Abstract
Background
Patients with chronic kidney disease (CKD) have considerable cardiovascular morbidity and mortality. Aortic stiffness is an independent predictor of cardiovascular risk and related to left ventricular remodeling and heart failure. Myocardial fibrosis is the pathophysiological hallmark of the failing heart.
Methods and results
An observational study of consecutive CKD patients (n = 276) undergoing comprehensive clinical cardiovascular magnetic resonance imaging. The relationship between aortic stiffness, myocardial fibrosis, left ventricular remodeling and the severity of chronic kidney disease was examined. Compared to age-gender matched controls with no known kidney disease (n = 242), CKD patients had considerably higher myocardial native T1 and central aortic PWV (p ≪ 0.001), as well as abnormal diastolic relaxation by E/e′ (mean) by echocardiography (p ≪ 0.01). A third of all patients had LGE, with similar proportions for the presence and the (ischaemic and non-ischaemic) pattern between the groups. PWV was strongly associated with and age, NT-proBNP and native T1 in both groups, but not with LGE presence or type; the associations were amplified in severe CKD stages. In multivariate analyses, PWV was independently associated with native T1 in both groups (p ≪ 0.01) with near two-fold increase in adjusted R2 in the presence of CKD (native T1 (10 ms) R2, B(95%CI) CKD vs. non-CKD 0.28, 0.2(0.15–0.25) vs. 0.18, 0.1(0.06–0.15), p ≪ 0.01).
Conclusions
Aortic stiffness and interstitial myocardial fibrosis are interrelated; this association is accelerated in the presence of CKD, but independent of LGE. Our findings reiterate the significant contribution of CKD-related factors to the pathophysiology of cardiovascular remodeling.
Keywords: Aortic stiffness, Chronic kidney disease, Native T1 mapping
1. Introduction
Premature cardiovascular disease (CVD) is the leading cause of death in patients with kidney disease (CKD) [[1], [2], [3]], which is only partially explained by the effects of traditional CV risk factors and atherothrombotic coronary vascular complications. The non-atherosclerotic myocardial processes, which are intrinsically linked to marked structural and functional abnormalities include profound hypertrophic and interstitial remodeling and deposition of myocardial fibrosis [1], often referred to as ‘uremic cardiomyopathy’ [2]. Progression of CKD is associated with marked increase of risk of adverse events, including arrhythmia, sudden cardiac death and heart failure (HF) [2,3]. Earlier recognition and timely management of structural myocardial changes represent essential steps towards improving the morbidity and mortality of CKD patients.
Aortic stiffness is an independent predictor of adverse CV events in numerous subpopulations with high CV risk including patients with CKD [4]. Markedly increased aortic stiffness is fundamentally an independent accelerator of pathophysiology; increased left ventricular (LV) afterload and wall stress induce hypertrophic response, extracellular matrix turnover and accumulation of diffuse myocardial fibrosis [[5], [6], [7]], decreasing the efficiency of aorto-ventricular interaction [[8], [9], [10], [11]]. Aortic stiffness is independently related with myocardial T1 mapping, an emerging non-invasive marker of diffuse myocardial fibrosis [12], and strong predictor of all cause and CV mortality and HF hospitalisation [[13], [14], [15]]. In this study, we examined the relationship between central pulse wave velocity (PWV) a measure of aortic stiffness and non-invasive imaging markers of diffuse fibrosis and LV remodeling, and their relationship with the presence and severity of CKD.
2. Methods
This is a prospective observational study of consecutive CKD patients (n = 276) undergoing routine clinical assessment of cardiac function and structure, and presence of ischaemia by CMR (NCT03749551). CKD was defined by estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease formula ≤60 ml/min/1.73 m2 [18,19]. An independent age/gender/CV risk profile-matched cohort of patients with no evidence of kidney disease (eGFR ≫60 ml/min/1.73 m2 or other markers of kidney disease) served as control group (n = 242); these patients have been included in previous publications [13,14]. Subjects with a known diagnosis of specific cardiomyopathy, either determined phenotypically by imaging, or by endomyocardial biopsy, including myocardial infiltration due to amyloidosis, iron accumulation, lipid-storage disease, hypertrophic or arrhythmogenic right ventricular cardiomyopathy, non-compaction cardiomyopathy) or significant (≥grade III) primary or secondary valvular heart disease, were excluded from this study (details in supplementary material). Clinical meta-data, including systolic/diastolic blood pressure (BP), body mass index (BMI), presence of traditional CV risk factors [20], NYHA class, symptoms, medication, and findings of transthoracic echocardiography (E/e′ mean) were recorded. Blood tests were performed on samples obtained prior to CMR using commercially available platforms. Exclusion criteria for all subjects were the generally accepted contraindications to CMR (implantable non-MR safe devices (n = 2), cerebral aneurysm clips (n = 0), cochlear implants (n = 1). All procedures were carried out in accordance with the Declaration of Helsinki (2013) and clinical management guidelines. All subjects were counselled on possible risks of nephrogenic systemic sclerosis (NSF) and the current state of the knowledge and formal recommendations for minimizing the risk of NSF prior to CMR [21,22].The study protocol was reviewed and approved by the institutional ethics committee and written informed consent was obtained from all participants.
2.1. CMR image acquisition and analysis
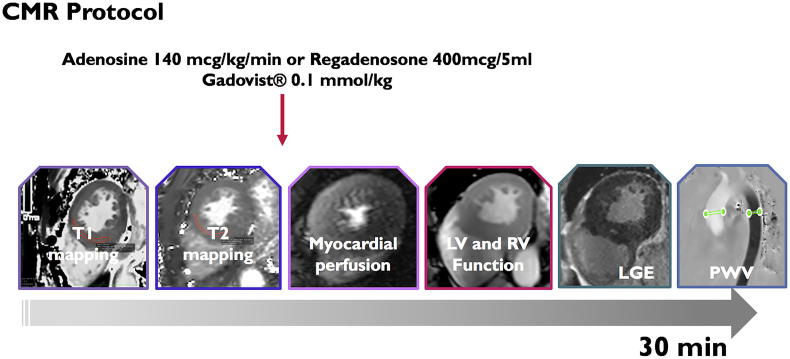
All subjects underwent a routine clinical scan protocol using a 3-Tesla clinical scanner (Skyra, Siemens Healthineers, Erlangen, Germany) for cardiac volumes and function, native T1 mapping (using Frankfurt Main (FFM)-MOLLI sequence), ischaemia imaging, scar imaging by late gadolinium enhancement (LGE) and in-plane flow acquisition with high-temporal resolution. All sequence parameters were reported previously [11,13], also included in supplementary material. Gadobutrol 0.1 mmol/kg (Gadovist®, Bayer, Leverkusen, Germany) was administered in all patients in line with the licensed recommendations for appropriate use of gadolinium-based contrast agents (GBCA) in diagnostic imaging [21,22] for stress-myocardial perfusion using vasodilatory test (regadenosone iv. bolus 400 μg/5 ml) [23,24]. Rest myocardial perfusion was omitted to minimize the total dose of GBCA [21,22]. Assessment of cardiac volumes, function and mass, interpretation of myocardial perfusion and LGE images has been performed following standardized recommendations [25,26]. LGE, an established marker of myocardial viability/hibernation with well-established prognostic significance, was characterized based on the presence and predominant pattern as ischaemic or non-ischaemic [25]. Central aortic PWV was calculated by dividing the length of the aorta between the locations used for aortic flow measurements with the time difference between the arrival of the pulse wave at these locations (Fig. 1). Relevant myocardial ischaemia was diagnosed by evidence of regional hypoperfusion affecting ≫10% myocardium (as 2 or more adjacent segments in 32 subsegment model) [23], whereas microvascular disease (MVD) was diagnosed as homogenous circumferential subendocardial hypoperfusion, lasting for over 6 consecutive beats, being often most pronounced in the midventricular slice. Inter- and intraobserver reproducibility and agreement of post-processing approaches have been reported previously [16,17], data specific to the present cohort is included in the supplementary material.
Fig. 1.
CMR imaging protocol, consisting of native T1 mapping, stress-myocardial perfusion for relevant myocardial ischaemia. Cine-imaging for cardiac volumes and LV mass, late gadolinium enhancement and PWV for central aortic stiffness. Rest myocardial perfusion was not performed in line with restricted allowance of GBCA in CKD [21,22].
2.2. Statistical analysis
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA, version 24.0). Departures from normality were examined using Shapiro-Wilk's test. Data is presented in counts (percentages), mean (standard deviation, SD) or median (interquartile range, IQR), as appropriate for the type of the data. Comparisons between groups were performed using Student t-test or one-way ANOVA for normally distributed variables, and chi2 and Mann-Whitney test for non-normally distributed variables. We used a modified CKD staging [18,19], with Group 4 inclusive of all patients with eGFR ≪15 ml/min/1.73 m2. The associations were analyzed by uni- and multivariate linear regression analyses and compared by Fisher r-to-z transformation. Multivariate regression analyses were used to examine the predictive associations between aortic stiffness and myocardial imaging variables. Collinearity diagnostics used to examine the variance inflation factor analysis. All tests were two-tailed and p-value of ≪0.05 was considered statistically significant.
3. Results
Characteristics of patient population are summarized in Table 1. Controls and CKD-patients were similar for age, gender, CV risk profile and background clinical history. CKD patients had lower hemoglobin/hematocrit and higher eGFR, hs-tropT, C-reactive protein and NT-proBNP (p ≪ 0.05). The groups were similar for most cardiac medications, except for the higher proportion of aldosterone inhibitors in the non-CKD group, whereas loop diuretics were more commonly used in the CKD group (p ≪ 0.05 for all). Common causes of CKD included hypertension (including polycystic kidney disease, 114, 42%), diabetes (103, 38%) and vasculitis (57, 21%); in 106(39%) was cause multifactorial. Compared to controls, CKD-patients had higher LV volumes and mass, and lower global systolic function (LV – ejection fraction, LV-EF, p ≪ 0.001). A third of all patients had LGE, with similar proportions for the presence and the pattern (ischaemic and non-ischaemic) between the groups. Non-ischaemic LGE was predominantly found as mid-myocardial septal striae (n = 24) followed by patchy diffuse intramyocardial LGE (n = 10). The mean extent of LGE (%) when present was similar between the groups, irrespectively of the LGE type (Fig. 2).
Table 1.
Subjects' characteristics. CMR measurements of function and structure and tissue characterization. Student t-test or Chi2 tests; all tests were two-tailed, p ≪ 0.05 was considered significant. BP – blood pressure, CAD – coronary artery disease, AF – atrial fibrillation, eGFR – estimated glomerular filtration rate, hs-TropT – high sensitive troponin T, CRP - C-reactive protein, NT-proBNP – N-terminal pro brain natriuretic peptide; RAS – renin-angiotensin system, LV – left ventricular, LGE – late gadolinium enhancement.
| Variable | Non-CKD controls (n = 242) | CKD patients (n = 276) | Sig. (p-value) |
|---|---|---|---|
| Age (years) | 56 ± 19 | 58 ± 21 | 0.131 |
| Male (n, %) | 145 (60) | 189(65) | 0.241 |
| BMI (kg/m2) | 27 ± 8 | 26 ± 9 | 0.185 |
| BP systolic (mm Hg) | 134 ± 17 | 137 ± 21 | 0.077 |
| BP diastolic (mm Hg) | 79 ± 10 | 78 ± 12 | 0.307 |
| Heart rate (bpm) | 73 ± 13 | 75 ± 14 | 0.094 |
| Active smokers (n, %) | 48 (20) | 66 (24) | 0.274 |
| Past smokers (n, %) | 86 (35) | 88 (32) | 0.470 |
| Hypertension (n, %) | 192 (91) | 262 (95) | 0.073 |
| Diabetes (n, %) | 116 (48) | 143 (52) | 0.364 |
| Type II (n, %) | 87 (36) | 112 (41) | 0.244 |
| Hyperlipidaemia (n, %) | 150 (62) | 188 (68) | 0.153 |
| Known CAD (n, %) | 68 (28) | 88 (32) | 0.322 |
| 3-vessel CAD or equivalent (n, %) | 32 (13) | 48 (17) | 0.205 |
| Previous revascularisation (n, %) | 53 (22) | 77 (28) | 0.117 |
| Previous diagnosis of HF (n, %) | 77 (32) | 108 (39) | 0.097 |
| Known AF (n, %) | 29 (12) | 50 (18) | 0.058 |
| Blood hemoglobin (g/dl) | 14.2 ± 1.2 | 12.6 ± 1.4 | ≪0.001 |
| Blood hematocrit (%) | 41.3 ± 5.2 | 39.8 ± 6.4 | 0.004 |
| eGFR (ml/min/m2) | 89 ± 15 | 55 ± 25 | ≪0.001 |
| hs C-reactive protein, mg/l | 3.9 ± 0.9 | 6.3 ± 1.8 | ≪0.001 |
| hs-TropT (ng/l) | 6 (4–10) | 14 (6–30) | 0.013 |
| NT-proBNP (pg/l) | 78 (38–207) | 582 (187–2192) | ≪0.001 |
| ≫300, n (%) | 46 (24) | 69 (62) | ≪0.001 |
| NYHA ≥III (n, %) | 68 (28) | 88 (32) | 0.322 |
| E/e′ (mean) | 8.3 ± 2.4 | 11.3 ± 4.5 | ≪0.001 |
| Cardiac medication | |||
| Beta blockers, n (%) | 138(57) | 174 (63) | 0.299 |
| RAS inhibitors, n (%) | 198(82) | 234(85) | 0.358 |
| Aldosterone inhibitors (n, %) | 68(28) | 33(12) | ≪0.001 |
| Neprilysin inhibitors (n, %) | 27(11) | 2(5) | 0.011 |
| Calcium antagonists (n, %) | 184(76) | 224(81) | 0.166 |
| Loop diuretics (n, %) | 68(28) | 199(72) | ≪0.001 |
| Platelet inhibition (n, %) | 138(57) | 136(51) | 0.172 |
| Statins (n, %) | 155(64) | 196(71) | 0.089 |
| CMR measures | |||
| LV-EDV index, ml/m2 | 83 ± 20 | 93 ± 33* | ≪0.001 |
| LV-ESV index, ml/m2 | 36 ± 19 | 46 ± 31* | ≪0.001 |
| LV-EF,% | 58 ± 11 | 54 ± 17* | ≪0.001 |
| LV mass index, g/m2 | 59 ± 14 | 70 ± 19* | ≪0.001 |
| RV-EF,% | 57 ± 9 | 56 ± 13 | 0.415 |
| LA area, cm2 | 23 ± 5 | 27 ± 7 | 0.002 |
| Myocardial LGE, n (%) | 70(29) | 97(35) | 0.145 |
| Ischemic type, n (%) | 34(14) | 44(16) | 0.636 |
| Non-ischemic, n (%) | 36(15) | 53(19) | 0.366 |
| LGE extent,% | 4.9(0.2–3.8) | 5.7(2.5–8.9) | 0.083 |
| Myocardial ischaemia, n (%) | 27(11) | 44(16) | 0.098 |
| Microvascular disease, n (%) | 16(7) | 50(18) | ≪0.001 |
| Pericardial enhancement, n (%) | 8(3) | 11(4) | 0.539 |
| Pericardial effusion (≫1 cm), n (%) | 16(7) | 29(11) | 0.115 |
| Native T1, ms | 1123 ± 31 | 1152 ± 43 | ≪0.001 |
| Central aortic PWV, m/s | 7.3 ± 2.4 | 9.2 ± 2.6 | ≪0.001 |
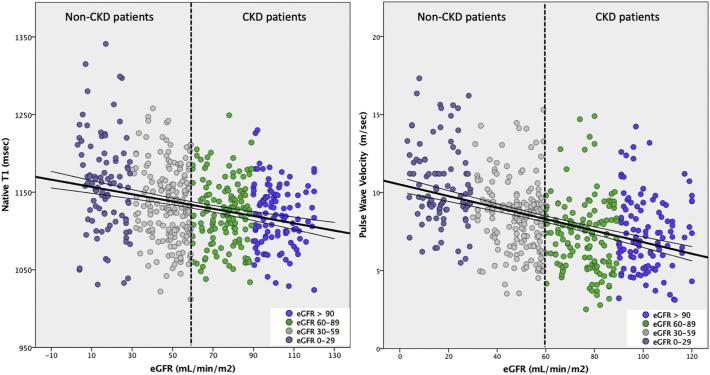
Fig. 2.
Aortic stiffness and diffuse myocardial fibrosis are negatively associated with severity of CKD. Bivariate associations between native T1 and PWV with eGFR (r = −0.31 and r = −0.44, p ≪ 0.001, respectively).
Patients with CKD had considerably higher myocardial native T1 and central aortic PWV (p ≪ 0.001), as well as abnormal diastolic relaxation by E/e′ (mean) by echocardiography (p ≪ 0.01). In the subgroup of controls with LGE, native T1 values and LV mass were higher and LV-EF was lower compared to those without LGE (native T1 (ms): 1138 ± 43 vs 1115 ± 37, LVmass (g/m2): 64 ± 14 vs 57 ± 13; LV-EF (%): 51 ± 13 vs 59 ± 8, p ≪ 0.001), whereas other parameters (PWV, E/e′, eGFR) did not differ. On the contrary, among CKD patients, native T1, PWV, E/e′ and LV mass were similar between patients with LGE and without, whereas LV-EF and eGFR were both markedly reduced in the former subgroup (LV-EF(%): 47 ± 16 vs. 59 ± 10; eGFR: 39 ± 16 vs. 33 ± 16, p ≪ 0.001).
3.1. Analysis of relationships
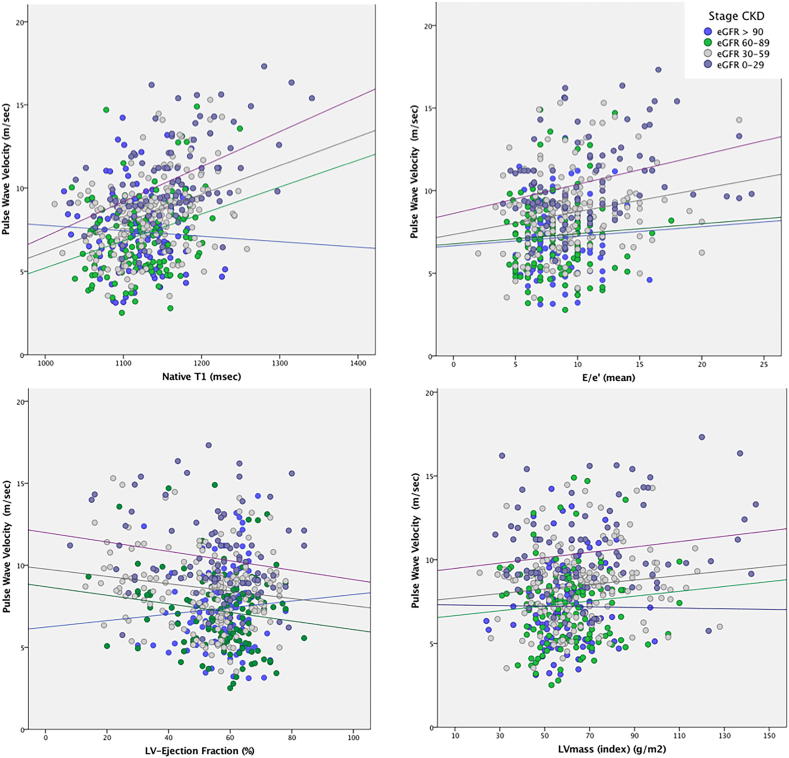
Group- (and CKD-stage-) specific associations with PWV are shown in Table 2 and Fig. 2, Fig. 3. There were significant association between PWV and age, NT-proBNP and native T1 in both groups. In the CKD group, there were also a significant association between PWV and eGFR, hematocrit and LV mass, global longitudinal strain and E/e′. Both groups showed significant associations between native T1 and measures of LV remodeling and stiffness. Furthermore, the associations between PWV and native T1, LV remodeling and stiffness were amplified in stages 3 and 4 (Table 3, Fig. 4). Controlling for age, gender, BMI, systolic BP, CV risk factors, native T1 showed a stronger relationship with markers of structural and functional LV remodeling and diastolic impairment compared to PWV (Table 4). In CKD group, dichotomizing for the presence and the type of LGE, the associations between PWV and native T1 were not significantly different (LGE-negative vs. LGE positive: r = 0.46 vs. r = 0.49, p ≪ 0.001, z-value −0.30, p = 0.763); ischemic vs non-ischemic LGE type: r = 0.44 vs. r = 0.53, p ≪ 0.001, z-value −0.56, p = 0.575. In multivariate stepwise linear regression analysis, accounting for CV risk factors and measures of LV remodeling, PWV was independently associated with native T1 in both groups (p ≪ 0.01); in CKD patients, this was followed by the model that also included eGFR (adjusted R2 = 0.28, p ≪ 0.01).
Table 2.
Bivariate correlations of PWV and native T1 with subjects' characteristics, LV geometry and function and tissue characterization. Pearson's (r, p-value) and Spearman (rho) coefficient, as appropriate for the type of the data. p-Value ≪0.05 was considered significant.
| Variable | Non-CKD controls |
CKD patients |
||
|---|---|---|---|---|
| PWV (m/s) | Native T1 (ms) | PWV (m/s) | Native T1 (ms) | |
| Age (years) | 0.31(≪0.001) | 0.13(0.05) | 0.26(≪0.001) | 0.14(0.048) |
| Gender (male) | −0.06(0.35) | 0.10(0.17) | 0.16(0.006) | 0.10(0.11) |
| Heart Rate (bpm) | 0.10(0.11) | −0.06(0.39) | 0.012(0.84) | 0.05(0.45) |
| BPsystolic (mm Hg) | 0.9(0.19) | 0.12(0.05) | 0.15(0.02) | 0.01(0.96) |
| eGFR (ml/min/m2) | 0.06(0.38) | 0.04(0.38) | −0.40(≪0.001) | −0.32(≪0.001) |
| Hematocrit (%) | 0.014(0.82) | 0.05(0.42) | −0.21(0.002) | −0.18(0.007) |
| hs-TropT | 0.02(0.76) | 0.02(0.79) | 0.03(0.21) | 0.14(0.028) |
| NT-proBNP | 0.14(0.03) | 0.25(≪0.001) | −0.29(≪0.001) | −0.30(≪0.001) |
| PWV (m/s) | / | 0.16(0.009) | / | 0.47(≪0.001) |
| Native T1 (ms) | 0.16(0.009) | / | 0.47(≪0.001) | / |
| LV-EDVi, ml/m2 | 0.20(≪0.001) | 0.24(≪0.001) | 0.10(0.09) | 0.29(≪0.001) |
| LV-ESVi, ml/m2 | 0.03(0.65) | 0.26 (≪0.001) | 0.09(0.13) | 0.33 (≪0.001) |
| LV-EF (%) | −0.061(0.33) | −0.22(≪0.001) | −0.16(0.069) | −0.33(≪0.001) |
| LV massi (g/m2) | 0.02(0.73) | 0.17(0.008) | 0.17(0.024) | 0.31(≪0.001) |
| RV-EF (%) | −0.10(0.611) | −0.10(0.11) | −0.023(0.31) | −0.15(0.024) |
| LA area, cm2 | 0.11(0.113) | 0.21(0.006) | 0.24(0.002) | 0.23(0.001) |
| E/e′ (mean) | 0.07(0.31) | 0.13(0.04) | 0.20(0.003) | 0.22(0.002) |
| LGE (present) | 0.04(0.56) | 0.11 (0.112) | 0.02(0.83) | 0.19(0.005) |
| LGE extent (%) | 0.09(0.37) | 0.11(0.06) | 0.03(0.23) | 0.07(0.19) |
Fig. 3.
Associations between aortic stiffness and markers of diffuse myocardial fibrosis, stiffness and remodeling are potentiated with severity of CKD. Bivariate correlations between PWV and native T1, E/e′ (mean), LV mass index and LV-EF.
Table 3.
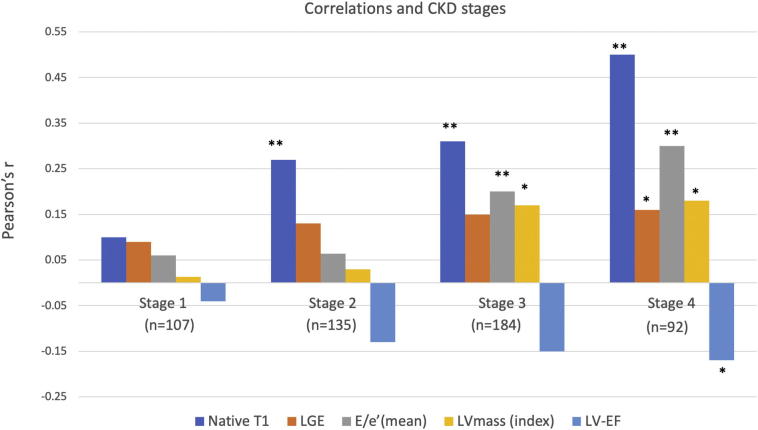
Pearson's associations between PWV and markers of LV remodeling increase with CKD stages. Comparisons are made against Stage 1 using r-to-z transformation (p ≪ 0.05*, p ≪ 0.01**).
| CKD stages | Stage 1 ≫90 (n = 107) | Stage 2 60–89 (n = 135) | z-Value | Stage 3 30–59 (n = 184) | z-Value | Stage 4 0–29 (n = 92) | z-Value |
|---|---|---|---|---|---|---|---|
| PWV, m/s (r/rho) | |||||||
| Native T1 | 0.10(0.32) | 0.27(0.002) | 1.4 | 0.31(≪0.001) | 2.3* | 0.50(≪0.001) | 3.5** |
| E/e′ (mean) | 0.06(0.56) | 0.06(0.46) | 0.0 | 0.20(0.007) | 1.2 | 0.30(0.004) | 1.7* |
| LVmass (index) | 0.01(0.19) | 0.03(0.77) | 0.1 | 0.17(0.02) | 1.3 | 0.18(0.01) | 1.3 |
| LV-EF (%) | −0.04(0.65) | −0.13(0.13) | 0.7 | −0.15(0.051) | 1.0 | −0.17(0.03) | 1.1 |
| LGE (present) | 0.09(0.39) | 0.13(0.13) | 0.3 | 0.15(0.052) | 0.5 | 0.16(0.04) | 0.6 |
Fig. 4.
Pulse wave velocity correlations and CKD Stages (Table 3). Native T1 has the strongest association with PWV in all stages, followed by E/e′, LVmass in stage 3 and LV-EF in stage 4. *p ≪ 0.05, **p ≪ 0.01.
Table 4.
Multivariate linear regression analysis of predictive associations with aortic stiffness (PWV).
| CKD patients | Adjusted R2 | B(95%CI) | Sig. (p-value) | VIF |
|---|---|---|---|---|
| Native T1 (10 ms) | 0.28 | 0.2(0.15–0.25) | ≪0.001 | 1.00 |
| All predictors in the model (p-value): age (0.22); gender (0.42), BMI (0.29), HTN (0.43), DM (0.62), smoking (0.22), hypercholesterolaemia (0.24), LV-EDV (0.13), LV-ESV (0.051); LV-EF (0.11), LV mass (0.06), E/e′ (mean) 0.013; LGE (presence) (0.25); | ||||
| Non-CKD controls | Adjusted R2 | B(95%CI) | Sig. (p-value) | VIF |
|---|---|---|---|---|
| Native T1 (10 ms) | 0.18 | 0.1(0.06–0.15) | 0.01 | 1.1 |
| All predictors in the model (p-value): age (0.39); gender (0.52), BMI (0.39), HTN (0.39), DM (0.32), smoking (0.61), hypercholesterolaemia (0.64); LV-EDV (0.62), LV-ESV (0.65); LV-EF (0.63), LV mass (0.93), LGE (presence) (0.91); | ||||
4. Discussion
Our results provide important novel insights into the pathophysiology of CVD in CKD by underlining the strong associations between aortic stiffness and accelerated myocardial hypertrophic-fibrotic remodeling. In CKD group, aortic stiffness and markers of diffuse myocardial fibrosis, by PWV and native T1, respectively, were significantly higher and strongly associated with eGFR, whereas no such association were found in the non-CKD cohort despite similar CV risk profile. Aortic stiffness and native T1 were associated with measures of myocardial stiffness and structural remodeling; these associations were amplified with increasing severity of CKD. Native T1 was an independent associate of PWV in both groups, with considerably stronger predictive relationship in the CKD-group. Our findings suggest the pathophysiological commonality of adverse vasculo-ventricular remodeling, which is potentiated in the presence of CKD.
To the best of our knowledge, our study is the largest observational prospective study using in-depth and comprehensive characterization of CVD in CKD, providing novel pathophysiological insights that might shed light to the excess of CVD in CKD [2]. Several previous studies reported on T1 mapping in the CKD patients, generally revealing significantly higher values compared to controls, which were reproducible and unaffected by the fluid status [27,28]. A further study in CKD highlighted the interrelatedness of T1 mapping markers with reduced LV-EF [29], which was not commensurate with the CV-risk factors. Our study expands on these previous observations by revealing strong associations between aortic stiffness, native T1 and markers of LV remodeling, all of which all have strong prognostic relevance in CKD patients [5,6,[9], [10], [11],13,14,30,31]. Whereas native T1 was the independent associate of PWV in both groups, the predictive association was intensified in the presence of CKD.
The prominent role of native T1 suggests an essential pathophysiological connection with the excessive structural remodeling, functional impairment and consequently poor prognosis in CKD [3]. The association with aortic stiffness builds upon the established concept of aorto-ventricular interdependence, postulating myocardial injury as a consequence of the increased LV afterload [7,11,[32], [33], [34], [35], [36], [37], [38], [39]]. However, compared to myocardial hypertrophy within physiological range in non-CKD group [12,40,41], the marked structural remodeling in CKD is accompanied with myocardial diffuse fibrosis and dysfunction. In CKD, native T1 is higher at any given PWV implying potentiation of the adverse remodeling with worsening stages of CKD [10,11,42,43] (Fig. 3). This effect appears inherently linked with the background presence of CKD likely related to neurohormonal overflow, which in addition to the chronic pressure and volume overload importantly accelerates LV remodeling with fibrosis, leading to development of HF [3,36,40,[44], [45], [46]].
4.1. Limitations
A few technical notes are necessary. Owing to an on-going discussion on the technically optimal approach to quantify myocardial abnormalities with T1 mapping techniques, ranging from a wide spectrum of sequences, various possible confounders, such as, but not limited to, hematocrit, partial volume, motion, magnetization transfer and fast water exchange to postprocessing pathways allowing precision, as well as focus on diffuse disease by exclusion of LGE (summarized in [[47], [48], [49]]), the findings obtained with FFM-MOLLI sequence are not immediately transferable to other choices of T1 mapping sequences. Generally, native T1 mapping has several valuable advantages, which are technical (simple acquisition, high precision, interstudy reproducibility, transferability (multicentre data)), and clinical (high discrimination between health and disease, short clinical scans, contrast-free follow ups). As for any diagnostic test, standardization of data acquisition and postprocessing, as well as predefined reference ranges, are prerequisite for application of T1 mapping in clinical routine. We achieved this by unifying the imaging parameters, performing the on-the fly quality control and by using centralized postprocessing. In addition, we reduced confounding blood partial volume by placing the region of interest conservatively in septal myocardium of midventricular slice [37].
Native T1 using the present sequence and the postprocessing approach (i.e. excluding LGE) has the highest correlation with collagen volume fraction in a model of chronically elevated LV pressure (r = 0.58, p = 0.027), as such relatable to the conditions of the present study [12,25] However, other important tissue influences, such as myocardial oedema, may have also been partially detected. Native T1 using this sequence/postprocessing approach also has a known relationship with outcome, which much stronger compared to ECV [13,14]. Finally, this paper intends to inform specifically about relationship between native T1 and the aortic stiffness in the context of the presence and absence of CKD, and based on a standardized acquisition protocol and postprocessing [16,17]. E/e′ mean measurements are known to be volume-load dependent and less reliable in patients with variable volume status, thus, may not be fully representative of diastolic impairment or increased LV loading pressures [45]. This is an ongoing study using standardized imaging protocols and data collection, which means that several subjects have been included in previous publications from our group [13,14].
In conclusion, aortic stiffness and interstitial myocardial fibrosis are interrelated; this association is accelerated in the presence of CKD, but independent of LGE. Our findings reiterate the significant contribution of CKD-related factors to the pathophysiology of cardiovascular remodeling. Our findings provide important novel mechanistic insights into the pathophysiology of CVD in CKD, underlying the strong associations with between aortic stiffness and accelerated myocardial hypertrophic-fibrotic remodeling in this population. Future studies on the role of native T1 mapping in identification and prognostication and therapy of uremic cardiomyopathy are needed.
Declaration of Competing Interest
No conflict of interests.
Footnotes
Statement: The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding: German Ministry of Education and Research via the German Centre for Cardiovascular Research (DZHK) to AZ, EN, VP.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100389.
Appendix A. Supplementary data
Supplementary material
References
- 1.Gross M.-L., Ritz E. Hypertrophy and fibrosis in the cardiomyopathy of uremia—beyond coronary heart disease. Semin. Dial. 2008;21:308–318. doi: 10.1111/j.1525-139X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 2.Mangion K., McDowell K., Mark P.B., Rutherford E. Characterizing cardiac involvement in chronic kidney disease using CMR-a systematic review. Curr. Cardiovasc. Imaging Rep. 2018;11:2. doi: 10.1007/s12410-018-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonelli M., Wiebe N., Culleton B., House A., Rabbat C., Fok M., McAlister F., Garg A.X. Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 4.Adenwalla S.F., Graham-Brown M.P.M., Leone F.M.T., Burton J.O., McCann G.P. The importance of accurate measurement of aortic stiffness in patients with chronic kidney disease and end-stage renal disease. Clin. Kidney J. 2017;10:503–515. doi: 10.1093/ckj/sfx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karras A., Haymann J.-P., Bozec E., Metzger M., Jacquot C., Maruani G., Houillier P., Froissart M., Stengel B., Guardiola P., Laurent S., Boutouyrie P., Briet M., Nephro Test Study Group Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–1457. doi: 10.1161/HYPERTENSIONAHA.112.197210. [DOI] [PubMed] [Google Scholar]
- 6.Verbeke F., Maréchal C., Van Laecke S., Van Biesen W., Devuyst O., Van Bortel L.M., Jadoul M., Vanholder R. Aortic stiffness and central wave reflections predict outcome in renal transplant recipients. Hypertension. 2011;58:833–838. doi: 10.1161/HYPERTENSIONAHA.111.176594. [DOI] [PubMed] [Google Scholar]
- 7.Chirinos J.A., Kips J.G., Jacobs D.R., Brumback L., Duprez D.A., Kronmal R., Bluemke D.A., Townsend R.R., Vermeersch S., Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redheuil A., Wu C.O., Kachenoura N., Ohyama Y., Yan R.T., Bertoni A.G., Hundley G.W., Duprez D.A., Jacobs D.R., Daniels L.B., Darwin C., Sibley C., Bluemke D.A., Lima J.A.C. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: the MESA study. J. Am. Coll. Cardiol. 2014;64:2619–2629. doi: 10.1016/j.jacc.2014.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohyama Y., Ambale-Venkatesh B., Noda C., Chugh A.R., Teixido-Tura G., Kim J.-Y., Donekal S., Yoneyama K., Gjesdal O., Redheuil A., Liu C.-Y., Nakamura T., Wu C.O., Hundley W.G., Bluemke D.A., Lima J.A.C. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging clinical perspective. Circ. Cardiovasc. Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonapace S., Rossi A., Cicoira M., Golia G., Zanolla L., Franceschini L., Conte L., Marino P., Zardini P., Vassanelli C. Aortic stiffness correlates with an increased extracellular matrix turnover in patients with dilated cardiomyopathy. Am. Heart J. 2006;152 doi: 10.1016/j.ahj.2006.04.026. (93.e1–6) [DOI] [PubMed] [Google Scholar]
- 11.Puntmann V.O., Arroyo Ucar E., Hinojar Baydes R., Ngah N.B., Kuo Y.S., Dabir D., Macmillan A., Cummins C., Higgins D.M., Gaddum N., Chowienczyk P., Plein S., Carr-White G., Nagel E. Aortic stiffness and interstitial myocardial fibrosis by native T1 are independently associated with left ventricular remodeling in patients with dilated cardiomyopathy. Hypertension. 2014;64:762–768. doi: 10.1161/HYPERTENSIONAHA.114.03928. [DOI] [PubMed] [Google Scholar]
- 12.Child N., Suna G., Dabir D., Yap M.L., Rogers T., Kathirgamanathan M., Arroyo Ucar E., Hinojar R., Mahmoud I., Young C., Wendler O., Mayr M., sandhu B., Morton G., Muhly-Reinholz M., Dimmeler S., Nagel E., Puntmann V.O. Comparison of MOLLI, shMOLLLI, and SASHA in discrimination between health and disease and relationship with histologically derived collagen volume fraction. Eur. Heart J. Cardiovasc. Imaging. 2017;119:277. doi: 10.1093/ehjci/jex309. [DOI] [PubMed] [Google Scholar]
- 13.Puntmann V.O., Carr-White G., Jabbour A., Yu C.-Y., Gebker R., Kelle S., Hinojar R., Doltra A., Varma N., Child N., Rogers T., Suna G., Arroyo Ucar E., Goodman B., Khan S., Dabir D., Herrmann E., Zeiher A.M., Nagel E. T1-mapping and outcome in nonischemic cardiomyopathy. JACC Cardiovasc. Imaging. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Puntmann V.O., Carr-White G., Jabbour A., Yu C.-Y., Gebker R., Kelle S., Rolf A., Zitzmann S., Peker E., D'Angelo T., Pathan F., Elen Valbuena S., Hinojar R., Arendt C., Narula J., Herrmann E., Zeiher A.M., Nagel E. International T1 Multicentre CMR Outcome Study. Native T1 and ECV of noninfarcted myocardium and outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 2018;71:766–778. doi: 10.1016/j.jacc.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Lee H., Park J.B., Yoon Y.E., Park E.A., Kim H.K., Lee W., Kim Y.J., Cho G.Y., Sohn D.W., Greiser A., Lee S.P. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc. Imaging. 2018;11:974–983. doi: 10.1016/j.jcmg.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Rogers T., Dabir D., Mahmoud I., Voigt T., Schaeffter T., Nagel E., Puntmann V.O. Standardization of T1 measurements with MOLLI in differentiation between health and disease – the ConSept study. J. Cardiovasc. Magn. Reson. 2013;15:78. doi: 10.1186/1532-429X-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabir D., Child N., Kalra A., Rogers T., Gebker R., Jabbour A., Plein S., Yu C.-Y., Otton J., Kidambi A., McDiarmid A., Broadbent D., Higgins D.M., Schnackenburg B., Foote L., Cummins C., Nagel E., Puntmann V.O. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2014;16:34. doi: 10.1186/s12968-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://www.nice.org.uk/guidance/cg182 (last accessed March 1st, 2019).
- 19.https://renal.org/information-resources/the-uk-eckd-guide/ckd-stages/ (last accessed March 1st 2019).
- 20.2016 European Guidelines on cardiovascular disease prevention in clinical practice. 2016;:1–78. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J.. 2016;37(29):2315–2381.
- 21.Reiter T., Ritter O., Prince M.R., Nordbeck P., Wanner C., Nagel E., Bauer W. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012;14:31. doi: 10.1186/1532-429X-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Gadolinium-containing_contrast_agents/human_referral_prac_000056.jsp&mid=WC0b01ac05805c516f (last accessed March 1st 2019).
- 23.Hussain S.T., Paul M., Plein S., McCann G.P., Shah A.M., Marber M.S., Chiribiri A., Morton G., Redwood S., MacCarthy P., Schuster A., Ishida M., Westwood M.A., Perera D., Nagel E. Design and rationale of the MR-INFORM study: stress perfusion cardiovascular magnetic resonance imaging to guide the management of patients with stable coronary artery disease. J. Cardiovasc. Magn. Reson. 2012;14:65. doi: 10.1186/1532-429X-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Task Force Members, Montalescot G., Sechtem U., Achenbach S., Andreotti F., Arden C., Budaj A., Bugiardini R., Crea F., Cuisset T., Di Mario C., Ferreira J.R., Gersh B.J., Gitt A.K., Hulot J.-S., Marx N., Opie L.H., Pfisterer M., Prescott E., Ruschitzka F., Sabaté M., Senior R., Taggart D.P., van der Wall E.E., CJM Vrints, ESC Committee for Practice Guidelines, Baumgartner H., Bax J.J., Bueno H., Dean V., Deaton C., Erol C., Fagard R., Ferrari R., Hoes A.W., Kirchhof P., Kolh P., Linhart A., Nihoyannopoulos P., Ponikowski P., Sirnes P.A., Tamargo J.L., Tendera M., Torbicki A., Windecker S., Reviewers Document, Knuuti J., Valgimigli M., Claeys M.J., Donner-Banzhoff N., Frank H., Funck-Brentano C., Gaemperli O., Gonzalez-Juanatey J.R., Hamilos M., Hasdai D., Husted S., James S.K., Kervinen K., Kristensen S.D., Lancellotti P., Maggioni A.P., Piepoli M.F., Pries A.R., Romeo F., Rydén L., Simoons M.L., Steg P.G., Timmis A., Wijns W., Yildirir A., Zamorano J.L. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur. Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 25.Puntmann V.O., Valbuena S., Hinojar R., Petersen S.E., Greenwood J.P., Kramer C.M., Kwong R.Y., McCann G.P., Berry C., Nagel E., SCMR Clinical Trial Writing Group Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: part I - analytical validation and clinical qualification. J. Cardiovasc. Magn. Reson. 2018;20(1):67. doi: 10.1186/s12968-018-0484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz-Menger J., Bluemke D.A., Bremerich J., Flamm S.D., Fogel M.A., Friedrich M.G., Kim R.J., Knobelsdorff-Brenkenhoff von F., Kramer C.M., Pennell D.J., Plein S., Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J. Cardiovasc. Magn. Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutherford E., Talle M.A., Mangion K., Bell E., Rauhalammi S.M., Roditi G., McComb C., Radjenovic A., Welsh P., Woodward R., Struthers A.D., Jardine A.G., Patel R.K., Berry C., Mark P.B. Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1 mapping. Kidney Int. 2016;90:845–852. doi: 10.1016/j.kint.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham-Brown M.P.M., Rutherford E., Levelt E., March D.S., Churchward D.R., Stensel D.J., McComb C., Mangion K., Cockburn S., Berry C., Moon J.C., Mark P.B., Burton J.O., McCann G.P. Native T1 mapping: inter-study, inter-observer and inter-center reproducibility in hemodialysis patients. J. Cardiovasc. Magn. Reson. 2017;19:21. doi: 10.1186/s12968-017-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards N.C., Moody W.E., Yuan M., Hayer M.K., Ferro C.J., Townend J.N., Steeds R.P. Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am. J. Cardiol. 2015;115:1311–1317. doi: 10.1016/j.amjcard.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Peterson G.E., de Backer T., Contreras G., Wang X., Kendrick C., Greene T., Appel L.J., Randall O.S., Lea J., Smogorzewski M., Vagaonescu T., Phillips R.A., African American Study of Kidney Disease Investigators Relationship of left ventricular hypertrophy and diastolic function with cardiovascular and renal outcomes in African Americans with hypertensive chronic kidney disease. Hypertension. 2013;62:518–525. doi: 10.1161/HYPERTENSIONAHA.111.00904. [DOI] [PubMed] [Google Scholar]
- 31.Mark P.B., Doyle A., Blyth K.G., Patel R.K., Weir R.A.P., Steedman T., Foster J.E., Dargie H.J., Jardine A.G. Vascular function assessed with cardiovascular magnetic resonance predicts survival in patients with advanced chronic kidney disease. J. Cardiovasc. Magn. Reson. 2008;10:39. doi: 10.1186/1532-429X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redheuil A., Yu W.-C., Mousseaux E., Harouni A.A., Kachenoura N., Wu C.O., Bluemke D., Lima J.A.C. Age-related changes in aortic arch geometry: relationship with proximal aortic function and left ventricular mass and remodeling. J. Am. Coll. Cardiol. 2011;58:1262–1270. doi: 10.1016/j.jacc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redheuil A., Yu W.-C., Wu C.O., Mousseaux E., de Cesare A., Yan R., Kachenoura N., Bluemke D., Lima J.A.C. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borlaug B.A., Kass D.A. Ventricular–vascular interaction in heart failure. Heart Fail. Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chemla D., Hébert J.L., Coirault C., Zamani K., Suard I., Colin P., Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am. J. Phys. 1998;274:H500–H505. doi: 10.1152/ajpheart.1998.274.2.H500. [DOI] [PubMed] [Google Scholar]
- 36.Puntmann V.O., Nagel E., Hughes A.D., Gebker R., Gaddum N., Chowienczyk P., Jahnke C., Mirelis J., Schnackenburg B., Paetsch I., Fleck E. Gender-specific differences in myocardial deformation and aortic stiffness at rest and Dobutamine stress. Hypertension. 2012;59:712–718. doi: 10.1161/HYPERTENSIONAHA.111.183335. [DOI] [PubMed] [Google Scholar]
- 37.Piechnik S.K., Ferreira V.M., Lewandowski A.J., Ntusi N.A., Banerjee R., Holloway C., Hofman M.B., Sado D.M., Maestrini V., White S.K., Lazdam M., Karamitsos T., Moon J.C., Neubauer S., Leeson P., Robson M.D. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J. Cardiovasc. Magn. Reson. 2013;15:13. doi: 10.1186/1532-429X-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ugander M., Oki A.J., Hsu L.Y., Kellman P., Greiser A., Aletras A.H., Sibley C.T., Chen M.Y., Bandettini W.P., Arai A.E. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur. Heart J. 2012;33:1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C.-Y., Liu Y.-C., Wu C., Armstrong A., Volpe G.J., van der Geest R.J., Liu Y., Hundley W.G., Gomes A.S., Liu S., Nacif M., Bluemke D.A., Lima J.A.C. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping. J. Am. Coll. Cardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinojar R., Varma N., Child N., Goodman B., Jabbour A., Yu C.-Y., Gebker R., Doltra A., Kelle S., Khan S., Rogers T., Arroyo Ucar E., Cummins C., Carr-White G., Nagel E., Puntmann V.O. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.115.003285. [DOI] [PubMed] [Google Scholar]
- 41.Dorn G.W. The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49:962–970. doi: 10.1161/HYPERTENSIONAHA.106.079426. [DOI] [PubMed] [Google Scholar]
- 42.Turkbey E.B., Redheuil A., J-YC Backlund, Small A.C., Cleary P.A., Lachin J.M., JAC Lima, Bluemke D.A., Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Aortic distensibility in type 1 diabetes. Diabetes Care. 2013;36:2380–2387. doi: 10.2337/dc12-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karp G., Wolak A., Baumfeld Y., Bar-Am N., Novack V., Wolak T., Fuchs L., Shalev A., Shelef I., Abu-Shakra M. Assessment of aortic stiffness among patients with systemic lupus erythematosus and rheumatoid arthritis by magnetic resonance imaging. Int. J. Card. Imaging. 2016;32:935–944. doi: 10.1007/s10554-016-0851-y. [DOI] [PubMed] [Google Scholar]
- 44.Dickhout J.G., Carlisle R.E., Austin R.C. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ. Res. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- 45.Franssen C.F.M., Navis G. Nat. Rev. Nephrol. 2013;9:190–192. doi: 10.1038/nrneph.2013.39. [DOI] [PubMed] [Google Scholar]
- 46.Borlaug B.A., Nishimura R.A., Sorajja P., Lam C.S.P., Redfield M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ. Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puntmann V.O., Peker E., Chandrashekhar Y., Nagel E. T1 mapping in characterizing myocardial disease. Circ. Res. 2016;119:277–299. doi: 10.1161/CIRCRESAHA.116.307974. [DOI] [PubMed] [Google Scholar]
- 48.Higgins D.M., Moon J.C. Review of T1 mapping methods: comparative effectiveness including reproducibility issues. Curr. Cardiovasc. Imaging Rep. 2014;7:9252. [Google Scholar]
- 49.Cameron D., Vassiliou V.S., Higgins D.M., Gatehouse P.D. Towards accurate and precise T 1 and extracellular volume mapping in the myocardium: a guide to current pitfalls and their solutions. MAGMA. 2017;52:1–21. doi: 10.1007/s10334-017-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material