 |
| 29 | 2.20 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 3.12 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.46 |
| 30 | 1.90 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 4.79 | 6.24 | 16.34 | 2.06 | 4.41 | 0.00 |
| 31 | 1.90 | 0.45 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.47 | 1.53 | 1.30 | ≤0.35 | 1.13 | 1.80 |
| 32 | 1.66 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.12 |
| 33 | 1.40 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.58 | 4.11 | 0.66 | 0.61 | 3.12 | 0.71 |
| 34 | 1.24 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 6.05 | 133.86 | 16.78 | ≤0.35 | 7.53 | 1.63 |
| 35 | 0.98 | ≤0.35 | 0.36 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.48 | ≤0.35 | ≤0.35 | 0.43 | 1.92 |
| 36 | 0.88 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.60 | 1.90 | 1.41 | ≤0.35 | 2.21 | 1.78 |
| 37 | 0.84 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.59 | ≤0.35 | ≤0.35 | 0.36 | 0.72 |
| 38 | 0.80 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 9.87 | 136.23 | 42.93 | ≤0.35 | 3.03 | 0.98 |
| 39 | 0.78 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.88 | 19.73 | 5.94 | 0.37 | 1.05 | 0.00 |
| 40 | 0.69 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.22 | 2.23 | 1.23 | ≤0.35 | 1.46 | 0.00 |
| 41 | 0.66 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.71 | 139.12 | 4.25 | ≤0.35 | 4.73 | 2.15 |
| 42 | 0.56 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.08 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.75 |
| 43 | 0.50 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.36 | 76.11 | 24.11 | ≤0.35 | 2.48 | 3.29 |
| 44 | 0.47 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.59 | 19.64 | 4.58 | ≤0.35 | 0.67 | n.d. |
| 45 | 0.43 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.60 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 126.62 | 4.76 | ≤0.35 | 2.39 | 1.97 |
| 46 | 0.42 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.93 |
| 47 | 0.42 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 75.80 | 4.19 | ≤0.35 | 3.30 | 2.34 |
| 48 | 0.41 | ≤0.35 | 0.69 | ≤0.35 | 0.72 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.71 | ≤0.35 | ≤0.35 | ≤0.35 | 0.00 |
| 49 | 0.36 | 0.65 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.43 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 4.44 |
| 50 | 0.30 | ≤0.35 | 0.42 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.42 |
| 51 | 0.30 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.55 | ≤0.35 | ≤0.35 | 1.97 |
| 52 | 0.26 | 2.96 | ≤0.35 | ≤0.35 | ≤0.35 | 1.86 | 1.66 | 1.30 | 3.52 | 2.36 | 1.04 | 3.93 | ≤0.35 | 0.99 | 43.77 | 3.01 | ≤0.35 | 2.05 | 1.34 |
| 53 | 0.20 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.37 | ≤0.35 | 0.71 | ≤0.35 | ≤0.35 | 4.45 |
| 54 | 0.10 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.63 | 4.65 | 3.15 | ≤0.35 | 3.98 | 2.34 |
| Median | 2.50 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.62 | 20.45 | 4.22 | ≤0.35 | 2.82 | 1.63 |
| Upper quartile | 10.20 | 0.40 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.82 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 3.18 | 101.20 | 17.67 | 0.93 | 6.68 | 2.34 |
| Lower quartile | 0.64 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.81 | 0.63 | ≤0.35 | 0.61 | 0.71 |
| 55 | 1300 | 26.31 | 22.65 | 2.28 | 14.4 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.56 | 1.04 | 0.9 | ≤0.35 | 1.94 | 11.39 |
| 56 | 770 | 49.95 | 40.51 | 2.72 | 11.14 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.52 | 20.86 |
| 57 | 660 | 100.5 | 40.23 | 5.09 | 28.87 | 5.37 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.99 | 0.46 | 7.45 | ≤0.35 | 1.64 | 13.34 |
| 58 | 420 | 8.99 | 1.43 | 0.63 | 6.13 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 16.70 |
| 59 | 400 | 20.71 | 6.16 | 0.78 | 9.94 | ≤0.35 | ≤0.35 | ≤0.35 | 0.43 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.46 | 0.47 | 0.94 | ≤0.35 | 2.14 | 9.93 |
| 60 | 400 | 19.37 | 4.68 | 4.15 | 9.12 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 17.60 |
| 61 | 380 | 72.71 | 13.63 | 7.6 | 12.46 | 12.91 | ≤0.35 | 2.71 | 16.29 | 10.07 | 1.49 | 13.58 | 1.87 | ≤0.35 | 1.33 | ≤0.35 | ≤0.35 | 9.18 | 16.68 |
| 62 | 380 | 35.31 | 23.21 | 5.36 | 17.05 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.38 | ≤0.35 | ≤0.35 | 0.62 | 19.18 |
| 63 | 370 | 65.8 | 28.21 | 3.02 | 9.51 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 4.92 | ≤0.35 | ≤0.35 | ≤0.35 | 10.80 |
| 64 | 350 | 2.68 | 1.99 | 4.49 | 14.51 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.44 | ≤0.35 | ≤0.35 | ≤0.35 | 2.09 |
| 65 | 320 | 3.81 | 9.18 | 1.98 | 4.61 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 23.59 |
| 66 | 300 | 23.04 | 8.54 | 4.2 | 7.9 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 15.26 |
| 67 | 280 | 34.02 | 21.36 | 1.45 | 17.75 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 10.35 |
| 68 | 270 | 58.84 | 9.12 | 4.69 | 15.36 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 18.37 |
| 69 | 250 | 38.11 | 15.44 | ≤0.35 | 6.41 | 0.42 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 22.36 | 0.78 | ≤0.35 | ≤0.35 | 4.04 |
| 70 | 140 | 51.64 | 10.09 | ≤0.35 | 8.04 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 4.12 | ≤0.35 | ≤0.35 | 2.85 | 14.96 |
| 71 | 98 | 34.03 | 7.73 | 0.63 | 2.06 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.68 | 2.88 | 2.78 | ≤0.35 | 1.35 | 7.93 |
| 72 | 98 | 30.1 | 4.87 | 0.69 | 2.36 | ≤0.35 | ≤0.35 | 0.53 | 3.81 | 2.49 | 0.54 | 2.62 | 0.81 | ≤0.35 | 0.57 | ≤0.35 | ≤0.35 | ≤0.35 | 9.29 |
| 73 | 95 | 13.23 | 1.72 | 0.59 | 2.48 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.74 | ≤0.35 | ≤0.35 | ≤0.35 | 7.41 |
| 74 | 75 | 9.79 | 2.91 | 1.34 | 4.39 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.81 | ≤0.35 | ≤0.35 | ≤0.35 | 4.50 |
| 75 | 71 | 15.66 | 4.43 | 2.31 | 7.66 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.32 | ≤0.35 | ≤0.35 | 0.92 | 13.30 |
| 76 | 71 | 10.82 | 10.51 | 10.56 | 2.62 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 10.47 |
| 77 | 67 | 8.8 | 25.48 | 0.75 | 25.03 | 1.43 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 8.32 |
| 78 | 60 | 67.55 | 9.75 | 4.59 | 14.38 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 4.9 | ≤0.35 | ≤0.35 | ≤0.35 | 13.77 |
| 79 | 28 | 2.39 | 0.61 | ≤0.35 | 2.41 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 5.14 | 1.63 |
| Median | 280 | 26.31 | 9.18 | 2.28 | 9.12 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.46 | ≤0.35 | ≤0.35 | ≤0.35 | 11.39 |
| Upper quartile | 390 | 50.8 | 22.01 | 4.54 | 14.46 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.57 | ≤0.35 | ≤0.35 | 1.5 | 16.69 |
| Lower quartile | 85 | 10.31 | 4.55 | 0.66 | 4.5 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 8.13 |
| ImmunoCAP (kUA/l) | ≤0.35 | Allergen-specfic IgE levels ISAC (ISU-E) | ≤0.35 | Mediator release in RBL-assay (%) | 0–5 |
| 0.35–1 | >0.35–1 | 5–10 | |||
| 1–15 | 1–15 | >10 | |||
| >15 | >15 |
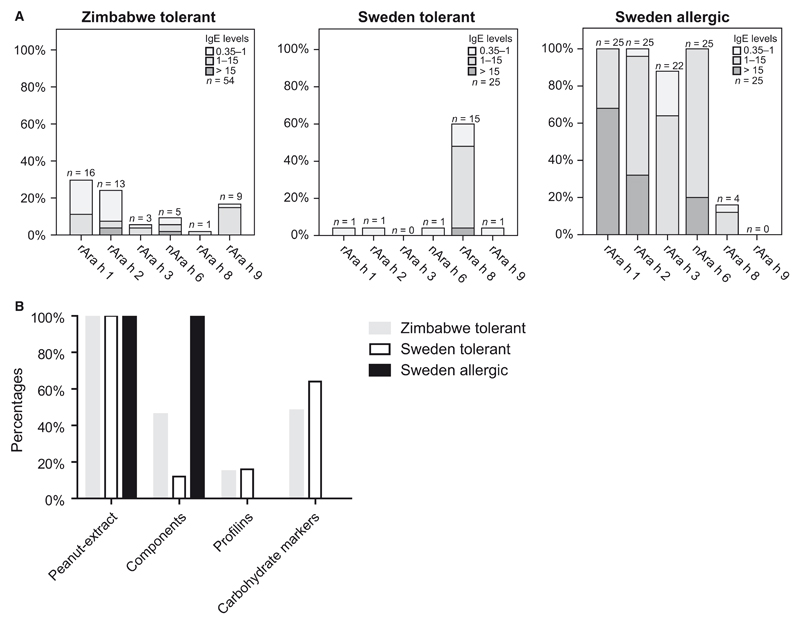
Figure 1.
Peanut allergen- and carbohydrate-specific IgE reactivities. (A), Percentages and number of African and Swedish asymptomatic and Swedish allergic patients with IgE to allergen components. Ranges of allergen-specific IgE levels are indicated. (B), Percentages of patients with IgE reactivity to peanut extract, to at least one of the allergenic peanut components (rAra h 1–3, 8, 9 and nAra h 6), to profilins, or to carbohydrate markers (MUXF3, nCup a 1, nCyn d 1, nPhl p 4) but not to the allergenic peanut components.
Peanut-allergic patients mount high IgE reactivity to peanut allergen components associated with systemic reactions
For comparison, we analyzed a group of 25 patients with doctor-diagnosed peanut allergy from Sweden (Fig. 1A). All patients were sensitized to rAra h 1, rAra h 2, and nAra h 6, and the IgE levels were high (medians 26.31, 9.18, and 9.12 ISU-E, respectively, Table 2). rAra h 3 was the fourth most commonly recognized component (22/25). None of these patients were sensitized against rAra h 9 (Fig. 1A). Seventeen patients mounted IgE to the carbohydrate marker pollen allergens and three to profilins. Compared to the African peanut-sensitized but asymptomatic patients, the IgE levels were much lower and none of the Swedish patients were solely sensitized to CCDs or profilins (Fig. 1B).
Swedish asymptomatic patients differ from African patients by their IgE reactivity to the Bet v 1-homologous peanut allergen rAra h 8
Sixty percent of the patients had IgE reactivity to the Bet v 1-homologous peanut allergen rAra h 8. Only 2 patients were sensitized to rAra h 1 and nAra h 6 or rAra h 2, and compared to the Zimbabwean patients, their IgE levels were even lower (Fig. 1A, Table S2). Seven patients showed IgE reactivity solely to CCDs (Fig. 1B and Table S2).
African peanut asymptomatic patients show strong IgG reactivity to peanut allergens
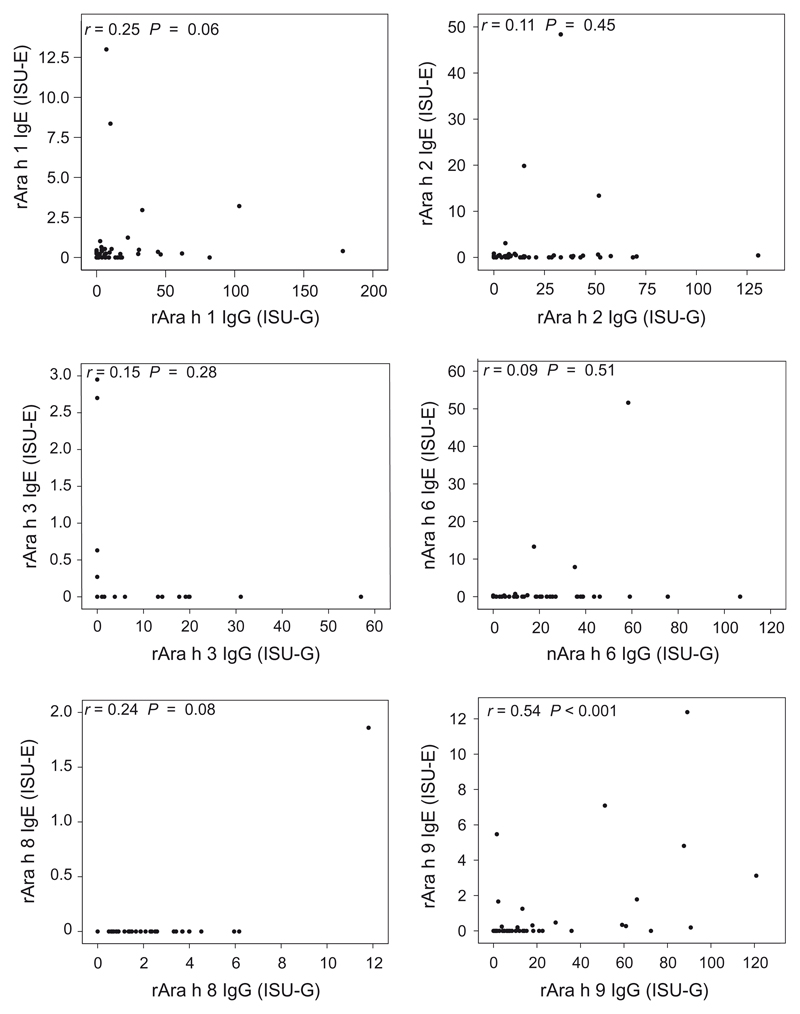
Peanut allergen-specific IgG levels were moderate/high (1–15 ISU-G) or very high (>15 ISU-G) in the majority of patients. IgG responses to rAra h 2 were most common (46/54). rAra h 9 and nAra h 6 also induced frequent (45/54 and 38/54; respectively) and high IgG responses (Table 3). All but one of the African patients with exclusive IgE reactivity to carbohydrate epitopes mounted IgG responses toward the highly allergenic peanut components. As the IgG recognition was much more frequent and intensive than the IgE recognition, no significant correlations were observed between allergen-specific IgE and IgG levels against the different peanut allergen components except for rAra h 9 (r = 0.54, P < 0.001, Fig. 2). By contrast, allergen-specific IgE and IgG levels against the clinically relevant house dust mite allergens, nDer p 1, rDer p 2, rDer p 5, rDer p 7, rDer p 21, and rDer p 23, correlated significantly in the African patients (Fig. S1). In line with the IgG responses, the IgG4 antibody reactivity was most frequent against rAra h 2 (31/54), rAra h 1 (23/54), and nAra h 6 22/54) and the levels were also the highest to these components. We observed no significant correlation between IgE and IgG4 levels to the different peanut allergen components except for rAra h 9 (r = 0.51, P < 0.001).
Table 3. Heat map of IgG reactivities to peanut allergen components, plant profilins, and carbohydrate markers. Ranges of allergen-specific IgG levels are indicated.
| Peanut | Profilins | CCD | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic Africa |
rAra h 1 | rAra h 2 | rAra h 3 | nAra h 6 | rAra h 8 | rAra h 9 | rBet v 2 | rHev b 8 | rMer a 1 | rPhl p 12 | rPru du 4 | rTri a 12 | nCry j 1 | nCyn d 1 | nCup a 1 | MUXF3 | nPhl p 4 |
| 1 | 10.05 | 51.85 | ≤0.35 | 35.31 | 1.67 | 87.58 | 8.56 | 68.41 | 37.12 | 4.14 | 16.27 | 11.67 | 12.6 | 94.31 | 102.6 | 43.58 | 93.05 |
| 2 | 3.89 | 2.82 | ≤0.35 | ≤0.35 | ≤0.35 | 89.13 | 35.86 | 130.3 | 75.59 | 5.92 | 49.41 | 41.62 | 13.6 | 108 | 103.8 | 32.77 | 80.31 |
| 3 | 1.23 | 33.03 | ≤0.35 | 58.4 | 2.09 | 3.82 | 22.49 | 67.15 | 66.6 | 1.22 | 45.1 | 18.12 | 14.18 | 19.93 | 69.01 | 8.09 | 22.2 |
| 4 | 0.62 | 27.22 | ≤0.35 | 59.1 | 4 | 28.58 | 16.16 | 2.29 | 1.6 | 1.81 | 3.55 | 0.86 | 29.01 | 47.13 | 61.56 | 12.82 | 33.69 |
| 5 | 18.38 | 39.19 | ≤0.35 | 37.84 | 1.4 | ≤0.35 | 19.95 | 114.6 | 52.01 | 2.65 | 43.63 | 4.11 | ≤0.35 | 47.55 | 100.3 | 5.41 | 9.34 |
| 6 | ≤0.35 | 14.93 | ≤0.35 | 17.63 | 2.36 | 1.53 | 13.19 | 41.2 | 23 | 5.29 | 18.87 | 10.35 | ≤0.35 | 17.53 | 23.86 | 7.51 | 7.1 |
| 7 | ≤0.35 | 5.69 | ≤0.35 | ≤0.35 | ≤0.35 | 11.03 | 18.37 | 40.74 | 24.95 | ≤0.35 | 18.32 | 4.65 | ≤0.35 | 19.13 | 46.29 | 8.71 | 12.31 |
| 8 | ≤0.35 | 5.83 | ≤0.35 | 3.71 | ≤0.35 | 51.23 | 45.19 | 4.52 | 54.24 | 59.95 | ≤0.35 | 3.47 | ≤0.35 | ≤0.35 | 23.35 | 3.17 | 2.48 |
| 9 | ≤0.35 | 8.33 | ≤0.35 | 5.16 | ≤0.35 | 13.31 | 5.11 | 2.96 | 4.19 | 3.4 | 3.33 | 6.66 | 3.52 | 63.65 | 60.45 | 38.48 | 40.22 |
| 10 | 30.7 | 33.05 | ≤0.35 | 24.63 | 0.82 | 0.91 | 3.12 | 7.88 | 2.14 | 0.96 | 1.33 | 1.52 | 2.27 | 39.9 | 33.24 | 12.37 | 33.95 |
| 11 | 7.11 | ≤0.35 | ≤0.35 | ≤0.35 | 1.35 | 1.38 | 2.32 | 2.95 | 1.68 | 1.03 | 1.23 | 1.8 | 2.23 | 40.6 | 32.84 | 1.87 | 5.94 |
| 12 | 178.3 | 130.5 | 13.13 | 106.8 | 2.51 | 17.92 | 6 | 16.46 | 3.03 | 1.68 | 5.08 | 4.66 | ≤0.35 | 26.89 | 20.55 | 7.22 | ≤0.35 |
| 13 | 10.87 | 8.04 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.14 | 1.15 | 0.75 | 0.42 | ≤0.35 | 1.37 | 1.94 | 25.49 | 20.4 | 9.75 | 15.58 |
| 14 | 8.86 | 20.84 | 3.8 | 18.44 | ≤0.35 | 60.93 | 0.72 | 5.38 | 1.1 | ≤0.35 | 1.13 | 0.48 | 32.42 | 39.83 | 41.42 | 5.07 | 13.64 |
| 15 | 2.35 | 2.28 | ≤0.35 | 10.31 | ≤0.35 | 1.67 | 4.52 | 20.14 | 15.79 | 2.04 | 14.4 | 8.15 | ≤0.35 | 25.05 | 39.19 | 3.96 | 8.22 |
| 16 | ≤0.35 | 1.06 | 1.53 | ≤0.35 | 3.99 | 1.01 | 4.03 | 7.78 | 2.97 | 1.67 | 3.24 | 2.6 | 1.21 | 34.28 | 23.15 | 16.89 | 43.18 |
| 17 | 13.66 | 10.3 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 3.68 | ≤0.35 | ≤0.35 | 14.26 | 19.61 | 3.59 | 5.22 |
| 18 | 9 | 42.78 | 19.95 | 36.47 | 0.7 | 22.55 | 1.08 | 5.77 | 1.6 | ≤0.35 | 0.89 | 1.49 | 51.6 | 56.83 | 69.28 | 1.62 | 8.68 |
| 19 | ≤0.35 | 14.94 | ≤0.35 | 10.04 | 1.49 | 7.03 | 17.55 | 47.4 | 56.25 | 1.61 | 29.86 | 12.22 | 10.9 | 20.26 | 61.7 | 8.34 | 23.56 |
| 20 | 103.3 | 51.46 | ≤0.35 | 46.08 | 3.7 | 90.72 | 18.41 | 81.26 | 36.71 | 6.19 | 21.4 | 16.96 | ≤0.35 | 39.01 | 24.12 | 5.21 | 8.08 |
| 21 | 15.33 | 28.5 | ≤0.35 | 6.92 | ≤0.35 | 13.58 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 25.73 | 26.75 | 20.18 | 7.57 | 11.72 |
| 22 | 1.55 | 68.64 | 57 | 75.53 | 3.42 | 59.16 | 3.34 | 10.47 | 9.79 | ≤0.35 | 5.09 | 1.55 | 22.09 | 32.65 | 39.02 | 1.99 | 5.14 |
| 23 | ≤0.35 | 13.94 | 14.05 | 20.23 | ≤0.35 | 65.95 | 4.1 | 2.04 | 4.55 | 1.31 | 2.5 | 1.54 | 22.42 | 34.23 | 68.46 | 16.46 | 29.49 |
| 24 | 22.68 | 38.2 | 19.11 | 25.64 | 0.81 | 11.91 | 1.72 | 1.84 | 2 | 1.5 | 0.97 | 2.9 | 6.27 | 115 | 150.8 | 8.42 | 18.43 |
| 25 | 2.62 | 10.85 | ≤0.35 | 14.79 | 0.64 | 4.23 | 1.35 | 3.71 | 1.29 | ≤0.35 | 1.49 | 1.08 | 21.48 | 8.49 | 9.65 | 0.54 | 2.3 |
| 26 | 6.02 | 5.77 | ≤0.35 | 4.74 | ≤0.35 | 6.14 | 2.78 | 1.76 | 0.8 | ≤0.35 | 1.18 | 1.4 | 10.26 | 18.62 | 18.32 | 0.78 | 4.71 |
| 27 | 17.24 | 17.31 | 31.03 | 23.18 | 6.17 | 20.97 | 1.21 | 3.46 | 2.52 | ≤0.35 | 1.14 | 1.03 | 15.47 | 19.33 | 36.57 | 4.43 | 14.57 |
| 28 | ≤0.35 | 1.19 | ≤0.35 | 1.35 | ≤0.35 | 1.14 | 1.44 | 1.95 | ≤0.35 | ≤0.35 | ≤0.35 | 0.71 | 12.03 | 14.35 | 14.6 | 4.56 | 9.31 |
| 29 | 5.85 | 14.76 | ≤0.35 | 13.3 | 3.32 | 120.9 | 5.49 | 4.03 | 2.61 | 1 | ≤0.35 | 1.97 | ≤0.35 | 40.69 | 24.85 | ≤0.35 | 6.68 |
| 30 | 3.96 | 1.08 | ≤0.35 | ≤0.35 | ≤0.35 | 15.24 | 0.91 | 8.65 | 1.69 | ≤0.35 | 1.66 | 1.32 | 22.85 | 11.46 | 10.38 | 2.64 | 9.42 |
| 31 | 3.74 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.6 | 5.88 | 1.17 | 0.71 | 1.11 | 2.16 | ≤0.35 | 22.77 | 18.14 | 1.83 | 4.05 |
| 32 | 6.48 | 3.81 | ≤0.35 | ≤0.35 | ≤0.35 | 35.9 | ≤0.35 | 6.65 | 2.12 | 1.12 | 1.78 | ≤0.35 | ≤0.35 | 15.24 | 1.84 | 1.32 | ≤0.35 |
| 33 | ≤0.35 | ≤0.35 | ≤0.35 | 1.25 | ≤0.35 | 7.54 | ≤0.35 | 2.88 | 1.8 | ≤0.35 | 1.35 | ≤0.35 | 12.53 | 18.75 | 16.3 | 3.44 | 9.55 |
| 34 | ≤0.35 | 5.36 | ≤0.35 | 9.04 | 1.17 | 2.69 | 8.19 | 2.41 | 2.07 | 1.38 | 1.65 | 6.58 | ≤0.35 | 10.13 | 72.05 | 4.78 | 10.88 |
| 35 | 44.45 | 44 | ≤0.35 | 38.83 | 2.59 | 72.4 | 2.71 | 18.72 | 2.4 | 1.73 | 3.24 | 3.75 | ≤0.35 | 6.91 | 6.93 | ≤0.35 | 1.82 |
| 36 | 6.32 | ≤0.35 | ≤0.35 | ≤0.35 | 4.52 | 14.2 | 7.02 | 7.39 | ≤0.35 | 1.91 | 4.58 | 4.43 | ≤0.35 | 19.08 | 25.25 | ≤0.35 | 9.53 |
| 37 | 61.87 | 57.72 | ≤0.35 | 38.66 | 0.49 | 0.52 | 1.15 | 5.91 | 1.61 | 0.59 | 1.16 | 0.97 | 6.38 | 71.99 | 77.83 | 16.78 | 37.29 |
| 38 | ≤0.35 | 6.9 | ≤0.35 | ≤0.35 | 2.3 | ≤0.35 | 2.41 | 3.14 | 3.37 | 1.79 | ≤0.35 | 5.26 | ≤0.35 | 20.27 | 11.37 | 10.88 | 6.87 |
| 39 | 6.71 | 15.21 | 19.8 | 18.76 | 1.88 | 0.63 | 8.76 | 5.08 | 4.24 | 1.69 | 4.29 | 2.77 | 16.9 | 13.57 | 72.23 | 9.67 | 26.53 |
| 40 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 0.59 | 8.49 | 0.82 | 1.96 | 1.63 | ≤0.35 | 0.68 | 1.13 | 16.94 | 19.34 | 16.95 | 7.29 | 10.74 |
| 41 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 1.87 | ≤0.35 | 2.88 | 1.63 | 3.12 | 1.46 | 1.52 | 3.86 | ≤0.35 | 2.34 | 29.11 | 1.89 | ≤0.35 |
| 42 | 30.14 | ≤0.35 | ≤0.35 | 46.09 | 1.37 | 4.42 | 19.33 | 5.65 | 9.17 | 7.19 | ≤0.35 | 12.72 | ≤0.35 | 12.41 | 7.43 | 5.02 | 7.79 |
| 43 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | ≤0.35 | 2.06 | 3.39 | 0.86 | 0.53 | 1.06 | 1.53 | ≤0.35 | 2.1 | 8.09 | 1.08 | 3.94 |
| 44 | 1.27 | 1.13 | 17.72 | 1.34 | ≤0.35 | 1.06 | 1.02 | 1.19 | 0.53 | ≤0.35 | 1.42 | 0.78 | 3.07 | 4.94 | 7.85 | 1.24 | 3.1 |
| 45 | 3.51 | 6.12 | ≤0.35 | ≤0.35 | 3.69 | 18.3 | 8.81 | 5.53 | 3.42 | 3.53 | 3.25 | 4.91 | ≤0.35 | 8.74 | 25.9 | 3.18 | 4.34 |
| 46 | ≤0.35 | 38.75 | ≤0.35 | 43.64 | ≤0.35 | 6.37 | 4.47 | 2.79 | 2.64 | 3.42 | ≤0.35 | 4.32 | ≤0.35 | ≤0.35 | 2.17 | 2.66 | 2.05 |
| 47 | ≤0.35 | 6.8 | ≤0.35 | 12.59 | 0.91 | 0.87 | 12.35 | 1.55 | 9.78 | 9.1 | 1.59 | 16.79 | ≤0.35 | ≤0.35 | 34.13 | ≤0.35 | ≤0.35 |
| 48 | ≤0.35 | 7.43 | 1.03 | 9.53 | ≤0.35 | 6.37 | 5.34 | 3.4 | 5.3 | ≤0.35 | 1.88 | 1.03 | 6.69 | 2.77 | 5.95 | 0.93 | 1.32 |
| 49 | 6.06 | 7.15 | ≤0.35 | ≤0.35 | ≤0.35 | 1.16 | 1.66 | 2.48 | 2.57 | 0.99 | 0.69 | 1.52 | 22.17 | 97.55 | 110.8 | 40.18 | 111.3 |
| 50 | 9.44 | 29.61 | ≤0.35 | 36.23 | ≤0.35 | ≤0.35 | 7.24 | 23.17 | 2.48 | 1.88 | 3.73 | 3.23 | ≤0.35 | 26.57 | 11.91 | ≤0.35 | ≤0.35 |
| 51 | 46.42 | 70.47 | ≤0.35 | 20.55 | ≤0.35 | ≤0.35 | 4.61 | 39.19 | 1.17 | 1.94 | 2.11 | 6.66 | ≤0.35 | 58.54 | 34.55 | 16.72 | 25.68 |
| 52 | 33.09 | 13 | 5.99 | 20.98 | 11.81 | 2.18 | ≤0.35 | 6.96 | 2.25 | 0.78 | 1.53 | 2.38 | ≤0.35 | 9.04 | 10.01 | 10.02 | 9.82 |
| 53 | 81.84 | 52.5 | ≤0.35 | 27 | 1.4 | 10.26 | 1.85 | 6 | 1.52 | 0.95 | 3.25 | 3.02 | 1.26 | 26.47 | 16.42 | 7.47 | 13.59 |
| 54 | 17.2 | 8.25 | ≤0.35 | 2.81 | 5.95 | 5.03 | 8.13 | 15.51 | 9.29 | 3.89 | 10.96 | 7.55 | 3.42 | 36.35 | 25.41 | 12.75 | 22.74 |
| Median | 4.91 | 9.32 | ≤0.35 | 10.18 | 0.76 | 6.26 | 3.69 | 5.46 | 2.50 | 1.27 | 1.66 | 2.69 | 2.09 | 20.27 | 25.05 | 5.05 | 9.38 |
| Upper quartile | 15.80 | 33.04 | ≤0.35 | 29.08 | 2.32 | 18.97 | 8.61 | 15.75 | 9.20 | 1.98 | 4.36 | 5.59 | 13.75 | 39.85 | 60.73 | 9.82 | 22.34 |
| Lower quartile | ≤0.35 | 2.69 | ≤0.35 | ≤0.35 | ≤0.35 | 0.99 | 1.32 | 2.46 | 1.58 | ≤0.35 | 1.10 | 1.27 | ≤0.35 | 12.17 | 15.88 | 1.86 | 4.27 |
| Peanut | Profilins | CCD | |||||||||||||||
| Peanut allergic Sweden |
rAra h 1 | rArah 2 | rAra h 3 | nArah 6 | rAra h 8 | rArah 9 | rBet v 2 | rHev b 8 | rMer a 1 | rPhl p 12 | rPru du 4 | rTri a 12 | nCry j 1 | nCyn d 1 | nCup a 1 | MUXF3 | nPhl p 4 |
| 55 | 173.5 | 14.49 | 4.45 | 11.2 | 2.15 | 1.67 | 3.7 | 7.73 | 4.12 | 4.38 | 2.17 | 3.51 | 11.71 | 20.28 | 18.81 | ≤0.35 | 22.21 |
| 56 | 82.52 | 24.32 | 4.6 | 12.47 | 4.16 | 5.21 | 2.79 | 8.55 | 2.97 | 3.9 | 8.8 | 1.76 | ≤0.35 | ≤0.35 | 2.38 | ≤0.35 | 2.89 |
| 57 | 67.43 | 15.13 | 2.45 | 25.38 | 2.45 | 2.04 | 1.74 | 1 | 1.85 | 1.41 | 1.3 | ≤0.35 | 7.87 | 0.62 | 10.06 | 1.39 | 3.14 |
| 58 | 117.3 | 30.13 | 6.54 | 48.79 | ≤0.35 | ≤0.35 | 1.1 | 2.72 | 1.01 | 0.75 | 1.28 | 1.35 | ≤0.35 | 2.01 | 7.65 | ≤0.35 | 0.54 |
| 59 | 25.32 | 8.83 | 3.95 | 6.76 | 4.07 | 66.89 | 2.78 | 26.24 | 3.81 | 2.44 | 28.26 | 1.27 | 9.3 | 32.46 | 13.35 | ≤0.35 | 33.81 |
| 60 | 154.5 | 13.06 | 10.66 | 15.49 | 0.79 | ≤0.35 | 2.39 | 1.86 | 1.95 | 3.01 | 1.53 | ≤0.35 | 12.66 | 14.46 | 20.42 | ≤0.35 | 23.81 |
| 61 | 28.42 | 23.33 | 16.22 | 14.06 | 2.22 | 3.87 | 10.25 | 77.25 | 31.27 | 5.29 | 39.56 | 10.35 | 3.18 | 1.94 | 9.23 | ≤0.35 | 21.03 |
| 62 | 133.9 | 33.15 | 18.75 | 28.53 | 0.57 | 3.44 | 20.84 | 4.21 | 12.41 | 8.63 | 2.51 | 7.73 | 10.07 | 24.04 | 13.68 | ≤0.35 | 64.52 |
| 63 | 19.8 | 9.82 | 4.16 | 4.28 | 0.98 | ≤0.35 | 1.57 | 1.06 | 1.19 | 1.05 | 0.81 | 1.24 | 3.59 | 1.88 | 7.09 | 0.89 | 4.85 |
| 64 | 164.5 | 14.72 | ≤0.35 | 12.41 | 2.48 | v | 0.92 | 3 | 0.95 | 1.3 | 3.67 | ≤0.35 | 0.91 | 8.34 | 3.04 | ≤0.35 | 7.81 |
| 65 | 164.9 | 43.81 | 22.14 | 11.35 | 1.06 | ≤0.35 | 4.38 | 2.21 | 2.66 | 4.07 | 1.77 | 2.44 | 11.69 | 6.2 | 5.99 | ≤0.35 | 8.96 |
| 66 | 74.43 | 9.08 | 7.09 | 23.55 | 1.48 | 2.37 | 0.87 | 3.87 | 1.74 | 1.91 | 1.46 | ≤0.35 | 2.71 | ≤0.35 | 2.57 | ≤0.35 | 0.77 |
| 67 | 27.28 | 14.89 | 4.11 | 9.02 | 2.3 | 1.05 | 2.11 | 6.03 | 4.14 | 2.29 | 4.23 | 2.95 | 1.74 | 4.64 | 3.73 | ≤0.35 | 6.7 |
| 68 | 91.17 | 8.77 | 6.89 | 10.54 | 2.35 | 1.35 | 3.9 | 5.38 | 3.96 | 2.96 | 6.76 | ≤0.35 | 13.1 | 19.89 | 27.04 | ≤0.35 | 30.41 |
| 69 | 34.27 | 17.35 | ≤0.35 | 5.55 | 2.09 | 1.18 | 11.72 | 2.64 | 12.46 | 12.52 | 1.56 | 1.61 | 4.05 | 17.15 | 3.8 | ≤0.35 | 3.19 |
| 70 | 102.9 | 5.58 | 26.17 | 28.2 | ≤0.35 | ≤0.35 | ≤0.35 | 2.26 | 1.31 | 2.99 | 0.94 | 1.13 | 2.2 | 12.66 | 0.77 | ≤0.35 | 6.29 |
| 71 | 72.79 | 17.57 | 11.13 | 13.3 | 1.77 | 22.21 | 1.23 | 10.05 | 1.54 | 0.79 | 2.42 | ≤0.35 | 5.91 | 8.29 | 29.45 | ≤0.35 | 28.29 |
| 72 | 38.48 | 15.28 | 0.69 | 11.69 | ≤0.35 | 1.71 | 13.52 | 108.3 | 70.9 | 4.6 | 85.55 | 8.29 | ≤0.35 | 5.93 | 3.1 | ≤0.35 | 8.87 |
| 73 | 51.02 | 14.67 | 3.63 | 9.44 | 1.63 | ≤0.35 | 2.23 | 1.08 | 1.32 | 1.02 | ≤0.35 | ≤0.35 | 1.18 | 3.04 | ≤0.35 | ≤0.35 | 2.39 |
| 74 | 23.52 | 8 | 1.5 | 8.55 | 1.11 | 34.89 | 10.53 | 24.57 | 12.54 | 4.04 | 15.54 | ≤0.35 | 17.78 | 5.32 | 56.91 | ≤0.35 | 58.15 |
| 75 | 111.7 | 77.95 | 12.08 | 8.95 | ≤0.35 | ≤0.35 | 2.01 | 2.18 | 2.32 | 3.13 | 1.92 | ≤0.35 | 2.06 | 29.17 | 4.64 | ≤0.35 | 31.86 |
| 76 | 161.7 | 29.05 | 5.8 | 9.15 | 3.35 | ≤0.35 | 1.43 | 1.52 | 1.83 | 1.99 | 2.16 | 1.83 | 6.22 | 2.21 | 0.93 | ≤0.35 | 1.39 |
| 77 | 9.37 | 11.75 | ≤0.35 | 8.34 | 4.69 | 1.05 | 1.05 | 2.42 | 1.43 | 1 | 2.92 | ≤0.35 | ≤0.35 | 0.38 | 3.84 | ≤0.35 | 4.04 |
| 78 | 112.3 | 31.69 | 13.36 | 21.74 | 0.58 | ≤0.35 | 3.62 | 4.16 | 4.15 | 2.58 | 3.28 | ≤0.35 | 3.59 | 1.74 | 6.97 | ≤0.35 | 7.66 |
| 79 | 2.9 | 27.21 | ≤0.35 | 17.7 | 0.52 | 2.76 | 0.62 | 0.73 | 1.3 | 1.49 | 0.61 | ≤0.35 | 4.13 | 3.27 | 2.36 | ≤0.35 | 18.46 |
| Median | 74.43 | 15.13 | 4.6 | 11.69 | 1.63 | 1.35 | 2.23 | 3 | 2.32 | 2.58 | 2.17 | 1.13 | 3.59 | 5.32 | 5.99 | ≤0.35 | 7.81 |
| Upper quartile | 125.6 | 28.13 | 11.61 | 19.72 | 2.4 | 3.44 | 4.14 | 8.14 | 4.15 | 4.06 | 5.5 | 2.14 | 9.69 | 15.81 | 13.52 | ≤0.35 | 26.05 |
| Lower quartile | 27.85 | 10.79 | 1.98 | 8.99 | 0.58 | ≤0.35 | 1.17 | 2.02 | 1.38 | 1.36 | 1.38 | ≤0.35 | 1.46 | 1.91 | 2.81 | ≤0.35 | 3.17 |
| Allergen-specific IgG levels |
ISAC (ISU-G) | ≤0.35 | 0.35-1 | 1-15 | >15 |
Figure 2.
Associations of IgE and IgG levels specific for Ara h 1, 2, 3, 6, 8, and 9 in African asymptomatic patients.
Swedish peanut-allergic patients show strong IgG reactivity to peanut allergens
All Swedish peanut-allergic patients mounted IgG responses to rAra h 1, rAra h 2, and nAra h 6 (Table 3). However, the IgG levels were much higher compared with the African asymptomatic patients (e.g., rAra h 1, median 74.43 ISU-G vs 4.91 ISU-G, P < 0.001). On the other hand, the Swedish peanut-allergic patients had significantly lower IgG levels to the glycosylated allergens compared with the African peanut asymptomatic patients (e.g., nCup a 1, median 5.99 ISU-G vs median 25.05 ISU-G, P < 0.001). The Swedish asymptomatic patients had the highest IgG levels against rAra h 8, the component against which they also had the highest IgE levels (Table S3). As in the African patients, we observed no significant correlations in the two Swedish control patient groups between allergen-specific IgE and IgG levels against the different peanut allergen components except for rAra h 8 (r = 0.61 P = 0.001) in the Swedish asymptomatic group. The allergen-specific IgG4 antibody responses in general followed the IgE pattern among the Swedish peanut-allergic as well as the asymptomatic patients. In the peanut-allergic group, the IgG4 levels to rAra h 1 and rAra h 2 correlated significantly with the specific IgE levels (r = 0.71, P < 0.001 and r = 0.58, P = 0.002, respectively). In the Swedish asymptomatic patient group, where IgE was directed against rAra h 8, the IgG4 levels were above all directed against this peanut allergen component (Table S4).
African and Swedish asymptomatic patients differ from peanut-allergic patients regarding IgE reactivity to Ara h 2 peptides
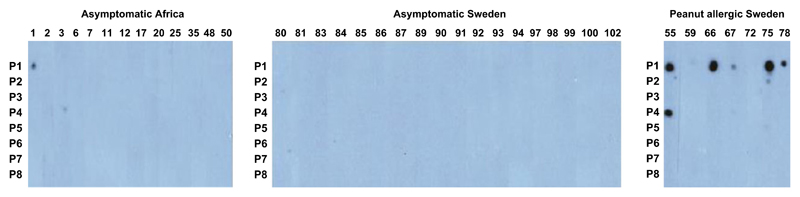
Only two of the 13 African Ara h 2-sensitized patients showed IgE reactivity to Ara h 2 peptides (Fig. 3). Furthermore, none of the Swedish asymptomatic patients showed IgE reactivity to Ara h 2 peptides; however, only one of them was sensitized to Ara h 2 (#92). By contrast, six of the seven Swedish Ara h 2-sensitized and allergic patients showed IgE reactivity to Ara h 2 peptides, with peptide 1 being the dominant peptide (Fig. 3).
Figure 3.
IgE reactivity to dot-blotted Ara h 2 peptides. Sera from asymptomatic African (left panel), asymptomatic Swedish (middle panel), and peanut-allergic Swedish patients (right panel) were tested for IgE reactivity to eight Ara h 2 peptides (Pep1-8). Bound IgE was detected with 125I-labeled anti-IgE and visualized by autoradiography.
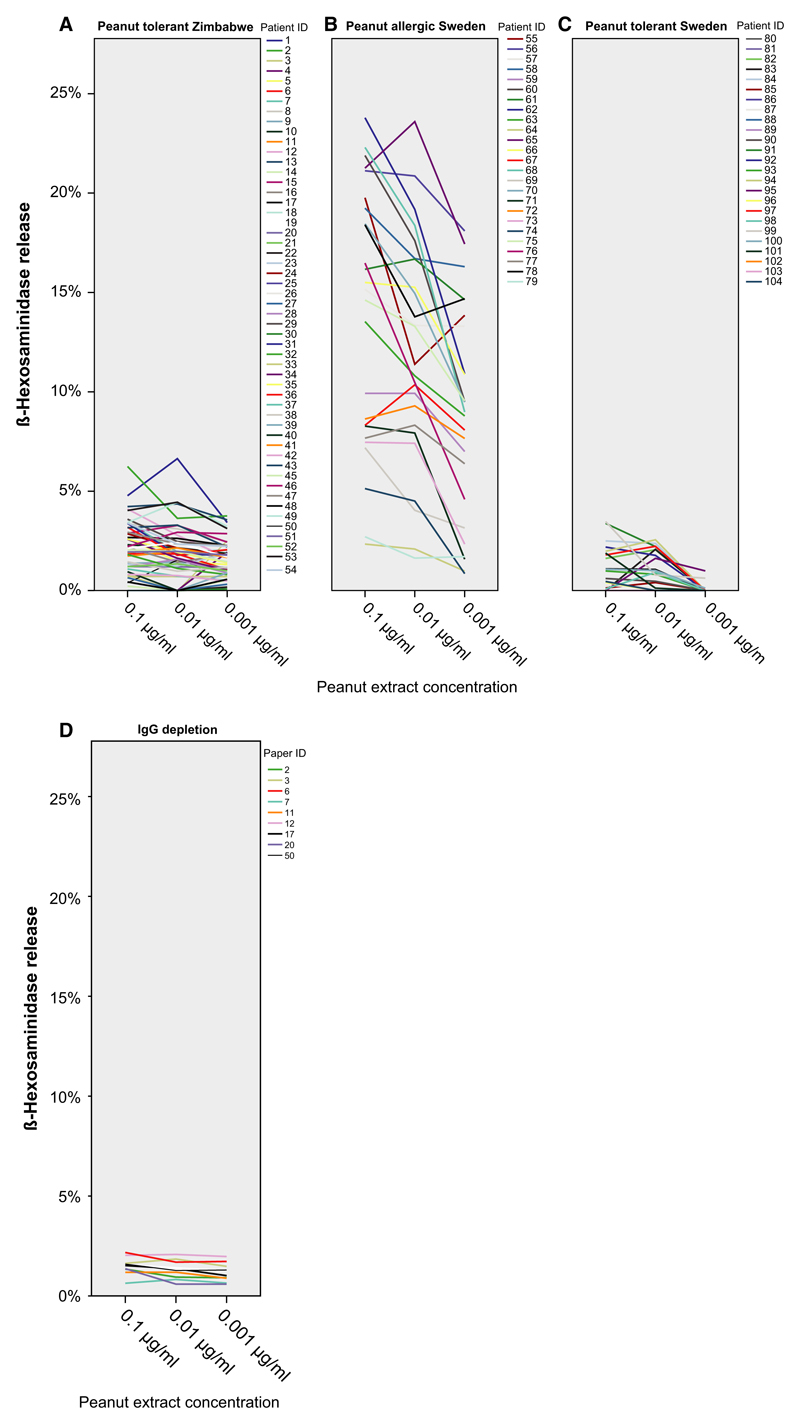
IgE from African asymptomatic patients induces little basophil activation to peanut extract
Sera from the African peanut asymptomatic patients induced no relevant basophil degranulation even though four of the sera had very high IgE levels to peanut extract (200, 79, 28, 18.6 kUA/l) as well as to rAra h 2 (13.48 ISU-E, 48.38 ISU-E, 19.84 ISU-E, and 3.08 ISU-E) (Fig. 4A). One of these sera mounted also very high IgE levels to the peanut allergen components, rAra h 2 (48.38 ISU-E) and nAra h 6 (51.61 ISU-E). Sera from the Swedish peanut asymptomatic patients induced likewise very little degranulation (median 0.78%). In contrast, sera from the Swedish peanut-allergic patients induced a high degranulation (median 11%) (Fig. 4B,C). Depletion of IgG from available African sera did not confer a higher degree of basophil activation (Fig. 4D).
Figure 4.
Induction of basophil degranulation. Displayed are the percentages of ß-hexosaminidase releases (y-axes) induced by three different concentrations of peanut allergen extract (x-axes) when RBL cells were loaded with sera from asymptomatic African (A), peanut-allergic Swedish (B), asymptomatic Swedish patients (C), or IgG-depleted African sera (D).
Discussion
This study shows that natural clinical tolerance to peanuts as it occurs in certain areas of the world can be based on several mechanisms. Unlike immunological tolerance which implies an active immunological process involving specific immune mechanisms, natural clinical tolerance is characterized by the presence of IgE antibodies but lack of symptoms (17, 28). We found that approximately half of the asymptomatic peanut-sensitized African patients were exclusively sensitized to cross-reactive carbohydrate epitopes with low allergenic activity, which is in line with the results from a recent paper from Ghana showing that peanut-specific IgE in asymptomatic patients was associated with cross-reactivity to clinical irrelevant CCDs (29). A few of our patients (8/54) had IgE antibodies solely to profilins, which normally do not induce severe allergic symptoms upon ingestion because they are sensitive to heat denaturation and gastric digestion (30, 31). In the other half of patients, we found IgE sensitizations against peanut allergens that are associated with systemic reactions (rAra h 1, rAra h 2, rAra h 3, nAra h 6, and rAra h 9) as they are heat-stable and resistant to digestion. The majority had IgE reactivity against the major peanut allergen rAra h 1 and 13 patients showed IgE to rAra h 2, which is thought to be the most allergenic peanut component and thus even suggested for improving the accuracy of peanut allergy diagnosis (32). Interestingly, nine of the patients who had IgE to the highly allergenic peanut components showed peanut allergen extract-specific IgE levels >15 kUA/l, which is considered as being highly indicative of generalized symptomatic reactions (22). For comparison, we analyzed a group of patients with doctor-diagnosed peanut allergy and a group of asymptomatic patients according to doctor’s diagnosis from Sweden and observed a different sensitization pattern. In contrast to the African patients, we found that all peanut-allergic patients had IgE against the storage proteins rAra h 1, rAra h 2, and nAra h 6 and that the IgE levels were high. The asymptomatic patients from Sweden differed from the African asymptomatic patients because they were mainly sensitized to the Bet v 1-homologous peanut allergen rAra h 8 with relatively low allergenic activity and their IgE levels to Ara h 1, 2, and 6 were even lower (11).
Another possible explanation for natural clinical tolerance to peanuts is the induction of high peanut allergen-specific IgG and IgG4 levels. However, we found that these antibodies could not discriminate between the African peanut-sensitized but asymptomatic patients and the Swedish peanut-allergic patients. Both groups had induced more frequent and intensive IgG responses than the IgE recognition. IgG responses to peanut allergen components even predominated over IgE responses. Thus, we found no significant correlations between IgE and IgG responses to clinically relevant peanut allergens. With respect to peanut-specific IgG4 reactivity, the responses in general followed the IgE reactivity, but they were less intense. Only a low correlation between IgE and IgG4 levels to Ara h 9 was noted, which could reflect the intake of LTP containing food. In the peanut-allergic and asymptomatic control groups from Sweden, the IgG responses against the peanut allergens followed the same pattern of being more common and intense than the IgE reactivity as in the African patients. Thus, the IgG responses to the peanut allergens did not seem to be the cause for clinical tolerance.
We next explored whether differences in IgE recognition to linear epitopes of Ara h 2, the component that has been shown to be particularly useful in predicting clinical sensitivity to peanut (33), could be responsible for natural clinical tolerance in the African patients. We found that almost each of the tested peanut-allergic Ara h 2-sensitized patients showed IgE reactivity mainly to peptide 1, whereas all but two of the African did not recognize the peptides. Furthermore, Swedish asymptomatic patients did not react with peptides. Notably, the reaction of the African patients was very weak. One explanation for the lack of peptide recognition could be that the patients’ IgE antibodies are directed against conformational epitopes and the Ara h 2 peptides only display linear epitopes. The results are in line with previous findings where patients with more severe allergic reactions to peanut and who outgrew their allergy can be characterized by peptide-specific IgE responses (34, 35). The area characterized by peptide 1 has earlier been defined as a major IgE epitope of Ara h 2 (36). Thus, it seems that exposure to peanuts in early childhood or even during pregnancy as in the African population may influence the peanut IgE epitope recognition.
Interestingly, when we analyzed the biological activity of peanut IgE in the different groups, we noted major differences. Sera from the African asymptomatic patients showed no or very poor allergenic activity to peanut extract as did the Swedish peanut-sensitized but asymptomatic patients. In contrast, IgE from the peanut-allergic patients was biologically very active. The β-hexosaminidase release was 7 times lower in the African peanut asymptomatic patients compared with the Swedish peanut-allergic patients. The poor biological activity of peanut-specific IgE in the African asymptomatic group can not only be explained by IgE to CCDs, as almost half were sensitized to highly allergenic peanut components. We found that sera from African patients (e.g., #22, #42) with IgE reactivity to highly allergenic components (i.e., Ara h 1, Ara h 2) did not induce relevant degranulation.
Finally, we investigated whether the low activity in the basophil activation assay could be due to blocking IgG antibodies. However, removal of IgG did not impair the degree of degranulation and thus could not explain the poor allergenic activity of peanut-specific IgE in the African asymptomatic patient group. The results are in line with a report by Segal and colleagues who found that the RBL cells released histamine through an IgE-mediated system and bound IgG aggregates could neither elicit histamine release nor have any effect upon IgE-induced release (37).
In conclusion, our study shows that natural clinical tolerance to peanuts in certain areas of the world can be based on several mechanisms, one being exclusive IgE reactivity to peanut components with low allergenic activity (e.g., cross-reactive carbohydrates, profilins, or PR-10 proteins) and the almost complete lack of IgE recognition of Ara h 2 peptides and for the first time the poor biological activity of peanut IgE. Allergen-specific IgG antibodies seem not to be involved. Thus, early and frequent peanut intake seems to induce IgE against epitopes with low affinity or monovalent epitopes at least in central Africa and would support the concept that it may prevent allergic symptoms to peanut.
Supplementary Material
Additional Supporting Information may be found in the online version of this article:
Funding
This study was supported by the FWF-funded doctoral program IAI and SFB F4605, the Swedish Research Council, the Stockholm County Council, the Swedish Heart-Lung Foundation, the Center for Inflammatory Diseases Karolinska Institutet, the Swedish Asthma and Allergy Association’s Research Foundation, the Swedish Cancer and Allergy Foundation, Konsul Th Bergs Foundation, the King Gustaf V 80th Birthday Foundation, the Hesselman Foundation, the Magnus Bergvall Foundation, Karolinska Institutet, and the FP7-funded program MeDALL of the European Union.
Abbreviations
- Ara h
Arachis hypogea
- CCD
cross-reactive carbohydrate determinants
- n
natural
- r
recombinant
- kUA/l
kilo units allergen per liter
- ISU
ISAC standardized units
- IQR
interquartile range
Footnotes
Author contribution
EW, CH, RV, and MvH designed the project, analyzed and interpreted the data, and wrote the manuscript. EW, MO, MFT, CL, TT, RW, DG, and JT performed the experiments. ES, AA, GL, and MW contributed with patients. AÖ contributed with interpretation of data, and all authors provided critical review of the manuscript.
Conflict of interest
Rudolf Valenta has received research grant support from Thermo Fisher, Uppsala, Sweden, and serves as a consultant for Thermo Fisher.
References
- 1.Green R, Luyt D. Clinical characteristics of childhood asthmatics in Johannesburg. S Afr Med J. 1997;87:878–882. [PubMed] [Google Scholar]
- 2.Hill DJ, Hosking CS, Heine RG. Clinical spectrum of food allergy in children in Australia and South-East Asia: identification and targets for treatment. Ann Med. 1999;31:272–281. doi: 10.3109/07853899908995890. [DOI] [PubMed] [Google Scholar]
- 3.Lee BW, Shek LP, Gerez IF, Soh SE, Van Bever HP. Food allergy-lessons from Asia. World Allergy Organ J. 2008;1:129–133. doi: 10.1097/WOX.0b013e31817b7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hourihane JO. Peanut allergy. Pediatr Clin North Am. 2011;58:445–458. doi: 10.1016/j.pcl.2011.02.004. xi. [DOI] [PubMed] [Google Scholar]
- 5.Burks AW, Williams LW, Helm RM, Connaughton C, Cockrell G, O'Brien TJ. Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J Allergy Clin Immunol. 1991;88:172–179. doi: 10.1016/0091-6749(91)90325-i. [DOI] [PubMed] [Google Scholar]
- 6.Burks AW, Williams LW, Connaughton C, Cockrell G, O'Brien TJ, Helm RM. Identification and characterization of a second major peanut allergen, Ara h II, with use of the sera of patients with atopic dermatitis and positive peanut challenge. J Allergy Clin Immunol. 1992;90:962–969. doi: 10.1016/0091-6749(92)90469-i. [DOI] [PubMed] [Google Scholar]
- 7.Eigenmann PA, Burks AW, Bannon GA, Sampson HA. Identification of unique peanut and soy allergens in sera adsorbed with cross-reacting antibodies. J Allergy Clin Immunol. 1996;98:969–978. doi: 10.1016/s0091-6749(96)80014-5. [DOI] [PubMed] [Google Scholar]
- 8.Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h 1, Ara h 2 and Ara h 3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h 2 is the most important peanut allergen. Clin Exp Allergy. 2004;34:583–590. doi: 10.1111/j.1365-2222.2004.1923.x. [DOI] [PubMed] [Google Scholar]
- 9.Kleber-Janke T, Crameri R, Appenzeller U, Schlaak M, Becker WM. Selective cloning of peanut allergens, including profilin and 2S albumins, by phage display technology. Int Arch Allergy Immunol. 1999;119:265–274. doi: 10.1159/000024203. [DOI] [PubMed] [Google Scholar]
- 10.Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, Wensing M, Becker WM, et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004;114:1410–1417. doi: 10.1016/j.jaci.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Asarnoj A, Nilsson C, Lidholm J, Glaumann S, Ostblom E, Hedlin G, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130:468–472. doi: 10.1016/j.jaci.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Asarnoj A, Ostblom E, Ahlstedt S, Hedlin G, Lilja G, van Hage M, et al. Reported symptoms to peanut between 4 and 8 years among children sensitized to peanut and birch pollen - results from the BAMSE birth cohort. Allergy. 2010;65:213–219. doi: 10.1111/j.1398-9995.2009.02138.x. [DOI] [PubMed] [Google Scholar]
- 13.Lauer I, Dueringer N, Pokoj S, Rehm S, Zoccatelli G, Reese G, et al. The non-specific lipid transfer protein, Ara h 9, is an important allergen in peanut. Clin Exp Allergy. 2009;39:1427–1437. doi: 10.1111/j.1365-2222.2009.03312.x. [DOI] [PubMed] [Google Scholar]
- 14.Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, et al. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009;124:771–778. doi: 10.1016/j.jaci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Aalberse JA, Meijer Y, Derksen N, van der Palen-Merkus T, Knol E, Aalberse RC. Moving from peanut extract to peanut components: towards validation of component-resolved IgE tests. Allergy. 2013;68:748–756. doi: 10.1111/all.12160. [DOI] [PubMed] [Google Scholar]
- 16.Eller E, Bindslev-Jensen C. Clinical value of component-resolved diagnostics in peanut-allergic patients. Allergy. 2013;68:190–194. doi: 10.1111/all.12075. [DOI] [PubMed] [Google Scholar]
- 17.Ruiter B, Knol EF, van Neerven RJ, Garssen J, Bruijnzeel-Koomen CA, Knulst AC, et al. Maintenance of tolerance to cow's milk in atopic individuals is characterized by high levels of specific immunoglobulin G4. Clin Exp Allergy. 2007;37:1103–1110. doi: 10.1111/j.1365-2222.2007.02749.x. [DOI] [PubMed] [Google Scholar]
- 18.Tay SS, Clark AT, Deighton J, King Y, Ewan PW. Patterns of immunoglobulin G responses to egg and peanut allergens are distinct: ovalbumin-specific immunoglobulin responses are ubiquitous, but peanut-specific immunoglobulin responses are up-regulated in peanut allergy. Clin Exp Allergy. 2007;37:1512–1518. doi: 10.1111/j.1365-2222.2007.02802.x. [DOI] [PubMed] [Google Scholar]
- 19.Sverremark-Ekstrom E, Hultgren EH, Borres MP, Nilsson C. Peanut sensitization during the first 5 yr of life is associated with elevated levels of peanut-specific IgG. Pediatr Allergy Immunol. 2012;23:224–229. doi: 10.1111/j.1399-3038.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 20.Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods. 2014;66:106–119. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulmeister U, Hochwallner H, Swoboda I, Focke-Tejkl M, Geller B, Nystrand M, et al. Cloning, expression, and mapping of allergenic determinants of alphaS1-casein, a major cow's milk allergen. J Immunol. 2009;182:7019–7029. doi: 10.4049/jimmunol.0712366. [DOI] [PubMed] [Google Scholar]
- 22.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 23.Vidal C, Sanmartin C, Armisen M, Rodriguez V, Linneberg A, Gonzalez-Quintela A. Minor interference of cross-reactive carbohydrates with the diagnosis of respiratory allergy in standard clinical conditions. Int Arch Allergy Immunol. 2012;157:176–185. doi: 10.1159/000324447. [DOI] [PubMed] [Google Scholar]
- 24.Han SH, Chang ZN, Chang HH, Chi CW, Wang JY, Lin CY. Identification and characterization of epitopes on Cyn d I, the major allergen of Bermuda grass pollen. J Allergy Clin Immunol. 1993;91:1035–1041. doi: 10.1016/0091-6749(93)90217-4. [DOI] [PubMed] [Google Scholar]
- 25.Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, et al. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–267. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 26.Okano M, Kimura Y, Kino K, Michigami Y, Sakamoto S, Sugata Y, et al. Roles of major oligosaccharides on Cry j 1 in human immunoglobulin E and T cell responses. Clin Exp Allergy. 2004;34:770–778. doi: 10.1111/j.1365-2222.2004.1948.x. [DOI] [PubMed] [Google Scholar]
- 27.Alisi C, Afferni C, Iacovacci P, Barletta B, Tinghino R, Butteroni C, et al. Rapid isolation, characterization, and glycan analysis of Cup a 1, the major allergen of Arizona cypress (Cupressus arizonica) pollen. Allergy. 2001;56:978–984. doi: 10.1034/j.1398-9995.2001.103125.x. [DOI] [PubMed] [Google Scholar]
- 28.Akdis M. Immune tolerance in allergy. Curr Opin Immunol. 2009;21:700–707. doi: 10.1016/j.coi.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Amoah AS, Obeng BB, Larbi IA, Versteeg SA, Aryeetey Y, Akkerdaas JH, et al. Peanut-specific IgE antibodies in asymptomatic Ghanaian children possibly caused by carbohydrate determinant cross-reactivity. J Allergy Clin Immunol. 2013;132:639–647. doi: 10.1016/j.jaci.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, et al. Profilins constitute a novel family of functional plant panallergens. J Exp Med. 1992;175:377–385. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauser M, Roulias A, Ferreira F, Egger M. Panallergens and their impact on the allergic patient. Allergy Asthma Clin Immunol. 2010;6:1. doi: 10.1186/1710-1492-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dang TD, Tang M, Choo S, Licciardi PV, Koplin JJ, Martin PE, et al. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012;129:1056–1063. doi: 10.1016/j.jaci.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Flinterman AE, Knol EF, Lencer DA, Bardina L, den HJC, Lin J, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121:737–743. doi: 10.1016/j.jaci.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Lin J, Bruni FM, Fu Z, Maloney J, Bardina L, Boner AL, et al. A bioinformatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129:1321–1328. doi: 10.1016/j.jaci.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997;245:334–339. doi: 10.1111/j.1432-1033.1997.t01-1-00334.x. [DOI] [PubMed] [Google Scholar]
- 37.Segal DM, Sharrow SO, Jones JF, Siraganian RP. Fc (IgG) receptors on rat basophilic leukemia cells. J Immunol. 1981;126:138–145. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.