Abstract
Objective:
To determine if pre-treatment non-linguistic cognition predicted language treatment outcomes and if so, which specific non-linguistic cognitive subskills predicted naming therapy outcomes.
Design:
Retrospective
Setting:
Research clinic
Participants:
Study 1 included data from 67 persons with aphasia who underwent language treatment and a pre-treatment cognitive-linguistic assessment battery. Study 2 included data from 27 Study 1 participants who completed additional pre-treatment non-linguistic cognitive assessments.
Interventions:
120-minute sessions of sentence comprehension (n=26) or naming treatment (n= 41) 2×/week for up to 10–12 weeks
Main Outcome Measure(s):
Proportion of potential maximal gain (i.e. PMG; assessed immediately after treatment [10–12 weeks]; formula = mean post-treatment score – mean pretreatment score/total number of trained items – mean pre-treatment score) and proportion of potential maximal gain maintained (i.e., PMGM; assessed 12 weeks after post-treatment [22–24 weeks]; formula = mean maintenance score – mean pre-treatment score/total number of trained items – mean pre-treatment score) as outcome variables; and pre-treatment assessment scores as predictor variables.
Results:
In study 1, 37% participants demonstrated non-linguistic cognitive deficits. Principal component analyses reduced assessment data to two components: linguistic and non-linguistic cognition. Backward elimination regression revealed that higher linguistic and non-linguistic cognitive function significantly predicted higher PMG after language therapy. In study 2, principal component analysis of only the non-linguistic cognitive measures identified three components: executive function, verbal short-term memory and visual short-term memory. Controlling for pre-treatment apraxia of speech and auditory comprehension deficits, regression analyses revealed that higher executive function and visual short-term memory significantly predicted higher PMG and PMGM after naming therapy.
Conclusions:
Pre-treatment non-linguistic cognitive function significantly influenced language treatment outcomes and maintenance of therapy gains.
Keywords: aphasia, cognition, rehabilitation, speech therapy
Approximately one-third of stroke survivors present with aphasia,1 a communication disorder traditionally described as impacting language while sparing non-linguistic cognitive abilities. Language processing certainly requires integrating linguistic skills with non-linguistic abilities, such as attention, memory, and executive function.2 Some investigators3 have suggested that language impairments in persons with post-stroke aphasia stem from misallocated non-linguistic cognitive resources rather than damaged linguistic representations. Previous evidence reveals that some persons with post-stroke aphasia exhibit attention,4 verbal and visual short-term memory5,6 and/or executive function impairments.7,8
While speech-language therapy has been shown to be effective for improving language functions,10 not all individuals respond to treatment. Recent studies11,12 have posited that non-linguistic cognitive deficits may explain treatment response variability in aphasia.13 Both pre-treatment linguistic and non-linguistic cognitive skills influence therapy outcomes.14,15 However, evidence regarding the impact of specific non-linguistic cognitive abilities on language therapy success has been mixed. Specifically, pre-treatment executive function, verbal short-term memory, and visuospatial processing skills have been linked to treatment outcomes in some studies, but not others.6,15,15–20
Non-linguistic cognition likely influences language rehabilitation outcomes for some persons with post-stroke aphasia, but to what extent remains unclear. Therefore, two retrospective studies were conducted with the following aims: 1) to ascertain the prevalence of non-linguistic cognitive deficits in persons with aphasia (n=67); 2) to investigate if pre-treatment non-linguistic cognitive skills predicted language gains following naming and sentence comprehension treatments; and 3) to determine which specific non-linguistic cognitive skills predicted naming therapy outcomes in a subsample of persons with aphasia (n=27). A comparable analysis focused on sentence comprehension treatment outcomes could not be investigated in this retrospective study due to limited availability of specific cognitive assessment data for patients who received sentence treatment.
METHODS – Study 1
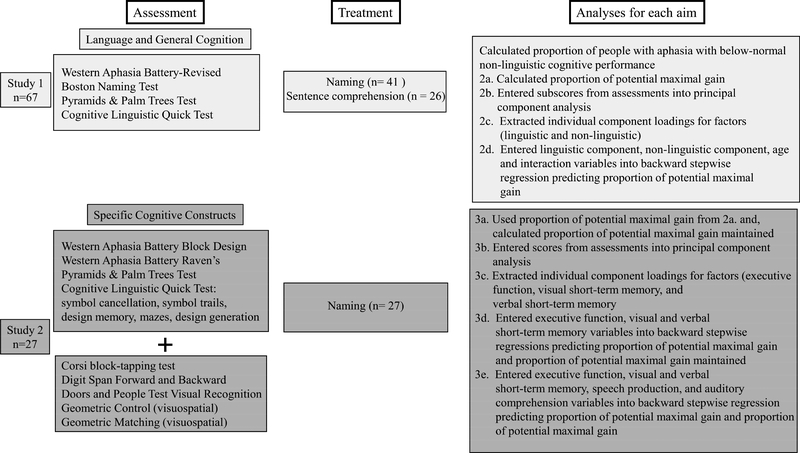
All data for both studies were collected from participants recruited to a research clinic for therapy studies from 2009–2017. Therapy study procedures were approved by the university’s Institutional Review Board. See Figure 1 for a flow chart of the current study’s methods and analyses. Refer to Supplementary Material for descriptions and normal cut-off scores for assessments.
Figure 1.
Flow chart of study 1 and 2 Note: Data from 27 participants in Study 1 who had undergone naming therapy were used in Study 2. No participants who had undergone sentence comprehension therapy were included in Study 2.
Sixty-seven persons with post-stroke aphasia (44 male; mean age=60.90; mean months post onset=53.58) were included in this study (see Table 1). Participants received language therapy (i.e., naming or sentence comprehension) for 120-minute sessions twice weekly for 1012 weeks in one of four studies and were administered a standardized cognitive-linguistic assessment battery. Individuals from these studies were enrolled in the present study if they completed the pre-treatment assessments and underwent the prescribed treatment protocol, excluding the possibility for missing data.
Table 1.
Participant demographics, pre-treatment cognitive-linguistic assessment scores, and treatment-related improvement scores (i.e., Proportion of potential maximal gain [PMG] and proportion of potential maximal gain maintained [PMGM])
| Construct/Test | Study 1 | Study 2 | |
|---|---|---|---|
| Sample size | 67(44 male) | 27(17 male) | |
| Age (years) | Mean±SD | 60.90±12.55 | 62.71±10.31 |
| Months Post Onset | Mean±SD | 53.58±47.78 | 54.49±51.92 |
| Range | 5-166 | 8-165 | |
| Aphasia Types (n per type) | Global | 1 | 1 |
| Broca’s | 19 | 9 | |
| Transcortical Motor | 2 | 1 | |
| Transcortical Sensory | 0 | 0 | |
| Wernicke’s | 7 | 2 | |
| Conduction | 5 | 2 | |
| Anomic | 31 | 11 | |
| Unable to be classified | 2 | 1 | |
| Pre-treatment | |||
| Mean±SD | |||
| Western Aphasia Battery-Revised | Aphasia Quotient | 65.43±25.59 | 58.85±25.66 |
| Language Quotient | 66.02±23.94 | 59.81±23.80 | |
| Spontaneous Speech* | 62.91±28.27 | 55.74±27.23 | |
| Auditory Verbal Comprehension* | 77.37±18.14 | 73.22±20.46 | |
| Repetition* | 61.88±32.05 | 55.56±32.69 | |
| Naming and Word Finding* | 62.06±31.06 | 54.00±31.32 | |
| Reading* | 70.54±23.41 | 65.48±23.99 | |
| Writing* | 57.70±29.74 | 49.83±28.90 | |
| Constructional, Visuospatial, Calculation* | 74.77±18.05 | 74.26±16.78 | |
| Block Design† | 81.48±23.27 | ||
| Raven’s Colored Progressive Matrices† | 74.37±16.97 | ||
| Cognitive Linguistic Quick Test | Composite Severity | 71.49±19.37 | 68.15±18.56 |
| Personal Facts* | 68.66±40.67 | 52.31±43.31 | |
| Symbol Cancellation*† | 75.75 ±37.96 | 70.99±39.72 | |
| Confrontation Naming* | 71.87±36.14 | 63.89±37.06 | |
| Story Retelling* | 38.21±24.12 | 31.11±21.90 | |
| Symbol Trails*† | 79.85±27.05 | 81.85±24.03 | |
| Generative Naming* | 26.53±18.45 | 23.05±19.23 | |
| Design Memory*† | 83.83±16.91 | 87.65±14.32 | |
| Mazes*† | 72.29±33.11 | 74.77±33.63 | |
| Design Generation*† | 42.59±17.27 | 40.17±18.64 | |
| Boston Naming Test* | 46.04±35.56 | 36.92±35.52 | |
| Pyramid and Palm Trees Test*† | 88.55±10.24 | 88.03±9.13 | |
| Corsi block-tapping test† | 53.17±16.01 | ||
| Digit span forward† | 23.15±19.90 | ||
| Digit span backward† | 12.43±13.08 | ||
| Doors visual recognition† | 60.80±17.46 | ||
| Geometric inclusion† | 88.52±13.57 | ||
| Geometric matching† | 88.15±7.49 | ||
| Diadochokinetic score (total produced) | 54.41±26.26 | ||
| Treatment Type | Naming | 41 | 27 |
| Sentence Comprehension | 26 | ||
| Baseline screener accuracy | Naming | 24.19±21.70 | 27.94±22.02 |
| Sentence Comprehension | 35.97±20.77 | ||
| Proportion of potential maximal gain (PMG) | Mean±SD | .53±.35 | .44±.38 |
| Range | −.18–1.00 | −.07–1.00 | |
| Proportion of potential maximal gain maintained (PMGM) | Mean±SD | .34±.31 −. | |
| Range | .06–.82 | ||
Note:
= test included in principal component analysis for Study 1
test included in principal component analysis for Study 1 † = test included in principal component analysis for Study 2 All pre-treatment assessment scores except Western Aphasia Battery-Revised Aphasia and Language Quotients reflect group-level percent correct (Mean±SD). The Aphasia and Language Quotients are weighted sums on a scale from 0–100, with higher scores suggesting more intact language function. While both metrics reflect overall language function, the Aphasia Quotient emphasizes auditory comprehension and verbal expression ability, whereas the Language Quotient generally highlights reading comprehension and written expression ability. Of note, the WAB summary scores were calculated as follows: Spontaneous Speech: XX/20; Auditory Verbal Comprehension: XX/200; Repetition: XX/100; Naming and Word Finding: XX/100; Reading: XX/100; Writing: XX/100; Constructional, Visuospatial, Calculation: XX/100; Block Design: XX/9; and Raven’s: XX/37. Proportion of potential maximal gain (PMG; assessed immediately after treatment phase ends [10–12 weeks after pre-treatment]) was calculated as follows: mean post-treatment trained item score – mean pre-treatment trained item score divided by total number of trained items – mean pre-treatment trained item score. Proportion of potential maximal gain maintained (PMGM; assessed 12 weeks after treatment phase ends [22–24 weeks after pre-treatment]) used the same formula, but mean post-treatment trained items score was replaced with mean maintenance trained items score. These metrics reflect the amount of improvement from pre- to post-treatment timepoints, while accounting for the participants’ ability at baseline. They are the only scores in this table that incorporate post-treatment data. All other scores reflect the pre-treatment timepoint only. Diadochokinetic score was used to capture pre-treatment apraxia of speech.
Before treatment, all participants were administered the following standardized assessments: Western Aphasia Battery-Revised21 to assess language function, the Boston Naming Test22 to measure verbal naming, the Cognitive-Linguistic Quick Test23 to assess cognitive-linguistic function, and the Pyramids and Palm Trees Test24 to measure semantic processing.
Data Analysis
First, to assess the prevalence of non-linguistic cognitive impairment, the number of participants with Cognitive Linguistic Quick Test Visuospatial Skills domain scores (i.e., composite of symbol cancellation, symbol trails, design memory, mazes and design generation tasks) below normal limits was calculated. These tasks include simple verbal and written directions with demonstrations and/or practice items to support task comprehension.
Per aim 2, to quantify treatment-related language improvement, individual proportion of potential maximal gain scores (PMG)15 were calculated for each participant based on the pre- and post-treatment assessments of their trained item sets as follows:
PMG, an alternative to relative change, reflects the magnitude of change while considering the number of items the participant could already name and/or comprehend at pre-treatment. It was utilized to standardize the amount of change across the four treatment studies contributing data to the analysis since all participants within a single study were trained on the same number of items/structures during therapy, but different studies trained different numbers of items. Data used in these analyses were derived from multiple baseline single-subject design studies; thus, participants were assessed on their trained items multiple times at each timepoint, and the timepoint average was used in the formula described above. Trained items included the items and/or structures targeted during therapy. See Supplementary Material for individual participants’ scores.
Eighteen pre-treatment standardized test sub-scores were identified as potential predictors of PMG (see Table 1). A principal component analysis with varimax rotation was performed to reveal the data structure. Component subscores were extracted for each participant and entered into a backward elimination linear regression with age and treatment type (i.e., naming or sentence comprehension). Two- and three-way interactions between each component score and the demographic variables were modeled as potential predictors of PMG.
RESULTS – Study 1
Aim 1: What is the prevalence of non-linguistic cognitive deficits in persons with poststroke aphasia?
According to the Cognitive Linguistic Quick Test Visuospatial Skills domain scores (i.e., metric of non-linguistic cognitive function; includes symbol cancellation, symbol trails, design memory, mazes, design generation tasks), 37.31% of participants scored below normal limits (i.e., normal cutoff for ages 18–60: < 78%; for ages 70–79: <59%).
Aim 2: Are non-linguistic cognitive skills predictive of language therapy outcomes?
Participants achieved an average PMG of 53% in the target skill (i.e., naming items, or comprehending sentence structures), indicating that they acquired approximately half of the items/structures on which they were incorrect at pre-treatment (see Table 1).
The principal component analysis, explaining 71% of the variance, revealed two components. Based on a criterion of a component loading of .5 or greater (see Table 2), component one consisted of all of the Western Aphasia Battery-Revised subscales and Cognitive Linguistic Quick Test subtests that involved overt linguistic processing, the Boston Naming Test and the Pyramids and Palm Trees Test. Component two included the Western Aphasia Battery-Revised subscale involving reasoning and problem-solving, subtests of the Cognitive Linguistic Quick Test measuring nonlinguistic cognition, and the Pyramids and Palm Trees Test. Thus, the assessment data were reduced to two distinct components: linguistic and non-linguistic cognition. Of note, the Pyramids and Palm Trees Test loaded strongly on both the linguistic (.52) and on the non-linguistic cognitive factor (.62). As this assessment measures both conceptual reasoning (non-linguistic cognition) and semantic access (linguistic cognition), a decision was made to retain it as a complex variable (i.e., contributes to both linguistic and non-linguistic component loadings).
Table 2.
Principal component loadings from full sample (n=67)
| Test/Subtest | Component 1 | Component 2 |
|---|---|---|
| Cognitive Linguistic Quick Test | ||
| Personal Facts | .89 | .05 |
| Symbol Cancellation | .10 | .53 |
| Confrontation Naming | .89 | .18 |
| Story Retelling | .83 | .14 |
| Symbol Trails | .26 | .79 |
| Generative Naming | .79 | .28 |
| Design Memory | .26 | .62 |
| Mazes | −.09 | .82 |
| Design Generation | .08 | .65 |
| Western Aphasia Battery-Revised | ||
| Spontaneous Speech | .87 | .21 |
| Auditory Verbal Comprehension | .82 | .21 |
| Repetition | .88 | .12 |
| Naming and Word Finding | .96 | .17 |
| Reading | .89 | .22 |
| Writing | .79 | .42 |
| Construction, Visuospatial, Calculation | .42 | .79 |
| Pyramids and Palm Trees Test | .52 | .62 |
| Boston Naming Test | .88 | .25 |
| Component Construct | Language Component | Cognitive Component |
Bold values indicate the component on which each test loads (i.e., component loadings ≥ .50). Pyramids and Palm Trees Test loaded above .50 on both components and was retained as a complex variable (i.e., contributes to both components when individual subject loadings are extracted).
The backward elimination regression analysis (n = 67) predicting PMG resulted in a significant best-fit model, explaining 58% of the variance. It included the linguistic component, non-linguistic cognitive component, treatment type, age, the interaction of the non-linguistic cognitive component with treatment type, and the interaction of the linguistic component with age, F(6,55)=12.48, p<.001. The linguistic (β = .49, SE=.16, t=3.08, p<.01) and the non-linguistic cognitive components (β = .42, SE=.10, t=4.07, p<.001) were both significant, with one-point increases predicting increases in PMG of .49 and .42, respectively. The non-linguistic cognitive component-by-treatment type interaction was also significant (β = −.23, SE=.07, t=−3.42, p<.001). Thus, pre-treatment non-linguistic cognitive skills were more influential for naming treatment than for sentence comprehension treatment.
METHODS – Study 2
Building on Study 1’s findings, a second study was conducted to identify which specific cognitive subskills influenced treatment outcomes in a subset of 27 participants from Study 1 who received a semantic-based naming treatment and more extensive cognitive assessment. In addition to Study 1’s assessments, these participants were given the following non-linguistic cognitive assessments before treatment: the Wechsler Adult Intelligence Scale Digit Span Forward25 and Backward to measure verbal short-term memory; the Visual Recognition subtest of the Doors and People Test26 and the Corsi block-tapping test27 to measure visual short-term memory; and two visuospatial tasks (i.e., Geometric Matching and Inclusion). While the Digit Span tasks required participants to repeat numbers and may have involved linguistic processing,28 they also required participants to temporarily maintain and manipulate information and are traditionally used to assess non-linguistic cognitive skills, such as attention and short-term memory. Thus, they will be referred to as non-linguistic cognitive tasks in this study to distinguish them from traditional language tasks (e.g., Boston Naming Test/lexical retrieval). Additionally, participants’ naming ability on trained items was assessed before treatment, immediately following the treatment phase (i.e., 12 weeks after pre-treatment assessment), and 12 weeks after the treatment phase ended (i.e., 24 weeks after pre-treatment assessment).
Data Analysis
PMG14,15 was used to capture therapy-related naming gains (i.e., 12 weeks after pre-treatment assessment). Proportion of potential maximal gain maintained (PMGM) was used to assess therapy-related naming gains maintained (i.e., 12 weeks after post-treatment assessment). It was calculated using the average score from the maintenance timepoint instead of post-treatment averages for 24 participants, as only 24/27 participants had completed follow-up testing at the time of analysis.
Scores on the non-linguistic cognitive assessment battery described above and scores on the tests that contributed to the non-linguistic cognitive component in Study 1 were entered into a principal component analysis to reduce the number of predictor variables. The participant-to-variable ratio of 1.93 may have resulted in an under-powered analysis; thus, two alternative analyses were conducted to compensate for this potential limitation. The results were largely consistent with those presented below and are available in the Supplementary Material.
Individual component scores derived from the principal component analysis were extracted for all 27 persons with aphasia and entered into two backward elimination linear regressions, one predicting PMG and one predicting PMGM. To account for the potential influence of pre-treatment apraxia of speech29 and/or auditory comprehension impairment30 on participants’ non-linguistic cognitive performance, the total sum of diadochokinetic productions and Western Aphasia Battery-Revised auditory verbal comprehension sub-scores were entered as regressors into two backward elimination models with the individual component scores.
RESULTS – Study 2
Aim 3: Which specific non-linguistic cognitive skills predict naming recovery?
In this sub-sample (n=27), naming treatment resulted in average PMG of about 44%, as shown in Table 1. Average proportion of PMGM was about 34%.
The principal component analysis revealed three components that explained 64% of the variance in the data. Tests with loadings of ≥ .5 for a component were considered to characterize the components according with specific neuropsychological constructs.31 Component one primarily represented executive function, component two reflected visual short-term memory, and component three comprised verbal short-term memory. See Table 3 for test loadings for each component.
Table 3.
Principal component analysis component loadings from subsample (n=27)
| Test/Subtest | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| Cognitive Linguistic Quick Test | |||
| Symbol Cancellation | 0.19 | 0.72 | 0.11 |
| Symbol Trails | 0.73 | 0.29 | 0.19 |
| Design Memory | 0.29 | 0.69 | 0.02 |
| Mazes | 0.71 | 0.15 | −0.20 |
| Design Generation | 0.04 | ||
| Western Aphasia Battery-Revised | |||
| Block Design | 0.72 | 0.29 | 0.22 |
| Raven’s Coloured Progressive Matrices | 0.75 | 0.32 | 0.15 |
| Pyramids and Palm Trees Test | 0.66 | 0.16 | 0.46 |
| Corsi | 0.17 | 0.72 | 0.11 |
| Digit Span Forward | 0.11 | 0.16 | 0.90 |
| Digit Span Backward | 0.11 | 0.04 | 0.90 |
| Doors Visual Recognition | 0.53 | 0.46 | −0.13 |
| Geometric Matching | 0.12 | 0.78 | 0.18 |
| Geometric Inclusion | 0.78 | 0.06 | 0.39 |
| Component Construct | Executive Function | Visual Short-term Memory | Verbal Short-term Memory |
Bold values indicate the component on which each test loads (i.e., component loadings ≥ .05).
The best-fit regression model significantly explained 56% of the variance in PMG (n=27), F(3,23)=9.83, p<.001. While executive function was retained in the model, only visual short-term memory and verbal short-term memory were significant predictors, with one-point increases predicting increases in PMG of .17 (p=.003) and .20 (p<.001), respectively.
For PMGM (n=24), the best-fit regression model explained 61% of the variance in treatment gains maintained, F(3,23)=12.23, p<.001. Once again, although executive function remained in the model, only visual short-term memory and verbal short-term memory were significant predictors with one-point increases in each predicting increases in PMGM of .13 (p<.01) and .18 (p<.001), respectively. In other words, patients with higher pre-treatment visual short-term memory and verbal short-term memory skills responded more favorably to semantic-based treatment--both in terms of immediate and maintained gains--than those with lower pretreatment skills in these domains.
Yet, these findings must be considered in the face of the challenges associated with nonlinguistic cognitive assessment in this population9 (e.g., repetition, lexical retrieval, and/or motor speech impairments may impact verbal short-term memory assessment; presence of hemiplegia and use of non-dominant hand may influence reaction time and/or quality of motor/written response, and visual deficits may affect visually-presented stimulus processing). Thus, two additional backward stepwise regression analyses were conducted to predict PMG and PMGM using executive function, visual short-term memory, and verbal short-term memory, while controlling for pre-treatment apraxia of speech and auditory comprehension impairment. The model predicting PMG (n=27) explained 57% of the variance (adjusted R2), F(4,22) = 9.7, p < .001. All variables were retained in the final model, but only executive function, visual short-term memory and auditory comprehension were significant predictors, with one-unit increases in each ability predicting .29 (p<.05), .30 (p<.05), and .41 (p <.05) increases in PMG, respectively. In the backward stepwise regression model predicting proportion of PMGM (n=24), the best-fit model significantly explained 62% of the variance (adjusted R2), F(2,21)= 9.19, p<.001. As with PMG, all variables remained in the final model, yet only executive function and visual short-term memory were significant predictors, with one-unit increases predicting increases in PMGM of .28 (p< .05) and .33 (p<.05), respectively.
These final analyses indicate that the digit span forward and backward tasks used in this study may have been capturing speech production ability as opposed to verbal short-term memory. Furthermore, non-linguistic cognitive task performance did not appear to be significantly influenced by auditory comprehension difficulty.30 The initial finding that verbal short-term memory was predictive of naming treatment outcomes was dampened, yet executive function and visual short-term memory were indeed influential of immediate semantic-based treatment success and longer-term maintenance of gains.
DISCUSSION
The analyses conducted in this study revealed a number of interesting relationships between aphasia, non-linguistic cognition, and treatment outcomes. First, we found that 37.31% of the participants exhibited non-linguistic cognitive deficits. Next, we found that pre-treatment standardized cognitive-linguistic assessment battery scores loaded onto two construct-specific factors: linguistic and non-linguistic cognition. Both factors predicted the magnitude of treatment-related change in sentence comprehension or naming. Additionally, there was an interaction between non-linguistic cognitive factors and treatment type, in that pre-treatment non-linguistic cognitive skills contributed less to sentence comprehension treatment than naming therapy outcomes. Finally, given the relationship between pre-treatment non-linguistic cognition and naming treatment response, we investigated this association further in 27 persons with aphasia, who had undergone additional pre-treatment non-linguistic testing. Executive function and visual short-term memory significantly predicted improvements immediately after treatment and gains maintained 12-weeks after stopping treatment.
Critically, study 1 revealed that assessment tasks commonly used in aphasia rehabilitation could be separated into two constructs, both of which were independently influential for language therapy success. While linguistic cognition was a stronger predictor than non-linguistic cognition, baseline non-linguistic cognition also predicted treatment gains. Consistent with previous work,14 these findings highlight the importance of non-linguistic cognitive skills in treatment management for individuals with aphasia. On closer inspection of the data, the interaction between treatment type and non-linguistic cognitive function may have been driven by a higher percentage of participants in the sentence comprehension group (46.2%) with nonlinguistic cognitive scores below normal limits than in the naming treatment group (31.7%), although this interpretation warrants further investigation.
Compelled by study 1’s results, study 2 investigated which non-linguistic cognitive subskills predicted semantic-based naming treatment outcomes. Based on prior work, it should not be surprising that specific non-linguistic cognitive abilities such as executive function,15–17 and visual short-term memory6 influenced naming therapy outcomes and maintenance of gains in this study. Semantic-based naming treatment steps required participants to integrate linguistic and non-linguistic skills. Executive function skills were likely employed by successful participants in different ways, such as when learning features of target items, initiating naming responses and self-correcting errors. Furthermore, participants may have relied on visual short-term memory to retain physical details of the pictured items they were trained to name and distinguish them from other items.
Based on these findings, pre-treatment non-linguistic cognitive skills were predictive of language therapy outcomes and, specifically, executive function and visual short-term memory were associated with naming treatment outcomes and maintenance of gains after a 12-week no-treatment phase. These findings and others14,15 emphasize that some of the heterogeneity seen in treatment response for persons with post-stroke aphasia may be explained by differences in pre-treatment non-linguistic cognition
There are several avenues for further research in this area. While targeting non-linguistic cognition has been shown to be effective for improving linguistic skills, 32 these studies had relatively small sample sizes and focused on the benefits of specific subskills. Future studies should investigate the effects of comprehensive non-linguistic cognitive rehabilitation on language recovery with larger participant samples. Another option is to evaluate non-linguistic cognitive skill improvement after language treatment, which has been studied less frequently,33 and would shed light on the relationship between linguistic and non-linguistic cognition. Lastly, it will be important to assess the benefit of simultaneous treatment of these processes and whether they co-improve with the ultimate goal of developing integrated cognitive-linguistic approaches to aphasia rehabilitation.
Study Limitations
The findings may have been impacted by sample size (i.e., underpowered principal components analyses), especially in study 2 (n = 27). Nonetheless, the reported findings were supported by supplemental analyses. Furthermore, there are currently no gold standard assessments for assessing non-linguistic cognition in aphasia. 30 Thus, participants’ performance on some non-linguistic cognitive assessments used in the present study may have been negatively impacted by speech (e.g., apraxia of speech may have influenced accurate production on digit span tasks), language (e.g., auditory comprehension may have hindered understanding instructions), or motor impairment (e.g., hemiplegia may have affected pen and paper timed tasks).
CONCLUSIONS
Consistent with emerging evidence, roughly 37.31% of individuals with chronic poststroke aphasia in this study presented with concomitant non-linguistic cognitive deficits. Pretreatment linguistic and non-linguistic cognitive abilities were predictive of language treatment outcomes. Participants with higher pre-treatment executive function and visual short-term memory skills demonstrated higher naming accuracy both immediately after semantic-based naming treatment and 12 weeks after treatment terminated.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Institutes of Health [1P50DC012283, 2013–2018; R21-R33DC010461, 2009–2015; 5F31DC011220, 2012–2014; 1K18DC011517, 2011–2013, NIH/NIDCD F31DC015940, 2017–2019 and 5T32DC013017, 2016–2018].
Abbreviation List:
- (PMG)
Proportion of potential maximal gain
- (PMGM)
Proportion of potential maximal gain maintained
Footnotes
No conflicts of interest were identified.
Presentations: American Congress of Rehabilitation Medicine Annual Conference (2017) Atlanta, GA; Academy of Aphasia Annual Meeting (2017) Baltimore, MD; American Speech-Language-Hearing Association Convention (2017) Los Angeles, CA; International Aphasia Rehabilitation Conference (2018)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dickey L, Kagan A, Lindsay MP, Fang J, Rowland A, Black S. Incidence and Profile of Inpatient Stroke-Induced Aphasia in Ontario, Canada. Arch Phys Med Rehabil. 2010;91(2):196–202. doi: 10.1016/j.apmr.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 2.Peach R Cognitive Approaches to Aphasia Treatment: Application of the Cognition of Language to Aphasia Intervention. Semin Speech Lang. 2017;38(01):003–004. doi: 10.1055/s-0036-1597259 [DOI] [PubMed] [Google Scholar]
- 3.McNeil M, Odell K, Tseng CH. Toward the integration of resource allocation into a general theory of aphasia. Clin Aphasiology. 1991;20:21–39. [Google Scholar]
- 4.Villard S, Kiran S. Between-session intra-individual variability in sustained, selective, and integrational non-linguistic attention in aphasia. Neuropsychologia. 2015;66:204–212. doi: 10.1016/j.neuropsychologia.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 5.Lang CJG, Quitz A. Verbal and nonverbal memory impairment in aphasia. J Neurol. 2012;259(8):1655–1661. doi: 10.1007/s00415-011-6394-1 [DOI] [PubMed] [Google Scholar]
- 6.Seniów J, Litwin M, Leśniak M. The relationship between non-linguistic cognitive deficits and language recovery in patients with aphasia. J Neurol Sci. 2009;283(1–2):91–94. doi: 10.1016/j.jns.2009.02.315 [DOI] [PubMed] [Google Scholar]
- 7.Purdy M Executive function ability in persons with aphasia. Aphasiology. 2002;16(4/6):549–557. [Google Scholar]
- 8.Kertesz A, McCabe P. Intelligence and aphasia: Performance of aphasics on Raven’s coloured progressive matrices (RCPM). Brain Lang. 1975;2:387–395. doi: 10.1016/S0093-934X(75)80079-4 [DOI] [PubMed] [Google Scholar]
- 9.Murray L, Salis C, Martin N, Dralle J. The use of standardised short-term and working memory tests in aphasia research: a systematic review. Neuropsychol Rehabil. 2018;28(3):309–351. doi: 10.1080/09602011.2016.1174718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke In: The Cochrane Collaboration, ed. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2016. http://doi.wiley.com/10.1002/14651858.CD000425.pub4. Accessed July 22, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonini MV, Radanovic M. Cognitive deficits in post-stroke aphasia. Arq Neuropsiquiatr. 2015;73(10):840–847. doi: 10.1590/0004-282X20150133 [DOI] [PubMed] [Google Scholar]
- 12.El Hachioui H, Visch-Brink EG, Lingsma HF, et al. Nonlinguistic cognitive impairment in poststroke aphasia: A prospective study. Neurorehabil Neural Repair. 2014;28(3):273–281. doi: 10.1177/1545968313508467 [DOI] [PubMed] [Google Scholar]
- 13.Code C, Torney A, Gildea-Howardine E, Willmes K. Outcome of a One-Month Therapy Intensive for Chronic Aphasia: Variable Individual Responses. Semin Speech Lang. 2010;31(01):021–033. doi: 10.1055/s-0029-1244950 [DOI] [PubMed] [Google Scholar]
- 14.Lambon Ralph MA, Snell C, Fillingham JK, Conroy P, Sage K. Predicting the outcome of anomia therapy for people with aphasia post CVA: Both language and cognitive status are key predictors. Neuropsychol Rehabil. 2010;20(2):289–305. doi: 10.1080/09602010903237875 [DOI] [PubMed] [Google Scholar]
- 15.Dignam J, Copland D, O’Brien K, Burfein P, Khan A, Rodriguez AD. Influence of Cognitive Ability on Therapy Outcomes for Anomia in Adults With Chronic Poststroke Aphasia. J Speech Lang Hear Res. 2017;60(2):406. doi: 10.1044/2016_JSLHR-L-15-0384 [DOI] [PubMed] [Google Scholar]
- 16.Fillingham JK, Sage K, Lambon Ralph MA. The treatment of anomia using errorless learning. Neuropsychol Rehabil. 2006;16(2):129–154. doi: 10.1080/09602010443000254 [DOI] [PubMed] [Google Scholar]
- 17.Kristensen LF, Steensig I, Pedersen AD, Pedersen AR, Nielsen JF. Constraint-induced aphasia therapy in subacute neurorehabilitation. Aphasiology. 2015;29(10):1152–1163. doi: 10.1080/02687038.2015.1028328 [DOI] [Google Scholar]
- 18.Purdy M, Koch A. Prediction of strategy usage by adults with aphasia. Aphasiology. 2006;20(2–4):337–348. doi: 10.1080/02687030500475085 [DOI] [Google Scholar]
- 19.Goldenberg G, Dettmers H, Grothe C, Spatt J. Influence of linguistic and non-linguistic capacities on spontaneous recovery of aphasia and on success of language therapy. Aphasiology. 1994;8(5):443–456. doi: 10.1080/02687039408248669 [DOI] [Google Scholar]
- 20.Yeung O, Law S-P. Executive functions and aphasia treatment outcomes: Data from an ortho-phonological cueing therapy for anomia in Chinese. Int J Speech Lang Pathol. 2010;12(6):529–544. doi: 10.3109/17549507.2011.516840 [DOI] [PubMed] [Google Scholar]
- 21.Kertesz A Western Aphasia Battery (Revised). San Antonio, TX: PsychCorp; 2006. [Google Scholar]
- 22.Goodglass H, Kaplan E, Weintraub S. The Revised Boston Naming Test. Philadelphia, PA: Lea & Febiger; 2001. [Google Scholar]
- 23.Helm-Estabrooks N Cognitive Linguistic Quick Test: Examiner’s Manual. Psychological Corporation; 2001. [Google Scholar]
- 24.Howard D, Patterson KE. The Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Pictures. Thames Valley Test Company; 1992. [Google Scholar]
- 25.Wechsler D Wechsler Adult Intelligence Scale - Fourth Edition (WAIS-IV). Pearson Education; 2008. [Google Scholar]
- 26.Baddeley AD, Emslie H, Nimmo-Smith I. The Doors and People Test. Bury St. Edmunds, UK: Thames Valley Test Company; 1994. [Google Scholar]
- 27.Kessels RP, Van Zandvoort MJ, Postma A, Kappelle LJ, De Haan EH. The Corsi block-tapping task: standardization and normative data. Appl Neuropsychol. 2000;7(4):252–258. http://www.tandfonline.com/doi/abs/10.1207/s15324826an0704_8. Accessed April 18, 2017. [DOI] [PubMed] [Google Scholar]
- 28.Martin N, Minkina I, Kohen FP, Kalinyak-Fliszar M. Assessment of linguistic and verbal short-term memory components of language abilities in aphasia. J Neurolinguistics. February 2018. doi: 10.1016/j.jneuroling.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabul B Apraxia Battery for Adults, Second Edition Austin, TX: Pro-Ed; 2000. [Google Scholar]
- 30.Wall KJ, Cumming TB, Copland DA. Determining the Association between Language and Cognitive Tests in Poststroke Aphasia. Front Neurol. 2017;8. doi: 10.3389/fneur.2017.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lezak MD. Neuropsychological Assessment. 3rd ed. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 32.Mayer JF, Mitchinson SI, Murray LL. Addressing concomitant executive dysfunction and aphasia: previous approaches and the new brain budget protocol. Aphasiology. 2016;31(7):837–860. doi: 10.1080/02687038.2016.1249333 [DOI] [Google Scholar]
- 33.Roches CAD, Balachandran I, Ascenso EM, Tripodis Y, Kiran S. Effectiveness of an impairment-based individualized rehabilitation program using an iPad-based software platform. Front Hum Neurosci. 2015;8 http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2015-26028-001&site=ehost-live&scope=site. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.