Abstract
Cadherins play a major role in mediating cell–cell adhesion, which shares many parallels with platelet–platelet interactions during aggregate formation and clot stabilization. Platelets express epithelial (E)-cadherin, but its contribution to platelet function and/or platelet production is currently unknown. To assess the role of E-cadherin in platelet production and function in vitro and in vivo, we utilized a megakaryocyte-specific E-cadherin knockout mouse model. Loss of E-cadherin in megakaryocytes does not affect megakaryocyte maturation, platelet number or size. However, platelet dysfunction in the absence of E-cadherin is revealed when conditional knockout mice are challenged with acute antibody-mediated platelet depletion. Unlike wild-type mice that recover fully, knockout mice die within 72 hours post-antibody administration, likely from haemorrhage. Furthermore, conditional knockout mice have prolonged tail bleeding times, unstable clot formation, reduced clot retraction and reduced fibrin deposition in in vivo injury models. Murine platelet aggregation in vitro in response to thrombin and thrombin receptor activating peptide is compromised in E-cadherin null platelets, while aggregation in response to adenosine diphosphate (ADP) is not significantly different. Consistent with this, in vitro aggregation of primary human platelets in response to thrombin is decreased by an inhibitory E-cadherin antibody. Integrin activation and granule secretion in response to ADP and thrombin are not affected in E-cadherin null platelets, but Akt and glycogen synthase kinase 3β (GSK3β) activation are attenuated, suggesting a that E-cadherin contributes to aggregation, clot stabilization and retraction that is mediated by phosphoinositide 3-kinase/Akt/GSK3β signalling. In summary, E-cadherin plays a salient role in platelet aggregation and clot stability.
Keywords: cadherins, clot stability, haemostasis, platelet aggregation
Introduction
Blood clots require tight adhesion of platelets to damaged endothelium and to each other. Platelets circulate in a resting state and express a variety of receptors including glycoprotein (GP) Ib-IX-V, GPVI and α2β1 that can bind to collagen and von Willebrand factor (VWF) on damaged endothelial surfaces to promote platelet adhesion and activation at sites of injury. Formation of the resultant stable clot requires thrombin generation and platelet-secreted factors such as adenosine diphosphate (ADP) and thromboxane A2 (TxA2), which promote platelet spreading, platelet aggregation, platelet plug formation and clot retraction.
Throughout this process, dynamic bi-directional signalling (outside-in and inside-out) is mediated by numerous signalling pathways, including the phosphoinositide 3-kinase (PI3K) pathway, and its downstream effector protein kinase B, also known as Akt. PI3K signalling is activated downstream of VWF binding to GPIb-IX-V.1 Additionally, PI3K/Akt/glycogen synthase kinase 3β (GSK3β) signalling modulates integrin αIIbβ3 ligand affinity and avidity,2 which is required for platelet spreading, aggregation and clot retraction.3,4
Examples of other cell surface proteins that contribute to contact-dependent signalling include ephrins and Cadherin 6 (CDH6), a type II cadherin expressed in platelets. CDH6 is a ligand for integrin αIIbβ3 (CD41/61), and may partially contribute to the mechanism of fibrinogen and VWF-independent platelet aggregation and thrombus formation.3,5,6 Interestingly, antibody-mediated inhibition of CDH6 reduces platelet aggregation and thrombus formation.7 Epithelial (E)-cadherin (CDH1), a type I cadherin, is also expressed in platelets,8 and was identified by mass spectrometry in the sheddome of phorbol 12-myristate 13-acetate activated platelets.9 Platelets also express downstream effector molecules of cadherin signalling, including β-catenin.10 The functional significance of E-cadherin expression in platelets has not previously been assessed. E-cadherin constitutive knockout animals are embryonic lethal with failure to form trophectodermal epithelium.11 Therefore, we generated a megakaryocyte/platelet-specific E-cadherin KO mouse model using the platelet factor 4 (Pf4)-Crerecombinase system crossed to E-cadherin floxed animals.12,13 In this study, we report a previously unappreciated role for E-cadherin in platelet function, specifically platelet aggregation and clot stability, likely mediated through PI3K/Akt/GSK3β signalling, by both in vitro and in vivo approaches.
Methods
Mice
All procedures were approved by the Institutional Animal Care and Use Committee of Yale University Institutional and the Children’s Hospital of Philadelphia and adhere to the European Convention on the Protection of Animals Used for Scientific Purpose. E-cadherin floxed mice (CDH1F/F)13 and Pf4-Cre expressing mice (C57BL/6-Tg(Pf4-Cre)Q3Rsko/J)12 were purchased from Jackson Laboratory and crossed in the Yale Animal Resource Center. For bone marrow (BM) transplantation studies, mice ubiquitously expressing enhanced green fluorescent protein (EGFP) (C57BL/6-Tg(CAG-EGFP)1Osb/J) were used as wild-type (WT) donors. For complete blood count (CBC) analysis, blood was drawn from the retro-orbital sinus into ethylenediaminetetraacetic acid containing tubes, and analysed on a Hemavet 950FS analyser (Drew Scientific, Texas, United States). Pf4-Cre positive mice that are homozygous for the CDH1-flox allele are referred to as cKO. Littermates lacking CDH1-flox alleles and/or Pf4-Cre are referred to as WT controls.
Cell Culture and Ribonucleic Acid Analysis
Mouse foetal liver cells were isolated from E12.5 to E13.5 livers by mechanical dissociation followed by filtration through 100 μm strainers. Red blood cells were lysed with Pharm Lyse (BD Biosciences). Cells were cultured for 4 days in StemSpan Expansion Media (Stem Cell Technologies) with 30% BIT9500 (Stem Cell Technologies), 50 ng/mL murine thrombopoietin (mTPO; ConnStem Inc.), L-glutamine (Life Technologies), penicillin/streptomycin (Life Technologies) and gentamicin (Gibco). To enrich megakaryocytes, a discontinuous bovine serum albumin (BSA; Sigma) gradient was performed as described,14 and the pellet was collected for downstream applications. Ribonucleic acid (RNA) was extracted with RNeasy (Qiagen) and complementary deoxyribonucleic acid (DNA) was synthesized with Superscript III (Life Technologies). Applied Biosystems (Life Technologies) TaqMan Gene Expression Assays were used for quantification on a CFX96 C1000 thermal cycler (Bio-Rad), with 18S RNA as internal control.
Flow Cytometry
DNA content analysis of megakaryocytes was performed as previously described.15 For platelet activation, whole blood was washed three times in Tyrode’s-HEPES buffer and resuspended in Tyrode’s-HEPES buffer with 2 mM CaCl2. Platelets were stimulated for 15 minutes at 37°C with indicated concentrations of ADP (Chrono-log), U-46619 (Cayman Chemical) or thrombin (Roche). Activation was terminated with 5 × volume 0.5% paraformaldehyde. Activated platelets were stained with fluorescein isothiocyanate (FITC)-labelled anti-CD41/61, phycoerythrin (PE)-labelled anti-JON/A or PE-labelled anti-P-selectin/CD62P (Emfret Analytics). For expression of other receptors, washed blood was stained with FITC-labelled anti-GPVI (Emfret Analytics) or FITC-labelled anti-CD42b (Emfret Analytics). Flow cytometry data were collected on FACSDiva software (BD), and analysed using FlowJo (Tree Star).
In Vivo Platelet Reduction
Blood was collected for baseline CBC from 4-week-old male mice prior to the experiment. Mice were weighed and injected via the retro-orbital sinus with 2 μg/g sterile antimouse CD42b (Emfret Analytics) in phosphate-buffered saline (PBS). Blood was collected from surviving mice at 7 hours and 3 or 7 days post-depletion for CBC to calculate the reduction of platelet numbers.
Bone Marrow Transplantation followed by Platelet Reduction
Adult male and female Ecad cKO mice underwent BM transplantation as previously described.16 Briefly, 8- to 10-week-old cKO recipients were lethally irradiated with 900 cGy using X-ray radiation, and transplanted with 107 unfractionated BM cells from either GFP-positive (WT) or E-cadherin cKO donors by intravenous injection. After 1 month, blood was collected, and donor engraftment was confirmed by the presence of GFP-positive cells detected by flow cytometry. Recipients were subjected to platelet reduction as described above with anti-mouse CD42b.
Histopathology
Mice were euthanized by terminal exsanguination after CO2 narcosis. Mice were examined for macroscopic and microscopic changes blind to experimental manipulation and genotype (by C.J.B.). Tissues were immersion fixed in 10% neutral-buffered formalin, trimmed, processed, sectioned and stained with haematoxylin and eosin (H&E) by the Mouse Research Pathology Unit, Department of Comparative Medicine, Yale University School of Medicine.
Murine Platelet Preparation
Blood was collected via the retro-orbital sinus into 3.2% sodium citrate (Medicago). Platelet-rich plasma (PRP) was obtained by centrifugation at 200 × g for 8 minutes. Washed platelets were prepared from PRP by washing at 100 × g in 140 mM NaCl, 5 mM KCl, 12 mM Na3C6H5O7, 10 mM dextrose and 12.5 mM sucrose, pH 6.0, and the platelet pellet isolated at 900 × g and re-suspended in Tyrode’s-HEPES (1 mM MgCl2, 5 mM HEPES, 140 mM NaCl, 2.7 mM KCl, 5.5 mM dextrose, 0.42 mM Na2HPO4, 12 mM NaHCO3, pH 7.4) with 2 mM CaCl2 and 0.02 U/mL apyrase (Sigma).
Static Adhesion Assays
To mimic E-cadherin homophilic interactions, wells were coated with recombinant chimeric Fc-E-cadherin (FcEcad; R&D Systems), consisting of an extracellular fragment of E-cadherin peptide fused to human immunoglobulin (Ig) G1 at concentrations indicated. PRP was washed at 100 g in Tyrode’s-HEPES supplemented with 2 mM CaCl2 and 0.5 μM prostaglandin I2 (Calbiochem) and rested. Final platelet concentration was adjusted to 2 × 108/mL. WT platelets were spun onto poly-L-lysine or Fc-E-cadherin-coated coverslips. To test platelet adhesion to fibrinogen, wells were coated with 1% BSA or fibrinogen (100 μg/mL; Sigma) overnight at 4°C in 96-well, flat bottom MaxiSorp plates (Thermo Scientific), then blocked in 1% BSA/PBS. Murine platelets (2 × 108/mL), were allowed to adhere for 30 minutes at 37°C. Non-adherent platelets were carefully removed by pipetting, and the wells washed with Tyrode’s-HEPES supplemented with 2 mM CaCl2. 100 μL p-nitrophenyl phosphate (Sigma) in 50 mM citrate buffer (pH 5.25–5.4) with 0.1% Triton-X, was added and incubated for 1 hour at 37°C. Next, 66 μL 2 N sodium hydroxide was added, and wells analysed at 405 nm (SpectraMax 190 microplate reader with SoftMax Pro 4.6 software, Molecular Devices).
Human Donors
Peripheral blood from consenting, healthy human donors was collected in compliance with a Yale University Investigational Review Board-approved protocol and in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects.
Platelet Aggregation and Adenosine Triphosphate Release Assay
Platelets were re-suspended at a concentration of 1 to 2 × 108/mL in either PRP, or aggregation wash buffer with apyrase as indicated. Platelet aggregation was performed using a Chrono-log Model 700 Whole Blood/Optical Lumi-Aggregometer with AGGRO/LINK software (Chrono-log, Pennsylvania, United States), stirring at 1,200 revolutions per minute. Platelet aggregation was recorded as per cent increase in light transmission after adding agonist. The maximum amplitude was used for recording the aggregation data. For murine platelet aggregation, platelets were resuspended in aggregation wash buffer with apyrase. ADP (Chrono-log), thrombin receptor-activating peptide-6 (TRAP6) (Bachem) and thrombin (Roche) agonists were used at concentrations indicated. For human platelet aggregation, venous blood was drawn from healthy adult volunteers. PRP was prepared from freshly drawn citrated blood by centrifugation at 250 relative centrifugal force for 12 minutes at 25°C. The PRP was incubated at 25°C for 5 minutes with either purified anti-E-cadherin antibody (clone HECD1; Life Technologies) or IgG isotype control antibody (BioLegend) at indicated concentrations. Agonist concentrations were determined by titer of each individual sample and chosen based on sub-maximal aggregation for the respective donor. For platelet adenosine triphosphate (ATP) release measurement, Luciferin-Luciferase reagent (Chrono-Log) was added to human PRP after antibody incubation. Indicated agonist concentration was added, and luminescence signal peaks were recorded according to the manufacturer’s instructions.
Bleeding Time
Bleeding time was assessed as described previously.17 After 12- to 20-week-old male mice were anaesthetized with xylazine (Lloyd Laboratories) with ketamine (Ketaset, Fort Dodge Animal Health), the tip of the tail was transected with a scalpel at a fixed tail diameter (1.5 mm) set using a drill gauge (American Tool Companies Inc.) and immersed in pre-warmed PBS. Primary bleeding time was measured as the time to first stoppage. Total bleeding time was the sum of all bleeding events within the first 25 minutes.
Ferric Chloride-Induced Injury
Experiments were performed as previously described.18 Briefly, the right carotid artery was exposed by blunt dissection, and a 1 × 2 mm piece of Whatman paper soaked in 10% ferric chloride was applied directly to the artery for 2 minutes. The chemical diffuses through the vessel wall and causes endothelial cell denudation, exposing basement membrane components such as collagen and VWF. The patch was then removed, and the artery washed with PBS. Stability of occlusions formed was scored based on the system of Eslin et al,18 with a score of 0 for no occlusion (no clot), 1 for a complete occlusion lasting less than 10 minutes (unstable) and 2 for a complete occlusion lasting greater than 10 minutes (stable).
Cremaster Injury Model
The cremaster injury model was performed as previously described.19 Briefly, the cremaster muscle is prepared and spread as shown on a microscope stage, and imaging is performed to visualize both the injury to the vessel by laser and the accumulation of platelets (labelled with fluorescently conjugated CD41 antibody injected into circulation prior to injury) forming a clot. Additionally, a fluorescently conjugated antibody to fibrin was injected to label and quantify fibrin deposition at the injury site. Intra-vital microscopy was performed as previously described using an Olympus BX61WI upright microscope with a 60 × (0.9 numerical aperture) water immersion objective.19,20 During thrombus formation, brightfield and fluorescent images were captured using a Cooke Sensicam charge-coupled device camera (Cooke) coupled to a DG4 wide-field excitation system. The microscope, camera and DG4 were controlled using the SlideBook 5.5 software (3I Intelligent Imaging Innovations). Anti-mouse CD41 Fab2 fragments (BD Biosciences) conjugated to Alexa-488 according to the manufacturer’s instructions (Life Technologies) and a murine anti-human fibrin antibody (59D8) conjugated to Alexa 647, which cross reacts with murine fibrin,21 were infused intrajugular prior to the first injury. Arterioles of 20 to 40 μm were selected. Vascular injury was induced with a pulsed nitrogen dye laser (SRS NL100, 440 nm) focused on the vessel wall through the microscope objective. Sta-tistical analysis was performed with the Prism 6.0 software (GraphPad Software) using a two-way analysis of variance (ANOVA) between groups, with p-values of < 0.05 considered significant.
Clot Retraction Assay
Murine assays were performed as previously described.22 Briefly, 10 × 107 platelets in PRP were added to 1 mL modified Tyrode’s buffer along with 5 × 106 red blood cells, 2 mM calcium and 2 mg/mL fibrinogen. Clotting was initiated with the addition of 0.01 U/mL of thrombin, and the tube was weighed immediately. A sealed pipette tip was inserted into each tube, and samples were incubated at room temperature with gentle shaking. Images were taken at 1, 10, 20, 30, 45 and 60 minutes, and the tip with the attached clot was removed from the tube and weighed after the final image. Human PRP was prepared as described above. Clot retraction assays were performed as previously described.23 Briefly, platelet clotting was induced by adding 0.025 M CaCl2 to freshly isolated human PRP pre-incubated with either anti-E-cadherin antibody or isotype control antibody at 37°C. Clots were monitored and imaged at 0, 15 and 30 minutes after addition of calcium. The released volume of serum was measured after maximal retraction at 30 minutes. Surface area of clots was measured with ImageJ.
Western Blot
Murine platelets (5 × 108/mL) were directly lysed in Laemmli sample buffer (Bio-Rad), resolved on Bio-Rad precast gradient gels and transferred onto polyvinylidene fluoride membranes. After blocking (5% dried milk in Tris-buffered saline, 0.1% Tween-20), primary antibodies were added: Akt (1:1,000; Cell Signaling), phospho-Akt (Ser473) (1:1,000; Cell Signaling), GSK3β (1:100, Santa Cruz), phospho-GSK3β (Ser9) (1:100, Santa Cruz) and secondary anti-bodies against rabbit and goat IgG conjugated to horseradish peroxidase (1:2,000). Blots were revealed using enhanced chemiluminescence substrate (Bio-Rad). The ChemiDoc imaging system with ImageLab software (Bio-Rad) was used for image acquisition and signal quantification.
Statistical Analysis
Statistical significance was assessed using the Prism 6.0 software (GraphPad Software, La Jolla, California, United States) using the Student’s t-test or two-way ANOVA as appropriate (*p < 0.05).
Results
E-cadherin is Expressed in Megakaryocytes and Platelets, and Validation of E-Cadherin cKO Mice
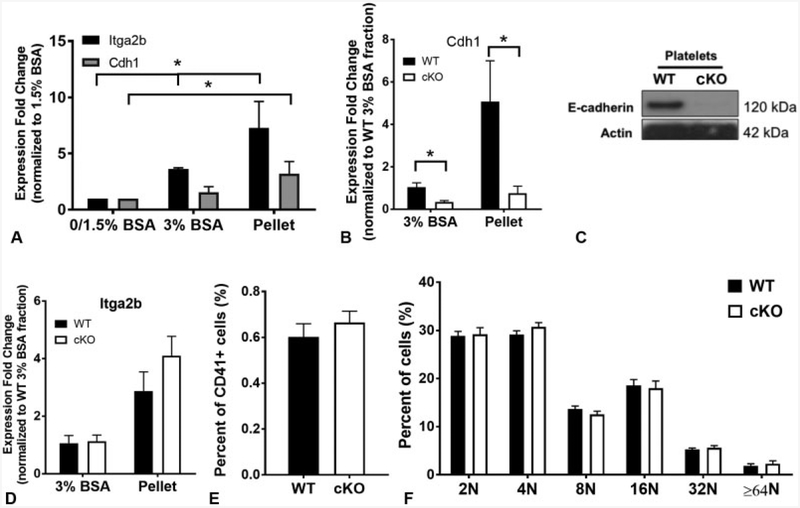
To assess E-cadherin expression in megakaryocytes, mouse foetal liver cells were cultured with mTPO to promote megakaryocyte differentiation.14 CD41-positive megakaryocytes were enriched in the 3% BSA and pellet fractions as expected (►Fig. 1A, p < 0.05). Similarly, E-cadherin messenger RNA was significantly higher in the pellet (most mature cells) fraction (►Fig. 1A, p < 0.05). To test the role of E-cadherin in megakaryopoiesis, we generated a mouse model of megakaryocyte-specific E-cadherin deletion (referred to hereafter as ‘Pf4/E-cadherin KO’ or simply ‘“cKO’ mice) using Pf4-Cre12 mice.11 cKO mice are viable and fertile. RNA analysis by quantitative reverse transcription-polymerase chain reaction on WT and cKO foetal liver-derived megakaryocytes confirmed decreased E-cadherin expression in cKO megakaryocytes (►Fig. 1B, p < 0.05). Western blot on WT and cKO platelets confirmed that E-cadherin is expressedin WT but notcKO platelets (►Fig. 1C). Unfortunately, attempts to determine the sub-cellular localization of E-cadherin in megakaryocytes and platelets by immunostaining were not successful due to non-specific binding of the anti-E-cad-herin antibodies to intracellular structures in fixed cKO and WT megakaryocytes and platelets (data not shown).
Fig. 1.
Epithelial (E)-cadherin is expressed in murine megakaryocytes. (A) Relative messenger ribonucleic acid (mRNA) expression in bovine serum albumin (BSA) gradient sub-fractions following in vitro differentiation of wild-type (WT) foetal liver cells. Itga2b (positive control) expression is significantly higher in the 3% and pellet fractions than the 0/1.5% BSA fraction (p < 0.05), and Cdh1 is significantly higher in the pellet fraction (p < 0.05). (B)Cdh1 mRNAis significantly reduced in differentiated foetal liver cells isolated from E-cadherin conditional knockout (cKO) embryos (p < 0.05). Data are presented as fold change normalized to the WT 3% BSA fraction ± standard deviation (SD) (n = 3). (C) Cropped Western blot of isolated murine platelets from WT and cKO mice demonstrating loss of E-cadherin protein in cKO platelet lysates. The blot was cut at 75 kDa, and the top portion blotted for E-cadherin, the bottom portion blotted for actin. This blot is representative of 5 repeats. (D) mRNA expression of Itga2b in BSA gradient sub-fractions following in vitro differentiation of foetal liver cells isolated from WT and cKO embryos. Data are presented as fold change normalized to the 3% BSA fraction ± SD (n = 3). (E) Flow cytometry on murine bone marrow (BM) of total per cent CD41+ cells, and (F) ploidy of BM-derived megakaryocytes (WT n = 5, cKO n = 4).
Megakaryocyte-Specific Pf4/E-Cadherin cKO Mice Showed no Defects in Megakaryopoiesis or Thrombopoiesis
There were no significant differences in the expression of Itga2b (►Fig. 1D), megakaryocyte numbers (per cent CD41+) (►Fig. 1E) or ploidy (►Fig. 1F) between BM-derived WT and cKO megakaryocytes, suggesting that loss of E-cadherin does not affect megakaryopoiesis.
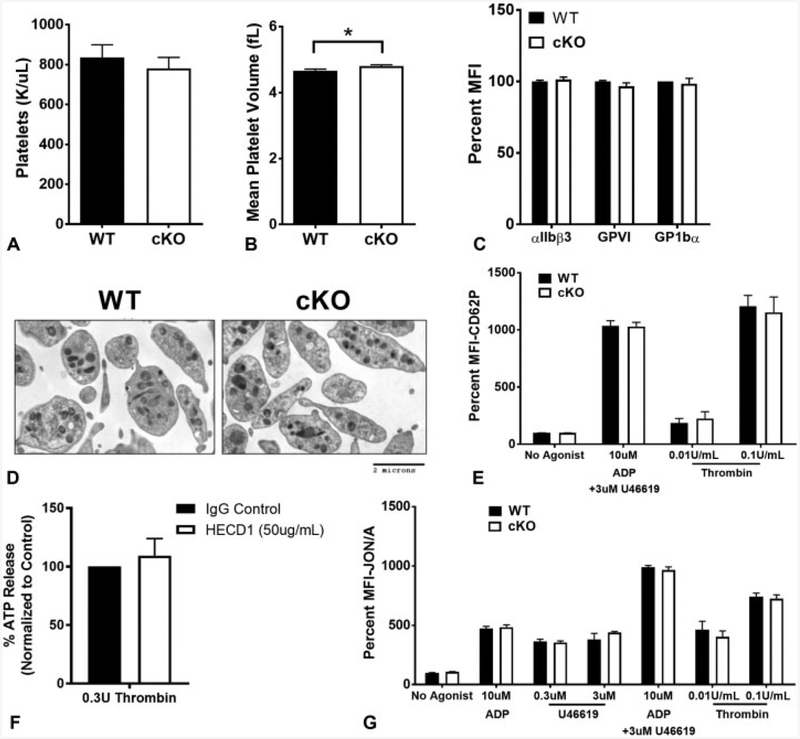
Platelet counts were indistinguishable between WT and cKO animals (►Fig. 2A), although there was a small, statistically significant increase in mean platelet volume in cKO mice (►Fig. 2B, p < 0.05) indicating normal platelet production in the absence of E-cadherin under steady-state conditions. Surface expression of other important platelet receptors, including αIIbβ3, GPVI and GP1bα, was unchanged in cKO platelets (►Fig. 2C).
Fig. 2.
Thrombocytopoiesis and granule secretion are not affected by epithelial (E)-cadherin. (A) Platelet counts in wild-type (WT) (n = 12) and conditional knockout (cKO) (n = 20) animals. Average absolute values ± standard deviation (SD) are shown. (B) Mean platelet volume between WT (n = 12) and cKO (n = 20) animals. There is a small, but significant increase in mean platelet volume in cKO mice (p < 0.05). Average absolute values ± SD are represented. (C) Cell surface expression by flow cytometry of platelet-critical in tegrins and glycoproteins. Data are presented as per cent mean fluorescence intensity (MFI) normalized to WT ± SD(n ≥ 6). (D) Electron microscopy images of resting platelets from WT and cKO mice show no obvious differences in granule number or distribution. (E) Alpha granule release in response to indicated agonists was measured by cell surface expression of P-selectin (CD62P). Data are presented as percent MFI normalized to WT without agonist ± SD (n ≥ 7). (F) Dense granule release in human platelets inhibited with an antibody against E-cadherin (HECD1) in response to thrombin was measured by cell adenosine triphosphate (ATP) release. Data are presented as per cent ATP release normalized to control-treated platelets ± SD(n = 3). (G) Washed platelets were stim ulated with the indicated concentrations of adenosine diphosphate (ADP), U46619 or thrombin and activation assessed via activated αIIbβ3 (JON/A). Data are presented as per cent MFI normalized to WT without agonist ± SD (n ≥ 6).
Platelet Granule Release is Unaffected by E-Cadherin
Basedon electron microscopy, therewere no striking differences observed in platelet morphology between unactivated platelets from WT and cKO mice (►Fig. 2D). Although there was no difference in number or distribution of granules, we also tested if E-cadherin is involved in granule secretion. Alpha granule secretion as measured by P-selectin surface exposure (CD62P) in response to ADP in combination with U46619, and thrombin was indistinguishable between WT and cKO platelets (►Fig. 2E). Dense granule secretion as measured by ATP release in response to thrombin was also tested. Due to technical challenges conducting ATP release in murine platelets, we instead used PRP from three healthy human donors for the assay. We did not observe a significant difference in ATP release between platelets treated with control IgG antibody versus treatment with an E-cadherin inhibitory antibody (►Fig. 2F). Collectively, these data suggest no role for E-cadherin in platelet granule secretion.
E-Cadherin does not Play a Significant Role in αIIbβ3 Activation
Although expression of the αIIbβ3 fibrinogen receptor is normal, we tested if platelet activation was perturbed in the absence of E-cadherin. Activated αIIbβ3 as measured by flow cytometric detection with Jon/A antibody was not significantly different between WT and cKO platelets stimulated by ADP, U46619 (a TxA2 receptor agonist) or thrombin (►Fig. 2G).
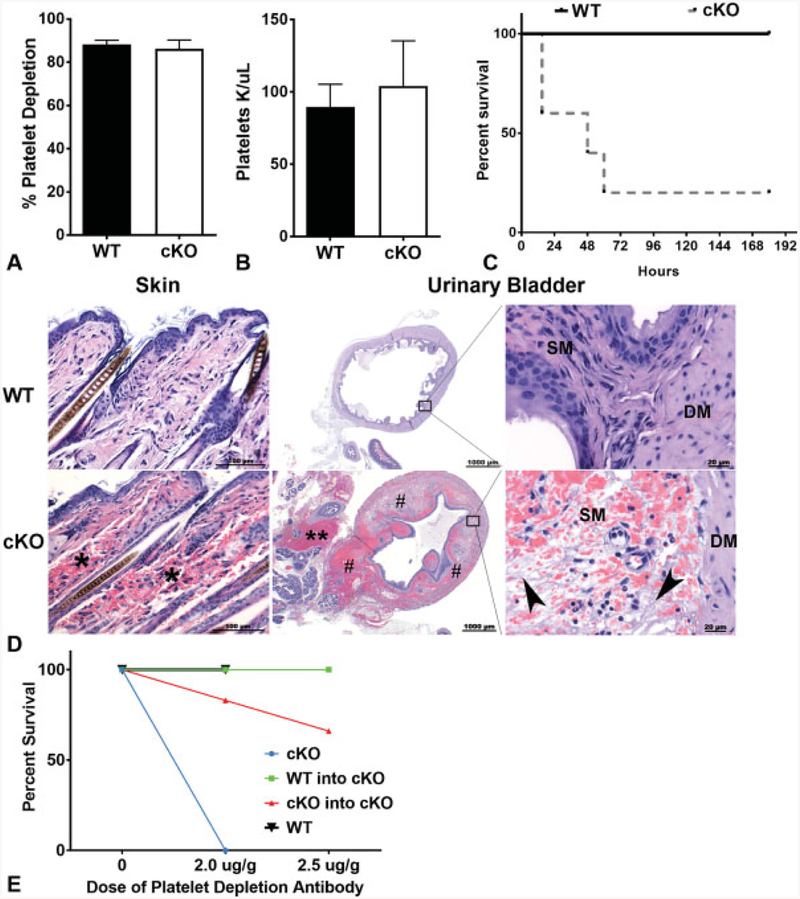
Acute Thrombocytopenia Causes Haemorrhage and Death in cKO, but not WT Mice
Despite a lack of a phenotype in cKO mice during steady-state haemostasis, we challenged the mice by inducing acute thrombocytopenia to assess for differences in the response of E-cadherin null mice to thrombocytopenia. Transient thrombocytopenia was induced by systemic administration of anti-CD42b antibody with comparable levels of depletion between cKO and WT mice as measured by both per cent platelet depletion (►Fig. 3A) and absolute number of platelets post-depletion (►Fig. 3B). However, the majority of cKO mice died within 50 hours of antibody administration, whereas all WT mice survived (►Fig. 3C). Necropsy analysis performed on additional mice sacrificed just 7 hours post-antibody treatment revealed regions of severe acute multifocal haemorrhage upon gross and histological examination predominantly in the cKO animals. These regions included: dermis, epididymis, gastric lamina propria and in the tissues adjacent to and within the urinary bladder, specifically within the submucosa and lamina propria (►Fig. 3D). Gross necropsy and microscopic analyses at later time points (36–48 hours) revealed similar observations, with haemorrhage becoming more pronounced in cKO animals. Although some WT animals had evidence of bleeding, especially at later time points, the frequency and severity were diminished relative to cKO animals (►Table 1). These findings suggest that the cause of death of cKO mice treated with anti-CD42b may have been hypovolemic shock caused by haemorrhage.
Fig. 3.
Epithelial (E)-cadherin conditional knockout (cKO) animals are sensitive to bleeding and death in response to immune-mediated thrombocytopenia. (A) Percent platelet depletion in wild-type (WT) and cKO mice after 6 to 10 hours of treatment with anti-CD42b (2 mg/g) (n ≥ 5). Data are presented as mean ± standard deviation (SD). (B) Absolute number of platelets remaining 6 to 10 hours after depletion with anti-CD42b (2 mg/g) between WT and cKO mice (n ≥ 5). Data are presented as mean ± SD. (C) Survival of animals following platelet depletion (n ≥ 5, p < 0.01). (D) Representative photomicrographs of haematoxylin and eosin (H&E)-stained sections of skin and urinary bladder (low and high magnification) in WT and cKO mice 7 hours post-antibody treatment. There is multi-focal marked dermal haemorrhage (*), massive haemorrhage within the adventitia (**) adjacent to the urinary bladder and marked and multi-focal haemorrhage (#) within the urinary bladder submucosa and detrusor muscle in cKO mice compared with WT mice. Higher power images of the boarder of the submucosa (SM) and detrusor muscle (DM) reveals there is fibrin (pale fibrillary material, arrow heads), haemorrhage and oedema (clear spaces) within submucosa of cKO mice compared with WT mice. (E) Survival of animals following platelet depletion with increasing doses of anti-CD42b in transplanted and untransplanted animals. cKO: untransplanted cKO mice (n = 3); WT: untransplanted WT mice (n = 3); WT-into-cKO: cKO mice transplanted with WT bone marrow (n = 5); cKO-into-cKO: cKO mice transplanted with cKO bone marrow (n = 6).
Table 1.
Frequency of histological haemorrhage in WT and cKO animals following anti-CD42b treatment
| Skin | Bladder | Brain, Skull | ||
|---|---|---|---|---|
| Early | WT | 0/3 | 0/3 | 1/3 |
| cKO | 1/3 | 1/3 | 3/3 | |
| Late | WT | 1/2 | 1/2 | 1/2 |
| cKO | 2/3 | 2/3 | 3/3 | |
Abbreviations: cKO, conditional knockout; H&E, haematoxylin and eosin; WT, wild-type.
Note: The number of animals displaying evidence of haemorrhage in skin, bladder and brain/skull by H&E is indicated for both the early (6–10 hour) and late (36–48 hour) time point necropsies.
To confirm that haemorrhage and death in cKO mice challenged with platelet depletion was not due to off-target effects of the Pf4 transgene or loss of E-cadherin expression from cells other than megakaryocytes and platelets, cKO mice underwent transplantation with WT (n = 5) or cKO (n = 6) BM, hereafter referred to as WT-into-cKO and cKO-into-cKO, respectively. One month post-transplant, we confirmed WT reconstitution of platelets in the WT-into-cKO by detecting GFP+ platelets in the circulation. In contrast to untransplanted cKO mice, only one cKO-into-cKO recipient died within 48 hours after antibody injection despite achieving similar levels of platelet depletion as untransplanted mice (►Fig. 3E). The remaining 5 WT-into-cKO recipients survived, and platelet levels returned to baseline in all surviving mice at the same rate within 7 days. We repeated the experiment on the surviving animals with a higher dose (2.5 ug/g) of anti-CD42b antibody. A second cKO-into-cKO recipient died within 48 hours of the injection (►Fig. 3E), while none of the WT-into-cKO recipients died. Thus, transplantation of WT BM into cKO mice decreases mortality due to acute thrombocytopenia. These findings confirm that, in the context of acute thrombocytopenia, platelet-specific KO of E-cadherin results in compromised platelet function leading to increased mortality.
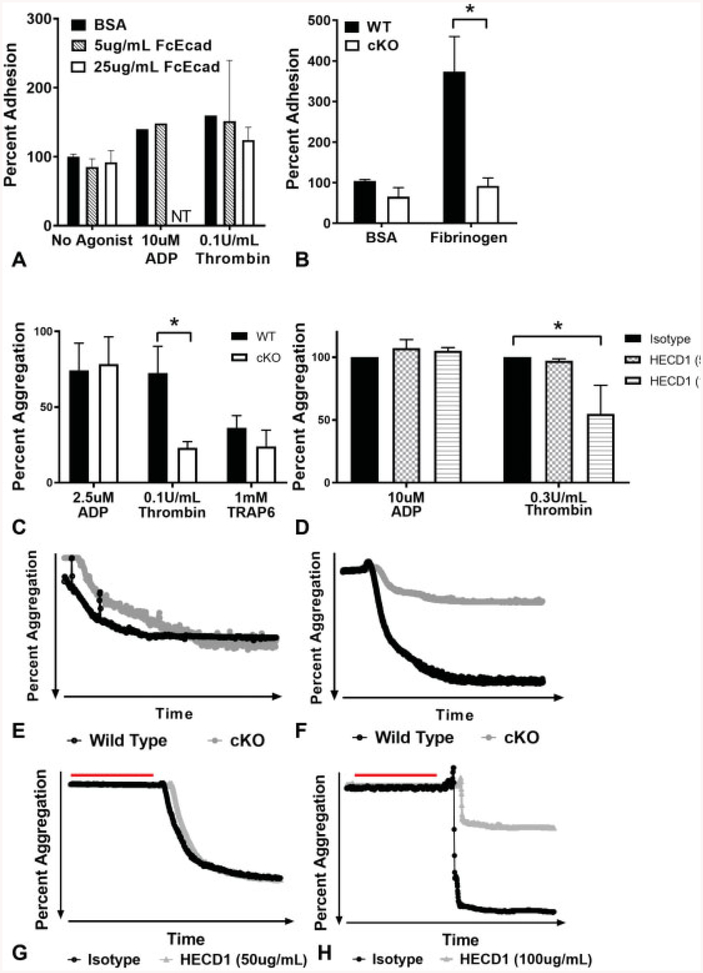
E-Cadherin Plays a Role in Fibrinogen Binding
Having established an important role for E-cadherin in platelet function and given the role of E-cadherin in intercellular adhesion in other tissues, we tested if activated platelets show adhesion to immobilized E-cadherin. However, static adhesion assays of WT platelets revealed no significant difference in adhesion to coverslips coated with increasing concentrations of E-cadherin compared with BSA controls regardless of agonist tested (►Fig. 4A). Because activated WT platelets bind to fibrinogen via αIIbβ3, and fibrinogen bridges mediate platelet–platelet interactions essential for clot formation, we compared the ability of WT and cKO platelets to adhere to fibrinogen. Platelets lacking E-cadherin showed a statistically significant decrease in adhesion to fibrinogen compared with WT platelets (►Fig. 4B, p < 0.05).
Fig. 4.
Fibrinogen binding is affected, and aggregation is impaired in the absence of epithelial (E)-cadherin. (A) Per cent adhesion of agonisttreated washed wild-type (WT) platelets to Fc-Ecad-coated wells. Data are presented as mean per cent adhesion normalized to bovine serum albumin (BSA) controls without agonist ± standard deviation (SD) (error bars indicate n = 3, lack of error bars indicates n = 1). (B) Per cent adhesion of washed WT or conditional knockout (cKO) platelets to BSA or fibrinogen. Data are presented as mean percent adhesion normalized to BSA controls ± SD (n ≥ 6, p < 0.05). (C) Per cent aggregation of washed platelets from WT and cKO mice treated with indicated agonists. Data are presented as mean per cent aggregation ± SD (n = 3, p < 0.05). (D) Per cent aggregation of human platelet-rich plasma treated with indicated agonists after incubation with control or anti-E-cadherin antibodies. (E) Representative aggregation trace of washed platelets from WT and cKO mice treated with adenosine diphosphate (ADP). (F) Representative aggregation trace of washed platelets from WT and cKO mice treated with thrombin. (G) Representative aggregation trace of human platelet-rich plasma treated with ADP after incubation with control or E-cadherin antibodies (red line). (H) Representative aggregation trace of human platelet-rich plasma treated with thrombin after incubation with control or E-cadherin antibodies (red line). Data are presented as mean per cent aggregation ± SD normalized to aggregation for the isotype control for each human donor (n = 3, p < 0.05). NT, not tested.
E-Cadherin cKO Platelets have an Aggregation Defect that can be Recapitulated In Vitro with Human Platelets
Given that E-cadherin contributes to intercellular adhesions in other cell types and platelet–fibrinogen binding, and CDH6 plays aroleinplatelet aggregation,7 we investigated aggregation in E-cadherin null platelets. The response ofcKO platelets to ADP (2.5 μM) was indistinguishable from WT platelets (►Fig. 4C, representative trace in Fig. 4E). However, a statistically significant twofold reduction in aggregation of cKO versus WT platelets in response to thrombin (0.1 U/mL) was observed (►Fig. 4C, p < 0.05, representative trace in Fig. 4F). Increasing doses of thrombin (0.4 and 1 U/mL) were also tested, and the highest dose of thrombin was able to overcome the aggregation defect in E-cad KO platelets (data not shown). Furthermore, this trend was also observed in platelets activated with TRAP6 (►Fig. 4C).
To extend analysis of the role of E-cadherin in platelets to the human context, we assessed the effects of an inhibitory anti-E-cadherin antibody on human platelet aggregation, analogous to previous studies exploring CDH6 function.7 Human PRP was incubated with an isotype negative control or the inhibitory E-cadherin antibody (clone HECD1) prior to agonist addition. Due to variations between healthy human donors, each agonist was first titered and then used at the lowest concentration that induced aggregation for each donor. Aggregation in the presence of the isotype control antibody for each donor was set as 100%. Similar to murine cKO platelets, human platelet aggregation in response to thrombin was significantly reduced by inhibitory E-cadherin antibody in a dose-dependent manner (►Fig. 4D and H, representative trace, p > 0.05). In contrast but consistent with murine cKO platelets, human platelet aggregation in response to ADP was not reduced by HECD1 (►Fig. 4D and G).
In Vivo Clot Retraction is Impaired in E-Cadherin cKO Mice
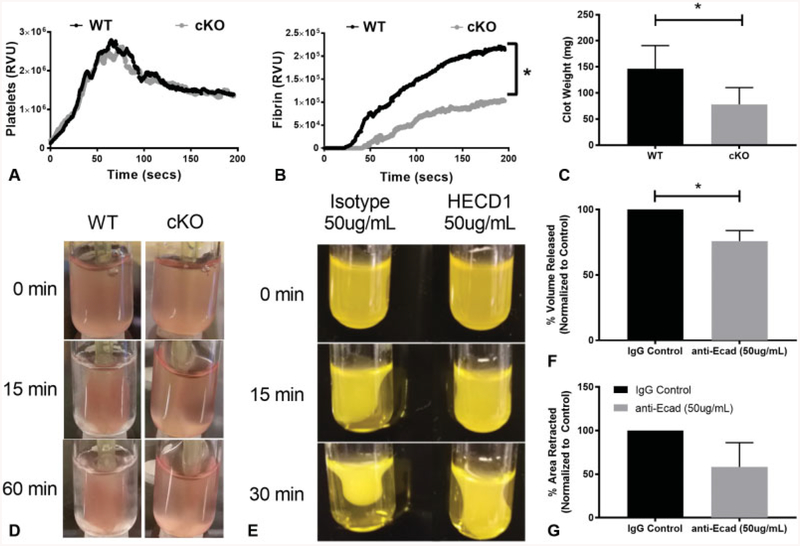
In light of impaired fibrinogen binding and aggregation in platelets lacking E-cadherin, we assayed platelet function in vivo by utilizing the cremaster laser injury model. Platelet recruitment and adhesion to the site of injury was comparable between WT and cKO mice (►Fig. 5A). However, the deposition of fibrin was significantly reduced in cKO mice (►Fig. 5B, p < 0.05), with a potential delay in initial fibrin deposition.
Fig.5.
Fibrin deposition is decreased, and clot retraction is impaired in the absence of epithelial (E)-cadherin. (A) The median relative value units (RVUs) representing platelet recruitment, and (B) fibrin deposition following laser-induced injury of the cremaster arterioles (30 injuries across n = 4 animals/genotype, p < 0.05). (C) Murine clot weight after 60 minutes of clot retraction. Data are presented as mean ± standard deviation (SD) (n > 6, p < 0.05). (D) Representative images of clots at 0, 15 and 60 minutes after incubation of wild-type (WT) and conditional knockout (cKO) platelets with fibrinogen, calcium and thrombin. (E) Representative images of clots formed by human platelet-rich plasma treated with control or anti-E-cadherin antibodies in high calcium at 0, 15 and 30 minutes after incubation. (F) Extruded plasma volume of human platelet-rich plasma treated with control or anti-E-cadherin antibodies after incubation in high calcium. Data are presented as per cent of extruded serum volume normalized to the extruded serum volume in control samples at 30 minutes ± SD (n = 3). (G) Human clot retraction measured by surface area of the clot calculated by ImageJ at 0 and 30 minutes. Data are presented as per cent of retracted clot area normalized to the area measured in control samples at 30 minutes (n = 3, p < 0.05).
Decreased fibrin deposition at the site of injury in the cremaster model lead us to test the hypothesis that clot retraction would also be impeded in cKO mice. We initiated clot retraction by exposure of murine platelets to a high concentration of calcium and fibrinogen, as described previously.22 To quantify changes in clot retraction rate and potency, platelet clots were imaged sequentially for 60 minutes (►Fig. 5D), and then weighed. We found a significant reduction in the mass of clots formed by cKO platelets compared with WT platelets (►Fig. 5C, p < 0.05). We confirmed the clot retraction defect in human samples (►Fig. 5E). There was a significant reduction in clot retraction in E-cadherin-inhibited samples as measured by volume of residual plasma remaining after 30 minutes of retraction (►Fig. 5F, p < 0.05), and a similar decreasing trend in the per cent area of the clot calculated by ImageJ (►Fig. 5G) implicating E-cadherin in clot retraction as well as platelet aggregation.
E-Cadherin cKO Animals have Impaired Haemostasis
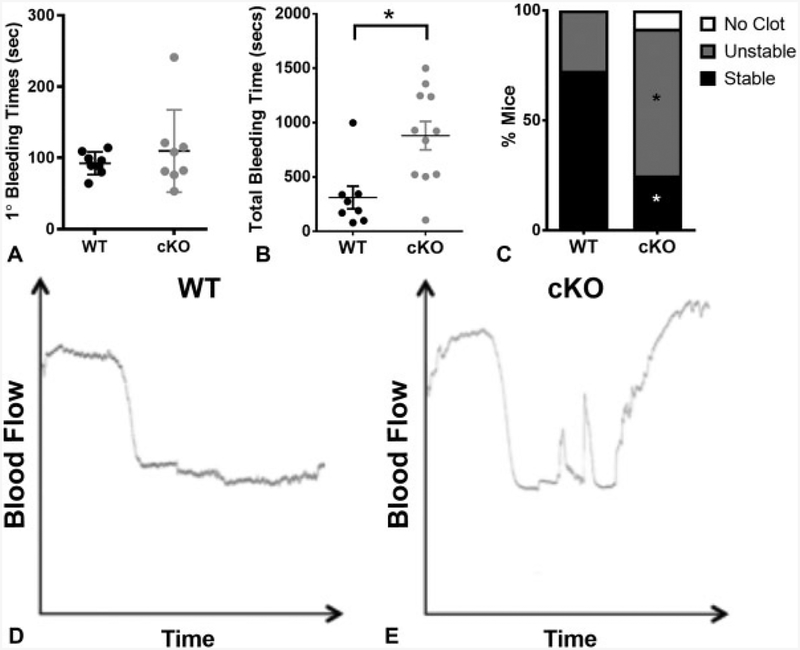
Based on our findings that E-cadherin is important for thrombin-mediated platelet aggregation and clot retraction, we assessed the haemostatic potential of platelets from cKO and WT mice in vivo by tail bleeding. Although the primary bleeding time as assessed by the time to first stoppage in bleeding was not significantly different between genotypes (►Fig. 6A), total bleeding times were significantly prolonged in cKO compared with WT controls (►Fig. 6B, p < 0.05) due to re-bleeding, which suggested decreased clot stability.
Fig. 6.
Epithelial (E)-cadherin conditional knockout (cKO) animals have impaired haemostatic potential. (A) Primary tail bleeding time in wild-type (WT) and cKO mice (n ≥ 7). (B) Total tail bleeding time in WT and cKO animals (n ≥ 8, p < 0.01). (C) The per cent of animals displaying specific clot behaviours (no clot, unstable or stable), assigned based on the duration of vessel occlusion in the ferric chloride carotid injury model (n = 12, p < 0.05). Representative traces of vessel occlusion after ferric chloride carotid injury in WT (D) and cKO (E) mice.
Next, we utilized the ferric chloride carotid injury model to further investigate clot behaviour in vivo based on vessel occlusion. Blood flow was monitored at the site of injury, and time to complete occlusive thrombus was recorded as well as the stability of the clot. All WT (11 of 11) and the majority of cKO (11 of12) animals formed initial platelet clots in response to the injury (representative traces, ►Fig. 6D and E). However, the clots in cKO animals were less stable than in WT, as indicated by restored blood flow (►Fig. 6E). Using a system developed by Eslin et al18 that correlates behaviour of the trace to the duration of time that the clot occludes the vessel, we categorized the response to injury as no clot, unstable or stable clot. In cKO mice, 9 of 11 formed either no clot or an unstable clot, versus just 3 of 11 WT animals (►Fig. 6C, p < 0.05). Together, the data from these in vivo assays provide evidence that E-cadherin contributes to the haemostatic potential of platelets.
Akt/GSK3β Signalling in Response to Thrombin is Attenuated in the Absence of E-Cadherin
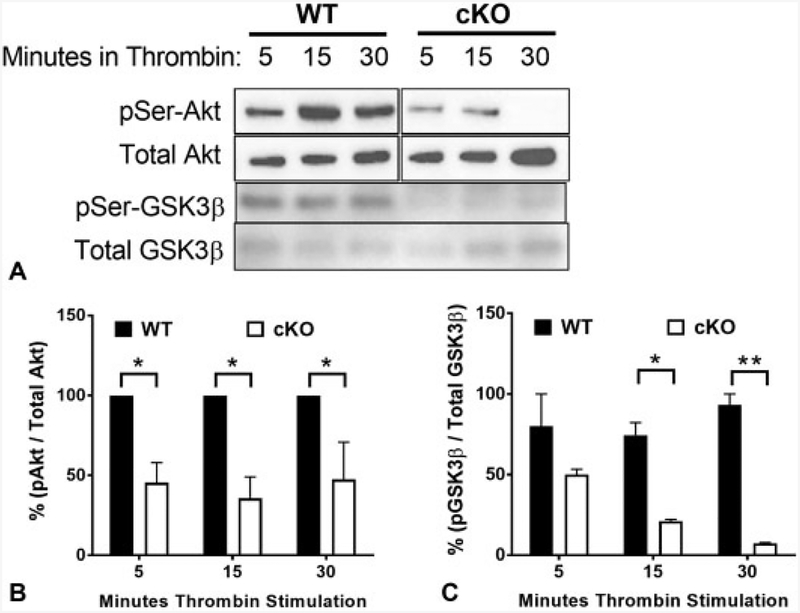
Given the defects in cKO platelet aggregation and clot retraction, and the fact that mice lacking expression of all three Akt isoforms also have defective platelet function,24–26 we investigated thrombin-induced Akt activation in murine platelets. In WT platelets, thrombin-induced Ser473 Akt phosphorylation occurs within 5 minutes of agonist addition, with a maximal signal at 15 minutes (►Fig. 7A). In cKO platelets, Ser473 phosphorylation was significantly diminished at all three time points, implying attenuation of the Akt signalling pathway (►Fig. 7B, p < 0.05). Given that GSK3β is a major effector of Akt signalling downstream of αIIbβ3, and plays an important role in clot retraction,27 we also measured levels of Ser9 GSK3β phosphorylation in response to thrombin. Similar to Akt, we find impaired phospho-GSK3β in response to thrombin at the same time points (►Fig. 7C, p < 0.05). Diminished Akt/GSK3β signalling could be, at least in part, responsible for the reduced aggregation and clot retraction observed in E-cadherin-deficient platelets. These observations together with our in vitro and in vivo data strongly suggest an important role for E-cadherin in platelet aggregation and clot stability.
Fig.7.
Attenuated thrombin-induced Akt and glycogen synthase kinase 3β (GSK3β) phosphorylation in epithelial (E)-cadherin conditional knockout (cKO) platelets. (A) Isolated platelets from wild-type (WT) and cKO mice were stimulated with 0.1 U/mL thrombin for 5, 1 5 and 30 minutes. Western blots of platelet lysates were performed with antibodies against phospho-Akt, total Akt, phospho-GSK3β and total GSK3β. Representative blots shown. (B) Data are presented as mean ratio of phospho-Akt/total Akt determined by densitometry normalized to WT ± standard deviation (SD) (n = 3, p < 0.05). (C) Data are presented as mean ± SD ratio of phospho-GSK3β/total GSK3β determined by densitometry (n = 3, p < 0.05).
Discussion
This study confirms the presence of E-cadherin protein in megakaryocytes and platelets and reveals a function for E-cadherin in haemostasis in vivo. We have demonstrated that although E-cadherin is not required for normal megakaryopoiesis, thrombopoiesis or maintenance of normal blood flow, it is required for haemostasis following acute thrombocytopenia or injury. The haemorrhage and death observed in cKO animals following platelet depletion appear to be a direct consequence of the decreased platelet numbers in the cKO animals. However, published data both in mouse models and human patients suggest that low platelet counts alone do not cause extensive bleeding. Morowski et al elegantly demonstrated in WT mice that platelet haemostatic functions are preserved at low platelet counts, with platelet depletion to levels ≤ 97.5% having no effect on either tail bleeding times, or the development of occlusive thrombi in small arterioles.28 They postulated that loss of normal haemostatic function with thrombocytopenia is more likely related to platelet function than platelet numbers.28 Platelet dysfunction in the absence of E-cadherin is revealed by a prolonged bleeding time, and is exacerbated when the platelet number is decreased. However, unchallenged cKO mice have no evidence of spontaneous bleeding. The regions of haemorrhage following induced thrombocytopenia tended to be in tissues with muscular movement and tension, which is consistent with data from the ferric chloride and cremaster injury models, where E-cadherin null platelets displayed impaired haemostatic function.
To confirm that the platelet-specific deletion of E-cadherin was responsible for haemorrhage after induced acute thrombocytopenia, we performed BM transplantation from WT donors into cKO recipients, as well as cKO donors into cKO recipients. While we did confirm that WT-derived platelets rescued the lethal haemorrhaging observed in thrombocytopenic cKO mice after platelet depletion, we were initially surprised to find that overall transplanted animals were more resistant to haemorrhage and death compared with non-transplanted mice. This may be due to impacts of lethal irradiation prior to BM transplantation on coagulation. For example, fibrinogen plasma levels are significantly elevated in direct proportion to the radiation dose and remain elevated for at least 1 week in mice after irradiation.29 One possible explanation for the increased survival of transplanted animals upon acute thrombocytopenia could be that plasma fibrinogen levels remain elevated even after 1 month of total body irradiation, which could potentially overcome the haemostatic defect in platelets lacking E-cadherin; however, this was not measured in our study.
E-cadherin was not required for normal platelet activation (as measured by Jon/A binding), or granule release (as measured by platelet surface expression of P-selectin, or ATP release) in response to multiple agonists. However, the results from the cremaster laser injury model show decreased deposition of fibrin at the site of injury in cKO mice, and our in vitro assays demonstrate that E-cadherin plays a role in fibrinogen binding and thrombin-mediated platelet aggregation. These findings are highly reminiscent of the established role of CDH6 in platelet aggregation.7 CDH6 acts as a ligand for αIIbβ3 and can drive fibrinogen-independent platelet aggregation. E-cad-herin may act by a similar mechanism. It is interesting that, in E-cadherin-deficient platelets, only thrombin-mediated aggregation was affected, but not ADP. Due to the lack of a good antibody for performing surface membrane E-cad expression by fluorescence-activated cell sorting, we were not able to assess whether stimulating platelets with strong agonists may increase expression of E-cadherin at the platelet surface, which could contribute to aggregation.
Our aggregation results implicate E-cadherin in αIIbβ3 signalling since ADP induces aggregation through activation of G-protein-coupled receptors,30–32 whereas thrombin can activate signalling events downstream of αIIbβ3 including Akt and GSK3β,33 which in turn regulate late-stage thrombus formation including aggregation and clot retraction.33,34 Although the second wave of platelet aggregation in response to ADP may require thrombin generation and subsequent protease-activated receptor signaling,3,35 E-cadherin-deficient platelets aggregate normally in response to ADP stimulation while showing a defect in response to thrombin. This is similar to findings in mice with KO of three Akt isoforms, which have impaired responses to thrombin, but not ADP.24–26 Furthermore, GSK3β is a major effector of Akt signalling downstream of αIIbβ3 in later stages of platelet aggregation and clot retraction, leading to stable clot formation.27 We found decreased Akt/GSK3β signalling (phospho-Ser473 Akt and phosphor-Ser9 GSK3β) in response to thrombin in cKO platelets; however, we did not detect defects in α or dense granule secretion. Given the known role for Akt in stable clot formation, we propose that lack of E-cadherin in platelets decreases Akt signalling in response to thrombin stimulation, causing clot instability via attenuated GSK3β activity. Further-more, regulation of the avidity (but not expression) of αIIbβ3 by PI3K/Akt signalling plays a critical role in regulating thrombin-stimulated fibrin clot retraction.2 Our study demonstrates that αIIbβ3 surface expression is not perturbed in E-cadherin cKO platelets, but adhesion to fibrinogen, aggregation in response to thrombin and TRAP6 and fibrin accumulation are impaired, and total bleeding times are extended in cKO platelets compared with WT. Together, these findings support the hypothesis that E-cadherin plays a role in clot stability downstream of PI3K/Akt/GSK3β, possibly by affecting the avidity of αIIbβ3, which could decrease the fibrinogen binding, and result in weaker platelet–platelet interactions.
In line with this hypothesis, our results show that E-cadherin is important for clot retraction and stable thrombus formation in the ferric chloride injury model. Alternative hypotheses that could explain E-cadherin’s role in stable clot formation could include sequestering thrombin at the platelet surface in close proximity to αIIbβ3, or its involvement in fibrinolysis through interactions with α2-antiplasmin, thrombin-activatable fibrinolysis inhibitor and plasminogen activator inhibitor-2 to fibrin. However, we could not detect these interactions due to a lack of specific E-cadherin antibodies. Contact-dependent signalling is a late event in haemostasis that regulates the growth and stability of thrombi, while preventing premature disaggregation. Receptors such as ephrins, integrins (specifically αIIbβ3) and cadherins contribute to contact-dependent signalling in platelets and promote thrombin-induced aggregation and clot retraction.4,7,36 The data from our cKO model suggest a previously unappreciated role of E-cadherin-mediated contact-dependent signalling in platelet aggregation and clot stability.
The ability to recapitulate the agonist-specific aggregation and clot retraction defect observed in E-cadherin cKO murine platelets and human platelets with anti-E-cadherin antibody-mediated inhibition of aggregation in vitro suggests that there are similar functions of E-cadherin in human platelets. Perhaps, E-cadherin could be a target for therapeutic anticoagulation. Presently, mutations in E-cadherin are associated with the development of carcinomas, with germline inactivating mutations described in familial gastric cancer.37 Considering our findings, we propose extending such studies to assess associations with bleeding tendencies and cardiovascular events in these individuals.
In conclusion, our data have identified a novel player in platelet biology. E-cadherin expression in platelets has a functional role that is likely linked to its ability to mediate interaction between platelets and extracellular factors, such as fibrinogen, that influence the ability to form and stabilize platelet clots.
What is known about this topic?
Cadherins are known to play a role in cell–cell junctions and adherence.
E-cadherin is expressed in platelets.
Platelet function requires appropriate adhesion, activation, spreading, aggregation and retraction to form stable clots to maintain haemostasis.
What does this paper add?
Loss of E-cad does not perturb megakaryopoiesis.
E-cad knockout platelets exhibit platelet dysfunction, specifically in aggregation and clot stability.
PI3K/Akt/GSK3β signalling is attenuated in platelets lacking E-cad.
Acknowledgement
The authors thank Stephanie Halene and Huiyan Jin for helpful discussions.
Funding
These studies were supported by NIH R01 DK094934, the Yale NIDDK-funded Cooperative Center of Excellence in Hematology (U54 DK106857), R01 DK086267, T32HL00 7974 and R01 HL121323.
Footnotes
Conflict of Interest
None declared.
References
- 1.Signaling Du X. and regulation of the platelet glycoprotein Ib-IX-V complex. Curr Opin Hematol 2007;14(03):262–269 [DOI] [PubMed] [Google Scholar]
- 2.Schoenwaelder SM, Ono A, Nesbitt WS, Lim J, Jarman K, Jackson SP. Phosphoinositide 3-kinase p110 beta regulates integrin alpha IIb beta 3 avidity and the cellular transmission of contractile forces. J Biol Chem 2010;285(04):2886–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Reheman A, Chen P, et al. Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo. J Thromb Haemost 2006;4(10):2230–2237 [DOI] [PubMed] [Google Scholar]
- 4.Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood 2011; 118(23):5996–6005 [DOI] [PubMed] [Google Scholar]
- 5.Ni H, Denis CV, Subbarao S, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest 2000;106(03):385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reheman A, Yang H, Zhu G, et al. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood 2009;113 (08):1809–1817 [DOI] [PubMed] [Google Scholar]
- 7.Dunne E, Spring CM, Reheman A, et al. Cadherin 6 has a functional role in platelet aggregation and thrombus formation. Arterioscler Thromb Vasc Biol 2012;32(07):1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elrod JW, Park JH, Oshima T, Sharp CD, Minagar A, Alexander JS. Expression of junctional proteins in human platelets. Platelets 2003;14(04):247–251 [DOI] [PubMed] [Google Scholar]
- 9.Fong KP, Barry C, Tran AN, et al. Deciphering the human platelet sheddome. Blood 2011;117(01):e15–e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steele BM, Harper MT, Macaulay IC, et al. Canonical Wnt signaling negatively regulates platelet function. Proc Natl Acad Sci U S A 2009;106(47):19836–19841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A 1994;91(17):8263–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 2007;109(04):1503–1506 [DOI] [PubMed] [Google Scholar]
- 13.Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 2002;115(1–2):53–62 [DOI] [PubMed] [Google Scholar]
- 14.Cheng EC, Luo Q, Bruscia EM, et al. Role for MKL1 in megakaryocytic maturation. Blood 2009;113(12):2826–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith EC, Thon JN, Devine MT, et al. MKL1 and MKL2 play redundant and crucial roles in megakaryocyte maturation and platelet formation. Blood 2012;120(11):2317–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharkis SJ, Cahill R, Ahmed A, Jedrzejczak WW, Sell KW. Genetic requirements for bone marrow transplantation for stem-cell-defective W/Wv mice. Transplant Proc 1979;11(01):511–516 [PubMed] [Google Scholar]
- 17.Mahooti S, Graesser D, Patil S, et al. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol 2000;157(01):75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eslin DE, Zhang C, Samuels KJ, et al. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood 2004;104(10):3173–3180 [DOI] [PubMed] [Google Scholar]
- 19.Neyman M, Gewirtz J, Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood 2008;112(04):1101–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med 2002;8(10):1175–1181 [DOI] [PubMed] [Google Scholar]
- 21.Weiler-Guettler H, Christie PD, Beeler DL, et al. A targeted point mutation in thrombomodulin generates viable mice with a pre-thrombotic state. J Clin Invest 1998;101(09):1983–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchtmann K, Park ER, Bergsma A, Segula J, Edick MJ, Miranti CK. Homozygous loss of mouse tetraspanin CD82 enhances integrin αIIbβ3 expression and clot retraction in platelets. Exp Cell Res 2015;339(02):261–269 [DOI] [PubMed] [Google Scholar]
- 23.Tyagi T, Ahmad S, Gupta N, et al. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced pro-thrombotic phenotype. Blood 2014;123(08):1250–1260 [DOI] [PubMed] [Google Scholar]
- 24.Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood 2004;104(06):1703–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest 2004;113(03): 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien KA, Stojanovic-Terpo A, Hay N, Du X. An important role for Akt3 in platelet activation and thrombosis. Blood 2011;118(15): 4215–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Zhang Y, Wang Y, et al. PDK1 regulates platelet activation and arterial thrombosis. Blood 2013;121(18):3718–3726 [DOI] [PubMed] [Google Scholar]
- 28.Morowski M, Vögtle T, Kraft P, Kleinschnitz C, Stoll G, Nieswandt B. Only severe thrombocytopenia results in bleeding and defective thrombus formation in mice. Blood 2013;121(24):4938–4947 [DOI] [PubMed] [Google Scholar]
- 29.Sproull M, Kramp T, Tandle A, Shankavaram U, Camphausen K. Serum amyloid A as a biomarker for radiation exposure. Radiat Res 2015;184(01):14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem 1998;273(04):2030–2034 [DOI] [PubMed] [Google Scholar]
- 31.Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001;409(6817):202–207 [DOI] [PubMed] [Google Scholar]
- 32.Zhang FL, Luo L, Gustafson E, et al. ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem 2001;276 (11):8608–8615 [DOI] [PubMed] [Google Scholar]
- 33.Moore SF, van den Bosch MT, Hunter RW, Sakamoto K, Poole AW, Hers I. Dual regulation of glycogen synthase kinase 3 (GSK3)α/β by protein kinase C (PKC)α and Akt promotes thrombin-mediated integrin αIIbβ3 activation and granule secretion in platelets. J Biol Chem 2013;288(06):3918–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent PA, Séverin S, Hechler B, Vanhaesebroeck B, Payrastre B, Gratacap MP. Platelet PI3Kβ and GSK3 regulate thrombus stability at a high shear rate. Blood 2015;125(05):881–888 [DOI] [PubMed] [Google Scholar]
- 35.Jiang L, Xu C, Yu S, et al. A critical role of thrombin/PAR-1 in ADP-induced platelet secretion and the second wave of aggregation. J Thromb Haemost 2013;11(05):930–940 [DOI] [PubMed] [Google Scholar]
- 36.Prevost N, Woulfe D, Tognolini M, Brass LF. Contact-dependent signaling during the late events of platelet activation. J Thromb Haemost 2003;1(07):1613–1627 [DOI] [PubMed] [Google Scholar]
- 37.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998;392 (6674):402–405 [DOI] [PubMed] [Google Scholar]