Abstract
Background and purposes:
Hematoma location within the cerebellum may help identify the dominant small vessel disease (SVD) type (cerebral amyloid angiopathy [CAA] vs. non-amyloid SVD). However, it is unknown whether this holds true for cerebellar microbleeds (CMBs). We tested the hypothesis that cerebellar CMBs restricted to the cortex and vermis (defined as superficial regions) are associated with clinically diagnosed and pathology-verified CAA.
Methods:
Three hundred and seven consecutive spontaneous intracerebral hemorrhage (ICH) patients with a baseline MRI that included susceptibility-weighted imaging or angiography were enrolled. Using a topographical template, cerebellar CMBs patterns were defined as strictly superficial vs. deep (cerebellar grey nuclei and white matter) or mixed (both regions involved). Thirty-six ICH patients with cerebellar CMBs and neuropathology data available were evaluated for presence of CAA.
Results:
One hundred and thirty-five (44%) ICH patients had CMBs in the cerebellum. In the patient group with cerebellar CMBs, 85 (63%) showed a superficial pattern and 50 (37%) had a deep/mixed pattern. Strictly superficial cerebellar CMBs were independently associated with a supratentorial pattern of probable CAA-ICH according to the Boston criteria (OR: 1.6, CI: 1.03–2.5) and deep/mixed cerebellar CMBs with a pattern of deep/mixed-ICH (OR: 1.8, CI: 1.2–2.7). Pathologically-verified CAA was present in 23/24 (96%) of patients with superficial cerebellar CMBs vs. 3/12 (25%) of patients with deep/mixed cerebellar CMBs (p <0.001).
Conclusion:
In ICH patients, cerebellar CMBs are relatively common and often restricted to superficial regions. A strictly superficial distribution of cerebellar CMBs is associated with clinically diagnosed as well as pathologically-verified CAA.
Keywords: microbleeds, cerebral amyloid angiopathy, small vessel disease, intracerebral hemorrhage, cerebellum
INTRODUCTION
Cerebral microbleeds (CMBs) are a radiological manifestation of cerebral small vessel disease (SVD) and are frequently recognized in spontaneous intracerebral hemorrhage (ICH) patients who undergo brain MRI with blood-sensitive sequences.1 The spatial distribution of supratentorial ICH and CMBs tends to parallel the different anatomical distribution of cerebral amyloid angiopathy (CAA; mainly affecting supratentorial cortical and leptomeningeal vessels) and hypertensive degenerative small vessel changes (i.e., arteriolosclerosis that mainly involves the vasculature supplying the supratentorial deep grey nuclei and white matter) and is thus considered a useful MRI biomarker to differentiate these two main sporadic SVD types.2-4
Both CAA and hypertensive microvascular degenerative changes have been reported as potential underlying pathologies leading to arteriolar rupture, and hence hemorrhagic manifestations in the cerebellum.5-7 For this reason, the Boston criteria do not count cerebellar-ICH as either for or against the diagnosis of CAA.2,3 However, limited pathology-based evidences suggest that the location of the hematoma within the cerebellum may also help identify the dominant SVD type (CAA vs. hypertensive degenerative small vessel changes).5-7 When CAA is the potential underlying mechanism of the ICH,6,7 the hematoma has been reported to be mostly restricted to the cerebellar cortex and vermis (i.e., superficial cerebellar regions).6,7 By contrast, when the hemorrhage is more likely to be related to hypertension, the primary hematoma has been reported mostly in the cerebellar grey nuclei and deep white matter (i.e., deep cerebellar regions).5 In line with these findings, we have recently reported that patients with spontaneous ICH located in superficial cerebellar regions more frequently have supratentorial lobar CMBs on MRI, a putative CAA marker.3,8,9 Conversely, patients with deeply located cerebellar ICH have higher rates of hypertension compared to patients with hematomas restricted to superficial cerebellar regions.8
It is not currently known whether the spatial distribution of CMBs within the cerebellum also suggests a particular SVD type in ICH patients. Therefore, we aimed to test whether CMBs restricted to superficial cerebellar regions will be associated with clinically diagnosed as well as pathology-confirmed CAA. Furthermore, we aimed to evaluate the association between presence of cerebellar CMBs and supratentorial SVD neuroimaging markers.
METHODS
Patients selection and baseline characteristics
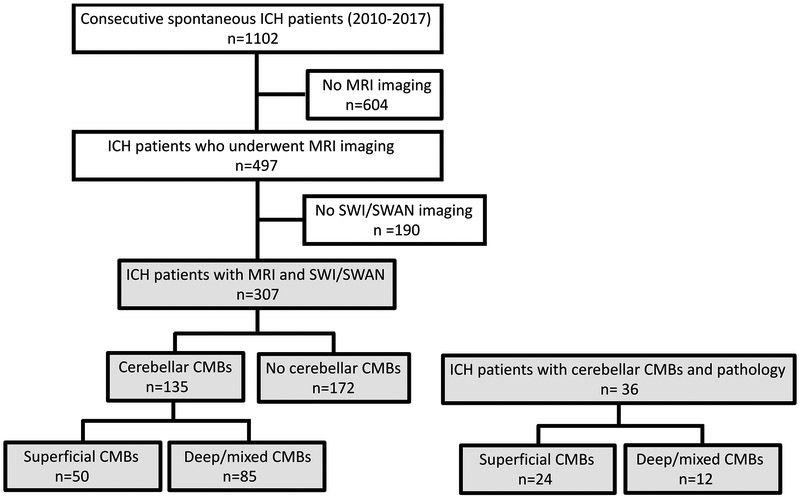
We retrospectively analyzed data from consecutive spontaneous supratentorial ICH patients admitted at Massachusetts General Hospital (MGH) between 2010–2017, as already extensively described in previous publications.4,10,11 For the current analysis, eligibility criteria among our ICH-MRI cohort included the availability of susceptibility weighted (SWI) imaging or susceptibility weighted angiography (SWAN) sequences within 3 months after the acute event. In order to increase the accuracy and consistency for CMBs detection, we elected to exclude from the current analysis patients whose MRI included only the less reliable blood-sensitive T2* gradient recall echo (GRE) techniques.12 A flow-chart of patient selection is reported in Figure 1. Secondary causes of ICH were ruled out in all patients by CT angiography, MR angiography or an MRI with gadolinium contrast.
Figure 1. Flow chart of study enrollment.
ICH: Intracerebral hemorrhage, SWI: Susceptibility-weighted images, SWAN: Susceptibility-weighted angiography, CMBs: cerebral microbleeds
This study was performed with approval and in accordance with the guidelines of the MGH institutional review board, which allows us to collect data on all ICH patients treated at MGH. The authors declare that all supporting data are available within the article [and its online supplementary files].
Baseline data collection was performed as described. Briefly, the following clinical variables were systematically recorded for each participant: age, sex, presence of hypertension, diabetes mellitus, hypercholesterolemia, warfarin and antiplatelet use.4,10,11
MRI evaluation
Images were obtained with a 1.5T MR scanner including whole-brain T2-weighted, T1-weighted and fluid-attenuated inversion recovery. Blood sensitive sequences included SWI MRI (TR/TE: 30/20 ms, slice thickness: 1.8 mm) or SWAN imaging (TR/TE: 78.3/49.8 ms; slice thickness: 3 mm) as part of the diagnostic work-up after spontaneous ICH.12
Presence of CMBs, ICH and cortical superficial siderosis (cSS) were evaluated according to current consensus criteria, as previously described.1,4,13 Based on the modified Boston criteria, patients with a combination ≥1 strictly lobar supratentorial ICH and either ≥1 strictly lobar supratentorial CMBs or presence of cSS were defined as probable CAA.3 Conversely, patients with strictly deep (defined as basal ganglia, thalamus, brainstem) ICH with or without strictly deep CMBs and patients with a combination of lobar and deep bleeds were defined as deep/mixed ICH (non-CAA ICH group).4 Enlarged perivascular spaces (EPVS) were rated on axial T2-weighted MRIs in the basal ganglia and centrum semiovale and dichotomized as high (score > 20) or low (score < 20).10 Lacunes were evaluated in the supratentorial region, and defined as round or ovoid, subcortical, fluid-filled (similar signal as CSF) cavities, between 3 mm and 15 mm in diameter, according to Standards for Reporting Vascular Changes on Neuroimaging criteria.14,15 White matter hyperintensities (WMH) volume was semi-automatically quantified as previously validated with a computer-assisted process.16 All MRI analyses were performed blinded to clinical data.
Cerebellar CMBs topographical pattern evaluation
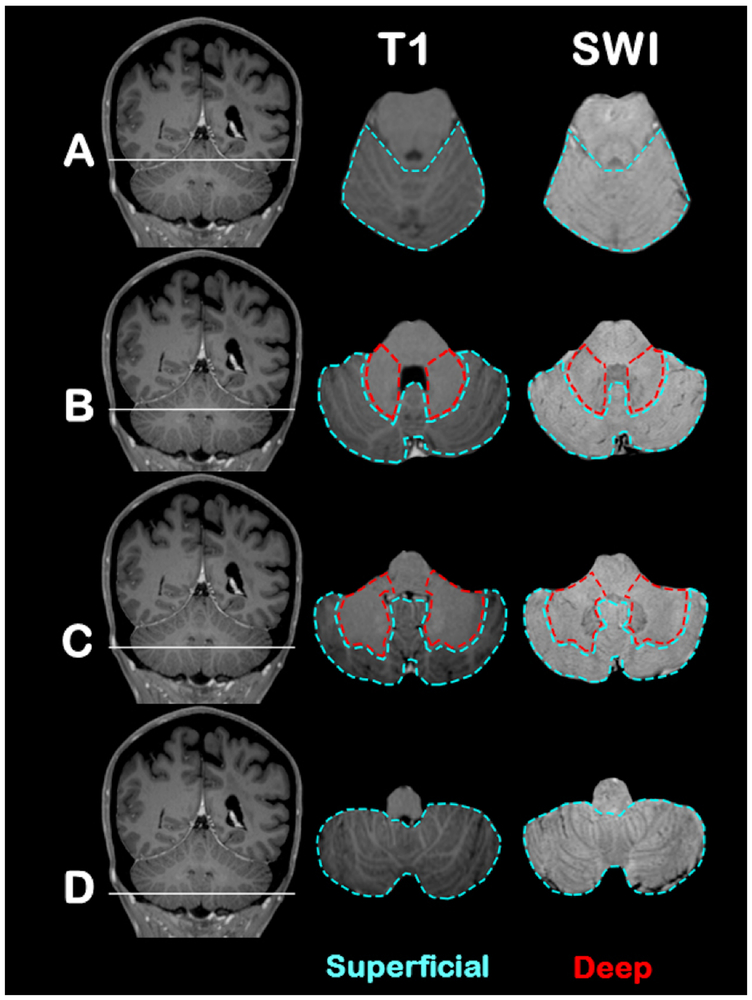
To test our hypothesis, we designed and further refined a topographical template that could reliably differentiate cerebellar cortex and vermis from deep cerebellar grey nuclei and white matter using MRI images. In order to create a cerebellar topographical template for CMBs classification, we used: a) post-contrast T1-MPRAGE (TR/TE 2530/1.64 ms, slice thickness 1 mm, Dotarem 12 mL); and b) SWI images of the cerebellum from high resolution MRI. The topographical template defines two main cerebellar regions: a) superficial region (outlined in blue in Figure 2) that includes cerebellar cortex and vermis; and b) deep region (outlined in red in Figure 2) that includes deep cerebellar grey nuclei and white matter. Cases with concomitant presence of cerebellar CMBs in both superficial and deep cerebellar regions were defined as having a mixed cerebellar CMBs pattern (see Figure 3 for examples of cerebellar CMBs distribution patterns). During the rating process we applied the topographical template to each patient. SWI and T1-weighted imaging sequences were co-aligned in the axial plane from a caudal to cranial direction and reviewed only up to the most cranial portion of the cerebellum. This process enabled the rater to be at least partly blinded to MRI supratentorial SVD lesions. Ratings of cerebellar CMBs location were performed separately by two independent raters (MP and TP) and cases of disagreement were resolved by consensus. Interrater agreement for superficial vs. deep/mixed pattern was calculated using all patients with cerebellar CMBs (n=135 MRIs) and was excellent (Cohen’s-kappa: 0.96, 95% confidence interval [CI] 0.92–1).
Figure 2. Cerebellar topographic template.
(A-D) Four representative cerebellar areas (through transversal plane in a cranial to caudal direction) have been selected to defined superficial (outlined in blue) and deep (outlined in red) cerebellar regions. MRI scans in the first column are coronal T1-MPRAGE sequences that localize the four regions. The second and third columns are axial T1-MPRAGE and susceptibility-weighted images sequences, respectively.
A: Most cranial portion of the cerebellum that mainly includes upper vermis and cerebellar cortex.
B-C: Regions of the cerebellar peduncles that include grey nuclei, surrounding white matter and cerebellar cortex.
D: Most caudal portion of the cerebellum that mainly includes lower vermis and cerebellar cortex.
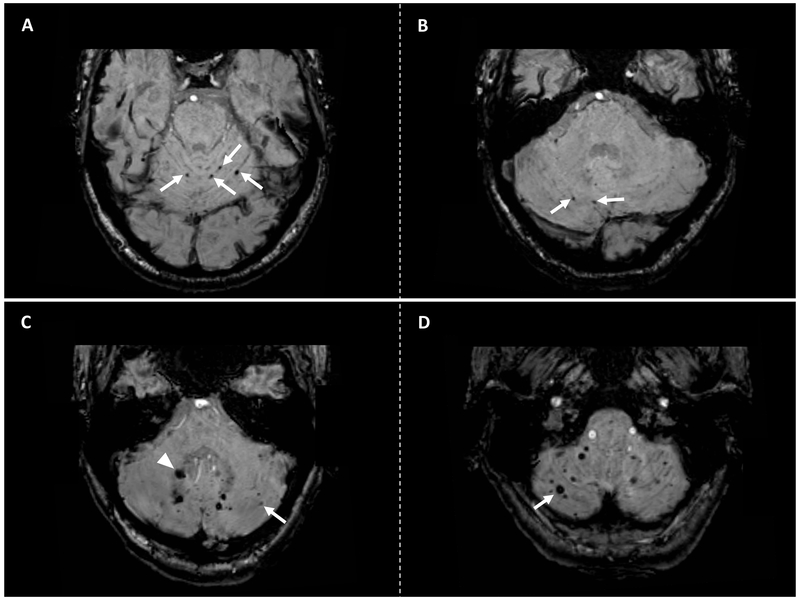
Figure 3. Examples of cerebellar CMBs distribution patterns.
Panel A-B: Axial susceptibility-weighted images (SWI) sequences of a cerebral amyloid angiopathy (CAA)-related ICH patient showing strictly superficial cerebellar microbleeds (arrows). Panel C-D: Axial SWI sequences of a non CAA-related ICH patient showing a mixed cerebellar CMBs pattern. Panel C shows cerebellar CMBs located in the deep cerebellar grey and white matter (arrowhead) and superficial (arrow) area. Panel D shows additional CMBs in the superficial cerebellar area (arrow) in the same patient.
Neuropathologic cohort and evaluation
We screened an established database of spontaneous ICH patients from MGH (1997–2018) with brain pathology ratings available, allowing assessment of CAA presence and severity. Neuropathology material included brain biopsy, brain tissue from hematoma evacuation, or full brain autopsy. Morphological assessment was performed by routine hematoxylin-eosin staining and the presence or absence and severity of vascular amyloid-β deposition was confirmed by immunohistochemical detection and/or congo red staining. CAA presence and severity was assessed in all available slides and vessels, based on the Vonsattel grading system.17,18 Cases were considered positive for CAA if they had at least mild (Vonsattel grade 1) vascular amyloid deposition on brain biopsy and hematoma evacuation material or moderate to severe (Vonsattel grades 2–4) vascular amyloid deposition on full brain autopsy.
For the current analysis, eligibility criteria among our neuropathology cohort included: (a) neuropathology assessment of CAA grade; (b) available brain MRI sequences of adequate quality including T2-weighted, FLAIR, and T2*-GRE and/or SWI/SWAN; and (c) presence of at least one cerebellar CMB in any distribution as defined in the neuroimaging section above. Among 113 screened cases of spontaneous ICH with neuropathology available and in vivo clinical brain MRI, 36 patients demonstrated cerebellar CMBs and were thus included in our analysis.
Statistical analysis
Univariate analyses were performed to evaluate differences in demographic, clinical, and neuroimaging characteristics between patients with and without cerebellar CMBs using χ2 and the Fisher’s exact tests for categorical variables and two-sample t tests or Mann–Whitney U tests depending on the distribution of continuous variables. To test for independent associations with presence of cerebellar CMBs, all variables which showed a p-value < 0.1 in univariate analyses (plus age and sex) were entered in a multivariable nominal regression analysis.
In the subgroup of ICH patients with cerebellar CMBs, univariate analyses were performed to evaluate differences between patients with strictly superficial cerebellar CMBs vs. those with deep/mixed cerebellar CMBs. To test for independent associations for presence of superficial cerebellar CMBs, all variables which showed a p-value < 0.1 in univariate analyses (plus age and sex) were entered in a multivariable nominal regression analysis. A similar model was performed to test for independent associations with presence of deep/mixed cerebellar CMBs. WMH volume was log-transformed for multivariable analyses to reduce the degree of skewness of the variable.
Finally, we performed analyses by Fisher’s exact test in the ICH pathology cohort to test for associations between presence of pathology-verified CAA and strictly superficial cerebellar CMBs pattern.
All analyses were performed with JMP Pro 13 software (SASInstitute Inc, Cary, NC).
RESULTS
From an initial cohort of 497 spontaneous ICH patients with MRI, 190 patients were excluded because they did not meet inclusion criteria for the current analysis (Figure 1). Baseline clinical characteristics, and 30-day mortality were similar between excluded and included ICH patients who undergo an MRI (supplementary Table I).
Out of 307 spontaneous ICH patients, 135 (44%) had at least one cerebellar CMB. In patients with cerebellar CMBs the median of cerebellar CMBs in any location was 2 (interquartile range [IQR] 1–3), the median of superficial cerebellar CMBs was 1 (IQR 1–3) and of deep cerebellar CMBs was 0 (IQR 0–1). Table 1 reports univariate comparisons between patients with vs. without cerebellar CMBs. When compared with patients without cerebellar CMBs, patients with cerebellar CMBs more frequently had deep/mixed ICH, higher rates of hypertension, more frequently showed supratentorial CMBs, lacunes and higher WMH volume, and were more frequently on warfarin at admission (p<0.05). In multivariable analyses, presence of cerebellar CMBs was independently associated with hypertension (odds ratio [OR] 1.5, 95% confidence interval [CI] 1.1–2.1, p=0.01), supratentorial CMBs (OR 1.4, CI 1.1–1.9, p=0.02) and higher LogWMH volumes (OR 1.3, CI 1.01–1.8, p=0.04).
Table 1.
Univariate comparisons between patients with and without cerebellar microbleeds.
| Patient without cerebellar CMBs N=172 |
Patient with cerebellar CMBs N=135 |
p-value | |
|---|---|---|---|
| Age, years (SD) | 70.4 (12.8) | 69.7 (12.6) | 0.6 |
| Sex (female), n (%) | 90 (52.3) | 84 (62.2) | 0.1 |
| Hypertension, n (%) | 121 (70.3) | 117 (86.7) | <0.001 |
| Diabetes, n (%) | 36 (20.9) | 35 (25.9) | 0.3 |
| Hypercholesterolemia, n (%) | 82 (47.7) | 56 (41.5) | 0.3 |
| Warfarin use, n (%) | 19 (11.0) | 28 (20.7) | 0.03 |
| Antiplatelets use, n (%) | 77 (44.8) | 57 (42.2) | 0.7 |
| Presence of supratentorial CMBs, n (%) | 88 (51.2) | 96 (71.1) | <0.001 |
| Lacunes presence, n (%) | 53 (30.8) | 58 (42.9) | 0.03 |
| Severe CSO EPVS > 20, n (%) | 70 (40.7) | 57 (42.2) | 0.8 |
| Severe BG EPVS > 20, n (%) | 36 (20.9) | 35 (25.9) | 0.3 |
| WMH volume mL, median (IQR) | 15 (8.2–29.7) | 24 (11–41.2) | <0.001 |
| cSS presence, n (%) | 13 (7.6) | 18 (13.3) | 0.3 |
| Diagnosis of Probable CAA*, n (%) | 49 (28.5) | 38 (28.1) | 1.0 |
| Diagnosis of Deep/Mixed ICH, n (%) | 72 (41.9) | 78 (57.8) | 0.006 |
SD: standard deviation, CMBs: cerebral microbleeds, CSO: Centrum Semiovale, EPVS: Enlarged perivascular spaces, cSS: cortical superficial siderosis, SVD: small vessel disease, WMH: white matter hyperintensities, IQR: interquartile range, CAA: cerebral amyloid angiopathy.
Probable CAA according to the modified Boston criteria
Cerebellar microbleeds location
Among 135 patients with cerebellar CMBs, 85 (63%) showed strictly superficial cerebellar CMBs and 50 (37%) had deep/mixed cerebellar CMBs. Table 2 reports univariate comparisons between patients with strictly superficial cerebellar CMBs vs. those with deep/mixed cerebellar CMBs. Diagnosis of probable CAA was more frequent in patients with superficial cerebellar CMBs than in those with deep/mixed cerebellar CMBs (35% vs. 16%, respectively; p=0.02). Conversely, diagnosis of deep/mixed ICH was more frequent in patients with deep/mixed cerebellar CMBs than in those with superficial cerebellar CMBs (76% vs 47%, respectively; p=0.001). In multivariable analyses, diagnosis of probable CAA was independently associated with strictly superficial cerebellar CMBs (OR: 1.6, CI: 1.03–2.5, p=0.03) while deep/mixed ICH diagnosis with deep/mixed cerebellar CMBs (OR: 1.8, CI: 1.2–2.7, p=0.001).
Table 2.
Univariate comparisons between patients with superficial cerebellar microbleeds and those with deep/mixed cerebellar microbleeds.
| Strictly superficial cerebellar CMBs N= 85 |
Deep/mixed cerebellar CMBs N= 50 |
p-value | |
|---|---|---|---|
| Age, years (SD) | 70.8 (12.1) | 68.1 (13.3) | 0.2 |
| Sex (female), n (%) | 55 (64.7) | 29 (58.0) | 0.5 |
| Hypertension, n (%) | 74 (87.1) | 43 (86.0) | 1 |
| Diabetes, n (%) | 22 (25.9) | 13 (26.0) | 1 |
| Hypercholesterolemia, n (%) | 37 (43.5) | 19 (38.0) | 0.6 |
| Warfarin use, n (%) | 15 (17.6) | 13 (26.0) | 0.2 |
| Antiplatelets use, n (%) | 38 (44.7) | 17 (34.0) | 0.3 |
| Presence of supratentorial CMBs, n (%) | 57 (67.1) | 39 (78.0) | 0.2 |
| Lacunes presence, n (%) | 36 (42.3) | 22 (44.0) | 0.8 |
| Severe CSO EPVS > 20, n (%) | 36 (42.3) | 21 (42.0) | 1.0 |
| Severe BG EPVS > 20, n (%) | 29 (34.1) | 19 (38.0) | 0.2 |
| WMH volume, median (IQR) | 23.6 (6.6-38.0) | 28.9 (12.1-48.7) | 0.3 |
| cSS presence, n (%) | 14 (16.4) | 4 (8.0) | 0.2 |
| Diagnosis of Probable CAA,* n (%) | 30 (35.3) | 8 (16.0) | 0.02 |
| Diagnosis of Deep/Mixed ICH, n (%) | 40 (47.0) | 38 (76.0) | 0.001 |
SD: standard deviation, CMBs: cerebral microbleeds, CSO: centrum semiovale, BG: basal ganglia, CMBs: cerebral microbleeds, EPVS: enlarged perivascular spaces, WMH: white matter hyperintensities, IQR: interquartile range, cSS: cortical superficial siderosis, ICH: intracerebral hemorrhage, CAA: cerebral amyloid angiopathy.
ICH cohort with pathology data
The pathology ICH cohort included 36 patients with cerebellar CMBs (Mean age: 70.1 standard deviation: 11.2, 54% were male, hypertension was present in 62% and 29% were diabetic). Neuropathology samples came from autopsy in 11 cases, brain biopsy in 5 cases and hematoma evacuation in 20 cases. Twenty-six out of thirty-six patients (72%) had pathology-verified CAA. Strictly superficial cerebellar CMBs were present in 24 (67%) patients while a deep/mixed pattern was present in 12 (33%) patients. See Table 3 for associations between cerebellar CMBs distribution patterns and presence of pathology-verified CAA. Out of 26 patients who showed CAA, 23 (88%) had strictly superficial CMBs and 3 (12%) showed deep/mixed CMBs. In the 10 patients without CAA, 9 (90%) had deep/mixed cerebellar CMBs and 1 (10%) strictly superficial cerebellar CMBs.
Table 3.
Association between strictly superficial and deep/mixed cerebellar microbleeds patterns according to pathologically-verified CAA.
| Patient with superficial cerebellar CMBs N= 24 |
Patient with deep/mixed cerebellar CMBs N= 12 |
p-value | |
|---|---|---|---|
| Pathologically-verified CAA | 23 (95.8) | 3 (25.0) | 0.001 |
| No pathologically-verified CAA | 1 (4.2) | 9 (75.0) |
CAA: cerebral amyloid angiopathy, CMBs: cerebral microbleeds
DISCUSSION
Our study showed that in spontaneous supratentorial ICH patients, CMBs within the cerebellum are relatively frequent, being present in approximately 40% of the participants. We proposed a simple, hypothesis-driven topographical classification of cerebellar CMBs into superficial (restricted to cortex and vermis) and deep (grey nuclei and white matter) patterns. The spatial differentiation of cerebellar CMBs is straightforward to perform with this tool and based on the current findings might help differentiate the underlying SVD type in ICH patients. CMBs restricted to superficial cerebellar area were independently associated with probable CAA diagnosis, while deep/mixed cerebellar CMBs associated with deep/mixed (i.e. non-CAA) ICH. The association between a strictly superficial cerebellar CMBs pattern and CAA was also supported by our pathology-based ICH dataset.
Previous detailed anatomical vasculature networks of the whole brain using injections with indian ink or low viscosity resin suggested that intracortical cerebellar arteries and veins share a structure and form a network similar to supratentorial cortical vessels.19 Furthermore, in analogy to supratentorial regions, pathologically-based observation suggest that when vascular amyloid deposition involves the cerebellum, it is usually confined to cerebellar cortical and leptomeningeal vessels.20,21 Conversely, hypertensive degenerative vascular changes have been mainly reported in the cerebellar grey nuclei and surrounding white matter.5,22 In line with these pathologic observations, we report that the spatial distribution of cerebellar CMBs within the cerebellum seems to reflect the different regional distribution of CAA and arteriolosclerosis within the cerebellum. The mechanistic basis for this predilection for leptomeningeal and cortical cerebellar vessels by CAA has not been extensively evaluated, but has been proposed to relate to similarities between the cerebellar and supratentorial cortical vasculature.6,20,21 In the region of cerebellar grey nuclei and surrounding white matter deep branches that originate from the major cerebellar arteries form an extensive network of anastomoses, potentially accounting for their vulnerability to hypertension.5
Although our sub-study with pathology data found a robust association between strictly superficial cerebellar CMBs pattern (Table 3) for CAA, this result may be partly driven by the high overall prevalence of CAA pathology in our patients with available brain specimens. In our MRI cohort, which is a larger and less selected group, strictly superficial cerebellar CMBs were not as frequent in patients with CAA-ICH, and with deep/mixed ICH diagnosis actually more frequently represented in patients with strictly superficial cerebellar CMBs (Table 2). The latter results support the idea that hypertension-related microvascular damage is the most common SVD type in the cerebellum and while preferentially affecting deep cerebellar penetrating territories, can involve superficial lobar territories as well.4,23,24 However, in the MRI cohort 16% of patients with deep/mixed cerebellar CMBs were probable CAA and, in the pathology cohort, 25% of patients with deep/mixed cerebellar CMBs had pathology verified CAA. These results suggest that a proportion of patients with deep/mixed cerebellar CMBs might reflect an interaction between hypertension and CAA.4 Our findings also suggest that, in isolation, topographical assessment of cerebellar CMBs may not be fully discriminative of the underlying pathology. It therefore remains to be determined whether the pattern of cerebellar CMBs, which we have shown to be reliably measurable, can be incorporated in the diagnostic criteria for CAA to improve sensitivity or specificity.25 Furthermore, whether cerebellar CMBs count in the superficial or deep/mixed territories carries additional diagnostic information or whether total cerebellar CMB count influences clinical outcome after ICH (i.e. mortality, functional outcome, cognitive impairment) should also be addressed in future studies.
Several limitations need to be considered. Loss of blinding to supratentorial lesions during rating of cerebellar CMBs cannot be fully excluded, though we believe this was minimized by our method of scrolling and reviewing MR images only up to the most cranial portion of the cerebellum. Another limitation is patients selection bias. For the imaging analyses, we elected a priori to increase uniformity of CMB detection by including only patients who underwent SWI/SWAN, thus excluding patients with T2* GRE sequences. Reassuringly, we did not find any differences in demographic, vascular risk factors and ICH clinical severity between the two groups (Supplementary Table 1). There was also substantial selection bias in the pathologic sub-study, resulting in a high overall prevalence of CAA pathology as noted above. The differences in patient selection across these two datasets make it difficult to estimate absolute sensitivity and specificity of the cerebellar CMB distributions, but both are supportive of the predilection of CAA-related CMBs for the superficial cerebellar territory. The topographical template we used to define superficial and cerebellar areas was selected a priori to differentiate cerebellar cortex from deep white and grey cerebellar matter. However, we cannot exclude that alternative templates, such as a more conservative delineation of superficial cerebellar area, could have been more discriminative.
In conclusion, cerebellar CMBs are relatively frequent in spontaneous ICH and are associated with supratentorial radiological markers of SVD, suggesting a possible association with advanced SVD pathology. CMBs within the cerebellum show different distribution patterns (strictly superficial vs. deep/mixed cerebellar pattern) that can be reliably evaluated on structural MRI with the use of a topographical template. Spontaneous ICH patients who harbor CAA as the dominant SVD type mainly show cerebellar CMBs restricted to the superficial cerebellar regions. By contrast, patients with deep/mixed (non-CAA) ICH show predominantly a deep/mixed cerebellar CMBs pattern. If externally replicated in other independent cohorts, CMBs location within the cerebellum might be helpful in clinical practice to distinguish spontaneous ICH patients with CAA from those related to hypertensive SVD.
Supplementary Material
Acknowledgments
Study funding: Dr. Rosand is supported by NIH grant 5U01NS036695; Dr. Viswanathan is supported by NIH grants R01AG047975, P50AG005134, and K23AG02872605; Dr. Gurol is supported by NIH grant NS083711; Dr. Greenberg is supported by NIH grants NIA R01AG026484 and R01NS070834.
Footnotes
Disclosures: Dr. Pasi, Dr. Pongpitakmetha, Dr. Charidimou, Dr. Singh, Dr. Tsai, Dr. Xiong, Dr. Boulouis, Mr. Warren, Dr. Frosh, Dr. Viswanathan, Dr. Gurol and Dr. Greenberg report no financial disclosures relevant to the manuscript. Dr. Rosand consults for Boehringer Ingelheim, New Beta Innovation and Pfizer.
REFERENCES
- 1.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Salman RA-S, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. The Lancet Neurology. 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston Criteria. Neurology. 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 3.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology. 2018;90:e119–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinsdale HB. Spontaneous hemorrhage in the posterior fossa: a study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Archives of neurology. 1964;10:200–217. [DOI] [PubMed] [Google Scholar]
- 6.Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J. Neurol. Sci. 1993;116:135–141. [DOI] [PubMed] [Google Scholar]
- 7.Cuny E, Loiseau H, Rivel J, Vital C, Castel J-P. Amyloid angiopathy-related cerebellar hemorrhage. Surgical Neurology. 1996;46:235–239. [DOI] [PubMed] [Google Scholar]
- 8.Pasi M, Marini S, Morotti A, Boulouis G, Xiong L, Charidimou A, et al. Cerebellar Hematoma Location: Implications for the Underlying Microangiopathy. Stroke. 2018;49:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Ramirez S, Romero J-R, Shoamanesh A, McKee AC, Van Etten E, Pontes-Neto O, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimer’s & Dementia. 2015;11:1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charidimou A, Boulouis G, Roongpiboonsopit D, Auriel E, Pasi M, Haley K, et al. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: A prospective study. Neurology. 2017;89:2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng A-L, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, et al. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke. 2013;44:2782–2786. [DOI] [PubMed] [Google Scholar]
- 13.Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron J-C, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138:2126–2139. [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. 2017;88:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma β-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 17.Vonsattel JPG, Myers RH, Tessa Hedley-Whyte E, Ropper AH, Bird ED, Richardson EP. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Annals of neurology. 1991;30:637–649. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg SM, Vonsattel J-PG. Diagnosis of Cerebral Amyloid Angiopathy: Sensitivity and Specificity of Cortical Biopsy. Stroke. 1997;28:1418–1422. [DOI] [PubMed] [Google Scholar]
- 19.Duvernoy H, Delon S, Vannson JL. The vascularization of the human cerebellar cortex. Brain Res. Bull. 1983;11:419–480. [DOI] [PubMed] [Google Scholar]
- 20.Yamada M, Tsukagoshi H, Otomo E, Hayakawa M. Cerebral amyloid angiopathy in the aged. J Neurol. 1987;234:371–376. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke. 1983;14:915–923. [DOI] [PubMed] [Google Scholar]
- 22.Fisher CM, Picard EH, Polak A, Dalal P, Pojemann RG. Acute hypertensive cerebellar hemorrhage: diagnosis and surgical treatment. J. Nerv. Ment. Dis. 1965;140:38–57. [DOI] [PubMed] [Google Scholar]
- 23.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. American Journal of Neuroradiology. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 24.Broderick J, Brott T, Tomsick T, Leach A. Lobar hemorrhage in the elderly. The undiminishing importance of hypertension. Stroke. 1993;24:49–51. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SM, Charidimou A. Diagnosis of Cerebral Amyloid Angiopathy: Evolution of the Boston Criteria. Stroke. 2018;49:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.