Abstract
The extracellular matrix proteoglycan decorin is well-known for its oncosuppressive activity. Here, decorin expression was examined in human vulva carcinoma tissue samples and in primary and commercial cell lines representing this malignant disease. Furthermore, the effect of adenovirus-mediated decorin cDNA (Ad-DCN) transduction on the viability, proliferation, and the expression and activity of the epidermal growth factor receptor (ErbB/HER) family members of the cell lines were investigated. Using in situ hybridization and immunohistochemistry for decorin, it was demonstrated that malignant cells in human vulva carcinoma tissues lack decorin expression. This result was true independently on tumor stage, grade or human papillomavirus status. RT-qPCR analyses showed that the human vulva carcinoma cell lines used in this study were also negative for decorin expression. Transduction of the cell lines with Ad-DCN caused a marked reduction in cell viability, while the proliferation of the cells was not affected. Experiments examining potential mechanisms behind the oncosuppressive effect of Ad-DCN transduction revealed that ErbB2/HER2 expression and activity in carcinoma cells were markedly downregulated. In conclusion, the results of this study showed that human vulva carcinoma cells lack decorin expression, and that Ad-DCN transduction of these cells induces oncosuppressive activity in part via downregulation of ErbB2/HER2.
Keywords: Decorin cDNA, ErbB2/HER2, oncosuppressive, transduction, vulva carcinoma
Introduction
The role of the extracellular matrix (ECM) is indisputable during all stages of tumorigenesis.1,2 Indeed, the tumor-associated ECM has been shown to be able to modulate all defined hallmarks of cancer, including evasion of growth suppression, death resistance and sustained proliferation.2 A crucial regulator of the ECM structure and function is a small leucine-rich proteoglycan, decorin, originally named for its ability to bind to and decorate collagen type I fibrils.3 Later on, decorin was discovered to modulate vital cell functions including proliferation, migration, apoptosis, and autophagy via its versatile interactions with growth factors and growth factor receptors such as transforming growth factor-ß (TGF-ß), epidermal growth factor receptor (EGFR), VEGF receptor-2 (VEGFR-2), and hepatocyte growth factor receptor (Met).4–8
Decorin’s antioncogenic role in cancer was identified when it was shown that lack of decorin was permissive for tumorigenesis, i.e., mice deficient of both decorin and the tumor suppressor gene p53 developed tumors at a faster rate than p53 null animals.9 Currently, decorin is generally recognized as a potent oncosuppressive molecule.10,11 The oncosuppressive action of decorin is known to be mediated via different pathways, including inhibition of several receptor tyrosine kinases (RTKs).4,7,11,12 Specifically, decorin has been shown to directly bind to, e.g., EGFR and to activate the mitogen-activated protein kinase (MAPK) signaling pathway, subsequently leading to induction of a potent inhibitor of cyclin-dependent kinases, p21WAF-1, and ultimately to cell cycle arrest.13
The expression of decorin in different cancer types has primarily been shown to be decreased.14 This also applies to gynecological malignancies such as endometrial and ovarian cancers.15,16 We have previously shown that human breast, bladder, and colon cancer cells do not express decorin.17–20 We have also shown that adenovirus-mediated decorin cDNA (Ad-DCN) transduction of cells representing the above cancers markedly decreases their malignant behavior.17–20 Furthermore, in our recent study, we have even been able to demonstrate that Ad-DCN transduction alters the cytological features of human metaplastic breast carcinoma tissue toward a less malignant phenotype.20
Vulva carcinoma, frequently associated with human papilloma virus (HPV), is the fourth most common gynecological cancer, and its incidence in women under 60 years is increasing worldwide.21 Although the initial prognosis of early stages of vulva carcinoma is good, improvements in the treatment of this disease in advanced stages and in its recurrence are needed.22 Currently, treatment alternatives for vulva carcinoma are limited, with radical vulvectomy being the principal treatment.22 In this study, the expression of decorin, a potent oncosuppressive proteoglycan, was examined in human vulva carcinoma tissue samples and in cultured cells representing this malignancy. In addition, the effects of Ad-DCN transduction on cell viability and proliferation, and on the expression and activity of the ErbB/HER family members, were investigated in cultured human vulva carcinoma cell lines.
Materials and Methods
Human Vulva Carcinoma Tissue Samples
Human vulva carcinoma tissue microarray (TMA) samples were derived from 100 patients, diagnosed and operated at the Turku University Hospital, Turku, Finland, between the years 2000 and 2013. The characteristics of the study population are presented in Table 1. A pathologist reviewed all slides from the resections. Both full patient records and paraffin-embedded tissue samples were available. Tissue cores (1 mm) from both the peripheral invasive front and the central primary tumor, adjacent mucous membrane, and the lymph nodes were collected for the TMA. HPV status of the samples was evaluated by p16INK4A immunostaining. Tissue samples with >25% tumor cell positivity for p16INK4A were considered HPV positive.23 The primary samples were obtained from the archives of Auria Biobank (Laboratory Division, Turku University Hospital, Turku, Finland). The use of the human primary tissue samples was approved by the local Ethics Committee (number ETMK 61/1802/2013) and Auria Biobank (number AB15-9293), and in accordance with the Finnish Biobank Act (688/2012). A separate informed consent from individual patients was waived.
Table 1.
Characteristics of the Study Population.
| Variable | Patients or Mean |
|---|---|
| Total patient number | 100 |
| Age at surgery (years) | 69.4 (range: 37.5–96.2) |
| Primary site of the tumor | |
| Vulva | 100 |
| Histology | |
| SCC | 94 |
| SCC in situ | 2 |
| SCC microinvasive | 3 |
| Verrucous carcinoma | 1 |
| Grade | |
| I | 38 |
| II | 39 |
| III | 19 |
| Unknown | 4 |
| HPV statusa | |
| HPV positive | 27 |
| HPV negative | 73 |
| Local recurrence | 11 |
| Lymph node metastasis | 28 |
Abbreviations: SCC, squamous cell carcinoma; HPV, human papilloma virus.
Estimation of HPV status by p16INK4A immunostaining. Tissue samples with >25% tumor cell positivity for p16INK4A were considered to be HPV positive.
Decorin In Situ Hybridization (ISH)
ISH for decorin was performed using antisense and sense single-stranded digoxigenin (DIG)-labeled RNA riboprobes for decorin on 5µm tissue sections as previously described in detail.24
Immunohistochemistry (IHC)
IHC staining for decorin was performed on 5µm tissue sections using a rabbit polyclonal decorin antibody (H-80; Santa Cruz Inc., Dallas, TX; diluted 1:50) as previously described in detail.24 IHC staining for Ki-67 with rabbit monoclonal antibody against Ki-67 (clone 30-9, Ventana/Roche Diagnostics, Tuscon, AZ; dilution 1:200) was performed with a Bench Mark XT immunostainer and ultraVIEW Universal DAB Detection Kit (Ventana/Roche).17
Imaging of Tissue Samples
The tissue samples were imaged with the Pannoramic Digital Slide scanner (The Pannoramic 250 Flash; 3DHISTECH Ltd., Budapest, Hungary). The images were viewed and edited with the Pannoramic Viewer (3DHISTECH Ltd.).
Cell Lines
Human primary vulva carcinoma cell lines UM-SCV-1B and UM-SCV-7 were derived from fresh tumor samples as previously described.25,26 The A431 vulva carcinoma cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA). The characteristics of the cell lines are presented in Table 2.25,26 All cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM; Gibco/Thermo Fisher Scientific, Waltham, MA) with 10% fetal bovine serum (FBS; Biochrom AG, Berlin, Germany), penicillin (100 IU/ml), and streptomycin (100 µg/ml) (Sigma Aldrich, St Louis, MO) at 37C with 5% CO2.
Table 2.
Characteristics of Vulva Carcinoma Cell Lines.
| Cell Line | Origin | Age | Histologic Gradea | TNM Classificationb | Survival After Initial Diagnosis | Doubling Time in Vitro (hr) |
|---|---|---|---|---|---|---|
| UM-SCV-1B | Pleural effusion | 62 | III | T3N2M1 | 2 months | 34 |
| UM-SCV-7 | Vulva | 77 | I-III | T2N2M0 | 1 month | 37 |
| A431 | Vulva | 85 | Unknown | Unknown | Unknown | 14 |
Patients’ age, histologic grade and TNM classification at the time of initial diagnosis, survival of the patient after initial diagnosis, and doubling time of the cell lines in vitro.
Histologic grading: well or poorly differentiated squamous cell carcinoma.
TNM status of primary tumors according to American Joint Committee of Staging.
Decorin cDNA Transduction and Cell Viability
Construction of the adenoviral vectors Ad-DCN and Ad-LacZ has been described earlier.17 Transductions of human vulva carcinoma cells were performed as previously described with minor modifications.17 First, 550 x 103 cells (UM-SCV-7 and UM-SCV-1B) or 2 x 106 cells (A431) were seeded to 25cm2 cell culture flasks. After 24 hr, the cells were transduced with medium containing 100 pfu/cell of Ad-DCN or Ad-LacZ. The transductions were repeated the next day. After the following 24 hr, all cells were given fresh medium and cultured for additional 24 hr. Non-transduced cultures were used as controls and were given fresh medium at the above time points.
To examine the effect of Ad-DCN transduction on the viability of cultured human primary UM-SCV-7 vulva carcinoma cells, attached and detached Trypan blue (Trypan Blue Dye; Bio-Rad Laboratories, Hercules, CA) positive cells were counted at the end of the experiments using Bio-Rad TC20 automated cell counter (Bio-Rad Laboratories).
Quantification of Ki-67
The effect of Ad-DCN transduction on the proliferation index of cultured human primary UM-SCV-7 vulva carcinoma cells was examined by quantification of Ki-67 staining. The cells were seeded to eight-well chamber slides (Thermo Scientific, Rochester, NY), 50 x 103 cells/well, and transduced as described above. At the end of the experiments (see above), the cells were fixed with 4% paraformaldehyde in PBS (Gibco/Thermo Fisher Scientific, Waltham, MA) for 48 hr, washed and stained for Ki-67. The wells of the chamber slides were photographed under a microscope using 10 x magnification. The percentage of Ki-67-positive cells was determined based on nine snapshots of triplicates of Ad-DCN transduced, Ad-LacZ transduced, or non-transduced control cultures using ImmunoRatio, a publicly available web application for Ki-67 analysis.27
Quantitative Reverse Transcriptase PCR
Total RNA of the UM-SCV-7, UM-SCV-1B, and A431 cell cultures was isolated using the Nucleospin RNA II kit (Marchery-Nagel, Bethlehem, PA) according to the manufacturer ’s protocol, and RNA concentrations were determined using a Nano-drop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The cDNA was synthesized from 1 µg of RNA using the SensiFAST cDNA synthesis kit (Bioline, London, UK) according to the manufacturer ’s instructions. Quantitative reverse transcriptase PCR (RT-qPCR) analyses for decorin and the housekeeping hRPL19 gene expression in human vulva carcinoma cells were performed with DyNAmo Flash SYBR Green qPCR kit (Thermo Fisher Scientific). RT-qPCR analyses for EGFR/HER1, ErbB2/HER2, ErbB3/HER3, ErbB4/HER4 (JM-a) isoform, and GAPDH expression were performed as previously described.28 All samples were run in triplicates. The primers and the probes are presented in Table 3. Thermal cycling was performed with QuantStudio 12K Flex Real-Time PCR System (Thermo Fisher Scientific). Relative changes in gene expression were calculated with the 2-∆∆CT method.29
Table 3.
Sequences for Specific Primers and TaqMan Probes.
| Gene | Primer/Probea | Sequence |
|---|---|---|
| decorin | primer forward | 5 ’-GGA ATT GAA AAT GGG GCT TT-3 ’ |
| primer reverse | 5 ’-GCC ATT GTC AAC AGC AGA GA-3 ’ | |
| hRPL19 | primer forward | 5 ’-AGG CAC ATG GGC ATA GGT AA-3 ’ |
| primer reverse | 5 ’-CCA TGA GAA TCC GCT TGT TT-3 ’ | |
| EGFR/HER1 | primer forward | 5 ’-CCA CCT GTG CCA TCC AAA CT-3 ’ |
| primer reverse | 5 ’-GGC GAT GGA CGG GAT CTT-3 ’ | |
| probe | 5 ’-CCA GGT CTT GAA GGC TGT CCA ACG AAT-3 ’ | |
| ErbB2/HER2 | primer forward | 5 ’-AGC CTT GCC CCA TCA ACT G-3 ’ |
| primer reverse | 5 ’-ATT GCC AAC CAC CGC AGA-3 ’ | |
| probe | 5 ’-CCA CTC CTG TGT GGA CCT GGA TGA CA-3 ’ | |
| ErbB3/HER3 | primer forward | 5 ’-CCC TGC CAT GAG AAC TGC AC-3 ’ |
| primer reverse | 5’-TCA CTG TCA AAG CCA TTG TCA GAT-3 ’ | |
| probe | 5 ’-GTT TGT CCT AAA CAG TCT TGA AGC TCT GGT C-3 ’ | |
| ErbB4/HER4 (JM-a) | primer forward | 5 ’-CCA CCC ATG CCA TCC AAA-3 ’ |
| primer reverse | 5 ’-CCA ATT ACT CCA GCT GCA ATC A-3 ’ | |
| probe | 5 ’-CAT GGA CGG GCC ATT CCA CTT TAC CA-3 ’ | |
| GAPDH | primer forward | 5 ’-AGC CAC ATC GCT CAG ACA C-3 ’ |
| primer reverse | 5 ’-GCC CAA TAC GAC CAA ATC C-3 ’ | |
| probe | universal fluorescent probe #60 (Universal ProbeLibrary, Roche) |
Probes were labelled with fluorescent reporter dye FAM. Abbreviations: EGFR, epidermal growth factor receptor.
Specific probes used for TaqMan RT-qPCR.
Western Blot Analyses for ErbB/HER Family Members, Akt, and p62
The human primary vulva carcinoma cell lines UM-SCV-1B, UM-SCV-7, and commercial A431 cells were cultured and transduced with Ad-DCN as described above. Thereafter, the cells were washed with PBS and lysed with lysis buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 % Triton X-100) with added protease and phosphatase inhibitors (Thermo Fisher Scientific). The protein concentration was measured with Bradford protein assay (Bio-Rad Laboratories). Equal amounts of protein samples were separated with SDS-PAGE and transferred to nitrocellulose membranes. Membranes were analyzed by Western blotting using the primary antibodies against EGFR/HER1 (#2232), ErbB2/HER2 (#2165), ErbB3/HER3 (#4754), phospho-EGFR/HER1 (#2220), phospho-ErbB2/HER2 (#2243), phospho-ErbB3/HER3 (#4791), Akt (pan) (#2920) (Cell Signaling Technology, Danvers, MA, USA), and p62/SQSTM1 (NBP1-48320) (Novus Biologicals, Centennial CO, USA). Anti-ß-actin was used as a loading control (A5441) (Sigma-Aldrich). The Western blots were developed using the WesternBright Quantum Western blotting detection kit (Advansta, Menlo Park, CA). Signals from Western blot analyses of three independent experiments were quantified using Image Studio software (LICOR, Lincoln, NE). The quantified data were normalized by actin signal in each sample.
Statistical Analyses
Student’s t-test was used in statistical analyses to compare continuous variables between the groups. All values p < 0,05 were considered statistically significant.
Results
Localization of Decorin mRNA and Immunoreactivity in Human Vulva Carcinoma Tissue Samples
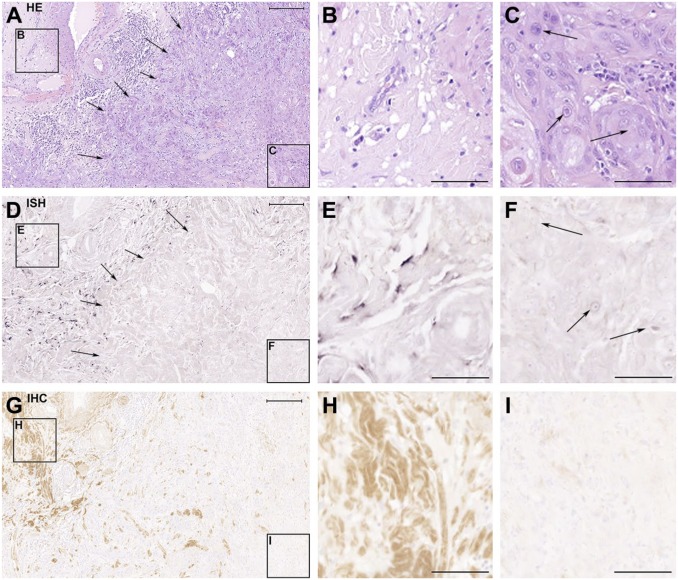
Serial sections of TMA samples from 100 vulva carcinoma patients were used to localize decorin mRNA and immunoreactivity. Based on the HE staining, the samples were shown to contain both non-malignant and malignant cell areas (Fig. 1A to C). The ISH results of the samples (Fig. 1D to F) demonstrated that malignant cells in human vulva carcinoma tissues lack decorin mRNA expression (Fig. 1D and F) and that decorin is expressed only in the areas populated by non-malignant stromal cells (Fig. 1D and E). These results were verified by IHC staining for decorin of consecutive tissue samples demonstrating that decorin immunoreactivity could merely be detected in non-malignant tissue areas (Fig. 1G and H), and not in areas populated solely by malignant cells (Fig. 1G and I). Identical results were obtained for all the vulva carcinoma tissue samples of this study, independently on the patient’s age, tumor stage, grade, or HPV status.
Figure 1.
Human vulva carcinoma cells are negative for decorin expression in vivo. The panel consists of serial sections of a representative human vulva carcinoma tissue sample analyzed with hematoxylin and eosin staining (A-C), ISH for decorin (D-F), and IHC for decorin (G-I). The frames on the left side of the panels A, D, and G mark non-malignant tissue areas, shown magnified in panels B, E, and H, respectively. The frames on the right side of the panels A, D, and G mark carcinoma areas, and they are shown magnified in panels C, F, and I, respectively. Arrows in A and D mark the border between malignant and non-malignant tissue areas. Arrows in C and F point to squamous carcinoma cells. Positive ISH signal for decorin can be seen in purple (D and E). Positive immunostaining for decorin can be seen in brown in G and H. Note that there is a total absence of decorin mRNA expression and immunoreactivity in panels F and I that represent tissue areas populated by malignant cells. Scale bar in A, D, and G 500 µm and in B, C, E, F, H, and I 200µm. Abbreviations: HE, hematoxylin and eosin; ISH, in situ hybridization; IHC, immunohistochemistry.
Expression of Decorin by Human Vulva Carcinoma Cell Lines
To verify the results obtained from analyses of tissue samples, decorin mRNA expression was also analyzed in the UM-SCV-1B, UM-SCV-7, and A431 human vulva carcinoma cell lines. RT-qPCR analyses revealed that decorin is not expressed by any of the above cell lines (data not shown).
Effects of Ad-DCN Transduction on Cultured Primary Human Vulva Carcinoma Cells
Ad-DCN transduction of human primary UM-SCV-7 vulva carcinoma cell cultures markedly increased the number of detached cells (Fig. 2A) compared to Ad-LacZ transduced cell cultures (Fig. 2B). The number of live cells was significantly lower in Ad-DCN transduced cell cultures compared to Ad-LacZ transduced cultures (Fig. 2C). Compared to Ad-LacZ transduction, Ad-DCN transduction caused an increase in the number of dead cells as shown by Trypan blue staining (Fig. 3). In contrast, the proliferation index determined by Ki-67 staining did not differ between Ad-DCN and Ad-LacZ transduced cultures (Fig. 4).
Figure 2.
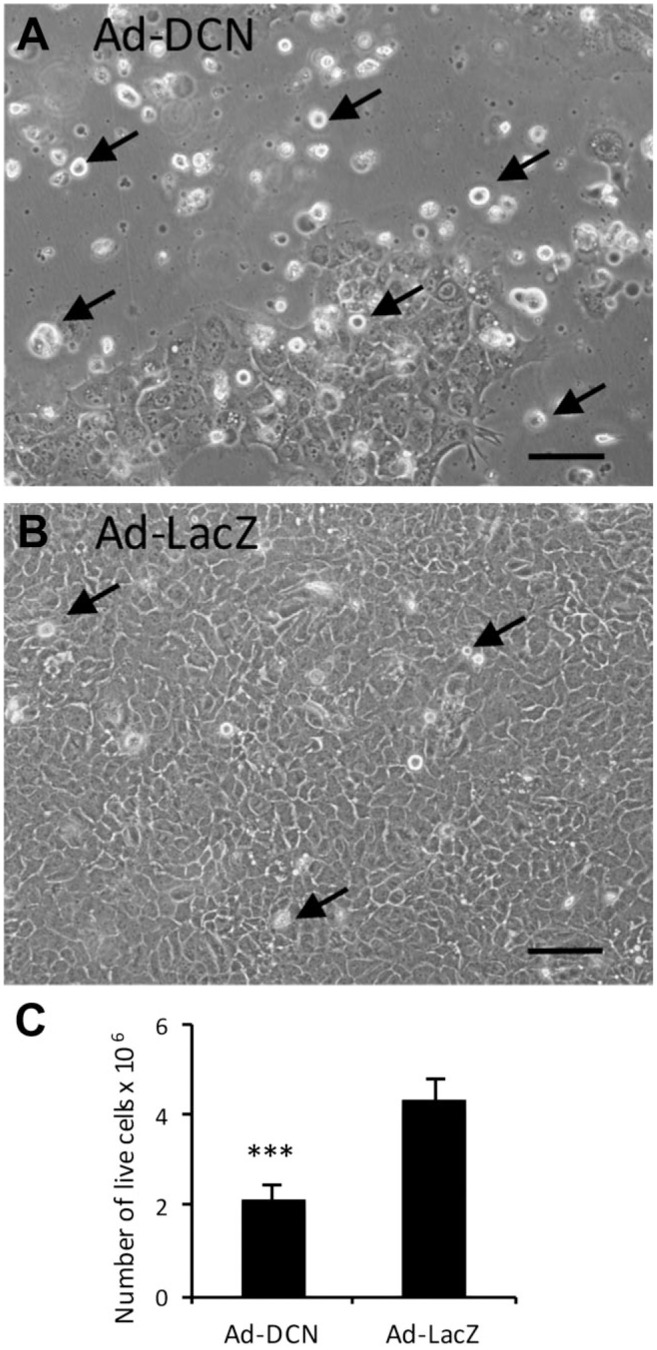
Ad-DCN transduction decreases the viability of cultured human primary UM-SCV-7 vulva carcinoma cells. Images of human primary vulva carcinoma cell cultures after Ad-DCN transduction (A) and Ad-LacZ transduction (B). Histogram (C) showing the number of live cells (Trypan blue negative cells) 3 days after Ad-DCN and Ad-LacZ transductions. Arrows in A and B mark representative non-viable (detached) cells. Capped bars on top of the columns mark standard deviation. ***p<0.001, Student’s t-test. Scale bars, 100 µm. Abbreviation: Ad-DCN, adenovirus-mediated decorin cDNA.
Figure 3.
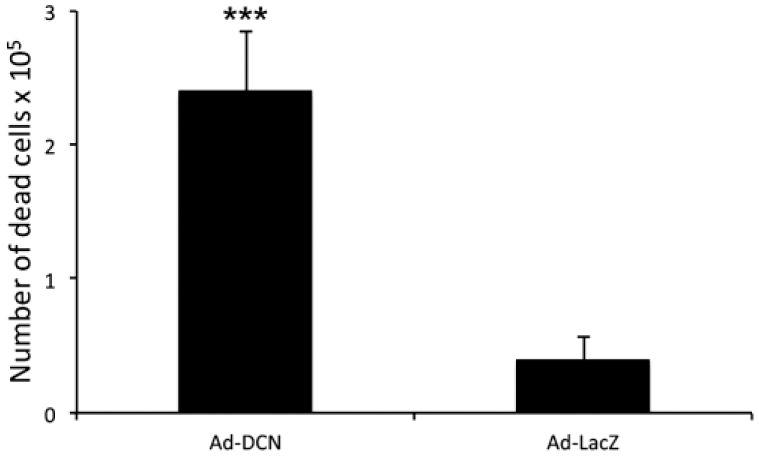
Ad-DCN transduction promotes death of human primary UM-SCV-7 vulva carcinoma cells. Histogram showing the number of dead cells (Trypan blue positive) 3 days after Ad-DCN and Ad-LacZ transductions. Capped bars on top of the columns mark standard deviation. ***p<0,001, Student’s t-test. Abbreviation: Ad-DCN, adenovirus-mediated decorin cDNA.
Figure 4.
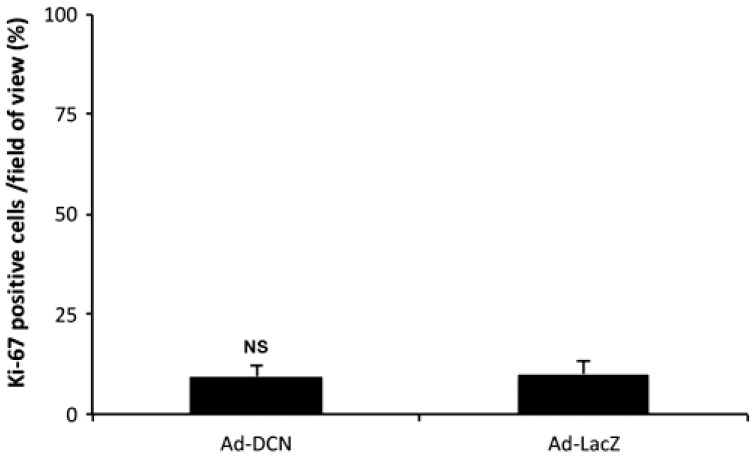
Proliferation index of cultured human primary UM-SCV-7 vulva carcinoma cells in Ad-DCN and Ad-LacZ transduced cultures. Histogram showing percentage of Ki-67-positive cells/field of view based on nine snapshots of Ad-DCN-transduced and Ad-LacZ-transduced cell cultures. Capped bars on top of the columns mark standard deviations. Student’s t-test. Abbreviation: Ad-DCN, adenovirus-mediated decorin cDNA; NS, non-significant.
mRNA Expression of ErbB/HER Family Members in Cultured Human Primary Vulva Carcinoma Cells
The ErbB/HER family members have been shown to be overexpressed in different cancers.30–32 Here, the expression of these receptors was examined in cultured human primary UM-SCV-7 vulva carcinoma cells. The results showed that these human vulva carcinoma cells express detectable amounts of three of the four ErbB/HER family members, namely EGFR/HER1, ErbB2/HER2 and ErbB3/HER3 (Fig. 5). EGFR/HER1 was found to be the receptor with the highest expression level in these cells (Fig. 5).
Figure 5.
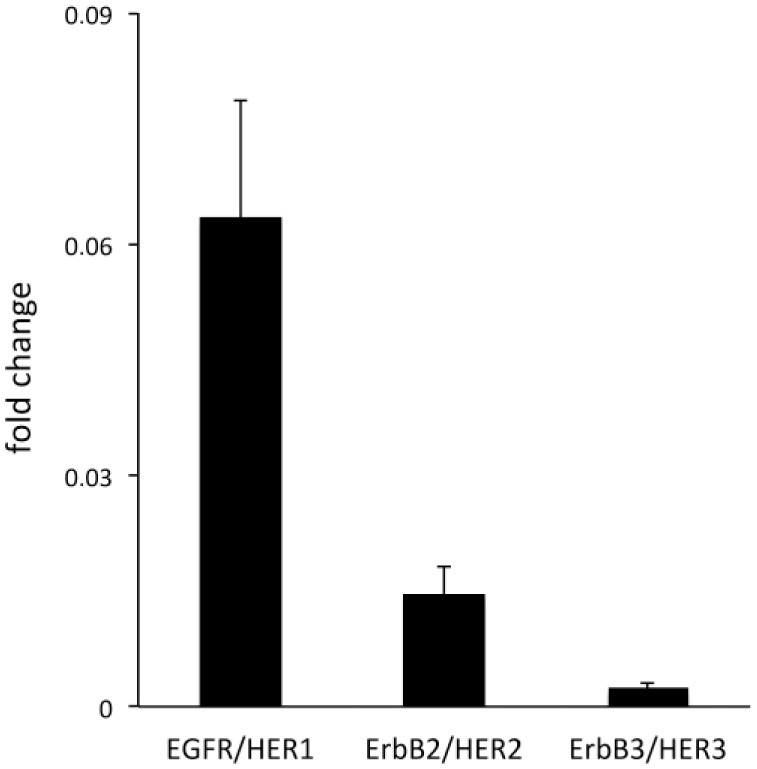
Expression of epidermal growth factor receptor family (ErbB/HER) members in human primary UM-SCV-7 vulva carcinoma cells. Relative mRNA expression of ErbB/HER family members in a human primary vulva carcinoma cell line (UM-SCV-7). Note that the cells express three of the four ErbB/HER family members, namely EGFR/HER1, ErbB2/HER2, and ErbB3/HER3, and not ErbB4/HER4. Abbreviation: EGFR, epidermal growth factor receptor.
Effect of Ad-DCN Transduction on the Expression and Activity of ErbB/HER Receptors in Human Vulva Carcinoma Cells
Decorin has previously been shown to downregulate the expression and activity of two members of the ErbB/HER family, namely EGFR/HER1 and ErbB2/HER2.12,33 The mRNA expression of these receptors, and also the expression of the ErbB3/HER3 receptor were studied in Ad-DCN and Ad-LacZ transduced human primary UM-SCV-7 cells. The RT-qPCR results revealed that Ad-DCN transduction causes a statistically significant downregulation of ErbB2/HER2 mRNA expression in these primary human vulva carcinoma cells (Fig. 6A). In contrast, no changes in the expression of EGFR/HER1 or ErbB3/HER3 could be detected (data not shown). Next, the protein levels of the EGFR/HER1, ErbB2/HER2, and ErbB3/HER3 and their phosphorylated forms were analyzed by Western blot in Ad-DCN transduced and Ad-LacZ transduced primary UM-SCV-7 and UM-SCV-1B vulva carcinoma cell lines and in the A431vulva carcinoma cell line. In concordance with the RT-qPCR results, Western blot analyses revealed that the amount of ErbB2/HER2 (Fig. 6B) and also its phosphorylated form (pErbB2/pHER2) (Fig. 6C) was decreased in Ad-DCN compared to Ad-LacZ transduced UM-SCV-7, UM-SCV-1B, and A431 vulva carcinoma cells. In contrast, no comparable consistent effects of Ad-DCN transduction on the expression and activity of EGFR/HER1 or ErbB3/HER3 were found (Supplemental Fig. 1). Decorin protein core has previously been shown to downregulate EGFR in the A431 cell line.33 In this study, the activity of this receptor was found to be downregulated in the Ad-DCN transduced A431 cell line (Supplemental Fig. 2A). Furthermore, the amount and activity of ErbB2/HER2 were reduced in the A431 cell line (Supplemental Fig. 2B).
Figure 6.
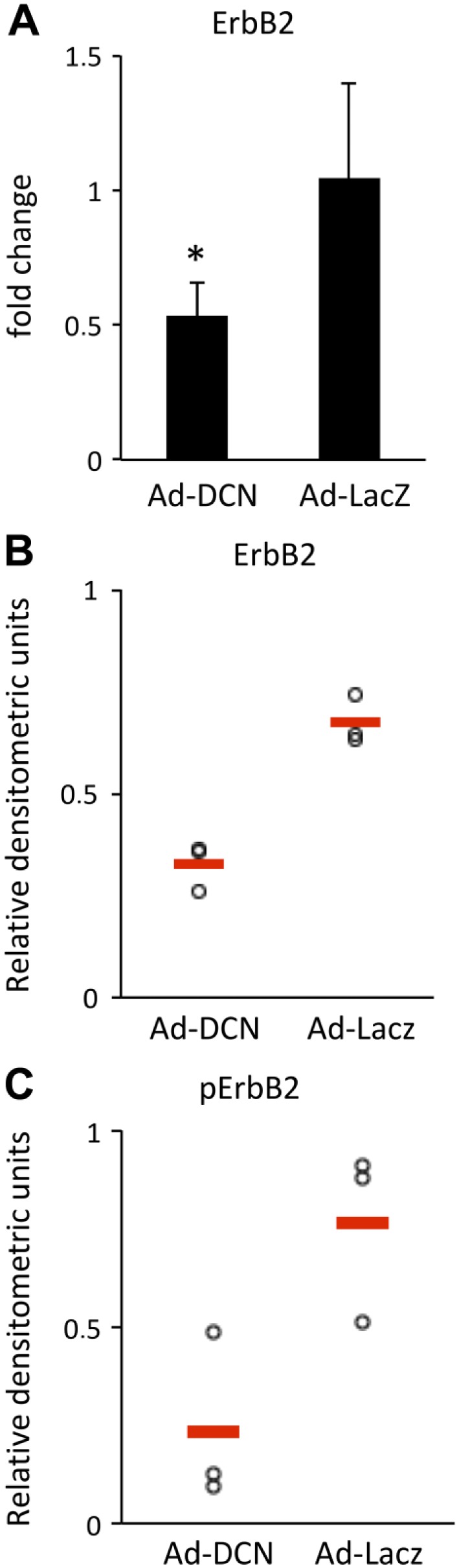
ErbB2/HER2 expression and activity are downregulated in response to Ad-DCN transduction. Histogram showing ErbB2/HER2 mRNA expression in Ad-DCN transduced and AD-LacZ transduced human primary UM-SCV-7 cells (A). Note that the ErbB2/HER2 mRNA expression is significantly downregulated in Ad-DCN transduced cells compared to Ad-LacZ transduced cells. Representative results from Western blot analyses of ErbB2/HER2 expression and activity in human vulva carcinoma cells (B, C). Quantification of signals from Western blot analyses of ErbB2/HER2 expression in Ad-DCN transduced and Ad-LacZ transduced human primary UM-SCV-7 vulva carcinoma cells from three independent experiments. Ad-DCN transduction decreases the total amount of ErbB2/HER2 protein (B) and its phosphorylated form (C) in human vulva carcinoma cells. Individual experiments are presented by dots. Means of the individual experiments are shown by line. Abbreviation: Ad-DCN, adenovirus-mediated decorin cDNA.
Effect of Ad-DCN Transduction on the Expression of Akt in Human Primary Vulva Carcinoma Cells
Because ErbB2/HER2 is known to influence cell survival via the PI3-Akt pathway, the effect of Ad-DCN transduction on the amount of Akt protein in human primary UM-SCV-7 vulva carcinoma cells was also examined.34 The results demonstrated that Ad-DCN transduction decreased the amount of Akt protein (Fig. 7) concomitantly to ErbB2/HER2 (see above).
Figure 7.
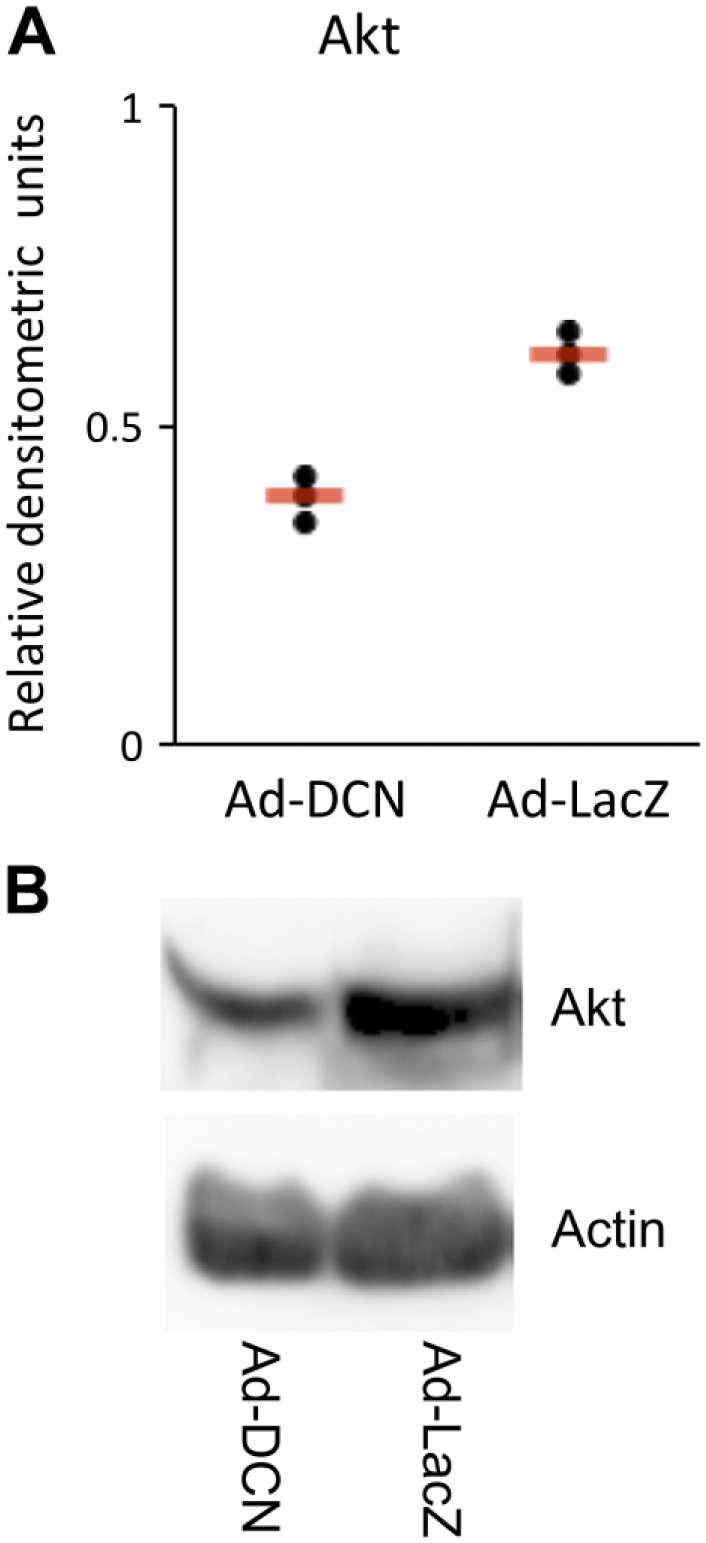
Ad-DCN transduction decreases the protein level of Akt in human primary UM-SCV-7 vulva carcinoma cells. Quantified signals from three independent Western blot analyses of Akt in Ad-DCN and Ad-LacZ transduced human primary UM-SCV7 vulva carcinoma cells (A). The amount of Akt protein is decreased in Ad-DCN transduced cells compared to Ad-LacZ transduced cells (A). Signals from individual experiments are presented as dots and the means of the experiments are shown by line. Representative Western blot image of Akt protein expression in Ad-DCN and Ad-LacZ transduced human primary UM-SCV7 vulva carcinoma cells (B). Abbreviation: Ad-DCN, adenovirus-mediated decorin cDNA.
Effect of Ad-DCN Transduction on the Expression of p62 in Human Vulva Carcinoma Cells
Decorin has previously been shown to provoke autophagy.8 Therefore, we wanted to investigate whether the Ad-DCN-induced decrease in cell viability is associated with changes in the amount of the validated autophagy marker P62 in human primary UM-SCV-7 vulva carcinoma cells. The results showed that the expression level of p62 was not affected by Ad-DCN transduction in UM-SCV-7 human primary vulva carcinoma cells (Supplemental Fig. 3).
Discussion
In the present study, decorin expression was analyzed in tissue samples from 100 vulva carcinoma patients. The results showed that malignant cells of this carcinoma were completely negative of decorin expression and that decorin expression could only be detected in tissue areas populated by non-malignant stromal cells. To the best of our knowledge, no previous studies have been published regarding decorin expression in human vulva carcinoma tissues. The results of this study are consistent with our previous studies demonstrating that malignant cells of various other human epithelial cancers, namely human breast, bladder, and colon carcinomas lack decorin expression.17–20 The expression of decorin was further studied in cultured human primary and commercial vulva carcinoma cell lines. In agreement with the above tissue sample data, no decorin expression could be detected in these cell lines.
The oncosuppressive potential of decorin has earlier been studied in various experimental settings targeting different cancer types.10,35–37 In this study, the effects of Ad-DCN transduction on the viability and proliferation of cultured human vulva carcinoma cells were examined. The results showed that Ad-DCN transduction considerably decreased cell viability of the cell lines.
Decorin is known to act as a potent inhibitor of RTKs, primarily by inhibiting the signaling pathways of EGFR/HER1, Met, and VEGFR-2.4,7,38 Decorin has also been shown to induce apoptosis via activation of caspase-3.33 Earlier, EGFR/HER1 has been shown to contribute to the progression of invasive phenotype of vulvar squamous lesions.39 Moreover, the expression of EGFR/HER1 together with the overexpression of EGF has been linked with the proliferation of neoplastic squamous cells in vulva.40 Furthermore, amplification of EGFR/HER1 gene has been found in vulva carcinomas that were associated with decreased survival of the patients.41 Therefore, in this study, we analyzed the expression of the EGFR/HER1 and ErbB2-4/HER2-4 in human vulva carcinoma cell lines. We also examined whether Ad-DCN transduction is able to modulate the expression and/or activity of these receptors. The results showed that human vulva carcinoma cells express three of the four members of the ErbB/HER family, namely EGFR/HER1, ErbB2/HER2, and ErbB3/HER3. Furthermore, the results showed that Ad-DCN transduction significantly downregulated ErbB2/HER2 mRNA expression. This downregulation was also detected at the protein level, i.e., Ad-DCN transduction decreased both ErbB2/HER2 protein and its phosphorylated form in all of the human vulva carcinoma cell lines used in this study. In contrast, no consistent effect on the expression and activity of EGFR/HER1 and ErbB3/HER3 could be established. Delivery of decorin protein core has previously been shown to downregulate EGFR in A431 vulva carcinoma cells.33 In accordance with this, in the present study, Ad-DCN transduction was found to downregulate the activity of EGFR/HER1. To the best of our knowledge, this is the first study reporting decorin-induced downregulation of ErbB2/HER2 in gynecological cancers.
The ErbB2/HER2 receptor signals primarily through two different pathways, the MAPK pathway that stimulates cell proliferation, and the PI3K-Akt pathway that promotes tumor cell survival by phosphorylation of pro-apoptotic molecules.34 Considering that Ad-DCN transduction decreased cell viability of vulva carcinoma cells, it can be speculated that this effect might to some extent be mediated through the PI3K-Akt signaling pathway. Indeed, our result from analyses regarding the amount of Akt protein in Ad-DCN-transduced human vulva carcinoma cells showed that in parallel with ErbB2/HER2 gene expression and protein activity also the amount of Akt protein was decreased in these cells. This implies that the PI3K-Akt signaling pathway is involved in the decreased cell viability seen in this study.
Decorin has recently been shown to induce autophagy and mitophagy in, e.g., breast cancer and glioma cells by activating proautophagic molecules downstream of VEGFR2 and Met.8,42–44 The degradation of p62 has previously been associated with decorin-provoked autophagy in glioma cells.43 Therefore, we wanted to explore whether the decrease in cell viability seen in this study could be associated with changes in this well-established marker of autophagy.45,46 However, the expression level of p62 was not affected by Ad-DCN transduction in vitro.
Because the treatment alternatives for vulva carcinoma are still limited, additional therapeutic approaches are needed. Erlotinib, a selective inhibitor of the EGFR/HER1, has been used in clinical trials on vulva carcinoma with promising but unfortunately only short-term effects.47,48 At present, 28 different clinical trials for vulva carcinoma are recruiting according to the ClinicalTrials.gov database (https://clinicaltrials.gov/). Indeed, with the increasing knowledge of the pathogenesis and molecular background of vulva carcinoma, more potent therapeutics ought to be developed. The results of the present study suggest that decorin cDNA transduction provides a potential additional candidate therapy for the treatment of human vulva carcinoma in the future. The results of this study revealed that human vulva carcinoma cells are devoid of decorin expression. The results also showed, that transduction of cultured human vulva carcinoma cells with Ad-DCN reduces cell viability. Moreover, Ad-DCN transduction significantly downregulated ErbB2/HER2 expression and activity in these cells. The results of this study further strengthen the role of decorin as an oncosuppressive molecule, and also emphasizes the importance of a continued development of decorin-based adjuvant therapies for human epithelial cancers such as human vulva carcinoma.
Supplemental Material
Supplemental material, DS_10.13690022155419845373 for Decorin Expression in Human Vulva Carcinoma: Oncosuppressive Effect of Decorin cDNA Transduction on Carcinoma Cells by Marie C. Nyman, Anne B. Jokilammi, Pia C. Boström, Samu H. Kurki, Annele O. Sainio, Seija E. Grenman, Katri J. Orte, Sakari H. Hietanen, Klaus Elenius and Hannu T. Järveläinen in Journal of Histochemistry & Cytochemistry
Acknowledgments
The authors thank Ms. Sinikka Kollanus for her excellent technical assistance and Mr. Jaakko Liippo for his valuable assistance with the images.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HJ conceived and designed the experiments. MN and AJ carried out the experiments. MN, AJ, PB, SK, KO, KE, and HJ analyzed the data. KO, SH, and SG were responsible for the tissue samples and the cell lines. MN, AS, and HJ were primarily responsible for writing the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the funds of Varsinais-Suomi Health District and Satakunta Central Hospital, The Cancer Society of Southwestern Finland, The Swedish Cultural Foundation in Finland, The Waldemar von Frenckell Foundation, the Medical Foundation for Life and Health in Finland, and the Finnish-Norwegian Medical foundation. The funding from the Jane and Aatos Erkko Foundation (grant number 170046) is acknowledged.
Contributor Information
Marie C. Nyman, Medical Biochemistry and Genetics, Institute of Biomedicine, University of Turku, Turku, Finland
Anne B. Jokilammi, Medical Biochemistry and Genetics, Institute of Biomedicine, University of Turku, Turku, Finland
Pia C. Boström, Department of Pathology, University of Turku, Turku, Finland Turku University Hospital, Turku, Finland.
Samu H. Kurki, Auria Biobank, University of Turku, Turku, Finland Turku University Hospital, Turku, Finland.
Annele O. Sainio, Medical Biochemistry and Genetics, Institute of Biomedicine, University of Turku, Turku, Finland
Seija E. Grenman, Department of Obstetrics and Gynecology, Turku University Hospital, Turku, Finland
Katri J. Orte, Department of Pathology, University of Turku, Turku, Finland Turku University Hospital, Turku, Finland.
Sakari H. Hietanen, Department of Obstetrics and Gynecology, Turku University Hospital, Turku, Finland
Klaus Elenius, Medical Biochemistry and Genetics, Institute of Biomedicine, University of Turku, Turku, Finland; Department of Oncology, Turku University Hospital, Turku, Finland; Medicity Research Laboratory, Turku, Finland.
Hannu T. Järveläinen, Medical Biochemistry and Genetics, Institute of Biomedicine, University of Turku, Turku, Finland; Department of Internal Medicine, Satakunta Central Hospital, Pori, Finland.
Literature Cited
- 1. Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017. May 1;7(5):1016–36. [PMC free article] [PubMed] [Google Scholar]
- 2. Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014. December;15(12):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988. June 1;252(2):313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998. January 15;101(2):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990. July 19;346(6281):281–4. [DOI] [PubMed] [Google Scholar]
- 6. Bi X, Yang W. Biological functions of decorin in cancer. Chin J Cancer. 2013. May;32(5):266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011. August;25(8):1431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci U S A. 2013. July;110(28):E2582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci U S A. 1999. March 16;96(6):3092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neill T, Schaefer L, Iozzo RV. Decorin as a multivalent therapeutic agent against cancer. Adv Drug Deliv Rev. 2016. February 1;97:174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sainio AO, Järveläinen HT. Decorin-mediated oncosuppression: a potential future adjuvant therapy for human epithelial cancers. Br J Pharmacol. 2019;176:5–15. doi: 10.1111/bph.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000. November 10;275(45):35153–61. [DOI] [PubMed] [Google Scholar]
- 13. Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999. February 19;274(8):4489–92. [DOI] [PubMed] [Google Scholar]
- 14. Bozoky B, Savchenko A, Guven H, Ponten F, Klein G, Szekely L. Decreased decorin expression in the tumor microenvironment. Cancer Med. 2014. June;3(3):485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shridhar V, Lee J, Pandita A, Iturria S, Avula R, Staub J, Morrissey M, Calhoun E, Sen A, Kalli K, Keeney G, Roche P, Cliby W, Lu K, Schmandt R, Mills GB, Bast RC, Jr, James CD, Couch FJ, Hartmann LC, Lillie J, Smith DI. Genetic analysis of early- versus late-stage ovarian tumors. Cancer Res. 2001. August 1;61(15):5895–904. [PubMed] [Google Scholar]
- 16. Smid-Koopman E, Blok LJ, Chadha-Ajwani S, Helmerhorst TJ, Brinkmann AO, Huikeshoven FJ. Gene expression profiles of human endometrial cancer samples using a cDNA-expression array technique: assessment of an analysis method. Br J Cancer. 2000. July;83(2):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boström P, Sainio A, Kakko T, Savontaus M, Söderström Järveläinen H. Localization of decorin gene expression in normal human breast tissue and in benign and malignant tumors of the human breast. Histochem Cell Biol. 2013. January;139(1):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sainio A, Nyman M, Lund R, Vuorikoski S, Boström P, Laato M, Boström PJ, Järveläinen H. Lack of decorin expression by human bladder cancer cells offers new tools in the therapy of urothelial malignancies. PLoS ONE. 2013. October 11;8(10):e76190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nyman MC, Sainio AO, Pennanen MM, Lund RJ, Vuorikoski S, Sundström JTT, Järvelainen HT. Decorin in human colon cancer: localization in vivo and effect on cancer cell behavior in vitro. J Histochem Cytochem. 2015. September;63(9):710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boström P, Sainio A, Eigėlienė N, Jokilammi A, Elenius K, Koskivuo I, Järveläinen H. Human metaplastic breast carcinoma and decorin. Cancer Microenviron. 2017. June 26;10(1–3):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Te Grootenhuis NC, Pouwer AW, de Bock GH, Hollema H, Bulten J, van der Zee AGJ, de Hullu JA, Oonk MHM. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol Oncol. 2017;148:622–31. [DOI] [PubMed] [Google Scholar]
- 22. Yap J, O’Neill D, Nagenthiran S, Dawson CW, Luesley DM. Current insights into the aetiology, pathobiology, and management of local disease recurrence in squamous cell carcinoma of the vulva. BJOG. 2017. May;12 4(6):946–54. [DOI] [PubMed] [Google Scholar]
- 23. Halec G, Alemany L, Quiros B, Clavero O, Höfler D, Alejo M, Quint W, Pawlita M, Bosch FX, de Sanjose S. Biological relevance of human papillomaviruses in vulvar cancer. Mod Pathol. 2017. April;30(4):549–62. [DOI] [PubMed] [Google Scholar]
- 24. Salomäki HH, Sainio AO, Söderström M, Pakkanen S, Laine J, Järveläinen HT. Differential expression of decorin by human malignant and benign vascular tumors. J Histochem Cytochem. 2008. July;56(7):639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grenman SE, Van Dyke DL, Worsham MJ, England B, McClatchey KD, Hopkins M, Babu VR, Grenman R, Carey TE. Phenotypic characterization, karyotype analysis and in vitro tamoxifen sensitivity of new ER-negative vulvar carcinoma cell lines, UM-SCV-1A and UM-SCV-1B. Int J Cancer. 1990. May 15;45(5):920–7. [DOI] [PubMed] [Google Scholar]
- 26. Raitanen M, Worsham MJ, Lakkala T, Carey TE, Van Dyke DL, Grenman R, Klemi P, Rantanen V, Isola J, Grenman S. Characterization of 10 vulvar carcinoma cell lines by karyotyping, comparative genomic hybridization and flow cytometry. Gynecol Oncol. 2004. April;93(1):155–63. [DOI] [PubMed] [Google Scholar]
- 27. Tuominen VJ, Ruotoistenmäki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and ki-67. Breast Cancer Res. 2010;12(4):R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Junttila TT, Laato M, Vahlberg T, Söderström K, Visakorpi T, Isola J, Elenius K. Identification of patients with transitional cell carcinoma of the bladder overexpressing ErbB2, ErbB3, or specific ErbB4 isoforms: real-time reverse transcription-PCR analysis in estimation of ErbB receptor status from cancer patients. Clin Cancer Res. 2003. November 1;9(14):5346–57. [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001. December;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 30. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. January 9;235(4785):177–82. [DOI] [PubMed] [Google Scholar]
- 31. Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer. 2003. February;2(4):246–51. [DOI] [PubMed] [Google Scholar]
- 32. Selvaggi G, Novello S, Torri V, Leonardo E, De Giuli P, Borasio P, Mossetti C, Ardissone F, Lausi P, Scagliotti GV. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004. January;15(1):28–32. [DOI] [PubMed] [Google Scholar]
- 33. Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006. September 8;281(36):26408–18. [DOI] [PubMed] [Google Scholar]
- 34. Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009. Jul;9(7):463–75. [DOI] [PubMed] [Google Scholar]
- 35. Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002. May 23;21(23):3688–95. [DOI] [PubMed] [Google Scholar]
- 36. Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005. February;24(6):1104–10. [DOI] [PubMed] [Google Scholar]
- 37. Tralhão JG, Schaefer L, Micegova M, Evaristo C, Schönherr E, Kayal S, Veiga-Fernandes H, Danel C, Iozzo R, Kresse H, Lemarchand P. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer. FASEB J. 2003;17(3):464–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the met receptor. J Cell Biol. 2009. May 18;185(4):743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brustmann H. Epidermal growth factor receptor is involved in the development of an invasive phenotype in vulvar squamous lesions, but is not related to MIB-1 immunoreactivity. Int J Gynecol Pathol. 2007. October;26(4):481–9. [DOI] [PubMed] [Google Scholar]
- 40. Wu X, Xin Y, Yao J, Hasui K, Tsuyama S, Yonezawa S, Murata F. Expression of epithelial growth factor receptor and its two ligands, transforming growth factor-alpha and epithelial growth factor, in normal and neoplastic squamous cells in the vulva: an immunohistochemical study. Med Electron Microsc. 2001. September;34(3):179–84. [DOI] [PubMed] [Google Scholar]
- 41. Growdon WB, Boisvert SL, Akhavanfard S, Oliva E, Dias-Santagata DC, Kojiro S, Horowitz NS, Iafrate AJ, Borger DR, Rueda BR. Decreased survival in EGFR gene amplified vulvar carcinoma. Gynecol Oncol. 2008. November;111(2):289–97. [DOI] [PubMed] [Google Scholar]
- 42. Goyal A, Neill T, Owens RT, Schaefer L, Iozzo RV. Reprint of: decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biol. 2014. April;35:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yao T, Zhang C, Gong M, Zhang M, Wang L, Ding W. Decorin-mediated inhibition of the migration of U87MG glioma cells involves activation of autophagy and suppression of TGF-β signaling. FEBS Open Bio. 2016;6(7):707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neill T, Torres A, Buraschi S, Owens RT, Hoek JB, Baffa R, Iozzo RV. Decorin induces mitophagy in breast carcino6ma cells via peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and mitostatin. J Biol Chem. 2014. February 21;289(8):4952–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015. December;282(24):4672–8. [DOI] [PubMed] [Google Scholar]
- 46. Bjørkøy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Meth Enzymol. 2009;452:181–97. [DOI] [PubMed] [Google Scholar]
- 47. Bacha OM, Levesque E, Renaud MC, Lalancette M. A case of recurrent vulvar carcinoma treated with erlotinib, an EGFR inhibitor. Eur J Gynaecol Oncol. 2011;32(4):423–4. [PubMed] [Google Scholar]
- 48. Horowitz NS, Olawaiye AB, Borger DR, Growdon WB, Krasner CN, Matulonis UA, Liu JF, Lee J, Brard L, Dizon DS. Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecol Oncol. 2012. October;127(1):141–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.13690022155419845373 for Decorin Expression in Human Vulva Carcinoma: Oncosuppressive Effect of Decorin cDNA Transduction on Carcinoma Cells by Marie C. Nyman, Anne B. Jokilammi, Pia C. Boström, Samu H. Kurki, Annele O. Sainio, Seija E. Grenman, Katri J. Orte, Sakari H. Hietanen, Klaus Elenius and Hannu T. Järveläinen in Journal of Histochemistry & Cytochemistry