Abstract
Background
Plasma volume expanders are used in connection to paracentesis in people with cirrhosis to prevent reduction of effective plasma volume, which may trigger deleterious effect on haemodynamic balance, and increase morbidity and mortality. Albumin is considered the standard product against which no plasma expansion or other plasma expanders, e.g. other colloids (polygeline , dextrans, hydroxyethyl starch solutions, fresh frozen plasma), intravenous infusion of ascitic fluid, crystalloids, or mannitol have been compared. However, the benefits and harms of these plasma expanders are not fully clear.
Objectives
To assess the benefits and harms of any plasma volume expanders such as albumin, other colloids (polygeline, dextrans, hydroxyethyl starch solutions, fresh frozen plasma), intravenous infusion of ascitic fluid, crystalloids, or mannitol versus no plasma volume expander or versus another plasma volume expander for paracentesis in people with cirrhosis and large ascites.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, CNKI, VIP, Wanfang, Science Citation Index Expanded, and Conference Proceedings Citation Index until January 2019. Furthermore, we searched FDA, EMA, WHO (last search January 2019), www.clinicaltrials.gov/, and www.controlled‐trials.com/ for ongoing trials.
Selection criteria
Randomised clinical trials, no matter their design or year of publication, publication status, and language, assessing the use of any type of plasma expander versus placebo, no intervention, or a different plasma expander in connection with paracentesis for ascites in people with cirrhosis. We considered quasi‐randomised, retrieved with the searches for randomised clinical trials only, for reports on harms.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We calculated the risk ratio (RR) or mean difference (MD) using the fixed‐effect model and the random‐effects model meta‐analyses, based on the intention‐to‐treat principle, whenever possible. If the fixed‐effect and random‐effects models showed different results, then we made our conclusions based on the analysis with the highest P value (the more conservative result). We assessed risks of bias of the individual trials using predefined bias risk domains. We assessed the certainty of the evidence at an outcome level, using GRADE, and constructed 'Summary of Findings' tables for seven of our review outcomes.
Main results
We identified 27 randomised clinical trials for inclusion in this review (24 published as full‐text articles and 3 as abstracts). Five of the trials, with 271 participants, assessed plasma expanders (albumin in four trials and ascitic fluid in one trial) versus no plasma expander. The remaining 22 trials, with 1321 participants, assessed one type of plasma expander, i.e. dextran, hydroxyethyl starch, polygeline, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus another type of plasma expander, i.e. albumin in 20 of these trials and polygeline in one trial. Twenty‐five trials provided data for quantitative meta‐analysis. According to the Child‐Pugh classification, most participants were at an intermediate to advanced stage of liver disease in the absence of hepatocellular carcinoma, recent gastrointestinal bleeding, infections, and hepatic encephalopathy. All trials were assessed as at overall high risk of bias. Ten trials seemed not to have been funded by industry; twelve trials were considered unclear about funding; and five trials were considered funded by industry or a for‐profit institution.
We found no evidence of a difference in effect between plasma expansion versus no plasma expansion on mortality (RR 0.52, 95% CI 0.06 to 4.83; 248 participants; 4 trials; very low certainty); renal impairment (RR 0.32, 95% CI 0.02 to 5.88; 181 participants; 4 trials; very low certainty); other liver‐related complications (RR 1.61, 95% CI 0.79 to 3.27; 248 participants; 4 trials; very low certainty); and non‐serious adverse events (RR 1.04, 95% CI 0.32 to 3.40; 158 participants; 3 trials; very low certainty). Two of the trials stated that no serious adverse events occurred while the remaining trials did not report on this outcome. No trial reported data on health‐related quality of life.
We found no evidence of a difference in effect between experimental plasma expanders versus albumin on mortality (RR 1.03, 95% CI 0.82 to 1.30; 1014 participants; 14 trials; very low certainty); serious adverse events (RR 0.89, 95% CI 0.10 to 8.30; 118 participants; 2 trials; very low certainty); renal impairment (RR 1.17, 95% CI 0.71 to 1.91; 1107 participants; 17 trials; very low certainty); other liver‐related complications (RR 1.10, 95% CI 0.82 to 1.48; 1083 participants; 16 trials; very low certainty); and non‐serious adverse events (RR 1.37, 95% CI 0.66 to 2.85; 977 participants; 14 trials; very low certainty). We found no data on heath‐related quality of life and refractory ascites.
Authors' conclusions
Our systematic review and meta‐analysis did not find any benefits or harms of plasma expanders versus no plasma expander or of one plasma expander such as polygeline, dextrans, hydroxyethyl starch, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus albumin on primary or secondary outcomes. The data originated from few, small, mostly short‐term trials at high risks of systematic errors (bias) and high risks of random errors (play of chance). GRADE assessments concluded that the evidence was of very low certainty. Therefore, we can neither demonstrate or discard any benefit of plasma expansion versus no plasma expansion, and differences between one plasma expander versus another plasma expander.
Larger trials at low risks of bias are needed to assess the role of plasma expanders in connection with paracentesis. Such trials should be conducted according to the SPIRIT guidelines and reported according to the CONSORT guidelines.
Plain language summary
Plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis
Background
People with cirrhosis (scarring of the liver tissue) can accumulate fluid (ascites) in their abdomen which may be hard or impossible to treat with diuretics (drugs that increase urinary excretion of water and salt). Abdominal paracentesis, evacuation of fluid from the abdomen through a needle, can be done. Paracentesis can alter the equilibrium between circulation and the abdomen fluid and lead to renal dysfunction and alteration of the fluid balance. We studied if infusion of special fluids, so called plasma expanders, could stop these alterations and reduce complications and mortality.
Objective
To assess the benefits and harms of any intravenous fluid infusion (acting as plasma expansion) in people with cirrhosis and ascites treated by paracentesis.
Review methods and criteria
The evidence is current up to 22 January 2019.
This systematic review assessed the role of plasma expanders evaluated in 27 trials including 1592 participants. Four trials compared albumin and one trial compared intravenous ascitic fluid infusion versus no plasma expander. Twenty‐one trials compared one plasma expander such as dextran, polygeline, hydroxyethyl starch, fresh frozen plasma, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus albumin. One trial compared intravenous ascitic fluid infusion versus polygeline. Primary outcomes were mortality due to any cause; serious adverse events; and health‐related quality of life. Secondary outcomes were refractory ascites (ascites that could not be treated medically); renal impairment; other complications due to liver cirrhosis such as gastrointestinal bleeding, hepatic encephalopathy (decline in brain function due to liver disease) or infections; and non‐serious adverse events.
Trial funding sources
Ten trials seemed not to have been funded by industry; twelve trials were considered unclear about funding; and five trials were considered funded by industry or a for‐profit institution.
Key results
Our systematic review could not show any benefit or harm of plasma expansion versus no plasma expansion or of one plasma expander like dextran, polygeline, hydroxyethyl starch, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus albumin on primary or secondary outcomes.
Certainty of the evidence
The data came from only few, small, mostly short‐term trials, at high risks of systematic errors (bias) and high risks of random errors (play of chance). Accordingly, we concluded that the certainty of evidence for each of our prespecified review outcomes was very low.
Summary of findings
Background
Description of the condition
Ascites is associated with increased mortality in people with cirrhosis (D'Amico 1986; Salerno 1991; D'Amico 2006). Ascites is graded as mild, moderate, and large (AASLD 2009; AASLD 2012; EASL 2018). It has been shown that people with mild or moderate ascites respond positively to dietary sodium restriction and diuretics (Runyon 1998; AASLD 2009; AASLD 2012; EASL 2018). However, people with large ascites may have respiratory problems, in which case paracentesis is usually used to provide relief (Ginés 1987). Paracentesis can be partial, defined as repeated sessions of paracentesis, or total, when performed in one session (Titó 1990). Abdominal paracentesis seems to be associated with lower incidence of adverse events and quicker resolution compared with diuretic treatment (Ginés 1987). Moreover, repeated paracentesis is used in people with large ascites who are unresponsive to intensive diuretic treatment and dietary sodium restriction, or in those who experience adverse effects from these treatments (e.g. hypotension, hyponatraemia, hyperkalaemia, renal impairment, or hepatic encephalopathy). This type of ascites is defined as refractory (Arroyo 1996; Runyon 1998; Moore 2003; AASLD 2009; AASLD 2012; EASL 2018).
Paracentesis may induce hypovolaemia and haemodynamic changes with circulatory dysfunction which is expressed by a marked increase of the plasma renin activity in about 27% to 40% of the people undergoing paracentesis, as reported by Ruiz‐del‐Arbol 1997 and Vila 1998. This syndrome, referred to as post‐paracentesis circulatory dysfunction (or paracentesis‐induced circulatory dysfunction), may be associated with shorter time to readmission, and shorter survival (Ginès 1996), but it is not clear if its prevention would reduce morbidity and mortality.
Description of the intervention
The intravenous infusion of fluids, acting as a plasma expander, could counterbalance the deleterious effects of paracentesis.
The results of haemodynamic (García‐Compeán 1993; Luca 1995) and clinical studies (Ginès 1988; Ginès 1996) suggest that the risk of hypovolaemia and circulatory dysfunction may be reduced by the intravenous infusion of albumin. As albumin is expensive, volume expansion is also carried out by administering cheaper colloids (polygeline, dextrans, hydroxyethyl starch solutions, fresh frozen plasma), crystalloids, mannitol or by intravenous infusion of the removed ascitic fluid. The effects of these interventions have been compared with that of albumin in randomised clinical trials, but the results have been heterogeneous (Planas 1990; Smart 1990; Salerno 1991; Bruno 1992; Fassio 1992; Ginès 1996; Moreau 2006; Al Sebaey 2012).
How the intervention might work
Paracentesis can increase arterial vasodilation in people with cirrhotic ascites, which may lead to reduced effective circulating plasma volume and may result in a reduction of arterial pressure and activation of the renin‐angiotensin‐aldosterone system (RAAS) as well as the sympathetic nervous system (SNS). This, in turn, may lead to increased sodium and water retention, and renal vasoconstriction (Ruiz‐del‐Arbol 1997; Saló 1997; Vila 1998).
Plasma expansion could prevent or improve the haemodynamic derangement induced by the paracentesis, by filling up the vascular system, and balancing the decreased vascular resistance ‐ thus, preventing the subsequent activation of vasoconstrictive systems (Ginès 1988). The use of albumin is based on its effect on intravascular volume and also on its anti‐inflammatory and vasoconstrictive properties (Garcia‐Martinez 2013; Garcia‐Tsao 2018).
Why it is important to do this review
The benefits of albumin and other colloids have been questioned for a long time. Many randomised clinical trials have been conducted to assess the role of albumin or colloids in the intensive care setting, and several Cochrane systematic reviews have been published on this topic (Cochrane Injuries Group Albumin Reviewers 1998; Schierhout 1998a; Alderson 2002; Roberts 2011; Perel 2013; Lewis 2018). In the Cochrane review by Perel and colleagues, no benefit was found for colloids, including albumin, when compared with crystalloids for fluid resuscitation in critically ill people with trauma, burns, or following surgery. However, the review found evidence of increased mortality due to the use of hydroxyethyl starch (Perel 2013). These results are not consistent with results of two other systematic reviews (He 2015; Qureshi 2016). More recently, Lewis and colleagues updated the Perel 2013 review, excluding the participants scheduled for elective surgery, and the review authors confirmed that colloids compared with crystalloids for fluid replacement probably made little or no difference in mortality of critically ill people. The review authors did not find increased mortality due to hydroxyethyl starch solutions either (Lewis 2018). hydroxyethyl starch seems to increase mortality in people with severe sepsis, following the studies by Haase 2013, Haase 2014, and Perner 2014. These results continue to raise a debate, above all, on the use of albumin and other plasma expanders in intensive care patients (Bapat 1998; Beale 1998; Chalmers 1998; Corder 1998; Frame 1998; Goodman 1998; Gosling 1998; Kaag 1998; Lawler 1998; Makin 1998; McAnulty 1998; Nadel 1998; Nel 1998; Offringa 1998; Petros 1998; Riordan 1998; Roberts 1998; Schierhout 1998b; Shwe 1998; Soni 1998; Watts 1998; Wyncoll 1998; Roberts 1999; Hartog 2014; Haase 2014).
In patients with cirrhosis, albumin has been used with different results in connection with paracentesis (Ginés 1987; Ginès 1988; Titó 1990), or in connection with diuretics (Gentilini 1999; Romanelli 2006), or in people having spontaneous bacterial peritonitis, or other bacterial infections (Sort 1999; Guevara 2012; Kwok 2013; Salerno 2013; Thévenot 2015), or in patients with hepatorenal syndrome with or without the use of vasoconstrictors (Martín‐Llahí 2008; Boyer 2016), or in patients with hyponatraemia (McCormick 1990; Jalan 2007; Bajaj 2018). Recently, an increased survival with long‐term albumin administration was observed in a large open‐label randomised trial including participants with decompensated cirrhosis and uncomplicated ascites treated by diuretics (Caraceni 2018) and in a non randomised study including cirrhotic participants with refractory ascites (Di Pascoli 2019). On the contrary, in participants with cirrhosis awaiting liver transplantation, treatment with albumin and midodrine neither prevented complications of cirrhosis nor improved survival (O’Brien 2018; Solà 2018). Conflicting opinions have been recently published on the use of albumin in this peculiar setting of patients (Bernardi 2019; O'Brien 2019).
A meta‐analysis of randomised clinical trials of albumin for a series of indications including treatment of people with cirrhosis and tense ascites (probably comparable to large ascites) showed no effect on mortality (Wilkes 2001). Several other meta‐analyses have followed, including only people with cirrhosis and large ascites (Wong 2008; Bernardi 2012; Wang 2015). In the meta‐analyses of Bernardi and colleagues in paracentesis‐treated people, albumin versus no treatment reduced post‐paracentesis circulatory dysfunction and hyponatraemia, and albumin versus alternative treatments (other plasma‐expanders and vasoconstrictors) reduced post‐paracentesis circulatory dysfunction, hyponatraemia, and mortality. There was no reduction of other complications (Bernardi 2012). Similar results were obtained in the meta‐analysis by Wang and colleagues, in which albumin was compared with other plasma expanders and with vasoconstrictors (Wang 2015). Wong and colleagues reported data on paracentesis performed with or without albumin, or another plasma expander, and they observed no consistent effect on morbidity or mortality between the interventions (Wong 2008). The latest meta‐analysis, published by Kütting and colleagues, concluded that there was insufficient evidence of benefit on mortality due to albumin substitution in hepatocellular cancer‐free cirrhotic participants undergoing large volume paracentesis (Kütting 2017).
Despite the conflicting conclusions of these systematic reviews, plasma expansion with albumin after large volume paracentesis is recommended in several guidelines (AASLD 2012; AISF 2016; EASL 2018) with a high grade of recommendations, referring to the Bernardi 2012 meta‐analysis.
The current systematic review will not assess the benefits and harms of plasma expanders in cirrhotic patients for long‐term administration or in people with spontaneous bacterial peritonitis or hepatorenal syndrome, or when used after paracentesis compared with diuretics, or transjugular intrahepatic portosystemic shunt (TIPS). Our objectives are described below.
Objectives
To assess the benefits and harms of any plasma volume expanders such as albumin, other colloids, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus no plasma volume expander or versus another plasma volume expander for paracentesis in people with cirrhosis and large ascites.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials examining plasma volume expanders administered in connection with abdominal paracentesis procedure in people with cirrhosis and large ascites. Trials could have been unpublished or published as full papers, abstracts, or poster presentations. We included trials irrespective of blinding, language, and date of publication. We considered quasi‐randomised studies that were retrieved with the searches for randomised clinical trials for report on harms only, as uncommon adverse events are rarely captured in randomised clinical trials (Storebø 2018). We are aware that by using these selection methods we are putting more focus on benefits than on harms of these interventions.
Types of participants
Adults with liver cirrhosis and large ascites, either diuretic‐responsive or refractory ascites. The diagnosis of cirrhosis could have been established by clinical and laboratory data, or liver histology. We excluded trials including people with cirrhosis having spontaneous bacterial peritonitis, acute‐on‐chronic liver failure (ACLF), or hepatocellular carcinoma, or people with prior surgical and para‐surgical therapy (surgical large‐caval anastomosis, liver transplantation, and transjugular intrahepatic portosystemic shunt (TIPS)).
Ascites is defined as refractory if it could not be mobilised, or the early recurrence of which could not be prevented because of a lack of response to sodium restriction and diuretic treatment (diuretic‐resistant ascites), or because of development of diuretic‐induced complications (e.g. hypotension, hepatic encephalopathy, functional renal impairment, hyponatraemia, etc.) that precluded the use of an effective diuretic dosage (diuretic‐intractable ascites) (Arroyo 1996; Moore 2003). We recorded the definition of refractory ascites used in the trials if it deferred from the provided definition above (Arroyo 1996; Moore 2003). We labelled 'trials without refractory ascites' those in which participants with refractory ascites were excluded or, if not clearly stated, those trials in which diuretic treatment had been reported until hospital admission, and the mean levels of blood urea, blood creatinine, and blood natrium were normal, because we expected that the proportion of refractory ascites, if present, was low.
Types of interventions
Plasma volume expansion using albumin, other colloids, intravenous infusion of ascitic fluid, crystalloids, or mannitol versus no plasma volume expander (i.e. placebo or no intervention), administered in connection with paracentesis.
Plasma volume expansion using one plasma volume expander versus another plasma volume expander, administered in connection with paracentesis.
We included randomised clinical trials with collateral interventions if used in the same way in the trial comparison groups.
Types of outcome measures
Primary outcomes
All‐cause mortality at the end of the maximal follow‐up.
Serious adverse events at the end of the maximal follow‐up, excluding those for which definition of other liver‐related complications could be applied (see below). We considered an event as a serious adverse event if it fulfilled the definition of serious adverse events of the International Conference on Harmonization (ICH) Guidelines (ICH 1997), that is, any event that leads to death, is life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability, congenital birth or anomaly, and any important medical event which may have jeopardised the patient or requires intervention to prevent it. We considered all other adverse events as non‐serious.
Health‐related quality of life as measured by the trials.
Secondary outcomes
Refractory ascites (see the definition above).
Renal impairment.
Other complications due to liver cirrhosis such as gastrointestinal bleeding, hepatic encephalopathy, or infections (which we defined as 'other liver‐related complications'). This outcome did not include the outcomes listed under exploratory outcomes.
Non‐serious adverse events.
Exploratory outcomes
Recurrence of ascites, defined as ascites that requires repeated paracentesis or hospitalisation, or both.
Hypotension as defined by the trial authors.
Hyponatraemia as defined by the trial authors.
Post‐paracentesis circulatory dysfunction, defined as an increase in the plasma renin activity of more than 50 per cent of the pretreatment value to a level of more than 4 ng/mL/h on the sixth day after paracentesis (Ginès 1996).
Search methods for identification of studies
Electronic searches
We identified relevant studies by searching the Cochrane Hepato‐Biliary Group Controlled Trials Register (January 2019), the Cochrane Central Register of Controlled Trials (CENTRAL in the Cochrane Library, Issue 1, 2019), MEDLINE (1946 to January 2019), Embase (1974 to January 2019), LILACS (1982 to January 2019), three Chinese database including CNKI, VIP, and Wanfang (to August 2015), Science Citation Index Expanded (1900 to January 2019), and Conference Proceedings Citation Index (1990 to January 2019).
We also searched databases of ongoing trials (www.clinicaltrials.gov/ and www.controlled‐trials.com/) (with links to several databases). In addition, we searched the European Medicines Agency (EMA, www.ema.europe.eu), US Food and Drug Administration (FDA, www.fda.gov), and the World Health Organization International Clinical Trials Registry Platform (ICTRP 2011) until January 2019. For detailed search strategies, see Appendix 1. We did not apply any language or document type restrictions. We contacted authors of the included trials to request missing information.
Searching other resources
We checked references of included trials, meta‐analyses, and other publications that were retrieved with the searches for randomised clinical trials in order to identify further trials of relevance to our review.
Data collection and analysis
We performed the systematic review and meta‐analyses following recommendations of Cochrane (Higgins 2011), and the Cochrane Hepato‐Biliary Group (Gluud 2015). In the case of cross‐over trials, we included data only from the first period (Higgins 2011). We performed the analyses using Review Manager 5.3 (RevMan 2014) and Trial Sequential Analysis version 0.9 (Wetterslev 2008; Thorlund 2011; TSA 2011). We assessed the evidence according to recommendations from Jakobsen and colleagues (Jakobsen 2014).
Selection of studies
Two of the authors, RGS and GP, independently of each other, identified the trials for inclusion in accordance with the inclusion criteria of the updated review protocol and listed the excluded studies with the reasons for their exclusion. RGS and GP resolved disagreements through discussions.
Data extraction and management
Two authors, RGS and GP, independently extracted data. We resolved disagreements by discussion. Data extraction encompassed: ‐ trial inclusion and exclusion criteria; ‐ the comparability between the groups randomised to alternative treatments regarding baseline prognostic variables: aetiology of cirrhosis; mean age; proportion of males/females; participants with Child‐Pugh stages A, B, or C (Pugh 1973); proportion of participants with hepatic encephalopathy, with previous gastrointestinal bleeding episodes, with type of ascites (that is, large ascites: either diuretic‐responsive, or refractory ascites), or with arterial hypotension; mean arterial pressure; renal impairment; hyponatraemia. Furthermore, we recorded plasma renin activity, plasma aldosterone levels, urinary sodium excretion, and information on liver biochemistry (serum bilirubin, albumin, and prothrombin time or international normalised ratio); ‐ treatments during the trial: type and dose of plasma expander, and timing of administration of plasma expander in connection to paracentesis; for intravenous infusion of ascitic fluid, whether or not the ascitic fluid was filtered and concentrated; type of paracentesis (partial or total paracentesis); total amount of ascitic fluid removed; length of the procedure; sodium restriction and diuretics (type and dose) before and after paracentesis; timing for clinical and laboratory assessment; ‐ sample size calculation performed and reported; ‐ completeness and length of follow‐up of treatment groups and reasons for withdrawals; ‐ presence of, absence of or unknown for‐profit support.
Assessment of risk of bias in included studies
Due to the risk of overestimation of beneficial intervention effects and underestimation of harmful intervention effects in randomised clinical trials at unclear risk of bias or at high risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b), we assessed the influence of the risk of bias on our results. We used the domains with definitions provided below to assess the risk of bias in the included trials (Higgins 2011; Gluud 2015).
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent research assistant not otherwise involved in the trial.
Unclear risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (for example, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, serious adverse events, refractory ascites, renal impairment, other complications due to liver cirrhosis such as gastrointestinal bleeding, hepatic encephalopathy, or infections, and non‐serious adverse events. If the original protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time when the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: the study authors did not report all predefined outcomes fully, or it was not clear whether data on this outcomes were recorded or not.
High risk of bias: the study authors did not report all‐cause mortality or more secondary predefined outcomes.
Other bias
Low risk of bias: the trial appeared to be free of other bias domains that could put it at risk of bias.
Unclear risk of bias: the trial might or might not have been free of other domains that could have put it at risk of bias.
High risk of bias: there are other factors in the trial that could put it at risk of bias.
RGS and GP judged a trial to be at an overall low risk of bias when the trial was assessed as having a low risk of bias in all the above domains. RGS and GP judged a trial to be at an overall high risk of bias when the trial was assessed as having an unclear risk of bias or a high a risk of bias in one or more of the above domains.
RGS and GP resolved disagreements by discussion.
Measures of treatment effect
Dichotomous outcomes We used the risk ratio (RR) with 95% CI. We used both fixed‐effect and random‐effects meta‐analysis models.
Continuous outcomes We planned to use the mean difference (MD) with 95% CI or the standard mean difference (SMD) with 95% CI, depending on whether the scales used in the trials were the same or different.
Unit of analysis issues
The participants as randomised to the intervention groups of the clinical trials. In trials with a two parallel group design, we compared the experimental intervention group versus the control group. In the trials with a parallel group design with more than two intervention groups, we compared separately each of the experimental groups with the control group divided proportionately according to number of experimental groups.
In cross‐over trials, we only included the data from the first trial period in order avoid residual effects from the treatment (Higgins 2011). In order to avoid repeated observations on trial participants, we used participant trial data at the longest follow‐up (Higgins 2011).
Dealing with missing data
We tried to obtain missing data from authors of the included trials. We investigated attrition bias (i.e. dropouts, losses to follow‐up, and withdrawals). We performed our analyses based on the intention‐to‐treat principle, whenever possible.
Regarding the primary outcomes, we included participants with incomplete or missing data in sensitivity analyses by imputing them according to the following two extreme case scenarios (Hollis 1999):
‐ Extreme case analysis favouring the experimental intervention ('best‐worst' case scenario): none of the dropouts/participants lost from the experimental arm, but all of the dropouts/participants lost from the control arm experienced the outcome, including all randomised participants in the denominator. ‐ Extreme case analysis favouring the control ('worst‐best' case scenario): all dropouts/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomised participants in the denominator.
Assessment of heterogeneity
We checked for heterogeneity through visual inspection of the forest plots by using a standard Chi2 test and a significance level of α = 0.1. In view of the low power of such tests, we also examined heterogeneity by using the I2 statistic (Higgins 2002); I2 values of 50% or more indicate a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual trial characteristics and subgroups. For heterogeneity adjustment of the required information size, we used diversity, the D2 statistic (Wetterslev 2009).
Assessment of reporting biases
Whenever we had 10 or more trials, we drew funnel plots to assess reporting biases from the individual trials by plotting the risk ratio (RR) on a logarithmic scale against its standard error (Egger 1997; Higgins 2011).
For dichotomous outcomes, we tested asymmetry using the Harbord test in case tau2 was less than 0.1 (Harbord 2006), and we used Rücker 2008 in case tau2 was more than 0.1. We planned to use the regression asymmetry test (Egger 1997) and the adjusted rank correlation coefficient (Begg 1994) for our continuous outcome, health‐related quality of life.
Data synthesis
Meta‐analysis
For the statistical analyses, we used Review Manager 5 (RevMan 2014). For dichotomous outcomes, we calculated the Mantel‐Haenszel risk ratios (RRs). We planned to use the mean difference (MD) for the continuous outcome, health‐related quality of life.
We performed the analyses using the intention‐to‐treat (ITT) principle, including all randomly assigned participants, irrespective of completeness of data.
Review Manager 5 does not include trials with zero events in both intervention groups when calculating RR (RevMan 2014). To account for trials with zero events, we used Trial Sequential Analysis with a continuity correction (Thorlund 2011; TSA 2011).
We compared the intervention effects in subgroups of trials using RevMan 2014.
We intended to calculate the number‐needed‐to‐treat for an additional beneficial outcome (NNTB).
Trial Sequential Analysis
As a sensitivity analysis for our GRADE assessment of imprecision (see below), we used Trial Sequential Analysis which considers choice of statistical model (fixed or random) and diversity (Thorlund 2011; TSA 2011). We calculated the diversity‐adjusted required information size (DARIS, i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Brok 2008; Wetterslev 2008; Brok 2009; Wetterslev 2009; Thorlund 2010; Wetterslev 2017).
The underlying assumption of Trial Sequential Analysis is that testing for statistical significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and, if more than one trial was published in a year, we added the trials alphabetically according to the last name of the first author. On the basis of the DARIS, we constructed the trial sequential monitoring boundaries for benefit, harm, and futility (Wetterslev 2008; Wetterslev 2009; Thorlund 2011; Wetterslev 2017). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the DARIS; if the trial sequential monitoring boundary for benefit or harm is crossed before the DARIS is reached, firm evidence may be established and further trials may be superfluous. However, if the boundaries for benefit or harm are not crossed, it is most probably necessary to continue doing trials in order to detect or reject a certain intervention effect. However, if the cumulative Z‐curve crosses the trial sequential monitoring boundaries for futility, no more trials may be needed.
In our Trial Sequential Analysis of the two primary dichotomous outcomes, we based the DARIS on the event proportion in the control group; assuming a plausible relative risk reduction for mortality of 10% and a relative risk reduction for serious adverse events of 5%; a risk of type I error of 2.5% due to the three primary outcomes (Jakobsen 2014); a risk of type II error of 20%; and the diversity of the included trials in the meta‐analysis. For the continuous outcome, health‐related quality of life, we planned to estimate the DARIS using a minimal relevant difference of 10% of the mean response observed in the control group; the standard deviation of the meta‐analysis; alpha of 2.5% due to the three primary outcomes (Jakobsen 2014); beta of 20%; and the diversity as estimated from the trials in the meta‐analysis (Wetterslev 2009). We also calculated the Trial Sequential Analysis‐adjusted confidence intervals (CI) (Thorlund 2011; Wetterslev 2017).
In our Trial Sequential Analysis of secondary outcomes, we based the DARIS for dichotomous outcomes on the event proportion in the control group; we made an assumption of a relative risk reduction of 10% for refractory ascites, renal impairment, other liver‐related complications, and non‐severe adverse events; a type I error risk of 2.0% due to the four secondary outcomes (Jakobsen 2014); a risk of type II error of 20%; and the diversity of the included trials in the meta‐analysis.
A more detailed description of Trial Sequential Analysis and software program can be found at www.ctu.dk/tsa/ (Thorlund 2011).
Assessment of significance based on the standard meta‐analysis method and Trials Sequential Analysis method
We conducted both fixed‐effect and random‐effects model meta‐analyses. If the fixed‐effect and random‐effects models showed different results, then the most conservative result (the analysis with the highest P value, i.e. closest to the null hypothesis) was chosen as the main result of the two analyses (Jakobsen 2014).
We considered a P value of 0.025 or less, two‐tailed, as statistically significant if the DARIS was reached due to our three primary outcomes (Jakobsen 2014). We considered a P value of 0.02 or less, two‐tailed, as statistically significant if the required information size was reached due to our four secondary outcomes. We used the eight‐step procedure to assess if the thresholds for significance were crossed (Jakobsen 2014). We presented heterogeneity using the I2 statistic (Higgins 2002). We presented the results of the individual trials and meta‐analyses in the form of forest plots.
Where data were only available from one trial, we used Fisher's exact test for dichotomous data (Fisher 1922). We planned to use Student's t‐test for continuous data such as 'health‐related quality of life' (Student 1908).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses:
risk of bias, analysing separately randomised clinical trials at low risk of bias and trials at high risk of bias;
type of plasma expanders, analysing separately randomised clinical trials according to the plasma expander used;
refractory ascites, analysing separately randomised clinical trials including participants with refractory ascites and trials including participants without refractory ascites;
modality of paracentesis, analysing separately randomised clinical trials in which partial paracentesis repeated until disappearance of ascites were used, and randomised clinical trials in which total, one‐session paracentesis were used;
length of follow‐up, analysing separately randomised clinical trials with up to one month follow‐up (short follow‐up trials) and trials with a follow‐up longer than one month (long follow‐up trials);
trials without for‐profit support compared to trials with or unknown for‐profit support (see Appendix 2 for definition) (Lundh 2017).
To determine whether a statistically significant subgroup difference was detected, we considered the P value from the test for subgroup differences. We used the test to assess the difference between the pooled effect estimates for each subgroup. A P value of less than 0.1 showed a significant subgroup effect.
Sensitivity analysis
For sensitivity analyses, see Dealing with missing data and 'Summary of findings' tables paragraphs.
'Summary of findings' tables
We assessed the certainty of the evidence using the GRADE system to present review results in 'Summary of findings' (SoF) tables, using GRADEPro 3.6 (http://ims.cochrane.org/revman/gradepro). In SoF tables, we included three Primary outcomes as well as four Secondary outcomes. We designed two Sof tables as we have two comparisons (Table 1; Table 2). A SoF table consists of three parts: information about the review, a summary of the statistical results, and the grade of the certainty of evidence. The assessment of certainty of the available evidence is comprised of the number of studies, the types of studies (randomised or observational), and five factors including within study risk of bias, inconsistency of results (heterogeneity), indirectness of evidence (population, intervention, control, outcomes), imprecision of results, and publication bias that affect the certainty of the evidence (Guyatt 2008; Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013). The five factors are used to judge whether the certainty of the collected evidence should be downgraded or upgraded.
Summary of findings for the main comparison. Plasma expanders versus no plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis.
| Plasma expanders versus no plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis: primary and secondary outcomes | ||||||
|
Patient or population: cirrhotic participants with large ascites treated by paracentesis Settings: specialised units in an intensive or semi‐intensive setting Intervention: plasma expander Comparison: no plasma expander | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No plasma expander | Plasma expander | |||||
|
All‐cause mortality mean follow‐up 64 days (1‐222) |
Medium risk population | RR 0.52 (0.06 to 4.83) | 248 (4) | ⊕⊝⊝⊝ very low1 | ||
| 180 per 1000 | 94 per 1000 (11 to 869) | |||||
|
Serious adverse events mean follow‐up 15 days (1‐30) |
See comment | See comment | See comment | 108 (2) | ⊕⊝⊝⊝ very low2 | Two trials reported that no serious adverse events occurred |
| Health‐related quality of life | No data provided in any of the six trials | |||||
| Refractory ascites | No data provided in any of the six trials | |||||
|
Other liver‐related complications mean follow‐up 64 days (1‐222) |
Medium risk population |
RR 1.61 (0.79 to 3.27) |
248 (4) | ⊕⊝⊝⊝ very low4 | ||
| 90 per 1000 | 145 per 1000 (71 to 294) | |||||
|
Non‐serious adverse events mean follow‐up 91 days (1‐222) |
Medium risk population |
RR 1.04 (0.32 to 3.4) |
158 (3) | ⊕⊝⊝⊝ very low5 | ||
| 62 per 1000 | 64 per 1000 (20 to 210) | |||||
| * Assumed risk is the risk in comparison group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); heterogeneity: high heterogeneity (76%) (‐1 level); imprecision: the required information size as calculated by GRADE was not reached (‐1 level) 2 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: there were no events (‐2 levels) 3 Downgraded 5 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); high heterogeneity (67%) (‐1 level); imprecision: there were few events and the CI included appreciable benefit and harm (‐2 levels) 4 Downgraded 3 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE was not met (‐1 level) 5 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: there were few events and the CI included appreciable benefit and harm (‐2 levels)
Summary of findings 2. Other plasma expanders versus albumin for people with cirrhosis and large ascites treated with abdominal paracentesis.
| Other plasma expanders versus albumin for people with cirrhosis and large ascites treated with abdominal paracentesis: primary and secondary outcomes | ||||||
|
Patient or population: cirrhotic participants with large ascites treated by paracentesis Settings: specialised units in an intensive or semi‐intensive setting Intervention: all plasma expanders except albumin Comparison: albumin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Albumin | Experimental plasma expanders | |||||
|
All‐cause mortality mean follow‐up 208 days (3‐638) |
Medium risk population | RR 1.03 (0.82 to 1.30) |
1014 (14) | ⊕⊝⊝⊝ very low 1 |
||
| 183 per 1000 | 188 per 1000 (150 to 238) | |||||
|
Serious adverse events mean follow‐up 93 days (6‐180) |
Medium risk population | RR 0.89 (0.10 to 8.30) | 118 (2) | ⊕⊝⊝⊝ very low 2 |
||
| 18 per 1000 | 16 per 1000 (1.8 to 149) | |||||
| Health‐related quality of life | No data provided in any of the 20 trials | |||||
| Refractory ascites | No data provided in any of the 20 trials | |||||
|
Renal impairment mean follow‐up 174 days (3‐628) |
Medium risk population | RR 1.17 (0.71 to 1.91) |
1107 (17) | ⊕⊝⊝⊝ very low 3 | ||
| 49 per 1000 | 57 per 1000 (35 to 94) | |||||
|
Other liver‐related complications mean follow‐up 147 days (3‐638) |
Medium risk population | RR 1.10 (0.82 to 1.48) |
1083 (16) | ⊕⊝⊝⊝ very low 4 | ||
| 185 per 1000 | 203 per 1000 (152 to 274) | |||||
|
Non‐serious adverse events mean follow‐up 194 days (3‐638) |
Medium risk population | RR 1.37 (0.66 to 2.85) |
977 (14) |
⊕⊝⊝⊝ very low 5 | ||
| 25 per 1000 | 34 per 1000 (16 to 71) | |||||
| * Assumed risk is the risk in comparison group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE was not reached (‐1 level); publication bias (‐1 level) 2 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: there were few events and the CI included appreciable benefit and harm (‐2 levels) 3 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE was not reached (‐1 level); publication bias (‐ 1 level) 4 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE was not reached (‐1 level); publication bias (‐ 1 level) 5 Downgraded 3 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE was not reached (‐1 level).
As sensitivity analysis, we compared imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on authors' choice of plausible relative risk reduction (RRR) and multiplicity correction, and according to our Trial Sequential Analysis (TSA) with a similar choice of plausible RRR and multiplicity correction, in addition to considering the choice of meta‐analytic model and diversity (Jakobsen 2014; Castellini 2018; Gartlehner 2018).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The reference flow is summarised in the study flow diagram (Figure 1). For detailed search strategies, see Appendix 1.
1.
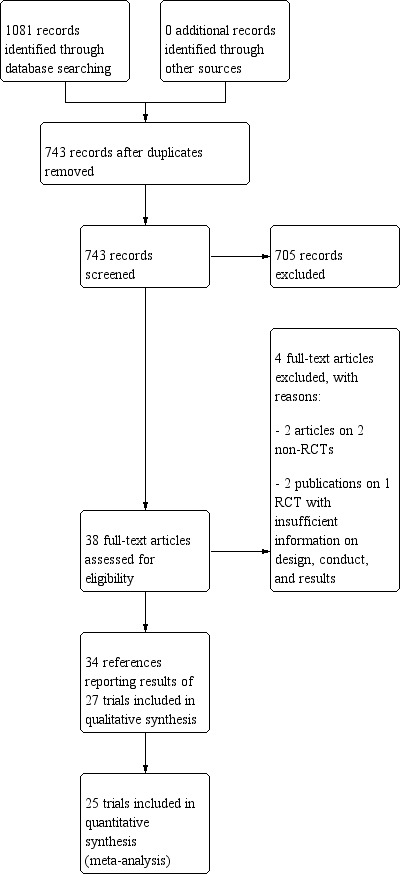
Study flow diagram.
We identified 1079 references through electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 86), Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (n = 146), MEDLINE (n = 98), Embase (n = 234), LILACS (n = 21), Science Citation Index EXPANDED and Conference Proceedings Citation Index – Science (Web of Science) (n = 212), and three Chinese database including CNKI (China National Knowledge Infrastructure) (n = 154), VIP (n = 88), and Wanfang (n = 40). We also searched databases of ongoing trials (www.clinicaltrials.gov/ and www.controlled‐trials.com/) (with links to several databases). One not yet recruiting trial was retrieved (NCT03202524). In addition, we searched European Medicines Agency (EMA), US Food and Drug Adminitration (FDA), and the World Health Organization International Clinical Trials Registry Platform (ICTRP 2011) until January 2019. One ongoing trial without interim data was retrieved (EudraCT 2010‐019783‐37). We did not apply any language or document type restrictions.
After the removal of 338 duplicates, we obtained 743 references. We then excluded 705 clearly irrelevant references through screening titles and reading abstracts. We retrieved 38 full‐text articles for further assessment. No references were identified through scanning reference lists of the identified randomised trials. Thirty‐four references were reports of 27 trials which fulfilled the inclusion criteria of our review.
Two trials were used only in a qualitative synthesis (García‐Compeán 2002; Degoricija 2003). García‐Compeán 2002 did not report the number of randomised participants for each group. Furthermore, they re‐randomised participants if readmitted for paracentesis during the follow‐up. Degoricija 2003 did not report the number of events per intervention group.
Included studies
Trial characteristics
We included 27 randomised clinical trials. Twenty‐four trials were published as full‐text articles and three trials as abstracts.
Two trial publications were in Korean (Kang 1998; Baik 2000) and two trials in Spanish (Bertrán 1991; Hernández Pérez 1995) languages. The remaining 23 trials were published in English.
The trials were conducted in Canada, China, Croatia, France, Germany, India, Italy, Korea, Pakistan, Russia, Spain, and the United States. All trials were performed in specialised units in intensive care or semi‐intensive care settings.
All trials had a parallel group design except one which used a cross‐over trial design (Sola‐Vera 2003). From the 26 trials with a parallel group design, one trial had three intervention groups (Ginès 1996), one had six intervention groups (Descos 1983), and two trials had five intervention groups (Degoricija 2003; Al Sebaey 2012). The remaining 22 trials had two intervention groups.
Ten trials seemed not to have been funded by industry (Descos 1983; Ginès 1988; Planas 1990; Simonetti 1991; Fassio 1992; Luca 1995; Sola‐Vera 2003; Moreau 2006; Al Sebaey 2012; Khan 2015). Twelve trials were considered unclear about funding (Bertrán 1991; Bruno 1992; Méndez 1991; García‐Compeán 1993; Hernández Pérez 1995; Kang 1998; Mehta 1998; Baik 2000; Zhao 2000; García‐Compeán 2002; Degoricija 2003; Abdel‐Khalek 2010). Five trials were considered funded by industry or a for‐profit institution (Smart 1990; Salerno 1991; Ginès 1996; Graziotto 1997; Altman 1998).
Participant characteristics
The trials included 1592 randomised participants with a mean sample size of 59 participants (range 12 to 289 participants). The mean age of the participants was 56.4 years with a mean range of 42.0 to 61.3 years. The mean proportion of males in the trial groups was 67.7%. The reported aetiology of cirrhosis was alcoholic in 60.9% of the participants (range 13% to 94%; 20 trials) and viral in 27.8% of the participants (range 2% to 70.6%; 17 trials). According to the Child‐Pugh classification, most participants had an intermediate to advanced stage of cirrhosis. Nine trials reported a mean Child‐Pugh score of 10.4 points (range 9.6 to 12), and seven trials reported that between 33% and 60% of the participants were in class B Child‐Pugh, and between 37% to 58% of the participants were in class C Child‐Pugh. One trial reported that 65% of the participants were in class C and for the remaining 35%, the class was not reported. In three trials, the proportion of participants in class A Child‐Pugh ranged between 2.9% and 8.3% (Bertrán 1991; Bruno 1992; Sola‐Vera 2003). In the remaining eleven trials, this information was not provided. Almost all trials excluded participants with hepatocellular carcinoma as well as recent gastrointestinal bleeding, infections, or hepatic encephalopathy. In Salerno 1991, 30% of participants had hepatocellular carcinoma. Seven trials assessed the effects of treatments in people with refractory ascites according to authors' diagnostic definitions (Smart 1990; Méndez 1991; Salerno 1991; Simonetti 1991; Graziotto 1997; Mehta 1998; Abdel‐Khalek 2010) (Characteristics of included studies). The remaining 20 trials included participants without refractory ascites because the mean value of blood urea was 21.86 + SD 8.58 mg/dL and the mean value of serum creatinine was 0.96 + SD 0.12 mg/dL (Characteristics of included studies). The proportion of people with renal impairment was reported in 10 trials (Ginès 1988; Planas 1990; Fassio 1992; Hernández Pérez 1995; Ginès 1996; Altman 1998; Zhao 2000; García‐Compeán 2002; Sola‐Vera 2003; Moreau 2006); it ranged from 0% in Altman 1998 and Fassio 1992 to 28% in García‐Compeán 2002, with a mean of 12%. Seventeen trials reported mean arterial pressure of 88 (SD 5.6) mmHg, and 15 trials reported the mean renin activity of 10.42 (SD 5.73) ng/mL/hour.
Paracentesis characteristics
All trial participants were treated with paracentesis. Total paracentesis completed in a single session was used in the experimental and control groups of 20 trials (Descos 1983; Planas 1990; Bertrán 1991; Méndez 1991; Salerno 1991; Bruno 1992; García‐Compeán 1993; Hernández Pérez 1995; Luca 1995; Ginès 1996; Graziotto 1997; Kang 1998; Mehta 1998; Baik 2000; García‐Compeán 2002; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010; Al Sebaey 2012; Khan 2015). Partial paracentesis, repeated until disappearance of ascites, was used in both intervention groups of four trials (Ginès 1988; Fassio 1992; Altman 1998; Zhao 2000). Single‐session paracentesis was used in the experimental group and partial paracentesis in the control group of two trials (Smart 1990; Simonetti 1991); the paracentesis was repeated until disappearance of ascites on alternate days in Simonetti 1991 and every day in Smart 1990. A single paracentesis of 6 L was performed in Degoricija 2003.
Intervention characteristics
Out of the 27 trials, five trials, including 271 participants, assessed plasma volume expansion versus no plasma volume expansion (Descos 1983; Ginès 1988; García‐Compeán 1993; Luca 1995; Baik 2000). Four of these trials used albumin as a plasma expander (Ginès 1988; García‐Compeán 1993; Luca 1995; Baik 2000) and the remaining trial with six trial groups assessed plasma volume expansion with intravenous infusion of filtrated ascitic fluid versus intravenous infusion of unmodified ascitic fluid versus no plasma expansion, or versus several different diuretic treatments (Descos 1983). For the purpose of our review, we put together, in the experimental group, the data from people treated with intravenous infusion of filtrated or unmodified ascitic fluid.
Twenty‐two trials, including 1321 participants, assessed the effect of a plasma volume expander versus another plasma volume expander. Overall, the experimental treatments were Dextran 70 in five trials (Planas 1990; Bertrán 1991; Fassio 1992; Hernández Pérez 1995; Ginès 1996) and Dextran 40 in one trial (García‐Compeán 2002); polygeline in five trials (Salerno 1991; Ginès 1996; Degoricija 2003; Moreau 2006; Khan 2015); hydroxyethyl starch in five trials (Méndez 1991; Altman 1998; Kang 1998; Abdel‐Khalek 2010; Al Sebaey 2012); fresh frozen plasma in one trial (Degoricija 2003); intravenous infusion of ascitic fluid in four trials (Smart 1990; Simonetti 1991; Bruno 1992; Graziotto 1997); saline solution in one trial (Sola‐Vera 2003); and mannitol(um) in one trial (Zhao 2000). In 21 trials, albumin was used as the control intervention. Dextran 70 and polygeline were assessed in a three‐armed trial compared with albumin (Ginès 1996). Albumin, fresh frozen plasma and polygeline plus bed rest, no plasma expanders without bed rest and diuretic treatment without paracentesis and bed rest were compared in a 5‐armed trial. For the purpose of this review, we used the data from the first three intervention groups (Degoricija 2003). Hydroxyethyl starch, terlipressin, midodrine, and albumin in two different doses (6 g/L of ascitic fluid in one group and 3 g/L in another group) were compared in a five‐armed trial (Al Sebaey 2012). For the purpose of this review, we compared the hydroxyethyl starch group with the two albumin groups, put together (Al Sebaey 2012). We did not use the data from the other two intervention groups of this trial. Intravenous infusion of ascitic fluid was compared with polygeline in one trial (Mehta 1998).
Overall, 175 participants were treated with Dextran 70, 209 with polygeline, 135 with hydroxyethyl starch, 77 with intravenous ascitic fluid infusion, 35 with 3.5% saline, and 32 with mannitol, and 10 participants with fresh frozen plasma versus 579 participants treated with albumin. The number of participants treated by Dextran 40 is unknown (see above, García‐Compeán 2002).
The dose of the plasma expanders for each litre of removed ascitic fluid was as follows: for albumin 2 g to 10 g, for dextran 6 g to 8 g, for hydroxyethyl starch 7.7 g to 13 g, for polygeline 4 g to 8 g, for 3.5% saline 170 mL, for mannitol 8 g to 16 g, and for fresh frozen plasma 100 mL.
The mean volume (± SD) of removed ascitic fluid reported in 24 trials was 8.1 L (SD 2.96) (range 4.0 L to 15.9 L). Diuretic treatment was used after paracentesis in 13 trials.
If recurrence of ascites occurred during the follow‐up period, the participants in seven trials were treated with the same schedule to which they were randomised originally (Ginès 1988; Planas 1990; Salerno 1991; Simonetti 1991; Fassio 1992; Ginès 1996; Abdel‐Khalek 2010). Participants were treated with an alternative treatment in one trial (Sola‐Vera 2003). As this trial was a cross‐over trial, we used the results from the first period of the trial on day 6 after paracentesis for all outcomes, except for the recurrence of ascites for which data were reported after discharge of trial participants.
Follow‐up and withdrawals
Fifteen trials reported analyses of outcomes within one month: at 24 hours in Luca 1995, at two days in Baik 2000, at three days in Kang 1998, at five days in Méndez 1991 and García‐Compeán 1993, at six days in Degoricija 2003, Sola‐Vera 2003, Al Sebaey 2012 and Khan 2015, at eight days in Bruno 1992, at 14 days in Hernández Pérez 1995, at 15 days in Altman 1998, and at one month in Descos 1983 and Bertrán 1991. In Mehta 1998, the median follow‐up was 17.5 days.
The other 12 trials had a follow‐up longer than a month (Ginès 1988; Planas 1990; Smart 1990; Salerno 1991; Simonetti 1991; Fassio 1992; García‐Compeán 2002; Ginès 1996; Graziotto 1997; Zhao 2000; Moreau 2006; Abdel‐Khalek 2010). In Sola‐Vera 2003 (a cross‐over trial), the follow‐up was longer than one month only for recurrence of ascites, whereas the other outcomes were recorded on day six.
Fifteen trials followed up the participants after their discharge from hospital (Descos 1983; Ginès 1988; Planas 1990; Smart 1990; Salerno 1991; Simonetti 1991; Fassio 1992; Ginès 1996; Graziotto 1997; Altman 1998; Mehta 1998; García‐Compeán 2002; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010).
In Ginès 1996 and Sola‐Vera 2003 trials, participants were followed up after discharge, but the authors reported data on the outcomes of interest only for the first hospitalisation (Ginès 1996), and on the sixth day after paracentesis (Sola‐Vera 2003). Therefore, we included them in the analysis of trials with a short follow‐up.
The mean follow‐up period was 136 days (range 1 to 638) in 25 trials (Descos 1983; Ginès 1988; Planas 1990; Bertrán 1991; Méndez 1991; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; García‐Compeán 1993; Hernández Pérez 1995; Luca 1995; Ginès 1996; Graziotto 1997; Altman 1998; Kang 1998; Baik 2000; Zhao 2000; García‐Compeán 2002; Degoricija 2003, Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010; Al Sebaey 2012; Khan 2015). The median follow‐up period was 231 days in Smart 1990 and 17.5 days in Mehta 1998.
In the five trials comparing plasma expansion versus no plasma expansion, the percentage of dropouts and withdrawals was 1.21%. In the twenty‐one trials comparing plasma expanders versus albumin, the percentage of reported dropouts and withdrawals was 5.27%.
Excluded studies
Characteristics of excluded studies table presents the excluded studies with the reason for their exclusion.
Three studies were excluded. Two studies were comparative, and not randomised trials (Zaak 2001; Nasr 2010). One study, published as abstract for the first time in 1990 (Antillon 1990), was still ongoing in 1991 (Antillon 1991). We could obtain no further information on the study.
Risk of bias in included studies
We based our assessment on published information and on that received from trial authors (Figure 2; Figure 3).
2.
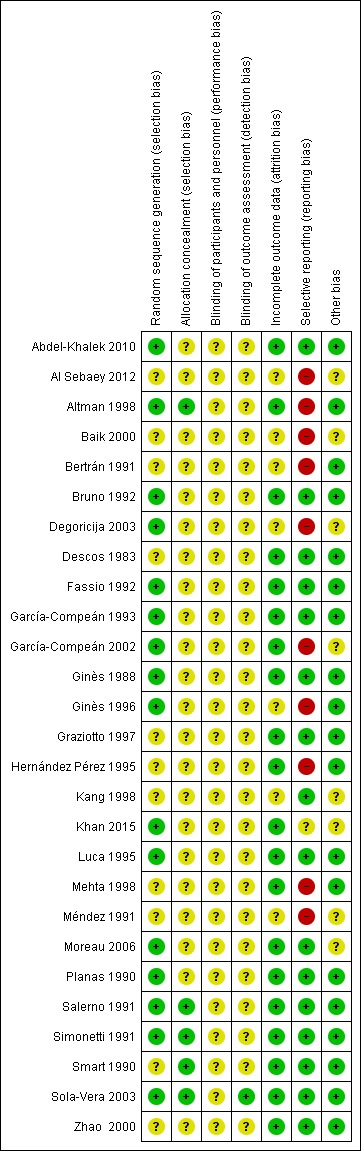
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
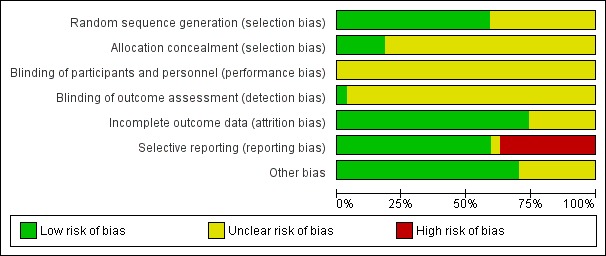
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation sequence generation
Sixteen trials were at low risk of bias regarding allocation sequence generation (Ginès 1988; Planas 1990; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; García‐Compeán 1993; Luca 1995; Ginès 1996; Altman 1998; García‐Compeán 2002; Sola‐Vera 2003; Degoricija 2003, Moreau 2006; Abdel‐Khalek 2010; Khan 2015). The remaining 11 trials were at unclear risk of bias.
Allocation concealment
Five trials were at low risk of bias regarding allocation concealment (Smart 1990; Salerno 1991; Simonetti 1991; Altman 1998; Sola‐Vera 2003). The risk of bias in the remaining 22 trials was unclear.
Blinding
Blinding of participants and personnel
All the 27 trials were at unclear risk of bias regarding blinding of participants and personnel.
Blinding of outcome assessment
We judged only one trial at low risk of bias regarding blinding of outcome assessment (Sola‐Vera 2003). The remaining 26 trials were at unclear risk of bias.
Incomplete outcome data
We judged twenty trials to be at low risk of bias regarding attrition bias. The remaining seven trials were at unclear risk of bias (Bertrán 1991; Méndez 1991; Ginès 1996; Kang 1998; Baik 2000; Degoricija 2003; Al Sebaey 2012).
Selective reporting
Twelve trials aimed to assess haemodynamic or neurohumoral changes after a short follow‐up period after paracentesis (Bertrán 1991; Méndez 1991; Bruno 1992; García‐Compeán 1993; Hernández Pérez 1995; Luca 1995; Altman 1998; Kang 1998; Baik 2000; Degoricija 2003; Al Sebaey 2012; Khan 2015).
Only four trials reported serious adverse events (Descos 1983; Luca 1995; Moreau 2006; Khan 2015). No trials reported data on refractory ascites. We followed the recommendation of the Cochrane Handbook, which stated that "review authors should look hard for the evidence of collection by study investigators of a small number of key outcomes that are routinely measured in the area in question”. In addition, most trials were published before a formal definition of serious adverse events and refractory ascites. So, the lack of the reporting of these two outcomes did not necessarily put the trials at high risk of bias.
Overall, we judged ten trials to be at high risk of bias of selective outcome reporting because information on mortality or more than one secondary outcome was missing (Bertrán 1991; Méndez 1991; Hernández Pérez 1995; Ginès 1996; Altman 1998; Mehta 1998; Baik 2000; García‐Compeán 2002; Degoricija 2003; Al Sebaey 2012). One trial was at unclear risk of bias (Khan 2015). The remaining sixteen trials were judged to be at low risk of bias (Descos 1983; Ginès 1988; Planas 1990; Smart 1990; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; García‐Compeán 1993; Luca 1995; Graziotto 1997; Kang 1998; Zhao 2000; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010).
Other potential sources of bias
We could suspect no other potential sources of bias in nineteen trials (Descos 1983; Ginès 1988; Planas 1990; Smart 1990; Bertrán 1991; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; García‐Compeán 1993; Hernández Pérez 1995; Luca 1995; Ginès 1996; Graziotto 1997; Altman 1998; Mehta 1998; Zhao 2000; Sola‐Vera 2003; Abdel‐Khalek 2010). We judged the remaining eight trials as having unclear risk because they were published as abstracts (Méndez 1991; Al Sebaey 2012), or the information was not enough (Kang 1998; Baik 2000; Moreau 2006; Khan 2015), or because of the characteristics of the design and analysis (García‐Compeán 2002; Degoricija 2003).
Overall risk of bias
We judged all trials to be at high risk of bias because they were assessed as having an uncertain risk of bias or a high risk of bias in one or more of the bias risk domains.
Effects of interventions
Plasma expanders versus no plasma expander
Primary outcomes
All‐cause mortality
Four trials provided data on mortality with a mean follow‐up of 64 days. Three trials had a follow‐up less than one month. No mortality occurred in two of the trials (García‐Compeán 1993; Luca 1995). The effect of plasma expanders (albumin and ascites infusion) compared with no plasma expander in terms of reduction in all‐cause mortality was very uncertain (RR 0.52, 95% CI 0.06 to 4.83; 248 participants; 4 trials; I2 = 76%; Analysis 1.1; Descos 1983; Ginès 1988; García‐Compeán 1993; Luca 1995).
1.1. Analysis.
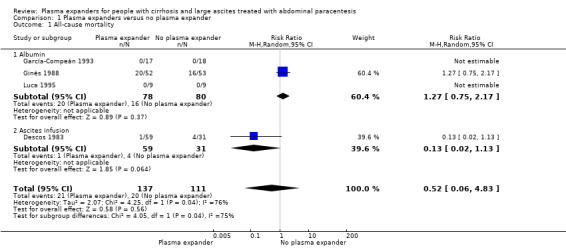
Comparison 1 Plasma expanders versus no plasma expander, Outcome 1 All‐cause mortality.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; there was high heterogeneity; and the required information size was not reached (Table 1; Table 17).
1. Comparison of imprecision by GRADE and Trial Sequential Analysis for the evaluation of primary and secondary outcomes in the comparison of plasma expanders versus no plasma expander.
| Comparison of imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on authors' choice of plausible relative risk reduction and multiplicity correction, and according to our Trial Sequential Analysis with a similar choice of plausible relative risk reduction and multiplicity correction, in addition to considering the choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size (OIS or DARIS) | Downgrading of evidence for imprecision based on required information size |
| All‐cause mortality ‐‐ GRADE Handbook | 18% | 25% | 5% | 20% | Not used | 2056 | One level |
| All‐cause mortality ‐‐ GRADE plausible RRR | 18% | 10% | 2.5% | 20% | Not used | 16,634 | One level |
| All‐cause mortality ‐‐ TSA | 18% | 10% | 2.5% | 20% | 88% | 143,664 | One level |
| Serious adverse events ‐‐ GRADE Handbook (1) |
|||||||
| Serious adverse events ‐‐ GRADE plausible RRR (1) | |||||||
| Serious adverse events ‐‐ TSA (1) | |||||||
| Health‐related quality of life ‐‐ GRADE Handbook | No data | ||||||
| Health‐related quality of life ‐‐ GRADE plausible RRR | No data | ||||||
| Health‐related quality of life ‐‐ TSA | No data | ||||||
| Refractory ascites ‐‐ GRADE Handbook | No data | ||||||
| Refractory ascites ‐‐ GRADE plausible RRR | No data | ||||||
| Refractory ascites ‐‐ TSA | No data | ||||||
| Renal impairment ‐‐ GRADE Handbook | 9.8% | 25% | 5% | 20% | Not used | 4100 | One level |
| Renal impairment ‐‐ GRADE plausible RRR | 9.8% | 10% | 2.00% | 20% | Not used | 35,290 | One level |
| Renal impairment ‐‐ TSA | 9.8% | 10% | 2.00% | 20% | 0% | 35,293 | One level |
| Other liver‐related complications ‐‐ GRADE Handbook | 9% | 25% | 5% | 20% | Not used | 4498 | One level |
| Other liver‐related complications ‐‐ GRADE plausible RRR | 9% | 10% | 2.00% | 20% | Not used | 38,750 | One level |
| Other liver‐related complications ‐‐ TSA | 9% | 10% | 2.00% | 20% | 0% | 38,752 | One level |
| Non‐serious adverse events ‐‐ GRADE Handbook | 6.25% | 25% | 5% | 20% | Not used | 6670 | One level |
| Non‐serious adverse events ‐‐ GRADE plausible RRR | 6.25% | 10% | 2.00% | 20% | Not used | 56,464 | One level |
| Non‐serious adverse events ‐‐ TSA | 6.25% | 10% | 2.00% | 20% | 0% | 56,467 | One level |
(1) Serious adverse events: 0/68 in plasma expander group and 0/40 in no plasma expander group, RR 1.00 (95% CI 0.00 to 217…)
OIS: optimal information size; DARIS: diversity‐adjusted required information size; RRR: relative risk reduction; TSA: Trial Sequential Analysis
Including the two trials with zero deaths in the Trial Sequential Analysis produced a comparable result (RR 0.64; 95% CI 0.14 to 2.93; P = 0.56; D2= 88%).
The Trial Sequential Analysis of this comparison was based on a mortality of 18% in the control group, a relative risk reduction of 10% with albumin or other plasma expanders, a type I error of 2.5%, a type II error of 20% (80% power), and 88% diversity. The DARIS was 143,664 participants. Due to the fact that only 248 participants were recruited (which is 0.17% of the DARIS of 143,664 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate Trial Sequential Analysis‐adjusted CIs.
Subgroup analysis
We could not perform subgroup analysis of trials according to their risk of bias because all the trials were assessed at high risk of bias; and according to participants with and participants without refractory ascites because all the trials included participants without refractory ascites.
Type of plasma expanders
The test for subgroup differences comparing the effects of each type of plasma expander (albumin and ascites infusion) suggested a difference between the plasma expanders used (P = 0.04, I² = 75.3%; Analysis 1.1). In trials comparing albumin versus no plasma expander, RR was 1.27, 95% CI 0.75 to 2.17; 158 participants; 3 trials; I2 not calculated because 2/3 of the trials had 0 events, Analysis 1.1.1; Ginès 1988; García‐Compeán 1993; Luca 1995) whereas in trials comparing intravenous ascitic fluid infusion versus no plasma expander RR was 0.13, 95% CI 0.02 to 1.13; 90 participants; 1 trial (Analysis 1.1.2; Descos 1983).
Modality of paracentesis
The test for subgroup differences comparing the effects of plasma expander versus no plasma expander in people treated by partial or total paracentesis suggested a difference between the two subgroups (P = 0.04, I² = 75.3%; Analysis 2.1). In trials in which partial paracentesis were used, RR was 1.27, 95% CI 0.75 to 2.17; 105 participants; 1 trial (Analysis 2.1.1; Ginès 1988) whereas the subgroup of trials in which total paracentesis were used, RR was 0.13, 95% CI 0.02 to 1.13; 143 participants; 3 trials; I2 not calculated because 2/3 of the trials had 0 events (Analysis 2.1.2; Descos 1983; García‐Compeán 1993; Luca 1995).
2.1. Analysis.
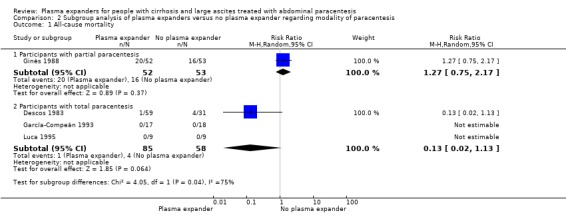
Comparison 2 Subgroup analysis of plasma expanders versus no plasma expander regarding modality of paracentesis, Outcome 1 All‐cause mortality.
Length of follow‐up
The test for subgroup differences comparing the effects of plasma expander versus no plasma expander in trials with a short follow‐up (up to one month) to trials with a long follow‐up (more than one month) suggested a difference (P = 0.04, I² = 75.3%; Analysis 3.1). In trials with a short follow‐up, RR was 0.13, 95% CI 0.02 to 1.13; 143 participants; 3 trials; I2 not calculated because 2/3 of the trials had 0 events (Analysis 3.1.1; Descos 1983; García‐Compeán 1993; Luca 1995) whereas in the trial with a long follow‐up RR was 1.27, 95% CI 0.75 to 2.17; 105 participants; 1 trial; I2 not applicable (Analysis 3.1.2; Ginès 1988).
3.1. Analysis.
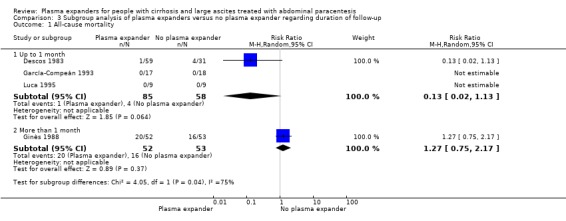
Comparison 3 Subgroup analysis of plasma expanders versus no plasma expander regarding duration of follow‐up, Outcome 1 All‐cause mortality.
For‐profit support
In the subgroup of trials without for‐profit funding, RR was 0.52, 95% CI 0.06 to 4.83; 213 participants; 3 trials; I2 = 76% (Analysis 4.1.1). In the only trial without information on for‐profit funding, no deaths were reported (García‐Compeán 1993).
4.1. Analysis.

Comparison 4 Subgroup analysis of plasma expanders versus no plasma expander regarding funding, Outcome 1 All‐cause mortality.
Sensitivity analysis
The best‐worst (RR 0.49, 95% CI 0.06 to 3.76; 248 participants; 4 trials; I2 = 73%; Analysis 5.1) and the worst‐best scenario analyses (RR 0.87, 95% CI 0.28 to 2.76; 248 participants; 4 trials; I2 = 60%; Analysis 6.1) both suggested neutral results.
5.1. Analysis.
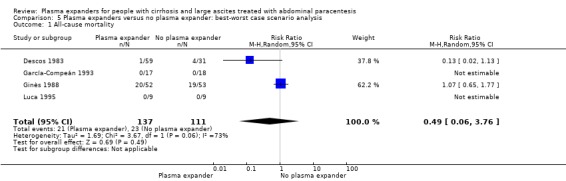
Comparison 5 Plasma expanders versus no plasma expander: best‐worst case scenario analysis, Outcome 1 All‐cause mortality.
6.1. Analysis.
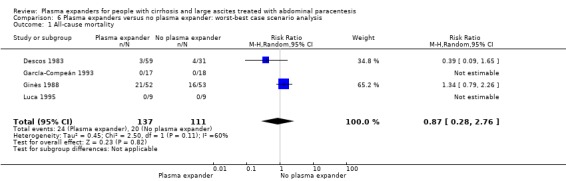
Comparison 6 Plasma expanders versus no plasma expander: worst‐best case scenario analysis, Outcome 1 All‐cause mortality.
Serious adverse events
Out of the five trials assessing plasma volume expansion versus no plasma volume expansion, two trials reported that there were no serious adverse events (Descos 1983; Luca 1995). The remaining three trials did not mention if serious adverse events occurred (Ginès 1988; García‐Compeán 1993; Baik 2000) (Analysis 1.2).
1.2. Analysis.
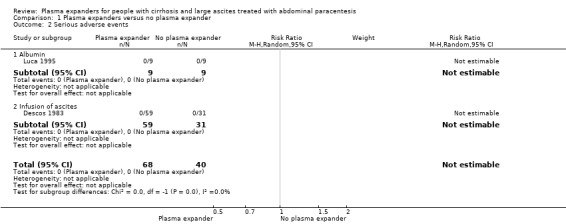
Comparison 1 Plasma expanders versus no plasma expander, Outcome 2 Serious adverse events.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; there was substantial imprecision due to the lack of events (Table 1). The required information size was not reached (Table 17).
Subgroup analysis
We could not perform subgroup analysis of trials according to their risk of bias because the two trials were assessed at high risk of bias; according to participants with and participants without refractory ascites because the two trials included participants without refractory ascites; according to modality of paracentesis because the two trials performed total paracentesis; according to length of follow‐up because the two trials had short follow‐up; and according to for‐profit funding because the two trials were without for‐profit funding (Subgroup analysis and investigation of heterogeneity).
Health‐related quality of life
None of the included trials reported health‐related quality of life.
Secondary outcomes
Refractory ascites
None of the included trials provided information on refractory ascites.
Renal impairment
Four trials reported data on renal impairment (Ginès 1988; García‐Compeán 1993; Luca 1995; Baik 2000). All four trials used albumin as a plasma expander. In two of the trials, renal impairment did not occur (Luca 1995; Baik 2000). The effect of albumin versus no plasma expander on renal impairment was uncertain (RR 0.32, 95% CI 0.02 to 5.88; 181 participants; 4 trials; I2 = 67%; Analysis 1.3).
1.3. Analysis.
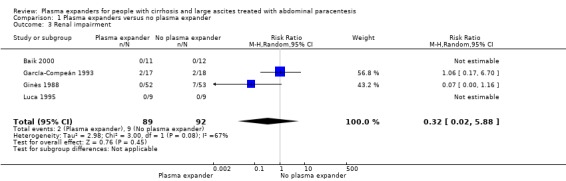
Comparison 1 Plasma expanders versus no plasma expander, Outcome 3 Renal impairment.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by five levels because all trials were at high risk of bias; there was high heterogeneity; there were few events and the CI included appreciable benefit and harm (Table 1). The required information size was not reached (Table 17).
Including the two trials with zero events in Trial Sequetial Analysis produced a comparable result (RR 1.02, 95% CI 0.16 to 6.43; P = 0.98).
The Trial Sequential Analysis of the four trials assessing plasma expander versus no plasma expander was constructed based on the renal impairment proportion of 9.8% in the control group, a relative risk reduction of 10.0% with plasma expander, a type I error of 2.00%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The DARIS was 35,293 participants. Due to the fact that only 181 participants were recruited (0.51% of the DARIS of 35,293 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate Trial Sequential Analysis‐adjusted CIs.
Subgroup analysis
None of the first three planned subgroup analyses could be performed because the risk of bias in the four trials providing data on renal impairment was high, all the four trials included participants without refractory ascites, and all the four trials used albumin.
Modality of paracentesis
The test for subgroup differences comparing the effects of plasma expander versus no plasma expander on renal impairment in participants treated by partial paracentesis and by total paracentesis showed no difference (P = 0.11, I² = 60.5%; Analysis 2.2). There was no evidence of a difference in renal impairment after partial paracentesis (RR 0.07, 95% CI 0.00 to 1.16; 105 participants; 1 trial; Analysis 2.2.1; Ginès 1988) and after total paracentesis (RR 1.06, 95% CI 0.17 to 6.70; 76 participants; 3 trials; I2 not applicable because 2/3 of the trials had 0 events; Analysis 2.2.2; García‐Compeán 1993; Luca 1995; Baik 2000).
2.2. Analysis.
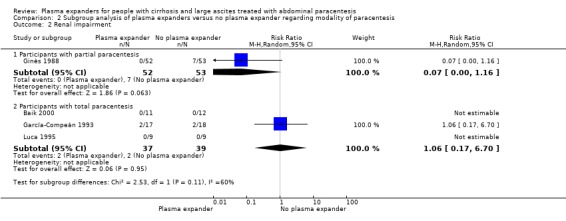
Comparison 2 Subgroup analysis of plasma expanders versus no plasma expander regarding modality of paracentesis, Outcome 2 Renal impairment.
Length of follow‐up
The test for subgroup differences comparing the effects of plasma expander versus no plasma expander in trials with a short follow‐up (up to one month) compared to trials with a long follow‐up (more than one month) showed no difference (P = 0.11, I² = 60.5; Analysis 3.2). We found no evidence of a difference in the effect of albumin‐treated participants and the untreated participants in trials with up to one month follow‐up in renal impairment (RR 1.06, 95% CI 0.17 to 6.70; 53 participants; 2 trials; I2 not applicable because 1/2 of the trials had 0 events; Analysis 3.2.1; García‐Compeán 1993; Luca 1995), and the same was observed between the albumin‐treated participants and the untreated participants in trials with a follow‐up of more than one month (RR 0.07, 95% CI 0.00 to 1.16; 105 participants; 1 trial; Analysis 3.2.2).
3.2. Analysis.
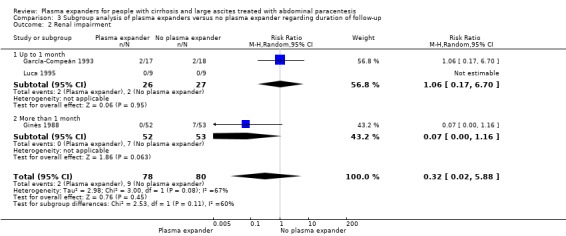
Comparison 3 Subgroup analysis of plasma expanders versus no plasma expander regarding duration of follow‐up, Outcome 2 Renal impairment.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no differences between the two subgroups (P = 0.11, I2 = 60.5%; Analysis 4.2). We found no difference in renal impairment either in the subgroup of trials without for‐profit funding (RR 0.07, 95% CI 0.00 to 1.16; 123 participants; 2 trials; I2 not applicable because 1/2 trial had 0 events; Analysis 4.2.1) or in the subgroup of trials without information on for‐profit funding (RR 1.06, 95% CI 0.17 to 6.70; 58 participants; 2 trials; I2 not applicable because 1/2 trial had 0 events; Analysis 4.2.2).
4.2. Analysis.
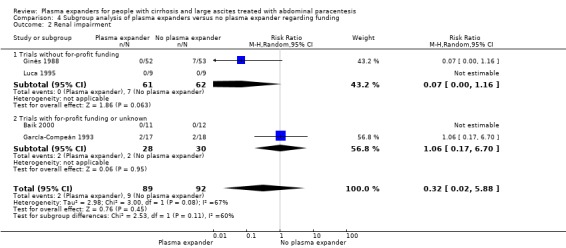
Comparison 4 Subgroup analysis of plasma expanders versus no plasma expander regarding funding, Outcome 2 Renal impairment.
Other liver‐related complications
Four trials reported data on other liver‐related complications such as gastrointestinal bleeding, hepatic encephalopathy, or infections (Descos 1983; Ginès 1988; García‐Compeán 1993; Luca 1995). The meta‐analysed results of these four trials showed no evidence of a difference in the effect of plasma expander (albumin and infusion of ascitic fluid) versus no plasma expander on other liver‐related complications (RR 1.61, 95% CI 0.79 to 3.27; 248 participants; 4 trials; I2 = 0%; Analysis 1.4).
1.4. Analysis.
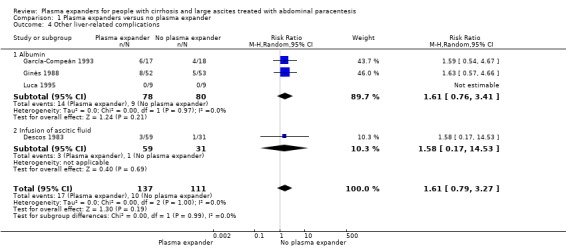
Comparison 1 Plasma expanders versus no plasma expander, Outcome 4 Other liver‐related complications.
Including the trial with zero events in Trial Sequential Analysis produced a comparable result (RR 1.61, 95% CI 0.79 to 3.27; P = 0.19).
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by three levels because all trials were at high risk of bias; and there was imprecision: the required information size was not reached (Table 1; Table 17).
Trial Sequential Analysis was constructed based on risk of other liver‐related complications of 9% in the control group, a relative risk reduction of 10% with plasma expander, a type I error of 2.00%, and a type II error of 20% (80% power). There was low diversity (D2 = 0%). The DARIS was 38,752 participants. Due to the fact that only 248 participants were recruited (0.64% of the DARIS of 38,752 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate Trial Sequential Analysis‐adjusted CIs.
Subgroup analysis
We could not perform two of the subgroup analyses because the risk of bias in the five trials was high, and because all trials included participants without refractory ascites (Subgroup analysis and investigation of heterogeneity).
Type of plasma expanders
The test for subgroup differences comparing the effects of each type of plasma expander showed no difference between the plasma expanders used (P = 0.99, I² = 0%) regarding other liver‐related complications: albumin versus the no plasma expander (RR 1.61, 95% CI 0.76 to 3.41; 158 participants; 3 trials; I2 = 0%; Analysis 1.4.1; Ginès 1988; García‐Compeán 1993; Luca 1995) and intravenous infusion of ascitic fluid versus the no plasma expander (RR 1.58, 95% CI 0.17 to 14.53; 90 participants; 1 trial; Analysis 1.4.2; Descos 1983).
Modality of paracentesis
The test for subgroup differences comparing the effects of the modality of paracentesis showed no difference (P = 0.97, I² = 0%; Analysis 2.3) regarding other liver‐related complications: participants with partial paracentesis (RR 1.63, 95% CI 0.57 to 4.66; 105 participants; 1 trial; Analysis 2.3.1; Ginès 1988) and participants with total paracentesis (RR 1.59, 95% CI 0.60 to 4.18; 143 participants; 3 trials; I2 = 0%; Analysis 2.3.2; Descos 1983; García‐Compeán 1993; Luca 1995).
2.3. Analysis.
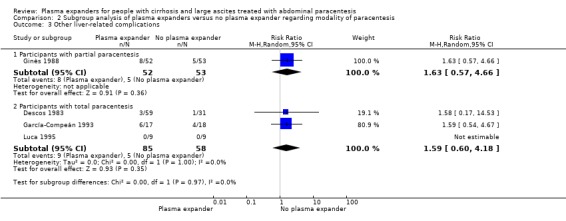
Comparison 2 Subgroup analysis of plasma expanders versus no plasma expander regarding modality of paracentesis, Outcome 3 Other liver‐related complications.
Length of follow‐up
The test for subgroup differences comparing the effects of duration of follow‐up showed no difference between the trials with a short follow‐up and the trials with a long follow‐up (P = 0.97; I² = 0%; Analysis 3.3) regarding other liver‐related complications: in trials with a follow‐up up to one month (RR 1.59, 95% CI 0.60 to 4.18; 143 participants; 3 trials; I2 = 0%; Analysis 3.3.1; Descos 1983; García‐Compeán 1993; Luca 1995) and in the single trial with more than one month follow‐up (RR 1.63, 95% CI 0.57 to 4.66; 105 participants; Analysis 3.3.2; Ginès 1988).
3.3. Analysis.
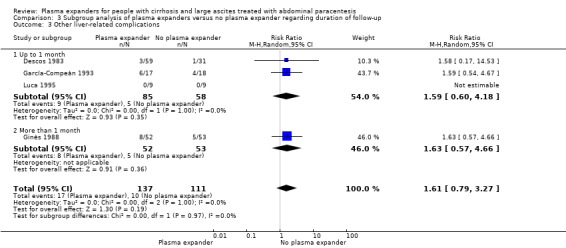
Comparison 3 Subgroup analysis of plasma expanders versus no plasma expander regarding duration of follow‐up, Outcome 3 Other liver‐related complications.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no difference between the two subgroups (P = 0.98, I2 = 0%; Analysis 4.3). We found no evidence of a difference in other liver‐related complications either in the subgroup of trials without for‐profit funding (RR 1.62, 95% CI 0.63 to 4.19; 213 participants; 3 trials; I2 = 0%; Analysis 4.3.1) or in the subgroup with the only trial without information on for‐profit funding (RR 1.59, 95% CI 0.54 to 4.67; 35 participants; 1 trial; Analysis 4.3.2).
4.3. Analysis.
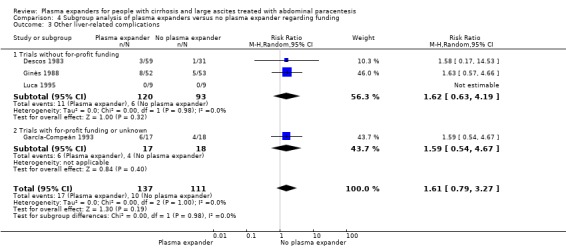
Comparison 4 Subgroup analysis of plasma expanders versus no plasma expander regarding funding, Outcome 3 Other liver‐related complications.
Non‐serious adverse events
Non‐serious adverse events were reported in three trials; they included local hematoma, fistula, transient fever, hyperkalaemia, leakage, or oedema of the abdominal wall (Ginès 1988; García‐Compeán 1993; Luca 1995). The analysis result of these three trials showed no evidence of a difference between plasma expander and no plasma expander (RR 1.04, 95% CI 0.32 to 3.40; 158 participants; I² = 0%; Analysis 1.5). Including the trial with zero deaths in the Trial Sequential Analysis produced a comparable result (RR 1.04; 95% CI 0.32 to 3.40; P = 0.95).
1.5. Analysis.
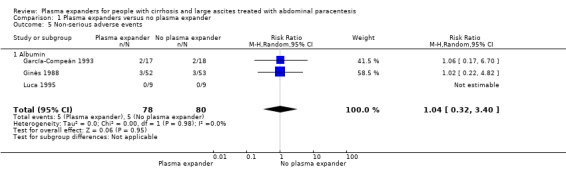
Comparison 1 Plasma expanders versus no plasma expander, Outcome 5 Non‐serious adverse events.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; and there was imprecision: there were few events and the CI included appreciable benefit and harm (Table 1). The required information size was not reached (Table 17).
Trial Sequential Analysis was constructed based on the risk in the control group of 6.25%, a relative risk reduction of 10% with plasma expansion, a type I error of 2.00%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The DARIS was 56,467 participants. Due to the fact that only 158 participants were recruited (which is 0.27% of the DARIS of 56,467 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate Trial Sequential Analysis‐adjusted CIs.
Subgroup analysis
We could not perform three of the subgroup analyses because the risk of bias in the three trials was high, all the three trials included participants without refractory ascites, and non‐serious adverse events were reported only in the trial that used albumin.
Modality of paracentesis
The test for subgroup differences comparing the effects of modality of paracentesis showed no difference between the two subgroups (P = 0.98; I² = 0%; Analysis 2.4): plasma expanders versus no plasma expander in trials with partial paracentesis (RR 1.02, 95% CI 0.22 to 4.82; 105 participants; 1 trial; Analysis 2.4.1) and in trials with total paracentesis (RR 1.06, 95% CI 0.17 to 6.70; 53 participants; 2 trials; I² not applicable because 1/2 trials had 0 events; Analysis 2.4.2).
2.4. Analysis.
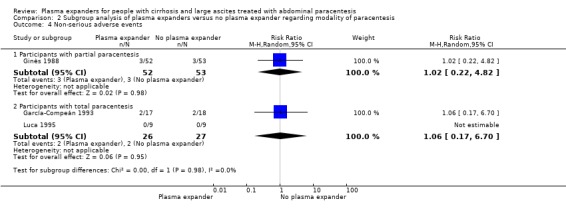
Comparison 2 Subgroup analysis of plasma expanders versus no plasma expander regarding modality of paracentesis, Outcome 4 Non‐serious adverse events.
Length of follow‐up
The test for subgroup differences comparing the effects of duration of follow‐up showed no difference between the trials with a short follow‐up and the trials with more than one month follow‐up (P = 0.98, I² = 0%; Analysis 3.4): trials with a short follow‐up (RR 1.06, 95% CI 0.17 to 6.70; 53 participants; 2 trials; I2 not applicable; Analysis 3.4.1; García‐Compeán 1993; Luca 1995) and trials with a long follow‐up (RR 1.02, 95% CI 0.22 to 4.82; 105 participants; 1 trial; I2 not applicable; Analysis 3.4.2; Ginès 1988).
3.4. Analysis.

Comparison 3 Subgroup analysis of plasma expanders versus no plasma expander regarding duration of follow‐up, Outcome 4 Non‐serious adverse events.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no difference between the two subgroups (P = 0.98, I2 = 0%; Analysis 4.4). We found no evidence of difference in non‐serious adverse events either in the subgroup of trials without for‐profit funding (RR 1.02, 95% CI 0.22 to 4.82; 123 participants; 2 trials; I2 not applicable because 1/2 trial had 0 events; Analysis 4.4.1) or in the subgroup with the only trial without information on for‐profit funding (RR 1.06, 95% CI 0.17 to 6.70; 35 participants; Analysis 4.4.2).
4.4. Analysis.
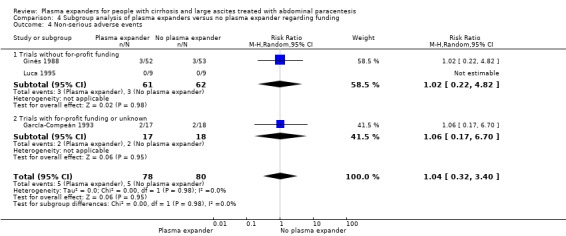
Comparison 4 Subgroup analysis of plasma expanders versus no plasma expander regarding funding, Outcome 4 Non‐serious adverse events.
Exploratory outcomes
Recurrence of ascites
Plasma expanders showed no evidence of a difference in effect on the recurrence of ascites (RR 1.30, 95% CI 0.49 to 3.42; 195 participants; 2 trials; I2 = 37%; Analysis 1.6).
1.6. Analysis.
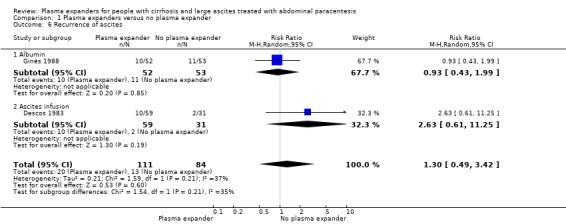
Comparison 1 Plasma expanders versus no plasma expander, Outcome 6 Recurrence of ascites.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by three levels because the two trials were at high risk of bias; and there was imprecision: the required information size was not reached (Table 18; Table 19).
2. Plasma expanders versus no plasma expander for people with cirrhosis and large ascites treated with abdominal paracentesis: exploratory outcomes.
| Plasma expanders versus no plasma expander for people with cirrhosis and large ascites treated with abdominal paracentesis: exploratory outcomes | ||||||
|
Patient or population: cirrhotic participants with large ascites treated by paracentesis Settings: specialised units in an intensive or semi‐intensive setting Intervention: plasma expander Comparison: no plasma expander | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No plasma expander | Plasma expander | |||||
|
Recurrence of ascites mean follow‐up 126 days (30‐222) |
Medium risk population | RR 1.30 (0.49 to 3.42) | 195 (2) | ⊕⊝⊝⊝ very low1 | ||
| 155 per 1000 | 201 per 1000 (76 to 529) | |||||
|
Hypotension follow‐up 2 days |
See comment | See comment | 23 (1) |
There was a single trial with 0 events in each group. | ||
|
Hyponatraemia mean follow‐up 57 days (1‐222) |
Medium risk population | RR 0.53 (0.05 to 5.65) | 181 (4) | ⊕⊝⊝⊝ very low2 | ||
| 130 per 1000 | 69 per 1000 (7 to 734) | |||||
| * Assumed risk is the risk in comparison group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1 Downgraded 3 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE not reached (‐1 levels) 2 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); high heterogeneity (67%) (‐1 level); imprecision: the required information size as calculated by GRADE was not reached (‐1 level)
3. Comparison of imprecision by GRADE and Trial Sequential Analysis for the evaluation of exploratory outcomes in the comparison of plasma expanders versus no plasma expander.
| Comparison of imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on authors' choice of plausible relative risk reduction and multiplicity correction, and according to our Trial Sequential Analysis with a similar choice of plausible relative risk reduction and multiplicity correction, in addition to considering the choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size (OIS or DARIS) | Downgrading of evidence for imprecision based on required information size |
| Recurrence of ascites ‐‐ GRADE Handbook | 15.5% | 25% | 5% | 20% | Not used | 2444 | One level |
| Recurrence of ascites ‐‐ GRADE plausible RRR | 15.5% | 10% | 2% | 20% | Not used | 20,980 | One level |
| Recurrence of ascites ‐‐ TSA | 15.5% | 10% | 2% | 20% | 51% | 43,013 | One level |
| Hypotension ‐‐ GRADE Handbook (1) | |||||||
| Hypotension ‐‐ GRADE plausible RRR (1) | |||||||
| Hypotension ‐‐ TSA (1) | |||||||
| Hyponatraemia ‐‐ GRADE Handbook | 13% | 25% | 5% | 20% | Not used | 2996 | One level |
| Hyponatraemia ‐‐ GRADE plausible RRR | 13% | 10% | 2% | 20% | Not used | 25,712 | One level |
| Hyponatraemia ‐‐ TSA | 13% | 10% | 2% | 20% | 10% | 28,526 | One level |
(1) There was a single trial with 0 events in each group
OIS: optimal information size; DARIS: diversity‐adjusted required information size; RRR: relative risk reduction; TSA: Trial Sequential Analysis
Trial Sequential Analysis was constructed based on the risk of 15.5% in the control group, a relative risk reduction of 10% in the plasma expander group, a type I error of 2.00%, and a type II error of 20% (80% power). Diversity was present (D2 = 51%). The DARIS was 43,013 participants. Due to the fact that only 195 participants were recruited (which is 0.45% of the DARIS of 43,013 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate Trial Sequential Analysis‐adjusted CIs.
Subgroup analysis
We did not conduct subgroup analysis because there were only two trials.
Hypotension
Only one of the trials reported that there was no occurrence of hypotension (Baik 2000) (Table 18; Table 19).
Hyponatraemia
Plasma expansion versus no plasma expansion showed no evidence of a difference in effect on the incidence of hyponatraemia (RR 0.53, 95% CI 0.05 to 5.65; 181 participants; 4 trials; I2 = 67%; Analysis 1.7). Including the trials with zero events in Trial Sequential Analysis produced a comparable result (RR 0.38, 95% CI 0.11 to 1.38; P = 0.15).
1.7. Analysis.
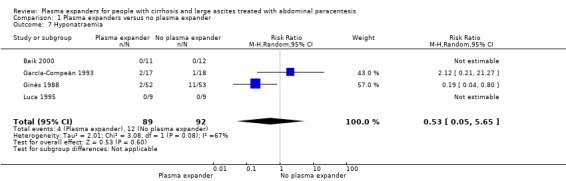
Comparison 1 Plasma expanders versus no plasma expander, Outcome 7 Hyponatraemia.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; there was high heterogeneity; and there was imprecision: the required information size was not reached (Table 18; Table 19).
Trial Sequential Analysis was based on risk of 13% in the control group, a relative risk reduction of 10% with plasma expansion, a type I error of 2.00%, and a type II error of 20% (80% power). Diversity (D2) was 10%. The DARIS was 28,526 participants. Due to the fact that only 181 participants were recruited (which is 0.63% of the DARIS of 28,526 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate Trial Sequential Analysis‐adjusted CIs.
Subgroup analyses
We could not perform three of the subgroup analysis because all the four trials were assessed at high risk of bias, included participants without refractory ascites, and used albumin.
Modality of paracentesis
The test for subgroup differences comparing the effects of modality of paracentesis showed a difference between the two subgroups (P = 0.08, I² = 67.3%; Analysis 2.5): trials using partial paracentesis (RR 0.19, 95% CI 0.04 to 0.80; 105 participants; 1 trial; Analysis 2.5.1; Ginès 1988) and trials using total paracentesis (RR 2.12, 95% CI 0.21 to 21.27; 76 participants; 3 trials; I2 not applicable because 2/3 trials had 0 events Analysis 2.5.2; García‐Compeán 1993; Luca 1995; Baik 2000).
2.5. Analysis.
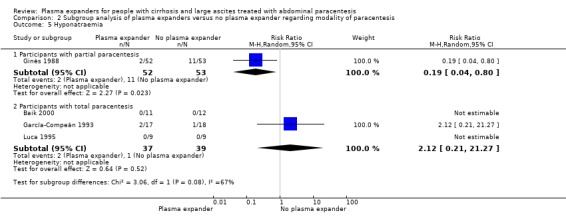
Comparison 2 Subgroup analysis of plasma expanders versus no plasma expander regarding modality of paracentesis, Outcome 5 Hyponatraemia.
Length of follow‐up
The test for subgroup differences comparing the effects of duration of follow‐up showed a difference between the two subgroups (P = 0.08, I² = 67.3% Analysis 3.5): trials with a short follow‐up (RR 2.12, 95% CI 0.21 to 21.27; 76 participants; 3 trials; I2 not applicable because 2/3 trials had 0 events; Analysis 3.5.1; García‐Compeán 1993; Luca 1995; Baik 2000) and trials with a long follow‐up (RR 0.19, 95% CI 0.04 to 0.80; 105 participants; Analysis 3.5.2; Ginès 1988).
3.5. Analysis.
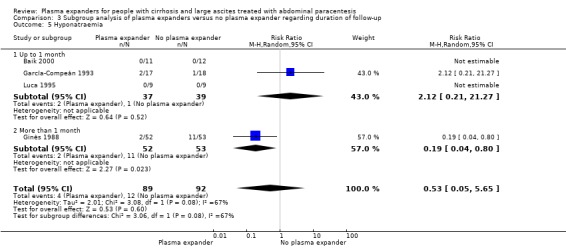
Comparison 3 Subgroup analysis of plasma expanders versus no plasma expander regarding duration of follow‐up, Outcome 5 Hyponatraemia.
For‐profit support
The test for subgroup differences comparing for‐profit support showed a difference between the two subgroups (P = 0.08, I2 = 67.3%; Analysis 4.5). Albumin versus no plasma expansion reduced hyponatraemia in the subgroup of trials without for‐profit funding (RR 0.19, 95% CI 0.04 to 0.80; 123 participants; 2 trials; I2 not applicable because 1/2 trial had 0 events; Analysis 4.5.1). No evidence of a difference was found in the subgroup of trials without information on for‐profit funding (RR 2.12, 95% CI 0.21 to 21.27; 58 participants; 2 trials; I2 not applicable because 1/2 trial had 0 events; Analysis 4.5.2).
4.5. Analysis.
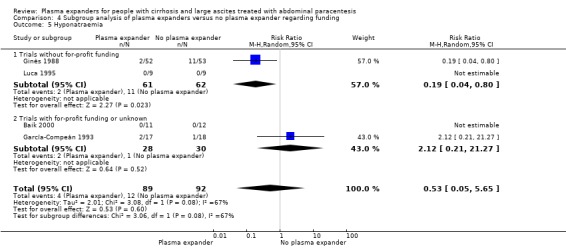
Comparison 4 Subgroup analysis of plasma expanders versus no plasma expander regarding funding, Outcome 5 Hyponatraemia.
Post‐paracentesis circulatory dysfunction
None of the included trials reported data on post‐paracentesis circulatory dysfunction.
Plasma expanders versus other plasma expanders
The trials compared different plasma expanders. Therefore, to achieve maximal homogeneity, we first analysed all the trials in which albumin was used as a control intervention, and then we analysed the trials in which both intervention groups used plasma expanders different from albumin.
Experimental plasma expanders versus albumin
Twenty‐one trials compared non‐albumin plasma expanders versus albumin (Planas 1990; Smart 1990; Bertrán 1991; Méndez 1991; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; Hernández Pérez 1995; Ginès 1996; Graziotto 1997; Altman 1998; Kang 1998; Zhao 2000; García‐Compeán 2002; Degoricija 2003; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010; Al Sebaey 2012; Khan 2015).
As one of these twenty‐one trials had three intervention groups, we performed the analysis splitting the trial as if there were two trials performed; i.e. polygeline versus albumin and dextran 70 versus albumin, using half of the participants in the albumin group for each of the comparisons (Ginès 1996). The trial by García‐Compeán 2002 did not report the number of participants allocated to the treatment groups, and, hence, we could not use their data for quantitative analysis. The trial by Degoricija 2003 did not report the number of events per intervention group, and, hence, we could not use it for quantitative analysis.
Primary outcomes
All‐cause mortality
Data on in‐hospital mortality were reported by five trials with albumin as the control intervention (Bruno 1992; Ginès 1996; Kang 1998; Sola‐Vera 2003; Khan 2015). In another five trials with a short follow‐up, mortality was not reported (Bertrán 1991; Méndez 1991; Hernández Pérez 1995; Altman 1998; Al Sebaey 2012).
Another nine trials provided mortality after discharge from hospital (Planas 1990; Smart 1990; Salerno 1991; Simonetti 1991; Fassio 1992; Graziotto 1997; Zhao 2000; Moreau 2006; Abdel‐Khalek 2010). Ginès and colleagues wrote that there was no difference in mortality between the two groups at follow‐up after discharge, but the trial did not report the number of events, and we received no reply from the trial authors to our data request (Ginès 1996).
There was no evidence of a difference in all‐cause mortality between the experimental plasma expanders group and the albumin group (RR 1.03, 95% CI 0.82 to 1.30; 1014 participants; 14 trials; I2 = 0%; Analysis 7.1; Figure 4). The intervention effect on all‐cause mortality did not change after inclusion of the two trials with zero deaths (RR 1.04; 95% CI 0.82 to 1.31; P = 0.75; D2 = 0%).
7.1. Analysis.
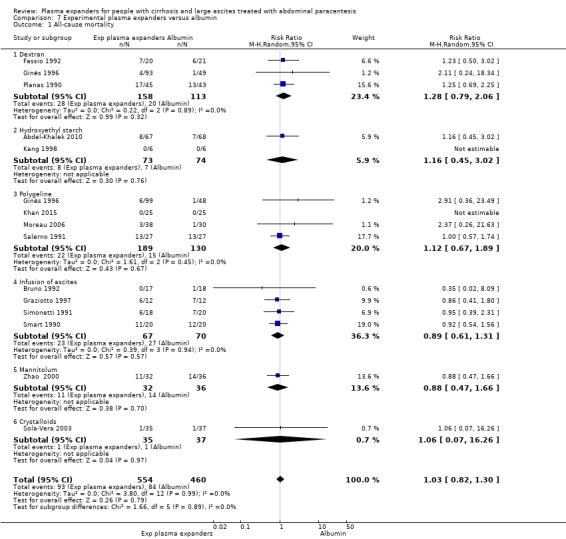
Comparison 7 Experimental plasma expanders versus albumin, Outcome 1 All‐cause mortality.
4.
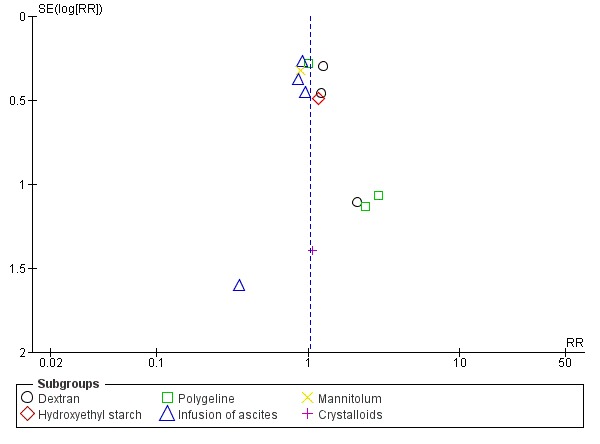
Funnel plot of comparison: 6 Experimental plasma expanders versus albumin, outcome: 6.1 All‐cause mortality.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; there was imprecision: the required information size was not reached; there was evidence of publication bias (Table 2; Table 20; Figure 4).
4. Comparison of imprecision by GRADE and Trial Sequential Analysis for the evaluation of primary and secondary outcomes in the comparison of plasma expanders versus albumin.
| Comparison of imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on authors' choice of plausible relative risk reduction and multiplicity correction, and according to our Trial Sequential Analysis with a similar choice of plausible relative risk reduction and multiplicity correction, in addition to considering the choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size (OIS or DARIS) | Downgrading of evidence for imprecision based on required information size |
| All‐cause mortality ‐‐ GRADE Handbook | 18.2% | 25% | 5% | 20% | Not used | 2030 | One level |
| All‐cause mortality ‐‐ GRADE plausible RRR | 18.2% | 10% | 2.5% | 20% | Not used | 16,415 | One level |
| All‐cause mortality ‐‐ TSA | 18.2% | 10% | 2.5% | 20% | 0% | 16,415 | One level |
| Serious adverse events ‐‐ GRADE Handbook | 1.8% | 25% | 5% | 20% | Not used | 24,032 | One level |
| Serious adverse events ‐‐ GRADE plausible RRR | 1.8% | 5% | 2.5% | 20% | Not used | 809,313 | One level |
| Serious adverse events ‐‐ TSA | 1.8% | 5% | 2.5% | 20% | 0% | 809,313 | One level |
| Health‐related quality of life ‐‐ GRADE Handbook | No data | ||||||
| Health‐related quality of life ‐‐ GRADE plausible RRR | No data | ||||||
| Health‐related quality of life ‐‐ TSA | No data | ||||||
| Refractory ascites ‐‐ GRADE Handbook | No data | ||||||
| Refractory ascites ‐‐ GRADE plausible RRR | No data | ||||||
| Refractory ascites ‐‐ TSA | No data | ||||||
| Renal impairment ‐‐ GRADE Handbook | 5% | 25% | 5% | 20% | Not used | 8404 | One level |
| Renal impairment ‐‐ GRADE plausible RRR | 5% | 10% | 2.00% | 20% | Not used | 72,650 | One level |
| Renal impairment ‐‐ TSA | 5% | 10% | 2.00% | 20% | 0% | 72,651 | One level |
| Other liver‐related complications ‐‐ GRADE Handbook | 18.5% | 25% | 5% | 20% | Not used | 1986 | One level |
| Other liver‐related complications ‐‐ GRADE plausible RRR | 18.5% | 10% | 2.00% | 20% | Not used | 16,990 | One level |
| Other liver‐related complications ‐‐ TSA | 18.5% | 10% | 2.00% | 20% | 0% | 16,992 | One level |
| Non‐serious adverse events ‐‐ GRADE Handbook | 2.5% | 25% | 5% | 20% | Not used | 16,904 | One level |
| Non‐serious adverse events ‐‐ GRADE plausible RRR | 2.5% | 10% | 2.00% | 20% | Not used | 148,922 | One level |
| Non‐serious adverse events ‐‐ TSA | 2.5% | 10% | 2.00% | 20% | 0% | 148,925 | One level |
OIS: optimal information size; DARIS: diversity‐adjusted required information size; RRR: relative risk reduction; TSA: Trial Sequential Analysis
Trial Sequential Analysis of this comparison was constructed on an all‐cause mortality of 18.2% in the albumin group, a relative risk reduction of 10% with the experimental plasma expanders, a type I error of 2.5%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The DARIS was 16,415 participants. In Trial Sequential Analysis, the information fraction was too small to produce an inner wedge futility area. The cumulative Z curve (blue line) did not approach the monitoring boundaries (red lines) for benefit or harm or futility (Figure 5).
5.
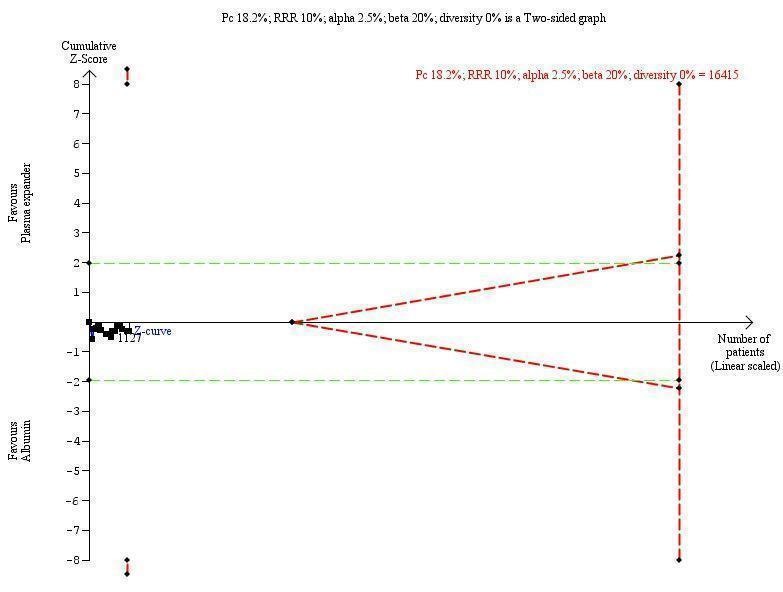
Experimental plasma expanders versus albumin ‐ 6.1 All‐cause mortality.
The diversity‐adjusted required information size of 16,415 participants was calculated based on a proportion of participants of 18.2% of participants dying in the control group; a relative risk reduction (RRR) of 10% in the plasma expander group; an alpha of 2.5%; a power of 80%; and a diversity of 0%.
The cumulative Z score did not cross borders for benefit, harm, or futility.
Subgroup analyses
We could not perform subgroup analysis of trials in terms of all‐cause mortality according to their risk of bias because all the trials were assessed at high risk.
Type of plasma expanders
The test for subgroup differences comparing the effects of distinct plasma expanders showed no difference between the six subgroups (P = 0.89, I² = 0%; Analysis 7.1).
The type of plasma expander compared with albumin showed no evidence of difference in effect on all‐cause mortality: dextran (RR 1.28, 95% CI 0.79 to 2.06; 271 participants; 3 trials; I2 = 0%; Analysis 7.1.1; Planas 1990; Fassio 1992; Ginès 1996); hydroxyethyl starch (RR 1.16, 95% CI 0.45 to 3.02; 147 participants; 2 trials; I2 not applicable because 1 out of 2 trials had 0 events; Analysis 7.1.2; Kang 1998; Abdel‐Khalek 2010); polygeline (RR 1.12, 95% CI 0.67 to 1.89; 319 participants; 4 trials; I2 = 0%; Analysis 7.1.3; Salerno 1991; Ginès 1996; Moreau 2006; Khan 2015); intravenous infusion of ascites (RR 0.89, 95% CI 0.61 to 1.31; 137 participants; 4 trials; I2 = 0%; Analysis 7.1.4; Smart 1990; Simonetti 1991; Bruno 1992; Graziotto 1997); mannitol (RR 0.88, 95% CI 0.47 to 1.66; Analysis 7.1.5; Zhao 2000); and crystalloids (RR 1.06, 95% CI 0.07 to 16.26; Analysis 7.1.6; Sola‐Vera 2003).
Refractory ascites
The test for subgroup differences comparing the effects of refractory ascites showed no difference between the two subgroups (P = 0.59, I² = 0%; Analysis 8.1): trials including participants without refractory ascites (RR 1.15, 95% CI 0.80 to 1.66; 723 participants; 9 trials; I2 = 0%; Analysis 8.1.1) and trials including participants with refractory ascites (RR 1.01, 95% CI 0.75 to 1.37; 291 participants; 5 trials; I2 = 0%; Analysis 8.1.2).
8.1. Analysis.
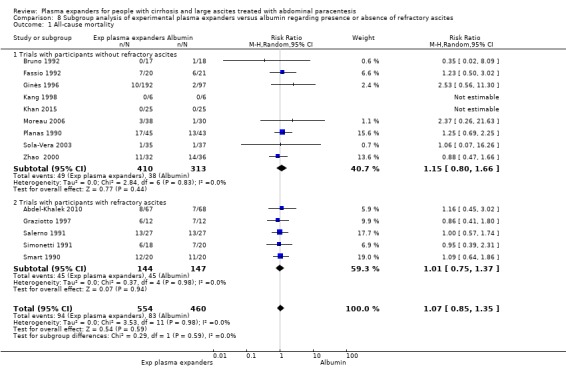
Comparison 8 Subgroup analysis of experimental plasma expanders versus albumin regarding presence or absence of refractory ascites, Outcome 1 All‐cause mortality.
Modality of paracentesis
The test for subgroup differences comparing the effects of modality of paracentesis showed no difference between the two subgroups (P = 0.73, I² = 0%; Analysis 9.1). We found no evidence of a difference in all‐cause mortality either in the partial paracentesis subgroup (RR 0.98, 95% CI 0.59 to 1.65; 109 participants; 2 trials; I2 = 0%; Analysis 9.1.1) or in the total paracentesis subgroup (RR 1.09, 95% CI 0.84 to 1.41; 905 participants; 12 trials; I2 = 0%; Analysis 9.1.2).
9.1. Analysis.
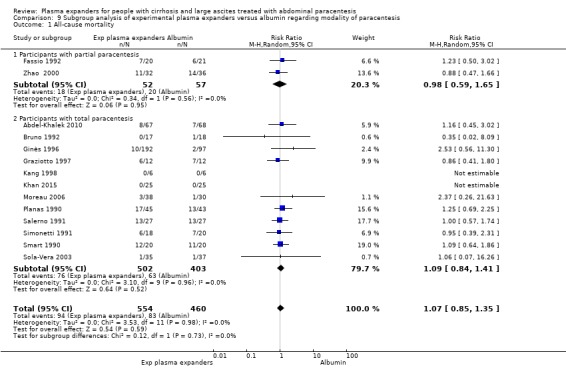
Comparison 9 Subgroup analysis of experimental plasma expanders versus albumin regarding modality of paracentesis, Outcome 1 All‐cause mortality.
Length of follow‐up
The test for subgroup differences comparing the effect of length of follow‐up showed no difference between the two subgroups (P = 0.48, I² = 0%; Analysis 10.1). In the trials with a short follow‐up, we found a RR 1.59, 95% CI 0.47 to 5.33; 458 participants; 5 trials; I2 = 0% (Analysis 10.1.1); and in the trials with a long follow‐up, we found a RR 1.02, 95% CI 0.80 to 1.29; 556 participants; 9 trials; I2 = 0% (Analysis 10.1.2).
10.1. Analysis.
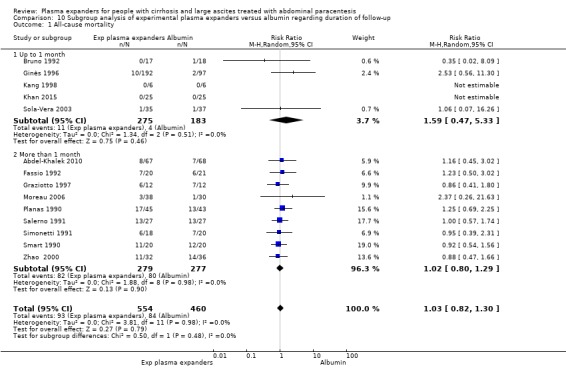
Comparison 10 Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up, Outcome 1 All‐cause mortality.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no difference (P = 0.41, I2 = 0%; Analysis 11.1). We found no evidence of a difference in all‐cause mortality either in the trials without for‐profit funding (RR 1.19, 95% CI 0.79 to 1.81; 357 participants; 6 trials; I2 = 0%; Analysis 11.1.1) or in the trials with or unknown for‐profit funding (RR 0.97, 95% CI 0.73 to 1.28; 657 participants; 8 trials; I2 = 0%; Analysis 11.1.2).
11.1. Analysis.
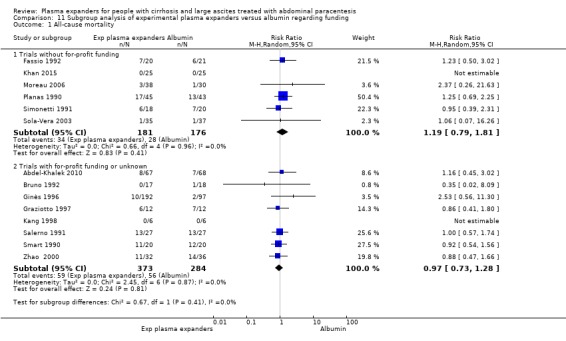
Comparison 11 Subgroup analysis of experimental plasma expanders versus albumin regarding funding, Outcome 1 All‐cause mortality.
Sensitivity analysis
Hypothesising the best‐worst scenario, mortality was increased by the other plasma expanders in comparison with albumin (RR 1.29, 95% CI 1.04 to 1.60; 1016 participants; 14 trials; I2 = 0%; Analysis 12.1). The worst‐best scenario analysis showed no evidence of a difference between other plasma expanders versus albumin (RR 0.99, 95% CI 0.96 to 1.03; 1016 participants; 14 trials; I2 = 8%; Analysis 13.1).
12.1. Analysis.
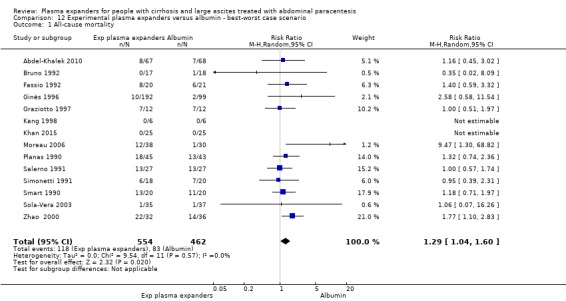
Comparison 12 Experimental plasma expanders versus albumin ‐ best‐worst case scenario, Outcome 1 All‐cause mortality.
13.1. Analysis.
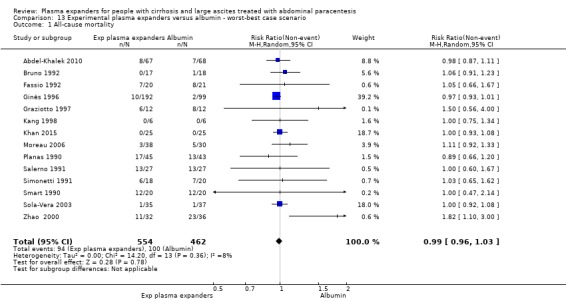
Comparison 13 Experimental plasma expanders versus albumin ‐ worst‐best case scenario, Outcome 1 All‐cause mortality.
Serious adverse events
Two trials reported data on serious adverse events. There was no evidence of a difference between other plasma expanders versus albumin in serious adverse events (Moreau 2006; Khan 2015) (RR 0.89, 95% CI 0.10 to 8.30; 118 participants; 2 trials; I2 = 0%; Analysis 7.2).
7.2. Analysis.
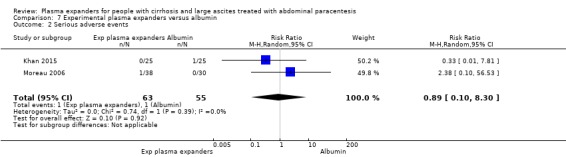
Comparison 7 Experimental plasma expanders versus albumin, Outcome 2 Serious adverse events.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by four levels because: all trials were at high risk of bias; and there was imprecision: there were few events and the CI included appreciable benefit and harm (Table 2). The optimal information size as calculated by GRADE was not reached (Table 20).
The Trial Sequential Analysis of this comparison was based on a risk of 1.8% in the control group, a relative risk reduction of 5% with experimental plasma expanders, a type I error of 2.5%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The DARIS was 809,313 participants. Due to the fact that only 118 participants were recruited (which is 0.01% of the DARIS of 809,313 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate Trial Sequential Analysis‐adjusted CIs.
Subgroup analyses
We could not perform subgroup analysis of trials according to their risk of bias, presence of refractory ascites, type of plasma expanders, modality of paracentesis, or for‐profit funding because both trials included participants with and without refractory ascites, used polygeline, performed total paracentesis, and were without for‐profit funding.
Length of follow‐up
The subgroup analysis comparing the effects of length of follow‐up showed no differences (P = 0.39, I² = 0%; Analysis 10.2): in the single trial with a short follow‐up (Khan 2015) (RR 0.33, 95% CI 0.01 to 7.81; 50 participants; Analysis 10.2.1) and in the single trial with a long follow‐up (Moreau 2006) (RR 2.38, 95% CI 0.10 to 56.53; 68 participants; Analysis 10.2.2).
10.2. Analysis.
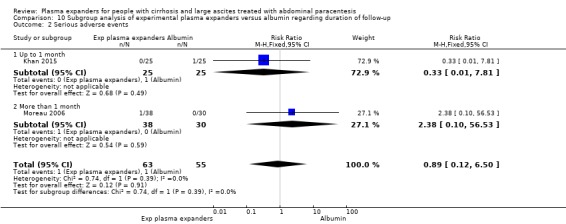
Comparison 10 Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up, Outcome 2 Serious adverse events.
Health‐related quality of life
None of the included trials assessed the health‐related quality of life.
Secondary outcomes
Refractory ascites
None of the included trials comparing a plasma expander versus albumin reported data on refractory ascites.
Renal impairment
Seventeen trials reported data on renal impairment (Planas 1990; Smart 1990; Bertrán 1991; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; Hernández Pérez 1995; Ginès 1996; Graziotto 1997; Altman 1998; Kang 1998; Zhao 2000; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010; Khan 2015). There was no evidence of a difference between experimental plasma expanders versus albumin in renal impairment (RR 1.17, 95% CI 0.71 to 1.91; 1107 participants; 17 trials; I2 = 0%; Analysis 7.3; Figure 6). Including the six trials with 0 events in Trial Sequential Analysis, renal impairment did not change significantly (RR 1.25, 95% CI 0.65 to 2.07; P = 0.39, D2 = 0%) (Bruno 1992; Graziotto 1997; Altman 1998; Kang 1998; Zhao 2000; Khan 2015).
7.3. Analysis.
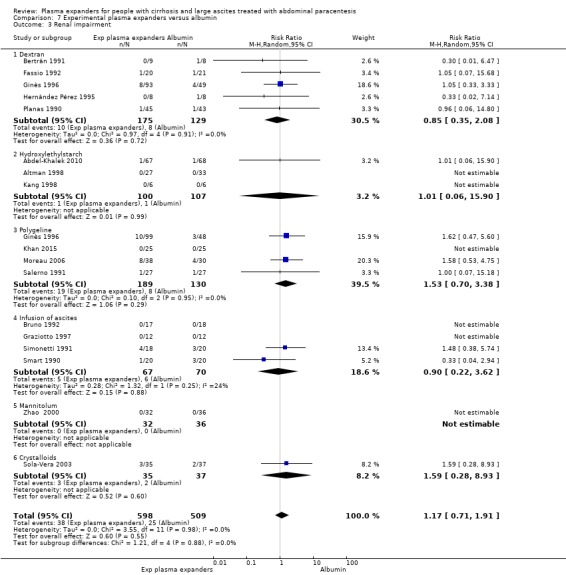
Comparison 7 Experimental plasma expanders versus albumin, Outcome 3 Renal impairment.
6.
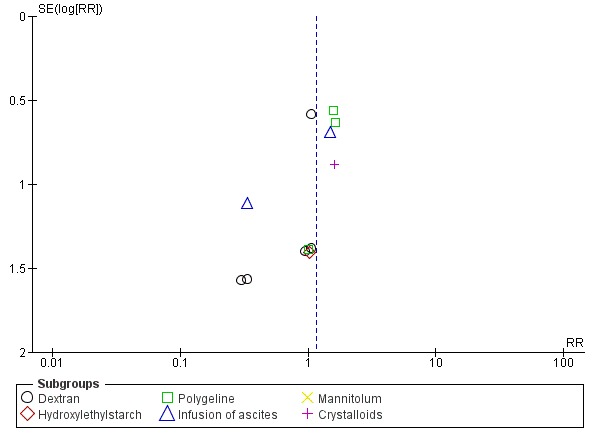
Funnel plot of comparison: 6 Experimental plasma expanders versus albumin, outcome: 6.3 Renal impairment.
We assessed the certainty of the evidence with GRADE as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; the required information size was not reached; and the funnel plot suggested publication bias (Table 2; Table 20; Figure 6).
Trial Sequential Analysis of this comparison was based on a renal impairment proportion of 5% in the albumin group, a relative risk reduction of 10% with experimental plasma expanders, a type I error of 2.00%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The DARIS was 72,651 participants. The Trial Sequential Analysis program could not construct an interpretable figure due to too little information (1.52%) (figure not shown) and Trial Sequential Analysis‐adjusted CIs could not be calculated.
Subgroup analysis
We could not perform subgroup analysis of trials in terms of renal impairment according to their risk of bias because all the trials were at high risk.
Type of plasma expanders
The test for subgroup differences comparing the effects of different plasma expanders showed no difference (P = 0.88, I² = 0%; Analysis 7.3): trials assessing versus albumin: dextran (RR 0.85, 95% CI 0.34 to 2.08; 304 participants; 5 trials; I2 = 0%; Analysis 7.3.1), hydroxyethyl starch (RR 1.01, 95% CI 0.06 to 15.90; 207 participants; 3 trials; I2 not applicable because 2/3 trials had 0 events; Analysis 7.3.2), polygeline (RR 1.53, 95% CI 0.70 to 3.38; 319 participants; 4 trials; I2 = 0%; Analysis 7.3.3), intravenous infusion of ascites (RR 0.90, 95% CI 0.22 to 3.62; 137 participants; 4 trials; I2 = 24%; Analysis 7.3.4), and crystalloids (RR 1.59, 95% CI 0.28 to 8.93; 72 participants; 1 trial; Analysis 7.3.6). No events were observed in the only trial in which mannitol was assessed (Zhao 2000) (Analysis 7.3.5).
Refractory ascites
The test for subgroup differences comparing the trials including participants without refractory ascites to the trials with participants with refractory ascites showed no difference (P = 0.69, I² = 0%; Analysis 8.2): trials including participants without refractory ascites (RR 1.24, 95% CI 0.70 to 2.20; 816 participants; 12 trials; I2 = 0%; Analysis 8.2.1) and trials including participants with refractory ascites (RR 0.98, 95% CI 0.37 to 2.65; 291 participants; 5 trials; I2 = 0%; Analysis 8.2.2).
8.2. Analysis.
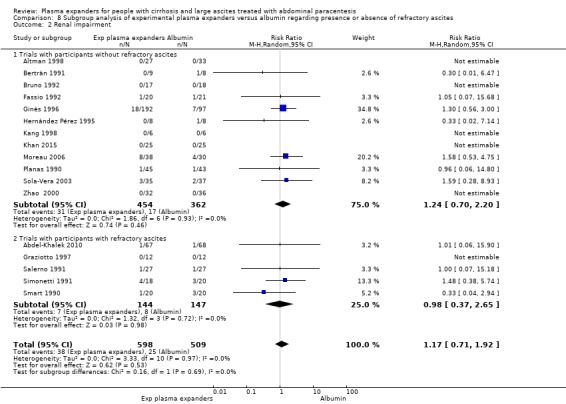
Comparison 8 Subgroup analysis of experimental plasma expanders versus albumin regarding presence or absence of refractory ascites, Outcome 2 Renal impairment.
Modality of paracentesis
The test for subgroup differences comparing different modality of paracentesis showed no difference (P = 0.99, I² = 0%; Analysis 9.2): trials in which partial paracentesis was used (RR 1.05, 95% CI 0.07 to 15.68; 169 participants; 3 trials; I2 not applicable because 2/3 trials had 0 events; Analysis 9.2.1) and trials in which total paracentesis was used (RR 1.04, 95% CI 0.59 to 1.84; 870 participants; 13 trials; I2 = 0%; Analysis 9.2.2).
9.2. Analysis.
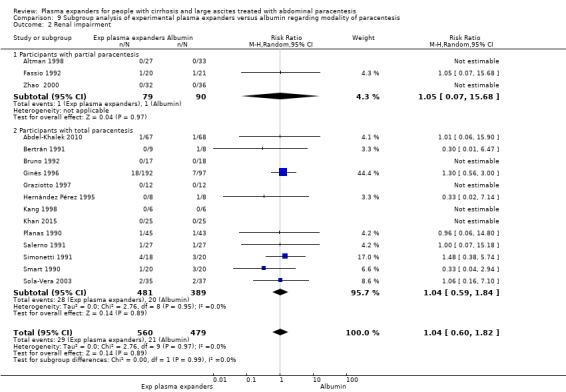
Comparison 9 Subgroup analysis of experimental plasma expanders versus albumin regarding modality of paracentesis, Outcome 2 Renal impairment.
Length of follow‐up
The test for subgroup differences comparing different lengths of follow‐up showed no difference between subgroups (P = 0.98, I² = 0%; Analysis 10.3): trials with a follow‐up up to one month (RR 1.13, 95% CI 0.56 to 2.25; 551 participants; 8 trials; I2 = 0%; Analysis 10.3.1) and trials with more than one month follow‐up (RR 1.14, 95% CI 0.58 to 2.22; 556 participants; 9 trials; I2 = 0%; Analysis 10.3.2).
10.3. Analysis.
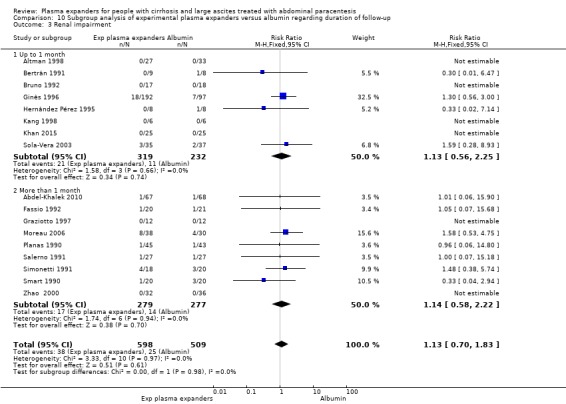
Comparison 10 Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up, Outcome 3 Renal impairment.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no difference between the two subgroups (P = 0.40, I2 = 0%; Analysis 11.2). We found no evidence of a difference in renal impairment either in the subgroup of trials without for‐profit funding (RR 1.46, 95% CI 0.72 to 2.97; 357 participants; 6 trials; I2 = 0%; Analysis 11.2.1) or in the subgroup of trials with or unknown for‐profit funding (RR 0.95, 95% CI 0.48 to 1.90; 750 participants; 11 trials; I2 = 0%; Analysis 11.2.2).
11.2. Analysis.
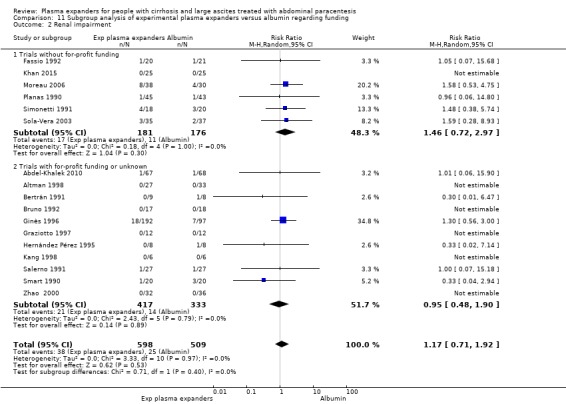
Comparison 11 Subgroup analysis of experimental plasma expanders versus albumin regarding funding, Outcome 2 Renal impairment.
Other liver‐related complications
Other liver‐related complications were reported In 16 trials (Planas 1990; Smart 1990; Bertrán 1991; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; Hernández Pérez 1995; Ginès 1996; Altman 1998; Kang 1998; Zhao 2000; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010; Khan 2015). There was no evidence of a difference between experimental plasma expanders versus albumin in other liver‐related complications (RR 1.10, 95% CI 0.82 to 1.48; 1083 participants; 16 trials; I2 = 19%; Analysis 7.4; Figure 7). The effect of the interventions on the occurrence of other liver‐related complications did not change by including the four trials with zero events (Bruno 1992; Hernández Pérez 1995; Kang 1998; Khan 2015) (RR 1.17, 95% CI 0.92 to 1.48; P = 0.20; D2 = 0%).
7.4. Analysis.
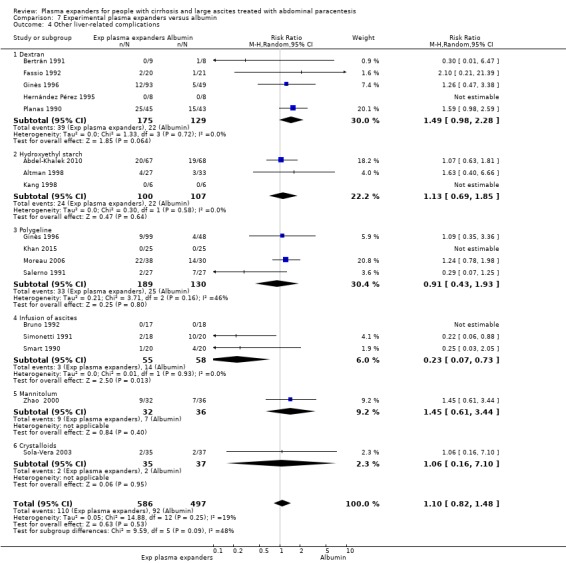
Comparison 7 Experimental plasma expanders versus albumin, Outcome 4 Other liver‐related complications.
7.
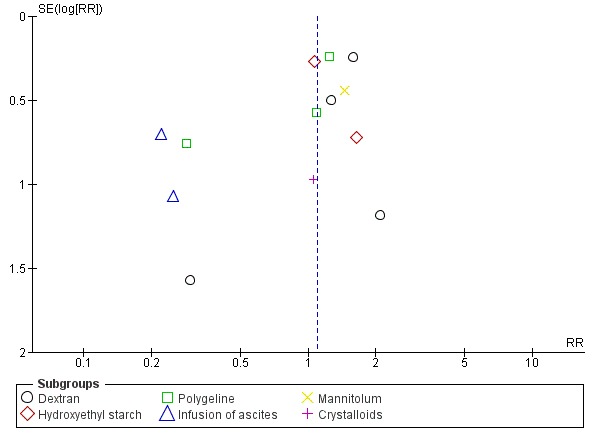
Funnel plot of comparison: 6 Experimental plasma expanders versus albumin, outcome: 6.4 Other liver‐related complications.
We assessed the certainty of the evidence for this outcome as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; there was imprecision: the required information size was not reached; and the funnel plot suggested publication bias (Table 2; Table 20; Figure 7).
Trial Sequential Analysis of this comparison was based on an incidence of other liver‐related complications of 18.5% in the albumin group, a relative risk reduction of 10% with experimental plasma expanders, a type I error of 2.00%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The diversity‐adjusted required information size was 16,992 participants. In Trial Sequential Analysis, the accrued information fraction was too small to produce the inner wedge futility area. The cumulative Z curve (blue line) did not reach the monitoring boundary (red line) suggesting that the results were not stable to adjustments for sparse data and multiple testing (Figure 8).
8.
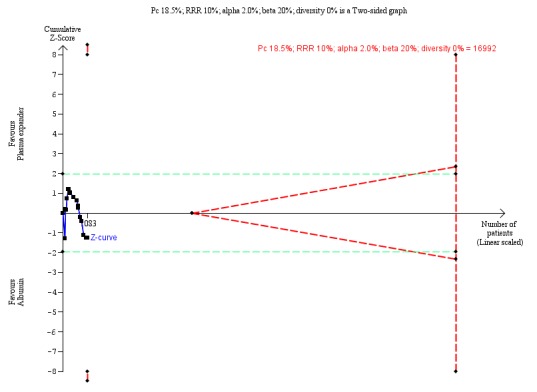
Experimental plasma expander versus albumin ‐ 6.4 Other liver‐related complications.
The diversity‐adjusted required information size of 16,992 participants was calculated based on a proportion of participants of 18.5% with other liver‐related complications in the control group; a relative risk reduction (RRR) of 10% in the plasma expander group; an alpha of 2.00%; a power of 80%; and a diversity of 0%.
The cumulative Z score did not cross borders for benefit, harm, or futility.
Subgroup analysis
We could not perform subgroup analysis of trials according to the risk of bias because all the trials were assessed at high risk.
Type of plasma expanders
The test for subgroup differences comparing the effects of different plasma expanders versus albumin showed a difference (P = 0.09, I² = 47.9%; Analysis 7.4): dextran (RR 1.49, 95% CI 0.98 to 2.28; 304 participants; 5 trials; I2 = 0%; Analysis 7.4.1); hydroxyethyl starch (RR 1.13, 95% CI 0.69 to 1.85; 207 participants; 3 trials; I2 = 0%; Analysis 7.4.2); polygeline (RR 0.91, 95% CI 0.43 to 1.93; 319 participants; 4 trials; I2 = 46%; Analysis 7.4.3); mannitol (RR 1.45, 95% CI 0.61 to 3.44; 68 participants; 1 trial; Analysis 7.4.5); and crystalloids (RR 1.06, 95% CI 0.16 to 7.10; 72 participants; 1 trial; Analysis 7.4.6)). Intravenous infusion of ascites reduced significantly other liver‐related complications in comparison with albumin (RR 0.23, 95% CI 0.07 to 0.73; 113 participants; 3 trials; I2 = 0%; Analysis 7.4.4).
Refractory ascites
The test for subgroup differences comparing separately the trials including participants without refractory ascites to trials including participants with refractory ascites showed a difference (P = 0.005, I² = 87%; Analysis 8.3): trials including participants without refractory ascites (RR 1.36, 95% CI 1.03 to 1.80; 766 participants; 12 trials; I2 = 0%; Analysis 8.3.1) and in trials including participants with refractory ascites (RR 0.64, 95% CI 0.41 to 1.00; 267 participants; 4 trials; I2 = 61%; Analysis 8.3.2).
8.3. Analysis.
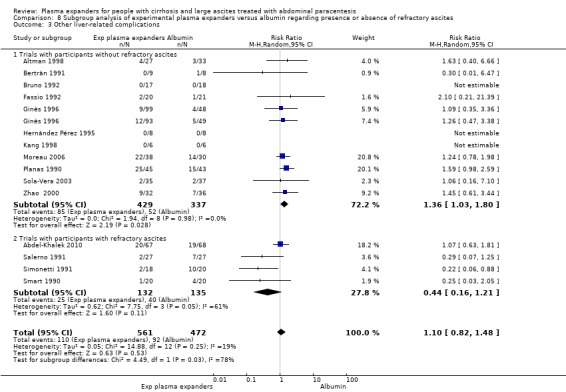
Comparison 8 Subgroup analysis of experimental plasma expanders versus albumin regarding presence or absence of refractory ascites, Outcome 3 Other liver‐related complications.
Modality of paracentesis
The test for subgroup differences comparing the effects of the experimental plasma expanders versus albumin in people treated by total compared to partial paracentesis showed no difference (P = 0.26, I² = 20.9%; Analysis 9.3): trials with partial paracentesis (RR 1.54, 95% CI 0.76 to 3.11; 169 participants; 3 trials; I2 = 0%; Analysis 9.3.1) and trials with total paracentesis (RR 0.98, 95% CI 0.67 to 1.42; 864 participants; 13 trials; I2 = 37%); Analysis 9.3.2)).
9.3. Analysis.
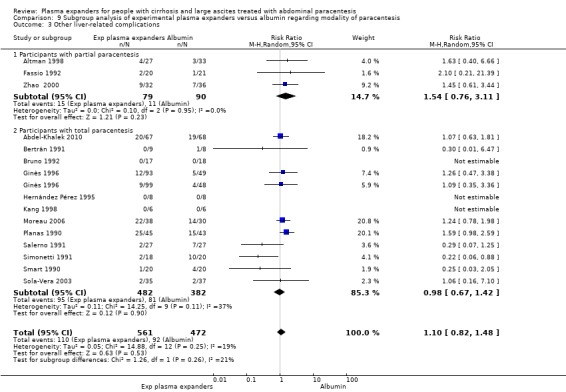
Comparison 9 Subgroup analysis of experimental plasma expanders versus albumin regarding modality of paracentesis, Outcome 3 Other liver‐related complications.
Length of follow‐up
The test for subgroup differences comparing separately the trials with a short follow‐up to the trials with a long follow‐up showed no difference (P = 0.66, I² = 0%; Analysis 10.4): trials with up to one month follow‐up (RR 1.17, 95% CI 0.64 to 2.16; 501 participants; 7 trials; I2 = 0%; Analysis 10.4.1) and trials with more than one month follow‐up (RR 0.99, 95% CI 0.65 to 1.53; 532 participants; 8 trials; I2 = 50%; Analysis 10.4.2).
10.4. Analysis.
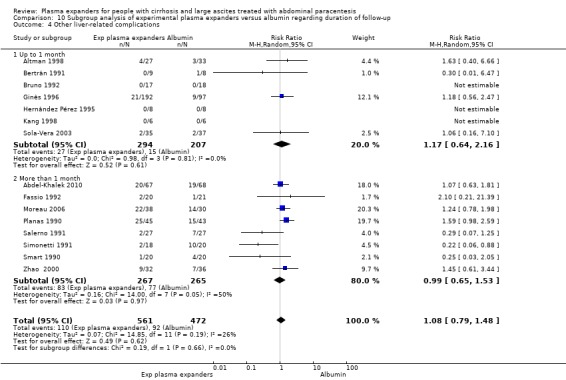
Comparison 10 Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up, Outcome 4 Other liver‐related complications.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no difference between the two subgroups (P = 0.20, I2 = 40.3%; Analysis 11.3). Experimental plasma expanders versus albumin led to more participants with other liver‐related complications in the subgroup of trials without for‐profit funding (RR 1.42, 95% CI 1.03 to 1.97; 357 participants; 6 trials; I2 = 0%; Analysis 11.3.1). We found no evidence of a difference in the subgroup of trials with or unknown for‐profit funding (RR 1.02, 95% CI 0.69 to 1.50; 726 participants; 10 trials; I2 = 7%; Analysis 11.3.2).
11.3. Analysis.
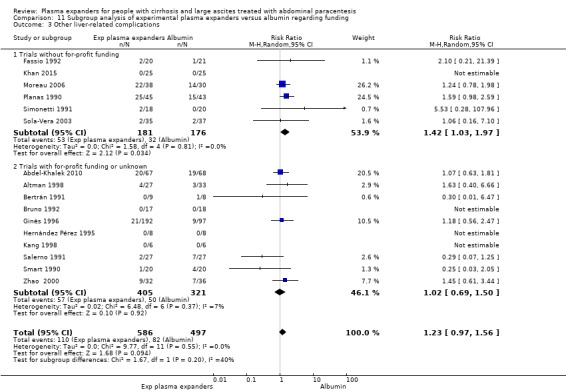
Comparison 11 Subgroup analysis of experimental plasma expanders versus albumin regarding funding, Outcome 3 Other liver‐related complications.
Non‐serious adverse events
Non‐serious adverse events were reported in 14 trials (Planas 1990; Smart 1990; Salerno 1991; Simonetti 1991; Bruno 1992; Hernández Pérez 1995; Ginès 1996; Graziotto 1997; Altman 1998; Kang 1998; Zhao 2000; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010). There was no evidence of a difference between the experimental plasma expanders group versus the albumin group in other liver‐related complications (RR 1.37, 95% CI 0.66 to 2.85; 977 participants; 14 trials; I2 = 0%; Analysis 7.5; Figure 9).
7.5. Analysis.
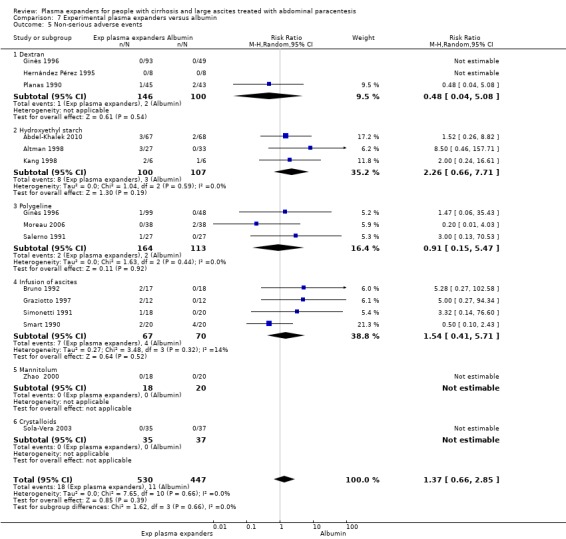
Comparison 7 Experimental plasma expanders versus albumin, Outcome 5 Non‐serious adverse events.
9.
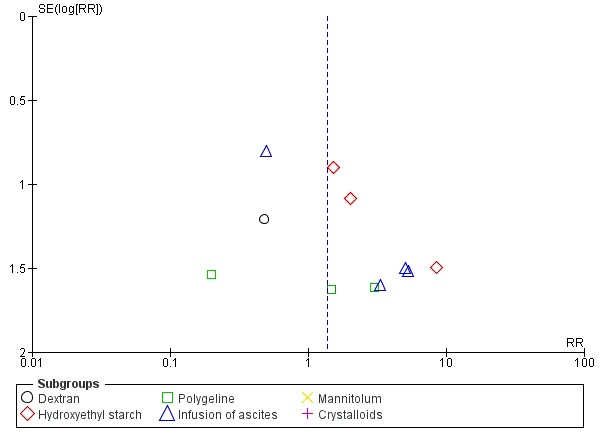
Funnel plot of comparison: 6 Experimental plasma expanders versus albumin, outcome: 6.5 Non‐serious adverse events.
Including the four trials with zero events produced a similar result (RR 0.92, 95% CI 0.36 to 2.35; P = 0.85; D2 = 0%) (Hernández Pérez 1995; Ginès 1996; Zhao 2000; Sola‐Vera 2003).
We assessed the level of certainty of the evidence for this outcome as very low. We downgraded the evidence by three levels because all trials were at high risk of bias; and there was imprecision: the required information size was not reached (Table 2; Table 20). There was no publication bias (Figure 9).
Trial Sequential Analysis of this comparison was based on a non‐serious adverse event proportion of 2.5% in the albumin group, a relative risk reduction of 10% with experimental plasma expanders, a type I error of 2.00%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The diversity‐adjusted required information size was 148,925 participants. Due to the fact that only 977 participants were recruited (which is 0.65% of the diversity‐adjusted required information size of 148,925 participants), the Trial Sequential Analysis program could not construct an interpretable figure and could not calculate the Trial Sequential Analysis‐adjusted confidence intervals.
Subgroup analysis
We could not perform subgroup analysis of trials according to the risk of bias because all the trials were at high risk.
Type of plasma expanders
The test for subgroup differences comparing the effects of different plasma expanders versus albumin showed no difference (P = 0.66, I² = 0%; Analysis 7.5): trials assessing dextran (RR 0.48, 95% CI 0.04 to 5.08; 246 participants; 3 trials; I2 not applicable because 2/3 trials had 0 events; Analysis 7.5.1); hydroxyethyl starch (RR 2.26, 95% CI 0.66 to 7.71; 207 participants; 3 trials; I2 = 0%; Analysis 7.5.2); polygeline (RR 0.91, 95% CI 0.15 to 5.47; 277 participants; 3 trials; I2 = 0%; Analysis 7.5.3); intravenous infusion of ascites (RR 1.54, 95% CI 0.41 to 5.71; 137 participants; 4 trials; I2 = 14%; Analysis 7.5.4). No events were observed in the mannitol and in the crystalloids subgroups.
Refractory ascites
The test for subgroup differences comparing separately the trials including participants without refractory ascites to trials including participants with refractory ascites showed no difference (P = 0.88, I² = 0%; Analysis 8.4): trials with participants without refractory ascites (RR 1.47, 95% CI 0.49 to 4.36; 686 participants; 10 trials; I2 = 0%; Analysis 8.4.1) and trials with participants with refractory ascites (RR 1.30, 95% CI 0.49 to 3.47; 291 participants; 5 trials; I2 = 0%; Analysis 8.4.2).
8.4. Analysis.
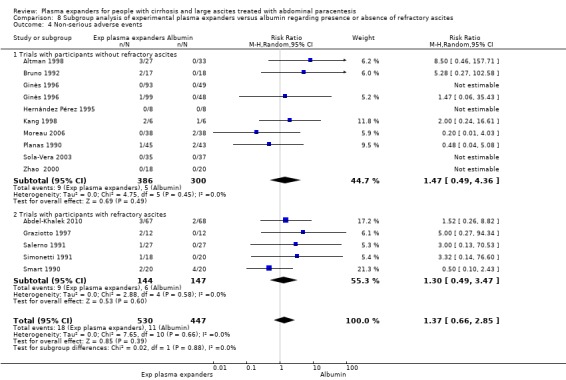
Comparison 8 Subgroup analysis of experimental plasma expanders versus albumin regarding presence or absence of refractory ascites, Outcome 4 Non‐serious adverse events.
Modality of paracentesis
The test for subgroup differences comparing the effects of experimental plasma expanders versus albumin in participants treated by partial or total paracentesis showed no difference (P = 0.21, I2 = 37.3%; Analysis 9.4): trials in which partial paracentesis was performed (RR 8.50, 95% CI 0.46 to 157.71; 98 participants; 2 trials; I2 not applicable because 1/2 trials had 0 events; Analysis 9.4.1) and trials in which total paracentesis was performed (RR 1.22, 95% CI 0.57 to 2.58; 879 participants; 13 trials; I2 = 0%; Analysis 9.4.2).
9.4. Analysis.
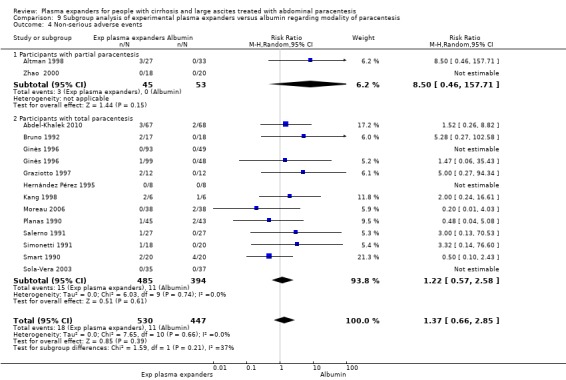
Comparison 9 Subgroup analysis of experimental plasma expanders versus albumin regarding modality of paracentesis, Outcome 4 Non‐serious adverse events.
Length of follow‐up
The test for subgroup differences comparing the effects of experimental plasma expanders versus albumin in participants with a short (up to one month) follow‐up compared to a long follow‐up showed no difference (P = 0.26, I² = 22.6%; Analysis 10.5): trials with a short follow‐up (RR 2.20, 95% CI 0.73 to 6.59; participants 484; 6 trials; I2 = 0; Analysis 10.5.1) and trials with a long follow‐up (RR 0.97, 95% CI 0.41 to 2.32; 493 participants; 8 trials; I2 = 0%; Analysis 10.5.2).
10.5. Analysis.
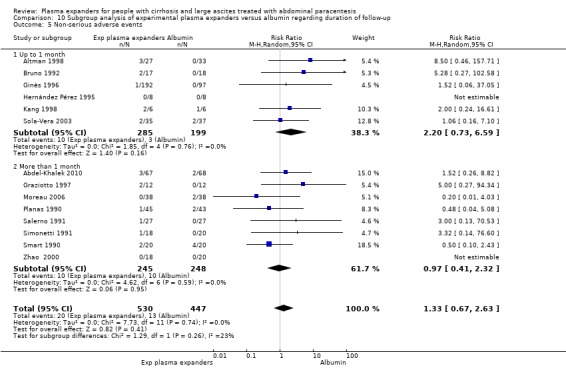
Comparison 10 Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up, Outcome 5 Non‐serious adverse events.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no difference between the two subgroups (P = 0.27, I2 = 18.2%; Analysis 11.4). We found no evidence of a difference in non‐serious adverse events either in the subgroup of trials without for‐profit funding (RR 0.62, 95% CI 0.12 to 3.05; 274 participants; 4 trials; I2 = 0%; Analysis 11.4.1) or in the subgroup of trials with or unknown for‐profit funding (RR 1.70, 95% CI 0.75 to 3.85; 703 participants; 10 trials; I2 = 0%; Analysis 11.4.2).
11.4. Analysis.
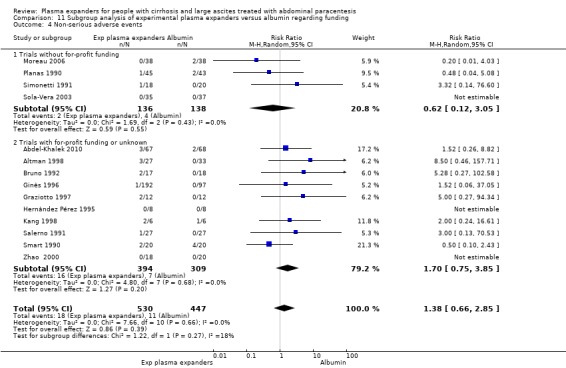
Comparison 11 Subgroup analysis of experimental plasma expanders versus albumin regarding funding, Outcome 4 Non‐serious adverse events.
Exploratory outcomes
Recurrence of ascites
Recurrence of ascites was reported in 12 trials (Planas 1990; Smart 1990; Salerno 1991; Simonetti 1991; Fassio 1992; Méndez 1991; Hernández Pérez 1995; Altman 1998; Zhao 2000; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010). Experimental plasma expanders had no effect in the recurrence of ascites in comparison with albumin (RR 1.14, 95% CI 0.96 to 1.36; 700 participants; 12 trials; I2 = 8%; Analysis 7.6; Figure 10).
7.6. Analysis.
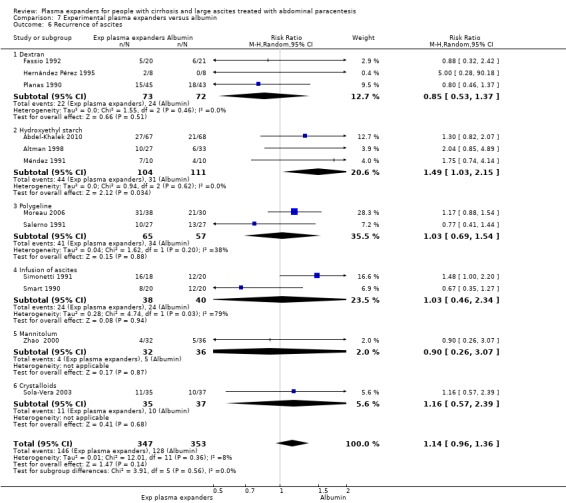
Comparison 7 Experimental plasma expanders versus albumin, Outcome 6 Recurrence of ascites.
10.
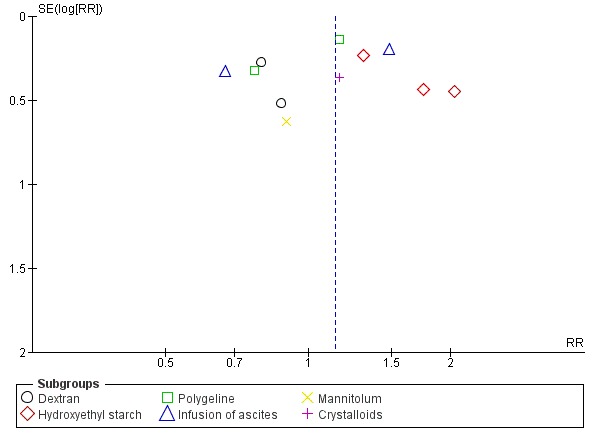
Funnel plot of comparison: 6 Experimental plasma expanders versus albumin, outcome: 6.6 Recurrence of ascites.
We assessed the level of certainty of the evidence with GRADE as very low. We downgraded the evidence by three levels because all trials were at high risk of bias; and there was imprecision: the required information size was not reached (Table 21; Table 22). There was no evidence of publication bias (Figure 10).
5. Plasma expanders versus albumin for people with cirrhosis and large ascites treated with abdominal paracentesis: exploratory outcomes.
| Plasma expanders versus albumin for people with cirrhosis and large ascites treated with abdominal paracentesis: exploratory outcomes | ||||||
|
Patient or population: cirrhotic participants with large ascites treated by paracentesis Settings: specialised units in an intensive or semi‐intensive setting Intervention: all plasma expanders except albumin Comparison: albumin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Albumin | Experimental plasma expanders | |||||
|
Recurrence of ascites mean follow‐up 169 days (5‐638) |
Medium risk population | RR 1.14 (0.96 to 1.36) | 700 (12) | ⊕⊝⊝⊝ very low1 | ||
| 363 per 1000 | 412 per 1000 (348 to 493) | |||||
|
Hypotension follow‐up 6 days |
88 per 1000 | 239 per 1000 (100 to 573) | RR 2.71 (1.13 to 6.50) | 135 (1) | Certainty of the evidence not assessed 2 | |
|
Hyponatraemia mean follow‐up 174 days (3‐638) |
Medium risk population | RR 1.49 (1.03 to 2.14) | 1107 (17) | ⊕⊝⊝⊝ very low3 | ||
| 73 per 1000 | 108 per 1000 (75 to 155) | |||||
|
Post‐paracentesis circulatory dysfunction mean follow‐up 100 days (6‐288) |
Medium risk population | RR 1.98 (1.31 to 2.99) | 432 (3) | ⊕⊝⊝⊝ very low4 | ||
| 148 per 1000 | 294 per 1000 (194 to 443) | |||||
| * Assumed risk is the risk in comparison group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1 Downgraded 3 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: required information size as calculated by GRADE not reached (‐1 level) 2 Not evaluated because there was only one trial 3 Downgraded 4 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE was not reached (‐1 level); publication bias(‐1 level) 4 Downgraded 3 levels because of within study risk of bias: all trials were at high risk of bias (‐2 levels); imprecision: the required information size as calculated by GRADE was not reached (‐1 level)
6. Comparison of imprecision by GRADE and Trial Sequential Analysis for the evaluation of exploratory outcomes in the comparison of plasma expanders versus albumin.
| Comparison of imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on authors' choice of plausible relative risk reduction and multiplicity correction, and according to our Trial Sequential Analysis with a similar choice of plausible relative risk reduction and multiplicity correction, in addition to considering the choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size (OIS or DARIS) | Downgrading of evidence for imprecision based on required information size |
| Recurrence of ascites ‐‐ GRADE Handbook | 36.3% | 25% | 5% | 20% | Not used | 824 | One level |
| Recurrence of ascites ‐‐ GRADE plausible RRR | 36.3% | 10% | 2% | 20% | Not used | 6882 | One level |
| Recurrence of ascites ‐‐ TSA | 36.3% | 10% | 2% | 20% | 3% | 7104 | One level |
| Hypotension ‐‐ GRADE Handbook | 8.8% | 25% | 5% | 20% | Not used | 4608 | One level |
| Hypotension ‐‐ GRADE plausible RRR | 8.8% | 10% | 2% | 20% | Not used | 39,712 | One level |
| Hypotension ‐‐ TSA (1) | |||||||
| Hyponatraemia ‐‐ GRADE Handbook | 7.3% | 25% | 5% | 20% | Not used | 5602 | One level |
| Hyponatraemia ‐‐ GRADE plausible RRR | 7.3% | 10% | 2% | 20% | Not used | 48,618 | One level |
| Hyponatraemia ‐‐ TSA | 7.3% | 10% | 2% | 20% | 0% | 48,620 | One level |
| Post‐paracentesis circulatory dysfunction ‐‐ GRADE Handbook | 14.8% | 25% | 5% | 20% | Not used | 2584 | One level |
| Post‐paracentesis circulatory dysfunction ‐‐ GRADE plausible RRR | 14.8% | 10% | 2% | 20% | Not used | 22,144 | One level |
| Post‐paracentesis circulatory dysfunction ‐‐ TSA | 14.8% | 10% | 2% | 20% | 0% | 22,146 | One level |
(1) TSA non calculated because there was only one trial
OIS: optimal information size; DARIS: diversity‐adjusted required information size; RRR: relative risk reduction; TSA: Trial Sequential Analysis
Trial Sequential Analysis of this comparison was based on a recurrence of ascites proportion of 36.3% in the albumin group, a relative risk reduction of 10% with experimental plasma expanders, a type I error of 2.00%, and a type II error of 20% (80% power). The diversity (D2) was 3%. The diversity‐adjusted required information size was 7104 participants. In Trial Sequential Analysis, the accrued information fraction was too small to produce an inner wedge futility area. The cumulative Z curve (blue line) did not approach the monitoring boundaries (red lines) for benefit or harm, demonstrating too few data (Figure 11).
11.
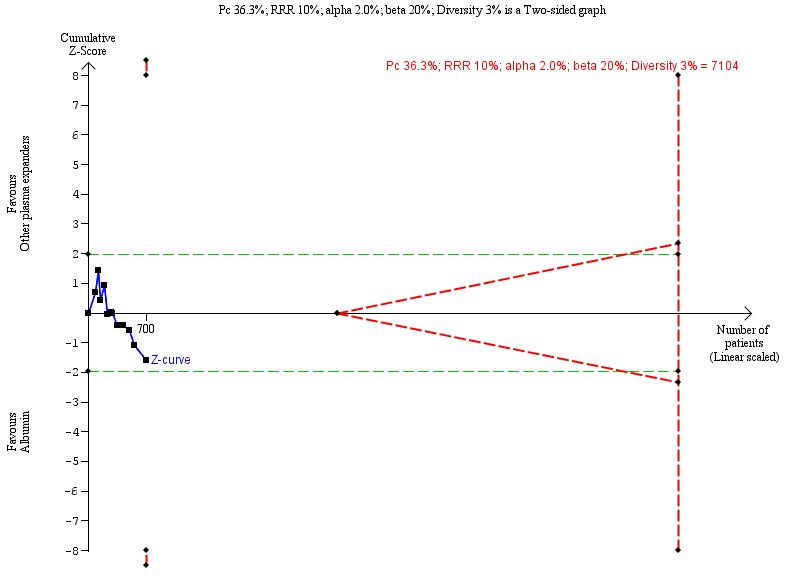
Experimental plasma expanders versus albumin ‐ 6.6 Recurrence of ascites.
The diversity‐adjusted required information size of 7104 participants was calculated based on a proportion of participants of 36.3% of participants suffering recurrence of ascites in the control group; a relative risk reduction (RRR) of 10% in the plasma expander group; an alpha of 2%; a power of 80%; and a diversity of 3%.
The cumulative Z score did not cross borders for benefit, harm, or futility.
Subgroup analysis
We could not perform subgroup analysis of trials according to the risk of bias because all the trials were at high risk of bias.
Type of plasma expanders
The test for subgroup differences comparing the effects of different plasma expanders versus albumin showed no differences between subgroups (P = 0.56, I² = 0%; Analysis 7.6): trials assessing dextran (RR 0.85, 95% CI 0.53 to 1.37; 145 participants; 3 trials; I2 = 0%; Analysis 7.6.1); polygeline (RR 1.03, 95% CI 0.69 to 1.54; 122 participants; 2 trials; I2 = 38%; Analysis 7.6.3); intravenous infusion of ascites (RR 1.03, 95% CI 0.46 to 2.34; 78 participants; 2 trials; I2 = 79%; Analysis 7.6.4); mannitol (RR 0.90, 95% CI 0.26 to 3.07; 68 participants; Analysis 7.6.5); and crystalloids (RR 1.16, 95% CI 0.57 to 2.39; 72 participants; Analysis 7.6.6). Hydroxyethyl starch increased the risk of recurrence of ascites in comparison with albumin (RR 1.49, 95% CI 1.03 to 2.15; 215 participants; 3 trials; I2 = 0%; Analysis 7.6.2).
Refractory ascites
The test for subgroup differences comparing the trials including participants without refractory ascites to trials including participants with refractory ascites did not show differences (P = 0.70, I² = 0%; Analysis 8.5): participants without refractory ascites (RR 1.10, 95% CI 0.90 to 1.35; 487 participants; 9 trials; I2 = 0%; Analysis 8.5.1) and participants with refractory ascites (RR 1.19, 95% CI 0.86 to 1.66; 235 participants; 4 trials; I2 = 30%; Analysis 8.5.2).
8.5. Analysis.

Comparison 8 Subgroup analysis of experimental plasma expanders versus albumin regarding presence or absence of refractory ascites, Outcome 5 Recurrence of ascites.
Modality of paracentesis
The test for subgroup difference comparing the effects of experimental plasma expanders versus albumin in people treated by partial or total paracentesis showed no differences (P = 0.60, I² = 0%; Analysis 9.5): participants treated by partial paracentesis (RR 1.28, 95% CI 0.72 to 2.26; 169 participants; 3 trials; I2 = 0%, Analysis 9.5.1) and participants treated by total paracentesis (RR 1.09, 95% CI 0.91 to 1.30; 533 participants; 9 trials; I2 = 8%; Analysis 9.5.2).
9.5. Analysis.
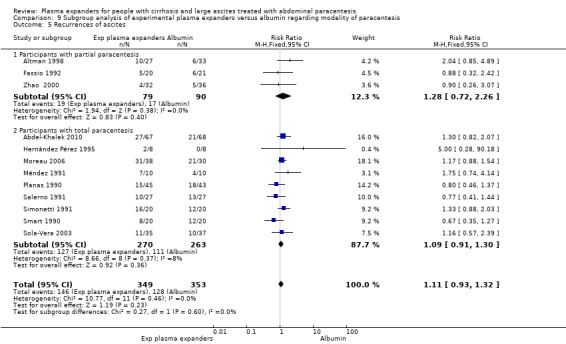
Comparison 9 Subgroup analysis of experimental plasma expanders versus albumin regarding modality of paracentesis, Outcome 5 Recurrences of ascites.
Length of follow‐up
The test for subgroup differences comparing the effects of experimental plasma expanders versus albumin in participants with a short (up to one month) follow‐up compared to a long (more than one month) follow‐up showed a difference between the subgroups (P = 0.03, I² = 77.9%; Analysis 10.6): trials with a short follow‐up (RR 2.07, 95% CI 1.12 to 3.83; 96 participants; 3 trials; I2 = 0%; Analysis 10.6.1) and trials with a follow‐up longer than one month (RR 1.03, 95% CI 0.86 to 1.24; 606 participants; 9 trials; I2 = 0%; Analysis 10.6.2).
10.6. Analysis.

Comparison 10 Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up, Outcome 6 Recurrence of ascites.
For‐profit support
The test for subgroup differences comparing for‐profit support showed no difference between the two subgroups (P = 0.87, I2 = 0%; Analysis 11.5). We found no evidence of difference in recurrence of ascites either in the subgroup of trials without for‐profit funding (RR 1.16, 95% CI 0.96 to 1.42; 307 participants; 5 trials; I2 = 0%; Analysis 11.5.1) or in the subgroup of trials with or unknown for‐profit funding (RR 1.13, 95% CI 0.79 to 1.60; 393 participants; 7 trials; I2 = 28%; Analysis 11.5.2).
11.5. Analysis.

Comparison 11 Subgroup analysis of experimental plasma expanders versus albumin regarding funding, Outcome 5 Recurrence of ascites.
Hypotension
One trial mentioned clinical hypotension that was more frequent in the hydroxyethyl starch group (16/67 participants, 24%) than in the albumin group (6/68 participants, 8%) (P = 0.02) (Abdel‐Khalek 2010) (Table 21; Table 22).
Hyponatraemia
Hyponatraemia was reported in seventeen trials (Planas 1990; Smart 1990; Bertrán 1991; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; Hernández Pérez 1995; Ginès 1996; Graziotto 1997; Altman 1998; Kang 1998; Zhao 2000; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010; Khan 2015). Hyponatraemia was more frequent in the experimental plasma expander group than in the albumin group (RR 1.49, 95% CI 1.03 to 2.14; 1107 participants; 17 trials; I2 = 0%; Analysis 7.7; Figure 12).
7.7. Analysis.
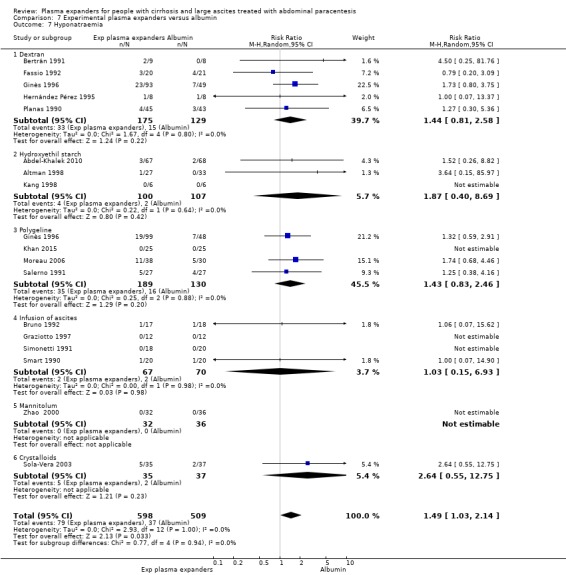
Comparison 7 Experimental plasma expanders versus albumin, Outcome 7 Hyponatraemia.
12.
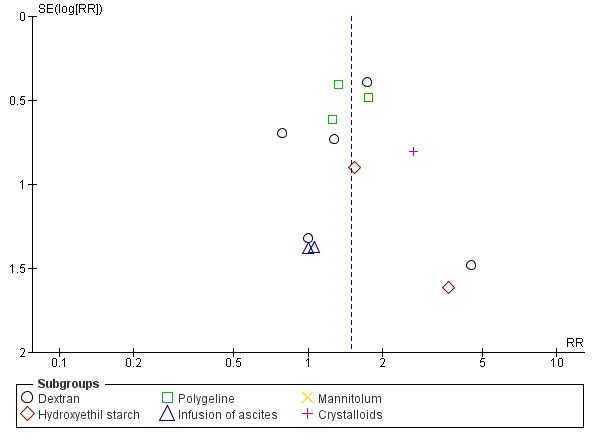
Funnel plot of comparison: 6 Experimental plasma expanders versus albumin, outcome: 6.7 Hyponatraemia.
Including the five trials with zero events (Simonetti 1991; Graziotto 1997; Kang 1998; Zhao 2000; Khan 2015), the result was similar (RR 1.45, 95% CI 1.00 to 2.09, P = 0.05).
We assessed the certainty of the evidence for this outcome as very low. We downgraded the evidence by four levels because all trials were at high risk of bias; there was imprecision: the required information size was not reached; and there was publication bias (Table 21; Table 22; Figure 12).
Trial Sequential Analysis of this comparison was based on a hyponatraemia proportion of 7.3% in the albumin group, a relative risk reduction of 10% with experimental plasma expanders, a type I error of 2.00%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The diversity‐adjusted required information size was 48,620 participants. The Trial Sequential Monitoring boundary could not be constructed due to too little information (2.28%) (figure not shown).
Subgroup analysis
We could not perform subgroup analysis of trials according to the risk of bias because all the trials were at high risk of bias.
Type of plasma expanders
The test for subgroup differences comparing the effects of different plasma expanders showed no difference between the subgroups (P = 0.94, I² = 0%; Analysis 7.7). No evidence of a difference in hyponatraemia was reported in the trials assessing dextran (RR 1.44, 95% CI 0.81 to 2.58; 304 participants; 5 trials; I2 = 0%; Analysis 7.7.1), hydroxyethyl starch (RR 1.87, 95% CI 0.40 to 8.69; 207 participants; 3 trials; I2 = 0%; Analysis 7.7.2), polygeline (RR 1.43, 95% CI 0.83 to 2.46; 319 participants; 4 trials; I2 = 0%; Analysis 7.7.3), intravenous infusion of ascites (RR 1.03, 95% CI 0.15 to 6.93; 137 participants; 4 trials; I2 = 0%; Analysis 7.7.4), and crystalloids (RR 2.64, 95% CI 0.55 to 12.75; 72 participants; Analysis 7.7.6) versus albumin. No events occurred in the mannitol trial (Zhao 2000) (Analysis 7.7.5).
Refractory ascites
The test for subgroup differences comparing the trials including participants without refractory ascites to the trials including participants with refractory ascites did not show a difference between the subgroups (P = 0.74, I² = 0%; Analysis 8.6). In the trials including participants without refractory ascites, the experimental plasma expanders increased the risk of hyponatraemia in comparison with albumin (RR 1.53, 95% CI 1.03 to 2.27; P = 0.04; 816 participants; 13 trials; I2 = 0%; Analysis 8.6.1). In the trials including participants with refractory ascites, no differences between the experimental plasma expanders and albumin were shown (RR 1.29, 95% CI 0.51 to 3.26; I2 = 0%; 291 participants; 5 trials; Analysis 8.6.2).
8.6. Analysis.
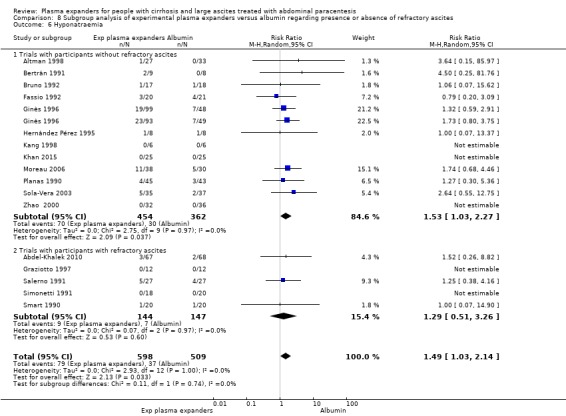
Comparison 8 Subgroup analysis of experimental plasma expanders versus albumin regarding presence or absence of refractory ascites, Outcome 6 Hyponatraemia.
Modality of paracentesis
The test for subgroup differences comparing the effects of experimental plasma expanders versus albumin in participants treated by total compared to partial paracentesis showed no difference (P = 0.52, I² = 0%; Analysis 9.6).
9.6. Analysis.
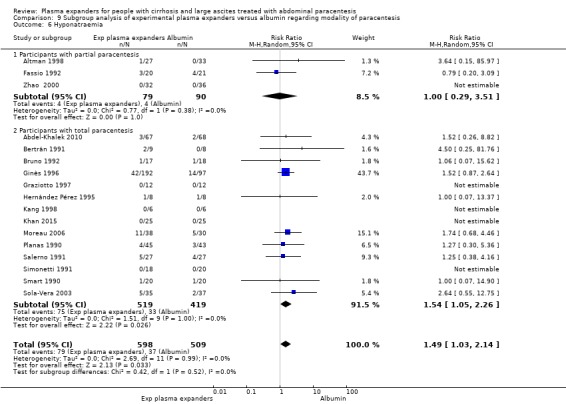
Comparison 9 Subgroup analysis of experimental plasma expanders versus albumin regarding modality of paracentesis, Outcome 6 Hyponatraemia.
Experimental plasma expanders did not affect hyponatraemia in comparison with albumin in the trials in which partial paracentesis was used (RR 1.00, 95% CI 0.29 to 3.51; 169 participants; 3 trials; I2 = 0%; Analysis 9.6.1). Experimental plasma expanders increased the risk of hyponatraemia in comparison with albumin in the trials in which total paracentesis was used (RR 1.54, 95% CI 1.05 to 2.26; 938 participants; 14 trials; I2 = 0%; Analysis 9.6.2).
Length of follow‐up
The test for subgroup differences comparing the effects of experimental plasma expanders versus albumin in people with a short (up to one month) follow‐up compared to a long follow‐up showed no difference (P = 0.56, I² = 0%; Analysis 10.7). Experimental plasma expanders versus albumin resulted in more participants with hyponatraemia in the trials with a short follow‐up (RR 1.64, 95% CI 1.01 to 2.68; 551 participants; 8 trials; I2 = 0%; Analysis 10.7.1). No difference was found in the trials with more than one month of follow‐up (RR 1.31, 95% CI 0.76 to 2.28; 556 participants; 9 trials; I2 = 0%; Analysis 10.7.2).
10.7. Analysis.
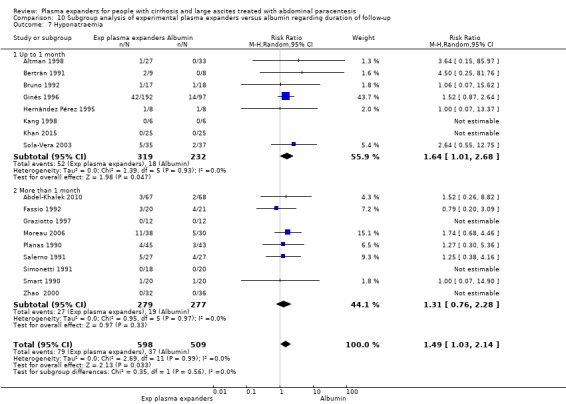
Comparison 10 Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up, Outcome 7 Hyponatraemia.
For‐profit support
The test for subgroup differences comparing funding support showed no difference between the two subgroups (P = 0.99, I2 = 0%; Analysis 11.6). We found no evidence of a difference in hyponatraemia either in the subgroup of trials without for‐profit funding (RR 1.48, 95% CI 0.79 to 2.77; 357 participants; 6 trials; I2 = 0%; Analysis 11.6.1) or in the subgroup of trials with or unknown for‐profit funding (RR 1.49, 95% CI 0.95 to 2.34; 750 participants; 11 trials; I2 = 0%; Analysis 11.6.2).
11.6. Analysis.
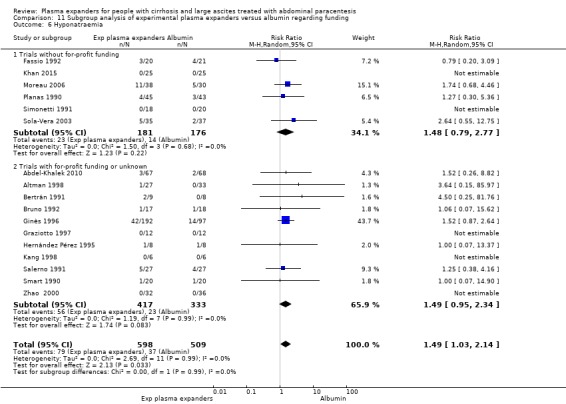
Comparison 11 Subgroup analysis of experimental plasma expanders versus albumin regarding funding, Outcome 6 Hyponatraemia.
Post‐paracentesis circulatory dysfunction
Post‐paracentesis circulatory dysfunction was assessed in three trials including 432 participants (Ginès 1996; Sola‐Vera 2003; Al Sebaey 2012). Post‐paracentesis circulatory dysfunction was more frequent in participants treated by experimental plasma expanders in comparison with albumin (RR 1.98, 95% CI 1.31 to 2.99; P = 0.001; I2 = 0%; Analysis 7.8).
7.8. Analysis.
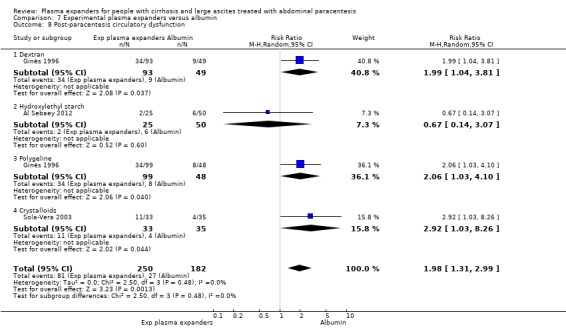
Comparison 7 Experimental plasma expanders versus albumin, Outcome 8 Post‐paracentesis circulatory dysfunction.
We assessed the level of certainty of the evidence for this outcome as very low. We downgraded the evidence by three levels because all trials were at high risk of bias; and imprecision: the required information size was not reached (Table 21; Table 22). Trial Sequential Analysis of this comparison was based on a post‐paracentesis circulatory dysfunction proportion of 14.8% in the albumin group, a relative risk reduction of 10% with experimental plasma expanders, a type I error of 2.00%, and a type II error of 20% (80% power). There was no diversity (D2 = 0%). The diversity‐adjusted required information size was 22,146 participants. The trial sequential monitoring boundary could not be constructed due to too little information (1.95%).
Subgroup analysis
We could not perform subgroup analysis of trials according to the risk of bias because all the trials were at high risk. In all three trials, the included participants were without refractory ascites and total paracentesis was used. So we could not perform subgroup analysis according to the presence of refractory ascites and to modality of paracentesis. Post‐paracentesis circulatory dysfunction by definition was assessed at six days after paracentesis. So we could not perform subgroup analysis according to length of follow‐up.
Type of plasma expanders
The test for subgroup differences comparing the effects of different plasma expanders showed no difference between the subgroups (P = 0.48; I² = 0%; Analysis 7.8). No evidence of a difference in post‐paracentesis circulatory dysfunction was reported in comparison with albumin in the trial assessing hydroxyethyl starch (RR 0.67, 95% CI 0.14 to 3.07; 75 participants; Analysis 7.8.2; Al Sebaey 2012). In comparison with albumin, there was an increased risk for post‐paracentesis circulatory dysfunction in the trials evaluating dextran (RR 1.99, 95% CI 1.04 to 3.81; 142 participants; Analysis 7.8.1; Ginès 1996), polygeline (RR 2.06, 95% CI 1.03 to 4.10; 147 participants; Analysis 7.8.3; Ginès 1996), and crystalloids (RR 2.92, 95% CI 1.03 to 8.26; 68 participants; Analysis 7.8.4; Sola‐Vera 2003).
For‐profit support
The test for subgroup differences comparing funding support showed no difference between the two subgroups (P = 0.73, I2 = 0%; Analysis 11.7). We found no evidence of a difference in post‐paracentesis circulatory dysfunction in the subgroup of trials without for‐profit funding (RR 1.56, 95% CI 0.37 to 6.51; 143 participants; 2 trials; I2 = 59%; Analysis 11.7.1). Experimental plasma expander versus albumin resulted in more participants with post‐paracentesis circulatory dysfunction in the single trial with for‐profit funding (RR 2.02, 95% CI 1.26 to 3.24; 289 participants; Analysis 11.7.2).
11.7. Analysis.

Comparison 11 Subgroup analysis of experimental plasma expanders versus albumin regarding funding, Outcome 7 Post‐paracentesis circulatory dysfunction.
Reinfusion of ascitic fluid versus polygeline
One trial including 10 participants per comparison group assessed intravenous infusion of ascitic fluid versus polygeline (Mehta 1998). No procedure‐related mortality was reported in the trial, but all‐cause mortality was not reported. The trial did not report data on serious adverse events, health‐related quality of life, renal impairment, hypotension, hyponatraemia, and post‐paracentesis circulatory dysfunction. There was one liver‐related complication (spontaneous bacterial peritonitis) in the intravenous infusion of ascitic fluid group, and no events occurred in the polygeline group (P = 1.00) (Analysis 14.1). Non‐serious adverse events were more frequent in the intravenous infusion of ascitic fluid group than in the polygeline group (7 and 0, respectively, P = 0.003) (Analysis 14.2). Recurrence of ascites was similar in both treatment groups (9 and 10, respectively, P = 1.00) (Analysis 14.3).
14.1. Analysis.
Comparison 14 Intravenous infusion of ascites versus polygeline, Outcome 1 Other liver‐related complications.
14.2. Analysis.
Comparison 14 Intravenous infusion of ascites versus polygeline, Outcome 2 Non‐serious adverse events.
14.3. Analysis.
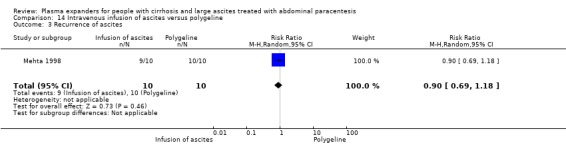
Comparison 14 Intravenous infusion of ascites versus polygeline, Outcome 3 Recurrence of ascites.
Dextran versus polygeline
Ginès 1996 assessed dextran (93 participants) versus polygeline (99 participants). There were no differences between the two treatments in mortality (4/93 (4%) versus 6/99 (6%) (P = 0.74)); renal impairment (8/93 (9%) versus 10/99 (10%) (P = 0.80)); other liver‐related complications (12/93 (13%) versus 9/99 (9%) (P = 0.48)); non‐serious adverse events (0/93 versus 1/99 (1%) (P = 1.00)); hyponatraemia (23/93 (25%) versus 19/99 (19%) (P = 0.38)); and post‐paracentesis circulatory dysfunction (34/93 (36%) versus 34/99 (34%) (P = 0.76).
Comparison of imprecision with GRADE and with Trial Sequential Analysis
The optimal information size (OIS) obtained with GRADE based on the GRADE Handbook, and with GRADE based on authors' choice of plausible relative risk reduction (RRR), and the diversity‐adjusted required information size (DARIS) obtained by Trial Sequential Analysis (TSA) were not reached in any primary, secondary, and exploratory outcomes. The agreement in the evaluation of imprecision, obtained by the TSA and GRADE methods, was substantial. We reported the comparison of imprecision evaluation for the primary, secondary and exploratory outcomes in Table 17; Table 19; Table 20; Table 22.
Number needed to treat for a beneficial outcome
Number needed to treat for a beneficial outcome (NNTB) was not calculated for each outcome because we judged it was useless to attempt to give an absolute measure of the effect based on a very low level of certainty.
Data from the two trials not included in the meta‐analysis
In the García‐Compeán 2002 trial, the authors reported outcomes referring to the number of paracentesis procedures (48 in dextran group and 48 in albumin group) and not to the number of included participants for each group. There were no deaths during the first hospitalisation, and 18 and 11, respectively during the follow‐up in the two groups. Recurrence was observed in 34 and 30 participants in each group. There were no differences between Dextran 40 and albumin in the number and type of complications: renal impairment; hyponatraemia; hyperkalaemia; and local haematoma.
In the Degoricija 2003 trial, it was not possible to extract numerical data on the outcomes of interest for the meta‐analysis. The authors reported that the different plasma expanders (albumin, fresh frozen plasma, or polygeline) after a single paracentesis of 6 L, associated with 24‐h bed rest before and after the procedure, did not induce significant changes in clinical measurements and laboratory parameters of hepatic and renal function, and plasma renin activity. No local complications related to the procedures were observed.
We judged both the two trials to be at high risk of bias (Figure 2). No data on benefit or harm were available. Thus, it was not possible to assess the certainty of the evidence.
Adverse events reported in non‐randomised studies retrieved with the searches for this review
In the Zaak 2001 study, intravenous infusion of ascitic fluid was compared to albumin. Among the 14 participants treated by intravenous infusion of ascitic fluid, there were 11 deaths (78%), 8 complications (57%), 4 cases of hyponatraemia (28%), 1 case of renal impairment (7%), and 3 cases of hepatic encephalopathy (21%). Among the 21 participants treated with albumin, there were 16 deaths (76%), 3 cases of hyponatraemia (14%), 2 cases of renal impairment (9%), and 2 cases of hepatic encephalopathy (9%).
In the Nasr 2010 study, Dextran 70 was compared to albumin. None of the study participants developed serious complications in the form of spontaneous bacterial peritonitis, sepsis, bleeding, or increase in the grade of hepatic encephalopathy until discharge after one week at least.
Adverse events reported in other studies with insufficient information on design, conduct, and results, retrieved with the searches for this review
Antillon and colleagues referred to a randomised trial comparing albumin infusion versus no plasma expander after large volume paracentesis in people with cirrhosis and refractory ascites in three publications (Antillon 1990 ‐ abstract; Antillon 1991 ‐ letter of comment to the Planas 1990 trial; Antillon 1993 ‐ comment to the Bruno 1992 trial). In the abstract, they reported three deaths out of seven participants (43%) in the albumin group and two deaths out of seven participants (29%) in the no plasma expander group (Antillon 1990). In the letter of comment to Planas 1990, Antillon reported that, in their "ongoing trial" including 28 participants, the one‐year survival was 45.0% in the albumin group and 41.6% in the no plasma expander group, and that the incidence of hepatorenal syndrome was 50% and 33% in the two groups, respectively (Antillon 1991). However, they did not report the number of participants in each group. In the comment to the Bruno 1992 trial, they referred to the trial again as "ongoing" without new results (Antillon 1993). No further information, requested by email and letter, was obtained from the authors.
Analyses not planned in the protocol
Trials comparing different amounts of albumin
We assessed the effect of two different doses of albumin in a separate analysis: 4 g/L versus 8 g/L of removed ascites (Alessandria 2011) and 2 g/L versus 6 g/L of removed ascites (Al Sebaey 2013).
The trial by Alessandria 2011 assessed low versus high dose of albumin in 35 participants in each group. There were no differences in the doses administered to the two groups in terms of mortality (3 events in each group, P = 1.00); renal impairment (0 events in each group); other complications (4 and 5 events, respectively, P = 1.00); and hyponatraemia (3 and 2 respectively, P = 1.00).
Post‐paracentesis circulatory dysfunction, assessed in the Al Sebaey 2013 and Alessandria 2011 trials, was similar between the two groups: 8/60 events in the low dose albumin group and 10/60 in high dose albumin group (RR 1.00, 95% CI 0.22 to 4.49; 120 participants; I2 = 0) (forest‐plot not shown).
Trials including participants with acute‐on‐chronic liver failure
One randomised clinical trial, published as abstracts (Arora 2018a; Arora 2018b), compared albumin with no plasma expander after a single < 5 L paracentesis in participants with acute‐on‐chronic liver failure (ACLF).
Forty participants received albumin (8 g/L of ascitic fluid) and forty received no plasma expander.
Albumin versus no plasma expansion reduced acute kidney injury (12/40 versus 25/40, P = 0.0067), hyponatraemia (9/40 versus 27/40, P = 0.0001), post‐paracentesis circulatory dysfunction (12/40 versus 28/40, P = 0.0007), but not hepatic encephalopathy (11/40 versus 20/40, P = 0.07). People without post‐paracentesis circulatory dysfunction (PPCD) had a higher mortality than people with PPCD (10/40 compared to 26/40, P = 0.0006).
Acute‐on‐chronic liver failure has peculiar pathophysiological, clinical, and prognostic features (Arroyo 2016).
Discussion
Summary of main results
The present systematic review with meta‐analyses assessed the role of plasma expanders after therapeutic paracentesis for large ascites in people with cirrhosis. The review aimed at answering two questions: whether plasma expanders are needed and whether there are differences between plasma expanders.
Five trials, including 271 participants, compared plasma expanders versus no plasma expander. Albumin was used in four trials (Ginès 1988; García‐Compeán 1993; Luca 1995; Baik 2000). Intravenous infusion of concentrated or unmodified ascitic fluid was used in one trial (Descos 1983). Most of the trials were only designed to assess outcomes during hospitalisation. Two trials assessed immediate, within 1 to 2 days, haemodynamic changes after paracentesis (Luca 1995; Baik 2000). Only one trial reported follow‐up after discharge (Ginès 1988).
We found very low‐certainty evidence that plasma expanders may make little or no difference to mortality. We found very low‐certainty evidence that plasma expanders may make little or no difference to serious adverse events, renal impairment, other liver‐related complications, non‐serious adverse events, recurrence of ascites, and hyponatraemia. We found very low‐certainty evidence that albumin decreases the risk of hyponatraemia in the subgroup of trials in which partial paracentesis was used, with a follow‐up more than one month, and without for‐profit funding. No evidence of a difference was detected for each of the other outcomes in subgroup analyses regarding the type of plasma expander, modality of paracentesis, or duration of follow‐up. No trials assessed health‐related quality of life, incidence of refractory ascites, or post‐paracentesis circulatory dysfunction.
Twenty‐one trials, including 1291 participants, assessed different plasma expanders versus albumin (Planas 1990; Smart 1990; Bertrán 1991; Salerno 1991; Simonetti 1991; Bruno 1992; Fassio 1992; Méndez 1991; Hernández Pérez 1995; Ginès 1996; Graziotto 1997; Altman 1998; Kang 1998; Zhao 2000; García‐Compeán 2002; Sola‐Vera 2003; Moreau 2006; Abdel‐Khalek 2010; Al Sebaey 2012; Khan 2015) or versus another plasma expander (Mehta 1998).
We found very low‐certainty evidence that experimental plasma expanders may make little or no difference to mortality. No evidence of a difference in mortality was found by analysing each experimental plasma expander (dextran, hydroxyethyl starch, polygeline, intravenous infusion of ascites, mannitol, saline solution) versus albumin. No evidence of a difference in mortality was found in the subgroups of trials including participants with refractory ascites or without refractory ascites, treated by different modality of paracentesis (total one session or partial repeated paracentesis), with different length of follow‐up (up to one month or more than one month), and type of funding. No trials assessed health‐related quality of life or incidence of refractory ascites. We found very low‐certainty evidence that experimental plasma expanders versus albumin may make little or no difference to serious adverse events, renal impairment, other liver related complications, non‐serious adverse events, and recurrence of ascites.
We found very low‐certainty evidence that experimental plasma expanders versus albumin increased incidence of hyponatraemia.
Hyponatraemia is associated with a significantly higher risk of death in cirrhosis (Ginés 2008; Mohanty 2015). Kim and colleagues demonstrated that serum sodium in patients on the waiting list for liver transplantation is an important predictor of mortality, independent of the model for end‐stage liver disease (MELD) score (Kim 2008). Whether this association is attributable to hyponatraemia itself or reflects the fact that patients with hyponatraemia tend to be ill in general, remains to be determined. The MELD‐Na, including serum Na, has been shown to predict survival more accurately than MELD alone (Biggins 2006) and it was proposed as a prognostic score in identifying patients for inclusion in the waiting list for liver transplantation. In addition, hyponatraemia is related to a higher risk of hepatic encephalopathy (Guevara 2009; Guevara 2010) and to an impaired health‐related quality of life (HRQOL) (Sola 2012). In this systematic review, there was an increased risk of hyponatraemia, but not of mortality, with experimental plasma expanders other than albumin, so the prognostic meaning of the increased risk of hyponatraemia is not clear.
Three trials provided very low certainty of evidence that experimental plasma expanders versus albumin increased the occurrence of post‐paracentesis circulatory dysfunction, addressed according to the formal definition proposed by Ginès 1996 and colleagues (Ginès 1996; Sola‐Vera 2003; Al Sebaey 2012). Post‐paracentesis circulatory dysfunction is defined as an increase in the plasma renin activity of more than 50% of the pretreatment value to a level of more than 4 ng/mL/h on the sixth day after paracentesis (Ginès 1996, Ruiz‐del‐Arbol 1997). Increase of renin activity suggests an activation of neurohumoral mechanism to maintain the correct homeostasis when reduction of effective plasma volume is induced by paracentesis (Ruiz‐del‐Arbol 1997), which interferes with the extremely sensible, precarious balance of the homeostatic mechanism in ascitic cirrhotic patients. However, the relationship of post‐paracentesis circulatory dysfunction with the prognosis is not definitely documented, as it is an unvalidated surrogate outcome for clinical effects (Gluud 2007; Jakobsen 2017; Jakobsen 2018). Moreover, in many trials, since renin activity is tested at different time points and by using different cut‐off values, the relationship with prognosis is totally lacking.
In the subgroup analysis, we found very low‐certainty evidence that experimental plasma expanders versus albumin increased incidence of other liver‐related complications in trials including people without refractory ascites and in trials without for‐profit funding, recurrence of ascites in trials with a short follow‐up, hyponatraemia in trials including people without refractory ascites, in trials in which total paracentesis was used and in those trials with a short follow‐up. Regarding the type of plasma expanders, we found very low‐certainty evidence that hydroxyethyl starch increased the risk of recurrence; polygeline, dextran, and crystalloids increased the risk of post‐paracentesis circulatory dysfunction; and intravenous infusion of ascitic fluid reduced the risk of other liver‐related complications. Experimental plasma expanders increased the risk of post‐paracentesis circulatory dysfunction in trials with for‐profit funding. No differences in the remaining subgroups were observed.
Overall, data to perform the subgroup analyses were lacking.
The differences between experimental plasma expanders versus albumin in some secondary and exploratory outcomes and in some subgroup analyses cannot be taken as proof of any intervention effect, considering the very low certainty of the evidence, the uncertain clinical meaning of the exploratory outcomes, and the number of comparisons that could generate false positive results. However, such differences can act as a stimulus to conduct more randomised clinical trials.
Overall completeness and applicability of evidence
The included trial participants had a wide range of characteristics, but their stage of cirrhosis was not very advanced, as exclusion criteria in some of the trials were presence of hepatocellular carcinoma, recent gastrointestinal bleeding, hepatic encephalopathy, and recent infection. In the majority of these trials, the participants were without renal failure and hyponatraemia. Some trials did not describe clearly if the trial participants had refractory ascites. So, based on our summary of the evidence, we conclude that the results are applicable to only people with intermediate to advanced stage of cirrhosis, usually treated by paracentesis in the clinics.
The present systematic review with meta‐analyses indicates insufficient evidence whether plasma expanders are better than no plasma expanders. This comparison should show that plasma expanders are superior to no plasma expanders before the comparison of different plasma expanders becomes of interest. Without such information, it becomes hard to interpret the comparison between the experimental plasma expanders versus albumin or versus other plasma expanders.
Quality of the evidence
Our current review has identified a number of methodological concerns. All trials were at high risk of bias. Moreover, there were high risks of random errors. The a priori required number of participants for a meta‐analysis to be conclusive was not reached, so this meta‐analysis has not enough information to reject or to accept the hypotheses.
Our assessments of risk of bias, and hence the certainty of the evidence, reflects lack of description or poor description of the trial design and performance, as well as incomplete reporting of results. We often failed in our attempts to obtain missing information from authors of the trial reports. Morever, there is a risk of chance effects due to multiple testing.
We used GRADE to consider the effect of study limitations on our outcomes. The conclusion is that the certainty of information is very low. When we assessed, in a sensitivity analysis, imprecision with Trial Sequential Analysis, then our assessment of the certainty of the evidence was also very low ‐ observations in accordance with previous studies (Castellini 2018; Gartlehner 2018).
Potential biases in the review process
We performed a comprehensive literature search for published and unpublished studies, and we combined electronic data searches with manual searches of the reference lists of the identified studies and also conference proceedings and abstract books from relevant national and international society meetings. We included trials regardless of their language of publication and whether they reported data on the outcomes we needed. We contacted relevant authors for additional information.
We consider it unlikely that we have failed to identify any published and unpublished trials. Two review authors independently assessed study eligibility, extracted data, and assessed risk of bias in included studies, and we believe that this has reduced potential biases in the review process. We included only quasi‐randomised trials and not observational studies for the assessment of adverse events. This may have biased our review towards assessment of benefits and might have overlooked late or rare harms. We did not seek translation of the full study reports for two studies that were reported in Korean (Kang 1998; Baik 2000); our judgements and data were limited to information available in the abstract or the tables.
Agreements and disagreements with other studies or reviews
Several meta‐analyses have been published on the use of albumin and other plasma expanders in cirrhotic patients with ascites undergoing paracentesis (Wong 2008; Bernardi 2012; Wang 2015; Kütting 2017).
The Wong 2008 meta‐analysis with nine trials found no difference in mortality and morbidity (renal impairment, hepatic encephalopathy, hyponatraemia) between plasma expansion versus no plasma expansion and between other plasma expanders versus albumin. The Bernardi 2012 meta‐analysis with 17 trials found that albumin in comparison with other plasma expanders and vasoconstrictors such as terlipressin, norepinephrine and midodrine, reduced mortality, hyponatraemia, and post‐paracentesis circulatory dysfunction and that albumin in comparison with no treatment reduced hyponatraemia and post‐paracentesis circulatory dysfunction. However, Chen 2014 and colleagues published a letter in which they presented the Trial Sequential Analysis of the data from the Bernardi's meta‐analysis which did not confirm their results and conclusions. On the other hand, Bernardi's reply confirmed the conclusions of the Bernardi 2012 meta‐analysis (Bernardi 2014). The Wang 2015 meta‐analysis with 13 trials found that albumin infusion had an advantage over alternative treatments in reducing hospital mortality, hyponatraemia, and post‐paracentesis circulatory dysfunction. Kütting and colleagues (Kütting 2017) instead, in their meta‐analysis of 21 trials, assessing albumin versus no plasma expanders, other plasma expanders, and vasoconstrictors, found insufficient evidence of benefit in mortality due to albumin substitution in hepatocellular carcinoma‐free cirrhotic patients undergoing large volume paracentesis.
We can explain the discrepant conclusions in the afore‐mentioned analyses and in our review by the differences in systematic review methodology, tested treatments, and selection of outcomes. Our review is the only one based on a pre‐published protocol, which was updated in conformity with the updated Cochrane methodology during the time of the review preparation. We used Trial Sequential Analysis to control the risks of random errors to which the conventional meta‐analysis is exposed (Wetterslev 2008; Thorlund 2010; Jakobsen 2014; Gluud 2015).
Our search for randomised clinical trials is current up to January 2019, which resulted in the inclusion of more trials than those included in the mentioned meta‐analyses.
We addressed, in two separate comparisons, the role of one plasma expander versus no plasma expander, and the role of different plasma expanders, similar to Wong (Wong 2008) and Bernardi (Bernardi 2012). Kutting and colleagues analysed together trials in which plasma expanders were tested against no treatment and against other plasma expanders (Kütting 2017). Wang did not include trials comparing plasma expanders versus no treatment (Wang 2015).
In this systematic review, in order to have a higher homogeneity of tested treatments, we chose to evaluate only substances aimed at maintaining a correct balance after paracentesis, by increasing intravascular volume (fluids and/or colloids). We excluded treatments which act by increasing peripheral resistance, such as the vasoconstrictors terlipressin, norepinephrine, and midodrine included in Bernardi's, Wang's and Kutting's meta‐analyses (Bernardi 2012, Wang 2015; Kütting 2017).
We included all the trials assessing every intravenous infusion of fluids which can act as plasma expanders and which have been tested in connection with paracentesis, including intravenous infusion of ascitic fluid after filtration, and in some cases, concentration, excluded in the other meta‐analyses. The rationale of its use is not different from that of other plasma expanders, such as colloids or saline solution.
After the publication of the trial by Ginès 1988, albumin was judged beneficial in connection with paracentesis, but expensive. Many trials were performed testing cheaper plasma expanders, or a modified system of intravenous infusion of ascitic fluid versus albumin. In our systematic review, we decided to test 'other than albumin plasma expanders' as the experimental treatment versus albumin as control treatment, in order to be more adherent to the real structure of the performed trials, whereas in the other meta‐analyses, albumin was tested as experimental treatment.
An important difference between this review and the meta‐analyses performed by others was the choice of outcomes, which we classified as primary, secondary, and exploratory according to their clinical importance. In particular, hyponatraemia and post‐paracentesis circulatory dysfunction were chosen as exploratory outcomes in the present review, whereas in Bernardi 2012 they were chosen as primary outcomes in addition to mortality, and in Kütting 2017 as secondary outcomes. Our choice is due to the putative surrogate character of hyponatraemia and of post‐paracentesis circulatory dysfunction (see above).
Morever, regarding post‐paracentesis circulatory dysfunction, we followed the Gines' definition (Ginès 1996), which is what the scientific community usually refers to. We included the Al Sebaey 2012 trial because the definition of post‐paracentesis circulatory dysfunction was only very slightly different from Ginès 1996. On the contrary, Bernardi 2012 and Kütting 2017 defined post‐paracentesis circulatory dysfunction in the methods section as an increase of plasma renin activity ≥ 50% (irrespective of observation time), but then in the results section they reported under the label of post‐paracentesis circulatory dysfunction any change of renin activity, irrespective of the rate of increase and timing of the assessment, and any change of aldosterone levels too. The relationship of these humoral changes and differences between treatments with robust clinical and prognostic outcomes has been not analysed and validated.
In a recent trial (Caraceni 2018), long‐term albumin administration in participants with cirrhosis and uncomplicated ascites treated by diuretics increased survival and reduced incidence of complications. This trial did not evaluate the role of albumin after therapeutic paracentesis. Albumin was administered in a weekly fixed schedule associated with the standard medical treatment for up to 18 months. In addition, the great majority of participants had moderate (grade 2) ascites, without refractory ascites. On the contrary, the trials of the present systematic review evaluated the benefits and harms of any plasma volume expanders versus no plasma volume expanders or versus another plasma volume expander after paracentesis, included mostly participants with large ascites, and some trials included participants with refractory ascites. So, Caraceni’s trial did not match the question of our systematic review, and its results cannot be applied to patients treated by paracentesis. However, they can raise stimulating issues on the role of albumin as a promising disease‐modifying treatment (Bernardi 2018).
.
Authors' conclusions
Implications for practice.
Based on the results of key clinical outcomes in this systematic review, we can neither demonstrate nor disprove any benefit of plasma expansion versus no plasma expansion, and differences between one plasma expander versus another plasma expander to be used after therapeutic paracentesis for large ascites in people with cirrhosis. So, the decision to use a plasma expander after large volume paracentesis, and the choice of the type could be based on the physician's and patient's values and preferences.
The increased risk of hyponatraemia and post‐paracentesis circulatory dysfunction with other plasma expanders compared to albumin, even considering the limits of their prognostic meaning and the very low level of evidence, could suggest caution in the choice of the plasma expanders in clinical practice.
Regulatory agencies introduced risk reduction minimisation measures, for hydroxyethyl starch. Its use is contraindicated in patients with sepsis, who are critically ill, with renal impairment or undergoing renal replacement therapy, with severe coagulopathy, or with impaired hepatic function and other severe conditions (FDA 2013, EMA 2018; AIFA 2018). According to some regulatory authorities, its use should be limited to managing hypovolaemia due to acute blood loss only when crystalloids are not considered sufficient (AIFA 2018; EMA 2018).
Albumin is considerably more expensive than the other intravenous fluids.
Intravenous infusion of ascitic fluid requires specialised staff and technical devices for filtration and concentration which can reduce its use.
The uncertainty of the results should suggest taking into account alternative treatments that improve the natural history of patients with decompensated cirrhosis, such as transjugular intrahepatic portosystemic shunt (TIPS) or orthotopic liver transplantation (OLT) when other criteria are satisfied (AASLD 2012; EASL 2016; EASL 2018).
Implications for research.
More large, high‐quality randomised clinical trials are necessary to assess the role of plasma expanders in connection with paracentesis in the treatment of ascites in cirrhotic participants. Such randomised clinical trials should be designed according to the SPIRIT guidelines (Standard Protocol Items: Recommendations for Interventional Trials; www.spirit‐statement.org) and reported according to the CONSORT guidelines (www.consort‐statement.org).
Acknowledgements
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
We thank the patients who participated in the reviewed trials and the researchers who conducted them.
We would like to thank Sarah L. Klingenberg from the Cochrane Hepato‐Biliary Group editorial office for her support and invaluable help.
We thank Arturo Marti‐Carvajal and Maoling Wei for their kind help in searching Lilacs and the Chinese data‐base, respectively.
Peer reviewer: Andrea Mancuso, Italy; Goran Poropat, Croatia Contact editor: Agostino Colli, Italy Sign‐off editor: Agostino Colli, Italy
Appendices
Appendix 1. Search strategies
| Database | Search date | Search strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | January 2019 (86 hits) |
(((volume OR plasma) AND expan*) OR diuretic* OR albumin* OR colloid* OR dextran OR h*mac*el OR polygeline OR reinfusion* OR hydroxy starch) AND paracentesis AND ascit* AND cirrho* |
| The Cochrane Central Register of Controlled Trials (Central) | January 2019, Issue 1 (146 hits) |
#1 MeSH descriptor: [Plasma Substitutes] explode all trees #2 MeSH descriptor: [Diuretics] explode all trees #3 MeSH descriptor: [Albumins] explode all trees #4 MeSH descriptor: [Colloids] explode all trees #5 MeSH descriptor: [Dextrans] explode all trees #6 MeSH descriptor: [Polygeline] explode all trees #7 MeSH descriptor: [Ascitic Fluid] explode all trees #8 (((volume or plasma) and expan*) or diuretic* or albumin* or colloid* or dextran or haemaccel or haemacel or hemaccel or hemacel or polygeline or reinfusion* or (hydroxy next starch)) #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 MeSH descriptor: [Paracentesis] explode all trees #11 paracentesis #12 #10 or #11 #13 MeSH descriptor: [Ascites] explode all trees #14 ascit* #15 #13 or #14 #16 MeSH descriptor: [Liver Cirrhosis] explode all trees #17 cirrho* #18 #16 or #17 #19 #9 and #12 and #15 and #18 |
| MEDLINE (Ovid SP) |
1946 to January 2019 (98 hits) | 1. exp Plasma Substitutes/ 2. exp Diuretics/ 3. exp Albumins/ 4. exp Colloids/ 5. exp Dextrans/ 6. exp Polygeline/ 7. exp Ascitic Fluid/ 8. (((volume or plasma) and expan*) or diuretic* or albumin* or colloid* or dextran or h*mac*el or polygeline or reinfusion* or "hydroxy starch").mp. [mp=title, original title, abstract, name of substance word, subject heading word] 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp Paracentesis/ 11. paracentesis.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 12. 11 or 10 13. exp Ascites/ 14. ascit*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 15. 13 or 14 16. exp Liver Cirrhosis/ 17. cirrho*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 18. 16 or 17 19. 18 and 9 and 12 and 15 20. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 21. 19 and 20 |
| Embase (Ovid SP) | 1974 to January 2019 (234 hits) | 1. exp Plasma Substitute/ 2. exp Diuretic Agent/ 3. exp Diuretic Therapy/ 4. exp Albumin/ 5. exp Colloid/ 6. exp Dextran/ 7. exp Ascites Fluid/ 8. (((volume or plasma) and expan*) or diuretic* or albumin* or colloid* or dextran or h*mac*el or polygeline or reinfusion* or "hydroxy starch").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp Paracentesis/ 11. paracentesis.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 12. 11 or 10 13. exp Ascites/ 14. ascit*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 15. 13 or 14 16. exp Liver Cirrhosis/ 17. cirrho*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 18. 16 or 17 19. 18 and 9 and 12 and 15 20. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 21. 19 and 20 |
| LILACS (Bireme) | 1982 to January 2019 (21 hits) | (((volume or plasma) and expan$) or diuretic$ or albumin$ or colloid$ or dextran or h$mac$el or polygeline or reinfusion$ or hydroxy starch) [Words] and paracentesis and ascit$ and cirrho$ [Words] |
| Science Citation Index Expanded Conference Proceedings Citation Index – Science (Web of Science) |
1900 to January 2019 1990 to January 2019 (212 hits) |
#7 #6 AND #5 #6 TS=(random* or blind* or placebo* or meta‐analysis) #5 #4 AND #3 AND #2 AND #1 #4 TS=cirrho* #3 TS=ascit* #2 TS=paracentesis #1 TS=(((volume or plasma) and expan*) or diuretic* or albumin* or colloid* or dextran or h*mac*el or polygeline or reinfusion* or hydroxy starch) |
| CNKI | 1979 to August 2015 (154 hits) | Liver Cirrhosis AND Ascites AND paracentesis, exact search in title OR key words, respectively. |
| VIP | 1989 to August 2015 (88 hits) | Liver Cirrhosis AND Ascites AND paracentesis, exact search in title OR key words, respectively |
| Wanfang | 1990 to August 2015 (40 hits) | Liver Cirrhosis AND Ascites AND paracentesis, exact search in title OR key words, respectively |
Appendix 2. Definition of for‐profit funding
Without for‐profit funding: The trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conductance, analysis, or reporting of trial results (industry‐sponsored studies overestimate the efficacy by about 25%) (Lundh 2017).
Unknown for‐profit funding: No information on clinical trial support or sponsorship was provided.
With for‐profit funding: The trial is sponsored by the industry or received any other type of for‐profit support.
Data and analyses
Comparison 1. Plasma expanders versus no plasma expander.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.06, 4.83] |
| 1.1 Albumin | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.75, 2.17] |
| 1.2 Ascites infusion | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 1.13] |
| 2 Serious adverse events | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Albumin | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Infusion of ascites | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Renal impairment | 4 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.02, 5.88] |
| 4 Other liver‐related complications | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.79, 3.27] |
| 4.1 Albumin | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.76, 3.41] |
| 4.2 Infusion of ascitic fluid | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.17, 14.53] |
| 5 Non‐serious adverse events | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.32, 3.40] |
| 5.1 Albumin | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.32, 3.40] |
| 6 Recurrence of ascites | 2 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.49, 3.42] |
| 6.1 Albumin | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.43, 1.99] |
| 6.2 Ascites infusion | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.61, 11.25] |
| 7 Hyponatraemia | 4 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.65] |
Comparison 2. Subgroup analysis of plasma expanders versus no plasma expander regarding modality of paracentesis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Participants with partial paracentesis | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.75, 2.17] |
| 1.2 Participants with total paracentesis | 3 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 1.13] |
| 2 Renal impairment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Participants with partial paracentesis | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.16] |
| 2.2 Participants with total paracentesis | 3 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.70] |
| 3 Other liver‐related complications | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Participants with partial paracentesis | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.57, 4.66] |
| 3.2 Participants with total paracentesis | 3 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.18] |
| 4 Non‐serious adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Participants with partial paracentesis | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.22, 4.82] |
| 4.2 Participants with total paracentesis | 2 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.70] |
| 5 Hyponatraemia | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Participants with partial paracentesis | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.04, 0.80] |
| 5.2 Participants with total paracentesis | 3 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.21, 21.27] |
Comparison 3. Subgroup analysis of plasma expanders versus no plasma expander regarding duration of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to 1 month | 3 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 1.13] |
| 1.2 More than 1 month | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.75, 2.17] |
| 2 Renal impairment | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.02, 5.88] |
| 2.1 Up to 1 month | 2 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.70] |
| 2.2 More than 1 month | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.16] |
| 3 Other liver‐related complications | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.79, 3.27] |
| 3.1 Up to 1 month | 3 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.18] |
| 3.2 More than 1 month | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.57, 4.66] |
| 4 Non‐serious adverse events | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.32, 3.40] |
| 4.1 Up to 1 month | 2 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.70] |
| 4.2 More than 1 month | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.22, 4.82] |
| 5 Hyponatraemia | 4 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.65] |
| 5.1 Up to 1 month | 3 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.21, 21.27] |
| 5.2 More than 1 month | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.04, 0.80] |
Comparison 4. Subgroup analysis of plasma expanders versus no plasma expander regarding funding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.06, 4.83] |
| 1.1 Trials without for‐profit funding | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.06, 4.83] |
| 1.2 Trials with for‐profit funding or unknown | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Renal impairment | 4 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.02, 5.88] |
| 2.1 Trials without for‐profit funding | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.16] |
| 2.2 Trials with for‐profit funding or unknown | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.70] |
| 3 Other liver‐related complications | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.79, 3.27] |
| 3.1 Trials without for‐profit funding | 3 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.63, 4.19] |
| 3.2 Trials with for‐profit funding or unknown | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.54, 4.67] |
| 4 Non‐serious adverse events | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.32, 3.40] |
| 4.1 Trials without for‐profit funding | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.22, 4.82] |
| 4.2 Trials with for‐profit funding or unknown | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.70] |
| 5 Hyponatraemia | 4 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 5.65] |
| 5.1 Trials without for‐profit funding | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.04, 0.80] |
| 5.2 Trials with for‐profit funding or unknown | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.21, 21.27] |
Comparison 5. Plasma expanders versus no plasma expander: best‐worst case scenario analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.06, 3.76] |
Comparison 6. Plasma expanders versus no plasma expander: worst‐best case scenario analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.28, 2.76] |
Comparison 7. Experimental plasma expanders versus albumin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 1014 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.82, 1.30] |
| 1.1 Dextran | 3 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.79, 2.06] |
| 1.2 Hydroxyethyl starch | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.45, 3.02] |
| 1.3 Polygeline | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.67, 1.89] |
| 1.4 Infusion of ascites | 4 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.31] |
| 1.5 Mannitolum | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.47, 1.66] |
| 1.6 Crystalloids | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.07, 16.26] |
| 2 Serious adverse events | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.10, 8.30] |
| 3 Renal impairment | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.71, 1.91] |
| 3.1 Dextran | 5 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.35, 2.08] |
| 3.2 Hydroxylethylstarch | 3 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.06, 15.90] |
| 3.3 Polygeline | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.70, 3.38] |
| 3.4 Infusion of ascites | 4 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.22, 3.62] |
| 3.5 Mannitolum | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Crystalloids | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.28, 8.93] |
| 4 Other liver‐related complications | 16 | 1083 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.48] |
| 4.1 Dextran | 5 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.98, 2.28] |
| 4.2 Hydroxyethyl starch | 3 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.69, 1.85] |
| 4.3 Polygeline | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.43, 1.93] |
| 4.4 Infusion of ascites | 3 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.73] |
| 4.5 Mannitolum | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.61, 3.44] |
| 4.6 Crystalloids | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.16, 7.10] |
| 5 Non‐serious adverse events | 14 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.66, 2.85] |
| 5.1 Dextran | 3 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.08] |
| 5.2 Hydroxyethyl starch | 3 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.66, 7.71] |
| 5.3 Polygeline | 3 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.15, 5.47] |
| 5.4 Infusion of ascites | 4 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.41, 5.71] |
| 5.5 Mannitolum | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Crystalloids | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Recurrence of ascites | 12 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.96, 1.36] |
| 6.1 Dextran | 3 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.53, 1.37] |
| 6.2 Hydroxyethyl starch | 3 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.03, 2.15] |
| 6.3 Polygeline | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.69, 1.54] |
| 6.4 Infusion of ascites | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.46, 2.34] |
| 6.5 Mannitolum | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.26, 3.07] |
| 6.6 Crystalloids | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.57, 2.39] |
| 7 Hyponatraemia | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.03, 2.14] |
| 7.1 Dextran | 5 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.81, 2.58] |
| 7.2 Hydroxyethil starch | 3 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.40, 8.69] |
| 7.3 Polygeline | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.83, 2.46] |
| 7.4 Infusion of ascites | 4 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.15, 6.93] |
| 7.5 Mannitolum | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 Crystalloids | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [0.55, 12.75] |
| 8 Post‐paracentesis circulatory dysfunction | 3 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.31, 2.99] |
| 8.1 Dextran | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.04, 3.81] |
| 8.2 Hydroxylethyl starch | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.14, 3.07] |
| 8.3 Polygeline | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.03, 4.10] |
| 8.4 Crystalloids | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 2.92 [1.03, 8.26] |
Comparison 8. Subgroup analysis of experimental plasma expanders versus albumin regarding presence or absence of refractory ascites.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 1014 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 1.1 Trials with participants without refractory ascites | 9 | 723 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.80, 1.66] |
| 1.2 Trials with participants with refractory ascites | 5 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.75, 1.37] |
| 2 Renal impairment | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.71, 1.92] |
| 2.1 Trials with participants without refractory ascites | 12 | 816 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.70, 2.20] |
| 2.2 Trials with participants with refractory ascites | 5 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.37, 2.65] |
| 3 Other liver‐related complications | 15 | 1033 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.48] |
| 3.1 Trials with participants without refractory ascites | 11 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.03, 1.80] |
| 3.2 Trials with participants with refractory ascites | 4 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.21] |
| 4 Non‐serious adverse events | 14 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.66, 2.85] |
| 4.1 Trials with participants without refractory ascites | 9 | 686 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.49, 4.36] |
| 4.2 Trials with participants with refractory ascites | 5 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.49, 3.47] |
| 5 Recurrence of ascites | 12 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.97, 1.34] |
| 5.1 Trials with participants without refractory ascites | 9 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.90, 1.35] |
| 5.2 Trials with participants with refractory ascites | 4 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.86, 1.66] |
| 6 Hyponatraemia | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.03, 2.14] |
| 6.1 Trials with participants without refractory ascites | 12 | 816 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.03, 2.27] |
| 6.2 Trials with participants with refractory ascites | 5 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.51, 3.26] |
Comparison 9. Subgroup analysis of experimental plasma expanders versus albumin regarding modality of paracentesis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 1014 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 1.1 Participants with partial paracentesis | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.59, 1.65] |
| 1.2 Participants with total paracentesis | 12 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.84, 1.41] |
| 2 Renal impairment | 16 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.82] |
| 2.1 Participants with partial paracentesis | 3 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.07, 15.68] |
| 2.2 Participants with total paracentesis | 13 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.59, 1.84] |
| 3 Other liver‐related complications | 15 | 1033 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.48] |
| 3.1 Participants with partial paracentesis | 3 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.76, 3.11] |
| 3.2 Participants with total paracentesis | 12 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.42] |
| 4 Non‐serious adverse events | 14 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.66, 2.85] |
| 4.1 Participants with partial paracentesis | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 8.5 [0.46, 157.71] |
| 4.2 Participants with total paracentesis | 12 | 879 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.57, 2.58] |
| 5 Recurrences of ascites | 12 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.32] |
| 5.1 Participants with partial paracentesis | 3 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.72, 2.26] |
| 5.2 Participants with total paracentesis | 9 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.91, 1.30] |
| 6 Hyponatraemia | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.03, 2.14] |
| 6.1 Participants with partial paracentesis | 3 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.29, 3.51] |
| 6.2 Participants with total paracentesis | 14 | 938 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.05, 2.26] |
Comparison 10. Subgroup analysis of experimental plasma expanders versus albumin regarding duration of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 1014 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.82, 1.30] |
| 1.1 Up to 1 month | 5 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.47, 5.33] |
| 1.2 More than 1 month | 9 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.80, 1.29] |
| 2 Serious adverse events | 2 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.12, 6.50] |
| 2.1 Up to 1 month | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 2.2 More than 1 month | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.10, 56.53] |
| 3 Renal impairment | 17 | 1107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.70, 1.83] |
| 3.1 Up to 1 month | 8 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.56, 2.25] |
| 3.2 More than 1 month | 9 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.58, 2.22] |
| 4 Other liver‐related complications | 15 | 1033 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.79, 1.48] |
| 4.1 Up to 1 month | 7 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.16] |
| 4.2 More than 1 month | 8 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.65, 1.53] |
| 5 Non‐serious adverse events | 14 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.67, 2.63] |
| 5.1 Up to 1 month | 6 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.73, 6.59] |
| 5.2 More than 1 month | 8 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.41, 2.32] |
| 6 Recurrence of ascites | 12 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.32] |
| 6.1 Up to 1 month | 3 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.12, 3.83] |
| 6.2 More than 1 month | 9 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.24] |
| 7 Hyponatraemia | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.03, 2.14] |
| 7.1 Up to 1 month | 8 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.01, 2.68] |
| 7.2 More than 1 month | 9 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.76, 2.28] |
Comparison 11. Subgroup analysis of experimental plasma expanders versus albumin regarding funding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Trials without for‐profit funding | 6 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.79, 1.81] |
| 1.2 Trials with for‐profit funding or unknown | 8 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.28] |
| 2 Renal impairment | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.71, 1.92] |
| 2.1 Trials without for‐profit funding | 6 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.72, 2.97] |
| 2.2 Trials with for‐profit funding or unknown | 11 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.48, 1.90] |
| 3 Other liver‐related complications | 16 | 1083 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.97, 1.56] |
| 3.1 Trials without for‐profit funding | 6 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.03, 1.97] |
| 3.2 Trials with for‐profit funding or unknown | 10 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.69, 1.50] |
| 4 Non‐serious adverse events | 14 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.66, 2.85] |
| 4.1 Trials without for‐profit funding | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.12, 3.05] |
| 4.2 Trials with for‐profit funding or unknown | 10 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.75, 3.85] |
| 5 Recurrence of ascites | 12 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.96, 1.36] |
| 5.1 Trials without for‐profit funding | 5 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.96, 1.42] |
| 5.2 Trials with for‐profit funding or unknown | 7 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.79, 1.60] |
| 6 Hyponatraemia | 17 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.03, 2.14] |
| 6.1 Trials without for‐profit funding | 6 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.79, 2.77] |
| 6.2 Trials with for‐profit funding or unknown | 11 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.95, 2.34] |
| 7 Post‐paracentesis circulatory dysfunction | 3 | 432 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [1.12, 3.32] |
| 7.1 Trials without for‐profit funding | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.37, 6.51] |
| 7.2 Trials with for‐profit funding or unknown | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.26, 3.24] |
Comparison 12. Experimental plasma expanders versus albumin ‐ best‐worst case scenario.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 1016 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.04, 1.60] |
Comparison 13. Experimental plasma expanders versus albumin ‐ worst‐best case scenario.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 1016 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.03] |
Comparison 14. Intravenous infusion of ascites versus polygeline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other liver‐related complications | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.14, 65.90] |
| 2 Non‐serious adverse events | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.0 [0.97, 231.84] |
| 3 Recurrence of ascites | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.18] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Descos 1983.
| Methods | RCT comparing paracentesis with reinfusion of ascitic fluid (concentrated or unmodified) and simple paracentesis in patients with liver cirrhosis | |
| Participants |
Inclusion criteria: patients with alcoholic or cryptogenetic cirrhosis with ascites confirmed by abdominal paracentesis and estimated to be equal to or greater than 5 litres Exclusion criteria: age under 20; impossibility of follow‐up; the need of emergency treatment for ascites; two recent gastrointestinal haemorrhages; haemorrhagic ascites; chronic hepatic encephalopathy and/or severe alcoholic hepatitis and/or tuberculosis and/or HCC, or the following complications persisting after three weeks (temporary exclusion criteria): active gastrointestinal haemorrhage, acute diarrhoea, hepatic encephalopathy, fever > 38 °C, infected ascites, blood urea > 8 mmol/L (0.050 g%), natraemia < 130 mmol/L, kaliaemia < 2.5 mmol/L or > 5.5 mmol/L, bilirubinaemia > 80 µmol/L or WBC > 12.000/µL We reported baseline data of the participants of the last 3 groups analysing the effect of different plasma expansion. Experimental group A (group 4 in the RCT) ‐ paracentesis with reinfusion of concentrated ascites: 36 participants Experimental group B (group 5 in the RCT) ‐ paracentesis with reinfusion of unmodified ascites: 23 participants Control group (group 6 in the RCT) ‐ simple paracentesis: 31 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 57, 56.7, and 57.3. Male (%): 52.7, 56.5, and 74.2. Alcohol consumption (g/d): 158, 170, and 199. Oedema (%): 66.6, 73.9, 87. Bilirubin (µmol/L): 46.8, 32.3 and 45.8. Albuminemia (g/L): 27.5, 28.1, and 27.0. Quick’s time (%): 50.7, 62, and 53. Ascites protein (g/L): 16.0, 15.1, and 14.8 |
|
| Interventions | All participants were submitted to a 4‐7 day period with low sodium diet (500 mg p/d) and water restriction (1 L/day). Group 1: sodium restriction (500 mg p/d) with spironolactone (maximal dose 400 mg p/d) Group 2: sodium restriction (500 mg p/d) with two diuretics (spironolactone, maximal dose 400 mg p/d, and furosemide, maximal dose 160 mg p/d, or amiloride and hydrochlorothiazide, maximal dose 20 mg and 200 mg p/d). Potassium chloride was associated according to kalaemia. Group 3: no sodium restriction and two diuretics (spironolactone, maximal dose 400 mg p/d, and furosemide, maximal dose 160 mg p/d, or amiloride and hydrochlorothiazide, maximal dose 20 mg and 200 mg p/d) Experimental group A ‐ Group 4 in the RCT (36 participants): sodium restriction (500 mg p/d) plus paracentesis with reinfusion of concentrated ascites. No diuretic treatment was used. If serum Na < 130‐135 mmol/L, a hypertonic solution of sodium was given. Experimental group B ‐ Group 5 in the RCT (23 participants): sodium restriction (500 mg p/d) plus paracentesis with reinfusion of unmodified ascites, over a period of eight hours. If shivering occurred, cortisone and diazepam with or without antibiotics were allowed. Furosemide 40 mg iv could be given, after half an hour, to increase diuresis. Control group ‐ Group 6 in the RCT (31 participants): sodium restriction (500 mg p/d) plus simple paracentesis (at a rate of 500 mL/h) until no further drainage occurred We analysed the last three groups, assessing the effect of different plasma expansion. |
|
| Outcomes | Results were assessed on discharge, or, in the case of hospitalisation of less than one month, one month after the beginning of treatment. At the time of discharge, the following clinical results of treatment were evaluated: ‐ weight loss; ‐ time in hospital; ‐ duration of treatment; ‐ ascites regression (total or partial); ‐ mortality; ‐ complications of cirrhosis; ‐ mechanical accidents; ‐ biochemical accidents. |
|
| Notes | During the therapeutic observation period, clinical and biochemical data were recorded twice a week. After mechanical drainage, the participant was kept under observation for 5 days. Further treatment and follow‐up (inpatient or outpatient basis) depended on clinical status. In the case of recurrence of ascites within 7 days, diuretics were given as in group 2; when this treatment proved inefficacious, a second mechanical drainage was performed. Mean time spent in hospital (days): 26.6 in experimental group A, 32.5 in experimental group B and 44.9 in control group In the results section, the number of participants with renal impairment or hyponatraemia for each group was unknown. Without for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method was not specified. The authors wrote that they used a table of random numbers, but it was not clear whether it was to generate the random sequence or to allocate participants. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report refractory ascites, but the trial was published before a formal definition of this condition. Other outcomes were reported. |
| Other bias | Low risk | No other bias identified |
Ginès 1988.
| Methods | Randomised controlled trial (RCT) of partial paracentesis with and without intravenous albumin in cirrhosis | |
| Participants | One hundred and five cirrhotic patients with tense ascites Exclusion criteria: HCC; hepatic encephalopathy, gastrointestinal bleeding or infection at entry; serum bilirubin > 10 mg/dL; prothrombin time < 40%; platelet < 40.000/µL; serum creatinine > 3 mg/dL; urinary sodium excretion > 10 mEq/day Before admission to the hospital, 72 participants were being treated with diuretics: 28 with spironolactone (50‐400 mg/day), 4 with triamterene (100‐300 mg/day) and 40 with furosemide (40‐160 mg/day) plus spironolactone (100‐400 mg/day). Experimental group – paracentesis and intravenous albumin infusion: 52 participants Control group – paracentesis without intervention: 53 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 56 and 58. Males (%): 61 and 73. Alcoholic cirrhosis (%): 63 and 49. Previous encephalopathy (%): 23 and 13. Previous gastrointestinal bleeding (%): 25 and 19. Previous episodes of ascites (%): 60 and 57. Refractory ascites: unknown. Peripheral oedema (%): 60 and 64. Pleural effusion (%): 8 and 13. Renal failure (%): 13 and 15. Serum bilirubin (mg/dL): 2.9 and 2.5. Prothrombin (%): 61 and 62. Serum albumin (g/L): 29 and 29; BUN (mg/dL) 18.5 and 18.7. Serum creatinine (mg/dL): 1.0 and 1.0. Serum sodium (mEq/L): 133 and 133. Urine sodium (mEq/day): 3.4 and 3.1. Mean arterial pressure (MAP) (mmHg): 85 and 85. PRA (only in 24 participants from each group, ng/mL/h): 6.2 and 5.4. PAC (only in 24 participants from each group, ng/dL): 98 and 81 |
|
| Interventions | After admission, diuretic treatment was withdrawn and participants were given a diet containing 50 mEq/day of sodium. In participants with serum sodium < 130 mEq/L, water ingestion was restricted to 500 mL/day. No NSAIDs and nephrotoxic agent within the 2 months before the study Experimental group: daily paracentesis (4‐6 L) until disappearance of ascites and intravenous albumin infusion (40 g) after each tap Control group: daily paracentesis (4‐6 L) until disappearance of ascites without intervention Volume of ascites removed, mean: 11.9 L in experimental group and 11.9 L in control group Number of paracentesis procedures performed per participant, mean: 2.8 in experimental group and 2.9 in control group Duration of hospitalisation, mean: 10.9 days in experimental group and 12 days in control group After ascites disappeared, participants were discharged from the hospital with diuretics (spironolactone, furosemide or both). |
|
| Outcomes | The authors did not specify in the methods the outcomes to be assessed. They reported data on: ‐ deaths during hospitalisation and follow‐up; ‐ occurrence of tense ascites during follow‐up; ‐ development of complications, renal impairment, and hyponatraemia during hospitalisation and during follow‐up; ‐ changes in plasma renin activity and aldosterone concentration. They did not report serious adverse events and refractory ascites incidence. |
|
| Notes | Randomisation independent in each hospital. Participants with and without renal failure were randomised separately. Plasma renin activity (PRA) and plasma aldosterone (PA) were measured in 24 participants from each group the day before and 48 h after completion of paracentesis treatment and were repeated 5 days after in 9 participants from control group. Participants developing tense ascites during follow‐up were readmitted to the hospital and treating according to their initial schedule. Renal impairment: ≥ 50% increase in serum creatinine or BUN, or both, to a level > 1.5 mg/dL and 30 mg/dL, respectively, after treatment Hyponatremia: decrease in serum sodium > 5 mEq/L to a level < 130 mEq/L after treatment or a decrease in serum sodium > 5 mEq/L in participants with a serum sodium concentration < 130 mEq/L before treatment Lost to follow‐up: 1 participant in experimental group (10 weeks after discharge from the hospital) and 3 participants in control group (21 and 40 weeks after discharge) Mean follow‐up period after discharge: experimental group, 29.1 weeks; control group, 34.4 weeks 10 participants from experimental group and 11 participants from control group required readmission for tense ascites. Without for‐profit funding No sample size calculation Letter sent on 2001. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events and refractory ascites, but the trial was published before a formal definition of these conditions. Other outcomes were reported. |
| Other bias | Low risk | No other bias identified |
Planas 1990.
| Methods | RCT comparing dextran‐70 versus albumin as plasma expander in cirrhotic patients with tense ascites treated with total paracentesis | |
| Participants | Eighty‐eight patients with cirrhosis and tense ascites Exclusion criteria: HCC; hepatic encephalopathy, gastrointestinal bleeding, infection at entry; serum bilirubin > 10 mg/dL; prothrombin time < 40%; platelet count < 40.000/mmc; serum creatinine > 3 mg/dL; urinary sodium excretion rate > 10 mEq/day Experimental group – total paracentesis and iv dextran‐70 infusion: 45 participants Control group – total paracentesis and iv albumin infusion: 43 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 59 and 59. Males (%): 67 and 58. Alcoholic cirrhosis (%): 71 and 63. Previous episodes of ascites (%): 64 and 58. Previous hepatic encephalopathy (%): 18 and 16. Previous episodes of gastrointestinal haemorrhage (%): 20 and 16. Peripheral oedema (%): 67 and 65. Renal failure (%): 15 and 21. BUN (mg/dL): 16 and 21. Serum creatinine (mg/dL): 0.9 and 1.1. Serum albumin (g/L): 27.6 and 27.9. Serum bilirubin (mg/dL): 2.9 and 3.5. Prothrombin (%): 52 and 55. Serum sodium (mEq/L): 130 and 130. Urine sodium (mL/day): 4 and 3. MAP (mmHg): 84 and 84. PRA (ng/mL/h): 10.5 and 9.3. PAC (ng/dL): 110 and 98 |
|
| Interventions | After admission, participants were given a diet containing 50 mEq/day of sodium. In participants with serum sodium < 130 mEq/L, liquid ingestion was restricted to 500 mL/day. Experimental group: total paracentesis and iv dextran‐70 infusion, 8 g/L of ascites removed (macrodex 6%, containing 6 g of dextran‐70 per 100 mL of dextrose solution). Control group: total paracentesis and iv infusion of albumin, 8 g/L of ascitic fluid removed Fifty percent of the dextran‐70 and albumin were infused immediately after paracentesis and the remaining 50% 6 hours later. Mean volume of ascites removed: experimental group, 9.4 L (range 3‐18.5); control group, 9.5 L (range 3‐17) Mean duration of paracentesis: experimental group, 48 min; control group, 51 min Duration of hospitalisation: experimental group, 9.9 days; control group, 9.4 days |
|
| Outcomes | The authors did not specify in the methods the outcomes to be assessed. They reported data on: ‐ deaths; ‐ complications during the first hospital stay: renal impairment (≥ 50% increase in serum creatinine or BUN or both to > 1.5 mg/dL and > 30 mg/dL, respectively, after treatment); hyponatraemia (decrease in serum sodium > 5 mEq/L to < 130 mEq/L or, for participants with serum concentration < 130 mEq/L before treatment, a decrease > 5 mEq/L during hospitalisation); encephalopathy; GI bleeding; severe infection; ‐ changes of standard liver function tests, renal function tests, mean arterial pressure, PRA, aldosterone at 48 hours and 6 days after paracentesis; ‐ probability of requiring readmission to hospital during follow‐up. |
|
| Notes | Participants with and without renal failure (renal failure was considered present when creatinine > 1.5 mg/dL) were randomised separately to ensure a similar number of cases with renal failure in both therapeutic groups. After treatment, participants remained in the hospital for 6 days without diuretics. Participants were discharged with diuretics (40 mg/day of furosemide and 200 mg/day of spironolactone in participants without renal failure; 80 mg/day of furosemide and 300 mg/day of spironolactone in participants with renal failure). Participants in whom tense ascites was developing during follow‐up were readmitted to the hospital and treated according to the initial schedule. One participant from each group was transplanted (15 and 2 weeks after inclusion; censored participants). Lost to follow‐up: 1 participant from experimental group (2 weeks after inclusion) Mean follow‐up period: 23.7 weeks from experimental group and 27.5 weeks from control group Thirty participants from experimental group and 24 from control group were readmitted during follow‐up. Mean volume of ascites removed: 9.6 L from experimental group and 9.8 L from control group Without for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events and refractory ascites, but the trial was published before a formal definition of these conditions. Other outcomes were reported. |
| Other bias | Low risk | No other bias identified |
Smart 1990.
| Methods | RCT comparing recirculation with partial paracentesis and iv albumin in refractory ascites | |
| Participants |
Inclusion criteria: forty patients with ascites due to portal hypertension and refractory to diuretic therapy. Refractory ascites was defined as failure to respond to fluid restriction (1500 mL/24 h), sodium restriction (50 mmol/24 h) and a minimum combination of furosemide 80 mg and spironolactone 200 mg daily. Patients were also included if this or lesser therapy was associated with significant hyponatraemia (serum Na < 130 mmol/L), a rising creatinine or the precipitation of encephalopathy. Exclusion criteria: liver cancer; within 2 weeks oesophageal variceal haemorrhage; ascitic infection (PMN > 250/mm3); severe hepatic encephalopathy. Experimental group ‐ total paracentesis and ascites recirculation: 20 participants. Control group ‐ repeated daily paracentesis of 3‐4 L and intravenous albumin: 20 participants. Baseline characteristics (values shown are mean where appropriate) Age (yr): 56 and 61. Males (%): 50 and 65. Alcoholic cirrhosis (%): 45 and 45. Peripheral oedema (%): 70 and 85. Renal impairment (%): 60 and 55. Child‐Pugh C (%): 60 and 70. Previous treatment for refractory ascites (%): 15 and 20. Previous diuretic complications (%): 60 and 65. Furosemide dose (mg/day): 60 and 60. Spironolactone dose (mg/day): 194 and 178. Serum urea (mmol/L): 9.0 and 10.7. Serum creatinine (µmol/L): 127 and 148. Serum bilirubin (µmol/L): 49 and 66. Serum albumin (g/L): 31 and 26. Serum sodium (mmol/L): 132 and 128. Serum potassium (mmol/L): 4.3 and 4.1 |
|
| Interventions | Experimental group: total paracentesis and intravenous infusion of filtrated and concentrated ascites (using Rhodiascit apparatus) at a rate of 250 mL/h approximately Control group: daily partial paracentesis (maximum 4 L) and intravenous infusion of 40 g of human albumin, given as 200 mL of salt‐poor albumin (total sodium content 28 mmol), over half an hour A mean of 6 L of waste resulting in a mean weight loss of 6.7 kg was observed in experimental group. Mean number of paracentesis procedures performed overall was 5, which removed a mean volume of ascites of 13.3 L with a resulting weight loss of 10.3 kg, in control group. |
|
| Outcomes | The authors did not specify in the methods the outcomes to be assessed. They reported data on: ‐ deaths; ‐ recurrence of ascites; ‐ complications; ‐ hospital stay length; ‐ costs. |
|
| Notes | Randomisation was stratified according to serum creatinine. Following the procedure, participants continued on diuretics and, if no fluid recurrence occurred, were discharged to be followed as outpatients. For the discharged participants (excluding those transplanted), the mean duration of hospital stay was 7 days in the experimental group and 11 days in the control group. Lost to follow‐up: one participant in each group The median follow‐up was 31 (max. 96) weeks in the ascites recirculation group and 35 (max 109) weeks in the paracentesis group. With for‐profit funding ("We would like to acknowledge the support of Armour Pharmaceutical Co Ltd. who supplied the 20% albumin solution used in this study") No sample size calculation Letter sent on Feb 2013. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method was not specified |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events and refractory ascites, but the trial was published before a formal definition of these conditions. Other outcomes were reported. |
| Other bias | Low risk | No other bias identified |
Salerno 1991.
| Methods | RCT comparing polygelin with albumin as plasma expander after total paracentesis in cirrhotic patients with refractory ascites | |
| Participants | Fifty‐four cirrhotic patients with refractory massive ascites (34 patients with diuretic‐resistant ascites and 20 with recurrent ascites). Inclusion criteria: Absolute unresponsiveness to diuretic drugs, defined as progressive increase of body weight and abdominal girth and positive sodium balance while the patient was on a low sodium diet and on the maximal‐tolerated doses of diuretics. Diuretic drugs were given stepwise with increasing doses up to 600 mg of spironolactone and 150 mg of furosemide, except for 16 patients for whom the administration was stopped al lower dosages (200‐300 mg of spironolactone and 25‐100 mg of furosemide) because of the rise of creatinine (> 2.5 mg/dL), the fall of plasma sodium (< 120 mEq/L) or both. Frequent recurrence of massive ascites (at least three episodes during the previous 9 months) in patients taking high doses of diuretic drugs (at least 300 mg of spironolactone plus 50 mg of furosemide) Exclusion criteria: Heart failure; primary kidney disease; infection; active gastrointestinal bleeding or severe encephalopathy Thirteen participants with renal failure (creatinine > 1.2 mg/dL and BUN > 25 mg/dL) and 11 participants with HCC were included. Experimental group – paracentesis and polygeline: 27 participants Control group– paracentesis and albumin: 27 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 53.2 and 55.9. Males (%): 74 and 63. Alcoholic cirrhosis (%): 48 and 44. Diuretic resistant ascites (%): 59 and 67. Renal impairment (%): 26 and 22. HCC (%): 22 and 18. Peripheral oedema (%): 37 and 52. Serum albumin (g/L): 30 and 33. Serum bilirubin (mg/dL): 4.6 and 3.9. Prothrombin time (ratio to normal): 1.4 and 1.4. BUN (mg/dL): 20 and 22.1. Serum creatinine (mg/dL): 0.98 and 1.26. Plasma sodium (mmol/L): 130.6 and 131.1. Urine sodium (mmol/day): 20.5 and 16.4. PRA (ng/mL/hr): 11.9 and 13.2. PAC (pg/mL): 801 and 1186. ANF (pg/mL): 30.1 and 37.1. MAP (mmHg): 90.3 and 92.3 |
|
| Interventions | During hospitalisation, participants were on a low‐sodium diet (40 mmol/day), with free access to water (except for participants with plasma sodium < 130 mEq/L, for whom water intake was restricted to 500 mL/day). Diuretics were discontinued at least 2 days before paracentesis for participants with diuretic‐resistant ascites but not for participants with relapsing ascites, who received 200 mg of spironolactone plus 25 mg of furosemide per day. Experimental group: total paracentesis (one session, large volume paracentesis) and iv infusion of haemaccel (polygelin 3.5%), 150 mL/L of ascites evacuated Control group: total paracentesis (one session, large volume paracentesis) and iv infusion of 20% human albumin solution, 30 mL/L of ascites evacuated Infusion was started just at the end of the evacuation and was completed within 4 hr. Amount of ascitic fluid removed (mean): experimental group, 8.3 L; control group, 8.9 L Duration of hospital stay (mean): experimental group, 24.5 days; control group, 26.8 days |
|
| Outcomes | The authors did not plan in the protocol the assessment of any outcome. They reported data on: ‐ deaths during hospitalisation and follow‐up; ‐ occurrence of tense ascites during follow‐up; ‐ development of complications during hospitalisation: kidney impairment (a 50% increase in creatinine to a level > 1.2 mg/dL), hyponatraemia (decrease in sodium of at least 5 mmol/L to a level < 130 mmol/L), hyperkalaemia (increase in potassium of at least 1.5 mmol/L to a level > 5.5 mmol/L); ‐ liver disease‐related morbidity; ‐ changes in plasma renin activity and aldosterone concentration. |
|
| Notes | Separate randomisation for participants with and without renal impairment Participants were discharged from the hospital while on a low‐sodium diet and standard diuretics dose (200 mg of spironolactone and 50 mg of furosemide) and subsequently adjusted. Mean follow‐up: 89.3 weeks for experimental group and 93.6 weeks for control group No dropouts or withdrawals were reported. Cost for an average infusion after one‐session total paracentesis: $21 for experimental group and $305 for control group With for‐profit funding ("We are indebted to Dr. Learco Mottola, Istituto Behring SpA, for support"). No sample size calculation We emailed Salerno and colleagues on Feb 2013. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events, but the trial was published before a formal definition of this condition. Other outcomes were reported. |
| Other bias | Low risk | No other bias identified |
Bertrán 1991.
| Methods | RCT comparing dextran‐70 versus albumin as plasma expander in cirrhotic patients with tense ascites treated with total paracentesis | |
| Participants | Seventeen cirrhotic patients with tense ascites Exclusion criteria: not reported Experimental group – paracentesis with dextran‐70: 9 participants Control group – paracentesis with albumin: 8 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 54 and 61. Male (%): 77 and 38. Alcoholism (%): 89 and 62. Child‐Pugh class (A‐B‐C) (number of participants): 1‐2‐6 and 0‐5‐3 |
|
| Interventions | After admission, the participants received a diet containing 50 mEq of sodium/day for 5 days. No diuretics were administered. Experimental group: total paracentesis with iv infusion of dextran‐70, 8 g/L of ascitic fluid removed Control group: total paracentesis with iv infusion of albumin, 8 g/L of ascitic fluid removed Half dose of albumin and dextran‐70 were infused immediately after paracentesis and half dose 6‐8 hours later. Volume of removed ascites (mean): 9.6 L in experimental group and 9.5 L in control group |
|
| Outcomes | The authors planned to evaluate the nutritional status of cirrhotic participants before and 2 days after paracentesis, by measuring triceps skinfold thickness, mid‐arm muscle circumference, and serum albumin. In addition, in 10 participants (6 from the experimental group and 4 from the control group) serum albumin was measured after 1 month from paracentesis. The authors reported data on: ‐ complications; ‐ mortality during hospitalisation. |
|
| Notes | Unknown for‐profit funding No sample size calculation A letter was sent on 21 October 2015. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method was not specified |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | The trial authors did not report mortality, serious adverse events, and non‐serious adverse events. |
| Other bias | Low risk | No other bias identified |
Simonetti 1991.
| Methods | RCT comparing total paracentesis and concentrated ascitic fluid reinfusion with repeated paracentesis and iv albumin in the treatment of cirrhotic patients with refractory ascites | |
| Participants |
Inclusion criteria: 38 patients with tense refractory ascites, defined as failure to respond to low‐sodium diet (10 mEq/d), water restriction (500‐700 mL/d) and previous diuretic treatment (K‐canrenoato up to 600 mg/day and furosemide up to 100 mg/day) and/or with clinical or humoral contraindications to the use of diuretics (e.g. hypotension, hepatic encephalopathy, functional renal failure – BUN > 37 mg/dL and/or serum creatinine > 1.5 mg/dL, hyponatraemia ≤ 125 mEq/L) Exclusion criteria: bilirubin > 6 mg/dL; prothrombin time < 40 %, hepatic encephalopathy > I grade; upper digestive bleeding in the last 3 months; infection of ascites in the last 3 months; HCC; platelet count < 40.000/mm3; azotaemia > 120 mg/dL and/or serum creatinine > 3 mg/dL; haemorrhagic ascitic fluid; severe cardiac or respiratory failure Experimental group – total paracentesis with reinfusion of filtered and concentrated ascitic fluid (TPRA): 18 participants Control group – courses of paracentesis and iv albumin infusion (RP): 20 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 62.7 and 57. Males (%): 50 and 75. Alcohol cirrhosis (%): 13 and 11. HBsAg positive cirrhosis (%): 5 and 5. HCV‐related cirrhosis (%): 65 and 65. HCV/alcohol‐related cirrhosis (%): 17 and 19. Child‐Pugh B (%): 72 and 40. Child‐Pugh C (%): 28 and 60. BUN (mg/dL): 30 and 30. Serum creatinine (mg/dL): 1.4 and 1.5. Plasma sodium (mEq/L): 134 and 135. Urine sodium (mEq/day): 24 and 16. PRA (ng/mL/h): 18.4 and 18.7. Plasmatic aldosterone (pg/d): 1695 and 775 |
|
| Interventions | Diuretics were stopped for 6 days before randomisation. Experimental group: total paracentesis with iv infusion of filtered and concentrated ascitic fluid in a shorter time (2‐4 h) and with a smaller final volume of intravenous infused fluid (800‐1000 mL) than previously reported (with Rhodiascit) Control group: partial paracentesis (4 L/day) every other day followed by iv infusion of 40 g albumin after each tap, until disappearance of ascites Volume of removed ascites (mean, L/course): 10 in experimental group and 11 in control group |
|
| Outcomes | The authors reported data on: ‐ deaths after 6 months of follow‐up; ‐ complications after 6 months of follow‐up; ‐ recurrence of ascites; ‐ length of hospitalisation; ‐ cost of treatment; ‐ failure: more than 2 courses/month required or incomplete removal of ascites during a single course. |
|
| Notes | No dropouts In experimental group, 68 courses were performed in a mean time of 129 ± 59 days; in control group , 58 courses were performed in a mean time of 88 ± 66 days. The mean time before a new course became necessary was 39 ± 33 days in the experimental group and 26 ± 13 days in the control group. Mean hospitalisation time necessary for single course was 1 day in the experimental group and 5 ± 2.3 days in the control group. Failures were one for each group. The treatment was stopped in: ‐ 7 participants of experimental group for: increasing hyperazotaemia (4 participants), liver failure (1 participant), umbilical hernia leakage (1 participant), hypertensive gastrointestinal haemorrhage (1 participant); ‐ 15 participants of control group for: increasing hyperazotaemia (3 participants), hypertensive gastrointestinal haemorrhage (4 participants), spontaneous bacterial peritonitis (3 participants), liver failure (3 participants), poor compliance (2 participants). There were 6 deaths in the experimental group and 7 deaths in the control group. Cost for each course: 200.000 lire in the experimental group and 420.000 lire in the control group Without for‐profit funding Sample size calculated Lacking information was directly provided from the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table number |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not possible to exclude that the possibility that unblinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not possible to exclude that the possibility that unblinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events, but the trial was published before a formal definition of these conditions. Other outcomes were reported. |
| Other bias | Low risk | No other bias identified |
Méndez 1991.
| Methods | RCT comparing hydroxyethyl starch and albumin as plasma expander after total paracentesis in cirrhotic patients with refractory ascites. | |
| Participants | Twenty cirrhotic patients with refractory ascites Experimental group – paracentesis with hydroxyethyl starch 450: 10 participants Control group – paracentesis with iv albumin: 10 participants |
|
| Interventions | Experimental group: total paracentesis and hydroxyethyl starch 450 (HES), 7.7 g/L of ascitic fluid removed Control group: total paracentesis and iv infusion of albumin, 8 g/L of ascitic fluid removed Total paracentesis was performed at admission and 5 days later. |
|
| Outcomes | The authors reported data on: ‐ changes in serum sodium, urea, creatinine, potassium, aldosterone and MAP after two and/or five days of total paracentesis; ‐ recurrence of ascitic fluid; ‐ changes in body weight; ‐ complications. |
|
| Notes | Abstract Both groups showed significant changes in serum Na, urea and creatinine after 2 and/or 5 days of total paracentesis. No significant changes were observed in K, ARP and MAP. The increase in aldosterone reached statistical significance only in experimental group on day 5. Reaccumulation of ascites was lower in the control group than in the experimental group (70.2% vs 43.7% P < 0.01). The difference was less marked in cirrhotic participants without oedema (55% vs 40.9% P < 0.05). No complications were observed. Unknown for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data |
| Selective reporting (reporting bias) | High risk | The trial authors did not report most predefined outcomes (mortality, non‐serious adverse events, other liver‐related complications, renal impairment). |
| Other bias | Unclear risk | Not evaluable |
Bruno 1992.
| Methods | RCT comparing spontaneous ascites filtration and reinfusion with total paracentesis and iv albumin infusion in cirrhotic patients with tense ascites | |
| Participants | Thirty‐five cirrhotic patients with recurrent tense ascites and urinary excretion < 20 mmol/day. Inclusion criteria: liver cirrhosis with tense ascites and urinary sodium excretion rate lower than 20 mmol/day; serum bilirubin concentration < 85 µmol/L; serum creatinine concentration < 265 µmol/L; prothrombin time < 18 s; platelet count > 50 x 109/L. Exclusion criteria: HCC; severe hepatic encephalopathy (III and IV stage); gastrointestinal bleeding or infection during the previous month; spontaneous bacterial peritonitis; treatment with nonsteroidal anti‐inflammatory drugs during the previous month On the first day of admission to hospital, diuretic treatment was discontinued and the participants were put on a low sodium diet (20 mmol/day), with fluid intake restricted to 500 mL/day in participants with serum sodium levels < 130 mmol/L and to 1 L/day in the remaining participants. Experimental group – spontaneous ascites filtration and reinfusion: 17 participants Control group – total paracentesis plus iv albumin infusion: 18 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 52 and 55. Males (%): 70 and 55. Alcohol (%): 65 and 50. Hepatitis B antigen positive (%): 18 and 22. Child Pugh A (%): 6 and 0. Child‐Pugh B (%): 59 and 61. Child‐Pugh C (%): 35 and 39. Na < 130 mmol/L (%): 29 and 50. Serum sodium (mmol/L): 132 and 131. Serum potassium (mmol/L): 4.0 and 4.1. Urine sodium (mmol/day): 8 and 3. Urine potassium (mmol/day): 28 and 24. Body weight (kg): 71.9 and 68.4 |
|
| Interventions | Experimental group: spontaneous ascites filtration and reinfusion (as described by Landini 1985) Control group: total paracentesis with iv albumin infusion, 4‐6 g/L of removed ascites Mean time needed to perform the procedure: 5 hours in the experimental group and 2 hours in the control group Mean volume of ascites removed: 7.0 L in the experimental group and 6.4 L in the control group |
|
| Outcomes | The authors reported data also on: ‐ haemodynamic, humoral and hormonal changes at 24 h and at 8 days; ‐ deaths during hospitalisation; ‐ development of complications during hospitalisation; ‐ treatment failure: increase in body weight of more than 50% of the weight loss after paracentesis; ‐ no resolution of ascites. |
|
| Notes | Baseline determinations were repeated on the eight day after treatment to assess the efficacy of both procedures. Time of observation: hospital stay Both procedures resulted in improvement of the subjective symptoms in all participants. Dilutional hyponatraemia occurred in one participant from each group. No participants dropped out from the study. Death: one participant control group (while in hospital 10 days after the procedure) Average cost per 10 L of ascites removed: $156 for spontaneous ascites filtration and reinfusion and $360 for total paracentesis with iv albumin infusion Unknown for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events, but the trial was published before a formal definition of this condition. Other outcomes were reported. |
| Other bias | Low risk | No other bias identified |
Fassio 1992.
| Methods | RCT comparing dextran‐70 with albumin as plasma expander after partial paracentesis in cirrhotic patients with tense ascites | |
| Participants | Forty‐one cirrhotic patients with tense ascites Exclusion criteria: liver cancer; gastrointestinal bleeding; hepatic encephalopathy; infection; serum urea > 60 mg/dL or serum creatinine > 1.5 mg/dL; prothrombin concentration < 40%; platelet count < 40.000/mm3; serum bilirubin > 10 mg/dL; urinary sodium concentration > 20 mEq/L Experimental group – paracentesis with dextran: 20 participants Control group – paracentesis with albumin: 21 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 54 and 54. Male (%): 70 and 81. Alcoholism (%): 75 and 90. Previous episodes of ascites (%): 65 and 52. Peripheral oedema (%): 70 and 62. Child‐Pugh score: 10.3 and 9.8. MAP (mmHg): 82 and 82. Serum bilirubin (mg/dL): 3.1 and 2.2. Prothrombin (%): 61 and 60. Serum albumin (g/dL): 2.6 and 2.7. Serum urea (mg/dL): 27 and 29. Serum creatinine (mg/dL): 1.0 and 1.0. Serum sodium (mEq/L): 132 and 132. PRA (measured in 15 participants from each group) (ng/mL/h): 6.9 and 7.7 |
|
| Interventions | After admission, the participants received a diet containing 50 mEq of sodium/day. Hyponatremic participants had water restriction. Diuretic treatment was stopped for at least 5 days before the paracentesis. Experimental group: daily paracentesis up to 5 L of ascitic fluid removed with iv infusion of dextran‐70 (6 g of dextran per 100 mL saline isotonic solution), 6 g for each litre of removed ascites Control group: daily paracentesis up to 5 L of ascitic fluid removed with iv infusion of albumin (20% solution), 6 g for each L of removed ascites Volume of removed ascites (mean): 12.9 L in the experimental group and 10.9 L in the control group |
|
| Outcomes | The authors did not plan in a protocol the assessment of any outcome. They reported data on: ‐ deaths during hospitalisation and follow‐up; ‐ no resolution of ascites; ‐ occurrence of tense ascites during follow‐up; ‐ development of complications during hospitalisation; ‐ changes in plasma renin activity and aldosterone concentration at 96 h; ‐ haemodynamic and hormonal effects during first hospitalisation. |
|
| Notes | In 12 participants of each group, PRA was repeated 96 h after the last paracentesis. Participants were given diuretics on the fifth day after paracentesis to prevent recurrence of ascites. Participants were discharged from the hospital and followed closely in the outpatient clinic. Participants who developed tense ascites during the follow‐up were readmitted to the hospital and treated according to the initial schedule. Development of renal impairment: serum creatinine and serum urea increased > 50% above 1.5 mg/dL or above 40 mg/dL Hyponatraemia: serum sodium dropped more than 5 mEq below 130 mEq/L One participant from the experimental group and 2 participants from the control group were lost to follow‐up. Mean follow‐up: experimental group, 32.4 weeks; control group, 26.9 weeks Cost for each participant: $15.48 for dextran and $364.27 for albumin (1 g of dextran = 0.20 dollars and 1 g of albumin = 5.37 dollars) Without for‐profit funding No sample size calculation We emailed Fassio and colleagues on Feb 2013. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to make treatment effects depart from plausible values. |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events and refractory ascites, but the trial was published before a formal definition of these conditions. They did not report non‐serious adverse events. |
| Other bias | Low risk | No other bias identified |
García‐Compeán 1993.
| Methods | RCT comparing total therapeutic paracentesis (TTP) with and without iv albumin in the treatment of cirrhotic tense ascites | |
| Participants | 35 cirrhotic patients with tense ascites (ascites which causes respiratory dysfunction) and the following criteria: no HCC; no hepatic encephalopathy, gastrointestinal haemorrhage, either local or systemic infection; serum bilirubin < 10 mg/dL; prothrombin time < 5 s over the control; platelet count > 50.000/mm3; serum creatinine < 3 mg/dL and serum sodium > 125 mEq/L Experimental group – total therapeutic paracentesis (TTP) plus iv albumin infusion: 17 participants Control group – total therapeutic paracentesis (TTP) without intervention: 18 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 54.6 and 56.7. Males (%): 65 and 55. Alcoholic cirrhosis (%): 70 and 72. Child‐Pugh B (%): 41 and 50. Child‐Pugh C (%): 59 and 50. Previous hepatic encephalopathy (%): 35 and 28. Previous gastrointestinal bleeding (%): 59 and 44. Previous ascites and/or oedema (%): 65 and 67. Refractory ascites (%): 0 and 0. Peripheral oedema (%): 88 and 83. BUN (mg/dL): 20.1 and 18. Creatinine (mg/dL): 0.92 and 1.01. Albumin (g/dL): 1.97 and 2.2. Bilirubin (mg/dL): 2.3 and 1.4. Prothrombin time (s/control): 5.2 and 4.7. Serum sodium (mEq/L): 134 and 135. Urinary sodium (mEq/L): 29 and 34. MAP (mmHg): 82 and 83. PRA not reported. PAC not reported |
|
| Interventions | Participants discontinued diuretics 3 days before treatment. A diet containing less than 50 mEq of sodium per day was prescribed. Experimental group: total paracentesis and iv albumin infusion (a solution of 25% human albumin containing a sodium concentration of 30 mEq/L), 5 g per litre of the removed ascites, in 1 hour Control group: total paracentesis without intervention Mean volume of extracted fluid (L): experimental group, 8.4; control group, 8.2 Mean time taken for TTP (min): experimental group, 64; control group, 60 min. Mean dose of infused albumin (g): 40 |
|
| Outcomes | The authors reported data on: ‐ biochemical, haemodynamic and hormonal changes; ‐ complications attributed to total paracentesis; ‐ mortality; ‐ plasma renin activity (PRA) and plasma aldosterone (PA) 6, 12, and 24 hours after treatment. |
|
| Notes | There was no confirmation of the refractoriness of ascites by previous intensive treatment with diuretics in any of the cases. Renal impairment: an increase > 50% in serum creatinine or BUN, or both, to a level > 1.5 mg/dL and 30 mg/dL, respectively, after treatment Hyponatremia: a serum sodium level < 130 mEq/L or a decrease > 5 mEq/L after treatment. Hyperkaliemia: an increase > 5 mEq/L Follow‐up: 5 days of hospitalisation Unknown for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The trial authors did not report serious adverse events, but the trial was published before a formal definition of this condition. Trial with a short follow‐up. Other outcomes were reported. |
| Other bias | Low risk | Other bias not identified |
Luca 1995.
| Methods | RCT evaluating haemodynamic and humoral changes after total paracentesis (TTP) with and without iv albumin in cirrhotic patients with tense ascites | |
| Participants | 18 cirrhotic patients with tense ascites Inclusion criteria: serum bilirubin < 5 mg/dL; prothrombin time > 40%; platelet count > 40,000/mm3; serum creatinine < 3 mg/dL; absence of hepatoma, portal thrombosis, hepatic encephalopathy, infections, and haemorrhage from gastroesophageal varices in the last 2 weeks Experimental group – total paracentesis (TTP) plus iv albumin infusion: 9 participants Control group – total paracentesis (TTP) without intervention: 9 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 59 and 58. Males (%): 78 and 67. Child‐Pugh score: 10.6 and 10.2. Alcoholic cirrhosis (%): 33 and 44. Serum bilirubin (mg/dL): 2.4 and 4.0. Prothrombin activity (%): 58 and 57. Plasma albumin (g/L): 25 and 27. Previous variceal bleeding (%): 22 and 11. Peripheral oedema (%): 78 and 78. Urine sodium (mEq/day): 12 and 7. PRA (ng/mL/h): 20.4 and 11.8. Plasma aldosterone (ng/dL): 231 and 159. Atrial natriuretic factor (fmol/mL): 33.4 and 26.1. Cardiac index (Lˑm2/min): 3.8 and 4.6. MAP (mmHg): 80 and 82. Femoral blood flow (mLˑm2/min): 207 and 240. HVPG (mmHg): 20.8 and 19.7. Azygos blood flow (mL/min): 681 and 885 |
|
| Interventions | After admission, participants were put on a low‐sodium diet (40 mEq/d) and did not receive diuretics or vasoactive drugs. Experimental group: total paracentesis and iv albumin (a solution of 20% albumin; sodium concentration 30 mEq/L) infusion, 8 g per L of the removed ascites. 50% of albumin within 1 hour at a rate of about 170 mL/hr and the other 50% during the following 6 hours Control group: total paracentesis without intervention Paracentesis: 8.6 L in experimental group and 9.1 L in control group. Time of paracentesis: 69 min in experimental group and 59 min in control group 2 |
|
| Outcomes | The authors reported data on: ‐ haemodynamic (systemic and hepatic) and neurohumoral changes at 24 hours; ‐ adverse effects. The authors gave us data on our predefined outcomes. |
|
| Notes | After paracentesis, before randomisation, there were no differences in systemic and splanchnic haemodynamics, vasoactive neurohumoral systems, and plasma volume between groups. Without for‐profit funding No sample size calculation We emailed Luca and colleagues on Feb 2013. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The authors did not report in the article information on mortality, serious adverse events, renal impairment, other liver‐related complications and non‐serious adverse events because aim of the trial was to assess haemodynamic and neuro‐humoral changes 24 h after paracentesis. This information was obtained from the authors. |
| Other bias | Low risk | No other bias identified |
Hernández Pérez 1995.
| Methods | RCT comparing dextran‐70 with albumin as plasma expander after total paracentesis in cirrhotic patients with tense ascites | |
| Participants | 16 cirrhotic patients with tense ascites and: no hepatic encephalopathy, gastrointestinal bleeding, infection, liver cancer, severe renal failure (serum creatinine > 3 mg/dL), electrolytes alteration; serum bilirubin < 10 mg/dL; prothrombin time < 3.5 s above the limit; platelet count > 40.000/mm3. Experimental group – large volume paracentesis with dextran: 8 participants Control group – large volume paracentesis with albumin: 8 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 59.38 and 59.88. Male (%): 25 and 50. Alcholic cirrhosis (%): 50 and 62. MAP (mmHg): 86.50 and 86.38. Serum albumin (g/dL): 3.16 and 3.14. Prothrombin (%): 61.24 and 66.49. Serum bilirubin (mg/dL): 2.28 and 2.28. Serum creatinine (mg/dL): 0.91 and 0.71. Serum urea (mg/dL): 28.75 and 32.17. Serum sodium (mEq/L): 136.64 and 136.34. Plasma renin (ng/mL/h): 1.91 and 1.58. Plasma aldosterone (pg/mL): 372.95 and 178.79 |
|
| Interventions | Participants received a diet containing 1 g of sodium daily. The allowed oral fluid intake did not exceed 1 L/day; fluid restriction of 500 mL/day was carried out in participants with serum sodium < 130 mEq/L. Diuretics were not allowed. Experimental group: large volume paracentesis, > 5 L of ascitic fluid removed, with iv infusion of dextran‐70 (6 g for each litre of removed ascites) Control group: large volume paracentesis, > 5 L of ascitic fluid removed, with iv infusion of albumin (6 g for each litre of removed ascites) 50% of the albumin and dextran‐70 were infused during paracentesis and the remaining 50% 6 hours later. The participants were discharged from the hospital on the sixth day with spironolactone 200 mg/die. Volume of removed ascites, mean: in the experimental group, 6.13 L; in the control group, 6.88 L |
|
| Outcomes | The authors reported data on: ‐ biochemical and hormonal effects 48 h after treatment; ‐ recurrence of ascites; ‐ complications. |
|
| Notes | If ascites recurred after discharge, the participants were treated using the same plasma expander. Follow‐up: two weeks Cost for plasma expander was $20.8 USD in the experimental group and $266 USD in the control group. Unknown for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | The study authors did not report mortality. The trial authors did not report serious adverse events, but the trial was published before a formal definition of these conditions. |
| Other bias | Low risk | No other bias identified |
Ginès 1996.
| Methods | RCT comparing dextran 70, polygeline, and albumin in cirrhotic patients with ascites treated by total paracentesis | |
| Participants | Two hundred eighty‐nine patients with cirrhosis who were admitted for tense ascites Exclusion criteria: HCC; current hepatic encephalopathy or bacterial infection; respiratory, renal, cardiac disease; gastrointestinal haemorrhage within the preceding month; serum bilirubin > 10 mg/dL; prothrombin time < 40%; platelet count < 40,000/mm3; serum creatinine > 3 mg/dL; treatment with propranolol for prophylaxis of variceal bleeding or re‐bleeding Experimental group A (group dextran‐70 in the RCT) – total paracentesis and iv dextran‐70: 93 participants Experimental group B (group polygeline in the RCT) – total paracentesis and iv polygeline: 99 participants Control group (group albumin in the RCT) – total paracentesis and iv albumin: 97 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 57, 57, and 59. Males (%): 68, 71, and 69. Alcohol abuse (%): 68, 70, and 72. Child‐Pugh score: 10, 10, and 10. Previous encephalopathy (%): 16, 18, and 13. Previous gastrointestinal bleeding (%): 20, 13, and 21. Previous ascites (%): 68, 60, and 66. Refractory ascites (%): unknown. Peripheral oedema (%): 60, 62, and 65. Renal impairment (creatinine > 1.5 mg/dL) (%): 16, 17, and 19. Serum bilirubin (mg/dL): 2.8, 2.7, and 2.8. Prothrombin time (%): 58, 61, and 58. Serum albumin (g/dL): 2.6, 2.6, and 2.7. Serum creatinine (mg/dL): 0.9, 1.0, and 0.9. Serum sodium (mEq/L): 133, 133, and 132. Urinary sodium (mEq/day): 6, 7, and 7. MAP (mmHg): 84, 84, and 82. PRA (ng/mL/h): 9.5, 8.6, and 9.2 |
|
| Interventions | Participants were studied after they received a low‐sodium diet (50 mmol/day) without diuretic therapy for 5 days. Experimental group A: total paracentesis (completed removal of ascites in a single tap) and iv dextran‐70 (8 g/L of ascitic fluid removed given as a glucose solution of dextran‐70) using the same schedule as for albumin Experimental group B: total paracentesis (completed removal of ascites in a single tap) and iv polygeline (8 g/L of ascitic fluid removed given as 3,5% saline solution of polygeline) using the same schedule as for albumin Control group: total paracentesis (complete removal of ascites in a single tap) and iv albumin infusion (8 g/L of ascitic fluid removed, with 50% of the dose within the first 2 hours and 50% 6‐8 hours after paracentesis; 20% albumin solution) Volume of ascites removed, mean (L): experimental group A, 7.7; experimental group B, 8.2; control group, 7.5 |
|
| Outcomes | Authors planned to analyse development of post‐paracentesis circulatory dysfunction as main outcome. Death and adverse events during first hospitalisation were also reported. |
|
| Notes | Separate randomisation for participants with and without renal impairment (serum creatinine > 1.5 mg/dL) After treatment, participants did not receive diuretics for 6 days. Participants were discharged from hospital with diuretics. The follow‐up period started at the end of the first hospitalisation. Participants were examined at least weekly during the first month, monthly for the next two months and bimonthly thereafter. PRA was measured 1 and 6 months after discharge in 122 and 58 participants, respectively. If tense ascites developed, participants were treated with total paracentesis and the same plasma expander assigned at inclusion. Post‐paracentesis circulatory dysfunction (PICD): increase in PRA of more than 50% of pretreatment value to a level > 4 ng/mL/h on the sixth day after paracentesis. The incidence of PCID was used to calculate sample size. The incidence of PICD was evaluated according to the plasma expander used and the volume of ascitic fluid drained. Of the 277 participants surviving to time of discharge, 27 were lost to follow‐up after a period ranging from 1 to 30 months. 14 participants underwent liver transplantation 1‐15 months after discharge (considered censored at the time of surgery). The remaining 236 participants were followed up to the end of the study or death. Mean follow‐up: experimental group A, 293 days; experimental group B, 269 days; control group, 302 days With for‐profit funding (educational grant from Hoechst Ibérica) Sample size calculation reported for PPCD Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | It was not reported if sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of the 277 patients surviving to time of discharge, 27 were lost to follow‐up after a period ranging from 1 to 30 months". |
| Selective reporting (reporting bias) | High risk | The study authors did not report mortality and serious adverse events during follow‐up. They stated "no significant differences were found among the groups in deaths". |
| Other bias | Low risk | No other bias identified |
Graziotto 1997.
| Methods | RCT comparing reinfusion of concentrated ascitic fluid (ARCA) with total paracentesis and iv albumin infusion (PARA) in cirrhotic patients with tense ascites | |
| Participants |
Inclusion criteria: patients with cirrhosis and tense ascites. None had responded to conventional treatment including low‐sodium diet and diuretics, K‐canrenoato or spironolactone up to 400 mg/day and furosemide up to 120 mg/day. Exclusion criteria: severe hepatic encephalopathy (grade III and IV), congestive heart failure, renal failure (serum creatinine > 250 mmol/L), recent major bleeding, neoplastic cell in the ascites and infected ascites (positive culture or PMN leukocytes > 300/microL). Experimental group – reinfusion of concentrated ascites: 12 participants Control group ‐ total paracentesis plus iv albumin: 12 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 54.5 and 59.7. Males (%): 67 and 75. Child‐Pugh B (%): 50 and 41.6. Child‐Pugh C (%): 50 and 58.4. Serum creatinine (µmol/L): 103.1 and 87.7. Blood urea nitrogen (g/L): 11.3 and 7.3. Serum albumin (g/dL): 3.2 and 3.0. Serum sodium (mmol/L): 132.5 and 130.7. Urinary sodium (mmol/day): 48.5 and 31. Serum bilirubin (µmol/L): 97.2 and 105.5. Prothrombin time (%): 53.8 and 42.9. PRA (mcg/L/hr) 17.4 and 10.6. Serum aldosterone (nmol/L): 1.4 and 1.5. Antidiuretic hormone (ng/L): 4.8 and 5.2. Atrial natriuretic factor (ng/L): 59.4 and 75 |
|
| Interventions | Experimental group: apheresis and reinfusion of concentrated ascites (ARCA) A mean of 8.8 L of ascites were removed and filtered over 80‐245 min and 220‐1020 mL of concentrated ascites, containing 23.3‐128.5 g of albumin (mean 59.8 g), was iv infused at the end of the apheresis. Control group: total paracentesis (PARA) plus iv infusion of human albumin (20% w/v, 6 g/L of removed ascites) A mean of 6.9 L of ascites were removed over 60‐270 min and 100‐450 mL of 20% w/v albumin equivalent to 20‐90 g of albumin was iv infused at the end of total paracentesis. |
|
| Outcomes | Death, liver transplantation and dropouts The following parameters were also recorded: reappearance of tense ascites, the period between the procedure and the need for repeated paracentesis, the number of paracentesis procedures and repeated admission to the hospital. |
|
| Notes | Lost to follow‐up (dropout): one participant per group The mean follow‐up was 577 days in the experimental group and 643 days in the control group. Liver transplantation: 2 participants in the experimental group and 1 participant in the control group. With for‐profit funding ("Technical assistance from Dideco Co.") No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The authors did not specify if the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients were lost at follow‐up (dropout), one for each group". |
| Selective reporting (reporting bias) | Low risk | The study authors did not report serious adverse events and other liver‐related complications. |
| Other bias | Low risk | No other bias identified |
Altman 1998.
| Methods | RCT comparing hydroxyethyl starch (HES) with albumin as plasma expander after partial paracentesis in cirrhotic patients with tense ascites | |
| Participants | Sixty‐five patients with cirrhosis admitted for ascites Inclusion criteria: cirrhosis with tense ascites; absence of severe associated disease; absence of gastrointestinal bleeding or infection within 2 days before entry; absence of previous porto‐systemic anastomosis or peritoneal‐venous shunt; serum creatinine < 1.4 mg/dL; serum sodium > 120 mEq/L; prothrombin time > 25% of normal; no diuretics in the last five days; and no plasma expansion and/or paracentesis within 10 days before entry Experimental group – repeated daily paracentesis (≤ 5 L) and iv 6.5% HES: 27 participants. Control group – repeated daily paracentesis (≤ 5 L) and iv albumin: 33 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 56.3 and 55.9. Males (%): 78 and 70. Alcoholic cirrhosis (%): 81 and 85. Previous episodes of ascites (%): 41 and 55. Peripheral oedema (%): 41 and 64. Pugh score: 9.3 and 9.8. Serum urea (mg/dL): 12 and 12.6. Serum creatinine (mg/dL): 0.89 and 0.84. Serum sodium (mEq/L): 134.3 and 134.2. Serum albumin (g/L): 27.3 and 26.3. PT (% of normal): 56.7 and 60.1. MAP (mmHg): 93 and 95 |
|
| Interventions | After hospital admission, participants were given a diet containing 20 mEq/day of sodium. Diuretics or anti‐inflammatory drugs were not permitted. Water ingestion was free. Beta‐blockers and nitrates were permitted. Experimental group: repeated daily large‐volume paracentesis (≤ 5 L) and iv infusion of 6.5% hydroxyethyl starch solution (HES; sodium chloride concentration 9 g/L), 32.5 g if removed ascites < 2 L and 65 g if removed ascites 2‐5 L of ascites Control group: repeated daily large‐volume paracentesis (≤ 5 L) and iv infusion with 20% human albumin solution (sodium chloride concentration 7 g/L), 20 g if removed ascites < 2 L and 40 g if removed ascites 2‐5 L Infusion was started at the end of the evacuation and was completed within 4 h. Volume of ascites removed (mean): 6.3 L in the experimental group and 7.7 L in the control group Volume of plasma expander infused (mean): 1538.5 mL in the experimental group and 387.9 mL in the control group |
|
| Outcomes | Primary outcome: development of renal failure or hyponatraemia within 15 days after paracentesis. Secondary outcome: tolerance of HES. Complications and cost were also evaluated. | |
| Notes | Sixty‐five participants were enrolled in the study. Five participants were not included in the final analysis: 3 participants (2 from the experimental group and 1 from the control group) in whom paracentesis was unsuccessful and ascites could not be drained, so these participants did not receive any infusion of plasma expander; 1 participant from the experimental group received diuretic treatment at the time of the inclusion; 1 participant from the experimental group developed renal impairment (creatinine 3.1 mg/dL) between randomisation and the first paracentesis. Thus, 60 participants were included in the final analysis. Renal failure: a 50% increase in plasma creatinine to a level > 1.4 mg/dL. Hyponatremia: decrease in sodium of at least 10 mmol/L to a level < 120 mmol/L PAC was analysed only in 19 participants. PAC increased (third day) in 5/8 (62%) in the experimental group and 3/11 (27%) in the control group. Mean cost of plasma expander per participant was: 154 FF (US$30.8) in the experimental group and 1427 FF (US$285) in the control group. With for‐profit funding ("This study was supported by Fresenius France") No sample size calculation We emailed Altman and colleagues on Feb 2013. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | The study authors did not report mortality and serious adverse events. |
| Other bias | Low risk | No other bias identified |
Mehta 1998.
| Methods | RCT comparing ascitic fluid filtration and iv infusion vs infusion of haemaccel after total‐volume paracentesis in cirrhotic patients with tense or intractable ascites | |
| Participants | Twenty cirrhotic patients with tense ascites or intractable ascites (if the ascites was uncontrolled despite 200 mg/day of spironolactone and 60 mg/day of furosemide, or if patients developed complications related to diuretics) Exclusion criteria: poor general condition; infected ascites (spontaneous bacterial peritonitis or its variants); pancreatic ascites; malignancy; recent gastrointestinal bleeding, grade III or IV hepatic encephalopathy; pregnancy All participants were put on a salt‐restricted diet (2 g or 88 mEq per day), with fluid intake restricted to 500 mL/day if serum Na < 130 mmol/L. Diuretics were discontinued at recruitment. Experimental group – total volume paracentesis and intravenous infusion of filtrated and concentrated ascitic fluid: 10 participants Control group – total volume paracentesis and intravenous infusion of haemaccel: 10 participants. Baseline characteristics (values shown are mean where appropriate) Age (yr): 50.8 and 54.7. Males (%): 80 and 80. Alcoholic cirrhosis (%): 70 and 60. Postnecrotic cirrhosis (%): 30 and 40. BUN (mg/dL): 11.4 and 11.2. Serum creatinine (mg/dL): 0.9 and 0.9. Serum sodium (mEq/L): 133.3 and 136. Urinary sodium (mEq/L): 34.2 and 34.8. Serum albumin (g/dL): 2.7 and 2.9. Serum bilirubin (mg/dL): 2.1 and 1.6. Prothrombin time (s, control of 13 s): 15.7 and 16.2. Body weight (kg): 68.6 and 58.4 |
|
| Interventions | Experimental group: total paracentesis with intravenous reinfusion of filtrated and concentrated ascitic fluid (AFI, performed by a modification of the method described by Landini 1985) Control group: total‐volume paracentesis and infusion of haemaccel, 150 mL for each litre of ascitic fluid removed Median time for the procedure (hours): 12 and 5.5 Median of ascitic fluid drained (L): 10.7 (including a median of 10.2 L of protein‐free filtrate and 0.5 L of concentrated ascitic fluid) and 8.0 Median volume of fluid infused (L): 0.5 and 1.1 Both the groups were off diuretics after the procedure. |
|
| Outcomes | The authors did not specify in the methods the outcomes to be assessed. They reported data on: ‐ procedure‐related mortality during hospitalisation; ‐ development of complications. |
|
| Notes | Median time for termination of the study: 3 weeks in the experimental group and 2 weeks in the control group Minor febrile reactions not requiring any specific therapy were common in the experimental group (7/10). Cost for procedure (in rupees): 295 for AFI and 440 for TVP Unknown for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | The authors reported procedure‐related mortality. So, it was not clear if they referred to all‐cause mortality. They did not report serious adverse events and renal impairment. |
| Other bias | Low risk | No other bias identified |
Kang 1998.
| Methods | RCT comparing hydroxyethyl starch (HES) with albumin after total paracentesis in cirrhotic patients with tense ascites | |
| Participants | Twelve cirrhotic patients with tense ascites Experimental group ‐ total paracentesis and iv HES: 6 participants Control group ‐ total paracentesis and iv albumin: 6 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 49 and 42. Males (%): 67 and 67. Alcoholic cirrhosis (%): 33 and 33. Viral cirrhosis (%): 67 and 67. Child‐Pugh class A‐B‐C (%): 17 ‐ 17 ‐ 66 and 0 ‐ 50 ‐ 50. Serum bilirubin (mg/dL): 3.8 and 4.1. PT (%): 54 and 49. Albumin (g/dL): 2.6 and 2.5. Serum creatinine (mg/dL): 0.8 and 1. BUN (mg/dL): 15 and 18.5. Serum sodium (mEq/L): 130 and 130. Serum potassium (mEq/L): 3.9 and 4.2. Urine sodium (mEq/L): 26 and 36. Mean arterial pressure (MAP): 100 and 100. HR (/min): 84 and 85 |
|
| Interventions | Experimental group: paracentesis and iv HES (8 g/L ascites removed) Control group: paracentesis and iv albumin (8 g/L of ascites removed) Volume of ascites removed (mean, L): 4.4 and 4.3 |
|
| Outcomes | Circulatory and renal dysfunction were the planned outcomes, monitored before, one, and three days after large paracentesis. Authors reported also complications during 72 h after paracentesis: dizziness, tachycardia, hyponatraemia (decrease more than 5 mEq/L below 130 mEq/L), hyperkalaemia (increase more than 1.5 mEq/L above 5.5 mEq/L), renal impairment (serum creatinine increase > 50% above 1.5 mg/dL), hepatic encephalopathy. |
|
| Notes | The article was written in Korean, except for the abstract, tables, and figures. Unknown for‐profit funding We emailed Kang and colleagues on 3 Jan 2017. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Data not obtainable |
| Allocation concealment (selection bias) | Unclear risk | Data not obtainable |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Data not obtainable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data not obtainable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data not obtainable |
| Selective reporting (reporting bias) | Low risk | This short follow‐up trial did not report any serious adverse events. |
| Other bias | Unclear risk | Data not obtainable |
Zhao 2000.
| Methods | RCT comparing mannitol with albumin as plasma expander after partial paracentesis in cirrhotic patients with tense ascites | |
| Participants | Sixty‐eight cirrhotic patients with tense ascites, fulfilling the following criteria: no liver cancer; no encephalopathy, gastrointestinal bleeding, or infection; serum bilirubin was less than 171 µmol/L, prothrombin time over 40%, platelet count above 40 x 109/L, serum creatinine below 264.99 µmol/L and the urinary sodium excretion was less than 10 mmol/L/d Experimental group – paracentesis and iv mannitol infusion: 32 participants Control group – paracentesis and iv albumin infusion: 36 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 48.6 and 51.9. Males (%): 69 and 67. HBsAg‐associated cirrhosis (%): 75 and 67. Peripheral oedema (%): 47 and 50. Renal failure (%): 19 and 22 |
|
| Interventions | The participants were given a diet with 50 mmol/L/d of sodium and the diuretic treatment was withdrawn. Experimental group: large volume paracentesis (3‐6 L/day) and iv infusion of 50 g of mannitol (20% mannitol 250 mL), at a rate of 1‐2 mL/min Control group: large volume paracentesis (3‐6 L/day) and iv infusion of 20 g of albumin Mean volume of ascites removed (L): experimental group, 3.8; control group, 4.2 |
|
| Outcomes | The authors did not specify in the methods the outcomes to be assessed. They reported data on: ‐ change in biochemical parameters 24 and 48 hours after treatment; ‐ complications during hospitalisation: renal impairment (≥ 50% increase in serum creatinine or BUN or both to > 132.45 µmol/L and > 10.71 mmol/L, respectively, after treatment); hyponatraemia (decrease in serum sodium > 5 mmol/L or serum sodium < 130 mmol/L after treatment); encephalopathy; gastrointestinal bleeding; severe infection; ‐ deaths at hospitalisation and during follow‐up. |
|
| Notes | Participants with and without renal failure (renal failure was considered to be present when serum creatinine concentration was > 132.45 µmol/L) were randomly separated to ensure a similar number of cases with renal failure in both therapeutic groups. After treatment, participants remained in hospital for 2 days without using diuretics. In the follow‐up, 2 participants from experimental group and 2 from control group were submitted to portocaval shunt. Of the remaining 64 participants discharged from the hospital, 11 in the experimental group and 9 in the control group were lost in the follow‐up. Mean follow‐up period after discharge from the hospital was 27.6 weeks in the experimental group and 30.5 weeks in the control group. Number of participants requiring readmission: 21 in the experimental group and 23 in the control group. 4 participants in the experimental group and 5 participants in the control group developed ascites again. Unknown for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The study authors did not report serious adverse events |
| Other bias | Low risk | No other bias identified |
Baik 2000.
| Methods | RCT comparing albumin with no plasma expansion after large volume paracentesis in cirrhotic patients with tense ascites | |
| Participants | 23 patients with cirrhosis and tense ascites treated by single large volume paracentesis were recruited. Experimental group ‐ paracentesis and iv albumin: 11 participants Control group ‐ paracentesis without iv albumin: 12 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 52.27 and 54.33. Males (%): 72.2 and 83.3. Alcoholic cirrhosis (%): 54.5 and 50. Viral cirrhosis (%): 27.3 and 33.3. Alcoholic + viral cirrhosis (%): 18.2 and 16.7. Child‐Pugh score: 9.82 and 10.33. Serum bilirubin (mg/dL): 6.13 and 5.03. Serum creatinine (mg/dL): 0.91 and 0.97. Urine sodium (mM/L): 119.45 and 91.36. Mean arterial pressure (MAP): 92.82 and 87.67. Plasma renin activity (ng/mL/h): 8.48 and 10.83. Plasma aldosterone (ng/dL): 25.11 and 52.09. Systemic vascular resistance index (dyn*s/cm5*m2): 1995.07 and 2369.17. Cardiac index (L/min*m2): 4.03 and 3.12 |
|
| Interventions | Experimental group: paracentesis and iv albumin (6 g/L of removed ascites) Control group: paracentesis without plasma expansion Volume of ascites removed (mean, L): 4.86 and 4.97 |
|
| Outcomes | Authors planned the evaluation of: ‐ systemic and renal haemodynamic parameters: mean arterial blood pressure, cardiac index, systemic vascular resistance index, resistive index of kidney and serum creatinine; ‐ indices associated with sodium homeostasis: urine sodium and osmolarity; ‐ neurohumoral factors: plasma renin activity and plasma concentration of aldosterone before and 48 h after a single large volume paracentesis. They reported also data on complications (hepatic encephalopathy, dizziness, tachycardia, hypotension) during 48 h after a single large volume paracentesis. |
|
| Notes | The article was written in Korean, except the abstract and tables. Unknown for‐profit funding We emailed Baik and colleagues on 3 Jan 2017. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Data not obtainable |
| Allocation concealment (selection bias) | Unclear risk | Data not obtainable |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Data not obtainable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data not obtainable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data not obtainable |
| Selective reporting (reporting bias) | High risk | The trial authors did not report in the article information on mortality, serious adverse events, other liver‐related complications and non‐serious adverse events, because the aim of the trial was to assess haemodynamic and neuro‐humoral changes 48 h after paracentesis. Data not obtained from the authors |
| Other bias | Unclear risk | Data not obtainable |
García‐Compeán 2002.
| Methods | RCT comparing dextran‐40 with albumin as plasma expanders after large volume paracentesis in cirrhotic patients with tense ascites | |
| Participants | Sixty‐nine cirrhotic patients with tense ascites (ascites causing respiratory dysfunction) 96 large‐volume paracentesis procedures were performed. Multiple punctures to the same patient were at least three months apart. In such cases, the patients were re‐randomised to be reassigned to one of the two treatments. Accordingly, the punctures were considered as independent events and were analysed separately. Therefore, clinical, biochemical, haemodynamic, and humoral parameters were assessed for each LVP. Inclusion criteria: absence of HCC; absence of hepatic encephalopathy, gastrointestinal bleeding, and either local or systemic infection; serum bilirubin level < 10 mg/dL; prothrombin time > 40%; platelet count > 50.000/microL; serum creatinine < 3 mg/dL; serum sodium > 125 mEq/L Experimental group – large‐volume paracentesis (LVP) with dextran‐40 Control group – large‐volume paracentesis (LVP) with albumin Baseline characteristics (in all included participants, n = 69) Males (%): 78. Mean age (yr): 58 ± 10. Alcohol cirrhosis (%): 83%. Viral cirrhosis (%): 7. PBC (%): 4. Autoimmune (%): 1. Crytgogenetic (%): 4 There was no confirmation in any of the participants of the refractoriness of the ascites by previous intensive treatment with diuretics. Baseline characteristics (distribution for procedures) (values shown are mean where appropriate) Age (yr): 59.4 and 58.9. Male (%): 73 and 71. Alcoholic cirrhosis (%): 79 and 81. Renal impairment (%): 31 and 25. MAP (mmHg): 87 and 89. Serum bilirubin (mg/dL): 2.8 and 3.32. Prothrombin (%): 56.6 and 57.2. Serum albumin (g/dL): 2.9 and 3. BUN (mg/dL): 32 and 34.1. Serum creatinine (mg/dL): 0.9 and 0.8. Serum sodium (mEq/L): 135.9 and 135.7. PRA (ng/mL/h, measured in 19 from group 1 and 16 from group 2): 11 and 11.8. PAC (pg/dL, measured in 21 from group 1 and 15 from group 2): 42.8 and 46.7. Extracted volume (L): 5.7 and 5.2. Child‐Pugh score: 10.3 and 10 |
|
| Interventions | Participants were admitted to the hospital at least 5 days before treatment. Diuretics were discontinued 3 days before treatment. A standard diet containing less than 50 mEq/day of sodium was prescribed, and fluid restriction (800 mL/d) was carried out in participants with serum sodium < 130 mEq/L. Other drugs were excluded, except for lactulose, antacids, H2 receptor blockers, and antibiotics. Experimental group: LVP plus an iv dextran‐40 infusion Control group: LVP plus an iv albumin infusion 8 g of either albumin or dextran were given for each litre of ascites removed. As soon as paracentesis was started, either a sodium‐free low‐molecular weight Dextran‐40‐sorbitol solution or a solution of 20% human albumin containing a sodium concentration of 80 mEq/L were infused for 1 or 2 h. Volume of ascites removed (mean): experimental group, 5.7 L; control group, 5.2 L Mean duration of paracentesis (min): 70 in experimental group and 63 in control group Duration of hospitalisation (days): 9.5 in experimental group and 9 in control group |
|
| Outcomes | The authors reported data on: ‐ clinical, biochemical, haemodynamic, and hormonal evaluations before and after paracentesis (24 and 48 h later); ‐ recurrence of ascites; ‐ complications during hospitalisation and during follow‐up; ‐ deaths during hospitalisation and during follow‐up. |
|
| Notes | Any repeat punctures on the same participant were at least three months apart. Renal impairment: an increase of > 50% in serum creatinine or BUN. Hyponatraemia: a decrease of > 5 mEq/L. PCD: an increase of > 50% in plasma renin activity from the pre‐treatment value to a level of > 4 ng/mL/h 2 days after paracentesis Thirty‐nine participants received at least one LVP with dextran‐40 infusion and 35 at least one LVP plus albumin infusion. Distribution of the 96 LVPs either with dextran‐40 or with albumin in the 69 participants was as follows: one LVP, 52 participants (29 in experimental group and 23 in control group); two LVPs, 10 participants (4 in experimental group, 4 in control group, and 2 in both groups consecutively). Multiple punctures on the same participant were at least three months apart. In such cases, the participants were re‐randomised to be reassigned to one of the two treatments. Accordingly, the punctures were considered as independent events and analysed separately. No participants died during hospitalisation. All participants were discharged from the hospital with prescriptions of a low sodium diet and for diuretics. The mean follow‐up period was: 13.7 months in the experimental group and 14.4 in the control group. Sixty‐four participants required readmission for ascites recurrence: 34 from the experimental group and 30 from the control group. There were no deaths during the first hospitalisation. Twenty‐nine participants died during the follow‐up period: 18 from the experimental group, and 11 from the control group (p > 0.05). In the absence of data on the number of randomised participants for each group, we couldn't include the data in the analysis. Unknown for‐profit funding No sample size calculation Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocation may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | The study authors did not report more predefined outcomes. In particular, the authors reported outcomes referring to the number of procedures and not to the number of participants. |
| Other bias | Unclear risk | Not evaluable |
Sola‐Vera 2003.
| Methods | RCT comparing saline with albumin as plasma expanders after total paracentesis in cirrhotic patients with tense ascites Cross‐over trial |
|
| Participants | 72 cirrhotic patients with tense ascites Exclusion criteria: prothrombin time less than 30%, platelet count less than 30.000/mm3, serum creatinine level greater than 240 µmol/L, urinary sodium excretion greater than 20 mEq/24 h, upper gastrointestinal bleeding, hepatic encephalopathy, bacterial infection within the preceding month, HCC, respiratory failure, cardiac failure, and organic renal disease, use of beta‐blockers with or without nitrates for preventing variceal bleeding Participants were on a low‐sodium diet (< 60 mmol/d) without diuretics for at least 3 days before paracentesis. Experimental group – paracentesis and saline: 35 participants Control group – paracentesis and albumin: 37 participants Baseline characteristics (values shown are mean where appropriate) Age (y): 62.9 and 59.9. Males (%): 57 and 73. Alcoholic cirrhosis (%): 48.6 and 62.1. Peripheral oedema (%): 77 and 76. Renal impairment (creatinine > 130 µmol/L) (%): 14 and 5. Hyponatremia (Na < 130 mEq/L) (%): 37 and 22. MAP (mmHg): 81.5 and 83.1. Serum bilirubin (mmol/L): 46.9 and 56.3. Serum albumin (g/L): 26.7 and 25.6. INR: 1.4 and 1.6. Serum creatinine (µmol/L): 95.6 and 83.4. Serum sodium (mEq/L): 131.1 and 132. Urinary sodium (mEq/d): 11.2 and 10.6. Child‐Pugh A (%): 0 and 0. Child‐Pugh B (%): 48 and 54. Child‐Pugh C (%): 52 and 46. PRA (ng/mL/h): 5.4 and 5.2. PAC (pmol/L): 1386.6 and 1899.6. NOx (nmol/L): 10.6 and 9.4 |
|
| Interventions | Experimental group: total volume paracentesis and iv infusion of saline, 170 mL of 3.5% saline solution per litre of ascites removed at 999 mL/h Control group: total volume paracentesis and iv albumin (20% albumin solution), 8 g/L of ascites removed Infusion of saline or albumin began 3 hours after starting the paracentesis. Participants did not receive diuretics in the forthcoming 6 days. Volume of ascites removed (mean): 6.4 L in the experimental group and 6.4 L in the control group Mean duration of paracentesis: 82.8 min in the experimental group and 91.1 min in the control group Volume of plasma expander infused (mL): experimental group, 1.071; control group, 259.2 |
|
| Outcomes | The authors reported data on: ‐ incidence of paracentesis‐induced circulatory dysfunction (PICD); ‐ mortality during hospital stay; ‐ complications during hospital stay. |
|
| Notes | Haematologic and biochemical analysis, PRA, PAC and serum nitritis/nitrates were valuated 6 days after paracentesis. Participants developing a second episode of tense ascites during follow‐up were readmitted and treated by total paracentesis and alternative plasma expander (11 participants initially treated with saline and 10 participants initially treated with albumin); mean time between first and second paracentesis was 69.0 days. PICD was estimated according to the changes in the activity of the renin‐angiotensin system and defined as an increase in PRA of more than 50% of the pre‐paracentesis value to a level of more than 4 ng/mL/h on the sixth day after paracentesis. PICD was also evaluated according to the volume of ascitic fluid removed. The effect of paracentesis on PRA and PAC was assessed in 33 participants from the experimental group and 35 participants from the control group. Duration of hospitalisation (from randomisation to discharge or death): 8.4 days in the experimental group and 10.6 days in the control group Cost of plasma expander per paracentesis: US$ 2.1 in the experimental group and US$ 186.4 in the control group Without for‐profit funding Sample size calculated to assess PPCD We emailed Sola‐Vera and colleagues on Feb 2013. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded. Personnel were not blinded. There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The study authors did not report serious adverse events. |
| Other bias | Low risk | No other bias identified |
Degoricija 2003.
| Methods | RCT comparing fresh frozen plasma, haemaccel and albumin in cirrhotic patients with ascites treated by paracentesis and bed rest | |
| Participants |
Inclusion criteria: alcohol‐induced Child‐Pugh C liver cirrhosis with tense ascites; sinus rhythm of the heart Exclusion criteria: hepatic encephalopathy grade III and IV; hepatocellular or metastatic liver carcinoma; gastrointestinal bleeding within 4 weeks; systemic infection (sepsis or peritonitis); serum creatinine ≥ 180 micromol/L; platelet count ≤ 20×109/L; serum sodium ≤ 125 mmol/L; chronic obstructive pulmonary disease; heart failure We reported baseline data of the participants of the first 3 groups analysing the effect of different plasma expanders. Group 1 ‐ paracentesis + albumin + bed rest: 10 participants Group 2 ‐ paracentesis + fresh frozen plasma + bed rest: 10 participants Group 3 ‐ paracentesis + polygeline + bed rest: 10 participants Baseline characteristics: Age (median, yr): 51, 54, and 52. Male (%): 80, 60, and 70. Alcohol aetiology (%): 60, 70, and 60. Alcohol + viral aetiology (%): 40, 30, and 40. Child‐Pugh C score (median): 12, 12.2, and 12.5. Prothrombin time (%): 54.3, 55, and 42.7. Serum bilirubin (micromol/L): 56.8, 57.2, and 70.2. Serum albumin (g/L): 28.44, 30.3, and 28.5. Serum urea (mmol/L): 4.5, 4.6, and 4.6. Serum creatinine (micromol/L): 100, 82, and 82. Serum sodium (mmol/L): 134, 135, and 135. Mean arterial pressure (mmHg, mean): 99.2, 105.3, and 105.2. Plasma renin activity (ng/mL/h): 8.0, 5.5, and 5.4 |
|
| Interventions | The participants were kept on a diet containing 40 mEq/day sodium; liquid ingestion was restricted to 1L/day in participants with peripheral oedema +2 and +3 or serum sodium < 130 mmol/L and diuretic treatment, cigarettes, alcohol, coffee, and tea were withdrawn. The use of diazepam, antibiotics, ranitidine, lactulose, and antacids was allowed where indicated. After diuretic discontinuation for 2‐7 days (5 days on average), participants were randomly allocated into 5 groups. Group 1: Paracentesis 6 L + infusion of 200 mL of 20% low sodium albumin (Human‐Albumin 20% Behring, Centeon Pharma GmbH, Marburg, Germany), with 6.6 g of albumin/L of ascites removed, and 25 mmol of sodium + bed rest Group 2: Paracentesis 6 L + 600 mL of fresh frozen plasma (Fresh Frozen Plasma, Croatian Institute of Transfusion Medicine; Zagreb, Croatia), with 3 g of albumin/L of ascites removed, and 81 mmol sodium + bed rest Group 3: Paracentesis 6 L + 900 mL of polygeline (haemaccel, Hoechst Marion Roussel, Horsholm, Denmark), with 5.25 g of polygeline/L of ascites removed, and 130.5 mmol of sodium + bed rest Group 4: Paracentesis 6 L; no bed rest Group 5: Furosemide 40 mg iv/day; no bed rest All participants were treated with spironolactone 200 mg/day. |
|
| Outcomes | ‐ Measurements of blood pressure, heart rate, body weight, and urine volume were done every morning on days 2‐6, and blood pressure and heart rate were measured on day 1 two, four, and six hours after the beginning of paracentesis. ‐ On day 1, 2, 3, and 6, urine was collected for 24 h to measure creatinine clearance. ‐ Blood samples for plasma renin activity, plasma aldosterone concentration, and atrial natriuretic peptide were obtained on days 1, 2, 3, and 6. |
|
| Notes | We have included in the analysis the first three groups, because they compared the role of different plasma expanders after a single paracentesis of 6 L, associated with bed rest. It was not possible to extract numerical data on the outcomes of interest for the meta‐analysis. The authors reported that the different plasma expanders (albumin, fresh frozen plasma, or polygeline) after a single paracentesis of 6 L, associated with 24‐h bed rest before and after the procedure, did not induce significant changes in clinical measurements and laboratory parameters of hepatic and renal function, and plasma renin activity. No local complications related to the procedures were observed. We emailed Degoricija and colleagues on 6th February 2019. Unknown for‐profit funding No sample size calculation Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results |
| Selective reporting (reporting bias) | High risk | The study authors did not report the predefined outcomes. No information obtained from the authors |
| Other bias | Unclear risk | Other bias not evaluable |
Moreau 2006.
| Methods | RCT comparing polygeline and albumin as plasma expander after paracentesis in cirrhotic patients with ascites | |
| Participants |
Inclusion criteria: cirrhotic patients with ascites who needed to receive a plasma expander for at least one of the following three indications: ascites removal by paracentesis, renal impairment (estimated creatinine clearance below 50 mL/min without findings of organic nephropathy) and marked hyponatraemia (serum sodium < 120 mmol/L). During the study, only patients with ascites were included. Other inclusion criteria: age 18 to 74 years, no allergy to albumin or gelatins, no asthma, no coronary disease, no cardiac failure, no respiratory disease, no HCC, and serum potassium < 6 mmol/L Exclusion criteria: septic shock, spontaneous bacterial peritonitis, gastrointestinal bleeding or dehydration within the previous 2 weeks; therapeutic paracentesis within the last week; serum creatinine > 2.49 mg/dL; treatment with nonsteroidal anti‐inflammatory drugs, aminoglycoside, vasopressin analogues, somatostatin or analogues; other disease that could affect short‐term prognosis; presence of TIPS or peritoneal‐venous shunt; liver transplantation scheduled within the next 3 months Experimental group – polygeline: 38 participants Control group ‐ albumin: 30 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 56 and 54. Males (%): 79 and 87. Alcoholic cirrhosis (%): 92 and 97. Alcohol and HCV infection (%): 8 and 3. Child‐Pugh score: 10.2 and 10. Previous gastrointestinal bleeding (%): 32 and 27. Duration of ascites (months): 24 and 18. Previous paracentesis (%): 76 and 72. Serum bilirubin (mg/dL): 3.6 and 3.2. Prothrombin time (%): 57 and 56. Serum albumin (g/L): 25 and 26. Serum sodium (mmol/L): 132 and 134. Serum creatinine (mg/dL): 0.9 and 0.9 |
|
| Interventions | Experimental group: 3.5% polygeline (haemaccel®) Control group: 20% human albumin One unit of colloid provided 17.5 of polygeline or 20 g of albumin. Treatment modalities at inclusion and during follow‐up: ‐ Ascites requiring paracentesis: after ascites removal, 1 U of the assigned colloid if < 4 L of removed ascites, 2 U if 4 to 6 L, 3 U if 6 to 8 L and 4 U if > 8 L ‐ Renal impairment or marked hyponatraemia (with or without paracentesis): 2 U of the assigned colloid if estimated dry body weight ≤ 50 kg, 3 U if 50‐66 kg and 4 U if > 66 kg. If the condition persisted > 2 days a second infusion was administered. Treatment modalities for other indications, during follow‐up: ‐ Spontaneous bacterial peritonitis: two infusions (at diagnosis and 2 days later) with a dose related to dry body weight ‐ Severe sepsis or conditions with decreased blood volume: a dose chosen by the investigator up to a maximum of 4 U of the assigned colloid If the complication persisted, additional infusion at the same dose could be administered after an interval of at least 2 days between each infusion and with no more than 3 infusions in 8 days. In participants with hepatorenal syndrome, according to centre’s protocol, the plasma expander used was only the assigned study colloid. NSAIDs and aminoglycoside were not allowed. A diuretic treatment and a low‐sodium diet were prescribed. Sixty‐eight participants were included for ascites removal by paracentesis. Among these, 5 participants had also renal impairment. |
|
| Outcomes | Primary end points planned in the protocol: ‐ a composite of renal impairment and marked hyponatraemia; ‐ survival. Because of the premature discontinuation of the trial (for safety concerns about bovine‐derived products), the new primary end point was the occurrence of a first liver‐related complication such as death, recurrent ascites requiring paracentesis, renal impairment (serum creatinine > 130 µmol/L with an increase > 50%), hyponatraemia (Na < 130 mmol/L with a decrease >5 mmol/L), bacterial infection, encephalopathy, portal hypertensive bleeding, and any other complication related to cirrhosis. Secondary end points: occurrence of each of the first liver‐related complications; first occurrence of renal impairment or hyponatraemia; incidence of liver‐related complications; incidence of recurrent ascites; total number of fluid‐loading sessions and total amount of colloid units; unblinded colloid administration; placement of TIPS or a peritoneal‐venous shunt, administration of vasopressin analogue, somatostatin or an analogue or liver transplantation; safety |
|
| Notes | RCT, double‐blind, with a 6‐month follow‐up period. Randomisation using stratification in the centres and hyponatraemia or renal impairment The trial was prematurely discontinued because of safety concerns about bovine‐derived products that emerged during the study period. Of the 81 participants enrolled, 78 were included in the safety population (44 in the experimental group and 34 in the control group), and 68 were included in the efficacy population (38 in the experimental group and 30 in the control group). Completed 6 months of follow‐up: 16% in the experimental group and 40% in the control group. Premature trial discontinuation: 29% in the experimental group and 33% in the control group. Unblinded colloid administration: 24% in the experimental group and 10% in the control group. Dropout (medical decision or withdrawal or consent): 24% in the experimental group and 7% in the control group. Lost to follow‐up: 7% in the control group. The total median cost adjusted to a 30‐day period was €4612 in the experimental group and €1915 in the control group. Without for‐profit funding An a priori sample size was calculated, but the trial was discontinued prematurely for safety concerns. Letter sent on Feb 2013. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generated by SAS V6.12 statistical software |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described, so the intervention allocation may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | In the Study design and treatment protocol section, the authors stated: "The 100 ml units of albumin and 500 ml units of polygeline were placed in identical carton boxes to mask the contents from investigators and patients. Only designated nurses at each centre were aware of the allocated treatment for each patient to regulate flow to obtain the same time of infusion per unit of colloid." But, as shown in table 2 of the RCT, 9/38 (24%) patients in the experimental group and 3/30 (10%) patients in the control group received unblinded colloid administration. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The study authors did not report only refractory ascites. |
| Other bias | Unclear risk | The trial was discontinued prematurely for safety concerns |
Abdel‐Khalek 2010.
| Methods | RCT comparing hydroxyethyl starch 6% with albumin as plasma expanders for treatment of patients with liver cirrhosis and tense ascites following total paracentesis | |
| Participants | One‐hundred and thirty‐five patients (60% with cirrhosis and schistosomal periportal fibrosis combined, 26.7% with posthepatitic cirrhosis, and 13.3% with schistosomal periportal fibrosis) with tense ascites Inclusion criteria: cirrhotic patients with tense ascites with absolute unresponsiveness to low‐sodium diet and maximal tolerated doses of diuretic therapy and need to receive a plasma expander for paracentesis for ascites Exclusion criteria: serum bilirubin level > 5 mg/dL, prothrombin activity < 40%, urinary sodium excretion > 10 mEq/day, severe hyponatraemia (< 125 mEq/L), serum creatinine > 2 mg/dL, platelet count <40,000/mm3, gastrointestinal haemorrhage within the previous 3 months, sepsis, hepatic encephalopathy, hepatic malignancy, terminal cardiac or respiratory disease, and treatment with propranolol for primary or secondary prophylaxis of portal hypertensive bleeding Experimental group – paracentesis and iv HES: 67 participants Control group – paracentesis and iv albumin: 68 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 46 and 47.97. Males (%): 80.6 and 76.5. Combined cirrhosis and fibrosis (%): 58.2 and 61.8. Posthepatitis (%): 28.4 and 25. Pure schistosomal (%): 13.4 and 13.2. Child‐Pugh score (mean): 10.98 and 11.01. MAP (mmHg): 90 and 90.5. Serum creatinine (mg/dL): 1.3 and 1.29. Serum sodium (mEq/L): 135 and 134.9. Serum albumin (g/dL): 2.9 and 2.9. Serum bilirubin (mg/dL): 1.50 and 1.49. Prothrombin activity (%): 67.4 and 67.5. PRA (ng/mL/h): 13.95 and 13.95. PAC (ng/dL): 148.8 and 147. Urine sodium (mEq/day): 5.85 and 5.55 |
|
| Interventions | On admission, participants were kept on a low‐sodium diet (50 mEq/day) and bed rest regimen. Diuretic administration was discontinued at least 5 days before paracentesis. No vasoactive drugs were allowed before or during the study. The allowed oral fluid intake did not exceed 1.5 L/day. Experimental group: one‐session nearly‐total paracentesis (performed in 4‐8 h) and simultaneous iv infusion of poly O‐2‐hydroxyethyl starch, average molecular weight 200,000, molar substitution 0.45‐0.55, in isotonic sodium chloride solution, Hemohes 6% (HES 6%). The infusion was given in a schedule similar to albumin infusion, using a solution containing 6 g per 100 mL of the solution. Control group: one‐session nearly‐total paracentesis (performed in 4‐8 h) and simultaneous iv albumin administration, 8 g/L of ascitic fluid removed, using 20% human albumin solution. Half of the required albumin was given during paracentesis and the other half was given 6‐8 h after paracentesis to avoid acute intravascular fluid overload. Volume of fluid removed (L): experimental group, 15.91; control group, 15.92. Duration of paracentesis (h): experimental group, 6.29; control group, 6.25 |
|
| Outcomes | The authors reported data on: ‐ death; ‐ recurrent episodes of massive ascites; ‐ renal impairment; ‐ hepatic encephalopathy; ‐ portal hypertensive bleeding; ‐ any other cirrhosis‐related complication. |
|
| Notes | Participants remained hospitalised for 7 days without diuretic therapy. Participants were then discharged from hospital on diuretic therapy. The 131 participants who were discharged from the hospital were followed closely until the end of the study or death. The follow‐up period started at the end of the first hospitalisation and lasted 6 months. Participants were examined at least weekly during the first month, monthly for the next two months, and bimonthly thereafter. If tense ascites developed, participants were treated with large‐volume paracentesis and the same plasma expander assigned at inclusion. Complications during hospitalisation: renal impairment (> 50% increase in plasma creatinine to a level > 1.5 mg/dL), hyponatraemia (decrease in sodium > 5 mEq/L to a level < 130 mEq/L), and hyperkalaemia (an increase > 1.5 mEq/L to a level > 5.5 mEq/L) Post‐paracentesis circulatory dysfunction: increase in PRA of more than 50% of pre‐treatment value to a level > 4 ng/mL/h on the first day after paracentesis Duration of hospital stay (days): experimental group, 12.01; control group, 11.98 Unknown for‐profit funding No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generated by statistical software |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described, so that intervention allocation may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The study authors did not report any serious adverse events. |
| Other bias | Low risk | No other bias identified |
Al Sebaey 2012.
| Methods | RCT comparing other plasma expanders or vasoconstrictors versus albumin for the prevention of paracentesis‐induced circulatory dysfunction in cirrhosis | |
| Participants | One hundred and twenty‐five cirrhotic patients (69 males, mean age 50 years) treated with large volume paracentesis for tense ascites Inclusion criteria: absence of hypertension, cardiac or respiratory disease, encephalopathy, sepsis, SBP, creatinine > 1.5 mg/dL and GI bleeding We analysed the three arms HES, low dose albumin, and standard dose albumin out of the five arms including also terlipressin and midodrine. Experimental group (group HES in the RCT) ‐ large volume paracentesis and iv HES: 25 participants Control group (standard dose albumin and low dose albumin in the RCT) ‐ large volume paracentesis and iv albumin: 50 participants (25 participants standard dose albumin and 25 participants low dose albumin) |
|
| Interventions | Experimental group: LVP and iv HES (130/0.4 6% iv solution, 8 g/L of ascites removed, 50% within 2 hours and 50% 6 hours after paracentesis) Control group: LVP and iv albumin (standard dose: 20% solution, 6 g/L of ascites removed, 50% within 2 hours and 50% 6 hours after paracentesis; low dose: 2 g/L of ascites removed) Volume of ascites removed was 13 ± 0.14 L/participant (all five arms) and was not different between groups. |
|
| Outcomes | Incidence of paracentesis‐induced circulatory dysfunction | |
| Notes | Abstract Plasma renin activity (PRA) was assessed at baseline and on day 6. Paracentesis‐induced circulatory dysfunction: increase in PRA > 50% of pretreatment value on day 6 Medication cost was significantly less in all groups compared to standard albumin. Without for‐profit funding No sample size calculation Letter sent. Reply not received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data |
| Selective reporting (reporting bias) | High risk | The study authors did not report most predefined outcomes. It was planned to assess PPCD and the trial had a follow‐up of 6 days. |
| Other bias | Unclear risk | Not evaluable |
Khan 2015.
| Methods | RCT comparing polygeline and albumin after paracentesis in cirrhotic patients | |
| Participants | Fifty cirrhotic patients with tense ascites were enrolled. Inclusion criteria: cirrhosis with tense ascites; serum bilirubin < 10 mg/dL; serum creatinine < 3 mg/dL; PT < 4 seconds prolonged (< 40% of normal); platelets > 40,000/microL Exclusion criteria: hepatocellular carcinoma; hepatic encephalopathy; recent upper GI bleed, or subacute bacterial peritonitis Experimental group ‐ paracentesis and iv polygeline: 25 participants Control group ‐ paracentesis and iv albumin: 25 participants Baseline characteristics (values shown are mean where appropriate) Age (yr): 51.2 and 53. Males (%): 53.2 and 26.6. Weight (kg): 49.8 and 52.4. HCV‐related cirrhosis (%): 100 and 100. Previous encephalopathy (%): 12 and 20. Previous upper gastrointestinal bleeding (%): 16 and 12. Recurrent ascites (%): 48 and 44. Serum bilirubin (mg/dL): 1.9 and 2.3. Prothrombin (sec): 14.3 and 14.6. Serum albumin (g/L): 31 and 29; Urea (mg/dL) 45.2 and 43.7. Serum creatinine (mg/dL): 1.3 and 1.4. Serum sodium (mEq/L): 134.9 and 134.5. Mean arterial pressure (MAP) (mmHg): 98.2 and 99 |
|
| Interventions | Experimental group: paracentesis followed by iv infusion of polygeline (125 ml/L of fluid removed) Control group: paracentesis followed by iv infusion of albumin (6 g/L of fluid removed) Half of the dose was given rapidly over half an hour and the remaining half slowly over another four hours. Volume of ascites removed (mean): experimental group, 4.8 L; control group, 3.5 L |
|
| Outcomes | One day before, the procedure samples for urea, creatinine, blood glucose, and electrolytes were taken and these were repeated six days later. Mean arterial pressure (MAP) and heart rate were measured before and after the procedure. | |
| Notes | The authors reported that in the control group, one participant developed signs of ileus and peritonitis 48 hours after the procedure. The participant was managed with iv antibiotics and conservative measures. The participant was discharged in good condition. Without for‐profit funding No sample size calculation We emailed Khan and colleagues on 3 Jan 2017. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess whether blinding was likely to induce bias on the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | The study was planned to assess events after a short follow‐up. Data were obtained from the authors. |
| Other bias | Unclear risk | Not evaluable |
AFI: ascitic fluid filtration and concentrate infusion ANF: atrial natriuretic factor ARCA: apheresis and reinfusion of concentrated ascites BUN: blood urea nitrogen HBsAg: hepatitis B surface antigen HCC: hepatocellular carcinoma HCV: hepatitis C virus HES: hydroxyethyl starch HVPG: hepatic venous pressure gradient iv: intravenous K: potassium LVP: large‐volume paracentesis MAP: mean arterial pressure Na: sodium NOx: nitric oxide metabolites PA: plasma aldosterone PAC: plasma aldosterone concentration PARA: total paracentesis plus intravenous albumin PICD: paracentesis‐induced circulatory dysfunction PMN: polymorphonuclear cells PPCD: post‐paracentesis circulatory dysfunction PRA: plasma renin activity PT: prothrombin time RCT: randomised clinical trial RP: repeated paracentesis plus albumin infusion SBP: spontaneous bacterial peritonitis TPRA: total paracentesis with reinfusion of filtered and concentrated ascitic fluid TTP: total therapeutic paracentesis TVP: total‐volume paracentesis U: urinary WBC: white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antillon 1990 | It was published as an abstract, but because of insufficient information provided in the abstract, we could not use any of the data. We could find no subsequent full text publication, reporting on the finalised trial. In Antillon 1991 (see below), the study was defined as an "ongoing" trial. The abstract read that albumin infusion was compared with no plasma expander after large volume paracentesis in people with cirrhosis and refractory ascites. The authors reported 3 deaths out of 7 participants (43%) in the albumin group and 2 deaths out of 7 participants (29%) in the no plasma expander group. Despite our email and letter inquiries, sent to the authors of the study, we could not obtain any further information on the trial. Letter sent on August 2015 |
| Antillon 1991 | A letter of comment to Planas 1990 study (included in our review). In this letter of comment, Antillon et al were referring to an “ongoing trial” with 28 participants. They reported one‐year survival of 45% in the albumin group and 41.6 % in the no plasma expander group as well as incidence of hepatorenal syndrome of 50% in the albumin group and 33% in the no plasma expander group. However, Antillon 1991 did not report the number of participants in each group. Because of insufficient information on design, trial conduct, and discrepant results with what was already published in the Antillon 1990 (abstract), we excluded the trial. Despite our email and letter inquiries, sent to the authors of the study, we could not obtain any further information on the trial. Letter sent on August 2015 |
| Nasr 2010 | Non‐RCT: Prospective study comparing albumin versus dextran‐70 after large volume paracentesis in patients with massive hepatic ascites |
| Zaak 2001 | Non‐RCT: Prospective study comparing human albumin versus reinfusion of ultrafiltrate‐ascitic fluid after total paracentesis in cirrhotic patients with tense ascites |
RCT: randomised clinical trial
Characteristics of ongoing studies [ordered by study ID]
EudraCT 2010‐019783‐37.
| Trial name or title | Two doses of albumin after large paracentesis in cirrhotics with refractory ascites: a randomized study |
| Methods | Randomised controlled trial, open‐label |
| Participants | Patients with decompensated liver cirrhosis and refractory ascites indicated for large (more then 5 L) volume paracentesis Principal inclusion criteria: subject is 18 years of age or older; male or non‐pregnant female; decompensated liver cirrhosis with refractory ascites and indication for paracentesis more then 5 L; informed consent Principal exclusion criteria: women who are pregnant or lactating; known or suspected hypersensitivity to albumin; acute coronary artery disease; pulmonary oedema, decompensation of heart failure; documented sustained severe hypertension (systolic blood pressure > 200 mmHg or diastolic blood pressure > 110 mmHg) at enrolment; subject has been previously enrolled in this study; renal and postrenal anuria; bleeding form oesophageal varices in last six months; severe anaemia (Hb < 80 g/L); haemorrhagic diathesis; known active malignancy or other serious medical comorbidity such that the subject's life expectancy is < 12 months; patients with ongoing significant active alcohol abuse; subject is part of the staff personnel directly involved with this study, or is a family member of the investigational staff |
| Interventions | Arm A Large volume paracentesis + iv albumin infusion, 5 g/L ascites removed Arm B Large volume paracentesis + iv albumin infusion, 10 g/L ascites removed |
| Outcomes | Survival; renal functions; Child‐Pugh, MELD score; albumin level; blood pressure, heart rate, vasopressor/s need; cardiac output/index, BNP level; time to readmission to the hospital during follow‐up |
| Starting date | Information not available |
| Contact information | Information not available |
| Notes | Planned number of subjects to be included: 100 participants Trial status: ongoing Sponsor information: Name of sponsor: Hospital Trebic; Country: Czech Republic; Status of the sponsor: non‐commercial |
NCT03202524.
| Trial name or title | Fresh frozen plasma as a substitute for albumin in patients receiving a large volume paracentesis |
| Methods | Randomised controlled trial, open‐label |
| Participants | Inclusion Criteria: age 18 years or older; cirrhosis of the liver based on biopsy or clinical and radiographic criteria; ability to provide informed consent (Grade 0 to 1 HE); grade 3 ascites or refractory ascites; ascites requiring frequent large volume paracentesis of at least 5 litres at least once a month; no diuretic use Exclusion Criteria: inability to obtain informed consent; age less than 18; hepatic encephalopathy grade > 1; septic shock; active infection; respiratory failure; heart failure with reduced ejection fraction of ≤ 50%; moderate or severe pulmonary hypertension; history of stroke; unstable coronary artery disease; chronic kidney disease (GFR < 60); GI bleed within 2 weeks; any licorice within 2 weeks of starting the study; any beta blocker use within the last 2 weeks; any diuretic use within 2 weeks; absence of paracentesis within 2 weeks; absence of volume expanders within 2 weeks; INR > 1.7 |
| Interventions | Active comparator: albumin
Participants undergoing large volume paracentesis will receive 50 mL of 25% albumin for every 2 L of ascites removed from their abdomen. Experimental: fresh frozen plasma Participants undergoing large volume paracentesis will receive 2 units of FFP for the first 4 L of removed ascites followed by 50 mL of 25% albumin for every additional 2 L of removed ascites. |
| Outcomes | Primary outcome measures: Incidence of post‐paracentesis circulatory dysfunction (PPCD) [time frame: 6 days], defined as an increase in plasma renin activity of more than 50% of baseline to > 4 ng/mL/h on the 6th day post paracentesis |
| Starting date | July 2017 |
| Contact information | Jacques C Beauvais, MD, 347‐610‐7305, jabeauva@montefiore.org Samuel Sigal, MD, 718‐920‐4768, ssigal@montefiore.org |
| Notes | Estimated enrolment: 100 participants Not yet recruiting Sponsors and Collaborators: Montefiore Medical Center Principal Investigator: Samuel Sigal, Montefiore Medical Center |
BNP: brain natriuretic peptide FFP: fresh frozen plasma GFR: glomerular filtration rate HE: hepatic encephalopathy INR: international normalised ratio MELD: model for end‐stage liver disease PPCD: post‐paracentesis circulatory dysfunction
Differences between protocol and review
The protocol part of the review was updated according to updated Cochrane and CHBG methodology and requirements and GRADE methodology.
We analysed other plasma expanders as experimental treatments and albumin as the control treatment despite the fact that we found no evidence that albumin provided any benefits.
According to suggestions of CHBG, we included quasi‐randomised trials, which were originally excluded for consideration of harms. This is because adverse events may be not caught in small or even large randomised clinical trials. We excluded observational studies.
Trials in people with spontaneous bacterial peritonitis (SBP) and acute‐on‐chronic liver failure (ACLF) are now excluded. Both conditions, the latter defined only recently, have peculiar pathophysiological, clinical, and prognostic features which could require a separate evaluation.
The types of plasma expander included have been increased. For example, mannitol and fresh frozen plasma are now included, because some trials using these treatments were retrieved by the systematic search.
In the definition of serious adverse events, we have excluded those for which definition of other liver‐related complications can be applied, and this outcome is now independently evaluated. In this way, we classified as independent outcomes (secondary and exploratory) renal impairment, recurrence of ascites, hypotension, hyponatraemia, and post‐paracentesis circulatory dysfunction. Morever, we have changed 'renal failure' to 'renal impairment', because the latter term is commonly used by the trials' authors. Renal impairment was defined more than 30 years ago, by a serum creatinine value greater than or equal to 1.5 mg/dl because this value was considered an index of glomerular filtration rate lower or equal to 40 mL/min
The number needed to treat for an additional beneficial outcome (NNTB) was chosen in the protocol to aid interpretation of the results. Considering the very low certainty of the evidence according to GRADE assessment, we abstained from calculating an adjunctive measure of effect.
According to CHBG, we planned to conduct Trial Sequential Analyses as a sensitivity analysis of our GRADE imprecision assessment.
To answer the specific questions regarding the effect of the plasma expanders in trials with or without refractory ascites, with different types of plasma expanders, modality of paracentesis, length of follow‐up, etc. we performed the subgroup analysis instead of a sensitivity analysis to obtain and compare the effect estimates in each subgroup.
Contributions of authors
RGS drafted the protocol, extracted the data, performed the analysis, and drafted the review. GP extracted the data, participated in the analysis, and revised the review. CG and DN revised the protocol and the review. GB revised the review. All authors approved the published review.
Sources of support
Internal sources
The Copenhagen Trial Unit, Copenhagen, Denmark.
Azienda Ospedaliera "Vincenzo Cervello", Palermo, Italy.
External sources
The Copenhagen Hospital Corporation's Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
The Danish Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
RGS: nothing to declare GP: nothing to declare CG: nothing to declare DN: nothing to declare GB: nothing to declare
New
References
References to studies included in this review
Abdel‐Khalek 2010 {published data only}
- Abdel‐Khalek EE, Arif S. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients With liver cirrhosis and tense ascites after large‐volume paracentesis. Journal of Hepatology 2010;52:S327. [Google Scholar]
- Abdel‐Khalek EE, Arif SE. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients with liver cirrhosis and tense ascites following large volume paracentesis. Arab Journal of Gastroenterology 2010;11:24‐9. [Google Scholar]
Al Sebaey 2012 {published data only}
- Al Sebaey A, Abdel‐Razek W, Bassuni A, Rewisha EA, Khalil M, Waked I, et al. Alternatives to albumin for prevention of paracentesis induced circulatory dysfunction (PICD) in cirrhosis. Hepatology 2012;56(S1):915A. [Google Scholar]
Altman 1998 {published data only}
- Altman C, Bernard B, Roulot D, Vitte R‐L, Ink O. Randomized comparative multicenter study of hydroxyethyl starch versus albumin as a plasma expander in cirrhotic patients with tense ascites treated with paracentesis. European Journal of Gastroenterology & Hepatology 1998;10:5‐10. [PUBMED: 9512946] [DOI] [PubMed] [Google Scholar]
Baik 2000 {published data only}
- Baik SK, Kim HS, Lee DK, Kim IH, Kwon SO. Hemodynamic changes after single large volume paracentesis (SLVP) in cirrhosis with tense ascites ‐ focusing on the effect of albumin as plasma expander. Korean Journal of Medicine 2000;58:276‐82. [Google Scholar]
Bertrán 1991 {published data only}
- Bertrán X, Fernández‐Bañares F, Planas F, Cabré E, Morillas R, León R, et al. Effect on the nutritional status of total paracentesis plus albumin or dextran‐70 i.v. infusion for the treatment of tense ascites in liver cirrhosis [Repercusión sobre el estado nutricional energético‐proteico de la paracentesis total asociada a la infusión de albúmina o dextano‐70 en el tratamiento de la ascitis a tensión en la cirrhosis hepática]. Revista Espanola de Enfermedades Digestivas 1991;79(5):320‐4. [PubMed] [Google Scholar]
Bruno 1992 {published data only}
- Bruno S, Borzio M, Romagnoni M, Battezzati PM, Rossi S, Chiesa A, et al. Comparison of spontaneous ascites filtration and reinfusion with total paracentesis with intravenous albumin infusion in cirrhotic patients with tense ascites. BMJ 1992;304:1655‐8. [PUBMED: 1633517] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Borzio M, Romagnoni M, Chiesa A, Rossi S, Pavesi S, et al. Spontaneous ascites filtration and reinfusion (SAFR) vs total paracentesis (TP) with i.v. albumin infusion in cirrhotic patients with tense ascites. Hepatology 1990;12:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Degoricija 2003 {published data only}
- Degoricija V, Zjacic‐Rotkvic V, Marout J, Sefer S, Troskot B. Clinical and neurohumoral response to posture, physical exercise, and ascites treatment in Child‐Pugh C liver cirrhosis: randomized prospective trial. Croatian Medical Journal 2003;44(2):178‐86. [PUBMED: 12698509] [PubMed] [Google Scholar]
- Degoricija V, Zjacic‐Rotkvic V, Troskot B, Šefer S. Postparacentesis neurohumoral changes and impairment of renal water excretion in Child‐Pugh C cirrhosis with and without volume replacement. Journal of Hepatology 2001;34:51. [Google Scholar]
Descos 1983 {published data only}
- Descos L, Gauthier A, Levy VG, Michel H, Quinton A, Rueff B, et al. Comparison of six treatments of ascites in patients with liver cirrhosis. A clinical trial. Hepato‐gastroenterology 1983;30:15‐20. [PUBMED: 6339343] [PubMed] [Google Scholar]
Fassio 1992 {published data only}
- Fassio E, Terg R, Landeira G, Abecasis R, Salemne M, Podesta A, et al. Paracentesis with dextran 70 vs paracentesis with albumin in cirrhosis with tense ascites. Results of a randomized study. Journal of Hepatology 1992;14(2‐3):310‐6. [PUBMED: 1380024] [DOI] [PubMed] [Google Scholar]
García‐Compeán 1993 {published data only}
- García‐Compeán D, Villareal JZ, Cuevas HB, Cantú DAG, Estrella M, Tamez EG, et al. Total therapeutic paracentesis (TTP) with and without intravenous albumin in the treatment of cirrhotic tense ascites: a randomized controlled trial. Liver 1993;13(5):233‐8. [PUBMED: 8259034] [DOI] [PubMed] [Google Scholar]
García‐Compeán 2002 {published data only}
- García‐Compeán D, Blanc P, Larrey D, Daures J‐P, Hirtz J, Mendoza E, et al. Treatment of cirrhotic tense ascites with dextran‐40 versus albumin associated with large volume paracentesis. A randomized controlled trial. Annals of Hepatology 2002;1(1):29‐35. [PUBMED: 15114293] [PubMed] [Google Scholar]
Ginès 1988 {published data only}
- Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology 1988;94(6):1493‐1502. [PUBMED: 3360270] [DOI] [PubMed] [Google Scholar]
Ginès 1996 {published data only}
- Ginès A, Fernández‐Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology 1996;111(4):1002‐10. [PUBMED: 8831595] [DOI] [PubMed] [Google Scholar]
- International Group for the Study of Ascites in Cirrhosis. Comparison of albumin, dextran‐70 and hemaccel in the prevention of effective hypovolemia in cirrhotic patients with ascites treated with total paracentesis. A randomized multicenter study. Hepatology 1995;22(4 Suppl):220A. [Google Scholar]
Graziotto 1997 {published data only}
- Graziotto A, Rossaro L, Inturri P, Salvagnini M. Reinfusion of concentrated ascitic fluid versus total paracentesis. A randomized prospective trial. Digestive Diseases and Sciences 1997;42(8):1708‐14. [PUBMED: 9286238] [DOI] [PubMed] [Google Scholar]
Hernández Pérez 1995 {published data only}
- Hernández Pérez RE, Aguilar Ramírez JR, Hernández Lopez JM, Gómez Maganda y Silva T. Total paracentesis and infusion of dextran 70 vs albumin in cirrhotic patients with tense ascites [Paracentesis masiva con reposición de dextran 70 vs albúmina en pacientes cirróticos con ascitis a tensión]. Revista de Gastroenterologia de Mexico 1995;60(1):22‐6. [PUBMED: 7543691] [PubMed] [Google Scholar]
Kang 1998 {published data only}
- Kang KE, Kim BH, Dong SH, Kim HJ, Chang YW, Lee JII, et al. The efficacy of hydroxyethyl starch as plasma expander in cirrhotic patients with tense ascites treated with large volume paracentesis. Korean Journal of Gastroenterology 1998;31:651‐60. [Google Scholar]
Khan 2015 {published data only}
- Khan MU, Rahim IU, Latif M. Hemaccel as a cheaper alternative to human albumin for plasma expansion during paracentesis in cirrhotic patients. Pakistan Journal of Medical and Health Sciences 2015;9(3):948‐51. [Google Scholar]
Luca 1995 {published data only}
- Luca A, García‐Pagán J, Bosch J, Feu F, Jiménez W, Ginés A, et al. Beneficial effects of intravenous albumin infusion on the hemodynamic and humoral changes after total paracentesis. Hepatology 1995;22(3):753‐8. [PUBMED: 7657279] [PubMed] [Google Scholar]
Mehta 1998 {published data only}
- Mehta NN, Abraham P, Ashar VJ, Shikare SS, Savant SS, Karnad DR, et al. Ascitic fluid filtration and intravenous infusion versus total‐volume paracentesis with infusion of plasma expander in cirrhosis with tense or intractable ascites. Indian Journal of Gastroenterology 1998;17(3):93‐6. [PubMed] [Google Scholar]
Méndez 1991 {published data only}
- Méndez C, Soriano G, Guarner C, Tomás A, Moyano D, Toro H, et al. Total paracentesis in cirrhotics with refractory ascites: albumin vs HES as plasmatic expanders. Journal of Hepatology 1991;13(Suppl 2):S145. [Google Scholar]
Moreau 2006 {published data only}
- Moreau R, Valla D‐C, Durand‐Zaleski I, Bronowicki J‐P, Durand F, Chaput J‐C, et al. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trial. Liver International 2006;26(1):46‐54. [PUBMED: 16420509] [DOI] [PubMed] [Google Scholar]
Planas 1990 {published data only}
- Planas R, Ginès P, Arroyo V, Llach J, Panés J, Vargas V, et al. Dextran‐70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology 1990;99(6):1736‐44. [PUBMED: 1699835] [DOI] [PubMed] [Google Scholar]
- Planas R, Llach J, Ginès P, Panés J, Vargas V, Salmeron JM, et al. Albumin versus dextran‐70 in cirrhotics with tense ascites treated with total paracentesis. Final results of a multicenter randomized comparative study. Journal of Hepatology 1990;11(Suppl 2):S49. [DOI] [PubMed] [Google Scholar]
Salerno 1991 {published data only}
- Salerno F, Badalamenti S, Lorenzano E, Moser P, Incerti P. Randomized comparative study of hemaccel vs albumin infusion after total paracentesis in cirrhotic patients with refractory ascites. Hepatology 1991;13(4):707‐13. [PUBMED: 1826281] [PubMed] [Google Scholar]
Simonetti 1991 {published data only}
- Rinaldi F, Simonetti RG, Maisano S, Dino O, Milazzo G, Luca A, et al. Refractory ascites in cirrhosis: treatment with total paracentesis and reinfusion of filtrated and concentrated ascitic fluid. Italian Journal of Gastroenterology 1990;22(4):261. [Google Scholar]
- Simonetti RG, Rinaldi F, Milazzo G, Dino O, Maisano S, Luca A, et al. Treatment of refractory ascites: a randomized prospective trial of total paracentesis with concentrated ascitic fluid reinfusion vs repeated paracentesis and albumin infusion. Italian Journal of Gastroenterology 1991;23(7):473. [Google Scholar]
Smart 1990 {published data only}
- Smart HL, Triger DR. A randomised prospective trial comparing daily paracentesis and intravenous albumin with recirculation in diuretic refractory ascites. Journal of Hepatology 1990;10(2):191‐7. [PUBMED: 2185298] [DOI] [PubMed] [Google Scholar]
Sola‐Vera 2003 {published data only}
- Sola‐Vera J, Minana J, Soriano G, Gonzalez B, Planella M, Lopez‐Balaguer JM, et al. Expansion with saline vs albumin in cirrhotic patients with tense ascites treated with total paracentesis. Result of a prospective randomized study. Gastroenterologia y Hepatologia 2001;24(2):90 A6. [Google Scholar]
- Sola‐Vera J, Miñana J, Ricart E, Planella M, González B, Torras X, et al. Randomized trial comparing albumin and saline in the prevention of paracentesis‐induced circultory dysfunction in cirrhotic patients with ascites. Hepatology 2003;37(5):1147‐53. [PUBMED: 12717396] [DOI] [PubMed] [Google Scholar]
Zhao 2000 {published data only}
- Zhao J, Yuan C, Wang D, LI X. Mannitolum infusion on cirrhotic patients with tense ascites treated by paracentesis. Chinese Medical Journal 2000;113(1):27‐30. [PUBMED: 11775204] [PubMed] [Google Scholar]
References to studies excluded from this review
Antillon 1990 {published data only}
- Antillon MR, Runyon BA, Steindel H, Montano AA. Is albumin infusion needed after large volume paracentesis in patients with refractory ascites?. Gastroenterology 1990;98:A565. [Google Scholar]
Antillon 1991 {published data only}
- Antillon MR, Runyon BA. Postparacentesis plasma expansion prevents asymptomatic laboratory abnormalities, but does it have any impact on morbidity or mortality?. Gastroenterology 1991;101(5):1455‐7. [PUBMED: 1936822] [DOI] [PubMed] [Google Scholar]
Nasr 2010 {published data only}
- Nasr G, Hassan A, Ahmed S, Serwah A. Predictors of large volume paracentesis induced circulatory dysfunction in patients with massive hepatic ascites. Journal of Cardiovascular Disease Research 2010;1(3):136‐44. [PUBMED: 21187868] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaak 2001 {published data only}
- Zaak D, Paquet KJ, Kuhn R. Prospective study comparing human albumin vs reinfusion of ultrafiltated‐ascitic fluid after total paracentesis in cirrhotic patients with tense ascites. Zeitschrift fur Gastroenterologie 2001;39(1):5‐10. [PUBMED: 11215366] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EudraCT 2010‐019783‐37 {unpublished data only}
- 2010‐019783‐37/CZ. Two doses of albumin after large paracentesis in cirrhotics with refractory ascites: a randomized study. clinicaltrialsregister.eu/ctr‐search/trial/2010‐019783‐37/CZ (first received 15 June 2010).
NCT03202524 {unpublished data only}
- NCT03202524. Fresh frozen plasma as a substitute for albumin in patients receiving a large volume paracentesis. clinicaltrials.gov/ct2/show/study/NCT03202524 (first received 28 June 2017).
Additional references
AASLD 2009
- Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49(6):2087‐107. [PUBMED: 19475696] [DOI] [PubMed] [Google Scholar]
AASLD 2012
- Runyon BA, AASLD. AASLD Practice Guideline. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology 2013;57(4):1651‐3. [PUBMED: 23463403] [DOI] [PubMed] [Google Scholar]
AIFA 2018
- Agenzia Italiana del Farmaco. Nota informativa importante sulla restrizione d’uso di HES. www.agenziafarmaco.gov.it/sites/default/files/IT_DHPC_HES_common.pdf (accessed prior to 16 June 2019).
AISF 2016
- Italian Association for the Study of the Liver (AISF), Italian Society of Transfusion Medicine and Immunohaematology (SIMTI). AISF‐SIMTI position paper: the appropriate use of albumin in patients with liver cirrhosis. Digestive and Liver Disease 2016;48(1):4‐15. [PUBMED: 26802734] [DOI] [PubMed] [Google Scholar]
Al Sebaey 2013
- Al Sebaey A, Razek WA, Bassuni A, Khalil M, Rewisha E, Waked I. Low‐dose albumin is equally effective as standard dose albumin in preventing PICD following large volume paracentesis (LVP). Hepatology International 2013;7 (Suppl 1):S451. [Google Scholar]
Alderson 2002
- Alderson P, Bunn F, Lefebvre C, Li Wan Po A, Li L, Roberts I, et al. the Albumin Reviewers. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001208; PUBMED: 11869596] [DOI] [PubMed] [Google Scholar]
Alessandria 2011
- Alessandria C, Elia C, Mazzabotta L, Risso A, Andrealli A, Spandra M, et al. Prevention of paracentesis‐induced circulatory dysfunction in cirrhosis: standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Digestive and Liver Disease 2011;43(11):881‐6. [PUBMED: 21741331] [DOI] [PubMed] [Google Scholar]
Antillon 1993
- Antillon MR, Runyon BA. Extracorporeal ultrafiltration and intravenous reinfusion of ascitic fluid vs. large‐volume paracentesis: a randomised controlled trial. Hepatology 1993;17(3):520‐2. [PUBMED: 8444427] [DOI] [PubMed] [Google Scholar]
Arora 2018a
- Arora V, Maiwall R, Thomas SS, Rajan V, Ali R, Kumar G, et al. Albumin decreases the incidence of paracentesis induced circulatory dysfunction with less than 5 litres of ascitic tap in acute on chronic liver failure (ACLF) patients: randomized controlled trial (NCT02467348). Journal of Hepatology 2018;68(S1):S42‐S43. [Google Scholar]
Arora 2018b
- Arora V, Maiwall R, Ali R, Kumar G, Thomas SS, Jain P, et al. Paracentesis induced circulatory dysfunction with modest paracentesis is reduced by albumin in acute‐on‐chronic liver failure: a randomized controlled trial (NCT02467348). Indian Journal of Gastroenterology 2018;37(S1):A17. [Google Scholar]
Arroyo 1996
- Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. International Ascites Club. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology 1996;23(1):164‐76. [PUBMED: 8550036] [DOI] [PubMed] [Google Scholar]
Arroyo 2016
- Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, et al. Acute‐on‐chronic liver failure in cirrhosis. Nature Reviews. Disease Primers 2016;2:16041. [PUBMED: 27277335] [DOI] [PubMed] [Google Scholar]
Bajaj 2018
- Bajaj JS, Tandon P, O'Leary JG, Biggins SW, Wong F, Kamath PS, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. American Journal of Gastroenterology 2018;113(9):1339. [PUBMED: 29880972] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Bapat 1998
- Bapat PP, Raine GJ. Fluid resuscitation with colloid or crystalloid solutions. Use of dextran‐70 for fluid resuscitation has been dying out. BMJ 1998;317(7153):278. [PUBMED: 9729073] [PubMed] [Google Scholar]
Beale 1998
- Beale RJ, Wyncoll DL, McLuckie A. Human albumin administration in critically ill patients. Analysis is superficial and conclusions exaggerated. BMJ 1998;317(7162):884. [PUBMED: 9786698] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
Bernardi 2012
- Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large‐volume paracentesis: a meta‐analysis of randomized trials. Hepatology 2012;55(4):1172‐81. [PUBMED: 22095893] [DOI] [PubMed] [Google Scholar]
Bernardi 2014
- Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Reply to PMID 22095893. Hepatology 2014;60(4):1443‐5. [PUBMED: 24585539] [DOI] [PubMed] [Google Scholar]
Bernardi 2018
- Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nature Reviews. Gastroenterology and Hepatology 2018;15(12):753‐64. [PUBMED: 30026556] [DOI] [PubMed] [Google Scholar]
Bernardi 2019
- Bernardi M, Zaccherini G, Caraceni P. Pro: the role of albumin in pre‐liver transplant management. Liver Transplantation 2019;25(1):128‐34. [PUBMED: 30346096] [DOI] [PubMed] [Google Scholar]
Biggins 2006
- Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence‐based incorporation of serum sodium concentration into MELD. Gastroenterology 2006;130(6):1652‐60. [PUBMED: 16697729] [DOI] [PubMed] [Google Scholar]
Boyer 2016
- Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, et al. Terlipressin plus albumin Is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 2016;150(7):1579‐89.e2. [PUBMED: 26896734] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Caraceni 2018
- Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long‐term albumin administration in decompensated cirrhosis (ANSWER): an open‐label randomised trial. Lancet 2018;391(10138):2417‐29. [PUBMED: 29861076] [DOI] [PubMed] [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7(1):110. [PUBMED: 30055658] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalmers 1998
- Chalmers I. Human albumin administration in critically ill patients. I would not want an albumin transfusion. BMJ 1998;317(7162):885. [PUBMED: 9786701] [PubMed] [Google Scholar]
Chen 2014
- Chen RP, Chen C, Yu JY, Huang XL, Zhou MT, Huang ZM. Trial sequence meta‐analysis can reject false‐positive result calculated from conventional meta‐analysis. Hepatology 2014;60(4):1442‐3. [PUBMED: 24585570] [DOI] [PubMed] [Google Scholar]
Cochrane Injuries Group Albumin Reviewers 1998
- Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 1998;317(7153):235‐40. [PUBMED: 9677209] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corder 1998
- Corder AP. Fluid resuscitation with colloid or crystalloid solutions. Virtually identical article had appeared in Cochrane Library. BMJ 1998;317(7153):279. [PUBMED: 9729075] [PubMed] [Google Scholar]
D'Amico 1986
- D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Digestive Diseases and Sciences 1986;31(5):468‐75. [PUBMED: 3009109] [DOI] [PubMed] [Google Scholar]
D'Amico 2006
- D’Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. [PUBMED: 16298014] [DOI] [PubMed] [Google Scholar]
Di Pascoli 2019
- Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Long‐term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver International 2019;39(1):98‐105. [PUBMED: 30230204] [DOI] [PubMed] [Google Scholar]
EASL 2016
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. Journal of Hepatology 2016;64(2):433‐85. [PUBMED: 26597456] [DOI] [PubMed] [Google Scholar]
EASL 2018
- European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology 2018;69(2):406‐60. [PUBMED: 29653741] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedure. BMJ 1997;315(7121):1533‐7. [PUBMED: 9432252] [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2018
- European Medicines Agency. Hydroxyethyl starch solutions: CMDh introduces new measures to protect patients. www.ema.europa.eu/documents/referral/hydroxyethyl‐starch‐article‐107i‐referral‐hydroxyethyl‐starch‐solutions‐cmdh‐introduces‐new‐measures_en.pdf (accessed prior to 16 June 2019).
FDA 2013
- Food, Drug Administration. FDA safety communication: boxed warning on increased mortality and severe renal injury, and additional warning on risk of bleeding, for use of hydroxyethylstarch solutions in some settings. fffenterprises.com/assets/downloads/Article‐FDASafetyCommunicationBoxedWarning6‐13.pdf (accessed prior to 16 June 2019).
Fisher 1922
- Fisher RA. On the interpretation of X2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87‐94. [Google Scholar]
Frame 1998
- Frame JD, Moiemem N. Human albumin administration in critically ill patients. Statisticians not trained in burns care should not evaluate data. BMJ 1998;317(7162):884‐5. [PUBMED: 9786700] [PubMed] [Google Scholar]
Garcia‐Martinez 2013
- Garcia‐Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013;58(5):1836‐46. [PUBMED: 23423799] [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2018
- Garcia‐Tsao G. Long‐term albumin in cirrhosis: is it the ANSWER?. Lancet 2018;391(10138):2391‐2. [PUBMED: 29861077] [DOI] [PubMed] [Google Scholar]
Gartlehner 2018
- Gartlehner G, Nussbaumer‐Streit B, Wagner G, Patel S, Swinson‐Evans T, Dobrescu A, et al. Increased risks for random errors are common in outcomes graded as high certainty of evidence. Journal of Clinical Epidemiology 2018;106:50‐9. [DOI: 10.1016/j.jclinepi.2018.10.009; PUBMED: 30342970] [DOI] [PubMed] [Google Scholar]
Gentilini 1999
- Gentilini P, Casini‐Raggi V, Fiore G, Romanelli RG, Buzzelli G, Pinzani M, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. Journal of Hepatology 1999;30(4):639‐45. [PUBMED: 10207805] [DOI] [PubMed] [Google Scholar]
Ginés 1987
- Ginés P, Arroyo V, Quintero E, Planas R, Bory F, Cabrera J, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology 1987;93(2):234‐41. [PUBMED: 3297907] [DOI] [PubMed] [Google Scholar]
Ginés 2008
- Ginés P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology 2008;48(3):1002‐10. [PUBMED: 18671303] [DOI] [PubMed] [Google Scholar]
Gluud 2007
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. [PUBMED: 17316871] [DOI] [PubMed] [Google Scholar]
Gluud 2015
- Gluud C, Nikolova D, Klingenberg SL, Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)). CHBG Module. hbg.cochrane.org (accessed prior to 16 June 2019).
Goodman 1998
- Goodman NW. Human albumin administration in critically ill patients. Paper failed to mention earlier review. BMJ 1998;317(7162):884. [PUBMED: 9786699] [PubMed] [Google Scholar]
Gosling 1998
- Gosling P. Fluid resuscitation with colloid or crystalloid solutions. Newer synthetic colloids should not be abandoned. BMJ 1998;317(7153):277. [PUBMED: 9729070] [PubMed] [Google Scholar]
Guevara 2009
- Guevara M, Baccaro ME, Torre A, Gómez‐Ansón B, Ríos J, Torres F, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time‐dependent analysis. American Journal of Gastroenterology 2009;104(6):1382‐9. [PUBMED: 19455124] [DOI] [PubMed] [Google Scholar]
Guevara 2010
- Guevara M, Baccaro ME, Ríos J, Martín‐Llahí M, Uriz J, Ruiz del Arbol L, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: relevance of serum sodium concentration. Liver International 2010;30(8):1137‐42. [PUBMED: 20602681] [DOI] [PubMed] [Google Scholar]
Guevara 2012
- Guevara M, Terra C, Nazar A, Solà E, Fernández J, Pavesi M. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. Journal of Hepatology 2012;57(4):759‐65. [PUBMED: 22732511] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Haase 2013
- Haase N, Perner A, Hennings LI, Siegemund M, Lauridsen B, Wetterslev M, et al. Hydroxyethyl starch 130/0.38‐0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta‐analysis and trial sequential analysis. BMJ 2013;346:f839. [PUBMED: 23418281] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haase 2014
- Haase NR. Hydroxyethyl starch in sepsis. Danish Medical Journal 2014;61(1):B4764. [PUBMED: 24393593] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] [DOI] [PubMed] [Google Scholar]
Hartog 2014
- Hartog CS, Natanson C, Sun J, Klein HG, Reinhart K. Concerns over use of hydroxyethyl starch solutions. BMJ 2014;349:g5981. [PUBMED: 25385352] [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2015
- He B, Xu B, Xu X, Li L, Ren R, Chen Z, et al. Hydroxyethyl starch versus other fluids for non‐septic patients in the intensive care unit: a meta‐analysis of randomized controlled trials. Critical Care 2015;19:92. [PUBMED: 25886952] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. available from handbook.cochrane.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [PUBMED: 10480822] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA) 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
ICTRP 2011
- World Health Organization. International Clinical Trials Registry Platform (ICTRP). www.who.int/ictrp/en/ (accessed prior to 16 June 2016).
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [PUBMED: 25416419] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2017
- Jakobsen JC, Nielsen EE, Feinberg J, Katakam KK, Fobian K, Hauser G, et al. Direct‐acting antivirals for chronic hepatitis C. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD012143.pub3; PUBMED: 28922704] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2018
- Jakobsen JC, Nielsen EE, Koretz RL, Gluud C. Do direct acting antivirals cure chronic hepatitis C?. BMJ 2018;361:K1382. [PUBMED: 29748173] [DOI] [PubMed] [Google Scholar]
Jalan 2007
- Jalan R, Mookerjee RP, Cheshire LM, Williams R, Davies NA. Albumin infusion for severe hyponatremia in patients with refractory ascites: a randomized clinical trial. Journal of Hepatology 2007;46(1 Suppl):S95. [PUBMED: 17571340] 17571340 [Google Scholar]
Kaag 1998
- Kaag M, Zoetmulder FA. Human albumin administration in critically ill patients. More research into proper use of albumin is needed. BMJ 1998;317(7162):883. [PUBMED: 9786696] [PubMed] [Google Scholar]
Kim 2008
- Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. New England Journal of Medicine 2008;359(10):1018‐26. [PUBMED: 18768945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and dicrepancies between small and large randomized trials in meta‐analysis. Annals of Internal Medicine 2001;135(11):982‐9. [PUBMED: 11730399] [DOI] [PubMed] [Google Scholar]
Kwok 2013
- Kwok CS, Krupa L, Mahtani A, Kaye D, Rushbrook SM, Phillips MG, et al. Albumin reduces paracentesis‐induced circulatory dysfunction and reduces death and renal impairment among patients with cirrhosis and infection: a systematic review and meta‐analysis. Biomed Research International 2013;2013:1‐8. [DOI: 10.1155/2013/295153; PUBMED: 24222902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kütting 2017
- Kütting F, Schubert J, Franklin J, Bowe A, Hoffmann V, Demir M, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC‐free cirrhotic patients undergoing large volume paracentesis. Journal of Gastroenterology and Hepatology 2017;32(2):327–38. [PUBMED: 27149296] [DOI] [PubMed] [Google Scholar]
Landini 1985
- Landini S, Coli U, Fracasso A, Morachiello P, Righetto F, Scanferla F, et al. Spontaneous ascites filtration and reinfusion (SAFR) in cirrhotic patients. International Journal of Artificial Organs 1985;8(5):227‐80. [PUBMED: 4086118] [PubMed] [Google Scholar]
Lawler 1998
- Lawler PG, Morgan GA. Human albumin administration in critically ill patients. Modified editorial might have restrained media response. BMJ 1998;317(7162):885. [PUBMED: 9786702] [PubMed] [Google Scholar]
Lewis 2018
- Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD000567.pub7; PUBMED: 30073665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3; PUBMED: 28207928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makin 1998
- Makin A, Plenderleith L. Fluid resuscitation with colloid or crystalloid solutions. One conclusion could be that hypertonic saline is better than colloids in trauma. BMJ. 1998;317(7153):277‐8. [PUBMED: 9729071] [PubMed] [Google Scholar]
Martín‐Llahí 2008
- Martín‐Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 2008;134(5):1352‐9. [PUBMED: 18471512] [DOI] [PubMed] [Google Scholar]
McAnulty 1998
- McAnulty GR, Grounds RM. Fluid resuscitation with colloid or crystalloid solutions. Eight studies should have been excluded. BMJ 1998;317(7153):278. [PUBMED: 9729072] [PubMed] [Google Scholar]
McCormick 1990
- McCormick PA, Mistry P, Kaye G, Burroughs AK, McIntyre N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut 1990;31(2):204‐7. [PUBMED: 2311979] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohanty 2015
- Mohanty A, Garcia‐Tsao G. Hyponatremia and hepatorenal syndrome. Gastroenterology and Hepatology 2015;11(4):220‐9. [PUBMED: 27099594] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003;38(1):258‐66. [PUBMED: 12830009] [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Nadel 1998
- Nadel S, Marriage S, Munter C, Britto J, Habibi P, Levin M. Human albumin administration in critically ill patients. Review did not provide recommendations for alternative treatment. BMJ 1998;317(7162):882‐3. [PUBMED: 9786694] [PubMed] [Google Scholar]
Nel 1998
- Nel R. Human albumin administration in critically ill patients. Critical analysis of original studies has to take place. BMJ 1998;317(7162):882. [PUBMED: 9786693] [PubMed] [Google Scholar]
O'Brien 2019
- O'Brien A. Con: the unclear benefit of albumin. Liver Transplantation 2019;25(1):135‐9. [PUBMED: 30447173] [DOI] [PubMed] [Google Scholar]
Offringa 1998
- Offringa M. Excess mortality after human albumin administration in critically ill patients. Clinical and pathophysiological evidence suggests albumin is harmful. BMJ 1998;317(7153):223‐4. [PUBMED: 9677204] [DOI] [PMC free article] [PubMed] [Google Scholar]
O’Brien 2018
- O’Brien A, Kamath PS, Trotter J. MACHT – Outpatient albumin infusions do not prevent complications of cirrhosis in patients on the liver transplant waiting list. Journal of Hepatology 2018;69(6):1217‐8. [DOI: 10.1016/j.jhep.2018.09.025; PUBMED: 30314843] [DOI] [PubMed] [Google Scholar]
Perel 2013
- Perel P, Robert I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD000567.pub6; PUBMED: 23450531] [DOI] [PubMed] [Google Scholar]
Perner 2014
- Perner A, Haase N, Winkel P, Guttormsen AB, Tenhunen J, Klemenzson G, et al. Long‐term outcomes in patients with severe sepsis randomised to resuscitation with hydroxyethyl starch 130/0.42 or Ringer's acetate. Intensive Care Medicine 2014;40(7):927‐34. [PUBMED: 24807084] [DOI] [PubMed] [Google Scholar]
Petros 1998
- Petros A, Schindler M, Pierce C, Jacobe S, Mok Q. Human albumin administration in critically ill patients. Evidence needs to be shown in paediatrics. BMJ 1998; Vol. 317, issue 7162:882. [PUBMED: 9748194] [PMC free article] [PubMed]
Pugh 1973
- Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery 1973;60(8):646‐9. [PUBMED: 4541913] [DOI] [PubMed] [Google Scholar]
Qureshi 2016
- Qureshi SH, Rizvi SI, Patel NN, Murphy GJ. Meta‐analysis of colloids versus crystalloids in critically ill, trauma and surgical patients. British Journal of Surgery 2016;103(1):14‐26. [PUBMED: 26522616] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riordan 1998
- Riordan FA, Williams A, Thomson AP. Human albumin administration in critically ill patients. Albumin has been used in meningococcal disease. BMJ 317;7162:883. [PUBMED: 9786695] [PubMed] [Google Scholar]
Roberts 1998
- Roberts I. Human albumin administration in critically ill patients. Authors’ response. BMJ 1998;317(7162):886. [PubMed] [Google Scholar]
Roberts 1999
- Roberts I, Edwards P, McLelland B. More on albumin. Use of human albumin in UK fell substantially when systematic review was published. BMJ 1999;318(7192):1214‐5. [PUBMED: 10221965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2011
- Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD001208.pub4; PUBMED: 22071799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romanelli 2006
- Romanelli RG, Villa G, Barletta G, Vizzutti F, Lanini F, Arena U, et al. Long‐term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World Journal of Gastroenterology 2006;12(9):1403‐7. [PUBMED: 16552809] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz‐del‐Arbol 1997
- Ruiz‐del–Arbol L, Monescillo A, Jimenén W, Garcia‐Plaza A, Arroyo V, Rodés J. Paracentesis‐induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology 1997;113(2):579‐86. [PUBMED: 9247479] [DOI] [PubMed] [Google Scholar]
Runyon 1998
- Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology 1998;27(1):264‐72. [PUBMED: 9425946] [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [PUBMED: 17592831] [DOI] [PubMed] [Google Scholar]
Salerno 2013
- Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta‐analysis of randomized trials. Clinical Gastroenterology and Hepatology 2013;11(2):123‐30.e1. [PUBMED: 23178229] [DOI] [PubMed] [Google Scholar]
Saló 1997
- Saló J, Ginès A, Ginès P, Piera C, Jiménez W, Guevara M, et al. Effect of therapeutic paracentesis on plasma volume and transvascular escape rate of albumin in patients with cirrhosis. Journal of Hepatology 1997;27(4):645‐53. [PUBMED: 9365040] [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] [DOI] [PubMed] [Google Scholar]
Schierhout 1998a
- Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ 1998;316(7136):961‐4. [PUBMED: 9550953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schierhout 1998b
- Schierhout GH, Roberts I. Authors’ reply. BMJ 1998;317(7153):279. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Shwe 1998
- Shwe KH, Bhavnani M. Human albumin administration in critically ill patients. Some patients may benefit. BMJ 1998;317(7162):885‐6. [PUBMED: 9786703] [PubMed] [Google Scholar]
Sola 2012
- Solà E, Watson H, Graupera I, Turón F, Barreto R, Rodríguez E, et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. Journal of Hepatology 2012;57(6):1199‐206. [PUBMED: 22824819] [DOI] [PubMed] [Google Scholar]
Solà 2018
- Solà E, Solé C, Simón‐Talero M, Martín‐Llahí M, Castellote J, Garcia‐Martínez R, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo‐controlled trial. Journal of Hepatology 2018;69(6):1250‐9. [PUBMED: 30138685] [DOI] [PubMed] [Google Scholar]
Soni 1998
- Soni N. Human albumin administration in critically ill patients. Validity of review methods must be assessed. BMJ 1998;317(7162):883‐4. [PUBMED: 9786697] [PubMed] [Google Scholar]
Sort 1999
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz‐del‐Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. New England Journal of Medicine 1999;341(6):403‐9. [PUBMED: 10432325] [DOI] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non‐randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD012069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Student 1908
- Student. The probable error of a mean. Biometrika 1908;6(1):1‐25. [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [PUBMED: 20865104] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). www.ctu.dk/tsa/files/TSA_manual.pdf (accessed prior to 16 June 2019).
Thévenot 2015
- Thévenot T, Bureau C, Oberti F, Anty R, Louvet A, Plessier A, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. Journal of Hepatology 2015;62(4):822‐30. [PUBMED: 25463545] [DOI] [PubMed] [Google Scholar]
Titó 1990
- Titó L, Ginès P, Arroyo V, Planas R, Planés J, Rimola A, et al. Total paracentesis associated with intravenous albumin management of patients with cirrhosis and ascites. Gastroenterology 1990;98(1):146‐51. [PUBMED: 2293573] [DOI] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet. TSA ‐ Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen, Denmark: Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, 2011.
Vila 1998
- Vila MC, Solà R, Molina L, Andreu M, Coll S, Gana J, et al. Hemodynamic changes in patients developing effective hypovolemia after total paracentesis. Journal of Hepatology 1998;28(4):639‐45. [PUBMED: 9566833] [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang Y‐C, Feng M‐l. A meta‐analysis of albumin infusion in patients undergoing large‐volume paracentesis [大量放腹水后输注人体白蛋白疗效的 Meta分析]. Chinese Hepatology 2015;20(5):381‐5. [DOI: 10.14000/j.cnki.issn.1008-1704.2015.05.010] [DOI] [Google Scholar]
Watts 1998
- Watts J. Fluid resuscitation with colloid or crystalloid solutions. Comparing different studies is difficult. BMJ 1998; Vol. 317, issue 7153:277. [PUBMED: 9677228] [PMC free article] [PubMed]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [PUBMED: 28264661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkes 2001
- Wilkes M, Navickis RJ. Patient survival after human albumin administration. A meta‐analysis of randomized, controlled trials. Annals of Internal Medicine 2001;135(3):149‐64. [PUBMED: 11487482] [DOI] [PubMed] [Google Scholar]
Wong 2008
- Wong CL, Holroyd‐Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results?. JAMA 2008;299(10):1166‐78. [PUBMED: 18334692] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wyncoll 1998
- Wyncoll DL, Beale RJ, McLuckie A. Fluid resuscitation with colloid or crystalloid solutions. Conditions and patient groups were too heterogeneous to allow meaningful comparisons. BMJ 1998;317(7153):278‐9. [PUBMED: 9729074] [PubMed] [Google Scholar]


