ABSTRACT
Background
Artificially sweetened beverages (ASBs) are commonly consumed and recommended for individuals at high risk for cardiometabolic diseases; however, the health effects of ASBs remain contradictory. Given that cross-sectional analyses are subject to reverse causation, prospective studies with long-term follow-up are needed to evaluate associations between ASBs and cardiometabolic health, especially among high-risk individuals.
Objective
The aim of this study was to examine associations of ASB intake and cardiometabolic health among high-risk women with prior gestational diabetes mellitus (GDM).
Methods
We included 607 women with GDM from the Danish National Birth Cohort (DNBC; 1996–2002) who completed a clinical exam 9–16 y after the DNBC pregnancy for the Diabetes & Women's Health (DWH) Study (2012–2014). We assessed ASB intake using FFQs completed during the DNBC pregnancy and at the DWH Study clinical exam. We examined cardiometabolic outcomes at the DWH clinical exam. We estimated percentage differences in continuous cardiometabolic markers and RRs for clinical endpoints in association with ASB intake both during pregnancy and at follow-up adjusted for prepregnancy BMI, diet, and lifestyle factors. Sensitivity analyses to account for reverse causation were performed.
Results
In pregnancy and at follow-up, 30.4% and 36.4% of women regularly (≥2 servings/wk) consumed ASB, respectively. Consumption of ASBs, both during pregnancy and at follow-up, was associated with higher glycated hemoglobin (HbA1c), insulin, HOMA-IR, triglycerides, liver fat, and adiposity and with lower HDL at follow-up. After adjustment for covariates, particularly prepregnancy BMI, the majority of associations between ASB intake in pregnancy and outcomes at follow-up became null with the exception of HbA1c. ASB intake at follow-up (≥1 serving/d compared with <1 serving/mo) was associated with higher HbA1c (6.5%; 95% CI: 1.9, 11.3; P-trend = 0.007); however, associations were not upheld in sensitivity analyses for reverse causation.
Conclusions
Among Danish women with a history of GDM, ASB intake was not significantly associated with cardiometabolic profiles.
Keywords: artificially sweetened beverages, nonnutritive sweeteners, soda, diet, obesity, diabetes, cardiometabolic health, gestational diabetes
Introduction
Artificially sweetened beverages (ASBs) are commonly consumed, with consumption increasing during the past decade in western European countries (1). ASBs have been purported as a low-calorie, healthier alternative to sugar-sweetened beverages and recommended for individuals at high risk for cardiometabolic diseases (2). Yet, scientific evidence on the short- and long-term health effects of ASBs remains conflicting. Many, but not all, animal studies suggest potential adverse effects of high doses of nonnutritive sweeteners on glucose intolerance, insulin resistance, and obesity that may be mediated through disruption of homeostatic metabolic systems via activation of sweet taste receptors and disruption of gut microbiota (3, 4). Well-designed randomized controlled trials on long-term impacts of nonnutritive sweeteners are sparse and generally have not observed beneficial or adverse effects on body weight overall (5–7), although there may be a benefit on body weight among overweight or obese individuals (6). Last, observational studies show potentially adverse associations with long-term consumption of nonnutritive sweeteners, reporting higher body weight and risk for cardiometabolic complications (5). Of particular note, findings from cross-sectional observational studies on ASBs and cardiometabolic health may be subject to reverse causation because individuals with an increased propensity for, or with, cardiometabolic complications or overweight/obesity may switch to foods and beverages containing nonnutritive sweeteners with the goal of reducing sugar intake.
In the current study, we aimed to examine associations of ASB consumption on a comprehensive panel of cardiometabolic markers among Danish women with prior gestational diabetes mellitus (GDM). This analysis addresses critical data gaps by examining ASB consumption collected twice over 9–16 y, by including a wide range of cardiometabolic endpoints, and by focusing on a high-risk population (8). Women with a history of GDM (i.e., hyperglycemia first recognized in pregnancy) have a high risk for developing type 2 diabetes (T2DM) and other cardiometabolic complications, such as cardiovascular disease and nonalcoholic fatty liver disease, later in life (9–11) and are therefore a good model for evaluating ASB intake in a high-risk population. We hypothesized that consumption of ASBs would be associated with poor cardiometabolic health in women with prior GDM.
Methods
Study population
This study utilized data from Danish women enrolled in the Diabetes & Women's Heath (DWH) Study (12), a long-term follow-up of women with GDM during the index pregnancy of the Danish National Birth Cohort (DNBC) (1996–2002) (13). All women with GDM in the DNBC (n = 1274 out of 91,827 women) were invited to participate in the DWH Study (2012–2014), of whom a total of 790 participated. The DWH Study follow-up consisted of questionnaires and a clinical exam; the clinical exam was completed by 607 women (Supplemental Figure 1). In the DNBC, women were classified as having a diagnosis of GDM if it was recorded in the Danish National Patient Register or women reported having GDM on the DNBC telephone interviews at 30 weeks of gestation or 6 mo postpartum (14). Women who participated in the DWH Study were largely comparable to the eligible source population with respect to age (31.6 y in the DWH Study compared with 31.3 y in the source population), prepregnancy BMI (in kg/m2; 27.5 compared with 27.7), nulliparity (54.9% compared with 56.7%), and smoking status (26.4% compared with 28.4%).
All women provided written informed consent. The study was approved by the Regional Scientific Ethical Committee of the Capital Region of Denmark (record no. H-4-2013-129). The Strengthening the Reporting of Observational Studies in Epidemiology cohort reporting guidelines were followed (15). The primary aim of the DWH Study was to identify genomic and environmental determinants underlying the development of T2DM and comorbidities among women with a history of GDM. The aims were not changed during the course of the study. The current analysis was not prespecified and is considered exploratory.
Exposure measures
Dietary intake was assessed at 2 time points: 1) during pregnancy during the conduct of the DNBC and 2) 9–16 y later as part of the DWH Study follow-up. Diet during the index pregnancy was assessed at 25 weeks of gestation using a validated semiquantitative 360-item FFQ, which collected information on habitual dietary intake during the previous month (16–18). Overall, the FFQ response rate in the DNBC was 74.2% (16), and the reliability of self-reported ASB intake assessed twice in a subsample was high (19). Similar to prior studies, dietary data for women with implausible daily energy intake (<956 or >4780 kcal/d; n = 4) were excluded (20). Diet at the follow-up clinical exam was assessed using a similar semiquantitative 360-item FFQ that collected information on habitual dietary intake during the previous year. Dietary data for women with implausible daily energy intake (<600 or >4000 kcal/d; n = 19) were excluded (21). Different cutoffs for implausible energy intake were applied to DNBC and the DWH Study because 1 represented a pregnant population and the other a nonpregnant population. A total of 438 (72.2%) women had complete dietary data at both time points. Missing dietary data were imputed as described later.
For both DNBC and DWH dietary data, a variable for total ASB intake was created by summing carbonated and noncarbonated sources of ASB. Specifically, on the DNBC FFQ, individuals reported intake of “Soda pop/Coca Cola without sugar (diet)” and “Lemonade without sugar (diet)” using 11 categories of intake ranging from “None per month” to “8 or more per day.” On the DWH FFQ, individuals reported their intake of “Soft drink and Coke, light (no sugar)” and “Lemonade, light (no sugar)” using 12 categories ranging from “Never during the last year” to “8 or more times per day.” For both time points, based on assumptions on standard portion sizes, reported frequency of intake was converted to grams/day using the FoodCalc program (22) combined with the Danish Food Composition Database (23). Servings per day of ASB were estimated based on the assumption that 1 serving was equivalent to 250 grams (20). Dietary data at both time points were utilized to calculate the Alternate Healthy Eating Index (AHEI) to assess overall dietary quality. The AHEI includes the following components: vegetables, fruits, whole grains, sugar-sweetened beverages, nuts and legumes, red/processed meat, trans fat, long-chain (n–3) fats (EPA + DHA), PUFAs as percentage of energy, sodium, and alcohol (24).
Outcome measures
Outcome measures were collected at follow-up clinical exam according to a standardized protocol, which consisted of anthropometric measurements; blood pressure measurements; fasting blood samples; and a 2-h, 75-g oral glucose tolerance test (in those without diabetes). Height (centimeters), weight (kilograms), and waist circumference (centimeters) midway between the lowest rib and the iliac crest were measured at least twice. BMI was calculated. Mean resting arterial blood pressure was calculated as [(systolic blood pressure) + 2 x (diastolic blood pressure)]/3. A whole-body DXA (GE Lunar Prodigy; GE Healthcare) scan was performed on a subset of women who had their clinical exam performed at Rigshospitalet (n = 192). Abdominal visceral adipose tissue mass was estimated using enCORE software (version 15; GE Healthcare) (25). At follow-up, women also self-reported heart disease, type 1 diabetes, T2DM, cancer, gout, elevated cholesterol, and elevated blood pressure.
Biospecimens were collected according to a standardized protocol. Fasting plasma glucose (millimoles per liter) and insulin (picomoles per liter) were measured immediately at the clinic after an ∼8- to 10-h overnight fast; all other sample aliquots were processed and immediately frozen at −80°C for assays at a later date. The following were measured in plasma using Roche Diagnostics reagents with assay CVs ≤4% unless otherwise noted: triglycerides (TGs; milligrams per decaliter), total cholesterol (milligrams per decaliter), HDL (milligrams per decaliter), alanine aminotransferase (ALT; international units per liter), aspartate aminotransferase (AST; international units per liter), γ-glutamyltransferase (international units per liter), total bilirubin (milligrams per decaliter; CV <7.3%), C-reactive protein–high sensitivity (CRP; milligrams per liter), and C-peptide (nanomoles per liter; Quansys Biosciences; CV <10.0%). LDL (milligrams per decaliter) was calculated as total cholesterol – HDL – (TG/5). Glycated hemoglobin (HbA1c; percentage; CV <1.2%) was measured in whole blood (Tosoh Bioscience).
Insulin resistance was determined by calculating HOMA-IR and homeostatic model assessment for β-cell function, which are calculated as follows (26):
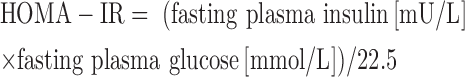 |
(1) |
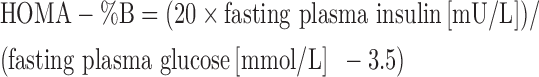 |
(2) |
Fatty liver was determined by calculating a liver fat score and liver fat percentage. Although not directly assessed by imaging, the liver fat score was previously validated for the prediction of nonalcoholic steatohepatitis assessed using proton magnetic resonance spectroscopy with a receiver operating characteristic––AUC of 0.87 (95% CI: 0.83–0.90) (27). The liver fat score utilizes the presence of metabolic syndrome (28) and T2DM and levels of insulin, AST, and ALT; it is calculated as follows (27):
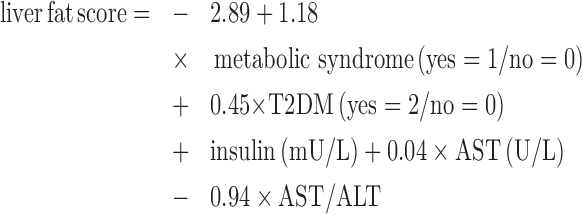 |
(3) |
Similarly, a previously validated continuous measure of liver fat percentage was calculated as follows (27):
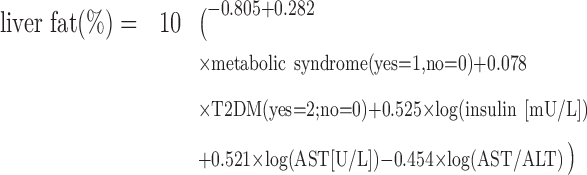 |
(4) |
Cardiometabolic endpoints included obesity (BMI ≥30.0), hyperglycemia (fasting glucose ≥7.0 mmol/L), hypertriglyceridemia (TG ≥200 mg/dL), T2DM, elevated liver function markers AST:ALT ratio (ratio ≥2), ALT (≥19.0 U/L), and an elevated liver fat score (> −0.640) (27). Diagnosis of T2DM was based on clinical exam results (HbA1c ≥6.5%, fasting glucose ≥7.0 mmol/L, or 2-h oral glucose tolerance test glucose ≥11.1 mmol/L) or self-report of physician diagnosis (29).
Additional variable measures
During the DNBC pregnancy, the following relevant variables were collected: current age, prepregnancy BMI (continuous) calculated from self-reported prepregnancy height and weight, high school education (yes/no), nulliparity (yes/no), ever smoker (yes/no), moderate or vigorous physical activity during pregnancy (minutes per week) (30), and prepregnancy chronic diseases (yes/no) including hypertension and cancer (no women had prepregnancy diabetes because the cohort consisted of all women with GDM during the index pregnancy). Physical activity was ascertained by asking women to report the types of exercise in which they engaged and, when relevant, the frequency and duration. The duration of moderate and vigorous activities was summed.
In addition to the clinical exam outcomes described previously, the following relevant variables were collected at the DWH Study follow-up: current age, employed (yes/no), high school education (yes/no), current smoker (yes/no), and moderate or vigorous physical activity (metabolic equivalent hours per week) (31).
Statistical analyses
ASB intake was classified by frequency as <1/mo (reference), 1–4/mo, 2–6/wk, and ≥1/d to allow for similar categories across the 2 time points, provide for public health relevant cut points, and follow prior articles on beverage intake (21). Descriptive analyses estimated the mean (SD) or median (IQR), as applicable, or frequency of the discrete covariates within each category of ASB exposure with statistical significance estimated using ANOVA or Wilcoxon 2-sample test statistic or chi-square tests, respectively. Models of continuous outcomes used log-transformed outcomes, and the final results were presented as the percentage change in each outcome. Models with binary clinical outcomes utilized modified Poisson regression with robust variance to estimate RRs and 95% CIs for each category of intake. A linear trend test across categories was performed using the median value within each category estimated as a continuous exposure. In order to rule out fatty liver due to alcohol intake, all models with liver-related outcomes (i.e., AST, ALT, γ-glutamyltransferase, bilirubin, AST:ALT ratio, calculated liver fat percentage, and elevated liver fat score) excluded women with high alcohol consumption reported at the clinical exam (52.4%; >24 g/d, approximately equivalent to 2 standard drinks/d) (32).
We used multiple imputation with 20 replicates to address missing exposure and covariate data for women who participated in the clinical exam (33, 34). We assumed that the data were missing at random. Each missing variable was imputed using dietary and covariate data at both time points (i.e., pregnancy and follow-up) and the log-transformed continuous outcomes. The distributions of the imputed variables were checked; the range of the continuous values was limited to the range in the original data.
The first set of analyses examined the association of the DNBC pregnancy ASB exposure with continuous cardiometabolic markers and clinical outcomes at DWH follow-up. The following covariates at the index pregnancy were selected a priori: prepregnancy age (continuous), prepregnancy BMI (continuous), nulliparous (yes/no), smoking (yes/no), moderate or vigorous physical activity (continuous), prepregnancy chronic diseases (yes/no), AHEI (continuous), coffee intake (continuous), and tea intake (continuous). The AHEI was chosen as a covariate because of its association with cardiometabolic disease (24). Because the AHEI included sugar-sweetened beverages as a component, they were not individually adjusted for in the analyses. We a priori included coffee and tea as covariates because of their associations with cardiometabolic health (35), and we hypothesized that women may replace artificially or sugar-sweetened beverages with either coffee or tea. Analyses were further adjusted for BMI at the follow-up exam (continuous) in a second model. Sensitivity analyses were performed stratifying by prepregnancy BMI (<25.0 compared with ≥25.0) with the goal of reducing the potential for reverse causality because the reason for ASB consumption may differ between women with and those without overweight or obesity.
The second set of analyses examined the association of the DWH Study follow-up ASB exposure with continuous cardiometabolic markers and clinical endpoints. Covariates selected a priori included current age (continuous), prepregnancy BMI at the index DNBC pregnancy (continuous), primiparous (yes/no), smoking (yes/no), moderate or vigorous physical activity (continuous), prepregnancy chronic diseases (yes/no), AHEI (continuous), coffee intake (continuous), and tea intake (continuous). To reduce the potential for residual confounding and reverse causation, all analyses were adjusted for prepregnancy BMI and the following sensitivity analyses were performed. First, sensitivity analyses were performed stratifying by prepregnancy BMI (<25.0 compared with ≥25.0) for 2 reasons: to further remove reverse causation and to further limit to higher risk women. Second, we excluded women with prevalent chronic diseases (i.e., self-reported type 1 or 2 diabetes, heart problems, cancer, elevated cholesterol, elevated blood pressure, or gout) at follow-up (n = 235). Third, we excluded women who reported not consuming any ASBs in pregnancy (n = 249). The latter 2 analyses were performed to further examine associations with current consumption levels, reducing the likelihood that women started consuming ASBs in response to an indication for health problems.
The third set of analyses examined the longitudinal pattern of ASB exposures by utilizing exposures both during pregnancy and at follow-up 9–16 y after the index pregnancy. Women were classified according to whether they consumed ASBs in the lower 2 categories (≤4/mo; i.e., no consumption or occasional) or the highest 2 categories (≥2/wk; i.e., regular consumption) at each time point, with the reference being women with no or occasional consumption at both time points. Covariates included those described previously for the pregnancy exposure models. Sensitivity analyses were performed stratifying by prepregnancy BMI (<25.0 compared with ≥25.0) and limiting analysis to women without chronic diseases at follow-up. At follow-up, women were asked if they added artificial sweeteners to their coffee or tea. Sensitivity analyses were performed combining coffee and tea with ASB in cases in which artificial sweeteners were added. Also, intake of artificial and sugar-sweetened beverages tended to be inversely correlated in our data. Therefore, we performed sensitivity analyses of ASB intake among women consuming <1 serving per month of sugar-sweetened beverages to fully remove any residual confounding. Last, sensitivity analyses utilized stabilized inverse probability weights to account for loss to follow-up.
All analyses were performed by using SAS software (version 9.4; SAS Institute), with P < 0.05 considered significant.
Results
During pregnancy, 30.4% (n = 139) of women reported consuming ASBs regularly (i.e., ≥2 servings per week); at the DWH Study follow-up 9–16 y later, 36.4% (n = 211) of women reported consuming ASBs regularly. Notably, 62.7% (n = 287) and 7.9% (n = 46) reported regular consumption of sugar-sweetened beverages across the same time period, respectively. Table 1 displays the participant characteristics according to ASB intake in pregnancy and at the DWH Study follow-up. Prepregnancy BMI increased significantly with higher consumption of ASB, ranging from mean values of 26.0 among women consuming <1 serving per month to 30.1 among women consuming ≥1 serving per day.
TABLE 1.
Participant characteristics according to artificially sweetened beverage intake in pregnancy and at follow-up 9–16 y later among women with a history of gestational diabetes1
| Artificially sweetened beverage intake in pregnancy (1996–2002)2 | |||||
|---|---|---|---|---|---|
| <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | ||
| Characteristics in pregnancy (1996–2002) | (n = 205) | (n = 72) | (n = 121) | (n = 60) | P 3 |
| Age, y | 31.6 (4.3) | 32.1 (4.7) | 31.0 (4.3) | 31.3 (4.5) | 0.38 |
| Prepregnancy BMI, kg/m2 | 26.0 (5.5) | 26.5 (5.2) | 27.6 (5.0) | 30.1 (6.2) | <0.001* |
| Nulliparous | 120 (58.5) | 42 (58.3) | 64 (52.9) | 35 (58.3) | 0.96 |
| High school education or greater | 72 (35.1) | 18 (25.0) | 40 (33.1) | 19 (31.7) | 0.84 |
| Smoker | 49 (23.9) | 17 (23.6) | 33 (27.3) | 20 (33.3) | 0.43 |
| Prepregnancy chronic diseases4 | 17 (8.3) | 9 (12.5) | 11 (9.1) | 4 (6.7) | 0.66 |
| Moderate/vigorous physical activity, MET-h/wk | 0.0 (0.0, 30.0) | 0.0 (0.0, 45.0) | 0.0 (0.0, 30.0) | 0.0 (0.0, 10.0) | 0.76 |
| Total energy, kcal/d | 2458.7 (638.3) | 2434.0 (598.3) | 2351.3 (667.7) | 2420.1 (622.6) | 0.53 |
| Coffee intake, g/d | 13.4 (0.0, 150.0) | 13.4 (0.0, 375.0) | 0.0 (0.0, 150.0) | 22.8 (0.0, 150.0) | 0.78 |
| Tea intake, g/d | 32.1 (13.4, 150.0) | 75.0 (13.4, 375.0) | 32.1 (0.0, 150.0) | 75.0 (6.7, 262.5) | 0.39 |
| Sugar-sweetened beverage intake, g/d | 145.0 (72.3, 253.2) | 102.4 (25.9, 203.0) | 92.1 (27.1, 197.7) | 70.5 (23.1, 234.6) | <0.001* |
| AHEI score5 | 47.9 (7.6) | 50.0 (8.2) | 49.5 (7.7) | 48.3 (7.6) | 0.13 |
| Habitual artificially sweetened beverage intake at follow-up (2012–2014)6 | |||||
|---|---|---|---|---|---|
| <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | ||
| Characteristics at follow-up (2012–2014) | (n = 197) | (n = 88) | (n = 214) | (n = 87) | P 3 |
| Age, y | 44.8 (4.9) | 44.0 (4.9) | 43.2 (4.3) | 42.6 (3.8) | <0.001* |
| BMI, kg/m2 | 26.8 (5.2) | 29.2 (5.6) | 29.6 (6.0) | 31.6 (5.6) | <0.001* |
| Primiparous | 25 (17.1) | 15 (13.1) | 28 (16.1) | 14 (0.0) | 0.71 |
| High school education | 197 (86.8) | 88 (83.0) | 214 (83.6) | 87 (81.6) | 0.67 |
| Smoker | 34 (17.3) | 11 (12.5) | 42 (19.6) | 18 (20.7) | 0.44 |
| Prevalent chronic diseases7 | 64 (32.5) | 31 (35.2) | 95 (44.4) | 45 (51.7) | 0.007* |
| Moderate/vigorous physical activity, MET-h | 24.9 (10.8, 51.7) | 22 (9.4, 56.8) | 25 (8.6, 41.9) | 16.5 (7.9, 40.0) | 0.14 |
| Energy intake, kcal/d | 1964 (613.4) | 1904.9 (594.4) | 1935.8 (515.1) | 1852.5 (600.2) | 0.48 |
| Coffee intake, g/d | 169.9 (11.9, 487.8) | 145.8 (15.5, 469.8) | 165.1 (9.7, 410.6) | 12.5 (0.0, 375) | <0.001* |
| Tea intake, g/d | 48.1 (9.3, 226.7) | 48.8 (10.6, 225) | 38.9 (3.3, 200) | 33.6 (3.3, 200) | 0.21 |
| Sugar-sweetened beverage intake, g/d | 8.3 (4.3, 25.3) | 16.9 (5.9, 20.3) | 5.4 (1.6, 21.4) | 4.6 (1.0, 12.7) | <0.001* |
| AHEI score5 | 52.4 (9.9) | 52.7 (9.8) | 50.9 (8.9) | 50.0 (9.2) | 0.09 |
Data presented as mean (SD), median (IQR), or n (%), as applicable. *Statistical significance, P < 0.05. AHEI, Alternative Healthy Eating Index–2010; MET-h, metabolic equivalent hours.
Characteristics not shown for the 149 women missing artificially sweetened beverage intake in pregnancy.
P values for parametric continuous variables calculated using ANOVA; P values for nonparametric continuous variables calculated using Wilcoxon 2-sample test statistic; and P values for categorical variables calculated using chi-square test statistic or Fisher's exact test, as applicable.
Prepregnancy chronic diseases include cancer and hypertension; no women had prepregnancy diabetes because the cohort consisted of all women with gestational diabetes during the index pregnancy.
AHEI score includes vegetables, fruit, whole grains, sugar-sweetened beverages and fruit juice, nuts and legumes, red/processed meat, trans-fat, long-chain (n–3) fats (EPA + DHA), PUFAs, sodium, and alcohol.
Characteristics not shown for the 28 women missing artificially sweetened beverage intake at follow-up.
The definition of prevalent chronic diseases was based on self-report of ever having a physician diagnosis of the following conditions: type 1 or type 2 diabetes, heart problems, cancer, gout, elevated cholesterol, or elevated blood pressure.
Overall consumption of ASBs both during pregnancy (Table 2) and at follow-up (Table 3) was associated with less favorable cardiometabolic profiles at follow-up. However, after adjustment for covariates including prepregnancy BMI, only women consuming ASBs weekly or daily in pregnancy had HbA1c levels that were 3–4% higher than in women consuming ASBs <1 serving per month (P-trend = 0.06); findings on other cardiometabolic phenotypes (e.g., insulin, C-peptide, HOMA-IR, waist circumference, hypertriglyceridemia, and obesity) either did not follow a significant trend with higher consumption or were not observed in the highest intake level (Table 4). When limited to women with normal weight before the index pregnancy, a significant trend (P = 0.01) was observed with higher ASB intake and HbA1c, such that daily intake was associated with 9.9% higher levels (95% CI: 1.2, 19.3) (Supplemental Table 1); however, among women with prepregnancy overweight or obesity, no significant trends were observed, and the majority of associations were null with the exception of higher C-peptide and liver fat percentage levels and an increased risk for elevated triglycerides among monthly consumers compared to nonconsumers (data not shown).
TABLE 2.
Unadjusted cardiometabolic outcomes at follow-up 9–16 y later according to artificially sweetened beverage intake in pregnancy among women with a history of gestational diabetes1
| Artificially sweetened beverage intake in pregnancy (1996–2002)2 | ||||||
|---|---|---|---|---|---|---|
| <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | |||
| n | (n = 205) | (n = 72) | (n = 121) | (n = 60) | P 3 | |
| Continuous outcomes (2012–2014) | ||||||
| HbA1C, % | 603 | 5.3 (5.1, 5.6) | 5.5 (5.0, 5.7) | 5.5 (5.2, 5.9) | 5.5 (5.1, 5.9) | 0.02* |
| Fasting glucose, mmol/L | 603 | 5.3 (5.0, 5.9) | 5.5 (5.1, 6.1) | 5.5 (5.0, 6.2) | 5.6 (5.0, 6.4) | 0.11 |
| Fasting insulin, pmol/L | 600 | 44.0 (31.0, 69.0) | 51.5 (38.0, 82.0) | 49.0 (36.0, 80.0) | 60.5 (47.0, 92.0) | <0.001* |
| C-peptide, pmol/L | 601 | 884.4 (686.4, 1221) | 1062.6 (765.6, 1343.1) | 983.4 (739.2, 1247.4) | 1042.8 (811.8, 1234.2) | 0.02* |
| HOMA-IR | 599 | 1.5 (1.0, 2.4) | 1.9 (1.3, 3.3) | 1.8 (1.2, 3.1) | 2.3 (1.5, 3.3) | <0.001* |
| HOMA-B | 599 | 63 (45.5, 101.2) | 78 (51.3, 108.8) | 75.1 (48.7, 101.7) | 85.6 (61.7, 112.9) | 0.04* |
| Triglycerides, mg/dL | 601 | 95.0 (73.0, 135.0) | 107.5 (80.5, 159.0) | 109.0 (85.0, 148.0) | 112.0 (91.0, 172.0) | 0.01* |
| HDL, mg/dL | 601 | 59.0 (49.0, 71.0) | 58.0 (48.5, 69.5) | 55.0 (47.0, 68.0) | 53.0 (46.0, 61.0) | 0.04* |
| LDL, mg/dL | 592 | 113.0 (96.0, 135.0) | 116.0 (90.0, 144.0) | 113.0 (98.0, 133.0) | 110.0 (84.0, 137.0) | 0.78 |
| BMI, kg/m2 | 606 | 26.7 (23.7, 31.3) | 27.7 (24.4, 31.6) | 29.9 (26.1, 33.3) | 30.8 (28.0, 34.3) | <0.001* |
| Waist circumference, cm | 606 | 95.6 (87.0, 105.8) | 98.3 (90.3, 106.8) | 101.2 (91.5, 111.6) | 104.4 (97.9, 115.7) | <0.001* |
| Visceral adipose tissue, cm3 | 192 | 624.0 (328.0, 1077.5) | 824.0 (245.0, 1432.0) | 771.0 (425.5, 1127.0) | 800.0 (353.0, 1609.0) | 0.48 |
| Mean arterial pressure, mm Hg | 607 | 89.8 (84.3, 101.2) | 93.3 (85.8, 100.7) | 91.3 (84.2, 101.2) | 93.1 (85.5, 102.6) | 0.65 |
| CRP, mg/L | 600 | 1.2 (0.6, 3.1) | 1.7 (0.7, 3.6) | 1.9 (0.7, 4.0) | 1.8 (0.6, 5.4) | 0.08 |
| ALT, U/L4 | 284 | 19.0 (16.0, 24.0) | 19.0 (15.0, 29.0) | 20.0 (17.0, 26.0) | 19.0 (14.5, 27.5) | 0.78 |
| AST, U/L4 | 283 | 27.5 (22.5, 33.5) | 28.0 (24.0, 36.0) | 30.0 (24.0, 34.0) | 26.5 (20.0, 35.0) | 0.52 |
| AST:ALT ratio4 | 283 | 1.4 (1.2, 1.8) | 1.5 (1.3, 1.8) | 1.4 (1.1, 1.6) | 1.4 (1.2, 1.6) | 0.27 |
| Liver fat, %4,5 | 280 | 2.5 (1.9, 5.4) | 2.6 (1.7, 7.3) | 3.8 (2.1, 8.0) | 5.0 (2.4, 7.5) | 0.04* |
| GGT, U/L4 | 286 | 17.0 (12.0, 28.0) | 16.0 (13.0, 30.0) | 18.0 (13.0, 30.0) | 22.0 (15.0, 35.0) | 0.27 |
| Bilirubin, mg/dL4 | 285 | 0.4 (0.3, 0.5) | 0.3 (0.3, 0.5) | 0.3 (0.2, 0.4) | 0.3 (0.3, 0.5) | 0.15 |
| Binary outcomes at follow-up (2012–2014)6 | ||||||
| Hyperglycemia | 603 | 18 (8.8) | 10 (13.9) | 16 (13.2) | 8 (13.3) | 0.48 |
| Type 2 diabetes | 607 | 45 (22.0) | 22 (30.6) | 33 (27.3) | 20 (33.3) | 0.23 |
| Hypertriglyceridemia | 601 | 13 (6.3) | 13 (18.1) | 11 (9.1) | 6 (10.0) | 0.04* |
| Obesity | 606 | 59 (28.8) | 26 (36.1) | 60 (49.6) | 33 (55.0) | <0.001* |
| Elevated ALT 4 | 284 | 52 (56.5) | 18 (52.9) | 38 (57.6) | 18 (52.9) | 0.96 |
| Elevated AST:ALT ratio4 | 284 | 12 (13.0) | 6 (17.7) | 4 (6.1) | 4 (11.8) | 0.35 |
| Elevated liver fat score4,7 | 280 | 34 (37.0) | 15 (44.1) | 34 (51.5) | 19 (55.9) | 0.11 |
Data presented as mean (SD), median (IQR), or n (%), as applicable. All values are unadjusted. *Statistical significance, P < 0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; fs, fasting serum; GGT, γ-glutamyltransferase; HOMA-B, homeostatic model assessment for β-cell function; T2DM, type 2 diabetes mellitus.
Outcomes not shown for the 149 women missing artificially sweetened beverage intake in pregnancy.
P values for parametric continuous variables calculated using ANOVA; P values for nonparametric continuous variables calculated using Wilcoxon 2-sample test statistic; and P values for categorical variables calculated using chi-square test statistic or Fisher's exact test, as applicable.
Outcomes related to liver function exclude women with habitual alcohol intake at follow-up of >24 g/d, approximately equivalent to 2 standard drinks/d.
Calculated liver fat % = 10(−0.805 + 0.282 × metabolic syndrome (yes = 1; no = 0) + 0.078 × T2DM (yes = 2; no = 0) + 0.525 × log(fs-insulin [mU/L]) + 0.521 × log(fs-AST [U/L]) – 0.454 × log(AST/ALT)).
The following definitions were used for the binary outcomes: elevated ALT, ≥19.0 U/L; elevated AST:ALT ratio, ratio ≥2; elevated liver fat score, >−0.640; hypertriglyceridemia, triglycerides ≥200 mg/dL; hyperglycemia, fasting glucose ≥7.0 mmol/L; type 2 diabetes, HbA1c ≥6.5%, fasting glucose ≥7.0 mmol/L, or 2-h oral glucose tolerance test glucose ≥11.1 mmol/L or self-report of physician diagnosis; obesity, BMI ≥30.0.
Liver fat score = −2.89 + 1.18 × metabolic syndrome (yes = 1/no = 0) + 0.45 × T2DM (yes = 2/no = 0) + insulin (mU/L) + 0.04 × AST (U/L) – 0.94 × AST/ALT.
TABLE 3.
Unadjusted cardiometabolic outcomes at follow-up according to artificially sweetened beverage intake at follow-up among women with a history of gestational diabetes1
| Habitual artificially sweetened beverage intake at follow-up (2012–2014)2 | ||||||
|---|---|---|---|---|---|---|
| n | (n = 197) | (n = 88) | (n = 214) | (n = 87) | P 3 | |
| Continuous outcomes (2012–2014) | ||||||
| HbA1c, % | 603 | 5.4 (5.2, 5.6) | 5.3 (5.1, 5.7) | 5.5 (5.2, 5.9) | 5.5 (5.2, 6.1) | 0.003* |
| Fasting glucose, mmol/L | 603 | 5.4 (5.0, 5.9) | 5.4 (5, 5.9) | 5.5 (5.1, 6.2) | 5.5 (5, 6.2) | 0.08 |
| Fasting insulin, pmol/L | 600 | 44.0 (31.0, 68.0) | 48.5 (36.0, 78.5) | 52.0 (36.0, 80.0) | 57.0 (43.0, 86.0) | <0.001* |
| C-peptide, pmol/L | 601 | 891.0 (699.6, 1181.4) | 973.5 (782.1, 1280.4) | 970.2 (755.7, 1240.8) | 1069.2 (844.8, 1221) | 0.09 |
| HOMA-IR | 599 | 1.5 (1.0, 2.3) | 1.6 (1.3, 2.7) | 1.9 (1.2, 3.1) | 2.1 (1.5, 3.6) | <0.001* |
| HOMA-B | 599 | 66.0 (44.0, 96.8) | 71.7 (54.1, 98.5) | 73.9 (48.3, 105.2) | 82.6 (58.5, 108.4) | 0.13 |
| Triglycerides, mg/dL | 601 | 95.0 (72.0, 136.0) | 93.5 (75.5, 133.5) | 107.0 (84.5, 155.5) | 116.0 (90.0, 173.0) | <0.001* |
| HDL, mg/dL | 601 | 60.0 (50.0, 71.0) | 59.0 (49.0, 70.0) | 55.0 (47.0, 67.0) | 54.0 (47.0, 62.0) | 0.003* |
| LDL, mg/dL | 592 | 120.0 (97.0, 143.0) | 107.5 (92.5, 128.0) | 111.0 (94.0, 133.0) | 114.0 (89.0, 134.0) | 0.06 |
| BMI, kg/m2 | 606 | 25.7 (23.1, 29.9) | 29.2 (24.9, 33.3) | 29.1 (25.4, 33.3) | 31.4 (27.0, 34.9) | <0.001* |
| Waist circumference, cm | 606 | 94.2 (86.3, 103.8) | 99.5 (92.2, 111.9) | 100.6 (90.6, 108.6) | 105.1 (96.6, 114.3) | <0.001* |
| Visceral adipose tissue, cm3 | 192 | 692.0 (348.0, 1113.0) | 677.0 (357.0, 1109.0) | 701.0 (413.0, 1319.0) | 821.5 (379.5, 1434.0) | 0.73 |
| Mean arterial pressure, mm Hg | 607 | 89.8 (83.7, 97.8) | 89.9 (84.2, 99.8) | 90.8 (84.5, 100.0) | 95 (87.7, 104.8) | 0.01* |
| CRP, mg/L | 600 | 1.3 (0.5, 2.9) | 1.3 (0.6, 3.7) | 1.8 (0.7, 4.3) | 2.5 (0.9, 4.6) | 0.008* |
| ALT, U/L4 | 284 | 19.0 (16.0, 24.0) | 18.0 (15.0, 29.0) | 21.0 (16.0, 25.0) | 19.0 (17.0, 28.0) | 0.22 |
| AST, U/L4 | 283 | 29.0 (24.0, 34.0) | 25.0 (21.0, 33.0) | 29.0 (24.0, 35.0) | 28.0 (24.0, 35.0) | 0.15 |
| AST:ALT ratio4 | 283 | 1.6 (1.2, 1.8) | 1.4 (1.0, 1.6) | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.7) | 0.049* |
| Liver fat, %4,5 | 280 | 2.6 (1.9, 5.3) | 2.6 (1.7, 6.2) | 3.7 (2.2, 7.3) | 4.3 (2.2, 7.3) | 0.04* |
| GGT, U/L4 | 286 | 17.0 (13.0, 26.0) | 18.0 (12.0, 38.0) | 19.0 (13.0, 28.0) | 22.0 (14.0, 35.0) | 0.44 |
| Bilirubin, mg/dL4 | 285 | 0.3 (0.3, 0.6) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) | 0.3 (0.3, 0.4) | 0.67 |
| Binary outcomes at follow-up (2012–2014)6 | ||||||
| Hyperglycemia | 603 | 11 (5.6) | 7 (8.0) | 31 (14.5) | 13 (14.9) | 0.01* |
| Type 2 diabetes | 607 | 39 (19.8) | 20 (22.7) | 68 (31.8) | 28 (32.2) | 0.02* |
| Hypertriglyceridemia | 601 | 13 (6.6) | 4 (4.6) | 22 (10.3) | 12 (13.8) | 0.08 |
| Obesity | 606 | 48 (24.4) | 36 (40.9) | 93 (43.5) | 54 (62.1) | <0.001* |
| Elevated ALT 4 | 284 | 40 (50.6) | 18 (46.2) | 62 (62.0) | 34 (58.6) | 0.23 |
| Elevated AST:ALT ratio4 | 284 | 14 (17.7) | 2 (5.1) | 10 (10.0) | 7 (12.1) | 0.20 |
| Elevated liver fat score4,7 | 280 | 25 (31.7) | 16 (41.0) | 48 (48.0) | 32 (55.2) | 0.03* |
Data presented as mean (SD), median (IQR), or n (%), as applicable. All values are unadjusted. *Statistical significance, P < 0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; fs, fasting serum; GGT, γ-glutamyltransferase; HOMA-B, homeostatic model assessment for β-cell function; T2DM, type 2 diabetes mellitus.
Outcomes not shown for the 28 women missing artificially sweetened beverage intake at follow-up.
P values for parametric continuous variables calculated using ANOVA; P values for nonparametric continuous variables calculated using Wilcoxon 2-sample test statistic; and P values for categorical variables calculated using chi-square test statistic or Fisher's exact test, as applicable.
Outcomes related to liver function exclude women with habitual alcohol intake at follow-up of >24 g/d, approximately equivalent to 2 standard drinks/d.
Calculated liver fat % = 10(−0.805 + 0.282 × metabolic syndrome (yes = 1; no = 0) + 0.078 × T2DM (yes = 2; no = 0) + 0.525 × log(fs-insulin [mU/L]) + 0.521 × log(fs-AST [U/L]) – 0.454 × log(AST/ALT)).
The following definitions were used for the binary outcomes: elevated ALT, ≥19.0 U/L; elevated AST:ALT ratio, ratio ≥2; elevated liver fat score, > −0.640; hypertriglyceridemia, triglycerides ≥200 mg/dL; hyperglycemia, fasting glucose ≥7.0 mmol/L; type 2 diabetes, HbA1c ≥6.5%, fasting glucose ≥7.0 mmol/L, or 2-h oral glucose tolerance test glucose ≥11.1 mmol/L or self-report of physician diagnosis; obesity, BMI ≥30.0.
Liver fat score = −2.89 + 1.18 × metabolic syndrome (yes = 1/no = 0) + 0.45 × T2DM (yes = 2/no = 0) + insulin (mU/L) + 0.04 × AST (U/L) – 0.94 × AST/ALT.
TABLE 4.
Consumption of artificially sweetened beverages during pregnancy and adjusted associations with cardiometabolic outcomes 9–16 y later among women with a history of gestational diabetes1
| Artificially sweetened beverage intake in pregnancy (1996–2002) | ||||||
|---|---|---|---|---|---|---|
| n | <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | P-trend | |
| Continuous outcomes (2012–2014)2 | ||||||
| HbA1c | 603 | 0.0 (Reference) | 2.2 (−1.6, 6.1) | 3.0 (0.3, 5.9)* | 4.2 (0.2, 8.3)* | 0.06 |
| Fasting glucose | 603 | 0.0 (Reference) | 0.6 (−6.6, 8.5) | 3.2 (−1.3, 8.0) | 4.0 (−2.4, 10.8) | 0.24 |
| Fasting insulin | 600 | 0.0 (Reference) | 21.1 (4.3, 40.7)* | 10.8 (−3.4, 27.0) | 9.2 (−8.1, 29.8) | 0.58 |
| C-peptide | 601 | 0.0 (Reference) | 14.8 (3.2, 27.6)* | 1.7 (−7.1, 11.4) | 0.0 (−10.9, 12.2) | 0.62 |
| HOMA-IR | 599 | 0.0 (Reference) | 24.5 (6.0, 46.1)* | 13.6 (−2.1, 31.8) | 11.7 (−6.8, 33.9) | 0.48 |
| HOMA-B | 599 | 0.0 (Reference) | 15.3 (−3.8, 38.3) | 4.3 (−10.2, 21.1) | 2.2 (−16.9, 25.7) | 0.94 |
| Triglycerides | 601 | 0.0 (Reference) | 10.4 (−1.3, 23.6) | 4.5 (−4.9, 14.9) | 4.5 (−7.6, 18.1) | 0.71 |
| HDL | 601 | 0.0 (Reference) | 0.2 (−5.6, 6.4) | −1.5 (−6.4, 3.7) | −1.1 (−6.9, 5.2) | 0.72 |
| LDL | 592 | 0.0 (Reference) | −0.2 (−8.2, 8.6) | 1.0 (−5.6, 8.0) | −3.9 (−11.8, 4.6) | 0.34 |
| BMI | 606 | 0.0 (Reference) | 1.5 (−1.9, 5.1) | 2.7 (−0.4, 5.9) | 1.0 (−2.9, 5.0) | 0.79 |
| Waist circumference | 606 | 0.0 (Reference) | 2.0 (−0.5, 4.5) | 2.6 (0.3, 4.9)* | 1.6 (−1.1, 4.5) | 0.40 |
| Visceral adipose tissue | 192 | 0.0 (Reference) | 27.6 (−14.4, 90.3) | 15.9 (−16.7, 61.4) | 18.7 (−20.7, 77.7) | 0.54 |
| Mean arterial pressure | 607 | 0.0 (Reference) | 1.1 (−1.8, 4.2) | −0.6 (−3.2, 2.0) | −0.8 (−3.6, 2.2) | 0.51 |
| CRP | 600 | 0.0 (Reference) | 17.6 (−13.5, 60.0) | 19.4 (−7.7, 54.3) | 9.9 (−17.5, 46.4) | 0.70 |
| ALT3 | 284 | 0.0 (Reference) | 3.7 (−11.1, 21.0) | 8.7 (−6.4, 26.2) | −1.7 (−17.1, 16.5) | 0.69 |
| AST3 | 283 | 0.0 (Reference) | 6.0 (−6.7, 20.5) | 4.6 (−6.5, 17.0) | −0.0 (−11.6, 13.1) | 0.80 |
| AST:ALT ratio3 | 283 | 0.0 (Reference) | 3.8 (−8.3, 17.4) | −3.7 (−12.7, 6.1) | 1.6 (−9.8, 14.4) | 0.82 |
| Liver fat %3,4 | 280 | 0.0 (Reference) | 13.4 (−11.7, 45.5) | 21.2 (−0.9, 48.3) | 14.6 (−10.9, 47.3) | 0.42 |
| GGT3 | 286 | 0.0 (Reference) | 3.0 (−18.9, 30.7) | 8.8 (−11.2, 33.4) | 13.6 (–11.0, 44.8) | 0.33 |
| Bilirubin3 | 285 | 0.0 (Reference) | −4.1 (–22.0, 17.9) | −11.9 (−23.6, 1.6) | −3.3 (−19.1, 15.6) | 0.87 |
| Binary outcomes (2012–2014)5 | ||||||
| Hyperglycemia | 603 | 1.00 (Reference) | 1.49 (0.73, 3.04) | 1.31 (0.70, 2.43) | 1.22 (0.56, 2.66) | 0.82 |
| Type 2 diabetes | 607 | 1.00 (Reference) | 1.29 (0.85, 1.94) | 1.11 (0.77, 1.59) | 1.17 (0.77, 1.77) | 0.63 |
| Hypertriglyceridemia | 601 | 1.00 (Reference) | 2.50 (1.27, 4.90)* | 1.21 (0.57, 2.56) | 1.13 (0.49, 2.63) | 0.72 |
| Obesity | 606 | 1.00 (Reference) | 1.20 (0.87, 1.64) | 1.43 (1.13, 1.82)* | 1.18 (0.89, 1.57) | 0.50 |
| Elevated ALT3 | 284 | 1.00 (Reference) | 0.92 (0.64, 1.33) | 1.06 (0.79, 1.41) | 1.05 (0.77, 1.43) | 0.70 |
| Elevated AST:ALT ratio3 | 284 | 1.00 (Reference) | 1.39 (0.53, 3.61) | 0.59 (0.22, 1.60) | 1.13 (0.43, 2.96) | 0.85 |
| Elevated liver fat score3,6 | 280 | 1.00 (Reference) | 1.20 (0.76, 1.89) | 1.24 (0.87, 1.77) | 1.22 (0.83, 1.81) | 0.43 |
Analyses adjusted for index pregnancy characteristics, including maternal age, prepregnancy BMI, parity, education, smoking, prepregnancy chronic diseases, moderate/vigorous physical activity, Alternative Healthy Eating Index–2010, intake of tea, and intake of coffee. Multiple imputation with 20 replicates was used for missing exposure and covariate data. *Statistical significance, P < 0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; fs, fasting serum; GGT, γ-glutamyltransferase; HOMA-B, homeostatic model assessment for β-cell function; T2DM, type 2 diabetes mellitus.
Continuous outcomes were log-transformed. Results are presented as the percentage difference (95% CI) calculated as the exponentiated β coefficient from the adjusted linear regression model, subtracting 1 and multiplying by 100.
Outcomes related to liver function exclude women with habitual alcohol intake at follow-up of >24 g/d, approximately equivalent to 2 standard drinks/d.
Calculated liver fat % = 10(−0.805 + 0.282 × metabolic syndrome (yes = 1; no = 0) + 0.078 × T2DM (yes = 2; no = 0) + 0.525 × log(fs-insulin [mU/L]) + 0.521 × log(fs-AST [U/L]) – 0.454 × log(AST/ALT)).
Binary outcomes are presented as RR (95% CI) calculated from a Poisson regression model with robust error variance. The following definitions were used for the binary outcomes: elevated ALT, ≥19.0 U/L; elevated AST:ALT ratio, ratio ≥2; liver fat score, > −0.640; hypertriglyceridemia, triglycerides ≥200 mg/dL; hyperglycemia, fasting glucose ≥7.0 mmol/L; type 2 diabetes, HbA1c ≥6.5%, fasting glucose ≥7.0 mmol/L, or 2-h oral glucose tolerance test glucose ≥11.1 mmol/L or self-report of physician diagnosis; obesity, BMI ≥30.0.
Liver fat score = −2.89 + 1.18 × metabolic syndrome (yes = 1/no = 0) + 0.45 × T2DM (yes = 2/no = 0) + insulin (mU/L) + 0.04 × AST (U/L) – 0.94 ×AST/ALT.
After adjusting for confounding variables including prepregnancy BMI, habitual ASB intake in the past year was not associated with the majority of cardiometabolic markers or clinical outcomes at follow-up except for ∼3–7% higher HbA1c levels among women consuming ASBs at least weekly compared to those consuming ASBs <1 serving per month (Table 5). When limited to women with prepregnancy normal weight, the significant trend with HbA1c was maintained, and an increased risk of hyperglycemia was also observed (Supplemental Table 2). Among women with prepregnancy overweight or obesity, no significant trends were observed, and the majority of associations were null with the exception of lower LDL levels among monthly and weekly consumers and a lower AST:ALT ratio among monthly consumers compared with nonconsumers (data not shown). When limited to women who were consumers of ASBs in pregnancy, the association with habitual intake in the past year of the follow-up and HbA1c levels was consistently observed (Supplemental Table 3). However, when limited to women without known chronic diseases at follow-up, the results were attenuated (Supplemental Table 4).
TABLE 5.
Consumption of artificially sweetened beverages during the past year and adjusted associations with cardiometabolic outcomes among women with a history of gestational diabetes1
| Habitual artificially sweetened beverage intake at follow-up (2012–2014) | ||||||
|---|---|---|---|---|---|---|
| n | <1 serving/mo | 1–4 servings/mo | 2–6 servings/wk | ≥1 servings/d | P-trend | |
| Continuous outcomes (2012–2014)2 | ||||||
| HbA1c | 603 | 0.0 (Reference) | −0.7 (−3.3, 1.9) | 3.0 (0.6, 5.5)* | 6.5 (1.9, 11.3)* | 0.007* |
| Fasting glucose | 603 | 0.0 (Reference) | 0.6 (−3.1, 4.4) | 4.5 (0.2, 9.1)* | 7.0 (−0.4, 14.9) | 0.11 |
| Fasting insulin | 600 | 0.0 (Reference) | 6.8 (−6.9, 22.5) | −3.0 (−14.4, 9.9) | −7.8 (−26.0, 14.9) | 0.39 |
| C-peptide | 601 | 0.0 (Reference) | 4.1 (−5.2, 14.4) | −2.9 (−10.7, 5.7) | −7.5 (−19.2, 5.9) | 0.20 |
| HOMA-IR | 599 | 0.0 (Reference) | 7.4 (−7.5, 24.8) | 2.4 (−10.4, 17.0) | −1.1 (−20.5, 22.9) | 0.74 |
| HOMA-B | 599 | 0.0 (Reference) | 4.3 (−9.3, 19.9) | −13.7 (−24.8, −1.0)* | −18.9 (−38.6, 7.1) | 0.17 |
| Triglycerides | 601 | 0.0 (Reference) | −2.2 (−11.3, 7.9) | 4.2 (−4.7, 14.0) | 5.8 (−8.1, 21.9) | 0.44 |
| HDL | 601 | 0.0 (Reference) | 2.7 (−2.9, 8.6) | 1.3 (−3.6, 6.4) | 1.1 (−6.0, 8.7) | 0.97 |
| LDL | 592 | 0.0 (Reference) | −5.6 (−12.2, 1.5) | −4.1 (−9.7, 1.8) | −1.8 (−9.8, 6.8) | 0.89 |
| BMI | 606 | 0.0 (Reference) | 3.0 (−0.2, 6.2) | 0.8 (−2.2, 4.0) | 2.5 (−2.8, 8.2) | 0.52 |
| Waist circumference | 606 | 0.0 (Reference) | 2.9 (0.3, 5.6)* | 0.3 (−1.7, 2.4) | 1.1 (−1.8, 4.2) | 0.79 |
| Visceral adipose tissue | 192 | 0.0 (Reference) | 10.4 (−21.7, 55.7) | 4.4 (−22.6, 40.8) | −7.4 (−37.5, 37.4) | 0.55 |
| Mean arterial pressure | 607 | 0.0 (Reference) | 0.3 (−2.6, 3.2) | −0.4 (−2.6, 1.9) | 2.6 (−0.9, 6.1) | 0.11 |
| CRP | 600 | 0.0 (Reference) | −5.8 (−29.3, 25.5) | −6.4 (−25.4, 17.4) | −9.8 (−33.7, 22.7) | 0.61 |
| ALT3 | 284 | 0.0 (Reference) | 2.6 (−12.7, 20.5) | 8.2 (−4.8, 22.9) | 2.4 (−12.4, 19.8) | 0.94 |
| AST3 | 283 | 0.0 (Reference) | −10.8 (−21.3, 1.2) | −2.1 (−10.8, 7.5) | −3.9 (−14.9, 8.5) | 0.90 |
| AST:ALT ratio3 | 283 | 0.0 (Reference) | −13.2 (−22.7, −2.5)* | −9.2 (−17.0, −0.6)* | −6.4 (−16.3, 4.8) | 0.92 |
| Liver fat %3,4 | 280 | 0.0 (Reference) | −6.4 (−26.3, 18.7) | 10.7 (−8.8, 34.3) | −1.0 (−23.5, 28.1) | 0.84 |
| GGT3 | 286 | 0.0 (Reference) | 8.9 (−18.5, 45.7) | −4.5 (−20.4, 14.6) | 4.7 (−16.4, 31.2) | 0.69 |
| Bilirubin3 | 285 | 0.0 (Reference) | 7.0 (−11.2, 29.0) | 5.2 (−8.7, 21.2) | −4.0 (−18.4, 13.0) | 0.31 |
| Binary outcomes (2012–2014)5 | ||||||
| Hyperglycemia | 603 | 1.00 (Reference) | 1.12 (0.47, 2.69) | 1.80 (0.95, 3.42) | 1.69 (0.74, 3.83) | 0.41 |
| Type 2 diabetes | 607 | 1.00 (Reference) | 1.03 (0.65, 1.65) | 1.34 (0.95, 1.91) | 1.13 (0.75, 1.71) | 0.94 |
| Hypertriglyceridemia | 601 | 1.00 (Reference) | 0.66 (0.21, 2.05) | 1.33 (0.67, 2.62) | 1.31 (0.57, 3.01) | 0.50 |
| Obesity | 606 | 1.00 (Reference) | 1.25 (0.91, 1.70) | 1.21 (0.93, 1.57) | 1.29 (0.95, 1.74) | 0.26 |
| Elevated ALT3 | 284 | 1.00 (Reference) | 0.87 (0.59, 1.30) | 1.17 (0.89, 1.53) | 1.05 (0.77, 1.45) | 0.83 |
| Elevated AST:ALT ratio3 | 284 | 1.00 (Reference) | 0.29 (0.07, 1.15) | 0.51 (0.24, 1.08) | 0.74 (0.29, 1.89) | 0.86 |
| Elevated liver fat score3,6 | 280 | 1.00 (Reference) | 1.11 (0.69, 1.78) | 1.39 (0.96, 2.00) | 1.29 (0.87, 1.93) | 0.43 |
Analyses adjusted for current characteristics, including maternal age, parity, education, smoking, moderate/vigorous physical activity, Alternative Healthy Eating Index–2010, intake of tea, intake of coffee, and prepregnancy chronic diseases at the index pregnancy. Multiple imputation with 20 replicates was used for missing exposure and covariate data. *Statistical significance, P < 0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; fs, fasting serum; GGT, γ-glutamyltransferase; HOMA-B, homeostatic model assessment for β-cell function; T2DM, type 2 diabetes mellitus.
Continuous outcomes were log-transformed. Results are presented as the percentage difference (95% CI) calculated as the exponentiated β coefficient from the adjusted model, subtracting 1 and multiplying by 100.
Outcomes related to liver function exclude women with habitual alcohol intake >24 g/d, approximately equivalent to 2 standard drinks/d.
Calculated liver fat % = 10(−0.805 + 0.282 × metabolic syndrome (yes = 1; no = 0) + 0.078 × T2DM (yes = 2; no = 0) + 0.525 × log(fs-insulin [mU/L]) + 0.521 × log(fs-AST [U/L]) – 0.454 × log(AST/ALT)).
Binary outcomes are presented as RR (95% CI). The following definitions were used for the binary outcomes: elevated ALT, ≥19.0 U/L; elevated AST:ALT ratio, ratio ≥2; liver fat score, > −0.640; hypertriglyceridemia, triglycerides ≥200 mg/dL; hyperglycemia, fasting glucose ≥7.0 mmol/L; type 2 diabetes, HbA1C ≥6.5%, fasting glucose ≥7.0 mmol/L, or 2-h oral glucose tolerance test glucose ≥11.1 mmol/L or self-report of physician diagnosis; obesity, BMI ≥30.0.
Liver fat score = −2.89 + 1.18 × metabolic syndrome (yes = 1/no = 0) + 0.45 × T2DM (yes = 2/no = 0) + insulin (mU/L) + 0.04 × AST (U/L) – 0.94 × AST/ALT.
Between pregnancy and follow-up, more women regularly consumed ASBs (30.4% compared with 36.4%), yet on average the weekly intake did not differ among the regular consumers [mean change: 0.2 servings per week (SD = 12.2); P = 89]. Comparatively, fewer women regularly consumed sugar-sweetened beverages (62.7% compared with 7.9%), and among regular consumers the average weekly intake also decreased [mean change: −4.8 servings per week (SD = 9.0); P = 0.02]. Table 6 displays associations between ASB consumption and cardiometabolic outcomes according to women's ASB consumption in pregnancy and in the past year at the follow-up visit. Compared with women with occasional or no consumption (i.e., ≤4 servings per month) in pregnancy and at follow-up, women with regular consumption (i.e., ≥2 servings per week) at both time points had 6–7% higher HbA1c and fasting glucose levels and a 37% increased risk in obesity. Among women with prepregnancy normal weight, regular consumption at both time points was associated with increased HbA1c, fasting glucose, triglyceride, and liver fat levels (Supplemental Table 5). However, among women with prepregnancy overweight or obesity (data not shown) or without known chronic diseases at follow-up, the results were attenuated (Supplemental Table 6).
TABLE 6.
Long-term consumption patterns of artificially sweetened beverages from pregnancy 9–16 y earlier to the past year and adjusted associations with current cardiometabolic outcomes among women with a history of gestational diabetes1
| Habitual artificially sweetened beverage intake | |||||
|---|---|---|---|---|---|
| n | ≤4 servings/mo in pregnancy and at follow-up | ≤4 servings/mo in pregnancy and ≥2 servings/wk at follow-up | ≥2 servings/wk in pregnancy and ≤4 servings/mo at follow-up | ≥2 serving/wk in pregnancy and at follow-up | |
| Continuous outcomes (2012–2014)2 | |||||
| HbA1c | 603 | 0.0 (Reference) | 4.3 (1.3, 7.4)* | 3.0 (−0.1, 6.1) | 6.0 (2.8, 9.1)* |
| Fasting glucose | 603 | 0.0 (Reference) | 3.5 (−2.2, 9.4) | 1.5 (−2.7, 5.9) | 7.1 (2.2,12.4)* |
| Fasting insulin | 600 | 0.0 (Reference) | −6.4 (−20.9, 10.8) | 8.6 (−8.2, 28.5) | −1.6 (−14.8, 13.7) |
| C-peptide | 601 | 0.0 (Reference) | −2.7 (−12.5, 8.2) | 1.3 (−10.0, 14.1) | −6.8 (−15.1, 2.4) |
| HOMA-IR | 599 | 0.0 (Reference) | −0.5 (−16.1, 18.1) | 10.5 (−8.3, 33.3) | 4.7 (−10.3, 22.2) |
| HOMA-B | 599 | 0.0 (Reference) | −16.9 (−31.9, 1.4) | 3.1 (−11.8, 20.5) | −14.3 (−27.3, 1.1) |
| Triglycerides | 601 | 0.0 (Reference) | 10.7 (0.0, 22.5) | 6.3 (−5.0, 19.0) | 5.2 (−4.9, 16.4) |
| HDL | 601 | 0.0 (Reference) | −0.6 (−6.6, 5.7) | −2.8 (−9.0, 3.9) | −0.6 (−5.8, 5.0) |
| LDL | 592 | 0.0 (Reference) | −1.7 (−8.2, 5.2) | −0.7 (−9.2, 8.7) | −2.0 (−8.5, 4.9) |
| BMI | 606 | 0.0 (Reference) | 1.5 (−2.7, 5.9) | 3.5 (−0.2, 7.3) | 1.5 (−1.7, 4.8) |
| Waist circumference | 606 | 0.0 (Reference) | −0.8 (−3.2, 1.6) | 1.7 (−1.4, 4.9) | 1.0 (−1.2, 3.3) |
| Visceral adipose tissue | 192 | 0.0 (Reference) | −0.2 (−30.8, 43.7) | 13.6 (−25.0, 72.1) | 3.7 (−26.4, 46.2) |
| Mean arterial pressure | 607 | 0.0 (Reference) | 0.2 (−2.6, 3.0) | −1.4 (−4.6, 1.9) | −0.5 (−3.0, 2.1) |
| CRP | 600 | 0.0 (Reference) | −2.7 (−26.6, 28.9) | 21.0 (−11.3, 65.1) | 3.5 (−19.8, 33.5) |
| ALT3 | 284 | 0.0 (Reference) | 8.9 (−5.7, 25.7) | 6.0 (−15.3, 32.5) | 6.5 (−8.4, 24.0) |
| AST3 | 283 | 0.0 (Reference) | 2.4 (−8.4, 14.5) | 1.8 (−12.8, 19.0) | 1.5 (−9.1, 13.4) |
| AST:ALT ratio3 | 283 | 0.0 (Reference) | −5.3 (−15.3, 5.8) | −3.9 (−16.6, 10.6) | −4.8 (−14.6, 6.0) |
| Liver fat %3,4 | 280 | 0.0 (Reference) | 8.0 (−12.8, 33.7) | 13.8 (−12.5, 48.1) | 19.8 (−3.3, 48.5) |
| GGT3 | 286 | 0.0 (Reference) | 2.6 (−17.9, 28.2) | 20.1 (−12.8, 65.4) | 3.0 (−16.4, 27.0) |
| Bilirubin3 | 285 | 0.0 (Reference) | −3.5 (−18.0, 13.6) | −14.2 (−28.9, 3.4) | −8.5 (−21.3, 6.4) |
| Binary outcomes (2012–2014)5 | |||||
| Hyperglycemia | 603 | 1.00 (Reference) | 1.86 (0.92, 3.78) | 1.20 (0.45, 3.21) | 1.80 (0.86, 3.75) |
| Type 2 diabetes | 607 | 1.00 (Reference) | 1.32 (0.90, 1.92) | 1.08 (0.65, 1.77) | 1.28 (0.88, 1.87) |
| Hypertriglyceridemia | 601 | 1.00 (Reference) | 1.77 (0.82, 3.78) | 0.92 (0.34, 2.45) | 1.23 (0.56, 2.68) |
| Obesity | 606 | 1.00 (Reference) | 1.18 (0.88, 1.59) | 1.41 (0.98, 2.02) | 1.37 (1.04, 1.81) |
| Elevated ALT3 | 284 | 1.00 (Reference) | 1.23 (0.88, 1.72) | 1.08 (0.68, 1.71) | 1.21 (0.87, 1.69) |
| Elevated AST:ALT ratio3 | 284 | 1.00 (Reference) | 0.88 (0.38, 2.04) | 0.84 (0.27, 2.62) | 0.62 (0.25, 1.57) |
| Elevated liver fat score3,6 | 280 | 1.00 (Reference) | 1.37 (0.92, 2.05) | 1.23 (0.73, 2.07) | 1.45 (0.97, 2.15) |
Analyses adjusted for current characteristics, including maternal age, parity, education, smoking, moderate/vigorous physical activity, Alternative Healthy Eating Index–2010, intake of tea, intake of coffee, and prepregnancy chronic diseases at the index pregnancy. Multiple imputation with 20 replicates was used for missing exposure and covariate data. *Statistical significance, P < 0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; fs, fasting serum; GGT, γ-glutamyltransferase; HOMA-B, homeostatic model assessment for β-cell function; T2DM, type 2 diabetes mellitus.
Continuous outcomes were log-transformed. Results are presented as the percentage difference (95% CI) calculated as the exponentiated β coefficient from the adjusted model, subtracting 1 and multiplying by 100.
Outcomes related to liver function exclude women with habitual alcohol intake >24 g/d, approximately equivalent to 2 standard drinks/d.
Calculated liver fat % = 10(−0.805 + 0.282 × metabolic syndrome (yes = 1; no = 0) + 0.078 × T2DM (yes = 2; no = 0) + 0.525 × log(fs-insulin [mU/L]) + 0.521 × log(fs-AST [U/L]) – 0.454 × log(AST/ALT)).
Binary outcomes are presented as RR (95% CI). The following definitions were used for the binary outcomes: elevated ALT, ≥19.0 U/L; elevated AST:ALT ratio, ratio ≥2; liver fat score, > −0.640; hypertriglyceridemia, triglycerides ≥200 mg/dL; hyperglycemia, fasting glucose ≥7.0 mmol/L; type 2 diabetes, HbA1c ≥6.5%, fasting glucose ≥7.0 mmol/L, or 2-h oral glucose tolerance test glucose ≥11.1 mmol/L or self-report of physician diagnosis; obesity, BMI ≥30.
Liver fat score = −2.89 + 1.18 × metabolic syndrome (yes = 1/no = 0) + 0.45 × T2DM (yes = 2/no = 0) + insulin (mU/L) + 0.04 × AST (U/L) – 0.94 × AST/ALT.
Sensitivity analyses utilizing inverse probability weights to account for loss to follow-up yielded similar results (data not shown). At follow-up, 6.9% and 9.4% of women added artificial sweeteners to their coffee and tea, respectively. Sensitivity analyses that included servings of coffee and tea with added artificial sweeteners to the ASB intake categories yielded similar results to the overall findings, with only a significant trend observed across ASB categories for HbA1c (Supplemental Table 7). Last, sensitivity analyses limited to nonconsumers of sugar-sweetened beverages in pregnancy (n = 82) or at follow-up (n = 319) also yielded findings that were null but generally in the same direction as the overall findings (Supplemental Tables 8 and 9).
Discussion
Using data collected at 2 time points 9–16 y apart among women with a history of GDM, we examined associations between ASB consumption and a comprehensive panel of cardiometabolic markers and clinical outcomes. After careful consideration for reverse causality and confounding, we found that ASB consumption was not significantly associated with either beneficial or detrimental cardiometabolic profiles in this sample of high-risk women. We observed that consumers of ASBs have an overall unfavorable cardiometabolic profile; however, this was largely attributable to existing adiposity at baseline, with the possible exception of an adverse impact on glucose homeostasis. Specifically, we observed that ASB intake was significantly associated with a small increase in HbA1c levels, particularly among women with normal prepregnancy weight, but these findings became statistically insignificant in sensitivity analyses to rule out the possibility of reverse causation. Furthermore, some adverse associations were observed with low levels of consumption, but the signals were not consistent across exposure time periods. Thus, the lack of consistent findings after accounting for other risk factors indicates that there were likely no adverse or favorable associations between ASB intake and cardiometabolic health among women with a history of GDM.
Women with a history of GDM have an exceptionally high risk for developing T2DM and other cardiometabolic complications later in life. Prior observational studies reported adherence to healthful dietary patterns was associated with a lower risk of progression from GDM to T2DM (36), yet there has been limited research on beverage intake in this high-risk population. We found that ∼30% of the women in our study were regularly consuming ASBs during pregnancy, which is much higher than in the general DNBC cohort and estimates for all US pregnant women during a similar period (19, 37), likely due to the fact that all women in our study had GDM during their pregnancy. At follow-up 9–16 y later, ∼36% were regular consumers. Notably, however, the amount that consumers were drinking was relatively low, with the majority of women consuming <1 serving per day. ASB consumption was strongly associated with less sugar-sweetened beverage intake, higher BMI prior to the index pregnancy and at follow-up, as well as prevalent chronic diseases at follow-up, which suggests that ASB intake may be a marker of high-risk women and thus the need for careful consideration of reverse causality.
Findings from many, but not all, animal studies have suggested an adverse association between high levels of ASBs and glucose intolerance, insulin resistance, and obesity (4, 38, 39). Yet, data in humans are conflicting. Observational studies have been mixed, with both beneficial and adverse associations observed between ASB intake and adiposity, metabolic syndrome, and T2DM; however, positive associations are largely confounded by reverse causality and attenuated with adjustment for BMI (4, 5, 7). Clinical trials tend to suggest either no effect or no beneficial effects on body weight with short-term replacement of sugar-sweetened beverages with ASBs, yet long-term evidence is lacking (4–6). Therefore, current recommendations for ASB intake are generally cautious given the limited evidence base; they suggest that ASBs can be used to reduce added sugar intake from sugar-sweetened beverages, particularly among individuals with diabetes, with the caveat that compensation with additional calories does not take place (2).
A major concern with observational studies on ASB consumption is confounding and reverse causality. For example, individuals who choose to drink ASBs may have switched from sugar-sweetened beverages given the historical perspective that ASBs were a benign low-calorie beverage option (36). We observed in our data that prepregnancy BMI was strongly and positively associated with ASB intake during pregnancy. Without adjustment for prepregnancy BMI, we would have observed an unfavorable cardiometabolic profile associated with ASB intake. Even after adjustment, we observed some positive associations with HbA1c and fasting glucose levels. However, with further consideration of reverse causality, particularly that the HbA1c findings were null among women with prepregnancy overweight or obesity, we suggest that ASBs are not associated with any of the studied cardiometabolic outcomes among women with a history of GDM.
To provide a more complete assessment of ASB intake, our analysis included a wide range of cardiometabolic outcomes across various domains. Associations between ASB intake and cardiometabolic health are biologically plausible given the wide array of systems that either have sweet taste (or similarly structured) receptors or have metabolic actions downstream of these pathways (8). A large cohort study examined components of metabolic syndrome and similarly observed null findings with blood pressure, HDL cholesterol, or triglycerides; significant associations with waist circumference and fasting glucose were observed, but these associations were not adjusted for baseline BMI (40). We also examined the outcome of fatty liver using previously validated liver fat scores (27). After adjustment for baseline BMI, we did not observe any significant findings with any liver outcome, which is consistent with the 1 prior study on ASBs and liver disease (41). The reporting of these null associations across multiple systems in the current study indicates that there are not overlooked pathways potentially affected by ASB intake and provides some of the first data in the high-risk population of women with prior GDM for whom informed recommendations are critically important.
Major strengths of our study are the use of prospective data and robust sensitivity analyses to reduce the potential for reverse causation. In addition, the wide range of outcomes in the study were assessed at a clinical exam performed according to a standard protocol. Furthermore, our follow-up data of 9–16 y adds to the literature on the potential long-term impacts of ASB consumption. One potential limitation of our study is that we utilized prepregnancy BMI to adjust for baseline BMI. Prepregnancy BMI was calculated from self-reported prepregnancy weight that was ascertained early in pregnancy. However, self-reported prepregnancy weight is highly correlated with measured prepregnancy weight (42). In addition, we did not have information on the type of artificial sweeteners in the drinks consumed by women in the study. However, during the same time period in Denmark, carbonated beverages usually contained a mixture of aspartame and acesulfame-K, and noncarbonated beverages usually contained cyclamate and saccharine (43). Cyclamate is approved in Europe but not in the United States or Canada (8), which may impact the generalizability of our findings beyond European women with prior GDM. Furthermore, we do not have data on food products containing nonnutritive sweeteners. Thus, overall consumption of nonnutritive sweeteners is likely underestimated. Another limitation was that we did not have data on how long women were consuming ASBs prior to baseline (i.e., pregnancy), which may have potentially led to obesity at baseline, in which case adjustment for prepregnancy BMI could be an overadjustment. Also, prior ASB intake may have contributed to the presence of chronic diseases at follow-up; therefore, excluding these women in sensitivity analyses could also be an overadjustment. However, we examined consumption patterns at both time points 9–16 y apart and also consumption at follow-up among women who were consumers at baseline, potentially indicating long-term consumption; these results were mostly null with the exception of HbA1C and also not robust to sensitivity analyses to address reverse causality. Last, our sample was moderately sized, which may have limited our power to detect true associations, especially among the highest consumers where the levels were quite low and for some of the select groups used for sensitivity analyses. Nonetheless, the current study represents the largest study among women at high risk with long-term follow-up and a comprehensive characterization of cardiometabolic profiles.
In conclusion, we observed that among Danish women with a history of GDM, a population at high risk for cardiometabolic diseases, ASB intake was not associated with either improvements in or worsening of cardiometabolic complications. Although high-risk individuals are recommended to consume ASBs instead of sugar-sweetened beverages as a means of reducing sugar intake and risk for cardiometabolic diseases, we did not observe significant improvements in or worsening of cardiometabolic health with higher ASB intake in this population.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows: CZ: obtained funding and led the Diabetes & Women's Health Study cohort design and data collection; SNH: designed the project conception and development of the overall research plan; SNH: analyzed the data and wrote the first draft of the manuscript; all authors: read the manuscript, interpreted the results, provided critical intellectual content, and approved the final manuscript. SNH and CZ: had primary responsibility for final content. The authors report no conflicts of interest.
Notes
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the NIH (contracts HHSN275201000020C, HHSN275201500003C, HHSN275201300026I, and HSN275201100002I), the March of Dimes Birth Defects Foundation (grants 6-FY-96-0240, 6-FY97-0553, 6-FY97-0521, and 6-FY00-407), Innovation Fund Denmark [grants 09-067124 and 11-115923, (Centre for Fetal Programming)], the Health Foundation (grant 11/263-96), the Heart Foundation (grant 96-2-4-83-22450), the European Union (grant FP7-289346-EarlyNutrition), and the Danish Diabetes Academy supported by the Novo Nordisk Foundation. YZ is supported by a career development award from the NIH Building Interdisciplinary Research Careers in Women's Health Program (grant 5K12HD05216). SHL is supported by grant P20GM109036 from the National Institute of General Medical Sciences of the NIH.
Data sharing: The analytic code used for the analysis described in this article is available on request by contacting SNH (hinklesn@mail.nih.gov). The data described in the article are not currently available for sharing; please contact CZ for more information (zhangcu@mail.nih.gov).
Supplemental Figure 1 and Supplemental Tables 1–9 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternate Healthy Eating Index; ALT, alanine aminotransferase; ASB, artificially sweetened beverage; AST, aspartate aminotransferase; CRP, C-reactive protein; DNBC, Danish National Birth Cohort; DWH, Diabetes & Women's Health Study; GDM, gestational diabetes mellitus; HbA1c, glycated hemoglobin; TG, triglyceride; T2DM, type 2 diabetes mellitus.
References
- 1. Popkin BM, Hawkes C.. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016;4(2):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson RK, Lichtenstein AH, Anderson CA, Carson JA, Després J-P, Hu FB, Kris-Etherton PM, Otten JJ, Towfighi A, Wylie-Rosett J. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018;138(9):e126–e40. [DOI] [PubMed] [Google Scholar]
- 3. Burke MV, Small DM.. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol Behav. 2015;152:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rogers P, Hogenkamp P, De Graaf C, Higgs S, Lluch A, Ness A, Penfold C, Perry R, Putz P, Yeomans M. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes. 2016;40(3):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiander M. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can Med Assoc J. 2017;189(28):E929–E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toews I, Lohner S, de Gaudry DK, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364:k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100(3): 765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rother KI, Conway EM, Sylvetsky AC. How non-nutritive sweeteners influence hormones and health. Trends Endocrinol Metab. 2018;29(7):455–67. [DOI] [PubMed] [Google Scholar]
- 9. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–8. [DOI] [PubMed] [Google Scholar]
- 10. Tobias DK, Stuard JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, Hu FB, Manson JA, Zhang C. History of gestational diabetes mellitus and long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12): 1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ajmera VH, Gunderson EP, VanWagner LB, Lewis CE, Carr JJ, Terrault NA. Gestational diabetes mellitus is strongly associated with non-alcoholic fatty liver disease. Am J Gastroenterol. 2016;111(5):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C, Hu FB, Olsen SF, Vaag A, Gore-Langton R, Chavarro JE, Bao W, Yeung E, Bowers K, Grunnet LG et al.. Rationale, design, and method of the Diabetes & Women's Health Study—a study of long-term health implications of glucose intolerance in pregnancy and their determinants. Acta Obstet Gynecol Scand. 2014;93(11):1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, Taxbol D, Hansen KD, Juhl M, Schow TB et al.. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29(4):300–7. [DOI] [PubMed] [Google Scholar]
- 14. Olsen SF, Houshmand-Oeregaard A, Granstrom C, Langhoff-Roos J, Damm P, Bech BH, Vaag AA, Zhang C. Diagnosing gestational diabetes mellitus in the Danish National Birth Cohort. Acta Obstet Gynecol Scand. 2017;96(5):563–9. [DOI] [PubMed] [Google Scholar]
- 15. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olsen SF, Mikkelsen TB, Knudsen VK, Orozova-Bekkevold I, Halldórsson TI, Strøm M, Østerdal ML. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21(1):76–86. [DOI] [PubMed] [Google Scholar]
- 17. Mikkelsen TB, Olsen SF, Rasmussen SE, Osler M. Relative validity of fruit and vegetable intake estimated by the food frequency questionnaire used in the Danish National Birth Cohort. Scand J Public Health. 2007;35(2):172–9. [DOI] [PubMed] [Google Scholar]
- 18. Mikkelsen TB, Osler M, Olsen SF. Validity of protein, retinol, folic acid and n–3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutr. 2006;9(6):771–8. [DOI] [PubMed] [Google Scholar]
- 19. Halldorsson TI, Strøm M, Petersen SB, Olsen SF. Intake of artificially sweetened soft drinks and risk of preterm delivery: a prospective cohort study in 59,334 Danish pregnant women. Am J Clin Nutr. 2010;92(3):626–33. [DOI] [PubMed] [Google Scholar]
- 20. Zhu Y, Olsen SF, Mendola P, Halldorsson TI, Rawal S, Hinkle SN, Yeung EH, Chavarro JE, Grunnet LG, Granström C. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int J Epidemiol. 2017;46(5):1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–34. [DOI] [PubMed] [Google Scholar]
- 22. Lauritsen J. FoodCalc. [Internet] [cited 2 October, 2018]. Available from: http://www.ibt.ku.dk/jesper/foodcalc/. [Google Scholar]
- 23. National Food Institute (Denmark). Danish Food Composition Databank (Version 6.02) [Internet] [cited 2 October, 2018]. Available from: http://www.foodcomp.dk/v6/fcdb_default.asp. [Google Scholar]
- 24. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity. 2012;20(6):1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 27. Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–72. [DOI] [PubMed] [Google Scholar]
- 28. Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet North Am Ed. 2005;366(9491):1059–62. [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–24.27979889 [Google Scholar]
- 30. Juhl M, Madsen M, Andersen AM, Andersen PK, Olsen J. Distribution and predictors of exercise habits among pregnant women in the Danish National Birth Cohort. Scand J Med Sci Sports. 2012;22(1):128–38. [DOI] [PubMed] [Google Scholar]
- 31. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 32. Danish Health Authority. Alcohol. [Internet].2014; [cited 7 March, 2019]. Available from https://www.sst.dk/en/health-and-lifestyle/alcohol. [Google Scholar]
- 33. Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9–10):1541–9. [DOI] [PubMed] [Google Scholar]
- 34. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169(22):2053–63. [DOI] [PubMed] [Google Scholar]
- 36. Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med. 2012;172(20):1566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sylvetsky AC, Figueroa J, Rother KI, Goran MI, Welsh JA. Trends in low-calorie sweetener consumption among pregnant women in the United States. Curr Dev Nutr. 2019;3(4):nzz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181. [DOI] [PubMed] [Google Scholar]
- 39. Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32(4):688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma J, Fox CS, Jacques PF, Speliotes EK, Hoffmann U, Smith CE, Saltzman E, McKeown NM. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. 2015;63(2):462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev. 2017;18(3):350–69. [DOI] [PubMed] [Google Scholar]
- 43. Leth T, Fabricius N, Fagt S. Estimated intake of intense sweeteners from non-alcoholic beverages in Denmark. Food Addit Contam. 2007;24(3):227–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


