The interactions between cognitive and sensorimotor impairments in schizophrenia remain largely unexplored. Using behavioural and neurophysiological techniques, Carment et al. show that inefficient attentional processing contributes to impaired sensorimotor control and altered task-related modulation of cortical excitability and inhibition in schizophrenia.
Keywords: schizophrenia, force control, attention, cortical excitability, eye movement
Abstract
Impairments in attentional, working memory and sensorimotor processing have been consistently reported in schizophrenia. However, the interaction between cognitive and sensorimotor impairments and the underlying neural mechanisms remains largely uncharted. We hypothesized that altered attentional processing in patients with schizophrenia, probed through saccadic inhibition, would partly explain impaired sensorimotor control and would be reflected as altered task-dependent modulation of cortical excitability and inhibition. Twenty-five stabilized patients with schizophrenia, 17 unaffected siblings and 25 healthy control subjects were recruited. Subjects performed visuomotor grip force-tracking alone (single-task condition) and with increased cognitive load (dual-task condition). In the dual-task condition, two types of trials were randomly presented: trials with visual distractors (requiring inhibition of saccades) or trials with addition of numbers (requiring saccades and addition). Both dual-task trial types required divided visual attention to the force-tracking target and to the distractor or number. Gaze was measured during force-tracking tasks, and task-dependent modulation of cortical excitability and inhibition were assessed using transcranial magnetic stimulation. In the single-task, patients with schizophrenia showed increased force-tracking error. In dual-task distraction trials, force-tracking error increased further in patients, but not in the other two groups. Patients inhibited fewer saccades to distractors, and the capacity to inhibit saccades explained group differences in force-tracking performance. Cortical excitability at rest was not different between groups and increased for all groups during single-task force-tracking, although, to a greater extent in patients (80%) compared to controls (40%). Compared to single-task force-tracking, the dual-task increased cortical excitability in control subjects, whereas patients showed decreased excitability. Again, the group differences in cortical excitability were no longer significant when failure to inhibit saccades was included as a covariate. Cortical inhibition was reduced in patients in all conditions, and only healthy controls increased inhibition in the dual-task. Siblings had similar force-tracking and gaze performance as controls but showed altered task-related modulation of cortical excitability and inhibition in dual-task conditions. In patients, neuropsychological scores of attention correlated with visuomotor performance and with task-dependant modulation of cortical excitability. Disorganization symptoms were greatest in patients with weakest task-dependent modulation of cortical excitability. This study provides insights into neurobiological mechanisms of impaired sensorimotor control in schizophrenia showing that deficient divided visual attention contributes to impaired visuomotor performance and is reflected in impaired modulation of cortical excitability and inhibition. In siblings, altered modulation of cortical excitability and inhibition is consistent with a genetic risk for cortical abnormality.
Introduction
Sensorimotor impairments are common in schizophrenia, even at an early stage of the disease (Manschreck et al., 2004, 2015). These impairments are characterized by a decreased ability to integrate and process sensory stimuli in order to execute a contextually appropriate motor action. They have, in part, been clinically operationalized as neurological soft signs, encompassing a set of discrete motor and sensorimotor abnormalities (Krebs et al., 2000). Sensorimotor impairments and neurological soft signs may, from the prodromal stage, predict the course of schizophrenia (Millan et al., 2016; Mittal, 2016; Walther et al., 2016; Caldani et al., 2017a; Térémetz et al., 2017). Impaired sensorimotor integration has been shown in a variety of tasks, including gait and posture (Kent et al., 2012; Bernard et al., 2014), fine motor function (Walther and Mittal, 2016) or gaze control (Calkins et al., 2008). In particular, patients with schizophrenia were less accurate in adjusting grip or finger force to a visual target (Rosen et al., 1991; Térémetz et al., 2014, 2017). These deficits constitute a non-negligible source of disability in everyday living for patients with schizophrenia (Bowie et al., 2006), may impact social functioning (Lehoux et al., 2003; Walther et al., 2015), and eventually lead to negative symptoms (Walther et al., 2016) or disorganization (Giersch et al., 2013).
Antipsychotic medication might account for some of the sensorimotor impairments in schizophrenia (Putzhammer et al., 2005; Nowak et al., 2013). However, growing evidence supports presence of sensorimotor impairments independent of medication. Impairments have been reported in first episode psychosis in a tapping task (Exner et al., 2006), in neuroleptic-naïve subjects when assessed for manual accuracy (Caligiuri and Lohr, 1994; Wolff and O’Driscoll, 1999; Ayehu et al., 2014; Térémetz et al., 2014) and also in subjects with ultra-high risk for developing a psychosis in terms of gaze control, e.g. saccades (Caldani et al., 2017b) and smooth pursuit eye movements (SPEMs) (van Tricht et al., 2010).
Schizophrenia is associated with altered attention, memory and executive functions (Fioravanti et al., 2005; Bowie et al., 2006). It has been proposed that impairments observed in complex motor tasks might be of cognitive rather than motor origin, i.e. due to altered allocation of attention (Delevoye-Turrell et al., 2006), poor sequence planning (Delevoye-Turrell et al., 2003; Grootens et al., 2009; Giersch et al., 2013), or deficient working memory (Lin et al., 2015). Together, these studies suggest a contribution of cognition to observed sensorimotor impairments in schizophrenia.
Cortical excitation-inhibition imbalance in primary motor cortex may contribute to sensorimotor impairments in schizophrenia. There is evidence for reduced short latency intracortical inhibition (SICI), measured using transcranial magnetic stimulation (TMS), during rest in schizophrenia (Daskalakis et al., 2002; for a review see Radhu et al., 2013). The cortical silent period, another TMS measure of inhibition, has also been shown to be prolonged or reduced (Wobrock et al., 2009). In contrast, TMS studies on corticospinal excitability (during rest) have not shown differences in schizophrenia (Pascual-Leone et al., 2002; Soubasi et al., 2010; Radhu et al., 2013). However, this does not exclude altered task-related modulation of cortical excitability and inhibition in schizophrenia, though studies addressing this issue are scarce. We recently showed reduced task-modulated SICI in schizophrenia using a motor inhibition paradigm, and this was related to greater prefrontal and premotor activation on functional magnetic resonance imaging (Lindberg et al., 2016). Similarly, computational modelling of grip force-tracking deficits suggested a contribution of altered task-modulated inhibition (Térémetz et al., 2014).
It has been shown in non-human primates that attention influences primary motor cortex excitability through modulation from parietal cortex (Wurtz et al., 1982). In healthy subjects, attention can modulate motor cortex excitability in a hand motor task, revealed using TMS (Conte et al., 2007; Hannah et al., 2018), but this remains largely unstudied in patients with schizophrenia. An EEG study revealed a reduced modulation of cortical activations, less focally organized, in an auditory odd-ball task requiring attention in schizophrenia (Gomez-Pilar et al., 2017). However, how cognitive demands in sensorimotor tasks modulate TMS measures of cortical excitability and inhibition remains unexplored. In this study, we investigated how cognitive load, experimentally manipulated using a dual-task paradigm, interacts with sensorimotor performance in schizophrenia. A visuomotor grip force-tracking task was first performed in a single-task condition. Then subjects performed grip force-tracking within a dual-task condition requiring simultaneous discrimination of peripheral visual cues (shapes or numbers). Subjects were instructed not to look at shapes while executing force-tracking (visual distractor trials with inhibition of saccades), but to look at numbers (perform a saccade) and add numbers in successive trials.
Behavioural hypotheses
On the behavioural level, we expected that force-tracking error would increase in the dual-task. Given the attentional deficits reported in schizophrenia (Kreither et al., 2017) and the difficulty to inhibit saccades (Calkins et al., 2008), our first hypothesis was that force-tracking error would increase only in patients in dual-task distractor trials. In contrast, given that subjects were required to perform saccades when adding numbers, we predicted an increase in tracking error in all groups in these trials, but expected that this effect would be greatest in patients (Lin et al., 2015). Our second hypothesis, in terms of task-related gaze, was that patients would show decreased smooth pursuit accuracy and failure to inhibit saccades to distractors, reflecting impaired visual attention (Calkins et al., 2008). The concomitant monitoring of tracking performance and gaze informs on eye-hand coordination, which is highly coupled in healthy subjects (Johansson et al., 2001), but yet uncharacterized in schizophrenia.
Neurophysiological hypotheses
Neuroimaging and EEG studies indicate altered structural and functional connectivity in schizophrenia (Gomez-Pilar et al., 2017; O’Donoghue et al., 2017). Given the impaired excitation-inhibition balance in schizophrenia (Gao and Penzes, 2015), we expected that altered distributed network activity in patients would lead to impaired task-related modulation of cortical excitability and inhibition, and this as a function of cognitive load. More specifically, given the hyperfocusing of attention in schizophrenia (Kreither et al., 2017), our third hypothesis was that patients would have increased motor cortex excitability and reduced inhibition (Lindberg et al., 2016) compared to control subjects during single-task force tracking. Our fourth hypothesis was that increased cognitive load (in the dual-task condition) would further increase excitability in healthy controls (Conte et al., 2007), but not in patients.
Materials and methods
Participants
Twenty-five patients [seven females, 18 males, mean age ± standard deviation (SD): 31 ± 9 years], fulfilling DSM-IV-R criteria for schizophrenia (American Psychiatric Association, 2000), were recruited in the university department (SHU) at Sainte-Anne Hospital, Paris, France. Patients with schizophrenia were all clinically stabilized and medicated with stable dose of atypical antipsychotics for at least one month. Patients on clozapine-based treatment were excluded as clozapine affects cortical excitability and inhibition (Daskalakis et al., 2008; Kaster et al., 2015). Twenty-five healthy control subjects, matched for age, hand dominance and gender (mean age: 30 ± 7 years), were recruited through a national healthy volunteer contact service and 17 non-psychotic siblings (12 females and five males, mean age: 36 ± 10 years; two siblings of the schizophrenia group) were recruited from family support groups. An approximated intelligence quotient was obtained [Wechsler Adult Intelligence Scale – third edition (WAIS-III); Grégoire and Wierzbicki, 2009] and subjects with a score <80 were excluded. Three subjects in each group were left-handed (Edinburg Handedness Inventory; Oldfield, 1971). All subjects were assessed with the Diagnostic Interview for Genetic Studies v3.0 to ascertain the diagnosis in patients and to preclude axis 1 and 2 diagnosis in healthy control subjects and siblings (Nurnberger et al., 1994). To control for potential confounders in the behavioural and physiological assessments, subjects were asked not to smoke or drink coffee before the assessment, and to report their smoking status. The study received ethical approval from the regional ethics committee (Ile de France VIII; Clinical Trials: NCT02826629) and all subjects provided written informed consent.
Clinical and neuropsychological assessments
For patients, clinical symptomatology (Table 1) was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) and complemented with the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962). All groups underwent clinical assessments of neurological soft signs (Krebs et al., 2000) defining three main functional subscores: (i) sensory integration; (ii) motor integration; and (iii) motor coordination. Extrapyramidal symptoms and abnormal involuntary movements were, respectively, assessed with the Simpson Angus Extra-Pyramidal Scale (SAS; Simpson and Angus, 1970) and the Abnormal Involuntary Movements Scale (AIMS; Munetz and Benjamin, 1988). Neuropsychological assessment included the Test battery for Attentional Performance (TAP; Zimmermann and Fimm, 2002) and the Stroop colour naming test (Stroop, 1935; Supplementary Table 1).
Table 1.
Clinical and demographic data
| PSZ ID | Age, y | Gender | Disease AAO | CPZ, mg/day | PANSS | BPRS (24–168) | SAS (0–44) | AIMS (0–40) | Neurological soft signs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total score (30–210) | Positive symptoms (7–49) | Negative symptoms (7–49) | General symptoms (16–112) | Disorganization symptoms (4–28) | Total score (0–105) | Sensory integration subscore (0–15) | Motor coordination subscore (0–21) | Motor integration subscore (0–18) | ||||||||
| 1 | 21 | F | 15 | 80 | 63 | 10 | 15 | 38 | 7 | 48 | 0 | 2 | 1.5 | 0 | 0.5 | 0 |
| 2 | 25 | M | 21 | 35 | 54 | 13 | 11 | 30 | 10 | 46 | 4 | 0 | 18.5 | 0.5 | 9 | 2 |
| 3 | 32 | F | 27 | 200 | 52 | 7 | 18 | 27 | 9 | 32 | 2 | 0 | 22 | 1.5 | 12.5 | 5 |
| 4 | 44 | M | 23 | 200 | 63 | 12 | 16 | 35 | 7 | 52 | 3 | 0 | 12.5 | 0.5 | 6.5 | 0 |
| 5 | 28 | M | 22 | 250 | 50 | 9 | 14 | 27 | 11 | 36 | 4 | 3 | 18.5 | 1 | 9.5 | 1 |
| 6 | 22 | M | 12 | 200 | 56 | 9 | 14 | 33 | 12 | 40 | 1 | 0 | 13 | 4.5 | 5.5 | 2 |
| 7 | 24 | M | 17 | 800 | 64 | 14 | 16 | 34 | 4 | 44 | 6 | 5 | 16.5 | 0 | 5.5 | 2.5 |
| 8 | 38 | M | 20 | 300 | 44 | 9 | 12 | 23 | 6 | 37 | 2 | 2 | 6 | 0.5 | 3.5 | 0 |
| 9 | 31 | F | 30 | 267 | 50 | 13 | 17 | 20 | 7 | 44 | 5 | 1 | 18.5 | 3.5 | 7 | 1 |
| 10 | 29 | M | 23 | 75 | 44 | 9 | 9 | 26 | 6 | 36 | 2 | 0 | 5.5 | 0 | 3.5 | 0 |
| 11 | 36 | M | 29 | 200 | 52 | 10 | 13 | 29 | 4 | 44 | 1 | 1 | 9 | 1 | 7 | 0 |
| 12 | 49 | F | 42 | 150 | 63 | 16 | 14 | 33 | 7 | 46 | 2 | 0 | 11.5 | 1.5 | 3 | 1 |
| 13 | 32 | M | 31 | 200 | 63 | 9 | 21 | 33 | 6 | 45 | 1 | 0 | 7.5 | 1 | 4 | 1 |
| 14 | 38 | F | 32 | 200 | 56 | 9 | 11 | 36 | 9 | 41 | 6 | 0 | 18 | 0.5 | 7.5 | 1.5 |
| 15 | 18 | F | 15 | 100 | 59 | 13 | 16 | 30 | 12 | 40 | 2 | 1 | 11.5 | 2 | 5 | 1 |
| 16 | 42 | M | 16 | 300 | 81 | 14 | 28 | 39 | 11 | 49 | 6 | 3 | 19.5 | 2 | 7.5 | 3 |
| 17 | 18 | M | 15 | 300 | 51 | 14 | 13 | 24 | 11 | 42 | 3 | 3 | 21 | 5.5 | 6 | 4 |
| 18 | 29 | M | 24 | 200 | 44 | 7 | 11 | 26 | 7 | 36 | 8 | 0 | 16.5 | 1 | 7 | 0 |
| 19 | 39 | M | 19 | 300 | 47 | 10 | 11 | 26 | 10 | 40 | 1 | 0 | 17.5 | 4 | 8 | 1 |
| 20 | 30 | M | 23 | 100 | 54 | 8 | 10 | 36 | 4 | 46 | 0 | 0 | 3 | 0 | 3 | 0 |
| 21 | 24 | M | 20 | 1067 | 61 | 10 | 17 | 34 | 9 | 48 | 2 | 1 | 14.5 | 2.5 | 5.5 | 2 |
| 22 | 25 | M | 25 | 67 | 52 | 10 | 19 | 23 | 4 | 44 | 13 | 7 | 21 | 4 | 1.5 | 1 |
| 23 | 32 | M | 21 | 267 | 64 | 14 | 14 | 36 | 8 | 64 | 1 | 0 | 13.5 | 2 | 4 | 0.5 |
| 24 | 20 | F | 19 | 250 | 57 | 9 | 21 | 27 | 9 | 45 | 3 | 0 | 11.5 | 4 | 3.5 | 0 |
| 25 | 44 | M | 23 | 1067 | 59 | 10 | 12 | 37 | 9 | 38 | 0 | 0 | 16 | 2 | 8.5 | 1.5 |
| PSZ, mean ± SD | 31 ± 9 | 7F 18M | 23 ± 7 | 287 ± 276 | 56 ± 8 | 11 ± 8 | 15 ± 4 | 31 ± 5 | 8 ± 3 | 43 ± 7 | 3.1 ± 2.9 | 1.2 ± 1.8 | 13.8 ± 5.8 | 1.8 ± 1.6 | 5.8 ± 2.7 | 1.2 ± 1.3 |
| HC, mean ± SD | 30 ± 7 | 7F 18M | - | - | - | - | - | - | - | - | 1.3 ± 1.1* | 0.3 ± 0.4 | 7.6 ± 3.5*** | 0.9 ± 1.1* | 3.8 ± 2.1** | 0.5 ± 0.7* |
| SIB, mean ± SD | 35 ± 10 | 12F 5M | - | - | - | - | - | - | - | - | 1.1 ± 1.3* | 0 ± 0* | 7.3 ± 3.8*** | 0.8 ± 0.8* | 3.8 ± 2.7* | 0.8 ± 0.7 |
Detailed clinical data for patients with schizophrenia. Disease onset relates to first hospital assignment including diagnosis. Patients were assessed for with the PANSS, brief psychiatric rating scale (BPRS), Simpson Angus Extra-Pyramidal Scale (SAS), Abnormal Involuntary Movements Scale (AIMS), and neurological soft signs. There was no difference in the demographic data between groups. Group differences for clinical data tested with Mann-Whitney U-tests are displayed as follow: *P < 0.05; **P < 0.01, ***P < 0.001. Patient 22 was identified as an outlier in SAS score, but not in other scores (behavioural or neurophysiological measures) and this did not affect group results (see Supplementary material for detailed analysis).
AAO = age at onset; CPZ = chlorpromazine; F = female; HC = healthy control group; M = male; PSZ = patients with schizophrenia; SIB = non-psychotic siblings.
Visuomotor grip force-tracking task
Subjects performed a manual visuomotor grip force-tracking task with their right hand in two conditions (Fig. 1). Since three participants in each group were left-handed, 9 of 67 subjects performed the visuomotor task with their non-dominant hand.
Figure 1.
Visuomotor grip force-tracking set-up and conditions. Set-up for the visuomotor task: subjects were seated in front of a 22” computer screen, set at eye level at a distance of 60 cm with head stabilized (forehead and chin rest). The screen displayed the visuomotor force-tracking tasks. Grip force was displayed as a red cursor moving vertically and in real-time as a function of the exerted grip force. The target force was displayed as a right-to-left scrolling coloured line. A trial consisted of a single ramp-hold-and-release sequence. Trials with different cognitive load were presented pseudo-randomly: (i) single-task trial (Single): grip force-tracking; (ii) dual-task distraction trial (Dual-DIST): during force-tracking, distractors, consisting of white-filled shapes (square, star, triangle, 2 × 2 cm), were randomly displayed for 500 ms in the four periods at specific times (1500 ms into Rest; 380 ms before Ramp onset; 1500 ms into Hold; and 380 ms before Release); (iii) dual-task addition trial (Dual-ADD): while subjects performed the force-tracking task, numbers (from 1 to 9, 2 × 2 cm) were displayed for 500 ms with onset times identical to the Dual-DIST trials. Subjects were instructed to focus on the tracking task and to inhibit saccades toward irrelevant visual stimuli (distractors), but to make saccades toward relevant stimuli (numbers). They were asked to mentally sum the successive numbers, and report the sum when given a cue.
Single-task condition
Grip force-tracking (Single; similar to: Térémetz et al., 2014): subjects had to accurately match their grip force, represented by a red cursor (moving vertically), to the target force, a right-to-left scrolling line. The target force followed a ramp-hold-and-release paradigm. A single trial consisted of four successive periods: rest (3 s baseline force, 0 N), ramp (2 s, linearly increasing force), hold (3 s, steady force) and release (instantaneous drop to baseline). Trials were organized in blocks of six trials to a force target level of 5 N or 10% maximum voluntary contraction (MVC). A block at each force level was repeated three times (for a total of 36 trials; duration: 5 min 3 s). This condition corresponds to the basic (lowest) level of cognitive load.
Dual-task condition
While subjects performed the force-tracking task two types of visual cues were displayed in pseudo-randomized order. (i) Distractors, consisting of white-filled shapes (square, star, triangle, 2 × 2 cm), were displayed for 500 ms (in pseudo-random positions at least 12 cm away from the cursor) in one of the four periods (Dual-DIST trials). Timing of appearance was unpredictable and in each of the four periods defined as follows: 1500 ms into REST; 380 ms before RAMP-onset; 1500 ms into HOLD; or 380 ms before RELEASE. A maximum of two distractors appeared per trial. Subjects had to focus on the tracking task and inhibit saccades toward distractors. (ii) Numbers were displayed (Dual-ADD trials; from 1 to 9, 2 × 2 cm) for 500 ms in pseudo-random positions and with onset-times identical to Dual-DIST trials. Subjects had to make a saccade toward the stimulus, mentally sum the numbers of successive trials, and report the sum after an auditory cue (given after six dual-task trials).
Thus, the dual-task condition contained trials sharing a discrimination component (subjects were required to either inhibit or exert a saccade according the type of the visual stimulus), as well as a memory retention component of the score from the added numbers, thus cognitive load was higher than in the single-task condition. Further, since the addition occurs during Dual-ADD trials only, while memory retention was required in both dual-task trials, Dual-DIST trials represent intermediate and Dual-ADD trials with highest cognitive load
The order of trials was: 36 successive single-task trials (5 min 30 s), followed by 72 pseudo-randomly intermingled Dual-DIST and Dual-ADD trials (2 × 5 min 30 s). For familiarization, all subjects performed a series of six consecutive single-task trials at 10%MVC before data recording. MVC was assessed using a power grip dynamometer. Subjects were instructed prior to the task: to accurately match their grip force to the target force at all times, to ignore (inhibit saccades to) distractors, but to make saccades to relevant visual stimuli (numbers), to mentally sum the successively appearing numbers, and to verbally report the sum after a cue. Thus, whether to inhibit (Dual-DIST trial) or make a saccade (Dual-ADD trial) to a visual extrafoveal stimulus depended on peripheral visual on-line information.
Force recording and analysis
Tracking force was measured using the Power Grip Manipulandum (www.sensix.fr) and sampled at 1 kHz using a CED Power1401 (www.ced.co.uk) connected to a computer running Spike2V6.
Visuomotor tracking performance was analysed using MATLABV9.1 (The MathWorks, Inc., Natick, MA, USA). Acquired grip force was down-sampled to 100 Hz, and smoothed by a 20 ms sliding window. The following measures were extracted trial-by-trial, grouped for each condition (single-task, Dual-DIST, Dual-ADD) and then averaged across 108 trials for each subject.
Root mean square error (RMSe, N) was calculated from the absolute summed error between the target force and the tracking force. Error was extracted in the ramp and hold periods.
Force onset (ms) was defined as the time of the positive peak value of the derivative of the tracking force in the interval: target ramp-onset −500 ms to ramp-onset +500 ms.
Force offset (ms) was defined as the time of the negative peak value of the derivative of tracking force in the interval of ±500 ms around target release.
Gaze recording and analysis
Eye movements were recorded during the visuomotor task, with head stabilized (forehead and chin rest) in the dark. Each eye was scanned at 300 Hz by the Mobile EBT Tracker (www.suricog.com). After calibration (Caldani et al., 2017b), eye position was sampled at 1 kHz using linear interpolation and filtered with a 30 ms moving average window. MATLABV9.1 was used to extract eye movements. Saccade criteria: onset between 100 ms and 600 ms following a stimulus; saccade direction toward stimulus position; ratio between saccade amplitude and stimulus position between 0.8 and 1.2; saccade onset: first point with velocity >30°/s. Offset: first point <30°/s (Duyck et al., 2016).
Saccade performance: saccadic inhibition (%): 100 – %saccades to distractors (72 stimuli); 100% represents full inhibition (=no saccades to distractors). Saccadic execution (%): %saccades to displayed numbers (36 stimuli).
SPEM: vertical SPEM was analysed during the ramp period (from target ramp onset −150 ms to target ramp offset +300 ms). Note that the ramp period corresponds to a constant vertical target force velocity.
SPEM latency: latency of SPEM initiation from the onset of tracking force increase (vertical cursor movement during ramp) defined as: time of maximal eye movement acceleration within 300 ms of tracking force onset.
SPEM gain: ratio of eye velocity to target force velocity.
Neurophysiological recordings
TMS was used to assess cortical excitability and inhibition during task performance. Motor evoked potentials (MEPs) were recorded from four hand muscles using surface EMG electrodes (www.adinstruments.com) including the first dorsal interosseous, abductor digiti minimi, flexor carpi radialis, and extensor carpi radialis. EMG signals were amplified with a CED 1902, sampled at 1 kHz using a CED Power1401 connected to a computer running Spike2V6 (www.ced.co.uk). TMS was applied over the cortical representation of the right first dorsal interosseous (contralateral hemisphere) using a figure-of-eight coil (7-cm diameter) connected to two synchronized Magstim 200 units (www.magstim.com). Optimal coil position was defined as the stimulation site inducing the largest first dorsal interosseous MEPs (i.e. MEPs >50 mV) at the lowest intensity. The neuronavigation system was used during the entire session and coil position was maintained at a maximum of ±5 mm and/or 5° shift from the target using default MRI scan (www.ant-neuro.com).
Baseline measures
Baseline measures of resting motor threshold, cortical excitability, and SICI were assessed at complete rest, prior to the visuomotor tasks. Resting motor threshold was measured as the lowest stimulator intensity (%) that elicited a MEP >50 mV in at least 5 of 10 stimulations (Rossini et al., 1994) and cortical excitability was calculated from average MEP amplitude of 15 single pulse stimulations at 120% resting motor threshold. SICI was measured as the % reduction of the conditioned MEP obtained by paired-pulse stimulation (subthreshold conditioned stimulation at 80% resting motor threshold, applied 2 ms prior from the test pulse at 120% resting motor threshold). The cortical silent period was assessed during active contraction (Tinazzi et al., 2003), i.e. during the hold phase of the visuomotor task at 10% MVC, and expressed as the duration from MEP onset to the return of inhibition to pre-stimulus EMG amplitude (Soubasi et al., 2010).
Task-related modulation of cortical excitability and inhibition
Cortical excitability (single-pulse TMS) and inhibition (paired-pulse TMS) were measured during rest and hold phases of force-tracking during Single-task, Dual-DIST and Dual-ADD conditions. We studied the degree of modulation by comparing measures obtained in the hold phase during Single-task, Dual-DIST and Dual-ADD (Fig. 4A illustrates single subject data). TMS was applied 80 ms after the appearance of a distractor or number. The strength of between-condition modulation was also tested between the single-task condition and dual-task condition (Dual-ADD trials). A delta score was obtained (MEPDual-ADD − MEPSingle) corresponding to the mean MEP amplitude in Dual-ADD trials minus that in Single-task trials.
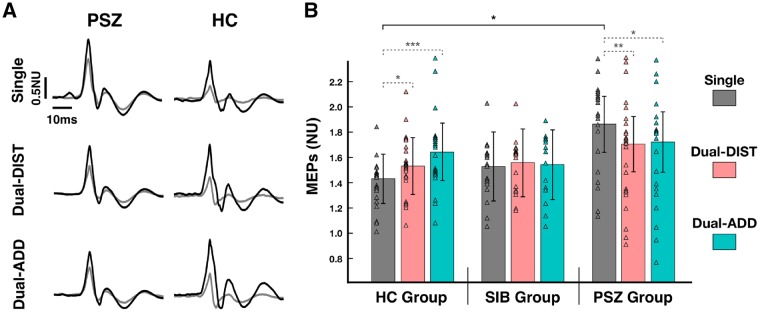
Figure 4.
Cortical excitability. (A) Raw data of MEPs recorded during the visuomotor grip force-tracking task (hold period) for a patient with schizophrenia (PSZ) and a healthy control subject (HC). Example trials show an unconditioned MEP (dark line) and a conditioned MEP (grey line) of the 1DI for (i) the single-task force-tracking condition (Single) and the dual-task condition with (ii) dual-task distraction trial (Dual-DIST) and (iii) dual-task addition trial (Dual-ADD). (B) Mean normalized amplitude of MEPs for the hold period (estimated marginal mean ± vertical bars: 95% CI) during Single (grey), Dual-DIST (pink) and Dual-ADD (cyan) trials for the three groups: patients with schizophrenia, healthy controls and siblings (SIB). Triangles represent data points of the individual subjects in each group and condition. Significant differences (LSD fisher post hoc tests for between-group comparisons are shown as horizontal black brackets and within-group comparisons as horizontal grey dashed bracket with: *P < 0.05; **P < 0.01, ***P < 0.001. For clarity, significant differences for between-group comparisons are only indicated between patients and healthy controls. Post hoc tests for between-group comparisons (not indicated) revealed that patients with schizophrenia showed an increased excitability only in single-task condition compared to healthy controls (patients with schizophrenia versus healthy controls: P = 0.02), this was not significantly different compared to siblings (patients with schizophrenia versus siblings: P = 0.08).
Twelve MEPs were obtained for each condition and pulse type. TMS interstimulus intervals varied with a minimum of 5 s.
Statistical analysis
Statistical analysis was performed on correct, artefact-free and non-outlier trials (Supplementary material). Statistical analyses (using Statistica10, StatSoft, Inc., USA) involved two-tailed paired t-test for assessing group differences in parametric measures (duration and latency of saccades, baseline TMS measures, control EGM measures, MVC and addition results) and Mann-Whitney U-tests for demographic and clinical outcomes. Group differences of behavioural (force-tracking and gaze) and physiological (TMS) measures were analysed using a general linear model repeated measures ANOVA with one Group factor (Schizophrenia, Healthy control, Non-psychotic siblings) and within-group factor Condition (Single-task, Dual-DIST, Dual-ADD). Fisher least significant difference post hoc test was used to investigate differences revealed by ANOVA. The level of significance was set to P < 0.05. Pearson’s correlation was used to assess the relation between modulation of cortical excitability and inhibition. Spearman’s rank-order correlation was used to independently assess relations between behavioural (tracking error; saccadic inhibition; SPEM gain) or neurophysiological (modulation of cortical excitability) variables and clinical measures PANSS (positive, negative and disorganization subscales), neurological soft signs (total score, sensori-integration, motor integration and motor coordination subscores), approximated intelligence quotient, Stroop (interference subscore), TAP (working-memory, incompatibility and divided attention subscores) and chlorpromazine equivalent (only for the schizophrenia group). The level of significance for correlation coefficients was corrected for multiple comparisons with false-discovery rate method (Benjamini and Hochberg, 1995). The relationship between attentional processing and force-tracking error was further tested using multiple linear regression including saccadic inhibition, Stroop (interference score) and incompatibility (TAP subscore) as predictors.
Data availability
All relevant data are within the paper and its Supplementary material.
Results
Behavioural results
Force control and gaze measures are detailed in Table 2. All 67 subjects successfully completed the visuomotor grip force-tracking tasks. No statistical difference was found between groups in reporting the correct sum of numbers shown in Dual-ADD: patients with schizophrenia 82%, healthy control subjects 89%, non-psychotic siblings 90% correct (patients with schizophrenia versus healthy control subjects: t(48) = −1.22, P = 0.23; patients with schizophrenia versus non-psychotic siblings: t(40) = −1.39, P = 0.17).
Table 2.
Visuomotor measures for the three groups
| Visuomotor measures | Patients with schizophrenia, Mean ± SD | Healthy control subjects Mean ± SD | Non-psychotic siblings Mean ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Single task | Dual-DIST | Dual-ADD | Single task | Dual DIST | Dual ADD | Single task | Dual-IST | Dual-ADD | |
| Force control | |||||||||
| Tracking error, RMSe | 0.12 ± 0.05 | 0.15 ± 0.07 | 0.17 ± 0.09 | 0.09 ± 0.03 | 0.10 ± 0.04 | 0.12 ± 0.04 | 0.09 ± 0.03 | 0.11 ± 0.03 | 0.13 ± 0.04 |
| Release duration, ms | 166 ± 46 | 171 ± 61 | 179 ± 89 | 148 ± 30 | 142 ± 45 | 145 ± 44 | 140 ± 33 | 135 ± 53 | 136 ± 54 |
| Force-onset, ms | 118 ± 22 | 119 ± 38 | 150 ± 120 | 115 ± 29 | 120 ± 46 | 132 ± 50 | 113 ± 35 | 105 ± 22 | 113 ± 22 |
| Force-offset, ms | 165 ± 50 | 166 ± 62 | 171 ± 59 | 161 ± 43 | 155 ± 47 | 160 ± 46 | 151 ± 33 | 136 ± 41 | 157 ± 39 |
| Eye tracking | |||||||||
| Saccade detection, % | 58 ± 24 | 34 ± 24 | 70 ± 23 | 36 ± 16 | 14 ± 9 | 74 ± 19 | 38 ± 21 | 17 ± 14 | 69 ± 20 |
| Saccade latency, ms | X | 217 ± 53 | 321 ± 65 | X | 182 ± 30 | 378 ± 72 | X | 180 ± 27 | 354 ± 68 |
| Saccade duration, ms | X | 161 ± 89 | 232 ± 71 | X | 157 ± 77 | 189 ± 50 | X | 117 ± 75 | 192 ± 57 |
| SPEM gain, ratio | 0.60 ± 0.34 | X | X | 0.86 ± 0.25 | X | X | 0.89 ± 0.22 | X | X |
| TMS | |||||||||
| MEPs, NU | 1.83 ± 0.54 | 1.70 ± 0.51 | 1.74 ± 0.55 | 1.43 ± 0.37 | 1.53 ± 0.46 | 1.60 ± 0.51 | 1.51 ± 0.54 | 1.56 ± 0.56 | 1.53 ± 0.51 |
| SICI, % | 30 ± 15 | 28 ± 13 | 29 ± 14 | 38 ± 14 | 44 ± 13 | 43 ± 14 | 39 ± 15 | 42 ± 20 | 43 ± 18 |
| CSP, ms | 745 ± 164 | 773 ± 122 | 810 ± 166 | 906 ± 177 | 887 ± 167 | 917 ± 174 | 848 ± 201 | 812 ± 188 | 793 ± 204 |
Main measures (mean ± SD) of performance in force control, eye tracking and TMS are detailed for all groups in (i) the Single-task tracking condition and the two Dual-task conditions with (ii) dual-task distraction trials (Dual-DIST) and (iii) dual-task addition trials (Dual-ADD).
Note that saccade occurrence in Dual-ADD did not reach 100% since subjects tended to avoid saccades to targets with small eccentricity (relative to the cursor). Saccade detection in the single-task condition is the occurrence of spontaneous non-required but task-related saccades during grip force-tracking (patients with schizophrenia versus healthy control subjects: P < 0.001; patients with schizophrenia versus non-psychotic siblings: P = 0.01).
CSP = cortical silent period; HC = healthy control group; NU = normalized unit (normalized on Rest values); PSZ = patients with schizophrenia; RMSe = root mean square error; SIB = non-psychotic siblings; X = absence of data in this condition.
Hypothesis 1: Visuomotor grip-force tracking task and increased attentional load
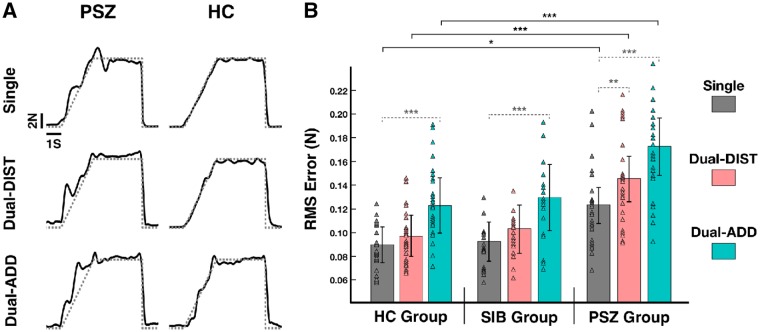
We tested whether force-tracking accuracy would decrease with increasing cognitive load, particularly in patients with schizophrenia during the Dual-DIST trials and in all three groups (but most strongly in patients with schizophrenia) during Dual-ADD trials. The ANOVA of force-tracking error (Table 2) showed a significant Group effect [F(2,62) = 6.16, P = 0.004] and post hoc tests revealed that patients with schizophrenia had a 40% increase in error compared to healthy control subjects (P = 0.002) and non-psychotic siblings (P = 0.01). The healthy control and non-psychotic siblings groups had similar performance in force-tracking error (P = 0.97). Tracking error also varied according to Condition [F(2,62) = 25.97, P < 0.001], showing an increase with cognitive load, i.e. increased error in Dual-DIST trials compared to Single-task tracking (P < 0.001), and a further increase in Dual-ADD compared to Dual-DIST trials (P < 0.001). We also explored interaction between Group and Condition since we found impaired gaze control in patients with schizophrenia in Dual-DIST trials (see below). Between group comparisons (Fig. 2) showed that patients had increased tracking error across conditions compared to control subjects (patients with schizophrenia versus healthy control subjects: Single, P = 0.03; Dual-DIST, P = 0.003; Dual-ADD, P < 0.001), and siblings (patients with schizophrenia versus non-psychotic siblings: Single, P = 0.04; Dual-DIST, P = 0.02; Dual-ADD, P < 0.001). Moreover, siblings and control subjects had similar tracking errors across conditions (healthy control subjects versus non-psychotic siblings, all P-values >0.50).
Figure 2.
Force control accuracy. (A) Raw data of single trials of grip force-tracking for a patient with schizophrenia (PSZ) and a healthy control subject (HC) for (i) the single-task force-tracking condition (Single) and the dual-task condition with (ii) dual-task distraction trial (Dual-DIST) and (iii) dual-task addition trial (Dual-ADD). Exerted force = solid line; target force (ramp-hold-and-release profile) = dotted grey line. Although the patient with schizophrenia seemed to perform the force matching task with less accuracy, the task was achieved; the overall modulation of force follows the target force throughout the trial, the force modulation is neither flat nor random, and the target hold-force level was reached (see Supplementary material for detailed analysis). (B) Mean RMS tracking-error (estimated marginal mean ± vertical bars: 95% confidence interval, CI) during Single (grey), Dual-DIST (pink) and Dual-ADD trials (cyan) for the three groups: patients with schizophrenia, healthy controls and siblings (SIB). Triangles represent data points of the individual subjects in each group and condition. Significant differences (LSD fisher post hoc tests for between group comparisons are shown as horizontal black brackets and within group comparisons as horizontal grey dashed brackets with: *P < 0.05; **P < 0.01, ***P < 0.001. For clarity, significant differences for between-group comparisons are only indicated between patients and healthy controls. Post hoc tests for between-group comparisons revealed that patients had increased error in the three conditions compared to controls (patients with schizophrenia versus healthy controls, Single: P = 0.03; Dual-DIST: P = 0.001; Dual-ADD: P < 0.001), but also to siblings (patients with schizophrenia versus siblings, Single: P = 0.04; DIST: P = 0.002; Dual-ADD: P < 0.001). Post hoc tests for within group comparisons revealed that DIST condition led to increased error compared to Single-task condition only in the patients with schizophrenia group (patient with schizophrenia: P = 0.003; healthy controls: P = 0.24; siblings: P = 0.88). However, Dual-ADD trials led to increased error compared to Single-task trials in all groups (patient with schizophrenia: P < 0.001; healthy controls: P < 0.001; siblings: P < 0.001).
Within group comparison showed that Dual-DIST led to increased error compared to Single-task only in patients (patients with schizophrenia: P = 0.003; healthy control subjects: P = 0.17; non-psychotic siblings: P = 0.11), whereas the Dual-ADD trials led to increased tracking error compared to Single-task in all three groups (patients with schizophrenia: P < 0.001; healthy control subjects: P = 0.002; non-psychotic siblings: P < 0.001).
Timing of tracking force onset and offset (Table 2) did not differ between groups [Onset: F(2,62) = 1.11, P = 0.34; Offset: F(2,62) = 0.89, P = 0.42]. No other interaction effects between within-group factors and Group were found. There was no effect of hand-dominance on performance.
Hypothesis 2: Visual attention during visuomotor grip-force tracking task
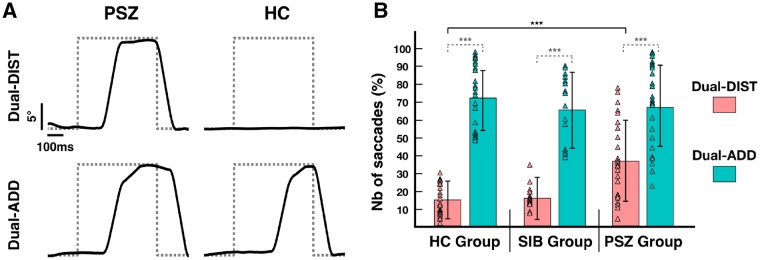
We examined whether patients with schizophrenia would show increased failure to inhibit saccades to distractors and would have decreased smooth pursuit accuracy. The ANOVA of saccadic inhibition (Dual-DIST) and saccadic execution (Dual-ADD) showed no significant effect of Group [F(2,64) = 2.14, P = 0.13, Fig. 3B], but a significant main effect of Condition, leading to an increased number of saccades toward numbers (Dual-ADD) compared to those towards distractors (Dual-DIST) in all groups [Condition: F(2,64) = 380.30, P < 0.001]. Moreover, a Condition × Group interaction was found [F(2,64) = 9, P < 0.001]. Post hoc testing revealed that when a distractor appeared in Dual-DIST trials, patients with schizophrenia failed to inhibit saccades in 34% ± 24 of the cases, whereas healthy control subjects and non-psychotic siblings failed in only 15% (healthy control subjects: 14% ± 9; non-psychotic siblings: 17% ± 14; schizophrenia versus healthy controls and schizophrenia versus non-psychotic siblings: P < 0.001). Control subjects and siblings had no significant difference in the number of non-inhibited saccades (healthy controls versus non-psychotic siblings: P = 0.61). In contrast, in Dual-ADD trials, the percentage of saccades toward the number was not significantly different between groups (schizophrenia: 70% ± 23, healthy controls: 74% ± 19, non-psychotic siblings : 69% ± 20; all P-values > 0.46).
Figure 3.
Saccadic execution. (A) Raw data of vertical eye position recorded during the visuomotor grip force-tracking task for a patient with schizophrenia (PSZ) and a healthy control subject (HC). Example trials show saccades (solid line) relative to the appearance of a visual stimulus (dotted grey line) during the dual-task condition: (i) dual-task distraction trial (Dual-DIST) where subjects had to inhibit saccades toward visual distractors; and (ii) dual-task addition trial (Dual-ADD) where subjects had to exert a saccade toward the stimulus (number). Initial gaze position corresponds to the vertical cursor (force-tracking) position; maximal gaze amplitude corresponds to vertical position of the number or distractor. (B) Mean percentage of exerted saccades toward visual stimuli (estimated marginal mean ± vertical bars: 95% CI) during Dual-DIST (displayed in pink) and Dual-ADD trials (displayed in cyan) for the three groups: patients with schizophrenia, healthy controls and siblings (SIB). Triangles represent data points of the individual subjects in each group and condition. Significant differences (LSD fisher post hoc tests for between group comparisons are shown as horizontal black brackets and within group comparisons as horizontal grey dashed brackets with: *P < 0.05; **P < 0.01, ***P < 0.001. For clarity, significant differences for between-group comparisons are only indicated between patients and healthy controls.
Saccade latency differed between groups and conditions (Table 2): patients with schizophrenia showed an increased latency for (erroneously executed) Dual-DIST saccades [t(48) = 2.92, P = 0.005] compared to healthy control subjects, but a decreased latency in (correctly executed) Dual-ADD saccades [t(48) = −2.88, P = 0.005]. Fixation duration on the target also varied: patients with schizophrenia showed longer duration in Dual-ADD [on numbers; t(48) = −2.46, P = 0.02], but no difference for duration in Dual-DIST [on distractors, t(48) = −0.12, P = 0.90] compared to controls.
The gain of SPEM showed a significant difference between groups [F(2,64) = 7.16, P = 0.002], and post hoc tests showed that patients with schizophrenia had lower gain compared to healthy control subjects (P = 0.002) and non-psychotic siblings (P = 0.002), while healthy control subjects and non-psychotic siblings had no significant difference (P = 0.76).
Relation between behavioural sensorimotor performance and attention
A number of results showed a relationship between attention and sensorimotor performance. First, when the ANOVA of force-tracking error was tested with saccadic inhibition (taken as a marker of attention derived from the Dual-DIST condition) as covariate, this explained the group differences. Thus, patients with schizophrenia no longer differed in force tracking performance when controlling for inhibition of saccades during Dual-DIST.
Second, we also found a positive correlation between tracking-error and Stroop interference score (r = 0.55, P = 0.004, corrected for multiple comparisons) and a negative correlation between tracking-error and performance in TAP subscore incompatibility (r = −0.60, P = 0.001, corrected for multiple comparisons) further suggesting a link to selective attention. In terms of gaze, a positive correlation was found between SPEM-gain and performance in TAP subscore incompatibility (r = 0.64, P < 0.001, corrected for multiple comparisons).
Finally, to clarify the relation between attention and force-tracking error with a complementary method, a multiple linear regression analysis was performed including the three above mentioned variables (saccadic inhibition; Stroop interference score; TAP subscore incompatibility). All three variables remained significant predictors of tracking error in the model: saccadic inhibition [F(1,23) = 4.84, P = 0.04], Stroop interference score [F(1,23) = 4.84, P = 0.01] and TAP subscore incompatibility [F(1,23) = 4.84, P = 0.02]. This multiple regression model explained 43% of force tracking error in the patients with schizophrenia [F(3,23) = 5.03, P = 0.009, R2 = 0.43].
Neurophysiological results
Resting motor threshold stimulus intensity at rest (P > 0.25) and MEP amplitude at rest (P > 0.68) did not differ between groups (Table 3). However, SICI at rest was significantly reduced in patients with schizophrenia compared to healthy control subjects [t(46) = − 2.23, P = 0.03] and non-psychotic siblings [t(39) = 2.90, P = 0.008]. There was no significant difference between groups in the level of EMG activity in the first dorsal interosseous prior to the stimulation onset at rest and at hold (EMG activity showed a fourfold increase for all groups during hold).
Table 3.
Neurophysiological data: non task-related baseline assessment for the three groups
| Neurophysiological assessments | Patients with schizophrenia Mean ± SD | Healthy control subjects Mean ± SD | Non-psychotic siblings Mean ± SD |
|---|---|---|---|
| MVC | |||
| Right hand, N | 384 ± 113 | 411 ± 124 | 326 ± 73 |
| Left hand, N | 346 ± 104 | 398 ± 112 | 334 ± 84 |
| TMS | |||
| Resting motor threshold, % stimulator | 54 ± 11 | 52 ± 8 | 50 ± 9 |
| Unconditioned MEP amplitude, mV | 1.80 ± 1.06 | 1.70 ± 0.98 | 1.98 ± 1.44 |
| Conditioned MEP amplitude, mV | 0.85 ± 0.53 | 0.53 ± 0.37* | 0.53 ± 0.35* |
| SICI, % reduction | 53 ± 17 | 68 ± 17* | 71 ± 19** |
| Control elements | |||
| REST EMG activity (mV) | 0.004 ± 0.003 | 0.003 ± 0.002 | 0.004 ± 0.002 |
| HOLD EMG activity (mV) | 0.018 ± 0.007 | 0.017 ± 0.010 | 0.016 ± 0.008 |
MVC for each hand was tested using force dynamometer. Resting motor threshold, MEPs for unconditioned and conditioned stimulus and SICI were obtained at baseline. Control elements were obtained 1500 ms prior to the stimulation during the rest and hold phases of the visuomotor tracking task. This to show that any changes in MEP amplitude cannot be attributed to group differences in EMG background activity. Group differences (t-tests) are displayed as follow: *P < 0.05; **P < 0.01, ***P < 0.001.
Hypothesis 3: Level of cortical excitability and inhibition during visuomotor grip-force tracking
We tested whether patients with schizophrenia had increased motor cortex excitability and reduced inhibition compared to healthy control subjects during single-task force tracking. Cortical excitability (MEP amplitude, Table 2) did not differ between groups [Group: F(2,62) = 1.31, P = 0.28] and conditions [Condition: F(2,62) = 0.93, P = 0.40], but showed a significant condition by group interaction [Condition × Group: F(4,124) = 6.42, P < 0.001]. Between group comparisons showed that, in single-task condition, patients with schizophrenia showed 80% increase in cortical excitability compared to 40% increase in healthy control subjects (schizophrenia versus healthy control: P = 0.02, Fig. 4B) and 50% increase in non-psychotic siblings (schizophrenia versus non-psychotic siblings: P = 0.08). This was, however, not the case in the dual-task condition: patients did not differ compared to control subjects (Dual-DIST: P = 0.32; Dual-ADD: P = 0.41) and to siblings (Dual-DIST: P = 0.44; Dual-ADD: P = 0.26). Control subjects and siblings had no significant difference in cortical excitability in all conditions (all P-values >0.66).
Importantly, within group comparisons showed that the dual-task condition led to decreased excitability compared to single-task condition in patients with schizophrenia (Dual-DIST: P = 0.001; Dual-ADD: P = 0.02), while healthy control subjects had increased excitability (Dual-DIST: P = 0.03; Dual-ADD: P < 0.001), and no significant difference was observed in the non-psychotic siblings group (Dual-DIST: P = 0.37; Dual-ADD: P = 0.67).
SICI showed a significant group effect [F(2,62) = 4.74, P = 0.01, Table 2]. Patients with schizophrenia had reduced SICI compared to healthy control subjects and non-psychotic siblings (P = 0.01 and P = 0.02, respectively), but SICI did not differ between healthy control subjects and non-psychotic siblings (P = 0.97). SICI did not differ between conditions [Condition: F(2,62) = 1.89, P = 0.16], but showed a significant Condition × Group interaction [F(4,124) = 2.85, P = 0.03]. Within group comparisons showed that the dual-task condition led to a strongest inhibition effect (SICI) compared to the single-task condition in healthy control subjects (Dual-DIST: P = 0.007; Dual-ADD: P = 0.03), but remained stable across conditions in patients with schizophrenia (Dual-DIST: P = 0.13; Dual-ADD: P = 0.31) and non-psychotic siblings (Dual-DIST: P = 0.27; Dual-ADD: P = 0.08).
Cortical silent period
The cortical silent period was not significantly different between groups [F(2,62) = 2.69, P = 0.08] and between task-conditions [F(2,124) = 0.58, P = 0.56]. However, a Group × Condition interaction was found [F(4,124) = 3, P = 0.02], indicating that in the single-task condition, patients with schizophrenia had a decreased cortical silent period compared to healthy control subjects (P = 0.008) but not compared to non-psychotic siblings (P = 0.09). No group differences in the cortical silent period were found in Dual-DIST and Dual-ADD trials. Moreover, healthy control subjects and non-psychotic siblings had no different cortical silent period duration across conditions (all P-values >0.16).
Hypothesis 4: Modulation of cortical excitability and inhibition with increased attentional load
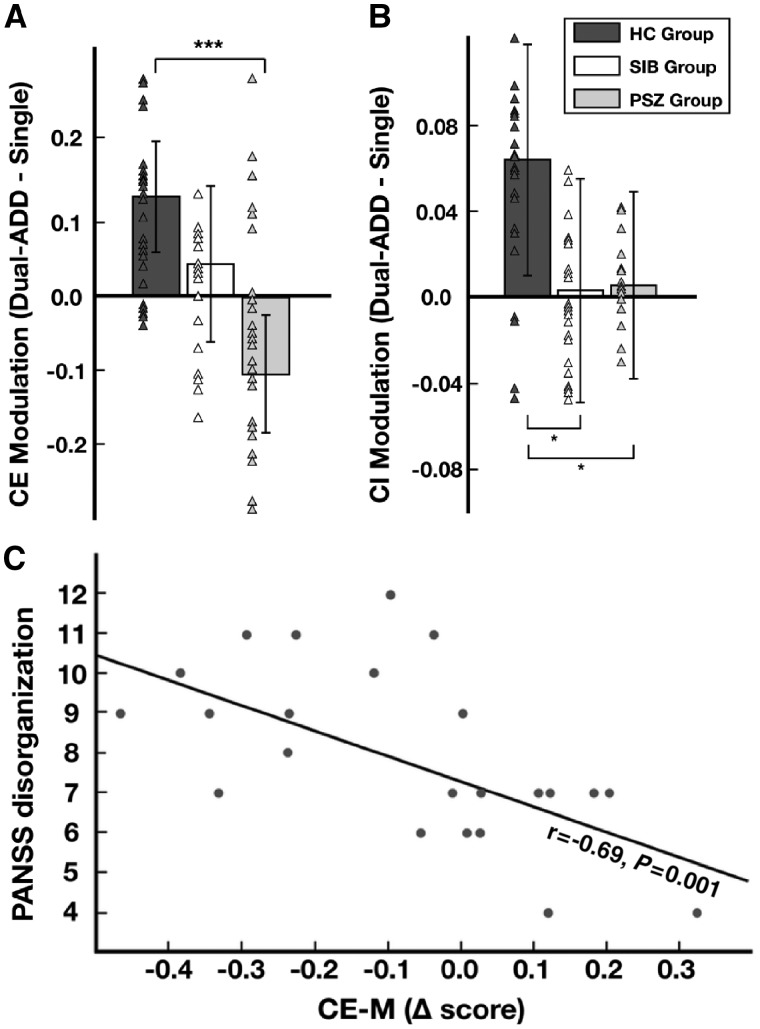
We assessed whether increased cognitive load in the dual-task condition would increase excitability in healthy control subjects, but not in patients with schizophrenia. The strength of condition-related MEP amplitude modulation varied significantly between groups (i.e. MEPDual-ADD − MEPSingle, P < 0.001, Fig. 5A). Healthy control subjects showed a strong positive modulation of excitability, whereas non-psychotic siblings showed a weak modulation, and patients with schizophrenia had a negative modulation. Similar patterns of condition-related modulation differences between groups were found in Dual-DIST trials.
Figure 5.
Modulation of cortical excitability and inhibition, relation to clinical score (PANSS). (A) Strength of modulation of cortical excitability (CE Modulation) for dual-task addition (Dual-ADD) compared to single-task trials (Single). This delta score (MEPDual-ADD − MEPSingle) corresponds to the mean MEP amplitude in Dual-ADD minus that in Single (estimated marginal mean ± vertical bars: 95% CI). For patients with schizophrenia (PSZ, grey), healthy control subjects (HC, dark grey) and siblings (SIB, white). Triangles represent data points of the individual subjects in each group. Significant differences between groups (LSD fisher post hoc tests) shown as horizontal brackets with: *P < 0.05, ***P < 0.001. (B) Strength of SICI modulation (CI Modulation); as in A but for (SICIDual-ADD − SICISingle). (C) Spearman’s correlation between delta score of cortical excitability (CE-M) and symptom severity: PANSS disorganization subscore for patients with schizophrenia (r = − 0.69, P = 0.001).
The strength of condition-related SICI modulation varied significantly between groups {SICIDual-ADD–SICISingle; Group: [F(2,62) = 4.30, P = 0.02]}. SICI modulation in patients with schizophrenia was significantly decreased in the dual-task condition compared to healthy control subjects (P = 0.01). Although siblings showed no significant difference in SICI amplitude compared to control subjects, they failed to show appropriate modulation of SICI (P = 0.03). Similar patterns of condition-related modulation differences between groups were found in Dual-DIST trials.
Investigating the relation between the modulation of cortical excitability and that of inhibition showed a correlation at a trend level for healthy controls (Pearson’s correlation; r = 0.50; P = 0.08) but not in patients (r = 0.18; P = 0.45) and siblings (r = 0.01; P = 0.97).
When the ANOVA was applied to the strength of modulation of cortical excitability including saccadic inhibition as covariate, this explained the group differences, this time in terms of cortical excitability.
Relation between tracking performance, cortical excitability and clinical scores
We also explored relations with clinical assessments to inform on clinical relevance of experimental measures. There was a positive correlation between tracking error and neurological soft signs subscore sensory integration (r = 0.64, P = 0.001, corrected for multiple comparisons).
The strength of modulation in cortical excitability correlated negatively with PANSS disorganization subscore (r = −0.69, P = 0.001, corrected for multiple comparisons; Fig. 5C). None of the visuomotor, gaze or neurophysiological measures correlated with CPZe (all P-values > 0.14). For siblings and healthy control subjects, no significant correlations were found between behavioural or neurophysiological measures and neuropsychological scores (all P-values > 0.07). No effect of smoking status was found in any TMS measures and results were similar when including smoking status as covariate (Supplementary material).
Discussion
In this study, we provide direct evidence that inefficient attentional processing contributes to both impaired sensorimotor performance and altered task-related modulation of cortical excitability in patients with schizophrenia. First, accuracy of visuomotor control of grip force decreased with increasing cognitive load in patients, in unaffected siblings and healthy control subjects. However, this decrease in accuracy was more pronounced in patients with schizophrenia, particularly in the dual-task condition requiring saccades away from the force-tracking task. Second, control of gaze was also affected in schizophrenia patients with a failure to inhibit saccades to visual distractors while controlling grip force. Third, physiologically, patients showed a clearly altered modulation of motor cortex excitability as a function of cognitive load (inversed modulation in the dual-task condition compared to control subjects) and reduced cortical inhibition (SICI) in all conditions compared to control subjects. Fourth, visual attention played a key role in visuomotor performance: group differences in both tracking performance and modulation of excitability were no longer significant when saccade inhibition (a marker of divided attention) was included as a covariate. In addition, the degree of sensorimotor impairment in patients correlated with two neuropsychological measures of attention (Stroop and TAP), and these two neuropsychological indicators of attention, as well as saccadic inhibition, predicted force tracking error in a multiple regression analysis in the patient group. This study also revealed that task-related modulation of cortical excitability correlated with symptoms of disorganization (PANSS). Furthermore, although siblings showed similar visuomotor and gaze performance to control subjects, their modulation of cortical excitability was weaker, though not inversed.
Attention and impaired eye-hand coordination in schizophrenia
In this study, we provide evidence for impaired sensorimotor control in schizophrenia in an eye-hand coordination paradigm. Patients with schizophrenia were markedly (∼40%) less accurate than control subjects and siblings in modulating grip force according to a moving visual target, confirming previous findings of grip and finger force control deficits in medicated and non-medicated patients with schizophrenia (Rosen et al., 1991; Caligiuri and Lohr, 1994; Delevoye-Turrell et al., 2002; Térémetz et al., 2014, 2017). We found that manual tracking error increased in conditions with higher cognitive load, i.e. in the presence of unpredictable visual stimuli that were either irrelevant (distractors) or relevant (numbers), and which required differential task-related control of gaze simultaneous to the ongoing visuomotor control of grip force. The presence of (erroneous) saccades to distractors in the Dual-task also explained group differences in tracking error, showing that impaired visual attention, probed using oculomotor function, contributes to deficient manual sensorimotor control. In this study, we found that three properties of gaze were perturbed in patients compared to control subjects: (i) distractors evoked more saccades (that should have been suppressed), and these erroneous saccades had longer latency; (ii) saccades to relevant stimuli (numbers) were of shorter latency, and the duration of fixation on the number was longer; and (iii) the gain of smooth pursuit, for foveal pursuit of the hand-controlled cursor during the ramp, i.e. when tight eye-hand coordination is required, was strongly reduced. The former two properties are consistent with deficient filtering of visual distractors during saccadic control (Calkins et al., 2008) and the latter with altered smooth pursuit observed in purely visual tasks (Kathmann et al., 2003; Arce et al., 2006; Caldani et al., 2017a; Bansal et al., 2018).
Deficient eye-hand coordination may be due to impaired divided visual attention (Jans et al., 2010). In our dual-task, when a number or a distractor (shape) was displayed, the subjects had to prepare an anticipatory saccade and then, inhibit or execute it depending on irrelevance (distractor) or relevance (number) of the visual stimulus. Healthy control subjects and siblings correctly inhibited their anticipatory saccades by use of peripheral vision for stimulus type identification, and could simultaneously maintain their attentional focus on controlling force. In contrast, patients with schizophrenia showed an increased failure rate in inhibiting anticipatory saccades, suggesting impaired distribution of attention. This result is in line with previous oculomotor studies (Reuter and Kathmann, 2004; Manoach et al., 2013) and with reports on deficient filtering (gating) of relevant/irrelevant information for saccadic control in schizophrenia (Calkins and Iacono, 2000; Calkins et al., 2008; Landgraf et al., 2008; Caldani et al., 2017a). The attentional deficit, revealed by altered gaze, likely contributes to deficient visuomotor manual control. Our results support this by showing that group differences in visuomotor control were abolished when accounting for the degree of saccade inhibition, and that attention measures (gaze and neuropsychological scores) significantly predicted impaired visuomotor performance (in both univariate and multiple regression analyses).
Although deficits in working memory have been reported in schizophrenia (Gold et al., 2003; Barch, 2005; Brandt et al., 2015a), we found a similar capacity to add numbers in Dual-ADD trials in all groups. This suggests a less important role of working memory in online sensorimotor adjustments consistent with recent findings showing intact tracking in patients with age-related cognitive decline (impaired working memory; Carment et al., 2018).
A recent study suggested that multiple aspects of cognitive dysfunction in schizophrenia, such as abnormal allocation of attention (Leonard et al., 2013) or impaired working memory (Luck et al., 2014), may have a common grounding in hyperfocusing of visual attention (Kreither et al., 2017). Although our experimental paradigms differ, decreased extrafoveal distinction between relevant and irrelevant stimuli during force-tracking indirectly supports hyperfocusing, i.e. impaired balance of attention in schizophrenia (Kreither et al., 2017).
Imbalance of task-related motor cortical excitability and inhibition
Patients with schizophrenia showed increased cortical excitability in the single-task force-tracking condition compared to control subjects, consistent with similar findings in other tasks (Gomez-Pilar et al., 2017; Sawaki et al., 2017). Since attention has been reported to influence cortical excitability in healthy controls (Conte et al., 2007), increased excitability may result from a dysfunctional allocation of attention (Delevoye-Turrell et al., 2006; Leonard et al., 2013) or hyperfocusing (Kreither et al., 2017), and may reflect the patients’ over-attentive commitment even in the single-task condition. In the dual-task condition with increasing cognitive load, when distractors or numbers appeared, patients with schizophrenia had decreased cortical excitability, the opposite pattern to that in healthy control subjects, who showed increased excitability. This opposite pattern of modulation in patients was present in both Dual-DIST and Dual-ADD trials, suggesting its dual-task dependence. Higher cognitive load was also related to increased SICI in healthy control subjects, whereas this modulation was weak in patients and sibling groups.
In summary, healthy subjects showed an increase of cortical excitability and SICI with increasing cognitive load (from the single-task, over the dual-task condition), but this was not the case in patients (inverted or no modulation). The cortical balance of excitation and inhibition (Dehghani et al., 2016) was probed in our study and we found an expected trend for balanced excitatory-inhibitory modulation in healthy control subjects. In contrast, we found a schizophrenia related imbalance in excitatory and inhibitory cortical activity consistent with previous studies during rest (Hasan et al., 2012; Radhu et al., 2013; Frantseva et al., 2014; Gao and Penzes, 2015). Few studies have explored this imbalance during sensorimotor tasks previously (Lindberg et al., 2016): our data suggest that altered (non-modulated) inhibitory mechanisms might interfere with the up- and downregulation of cortical excitation as a function of cognitive load.
Furthermore, the concept of hyperfocusing (Kreither et al., 2017) in schizophrenia seems consistent with our TMS data on cortical excitation: abnormally high attention in the Single-task (low cognitive load) condition would be consistent with abnormally increased excitation in patients with schizophrenia, which was the case. Abnormally low excitation under high cognitive load in patients with schizophrenia may be explained by reduced hyperfocusing in Dual-tasks (relative to Single-task) which may have led to reduced cortical excitation, whereas control subjects increased their attention in the Dual-task condition and showed increased excitation.
Our TMS findings are largely compatible with functional (fMRI/EEG) and anatomical neuroimaging studies showing more segmented activity (Gomez-Pilar et al., 2017) and altered cortical connectivity (Arce et al., 2006; Brandt et al., 2015b), pointing more generally to network abnormalities affecting manual control (Mouchet-Mages et al., 2007, 2011; Gay et al., 2013; Stegmayer et al., 2016).
Clinical relevance: patients versus siblings
We provide evidence that altered gaze, and by inference altered attentional processing, impacts manual sensorimotor performance in schizophrenia and was associated with abnormal modulation (imbalance) of cortical excitation and inhibition. Interestingly, the ability to modulate cortical activity was also associated with disorganization symptoms suggesting a link between behavioural performance and physiological mechanisms in schizophrenia. Moreover, oculomotor and force control deficits, related to neurological soft signs scores, suggest their potential use as state markers, although their specificity needs corroboration.
In unaffected siblings, manual and oculomotor performance was similar to healthy subjects, as were their neurological soft signs scores. Nonetheless, we show that siblings had altered cortical functioning. Modulation of cortical excitability was weak, i.e. at an intermediate level between that of control subjects and that of patients, and modulation of SICI was also less strong. This suggests that siblings, through a genetic risk component (Moran et al., 2013), present with an impairment in task-related cortical processing, though without behavioural manifestation, in line with normal neurological soft signs scores.
Limitations
In our study, patients with schizophrenia were all medicated and stabilized and they can be considered as well-functioning with respect to clinical (PANSS or neurological soft signs) and neuropsychological (WAIS) scores. Thus, to generalize our findings, replication in more severe patients or in early psychosis (disease evolution <2 years) is indicated. Recruitment of siblings was difficult: few were directly related to one of the patients, which may have weakened the strength of this cohort and statistical power. Nonetheless, highly significant statistical differences were found in key variables, suggesting adequate power. Fatigue or learning due to large number of (required) trials might have contributed to the observed deficits. However, each condition was pseudo-randomized and type of randomization did not explain any group differences. Furthermore, all groups showed similar success rate for the result of additions.
Conclusions
We found that patients with schizophrenia had impaired accuracy in visuomotor grip force control, concomitant with deficient control of gaze. Abnormal eye-hand coordination was particularly distinct in the dual-task condition. Increasing cognitive load induced the strongest impairment of manual sensorimotor performance in patients, who also showed altered attention-related modulation of cortical excitability and inhibition. Deficient gaze, a proxy for attention, explained a major portion of the behavioural impairments and of altered modulation of cortical excitability. Unaffected siblings showed normal behavioural performance but altered cortical excitability, consistent with a genetic risk for cortical abnormality. These behavioural and neurophysiological results pinpoint a key role for altered attentional processing (divided attention, filtering of irrelevant information) in sensorimotor impairments and modulation of cortical excitability/inhibition in schizophrenia.
Supplementary Material
Acknowledgements
We thank the ‘Delegation à la Recherche Clinique et l’Innovation’ (DRCI) and the Clinical Research Center (CRC) of Sainte-Anne Hospital, and the URC/CIC Paris Descartes of Necker - Cochin Hospital for their help in conducting this study. Thanks also to Maria Pia Bucci, Sophie Rivaud-Pechoux and Narjes Bendjemaa for their helpful contribution to this work.
Glossary
Abbreviations
- MEP
motor evoked potential
- MVC
maximum voluntary contraction
- PANSS
Positive and Negative Syndrome Scale
- SICI
short latency intracortical inhibition
- SPEM
smooth pursuit eye movement
- TAP
Test battery for Attentional Performance
- TMS
transcranial magnetic stimulation
Funding
This study was supported by the “Fondation pour la Recherché Médicale” (FRM - DPP20151033970). L.C. received a PhD-fellowship from Sorbonne Université. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests
The authors declare no competing financial interests.
References
- Arce E, Leland DS, Miller DA, Simmons AN, Winternheimer KC, Paulus MP. Individuals with schizophrenia present hypo- and hyperactivation during implicit cueing in an inhibitory task. NeuroImage 2006; 32: 704–13. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition: DSM-IV-TR®. American Psychiatric Association; 2000. [Google Scholar]
- Ayehu M, Shibre T, Milkias B, Fekadu A. Movement disorders in neuroleptic-naïve patients with schizophrenia spectrum disorders. BMC Psychiatry 2014; 14: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S, Ford JM, Spering M. The function and failure of sensory predictions. Ann N Y Acad Sci 2018; 1426: 199–220. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol 2005; 1: 321–53. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995; 57: 289–300. [Google Scholar]
- Bernard JA, Dean DJ, Kent JS, Orr JM, Pelletier-Baldelli A, Lunsford-Avery JR, et al. Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum Brain Mapp 2014; 35: 4064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry 2006; 163: 418–25. [DOI] [PubMed] [Google Scholar]
- Brandt CL, Doan NT, Tønnesen S, Agartz I, Hugdahl K, Melle I, et al. Assessing brain structural associations with working-memory related brain patterns in schizophrenia and healthy controls using linked independent component analysis. NeuroImage Clin 2015a; 9: 253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CL, Kaufmann T, Agartz I, Hugdahl K, Jensen J, Ueland T, et al. Cognitive effort and schizophrenia modulate large-scale functional brain connectivity. Schizophr Bull 2015b; 41: 1360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldani S, Amado I, Bendjemaa N, Vialatte F, Mam-Lam-Fook C, Gaillard R, et al. Oculomotricity and neurological soft signs: can we refine the endophenotype? A study in subjects belonging to the spectrum of schizophrenia. Psychiatry Res 2017a; 256: 490–7. [DOI] [PubMed] [Google Scholar]
- Caldani S, Bucci MP, Lamy J-C, Seassau M, Bendjemaa N, Gadel R, et al. Saccadic eye movements as markers of schizophrenia spectrum: exploration in at-risk mental states. Schizophr Res 2017b; 181: 30–7. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB. A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol Psychiatry 1994; 35: 104–11. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG. Eye movement dysfunction in schizophrenia: a heritable characteristic for enhancing phenotype definition. Am J Med Genet 2000; 97: 72–6. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG, Ones DS. Eye movement dysfunction in first-degree relatives of patients with schizophrenia: a meta-analytic evaluation of candidate endophenotypes. Brain Cogn 2008; 68: 436–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carment L, Abdellatif A, Lafuente-Lafuente C, Pariel S, Maier MA, Belmin J, et al. Manual dexterity and aging: a pilot study disentangling sensorimotor from cognitive decline. Front Neurol 2018; 9: 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A, Gilio F, Iezzi E, Frasca V, Inghilleri M, Berardelli A. Attention influences the excitability of cortical motor areas in healthy humans. Exp Brain Res 2007; 182: 109–17. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry 2002; 59: 347–54. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Moller B, Fountain SI, Chen R. Increased cortical inhibition in persons with schizophrenia treated with clozapine. J Psychopharmacol 2008; 22: 203–9. [DOI] [PubMed] [Google Scholar]
- Dehghani N, Peyrache A, Telenczuk B, Le Van Quyen M, Halgren E, Cash SS, et al. Dynamic balance of excitation and inhibition in human and monkey neocortex. Sci Rep 2016; 6: 23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye-Turrell Y, Giersch A, Danion J-M. A deficit in the adjustment of grip force responses in schizophrenia. Neuroreport 2002; 13: 1537–9. [DOI] [PubMed] [Google Scholar]
- Delevoye-Turrell Y, Giersch A, Danion J-M. Abnormal sequencing of motor actions in patients with schizophrenia: evidence from grip force adjustments during object manipulation. Am J Psychiatry 2003; 160: 134–41. [DOI] [PubMed] [Google Scholar]
- Delevoye-Turrell YN, Thomas P, Giersch A. Attention for movement production: abnormal profiles in schizophrenia. Schizophr Res 2006; 84: 430–2. [DOI] [PubMed] [Google Scholar]
- Duyck M, Collins T, Wexler M. Masking the saccadic smear. J Vis 2016; 16: 1. [DOI] [PubMed] [Google Scholar]
- Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr Res 2006; 84: 386–96. [DOI] [PubMed] [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev 2005; 15: 73–95. [DOI] [PubMed] [Google Scholar]
- Frantseva M, Cui J, Farzan F, Chinta LV, Perez Velazquez JL, Daskalakis ZJ. Disrupted cortical conductivity in schizophrenia: TMS-EEG study. Cereb Cortex 2014; 24: 211–21. [DOI] [PubMed] [Google Scholar]
- Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med 2015; 15: 146–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay O, Plaze M, Oppenheim C, Mouchet-Mages S, Gaillard R, Olié J-P, et al. Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull 2013; 39: 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giersch A, Wilquin H, Capa RL, Delevoye-Turrell YN. Combined visual and motor disorganization in patients with schizophrenia. Front Psychol 2013; 4: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. J Abnorm Psychol 2003; 112: 61–71. [PubMed] [Google Scholar]
- Gomez-Pilar J, Lubeiro A, Poza J, Hornero R, Ayuso M, Valcárcel C, et al. Functional EEG network analysis in schizophrenia: evidence of larger segregation and deficit of modulation. Prog Neuropsychopharmacol Biol Psychiatry 2017; 76: 116–23. [DOI] [PubMed] [Google Scholar]
- Grégoire J, Wierzbicki C. Comparison of four short forms of the Wechsler Adult Intelligence Scale—third edition (WAIS-III). Rev Eur Psychol Appliquée 2009; 59: 17–24. [Google Scholar]
- Grootens KP, Vermeeren L, Verkes RJ, Buitelaar JK, Sabbe BGC, van Veelen N, et al. Psychomotor planning is deficient in recent-onset schizophrenia. Schizophr Res 2009; 107: 294–302. [DOI] [PubMed] [Google Scholar]
- Hannah R, Rocchi L, Rothwell JC. Observing without acting: a balance of excitation and suppression in the human corticospinal pathway? Front Neurosci 2018; 12: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Wobrock T, Grefkes C, Labusga M, Levold K, Schneider-Axmann T, et al. Deficient inhibitory cortical networks in antipsychotic-naive subjects at risk of developing first-episode psychosis and first-episode schizophrenia patients: a cross-sectional study. Biol Psychiatry 2012; 72: 744–51. [DOI] [PubMed] [Google Scholar]
- Jans B, Peters JC, De Weerd P. Visual spatial attention to multiple locations at once: the jury is still out. Psychol Rev 2010; 117: 637–84. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci 2001; 21: 6917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaster TS, de Jesus D, Radhu N, Farzan F, Blumberger DM, Rajji TK, et al. Clozapine potentiation of GABA mediated cortical inhibition in treatment resistant schizophrenia. Schizophr Res 2015; 165: 157–62. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry 2003; 160: 696–702. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261–76. [DOI] [PubMed] [Google Scholar]
- Kent JS, Hong SL, Bolbecker AR, Klaunig MJ, Forsyth JK, O’Donnell BF, et al. Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One 2012; 7: e41808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs MO, Gut-Fayand A, Bourdel M, Dischamp J, Olié J. Validation and factorial structure of a standardized neurological examination assessing neurological soft signs in schizophrenia. Schizophr Res 2000; 45: 245–60. [DOI] [PubMed] [Google Scholar]
- Kreither J, Lopez-Calderon J, Leonard CJ, Robinson BM, Ruffle A, Hahn B, et al. Electrophysiological evidence for hyperfocusing of spatial attention in schizophrenia. J Neurosci 2017; 37: 3813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf S, Amado I, Bourdel M-C, Leonardi S, Krebs M-O. Memory-guided saccade abnormalities in schizophrenic patients and their healthy, full biological siblings. Psychol Med 2008; 38: 861–70. [DOI] [PubMed] [Google Scholar]
- Lehoux C, Everett J, Laplante L, Emond C, Trépanier J, Brassard A, et al. Fine motor dexterity is correlated to social functioning in schizophrenia. Schizophr Res 2003; 62: 269–73. [DOI] [PubMed] [Google Scholar]
- Leonard CJ, Kaiser ST, Robinson BM, Kappenman ES, Hahn B, Gold JM, et al. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex 2013; 23: 1582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Wu Y, Chen IC, Tsai P, Wu C, Chen C. Dual-task performance involving hand dexterity and cognitive tasks and daily functioning in people with schizophrenia: a pilot study. Am J Occup Ther 2015; 69: 6903250020p1–7. [DOI] [PubMed] [Google Scholar]
- Lindberg PG, Térémetz M, Charron S, Kebir O, Saby A, Bendjemaa N, et al. Altered cortical processing of motor inhibition in schizophrenia. Cortex 2016; 85: 1–12. [DOI] [PubMed] [Google Scholar]
- Luck SJ, McClenon C, Beck VM, Hollingworth A, Leonard CJ, Hahn B, et al. Hyperfocusing in schizophrenia: evidence from interactions between working memory and eye movements. J Abnorm Psychol 2014; 123: 783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Lee AKC, Hämäläinen MS, Dyckman KA, Friedman JS, Vangel M, et al. Anomalous use of context during task preparation in schizophrenia: a magnetoencephalography study. Biol Psychiatry 2013; 73: 967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschreck TC, Chun J, Merrill AM, Maher BA, Boshes RA, Glatt SJ, et al. Impaired motor performance in adolescents at familial high-risk for schizophrenia. Schizophr Res 2015; 168: 44–9. [DOI] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Candela SF. Earlier age of first diagnosis in schizophrenia is related to impaired motor control. Schizophr Bull 2004; 30: 351–60. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov 2016; 15: 485–515. [DOI] [PubMed] [Google Scholar]
- Mittal VA. Cross-cutting advancements usher in a new era for motor research in psychosis. Schizophr Bull 2016; 42: 1322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran ME, Hulshoff Pol H, Gogtay N. A family affair: brain abnormalities in siblings of patients with schizophrenia. Brain 2013; 136: 3215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchet-Mages S, Canceil O, Willard D, Krebs M-O, Cachia A, Martinot J-L, et al. Sensory dysfunction is correlated to cerebellar volume reduction in early schizophrenia. Schizophr Res 2007; 91: 266–9. [DOI] [PubMed] [Google Scholar]
- Mouchet-Mages S, Rodrigo S, Cachia A, Mouaffak F, Olie JP, Meder JF, et al. Correlations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr Scand 2011; 123: 451–8. [DOI] [PubMed] [Google Scholar]
- Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry 1988; 39: 1172–7. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Dafotakis M, Fink GR. Kinematic analysis of grasping in focal dystonia of the face and neck. Neuroscience 2013; 237: 216–22. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51: 849–59. [DOI] [PubMed] [Google Scholar]
- O’Donoghue S, Holleran L, Cannon DM, McDonald C. Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: a selective review of structural network analyses using diffusion MRI. J Affect Disord 2017; 209: 217–28. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962; 10: 799–812. [Google Scholar]
- Pascual-Leone A, Manoach DS, Birnbaum R, Goff DC. Motor cortical excitability in schizophrenia. Biol Psychiatry 2002; 52: 24–31. [DOI] [PubMed] [Google Scholar]
- Putzhammer A, Perfahl M, Pfeiff L, Hajak G. Correlation of subjective well-being in schizophrenic patients with gait parameters, expert-rated motor disturbances, and psychopathological status. Pharmacopsychiatry 2005; 38: 132–8. [DOI] [PubMed] [Google Scholar]
- Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol 2013; 124: 1309–20. [DOI] [PubMed] [Google Scholar]
- Reuter B, Kathmann N. Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychol (Amst) 2004; 115: 255–69. [DOI] [PubMed] [Google Scholar]
- Rosen AJ, Lockhart JJ, Gants ES, Westergaard CK. Maintenance of grip-induced muscle tension: a behavioral marker of schizophrenia. J Abnorm Psychol 1991; 100: 583–93. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994; 91: 79–92. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Kreither J, Leonard CJ, Kaiser ST, Hahn B, Gold JM, et al. Hyperfocusing of attention on goal-related information in schizophrenia: evidence from electrophysiology. J Abnorm Psychol 2017; 126: 106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212: 11–9. [DOI] [PubMed] [Google Scholar]
- Soubasi E, Chroni E, Gourzis P, Zisis A, Beratis S, Papathanasopoulos P. Cortical motor neurophysiology of patients with schizophrenia: a study using transcranial magnetic stimulation. Psychiatry Res 2010; 176: 132–6. [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Bohlhalter S, Vanbellingen T, Federspiel A, Moor J, Wiest R, et al. Structural brain correlates of defective gesture performance in schizophrenia. Cortex 2016; 78: 125–37. [DOI] [PubMed] [Google Scholar]
- Stroop RJ. Studies of interference in serial verbal reactions. J Exp Psychol 1935: 643–62. [Google Scholar]
- Térémetz M, Amado I, Bendjemaa N, Krebs M-O, Lindberg PG, Maier MA. Deficient grip force control in schizophrenia: behavioral and modeling evidence for altered motor inhibition and motor noise. PLoS One 2014; 9: e111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Térémetz M, Carment L, Brénugat-Herne L, Croca M, Bleton J-P, Krebs M-O, et al. Manual dexterity in schizophrenia—a neglected clinical marker? Front Psychiatry 2017; 8: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M, Farina S, Tamburin S, Facchini S, Fiaschi A, Restivo D, et al. Task-dependent modulation of excitatory and inhibitory functions within the human primary motor cortex. Exp Brain Res 2003; 150: 222–9. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Bour LJ, Boerée T, Koelman JHTM, de Haan L, et al. Increased saccadic rate during smooth pursuit eye movements in patients at Ultra High Risk for developing a psychosis. Brain Cogn 2010; 73: 215–21. [DOI] [PubMed] [Google Scholar]
- Walther S, Eisenhardt S, Bohlhalter S, Vanbellingen T, Müri R, Strik W, et al. Gesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr Bull 2016; 42: 1326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Mittal VA. Why we should take a closer look at gestures. Schizophr Bull 2016; 42: 259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Sulzbacher J, Vanbellingen T, Müri R, Strik W, et al. Nonverbal social communication and gesture control in schizophrenia. Schizophr Bull 2015; 41: 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Schneider-Axmann T, Retz W, Rösler M, Kadovic D, Falkai P, et al. Motor circuit abnormalities in first-episode schizophrenia assessed with transcranial magnetic stimulation. Pharmacopsychiatry 2009; 42: 194–201. [DOI] [PubMed] [Google Scholar]
- Wolff AL, O’Driscoll GA. Motor deficits and schizophrenia: the evidence from neuroleptic-naïve patients and populations at risk. J Psychiatry Neurosci 1999; 24: 304–14. [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME, Robinson DL. Brain mechanisms of visual attention. Sci Am 1982; 246: 124–35. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Fimm B. A test battery for attentional performance. In: Leclercq M, Zimmermann P, editors. Applied neuropsychology of attention. London: Psychology Press; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its Supplementary material.