Abstract
Motivational incentives play an influential role in value-based decision-making and cognitive control. A compelling hypothesis in the literature suggests that the brain integrates the motivational value of diverse incentives (e.g., motivational integration) into a common currency value signal that influences decision-making and behavior. To investigate whether motivational integration processes change during healthy aging, we tested older (N=44) and younger (N=54) adults in an innovative incentive integration task paradigm that establishes dissociable and additive effects of liquid (e.g., juice, neutral, saltwater) and monetary incentives on cognitive task performance. The results reveal that motivational incentives improve cognitive task performance in both older and younger adults, providing novel evidence demonstrating that age-related cognitive control deficits can be ameliorated with sufficient incentive motivation. Additional analyses revealed clear age-related differences in motivational integration. Younger adult task performance was modulated by both monetary and liquid incentives, whereas monetary reward effects were more gradual in older adults and more strongly impacted by trial-by-trial performance feedback. A surprising discovery was that older adults shifted attention from liquid valence toward monetary reward throughout task performance, but younger adults shifted attention from monetary reward toward integrating both monetary reward and liquid valence by the end of the task, suggesting differential strategic utilization of incentives. Together these data suggest that older adults may have impairments in incentive integration, and employ different motivational strategies to improve cognitive task performance. The findings suggest potential candidate neural mechanisms that may serve as the locus of age-related change, providing targets for future cognitive neuroscience investigations.
Keywords: aging, motivation, cognitive control, reward, decision-making
Introduction
Healthy aging is associated with a myriad of changes across cognitive and motivational/affective processes (Mather, 2016; Salthouse, 2005); these psychological changes are accompanied by functional, structural and neuromodulatory alterations in the brain (Bäckman, Nyberg, Lindenberger, Li, & Farde, 2006; Guitart-Masip et al., 2016; Li & Rieckmann, 2014; Persson et al., 2006; Reuter-Lorenz & Park, 2010; Volkow et al., 1998). Despite the overwhelming evidence for the socioemotional, motivational, and cognitive changes that occur throughout lifespan development (Hess, 2014; Kensinger & Gutchess, 2017; Nielsen & Mather, 2011), researchers have only recently begun to investigate how the complex interactions between age, cognition, and motivation/affect influence decision-making and goal-directed behavior (Braver et al., 2014; Carstensen & Mikels, 2005; Denburg et al., 2007; Ferdinand & Czernochowski, 2018; Samanez-Larkin & Knutson, 2015). Much remains to be elucidated about how cognitive and motivational processes, both independently and through their interaction, influence decision-making and goal-directed behavior across the lifespan.
In particular, humans on a daily basis appear to seamlessly and intuitively integrate different types of motivational incentives to drive their cognitive and behavioral goals. For example, an individual who is expending mental effort to file their taxes to earn a monetary refund ma y be additionally motivated to more expediently complete this cognitively demanding task if they received a bonus chocolate reward for completing the task in a timely manner. However, few studies to date have systematically examined whether and how different categories of motivational incentives influence cognitive processes across the adult lifespan. Researchers are increasingly appreciating the utility of incorporating more primary incentives (e.g., food, sex, social, emotional) in studies (Chiew & Braver, 2016; Krug & Braver, 2014; Licen, Hartmann, Repovs, & Slapnicar, 2016), although to our knowledge, no studies to date have yet examined the effects of such primary incentives on cognition in healthy aging. This important gap in the literature reveals the clear need to explore the effects of non-monetary and biologically relevant incentives on cognition, which can also demonstrate whether such incentives are more effective for modulating cognitive processing in old age.
We had two main objectives for this study. First, we aimed to address the following compelling question: can age-related cognitive impairments be remediated via a “non-cognitive” route? Second, we asked whether and how the integration of value across diverse motivational incentives is altered in older adults. In particular, we address a major gap in the literature by explicitly comparing the effects of primary (e.g., food, drink, shocks) and secondary (e.g., money, points) motivational incentives on cognitive processing in healthy aging. Here, we propose a key distinction: primary incentives are tangible and have an immediate physical impact on the biological system, whereas secondary incentives are abstract and have a delayed impact on the same system. Critically, the motivational value of secondary incentives is typically associated with the knowledge that such incentives can be used to acquire primary rewards later in time. These key questions are discussed in greater detail in the following paragraphs.
Motivational Enhancement of Cognitive Control: Amelioration of Age-Related Cognitive Impairments in Healthy Aging?
A recent compelling hypothesis posits that motivationally relevant contexts can ameliorate the well established cognitive impairments often associated with healthy aging (Castel, 2007; Cohen, Rissman, Suthana, Castel, & Knowlton, 2014; Ferdinand & Czernochowski, 2018). Converging evidence in the cognitive aging literature suggests that age-related differences in task performance can be attributed to a decline in older adults’ ability to exert cognitive control (Braver & Barch, 2002; Kray & Ferdinand, 2014). Specifically, older adults demonstrate reduced ability to actively maintain internal representations of relevant task goals in working memory in a sustained and preparatory manner, and continually update these representations throughout dynamically changing environments or contexts (Braver & West, 2008; Jong, 2001; Manard, Carabin, Jaspar, & Collette, 2014). This ability to optimally utilize contextual information (e.g., task instructions or relevant information conveyed by stimulus cues) to anticipate task demands is referred to proactive control (Braver, 2012; Redick, 2014); older adults in particular appear to have greater difficulty with employing a proactive control strategy compared to younger adults. Notably, such age-related impairments are associated with reduced activation in the dorsolateral prefrontal cortex in functional MRI (Jimura & Braver, 2010; Lamichhane, McDaniel, Waldum, & Braver, 2018; Paxton, Barch, Racine, & Braver, 2008; Rypma & D’Esposito, 2000) and event-related potential studies (Adrover-Roig & Barceló, 2010; Karayanidis, Whitson, Heathcote, & Michie, 2011; Kopp, Lange, Howe, & Wessel, 2014), bolstering the hypothesis that healthy aging is associated with context processing deficits that involve proactive control.
Recent evidence also supports the provocative idea that motivational incentives can selectively enhance cognitive control (Botvinick & Braver, 2015; Yee & Braver, 2018). Prior work has consistently shown that performance-contingent reward incentives are likely to activate a proactive control mode promoting advance preparation to enhance performance on cognitively demanding tasks in younger adults (Chiew & Braver, 2016; Frober & Dreisbach, 2014). Thus, one promising potential route by which age-related cognitive deficits can be overcome is via the use of motivational incentives, a primary question targeted in this study. With regard to aging, such motivational interventions are hypothesized to be most effective if they provide positive feedback on task performance and with incentive cues presented in advance, to allow for sufficient preparation to overcome less efficient cognitive processing in old age (Ferdinand & Czernochowski, 2018; Schmitt, Kray, & Ferdinand, 2017). Moreover, older adult cognition may not be impaired per se, but rather it may be that older adults require greater motivation to attain optimal task performance levels. Thus, if incentives can improve performance in a cognitive control task in older adults, it would reveal that age-related cognitive decline is related to a strategic bias away from using proactive control. More optimistically, it would provide evidence that motivation can ameliorate cognitive impairments associated with aging, and that incentives can enhance and restore older adult cognitive task performance. Alternatively, if incentives do not affect cognitive task performance in older adults, then age-related cognitive deficits instead may relate to general decreased processing efficiency arising from an overall slowing across multiple processing operations critical for cognitive function (Eckert, Keren, Roberts, Calhoun, & Harris, 2010; Finkel, Reynolds, McArdle, & Pedersen, 2007; Salthouse, 1996, 2000).
A related follow-up question focuses on whether older and younger adults show similar characteristic patterns of improvement in cognitive task performance in relation to motivational value. In other words, if both older and younger adults are presented with motivational incentives and increased task demands to earn those incentives, do both groups show similar improvements in task performance? If so, then older adults may be applying different strategies and/or compensatory mechanisms to achieve the same level of performance as younger adults. Alternatively, if it were simply too demanding for older adults to modulate task performance based on changing motivational value, they would show a reduced effect of motivational incentives. Such a pattern would imply that the locus of age-related deficits precisely target the interaction between motivation and cognition control, rather than from either process in isolation.
Motivational Integration and Cognitive Control Interactions in Healthy Aging: Examining the Effects of Primary and Secondary Incentives
A second outstanding question relates to how the integration of different categories of motivational incentives (i.e., motivational integration) is altered throughout the lifespan. A well-established hypothesis for motivational integration is the common currency account, which posits that different types of ‘options’ or ‘goods’ are represented in the brain via a common coding format, to enable comparison of dissimilar goods (e.g., primary vs. secondary incentives) in terms of their subjective desirability (Loewenstein, Rick, & Cohen, 2008; Montague, King-casas, & Cohen, 2006; Rangel, Camerer, & Montague, 2008). Many studies have converged upon the finding that ventromedial prefrontal cortex, orbitofrontal cortex, and ventral striatum are central to the representation of subjective value (Chib, Rangel, Shimojo, & O’Doherty, 2009; this common currency mechanism is preserved across the lifespan is currently unknown.
In the aging literature, although converging evidence suggests that older adults prioritize meaningful and self-relevant behavioral goals that maximize emotional satisfaction (e.g., social and emotional outcomes that benefit well-being over monetary reward or increased knowledge; Carstensen, 2006), surprisingly few studies have examined the cognitive and neural mechanisms underlying how different incentive types putatively alter cognitive processes throughout the lifespan. Most studies of motivation in healthy aging have focused on the influence of monetary incentives on cognition and behavior (Harsay, Cohen, Reneman, & Ridderinkhof, 2011; Schmitt, Ferdinand, & Kray, 2015; Spaniol, Bowen, Wegier, & Grady, 2015; Spaniol, Voss, Bowen, & Grady, 2011), and have rarely considered the effects of non-monetary or biologically relevant incentive types (e.g., primary reinforcers; Strough, Bruin, & Peters, 2015).
Older adults may place higher subjective value upon non-monetary or biologically relevant incentives compared to younger adults, and thus perform better with primary motivational incentives in cognitive tasks. Studies from the neuroeconomic decision-making literature have consistently shown age-related differences in the valuation across different motivational incentive types (Brown & Ridderinkhof, 2009; Mohr, Li, & Heekeren, 2010). For example, in temporal discounting tasks, older adults are equally likely to discount monetary rewards compared younger adults, but are more likely to discount social and health-related rewards (Eppinger, Nystrom, & Cohen, 2012; Green, Fry, & Myerson, 1994; Green, Myerson, & Ostaszewski, 1999; Löckenhoff O’Donoghue, & Dunning, 2011; Seaman et al., 2016). In other words, older adults tend value the receipt of social and health-related rewards over monetary rewards compared to younger adults. However, neural evidence for these age-related differences in temporal discounting of monetary rewards is mixed (Samanez-Larkin et al., 2011; Seaman, Brooks, et al., 2018), and age effects may interact with income (Green, Myerson, Lichtman, Rosen, & Fry, 1996). Given the prior literature, one might hypothesize that older adult task performance would be more influenced by liquid incentives than monetary rewards; however, to what extent such motivational effects would be observed in the brain is unclear.
Regarding motivational integration, it remains unanswered as to whether older adults demonstrate the ability to integrate different motivational incentive types, specifically in the case of primary (liquid) and secondary (monetary) incentives, and how these valuation processes affect cognitive control processes. If older adults demonstrate a similar incentive integration pattern as younger adults, it would reveal that this motivational integration mechanism is preserved throughout the lifespan. Alternatively, incentive valuation processes may be intact in older adults (Samanez-Larkin et al., 2007; Seaman, Brooks, et al., 2018; Wu, Samanez-Larkin, Katovich, & Knutson, 2014), but the incentive integration process itself may be cognitively demanding, and thus this ability would be subject to age-related decline. If so, then older adults may fail to integrate the motivational value of monetary and liquid incentives in modulating task performance or demonstrate a distinct motivational integration pattern from younger adults. Subsequently, older adults may be limited in their ability to simultaneously attend to information regarding monetary reward, liquid feedback valence (i.e., appetitive or aversive), and task rules.
Although no existing theories currently provide specific predictions about how motivational incentive integration is affected by age per se, the recent ‘affect-integration-motivation’ (AIM) framework provides a useful framework for conceptualizing the interaction between affective and motivational neural circuits (Samanez-Larkin & Knutson, 2015). Specifically, Samanez-Larkin and Knutson’s theory predicts that older adults would not integrate affective and cognitive inputs as well as younger adults in a decision-making task, and that this deficit may be mediated by reduced frontrostriatal connectivity between the thalamus, medial prefrontal cortex (mPFC), and ventral striatum (VS) in old age. However, this hypothesis is based on findings from the probabilistic reward learning literature, in which older adults showed reduced activation in the nucleus accumbens (NAc) and mPFC in response to reward prediction errors during incentive learning (Eppinger, Schuck, Nystrom, & Cohen, 2013; Samanez-Larkin, Worthy, Mata, McClure, & Knutson, 2014). It remains unknown whether the neural mechanism underlying incentive learning is similar to motivational integration. To our knowledge, no studies have yet examined motivational integration in healthy aging, and more importantly, whether/how integrated value influences cognitive control. Thus, a main study objective is to develop an experimental assay to probe this open question regarding motivational integration and cognitive control in healthy aging, which we hope will eventually engender further investigation of how underlying neural mechanisms are putatively altered throughout the human lifespan.
Incentive Integration Task Paradigm
A promising approach for operationalizing the effects of motivational incentives on cognitive control arises from the experimental psychology tradition, which systematically quantifies how high motivational value conditions selectively influences cognitive control measures in cognitive task paradigms (Botvinick & Braver, 2015; Yee & Braver, 2018). Several have proposed that incentives can boost cognitive processing via activation of motivational neural circuits, and these ideas have been supported by numerous studies demonstrating that monetary incentives enhance cognitive control by improving task-relevant processing in both younger adults (Braem, Hickey, D uthoo, & Notebaert, 2014; Chiew & Braver, 2013; Hefer & Dreisbach, 2017; Kang, Wang, & Zhou, 2017; Padmala & Pessoa, 2011) and older adults (Williams, Ryan S., Kudus, Farrah, Dyson, Benjamin J., & Spaniol, 2017).
Here, we introduce an innovative and powerful new experimental paradigm to probe the following outstanding question: how is the interaction between motivational integration and cognitive control affected throughout healthy aging? Recent evidence has revealed that healthy young adults appear to integrate the motivational value of primary and secondary incentives to modulate cognitive control task performance (Yee, Krug, Allen, & Braver, 2016). In particular, the use of proactive cognitive control – the ability to actively maintain contextual information, such as task instructions or relevant information conveyed by task cues – is encouraged through the use of a cued task-switching paradigm, in which advance task cues, which randomly vary between the two tasks, enable preparation for the upcoming trial. Furthermore, this task paradigm enables straightforward quantification of the dissociable and integrative effects of primary and secondary motivational incentives on cognitive task performance. Specifically, the motivational manipulations involve the utilization of monetary reward cues that vary on a trial-by-trial basis to indicate potential monetary rewards earned for fast and accurate performance, as well as oral liquid delivery to the participant’s mouth as post-trial performance feedback for successful attainment of monetary reward. Participants only receive liquid feedback if they successfully earned monetary reward in a given trial, and do not receive any liquid if they were incorrect, too slow, or did not respond. Critically, although the type of liquid received is manipulated in a blocked fashion (e.g., appetitive, neutral, aversive), the symbolic meaning of the liquid is the same throughout the entire experiment. Thus, any behavioral differences observed in task performance between liquid types can be attributed to differences in subjective valuation of the liquid feedback, and simultaneous consideration of both monetary rewards and liquid incentives during task performance (more details in Methods).
The paradigm was adapted from our prior study to evaluate whether and how older adults integrate the motivational value of primary and secondary incentives in a similar or different manner compared to younger adults, and critically, whether the combined motivational incentives can enhance the use of preparatory and proactive control strategy in healthy older adults. In particular, we hypothesized that older adults would show clear evidence of cognitive enhancement under incentive conditions relative to baseline. Moreover, we predicted even more direct parametric performance benefits in relationship to at least one of the experimentally manipulated incentive cues (monetary amount, liquid feedback). However, we expected that older adults might show evidence of impaired incentive integration, as this may be higher-order cognitive/motivational process that is likely decline in older age. In sum, the application of this powerful novel experimental assay can address the critical questions posed in this study, as well as provide a fundamental step towards understanding how motivation-control interactions are altered across the lifespan.
Methods
Participants
Older Adult Sample
55 adults (39 females; 66–92 years; M=77.2; SD=6.9) were recruited from the Older Adult Volunteer Pool at Washington University in St. Louis. All participants provided written consent approved by the Washington University IRB, and received payment for their participation ($10 per hour), plus additional earnings of up to seven dollars based on performance. Eleven participants were excluded from analyses due to technical error, participant inability to complete the task, or participant noncompliance with task instructions. The final sample consisted of 44 older adults (33 females; ages 66–90, M=76.6, SD=6.4).
Young Adult Sample
60 adults (33 females, 18–39 years; M=20.4; SD=3.5) were recruited from the Washington University Psychological and Brain Sciences Department Experimetrix Subject Pool and the St. Louis community. All participants provided written consent approved by the Washington University IRB, and received payment ($10 per hour) or equivalent course credit for their participation, plus additional earnings of up to seven dollars based on their performance. Six participants were excluded from the analyses due to technical error and/or participant noncompliance with task instructions. The final sample consisted of 54 younger adults (31 females; 18–39 years, M=20.4; SD=3.6). For both older and younger adult participants, data were collected and managed using a secure web-based application, Research Electronic Data Capture (REDCap), hosted at Washington University (Harris et al., 2009).
Task Paradigm
The behavioral task in the study is identical to the cued task-switching paradigm from Yee et al., (2016). Participants performed the consonant-vowel odd-even (CVOE) switching task (Minear & Shah, 2008; Rogers & Monsell, 1995), which entailed being presented with a letter-number pair (one letter and one number) and then asked to categorize the target symbol based on the assigned task in each trial - the letter as either a vowel or a consonant, or the number as odd or even. Participants maintained both task rules in working memory, and the task for a given trial was indicated by a cue display, which preceded the number-letter pair that read either “Attend Letter” or “Attend Number”. A reward cue was placed above and below each instruction cue, which indicated a low, medium, or high reward value (displayed as “$”, “$ $”, or “$ $ $ $”). The values of the monetary reward cues were randomized across trials. Although reward cues were visually displayed alongside the task cues in practice, baseline, and incentive blocks, participants could only earn monetary rewards during incentive blocks.
During incentive blocks, participants could earn monetary rewards for fast and accurate task performance (de tails about how the criterion response time (RT) is calculated are given in Procedure). A key aspect of the experimental design was the use of liquids as performance feedback for successful attainment of the monetary reward. Specifically, at the end of trials in which participants were accurate and faster than the criterion RT, they received a 2 mL drop of liquid directly to their mouths. Conversely, if participants answered incorrectly, too slowly, or not at all, they neither received money nor liquid. Critically, the type of liquid received was manipulated in a blocked fashion, such that it could be either positive / appetitive (apple juice), neutral (isotonic tasteless solution), or negative / aversive (saltwater). Importantly, because receipt of both monetary reward and liquid feedback are performance-contingent, participants must integrate the value of both types of motivational incentives (i.e., motivational integration) when performing this cognitive task. Thus, this paradigm enables straightforward comparison of the parametric effects of value on task performance for each type of motivational incentive (e.g., low vs. medium vs. high monetary rewards), as well as for “bundled” incentives (e.g., juice + high monetary reward vs. neutral + high monetary reward) that reflect the effect of integrated motivational value on cognitive task performance.
The paradigm is shown in Figure 1a. The task paradigm was programmed in E-Prime Version 2.0.10.242 (Psychology Software Tools, Pittsburgh PA; www.pstnet.com). Each trial consisted of a fixation display (200 ms), a fixation flicker to signal the upcoming cue (100 ms), a cue display (500 ms), a blank display (185 i0 ms), a target display of the number-letter pair (up to 2000 ms), a second fixation display (1000 ms), and a feedback display (2000 ms). Participant responses were recorded using an E-prime SR box, and response mappings were counterbalanced between participants.
Figure 1: Schematic of Incentive Integration Task Paradigm.
Subjects performed a consonant-vowel odd-even (CVOE) switching task, which entailed being presented with an ambiguous letter-number pair, and being asked to categorize the target symbol based on the task cue preceding the target (e.g., “Attend Number” or “Attend Letter). A reward cue was placed above and below each instruction cue, which indicated low ($), medium ($ $), or high ($ $ $ $) reward. Monetary reward cues were randomized across trials within each block. If subjects were accurate and faster than a subject criterion response time (30% of fastest correct response times for all trials during the baseline block), then they received 2 mL of liquid as performance feedback at the end of the trial. If subjects answered incorrectly, too slowly, or not at all, they neither received monetary reward or liquid. Liquid type was manipulated in a blocked fashion, counterbalanced across subjects, and was positive (apple juice), neutral (isotonic tasteless solution), or negative (saltwater).
Procedure
Participants were asked to abstain from eating or drinking anything besides water for the two hours prior to their scheduled session. Upon arrival, participants completed a contact information questionnaire and the Behavioral Inhibition & Avoidance Scales (BIS/BAS), a self-report survey often used to measure individual differences in motivation to avoid aversive outcomes and approach goal-oriented outcomes (Carver & White, 1994). We included this survey to test for associations between the effects of motivational incentives on cognitive task performance and this self-report measure of motivation.
Next, participants practiced the task paradigm. Each participant performed three practice blocks; two single task blocks (letter only or digit only, counterbalanced) followed by a third task-switching block. The three practice blocks consisted of 24, 24, and 48 trials. During each block, participants were given visual performance feedback on every trial (e.g., “Correct!”, “Incorrect!”, or “Too Slow!”). If the participant responded when the letter-number pair was presented, the button press ended the target display. If the participant was too slow or did not respond, then the target display remained on the screen for the complete 2000 ms.
After practicing the task, participants performed three baseline blocks. These were identical to the practice blocks, except that they were longer and participants no longer received any visual performance feedback. The baseline blocks were completed in the same task order as in practice, and consisted of 48, 48, and 96 trials (the last block included a break halfway through). In lieu of performance feedback at the end of each trial, participants saw a visual display with the text “Next Trial Coming Up” for 2000 ms. Participants were instructed to make responses as quickly and accurately as possible. After the last baseline block, a criterion cut-off time was calculated for each participant. This criterion RT was computed as the top 30 percentile of fastest correct response times for all trials (including no response trials) in the task-switching baseline block.
During the three incentive blocks, participants performed the same cued task-switching paradigm (96 trials; with break), but could now earn monetary reward in addition to their hourly pay compensation (up to $7 total). Participants were informed that the dollar signs visually displayed on the screen surrounding the task cue indicated the relative potential monetary value for a given trial, which they could earn if they were accurate and fast enough (specifically, faster than their criterion RT calculated from the task-switching baseline block). Critically, to earn the monetary reward, participants would need to significantly improve their performance on the task, i.e., increasing speed while maintaining accuracy. This is important for comparing performance between age groups, as this individualized criterion RT calculations are titrated to personal baseline performance. Thus, regardless of potential differences in baseline performance, if reward incentives improve performance equivalently for both age groups, then we would observe similar reward rates for both older and younger adults.
As mentioned previously, a key motivational manipulation was performance-contingent liquid feedback received for successful attainment of monetary reward. Specifically, participants received a 2 mL drop of oral liquid delivery if they were accurate and responded faster than their subjective criterion RT, but received neither money nor liquid if they responded incorrectly, too slow, or did not respond. Liquid feedback was dispensed using a digital infusion pump (model SP210iw, World Precision Instruments, Inc.) triggered by an output signal from the E-Prime script and delivered via Tygon tubing directly into the participant’s mouth. The type of liquid received was manipulated in a blocked fashion, and counterbalanced across participants, such that on a given block participants would receive positive / appetitive (apple juice), neutral (isotonic tasteless solution), or negative / aversive (saltwater) as performance feedback.
Critically, because receipt of monetary reward was always paired with oral liquid delivery, it is possible to examine the motivational value of bundled incentives on cognitive task performance. Furthermore, as the liquid feedback serves a symbolic incentive (i.e., oral liquid delivery provides the same information in a given trial regardless of valence), any observed differences in task performance between the liquid conditions would indicate incidental subjective valuation of the liquid feedback, which could be integrated with the motivational value of the monetary reward cue presented in that trial.
After completing the task, participants filled out a post-task survey, indicating on 7-point Likert-type scale the degree to which they liked or disliked each liquid, and how intense they would rate the taste of each liquid. They were also asked to rate their subjective motivation and liking of trials for every liquid-reward value pairing (e.g., “$ $” trials when receiving saltwater). At the end of the survey, participants were presented with four short-response questions that asked participants to describe various strategies they may have used for di fferent reward values and liquids. Older adult participants also completed the Short Blessed Test, a weighted six-item instrument originally designed to identify dementia, as a check of memory and concentration (Carpenter et al., 2011; Katzman et al., 1983). All older adults reported a score of 4 or less, indicating normal cognition (M=0.77, SD=1.20). Finally, after completing all surveys, participants were informed of their additional task earnings, paid, and debriefed.
Results1
Monetary Rewards Improve Cognitive Task Performance for Both Older and Younger Adults
The primary dependent measure used in the study was reward rate, though results are also described in terms of reaction time and accuracy (See Figure 2). Reward rate is defined as the percentage of rewarded trials in each condition of the experiment (when the participant was accurate and faster than their criterion RT cut-off), which arguably reflects the individual’s subjective motivation to implement cognitive control to earn monetary rewards. Both older and younger adults generally performed above the expected reward rate of 30%, indicating that performance was significantly improved relative to baseline levels (41/44 older adults and 52/54 younger adults exhibited significant improvement according to a binomial test; successes=86, trials=288, p=.05). Furthermore, when examining performance in the incentive blocks relative to baseline, older adults showed a significant reduction in reaction time [t(43)=12.339, p<.001; Cohen’s dz=1.86, CI95=(1.35,2.36,)] with no change in accuracy [t(43)=.587, p=.560; Cohen’s dz=0.09, CI95=(−.34,.51)], revealing clear motivation-related improvements. Younger adults also showed the expected RT reduction [t(53)=22.812, p<.001; Cohen’s dz=3.10, CI95=(2.54,3.67,)], but were significantly less accurate [t(53)=5.038, p<.001; Cohen’s dz=0.69, CI95=(.29,1.08)], suggesting they shifted down the speed-accuracy curve to increase their reward rate. RTs were log transformed to comply with normality assumptions for the paired t-tests, and Cohen’s dz effect sizes were calculated with the effsize package in R (Lakens, 2013; Torchiano, 2018).
Figure 2: Monetary Reward Effects on Reward Rate by Age Group.
In Figure 2a, reward rate is plotted against age group (OA=older adults, YA=younger adults). The dashed line represents the expected reward rate of 30%, assuming subjects did not improve their performance between baseline and incentive blocks. The solid lines represent 95% confidence intervals calculated by a binomial test [CIlower=.25, CIhigher= .35]. Each dot represents a single subject’s average reward rate. Figure 2b illustrates that while older adults are generally slower than younger adults, motivational incentives are associated with a significant reduction in response times (ms) between baseline and incentive blocks for both older and younger adults. Figure 2c shows accuracy between baseline and incentive blocks. Here, older adults maintained their accuracy between baseline and incentive blocks, whereas younger adults showed a significant drop in accuracy (i.e., they shifted down the speed-accuracy curve to increase reward rate). All error bars in all plots indicate standard error of the mean.
In terms of age effects on task-switching, a critical measure related to cognitive control, RT switch costs between baseline and incentive blocks were significantly reduced for older adults [t(43)=2.77, p=.008; Cohen’s dz=.42, CI95=(−.01,.85); Mbase=48 ms, Minc=20.35 ms], but not younger adults [t(53)=.256, p=.799; Cohen’s dz=.03, CI95=(−.35.42); Mbase=14.89 ms, Minc=12.49 ms], providing further evidence of motivation-related improvements in older adults. Accuracy switch costs were not significantly different between baseline and incentive blocks for either older adults [t(43)=−.564, p=.577; Cohen’s dz=−.08, CI95=(−.51,.34)] or younger adults [t(53)=1.403, p=.166; Cohen’s dz=−.19, CI95=(−.19,.57)].
We next examined performance within the incentive conditions, to identify the more selective, parametric incentive effects and their interaction with aging. A generalized linear mixed-effects model was conducted to test the effects of monetary reward and age group on reward rate. In the model, age (young, old) and monetary reward (low, medium, high) were treated as fixed effects, while subject was treated as a random effect. Additionally, random slopes of monetary reward by subject with correlated intercepts were included in the model (Magezi, 2015). Age was dummy coded by group (young=0, old=1), whereas money was contrast coded by value (low=−1, med=0, high=1). These models were conducted using the lmerTest (Kuznetsova, Brockoff, & Christensen, 2015) and LME4 (Bates et al., 2015) packages in RStudio using the R statistical language (R Core Team, 2017; RStudioTeam, 2016). The residuals were normally distributed, indicating that model assumptions were not violated. Due to the lack of consensus regarding the inclusion and decomposition of variance from random effects in general(ized) linear mixed models, it is currently challenging to accurately estimate canonical effect sizes for fixed effects in such models (e.g., Cohen’s d or f). However, to facilitate comparability across studies and meta-analyses, in conjunction with acknowledging the limitations of NHST approaches (Cumming, 2014), we report tables including fixed effects and 95% confidence intervals for these coefficient estimates for the subsequent models in the Supplement (Hlavac, 2018). Additionally, we report marginal R2 (variance explained by the fixed effects only) and conditional R2 (variance explained by fixed and random effects) for each model (Ludecke, 2019; Nakagawa, Johnson, Schielzeth, Building, & Glasgow, 2017).
The model revealed significant main effects of age [b=−.309, CI95=(−.509,−.108), z=− 3.021, p=.003] and monetary reward level [b=.249, CI95=(.198,.300), z=9.536, p<.001] and a significant interaction [b=−.167, CI95=(−.242,−.092), z=−4.358, p<.001]. However, older adults overall attained lower reward rates than younger adults [Mold=.58, SDold=.11; Myoung=.64, SDyoung=.12], indicating a lower degree of incentive-related improvement than younger adults, thus reducing overall monetary reward earnings [Mold=$3.94, SDold=$0.77; Myoung=$4.50, SDyoung=$0.77] (See Figure 2a). Despite the observed age group differences in task performance, higher monetary reward value boosted task performance in both older [b=.082, CI95=(.032,.132), z=3.218, p=.001] and younger adults [b=.251, CI95=(.195,.306), z=8.904, p<.001], though monetary reward value more effectively modulated younger adult performance, as indicated by the larger coefficient estimate and z-value for younger adults (See Table 1).
Table 1: Reward Rate, Response Times, and Accuracy.
Table of reward rates, response times, and accuracy by three monetary reward conditions. The mean score for each condition is listed, with standard deviation in parenthesis. Reward rate is the percentage of incentive trials for which monetary reward was received (i.e., if the subject was both accurate and faster than a reward criterion established from baseline block). Response times (in milliseconds) include only correct trials. Accuracy is the percentage of incentive trials that the subject answered correctly.
| Age Group | Monetary Reward | ||||
| Low ($) | Medium ($$) | High ($$$$) | |||
| Older Adults (n=44) | Reward Rate | .566 (.049) | .560 (.053) | .605 (.061) | |
| Response Time | 767 (31) | 772 (29) | 752 (36) | ||
| Accuracy | .861 (.039) | .864 (.039) | .875 (.029) | ||
| Younger Adults (n=54) | Reward Rate | .604 (.062) | .618 (.049) | .712 (.060) | |
| Response Time | 572 (41) | 572 (33) | 531 (35) | ||
| Accuracy | .783 (.046) | .803 (.033) | .830 (.053) | ||
Since the reward rate measure is determined by both response time (RT) and accuracy of the subjects, we examined these subcomponents separately to identify which measure(s) influenced reward rate. A general linear mixed effects model of response times (accurate trials only) revealed that, along with significant effects of monetary reward level [b=−21.130, CI95=(−28.254,−14.007), t=−5.814, p<.001], older adults were significantly slower than younger adults [b=205.569, CI95=(−151.226,249.912), t=7.414, p<.001], and there was a significant interaction between these factors [b=13.919, CI95=(−3.375,24.463), t=2.587, p=.011]. Higher valued rewards significantly improved RT in younger [b=−21.023, CI95=(−28.574,−13.473), t=−5.457, p<.001], but not older adults [b=−3.792, CI95=(−10.981,3.397), t=−1.034, p=.307]. A generalized linear mixed effects model for accuracy revealed that, in addition to a significant main effect of monetary reward level [b=.145, CI95=(.085,.205), z=4.759, p<.001], older adults were more accurate than younger adults [b=.538, CI95=(.234,.841), z=3.473, p=.001], and the two factors interacted [b=−.108, CI95 =(−.202,−.013), z=−2.228, p=.026]. Within-group analyses revealed that higher monetary reward improved accuracy in younger [b=.141, CI95=(.075,.206), z=4.19, p<.001], but not older adults [b=.045, CI95=(−.029,.120), z=1.189, p=.234]. All of the reward rate, response time, and accuracy measures by monetary reward level are listed in Table 1.
These data reveal that monetary incentives can indeed enhance cognitive control task performance in older adults, providing evidence that cognitive control impairments putatively associated with healthy aging can be overcome with sufficient incentive motivation. Notably, older adults during the incentive block are equally as fast [t(95)=−1.449, p=.151; Cohen’s dz=.08, CI95=(−.34,.51)] and no less accurate [t(93)=−1.111, p=.270; Cohen’s dz=−.22, CI95=(−.63,.18)] than younger adults during the baseline block, and also had similar RT switch costs [t(92)=.549, p=.584; Cohen’s dz=.11, CI95=(−.29,.51)], suggesting that older adults can perform as well as younger adults under normal conditions. This pattern is striking, as it suggests that the apparent “impairments” in task performance observed in the baseline block (RT slowing, higher switch costs) may have reflected motivational rather fixed cognitive limitations among older adults.
Interestingly, while older adult task performance significantly improved with higher monetary reward level, these reward rate effects appear to arise from a combination of reduced RT and increased accuracy, rather than independent modulation of these subcomponents. Conversely, higher monetary reward level significantly improved both RT and accuracy in younger adults. Furthermore, although older adults were generally slower than younger adults, they were also simultaneously more accurate overall. Moreover, introducing motivational incentives (and consequently increased task demands) appeared to distinctly influence older and younger adults task performance, affecting RT in the former group and both RT and accuracy in the latter group. Thus, older adults and younger adults appear to employ different task strategies to improve cognitive task performance. In particular, older adults prioritized accuracy over speed, consistent with an age-related accuracy bias under speeded cognitive task conditions (Rabbitt, 1979; Smith & Brewer, 1995; Starns & Ratcliff, 2010). Consequently, older adults were less able to modulate either of these performance dimensions according to the current monetary reward level.
Motivation and Cognitive Control Interactions: Age-Related Differences in Task Performance
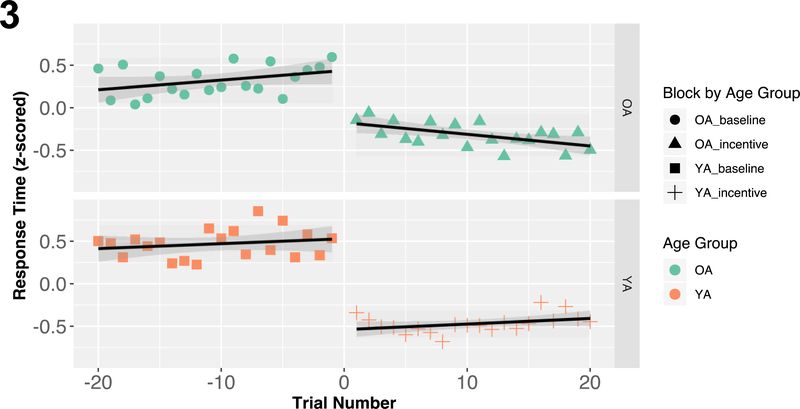
To examine what may be driving age-related differences in task performance during the incentive blocks (i.e., when motivational incentives are presented and increased task demands are required to earn those incentives), we examined transition effects from the last baseline block (when no rewards were available) to the first incentive block (when rewards became available). These transition effects are illustrated in Figure 3.
Figure 3: Transition Effects Between Baseline to Incentive Blocks.
Averaged response times across the last 20 trials of the baseline block and first 20 trials of the first incentive block. Younger adults were able to instantaneously reduce their RT when transitioning between baseline and incentive blocks, and this resulted in relatively stable performance during the incentive block. Conversely, older adults both significantly reduced their RT between blocks and across trials in the incentive block, revealing that OA progressively also sped up during the start of the incentive block. These age differences reveal that younger and older adults implement distinct strategies under motivated task conditions.
We performed a linear mixed effects model on response times (z-scored) from, with trial number (last 20 of baseline block coded from −20 to −1 and first 20 of incentive blocks coded from 1 to 20), block (baseline vs. incentive), and age group, treated as fixed effects. Block and age group were dummy coded (baseline=1, incentive=0; OA=1, YA=0), and subject was treated as a random effect. The full model revealed a significant three-way interaction between trial number, block, and age group [b=.026, CI95=(.006,.045), t=2.578, p=.010]. There were also significant main effects of block [b=1.609, CI95=(.911,1.226), t= 13.315, p<.001] and age group [b=.362, CI95=(.196,.528), t=4.280, p<.001], as well as significant two-way interactions between trial and age group [b=−.020, CI95=(−.034,−.006), t=−2.866, p=.004], and block and age group [b=− .454, CI95=(−.689,−.219), t=−3.792, p<.001]. Follow-up analyses within each age group clarified how the transition between baseline and incentive blocks influenced task performance. Younger adults immediately reduced their RT when transitioning between blocks, as evidenced by a significant main block effect [b=1.069, CI95=(.916,1.221), t=13.760, p<.001], and no trial number effect [b=.007, CI95=(−.002,.016), t=1.427, p=.154], indicating that beyond the instantaneous block effect, YA performance was relatively stable during the incentive block. In contrast, although older adults significantly reduced their RT during the incentive block [b=.614, CI95=(.433,.795), t=6.656, p<.001], the model also revealed a significant negative effect of trial number [b=−.014, CI95=(−.024,−.003), t=−2.518, p=.012] and a significant interaction [b=.025, CI95=(.010,040), t=3.250, p=.001]. Thus, while older adult performance was fairly stable at the end of the baseline block [b=.011, CI95=(−.001,.024), t=1.809, p=.071], their RTs progressively sped up at the start of the incentive block [b=−.014, CI95=(−.022,−.005), t=−3.089, p=.002].
Similar analyses on accuracy revealed a non-significant block effect [b=.442, CI95=(−.074,.958), z=1.681, p=.093] and an age group effect that approached statistical significance [b=.643, CI95=(−.012,1.298), z=1.923, p=.055]. Within-group analyses revealed that while younger adult accuracy did not significantly differ between blocks [b=.0440, CI95=(−.075,.955), z=1.674, p=.094], older adult accuracy significantly interacted with trial number [b=.055, CI95=(.003,.106), z=2.072, p=.038]. Older adults’ accuracy continued to improve during the end of the baseline block [b=.050, CI95=(.011,.089), z=2.503, p=.012], but significantly decreased at the start of the incentive block [b=−.008, CI95=(−.012,−.006), z=−6, p<.001].
It is evident that both age groups improved their task performance when presented with motivational incentives and increased cognitive task demands via distinct characteristic behavioral patterns. However, whereas younger adults instantaneously and optimally up-regulated the speed of their responses when transitioning to the incentive block, older adults utilized both instantaneous and more gradual, incremental strategies to optimize task performance under motivated task conditions.
Integration of Primary and Secondary Motivational Incentives in Older and Younger Adults
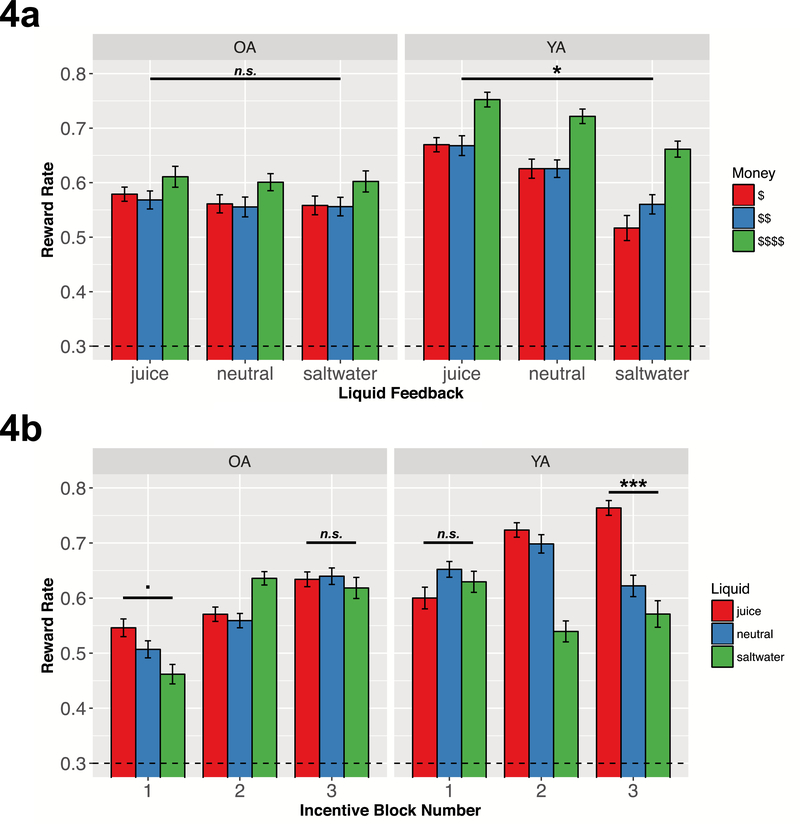
To test the whether motivational incentive integration occurs similarly in older and younger adults, we examined reward rate effects by both monetary reward and liquid feedback (See Figure 4a). We ran a similar generalized linear mixed effects model as before, except now adding liquid valence as a contrast coded fixed effect (Juice=1, Neutral=0, Saltwater=−1), in addition to the monetary reward and age group effects. The model also included random slopes and correlated intercepts for monetary reward and liquid by subject. The omnibus model showed a significant liquid effect [b=.283, CI95=(.183,.382), z=5.564, p<.001], as well as significant two-way interactions between liquid and monetary reward [b=−.060, CI95=(−.113,−.008), z=−2.265, p=.024], and liquid and age group [b=−.253, CI95=(−.401,−.105), z=−3.357, p<.001].
Figure 4: Motivation Incentive Integration Effects by Age Group.
Figure 4a illustrates reward rate by monetary reward level and liquid type for both age groups. Younger adults performed significantly better with juice compared to neutral solution feedback, as well as with neutral solution compared to saltwater feedback, and these liquid effects interacts with monetary reward level (see text for statistics). Older adult task performance was not modulated by liquid. Figure 4b illustrates reward rate effects by incentive block and liquid type. Younger adult task performance did not differ by liquid type in block 1, but these liquid effects were significant in the third block. In contrast, older adults show the opposite pattern - with marginally significant liquid effects in the first, but not the last, incentive block. All error bars in all plots indicate standard error of the mean.
To better understand how the liquid valence effects on reward rate differed by age, we conducted separate models for each age group. Younger adults performed better with juice compared to neutral or saltwater feedback [b=.284, CI95=(.172,.397), z=4.951, p<.001], and this liquid effect interacted with monetary reward level [b=−.060, CI95=(−.112,−.007), z=−2.228, p=.024]. When examining only juice and neutral blocks, there were main effects of monetary reward [b=179, CI95=(.137,.222), z=8.292, p<.001] and liquid [b=170, CI95=(.092,.247), z=4.276, p<.001], with their interaction approaching statistical significance [b=−.037, CI95=(−.075,.001), z=−1.920, p=.055]. When comparing saltwater and neutral blocks, the model revealed significant main effects of monetary reward [b=.232, CI95=(.135,.329), z=4.679, p<.001] and liquid [b=.363, CI95=(.182,.543), z=3.945, p<.001], and a significant interaction [b=−.111, CI95=(−.216,−.007), z=−2.096, p=.036]. These interactions reveal that at low monetary reward levels, liquid feedback had a higher impact on reward rate, suggesting that younger adults integrated the motivational value of the liquid feedback and monetary rewards to modulate task performance In contrast, older adults task performance was not affected by the valence of the liquid feedback [b=.030, CI95=(−.061,.122), z=.649, p=.516]. Thus, although older adults were motivated by higher monetary reward, they appeared to not implement the same motivational integration process as younger adults, as liquid valence had negligible impact on reward rate.
Reward Rate Effects by Incentive Block: Older Adult Task Performance Modulated by One Motivational Dimension at a Time
Additional exploratory analyses revealed a surprising discovery: older adult task performance not only improved during the incentive blocks, but also liquid incentives had distinct effects on early versus later task performance that varied by age group (Figure 4b). A generalized linear mixed effects model with reward rate predicted by monetary reward level, liquid valence, age group, and block number (contrast coded; block1=−1, block2=0, block3=1), with random slopes and correlated intercepts for monetary reward level and liquid valence, revealed a significant three-way interaction between liquid valence, age group, and block number [b=−.257, CI95=(−389,−.124), z=−3.803, p<.001]. Follow-up analyses revealed that older adults attained higher reward rates during later incentive blocks [b=.272, CI95=(.219,.326), z=10.042, p<.001]. When examining incentive effects for each block, the models revealed a trend-level effect of liquid valence in the first incentive block [b=.186, CI95=(−.027,.398), z=1.714, p=.087] and no monetary reward effect [b=.031, CI95=(−.047,.109), z=.787, p=.432], but a significant effect of only monetary reward level [b=.184, CI95=(.102,.267), z=4.388, p<.001] in the last incentive block (no hint of liquid effect [b=.036, CI95=(−.196,.269), z=.306, p=.76]). Conversely, though younger adults al so improved their reward rate across incentive blocks [b=.069, CI95=(.014,.123), z=2.474, p=.013], they showed a different pattern of incentive effects. Specifically, while task performance was influenced by monetary reward level only [b=.203, CI95=(.113,.292), z=4.447, p<.001] in the first incentive block (no hint of liquid effect [b=−.092, CI95=(−.263,.078), z=−1.062, p=.288]), it was influenced by both monetary reward level [b=.390, CI95=(−.277,.503), z=6.779, p<.001] and liquid valence [b=429, CI95=(.183,.676), z=3.411, p<.001] in the last incentive block.
These results suggest that older adult task performance transitions from selective sensitivity to liquid valence at the start of the session to selective sensitivity to monetary rewards by the end of the session, whereas younger adult task performance is initially sensitive only monetary reward, but is sensitive to both monetary reward and liquid valence by the end. Thus, whereas younger adults appear to integrate both incentive types after sufficient experience, older adults appear to attend to one motivational dimension at a time (i.e., a shift from sensitivity to liquids to monetary rewards). An additional interesting observation is that older adults gradually improved their performance during the incentive blocks, enough so that their reward rate in the third block matched younger adult performance in the first block [t(83)=.203, p=.839; Cohen’s dz=.04, CI95=(−.36,.45)]. Thus, it may be that older adults can earn the same level of reward as younger adults, but may need more practice (i.e., slower learning rates) to improve their task performance sufficiently to do so.
Trial-wise Value Estimates of Performance-Contingent Liquid Feedback
Given the prior work demonstrating that motivational interventions are most effective for older adults when they provide positive performance feedback (Ferdinand & Czernochowski, 2018), we aimed to test whether age group effects may be due to differential processing of the performance-contingent liquid feedback during the incentive blocks. Whereas younger adults integrated the motivational value of the liquid incentives with monetary rewards in a prospective manner to adjust cognitive control processing and modulate behavioral responses, older adults may have instead utilized the liquid reinforcement as an immediate and informative feedback signal to modulate motivational value on a trial-by-trial basis and improve cognitive task performance. That is, if the delivery (or absence) of liquid feedback facilitates improved task performance during the incentive blocks for older adults, it would reveal dissociable mechanisms by which younger and older adults process the liquid feedback in the task.
To formally test the hypothesis that younger and older adults process the liquid incentives distinctly, we implemented a simple reinforcement learning model (Sutton & Barto, 1998) to calculate trial-wise expected value estimates based on local reward history (i.e., whether a drop of liquid was delivered to signal successful attainment of monetary reward). Lambda (X) is a constant that represents learning rate and was set to 0.5. The trial-wise value estimate is represented as continuous variable V(t), and was initialized to 0.5 (range is between 0 and 1). Finally, r(t) is a binary variable that indicates whether the subject was rewarded (and received liquid feedback) on the previous trial. The model equation for value estimation is shown below.
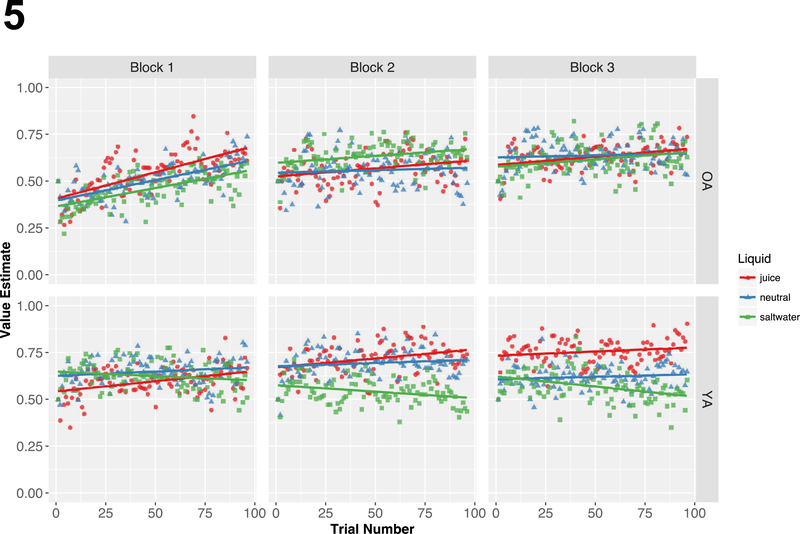
We used this model to calculate trial-wise value estimates for each liquid (juice, neutral, saltwater) for each subject. These averaged estimates are illustrated in Figure 5. We first applied a linear mixed effects model to confirm that this value estimate variable predicted reward rate [b=.418, CI95=(.330,.506), z=9.314, p<.001]. Next, we ran generalized linear mixed effects models to examine whether reward rate was significantly predicted by trial-wise value estimates over and above the experimentally manipulated motivational variables (e.g., monetary reward, liquid valence) for each age group. We included block number as a fixed effect, as well as random slopes for monetary reward and liquid valence and their correlated random intercepts.
Figure 5: Trial-by-Trial Value Estimates of Liquid Reinforcement Delivery.
A reinforcement learning model was implemented to calculate trial-by-trial value estimates of the liquid reinforcement, which are plotted by block, liquid, and age group. These value estimates were submitted to a generalized linear mixed effects model, which revealed that this variable was a unique predictor of older adult task performance over and above the experimentally manipulated motivational variables. Thus, the receipt of liquid reinforcement has a general performance feedback effect modulating trial-by-trial performance in older, but not younger, adults.
Younger adult task performance was not predicted by the trial-wise value estimate [b=.045, CI95=(−.086,.177), z=.677, p=.499], but was predicted by monetary reward, liquid valence, and their interaction (previously reported). There was no significant block effect. In contrast, older adult task performance was significantly predicted by the trial-wise value estimate [b=.239, CI95=(.106,.372), z=3.531, p<.001], as well as monetary reward and block number (previously reported). Notably, not only was there was no effect of liquid valence, but also no liquid valence by trial-wise value estimate interaction. Together, these data provide evidence that the liquid reinforcement served as an informative feedback signal that modulated motivational value on a trial-wise basis - improving task performance in older, but not younger, adults.
Self-Report Ratings
A potential concern is that the lack of liquid effects in older adults may be attributed to decreased taste sensitivity in aging (Boyce & Shone, 2006). To address this concern, we examined the self-report ratings to confirm whether older adults can detect and/or report differences between the liquid types. These self-report ratings are illustrated in Figure 6. The supplement contains detailed analyses demonstrating that older adults indeed discriminate between and show transitive preferences for the different liquids. Additionally, as previously shown in the Yee et al. (2016) study, self-report motivation ratings predicted unique variance over and above experimentally manipulated motivational variables for both older and younger adults [O A: χ2(1,9)=5.132, p=023; YA: χ2(1,9)=82.401, p<.001].
Figure 6: Self-Report Ratings.
Figure 6a shows the liking ratings for each liquid (e.g., “How much did you like the juice?”). Although both older and younger adults show transitive preferences (juice > neutral > saltwater), older adults reported the neutral solution and saltwater to be less aversive than younger adults. Figure 6b shows the intensity ratings for each liquid (e.g., “How intense did you find the juice?”), which were similar for older and younger adults. Figure 6c shows each subject’s z-scored motivation ratings for each of the 9 trial types (e.g., “How motivated were you on the juice $ $ $ $ trials?”) plotted against their z-scored reward rate. Self-report motivation ratings predicted unique variance over and above experimentally manipulated motivational variables for both age groups. All error bars in all plots indicate standard error of the mean.
BIS/BAS Survey
We examined whether reward rate was predicted by subcomponents of the BIS/BAS survey (Carver & White, 1994), a self-report questionnaire designed to measure two motivational systems: a behavioral inhibition system ideally targeting motivation to avoid aversive outcomes (BIS) and a behavioral activation system ideally targeting motivation to approach goal-oriented outcomes (BAS). A linear mixed model revealed that none of these questionnaire measures were associated with reward rate [BIS: b=.003, CI95=(−.006,.012), t=.575, p=.567; BASreward: b=−.008, CI95=(−.024,.008), t=−1.015, p=.313; BASfun: b=.006, CI95=(−.008,.020), t=−.882, p=.380; BASdrive: b=−.001, CI95=(−.013,.011), t=−.110, p=.912]. In terms of age group differences, older adults reported significantly lower ratings of BIS [t(95)=−5.301, p<.001; Cohen’s dz=−1.06, CI95=(−1.49,−.63)]. Interestingly, older adults also reported lower ratings of BAS reward [t(81)=− 2.058, p=043; Cohen’s dz=−.42, CI95=(−.84,−0.02)], BAS fun [t(95)=−1.697, p=.092; Cohen’s dz=−0.34, CI95=(−.74,−.07)], and BAS drive [t(85)=−1.996, p=.049; Cohen’s dz=−.42, CL95=(−.83,− 0.00)]. To rule out the possibility that the lack of liquid effects in older adults were due to these lower BIS/BAS ratings, we ran correlation analyses between differences in reward rate between liquid conditions (e.g., juice vs. neutral, saltwater vs. neutral) and the BIS/BAS ratings. Our results revealed only a trend-level relationship between saltwater vs. neutral reward rates and BIS [r(96)=−.188, p=.063], and no significant associations between juice vs. neutral reward rates and BAS reward [r(96)=−.147 p=.149], BAS fun [r(96)=−.016, p=.880], and BAS drive [r(95)=− .054, p= 598].
Discussion
In this study, we developed a novel experimental paradigm with primary and secondary incentives to probe the role of healthy aging in motivation-cognition interactions. The results provide important answers to each question posed at the outset. First, we found clear evidence that older adults can achieve substantial motivation-related performance enhancements even under task conditions with high cognitive control demands. Specifically, the reduction of RT switch costs in older adults between baseline and incentive conditions bolster the hypothesis that motivational manipulations can be used ameliorate cognitive control impairments. Interestingly, these motivational effects were qualitatively different between age groups, in that older adults exhibited a more gradual enhancement pattern, marked by a greater resistance to speeding of reaction times and a seemingly greater reliance on trial-by-trial reinforcement learning effects. Second, older adults did not reliably integrate the motivational valence of the liquid feedback, even though they reported distinct subjective liking, intensity, and motivation ratings for the liquids. Together, these results not only establish the presence of motivational influences on cognitive control in healthy aging, but also point to age-related differences in the nature of motivation-cognition interactions. In the following sections, we discuss these results in the context of the broader literature, address study limitations, and suggest potential future directions that may help elucidate neural mechanisms that underlie these age-related changes.
Motivational Enhancement of Cognitive Control in Older vs. Younger Adults
These results provide exciting novel evidence demonstrating that older adults, like younger adults, can exhibit significant motivation-related improvements in performance in tasks with high cognitive control demands. Three sets of findings provide convergent evidence in this regard. First, when compared to baseline performance, under incentive conditions, older adults significantly reduced reaction times by over 150 ms (a nearly 20% speed-up) without sacrificing accuracy. Moreover, older adult task performance during incentive conditions appears to be comparable to younger adult performance during baseline conditions, suggesting that age-related differences may not necessarily reflect a true cognitive impairment; rather, it suggests that with sufficient incentives older adult performance can be enhanced to match younger adult performance levels. Second, older adults’ rate of attaining rewards increased with motivational value (high monetary reward), as a function of decreased reaction time and increased accuracy. Third, self-report motivation levels predicted better task performance (increased reward rates) beyond the experimental factors. In sum, these findings add to a growing, but still sparse, literature suggesting that incentive motivation can effectively enhance cognitive performance in older adults, and additionally remediate age-related declines in especially highly demanding task domains (Braver et al., 2014; Di Rosa, Schiff, Cagnolati, & Mapelli, 2015; Harsay, Buitenweg, Wijnen, Guerreiro, & Richard, 2010; Spaniol, Schain, & Bowen, 2014). Although much work has examined the potential effectiveness (or lack thereof) for cognitive training interventions in remediating age-related cognitive decline (Bamidis et al., 2014; Kelly et al., 2014; Zhu, Yin, Lang, He, & Li, 2016), our findings suggest the promise of motivation-based interventions as an alternative to cognitive-based interventions. However, whether motivation-related remediating enhancements in performance are purely transient in nature or could be sustained over time (potentially combined with other forms of training) remains to be addressed.
Nevertheless, the results also suggest that reward motivation influences on cognitive processing may be qualitatively distinct for older adults. A key finding was that older adults showed a smaller increase in reward rate than younger adults, as evidenced by a more gradual adjustment in performance in response to the change in motivational context. Younger adults showed a substantial instantaneous drop in reaction time when rewards were initially introduced, whereas in older adults this effect was much smaller, and reaction times progressively decreased across trials. Likewise, older adults demonstrated higher accuracy and less overall speeding than younger adults. This pattern is consistent with prior literature showing that older adults tend to exhibit an accuracy-biased decision-making strategy when performing speeded cognitive tasks. In other words, older adults appeared to be more resistant to speeding up if this strategy would also worsen their accuracy, even if this speed-accuracy trade-off is favorable in terms of the potential rewards obtained (Rabbitt, 1979; Smith & Brewer, 1995; Starns & Ratcliff, 2010).
Finally, older adults appeared to rely more on performance feedback than younger adults, resulting in a more gradual trial-wise adjustment in reaction times, rather than an instantaneous shift in response to changes in motivational context. Older adults may be just as capable as young adults in achieving motivation-related enhancement in cognitive performance, but may require more time and practice to accomplish such effects. Although prior literature has assumed that such effects are primarily strategic and volitional in nature, indicating that older adults may simply be more averse to adopting fast speed-accuracy tradeoff strategies, recent work has suggested that such effects could be due to age-related degeneration of cortico-striatal white matter tracts (Forstmann et al., 2011).
Age-Related Differences in Motivational Integration: Motivational, Sensory, or Cognitive?
In younger adults, we clearly replicated findings from Yee et al. (2016), observing both main effects monetary reward and liquid valence on reward rate. We also replicated other previous findings in which se, report motivation and liking ratings predicted variance in reward rate over and the experimental factors. Together, these data point to a relatively robust pattern in which younger adults are able to integrate diverse motivational incentives into a unified estimate of subjective value, which then modulates cognitive task performance accordingly.
A key and unexpected finding was that older adults showed reduced sensitivity to liquid feedback, even though it was a “primary” motivational reinforcer. There are several potential explanations for this null effect of liquid. One possibility is that older adults simply did not find the liquid incentives as motivating as the younger adults. This interpretation would be somewhat surprising, given that much of the literature would posit that biological/immediate incentives are more influential on older adult cognition compared to symbolic/delayed incentives (Charles & Carstensen, 2010). If older adults were indeed more motivated by social and/or self-relevant incentives, a potential approach would be to use the same task with carefully targeted and age-appropriate incentives as performance feedback (e.g., social rewards; compliments or insults).
A second hypothesis relates to sensory processing changes that often accompany aging (Hoogeveen, Dalenberg, Renken, ter Horst, & Lorist, 2015; Jacobson, Green, & Murphy, 2010). If older adults are simply less sensitive to the taste of the liquids (i.e., they find saltwater less aversive than younger adults), this could explain the lack of liquid effects. However, self-report ratings and initial liquid effects provide some evidence against this account. Since older adults reported different preferences for the liquids, it seems unlikely that the null liquid effects are simply due to sensory deficits. Also, the block-related analyses yielded a surprising pattern in which liquid effects were present in older adults during the first incentive block, but yet Such a pattern seems inconsistent with a pure sensory account, given the dynamically changing sensitivity to liquid in older adults. However, it may be that older adults more quickly habituate to the taste of the liquids, resulting in decreasing sensory sensitivity across the experimental session. Further work is needed to directly evaluate the degree to which age-related changes in sensation and perception might moderate motivation-control interactions in older adults.
A final hypothesis is that age-related differences may reflect older adults’ difficulty with motivational integration, i.e., instantaneously integrating numerous cognitive demands to modulate task performance (Ennis, Hess, & Smith, 2013; Hess & Ennis, 2011). Whereas younger adults can integrate multiple task demands simultaneously (e.g., increasing speed while maintaining accuracy, attending to monetary reward values and task rules in each trial), older adults may prefer to trade-off attending to individual task demands to optimize performance throughout the entire experiment. The block-related effects are consistent with this hypothesis. Although older adults may have initially been more sensitive to liquid than monetary incentives (even though this pattern is sub-optimal with respect to the explicit task demands and would have yielded greatly reduced earnings), they may have strategically decided to filter out the liquid effects to direct more attention to the monetary reward cues (as supported by their eventual transition to modulate their performance based on monetary reward levels). Thus, when faced with age-related impairments in motivational integration, older adults may have adaptively compensated by up-regulating attention to monetary rewards at the expense of attention to liquid feedback. However, this interpretation is rather speculative and remains to be empirically tested.
Finally and intriguingly, a paradoxical consequence of older adults’ insensitivity to liquid valence is that their task performance was not as impaired by the aversive saltwater feedback compared to younger adults. Although older adults generally achieved lower reward rates, in the condition for which younger adults did the poorest (saltwater block on trials with lowest monetary rewards), older adults achieved numerically, even if not significantly, higher reward rates (OA mean=.558, YA mean=.517). This finding is counter-intuitive and contradicts what is more typically found in standard cognitive tasks, in which older adults generally have greater difficulty with ignoring task-irrelevant and potentially interfering stimulus dimensions (e.g., Stroop; Milham et al., 2002; Mutter, Naylor, & Patterson, 2005). In the current task design, the liquid feedback might play an analogous role: if the explicit goal is to maximize monetary reward, then ignoring the valence of liquid feedback would be the optimal strategy. It is intriguing that older adults appeared to adopt such a strategy while younger adults did not. Why such age differences are present is unclear, but it optimistically reveals a potential beneficial side effect of age-related deficits in motivational integration - specifically, older adults may be less sensitive to effects of task-irrelevant motivational dimensions when pursing cognitive task goals.
Prospective vs. Reinforcement Mechanisms of Performance-Contingent Liquid Feedback
A compelling finding based on analyses incorporating value estimates calculated from our reinforcement learning model was the observation that older adults appeared to learn from and use the performance-contingent liquid feedback to improve their task performance across incentive blocks. Critically, trial-wise value estimates derived from the performance feedback significantly predicted variation in trial-by-trial performance for older, but not younger, adults. Thus, consistent with the prior literature on the effectiveness of incentive motivation as performance feedback in healthy aging (Ferdinand & Czernochowski, 2018), these data suggest that when older adults encounter increased cognitive demands, the presence or absence of performance feedback can help them adaptively adjust their strategy to improve individual task performance. Further, these models provide a possible explanation for how older adults gradually incorporate the informational value of liquid feedback to modulate their task performance throughout the experiment, in contrast to the younger adults who appeared to prospectively and instantaneously combine the motivational value of the liquid feedback with monetary rewards to modulate task performance. Taken together, it is evident that younger and older adults pursued distinct prospective and reinforcement strategies for incorporating the liquid incentives to influence their overall task performance. Future work should aim to identify the boundary conditions of when and how incentives can effectively modulate older adult task performance.
Self-Report Likert Ratings Reveal Access to Subjective Motivational State
To alleviate concerns regarding reduce sensitivity to liquid valence in aging, we examined self-reported ratings of preference and intensity for the liquids (detailed analyses presented Supplemental Materials). Interestingly, these ratings revealed that although older adults performed similarly across the liquid conditions, they reported distinct preferences and motivation for the different liquids. Older adults demonstrate transitive preferences for the liquids (e.g., juice > neutral > saltwater), though they also foun d the neutral solution and saltwater less aversive than the younger adults. Older and younger adults both reported similar patterns of liquid intensity, though again more subtle age differences were present, in that older adults reported that the neutral solution was slightly more intense and the saltwater significantly less intense than the younger adults.
Additionally, in both age groups different levels of motivation were reported for the 9 different trial types, and these motivation ratings explained unique variance in task performance over and above the effects from the experimental variables (liquid feedback and monetary reward amount). The dissociable effects between self-report motivation ratings and experimentally manipulated motivational variables on reward rate are particularly intriguing, as they indicate that both older and younger adults may have explicit access to their own subjective motivational state, which influences their task performance (consistent with Yee et al. 2016). A hierarchical regression showed that adding these self-report ratings improved the model from that including only experimental manipulation effects, revealing the utility of incorporating self-report data in future studies. Importantly, these ratings may provide behavioral targets for neural investigation into the source of age-related changes in motivation-cognition interactions.
Neural Mechanisms of Motivation-Control Interactions: Aging, Dopamine, and Cognition
These compelling results provide a foundational framework and unique experimental paradigm for investigating the neural mechanisms underlying age-related differences in motivational integration – cognitive control interactions. In particular, we are optimistic that this novel task paradigm demonstrates clear utility for elucidating how humans process and integrate the motivational values of diverse incentives, and more importantly, how that valuation process can help individuals pursue cognitive task goals. Moreover, our behavioral results reveal intriguing age-related differences in motivational processing that can provide insight into how such valuation processes are affected throughout the lifespan.
A prevalent and continuously evolving hypothesis posits that the age-related changes in motivation and cognition can be attributed to the dysregulation of dopaminergic (DA) neuromodulation in the striatum and the frontal cortex (Bäckman et al., 2006; Berry et al., 2016; Braver & Barch, 2002; Li & Rieck mann, 2014). Although it is well known that dopamine (DA) plays a central role in modulating both motivational/affective and higher-order cognitive and functions (Aarts et al., 2010; Cools, 2008; Nieoullon, 2002), the exact boundary conditions for how these variables relate to one another remains somewhat elusive. Several have hypothesized that slower tonic dopamine signals are specifically involved in modulating the stability or maintenance of working memory representations via projections to the PFC (Braver & Cohen, 2000; Cools, 2016; Durstewitz & Seamans, 2002; Goldman-Rakic, 1992), and that incentive-driven DA release can promote maintenance of these task representations in PFC (Westbrook & Braver, 2016; Yee & Braver, 2018). However, there is currently limited empirical evidence to identify the functional relevance of tonic DA release that may occur in relation to the motivational context, and there is not yet a consensus regarding how changes in DA release throughout the lifespan interacts with PFC neural mechanisms.
Furthermore, both the heterogeneous effects on distinct components of the DA system within individuals (e.g., presynaptic verses postsynaptic) and as well as the substantial variability in DA function between individuals make it especially challenging to pinpoint the precise underlying root cause(s) for why and how dopaminergic mechanisms change with age (Berry, Jagust, & Hsu, 2018). Although numerous studies have reported a wide array of findings regarding changes in the DA system across adulthood (Bäckman, Lindenberger, Li, & Nyberg, 2010; Kaasinen et al., 2000; Kaasinen & Rinne, 2002; Volkow et al., 1998), as well as regional heterogeneity of age-related declines in DA binding potential (Seaman, Smith, et al., 2018), a recent meta-analysis of the literature found healthy aging to be associated with reduced DA receptors and transporters, but no differences in DA synthesis capacity (Karrer, Josef, Mata, Morris, & Samanez-Larkin, 2017). Conversely, some have observed a positive association between elevated striatal DA synthesis capacity (caudate) and working memory in healthy older adults, which is also associated with increased fMRI BOLD signal in dorsolateral PFC during the delay period of a working memory task (Landau, Lal, O’Neil, Baker, & Jagust, 2009), whereas others have observed older adults have higher DA synthesis capacity without a clear association with cognitive function (Berry et al., 2016; Braskie et al., 2008). Thus, one conceivable hypothesis may be that older adults up-regulate DA synthesis to compensate for reduced DA receptor availability (and lower binding potential) in striatum and PFC, and that the relationship between DA synthesis and behavioral cognitive measures may be mediated by striatal-PFC connectivity (Berry, Shah, & Jagust, 2018). If so, this would explain the opposing trends often observed in PET studies and potentially help reconcile the numerous mixed findings on the DAcognition relationships in healthy aging.
In the cognitive control literature, several fMRI studies have found evidence for age-related differences in DLPFC activation during cognitive control and working memory tasks (Jimura, Locke, & Braver, 2010; MacPherson, Phillips, & Della Sala, 2002; Manard, François, Phillips, Salmon, & Collette, 2017; Marschner et al., 2005). Some have theorized that healthy aging may be associated with alterations of incentive-driven modulation of cortical networks that influence multiple cognitive domains (Spaniol et al., 2015). Thus, if DA synthesis is maintained (or even increased) in older adults, but there are also simultaneously deficits in PFC modulation during cognitive control tasks, it would suggest that the locus of age-related decline may relate to the interaction between motivational and cognitive control processes in the brain. Thus, we would expect that, in healthy aging, reward learning performance (especially in task contexts with low cognitive demands) will be relatively intact, whereas performance on motivation-cognition tasks that require integration of value information to up-regulate cognitive processes would be impaired. However, no neuroimaging studies to date have examined whether and how diverse types of motivational incentives are integrated with cognitive processes in the brain. Therefore, a promising future direction would be to investigate how the relationships between DA, motivational integration, and cognitive control are influenced across the lifespan.
Study Limitations and Future Directions: Considering Age-Related Differences in Processing Motivational Cues
An important limitation to acknowledge in this study is that incentive type (monetary reward vs. liquid) was confounded with when incentive cues were presented (anticipation vs. outcome). That is, because liquid feedback was blocked and monetary reward level varied on a trial-by-trial basis, it is difficult to determine whether motivational incentive integration effects observed in this study would generalize to other task configurations with different delivery schedules with the same incentives. Given the neural evidence showing differences between anticipatory and outcome related effects of monetary rewards (Knutson, Fong, Adams, Varner, & Hommer, 2001), it is important for future studies to explore different task configurations to test if age-related differences in motivation incentive integration are similar with other task designs, as well as target such effects on more direct measures of cognitive control (e.g., mixed costs).
Likewise, although our results provide novel insights into motivational integration-cognitive control interactions in healthy aging, many open questions remain regarding the nature of how and when different types of motivational incentives can modulate cognition affected throughout the lifespan. For instance, motivational valence (appetitive vs. aversive) is an important factor in terms of its influence on older adult cognition, and should be investigated more thoroughly in future studies. In the decision-making literature, several studies have observed that older adults anticipate monetary gains similarly to younger adults, but are less sensitive to the anticipation of monetary losses compared to younger adults (Samanez-Larkin et al., 2007; Wu et al., 2014 ). Moreover, the literature on aging and risk preferences for gains versus losses appears to be mixed. Some studies have observed that older adults exhibit similar risk preferences to younger adults in monetary decision-making in the gain domain, but greater risk aversion concerning monetary losses compared to younger adults (Kurnianingsih, Sim, Chee, & Mullette-Gillman, 2015; Mikels & Reed, 2009; Tymula, Belmaker, Ruderman, Glimcher, & Levy, 2013). However, a recent large-scale smartphone based study observed age-related reductions in the gain domain, but not the loss domain (Rutledge et al., 2016). Given the established age-related differences in processing monetary gains vs. losses, a clear future direction would be to test whether these age-related differences in motivational integration-cognition interactions persist in the domain of monetary losses.
In the social/affective literature, researchers have long observed that older adults show heightened attention towards and better memory for positive compared to negative material, and this shift from a negativity bias to a positivity bias has since been coined the “age-related positivity effect” (Mather & Carstensen, 2005; Reed & Carstensen, 2012). It is unresolved whether this effect arises due to increased attention towards positive goal-relevant stimuli or strategic management of negative emotional states. Critically, however, this pattern of behavior lends support to the Socioemotional Selectivity Theory (SST), a lifespan theory of motivation which postulates that perceived shortening of time horizons is associated with a systemic motivational shift towards social and emotional goals that optimize affective well-being and center on maintenance or loss prevention, rather than acquisition or growth (Carstensen, 2006). In other words, because older adults become aware that time is limited, they consequently prioritize and pursue meaningful and self-relevant behavioral goals that maximize emotional satisfaction. Some have argued that these enhanced emotion-regulatory capacities contribute to greater positive affect and life satisfaction (Carstensen et al., 2011; English & Carstensen, 2014; Urry & Gross, 2010). Thus, a potentially interesting future research direction would be to incorporate more self-relevant incentives (e.g., social feedback, emotional stimuli) to determine if they are more effective for enhancing cognitive processes for older adults, as well as test whether this positivity bias is present in the context of motivation-cognition interactions.
Conclusion
Despite the growth of an aging population, an understudied question relates to whether and how motivational and cognitive functions, both independently and via their interaction, change throughout the lifespan. We report results from a novel experimental paradigm that demonstrates that sufficient incentive motivation can help enhance cognition and overcome cognitive control impairments among older adults, enough so to match younger adult task performance. Furthermore, older and younger adults were found to apply distinct strategies and approaches for processing motivational incentives and utilizing them to influence their pursuit of cognitive task goals. Importantly, these results provide a strong foundation to foster future investigation of the neural mechanisms underlying how motivational integration changes with age. Such knowledge can be used to accomplish broader objectives of informing public policy and enhancing clinical intervention, and potentially contribute to the development of promising motivational interventions that can ameliorate the cognitive impairments in healthy aging.
Supplementary Material
Acknowledgements
We would like to thank our research assistants Katie Shapiro, Roderick Seow, and Jessica Weiss for data collection. We additionally appreciate Tammy English, Denise Head, Dave Balota, and members of the CCP lab, for their insightful perspectives on theoretical questions and expertise for data analyses.
Funding
This work was supported by the National Institutes of Health grants R01AG043461, R21MH105800, and R37MH066078 to TSB. Additionally, DMY was supported by T32NS073547, T32AG000030, and F31DA042574.
Footnotes
All relevant experimental scripts, subject data, and data analyses located in the online repository in Open Science Framework: https://osf.io/3mztb/
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernández G, Helmich RC, & Cools R (2010). Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology, 35(9), 1943–1951. 10.1038/npp.2010.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrover-Roig D, & Barceló F (2010). Individual differences in aging and cognitive control modulate the neural indexes of context updating and maintenance during task switching. Cortex, 46(4), 434–450. http://doi.org/10.1016Zj.cortex.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li S, & Nyberg L (2010). Linking cognitive aging to alterations in dopamine neurotransmitter functioning : Recent data and future avenues. Neuroscience andBiobehavioral Reviews, 34(5), 670–677. http://doi.org/10.1016Zj.neubiorev.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, & Farde L (2006). The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience and Biobehavioral Reviews, 30(6), 791–807. 10.1016/j.neubiorev.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Bamidis PD, Vivas AB, Styliadis C, Frantzidis C, Klados M, Schlee W, … Papageorgiou SG (2014). A review of physical and cognitive interventions in aging. Neuroscience and Biobehavioral Reviews, 44, 206–220. 10.1016/j.neubiorev.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Bates DM, Maechler M, Ben B, Walker S, Bojesen, Christensen Rune Singmann H, Singmann H, & Dai B (2015). Linear mixed-effects models using Eigen and S4. R package version (2015). Retrieved from http://cran.r-project.org/web/packages/lme4/lme4.pdf [Google Scholar]
- Berry AS, Jagust WJ, & Hsu M (2018). Age-related variability in decision-making : Insights from neurochemistry. Cognitive Affective & Behavioral Neuroscience. 10.3758/s13415-018-00678-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Baker SL, Vogel JW, O’Neil JP, Janabi M, … Jagust WJ (2016). Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. The Journal of Neuroscience, 36(50), 12559–12569. 10.1523/JNEUROSCI.0626-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, & Jagust WJ (2018). The Influence of Dopamine on Cognitive Flexibility Is Mediated by Functional Connectivity in Young but Not Older Adults. Journal of Cognitive Neuroscience, 30(9), 1330–1344. 10.1162/jocn [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, & Braver TS (2015). Motivation and Cognitive Control: From Behavior to Neural Mechanism. Annual Review of Psychology, 66, 83–113. 10.1146/annurev-psych-010814-015044 [DOI] [PubMed] [Google Scholar]
- Boyce JM, & Shone GR (2006). Effects of ageing on smell and test. Postgraduate Medical Journal, 82(967), 301–4. 10.1136/pgmj.2005.039651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S, Hickey C, Duthoo W, & Notebaert W (2014). Reward Determines the Context-Sensitivity of Cognitive Control. Journal of Experimental Psychology: Human Perception and Performance, 40(5), 1769–1778. 10.1037/a0037554 [DOI] [PubMed] [Google Scholar]
- Braskie MN, Wilcox CE, Landau SM, Neil JPO, Baker SL, Madison CM, … Jagust WJ (2008). Relationship of Striatal Dopamine Synthesis Capacity to Age and Cognition. Journal of Neuroscience, 28(52), 14320–14328. 10.1523/JNEUROSCI.3729-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. 10.1016/j.tics.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, & Barch DM (2002). A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience andBiobehavioralReviews, 26(7), 809–817. 10.1016/S0149-7634(02)00067-2 [DOI] [PubMed] [Google Scholar]
- Braver TS, & Cohen JD (2000). On the Control of Control: The Role of Dopamine in Regulating Prefrontal Function and Working Memory In Monsell S & Driver J (Eds.), Attention and performance XVIII (pp. 713–737). Cambridge, Massachusetts: MIT Press; 10.1016/S0165-0173(03)00143-7 [DOI] [Google Scholar]
- Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA, Clement NJ, … Somerville LH (2014). Mechanisms of motivation-cognition interaction: challenges and opportunities. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 443–72. 10.3758/s13415-014-0300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, & West R (2008). Memory, Working Executive Control, and Aging. In The Handbook of Aging and Cognition (pp. 311–372). [Google Scholar]
- Brown SBRE, & Ridderinkhof KR (2009). Aging and the neuroeconomics of decision making : A review. Cognitive, Affective, & Behavioral Neuroscience, 9(4), 365–379. 10.3758/CABN.9A365 [DOI] [PubMed] [Google Scholar]
- Carpenter CR, Bassett ER, Fischer GM, Shirshekan J, Galvin JE, & Morris JC (2011). Four Sensitive Screening Tools to Detect Cognitive Dysfunction in Geriatric Emergency Department Patients : Brief Alzheimer’s Screen, Short Blessed Test, Ottawa 3DY, and the Caregiver-completed AD8. Society for Academic Emergency Medicine, 18(4), 374–384. 10.1111/j.1553-2712.2011.01040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL (2006). The Influence of a Sense of Time on Human Development. Science, 312, 1913–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, & Mikels JA (2005). At the Intersection of Emotion and Cognition. Current Directions in Psychological Science, 14(3), 117–121. 10.1111/j.0963-7214.2005.00348.x [DOI] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, … Nesselroade JR (2011). Emotional Experience Improves With Age: Evidence Based on Over 10 Years of Experience Sampling. Psychology and Aging, 26(1), 21–33. 10.1037/a0021285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/Z0022-3514.67.2.319 [DOI] [Google Scholar]
- Castel AD (2007). The Adaptive and Strategic Use of Memory By Older Adults: Evaluative Processing and Value-Directed Remembering. Psychology of Learning and Motivation - Advances in Research and Theory, 48, 225–270. 10.1016/S0079-7421(07)48006-9 [DOI] [Google Scholar]
- Charles ST, & Carstensen LL (2010). Social and emotional aging. Annual Review of Psychology, 61(383–409), 383–409. 10.1146/annurev.psych.093008.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, & O’Doherty JP (2009). Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(39), 12315–20. 10.1523/JNEUROSCI.2575-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, & Braver TS (2013). Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Frontiers in Psychology, 4(January). 10.3389/fpsyg.2013.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, & Braver TS (2016). Reward Favors the Prepared: Incentive and Task-Informative Cues Interact to Enhance Attentional Control. Journal of Experimental Psychology: Human Perception and Performance, 42(1), 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Rissman J, Suthana NA, Castel AD, & Knowlton BJ (2014). Value-based modulation of memory encoding involves strategic engagement of fronto-temporal semantic processing regions. Cognitive, Affective and Behavioral Neuroscience, 14(2), 578–592. 10.3758/s13415-014-0275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R (2008). Role of dopamine in the motivational and cognitive control of behavior. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 14(4), 381–95. 10.1177/1073858408317009 [DOI] [PubMed] [Google Scholar]
- Cools R (2016). The costs and benefits of brain dopamine for cognitive control. WIREs Cogn Sci, 7, 317–329. 10.1002/wcs.1401 [DOI] [PubMed] [Google Scholar]
- Cumming G (2014). The New Statistics : Why and How. Psychological Science, 25(1), 7–29. 10.1177/0956797613504966 [DOI] [PubMed] [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, & Wallace RB (2007). The orbitofrontal cortex, real-world decision making, and normal aging. Annals of the New York Academy of Sciences, 1121, 480–498. 10.1196/annals.1401.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa E, Schiff S, Cagnolati F, & Mapelli D (2015). Motivation-cognition interaction: how feedback processing changes in healthy ageing and in Parkinson’s disease. Aging Clinical and Experimental Research, 27(6), 911–920. 10.1007/s40520-015-0358-8 [DOI] [PubMed] [Google Scholar]
- Durstewitz D, & Seamans JK (2002). The computational role of dopamine D1 receptors in working memory. Neural Networks, 15, 561–572. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Keren NI, Roberts DR, Calhoun VD, & Harris KC (2010). Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Frontiers in Human Neuroscience, 4(March), 1–14. 10.3389/neuro.09.010.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English T, & Carstensen LL (2014). Emotional experience in the mornings and the evenings: Consideration of age differences in specific emotions by time of day. Frontiers in Psychology, 5, 1–9. 10.3389/fpsyg.2014.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis GE, Hess TM, & Smith BT (2013). The impact of age and motivation on cognitive effort: Implications for cognitive engagement in older adulthood. Psychology and Aging, 28(2), 495–504. 10.1037/a0031255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, & Cohen JD (2012). Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS ONE, 7(5), 1–10. 10.1371/journal.pone.0036953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Schuck NW, Nystrom LE, & Cohen JD (2013). Reduced striatal responses to reward prediction errors in older compared with younger adults. The Journal of Neuroscience, 55(24), 9905–12. 10.1523/JNEUROSCI.2942-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand NK, & Czernochowski D (2018). Motivational influences on performance monitoring and cognitive control across the adult lifespan. Frontiers in Psychology, 9(JUN), 1–19. 10.3389/fpsyg.2018.01018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, & Pedersen NL (2007). Age Changes in Processing Speed as a Leading Indicator of Cognitive Aging. Psychology and Aging, 22(3), 558–568. 10.1037/0882-7974.22.3.558 [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Tittgemeyer M, Wagenmakers E-J, Derrfuss J, Imperati D, & Brown S (2011). The Speed-Accuracy Tradeoff in the Elderly Brain: A Structural Model-Based Approach. Journal of Neuroscience, 31(47), 17242–17249. 10.1523/JNEUROSCI.0309-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frober K, & Dreisbach G (2014). The differential influences of positive affect, random reward, and performance-contingent reward on cognitive control. Cogn Affect Behav Neurosci, 14, 530–547. 10.3758/s13415-014-0259-x [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1992). Dopamine-mediated mechanisms of the prefrontal cortex. Seminars in Neuroscience, 4(2), 149–159. 10.1016/1044-5765(92)90013-R [DOI] [Google Scholar]
- Green L, Fry AF, & Myerson J (1994). Discounting of delayed rewards: A Life-Span Comparison. Psychological Science, 5(1), 33–36. 10.1111/j.1467-9280.1994.tb00610.x [DOI] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, & Fry AF (1996). Temporal discounting in choice between delayed rewards: the role of age and income. Psychology and Aging, 11(1), 79–84. 10.1037/0882-7974.11.L79 [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, & Ostaszewski P (1999). Discounting of delayed rewards across the life span: Age differences in individual discounting functions. Behavioural Processes, 46(1), 89–96. 10.1016/S0376-6357(99)00021-2 [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Salami A, Garrett D, Rieckmann A, Lindenberger U, & Bäckman L (2016). BOLD Variability is Related to Dopaminergic Neurotransmission and Cognitive Aging. Cerebral Cortex, 26(5), 2074–2083. 10.1093/cercor/bhv029 [DOI] [PubMed] [Google Scholar]
- Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/jjbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Buitenweg JIV, Wijnen JG, Guerreiro MJS, & Richard K (2010). Remedial effects of motivational incentive on declining cognitive control in healthy aging and Parkinson ‘ s disease. Frontiers in Aging Neuroscience, 2(October), 1–12. 10.3389/fnagi.2010.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Cohen MX, Reneman L, & Ridderinkhof KR (2011). How the aging brain translates motivational incentive into action: The role of individual differences in striato-cortical white matter pathways. Developmental Cognitive Neuroscience, 1(4), 530–539. http://doi.org/10.1016Zj.dcn.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefer C, & Dreisbach G (2017). How Performance-Contingent Reward Prospect Modulates Cognitive Control: Increased Cue Maintenance at the Cost of Decreased Flexibility. Journal of Experimental Psychology: Learning, Memory, and Cognition. [DOI] [PubMed] [Google Scholar]
- Hess TM, & Ennis GE (2011). Age differences in the effort and costs associated with cognitive activity. The Journals of Gereontology, Series B: Psychological Sciences and Social Sciences, 67(June), 447–455. 10.1093/geronb/gbr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavac M (2018). stargazer: Well-Formatted Regression and Summary Statistics Tables. R package version 5.2.2.
- Hoogeveen HR, Dalenberg JR, Renken RJ, ter Horst GJ, & Lorist MM (2015). Neural processing of basic tastes in healthy young and older adults - an fMRI study. NeuroImage, 119, 1–12. 10.1016/j.neuroimage.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Jacobson A, Green E, & Murphy C (2010). Age-related functional changes in gustatory and reward processing regions: An fMRI study. NeuroImage, 53(2), 602–610. http://doi.org/10.1016Zj.neuroimage.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, & Braver TS (2010). Age-Related Shifts in Brain Activity Dynamics during Task Switching. Cerebral Cortex, 20(June), 1420–1431. 10.1093/cercor/bhp206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Locke HS, & Braver TS (2010). Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8871–6. 10.1073/pnas.1002007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong R De. (2001). Adult age differences in goal activation and goal maintenance. European Journal of Cognitive Psychology, 13(1–2), 71–89. 10.1080/09541440042000223 [DOI] [Google Scholar]
- Kaasinen V, & Rinne JO (2002). Functional imaging studies of dopamine system and cognition in normal aging and Parkinson ‘ s disease. Neuroscience andBiobehavioral Reviews, 26, 785–793. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, … Rinne JO (2000). Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiology of Aging, 21(5), 683–688. 10.1016/S0197-4580(00)00149-4 [DOI] [PubMed] [Google Scholar]
- Kang G, Wang L, & Zhou X (2017). Reward interacts with modality shift to reduce cross-modal conflict. Journal of Vision, 17(1)(19), 1–14. 10.1167/17.E19.doi [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Whitson LR, Heathcote A, & Michie PT (2011). Variability in proactive and reactive cognitive control processes across the adult lifespan. Frontiers in Psychology, 2(NOV), 1–19. 10.3389/fpsyg.2011.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer TM, Josef AK, Mata R, Morris ED, & Samanez-Larkin GR (2017). Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiology of Aging, 57, 36–46. http://doi.org/10.10167j.neurobiolaging.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, & Schimmel H (1983). Validation of a Short Orientation-Memory-Concentration Test of Cognitive Impairment. The American Journal of Psychiatry, 140(6), 734–739. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, & Brennan S (2014). The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: A systematic review and meta-analysis. Ageing Research Reviews, 15(1), 28–43. 10.1016/j.arr.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong CAGW, Adams CM, Varner JL, & Hommer D (2001). Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport, 12(17), 3683–3687. [DOI] [PubMed] [Google Scholar]
- Kopp B, Lange F, Howe J, & Wessel K (2014). Age-related changes in neural recruitment for cognitive control. Brain and Cognition, 55(1), 209–219. 10.1016/j.bandc.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Kray J, & Ferdinand NK (2014). Task Switching and Aging In Grange JA & Houghton G (Eds.), Task switching and cognitive control (pp. 350–371). New York, NY: Oxford University Press; 10.1093/acprof:osobl/9780199921959.003.0014 [DOI] [Google Scholar]
- Krug M, & Braver TS (2014). Motivation and Cognitive Control: Going Beyond Monetary Incentives. In The Psychological Science of Money (pp. 137–158). 10.1007/978-1-4939-0959-9_10 [DOI] [Google Scholar]
- Kurnianingsih YA, Sim SKY, Chee MWL, & Mullette-Gillman OA (2015). Aging and loss decision making: increased risk aversion and decreased use of maximizing information, with correlated rationality and value maximization. Frontiers in Human Neuroscience, 9(May), 1–12. 10.3389/fnhum.2015.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockoff PB, & Christensen RH (2015). lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version (2015). Retrieved from http://cran.r-project.org/web/packages/lmerTest/lmerTest.pdf [Google Scholar]
- Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science : a practical primer for t -tests and ANOVAs, 4(November), 1–12. 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane B, McDaniel MA, Waldum ER, & Braver TS (2018). Age-related changes in neural mechanisms of prospective memory. Cognitive, Affective, & Behaviorial Neuroscience. 10.3758/s13415-018-0617-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lal R, O’Neil JP, Baker S, & Jagust WJ (2009). Striatal dopamine and working memory. Cerebral Cortex, 19(2), 445–454. 10.1093/cercor/bhn095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, & Rieckmann A (2014). Neuromodulation and aging: Implications of aging neuronal gain control on cognition. Current Opinion in Neurobiology, 29, 148–158. http://doi.org/10.1016Zj.conb.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Licen M, Hartmann F, Repovs G, & Slapnicar S (2016). The Impact of Social Pressure and Monetary Incentive on Cognitive Control. Frontiers in Psychology, 7(February), 1–16. 10.3389/fpsyg.2016.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff CE, O’Donoghue T, & Dunning D (2011). Age differences in temporal discounting: The role of dispositional affect and anticipated emotions. Psychology and Aging, 26(2), 274–284. 10.1037/a0023280 [DOI] [PubMed] [Google Scholar]
- Loewenstein G, Rick S, & Cohen JD (2008). Neuroeconomics. Annual Review of Psychology, 59(1), 647–672. 10.1146/annurev.psych.59.103006.093710 [DOI] [PubMed] [Google Scholar]
- Lüdecke D (2019). sjstats: Statistical Functions for Regression Models (version 0.17.13). 10.5281/zenodo.1284472 [DOI]
- MacPherson SE, Phillips LH, & Della Sala S (2002). Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychology and Aging Aging, 17(4), 598–609. 10.1037//0882-7974.17.4.598 [DOI] [PubMed] [Google Scholar]
- Magezi D. a. (2015). Linear mixed-effects models for within-participant psychology experiments: an introductory tutorial and free, graphical user interface (LMMgui). Frontiers in Psychology, 6(January), 1–7. 10.3389/fpsyg.2015.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manard M, Carabin D, Jaspar M, & Collette F (2014). Age-related decline in cognitive control: the role of fluid intelligence and processing speed. BMC Neuroscience, 15, 7 10.1186/1471-2202-15-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manard M, François S, Phillips C, Salmon E, & Collette F (2017). The neural bases of proactive and reactive control processes in normal aging. Behavioural Brain Research, 320, 504–516. 10.1016/j.bbr.2016.10.026 [DOI] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, & Heekeren HR (2005). Reward-based decision-making and aging. Brain Research Bulletin, 67(5), 382390 http://doi.Org/10.1016/j.brainresbull.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Mather M (2016). The Affective Neuroscience of Aging. Annual Review of Psychology, 67(1), 213–238. 10.1146/annurev-psych-122414-033540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Carstensen LL (2005). Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences, 9(10), 496–502. http://doi.org/10.1016Zj.tics.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Mikels JA, & Reed AE (2009). Monetary losses do not loom large in later life: Age differences in the framing effect. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 64(4), 457–460. 10.1093/geronb/gbp043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, & Cohen NJ (2002). Attentional Control in the Aging Brain: Insights from an fMRI Study of the Stroop Task. Brain and Cognition, 49(3), 277–296. 10.1006/brcg.2001.1501 [DOI] [PubMed] [Google Scholar]
- Minear M, & Shah P (2008). Training and transfer effects in task switching. Memory & Cognition, 36(8), 1470–1483. 10.3758/MC.336.8.1470 [DOI] [PubMed] [Google Scholar]
- Mohr PNC, Li S, & Heekeren HR (2010). Neuroeconomics and aging: Neuromodulation of economic decision making in old age. Neuroscience and Biobehavioral Reviews, 34, 678–688. 10.1016/j.neubiorev.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Montague PR, King-casas B, & Cohen JD (2006). Imaging Valuation Models in Human Choice. Annual Review of Neuroscience, 29, 417–448. 10.1146/annurev.neuro.29.051605.112903 [DOI] [PubMed] [Google Scholar]
- Mutter SA, Naylor JC, & Patterson ER (2005). The effects of age and task context on Stroop task performance. Memory and Cognition, 33(3), 514–530. 10.3758/BF03193068 [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Johnson PCD, Schielzeth H, Building GK, & Glasgow G (2017). The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of the Royal Society Interface, 14(20170213), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A (2002). Dopamine and the regulation of cognition and attention. Progress in Neurobiology, 67(1), 53–83. 10.1016/S0301-0082(02)00011-4 [DOI] [PubMed] [Google Scholar]
- Padmala S, & Pessoa L (2011). Reward Reduces Conflict by Enhancing Attentional Control and Biasing Visual Cortical Processing. Journal of Cognitive Neuroscience, 23(11), 3419–3432. 10.1162/jocn_a_00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, & Braver TS (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex, 18(5), 1010–1028. 10.1093/cercor/bhm135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, & Buckner RL (2006). Structure-function correlates of cognitive decline in aging. Cerebral Cortex, 16(7), 907–915. 10.1093/cercor/bhj036 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rabbitt P (1979). How old and young subjects monitor and control responses for accuracy and speed. British Journal of Psychology. 10.1111/j.2044-8295.1979.tb01687.x [DOI] [Google Scholar]
- Rangel A, Camerer C, & Montague PR (2008). A framework for studying the neurobiology of value-based decision making. Nature Reviews. Neuroscience, 9(7), 545–556. 10.1038/nrn2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick TS (2014). Cognitive control in context: Working memory capacity and proactive control. Acta Psychologica, 145(1), 1–9. http://doi.org/10.10167j.actpsy.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Reed AE, & Carstensen LL (2012). The theory behind the age-related positivity effect. Frontiers in Psychology, 3(SEP), 1–9. 10.3389/fpsyg.2012.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Park DC (2010). Human Neuroscience and the Aging Mind: at Old Problems A New Look. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 65(4), 405–415. 10.1093/geronb/gbq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, & Monsell S (1995). Costs of a predictible switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124(2), 207–231. 10.1037/0096-3445.124.2.207 [DOI] [Google Scholar]
- RStudioTeam. (2016). RStudio: Integrated Development for R. Boston, MA: Rstudio, Inc; Retrieved from http://www.rstudio.com/ [Google Scholar]
- Rutledge RB, Smittenaar P, Zeidman P, Brown HR, Adams RA, Lindenberger U, … Dolan RJ (2016). Risk Taking for Potential Reward Decreases across the Lifespan. Current Biology, 26(12), 1634–1639. 10.1016/jxub.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, & D’Esposito M (2000). Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience, 3(5), 509–515. 10.1038/74889 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996). The Processing-Speed Theory of Adult Age Differences in Cognition. Psychological Review, 103(3), 403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2000). Aging and measures of processing speed. Biological Psychology, 54(1–3), 35–54. 10.1016/S0301-0511(00)00052-1 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2005). What and when of cognitive aging. Current Directions in Psychological Science, 13(4), 140–144. 10.1111/j.0963-7214.2004.00293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, & Knutson B (2007). Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience, 10(6), 787–791. 10.1038/nn1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, & Knutson B (2015). Decision making in the ageing brain: changes in affective and motivational circuits. Nature Reviews Neuroscience, 16(May). 10.1038/nrn3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Mata R, Radu PT, Ballard IC, Carstensen LL, & McClure SM (2011). Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in Neuroscience, 5(NOV), 1–12. 10.3389/fnins.2011.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Worthy DA, Mata R, McClure SM, & Knutson B (2014). Adult age differences in frontostriatal representation of prediction error but not reward outcome. Cognitive Affective & Behavioral Neuroscience, 672–682. 10.3758/s13415-014-0297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H, Ferdinand NK, & Kray J (2015). The influence of monetary incentives on context processing in younger and older adults: an event-related potential study, 416–434. 10.3758/s13415-015-0335-x [DOI] [PubMed] [Google Scholar]
- Schmitt H, Kray J, & Ferdinand NK (2017). Does the Effort of Processing Potential Incentives Influence the Adaption of Context Updating in Older Adults? Frontiers in Psychology, 5(1969), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman KL, Brooks N, Karrer TM, Castrellon JJ, Perkins SF, Dang L, … Samanez-Larkin GR (2018). Subjective Value Representations during Effort, Probability, and Time Discounting across Adulthood. Social Cognitive and Affective Neuroscience, (May), nsy021–nsy021. 10.1093/scan/nsy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman KL, Gorlick M, Kruti V, Hsu M, Zald D, & Samanez-Larkin G (2016). Adult age differences in decision making across domains: Increased discounting of social and health-related rewards. Psychology and Aging, 31(7), 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman KL, Smith CT, Juarez EJ, Dang LC, Castrellon JJ, & Samanez-Larkin GR (2018). Differential regional decline in dopamine receptor availability across adulthood: Linear and nonlinear effects of age. BioRxiv. [DOI] [PMC free article] [PubMed]
- Sescousse G, Caldu X, Segura B, & Dreher J-C (2013). Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience andBiobehavioral Reviews, 37(4), 681–96. 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Sescousse G, Li Y, & Dreher JC (2015). A common currency for the computation of motivational values in the human striatum. Social Cognitive and Affective Neuroscience, 10(4), 467–473. 10.1093/scan/nsu074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, & Brewer N (1995). Slowness and Age: Speed-Accuracy Mechanisms. Psychology and Aging, 10(2), 238–247. 10.1037/0882-7974.10.2.238 [DOI] [PubMed] [Google Scholar]
- Spaniol J, Bowen HJ, Wegier P, & Grady C (2015). Neural responses to monetary incentives in younger and older adults. Brain Research, 1612, 70–82. http://doi.org/10.1016Zj.brainres.2014.09.063 [DOI] [PubMed] [Google Scholar]
- Spaniol J, Schain C, & Bowen HJ (2014). Reward-enhanced memory in younger and older adults. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 69(5), 730–740. 10.1093/geronb/gbt044 [DOI] [PubMed] [Google Scholar]
- Spaniol J, Voss A, Bowen HJ, & Grady CL (2011). Motivational incentives modulate age differences in visual perception. Psychology and Aging, 26(4), 932–939. 10.1037/a0023297 [DOI] [PubMed] [Google Scholar]
- Starns JJ, & Ratcliff R (2010). The effects of aging on the speed-accuracy compromise: Boundary optimality in the diffusion model. Psychology and Aging, 25(2), 377–390. 10.1037/a0018022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strough J, Bruin W. B. de, & Peters E (2015). New perspectives for motivating better decisions in older adults. Frontiers in Psychology, 6(June), 1–10. 10.3389/fpsyg.2015.00783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, & Barto A (1998). Reinforcement Learning: An Introduction. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Torchiano M (2018). effsize: Efficient Effect Size Computation, R package version 0.7.4. 10.5281/zenodo.1480624 [DOI]
- Tymula A, Belmaker LAR, Ruderman L, Glimcher PW, & Levy I (2013). Like cognitive function, decision making across the life span shows profound age-related changes. Proceedings of the National Academy of Sciences, 110(42), 17143–17148. 10.1073/pnas.1517212112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, & Gross JJ (2010). Emotion Regulation in Older Age. Current Directions in Psychological Science, 19(6), 352–357. 10.1177/0963721410388395 [DOI] [Google Scholar]
- Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding Y-S, … Logan J (1998). Association Between Declines in Brain Dopamine Activity with Age and Cognitive and Motor Impairment in Healthy Individuals. American Journal Of Psychiatry, 155(3), 344–349. http://doi.Org/10.1176/ajp.155.3.344 [DOI] [PubMed] [Google Scholar]
- Westbrook A, & Braver TS (2016). Dopamine Does Double Duty in Motivating Cognitive Effort. Neuron, 89(4), 695–710. http://doi.org/10.1016Zj.neuron.2015.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Ryan S., Kudus Farrah, Dyson Benjamin J., & Spaniol J. (2017). Transient and sustained incentive effects on electrophysiological indices of cognitive control in younger and older adults. Cognitive Affective & Behavioral Neuroscience. [DOI] [PubMed] [Google Scholar]
- Wu CC, Samanez-Larkin GR, Katovich K, & Knutson B (2014). Affective traits link to reliable neural markers of incentive anticipation. Neuroimage, 84, 279–289. 10.1016/j.neuroimage.2013.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DM, & Braver TS (2018). Interactions of motivation and cognitive control. Current Opinion in Behavioral Sciences, 19, 83–90. 10.1016/j.cobeha.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DM, Krug MK, Allen A, & Braver TS (2016). Humans Integrate Monetary and Liquid Incentives to Motivate Cognitive Task Performance. Frontiers in Psychology, d(January), 1–17. 10.3389/fpsyg.2015.02037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Yin S, Lang M, He R, & Li J (2016). The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Research Reviews, 31, 67–79. 10.1016/j.arr.2016.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.