Abstract
Background
Dysbiosis has been recently demonstrated In patients with ankylosing spondylitis (AS) but its implications in the modulation of intestinal immune responses have never been studied. The aim of this study was to investigate the role of ileal bacteria in modulating local and systemic immune responses in AS.
Methods
Ileal biopsies were obtained from 50 HLA-B27+ patients with AS and 20 normal subjects. Silver stain was used to visualise bacteria. Ileal expression of tight and adherens junction proteins was investigated by TaqMan real-time (RT)-PCR and immunohistochemistry. Serum levels of lipopolysaccharide (LPS), LPS-binding protein (LPS-BP), intestinal fatty acid-BP (iFABP) and zonulin were assayed by ELISA. Monocyte immunological functions were studied in in vitro experiments. In addition the effects of antibiotics on tight junctions in human leukocyte antigen (HLA)-B27 transgenic (TG) rats were assessed.
Results
Adherent and invasive bacteria were observed in the gut of patients with AS with the bacterial scores significantly correlated with gut inflammation. Impairment of the gut vascular barrier (GVB) was also present in AS, accompanied by significant upregulation of zonulin, and associated with high serum levels of LPS, LPS-BP, iFABP and zonulin. In in vitro studies zonulin altered endothelial tight junctions while its epithelial release was modulated by isolated AS ileal bacteria. AS circulating monocytes displayed an anergic phenotype partially restored by ex vivo stimulation with LPS+sCD14 and their stimulation with recombinant zonulin induced a clear M2 phenotype. Antibiotics restored tight junction function in HLA-B27 TG rats.
Conclusions
Bacterial ileitis, increased zonulin expression and damaged intestinal mucosal barrier and GVB, characterises the gut of patients with AS and are associated with increased blood levels of zonulin, and bacterial products. Bacterial products and zonulin influence monocyte behaviour.
INTRODUCTION
In healthy subjects, the gastrointestinal tract is colonised by a broad range of microbes, termed the gut microbiota.1 In heathy individuals a gut epithelial barrier2 and a gut vascular barrier (GVB)3 control the translocation of bacteria and bacterial antigens into the bloodstream. The homoeostasis of normal microbial flora in the gut is essential for intestinal health and its altered balance, termed dysbiosis, may influence intestinal permeability through the release of zonulin,4 a protein that modulates the permeability of epithelial tight junctions of the digestive tract.
Dysbiosis has been recently demonstrated in the terminal ileum of patients with ankylosing spondylitis (AS) together with the presence of subclinical gut inflammation.5,6 It is unclear, however, whether this dysbiosis is a cause or consequence of the inflammation and whether dysbiosis modulates immune responses in AS. The aim of the present study was to study the tissue localisation of bacteria in the gut of patients with AS and the eventual changes in gut-epithelial barrier and GVB integrity. We also assessed the role of zonulin in modulating intestinal permeability and monocyte activation. Finally, we analysed whether alterations in gut permeability and microbiota composition are associated with systemic immune responses.
METHODS
For more details about patients and controls see supplemental methods and online supplementary table S1.
Histomorphological grading and immunohistochemistry
One hundred and sixty-five biopsies were obtained from the 50 patients with AS enrolled. Gut specimens from patients with AS were histologically divided as previously described in: normal gut histology, acute and chronic inflammation.7,8 The degree of gut inflammation was also evaluated by using interleukin (IL)-8 as a general marker of inflammation.9 For more details about bacteria characterisation and immunohistochemistry see supplemental methods.
Isolation of bacteria
Ileal biopsy specimens from patients and controls enrolled at the University of Palermo, were immediately processed for bacteriological study in the Microbiology Laboratory, Azienda Ospedaliera Villa Sofia Cervello, Palermo, Italy according to Conte et al.10 For more information, see supplemental methods.
Cultures for aerobic and facultative anaerobic bacteria
For bacterial cultures only ileal biopsies obtained from patients with AS and controls enrolled at the University of Palermo were used. For more information, see online supplementary methods.
RNA extraction and quantitative TaqMan real-time (RT)-PCR
Total RNA was extracted using the Qiagen RNeasy Mini kit, with on-column DNase I digestion as previously described.8 For more information, see online supplementary methods.
Flow cytometry analysis of surface and intracellular antigens
Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of 20 patients with AS and 10 healthy controls as previously described.8 A list of the antibodies used is provided in online supplementary table S2.
ELISA for circulating LPS, iFABP and zonulin
Levels of lipopolysaccharide (LPS), LPS-binding protein (BP), intestinal fatty acid-BP (iFABP) and zonulin proteins were analysed in sera of all patients with AS and controls. For more information, see online supplementary methods.
Cell cultures
In order to evaluate the role of intestinal bacteria isolated from patients with AS in modulating epithelial zonulin levels, bacteria were isolated from ileal AS samples obtained from patients enrolled at the University of Palermo as described by Conte et al10 and incubated with Caco-2 epithelial cells. The modulation of zonulin mRNA was then assessed by RT-PCR. The effect of zonulin on human umbilical vein endothelial cells and PBMCs was evaluated as previously described.10 For more information, see supplemental methods.
Human leukocyte antigen (HLA)-B27 TG rats
HLA-B*2705 transgenic (TG) rats of line 33–3 (B27-TG) on a Fischer background (F344/NTac-Tg [HLA-B*2705, β2M]) (Taconic, Hudson, New York, USA) were backcrossed with Piebald Virol Glaxo (PVG) rats (PVG/OlaHsd) (Harlan, UK) for a minimum of 10 generations before their use in experiments as previously described.11 For more information, see online supplementary methods.
Statistical analysis
The non-parametrical Mann-Whitney test was used to calculate the statistical significance between groups. Spearman’s rank correlation was used to calculate the correlation between different variables in AS. p Values <0.05 were considered statistically significant.
RESULTS
Assessment of intestinal gut inflammation in AS
IL-8 was overexpressed in patients with AS with chronic inflammation (see online supplementary figure S1C,D) compared with those with acute inflammation (see online supplementary figure S1B,D) and without inflammation (see online supplementary figure S1D) and controls (see online supplementary figure S1A, D). In patients with AS, the number of IL-8 positive cells was correlated with the degree of intestinal inflammation (see online supplementary figure S1D).
Adherent and invasive bacteria are present in the gut of patients with AS
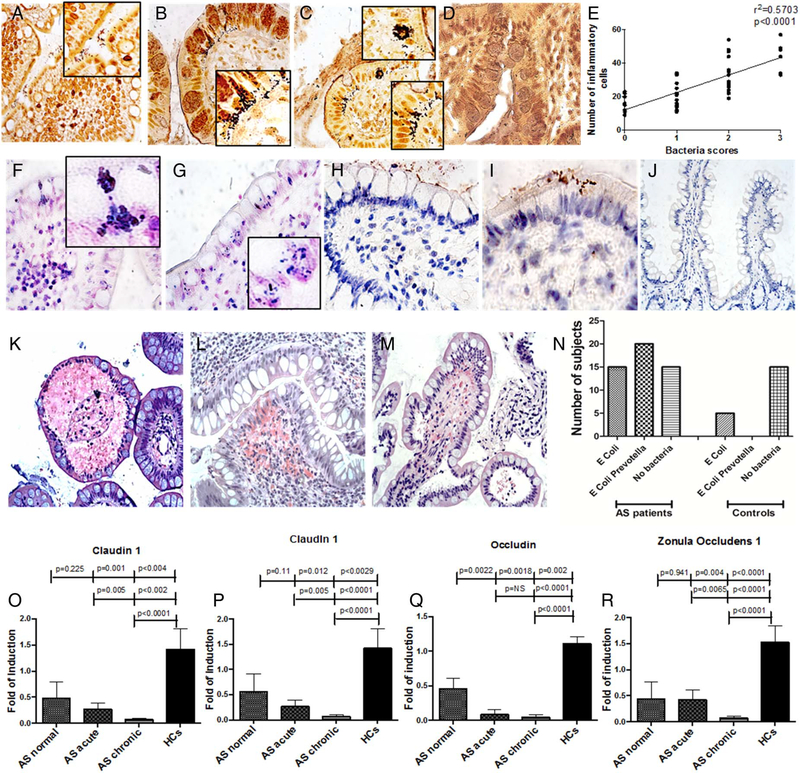
Adherent and invading rod-shaped bacteria were observed in 35 out of 50 patients with AS (25/33 of the Palermo cohort and 10/17 of the Ghent cohort) independent of the presence of acute or chronic inflammation. Among these patients, only four showing acute inflammation and four showing chronic inflammation were taking sulfasalazine. Of these, two out of four patients with acute inflammation and one out of four with chronic inflammation did not show cultivable bacteria. Bacteria were mainly detected within the epithelium and rarely in the context of lamina propria (figure 1A–C). Absence of adherent and/or invasive bacteria was observed in normal ileum (figure 1D). In particular, invasive bacteria, sometimes aggregated in clusters, were observed in 12 patients with AS of the Palermo’s cohort and in 7 patients with AS of the Ghent cohort. The bacterial scores significantly correlated with the percentages of infiltrating inflammatory cells (r2 = 0.57, p<0.0001) (figure 1E). Gram-positive (F-G) and Gram-negative (H-I) bacteria were confirmed to be both adherent and invasive in patients with AS. The presence of invasive bacteria in AS was invariably associated with histological changes characterised by the detachment of basal membranes from the lamina propria, forming vacuoles inside the villi, and oedematous lamina propria with extravasated red blood cells (figure 1K–M and see online supplementary table S3). Isolated oedematous lamina propria, without detachment of basal membranes and/or vasculitis, was observed in the intestine of all patients displaying adherent bacteria (see online supplementary table S3). Identification of the bacteria from culture of ileal samples showed that all the patients with AS of the Palermo’s cohort had cultivable bacteria essentially the Gram-negative bacteria Escherichia coli and Prevotella spp (figure 1N). Conversely, only 5 out of 20 control samples displayed cultivable bacteria (25%), E. coli being the only Gram-negative species found (figure 1N). No culture of ileal samples was performed in ileal samples from the Ghent cohort. Cultures of Prevotella spp and E. coli were confirmed by PCR.
Figure 1.
Invasive and adherent bacteria are present in the ileum of patients with ankylosing spondylitis (AS) and are associated with alterations of tight junction proteins. (A-D) Representative microphotographs showing adherent (A) and invasive (B and C) bacteria in AS but not in controls (D). (E) Bacterial scores are directly correlated with the number of infiltrating mononuclear cells. (F-G) Representative images showing Gram staining in patients with AS demonstrating the presence of invading Gram-positive bacteria. (H-J) Representative images showing immunohistochemistry for lipopolysaccharide (LPS) in patients with AS (H and I) and controls (J). (K-M) Histological alterations are associated with the presence of bacteria such as haemorrhages (K and L) and detachment of epithelium from basal membrane (M). (N) Cultures of isolated bacteria displayed mainly Escherichia coli and Prevotella spp. (O–R) relative m-RNA levels of claudin1 (O), claudin 4 (P), occludin (Q) and zonula occludens 1 (R) were assessed by quantitative real-time (RT)-PCR in ileal samples obtained from all the patients and all the controls. Data are expressed as mean (SEM). (A-D, F-J): original magnification×250. Insert in (A-C) and (F-G) original magnification×630.
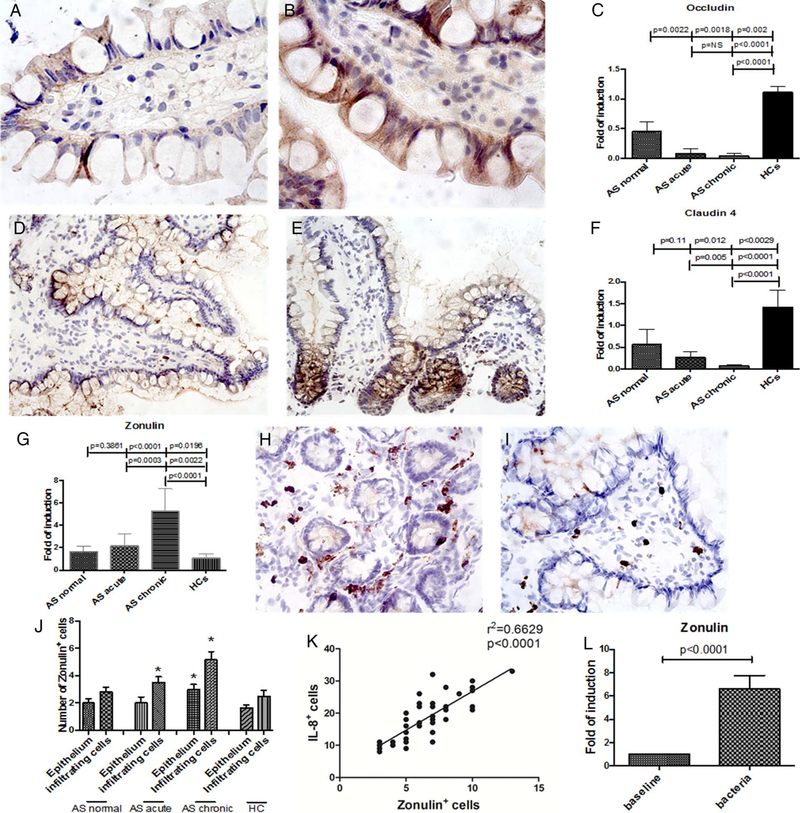
We next studied the expression of intestinal tight junction proteins. A significant downregulation of claudin 1 (figure 1O), claudin 4 (figure 1P), occludin (figure 1Q) and zonula occludens 1 (figure 1R) was observed in the gut of patients with AS (expecially in those with chronic gut inflammation) compared with controls. The significant downregulation of the tight junction proteins in AS was confirmed by immunohistochemistry (IHC) demonstrating the reduced expression in AS of occludin (figure 2A,C) and claudin 4 (figure 2D,F) compared with controls (figure 2B,C,E and F).
Figure 2.
Occludin, claudin 4 and zonulin 1 tissue expression is altered on patients with ankylosing spondylitis (AS) and modulated by intestinal bacteria. (A and B) representative imaging showing occludin expression in the gut of patients with AS (A) and controls (B). (C) Higher numbers of occludin positive cells were observed in healthy controls compared with AS. (D and E) Representative imaging showing claudin 4 expression in the gut of patients with AS (D) and controls (E). (F) Higher numbers of claudin 4 positive cells were observed in healthy controls compared with AS. (G) relative m-RNA levels of zonulin 1 were assessed by real-time (RT)-PCR in the ileal samples obtained from all the patients with AS and HCs. (H and I) Representative imaging showing zonulin 1 expression in the gut of patients with AS (H) and controls (I). (J) Quantification of zonulin 1 positive cells was performed in the ileal biopsies of all the patients and the controls showing higher numbers of zonulin 1 positive cells in patients with AS. (K) The number of zonulin positive cells was significantly and directly correlated with the number of IL-8 positive cells. (L) Caco-2 cells were incubated with bacteria isolated from ileal biopsies obtained from five patients with AS and the modulation of zonulin expression assessed by RT-PCR. Data are expressed as mean (SEM) of five independent experiments. (A and B) Original magnification×630. (D and E) Original magnification×250. (H and I) Original magnification×400. Data are expressed as mean (SEM).
Zonulin is upregulated in the gut of patients with AS and modulated by ileal bacteria
We next evaluated zonulin expression in the biopsies of all patients with AS and controls. Significant upregulation of zonulin mRNA was observed in the ileal samples of patients with AS, expecially in those with chronic gut inflammation, (figure 2G), inversely correlated with the expression levels of claudin 1 (r2=0.28, p<0.0001) (see online supplementary figure S1E), claudin 4 (r2=0.324, p<0.0001) (see online supplementary figure S1F), occludin (r2=0.654, p>0.0001) (see online supplementary figure S1G) and zonula occludens 1 (r2=0.245, p<0.001) (see online supplementary figure S1H). Zonulin has been identified as prehaptoglobin 2, one of the two genetic variants (together with haptoglobin 1) of human haptoglobins.3 Since we cannot completely discriminate between pre-HP2 and HP2 by RT-PCR,12 overexpression of zonulin was also confirmed by immunohistochemistry in frozen ileal samples obtained from patients with AS by using a specific antizonulin antibody (figure 2H–J). Analysis of tissue distribution of zonulin demonstrated its expression among epithelial cells and infiltrating mononuclear cells (figure 2H,I). Interestingly, the number of zonulin+ cells correlated with the number of IL-8+ cells (figure 2K). We next evaluated in vitro the role of isolated ileal bacteria from patients with AS in modulating zonulin expression. As shown in figure 2L, co-culture of Caco-2 cells with bacteria isolated from ileal biopsies of five patients with AS of the Palermo’s cohort induced significant upregulation of zonulin.
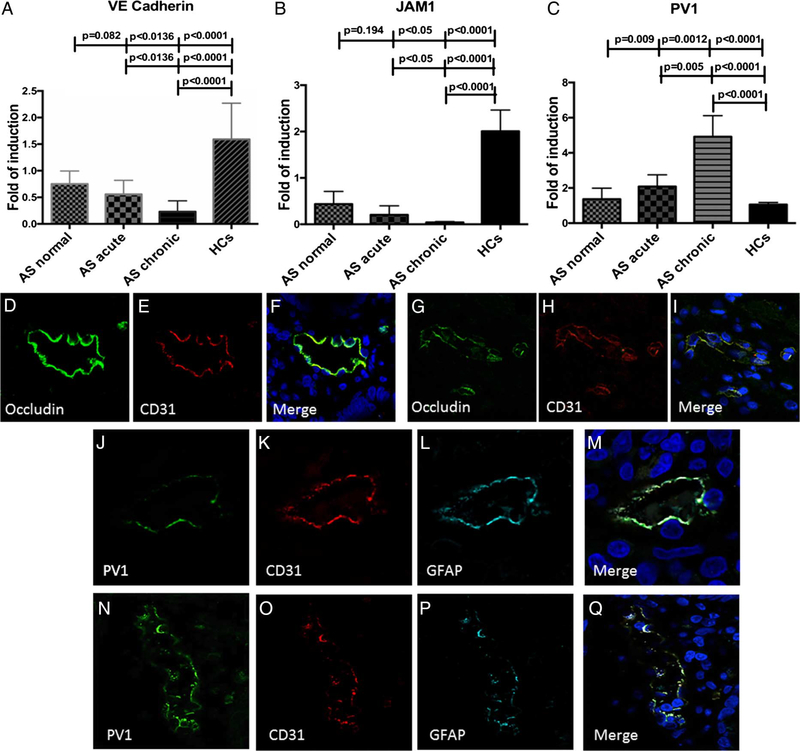
Impairment of the GVB occurs in patients with AS
In order to evaluate whether increased intestinal permeability was paralleled by impairment of the GVB,2 RT-PCR for junctional adhesion molecule-A (JAM-A), a vascular tight junctions protein, vascular endothelial (VE)-cadherin, a vascular adherens junctions protein, and PV1, a marker of endothelial cells permeability, was performed. VE-cadherin and JAM-A (figure 3A,B), were significantly downregulated in the inflamed ileum of patients with AS together with a significant upregulation of PV1 expecially in those patients with chronic gut inflammation compared with controls (figure 3C). To confirm the alteration of GVB, confocal microscopy analysis of occludin and CD31 (a specific endothelial cell marker) expression and of CD31/glial fibrillary acidic protein (GFAP)/PV1 was performed next in ileal samples from patients with AS and controls. As shown in figure 3D–F, endothelial occludin expression in healthy controls (HC) showed a continuous staining of the junctional protein that surrounded cell borders. In comparison, endothelial cells from patients with AS exhibited the disappearance of the classic occludin continuous staining, showing a jagged and broken vascular distribution (figure 3G–I). Analysis of GVB showed a higher expression of PV1 in AS (figure 3N) compared with HC (figure 3J) and confirmed the disorganised staining for CD31 (figure 3O) and GFAP (figure 3P).
Figure 3.
Gut vascular barrier (GVB) in patients with ankylosing spondylitis (AS). (A-C) relative m-RNA levels of VE-cadherin (A), junctional adhesion molecule (JAM)-1 (B) and PV1 (C) were assessed by RT-PCR in AS and HC ileal samples. (D-F) and (G-I) Representative confocal microscopy images showing CD31 and occludin co-localisation in HCs (D-F) and AS (G-I). (J-M and N-Q): Representative confocal microscopy images showing PV1, CD31 and GFAP co-localisation in HCs (J-M) and AS (N-Q). (D-Q): Original magnification×400. Data are expressed as mean (SEM).
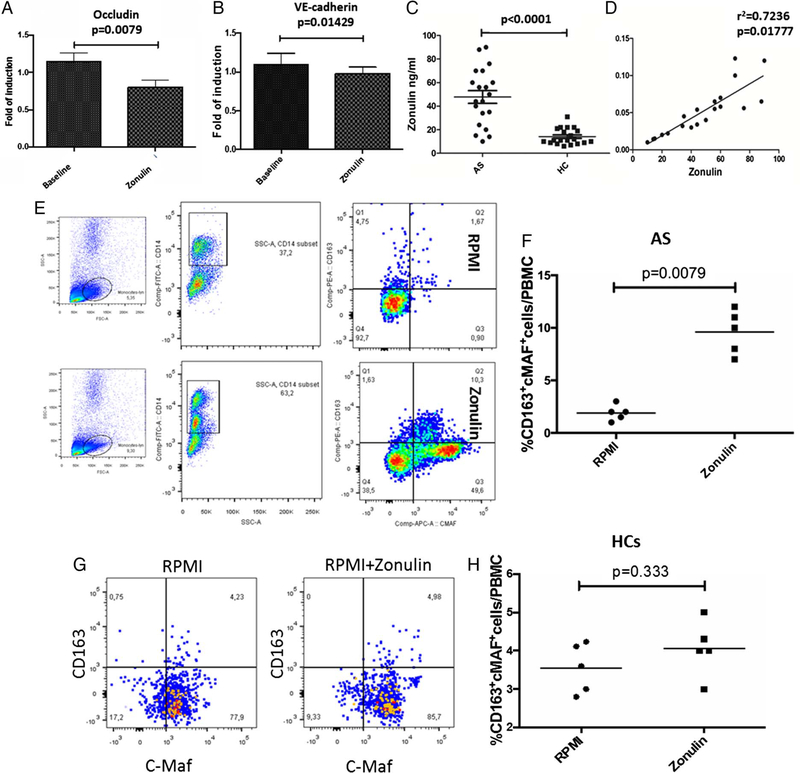
Zonulin alters the expression of endothelial tight junctions
We next evaluated in vitro whether zonulin may influence the expression of endothelial tight junction proteins. As shown in figure 4, zonulin induced a significant downregulation of occludin (figure 4A) and VE-cadherin (figure 4B). Corresponding with the alteration of the GVB, increased serum zonulin levels (figure 4C) were observed in AS. To establish whether serum zonulin levels are correlated with intestinal permeability, lactulose (LA)/mannitol (MA) urine ratio was determined in 20 patients with AS and 20 controls, all enrolled at the University of Palermo. An increased intestinal permeability, significantly correlated with serum zonulin, was present in patients with AS (LA/MA 0.052±0.002, r2=0.7236, p=0.01777) (figure 4D) but not in healthy controls (LA/MA 0.021±0.0011; r2=0.1858, p>0.05) (data not shown). Zonulin has a CD163 binding motif identical to that present in mature haptoglobin 210,13 In order to assess the potential functional relevance of zonulin interaction with CD163, isolated PBMCs from patients with AS and controls were incubated with recombinant zonulin. As shown in figure 4E,F,G and H, incubation with zonulin induced a significant expansion of c-MAF+CD163+ cells identified as M2 polarised macrophages14 in AS (figure 4E,F) but not in controls (figure 4G,H).
Figure 4.
Serum levels of zonulin in patients with ankylosing spondylitis (AS) and in vitro effects of zonulin on human umbilical vein endothelial cells (HUVECs) and peripheral monocytes. (A and B) MRNA expression of occludin (A) and VE-cadherin (B) was assessed in HUVEC cells treated or not with recombinant human zonulin by RT-PCR. Significant downregulation of Occludin and VE-cadherin was observed in HUVEC after incubation with zonulin. (C and D) Serum levels of zonulin were evaluated in 20 patients with AS and 20 controls (C) and correlated with LA/MA ratio (D). (E-H) Peripheral blood mononuclear cells (PBMCs) obtained from five patients with AS (E) and five controls (G) were incubated with recombinant zonulin and the percentage of CD163+c-MAF+ cells evaluated by flow cytometry; percentages of AS (F) and controls (H) CD163+c-MAF+ cells before and after zonulin stimulation. (A-B) Data are expressed as mean (SEM). (C, D, F and H): Data are expressed as individual data points.
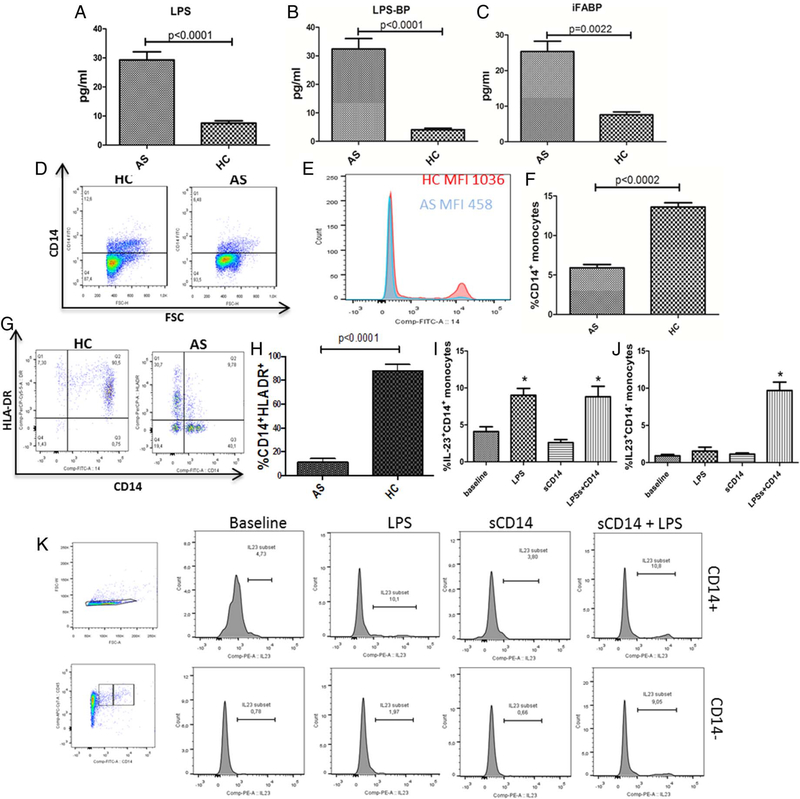
Increased serum levels of iFABP, LPS and LPS-BP are found in patients with AS
Since the alterations of epithelial and endothelial permeability, we next evaluated the serum levels of LPS, LPS-BP and iFABP in all the patients with AS and controls. As shown in figure 5, significantly increased levels of LPS (figure 5A), LPS-BP (figure 5B) and iFABP (figure 5C) were observed in patients with AS. Since it has been demonstrated that the presence of high LPS concentration downregulates the expression of CD14,15 we examined by flow cytometry, the expression of CD14 in circulating monocytes and the effects of LPS and soluble CD14 stimulation on IL-23 production. A significant reduction of CD14+ monocytes (figure 5D–F) and of HLADR+ monocytes (figure 5G,H) was observed only in AS monocytes. Since the soluble form of CD14 (sCD14) has been demonstrated to enable CD14− cells to respond to LPS,16 we next evaluated whether sCD14 might rescue AS CD14− cells from their anergic state. Among AS CD14+ cells, stimulation with LPS, but not with sCD14, modified the expression of IL-23 that was not further modified by the combination of LPS and sCD14 (figure 5I–K). Conversely, only the combination of sCD14 and LPS strongly upregulated the production of IL-23 in AS CD14− monocytes (figure 5I–K).
Figure 5.
Intestinal bacterial products translocate into ankylosing spondylitis (AS) bloodstream and modulate monocyte behaviour. (A-C) Serum levels of lipopolysaccharide (LPS) (A), LPS-binding protein (BP) (B) and intestinal fatty acid-BP (iFABP) (C) are increased in the sera obtained from patients with AS compared with controls. (D-F) Percentages of CD14+ cells is reduced in peripheral blood mononuclear cells (PBMCs) from patients with AS. (D) Representative dot plot showing the percentage of CD14+ cells gated on CD45 region among PBMCs in patients with AS and controls, (E) representative histogram showing CD14 MFI in patients with AS and HCs. (F) percentages of CD14+ cells in patients with AS and controls. (G and H) Percentage of HLA-antigen D Related (DR)+ cells is reduced in PBMCs from patients with AS. (G) Representative dot plot showing the percentage of HLA-DR+ cells gated on the monocytes region among PBMCs in patients with AS and controls, (H) percentages of CD14+ cells in patients with AS and controls. (I-K) Effects of monocyte stimulation with LPS alone, sCD14 alone or sCD14+LPS on CD14+ (H) and CD14− monocytes. Combination of LPS+sCD14 increased IL-23 production only in CD14− cells (I and J). (K) Representative dot plots showing the gating strategy and the percentage of IL-23 expressing cells. Results are showed as mean (SEM).
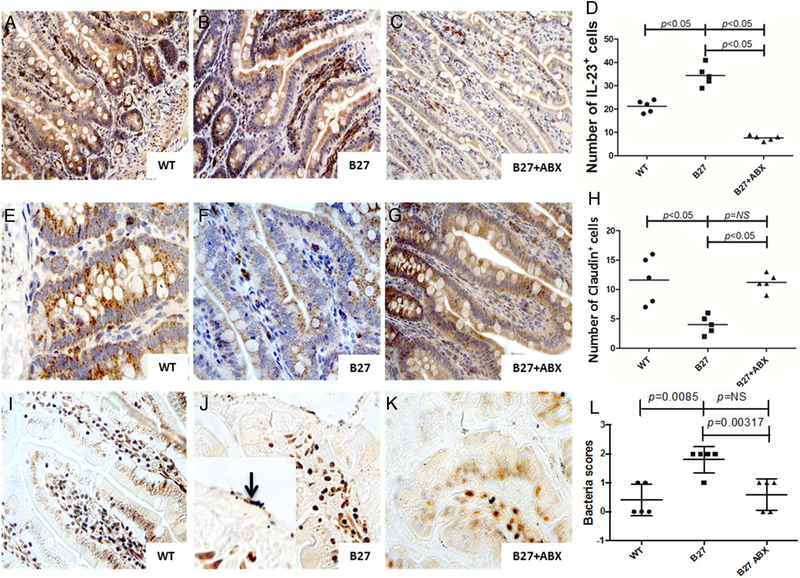
Alteration of epithelial tight junctions occurs in HLA-B27 rats and is restored by antibiotic treatment
In human HLA-B27 and β2-microglobulin TG rats (B27-TG), ileitis develops spontaneously.17 In order to study whether alteration of tight junctions is present in the ileal samples of these rats, ileal samples from five HLA-B27 TG and five wild type (WT) rats were evaluated. HA-B27 rats displayed ileal inflammation characterised by IL-23 increased expression (figure 6B), occludin downregulation (figure 6F) and the presence of adherent bacteria (figure 6J). Antibiotic treatment caused a significant amelioration of signs of intestinal inflammation as previously described,18 the reduction of IL-23 expression (figure 6C, D), the normalisation of occludin expression (figure 6G,H) and the disappearance of adherent bacteria (figure 6K,L).
Figure 6.
Ileal inflammation and dysbiosis in HLAB27 transgenic rats is modified by antibiotic treatment. (A-C) Representative images showing IL-23 staining in ileal samples obtaining from wild type (WT) rats (A), HLA-B27 transgenic (TG) rats (B) and HLA-B27 TG rats after antibiotic treatment (C). (D) Semiquantitative evaluation of IL-23+ cells. (E-F) Representative images showing IL-23 staining in ileal samples obtained from WT rats (E), HLA-B27 TG rats (F) and HLA-B27 TG rats after antibiotic treatment (G). (H) Semiquantitative evaluation of IL-23+ cells. (I-K) representative images showing Warthin starry staining for identifying bacteria in ileal samples obtained from WT rats (I), HLA-B27 TG rats (J) and HLA-B27 TG rats after antibiotic treatment (K). Higher numbers of adhering and sometimes invading bacteria were observed in HLA-B27 rats (J and insert in J). (L) Semiquantitative evaluation of bacteria in rats ileal samples. (A-C, E-G, I-K) original magnification ×250; J insert: original magnification ×630. Data are expressed as individual data points.
DISCUSSION
In this study we demonstrate that adherent and invading bacteria are present in the ileum of patients with AS and are associated with the alteration of the epithelial barrier and the GVB. The presence of leaky epithelium and endothelium in AS ileum is accompanied by the translocation of zonulin and bacterial products into the bloodstream possibly inducing the modulation of the innate immune system in AS.
The intestinal microbiota plays a critical role in modulating the immune response of the gut.19 The potential role of intestinal bacteria in the pathogenesis of gut inflammation in patients with Spondyloarthritis (SpA) has been highlighted by the identification of dysbiosis in different SpA subsets, including patients with AS.5,20,21 However, the question of how dysbiosis can influence local and systemic immune responses in AS has not yet been explored.
In this study we confirm and expand our previous results5 by demonstrating that Gram-negative bacteria, essentially E. Coli and Prevotella spp, and Gram-positive bacteria are present in AS ileal samples, displaying both adherent and invasive behaviour. Interestingly, the presence of invasive bacteria was associated with specific histological alterations mainly characterised by the detachment of basal membrane from the lamina propria, leading to the formation of vacuoles inside the villi and haemorrhagic extravasation. These histological findings seem to be directly attributable to the presence of bacteria since similar histological alterations have previously been reported in mice infected with enteropathogenic E. coli.22
In the presence of pathogenic or non-pathogenic enteric bacteria, mammalian small intestines activate the zonulin pathway3 that is involved in the regulation of the permeability of epithelial tight junctions.4 In our study, tissue levels of zonulin were significantly upregulated in AS ileal samples and accompanied by IL-8 overexpression and a profound reduced expression of tight junction proteins by epithelial cells. These alterations were dependent on the degree of intestinal inflammation, associated with both adherent and invasive bacteria and apparently related to a reduced expression by epithelial cells. We, however, cannot exclude that loss of epithelial cells may also contribute to the reduced tight junction protein expression. Serum zonulin increase was also observed in patients with AS with more pronounced gut inflammation, accompanied by an increased intestinal permeability evaluated by LA/MA urine ratio. Interestingly, isolated bacteria from AS ileal biopsies significantly upregulated zonulin expression in cultured epithelial cells, apparently indicating a specific effect of AS-associated bacteria. It is unclear whether these alterations are the cause or the consequence of intestinal dysbiosis. However, here we demonstrated that alterations of tight junctions, also present in HLA-B27 TG rats, are restored after antibiotic treatments and that antibiotics therapy reduced epithelium-adherent bacteria, suggesting that intestinal dysbiosis might be responsible for the impairment of the epithelial barrier. The reduced number of adherent intestinal bacteria we observed is consistent with previous studies demonstrating that antibiotic treatment reduces mucosal adherent bacteria in mice.18
Together with the gut epithelial barrier, a GVB has been recently demonstrated in mice and humans, that acts by preventing the entry into the bloodstream of microbiota-derived products.2 The GVB shows adherens junctions and tight junctions that seem to be modulated or downregulated, as demonstrated in our in vitro experiments, by zonulin. Increased zonulin expression was in fact accompanied by a significant downregulation of endothelial tight junction proteins, such as occludin, and vascular adherens proteins such as VE-cadherin and by the upregulation of PV1, a marker of increased endothelial permeability.23 The presence of a ‘leaky endothelium’ was also confirmed by confocal microscopy experiments showing disorganised staining for CD31, occludin and GFAP and by the demonstration of increased serum levels of zonulin and bacterial products such as LPS, iFABP and LPS-BP in serum of patients with AS. Overall, our results point to a zonulin-dependent epithelial and endothelial loss of barrier function. The fact that gene expression analysis cannot distinguish between pre-HP2 (alias zonulin) and Hp2 and that antibodies used for the IHC experiments may not be specific enough to exclusively detect zonulin and not mature HP2 may raise the possibility that HP2 rather than zonulin is upregulated. However, the decreased expression of tight junction protein and, most importantly, the direct correlation between zonulin and LA/MA point clearly to the involvement of zonulin and not the mature HP2 that has never been reported to have an effect on barrier function.
Zonulin has a CD163 binding motif identical to that present in mature haptoglobin 210 that has been shown to bind the haptoglobin receptor CD163.13 Therefore, it is conceivable that zonulin binds to CD163 as well as to haptoglobin. The potential functional relevance of this binding in the regulation of monocytes’ behaviour, however, has been not previously studied. Here we demonstrated that zonulin induces a significant in vitro expansion of CD163+c-MAF+ monocytes, compatible with the M2 phenotype, and that these cells were expanded in the peripheral blood of patients with AS. Macrophages play essential activities in homoeostasis maintenance during different organism conditions and may be polarised according to various stimuli into distinct populations. M2 macrophages are macrophages essentially involved in the pathogenesis of asthma, fibrosis, atopic dermatitis, cancer and granuloma formation.24 Furthermore, an increased frequency of CD163+M2 monocytes, producing IL-23, has been previously demonstrated to be expanded in the peripheral blood and inflamed gut and synovial tissues of patients with AS.25,26 The in vitro stimulation of AS PBMCs with recombinant zonulin, was also accompanied by a significant expansion of c-MAF+CD163+ M2 cells. We also observed the zonulin-dependent expansion of c-MAF+CD163− cells. Beyond its role in modulating macrophage differentiation, c-MAF is also involved in the differentiation of T helper cells27,28 and we cannot exclude that zonulin might also induce the expansion of c-MAF+ T cells.
We also studied the functional relevance of the increased circulating levels of bacterial products in AS. In the gut, the presence of high LPS concentrations downregulates the monocyte expression of CD14, the receptor involved in the binding of the LPS/LPS-BP complex.15 Increased LPS levels in AS, were accompanied by the downregulation of CD14 on the surface of monocytes together with the reduced expression of HLA-antigen D Related (DR). CD14−HLADR− monocytes have been demonstrated to be functionally anergic29 and this anergic phenotype was rescued, at least in part, by the co-incubation of these cells with LPS+sCD14 leading to an increased expression of IL-23.
In conclusion, in this study we provide the first evidence that adherent and invasive bacteria are present in the inflamed gut of patients with AS and that these bacteria, through the release of zonulin, may induce a leaky gut epithelial and endothelial barrier, leading to the translocation of intestinal-derived proteins into the bloodstream, ultimately inducing systemic immune alterations that might participate in AS pathogenesis.
Supplementary Material
Acknowledgements
The authors thank Dr Francesca Raiata (Sezione di Anatomia Patologica, Azienda Ospedaliera Ospedali riuniti Villa Sofia Cervello, Palermo, Italy) for her technical support in immunohistochemical experiments. The authors also thank Dr Angelo Ferrante for his help in preparing the overview figure.
Funding This study was in part supported by a grant of Ministero dell’Istruzione, dell’Università e della ricerca Scientifica from Italy.
Footnotes
Competing interests None declared.
Patient consent Obtained.
Ethics approval University of Palermo and Ghent.
Twitter Follow Simon Milling @s_milling
REFERENCES
- 1.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014;14:141–53. [DOI] [PubMed] [Google Scholar]
- 2.Spadoni I, Zagato E, Bertocchi A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015;350:830–4. [DOI] [PubMed] [Google Scholar]
- 3.El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002;123:1607–15. [DOI] [PubMed] [Google Scholar]
- 4.Zonulin Fasano A. and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011;91:151–75. [DOI] [PubMed] [Google Scholar]
- 5.Costello ME, Ciccia F, Willner D, et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol 2015;67:686–91. [DOI] [PubMed] [Google Scholar]
- 6.Tito RY, Cypers H, Joossens M, et al. Dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol 2017;69:114–21. [DOI] [PubMed] [Google Scholar]
- 7.Ciccia F, Bombardieri M, Principato A, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheumatol 2009;60:955–65. [DOI] [PubMed] [Google Scholar]
- 8.Ciccia F, Accardo-Palumbo A, Rizzo A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with Ankylosing Spondylitis and subclinical gut inflammation. Ann Rheum Dis 2014;73:1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daig R, Andus T, Aschenbrenner E, et al. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 1996;38:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte MP, Schippa S, Zamboni I, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 2006;55:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utriainen L, Firmin D, Wright P, et al. Expression of HLA-B27 causes loss of migratory dendritic cells in a rat model of spondylarthritis. Arthritis Rheum 2012;64:3199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA 2009;106:16799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graversen JH, Madsen M, Moestrup SK. CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol 2002;34:309–14. [DOI] [PubMed] [Google Scholar]
- 14.Barros MHM, Hauck F, Dreyer JH, et al. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013;8:e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith PD, Smythies LE, Mosteller-Barnum M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for lps- and iga-mediated activities. J Immunol 2001;167:2651–6. [DOI] [PubMed] [Google Scholar]
- 16.Frey EA, Miller DS, Jahr TG, et al. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med 1992;176:1665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiko S, Grisham MB. Spontaneous intestinal inflammation and nitric oxide metabolism in HLA-B27 transgenic rats. Gastroenterology 1995;109:142–50. [DOI] [PubMed] [Google Scholar]
- 18.Dieleman LA, Goerres MS, Arends A, et al. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut 2003;52:370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada N, Seo SU, Chen GY, et al. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 2013;13:321–35. [DOI] [PubMed] [Google Scholar]
- 20.Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Nat Rev Immunol 2015;67:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoll ML, Kumar R, Morrow CD, et al. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther 2014;16:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vulcano AB, Tino-De-Franco M, Amaral JA, et al. Oral infection with enteropathogenic Escherichia coli triggers immune response and intestinal histological alterations in mice selected for their minimal acute inflammatory responses. Microbiol Immunol 2014;58:352–9. [DOI] [PubMed] [Google Scholar]
- 23.Stan RV, Tse D, Deharvengt SJ, et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell 2012;23:1203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeten D, Møller HJ, Delanghe J, et al. Association of CD163+ macrophages and local production of soluble CD163 with decreased lymphocyte activation in spondylarthropathy synovitis. Arthritis Rheumatol 2004;50: 1611–23. [DOI] [PubMed] [Google Scholar]
- 26.Ciccia F, Alessandro R, Rizzo A, et al. Macrophage phenotype in the subclinical gut Inflammation of patients with ankylosing spondylitis. Rheumatology (Oxford) 2014;53:104–13. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Suto A, Iwamoto T, et al. Sox5 and c-Maf cooperatively induce Th17 cell differentiation via RORγt induction as downstream targets of Stat3. J Exp Med 2014;211:1857–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haribhai D, Ziegelbauer J, Jia S, et al. Alternatively activated macrophages boost induced regulatory T and Th17 Cell responses during immunotherapy for colitis. J Immunol 2016;196:3305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams MA, Withington S, Newland AC, et al. Monocyte anergy in septic shock is associated with a predilection to apoptosis and is reversed by granulocyte-macrophage colony-stimulating factor ex vivo. J Infect Dis 1998;178:1421–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.