Abstract
Background
Propolis from apiculture is known for wide range of medicinal properties owing to its vast chemical constituents including polyphenols, flavonoids and anticancer agent Caffeic acid phenethyl ester (CAPE).
Objectives
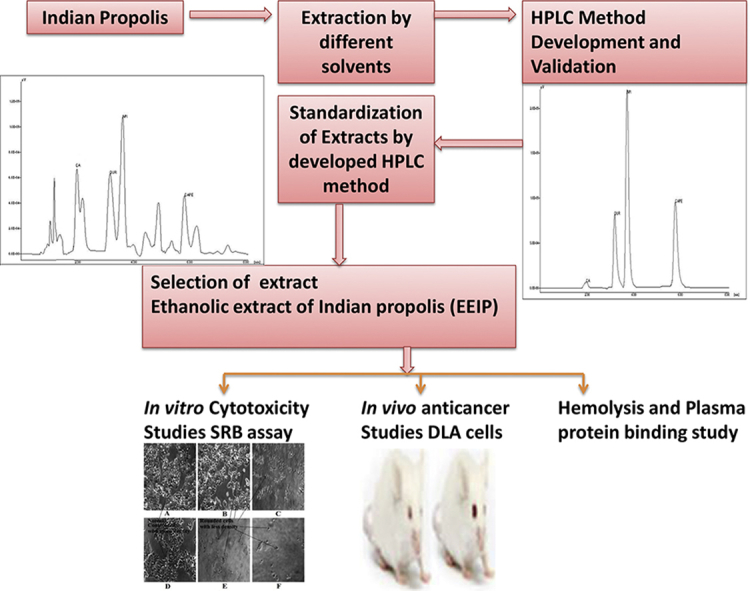
The objective of the study was to extract and standardize Indian propolis (IP) with respect to selected markers by newly developed High performance liquid chromatography (HPLC) method, to evaluate in vitro and in vivo anticancer activity and biosafety of Indian propolis.
Materials and methods
IP was extracted, optimized and standardized using a newly developed and validated HPLC method for simultaneous estimation of caffeic acid, apigenin, quercetin and CAPE. The standardised ethanolic extract of IP (EEIP) was screened for in vitro cytotoxicity using sulforhodamine B (SRB) assay, in vivo anti-carcinogenic effect against Dalton’s Lymphoma ascites (DLA) cells, hemolytic effect and pesticide analysis.
Results
The EEIP was found to contain more amount of total flavonoids (23.61 ± 0.0452 mg equivalent of quercetin/g), total polyphenolics (34.82 ± 0.0785 mg equivalent of gallic acid/g) and all selected markers except caffeic acid compared to all other extracts. EEIP showed better anti-cancer potential than CAPE on MCF-7 and HT-29 cell line and significant (p < 0.01) in vivo anti-carcinogenic effects against DLA in comparison with 5-fluorouracil. EEIP was found to be non-hemolytic.
Conclusion
From in vitro cytotoxicity, in vivo anti-carcinogenicity and biosafety studies it can be concluded that the standardized EEIP is safe and can be considered for further development as a biomedicine.
Keywords: Indian propolis, Caffeic acid phenethyl ester, Anticancer, HPLC
Graphical abstract
1. Introduction
Natural products have proved to be a rich source of various constituents that have found to possess antitumor activity and applications in cancer chemotherapy [1]. One such natural product, propolis is an important healthy food ingredient which has nutritional and medicinal properties obtained from apiculture [2], [3]. Physical appearance of propolis depends upon plants from which this resinous substance is collected. Generally propolis is yellowish brown to almost black in color. Propolis is naturally available in resinous and wax form. It is a sticky and gummy form derived from honey bees used as building and insulating material to honey combs. Its smell is pleasant due to honey, wax and vanilla but has a bitter taste. Its melting point is usually 60–70 °C [4]. The various complex chemical constituents possessed by propolis varies according to geographical origin and depends greatly upon bee-released and plant-derived compounds. Generally raw propolis contains resins (50%), waxes (30%), essential oils (10%), pollen (5%), and various organic compounds (5%). Till now, more than 300 different constituents were reported from propolis. Composition of propolis also varies depending upon collection time and place [5]. Propolis has been widely used for its nutritional and medicinal values since ancient time in Greece, Roman empire, Egypt and various countries. Even today it continues to be a popular remedy, as natural product and as a healthy food [6]. Stingless bees belong to Apidae family and these are exclusively observed in tropical and subtropical regions. They act as pollinators. They collect pollens and nectar from various medicinal plants which include Coco, palm, tulsi etc. [7]. Indian propolis is available throughout the India and according to different geographical origin its chemical composition varies. Various studies are reported on Indian propolis of different geographical region which includes Karnataka, West Bengal, Gujarat, Madhya Pradesh, Maharashtra and Rajasthan [5].
Apart from the use of propolis as a nutritional and healthy food constituent, its use as folk medicine in human health [8] has been reported from ancient time. Propolis of various regions is known to exhibit various activities including anti-fungal, antibacterial and anti-cancer activity [9], [10], [11]. Use of propolis as an anti-inflammatory, anti-fungal, anti-viral, anti-ulcer has been reported in Ayurveda, Homeopathy and acupuncture [12]. In Egypt, traditionally propolis was used as an anti-putrefactive and anti-pyretic agent. Greeks and Romans used propolis as a mouth disinfectant, antiseptic and wound healing agent. Therapeutic use of propolis was also continued by Arab physicians until Middle Age. London pharmacopoeia listed propolis as official drug in 17th century and it became popular as an antibacterial agent in Europe in 17th and 20th centuries. In World War II, propolis was employed for tuberculosis treatment. Apart from this propolis was also reported in treatment of wounds, healing, burns, sore throat and stomach ulcer. First scientific work related to chemical properties and composition of propolis was published and indexed to chemical abstracts in 1908 [13].
Various in vitro and in vivo studies on propolis and its isolated constituents [14] have been reported for anti-cancer activity. The anti-cancer activity of propolis is attributed to Caffeic acid phenethyl ester (CAPE) in addition to polyphenols and flavonoids [15], [16] CAPE is a specific inhibitor of NF-κB [16]. But isolation, purification or synthesis of CAPE is costly and tedious process. Various analytical methods have been reported for identification, separation of chemical constituents and standardization of Indian propolis extracts including HPLC, HPTLC and GC–MS. Chemical analysis methods have been also reported for quantification of polyphenols, flavonoids etc. [5], [17], [18], [19].
Raw propolis cannot be used for delivery due to its complex structure. Various solvents have been reported for commercial extraction which includes water, methanol, ethanol, dichloromethane, ether etc. These solvent systems are used for removal of inert material as well as extract specific compounds. Biological activity varies depending upon extraction of solvent and method used [13], [20], [21], [22], [23].
Although propolis is extensively studied globally, the research on Indian propolis is at infancy. There is lack of studies on extraction optimization, analytical method and biosafety study on Indian propolis except few [7], [18], [24], [25], [26], [27]. Hence, in the present study, an attempt has been made for extraction of Indian propolis by suitable method, standardization with respect to selected markers by newly developed reversed phase high performance liquid chromatography (RP-HPLC) method, evaluation of in vitro and in vivo anti-cancer activity to study synergistic effects of CAPE with other polyphenols and flavonoids and biosafety to explore Indian propolis as a biomedicine.
2. Materials and methods
2.1. Material
The Indian propolis sample collected in the month of December was purchased from local bee keeper from the Bharatpur region of Rajasthan, India and authenticated by Central Bee Research and Training Institute (CBRTI), Pune. Apigenin (> 99% purity) was purchased from Natural Remedies India Private Limited, Bangalore, India. Caffeic acid and CAPE (> 99% purity) were purchased from Sigma Aldrich, Bangalore, India. All reagents used were of analytical grade from Merck, India.
2.2. Total balsam content
1 g of crude propolis was accurately weighed and dissolved in 10 ml of ethanol, filtered and filtrate was evaporated to dryness until constant weight was obtained, and the ethanol soluble fraction was taken as percentage of balsam in the crude propolis sample [28].
2.3. Extraction and characterization of Indian propolis
2.3.1. Extraction
10 g of crude propolis was extracted with 30 ml of hexane by sonication for 30 min to remove the wax and filtered to obtain hexane extract of Indian propolis (HEIP). The mark was further extracted with ethyl acetate by sonication method and ethanol by Soxhlet method at 60 °C to obtain ethyl acetate extract of Indian propolis (EAEIP) and ethanolic extract of Indian propolis (EEIP) respectively. Further, mark was extracted with water by Soxhlet extraction at 100 °C to obtain water extract of Indian propolis (WEIP). All extracts were stored in 2–8 °C and used for further evaluation [24].
2.3.2. Total flavonoids and total polyphenol contents
Total flavonoids and polyphenol contents were determined by following methods reported by Marinova et al. and results were expressed in the form of mg equivalence of quercetin/g for flavonoids and mg equivalence of gallic acid/g for polyphenols [29], [30], [31].
2.4. Method development and validation
Individual stock solutions of caffeic acid, apigenin, quercetin and CAPE were prepared in ethanol to obtain 1 mg/mL solutions, diluted suitably to obtain working standards and stored in refrigerator (4 °C). 3 mg of extract was accurately weighed and dissolved in 1 ml of ethanol and diluted suitably to achieve concentration of 3000 μg/mL solution. HPLC separation was optimized using the aliquots of standard solutions ranging from 20–70 μg/mL for apigenin, caffeic acid and CAPE and 30–80 μg/mL for quercetin and analyzed. The proposed method was validated as per ICH guidelines (ICH Q2 (R1) 2005). Accuracy is determined by adding of known amount of analyte in the sample and expressed as % recovery. It was determined by calculating recovery of caffeic acid, apigenin, quercetin and CAPE by standard spiking method. For determination of intra-day and inter-day precision, solutions of 3 different concentrations were analyzed at 3 different time intervals in same day and different days and percent RSD was calculated.
Robustness of the method was determined by measuring the effect of small and deliberate changes in the analytical parameter on the retention time and peak area. The parameters selected were mobile phase concentration, flow rate and wavelength. While one parameter was altered remaining were kept constant. Standard deviation and percent standard deviation of peak area were calculated.
The limit of detection (LOD) and limit of quantitation (LOQ) were calculated as per ICH guidelines based on standard deviation of the response and the slope. All extracts were standardized for caffeic acid, apigenin, quercetin and CAPE using developed HPLC method [32], [33], [34], [35].
2.5. Total moisture content and pesticide analysis
Total moisture content of EEIP was determined using Mettle Toledo HB 43 Moisture analyzer. 3 g of raw propolis was kept on pan at 100 °C until constant weight was obtained.
Total pesticide content of EEIP was analyzed by Marco et al. using 410 Proster binary LC with 500 MS IT PDA detectors and EEIP was analyzed to check various types of pesticides [36].
2.6. In vitro anti-cancer study
In vitro anti-cancer study was carried out at Advanced Centre for Treatment, Research and Education in Cancer (ACTREC, Navi Mumbai). The study was carried out by in vitro Sulforhodamine B assay method. The cell lines of MCF-7 (Human breast cancer) and HT-9 (Colon cancer) were procured from NCCS, Pune, India. The cytotoxicity study protocol for in vitro Sulforhodamine B assay was followed by method described by Bothiraja et al.
EEIP and CAPE were diluted in the concentration ranges of 10, 20, 40 and 80 μg/mL which were analyzed for cytotoxicity using SRB assay. The cells were cultured in RPMI 1640 medium, supplemented with 10% v/v fetal bovine serum (FBS) and 2 mM L-glutamate. Cells were seeded at the density of 5 × 103 cells per well in 96-well plates using in situ fixing agent trichloroacetic acid (TCA). After 24 h of incubation at 37 °C with 100% relative humidity (RH), the growth medium was replaced with 100 μL of fresh medium containing various concentrations (10–80 μg/mL) of EEIP and CAPE. The culture medium without any drug formulation was used as a control. After 48 h incubation, assay was terminated by adding 50 μL of the cold TCA and incubated for 60 min at 4 °C. The medium was removed and washed with sterile PBS and dried. 50 μL of SRB solution (0.4% w/v in 1% acetic acid) was added to each well and further incubated for 20 min at room temperature. After staining, unbound dye was removed by washing with 1% acetic acid and plates were air dried. Bound stain was eluted with 10 mM trizma base and the absorbance was measured on an ELISA plate reader at a wavelength of 540 nm with 640 nm reference wavelength. Percent growth was calculated on a plate-by-plate basis for test results relative to control wells using the following equation: [37].
2.7. Hemolysis and plasma protein binding study
Hemolytic effect and the plasma protein binding ability of EEIP were evaluated using method described by Bothiraja et al. [37].
2.8. In vivo anti-cancer study
The study protocol was approved by Institutional Animal Ethics Committee of Bharati Vidyapeeth University, Poona college of Pharmacy, Pune, as per approval number CPCSEA/QA/06/2015-16. The study was carried out in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Male Swiss albino mice (20–25 g) were used for the study. They were acclimatized to laboratory environment at temperature 25 ± 2 °C and 12 h dark/light cycle. Diet and water was supplied. Amla Cancer Research Center, Trissur, Kerala, India supplied the Dalton's Lymphoma ascites (DLA) cells. The cells were intraperitoneally (i.p) transplanted and maintained in vivo in Swiss albino mice. DLA cells were aspirated from peritoneal cavity of the mice using saline while transforming the tumor cells to the grouped animal. Cell counts were conducted and further dilutions were made so that total cell count should be 1 × 106. Before starting treatments, tumor growth in the mice was allowed for minimum seven days. Animals were divided in to six groups of six each. Group 1 (G1) and Group 2 (G2) were named as the normal control and tumor control respectively. G1 and G2 were supplied with normal diet and water. Group 3 (G3) served as the positive control which was treated with injection 5-fluorouracil at 20 mg/kg body weight, intraperitoneally. G2–G6 were injected with DLA cells (1 × 106 cells per mouse) intraperitoneally. Group 4 (G4), Group 5 (G5) and Group 6 (G6) were treated with EEIP at a dose of 100, 200 and 400 mg/kg intraperitoneally.
After 24 h of inoculation, treatment was given once daily for 14 days. After treatment, all animals from each group were sacrificed by euthanasia. Retro-orbital plexus bleeding method was used for blood withdrawal from each mouse. Hematological parameters like RBC count, WBC count, platelet count, Hb content and packed cell volume; serum enzyme and lipid profile which include alkaline phosphatase (ALP), total cholesterol (TC), aspartate amino transferase (AST), triglycerides (TG), and alanine amino transferase (ALT); and derived parameters like life span (%), body weight, and cancer cell count were evaluated [38], [39], [40].
2.9. Statistical analysis
The results are expressed as mean ± SD. One way ANOVA followed by Newman–Keuls multiple comparison test was used for evaluation of the in vivo study data; p < 0.01 implied significance.
3. Results
3.1. Characterization of Indian propolis sample
Preliminary analysis revealed that crude Indian propolis was yellowish brown in color, and sticky. It had a typical odor with a bitter taste. The pollens of Brassica campestris, Eucalyptus species, Cocos nucifera, Punica grantanum and few grains belonging to Asterace family were present in Indian stingless bee propolis. Total balsam content was found to be 46% w/w. The total extraction yield of each extract was 18.23% w/w, 7.11% w/w, 49.00% w/w and 10.40% w/w for HEIP, EAEIP, EEIP and WEIP respectively. Total polyphenol content was found to be 18.06 ± 0.064 and 34.82 ± 0.078 mg equivalent of gallic acid/g in EAEIP and EEIP respectively. Flavonoid content of EEIP and EAEIP was found to be 23.61 ± 0.045 and 11.30 ± 0.011 mg equivalent of quercetin/g respectively.
3.2. Method development and validation
A mobile phase consisting of methanol and water (80:20 v/v) at flow rate of 1.0 mL/min using BDS Hypersil C18 (250 mm × 4.6 mm; 5 μ particle size) Thermo Scientific column was found to give desirable separation. Injection volume used was 10 μL, and the detection wavelength was set at 331 nm. Temperature was maintained at 25 °C ± 2 °C.
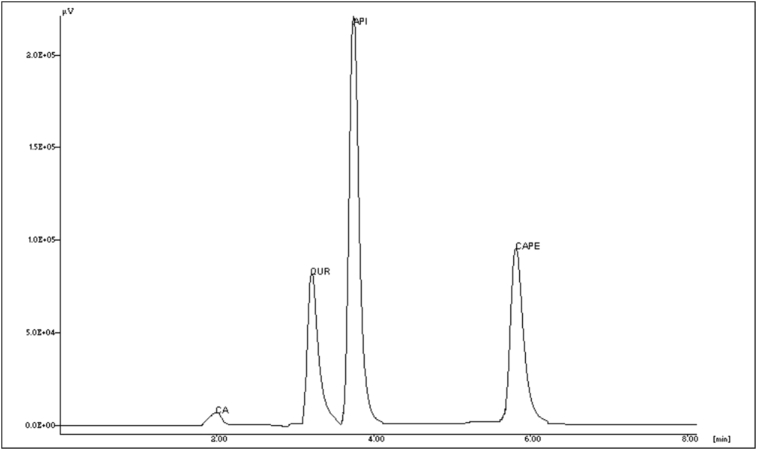
Acceptable response and detection of selected markers were obtained at wavelength 331 nm. Each run was followed by 10 min wash with methanol. Calibration curves were plotted which were found to (n = 3 repetitions of each point) be linear in the range of 20–70 μg/ml for caffeic acid, apigenin and CAPE, 30–80 μg/mL for quercetin and with good correlation co-efficient. Linear regression data, average retention time, LOD and LOQ for all markers is shown in Table 1. Representative chromatogram is shown in Fig. 1.
Table 1.
Summary of validation parameters.
| Parameters | Caffeic acid | Quercetin | Apigenin | CAPE |
|---|---|---|---|---|
| Linearity range (μg/mL) | 20–70 | 30–80 | 20–70 | 20–70 |
| Regression equation | y = 5024x + 1464 | y = 37,619x − 79,941 | y = 70,037x − 1,377,000 | y = 56,240x − 60,582 |
| r2 | 0.998 | 0.999 | 0.999 | 0.998 |
| Slope | 5024 | 37,619 | 70,037 | 56,240 |
| Intercept | 1464 | 79,941 | 1,377,000 | 60,582 |
| Retention time (min) | 1.993 ± 0.125 | 3.153 ± 0.073 | 3.757 ± 0.138 | 5.804 ± 0.069 |
| Theoretical plates | 2795 ± 0.088 | 3496 ± 0.078 | 5202.8 ± 0.898 | 7359.5 ± 0.188 |
| LOD (μg/mL) | 2.413 | 1.649 | 1.752 | 2.499 |
| LOQ (μg/mL) | 7.312 | 4.997 | 5.309 | 7.572 |
| Precision | ||||
| Intra-day | ≤ 2 | ≤ 2 | ≤ 2 | ≤ 2 |
| Inter-day | ≤ 2 | ≤ 2 | ≤ 2 | ≤ 2 |
| Accuracy (%) | 97.410–99.520 | 98.920–99.820 | 97.750–99.330 | 98.350–99.720 |
| Robustness | ≤ 2 | ≤ 2 | ≤ 2 | ≤ 2 |
r2 – square of correlation coefficient, LOD – limit of detection, LOQ – limit of quantitation, μg/mL – microgram per milliliter, % RSD – percent relative standard deviation.
Fig. 1.
RP-HPLC chromatogram of caffeic acid (CA), quercetin (QUR), apigenin (API) and caffeic acid phenethyl ester (CAPE).
The percent relative standard deviation (% RSD) for intra-day and inter-day precision for all four markers was found to be less than ≤ 2. Satisfactory recoveries for all four markers were obtained as shown in Table 1. The robustness result showed that the peak areas remain unaffected (% RSD ≤ 2) which indicates that the proposed method is robust.
3.3. Standardization of extracts
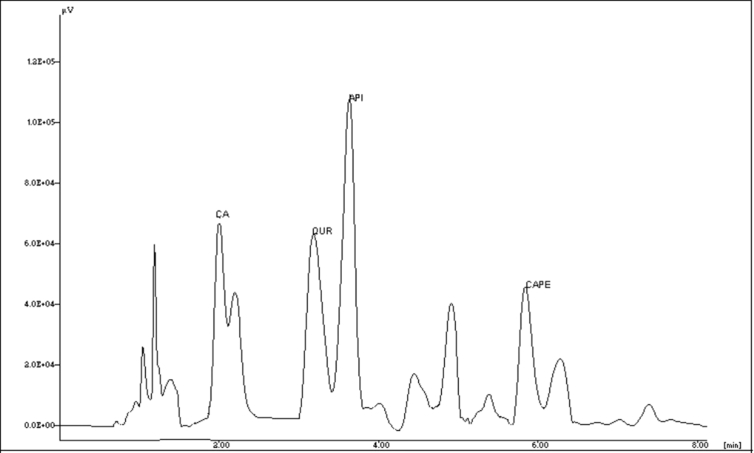
The percent amount of apigenin, quercetin and CAPE in EEIP was found to be 1.005 ± 0.070, 1.344 ± 0.021 and 0.677 ± 0.002 respectively. Amount of caffeic acid in WEIP was found to be 1.019% ± 0.016. Representative chromatograms are shown in Fig. 2.
Fig. 2.
RP-HPLC chromatogram of ethanolic extract of Indian propolis (EEIP) showing presence of caffeic acid (CA), quercetin (QUR), apigenin (API) and caffeic acid phenethyl ester (CAPE).
3.4. Moisture content and pesticide analysis
Total moisture content in crude propolis was 3.5% w/w. About 113 pesticides were tested including phorate, ediphenphos, dimethoate and tricyclazole. All pesticides were found to be absent in EEIP.
3.5. In vitro cell line study
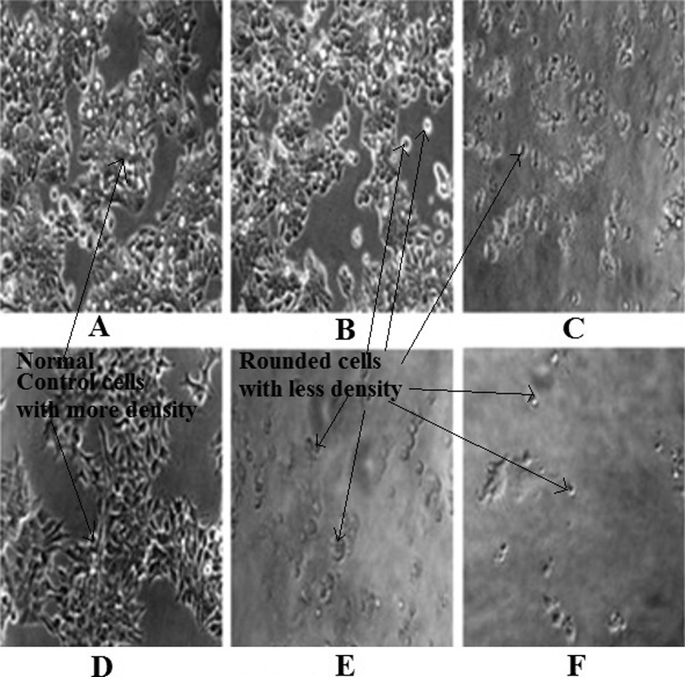
In vitro anti-cancer activity of EEIP was investigated and compared with the pure CAPE against human breast cancer MCF-7 and colon cancer HT-29 cells using in vitro SRB assay. The result illustrated in Table 2 indicates that EEIP showed better activity than pure CAPE. The total growth inhibition (TGI) concentration value of EEIP was found to be (31.10 μg/mL) and (39.90 μg/mL) whereas CAPE was 46.00 μg/mL and 47.20 μg/mL on MCF-7 breast cancer cells and HT-29 colon cancer cell lines respectively. Fig. 3(A–F) is the representative microscopic images obtained from in vitro anti-cancer studies on colon cancer cell line HT-29 and breast cancer cell line MCF-7. Results and microscopic images (Fig. 3(A–F)) showed that in Fig. 3(A&D) cells appeared more dense which are of normal control group of HT-9 and MCF-7 respectively. CAPE treated group on HT-9 and MCF-7 (Fig. 3B&E) showed less dense and rounded cells. Similarly more reduction in density and compact rounded cells were observed in EEIP treated group (Fig. 3C&F) on HT-29 and MCF-7 cells respectively.
Table 2.
TGI and GI50 values of ADR, CAPE and EEIP on HT-29 colon cancer cell line and MCF-7 breast cancer cell line.
| Samples | HT-29 cell line | HT-29 cell line | MCF-7 cell line | MCF-7 cell line |
|---|---|---|---|---|
| TGI (μg/mL) | GI50 (μg/mL) | TGI (μg/mL) | GI50 (μg/mL) | |
| ADR | < 10 | < 10 | < 10 | < 10 |
| CAPE | 47.20 ± 0.10 | 20.10 ± 0.050 | 46.00 ± 0.020 | 12.1 ± 0.010 |
| EEIP | 39.90 ± 0.020 | 16.50 ± 0.010 | 31.10 ± 0.015 | < 10 |
Values are presented as mean (n = 3).
EEIP – ethanolic extract of Indian Propolis; CAPE – caffeic acid phenethyl ester; ADR – adriamycin positive control; TGI – concentration of drug that produce total inhibition of cells; GI50 – concentration of drug that produce 50% inhibition of cells; MCF 7 – human breast cancer cell line; HT-29 – human colon cancer cell line; μg/mL – microgram per milliliter.
Fig. 3.
In vitro cytotoxicity study on HT-29 cell line: A) Normal control cells (HT-29), B) CAPE treated, C) EEIP treated and in vitro cytotoxicity study on MCF-7 cell lines, D) normal control cells (MCF-7), E) CAPE treated and F) EEIP treated.
3.6. Hemolysis study and plasma protein binding
The hemolytic potential of the EEIP was evaluated using optical density method. The result indicates that the hemolysis rates for 0.25, 0.5, 0.75, 1.0 and 1.5 mg/mL concentration of EEIP were 1.5, 2, 2.8, 3.4 and 4.1% respectively.
The plasma protein binding rate for EEIP was obtained as 57.34 ± 1.36%.
3.7. In vivo anti-cancer study
Average life span of animal was found to be 48% in the DLA tumor control group, whereas average life span after 5-FU treatment was found to be 96%. EEIP at a dose of 100, 200, 400 mg/kg body weight showed percent increase in life span (Table 3) and these values were significant (p < 0.01). EEIP at a dose of 100, 200, 400 mg/kg body weight showed significant reduction (p < 0.01) in percent increase in body weight, packed cell volume and viable tumor cell count of animals when compared to DLA tumor bearing mice. As shown in Table 4, WBC count increased and Hb count, RBC count and platelets count decreased in the DLA control group (G2) as compared to normal control group (G1). Treatment with EEIP at a dose of 100, 200, 400 mg/kg body weight showed reversed changes in these values to about normal level.
Table 3.
Effect of EEIP on the life span, body weight and cancer cell count of tumor induced mice.
| Treatment groups | Number of animals | Percent increase in life span | Increase in body weight (g) | Cancer cell count (mL × 106) |
|---|---|---|---|---|
| G1 | 6 | ≫30 days | 02.22 ± 0.68 | – |
| G2 | 6 | 48% | 9.44 ± 1.86a | 2.75 ± 0.80a |
| G3 | 6 | 96% | 5.66 ± 0.42b | 1.30 ± 0.22b |
| G4 | 6 | 88% | 5.45 ± 0.32b | 1.42 ± 0.30b |
| G5 | 6 | 89% | 5.36 ± 0.30b | 1.40 ± 0.28b |
| G6 | 6 | 90% | 5.30 ± 0.28b | 1.38 ± 0.24b |
G1 – normal control, G2 – cancer control, G3 – positive control, G4–G6– treatment control EEIP 100, 200, 400 mg/kg respectively.
All values are expressed as mean ± SD for 6 animals in each group.
One-way ANOVA followed by Newman–Keuls multiple comparison test.
Values are significantly different from normal control (G1) at p < 0.01.
Values are significantly different from cancer control (G2) at p < 0.01.
Table 4.
Effect of EEIP on Hematological parameters.
| Treatment groups | Total WBC (cells/ml × 103) | RBC count (millions/mm3) | Hb (g/Dl) | Packed cell volume (%) | Platelets (Lakhs/mm3) |
|---|---|---|---|---|---|
| G1 | 10.35 ± 1.05 | 4.55 ± 1.95 | 12.90 ± 1.95 | 14.25 ± 2.44 | 3.60 ± 0.95 |
| G2 | 15.30 ± 2.60a | 2.70 ± 0.98a | 6.80 ± 0.95a | 38.36 ± 3.35a | 1.70 ± 0.42a |
| G3 | 12.30 ± 1.34b | 4.05 ± 1.62b | 11.90 ± 1.48b | 16.40 ± 1.40b | 2.94 ± 0.50b |
| G4 | 12.12 ± 1.26b | 4.06 ± 1.50b | 12.22 ± 1.52b | 17.30 ± 2.36b | 3.30 ± 0.65b |
| G5 | 12.05 ± 1.22b | 4.08 ± 1.60b | 12.25 ± 1.55b | 17.24 ± 2.30b | 3.36 ± 0.68b |
| G6 | 11.85 ± 1.18b | 4.12 ± 1.65b | 12.35 ± 1.60b | 17.20 ± 2.26b | 3.40 ± 0.70b |
G1 – Normal control, G2 – Cancer control, G3 – Positive control, G4–G6 – Treatment control EEIP 100, 200, 400 mg/kg respectively.
All values are expressed as mean ± SD for 6 animals in each group.
One-way ANOVA followed by Newman–Keuls multiple comparison test.
Values are significantly different from normal control (G1) at p < 0.01.
Values are significantly different from cancer control (G2) at p < 0.01.
The inoculation of DLA cells caused significant (p < 0.01) increase in the level of serum enzyme parameters in the tumor control group (G2) in comparison with the normal control group (G1). The treatment with EEIP at the dose of 100, 200 and 400 mg/kg body weight showed reversed changes in these values towards the normal level (Table 5). The treatment with standard 5-FU gave similar results.
Table 5.
Effect of EEIP on serum enzymes and lipid proteins.
| Treatment groups | Cholesterol (mg/dl) | TGL (mg/dl) | AST (U/L) | ALT (U/L) | ALP (U/L) |
|---|---|---|---|---|---|
| G1 | 108.85 ± 3.05 | 136.85 ± 2.55 | 36.40 ± 1.65 | 31.28 ± 1.45 | 132.28 ± 2.08 |
| G2 | 146.95 ± 4.34a | 220.28 ± 4.40a | 78.6 ± 2.94a | 62.32 ± 2.60a | 265.30 ± 4.35a |
| G3 | 126.30 ± 3.84b | 169.15 ± 2.65b | 44.40 ± 1.72b | 34.52 ± 1.70b | 154.45 ± 2.40b |
| G4 | 117.26 ± 3.42b | 160.08 ± 2.55b | 42.44 ± 2.30b | 35.28 ± 1.55b | 162.45 ± 2.22b |
| G5 | 115.18 ± 3.38b | 156.25 ± 2.50b | 41.60 ± 2.20b | 34.90 ± 1.42b | 160.48 ± 2.18b |
| G6 | 113.36 ± 3.26b | 153.30 ± 2.46b | 40.90 ± 2.16b | 34.80 ± 1.38b | 158.45 ± 2.15b |
G1 – Normal control, G2 – Cancer control, G3 – Positive control, G4–G6 – Treatment control EEIP 100, 200, 400 mg/kg respectively, Total Cholesterol (TC), Triglycerides (TGL), Aspartate amino Transferase (AST), Alanine amino Transferase (ALT), Alkaline phosphatase (ALP), U/L – units per liter.
All values are expressed as mean ± SD for 6 animals in each group.
One-way ANOVA followed by Newman–Keuls multiple comparison test.
Values are significantly different from normal control (G1) at p < 0.01.
Values are significantly different from cancer control (G2) at p < 0.01.
4. Discussion
The new HPLC method was developed and validated for simultaneous estimation of selected markers. From the linear regression data it was found that the developed method is linear and sensitive. Baseline did not show any significant noise and there were no other interfering peaks around the retention time of caffeic acid, apigenin, quercetin and CAPE, indicating proposed RP-HPLC method is specific. The relative standard deviation values of the intra-day and interday precision study were within limit as per ICH guideline and method showed good precision. The proposed RP-HPLC method was found to be reliable for simultaneous quantification of selected markers and validation parameters are in the limits of ICH guidelines.
Results showed that the EEIP contains presence of apigenin, quercetin and CAPE whereas WEIP showed the presence of caffeic acid. Absence of markers was observed in hexane and ethyl acetate extracts.
In vitro anti-cancer activity of EEIP was investigated and compared with pure CAPE against human breast cancer MCF-7 and colon cancer HT-29 cells using in vitro Sulforhodamine B (SRB) assay. SRB assay is a well known and sensitive method for evaluating cytotoxic activity against both cancer and non-cancerous cell lines. It is advantageous over other contemporary cytotoxicity assays; it is independent of cell metabolic activity and also not interfered by test compounds. The GI 50 and TGI of EEIP were determined for two cancer cell lines by SRB assay. The cytotoxic ability of crude extracts can be attributed to their phytochemical constituents. The results obtained from GI 50, reveal that activity of both CAPE and EEIP was comparable to adriamycin and can be considered to have anti-cancer potential. The results showed that EEIP possesses comparatively better anti-cancer potential on MCF-7 breast cancer cell line than HT-29 human colon cancer cell line. Also, in both cell lines EEIP exhibited better anti-cancer potential than CAPE that may be because of synergistic activity of other polyphenols and flavonoids present in EEIP.
It has been postulated that polyphenols and flavonoids possess anti-cancer activity by several mechanisms including decrease of ROS, modulation of signaling pathways and down regulation of nuclear transcription factor kappa B (NF-κB). The reason for better growth inhibition on MCF-7 and HT-29 cell line might be due to synergistic effect of various polyphenols and flavonoids present in EEIP.
The acceptable hemolysis rate (less than 3%) [37] shown by EEIP denotes its non-hemolytic property up to 750 μg/ml. EEIP showed no or less effect on red blood cells. So the EEIP may be considered as biosafe for internal use. The plasma protein binding rate of EEIP was found in slightly higher range and indicates the need for development of suitable formulation to use EEIP internally as drug delivery system.
In in vivo anti-cancer activity, rapid increase in ascitic tumor volume was observed in DLA tumor bearing control group (G2). This ascitic fluid acts as a nutritional source for the growth of tumor cells [40]. Results showed that EEIP at a dose of 100, 200, 400 mg/kg body weight decreases the nutritional fluid volume, arrests the tumor growth and increases life span of DLA bearing mice which supports anti-tumor nature of EEIP. Myelosuppression and anemia are the common problems in cancer chemotherapy. Reduction in hemoglobin content results in anemia in tumor bearing mice because of iron deficiency, hemolysis or myelopathic conditions [41]. After treatment with EEIP at the dose of 100, 200 and 400 mg/kg, hemoglobin (Hb) content, RBC count, WBC count came to normal levels significantly. It indicates the protective action of EEIP at the dose of 100, 200 and 400 mg/kg on the hemopoietic system. The significantly elevated level of total cholesterol, TG, AST, ALT, ALP in serum of tumor inoculated animals indicated liver damage. EEIP at the dose of 100, 200 and 400 mg/kg significantly changed their levels to normal. Overall data supports the anti-tumor nature of EEIP. EEIP showed better in vitro and in vivo cytotoxicity potential on MCF-7 and HT-29 cell line as compared to CAPE which may be attributed to synergistic effects of various polyphenols and flavonoids in its composition.
5. Conclusion
New, simple, precise and reliable HPLC method for simultaneous estimation of caffeic acid, apigenin, quercetin and CAPE was developed and different extracts of Indian propolis have been standardized. EEIP was selected on basis of standardization and chemical analysis. The polyphenols and flavonoid rich EEIP exhibited better in vitro anti-cancer activity than pure CAPE, a potent anti-cancer constituent of propolis. Antitumor activity in vivo reveals that EEIP was effective in inhibiting the tumor progression, most likely because of synergistic activity of constituents present in the extract. However, the exact molecular mechanism by which EEIP mediates its anti-tumor activity is to be studied. From pesticidal analysis, hemolysis and plasma protein binding studies it can be concluded that the EEIP is safe for internal use and can be considered for development of suitable formulation. Based on the above promising results, further development of suitable formulation for CAPE and EEIP and its in vivo antitumor study are in process.
Sources of funding
This work was supported by University Grants Commission, Government of India for financial support through Major Research project (UGC-MRP) [grant numbers UGC-MRP, F.No.42-699/SR 2013].
Conflict of interest
None
Acknowledgement
Authors are thankful to Central Bee Research and Training Institute (CBRTI), Pune, India for authentication of Indian propolis and Advanced Centre of Treatment Research and Education in Cancer (ACTREC), Mumbai, India for in vitro cytotoxicity study.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Watanabe M.A.E., Amarante M.K., Conti B.J., Sforcin J.M. Cytotoxic constituents of propolis inducing anticancer effects: a review. J Pharm Pharmacol. 2011;63:1378–1386. doi: 10.1111/j.2042-7158.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Ajibola A., Chamunorwa J.P., Erlwanger K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr Metab (Lond) 2012;9(61):1–12. doi: 10.1186/1743-7075-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viuda-Martos M., Ruiz-Navajas Y., Fernandez-Lopez J., Perez-Alvarez J.A. Functional properties of honey, propolis and royal jelly. J Food Sci. 2008;73(9):R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 4.Jain A., Deepti D., Sonkusre S., Singh A., Taunk T., Sood P. Propolis: a boon of nature – an overview. Sch J Appl Med Sci. 2015;3(2E):873–877. [Google Scholar]
- 5.Wagh V.D. Propolis: a wonder bees product and its pharmacological potentials. Adv Pharmacol Sci. 2013;2013:11. doi: 10.1155/2013/308249. Article ID 308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan J., Lu Y., Abula S., Hu Y., Liu J., Fan Y. Optimization on preparation condition of propolis flavonoids liposomes by response surface methodology and research of its immunoenhancement activity. Evid Based Complement Altern Med. 2013;2013:1–8. doi: 10.1155/2013/505703. Article ID 505703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kothai S., Jayanthi B. A study on propolis of stingless bees reared from the most commercial hub of Chennai, Tamilnadu, India. Int Res J Environ Sci. 2015;4(7):39–47. [Google Scholar]
- 8.Mathivanan V., Shah G.N., Manzoor M., Mir G.M., Selvisabhanayakam A review on propolis – as a novel folk medicine. Indian J Sci. 2013;2(3):23–30. [Google Scholar]
- 9.Ngatu N.R., Saruta T., Hirota R., Eitoku M., Muzembo B.A., Nangana L.S. Antifungal efficacy of Brazilian green propolis extracts and honey on Tinea capitis and Tinea versicolor. Eur J Integr Med. 2011;3(4):e281–e287. [Google Scholar]
- 10.Seidel V., Peyfoon E., Watson D.G., Fearnley J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother Res. 2008;22(9):1256–1263. doi: 10.1002/ptr.2480. [DOI] [PubMed] [Google Scholar]
- 11.Choudhari M.K., Haghniaz R., Rajwade J.M., Paknikar K.M. Anticancer activity of Indian stingless bee propolis: an in vitro study. Evid Based Complement Altern Med. 2013;2013:1–10. doi: 10.1155/2013/928280. Article ID 928280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miguel M.G., Antunes M.D. Is propolis safe as an alternative medicine? J Pharm Bioallied Sci. 2011;3:479–495. doi: 10.4103/0975-7406.90101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagh V.D., Borkar R.D. Indian propolis: a potential natural antimicrobial and antifungal agent. Int J Pharm Pharm Sci. 2012;4(4):12–17. [Google Scholar]
- 14.Sawicka D., Car H., Halina M., Borawska H.M., Niklinski J. The anticancer activity of propolis. Folia Histochem Cytobiol. 2012;50(1):25–37. doi: 10.2478/18693. [DOI] [PubMed] [Google Scholar]
- 15.Orsolic N., Horvat A., Basic I. Proceedings of 37th international apiculture congress, Durban, South Africa. 2001. A comparison of antitumor activity of propolis and its related flavonoids. [Google Scholar]
- 16.Ozturk G., Ginis Z., Akyol S., Erden G., Gurel A., Akyol O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. Eur Rev Med Pharmacol Sci. 2012;16:2064–2068. [PubMed] [Google Scholar]
- 17.Naik D.G., Vaidya H.S., Namjoshi T.P. Essential oil of indian propolis: chemical composition and repellency against the honeybee Apis florea. Chem Biodivers. 2013;10:649–657. doi: 10.1002/cbdv.201200165. [DOI] [PubMed] [Google Scholar]
- 18.Thirugnanasampandan R., Raveendran S.B., Jayakumar R. Analysis of chemical composition and bioactive property evaluation of Indian propolis. Asian Pac J Trop Biomed. 2012;2(8):651–654. doi: 10.1016/S2221-1691(12)60114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar N., Mueen Ahmed K.K., Dang R., Shivananda T.N., Das K. GC–MS analysis of propolis of Indian origin. J Young Pharm. 2009;1:46–48. [Google Scholar]
- 20.Krol W., Czuba Z., Scheller S., Gabrys J., Grabiec S., Shani J. Anti-oxidant property of ethanolic extract of propolis (EEP) as evaluated by inhibiting the chemiluminescence oxidation of luminol. Biochem Int. 1990;21(4):593–597. [PubMed] [Google Scholar]
- 21.Li H., Kapur A., Yang J.X., Srivastava S., McLeod D.G., Paredes-Guzman J.F. Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its biological origin. Int J Oncol. 2007;31:601–606. [PubMed] [Google Scholar]
- 22.Kumar N., Ahmad M., Dang R., Husain A. Antioxidant and antimicrobial activity of propolis from Tamil Nadu zone. J Med Plant Res. 2008;2(12):361–364. [Google Scholar]
- 23.Kumar M.R., Bose V.S.C., Sathyabama S., Priyadarshini V.B. Antimicrobial and DPPH free radical-scavenging activities of the ethanol extract of propolis collected from India. J Ecobiotechnol. 2011;3(1):8–13. [Google Scholar]
- 24.Naik D.G., Vaidya H.S. Antioxidant properties of volatile oil of Indian propolis. J ApiProduct ApiMed Sci. 2011;3(2):89–93. [Google Scholar]
- 25.Kothai S., Jayanthi B. Anti cancer activity of silver nano particles bio-synthesized using stingless bee propolis (Tetragonula iridipennis) of Tamilnadu. Asian J Biomed Pharm Sci. 2014;4(40):30–37. [Google Scholar]
- 26.Shubharani R., Sivaram V., Kishore B.R. In-vitro cytotoxicity of Indian bee propolis on cancer cell lines. Int J Pharm Bio Sci. 2014;5(4):698–706. [Google Scholar]
- 27.Turnia I., Nongkhlaw F.M.W., Joshi S.R., Prasad S.B. Antibacterial and antitumor activity of methanolic extract of propolis from Meghalaya. World J Pharm Pharm Sci. 2015;4(11):1809–1821. [Google Scholar]
- 28.Popova M.P., Bankova V.S., Bogdanov S., Tsvetkova I., Naydensky C., Marcazzan G.L. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie. 2007;38(3):306–311. [Google Scholar]
- 29.Marinova D., Ribarova F., Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall. 2005;40(3):255–260. [Google Scholar]
- 30.Lagouri V., Prasianaki D., Krysta llI F. Antioxidant properties and phenolic composition of Greek propolis extracts. Int J Food Prop. 2014;17(3):511–522. [Google Scholar]
- 31.Rezzan A., Huseyin S., Omer E., Esra U., Sevgi K. Properties of phenolic composition and biological activity of propolis from Turkey. Int J Food Prop. 2013;16(2):277–287. [Google Scholar]
- 32.International Conference on Harmonization . 2005. ICH Q2 (R1), Validation of analytical procedures: text and methodology. [Google Scholar]
- 33.Singh R. HPLC method development and validation – an overview. J Pharm Educ Res. 2013;4(1):26–33. [Google Scholar]
- 34.Bruschi M.L., Franco S.L., Gremiao M.P.D. Application of a HPLC method for analysis of propolis extract. J Liq Chromatogr Relat Technol. 2003;26(14):2399–2409. [Google Scholar]
- 35.Rivero-Cruz B., Martinez-Chavez A. Development and validation of a RP-HPLC method for the simultaneous quantification of flavonoids markers in Mexican propolis. Food Anal Methods. 2015;8(2):413–419. [Google Scholar]
- 36.Marco G.D., Canuti L., Impei S., Leonardi D., Canini A. Nutraceutical properties of honey and pollen produced in a natural park. Agric Sci. 2012;3:187–200. [Google Scholar]
- 37.Bothiraja C., Kapare H.S., Pawar A.P., Shaikh K.S. Development of plumbagin-loaded phospholipid–Tween® 80 mixed micelles: formulation, optimization, effect on breast cancer cells and human blood/serum compatibility testing. Ther Deliv. 2013;4(10):1247–1259. doi: 10.4155/tde.13.92. [DOI] [PubMed] [Google Scholar]
- 38.Unnikrishnan M.C., Kuttan R. Tumor reducing and anti-carcinogenic activity of selected species. Cancer Lett. 1990;51:85–89. doi: 10.1016/0304-3835(90)90235-p. [DOI] [PubMed] [Google Scholar]
- 39.Sathiyanarayanan L., Shinnathambi A., Chidhambarnathan N. Anticarcinogenic activity of Leptadenia reticulata against Dalton's ascitic lymphoma. Iran J Pharmacol Ther. 2006;6:133–136. [Google Scholar]
- 40.Prasad S.B., Giri A. Anti tumor effect of cisplatin against murine ascites Dalton's lymphoma. Indian J Exp Biol. 1994;32:155–162. [PubMed] [Google Scholar]
- 41.Fenninger L.D., Mider G.B. Energy and nitrogen metabolism in cancer. Adv Cancer Res. 1954;2:229–253. doi: 10.1016/s0065-230x(08)60496-0. [DOI] [PubMed] [Google Scholar]