Abstract
Testicular tissue cryopreservation is an experimental method to preserve the fertility of prepubertal patients before they initiate gonadotoxic therapies for cancer or other conditions. Here we provide the proof of principle that cryopreserved prepubertal testicular tissues can be autologously grafted under the back skin or scrotal skin of castrated pubertal Rhesus macaques and matured to produce functional sperm. During the eight-to twelve-month observation period, grafts grew and produced testosterone. Complete spermatogenesis was confirmed in all grafts at the time of recovery. Graft-derived sperm were competent to fertilize Rhesus oocytes, leading to preimplantation embryo development, pregnancy, and the birth of a healthy female baby. Pending the demonstration that similar results are obtained in non-castrated recipients, testicular tissue grafting may be applied in the clinic.
Chemotherapy and radiation treatments for cancer or other conditions can deplete spermatogonial stem cells (SSCs) in the testis, resulting in permanent infertility (1–4). Adult men can cryopreserve sperm prior to gonadotoxic treatment that can be used in the future to have biological children using established assisted reproductive technologies. Sperm freezing is not an option for prepubertal boys who are not yet producing sperm (5, 6). This is an important human health concern because the survival rate of children with cancer is over 80% (7, 8), and 30% of childhood cancer survivors will be infertile as adults (9). The only fertility preservation option available for prepubertal boys is cryopreservation of testicular tissues, which contain SSCs (10, 11).
There are several cell-and tissue-based therapies in the research pipeline that may allow patients to use their cryopreserved testicular tissues to produce sperm and generate biological children (5, 12). Testicular tissue grafting and xenografting are mature technologies in which immature testicular tissues, containing SSCs, are grafted ectopically under the skin. Immature testicular tissues from mice, pigs, goats, rabbits, hamsters, dogs, cats, horses, cattle, and monkeys have been grafted under the back skin of immune-deficient nude mice and matured to enable spermatogenesis (reviewed (13)), and produce fertilization-competent sperm (mice, pigs, goats and monkeys (14–16)) and generate live offspring (mice, pigs, and monkeys (16–19)). Therefore, it is theoretically possible to graft immature testicular tissue from a childhood cancer survivor into an animal host to produce sperm that can be used in the IVF clinic to achieve pregnancy. However, the possibility that viruses or other xenobiotics could be transmitted from the animal host to humans needs to be carefully considered (20–22).
Three studies have reported autologous grafting of immature testicular tissue in nonhuman primates (23–25). Complete spermatogenesis was reported for fresh (24) or cryopreserved (25) testicular tissue grafts in the scrotum, but not under the back skin. Recovery of cryopreserved grafts was low (5%), with complete spermatogenesis observed in only 13 and 17% of seminiferous tubules in two surviving scrotal grafts (25). Sperm function was not tested by fertilization or production of offspring in those studies. Cryopreservation, perhaps for many years, is an essential component of the fertility preservation paradigm for prepubertal cancer survivors.
We modeled the prepubertal cancer survivor in Rhesus macaques and report that autologously grafted, frozen, and thawed prepubertal Rhesus testicular tissues can be matured to produce sperm that were competent to fertilize Rhesus oocytes, establish a pregnancy, and produce a healthy graft-derived baby (Grady).
Experimental design
Five prepubertal Rhesus macaques were hemicastrated (removal of one testis); testis tissues were cut into small pieces (9–20 mm3) and cryopreserved (Fig. 1A, see methods). Five to seven months after hemicastration, the remaining testis was removed and cut into small pieces (9–20 mm3). Some of that tissue was designated for fresh tissue grafting and the remaining tissue was cryopreserved. Immediately after removal of the second testis, fresh and cryopreserved tissue fragments (cryopreservation time ranged from 5 hours to 5 months) were autologously grafted under the back skin (3 sites fresh; 3 sites cryopreserved) and under the scrotal skin (1 side fresh and 1 side cryopreserved; Figure 1B). Surgical scissors were used to create a skin flap at each site and four testicular tissue fragments were independently sutured to the subcutaneous aspect of each skin flap (Fig. 1C). Average testicular tissue fragment weight at the time of grafting was 15.34 ± 1.54 mg. Matrigel was injected into four of the six sites on the back (2 cryopreserved and 2 fresh) and both scrotal sites to stimulate angiogenesis (Fig. 1B).
Figure 1: Autologous grafting ofprepubertal testicular tissue fragments.
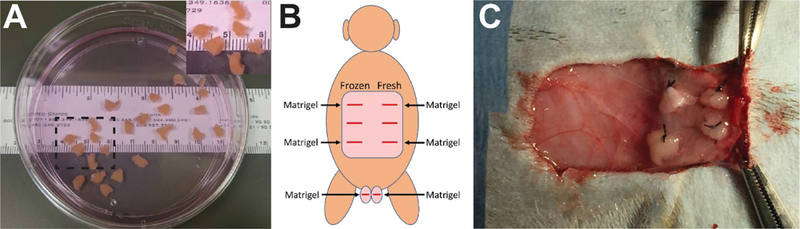
Fresh or cryopreserved testicular tissue fragments (9–20 mm3) from prepubertal monkeys (A). (A, Inset) Higher magnification image of area demarcated with dashed black box in (A). Testicular tissue fragments were grafted under the back skin or scrotal skin, as indicated in (B; viewed from the back of animal). Matrigel was added to four of the six graft sites on the back and both scrotal sites (B). Four pieces offresh or frozen-thawed testis tissue were sutured to the subcutaneous aspect of the skin at each graft site (C).
Pre-graft testicular tissue is immature, lacking spermatogenesis
Histological examination confirmed that testicular tissues of all animals were immature at the time of hemicastration and at the time of castration (Fig. 2A and B; Fig. S1). Undifferentiated stem/progenitor spermatogonia (types Adark and Apale) and differentiated spermatogonia (type B) were the only germ cells seen in the seminiferous tubules of four animals (13–022, 13–024, 13–026, and 13–030), whereas early meiotic cells (pachytene spermatocytes) were also observed in 0.8% of tubules of 13–008. Adult tissue cross sections are included as a control for comparison (Fig. 2C).
Figure 2: Histological and immunofluorescent analyses ofprepubertal testicular tissue prior to grafting.
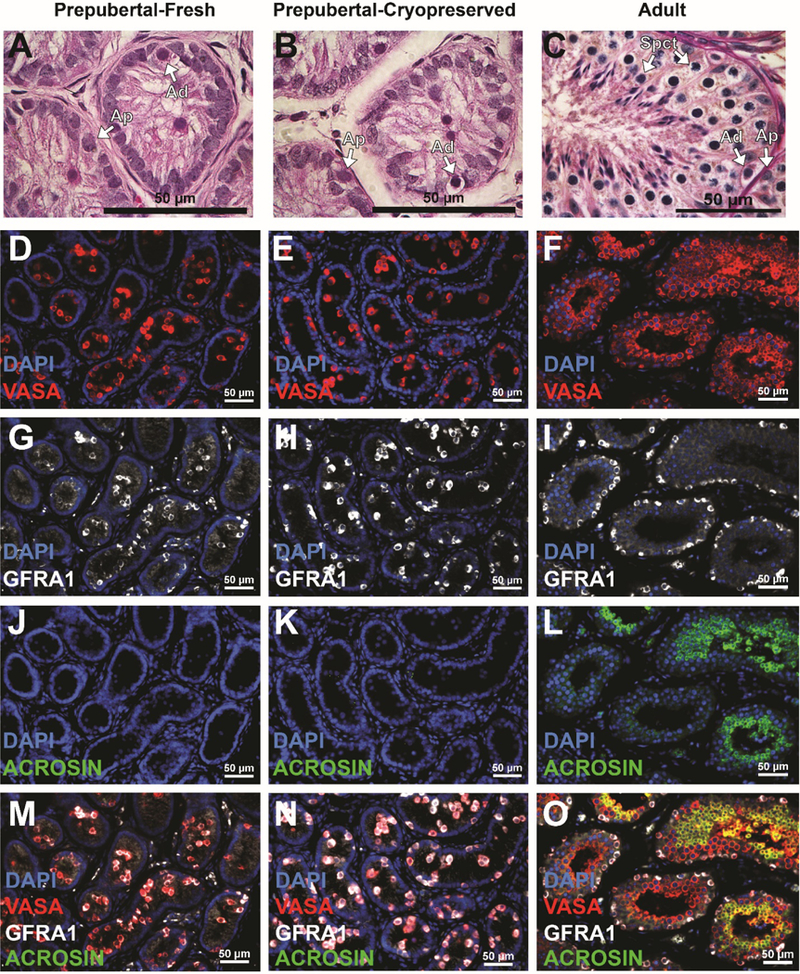
Hematoxylin and Eosin staining indicate thatpre-graftedfresh (A) and frozen-thawed (B) testis tissues are immature. In contrast, multiple layers of germ cells with complete spermatogenesis were seen in adult testis tissue controls (C). Arrows indicate undifferentiated type Adark (Ad) and Apale (Ap) stem/progenitor spermatogonia and spermatocytes (Spct). Immunofluorescence staining for VASA+ germ cells (red; D-F); GFRA1 + undifferentiated spermatogonia (white; G-I) and ACROSIN+ post-meiotic germ cells (green; J-L). Merged images are shown in (M-O). Scale bar = 50μm. Please see additional staining for UTF1, BOULE and CREM in Supplementary Figure S2.
Immunofluorescent staining revealed that VASA+ germ cells were located in the lumen and on the basement membrane of the seminiferous tubules in pre-graft fresh (Fig. 2D) and pre-graft frozen-thawed testis tissue (Fig. 2E). Pre-graft tissues contained GFRA1+ undifferentiated spermatogonia (Fig. 2G,H, M, N), but no ACROSIN+ post-meiotic cells (Fig. 2J, K, M, N). Seminiferous tubules of adult controls contained multiple layers of VASA+ germ cells (Fig. 2F), including GFRA1+ undifferentiated spermatogonia (Fig. 2I) and ACROSIN+ post-meiotic spermatids (Fig. 2L). Additional markers of undifferentiated spermatogonia (UTF1), spermatocytes (BOULE), and spermatids (CREM) are shown in Figure S2.
Graft growth and endocrine function
A few months after grafting, palpable masses were observed at graft sites on the back (Fig. 3A) and scrotum (Fig. 3B). Graft growth was monitored using calipers and graft area measurements (length x width) were recorded (Fig. 3C–F). Graft sizes (back or scrotum) were not impacted by cryopreservation (Fig. 3D and E; p>0.05) or addition of Matrigel (comparisons from back only; Fig. 3F; p>0.05).
Figure 3: Testicular tissue grafts increase in size during the 8-to 12-month in vivo incubation period.
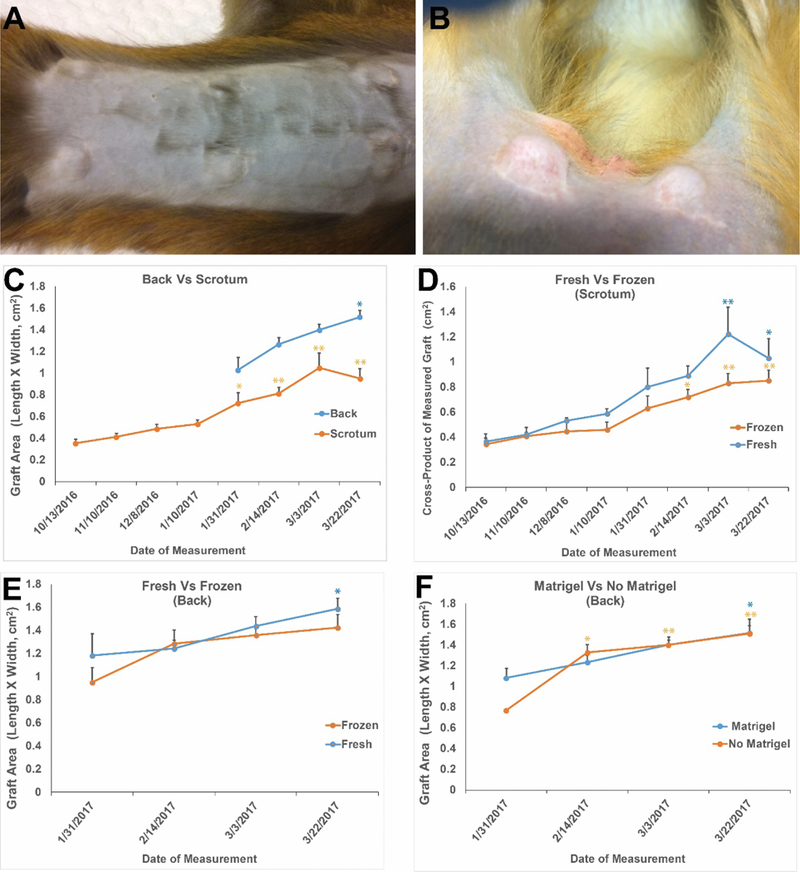
By 4–5 months after grafting, grafts were easily visualized under the back skin (A) and scrotal skin (B). Calipers were used to monitor graft growth (C-F). All grafts grew during the 8-to 12-month incubation period. Grafts on the back and in the scrotal area grew significantly over time relative to the first graft size measurement (C). Graft sizes (length x width) on each analysis date were not impacted by processing (fresh versus frozen-thawed) in the scrotum (D) or on the back (E) or by addition of Matrigel to the back sites (F). Frozen grafts on the back exhibited a trend toward increasing size through the experiment (E), but that increase was not statistically significant. All other grafts exhibited statistically significant increases in size through the experiment. Data points are presented as mean ± standard error of the mean. * indicates P<0.05 compared with initial graft size measurement and ** indicates P<0.01 compared with initial graft size measurement within each treatment group.
After grafting, and as animals entered puberty, circulating testosterone (T) levels increased in all monkeys and remained elevated above baseline in 4/5 recipients, indicating a functional hypothalamic-pituitary-testicular axis (Fig. S3A, C, E, G, I). Normal pubertal levels of T in Rhesus macaques are about 2 ng/ml (26, 27). Circulating FSH levels of all animals were in the normal range (not castrate range) for pubertal and adult Rhesus macaques (26, 28), indicating a functional negative feedback loop from the grafted testicular tissue to the hypothalamus and pituitary (Fig. S3B, D, F, H, J).
Graft recovery and analysis
Eight to twelve months after grafting, testicular tissues were recovered from all (39/39; 100%) graft sites. There was no graft in the left scrotum of 13–030 because he ripped open that incision immediately after surgery and destroyed the graft. It was not possible to isolate grafts from individual tissue fragments because the four tissue fragments grafted at each site grew and fused into single large masses weighing an average of 308.61 mg (Fig. 4A; Table S1). This represents an approximate five-fold increase in graft weight compared with the ~60 mg of tissue initially grafted at each site (4 fragments × 15.34 mg/fragment). Seminiferous tubules that were approximately 150–200 μm in diameter were observed in all grafts (Fig 4B and C). Grafts from all animals were fixed for histology and immunohistochemical analyses and, in most cases (32/39 grafts) manually dissected and/or digested with collagenase IV to release sperm for fertilization experiments (Fig. 4D). Although the weight of testicular tissue grafts recovered from scrotal skin is greater (P<0.01) than those from the back skin (Fig. 4E), there were no differences in grafts weights from fresh versus frozen testicular tissue (Fig. 4F) and graft weights were not impacted by the addition of Matrigel (comparing back grafts only, Fig. 4G).
Figure 4: Recovery of testicular tissue grafts.
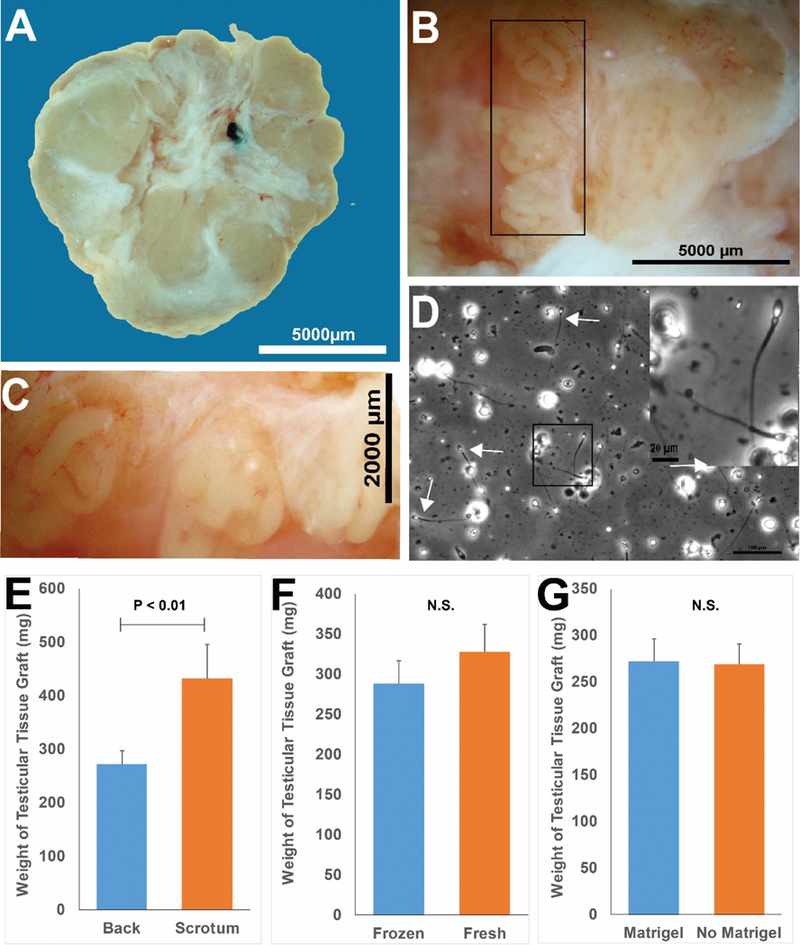
Grafts were recovered as one fused tissue, but white fibrous tissue may demarcate margins between individual testis tissue pieces that were originally placed at each graft (A). Seminiferous tubules could be distinguished after gentle teasing apart of the graft tissue with forceps (B). (C) Higher magnification of black box area in B. Panel D (white arrows) depicts sperm that were released by mechanical dissection of a scrotal graft from animal 13–030. (D, Inset) Higher magnification of black box region in (D). Grafts recovered from under the scrotal skin were larger than those recovered from under the back skin (E). Graft size was not impacted by processing (fresh versus frozen, (E)) or by addition of Matrigel (F). Bar graphs are presented as mean ± standard error of the mean. P<0.05 was considered by be significant. N.S. = not significant.
Complete spermatogenesis from autologous testicular tissue grafts
Seminiferous tubules from all grafts exhibited complete spermatogenesis with multiple layers of VASA+ germ cells (Fig. 5A and C) and ACROSIN+ post-meiotic spermatids (Fig. 5B and C). Additional staining for undifferentiated stem/progenitor spermatogonia (UTF1), spermatocytes (BOULE), and spermatids (CREM) is shown in Figure S4. Hematoxylin and eosin staining confirmed complete spermatogenesis in grafts from all experimental animals (Fig. 5D and Fig S5). Most seminiferous tubules (≥70%) contained complete spermatogenesis with elongated spermatids and/or sperm (Fig. 5E–G, Fig. S5). Complete spermatogenesis with sperm was confirmed in tissues recovered from 100% of graft sites (39/39; Data Table S1). There was no impact of Matrigel, cryopreservation, or graft location on the percentage of tubules with complete spermatogenesis (spermatids/sperm) (P>0.05).
Figure 5: Histological evaluation of spermatogenic development in grafts.
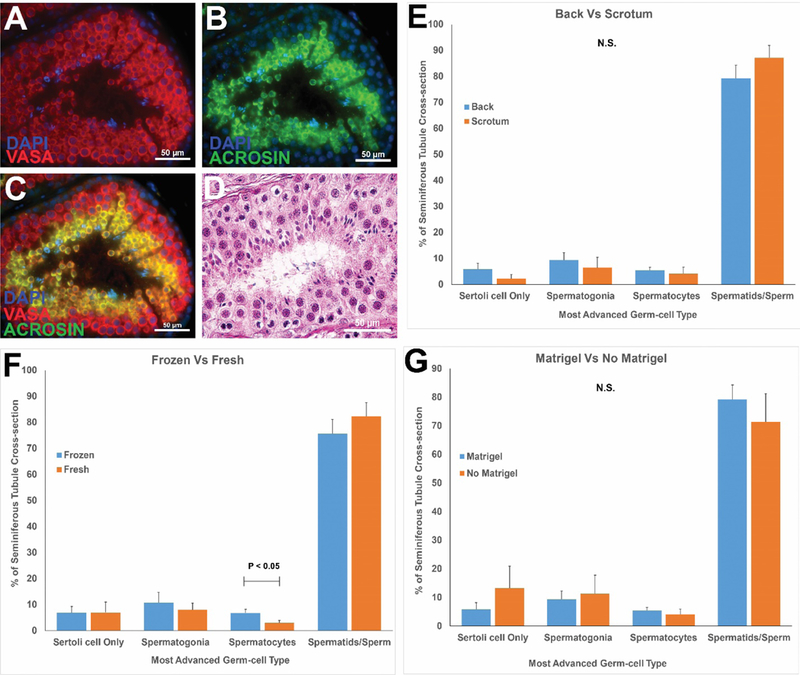
Immunofluorescence staining of recovered grafted tissue for VASA+ germ cells (red, A) and ACROSIN+ post-meiotic cells (green, B). DAPI counterstain marks all cell nuclei (blue). The merged VASA/ACROSIN/DAPI co-stain is shown in (C). Please see Figure S4 for additional markers of undifferentiated spermatogonia (UTF1), spermatocytes (BOULE) and spermatids (CREM). Hematoxylin and Eosin staining of post-graft tissues (D). Please see H and E staining for grafts from each individual animal in Figure S5. Quantification of most advanced germ cell type in graft seminiferous tubules (E-G). Bars represent mean ± standard error of the mean. N.S. = not significant; P<0.05 was considered statistically significant.
Grafts were fibrotic and difficult to dissect entirely with forceps. After manual dissection, some grafts were digested with collagenase IV to release the remaining sperm. Live sperm were recovered from the majority of grafts (26/32). When sperm were recovered and quantified (19 grafts), counts ranged from 60 sperm to 21 million sperm per graft (Table S1). Sperm were recovered from fresh and cryopreserved grafts; grafts on the back and in the scrotum; and in grafts with or without Matrigel (Data Table S1).
Fertilization and preimplantation embryo development from graft-derived sperm
To model the prepubertal cancer survivor, we selected sperm for functional testing from cryopreserved grafts recovered from under the scrotal skin of monkey 13–008 (Table S1). Graft sperm released by mechanical dissection and collagenase IV digestion were suspended separately in human tubal fluid. An aliquot of mechanically dissected sperm was shipped at ambient temperature to the Oregon National Primate Research Center for functional testing by intracytoplasmic sperm injection (ICSI) in May 2017 (Table S2). The remaining pre-digest and post-digest sperm were cryopreserved for future ICSI experiments performed in October and November 2017. A total of 138 eggs were fertilized by ICSI; 39 (28%) cleaved (progressed to 2-cell stage) and 16 (41%) cleavage stage embryos developed into blastocyst stage embryos (Fig. 6A–F and Data Table S2).
Figure 6: Functional evaluation of graft-derived sperm.
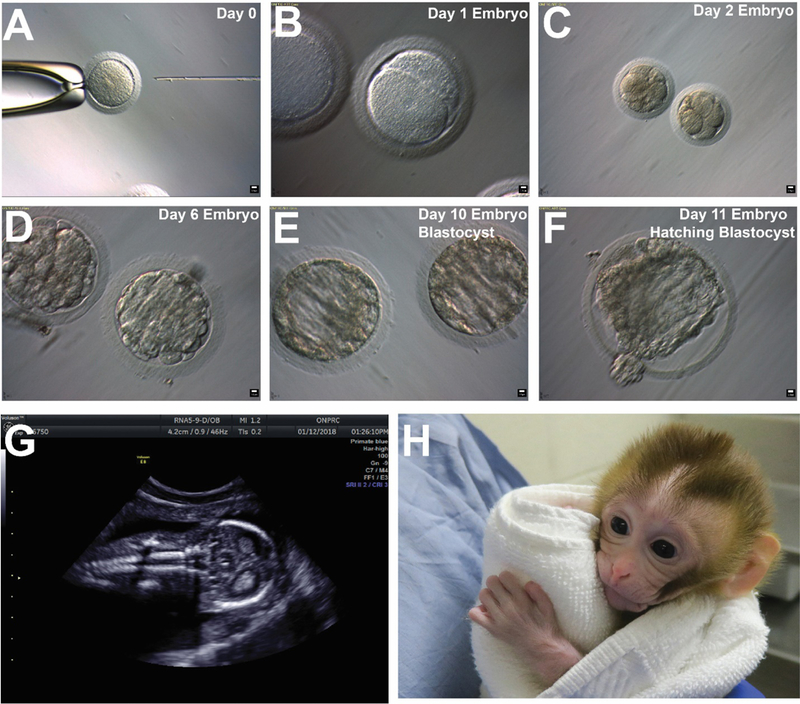
Sperm were derived from a cryopreserved graft retrieved from the left scrotum of13–008 that was recovered nine months after grafting. Fresh graft-derived sperm were used for the May 2017ICSI trial. The remaining sperm were cryopreserved and used for the October 2017 and November 2017 trials (See Table S2). Graft-derived sperm were used to fertilize Rhesus oocytes by ICSI (A). The resulting embryos attained 2-cell stage by day 1 (B); 8-cell stage by day 2 (C); morula stage by day 6 (D); blastocyst stage by day 10 (E); and hatching blastocyst by day 11 in culture (F). Blastocyst embryos were transferred to recipient females and a pregnancy was confirmed by ultrasound on December 12, 2017. Normal fetal development was confirmed by ultrasound on January 15, 2018 (G) and a graft-derived baby (Grady) was born by c-section on April 16, 2018 (H, image from two-week check-up).
Cleavage rates (2-cell embryo rate) of 28% after ICSI with graft-derived testicular sperm were lower than previous reports with ejaculated sperm in Rhesus, but the pace of embryo development (29) and blastocyst development rates were in the expected range (30). The reduced cleavage rate may reflect sperm quality deficits caused by cryopreservation of the testicular tissue before grafting or cryopreservation of graft-derived sperm after graft recovery and before ICSI. However, fresh and frozen testicular sperm produce similar ICSI cleavage rates in the human clinic (31, 32). We observed sperm quality deficits caused by the enzymatic digestion of testicular tissue to retrieve sperm (detached heads, fragile sperm) and the corresponding cleavage rates in those ICSI trials were 13% and 10%, respectively (Table S2, November trial). Thus, mechanical dissection is preferred; and/or future studies are needed to optimize enzymatic digestion protocols. Egg quality deficits have been reported for Rhesus cycles performed at the beginning (October) and end (May) of the breeding season (30). In this regard, the ICSI trial that was performed in November with mechanically dissected sperm, produced more mature eggs; the cleavage rate was 60%; the blastocyst rate was 67% and the pregnancy rate was 25% (Table S2), which are comparable to previous reports for the Rhesus macaque with ejaculated sperm (30).
Pregnancy and live offspring from graft-derived sperm
A total of 11 blastocyst stage embryos (10 fresh and 1 frozen) were transferred into 6 recipient females (Table S1 and Table S2). A pregnancy from a fresh blastocyst transfer was confirmed by ultrasound of one recipient female on December 15, 2017 (Table S1). A follow up ultrasound confirmed normal fetal development on January 12, 2018 (Fig. 6G) and a caesarean section was scheduled. Grady (graft-derived baby) was born on April 16, 2018 (Fig. 6H). Grady weighed 471 grams at birth and the fetal/placental weight ratio was 3, which are normal for a near term female Rhesus macaques (33–36). APGAR scores were 5 at 1 minute; 6 at 5 minutes and 7 at 30 minutes after delivery, which are consistent with previous reports for surgically delivered near term macaque infants (37). Grady’s three-month and six-month cage side behavioral assessments revealed normal social distance behavior with her Mom as well as normal social/object play activities (Fig. S6).
Discussion
Autologous testicular tissue grafting is an experimental approach that might be used to produce sperm from human cryopreserved prepubertal testis tissues. This approach was initially described in mice with the production of sperm from fresh and cryopreserved grafts that were competent to fertilize eggs and produce live offspring (14, 17, 18). Those results have been replicated to some extent in higher primates, but graft survival from autologous cryopreserved primate tissues was low and sperm were not tested functionally by fertilization or with the production of live offspring (23–25). These gaps need to be addressed to justify translating autologous testicular tissue grafting to the human clinic (e.g., for childhood cancer survivors).
Our experimental design was similar to previous studies (23–25), but a few key differences may be noteworthy. First, we used a lower concentration of DMSO (5%, 0.7M) than previous nonhuman primate autograft studies (1.4M). Differences in DMSO concentration may have implications for tissue toxicity and/or protection from cryopreservation damage. Controlled slow rate freezing in 0.7M DMSO is the condition we use in the fertility preservation clinic of the University of Pittsburgh Medical Center (https://fertilitypreservationpittsburgh.org/ ) and is based on a protocol described by Keros and colleagues (38, 39) for freezing prepubertal human testicular tissues. We did not attempt to optimize freezing conditions in this Rhesus grafting study but acknowledge that Jahnukainen and colleagues found that survival of Rhesus testicular tissue grafts cryopreserved in 1.4M DMSO was better than those cryopreserved in 0.7M DMSO (40). Complete spermatogenesis was not observed under either freezing condition in that study, perhaps due to the relatively short xenograft incubation time (3–5 months). Second, testicular tissue pieces were larger (9–20 mm3) in this study than previous studies (0.5–1 mm3). It may seem counterintuitive that larger tissue fragments could vascularize and survive ischemic injury better than smaller tissue fragments, unless there are critical autocrine/paracrine factors in the graft itself that contribute to survival of the tissue. Third, we individually sutured each testicular tissue fragments (4 per graft site) to the subcutaneous aspect of the skin rather than depositing as a slurry of small pieces in the subcutaneous space. Previous studies indicated that survival of ectopic ovarian tissue xenografts was enhanced by grafting into an angiogenic granulation zone created by injury of the underlying muscle tissue (41). The subcutaneous layer of the skin has a high capillary density (42), and perhaps injury caused by the suture needles and/or skin flap incision was sufficient to promote angiogenic granulation and vascularization of the apposed testicular tissue grafts.
The “gold standard” proof of concept for any reproductive technology is the production of functional gametes and live offspring. In this study, we demonstrated that frozen and thawed prepubertal testicular tissue could be matured in vivo by grafting under the back skin or scrotal skin of the same animal to produce functional sperm and a healthy baby. One caveat to our study is that testicular tissues were grafted into castrated animals, which would not usually be the situation with prepubertal cancer survivors. We castrated animals in this study because that is the only condition that produced sperm in previous nonhuman primate autologous testicular tissue grafting studies (24, 25). Future studies are needed to confirm that graft development proceeds in a similar fashion, in the scrotum or under the skin of individuals with intact testes. A second caveat is that testicular tissues collected prior to cancer treatment might harbor malignant cells. Therefore, the autologous grafting approach may not be appropriate for childhood leukemia, lymphoma, or testicular cancer survivors. On the other hand, the autologous testicular tissue grafting could be appropriate for patients receiving bone marrow transplants for non-malignant conditions (e.g., β-Thalassemia, sickle cell anemia) or patients with solid tumors that do not metastasize to the testes, including sarcomas and neuroblastomas, which comprise >60% of young patients who have frozen testicular tissues at the Fertility Preservation Program in Pittsburgh and coordinated recruitment sites (https://fertilitypreservationpittsburgh.org/ ; Fig. S7).
Testicular tissue grafting and xenografting are mature technologies that have been replicated in numerous mammalian species and may be ready for translation to the human clinic pending demonstration that similar results can be obtained in non-castrated recipients. Complete spermatogenesis from grafted human tissues has not yet been achieved (reviewed in (12)). Human tissue studies as well as studies addressing the caveats outlined above are needed to understand the scope, safety, and feasibility of testicular tissue grafting in patients.
Supplementary Material
Acknowledgments:
We thank the lab animal research resource (LARR) staff of Magee-Womens Research Institute for animal husbandry and technical support. Histology was performed by the Histology core of Magee-Womens Research Institute. FSH assays were performed by the Endocrine Technologies Support Core at the Oregon National Primate Research Center.
Funding: This work was supported by NICHD grants P01 HD075795 and R01 HD076412 to KEO; a diversity supplement to HD076412 for APF; NIH P51 OD011092 to the Oregon National Primate Research Center (JDH); and the Magee-Womens Research Institute and Foundation.
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: All data is available in the main text or the supplementary materials.
REFERENCES AND NOTES
- 1.Green DM et al. , Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 332–339 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine J, Canada A, Stern CJ, Fertility preservation in adolescents and young adults with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 4831–4841 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Meistrich ML, Male gonadal toxicity. Pediatric Blood & Cancer 53, 261–266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace WH, Anderson RA, Irvine DS, Fertility preservation for young patients with cancer: who is at risk and what can be offered? The lancet oncology 6, 209–218 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Goossens E, Van Saen D, Tournaye H, Spermatogonial stem cell preservation and transplantation: from research to clinic. Human Reproduction 28, 897–907 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Valli H et al. , Germline stem cells: toward the regeneration of spermatogenesis. Fertility and Sterility 101, 3–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson MM, Reproductive outcomes for survivors of childhood cancer. Obstetrics and gynecology 116, 1171–1183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.N. A. Howlader N, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (National Cancer Institute., Bethesda, MD, 2017), vol. 2018.
- 9.Thomson AB et al. , Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case-control study. Lancet 360, 361–367 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Paniagua R, Nistal M, Morphological and histometric study of human spermatogonia from birth to the onset of puberty. Journal of anatomy 139 ( Pt 3), 535–552 (1984). [PMC free article] [PubMed] [Google Scholar]
- 11.Onofre J, Baert Y, Faes K, Goossens E, Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Human Reproduction Update 22, 744–761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gassei K, Orwig KE, Experimental methods to preserve male fertility and treat male factor infertility. Fertil Steril 105, 256–266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arregui L, Dobrinski I, Xenografting of testicular tissue pieces: 12 years of an in vivo spermatogenesis system. Reproduction 148, R71–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honaramooz A et al. , Sperm from neonatal mammalian testes grafted in mice. Nature 418, 778–781 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Honaramooz A, Li MW, Penedo MCT, Meyers S, Dobrinski I, Accelerated maturation of primate testis by xenografting into mice. Biology of Reproduction 70, 1500–1503 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Kaneko H et al. , Generation of Live Piglets for the First Time Using Sperm Retrieved from Immature Testicular Tissue Cryopreserved and Grafted into Nude Mice. PLOS ONE 8, e70989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I, Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biology of Reproduction 68, 2331–2335 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Shinohara T et al. , Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod 17, 3039–3045 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Liu Z et al. , Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell research 26, 139–142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss RA, The discovery of endogenous retroviruses. Retrovirology 3, 67 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimsa MC et al. , Porcine endogenous retroviruses in xenotransplantation--molecular aspects. Viruses 6, 2062–2083 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.in CDC: Diseases directly transmitted by rodents (Centers for Disease Control, 2017), pp. https://www.cdc.gov/rodents/diseases/direct.html .
- 23.Wistuba J et al. , Meiosis in autologous ectopic transplants of immature testicular tissue grafted to Callithrix jacchus. Biology of Reproduction 74, 706–713 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Luetjens CM, Stukenborg J-B, Nieschlag E, Simoni M, Wistuba J, Complete Spermatogenesis in Orthotopic But Not in Ectopic Transplants of Autologously Grafted Marmoset Testicular Tissue. Endocrinology 149, 1736–1747 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S, Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res 72, 5174–5178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arslan M et al. , Changes in circulating levels of immunoreactive follicle stimulating hormone, luteinizing hormone, and testosterone during sexual development in the rhesus monkey, Macaca mulatta. J Med Primatol 15, 351–359 (1986). [PubMed] [Google Scholar]
- 27.Bernstein IS, Ruehlmann TE, Judge PG, Lindquist T, Weed JL, Testosterone changes during the period of adolescence in male rhesus monkeys (Macaca mulatta). American journal of primatology 24, 29–38 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Attardi B et al. , Effects of orchidectomy on gonadotropin and inhibin subunit messenger ribonucleic acids in the pituitary of the rhesus monkey (Macaca mulatta). Endocrinology 130, 1238–1244 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Hewitson L, Takahashi D, Dominko T, Simerly C, Schatten G, Fertilization and embryo development to blastocysts after intracytoplasmic sperm injection in the rhesus monkey. Hum Reprod 13, 3449–3455 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Nusser KD et al. , Developmental competence of oocytes after ICSI in the rhesus monkey. Human Reproduction 16, 130–137 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Habermann H et al. , In vitro fertilization outcomes after intracytoplasmic sperm injection with fresh or frozen-thawed testicular spermatozoa. Fertil Steril 73, 955–960 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Tavukcuoglu S et al. , Using Fresh and Frozen Testicular Sperm Samples in Couples Undergoing ICSI-MicroTESE Treatment. Journal of reproduction & infertility 14, 79–84 (2013). [PMC free article] [PubMed] [Google Scholar]
- 33.Van Wagenen G, Vital statistics from a breeding colony. Reproduction and pregnancy outcome in Macaca mulatta. J Med Primatol 1, 2–28 (1972). [PubMed] [Google Scholar]
- 34.Hopper KJ, Capozzi DK, Newsome JT, Effects of maternal and infant characteristics on birth weight and gestation length in a colony of rhesus macaques (Macaca mulatta). Comparative medicine 58, 597–603 (2008). [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson ME, Ford SP, Comparative aspects of placental efficiency. Reproduction (Cambridge, England) Supplement 58, 223–232 (2001). [PubMed] [Google Scholar]
- 36.Digiacomo RF, Shaughnessy PW, Tomlin SL, Fetal-placental weight relationships in the rhesus (Macaca mulatta). Biol Reprod 18, 749–753 (1978). [DOI] [PubMed] [Google Scholar]
- 37.Jacobson Misbe EN, Richards TL, McPherson RJ, Burbacher TM, Juul SE, Perinatal Asphyxia in a Nonhuman Primate Model. Developmental Neuroscience 33, 210–221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keros V et al. , Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Human Reproduction 20, 1676–1687 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Keros V et al. , Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod 22, 1384–1395 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S, Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Human Reproduction 22, 1060–1067 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Israely T, Nevo N, Harmelin A, Neeman M, Tsafriri A, Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod 21, 1368–1379 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Rucker M, Roesken F, Vollmar B, Menger MD, A novel approach for comparative study of periosteum, muscle, subcutis, and skin microcirculation by intravital fluorescence microscopy. Microvascular research 56, 30–42 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Tachibana M, Sparman M, Mitalipov S, Chromosome transfer in mature oocytes. Nature protocols 5, 1138–1147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL, In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev 27, 261–280 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


