Abstract
Genetics provides two major opportunities for understanding human disease—as a transformative line of etiological inquiry and as a biomarker for heritable diseases. In psychiatry, biomarkers are very much needed both for research and treatment given the heterogenous populations identified by current phenomenologically-based diagnostic systems. To date, however, useful and valid biomarkers have been scant due to the inaccessibility and complexity of human brain tissue and consequent lack of insight into disease mechanisms. Genetic biomarkers are therefore especially promising for psychiatric disorders. Genome-wide association studies (GWAS) of common diseases have matured over the last decade, generating the knowledge base for increasingly informative individual-level genetic risk prediction. In this review, we discuss fundamental concepts involved in computing genetic risk with current methods, strengths and weaknesses of various approaches, assessments of utility, and applications various psychiatric disorders and related traits. Although genetic risk prediction has become increasingly straightforward to apply and common in published studies, there are important pitfalls to avoid. At present, the clinical utility of genetic risk prediction is still low; however, there is significant promise for future clinical applications as the ancestral diversity and sample sizes of GWAS increase. We discuss emerging data and methods aimed at improving the value of genetic risk prediction for disentangling disease mechanisms and stratifying subjects for epidemiological and clinical studies. For all applications, it is absolutely critical that polygenic risk prediction is applied with appropriate methodology and control for confounding to avoid repeating some mistakes of the candidate gene era.
Keywords: polygenic risk scores, complex traits, psychiatric disorders, precision medicine, population genetics, statistical genetics, heritability, psychiatric genetics, liability threshold model
Historical context and background for modeling complex traits
A brief history of the foundation of the theories underlying statistical genetics
Genetic risk prediction is rooted in complex trait theory and biometry (the application of statistics to biological measures), which emerged in the 19th century. Darwin’s concepts of selection based on continuous phenotypic variation were reconciled with Mendel’s laws of inheritance proposing discontinuous steps, which were initially interpreted as contradictory. In this context, in the mid-1870s Sir Francis Galton promoted use of twin and family studies to investigate inheritance. He recognized the utility of sum of squares based on Carl Friedrich Gauss’s and Adrien-Marie Legendre’s work at the turn of the 17th century for studies of heredity (Table S1); he applied regression to the mean, the workhorse of modern genome-wide association studies (GWAS). Karl Pearson developed fundamental statistical concepts for biometry, such as correlation and regression coefficients, and helped develop mathematical models of inheritance around the turn of the 20th century. In 1918, Ronald Fisher harmonized these concepts with the introduction of the biometrical model (Table S1) (1). Fisher’s framework introduced the infinitesimal model, in which large numbers of discrete genetic loci, each transmitted in Mendelian fashion, contribute additively to continuous phenotypic variation; thus, each individual variant explains a small fraction of heritable variation in a phenotype.
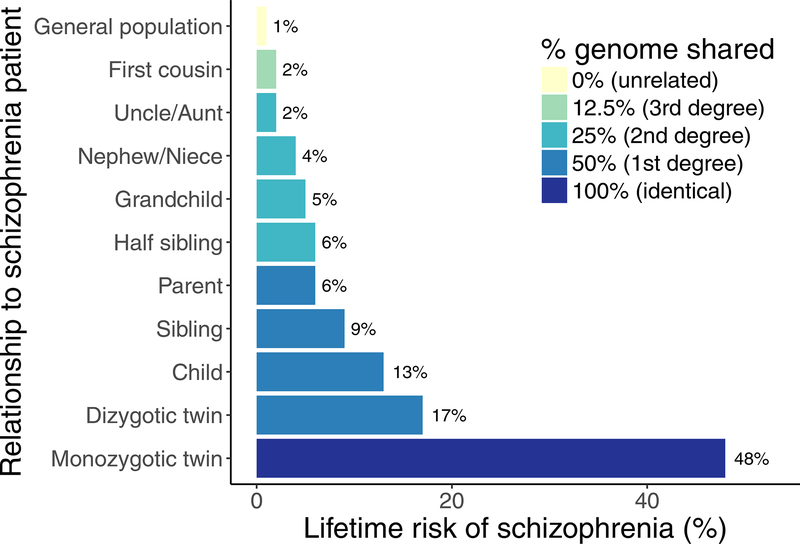
Ultimately, this synthesis of statistical and evolutionary theory to complex traits established the fundamental models still used today. In 1901, Pearson and Lee proposed the liability threshold model, asserting a normal risk distribution for binary outcomes (2), and Wright carried its application forward to genetics (3). These models proposed that many small genetic and environmental factors combine additively to give rise to phenotypic variation. Advances to Wright’s work considered additive genetic variation along a continuum for binary human diseases and traits, introducing the statistical theory of the modern liability-threshold model (4). Such models are relevant to essentially all common psychiatric disorders. Remarkably prescient work conducted 50 years ago by Gottesman and Shields compiled incidence data for schizophrenia in families, and proposed a then-overlooked but now widely-accepted argument that risk for schizophrenia is both highly heritable and polygenic (5). That is, many genetic risk factors of small effects across the genome account, in aggregate, for a substantial fraction of total psychiatric disease risk (Figure 1).
Figure 1. Proportion of DNA shared influences risk of heritable disease.
Relationship with schizophrenia patients predicts lifetime risk of schizophrenia in family members (adapted from Gottesman, 1991).
GWAS in the modern era and fundamental concepts in genetic risk prediction
The Human Genome Project, HapMap, and other large collaborative projects motivated the development of new technologies and directly contributed to shared computational and genomic data resources that dramatically accelerated the ability to perform GWAS. DNA microarrays enabled cost-effective association testing of common genetic variants across the whole genome with a disease or other trait (Table S1). The success of GWAS prompted the development of analytic methods for leveraging genome-wide variation to estimate heritability and individual-level genetic risk, among other applications (6). Here we focus on risk prediction, but the goals, approaches, and advances in GWAS have also been reviewed (7; 8).
The primary output of GWAS is a set of summary statistics for the association between a trait and each of the genotyped or imputed SNPs in the study. Summary statistics typically include: variant ID, position in the genome, risk and protective or effect alleles, sample size, p-value, effect size, and confidence in this estimate (e.g. standard error). Such summary statistics have been made publicly available by many large-scale GWAS consortia (9–12).
Genetic risk prediction has long had a role in agriculture, with estimated breeding values predating genetic risk prediction in humans. While the fundamental concepts are similar, the contexts are quite different for many reasons, ethical and otherwise. When considering plant and animal breeding values in agricultural contexts, humans apply genomic prediction by enforcing artificial selection over many generations of breeding in strictly controlled environments not germane to human disease applications (13).
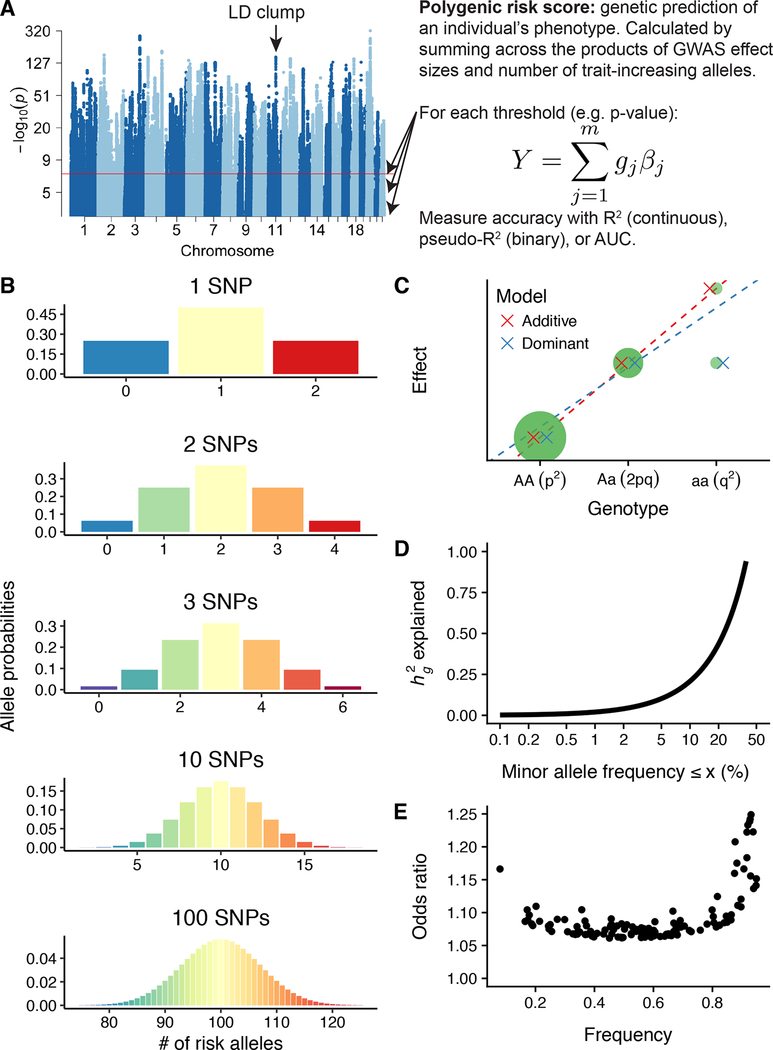
The most commonly applied approach for predicting genetic risk of human disease is computing polygenic risk scores (PRS) from GWAS summary statistics (Figure 2A) (14). This approach was introduced early in the GWAS era, developed first in the context of psychiatric disease. Researchers recognized that insufficient sample sizes in early studies produced few robust associations, but the aggregation of many loci below the genome-wide significance threshold could significantly predict disease risk in new studies. These analyses were consistent with a polygenic mode of inheritance from variants tagging causal risk (15; 16). At their core, PRS are simply calculated by multiplying the number of risk alleles a person carries by the effect size of each variant, and then summing each of these products across all risk loci (Figure 2A) (17). To ensure the validity of these scores, it is essential that the effect sizes are estimated in an independent cohort. This approach has now provided an empirical demonstration of early theoretical models from Gottesman & Shields for schizophrenia and even earlier from Fisher for quantitative traits (1; 5).
Figure 2. Normal genetic risk in a population with an additive genetic architecture.
A) Definition and illustration of polygenic risk score calculation. Using a set of existing GWAS summary statistics, the polygenic risk score is computed in a target cohort as where j is a SNP in m independent SNPs associated with the phenotype of interest, g is the number of trait-increasing alleles for a particular SNP, and β is the corresponding GWAS effect size estimate. An LD clump is an associated locus with one or few causal loci but a linkage peak of associated variants due to LD correlation in the region. The signal-to-noise ratio can be tuned to maximize prediction accuracy in a target cohort by modifying the maximum p-value threshold for SNP inclusion. B) Large numbers of SNPs contributing to complex traits can be modeled accurately with genetic liability as a normal distribution. Here, we demonstrate this by showing the genetic risk distribution for increasing numbers of SNPs with an allele frequency of 0.5 (although normality is expected regardless of allele frequency when larger numbers of SNPs are causal). The best-powered GWAS of complex traits such as height, schizophrenia, and educational attainment have identified hundreds to thousands of independent, genome-wide significant loci. This phenomenon can be explained by the central limit theorem as demonstrated previously (107). C) Additive GWAS regression models tend to work well for genetic associations across a range of allele frequencies, even in the presence of dominance. D) Previous work in the UK Biobank has demonstrated that across 25 complex traits and diseases, most of the heritable variation in complex traits can be explained by common variants (e.g., ≥ 5% allele frequency). While the exact proportion can vary, the curve illustrates h2 = 2*p*(1-p)α, where α = −0.38 ± 0.02 across complex traits (108). E) A previous GWAS of schizophrenia identified 128 independent genome-wide significant loci, shown here (11). These loci illustrate a relationship between frequency and corresponding odds ratios, in which lower frequency variants can have larger effect sizes, reflecting the impact of natural selection on genetic architecture.
Methods to assess genetic risk
Concepts and methods to interpret ranges of genetic prediction accuracy
The key elements that influence the accuracy of a PRS for a specific trait are its SNP heritability, genetic architecture, and sample size of the discovery GWAS (Table S1, see also Supplemental Note). SNP heritability is estimated as the additive contribution of common genetic variation to trait variability (8; 18–22) (Table 1). It is typically a fraction of family-based heritability, which captures contributions from all types of genetic variation.
Table 1.
Overview of statistical/computational methods for estimating or predicting genetic risk, heritability, and genetic correlation.
| Estimate | Method | Description | Training data | Reference | |
|---|---|---|---|---|---|
| Genetic risk prediction |
Risk profile (PLINK) | Variants included in a GWAS are typically filtered on MAF, missingness, and LD from an ancestry-matched cohort using a greedy pruning approach (--clump). Then in an independent dataset with individual-level genotypes, effect sizes of variants meeting varying p-value thresholds are multiplied by the number of risk genotypes at each locus, then summed across the genome (--profile). This approach is the simplest but most heuristic. | Summary statistics |
(15) | |
| LDPred | Bayesian model that computes genetic risk scores using posterior mean effect sizes estimated with GWAS summary statistics by conditioning on a prior genetic architecture and a proxy LD reference panel. | Summary statistics |
(32) | ||
| MultiPRS | Builds on the commonly used risk profile approach to combine training data from multiple populations for improved risk prediction, particularly in admixed populations. PRS are weighted as a mixture of each training set by tuning p-value and LD thresholds to optimize prediction accuracies. | Summary statistics |
(109) | ||
| GeRSI | This linear mixed model framework predicts genetic risk by fitting a mixture of accurately estimated large fixed effects alongside smaller, less precisely estimated random effects while accounting for case-control ascertainment | Individual-level genotypes |
(28) | ||
| BLUP/gBLUP (also in GCTA) | Best linear unbiased prediction is computed by fitting linear mixed models with principal components as covariates that adjust for population structure. This approach assumes an infinitesimal distribution of effect sizes by fitting all genome-wide SNP effects simultaneously. | Individual-level genotypes |
(29) | ||
| XP-BLUP | Similar to BLUP, uses a multiple-component LMM model specifically designed for prediction in admixed populations by selecting SNPs from a trans-ethnic GWAS, then uses effect sizes from an ethnic-specific training GWAS for prediction in a target cohort. It also assumes an infinitesimal distribution of effect sizes by fitting all genome-wide SNP effects simultaneously. | Individual-level genotypes |
(30) | ||
| LASSO | A sparse penalized regression model is used to predict continuous or binary phenotypes. | Individual-level genotypes |
(110; 111) | ||
| BSLMM (also h2) | Bayesian Sparse Linear Mixed Model is a hybrid between a linear mixed model and a Bayesian variable selection regression (BVSR), which fits all SNPs simultaneously assuming noninfinitesimal priors, which account for LD between SNPs to provide greater power for detection and to improve risk prediction. It is used for discovery, to estimate heritability, and to predict phenotypes. BVSR assumes a relatively small proportion of all variants affect the phenotype. Similar to BayesR, but assume a mixture of two normal distributions with different variances of SNP effect sizes. | Individual-level genotypes |
(112) | ||
| BayesR (also h2) | Similar to BSLMM in theory and prediction accuracy, this hierarchical Bayesian mixture model fits all SNPs simultaneously for discovery, estimation, and prediction analysis of complex traits. BayesR can also be used to infer genetic architecture by assuming a mixture of four normal distributions of SNP effect sizes with means of zero and fixed relative variances. | Individual-level genotypes |
(113; 114) | ||
| Heritability (h2) |
GCTA (also p) | Estimates the proportion of phenotypic variance of a complex trait explained by all genome-wide SNPs in an additive model using the restricted maximum likelihood (GREML) method | Individual-level genotypes |
(18) | |
| BOLT-LMM | Like GCTA, this Bayesian mixed model association method was optimized to scale more efficiently by approximating variance components, circumventing the costly genetic relationship matrix calculation. | Individual-level genotypes |
(21) | ||
| LDSC (also p) | Assumes that under a polygenic architecture, SNPs that are in high LD with many other SNPs are more likely to tag a causal variant, and thus expects higher test statistics (i.e. x2 statistics). Computes heritability vs stratification from linear regression between mean j2 and LD score bin. Extensions to the initial method also partition heritability into functional categories of the genome. | Summary statistics |
(19; 33) | ||
| HESS | Estimates total heritability from genotyped SNPs at a single locus by accounting for linkage disequilibrium (LD) among variants. | Summary statistics |
(20) | ||
| Genetic correlation (P) |
POPCORN | This trans-ethnic genetic correlation method estimates the correlation of causal variant effect sizes across populations by computing the similarity in effect size estimates with consideration to population-specific LD at each SNP. | Summary statistics |
(115) | |
PRS have become much more valuable in recent years as sample sizes have increased to produce numerous independent1 genome-wide significant associations for many phenotypes. For multi-trait or trans-ethnic analyses, PRS utility also depends on phenotype consistency (often measured by genetic correlation) across cohorts.
Whereas PRS provide individual-level genetic trait predictions, genetic correlation measures the heritable overlap among traits that share a genetic basis. Multi-trait analysis, particularly for traits with high genetic correlation such as schizophrenia and bipolar disorder, can improve prediction power for each trait (23).
The genetic architecture of a trait is determined by the number of causal genetic variants, and their corresponding effect sizes. Assuming constant heritability, more causal genetic variants reduce the average effect size. Most complex traits are highly polygenic, meaning that many causal SNPs have small effect sizes that are not yet genome-wide significant (i.e., p < 5e-8). Because smaller effects are more difficult to accurately estimate, highly polygenic traits are more difficult to predict (24; 25). As discovery sample sizes increase, the estimation error for each SNP effect shrinks, improving predictive power (26).
The key steps of computing PRS are determining which variants to include2 and their weights (Table S1). One of the most widely-adopted methods is genetic risk profiling in plink, a commonly used computational toolkit. In this approach, semi-independent SNP effects are multiplied by the number of risk alleles, starting with the most to least significant associations; these effects are then summed across the genome. An important consideration is the choice of the optimal p-value threshold, analogous to a tuning parameter that balances a signal and noise tradeoff. This tradeoff arises because more significant p-value thresholds have higher proportions of causal variants, but the total number of variants is smaller than with more permissive thresholds. There is not simply one optimal p-value threshold for a discovery GWAS dataset; rather, it varies based on SNP overlap, genetic divergence3, genetic correlation, and other differences between the discovery and target data. The standard PRS approach is to calculate several scores from SNPs meeting various p-value thresholds on a log scale ranging from genome-wide significant (p < 5e-8) to all independent SNPs (p < 1), then compute and report accuracy for each PRS4. In nearly all modern GWAS of complex traits, PRS computed using permissive p-value thresholds that aggregate the effects of 1,000s to 100,000s of independent SNPs typically explain more phenotypic variation than loci strictly meeting genome-wide significance. For example, no single common variant explains more than 0.1% of schizophrenia risk; however, ~10,000 SNPs together explain 18% of the variance between schizophrenia cases and controls, whereas genome-wide significant variants explain only 3% of risk (11). Table 1 describes several genetic risk prediction methods that have been developed and applied across diseases (15).
Statistical methods overview for genetic risk prediction and architecture
Empirically, GWAS suggest that there is considerable variability in the number and distribution of causal effects across complex traits, so some models are more appropriate for predicting a given complex trait than others. For example, an infinitesimal model that considers a large number of small effects performs best when predicting risk of schizophrenia, which is highly polygenic (Figure 2E). In contrast, autoimmune diseases typically have simpler genetic architectures that can be modeled well with linear mixed models; this approach models large effects primarily in the MHC region (28) as accurately estimated fixed effects, and a larger number of small, imprecise effect estimates as random effects.
A general issue for genetic risk prediction is that individual-level genotype and phenotype data are often subject to strict ethical and regulatory protections that limit access. Summary statistics from GWAS consortia are more commonly available, and some methods can predict genetic risk using these statistics rather than individual-level data. Methods that use individual-level genotype data can slightly outperform methods that use summary statistics as input, in part because they model data jointly rather than as marginal summary statistics, providing direct access to precise measures of correlation between variants rather than from reference panel estimates (29; 30). However, because this accuracy gain tends to be small, computationally efficient methods relying on summary statistics5 (Table 1) have been favored.
PRS are increasingly being employed to assess genetic relationships among phenotypes, especially those not measured at GWAS scale. PRS can hypothetically be used to examine cross-trait correlation and dissect biological pathways for phenotypes that have been measured at sufficient scale to generate well-powered GWAS summary statistics, but more appropriate methods have been specifically designed for these purposes. For example, heritability can be partitioned into functional elements with any biological annotation using LD score regression (33). For all analyses, it is important to consider sample ascertainment, and potential biases relevant to inferring the relationships between phenotypes. A summary of the methodological approaches used by various software packages to predict genetic risk, assess heritability, and compute genetic correlation among traits is described in Table 1.
Limitations and misunderstandings of clinical, translational, and research applications of PRS
There are several current limitations that restrict the broad utility and applicability of genetic risk prediction in research and clinical contexts, including insufficiently powered GWAS sample sizes for most complex traits, potential confounding in causal inference, and a lack of ancestral diversity in current studies. While the scale of GWAS has rapidly expanded over the last decade, most diseases still lack sufficient sample sizes for clinically relevant power from PRS (34). Furthermore, PRS comparing traits using GWAS for genetically uncorrelated phenotypes can lead to incorrect study conclusions from Type 1 error.
These limitations mean that PRS are not yet clinically useful in psychiatry. Nonetheless, genetics is beginning to aid our understanding of the pleiotropic relationships among psychiatric disorders, cognitive, and behavioral phenotypes. Because there are few, if any well-validated biomarkers in psychiatry (in contrast with most other areas of medicine), significant challenges remain. While the predictive utility of PRS is growing and useful in a research context, it is still low enough for most disorders that it is still premature to use PRS to influence how patients are currently treated. To understand how to incorporate PRS into clinical practice for patients with heritable psychiatric disorders, studies will need to assess health outcomes for various behavioral interventions, treatment regimens, and/or differential diagnoses.
Pleiotropy, confounding, and causal inference
Recent methods have been developed to enable a deeper understanding of pleiotropic effects, in which the same genetic variant is associated with multiple outcomes. A recent method for multi-trait analysis of summary statistics models sample overlap and genetic correlation between studies to improve effect size estimation for SNPs associated with each trait, thereby improving prediction accuracy (19; 23). Consequently, researchers have jointly analyzed genetically correlated traits including schizophrenia, bipolar disorder, and major depressive disorder to improve prediction accuracy for each disorder (35; 36). To better understand the biological pathways underlying polygenic signals, extensions of LD score regression methods have been developed that partition heritability from summary statistics into functional annotations that disproportionately contribute to the association signal, such as cell-type specific gene expression (33; 37). These statistical tools aid in the biological interpretation of polygenic signals and genetically correlated phenotypes.
While PRS are useful for studying the correlation between pairs of genotype-phenotype associations, they cannot be taken as evidence of causality, in part because PRS are a weak epidemiological instrument. A major reason is that the large number of SNPs typically used in their calculation usually have highly pleiotropic influences, in which they influence two or more different biological processes (e.g., calcium channel function in the brain and heart), which may be indirectly associated with the outcome of interest. Pleiotropy is a widespread phenomenon (38; 39) that PRS are especially sensitive to given their construction from many SNPs.
Instead, Mendelian randomization (MR) or related methods (40) often complement PRS analyses by providing a useful approach to disentangle causal relationships among phenotypes (41). One of the most important requirements of MR is a strong instrument—one or more genetic variants that are robustly associated with the exposure of interest. MR tests whether the exposure-associated variants result in proportional effects on the outcome, assuming no confounding factors such as population stratification, pleiotropy, or other confounding. Such instruments are rare in psychiatry due to their highly polygenic nature, overlapping biology, and noisy phenotyping. Several methods attempt to account for pleiotropic bias by correcting the dose-response slope between the exposure and outcome either for the average variant or for some subset of variants. A typical MR approach to meet such strong assumptions is to require independent genome-wide significant variants (41; 42). Rigorous MR analyses with smaller numbers of highly significant variants using methods designed to account for pleiotropic bias enable causal inference that is less likely to be confounded (41). Resources such as MR-Base enable causal inference with GWAS summary statistics (43).
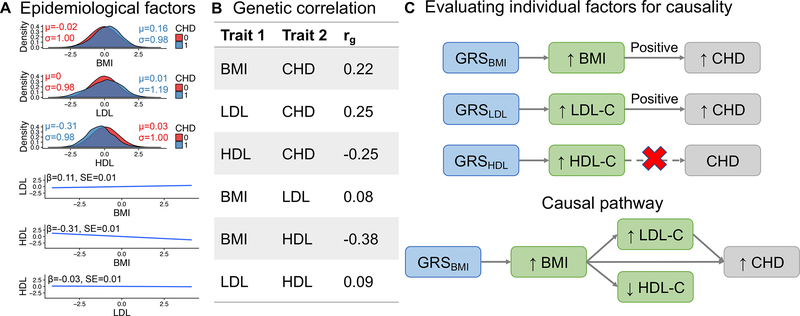
Prior work has clarified causality for many phenotypes. One of the most instructive examples of MR relates to coronary heart disease (CHD). CHD is genetically and epidemiologically correlated with elevated BMI, high levels of low-density lipoprotein (LDL) cholesterol and triglycerides, and low levels of high-density lipoprotein (HDL) cholesterol. However, notwithstanding these correlations, MR shows that HDL does not causally impact CHD risk (44; 45) (Figure 3). This explains why previous clinical trials with drugs aimed at raising HDL levels failed to decrease CHD risk. This demonstrates that MR can shape therapeutic strategies—while precisely measured biomarkers such as HDL that are correlated with disease outcomes such as CHD have some value for predictive modeling, perturbing HDL levels is an invalid strategy for reducing CHD risk. In psychiatry, MR has demonstrated the protective influence of accelerometer-based physical activity on major depression but no opposite relationship of depression influencing physical activity (46).
Figure 3. Correlated epidemiological and genetic factors can be causally dissected with Mendelian randomization.
GRS = genetic risk scores from independent genome-wide significant SNPs. BMI = body mass index, LDL-C = LDL cholesterol, HDL-C = HDL cholesterol, CHD = coronary heart disease. A) Epidemiological factors from FINRISK and their associations with risk of CHD after all evaluated factors have been normalized, and age and sex have been regressed out. Test statistics for each of the panel comparisons are written in plot corners and are as follows (t-tests for the top 3 panels, ANOVA for the bottom 3 panels): BMI: p=3.5e-18; LDL: p=0.79; HDL: p=5.3e-62; LDL and BMI: p=2.7e-73, HDL and BMI: p<1e-100, HDL and LDL: p=2.0e-7. B) Genetic factors associated with LDL-C, HDL-C, and BMI enable causal inference for CHD. Whereas genetic risk of increased LDL-C and BMI are causally associated with increased risk of CHD, HDL-C is genetically anti-correlated with CHD but is not causal. Genetic correlations (ρg) are from LD Hub (12).
While causal inference with genetic data can be highly valuable, a notable caveat relates to collider bias. This describes the phenomenon in which conditioning on a common effect of exposure and outcome results in an over- or under-identification of genetic risk factors influencing disease outcome (42; 47; 48). A logical example arises from the fact that sex and autosomal variants both influence height but are intrinsically unrelated, as no autosomal variants determine sex. However, autosomal loci are spuriously associated with sex when modeled with SNPs and height covariates (i.e.: sex ~ SNP + height) (49).
Eurocentric GWAS biases limit the generalizability of genetic risk prediction
The vast majority of GWAS have been conducted in individuals of European descent (50–55), limiting diverse applications of PRS, and introducing unpredictable directional biases across populations (56). Importantly, trans-ethnic PRS often explain many-fold less of the heritable variation than in the study population (24; 55–57), as has been demonstrated for psychosis in African Americans when predicted from European GWAS (32; 58). Previous studies have shown a roughly 2- to 5-fold reduction in heritable variation explained in East Asians and African Americans relative to Europeans, respectively (55; 59; 60). Spurious GWAS associations are also possible within Europe if population stratification is not properly accounted for (61), creating downstream interpretation challenges when PRS are computed from confounded GWAS. Further, differ environmental factors can non-causally associate with genetic divergence, such as access to clean drinking water during development. While many psychiatric disorders are highly heritable, some environmental factors can dwarf individual genetic effect sizes and create issues of comparability across diverse populations. Critically needed statistical methods are being developed to improve the generalizability of genetic risk scores across diverse populations, including our own and others (62). Analytical methods alone are unlikely to provide a complete fix, however. Without a massive investment to perform similarly sized GWAS in globally diverse populations, PRS across all diseases are much more likely to benefit European ancestry populations already on the positive end of health disparities.
Uneven common variant risk contributions across the phenotypic spectrum
High predicted genetic risk for the same disease across individuals does not necessarily correspond to homogeneously dysregulated biology—instead, disease-relevant pathways may be affected in different ways in individuals with similarly high polygenic risk. Importantly for precision medicine, schizophrenia patients with high common and rare variant risk have inherited different rates of variants in known and predicted gene targets of antipsychotic drugs, likely contributing to the variable treatment responses among patients (63). This and other work highlights the eventual utility of genomic medicine for predicting treatment trajectories (63–66), while cautioning that in the absence of more granular pathway insights, heterogeneous biological perturbations can limit the utility of PRS analyses jointly with other clinical and experimental tools (67). Further, the pervasiveness, ease of use, and potentially unrecognized limitations of PRS warns of issues reminiscent of the candidate gene era, in which well-characterized phenomena are repeatedly studied while unknown biology remains undiscovered (68).
For many traits, risk is additively conferred by variants across the frequency spectrum, ranging from de novo (i.e., newly arising) to common, only the latter of which is captured by PRS. While rare variants can have larger effects than common variants (Figure 2E), most heritable variation in a population is explained by the latter (Figure 2D) (69–75). Some autism spectrum disorders (ASD) and intellectual disability (ID) patients have high polygenic risk and large effect rare variants contributing to their case status (69), but relatively few have solely pathogenic de novo causes (73). These variants can contribute more to syndromic features (e.g. for ASD and ID); for example, developmental disability patients with severe ID (76) or craniofacial dysmorphia (71) are more likely to carry a strong acting de novo variant than individuals with mild developmental delays and more typical physical features.
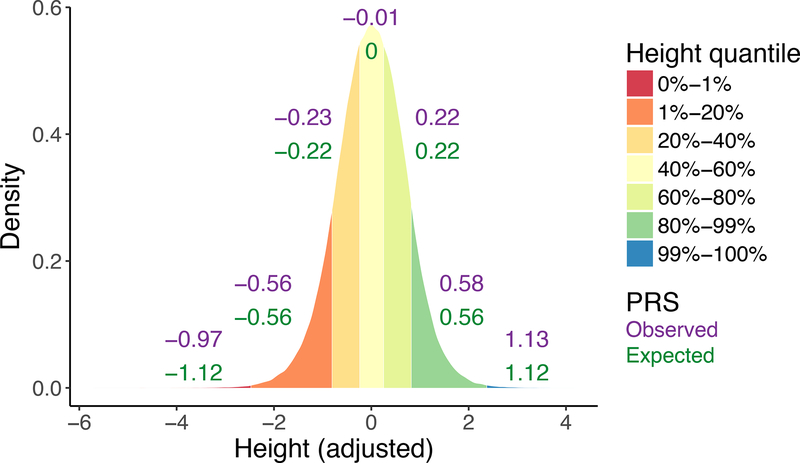
Individuals with phenotypic extremes may be more likely to have average PRS than expected given the individuals’ deviation from the mean. This asymmetric observation about the imbalanced environmental or rare genetic contribution in phenotypic outliers is perhaps best illustrated by height, a continuous phenotype. We demonstrate this phenomenon by computing PRS for height in the UK Biobank using summary statistics from the GIANT consortium and comparing observed versus expected scores along the height distribution (77). Extremely short individuals are more likely to have monogenic or environmental factors contributing to their height than others, as demonstrated by less predictive polygenic scores (78) (Figure 4). As an analogy for this phenomenon in psychiatric disorders, very large contributing environmental effects, such as syphilis or toxic exposure to metals, potentially render polygenic risk irrelevant.
Figure 4. Predictive accuracy of polygenic risk scores for height at intervals along the measured height distribution in the UK Biobank.
Using summary statistics from the GIANT Consortium, we computed polygenic risk scores for height and compared them to the distribution of standardized height in the UK Biobank after adjusting for sex and the first 10 principal components. However, prediction accuracy is not distributed evenly; it performs particularly poorly at the extreme short end of the height distribution, indicating a larger contribution of environmental factors, large-effect rare variants, and/or other factors in these individuals. Numbers in the plot indicate observed versus expected polygenic risk scores within corresponding breakpoints along the adjusted height distribution. Expected polygenic risk scores comes from multivariate normal simulations assuming the same correlation between adjusted height and observed polygenic risk scores.
Current applications and promising future directions
Applications of GWAS and PRS to psychiatric disorders and related traits
Although PRS hold especially great promise in psychiatry due to inaccessibility and complexity of brain tissue and lack of clinical biomarkers, they are not yet clinically useful, but are useful for research. Applications of PRS to psychiatric disorders have provided insight into disease outcomes, correlated phenotypes, and biological mechanisms. We review recent large GWAS and PRS of psychiatric disorders and relevant phenotypes in Table 2.
Table 2. Recent gene discovery and PRS study highlights in psychiatric disorders and related behavioral and cognitive traits.
SCZ=schizophrenia, BP = bipolar disorder, ASD=autism spectrum disorders, DD=developmental delay, ID=intellectual disability, MDD = major depressive disorder, EA = educational attainment.
| Phenotype | Description | Reference |
|---|---|---|
| Cross-disorder | Common variant risk for psychiatric disorders was significantly correlated, especially among ADHD, BP, MDD, and SCZ, whereas neurological disorders are genetically more distinct. | (116) |
| SCZ | This GWAS of unprecedented scale for psychiatric disorders at the time clearly demonstrated the highly heritable, polygenic nature of inheritance for schizophrenia and the continuous genetic risk spectrum in the general population. PRS explain 7% of the variance on the liability scale. | (11) |
| This trans-ethnic GWAS of East Asian and European populations showed that there is no significant difference between populations (rg=0.98), improved power for fine-mapping, and demonstrated that polygenic risk prediction in East Asians with ancestry-matched summary statistics outperformed prediction with summary statistics from several-fold larger summary statistics in Europeans. | (117) | |
| Schizophrenia is associated with PRS, socioeconomic status, and family psychiatric history, with family history partially mediated through PRS. While each factor is interdependent, they each account for a sizeable fraction of cases, but a modest part of total variation | (79) | |
| BP | This GWAS of highly heritable BP identified 30 genome-wide significant loci. BP shows significant genetic correlation with several other psychiatric-relevant traits. Subphenotype analysis indicated that BP type 1 (manic episodes) is more genetically correlated with SCZ, whereas BP type 2 (hypomanic) is more genetically correlated with MDD. PRS explain 4% of the variance on the liability scale. | (118) |
| BP/SCZ | By comparing and contrasting BP and SCZ cases, this study identified primarily shared loci between these disorders implicating synaptic and neuronal pathways. By comparing and contrasting polygenic signals of subphenotypes in SCZ and BP, it also identified correlations between BP pRs and manic symptoms in SCZ cases, BP PRS and psychotic features in BP patients, SCZ PRS and BP cases with versus without psychotic features, and SCZ PRS and SCZ patients with increased negative symptoms. | (119) |
| Anorexia | The largest existing GWAS of 3,495 cases and 10,982 controls identified one genome-wide significant locus and significant heritability. GWAS results show genetic correlation with other psychiatric and metabolic phenotypes. | (120) |
| ASD | The largest ASD GWAS to date recapitulates high heritability, qualitative and quantitative heterogeneity in subtypes, and some shared architecture with correlated phenotypes (e.g. SCZ, MDD, and EA). PRS accuracy is not reported on the liability scale, but Nagelkerke’s R2 is 2.5%. | (92) |
| Developed and applied polygenic transmission disequilibrium tests in families with a child with ASD to disentangle the contribution of polygenic and de novo variants to overall risk. Findings show that polygenic risk contributes additively with strong acting de novo variants | (70) | |
| Both through common polygenic signal and de novo variant analysis, genome-wide links are evident between ASD, typical variation in social behavioral and developmental traits, and adaptive functioning. | (69) | |
| Compared social communication difficulties throughout developmental ages in typically- developing youths as well as ASD and SCZ patients. Genetic influences on social communication difficulties decreased with age in ASD patients, but persisted across age with an increase in late adolescence in SCZ patients. | (121) | |
| By constructing polygenic risk scores for ASD, ADHD, and cognitive ability in three general population cohorts, this study finds a positive correlation between ASD and general cognitive ability, indicating that these genetic relationships are partially independent of clinical state. | (93) | |
| DD/ID | In a cohort of children with severe neurodevelopmental disorders expected to be almost entirely monogenic, 7.7% of risk is attributable to common genetic variants, with polygenic burden over-transmitted by parents. | (74) |
| ADHD | The largest ADHD GWAS to date identifies associated variants enriched in evolutionarily constrained genes, loss-of-function intolerant genes, and brain-expressed regulatory marks. Findings support a polygenic architecture and that clinical diagnosis is an extreme expression of one or more heritable traits. PRS accuracy is not reported on the liability scale, but Nagelkerke’s R2 is 5.5%. | (122) |
| Using PRS for ADHD in a general population cohort, findings indicate that risk for ADHD is positively associated with hyperactive-impulsive and inattentive traits, negatively associated with pragmatic language abilities, and that diagnosed girls have a higher polygenic burden than boys. | (89) | |
| MDD | This GWAS of clinically selective MDD cases identifies associations enriched in brain- expressed regions that tend to occur in highly conserved regions. In both genetic correlation and Mendelian randomization analyses, MDD is positively related to BMI, and negatively related to education. PRS explain 1.9% of the variance on the liability scale. | (123) |
| In a Han Chinese cohort of women with and without MDD including ascertainment of key environmental risk factors, GWAS results suggest etiological heterogeneity as a function of environmental exposure, particularly among those who did/did not report exposure to adversity. | (124) | |
| Mood disorders |
GWAS of mood disorders, including meta-analysis of BP and MDD, showed more genetic similarity with MDD. In subtype analyses, MDD showed the strongest correlation with type II BP disorder. Additionally, while BP is positively associated and MDD is negatively associated with EA, neither is associated with IQ. | (125) |
| PTSD | This multi-ethnic GWAS analysis identified suggestive evidence of heritability in females but not males, perhaps due to differences in trauma exposure and/or environmental factors. It did not identify any genome-wide significant associations. This result coupled with the relatively low heritability compared to other psychiatric disorders suggests that larger sample sizes are needed for further biological insights. PRS accuracy is not reported. | (126) |
| IQ | This GWAS of 269,867 individuals identified 205 independent associations enriched in conserved and coding regions. Mendelian randomization results suggest protective effects against Alzheimer’s disease and ADHD and pleiotropic effects for schizophrenia. PRS explain 5.2% of the variance. | (127) |
| Meta-analysis of human intelligence is significantly heritability (/ij=0.21, se=0.01), with high polygenicity and substantial genetic correlation between children and adults. Associated loci are enriched in coding regions, with heritability partitioning signals highly enriched in brain tissue, especially those regulating cell development. PRS from this study explain 4.8% of the variance in intelligence on average. | (128) | |
| EA | This large-scale GWAS included 1.1 million individuals and identified 1,271 independent genome-wide significant associations that are functionally enriched in brain-expressed regions. PRS from multi-phenotype analyses of EA with cognitive performance, math ability, and highest math class taken together explain 11–13% of variation in EA. | (129) |
| This GWAS of educational attainment in 293,723 individuals identified 75 independent genome-wide significant associations. These associated loci are disproportionately expressed in fetal brain tissue, and are significantly associated with several neuropsychiatric, behavioral, and anthropometric traits. | (130) | |
| General cognitive function |
This GWAS of over 300,000 individuals identified 148 independent loci associated with general cognitive function and 42 associations with reaction time. These loci include associations with neurodevelopmental and neurodegenerative phenotypes, as well as psychiatric illnesses and brain structure. Signals are enriched for brain-expressed regions. PRS explain up to 4.3% of the variance. | (131) |
PRS studies have been especially abundant in schizophrenia, where GWAS sample sizes have thus far been the largest (11). For example, family history, socioeconomic status, and PRS explain a similar fraction of overall schizophrenia risk, with family history mediated partially through PRS (79). PRS that predict psychiatric disorder risk are associated with behavioral and cognitive differences in the general population among individuals that are typically referred to as ‘controls’ (69). High schizophrenia PRS is associated with higher childhood cognitive, social, behavioral, and emotional impairments, although the great majority of children exhibiting such deficits do not later develop schizophrenia (80). Among diagnosed schizophrenia patients, outcomes such as chronicity and hospital admission rates have also been significantly correlated with schizophrenia PRS, although chronic readmission could bias ascertainment in GWAS (81). Other cognitive phenotypes are also associated with schizophrenia risk, including lower IQ and educational attainment, and higher creativity (82–84). Geographical risk from serial founder effects are correlated with elevated schizophrenia risk in northern Finland (85–87), although additional work is needed to disentangle the role of population structure (56).
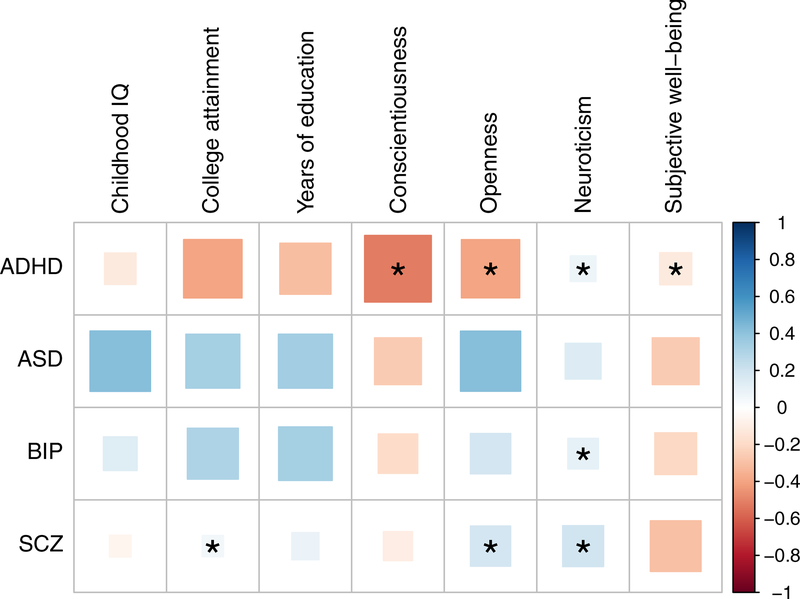
The prevalence, comorbidity with other developmental conditions, demographic factors, social communication traits, and behavioral outcomes have been queried with PRS for developmental disorders such as ASD and ADHD in deeply phenotyped longitudinal cohort studies (88–90). Variants across the full spectrum of allele frequencies typically act in an additive manner, indicating that PRS alone are insufficient to fully understand genetic risk, particularly in patients with syndromic presentations (71; 73; 91). The genetic architecture of ASD and its associated risk with other psychiatric disorders have been queried both through genetic correlation and PRS (83; 84; 92). Interestingly, several previous studies have identified a positive genetic correlation between higher IQ, educational attainment, and ASD diagnosis (70; 93), despite much lower than average observed IQ and educational attainment in ASD patients (72) (Figure 5). PRS and related methods provide tools to query the pleiotropic relationship between these disorders and cognitive, behavioral, physical, and other traits.
Figure 5. Genetic correlation between psychiatric disorders and cognitive/behavioral phenotypes.
Measures are from LD Hub (12). ASD = autism spectrum disorders, BIP = bipolar disorder, SCZ = schizophrenia. Legend indicates genetic correlation, ρg.
Growing data resources and applications aid genetic risk interpretability
Genetic insights into disease risk are becoming increasingly meaningful and precise with larger sample sizes, greater phenotypic dimensionality, and increased resolution of biological resources (e.g., single cell RNA sequencing of various cell types) (94). National health record studies provide leading examples, such as in a study involving 2.3 million Swedes, which found that fecundity is dramatically reduced among patients with schizophrenia, ASD, anorexia, and other disorders relative to unaffected siblings (95). This reduced fecundity indicates that negative selection rapidly purges even weakly deleterious variants from the genome (96). Leading efforts to provide easy, unified access linking genetic data, clinical records, and other national registry data such as in the UK Biobank, the BioBank Japan, the China Kadoorie Biobank, Nordic biobanks, and others will provide invaluable insights into otherwise obscured biological mechanisms and therapeutic targets. Further gains will be made as global initiatives expand the genetic diversity in psychiatric cases, aiding fine-mapping endeavors, research capacity, and the discovery of novel population-specific risk variants (97–100).
The future of genetic risk prediction is anticipated to benefit several areas of research and clinical practice, and democratize the interpretation of personalized medicine. For example, user interfaces such as KardioKompassi in Finland and a mobile app, MyGeneRank, have recently been developed to provide personalized polygenic risk scores for coronary artery disease (101). Contrasting these tools, however, highlights the differences in predictive power; the former tool integrates a risk score computed from the largest genetic scan of cardiovascular disease using genome-wide clumped SNPs (N ~ 50,000) and integrates measured clinical and environmental risk factors. The latter tool, in contrast, is based on only 57 SNPs, meaning the PRS alone is expected to explain ~50% less phenotypic variation (15). While KardioKompassi is therefore expected to be much more informative, because the clinical and environmental effects are learned in Finland, they may have lower generalizability to non-Finnish individuals where environments and medical systems differ, even if the genetic score is equally valid in some countries. Considerable caution is urged with broad public deployment as lack of diversity straightforward explanations provide unmet challenges (56). Researchers have a responsibility to explain the predictive power (or lack thereof) of genetic tools to the broader public.
With large-scale retrospective studies and disease course trajectory data, PRS may help articulate longitudinal timelines relevant to phenotypic variation. For example, the apolipoprotein E4 (ApoE4) allele is the strongest genetic risk factor for late-onset Alzheimer’s disease with a large effect on cognitive decline among individuals in their 50s and 60s. It has no effect on educational attainment in youth, however, and greater resolution into genetic timelines will become increasingly available with larger studies and novel methods. By aggregating PRS with clinical features such as current health, family history, cognitive, and behavioral measures that predict disease in patients’ coming years of life, preventative trials can be more productive (102). Alzheimer’s disease, dementia with Lewy bodies, and Parkinson’s disease have symptomatic overlap with some diagnoses only confirmed post mortem. These three dementia disorders are genetically correlated, but some distinctive genetic signatures relating to each (103; 104) may provide granularity into differential diagnosis, enabling more rapid success in clinical trials through streamlining patient selection and reduced overall costs (105).
New statistical methods are being rapidly developed to meet the needs of increasing GWAS sample sizes and ancestral diversity, estimating effect sizes more precisely and increasing the accuracy and generalizability of genetic risk prediction. A potential future use of PRS is in clinical trials, potentially enabling more effective drug treatment targeting in high-risk patients (106). PRS can provide an estimate of how many at risk individuals will be expected to exhibit clinical symptoms or be diagnosed, providing a measurable change from expectation for drug trials. Another promising area is in early intervention, in which at-risk patient populations are more efficiently identified with PRS, for example prior to a prodromal period in schizophrenia. Given high heritability for many disorders and the lack of existing biomarkers due to the inaccessibility of human brain tissue, genetic risk prediction holds especially great promise for psychiatry, and we recommend careful consideration of emerging methods and applications in genetic risk prediction.
Supplementary Material
Acknowledgments
We thank Eric Minikel for helpful feedback and discussions during the preparation of this manuscript. We also thank Sanni Ruotsalainen for plotting epidemiological cardiovascular factors using FINRISK data. This work was supported by funding from the National Institutes of Health (K99MH117229 to A.R.M.). UK Biobank analyses were conducted via application 31063.
Financial Disclosures
ARM, MJD, and ERB reported no biomedical financial interests or potential conflicts of interest. SEH is a Director of Voyager Therapeutics and Q-State Biosciences, and serves on the Scientific Advisory Boards of Janssen, BlackThorn, and F-Prime. BMN is a member of Deep Genomics Scientific Advisory Board, has received travel expenses from Illumina, and also serves as a consultant for Avanir and Trigeminal solutions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SNPs located in the same region of the genome tend to be inherited together due to low proximal recombination. This genomic correlation is referred to as linkage disequilibrium (LD).
This is based on SNPs meeting several p-value thresholds and independence from other significant variants (e.g. LD R2 greater than some threshold)
Genetic divergence (often quantified by FST or fixation index) measures population differences due to an accumulation of genetic changes over time
PRS accuracy is typically computed by assessing phenotypic variance (i.e. partial R2 for the PRS). This is computed by comparing two linear models with established covariates, including one with and one without the PRS term (27).
References
- 1.Fisher RA (1918): XV.—The Correlation between Relatives on the Supposition of Mendelian Inheritance. Transactions of the Royal Society of Edinburgh. 52: 399–433. [Google Scholar]
- 2.Pearson K, Lee A (1900): Mathematical Contributions to the Theory of Evolution. VIII. On the Inheritance of Characters not Capable of Exact Quantitative Measurement. Part I. Introductory. Part II. On the Inheritance of Coat-Colour in Horses. Part III. On the Inheritance of Eye-Colour in Man. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 195: 79–150. [Google Scholar]
- 3.Wright S (1934): An Analysis of Variability in Number of Digits in an Inbred Strain of Guinea Pigs. Genetics. 19: 506–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falconer DS (1965): The inheritance of liability to certain diseases, estimated from the incidence among relatives. Annals of Human Genetics, 2nd ed. 29: 51–76. [Google Scholar]
- 5.Gottesman II, Shields J (1967): A polygenic theory of schizophrenia. Proc Natl Acad Sci USA. 58: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The International HapMap Consortium (2005): A haplotype map of the human genome. Nature. 437: 1299–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J (2017): 10 Years of GWAS Discovery: Biology, Function, and Translation. 101: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Zeng J, Goddard ME, Wray NR, Visscher PM (2017): Concepts, estimation and interpretation of SNP-based heritability. Nat Genet. 49: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 9.Howrigan D (2017, September 20): Details and Considerations of the Uk Biobank GWAS. http://wwwnealelabis/blog/2017/9/11/details-and-considerations-of-the-uk-biobank-gwas.
- 10.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. (2015): Genetic studies of body mass index yield new insights for obesity biology. Nature. 518: 197206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA, et al. (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. (2017): LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 33: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey JM, Chiurugwi T, Mackay I, Powell W, Implementing Genomic Selection in CGIAR Breeding Programs Workshop Participants (2017): Genomic prediction unifies animal and plant breeding programs to form platforms for biological discovery. Nature Publishing Group. 49: 1297–1303. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee N, Shi J, García-Closas M (2016): Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 17: 392406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al. (2009): Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 460: 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wray NR, Goddard ME, Visscher PM (2007): Prediction of individual genetic risk to disease from genome-wide association studies. Genome Research. 17: 1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, Middeldorp CM (2014): Research Review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatr. 55: 1068–1087. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. (2010): Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 42: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. (2015): LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 47: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi H, Kichaev G, Pasaniuc B (2016): Contrasting the Genetic Architecture of 30 Complex Traits from Summary Association Data. The American Journal of Human Genetics. 99: 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh P-R, Tucker G, Bulik-Sullivan BK, Vilhjálmsson BJ, Finucane HK, Salem RM, et al. (2015): Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet. 47: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golan D, Lander ES, Rosset S (2014): Measuring missing heritability: Inferring the contribution of common variants. Proc Natl Acad Sci USA. 111: E5272–E5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, et al. (2018): Multi-trait analysis of genome-wide association summary statistics using MTAG. Nature Publishing Group. 50: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scutari M, Mackay I, Balding D (2016): Using Genetic Distance to Infer the Accuracy of Genomic Prediction. (Hickey JM, editor) PLoS Genet. 12: e1006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wray NR, Yang J, Ben J Hayes, Price AL, Goddard ME, Visscher PM (2013): Pitfalls of predicting complex traits from SNPs. Nature Publishing Group. 14: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee N, Wheeler B, Sampson J, Hartge P, Chanock SJ, Park J-H (2013): Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nature Publishing Group. 45: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Euesden J, Lewis CM, O’Reilly PF (2015): PRSice: Polygenic Risk Score software. Bioinformatics. 31: 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golan D, Rosset S (2014): Effective Genetic-Risk Prediction Using Mixed Models. The American Journal of Human Genetics. 95: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C-Y, Han J, Hunter DJ, Kraft P, Price AL (2015): Explicit Modeling of Ancestry Improves Polygenic Risk Scores and BLUP Prediction. Genet Epidemiol. 39: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coram MA, Fang H, Candille SI, Assimes TL, Tang H (2017): Leveraging Multi-ethnic Evidence for Risk Assessment of Quantitative Traits in Minority Populations. Am J Hum Genet. 101: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquez-Luna C, Loh P-R, South Asian Type 2 Diabetes (SAT2D) Consortium, SIGMA Type 2 Diabetes Consortium, Price AL (2017): Multiethnic polygenic risk scores improve risk prediction in diverse populations. Genet Epidemiol. 41: 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilhjálmsson BJ, Yang J, Finucane HK, Gusev A, Lindström S, Genovese G, et al. (2015): Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. The American Journal of Human Genetics. 97: 576–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh P-R, et al. (2015): Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 47: 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudbridge F (2013): Power and Predictive Accuracy of Polygenic Risk Scores. (Wray NR, editor) PLoS Genet. 9: e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier R, Moser G, Chen G-B, Ripke S, Coryell W, Potash JB, et al. (2015): Joint Analysis of Psychiatric Disorders Increases Accuracy of Risk Prediction for Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. The American Journal of Human Genetics. 96: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maier RM, Zhu Z, Lee SH, Trzaskowski M, Ruderfer DM, Stahl EA, et al. (2018): Improving genetic prediction by leveraging genetic correlations among human diseases and traits. Nature Communications. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gusev A, Trynka G, Finucane H, Vilhjálmsson BJ, Xu H, Zang C, et al. (2014): Partitioning heritability of regulatory and cell-type-specific variants across 11 common diseases. Am J Hum Genet. 95: 535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA (2016): Detection and interpretation of shared genetic influences on 42 human traits. Nature Publishing Group. 48: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verbanck M, Chen C-Y, Neale B, Do R (2018): Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Publishing Group. 50: 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor LJ, Price AL (2018): Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat Genet. 32: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes MV, Ala-Korpela M, Smith GD (2017): Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nature Publishing Group. 14: 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paternoster L, Tilling K, Davey Smith G (2017): Genetic epidemiology and Mendelian randomization for informing disease therapeutics: Conceptual and methodological challenges. (Barsh GS, editor) PLoS Genet. 13: e1006944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemani G, Zheng J, Wade KH, Laurin C, Elsworth B, Burgess S, et al. (2016): MR-Base: a platform for systematic causal inference across the phenome using billions of genetic associations. 1–32. [Google Scholar]
- 44.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. (2013): Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 45: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. (2012): Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 380: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi KW, Chen C-Y, Stein MB, Klimentidis YC, Wang M-J, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, et al. (2018): Testing Causal Bidirectional Influences between Physical Activity and Depression using Mendelian Randomization. 1–19. [Google Scholar]
- 47.Martin J, Tilling K, Hubbard L, Stergiakouli E, Thapar A, Davey Smith G, et al. (2016): Association of Genetic Risk for Schizophrenia With Nonparticipation Over Time in a Population-Based Cohort Study. Am J Epidemiol. 183: 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dudbridge F (2016): Polygenic Epidemiology. Genet Epidemiol. 40: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Day FR, Loh P-R, Scott RA, Ong KK, Perry JRB (2016): A Robust Example of Collider Bias in a Genetic Association Study. Am J Hum Genet. 98: 392–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Need AC, Goldstein DB (2009): Next generation disparities in human genomics: concerns and remedies. Trends in Genetics. 25: 489–494. [DOI] [PubMed] [Google Scholar]
- 51.Popejoy AB, Fullerton SM (2016): Genomics is failing on diversity. Nature. 538: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hindorff LA, Bonham VL, Brody LC, Ginoza MEC, Hutter CM, Manolio TA, Green ED (2017): Prioritizing diversity in human genomics research. Nature Publishing Group. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bustamante CD, La Vega De FM, Burchard EG (2011): Genomics for the world. Nature. 475: 163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morales J, Welter D, Bowler EH, Cerezo M, Harris LW, McMahon AC, et al. (2018): A standardized framework for representation of ancestry data in genomics studies, with application to the NHGRI-EBI GWAS Catalog. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ (2018): Hidden “risk” in polygenic scores: clinical use today could exacerbate health disparities. bioRxiv. doi: 10.1101/441261. [DOI] [PubMed] [Google Scholar]
- 56.Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, et al. (2017): Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am J Hum Genet. 100: 635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duncan L, Shen H, Gelaye B, Ressler K, Feldman M, Peterson R, Domingue B (2018): Analysis of Polygenic Score Usage and Performance across Diverse Human Populations. doi: 10.1101/398396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vassos E, Di Forti M, Coleman J, Iyegbe C, Prata D, Euesden J, et al. (2017): An Examination of Polygenic Score Risk Prediction in Individuals With First-Episode Psychosis. Biological Psychiatry. 81: 470–477. [DOI] [PubMed] [Google Scholar]
- 59.Ware EB, Schmitz LL, Faul JD, Gard A, Mitchell C, Smith JA, et al. (2017): Heterogeneity in polygenic scores for common human traits. 1–13. [Google Scholar]
- 60.Akiyama M, Okada Y, Kanai M, Takahashi A, Momozawa Y, Ikeda M, et al. (2017): Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet. 49: 1458–1467. [DOI] [PubMed] [Google Scholar]
- 61.Campbell CD, Ogburn EL, Lunetta KL, Lyon HN, Freedman ML, Groop LC, et al. (2005): Demonstrating stratification in a European American population. Nat Genet. 37: 868–872. [DOI] [PubMed] [Google Scholar]
- 62.Grinde KE, Qi Q, Thornton TA, Liu S, Shadyab AH, Chan KHK, et al. (2018): Generalizing Genetic Risk Scores from Europeans to Hispanics/Latinos. doi: 10.1101/242404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruderfer DM, Charney AW, Readhead B, Kidd BA, Kähler AK, Kenny PJ, et al. (2016): Polygenic overlap between schizophrenia risk and antipsychotic response: a genomic medicine approach. The Lancet Psychiatry. 3: 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kullo IJ, Jouni H, Austin EE, Brown S-A, Kruisselbrink TM, Isseh IN, et al. (2016): Incorporating a Genetic Risk Score Into Coronary Heart Disease Risk Estimates: Effect on Low-Density Lipoprotein Cholesterol Levels (the MI-GENES Clinical Trial). Circulation. 133: 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paquette M, Chong M, Thériault S, Dufour R, Paré G, Baass A (2017): Polygenic risk score predicts prevalence of cardiovascular disease in patients with familial hypercholesterolemia. Journal of Clinical Lipidology. 11: 725–732.e5. [DOI] [PubMed] [Google Scholar]
- 66.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. (2016): Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med. 375: 2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linden DEJ (2012): The Challenges and Promise of Neuroimaging in Psychiatry. Neuron. 73: 8–22. [DOI] [PubMed] [Google Scholar]
- 68.Duncan LE, Keller MC (2011): A Critical Review of the First 10 Years of Candidate Gene-by-Environment Interaction Research in Psychiatry. AJP. 168: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, et al. (2016): Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 48: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiner DJ, Wigdor EM, Ripke S, Walters RK, Kosmicki JA, Grove J, et al. (2017): Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet. 49: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deciphering Developmental Disorders Study (2017): Prevalence and architecture of de novo mutations in developmental disorders. Nature. 542: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH, Daly MJ (2014): Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc Natl Acad Sci USA. 111: 15161–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, et al. (2012): Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 485: 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niemi ME, Martin HC, Rice DL, Gallone G, Gordon S, Kelemen M, et al. (2018): Common genetic variants contribute to risk of rare severe neurodevelopmental disorders. bioRxiv. doi: 10.1101/309070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Study TDDD (2015): Large-scale discovery of novel genetic causes of developmental disorders. 519: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reichenberg A, Cederlöf M, McMillan A, Trzaskowski M, Kapra O, Fruchter E, et al. (2016): Discontinuity in the genetic and environmental causes of the intellectual disability spectrum. Proc Natl Acad Sci USA. 113: 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. (2014): Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 46: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan Y, Holmen OL, Dauber A, Vatten L, Havulinna AS, Skorpen F, et al. (2011): Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals. (Gibson G, editor) PLoS Genet. 7: e1002439–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agerbo E, Sullivan PF, Vilhjálmsson BJ, Pedersen CB, Mors O, Børglum AD, et al. (2015): Polygenic Risk Score, Parental Socioeconomic Status, Family History of Psychiatric Disorders, and the Risk for Schizophrenia. JAMA Psychiatry. 72: 635–7. [DOI] [PubMed] [Google Scholar]
- 80.Riglin L, Collishaw S, Richards A, Thapar AK, Maughan B, O’Donovan MC, Thapar A (2017): Schizophrenia risk alleles and neurodevelopmental outcomes in childhood: a population-based cohort study. The Lancet Psychiatry. 4: 57–62. [DOI] [PubMed] [Google Scholar]
- 81.Meier SM, Agerbo E, Maier R, Pedersen CB, Lang M, Grove J, et al. (2015): High loading of polygenic risk in cases with chronic schizophrenia. Molecular Psychiatry. 21: 969–974. [DOI] [PubMed] [Google Scholar]
- 82.Power RA, Steinberg S, Bjornsdottir G, Rietveld CA, Abdellaoui A, Nivard MM, et al. (2015): Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat Neurosci. 18: 953–955. [DOI] [PubMed] [Google Scholar]
- 83.Anttila V, Bulik-Sullivan B, Finucane HK, Walters R, Bras J, Duncan L, et al. (2017): Analysis of shared heritability in common disorders of the brain. bioRxiv. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DCM, Ritchie, et al. (2016): Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. 21: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stoll G, Pietiläinen OPH, Linder B, Suvisaari J, Brosi C, Hennah W, et al. (2013): Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci. 16: 1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kerminen S, Havulinna AS, Hellenthal G, Martin AR, Sarin A-P, Perola M, et al. (2017): Fine-Scale Genetic Structure in Finland. G3. 7: 3459–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin AR, Karczewski KJ, Kerminen S, Kurki M, Sarin A-P, Artomov M, et al. (2017): Haplotype sharing provides insights into fine-scale population history and disease in Finland. 1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams E, Thomas K, Sidebotham H, Emond A (2008): Prevalence and characteristics of autistic spectrum disorders in the ALSPAC cohort. Developmental Medicine & Child Neurology. 50: 672–677. [DOI] [PubMed] [Google Scholar]
- 89.Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, Thapar A (2014): Genetic Risk for Attention-Deficit/Hyperactivity Disorder Contributes to Neurodevelopmental Traits in the General Population. Biological Psychiatry. 76: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stergiakouli E, Smith GD, Martin J, Skuse DH, Viechtbauer W, Ring SM, et al. (2017): Shared genetic influences between dimensional ASD and ADHD symptoms during child and adolescent development. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. (2015): Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 87: 1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grove J, Ripke S, Als TD, Mattheisen M, Walters R, Won H, et al. (2017): Common risk variants identified in autism spectrum disorder. 1–42. [Google Scholar]
- 93.Clarke T-K, Lupton MK, Fernandez-Pujals AM, Starr J, Davies G, Cox S, et al. (2015): Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. 21: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rozenblatt-Rosen O, Stubbington MJT, Regev A, Teichmann SA (2017): The Human Cell Atlas: from vision to reality. Nature. 550: 451–453. [DOI] [PubMed] [Google Scholar]
- 95.Power RA, Kyaga S, Uher R, MacCabe JH, Långström N, Landen M, et al. (2013): Fecundity of Patients With Schizophrenia, Autism, Bipolar Disorder, Depression, Anorexia Nervosa, or Substance Abuse vs Their Unaffected Siblings. JAMA Psychiatry. 70: 22. [DOI] [PubMed] [Google Scholar]
- 96.Daly MJ, Robinson EB, Neale BM (2016): Chapter 3 – Natural Selection and Neuropsychiatric Disease: Theory, Observation, and Emerging Genetic Findings. Genomics, Circuits, and Pathways in Clinical Neuropsychiatry. Elsevier, pp 51–61. [Google Scholar]
- 97.Kichaev G, Pasaniuc B (2015): Leveraging Functional-Annotation Data in Trans-ethnic Fine-Mapping Studies. Am J Hum Genet. 97: 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carlson CS, Matise TC, North KE, Haiman CA, Fesinmeyer MD, Buyske S, et al. (2013): Generalization and Dilution of Association Results from European GWAS in Populations of Non-European Ancestry: The PAGE Study. (Gibson G, editor) PLoS Biol. 11: e1001661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, Ferreira T, et al. (2014): Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 46: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaitlen N, Pasaniuc B, Gur T, Ziv E, Halperin E (2010): Leveraging Genetic Variability across Populations for the Identification of Causal Variants. The American Journal of Human Genetics. 86: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muse ED, Wineinger NE, Schrader B, Molparia B, Spencer EG, Bodian DL, et al. (2017): Moving Beyond Clinical Risk Scores with a Mobile App for the Genomic Risk of Coronary Artery Disease. 1–14. [Google Scholar]
- 102.Ruderfer DM, Korn J, Purcell SM (2010): Family-based genetic risk prediction of multifactorial disease. Genome Med. 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guerreiro R, Ross OA, Kun-Rodrigues C, Hernandez DG, Orme T, Eicher JD, et al. (2018): Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. The Lancet Neurology. 17: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. (2017): Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. The Lancet Neurology. 16: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K (2016): The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 18: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Natarajan P, Young R, Stitziel NO, Padmanabhan S, Baber U, Mehran R, et al. (2017): Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention SettingClinical Perspective. Circulation. 135: 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plomin R, Haworth CMA, Davis OSP (2009): Common disorders are quantitative traits. Nat Rev Genet. 10: 872–878. [DOI] [PubMed] [Google Scholar]
- 108.Schoech A, Jordan D, Loh P-R, Gazal S, O’Connor L, Balick DJ, et al. (2017): Quantification of frequency-dependent genetic architectures and action of negative selection in 25 UK Biobank traits. doi: 10.1101/188086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marquez-Luna C, Loh P-R, South Asian Type 2 Diabetes (SAT2D) Consortium, The SIGMA Type 2 Diabetes Consortium, Price AL (2016): Multi-ethnic polygenic risk scores improve risk prediction in diverse populations. 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abraham G, Kowalczyk A, Zobel J, Inouye M (2012): Performance and Robustness of Penalized and Unpenalized Methods for Genetic Prediction of Complex Human Disease. Genet Epidemiol. 37: 184–195. [DOI] [PubMed] [Google Scholar]
- 111.Makowsky R, Pajewski NM, Klimentidis YC, Vazquez AI, Duarte CW, Allison DB, de los Campos G (2011): Beyond Missing Heritability: Prediction of Complex Traits. (Gibson G, editor) PLoS Genet. 7: e1002051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou X, Carbonetto P, Stephens M (2013): Polygenic modeling with bayesian sparse linear mixed models. PLoS Genet. 9: e1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moser G, Lee SH, Hayes BJ, Goddard ME, Wray NR, Visscher PM (2015): Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model. (Haley C, editor) PLoS Genet. 11: e1004969–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Erbe M, Hayes BJ, Matukumalli LK, Goswami S, Bowman PJ, Reich CM, et al. (2012): Improving accuracy of genomic predictions within and between dairy cattle breeds with imputed high-density single nucleotide polymorphism panels. Journal of Dairy Science. 95: 4114–4129. [DOI] [PubMed] [Google Scholar]
- 115.Brown BC, Ye CJ, Price AL, Zaitlen N (2016): Transethnic Genetic-Correlation Estimates from Summary Statistics. The American Journal of Human Genetics. 99: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Consortium Brainstorm, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. (2018): Analysis of shared heritability in common disorders of the brain. Science. 360. doi: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lam M, Chen C-Y, Li Z, Martin A, Bryois J, Ma X, et al. (2018): Comparative genetic architectures of schizophrenia in East Asian and European populations. 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stahl E, Breen G, Forstner A, McQuillin A, Ripke S, Bipolar Disorder Working Group of the Psychiatric Genomics Consortium, et al. (2018): Genomewide association study identifies 30 loci associated with bipolar disorder. 1–41. [Google Scholar]
- 119.Ruderfer DM, Ripke S, McQuillin A, Boocock J, Stahl EA, Pavlides JMW, et al. (2018): Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell. 173: 1705–1715.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duncan L, Yilmaz Z, Gaspar H, Walters R, Goldstein J, Anttila V, et al. (2017): Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am J Psychiatry. 174: 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pourcain BS, Robinson EB, Anttila V, Sullivan BB, Maller J, Golding J, et al. (2016): ASD and schizophrenia show distinct developmental profiles in common genetic overlap with population-based social communication difficulties. Molecular Psychiatry. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. (2017): Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. (2018): Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 50: 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peterson RE, Cai N, Dahl AW, Bigdeli TB, Edwards AC, Webb BT, et al. (2018): Molecular Genetic Analysis Subdivided by Adversity Exposure Suggests Etiologic Heterogeneity in Major Depression. AJP. 175: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coleman J, Bipolar Disorder Working Group of the Psychiatric Genomics Consortium, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Breen G (2018): The genetics of the mood disorder spectrum: genome-wide association analyses of over 185,000 cases and 439,000 controls. bioRxiv. 1–56. [Google Scholar]
- 126.Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, et al. (2017): Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Molecular Psychiatry. doi: 10.1038/mp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. (2018): Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JRI, Krapohl E, et al. (2017): Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet. 49: 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wedow R, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, Bowers P, et al. (2018): Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 92: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. (2016): Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 533: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, et al. (2018): Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nature Communications. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.