Abstract
The diamondback moth (DBM), Plutella xylostella (Lepidoptera: Plutellidae) is a very destructive crucifer-specialized pest that has resulted in significant crop losses worldwide. DBM is well attracted to glucosinolates (which act as fingerprints and essential for herbivores in host plant recognition) containing crucifers such as wintercress, Barbarea vulgaris (Brassicaceae) despite poor larval survival on it due to high-to-low concentration of saponins and generally to other plants in the genus Barbarea. B. vulgaris build up resistance against DBM and other herbivorous insects using glucosinulates which are used in plant defense. Aside glucosinolates, Barbarea genus also contains triterpenoid saponins, which are toxic to insects and act as feeding deterrents for plant specialist herbivores (such as DBM). Previous studies have found interesting relationship between the host plant and secondary metabolite contents, which indicate that attraction or resistance to specialist herbivore DBM, is due to higher concentrations of glucosinolates and saponins in younger leaves in contrast to the older leaves of Barbarea genus. As a response to this phenomenon, herbivores as DBM has developed a strategy of defense against these plant biochemicals. Because there is a lack of full knowledge in understanding bioactive molecules (such as saponins) role in plant defense against plant herbivores. Thus, in this review, we discuss the role of secondary plant metabolites in plant defense mechanisms against the specialist herbivores. In the future, trials by plant breeders could aim at transferring these bioactive molecules against herbivore to cash crops.
Keywords: bioactive molecule, biological management, host plant resistance, plant immunity, plant secondary metabolites, triterpenoids
1. Introduction
The capacity of individual plant species to develop novel metabolites has been affirmed in charge of their imperviousness to plant herbivores. Plants have developed surprising diversity of substance protections against plant herbivores in light of bioactive mixtures of low atomic weight. A case of the bioactive mixtures utilized by plants in this regard are the triterpenoid saponins (Figure 1); which encourages plant immunity against a wide range of insect pests, pathogens, as well as other herbivores.
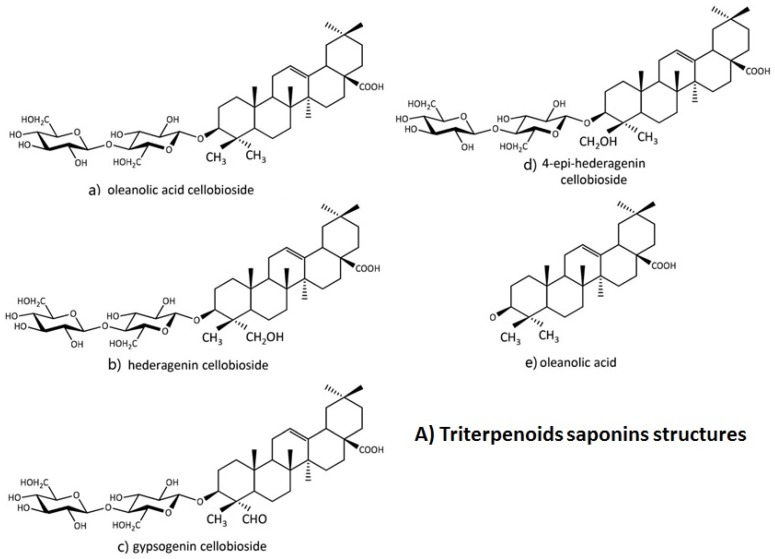
Figure 1.
Triterpenoids saponins identified in Barbarea vulgaris.
Triterpenoid saponins are mostly found in dicotyledonous species whereas monocots mainly synthesis steroidal saponins. Some leguminous crops such as: Pea, sugar beet, soybeans, cowpea, asparagus, and capsicum peppers have been reported to contain saponins [1]. Saponins are considered one of the immeasurable and distinct groups of bio-plant items, and categorize secondary plant metabolites with particular natural properties [2,3]. Saponins content in plants is dynamic, and it influences various biotic stimuli that are related to pest attack, pathogenic infection, plant mutualistic symbioses with rhizobial bacteria and mycorrhizal fungi [2]. About over 200 different structures of saponins had so far been described [4]. Likewise, Khakimov, et al. [5] reported that blends of different chemical structures are accumulated by saponin producing plants. The biological activity of saponins can be attributed to the amphipathic properties of the constituting molecules, which consist of a hydrophobic triterpene or sterol backbone and a hydrophilic carbohydrate chain. Some saponins have potent biological activities that are influenced by other aspects of their structure.
Saponins have been reported from different and unrelated plant families [5]. Whereas cereals are insufficient in saponins, aside from a few species of grass, such as Panicum virgatum, Panicum coloratum, and Avena spp. [6,7,8]. Additional gene families which have been reported to be involved in saponin biosynthesis and diversification are methyl and acyltransferases [9,10]. Aside crucifers, saponins occur constitutively in many other plant species as part of their defense system. For Brassicaceae, just a couple of species are known to yield saponins [11,12]. For example, the species from genus–rea {wintercress, B. vulgaris R. Br. (Brassicaceae)} are identified to create saponin; which are directly related to the plant defense against specialist herbivore, such as the diamondback moth (Plutella xylostella) (Lepidoptera: Plutellidae) [5]. P. xylostella is a typical crucifer specialist that is known worldwide as a severe pest of cruciferous crops, such as cabbage (Brassica oleracea) and oilseed rape (Brassica napus) [13,14].
Most of the glucosinolates–containing crucifers are all suitable hosts for the plant pest. The attractiveness of P. xylostella to these plant species is as a result of the glucosinolates content and its secondary products; such as isothiocyanates [15]. These compounds have been found to stimulate oviposition by P. xylostella adults, as well as, feeding by the larvae [16]. A similar observation has also been reported in cabbage butterfly (Pieris rapae); another crucifer specialist by Huang, Renwick and Sachdev-Gupta [15]. In another related report, a highly feeding deterrent activity to P. xylostella larvae was recorded in a chloroform extract of B. vulgaris leaves [17,18].
The management of P. xylostella has recorded minimum success as a result of its notorious ability to develop resistance to synthetic insecticides [13]. The ability of the pest to adapt plant secondary metabolites for host plant recognition, feeding, and oviposition stimulants has also been reported [19,20,21]. Moreover, inadequate knowledge of the biosynthetic paths and conducting systems of saponins has additionally complicated its application for pest control. However, the prospect of saponins modification as direct plant defense strategies against pests has offered alternative control measure for inclusion in an integrated pest management program for P. xylostella.
2. Plant Defense and Evolution
A variety of plants is susceptible to environmental disputes, but could not escape. In spite of this evident exposure, the Earth’s flora has developed to be highly abundant and diverse. It’s a reality that not all plants are entirely consumed, this could be as a result of top-down control [22], also to bottom-up mechanisms such as the direct defense of plants in response to herbivores [23]. Plants might play a major role in top-down control of herbivores by enrolling natural enemies of their enemies as an indirect defense [24]. Regarding a wide range of herbivores, direct plant defense mechanisms can demand structural adjustments, for example, trichomes, thistles, and silica bodies or assistance some other natural products. Furthermore, the auxiliary metabolites that potentially built up are lethal to herbivores, or attract the natural foes of the herbivores [25]. Disregarding their name, derivative metabolites have a vital impact on the chemical communication between plants and their surroundings. They are of basic significance for the appeal of pollinators (terpenes), protecting the plant as opposed to UV light (flavonoids), pathogens and herbivores (alkaloids, glucosinolates, saponins). The majority of plants comprises a significant range of plant derivatives [26]. From a developmental point, this range is mystified however even ineffectively understood.
The reciprocal process of adaptation within plants and their insect herbivores was observed by Stahl [27], and he proposed that the synthetic mixtures may be included. These above thoughts were advanced by Ehrlich and Raven [23] to deliver a hypothetical background for the compound nature of insect and plant communications. They proposed a well-ordered biochemical co-advancement amongst plants and bugs. Unexpectedly, some herbivore species build up a resistance against biochemical compounds that are dangerous and distasteful to different insect pests. Gradually these biocompounds may possibly act as feeding stimulant or attractant for a particular insect, which has changed according to certain conditions and even utilizes some biochemicals as a guard, from the respective plant. It is useful for the insect pest as the plant constitutes a habitation which is limited for other generalist insect pests that are dissuaded by the biochemical compounds [28,29]. As a result, plants require new chemical admixtures to be ensured against these particular groups of insects. This procedure may bring about a proportional, well ordered “arms race” inside insect pests and its host plant, driving a wide range of biochemical-barrier mixtures [30].
3. Chemical Variety of Secondary Metabolites
Recently different ecological and evolutionary theories explain the chemical variety of secondary plant compounds. Generally, plants are required to be able to compete for the vast range of aboveground and belowground specialist herbivore. Consequently, they may incidentally be in a similar place; and compete with phytophagous arthropods, and other microorganisms, like virus, bacteria, as well as fungi [31,32,33]. As a result of insect pest’s diversity and the co-occurrence scope of bolstering plans, the plant requires mixtures of biochemicals for its defense. Thus, a wide range of biochemicals may give such protection [34]. Additionally, a variety of biochemical compounds is required by plants for producing strong physiological and biochemical effects to fight against different kinds of herbivores (see Table 1). Moreover, very lethal admixtures may have an adverse effect on many beneficial insects which are factually valuable for plants. Examples are pollinators and parasitoids [35,36]. Thus, the plant should have the capacity, to recognize phytophagous insect pests with comparable characters, as well as focus on its defense towards an exact body to maintain a strategic distance from such contrary impact on beneficial insects. In this way, a substantial diversity of chemicals would be required with a high specificity [37]. As a result, chemical variety is strongly motivated by the development of phytophagous insect pests. Since development is well on the way to request just a single or a couple of phytophagous insect pests, alternate phytophagous arthropod will remain prevented by the old biochemical. As an outcome, a compound has a capacity to protect, and it might be helpful to extend the biochemical range of a particular plant, in spite of substituting the old synthetic compounds with a recent chemical. Thus, the ethical force of the plant and phytophagous insect co-operations involves a distinct and dynamic arrangement of biochemical compounds.
Table 1.
Biochemical compounds responsible for plant defense against herbivores.
| Family | Plant | Secondary Metabolite Form | Reference |
|---|---|---|---|
| Aceraceae | Acer velutinum | Td.S | [38] |
| Agavaceae | Agave sisalana | S.A | [39] |
| Amaranthaceae | Achyranthes bidentata | Te.S; Bidentatoside II and chikusetsusaponin V methyl ester. | [40] |
| Chenopodium quinoa | Td.S | [1,41] | |
| Beta vulgaris | Td.S | [42,43] | |
| Apiaceae | Steganotaenia araliacea | Td.S | [44] |
| Aquifoliaceae | Ilex opaca | [45] | |
| Araliaceae | Panax ginseng | Te.S; Ginsenosides, glycosides of triterpenoid aglycones | [46,47] |
| Asparagaceae | Yucca schidigera | S.S | [48,49] |
| Asparagus officinalis | S.S | [1,41] | |
| Asteraceae | Atractylis flava | Td.S | [50] |
| Brassicacea | B. vulgaris | Td.S; hederagenincellobioside, oleanolic acid cellobioside, epihederagenincellobioside, and gypsogenincellobioside | [4,51,52,53] |
| Campanulaceae | Platycodon grandiflorum | Td.S | [54] |
| Caryophyllaceae | Saponaria officinalis | Td.S | [55,56,57] |
| Columelliaceae | Desfontainia spinose | Triterpenoids | [58] |
| Combretaceae | Combretum nigricans | Cytotoxic pentacyclic triterpenes | [59] |
| Compositae | Aster auriculatus | Td.S | [60] |
| As. tataricus | Triterpene glycoside | [61] | |
| As. ageratoides | Td.S | [62] | |
| As. batagensis | Td.S | [63,64,65] | |
| As. bellidiastrum | Td.S | [66,67] | |
| As. lingulatus | Td.S | [68,69] | |
| As. scaber | Td.S | [70] | |
| As. sedifolius | Oleane-type saponins; Astersedifolioside A, B and C | [71] | |
| As. yunnamensis | Td.S | [64,72,73] | |
| Cucurbitaceae | Gynostemma pentaphyllum | Gypenosides | [74,75] |
| Momordica charantia | Td.S | [76] | |
| Dioscoreae | Dioscorea spp. | Te.S, Dioscin | [77,78] |
| Fabaceae | Glycyrrhiza spp. | Glycyrrhizin; Td.S | [79,80] |
| Medicago sativa | Td.S | [81,82,83] | |
| Desmodium adscendens | Td.S | [84,85] | |
| Flacourtiaceae | Aphloia madagascariensis | Te.S | [86] |
| Flacourtiaceae | Aphloia theiformis | Te.S | [87] |
| Hippocastanaceae | Aesculus spp. | Td.S; Escins Polyhydroxyoleanene pentacyclic triterpenoid saponins; Aesculiosides | [88,89,90,91,92] |
| Lamiaceae | Salvia staminea | Td.S, salvistamineol | [93] |
| Lecythidaceae | Petersianthus macrocarpus | Td.S | [94,95] |
| Barringtonia acutangula | Monodesmosidic glucuronide saponins; Barringtosides A, B and C | [96] | |
| Liliaceae | Allium aflatunense | S.S | [97,98] |
| A. albanum | S.S | [99] | |
| A. albiflorus | S.S | [100] | |
| A. albopilosum | S.G | [101] | |
| A. ampeloprasum | S.S | [102,103,104] | |
| A. ascalonicum | S.S | [105] | |
| A. cepa | S.S; furostanol saponins, ceposide A, B, and C | [106,107,108,109] | |
| A. chinense | S.S | [110,111,112] | |
| A. elburzense | S.S | [113] | |
| A. erubescens | S.S | [114,115] | |
| A. fistulosum | S.S | [116] | |
| A. giganteum | S.S | [97,117,118,119] | |
| A. jesdianum | S.G | [120] | |
| A. karataviense | S.S | [121,122] | |
| A. macleanii | S.G | [123] | |
| A. macrostemon | Furostanol glycosides | [124] | |
| A. narcissiflorum | S.S | [125,126,127] | |
| A. nutans | S.S | [128,129] | |
| A. ostrowskianum | S.G | [101] | |
| A. porrum | Spirostane-type saponin | [130,131,132] | |
| A. sativum | S.S | [111,133,134,135] | |
| A. schubertii | S.S | [136] | |
| A. sphaerosephalon | Furostanol saponin | [137] | |
| A. senescens | S.G | [123] | |
| A. triquetrum | S.S | [138] | |
| A. tuberosum | S.S | [139,140] | |
| A. turcomanicum | S.S | [141] | |
| A. vineale | Molluscicidal saponins | [142] | |
| A. waldstenii | Steroids of spirostan and furostan series | [115] | |
| Loganiaceae | Antonia ovata | Td.S | [143] |
| Myrsinaceae | Myrsine pellucida | Te.S | [144] |
| Tapeinosperma clethroides | Glucuronide saponins: Desacyl-jegosaponin, desacylboninsaponin A, and sakuraso-saponin | [145,146] | |
| Nyctaginaceae | Pisonia umbellifera | Oleanolic acid saponins and Seco-glycopyranosyl moiety. | [147] |
| Phyllanthaceae | Glochidion eriocarpum | Cytotoxic oleane-type triterpene saponins | [148] |
| Phytolaccaceae | Phytolacca bogotensis | Te.S | [149] |
| Poaceae | Avena sativa | S.S | [1] |
| Quillajaceae | Quillaja saponaria | Te.S | [150,151] |
| Ranunculaceae | Anemone flaccida | Te.S | [152,153] |
| Rhamnaceae | Ziziphus joazeiro | Triterpenicaglycone | [39] |
| Rosaceae | Rosa laevigata | Triterpene glucosides | [154] |
| Sapindaceae | Smelophyllum capense | Te.S | [155] |
| Filicium decipiens | Te.S | [156] | |
| Harpullia cupanioides | Triterpenoïdes | [157,158] | |
| Sapindus mukorossi | [159] | ||
| Sapotaceae | Tridesmostemon claessenssi | Tridesmosaponin A and B | [160] |
| Gambeya boukokoensis | Gamboukokoensides A and B | [161] | |
| Mimusops spp. | Td.S | [162] | |
| Solanaceae | Solanum tuberosum | S.S | [1] |
| S. melongena | S.S | [1,41] | |
| Capsicum species | S.S; four glucose moieties and three glucose moieties | [1,163] | |
| Symplocaceae | Symplocos chinensis | Td.S | [164,165,166] |
| Theaceae | Camellia sinensis | Td.S | [41] |
S.A = Steroidal aglycone; S.S = Steroid saponins; S.G = Steroidal glycosides; St.S = Steroidal saponins; Td.S = Triterpenoid Saponins; Te.S = Triterpene saponins
4. Balance of Costs and Benefits by Formation of New Compounds
If a gene is changed in an individual plant, the fortune of this gene relies on how it affects the plant’s fitness. A change regarding mutation can be deleterious, neutral or beneficial. If mutations are deleterious they will quickly diminish, but on the other hand, useful ones will soon be changed in the population by natural selection. When the “new” and “old” gene are selectively neutral, polymorphisms can become balanced, and the selection keeps segregating alleles for extended periods of time [167]. Subsequent of the aggregate adjustment of expenses and advantages in the natural ecosystem of the plant portrays the variety among various groups of population and species in amount and kind of protection. Such modification can affect the competitiveness amongst genotypes and as a result the choice for a specific genotype [168]. Beside the undeniable advantages clarified in the previous, large amounts of protection, without enemy violence, are thought to be expensive [169,170]. The defense expenses are mostly visualized regarding the distribution of minimal assets from different vigor upgrading capacities inside a plant, for example, photosynthesis, development, as well as new generation [171,172]. Though, those expenses are not evident, as were assumed for plant biochemicals, especially volatiles along with a particular amount of terpenoids by Dicke and Sabelis [24], and Gershenzon [173]. Some defenses may demand ecological exchanges [168], so when supplies are distributed to protect against a particular phytophagous insect, it can decrease the vigor of the plant when harm triggered by other non-target phytophagous insect increments. Eventually, it is expensive when protection admixtures discourage advantageous living bodies, for example, crop pollinators and expected enemies of the phytophagous insect pests [174].
A diversity of plant defense chemical compounds can act as shields in contrast to insects, involving alkaloids, flavonoids, glucosinolates, and phenolic acids [175]. Mostly chemical compounds production is prompted by certain biotic or abiotic factors. Such a schematic arrangement is considered as fight against pathogens and frame insurance economically. Various chemical admixtures involved against insects are the saponins, which have distinctive chemical configurations commonly containing a triterpenoid and steroidal core with a differing quality of glycosylation structures. Saponins are presented in References [176,177] particular 100 various plant categories, even though they mostly are general in species from distinct families or genre, for example, Leguminosae and Liliaceae [178]. Saponins are acquired independent from outside signals and lead to the innate immunity, so named as hypo anticipations because they introduced in individual plants.
The positive aspect of cumulating saponins is primary protection, which is not just for huge measures of vitality, as well as for pathogens to develop mod additionally that it makes feasible for pathogens to develop moderation. It voided when saponin antecedents cumulate and saponin stuff raises resultantly chemical changes of precursor molecules, which incited by pathogenic contamination [179]. Perhaps, the saponin substance may build ideally to the limited quantity due to the chemical response of deposited precursors for biochemical compound safety system or because of pathogen given debasement [180,181].
In the beginning, several studies data on the specific activity of saponins against insects were limited to leguminous origins and extracts [182]. Hostettmann and Marston [183] indicated that several high saponin plant parts from various families, including Aquifoliaceae, Theaceae, as well as Leguminosae, are resistant to insects. Recently, a lot of studies showing the structural activity of concentrated or pure saponin fractions against insects have widely elaborated, and have influenced insects such as aphids, beetles, caterpillars, and flies [1,184]. Nevertheless, the relevant studies of the consequences of various saponins from different origins against insects of different feeding differentiation are still limited.
The behavior of insects changes with individual components of host food, some nutrients attract the insects, while others repel. Hence, plants can synthesize some substances that are important for their significant exercises, while the auxiliary metabolites are included during the time spent co-development amongst plants and other living organisms, for example, insects [185,186]. P. xylostella is a serious pest of cruciferous crops with a cosmopolitan distribution [187]. P. xylostella has developed resistance to existing chemical insecticides including the Bt toxin [188], making it increasingly difficult to control [189]. The capacity of P. xylostella to quickly create imperviousness to insecticides, joined with typically ecological and suitability risks, have fortified enthusiasm for optional controlling systems, for example, trap crops [190]. A trap crop proposed for P. xylostella is wintercress, B. vulgaris [191,192,193,194]. It is a biennial or short-lived perennial plant native to temperate regions worldwide [195]. According to the findings of Shinoda, Nagao, Nakayama, Serizawa, Koshioka, Okabe and Kawai [16], the response of P. xylostella larvae is to be suspected that there is a feeding-deterrent in a crucifer-B. vulgaris. They recorded an adverse effect of the plant volatile compounds on the specialist pest larvae, as the feeding rate of larvae of P. xylostella was reduced on the plant. The feeding deterrent was isolated from B. vulgaris leaves and was identified through the structure to be a monodesmosidic triterpenoid saponin.
5. Larval Feeding Preference and Adult Oviposition Behavior
Larval feeding choice and adult oviposition for younger leaves when contrasted with more seasoned leaves of a specific accommodating plant is a general pattern common with numerous phytophagous insects, particularly in connoisseurs, encompassing P. xylostella [196]. Whenever P. xylostella adults have an option of B. vulgaris and different cruciferous crops, despite the fact P. xylostella larvae cannot continue their lives on a limited range of B. vulgaris, as such as plants being much supportive for oviposition of P. xylostella adults [16,197]. This non survivorship is thought to be as a result of saponins [196].
5.1. P. xylostella Larval Survival on Cotyledons and True Leaves within the Same Plant
Cotyledons represent the capacity of food storage for the improvement of plant, which is the primary photosynthetic network for the plant after germination [198], cotyledons of brassicaceous plants contain varying contents of glucosinolates [199,200]. In Barbarea plants, glucosinolates that might protect the plants against generalist herbivores, were present in the cotyledons, while saponins, which could defend the plant against specialist herbivores like P. xylostella. Similarly, some saponins were not present in cotyledons, indicating that there might be some other biochemical compounds which are responsible for plant defense against herbivores.
5.2. Saponins Presentation in B. Vulgaris Var Arcuata (Isolation and Identification)
The isolation and identification of a triterpenoid saponin, from the leaves of B. vulgaris, which strongly deters feeding of P. xylostella larvae and also the oleane type saponin was studied by Shinoda, et al. [201]. Nielsen, et al. [202]) and Augustin, et al [4] found five triterpenoid saponins in B. vulgaris namely; 3-O-cellobiosyl-hederagenin (hederagenin cellobioside), 3-O-cellobiosyl-oleanoic acid (oleanolic acid), 3-O-cellobiosyl-gypsogenin (gypsogenincellobioside), 3-O-cellobiosylcochalic acid (cochalic acid cellobioside) and 3-O-cellobiosyl-4-epihederagenin (4-epihegragenin cellobioside) Hederagenin cellobioside and oleanolic acid (Figure 1), which make B. vulgaris resistant to P. xylostella and are correlated with deterrence of adult P. xylostella females [4,52,203]. Shinoda, et al [16] discovered that this is not only the first feeding deterrent to P. xylostella found in the family Brassicaceae, but also the first oleanane-type saponin found in this family. So, advance clarification of the chemical configuration of saponins could enhance the development of hydrophobic analogs which may be characterized as fascinating insecticides and herbicides, which potentially required for ecologically more suitable than present synthetic pesticide and herbicides.
6. Biological Significance of Saponins
Saponins are biochemical compounds or otherwise depicted as natural products, which have an extensive spectrum of natural performances. Numerous biological roles have been indicated for various saponins, including anti-inflammatory allelopathic action, anti-carcinogenic, mitigating cell reinforcement, heamolytic, hypocholesterolemic resistance stimulators, cell layer permeabilizing characteristics, as well as can influence feeding behavior, development, and cause mortality, development hindrance, limit the insects’ productiveness and protection against insects and other micro-organisms.
6.1. Saponins Interference with the Feeding Behavior
Some previous reports are available indicating the inability of insect pests larvae to attack Brassicaceae species (B. vulgaris) due to triterpene (saponin), along with two sugars at the position of C3, which restrain the prosecution of the food uptake [201]. Saponins also showed strong effects against other pathogens like fungi mollusks certain bacteria and viruses. In general, it is believed that such biochemicals operate crucially in the plant protection against biotic, as well as abiotic factors, as reported from soybean saponins, which had shown detrimental effects against Tribolium castaneum, Bufo viridis and Lebistes reticulatus. Similarly, saponins were also observed to check the cholinesterases, as well as the proteolytic drive of other enzymes, like trypsin, chymotrypsin and papain, which leads towards non-specific communication with other protein. Moreover, some studies reported that Quillaja Saponaria saponins induce fatality in living insects, and a potent cytotoxic activity on other insects like Drosophila melanogaster cells [204].
6.2. Saponins Effects on Protein Digestion
The toxicity of saponins to various organisms linked to their interaction with biological membranes. Some saponins form complexes with proteins [205] and by this action, they apparently inhibit proteinases and affect digestion in insect gut [204,206,207]. The capability of saponins to penetrate the cell membrane and to induce apoptosis makes saponins cytotoxic to lepidopteran cells [204].
6.3. Enterotoxicity
Saponins are a group of steroidal or triterpenoid secondary plant metabolites, with divergent biological activities [208,209,210], they are responsible for plant defense against antagonists; such as mollusks, pathogens and insects [211,212]. The combination of hydrophilic sugars and hydrophobic sapogenin enable saponins to incorporate into biological membranes. Toxicity of saponins to different organisms seems to be related to their interaction with biological membranes and might be related to their soap-like properties. As a result, detoxification of saponins is probably regarded as enzymatic hydrolysis of the glycosidic bonds, as already produced for fungi [213,214].
Many crucifer specialist insects, such as Pieris brassicae and Pieris rapae and Pieris nemorum with R-genes, are insusceptible to the defenses of B. vulgaris. By finding out the structures of saponins in B. vulgaris [16,215] has allowed for investigations into the mechanism by which these in susceptible insects can deal with the potentially toxic saponins. Badenes-Perez, Reichelt, Gershenzon and Heckel [196] reported that the struggle of B. vulgaris to the diamondback moth (DBM) is prompted by two different saponins; I) 3-0-b-cellobiosylhederagenin and II) 3-0-cellobiosyloleanolic acid, which prevents the feeding of P. xylostella. Likewise, it had been reported that the combination of feeding deterrents showed feeding deterrent habituation in other insects and the combination of saponins I and II may also slow down feeding deterrent habituation in P. xylostella. Nevertheless, saponins I and II contain similar chemical structures; cross habituation might be easier as compared to compounds with different chemical structures, which also indicate the synthesis of saponin-II could be after that of saponin-I [216,217,218,219].
Idris and Grafius [220] and Badenes-Pérez, et al. [221] showed that a small percentage of larvae of a P. xylostella population collected from the field were able to survive on B. vulgaris, even though they did not report the concentration of saponins in these plants. Further researches are required to verify in any case being feeding deterrents, saponins I and II, might have a toxic effect on P. xylostella larvae. Badenes-Perez, Reichelt, Gershenzon and Heckel [196] observed that continuous feeding of neonates of P. xylostella usually on resistant B. vulgaris, results in feeding signs [192].
Dissimilarly to glucosinolates, saponins I and II do not have all the earmarks of being expressed on the leaf covering of Barbarea [222]. Therefore, it is probably that neonates of P. xylostella encounter glucosinolates on the leaf surface and start feeding, while feeding is reduced when insects come into contact with the saponins in the leaf tissue. Likewise saponins I and II, other saponins have been segregated from P-type B. vulgaris var. arcuata, which are responsible for the resistance of this plant to P. nemorum [53,223]. Given the similarity in the resistance mechanisms of G-type B. vulgaris var. arcuata to both P. nemorum and P. xylostella, these saponins might be required in the resistance of Barbarea to P. xylostella. Saponins display higher toxicity, even though the precise mode of action of saponins remains unresolved, it was reported by Badenes-Perez, et al. [196] that saponins specifically target pest insects: Both the continuous insect cells and the primary midgut cells of Spodoptera littoralis showed high sensitivity to Q. saponaria saponin. The phenomenon behind such synergistic mechanisms are unknown, but may include the ability of one biochemical to inhibit the detoxification of other components or to up regulate the absorption of others from the gut.
More significantly, the saponins can cause great and quick in vivo enterotoxin results on the larvae of S. littoralis, and with contents likewise those that can be presented in nature. Therefore, saponins showed substantial evidence for the potency in the control of pest insects, especially insect midgut epithelium as the primary target tissue. So, the insect midgut is an attractive target, as any damaging effect on the midgut epithelial cells will result in starvation, leading towards slow insect mortality. As this component is not the same in midgut cells as the approach of Bacillus thuringiensis (Bt), it can likewise be of assistance in the management of imperviousness to Bt. Furthermore, as aphids are not perceptive to the poisons of Bt, all observations propose that saponins may represent a noteworthy outcome in developing new, substitute, environmentally favorable aphid control agents amongst integrated pest management.
7. Limits of the Use of Saponins in Pest Management Control
Some saponins have heamolytic and cytotoxic effects which have the potential of inhibiting the protease activity. Due to this constraint, it is difficult to apply in the field, as they might also be toxic to humans. The saponins function to protect host plant and to discourage phytophagous insects usually is explained according to their performance in the body of the exposed organism, such as less food consumption, obstructions as well other poisons [224,225]. Mostly, saponins are known as disincentives against insect pests, but their mode of action is yet relatively obscure, however it is identified to interrupt cell sheets [213,226].
Moreover, it was assumed with respect to insects that insect resistance, on the base of ecdysteroid receptor complex (EcR), may be due to particular steroidal saponins, which have resemblance with 20-hydroxyecdysone (molting hormone) [227,228]. Even though, the saponins performance was not supported by real resistance reaction to EcR communication, yet rather than loss of cellular unity considerably, due to the pervasion of the insect cell layer, as described by De Geyter, Swevers, Caccia, Geelen and Smagghe [204]. Plant-derived triterpenoid and steroidal saponins are very promising for the development of botanical insecticides. Aside from cellular poisoning quality, saponins additionally exhibited hindrance or anti-feedant drive against herbivores, especially insects. In a previous study, it was reported that saponins (aginosid) extracted from leek (Allium porrum) caused a noteworthy obstruction in response to two Lepidopteran insect pests; Peridroma saucia and Mamestra configurata [229], as well as in sucking insect pests [230]. In another related study, extracts from the roots of Saponaria officinalis induced a reduction in the rate of oviposition by females of Tetranychus urticae [231]. The plant extracts were found to contain a mix of various saponins [232,233,234] which are suggested to be responsible for the acaricidal efficacy. However, the mechanism of action on mites needs to be further explored.
8. Conclusions and Recommendations
Glucosinolates and saponins play an important role in the plant defense against specialist herbivores. Preliminary data on the saponins’ performance was constrained to reports of leguminous reserves as well other by-products [182]. Thus, comparative studies on the role of saponins are still limited. The chemical basis of previously reported flea beetle resistance in the G-type of B. vulgaris var. arcuata is unknown, but resistance is not correlated to glucosinolates or glucosinolate levels [235]. Resistance may be due to the occurrence of a triterpenoid saponin, which made resistant to B. vulgaris against DBM [16]. Development in the interpretation of saponins biosynthetic system has been obstructed due to a distinctive molecular configuration along with the complication of enzymes, related to two major superfamilies, such as I) cytochrome (P450) and II) glycosyltransferase (GT). The greater part of the Allium and Calamus species consist of saponins, which have a crucial role in health; as such saponins are responsible to decrease the level of garlic cholesterol, as well as enhance the anti-fungal function of garlic [236,237]. They display higher toxicity, even though the precise mode of action of saponins remains unresolved. It has been reported by Badenes-Perez, et al. [196] that saponins from Q. saponaria approach S. littoralis directly by affecting consistent insect midgut cells. It might be exemplified significant results in developing new, substitute, environmentally favorable control agents amongst integrated pest management.
As the discovery of plant defense chemicals continues at its present rapid pace, the present studies discussed above represent the role of plant secondary metabolites in plant defense against herbivores. Given the complex chemical structures of plants, which are not easy to fully understand, play the actual role in defense mechanism (adaptations or counteradaptations) in plant–herbivore interactions. Thus, the structure activity studies of saponins as deterrents for specialist herbivore (such as P. xylostella), therefore, are useful for the deeper understanding of the components and the systems concerned with insect resistance. However, a targeted isolation of these insect repellants will elucidate their structures. Therefore, the improvement of hydrophobic analogs might be regulated by a particular chemical structure of saponins, which may be characterized as interesting chemical sprays, for a particular range of plants, and are (potentially) more natural, compared to the present synthetic herbicide used against herbivores.
Acknowledgments
We are grateful to the researchers who have contributed to this field.
Author Contributions
M.H. conceived, designed and written the manuscript. M.Q. revised and helped in figure preparation. B.D., W.I., M.S.H., and B.S.B. helped in the collection of information and writing the manuscript. L.W. and D.Q. conceived, revised and finalized the manuscripts. All authors approved the submission version.
Funding
This study was supported by National Natural Science Grant of China (Award no. 30400061), Natural Science Foundation of Fujian Province, China (2011J01082) and Special Fund for Science and Technology Innovation of FAFU (CXZX2016107). And National Key Project of R&D of China (2018YFD0201500), Key Projects of Science and Technology of Fujian Province (2016N0005), and Research Fund for the International Collaborative Program (KXGH17004) with (CXZX2017211) from Fujian Agriculture and Forestry University (FAFU).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.De Geyter E., Lambert E., Geelen D., Smagghe G. Novel advances with plant saponins as natural insecticides to control pest insects. Pest Technol. 2007;1:96–105. [Google Scholar]
- 2.Mugford S.T., Osbourn A. Isoprenoid Synthesis in Plants and Microorganisms. Springer; New York, NY, USA: 2012. Saponin synthesis and function; pp. 405–424. [Google Scholar]
- 3.Moses T., Papadopoulou K.K., Osbourn A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014;49:439–462. doi: 10.3109/10409238.2014.953628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustin J.M., Drok S., Shinoda T., Sanmiya K., Nielsen J.K., Khakimov B., Olsen C.E., Hansen E.H., Kuzina V., Ekstrøm C.T. UDP-glycosyltransferases from the UGT73C subfamily in Barbarea vulgaris catalyze sapogenin 3-O-glucosylation in saponin-mediated insect resistance. Plant Physiol. 2012;160:1881–1895. doi: 10.1104/pp.112.202747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khakimov B., Kuzina V., Erthmann P.Ø., Fukushima E.O., Augustin J.M., Olsen C.E., Scholtalbers J., Volpin H., Andersen S.B., Hauser T.P. Identification and genome organization of saponin pathway genes from a wild crucifer, and their use for transient production of saponins in Nicotiana benthamiana. Plant J. 2015;84:478–490. doi: 10.1111/tpj.13012. [DOI] [PubMed] [Google Scholar]
- 6.Patamalai B., Hejtmancik E., Bridges C., Hill D., Camp B. The isolation and identification of steroidal sapogenins in Kleingrass. Vet. Hum. Toxicol. 1990;32:314–318. [PubMed] [Google Scholar]
- 7.Lee S.T., Stegelmeier B.L., Gardner D.R., Vogel K.P. The isolation and identification of steroidal sapogenins in switchgrass. J. Nat. Toxins. 2001;10:273–281. [PubMed] [Google Scholar]
- 8.Osbourn A.E. Saponins in cereals. Phytochemistry. 2003;62:1–4. doi: 10.1016/S0031-9422(02)00393-X. [DOI] [PubMed] [Google Scholar]
- 9.Thimmappa R., Geisler K., Louveau T., O’Maille P., Osbourn A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014;65:225–257. doi: 10.1146/annurev-arplant-050312-120229. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S. Biosynthesis of Structurally Diverse Triterpenes in Plants: The Role of Oxidosqualene Cyclases. Proc. Indian Natl. Sci. Acad. 2016;82:1189–1210. doi: 10.16943/ptinsa/2016/48578. [DOI] [Google Scholar]
- 11.Nielsen J.K., Nagao T., Okabe H., Shinoda T. Resistance in the plant, Barbarea vulgaris, and counter-adaptations in flea beetles mediated by saponins. J. Chem. Ecol. 2010;36:277–285. doi: 10.1007/s10886-010-9758-6. [DOI] [PubMed] [Google Scholar]
- 12.Badenes-Perez F.R., Gershenzon J., Heckel D.G. Insect attraction versus plant defense: Young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. Plos One. 2014;9:e95766. doi: 10.1371/journal.pone.0095766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talekar N., Shelton A. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993;38:275–301. doi: 10.1146/annurev.en.38.010193.001423. [DOI] [Google Scholar]
- 14.Li Z., Feng X., Liu S.-S., You M., Furlong M.J. Biology, Ecology, and Management of the Diamondback Moth in China. Annu. Rev. Entomol. 2016;61:277–296. doi: 10.1146/annurev-ento-010715-023622. [DOI] [PubMed] [Google Scholar]
- 15.Huang X., Renwick J., Sachdev-Gupta K. Oviposition stimulants in Barbarea vulgaris for Pieris rapae and P. napi oleracea: Isolation, identification and differential activity. J. Chem. Ecol. 1994;20:423–438. doi: 10.1007/BF02064448. [DOI] [PubMed] [Google Scholar]
- 16.Shinoda T., Nagao T., Nakayama M., Serizawa H., Koshioka M., Okabe H., Kawai A. Identification of a triterpenoid saponin from a crucifer, Barbarea vulgaris, as a feeding deterrent to the diamondback moth, Plutella xylostella. J. Chem. Ecol. 2002;28:587–599. doi: 10.1023/a:1014500330510. [DOI] [PubMed] [Google Scholar]
- 17.Serizawa H., Shinoda T., Kawai A. Occurrence of a feeding deterrent in Barbarea vulgaris (Brassicales: Brassicaceae), a crucifer unacceptable to the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Appl. Entomol. Zool. 2001;36:465–470. [Google Scholar]
- 18.Newman K. Feeding and oviposition preferences of the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) on six Brassicaceae host plant species. M.Sc Thesis. 2014 [Google Scholar]
- 19.Hopkins R.J., van Dam N.M., van Loon J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009;54:57–83. doi: 10.1146/annurev.ento.54.110807.090623. [DOI] [PubMed] [Google Scholar]
- 20.Ratzka A., Vogel H., Kliebenstein D.J., Mitchell-Olds T., Kroymann J. Disarming the mustard oil bomb. Proc. Natl. Acad. Sci. USA. 2002;99:11223–11228. doi: 10.1073/pnas.172112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renwick J.A.A., Haribal M., Gouinguené S., Städler E. Isothiocyanates stimulating oviposition by the diamondback moth, Plutella xylostella. J. Chem. Ecol. 2006;32:755–766. doi: 10.1007/s10886-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 22.Hairston N.G., Smith F.E., Slobodkin L.B. Community structure, population control, and competition. Am. Nat. 1960;94:421–425. doi: 10.1086/282146. [DOI] [Google Scholar]
- 23.Ehrlich P.R., Raven P.H. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608. doi: 10.1111/j.1558-5646.1964.tb01674.x. [DOI] [Google Scholar]
- 24.Dicke M., Sabelis M.W. Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants. SPB Academic Publishing; The Hague, The Netherlands: 1989. Does it pay plants to advertise for bodyguards? Towards a cost-benefit analysis of induced synomone production; pp. 341–358. [Google Scholar]
- 25.Renwick J.A.A. The chemical world of crucivores: Lures, treats and traps. Entomol. Exp. Et Appl. 2002;104:35–42. doi: 10.1046/j.1570-7458.2002.00988.x. [DOI] [Google Scholar]
- 26.Jones C.G., Firn R.D., Malcolm S.B. On the evolution of plant secondary chemical diversity [and discussion] Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991;333:273–280. [Google Scholar]
- 27.Stahl E. Pflanzen und Schnecken: Eine Biologische Studie über die Schutzmittel der Pflanzen gegen Schneckenfrass. Vol. 1 G. Fischer; Germany: 1888. [Google Scholar]
- 28.Cornell H.V., Hawkins B.A. Herbivore responses to plant secondary compounds: A test of phytochemical coevolution theory. Am. Nat. 2003;161:507–522. doi: 10.1086/368346. [DOI] [PubMed] [Google Scholar]
- 29.Van der Putten W.H. Plant defense belowground and spatiotemporal processes in natural vegetation. Ecology. 2003;84:2269–2280. doi: 10.1890/02-0284. [DOI] [Google Scholar]
- 30.Iwao K., Rausher M.D. Evolution of plant resistance to multiple herbivores: Quantifying diffuse coevolution. Am. Nat. 1997;149:316–335. doi: 10.1086/285992. [DOI] [Google Scholar]
- 31.Van der Putten W.H., Vet L.E.M., Harvey J.A., Wäckers F.L. Linking above-and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol. Evol. 2001;16:547–554. doi: 10.1016/S0169-5347(01)02265-0. [DOI] [Google Scholar]
- 32.Van Dam N.M., Harvey J.A., Wäckers F.L., Bezemer T.M., van der Putten W.H., Vet L.E. Interactions between aboveground and belowground induced responses against phytophages. Basic Appl. Ecol. 2003;4:63–77. doi: 10.1078/1439-1791-00133. [DOI] [Google Scholar]
- 33.Bezemer T.M., van Dam N.M. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 2005;20:617–624. doi: 10.1016/j.tree.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Rask L., Andréasson E., Ekbom B., Eriksson S., Pontoppidan B., Meijer J. Plant Molecular Evolution. Springer; The Netherlands: 2000. Myrosinase: Gene family evolution and herbivore defense in Brassicaceae; pp. 93–113. [PubMed] [Google Scholar]
- 35.Poveda K., Steffan-Dewenter I., Scheu S., Tscharntke T. Effects of below-and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia. 2003;135:601–605. doi: 10.1007/s00442-003-1228-1. [DOI] [PubMed] [Google Scholar]
- 36.Soler R., Harvey J.A., Kamp A.F.D., Vet L.E.M., Van der Putten W.H., Van Dam N.M., Stuefer J.F., Gols R., Hordijk C.A., Martijn Bezemer T. Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant-volatile signals. Oikos. 2007;116:367–376. doi: 10.1111/j.0030-1299.2007.15501.x. [DOI] [Google Scholar]
- 37.Fritz R.S., Simms E.L. Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics. University of Chicago Press; Chicago, IL, USA: 1992. [Google Scholar]
- 38.Glénsk M., Włodarczyk M., Bassarello C., Pizza C., Stefanowicz P., Świtalska M. A Major Saponin from Leaves Extract of Acer velutinum. Z. Für Nat. B. 2009;64:1081–1086. doi: 10.1515/znb-2009-0915. [DOI] [Google Scholar]
- 39.Ribeiro B.D., Alviano D.S., Barreto D.W., Coelho M.A.Z. Functional properties of saponins from sisal (Agave sisalana) and juá (Ziziphus joazeiro): Critical micellar concentration, antioxidant and antimicrobial activities. Colloids Surf. A Physicochem. Eng. Asp. 2013;436:736–743. doi: 10.1016/j.colsurfa.2013.08.007. [DOI] [Google Scholar]
- 40.Mitaine-Offer A.-C., Marouf A., Hanquet B., Birlirakis N., Lacaille-Dubois M.-A. Two Triterpene Saponins from Achyranthes bidentata. Chem. Pharm. Bull. 2001;49:1492–1494. doi: 10.1248/cpb.49.1492. [DOI] [PubMed] [Google Scholar]
- 41.Francis G., Kerem Z., Makkar H.P., Becker K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 42.Ridout C.L., Price K.R., Parkin G., Dijoux M.G., Lavaud C. Saponins from sugar beet and the floc problem. J. Agric. Food Chem. 1994;42:279–282. doi: 10.1021/jf00038a010. [DOI] [Google Scholar]
- 43.Massiot G., Dijoux M.-G., Lavaud C., Le Men-Olivier L., Connolly J.D., Sheeley D.M. Seco-glycosides of oleanolic acid fromBeta vulgaris. Phytochemistry. 1994;37:1667–1670. doi: 10.1016/S0031-9422(00)89589-8. [DOI] [PubMed] [Google Scholar]
- 44.Lavaud C., Massiot G., Le Men-Olivier L., Viari A., Vigny P., Delaude C. Saponins from Steganotaenia araliacea. Phytochemistry. 1992;31:3177–3181. doi: 10.1016/0031-9422(92)83470-J. [DOI] [PubMed] [Google Scholar]
- 45.Potter D.A., Kimmerer T.W. Inhibition of herbivory on young holly leaves: Evidence for the defensive role of saponins. Oecologia. 1989;78:322–329. doi: 10.1007/BF00379105. [DOI] [PubMed] [Google Scholar]
- 46.Shin B.-K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo H., Sun C., Sun Y., Wu Q., Li Y., Song J., Niu Y., Cheng X., Xu H., Li C., et al. Analysis of the transcriptome of Panax notoginseng root uncovers putative triterpene saponin-biosynthetic genes and genetic markers. Bmc Genom. 2011;12:S5. doi: 10.1186/1471-2164-12-S5-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyakoshi M., Tamura Y., Masuda H., Mizutani K., Tanaka O., Ikeda T., Ohtani K., Kasai R., Yamasaki K. Antiyeast steroidal saponins from Yucca schidigera (Mohave Yucca), a new anti-food-deteriorating agent. J. Nat. Prod. 2000;63:332–338. doi: 10.1021/np9904354. [DOI] [PubMed] [Google Scholar]
- 49.Piacente S., Pizza C., Oleszek W. Saponins and phenolics of Yucca schidigera Roezl: Chemistry and bioactivity. Phytochem. Rev. 2005;4:177–190. doi: 10.1007/s11101-005-1234-5. [DOI] [Google Scholar]
- 50.Chabani S., Lavaud C., Benkhaled M., Harakat D., Long C., Haba H. Three new oleanane-type triterpene saponins from Atractylis flava. Phytochem. Lett. 2016;15:88–93. doi: 10.1016/j.phytol.2015.11.017. [DOI] [Google Scholar]
- 51.Khakimov B., Amigo J.M., Bak S., Engelsen S.B. Plant metabolomics: Resolution and quantification of elusive peaks in liquid chromatography–mass spectrometry profiles of complex plant extracts using multi-way decomposition methods. J. Chromatogr. A. 2012;1266:84–94. doi: 10.1016/j.chroma.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Kuzina V., Ekstrøm C.T., Andersen S.B., Nielsen J.K., Olsen C.E., Bak S. Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol. 2009;151:1977–1990. doi: 10.1104/pp.109.136952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuzina V., Nielsen J.K., Augustin J.M., Torp A.M., Bak S., Andersen S.B. Barbarea vulgaris linkage map and quantitative trait loci for saponins, glucosinolates, hairiness and resistance to the herbivore Phyllotreta nemorum. Phytochemistry. 2011;72:188–198. doi: 10.1016/j.phytochem.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Ma C.-H., Gao Z.-J., Zhang J.-J., Zhang W., Shao J.-H., Hai M.-R., Chen J.-W., Yang S.-C., Zhang G.-H. Candidate Genes Involved in the Biosynthesis of Triterpenoid Saponins in Platycodon grandiflorum Identified by Transcriptome Analysis. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hostettmann K., Marston A. Saponins. Cambridge University Press; New York, NY, USA: 2005. [Google Scholar]
- 56.Koike K., Jia Z., Nikaido T. New triterpenoid saponins and sapogenins from Saponaria officinalis. J. Nat. Prod. 1999;62:1655–1659. doi: 10.1021/np990311r. [DOI] [PubMed] [Google Scholar]
- 57.Jia Z., Koike K., Sahu N.P., Nikaido T. Triterpenoid saponins from Caryophyllaceae family. In: Atta ur R., editor. Studies in Natural Products Chemistry. Volume 26. Elsevier; Athens, GA, USA: 2002. pp. 3–61. Part G. [Google Scholar]
- 58.Houghton P.J., Lian L.M. Triterpenoids from Desfontainia spinosa. Phytochemistry. 1986;25:1939–1944. doi: 10.1016/S0031-9422(00)81179-6. [DOI] [Google Scholar]
- 59.Simon G., Dewelle J., Nacoulma O., Guissou P., Kiss R., Daloze D., Braekman J.C. Cytotoxic pentacyclic triterpenes from Combretum nigricans. Fitoterapia. 2003;74:339–344. doi: 10.1016/S0367-326X(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 60.Wang C.Z., Yu D.Q. Triterpenoid Saponins from Aster auriculatus. J. Asian Nat. Prod. Res. 1998;1:1–14. doi: 10.1080/10286029808039839. [DOI] [PubMed] [Google Scholar]
- 61.Dongliang C., Yu S. Terpenoid glycosides from the roots of Aster tataricus. Phytochemistry. 1993;35:173–176. doi: 10.1016/S0031-9422(00)90528-4. [DOI] [PubMed] [Google Scholar]
- 62.Sakai K., Nagao T., Okabe H. Triterpenoid saponins from the ground part of Aster ageratoides var. ovatus. Phytochemistry. 1999;51:309–318. doi: 10.1016/S0031-9422(98)00766-3. [DOI] [Google Scholar]
- 63.Shao Y., Zhou B.-N., Lin L.-Z., Cordell G.A. Two new triterpenoid saponins, asterbatanoside F and G, from Aster batangensis. Nat. Prod. Lett. 1994;5:233–240. doi: 10.1080/10575639408044065. [DOI] [Google Scholar]
- 64.Shao Y., Zhou B.-N., Lin L.-Z., Cordell G.A. Triterpene saponins from Aster yunnanensis. Phytochemistry. 1995;38:1487–1492. doi: 10.1016/0031-9422(94)00794-T. [DOI] [PubMed] [Google Scholar]
- 65.Shao Y., Zhou B., Ma K., Wu H., Lin L., Cordell G.A. Medicagenic acid saponins from Aster batangensis. Phytochemistry. 1995;39:875–881. doi: 10.1016/0031-9422(95)00016-Z. [DOI] [PubMed] [Google Scholar]
- 66.Schöpke T., Al-Tawaha C., Wray V., Nimtz M., Meyer A., Hiller K. Triterpenoid saponins from the aerial parts of Aster bellidiastrum. Phytochemistry. 1995;40:1489–1492. doi: 10.1016/0031-9422(95)00432-7. [DOI] [PubMed] [Google Scholar]
- 67.Schöpke T., Al-Tawaha C., Wray V., Nimtz M., Hiller K. Triterpenoid saponins from Aster bellidiastrum. Phytochemistry. 1997;45:125–132. doi: 10.1016/S0031-9422(96)00776-5. [DOI] [PubMed] [Google Scholar]
- 68.Shao Y., Ho C.-T., Chin C.-K., Poobrasert O., Yang S.-W., Cordell G.A. Asterlingulatosides C and D, cytotoxic triterpenoid saponins from Aster lingulatus. J. Nat. Prod. 1997;60:743–746. doi: 10.1021/np970080t. [DOI] [PubMed] [Google Scholar]
- 69.Shao Y., Ho C.-T., Chin C.-K., Rosen R.T., Hu B., Qin G.-W. Triterpenoid saponins from Aster lingulatus. Phytochemistry. 1997;44:337–340. doi: 10.1016/S0031-9422(96)00551-1. [DOI] [PubMed] [Google Scholar]
- 70.Nagao T., Tanaka R., Okabe H. Saponins Used in Traditional and Modern Medicine. Springer; Boston, MA, USA: 1996. Saponins from the compositae plants: Structures of the saponins from Aster scaber Thunb; pp. 297–307. [DOI] [PubMed] [Google Scholar]
- 71.Corea G., Iorizzi M., Lanzotti V., Cammareri M., Conicella C., Laezza C., Bifulco M. Astersedifolioside A–C, three new oleane-type saponins with antiproliferative activity. Bioorganic Med. Chem. 2004;12:4909–4915. doi: 10.1016/j.bmc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 72.Shao Y., Zhou B.-N., Lin L.-Z., Cordell G.A. Asteryunnanosides F and G: Two new triterpenoid saponins from Aster yunnanensis. Planta Med. 1995;61:446–449. doi: 10.1055/s-2006-958133. [DOI] [PubMed] [Google Scholar]
- 73.Shao Y., Zhou B.-N., Ma K., Wu H.-M. Echinocystic acid saponins from Aster yunnanensis. J. Nat. Prod. 1995;58:837–842. doi: 10.1021/np50120a003. [DOI] [Google Scholar]
- 74.Shi L., Cao J.-Q., Shi S.-M., Zhao Y.-Q. Triterpenoid saponins from Gynostemma pentaphyllum. J. Asian Nat. Prod. Res. 2011;13:168–177. doi: 10.1080/10286020.2010.547029. [DOI] [PubMed] [Google Scholar]
- 75.Yang F., Shi H., Zhang X., Yang H., Zhou Q., Yu L.L. Two new saponins from tetraploid jiaogulan (Gynostemma pentaphyllum), and their anti-inflammatory and α-glucosidase inhibitory activities. Food Chem. 2013;141:3606–3613. doi: 10.1016/j.foodchem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 76.Keller A.C., Ma J., Kavalier A., He K., Brillantes A.-M.B., Kennelly E.J. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine. 2011;19:32–37. doi: 10.1016/j.phymed.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waller G.R., Yamasaki K. Saponins Used in Traditional and Modern Medicine. Volume 404 Springer Science & Business Media; Chicago, IL, USA: 2013. [Google Scholar]
- 78.Qin Y., Wu X., Huang W., Gong G., Li D., He Y., Zhao Y. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis CH Wright in rodents. J. Ethnopharmacol. 2009;126:543–550. doi: 10.1016/j.jep.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 79.Xu R., Zhao W., Xu J., Shao B., Qin G. Studies on Bioactive Saponins from Chinese Medicinal Plants. In: Waller G.R., Yamasaki K., editors. Saponins Used in Traditional and Modern Medicine. Springer US; Boston, MA, USA: 1996. pp. 371–382. [DOI] [PubMed] [Google Scholar]
- 80.Augustin J.M., Kuzina V., Andersen S.B., Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry. 2011;72:435–457. doi: 10.1016/j.phytochem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 81.Massiot G., Lavaud C., Guillaume D., Le Men-Olivier L. Reinvestigation of the sapogenins and prosapogenins from alfalfa (Medicago sativa) J. Agric. Food Chem. 1988;36:902–909. doi: 10.1021/jf00083a005. [DOI] [Google Scholar]
- 82.Massiot G., Lavaud C., Besson V., Le Men-Olivier L., Van Binst G. Saponins from aerial parts of alfalfa (Medicago sativa) J. Agric. Food Chem. 1991;39:78–82. doi: 10.1021/jf00001a014. [DOI] [Google Scholar]
- 83.Oleszek W., Price K.R., Colquhoun I.J., Jurzysta M., Ploszynski M., Fenwick G.R. Isolation and identification of alfalfa (Medicago sativa L.) root saponins: Their activity in relation to a fungal bioassay. J. Agric. Food Chem. 1990;38:1810–1817. doi: 10.1021/jf00099a006. [DOI] [Google Scholar]
- 84.Rastogi S., Pandey M.M., Rawat A.K.S. An ethnomedicinal, phytochemical and pharmacological profile of Desmodium gangeticum (L.) DC. and Desmodium adscendens (Sw.) DC. J. Ethnopharmacol. 2011;136:283–296. doi: 10.1016/j.jep.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 85.McManus O., Harris G., Giangiacomo K., Feigenbaum P., Reuben J., Addy M., Burka J.F., Kaczorowski G., Garcia M. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32:6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- 86.Dijoux M.-G., Lavaud C., Massiot G., Le Men-Olivier L., Sheeley D.M. A saponin from leaves of Aphloia madagascariensis. Phytochemistry. 1993;34:497–499. doi: 10.1016/0031-9422(93)80037-S. [DOI] [Google Scholar]
- 87.Gopalsamy N., Vargas D., Guého J., Ricaud C., Hostettmann K. Saponins from leaves of Aphloia theiformis. Phytochemistry. 1988;27:3593–3595. doi: 10.1016/0031-9422(88)80774-X. [DOI] [Google Scholar]
- 88.Kimura H., Ogawa S., Jisaka M., Kimura Y., Katsube T., Yokota K. Identification of novel saponins from edible seeds of Japanese horse chestnut (Aesculus turbinata Blume) after treatment with wooden ashes and their nutraceutical activity. J. Pharm. Biomed. Anal. 2006;41:1657–1665. doi: 10.1016/j.jpba.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 89.Kimura H., Ogawa S., Katsube T., Jisaka M., Yokota K. Antiobese effects of novel saponins from edible seeds of Japanese horse chestnut (Aesculus turbinata Blume) after treatment with wood ashes. J. Agric. Food Chem. 2008;56:4783–4788. doi: 10.1021/jf800340s. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Z., Li S. Cytotoxic triterpenoid saponins from the fruits of Aesculus pavia L. Phytochemistry. 2007;68:2075–2086. doi: 10.1016/j.phytochem.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z., Li S., Zhang S., Gorenstein D. Triterpenoid saponins from the fruits of Aesculus pavia. Phytochemistry. 2006;67:784–794. doi: 10.1016/j.phytochem.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 92.Yoshikawa M., Murakami T., Matsuda H., Yamahara J., Murakami N., Kitagawa I. Bioactive saponins and glycosides. III. Horse chestnut.(1): The structures, inhibitory effects on ethanol absorption, and hypoglycemic activity of escins Ia, Ib, IIa, IIb, and IIIa from the seeds of Aesculus hippocastanum L. Chem. Pharm. Bull. 1996;44:1454–1464. doi: 10.1248/cpb.44.1454. [DOI] [PubMed] [Google Scholar]
- 93.Topcu G., Altiner E.N., Gozcu S., Halfon B., Aydogmus Z., Pezzuto J., Zhou B.-N., Kingston D.G. Studies on di-and triterpenoids from Salvia staminea with cytotoxic activity. Planta Med. 2003;69:464–467. doi: 10.1055/s-2003-39705. [DOI] [PubMed] [Google Scholar]
- 94.Massiot G., Xiang-Fei C., Lavaud C., Le Men-Olivier L., Delaude C., Viari A., Vigny P., Duval J. Saponins from stem bark of Petersianthus macrocarpus. Phytochemistry. 1992;31:3571–3576. doi: 10.1016/0031-9422(92)83729-I. [DOI] [PubMed] [Google Scholar]
- 95.El Izzi A., Benie T., Thieulant M.-L., Le Men-Olivier L., Duval J. Stimulation of LH release from cultured pituitary cells by saponins of Petersianthus macrocarpus: A permeabilizing effect. Planta Med. 1992;58:229–233. doi: 10.1055/s-2006-961441. [DOI] [PubMed] [Google Scholar]
- 96.Pal B.C., Chaudhuri T., Yoshikawa K., Arihara S. Saponins from Barringtonia acutangula. Phytochemistry. 1994;35:1315–1318. doi: 10.1016/S0031-9422(00)94845-3. [DOI] [PubMed] [Google Scholar]
- 97.Kawashima K., Mimaki Y., Sashida Y. Steroidal saponins from Allium giganteum and A. aflatunense. Phytochemistry. 1991;30:3063–3067. doi: 10.1016/S0031-9422(00)98253-0. [DOI] [Google Scholar]
- 98.Mimaki Y., Kuroda M., Sashida Y. Steroidal saponins from the bulbs of Allium aflatunense. Natural Med. 1999;53:88–93. [Google Scholar]
- 99.Ismailov A., Tagiev S., Rasulov E. Steroid saponins and sapogenins from Allium rubellum and Allium albanum. Khimiia Prir. Soedin. Chem. Natural Compound. 1976;12:495. doi: 10.1007/BF00564837. [DOI] [Google Scholar]
- 100.Ismaĭlov A., Aliev A. Determination of the steroid saponins in the onion (Allium albiflorus) that grows in Azerbaijan. Farmatsiia. 1976;25:17. [PubMed] [Google Scholar]
- 101.Mimaki Y., Kawashima K., Kanmoto T., Sashida Y. Steroidal glycosides from Allium albopilosum and A. ostrowskianum. Phytochemistry. 1993;34:799–805. doi: 10.1016/0031-9422(93)85362-U. [DOI] [PubMed] [Google Scholar]
- 102.MoRITA T., USHIROGUCHI T., HAYASHI N., MATSUURA H., ITAKURA Y., Fuwa T. Steroidal saponins from elephant garlic, bulbs of Allium ampeloprasum L. Chem. Pharm. Bull. 1988;36:3480–3486. doi: 10.1248/cpb.36.3480. [DOI] [PubMed] [Google Scholar]
- 103.Matsunaga S., Fusetani N., Nishikawa H., Takamura S., Saito T. New antifungal and cytotoxic steroidal saponins from the bulbs of an elephant garlic mutant. Biosci. Biotechnol. Biochem. 1998;62:1904–1911. doi: 10.1271/bbb.62.1904. [DOI] [PubMed] [Google Scholar]
- 104.Mimaki Y., Kuroda M., Sashida Y. Steroidal saponins from the bulbs of Allium ampeloprasum. Natural Med. 1999;53:134–137. [Google Scholar]
- 105.Fattorusso E., Iorizzi M., Lanzotti V., Taglialatela-Scafati O. Chemical composition of shallot (Allium ascalonicum Hort.) J. Agric. Food Chem. 2002;50:5686–5690. doi: 10.1021/jf020396t. [DOI] [PubMed] [Google Scholar]
- 106.Corea G., Fattorusso E., Lanzotti V., Capasso R., Izzo A.A. Antispasmodic saponins from bulbs of red onion, Allium cepa L. var. Tropea. J. Agric. Food Chem. 2005;53:935–940. doi: 10.1021/jf048404o. [DOI] [PubMed] [Google Scholar]
- 107.Dini I., Tenore G.C., Trimarco E., Dini A. Furostanol saponins in Allium caepa L. Var. tropeana seeds. Food Chem. 2005;93:205–214. doi: 10.1016/j.foodchem.2004.09.015. [DOI] [Google Scholar]
- 108.Li C.-J., Yuan L., Ji T.-F., Yang J.-B., Wang A.-G., Su Y.-L. Furostanol saponins from the seeds of Allium cepa L. Fitoterapia. 2014;99:56–63. doi: 10.1016/j.fitote.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 109.Lanzotti V., Romano A., Lanzuise S., Bonanomi G., Scala F. Antifungal saponins from bulbs of white onion, Allium cepa L. Phytochemistry. 2012;74:133–139. doi: 10.1016/j.phytochem.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 110.Kuroda M., Mimaki Y., Kameyama A., Sashida Y., Nikaido T. Steroidal saponins from Allium chinense and their inhibitory activities on cyclic AMP phosphodiesterase and Na+ K+ ATPase. Phytochemistry. 1995;40:1071–1076. doi: 10.1016/0031-9422(95)00423-5. [DOI] [PubMed] [Google Scholar]
- 111.Peng J.-P., Yao X.-S. Saponins Used in Traditional and Modern Medicine. Springer; Boston, MA, USA: 1996. 19 new steroidal saponins from Allium plants: Isolation, structural elucidation and effect on blood coagulability; pp. 511–526. [DOI] [PubMed] [Google Scholar]
- 112.Jiang Y., Wang N.-L., Yao X.-S., Kitanaka S. Steroidal saponins from the bulbs of Allium chinense. Stud. Plant Sci. 1999;6:212–219. [Google Scholar]
- 113.Barile E., Zolfaghari B., Sajjadi S.E., Lanzotti V. Saponins of Allium e lburzense. J. Nat. Prod. 2004;67:2037–2042. doi: 10.1021/np0497752. [DOI] [PubMed] [Google Scholar]
- 114.Chincharadze D., Kel’Ginbaev A., Gorovits M., Eristavi L., Gorovits T., Abubakirov N. Steroid saponins and sapogenins of Allium. XV. Eruboside B from Allium erubescens. Chem. Nat. Compd. 1979;15:442–446. doi: 10.1007/BF00565042. [DOI] [Google Scholar]
- 115.Kravets S., Vollerner Y.S., Gorovits M., Abubakirov N. Steriods of the spirostan and furostan series from plants of the genus Allium. Chem. Nat. Compd. 1990;26:359–373. doi: 10.1007/BF00598985. [DOI] [Google Scholar]
- 116.Do J.C., Jung K.Y., Son K.H. Steroidal saponins from the subterranean part of Allium fistulosum. J. Nat. Prod. 1992;55:168–173. doi: 10.1021/np50080a003. [DOI] [Google Scholar]
- 117.KELGINBAEV A., Gorovits M., Gorovits T., Abubakirov N. Steroidal saponins and sapogenins of Allium. 9. Aginoside structure. Khimiya Prir. Soedin. 1976;4:480–486. [Google Scholar]
- 118.SAsHiDA Y., KAwASHIMA K., MIMAKI Y. Novel polyhydroxylated steroidal saponins from Allium giganteum. Chem. Pharm. Bull. 1991;39:698–703. doi: 10.1248/cpb.39.698. [DOI] [Google Scholar]
- 119.MIMAKI Y., NIKAIDO T., MATSUMOTO K., SASHIDA Y., OHMOTO T. New steroidal saponins from the bulbs of Allium giganteum exhibiting potent inhibition of cAMP phosphodiesterase activity. Chem. Pharm. Bull. 1994;42:710–714. doi: 10.1248/cpb.42.710. [DOI] [PubMed] [Google Scholar]
- 120.Mimaki Y., Kuroda M., Fukasawa T., Sashida Y. Steroidal glycosides from the bulbs of Allium jesdianum. J. Nat. Prod. 1999;62:194–197. doi: 10.1021/np980346b. [DOI] [PubMed] [Google Scholar]
- 121.Mimaki Y., Kuroda M., Fukasawa T., SASHIDA Y. Steroidal saponins from the bulbs of Allium karataviense. Chem. Pharm. Bull. 1999;47:738–743. doi: 10.1248/cpb.47.738. [DOI] [PubMed] [Google Scholar]
- 122.Vollerner Y.S., Abdullaev N., Gorovits M., Abubakirov N. Steroid saponins and sapogenins of Allium. XX. Structure of karatavisoides E and F. Chem. Nat. Compd. 1984;20:64–68. doi: 10.1007/BF00574793. [DOI] [Google Scholar]
- 123.Inoue T., Mimaki Y., Sashida Y., Nishino A., Satomi Y., Nishino H. Steroidal glycosides from Allium macleanii and A. senescens, and their inhibitory activity on tumour promoter-induced phospholipid metabolism of HeLa cells. Phytochemistry. 1995;40:521–525. doi: 10.1016/0031-9422(95)00223-T. [DOI] [PubMed] [Google Scholar]
- 124.Peng J., Yao X., Kobayashi H., Ma C. Novel furostanol glycosides from Allium macrostemon. Planta Med. 1995;61:58–61. doi: 10.1055/s-2006-958000. [DOI] [PubMed] [Google Scholar]
- 125.Lazur’Evskii G., Krokhmalyuk V., Kintya P. Structure of steroid glycosides of Allium narcissiflorum Wills. Dokl. Biochem. 1975;221:151. [PubMed] [Google Scholar]
- 126.Krokhmalyuk V., Kintya P. Steroid saponins. XIII. The structure of alliumosides D and E from Allium narcissiflorum. Chem. Nat. Compd. 1976;12:165–168. doi: 10.1007/BF00566337. [DOI] [Google Scholar]
- 127.Mimaki Y., Satou T., Ohmura M., Sashida Y. Steroidal saponins from the bulbs of Allium narcissiflorum. Nat. Med. 1996;50:308. [Google Scholar]
- 128.Akhov L., Musienko M., Piacente S., Pizza C., Oleszek W. Structure of steroidal saponins from underground parts of Allium nutans L. J. Agric. Food Chem. 1999;47:3193–3196. doi: 10.1021/jf9901800. [DOI] [PubMed] [Google Scholar]
- 129.Akhov L., Musienko M., Shishova Y., Polishuk V., Oleszek W. Saponins in Food, Feedstuffs and Medicinal Plants. Springer; Dordrecht, The Netherlands: 2000. Biological Activity of Deltoside from Allium Nutans L. pp. 227–231. [Google Scholar]
- 130.Harmatha J., Mauchamp B., Arnault C., Sláma K. Identification of a spirostane-type saponin in the flowers of leek with inhibitory effects on growth of leek-moth larvae. Biochem. Syst. Ecol. 1987;15:113–116. doi: 10.1016/0305-1978(87)90089-5. [DOI] [Google Scholar]
- 131.Carotenuto A., Fattorusso E., Lanzotti V., Magno S. Spirostanol saponins of Allium porrum L. Phytochemistry. 1999;51:1077–1082. doi: 10.1016/S0031-9422(98)00712-2. [DOI] [PubMed] [Google Scholar]
- 132.Fattorusso E., Lanzotti V., Taglialatela-Scafati O., Di Rosa M., Ianaro A. Cytotoxic saponins from bulbs of Allium porrum L. J. Agric. Food Chem. 2000;48:3455–3462. doi: 10.1021/jf000331v. [DOI] [PubMed] [Google Scholar]
- 133.MATSUURA H., USHIROGUCHI T., ITAKURA Y. Further studies on steroidal glycosides from bulbs, roots and leaves of Allium sativum L. Chem. Pharm. Bull. 1989;37:2741–2743. doi: 10.1248/cpb.37.2741. [DOI] [Google Scholar]
- 134.Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J. Nutr. 2001;131:1000S–1005S. doi: 10.1093/jn/131.3.1000S. [DOI] [PubMed] [Google Scholar]
- 135.Lanzotti V. Bioactive Saponins from Allium and Aster Plants. Phytochem. Rev. 2005;4:95–110. doi: 10.1007/s11101-005-1254-1. [DOI] [Google Scholar]
- 136.Kawashima K., Mimaki Y., Sashida Y. Steroidal saponins from the bulbs of Allium schubertii. Phytochemistry. 1993;32:1267–1272. doi: 10.1016/S0031-9422(00)95103-3. [DOI] [PubMed] [Google Scholar]
- 137.Mimaki Y., Satou T., Kuroda M., Kameyama A., Sashida Y., Li H.-y., Harada N. A new furostanol saponin with six sugars from the bulbs of Allium sphaerosephalon structural elucidation by modern NMR techniques. Chem. Lett. 1996;25:431–432. doi: 10.1246/cl.1996.431. [DOI] [Google Scholar]
- 138.Corea G., Fattorusso E., Lanzotti V. Saponins and Flavonoids of Allium triquetrum. J. Nat. Prod. 2003;66:1405–1411. doi: 10.1021/np030226q. [DOI] [PubMed] [Google Scholar]
- 139.Zou Z.-M., Yu D.-Q., Cong P.-Z. A steroidal saponin from the seeds of Allium tuberosum. Phytochemistry. 2001;57:1219–1222. doi: 10.1016/S0031-9422(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 140.Sang S., Mao S., Lao A., Chen Z., Ho C.-T. New steroid saponins from the seeds of Allium tuberosum L. Food Chem. 2003;83:499–506. doi: 10.1016/S0308-8146(03)00131-6. [DOI] [PubMed] [Google Scholar]
- 141.Pirtskhalava G., Gorovits M., Gorovits T., Abubakirov N. Steroid saponins and sapogenins of Allium. XVI. Turoside C from Allium turcomanicum. Chem. Nat. Compd. 1979;15:446–452. doi: 10.1007/BF00565043. [DOI] [Google Scholar]
- 142.Chen S., Snyder J.K. Diosgenin-bearing, molluscicidal saponins from Allium vineale: An NMR approach for the structural assignment of oligosaccharide units. J. Org. Chem. 1989;54:3679–3689. doi: 10.1021/jo00276a033. [DOI] [Google Scholar]
- 143.Magid A.A., Bobichon H., Borie N., Lalun N., Long C., Moretti C., Lavaud C. Cytotoxic triterpenoid saponins from the stem bark of Antonia ovata. Phytochemistry. 2010;71:429–434. doi: 10.1016/j.phytochem.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 144.Lavaud C., Massiot G., Moretti C., Le Men-Olivier L. Triterpene saponins Frommyrsine pellucida. Phytochemistry. 1994;37:1671–1677. doi: 10.1016/S0031-9422(00)89590-4. [DOI] [PubMed] [Google Scholar]
- 145.Kitagawa I., Yoshikawa M., Kobayashi K., Imakura Y., Im K., Ikenishi Y. Saponin and sapogenol. XXVIII. Reinvestigation of the branching positions in the glucuronide moieties of three glucuronide saponins: Desacyl-jegosaponin, desacyl-boninsaponin A, and sakuraso-saponin. Chem. Pharm. Bull. 1980;28:296–300. doi: 10.1248/cpb.28.296. [DOI] [Google Scholar]
- 146.Lavaud C., Pichelin O., Massiot G., Le Men-Olivier L., Sevenet T., Cosson J. Sakuraso-saponin from Tapeinosperma clethroides. Fitoterapia. 1999;70:116–118. doi: 10.1016/S0367-326X(98)00034-3. [DOI] [Google Scholar]
- 147.Lavaud C., Beauvière S., Massiot G., Le Men-Olivier L., Bourdy G. Saponins from Pisonia umbellifera. Phytochemistry. 1996;43:189–194. doi: 10.1016/0031-9422(96)00253-1. [DOI] [PubMed] [Google Scholar]
- 148.Nhiem N.X., Thu V.K., Van Kiem P., Van Minh C., Tai B.H., Quang T.H., Cuong N.X., Yen P.H., Boo H.-J., Kang J.-I., et al. Cytotoxic oleane-type triterpene saponins from Glochidion eriocarpum. Arch. Pharmacal Res. 2012;35:19–26. doi: 10.1007/s12272-012-0102-2. [DOI] [PubMed] [Google Scholar]
- 149.Montoya G., Arango G.J., Unger M., Holzgrabe U. Saponins from Phytolacca bogotensis using HPLC-ESI/multi-stage tandem mass spectrometry. Phytochem. Anal. 2009;20:465–474. doi: 10.1002/pca.1148. [DOI] [PubMed] [Google Scholar]
- 150.Bankefors J., Nord L.I., Kenne L. Structural classification of acyl-substituted Quillaja saponins by electrospray ionisation ion trap multiple-stage mass spectrometry in combination with multivariate analysis. Rapid Commun. Mass Spectrom. 2008;22:3851–3860. doi: 10.1002/rcm.3803. [DOI] [PubMed] [Google Scholar]
- 151.Kensil C.R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 152.Zhan C., Li X., Zhao Z., Yang T., Wang X., Luo B., Zhang Q., Hu Y., Hu X. Comprehensive Analysis of the Triterpenoid Saponins Biosynthetic Pathway in Anemone flaccida by Transcriptome and Proteome Profiling. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao L., Chen W.-M., Fang Q.-C. Triterpenoid saponins from Anemone flaccida. Planta Med. 1990;56:92–93. doi: 10.1055/s-2006-960894. [DOI] [PubMed] [Google Scholar]
- 154.Yuan J.-Q., Yang X.-Z., Miao J.-H., Tang C.-P., Ke C.-Q., Zhang J.-B., Ma X.-J., Ye Y. New triterpene glucosides from the roots of Rosa laevigata Michx. Molecules. 2008;13:2229–2237. doi: 10.3390/molecules13092229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lavaud C., Voutquenne L., Massiot G., Le Men-Olivier L., Delaude C. Saponines triperpéniques de Smelophyllum capense (Sapindaceae) Bull. De La Société R. Des Sci. De Liège. 1994;63:455–463. [Google Scholar]
- 156.Lavaud C., Voutquenne L., Massiot G., Le Men-Olivier L., Das B.C., Laprévote O., Serani L., Delaude C., Becchi M. Saponins from the stem bark of Filicium decipiens. Phytochemistry. 1998;47:441–449. doi: 10.1016/S0031-9422(97)00586-4. [DOI] [PubMed] [Google Scholar]
- 157.Dimbi M.Z., Warin R., Delaude C., Huls R., Mpuza K. Triterpenoides de Harpullia cupanioides. Bull. Des Sociétés Chim. Belg. 1983;92:473–484. doi: 10.1002/bscb.19830920509. [DOI] [Google Scholar]
- 158.Voutquenne L., Lavaud C., Massiot G., Delaude C. Saponins from Harpullia cupanioides. Phytochemistry. 1998;49:2081–2085. doi: 10.1016/S0031-9422(98)00406-3. [DOI] [PubMed] [Google Scholar]
- 159.Shiau I.-L., Shih T.-L., Wang Y.-N., Chen H.-T., Lan H.-F., Lin H.C., Yang B.-Y., Ko C.-H., Murase Y. Quantification for saponin from a soapberry (Sapindus mukorossi Gaertn) in cleaning products by a chromatographic and two colorimetric assays. J. Fac. Agr. Kyushu Univ. 2009;54:215–221. [Google Scholar]
- 160.Massiot G., Lavaud C., Delaude C.M., Van Binst G., Miller S.P.F., Fales H.M. Saponins from Tridesmostemon claessenssi. Phytochemistry. 1990;29:3291–3298. doi: 10.1016/0031-9422(90)80202-R. [DOI] [Google Scholar]
- 161.Wandji J., Tillequin F., Mulholland D.A., Shirri J.C., Tsabang N., Seguin E., Verite P., Libot F., Fomum Z. Pentacyclic triterpenoid and saponins from Gambeya boukokoensis. Phytochemistry. 2003;64:845–849. doi: 10.1016/S0031-9422(03)00495-3. [DOI] [PubMed] [Google Scholar]
- 162.Lavaud C., Massiot G., Becchi M., Misra G., Nigam S. Saponins from three species of Mimusops. Phytochemistry. 1996;41:887–893. doi: 10.1016/0031-9422(95)00692-3. [DOI] [PubMed] [Google Scholar]
- 163.De Lucca A., Boue S., Palmgren M., Maskos K., Cleveland T. Fungicidal properties of two saponins from Capsicum frutescens and the relationship of structure and fungicidal activity. Can. J. Microbiol. 2006;52:336–342. doi: 10.1139/w05-137. [DOI] [PubMed] [Google Scholar]
- 164.Li X.-H., Shen D.-D., Li N., Yu S.-S. Bioactive triterpenoids from Symplocos chinensis. J. Asian Nat. Prod. Res. 2003;5:49–56. doi: 10.1080/1028602031000080469. [DOI] [PubMed] [Google Scholar]
- 165.Li B., Abliz Z., Tang M., Fu G., Yu S. Rapid structural characterization of triterpenoid saponins in crude extract from Symplocos chinensis using liquid chromatography combined with electrospray ionization tandem mass spectrometry. J. Chromatogr. A. 2006;1101:53–62. doi: 10.1016/j.chroma.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 166.Fu G.-M., Wang Y.-H., Gao S., Tang M.-J., Yu S.-S. Five new cytotoxic triterpenoid saponins from the roots of Symplocos chinensis. Planta Med. 2005;71:666–672. doi: 10.1055/s-2005-871274. [DOI] [PubMed] [Google Scholar]
- 167.Mitchell-Olds T., Clauss M.J. Plant evolutionary genomics. Curr. Opin. Plant Biol. 2002;5:74–79. doi: 10.1016/S1369-5266(01)00231-X. [DOI] [PubMed] [Google Scholar]
- 168.Simms E.L., Rausher M.D. Costs and benefits of plant resistance to herbivory. Am. Nat. 1987;130:570–581. doi: 10.1086/284731. [DOI] [Google Scholar]
- 169.Vrieling K., Smit W., van der Meijden E. Tritrophic interactions between aphids (Aphis jacobaeae Schrank), ant species, Tyria jacobaeae L. and Senecio jacobaea L. lead to maintenance of genetic variation in pyrrolizidine alkaloid concentration. Oecologia. 1991;86:177–182. doi: 10.1007/BF00317529. [DOI] [PubMed] [Google Scholar]
- 170.Cipollini D. Variation in the expression of chemical defenses in Alliaria petiolata (Brassicaceae) in the field and common garden. Am. J. Bot. 2002;89:1422–1430. doi: 10.3732/ajb.89.9.1422. [DOI] [PubMed] [Google Scholar]
- 171.Van der Meijden E., Wijn M., Verkaar H.J. Defence and regrowth, alternative plant strategies in the struggle against herbivores. Oikos. 1988;51:355–363. doi: 10.2307/3565318. [DOI] [Google Scholar]
- 172.Herms D.A., Mattson W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992;67:283–335. doi: 10.1086/417659. [DOI] [Google Scholar]
- 173.Gershenzon J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994;20:1281–1328. doi: 10.1007/BF02059810. [DOI] [PubMed] [Google Scholar]
- 174.Strauss S.Y., Siemens D.H., Decher M.B., Mitchell-Olds T. Ecological costs of plant resistance to herbivores in the currency of pollination. Evolution. 1999;53:1105–1113. doi: 10.1111/j.1558-5646.1999.tb04525.x. [DOI] [PubMed] [Google Scholar]
- 175.Rosenthal G., Janzen D. Herbivores: Their interaction with plant secondary metabolites. Academic Press; New York, NY, USA: 1979. 718p [Google Scholar]
- 176.Hostettmann K., Marston A., Wolfender J.L. Proceedings-Phytochemical Society of Europe. Oxford University Press Inc.; Oxford, UK: 1995. In Strategy in the search for new biologically active plant constituents; p. 17. [Google Scholar]
- 177.Abreu A.C., McBain A.J., Simoes M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012;29:1007–1021. doi: 10.1039/c2np20035j. [DOI] [PubMed] [Google Scholar]
- 178.Harborne J.B., Baxter H. Dictionary of Plant Toxins. John Wiley & Sons; UK: 1996. [Google Scholar]
- 179.Morrissey J.P., Osbourn A.E. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 1999;63:708–724. doi: 10.1128/mmbr.63.3.708-724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Szakiel A., Pączkowski C., Henry M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011;10:471–491. doi: 10.1007/s11101-010-9177-x. [DOI] [Google Scholar]
- 181.Sampaio B.L., Edrada-Ebel R., Da Costa F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016;6:29265. doi: 10.1038/srep29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Applebaum S., Birk Y. Herbivores: Their Interactions with Secondary Plant Metabolites. Acad. Press; New York, NY, USA: 1979. pp. 539–566. [Google Scholar]
- 183.Hostettmann K., Marston A. Chemistry and Pharmacology of Natural Products, Saponin. Cambridge University Press; London, UK: 1995. [Google Scholar]
- 184.Purkayastha J., Arora R., Singh L. Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization. Springer; New Delhi, India: 2016. Sustainable and Novel Eco-friendly Approaches Towards Integrated Disease and Vector Management; pp. 11–23. [Google Scholar]
- 185.Alias E.E.M., Zeinelabdin M.H., Bashir N.H.H., Assad Y.O.H., Abdelbagi O.M. Hypoglycemic and toxic effects of saponins from the fruit of bitter apple [Citrullus colocynthis (L.) Schrad] on the internal organs of Norway rat [Rattus norvegicus (Berkenhout)] Gezira J. Agric. Sci. 2015;13:1–17. [Google Scholar]
- 186.Fuenzalida T. Plant natural defense against insects: Role of secondary metabolites. Santiago. 2015:1–24. [Google Scholar]
- 187.Furlong M.J., Wright D.J., Dosdall L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu Rev Entomol. 2013;58:517–541. doi: 10.1146/annurev-ento-120811-153605. [DOI] [PubMed] [Google Scholar]
- 188.Heckel D.G., Gahan L.J., Liu Y.B., Tabashnik B.E. Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc. Natl. Acad. Sci. USA. 1999;96:8373–8377. doi: 10.1073/pnas.96.15.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tabashnik B.E., Huang F., Ghimire M.N., Leonard B.R., Siegfried B.D., Rangasamy M., Yang Y., Wu Y., Gahan L.J., Heckel D.G., et al. Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat. Biotechnol. 2011;29:1128–1131. doi: 10.1038/nbt.1988. [DOI] [PubMed] [Google Scholar]
- 190.Shelton A.M., Badenes-Perez F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006;51:285–308. doi: 10.1146/annurev.ento.51.110104.150959. [DOI] [PubMed] [Google Scholar]
- 191.Badenes-Perez F.R., Shelton A.M., Nault B.A. Evaluating trap crops for diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) J. Econ. Entomol. 2004;97:1365–1372. doi: 10.1093/jee/97.4.1365. [DOI] [PubMed] [Google Scholar]
- 192.Lu J.H., Liu S.S., Shelton A.M. Laboratory evaluations of a wild crucifer Barbarea vulgaris as a management tool for the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) Bull. Entomol. Res. 2004;94:509–516. doi: 10.1079/BER2004328. [DOI] [PubMed] [Google Scholar]
- 193.Shelton A.M., Nault B.A. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Crop Prot. 2004;23:497–503. doi: 10.1016/j.cropro.2003.10.005. [DOI] [Google Scholar]
- 194.Badenes-Perez F.R., Shelton A.M., Nault B.A. Using yellow rocket as a trap crop for diamondback moth (Lepidoptera: Plutellidae) J. Econ. Entomol. 2005;98:884–890. doi: 10.1603/0022-0493-98.3.884. [DOI] [PubMed] [Google Scholar]
- 195.MacDonald M.A., Cavers P.B. The biology of Canadian weeds: 97. Barbarea vulgaris R. Br. Can. J. Plant Sci. 1991;71:149–166. doi: 10.4141/cjps91-016. [DOI] [Google Scholar]
- 196.Badenes-Perez F.R., Reichelt M., Gershenzon J., Heckel D.G. Using plant chemistry and insect preference to study the potential of Barbarea (Brassicaceae) as a dead-end trap crop for diamondback moth (Lepidoptera: Plutellidae) Phytochemistry. 2014;98:137–144. doi: 10.1016/j.phytochem.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 197.Newman K., You M., Vasseur L. Diamondback Moth (Lepidoptera: Plutellidae) Exhibits Oviposition and Larval Feeding Preferences Among Crops, Wild plants, and Ornamentals as Host Plants. J. Econ. Entomol. 2016:tow002. doi: 10.1093/jee/tow002. [DOI] [PubMed] [Google Scholar]
- 198.Boege K., Marquis R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 199.Petersen B., Chen S., Hansen C., Olsen C., Halkier B. Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta. 2002;214:562–571. doi: 10.1007/s004250100659. [DOI] [PubMed] [Google Scholar]
- 200.Wallace S.K., Eigenbrode S.D. Changes in the glucosinolate–myrosinase defense system in Brassica juncea cotyledons during seedling development. J. Chem. Ecol. 2002;28:243–256. doi: 10.1023/A:1017973005994. [DOI] [PubMed] [Google Scholar]
- 201.Agerbirk N., Olsen C.E., Bibby B.M., Frandsen H.O., Brown L.D., Nielsen J.K., Renwick J.A.A. A Saponin Correlated with Variable Resistance of Barbarea vulgaris to the Diamondback Moth Plutella xylostella. J. Chem. Ecol. 2003;29:1417–1433. doi: 10.1023/A:1024217504445. [DOI] [PubMed] [Google Scholar]
- 202.Nielsen N.J., Nielsen J., Staerk D. New resistance-correlated saponins from the insect-resistant crucifer Barbarea vulgaris. J. Agric. Food Chem. 2010;58:5509–5514. doi: 10.1021/jf903988f. [DOI] [PubMed] [Google Scholar]
- 203.van Mölken T., Kuzina V., Munk K.R., Olsen C.E., Sundelin T., van Dam N.M., Hauser T.P. Consequences of combined herbivore feeding and pathogen infection for fitness of Barbarea vulgaris plants. Oecologia. 2014;175:589–600. doi: 10.1007/s00442-014-2928-4. [DOI] [PubMed] [Google Scholar]
- 204.De Geyter E., Swevers L., Caccia S., Geelen D., Smagghe G. Saponins show high entomotoxicity by cell membrane permeation in Lepidoptera. Pest Manag. Sci. 2012;68:1199–1205. doi: 10.1002/ps.3284. [DOI] [PubMed] [Google Scholar]
- 205.Potter S.M., Jimenez-Flores R., Pollack J., Lone T.A., Berber-Jimenez M.D. Protein-saponin interaction and its influence on blood lipids. J. Agric. Food Chem. 1993;41:1287–1291. doi: 10.1021/jf00032a023. [DOI] [Google Scholar]
- 206.Soetan K., Ajibade T., Akinrinde A. Saponins; A Ubiquitous Phytochemical: A Review of its Biochemical, Physiological and Pharmacological Effects. Recent Prog. Med. Plants. 2014;43:1–24. [Google Scholar]
- 207.Amtul J.S., Shakoori A.R. Potential of Azadirachtin and Neem (Azadirachta indica) Based Saponins as Biopesticides for In vitro Insect Pests Cellulase (Beta-1, 4-Endoglucanase) Enzyme Inhibition and In vivo Repellency on Tribolium castaneum. Br. Biotechnol. J. 2014;4:904–917. [Google Scholar]
- 208.Goławska S., Łukasik I., Wójcicka A., Sytykiewicz H. Relationship between saponin content in alfalfa and aphid development. Acta Biol. Crac. Ser. Bot. 2012;54:39–46. doi: 10.2478/v10182-012-0022-y. [DOI] [Google Scholar]
- 209.Tava A., Odoardi M. Saponins Used in food and Agriculture. Springer; Boston, MA, USA: 1996. Saponins from Medicago spp.: Chemical characterization and biological activity against insects; pp. 97–109. [DOI] [PubMed] [Google Scholar]
- 210.Vincken J.-P., Heng L., de Groot A., Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275–297. doi: 10.1016/j.phytochem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 211.Lee H.-A., Lee H.-Y., Seo E., Lee J., Kim S.-B., Oh S., Choi E., Choi E., Lee S.E., Choi D. Current Understandings on Plant Nonhost Resistance. Mol. Plant-Microbe Interact. 2017;30:5–15. doi: 10.1094/MPMI-10-16-0213-CR. [DOI] [PubMed] [Google Scholar]
- 212.Dowd P.F., Berhow M.A., Johnson E.T. Differential activity of multiple saponins against omnivorous insects with varying feeding preferences. J. Chem. Ecol. 2011;37:443–449. doi: 10.1007/s10886-011-9950-3. [DOI] [PubMed] [Google Scholar]
- 213.Osbourn A. Saponins and plant defence—A soap story. Trends Plant Sci. 1996;1:4–9. doi: 10.1016/S1360-1385(96)80016-1. [DOI] [Google Scholar]
- 214.Osbourn A.E., Wubben J.P., Melton R.E., Carter J.P., Daniels M.J. Phytochemical Signals and Plant-Microbe Interactions. Springer; Boston, MA, USA: 1998. Saponins and plant defense; pp. 1–15. [Google Scholar]
- 215.Chaieb I. Saponins as insecticides: A review. Tunis. J. Plant Prot. 2010;5:39–50. [Google Scholar]
- 216.Akhtar Y., Isman M.B. Binary mixtures of feeding deterrents mitigate the decrease in feeding deterrent response to antifeedants following prolonged exposure in the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae) Chemoecology. 2003;13:177–182. doi: 10.1007/s00049-003-0246-0. [DOI] [Google Scholar]
- 217.Akhtar Y., Isman M.B. Larval exposure to oviposition deterrents alters subsequent oviposition behavior in generalist, Trichoplusia ni and specialist, Plutella xylostella moths. J. Chem. Ecol. 2003;29:1853–1870. doi: 10.1023/A:1024802328458. [DOI] [PubMed] [Google Scholar]
- 218.Kumar R., Bhardwaj U., Kumar P., Mazumdar-Leighton S. Midgut serine proteases and alternative host plant utilization in Pieris brassicae L. Front. Physiol. 2015;6:95. doi: 10.3389/fphys.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Frisch T., Agerbirk N., Davis S., Cipollini D., Olsen C.E., Motawia M.S., Bjarnholt N., Møller B.L. Glucosinolate-related glucosides in Alliaria petiolata: Sources of variation in the plant and different metabolism in an adapted specialist herbivore, Pieris rapae. J. Chem. Ecol. 2014;40:1063–1079. doi: 10.1007/s10886-014-0509-y. [DOI] [PubMed] [Google Scholar]
- 220.Idris A.B., Grafius E. The potential of using Barbarea vulgaris in insecticide-resistant diamondback moth management. Resist. Pest Manag. (USA) 1994;6:7–8. [Google Scholar]
- 221.Badenes-Pérez F.R., Márquez B.P., Petitpierre E. Can flowering Barbarea spp. (Brassicaceae) be used simultaneously as a trap crop and in conservation biological control? J. Pest Sci. 2016;4:1–11. doi: 10.1007/s10340-016-0815-y. [DOI] [Google Scholar]
- 222.Badenes-Pérez F.R., Reichelt M., Gershenzon J., Heckel D.G. Phylloplane location of glucosinolates in Barbarea spp. (Brassicaceae) and misleading assessment of host suitability by a specialist herbivore. New Phytol. 2011;189:549–556. doi: 10.1111/j.1469-8137.2010.03486.x. [DOI] [PubMed] [Google Scholar]
- 223.Vermeer K.M.C.A. Genetics of Insect Resistance to Plant Defence. Wageningen University; Wageningen, The Netherlands: 2014. [Google Scholar]
- 224.Wittstock U., Gershenzon J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 2002;5:300–307. doi: 10.1016/S1369-5266(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 225.Mithöfer A., Maffei M.E. Plant Toxins. Springer AG; Switzerland: 2016. General mechanisms of plant defense and plant toxins; pp. 1–22. [Google Scholar]
- 226.Sparg S.G., Light M.E., Van Staden J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004;94:219–243. doi: 10.1016/j.jep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 227.Dinan L. Phytoecdysteroids: Biological aspects. Phytochemistry. 2001;57:325–339. doi: 10.1016/S0031-9422(01)00078-4. [DOI] [PubMed] [Google Scholar]
- 228.Harder M.J., Tello V.E., Giliomee J.H. The acaricidal effect of ethanolic extracts of Chenopodium quinoa Willd. on Tetranychus urticae Koch (Acari: Tetranychidae) Afr. Entomol. 2016;24:50–60. doi: 10.4001/003.024.0050. [DOI] [Google Scholar]
- 229.Nawrot J., Koul O., Isman M., Harmatha J. Naturally occurring antifeedants: Effects on two polyphagous lepidopterans. J. Appl. Entomol. 1991;112:194–201. doi: 10.1111/j.1439-0418.1991.tb01046.x. [DOI] [Google Scholar]
- 230.Goławska S., Sprawka I., Łukasik I. Effect of saponins and apigenin mixtures on feeding behavior of the pea aphid, Acyrthosiphon pisum Harris. Biochem. Syst. Ecol. 2014;55:137–144. doi: 10.1016/j.bse.2014.03.010. [DOI] [Google Scholar]
- 231.Pavela R. Extract from the roots of Saponaria officinalis as a potential acaricide against Tetranychus urticae. J. Pest Sci. 2017;90:683–692. doi: 10.1007/s10340-016-0828-6. [DOI] [Google Scholar]
- 232.Wina E., Muetzel S., Becker K. The impact of saponins or saponin-containing plant materials on ruminant production—A review. J. Agric. Food Chem. 2005;53:8093–8105. doi: 10.1021/jf048053d. [DOI] [PubMed] [Google Scholar]
- 233.Tamura Y., Miyakoshi M., Yamamoto M. Alternative Medicine. InTech; London, UK: 2012. Application of saponin-containing plants in foods and cosmetics. [Google Scholar]
- 234.Jia Z., Koike K., Nikaido T. Major triterpenoid saponins from Saponaria officinalis. J. Nat. Prod. 1998;61:1368–1373. doi: 10.1021/np980167u. [DOI] [PubMed] [Google Scholar]
- 235.Agerbirk N., Olsen C.E., Nielsen J.K. Seasonal variation in leaf glucosinolates and insect resistance in two types of Barbarea vulgaris ssp. arcuata. Phytochemistry. 2001;58:91–100. doi: 10.1016/S0031-9422(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 236.Borah B., Phukon P., Hazarika M.P., Ahmed R., Sarmah D.K., Wann S.B., Das A., Bhau B.S. Calamus leptospadix Griff. a high saponin yielding plant with antimicrobial property. Ind. Crop. Prod. 2016;82:127–132. doi: 10.1016/j.indcrop.2015.11.075. [DOI] [Google Scholar]
- 237.Lanzotti V., Barile E., Antignani V., Bonanomi G., Scala F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera. Phytochemistry. 2012;78:126–134. doi: 10.1016/j.phytochem.2012.03.009. [DOI] [PubMed] [Google Scholar]