Abstract
The present study is to investigate the antitumor, antioxidant and antibacterial potential of silver nanoparticles (Ag NPs) synthesized from a phenolic derivative 4-N-methyl benzoic acid, isolated from a medicinal plant (Memecylon umbellatum Burm F). The Bio-inspired nanoparticles (NPs) were analyzed by using UV–vis spectroscopy, FTIR, HRTEM, Zeta potential and XRD techniques. The UV–vis spectroscopy study at the band of 430 nm confirmed the nanoparticles formation. HRTEM report showed that the AgNPs synthesized were in the size range 7–23 nm. The harvested nanoparticles were subjected to anti-bacterial assay and a dose dependent inhibitory action was observed against the tested human pathogens. Among the tested bacteria, Acinetobacter baumannii was found to be highly sensitive to AgNPs (diameter of zone of inhibition was 31 mm). Further, the silver nanoparticles exhibited a good anti-tumor activity against the breast cancer cell line (MCF 7) with an IC50 value of 42.19 µg/mL. As the present study confirmed a good antibacterial, antioxidant and antitumor activity in the nanoparticles synthesized using 4-N-methyl benzoic acid derived from a medicinal plant, the product can be further tested to formulate a good lead compound for biomedical applications.
Keywords: Memecylon, Bio inspired AgNPs, Antibacterial, Antioxidant and antitumor
1. Introduction
Nano based research is a promising area in biomedical approach especially in malignant therapy and diagnostics. Cancer is the second leading causes of death globally. The development of new drugs for cancer treatment still remains a great challenge to scientific community and pharmaceutical industries. Among women breast cancer is a rising problem today. The major risk factors for breast cancer include family history, reproductive functional changes, food and nutrition and hormonal changes (Gerber et al., 2003). To study the breast cancer cells and its vulnerability to apoptosis, MCF-7 cell lines are recommended (Levenson and Jordan, 1997). Nanoparticles with the size range 100 nm are reported to be effective in biomedical applications (Devanesan et al., 2018, Alfuraydi et al., 2019). Magnetic nanoparticles are reported to be used to treat patients with different cancer (Johannsen et al., 2010). The major challenge to overpower breast cancer is the heterogeneity of breast cancer tissues and non-availability of right medicine (Ali et al., 2019). This situation imposes an urgent need to develop an effective and less toxic cancer drug. The perusal of available report shows that the bioactive compounds from natural products can be used to develop lead compounds for several oncological problems (Mahassni and Al-Reemi, 2013). In this aspect the present study is planned to synthesize silver nanoparticles using the bioactive compound isolated from a medicinal plant and to evaluate its efficacy to deal tumor cells and also to overcome oxidative stress in cells.
Further nanomaterial are reported as a good antibacterial agent like the standard antibiotics. Sungkaworn et al. (2008) reported nanomaterial to inhibit the growth of more than 600 micro pathogens. Magnetic nanoparticles prepared using silver, gold and platinum are also used in several medical applications (Kumar et al., 2014, Jose and Sakthivel, 2014). Among the different NPs, the silver nanoparticles have gained more attention than others because of the presence of unique and alterable surface Plasmon resonance (Veena et al., 2019, Jose et al., 2016a, Jose et al., 2016b). Silver has been reported to be an effective antimicrobial agent because of its less human toxicity and also its promising application in in-vitro and in-vivo intervention (Roy et al., 2017; Zaheer, 2018). The silver ion and other silver modified inorganic materials are reported to inhibit the bacterial growth by interfering with the functioning of DNA (Jiraroj et al., 2014).
The AgNPs produced by chemical techniques may lead to the adsorption of chemicals with toxic nature and that may cause toxic problem. Hence, the green syntheses of NPs have become a major focus for researchers (Hebeish et al., 2013). Nanoparticles can be synthesized at low cost by using the extracts from plants and products from microbes (Aswathy and Philip, 2012). The potential of the green synthesized AgNPs depends on the solvent, reducing agent used and stability of AgNPs (Raveendran et al., 2006).
Nature has devised ingenious and elegant way of developing efficient functional materials. The natural bioactive compounds like D-Glucose (Sen et al., 2013), glucan, sodium para-hydroxy benzoate tetra hydrate (Durai et al., 2014) and saponins (Geethalakshmi and Sarada, 2013) were also used to synthesize AgNPs. The size of AgNPs help in easy bio absorption and the surface enhanced resonance properties of AgNPs enhances its application in different biomedical tools either as a single compound or in combination with other smart materials (Sarkar et al., 2012). The shape, size, distribution and morphological features of NPs influence their applications in human health care system (AlSalhi et al., 2016, Alfuraydi et al., 2019).
Nickavar and Esbati (2012) reports that the antioxidant free radical scavenging activity seen in polyphenol and their ability to inhibit peroxidation along with the chelation of transition metals promotes its biomedical importance. The phenolic compounds also are reported to reduce lipid peroxidation and prevent oxidative damages in DNA by scavenging free oxygen radicals (Cao et al., 2013). The antimicrobial activity of AgNPs has led to the growth of innovative benefits as an alternative to antibiotics (Jeeva et al., 2014). The present study proposes a fast and low cost method of synthesis of green AgNPs using 4-N-methyl benzoic acid a phenolic derivative extracted from a medicinal plant. Hitherto there is no study on anti-proliferative, antioxidant and antibacterial activities of AgNPs synthesized using 4-N-methyl benzoic acid. With these objectives to find out a novel biomedical compound, the present study is designed.
2. Materials and methods
2.1. Reagents
The experimental reagents were purchased from Sigma and the experiments were conducted in aseptic laboratory conditions.
2.2. Isolation of bioactive compound from M. umbellatum (leaf)
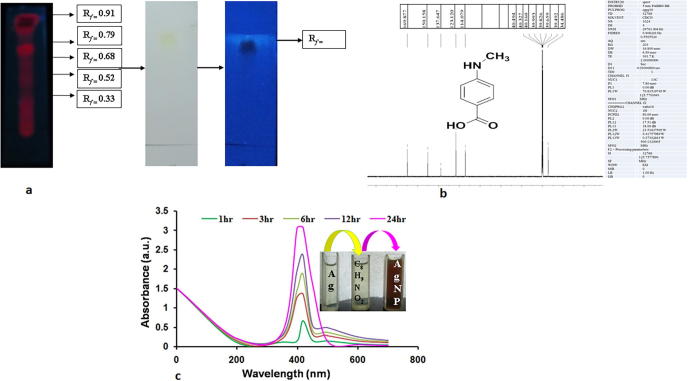
Column chromatography is a widely used technique in organic chemistry and plant biochemistry, to isolate and identify the bioactive compounds. Fourteen grams of methanolic extract of the leaves of M. umbellatum was loaded in to a silica gel for column chromatography separation and eluted with the solvents hexane, hexane in ethyl acetate, chloroform in methanol and methanol in different ratio. The obtained fractions were observed using TLC and the spots were visualized under UV–vis spectroscope in short and long wavelength (254 and 366 nm). Among the fractions, the active fraction (MUME 1) was separated and subjected to further analyses. MUME 1 was spotted on TLC plates, dried and the developed band was visualized under UV light. Rf value was calculated. Further, C Nuclear Magnetic Resonance (NMR) spectroscopy was carried out at 100 and 400 MHz-.
2.2.1. Synthesis AgNPs
Silver nitrate (AgNO3) (0.01 mM) was freshly prepared with milli Q water. Ten milligrams of bioactive compound was added to 10 mL of milli Q water and filtered using syringe filter (0.2 µm). Compound mixture of 3 mL was added with 97 mL of 0.01 mM of AgNO3 solution and kept at room temperature for few hours. Then it was centrifuged at 15,000 RPM for 30 min and the sediment was collected and it was again dispersed in DH2O, centrifuged and the process was continued three times to wash off any absorbed substances on the surface of AgNPs.
2.2.2. Characterization of AgNPs
Visual observation and optical density of AgNPs was measured at 200–800 nm using spectrophotometer (PerkinElmer-USA) and further characterization made as followed by (Devanesan et al., 2018, AlSalhi et al., 2016).
2.3. Antimicrobial analysis
In vitro antibacterial activity of the bio-inspired AgNPs was tested against G (+) and Gram (−) bacteria, Bacillus subtilis (ATCC 19659), Acenetobacter baumannii (ATCC 19606), Salmonella paratyphi (ATCC 9150) and Staphylococcus aureus (ATCC 25923).
2.3.1. Preparation of inoculum
The identified bacterial pathogens were cultured in nutrient agar for 24 hrs at 37 °C. Then the culture was inoculated in to nutrient broth and kept undisturbed at 4 °C. From the broth overnight grown cultures were taken out and the turbidity of the culture was made equivalent to 0.5 McFarland standards. About 0.2 mL of cultured microbes were taken into 20 mL sterile nutrient broth at 105–107 CFU/mL an optical density of 0.1 at 600 nm
MHA media was poured into a Petridish, and 0.1 mL of active growth bacterial culture was taken in and it was spread over the solidified media uniformly. Eight mm diameter well was made and each well was filled with different doses (10, 25, 50 and 100 µg/mL) of samples (Varghese et al., 2019, Jalal et al., 2018).
2.4. Antioxidant measurement
2.4.1. Free radical scavenging assay using DPPH
The free radical scavenging ability of AgNPs was assayed according to Guntur et al. (2018). In this study 0.1 mM solution of DPPH was prepared using methanol and the absorbance of DPPH was measured at 517 nm. About 100 μL aqueous solution were poured to 3 mL of DPPH mixtures. After 30 min of incubation, the discoloration of purple color was measured at 517 nm. Methanol served as blank whereas DPPH in methanol with standard served as positive control and the free radical scavenging percentage was calculated as follows:
2.4.2. Superoxide radical scavenging assay
The effect of synthesized AgNPs on superoxide radical scavenging was measured using spectrophotometer (Gow-Chin and Hui-Yin, 1995).
2.5. Metal chelating effect of AgNPs
The chelation of ferrous ions by AgNPs was determined by ferrous ion chelation method (Dinis et al., 1994).
2.6. In vitro anti-proliferative activity of AgNPs
AgNPs induced anti-proliferation of tumor cell line was measured by MTT assay (Mosmann, 1983). Cell death ratio was calculated using the formula given below
2.6.1. Determination of apoptotic cell death using AO/EB staining
The morphological changes in cancer cells treated with AgNPs were observed (Mosmann, 1983, AlSalhi et al., 2016). Overnight culture of MCF-7 cells (3 × 106) were used to measure the apoptotic activity of AgNPs (10, 25 and 50 µg/mL) and incubated for 24 h at 37 °C.
3. Results and discussion
The leaf sample loaded to silica column was first eluted using absolute hexane. The mobile phase was gradually co-mixed with hexane in ethyl acetate, followed by chloroform in methanol and conclusively eluted with absolute methanol. The amassed fractions were concentrated using rotary evaporator and visualized under TLC to spot the presence of active compounds. The Rf value was calculated. In the mobile phase, the solvent ratio of hexane and methanol (9.0:1.0 mL) showed a single band with Rf value 0.68 (Fig. 1a) and it was named as MUME 1. It was further characterized using 13C NMR spectroscopy. The results showed eight carbon atoms with four carbon signals for two methine δ114.1 (2C-3, 5) and δ123.1 (2C, C-2, 6). The aromatic quaternary carbon signal showed resonances at δ137.6 (C-1) and δ150.1 (C-4). The downfield signed at δ169.8 was assigned to the acid carbonyl carbon. The signal at δ34.48 was assigned to N-methyl carbon (Fig. 1b). Based on the spectral analyses, molecular structure of the bioactive compound was identified as a phenolic derivative, 4-N-methyl benzoic acid with an empirical formula C8H9NO2. To prepare AgNPs 3 mL of freshly prepared bioactive compound solution was mixed with 97 mL of AgNO3 (0.01 mM) solution. In this study, Ag+ ions were converted into silver oxide within 3 min of reaction time at ambient temperature. This prepared AgNPs exhibited a minimum UV–vis absorption within the range 410–430 nm (Fig. 1c). When the bioactive compound was added into silver nitrate solution, the surface Plasmon peak dominates and becomes broadened and it altered the electron density. As phenolic compounds possess hydroxyl and carboxyl groups, it easily binds to the metals as reported (Devanesan et al., 2017, Singh et al., 2013). AgNPs exhibited an absorption peak at 420 nm, as reported earlier (Sen et al., 2013).
Fig. 1.
(a) Thin layer chromatography of bioactive compound (MUME 1); (b) 13C NMR spectrum of bioactive compound; (c) UV–vis spectra of Silver nanoparticles at various time intervals.
FTIR spectrum of AgNPs was observed based on the peak at 3427, 2729, 2393, 2061, 1762 and 1388 cm−1 (Fig. 2). The observed peak around 3427, 2729, 2393 cm−1 notifies carboxylic group of O—H stretching vibration of NPs. The 2393 cm−1 is C tribble band C stretching vibration of nanoparticles. In the compound the presence of aldehyde (1762 cm−1) was reported. The silver ions join with biomolecules containing carboxyl and hydroxyl groups in acidic condition. Further the aldehydes and ketone present in the molecules reduce silver ions into silver nanoparticles and get oxidized (1762, 1388 cm−1). The carboxyl and hydroxyl ions in the biomolecules develop a protective layer on the surface of AgNPs. This protective layer helps to develop stability (Li et al., 2007). The present result indicate that the amides, phenols, carboxyl and amine group residues are also involved in AgNPs synthesis.
Fig. 2.
FT-IR spectrum of Ag NPs.
The formed AgNPs are crystalline as per XRD report (Fig. 3). The XRD pattern was exhibited four peaks at 2θ ranges of 38.28°, 46.66°, 63.26° and 77.33° can be labeled to the 111, 200, 220 and 311. The results obtained was associated by the data base JCPDS no 4-0783. The XRD patterns displayed were consistent with the earlier reports (Raj et al., 2018).
Fig. 3.
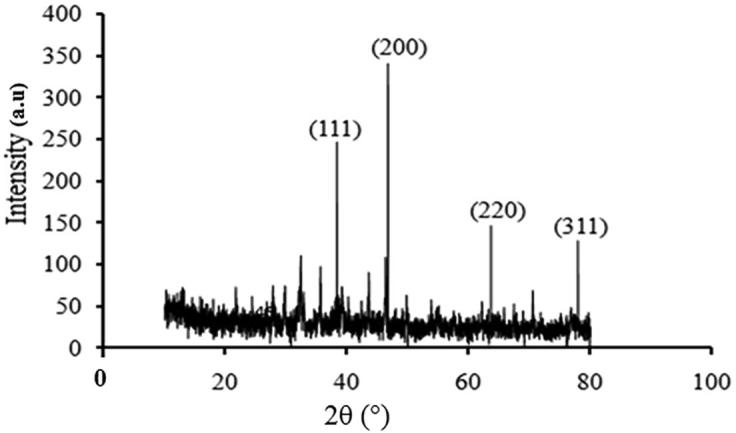
XRD patterns of green synthesized Ag NPs.
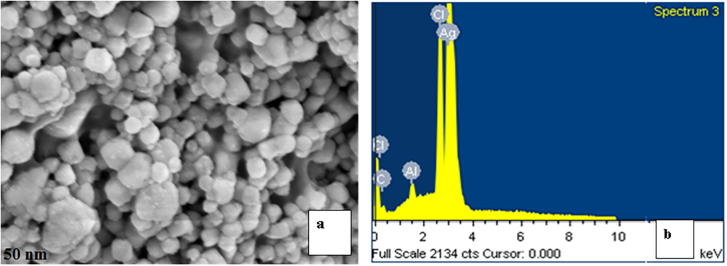
The morphology and structure of the prepared AgNPs was observed using FESEM (Fig. 4a), which showed that the formed AgNPs were in the range 7–23 nm in size and average size is 15 nm. The Energy Dispersive X-ray analysis (EDX) revealed a strong signal in the silver zone confirmed the AgNPs presence (Fig. 4b). Similar result was reported earlier (Gorbe et al., 2016, Jose et al., 2016a, Jose et al., 2016b). To confirm the average, further analysis was made using Electron Microscope.
Fig. 4.
(a) Morphological and Elemental composition analysis of AgNPs by FESEM (b) Energy Dispersive X-ray analysis of AgNPs.
HRTEM micrograph of the synthesized AgNPs was observed magnification at 10 nm scales as shown in Fig. 5a. The formation of silver atoms in the fabricated nanoparticles was confirmed by SAED analysis in Fig. 5b. Results showed that; AgNPs were poly-dispersed in nature, spherical in shape with particles size ranges between 7 and 22 nm. It is reported that AgNPs in the size range 1–10 nm can easily attach to the surface of cell membrane. This potentiality of AgNPs interfere with cell respiration and its permeability (Senthilkumar et al., 2017, Jose et al., 2016a, Jose et al., 2016b). Kavitha et al. (2014) reported that NPs with 7 nm size was also synthesized using sodium borohydride (NaBH4), but in the present study AgNPs with similar size was prepared using green synthesis method and this bio inspired NPs are more safer than that prepared by chemical methods.
Fig. 5.
(a) HR-TEM analysis of Ag NPs; (b) SAED analysis of Ag NPs.
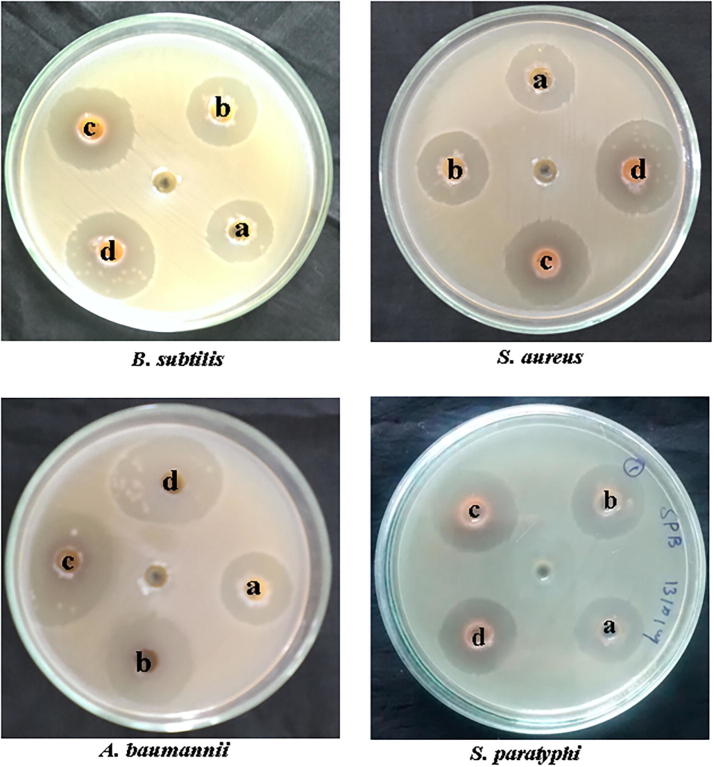
The anti-bacterial activity of AgNPs was studied using well diffusion method (Fig. 6). All the concentrations displayed a measurable size of zone of inhibition. The maximum activity was found for A. baumannii (31 mm) and S. aureus (26) and minimum activity was seen against S. paratyphi (21 mm) and Bacillus subtilis (19 mm). The results showed that, AgNPs exhibited a broad spectral antibiotic activity against G+ and G− bacteria. Amongst all the pathogens tested, Acinetobacter baumannii was found to be the most sensitive to various concentrations of AgNPs. The mechanism of action of AgNPs on bacterial cells involve multiple targets. Nanoparticles combined with drugs can be easily suspended in liquids and thereby they can easily penetrate into tissue and deep organ easily and also show an extended half-life like any potential drug (Sahoo et al., 2007). In earlier reports, the nanoparticles combined with antibiotics showed synergistic anti-bacterial activity against microorganisms, particularly P. aeruginosa, E. coli and A. baumannii (Ghosh et al., 2012). The AgNPs are reported to interfere with the permeability mechanism in the membrane of bacterial cells (Korshed, et al., 2018) and inactivate the proteins thus interfacing with the replication of DNA (Chaloupka et al., 2010). Smaller sized nanoparticles have huge impact because of its improved biocompatibility (Elangovan et al., 2015, Devanesan et al., 2018, AlSalhi et al., 2016 Alfuraydi et al., 2019). The AgNPs synthesized in the present study from a phenolic derivative, 4-N-methyl benzoic acid (a potential nontoxic reducing agent) can be used for many biomedical applications.
Fig. 6.
In vitro antibacterial activity of Ag NPs on human pathogenic bacteria.
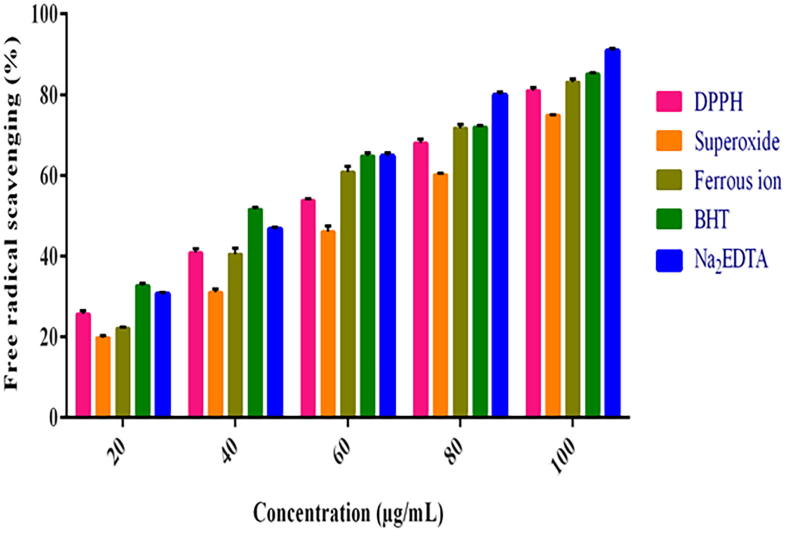
The scavenging efficacy of AgNPs is represented in (Fig. 7). The present study shows that AgNPs was effective against DPPH radical scavenging (81.57%) with EC50 range of 53.46 µg/mL and BHT showed 85.39% with EC50 range of 37.92 µg/mL. The study showed that synthesized AgNPs exhibited better results compared to previous reports (Varghese et al, 2019), It might be due to the phenolic nature. Phenolic complexes are always associated with antioxidant mechanisms, which will donate hydrogen ion or an electron to stabilize and to scavenge the free radicals (Ali et al., 2013). Most of the bioactive compounds from plants are multifunctional; hence it is necessary to invoke more than one antioxidant activities (Wong et al., 2006). AgNPs showed maximum scavenging of superoxide radicals (Fig. 7) with an EC50 value of 66.68 μg/mL (74.76%), while the standard showed an EC50 value of 53.39 μg/mL (80.71%). Ramamurthy et al. (2013), have reported that 52% superoxide radical scavenging activity was observed for AgNPs whereas, the AuNPs exhibited 47% only. The results concluded that AgNPs exhibited an impressive antioxidant activity when compared to other metal nanoparticles. The ferrous ion chelation of AgNPs was determined by measuring the iron-ferrozine complex. It exhibited better results from at 10–100 μg/mL, ranged from 21.90 to 82.49% with EC50 value of 50.37 μg/mL while, BHT showed 30.45–90.31% with EC50 value of 44.10 μg/mL (Fig. 7). The metal chelating activity was high when the concentration of AgNPs increased (Deng et al., 2016). Metal chelating ability in the AgNPs further helps to reduce the concentration of catalyzing reaction in the transition metals present in lipid peroxides (Moukette et al., 2015). The in-vitro antioxidant assays, indicated that AgNPs exhibited a dose dependent radical scavenging activity.
Fig. 7.
In vitro antioxidant activity of Ag NPs including DPPH free radical scavenging assay Superoxide radical scavenging assay and Ferrous ion chelation effect.
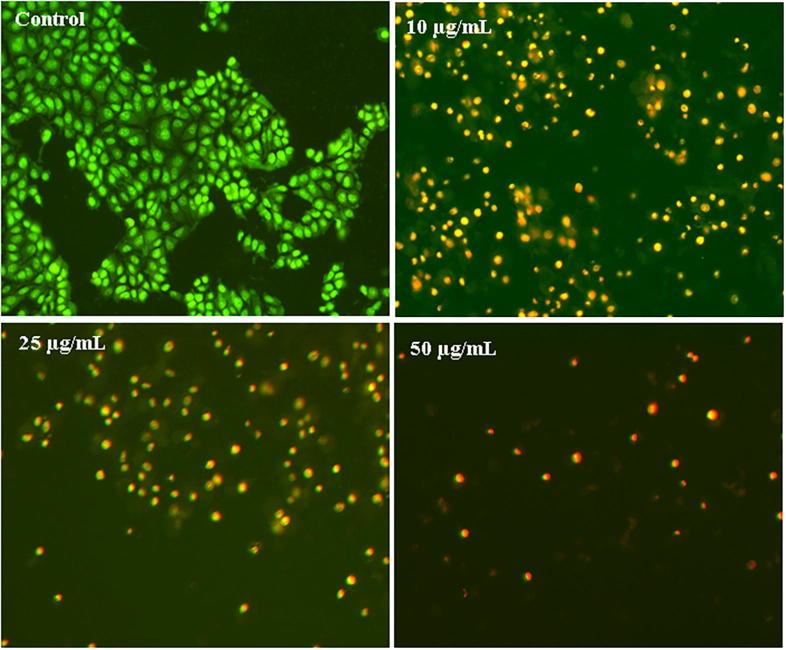
Anti-proliferative effect was investigated by incubating human breast cancer cell lines (MCF-7) (10, 25 and50 µg/mL). AgNPs exhibited maximum cytotoxicity on breast cancer cell with an IC50 value of 42.19 µg/mL along with cell shrinkage, blebbing and restricted cell spreading patterns compared to control cells (Fig. 8). The nuclei of control cells did not show any apparent morphological changes. However, AgNPs treated cells showed chromatin condensation followed by formation of apoptotic bodies that, AgNPs showed as orange-stained nuclei with fragmentation (Fig. 9). Earlier studies (Vivek et al., 2012, Elshawy et al., 2016, Venugopal et al., 2017, Alfuraydi et al., 2019), have reported that, AgNPs is a good anticancer agent, on MCF-7 cells with an IC50 value of 50 µg/mL for 24 h incubation.
Fig. 8.
Antiproliferative activity of Ag NPs on MCF-7 cell lines.
Fig. 9.
Fluorescence-microscopic images of Ag NPs treated MCF-7 cell lines.
4. Conclusions
The medicinal plant Memecylon umbellatum derived 4-N-methyl-benzoic acid is an efficient bioactive compound to fabricate silver nanoparticles and it possesses greater anti-bacterial effect for tested all pathogenic microorganisms. Besides, AgNPs displayed a good free-radical scavenging properties. The AgNPs also showed a good cytotoxicity on breast cancer cell lines (MCF-7). The study confirmed that the carboxyl and hydroxyl groups of 4-N- methyl benzoic acid played a significant role in the synthesis of AgNPs, The duration of the synthesis of AgNPs is quite fast in this method when compared with other plant extracts. Hence, the present study paved a way for an new approach towards the synthesis of AgNPs using natural products and these NPs can be used in various biomedical applications.
Author contributions
K. A, A.J.A, S.D, M.S.A. were conceptualization in the present study, characterization and writing original draft. P.M were involved data analyses and helped to improve the original draft.
Acknowledgments
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Conflicts of interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Kannan Elangovan, Email: itsmeelangok84@gmail.com.
Sandhanasamy Devanesan, Email: dsandhanasamy@ksu.edu.sa.
References
- Alfuraydi A.A., Devanesan S., Al-Ansari M., AlSalhi M.S., Ranjitsingh A.J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B. 2019;192:83–89. doi: 10.1016/j.jphotobiol.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Ali A.Q., Mohammad A.F., Faisal M.A.T., Khalid M.A., Ajmal Ali M., Joongku L., Waleed A.Q.H., Ahmed H.M. Cytogenotoxic effects of Adenium obesum seeds extracts on breast cancer cells. Saudi J. Biol. Sci. 2019;26:547–553. doi: 10.1016/j.sjbs.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.S., Amin M.R., Kamal C.M., Hossain M.A. In-vitro antioxidant, cytotoxic, thrombolytic activities and phytochemical evaluation of methanol extract of the A. philippense L. leaves. Asian Pac. J. Trop Biomed. 2013;3:464–469. doi: 10.1016/S2221-1691(13)60097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSalhi M.S., Devanesan S., Alfuraydi A.A., Vishnubalaji R., Munusamy M.A., Murugan K., Nicoletti M., Benelli G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 2016;11:4439–4449. doi: 10.2147/IJN.S113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswathy A.S., Philip D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;97:1–5. doi: 10.1016/j.saa.2012.05.083. [DOI] [PubMed] [Google Scholar]
- Cao J., Xia X., Dai X., Xiao J., Wang Q., Andrae-Marobela K., Okatch H. Flavonoids profiles, antioxidant, acetylcholinesterase inhibition activities of extract from Dryoathyrium boryanum (Willd) Ching. Food Chem. Toxicol. 2013;55:121–128. doi: 10.1016/j.fct.2012.12.051. [DOI] [PubMed] [Google Scholar]
- Chaloupka K., Malam Y., Seifalian A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Deng H., McShan D., Zhang Y., Sinha S.S., Arslan Z., Ray P.C., Yu H. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ. Sci. Technol. 2016;50(2016):8840–8848. doi: 10.1021/acs.est.6b00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanesan S., AlSalhi M.S., Balaji R.V., Ranjitsingh A.J.A., Ahamed A., Alfuraydi A.A., AlQahtani F.Y., Aleanizy F.S., Othman A.H. Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum peel extract. Nanoscale Res. Lett. 2018;13:e315. doi: 10.1186/s11671-018-2731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanesan S., AlSalhi M.S., Vishnubalaji R., Alfuraydi A.A., Nehad M.A.M., Murugan K., Shaban R.M.M., Nicoletti M., Benelli G. Rapid biological synthesis of silver nanoparticles using plant seed extracts and their cytotoxicity on colorectal cancer cell lines. J. Clust. Sci. 2017;28:595–605. [Google Scholar]
- Dinis T.C., Maderia V.M., Almeida L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Durai P., Chinnasamy A., Gajendran B., Ramar M., Pappu S., Kasivelu G., Thirunavukkarasu A. Synthesis and characterization of silver nanoparticles using crystal compound of sodium para-hydroxybenzoate tetrahydrate isolated from Vitex negundo. L leaves and its apoptotic effect on human colon cancer cell lines. Eur. J. Med. Chem. 2014:90–99. doi: 10.1016/j.ejmech.2014.07.012. 84 84. [DOI] [PubMed] [Google Scholar]
- Elangovan K., Elumalai D., Anupriya S., Shenbhagaraman R., Kaleena P.K., Murugesan K. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographis echioides and its bio-efficacy on anticancer and antibacterial activities. J. Photochem. Photobiol. B. 2015;151:118–124. doi: 10.1016/j.jphotobiol.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Elshawy O.E., Helmy E., Rashed L.A. Preparation, characterization and in vitro evaluation of the antitumor activity of the biologically synthesized silver nanoparticles. Adv. Nanopart. 2016;5:149–166. [Google Scholar]
- Geethalakshmi R., Sarada D.V.L. Characterization and antimicrobial activity of gold and silver nanoparticles synthesized using saponin isolated from Trianthema Ind. Crop. Prod. 2013;51:107–115. [Google Scholar]
- Gerber B., Müller H., Reimer T., Krause A., Friese K. Nutrition and lifestyle factors on the risk of developing breast cancer. Breast Cancer Res Treat. 2003;79:265–276. doi: 10.1023/a:1023959818513. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Patil S., Ahire M., Kitture R., Kale S., Pardesi K., Cameotra S.S., Bellare J., Dhavale D.D., Jabgunde A., Chopade B.A. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012;7:483–496. doi: 10.2147/IJN.S24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbe M., Bhat R., Aznar E., Sancenón F., Marcos M.D., Herraiz F.J., Prohens J., Venkataraman A., Martínez-Máñez R. Rapid biosynthesis of silver nanoparticles using pepino (solanum muricatum) leaf extract and their cytotoxicity on Hela cells. Materials. 2016;9:e325. doi: 10.3390/ma9050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow-Chin Y., Hui-Yin C. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43:27–32. [Google Scholar]
- Guntur S.R., Kumar N.S., Hegde M.M., Dirisala V.R. In-vitro studies of the antimicrobial and free-radical scavenging potentials of silver nanoparticles biosynthesized from the extract of Desmostachya bipinnata. Anal. Chem. Insights. 2018;13:1–9. doi: 10.1177/1177390118782877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeish A., Hashem M., El-Hady M.M., Sharaf S. Development of CMC hydrogels loaded with silver nano-particles for medical applications. Carbohydr. Polym. 2013;92:407–413. doi: 10.1016/j.carbpol.2012.08.094. [DOI] [PubMed] [Google Scholar]
- Jalal M., Ansari M.A., Alzohairy M.A., Ali S.G., Khan H.M., Almatroudi A., Raees K. Biosynthesis of silver nanoparticles from oropharyngeal candida Glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials. 2018;8:e586. doi: 10.3390/nano8080586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeeva K., Thiyagarajan M., Elangovan V., Geetha N., Venkatachalam P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind. Crop. Prod. 2014;52:714–720. [Google Scholar]
- Jiraroj D., Tungasmita S., Tungasmita D.N. Silver ions and silver nanoparticles in zeolite A composites for antibacterial activity. Powder Technol. 2014;264:418–422. [Google Scholar]
- Johannsen M., Thiesen B., Wust P., Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperthermia. 2010;26:790–795. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- Jose M., Sakthivel M. Synthesis and characterization of silver nanospheres in mixed surfactant solution. Mater Lett. 2014;117:78–81. [Google Scholar]
- Jose M., Thanal R., Prince Joshu J., Martin Britto Dhas S.A., Jerome Das S. Anisotropic growth of silver nanostructures from silver spheres by a simple chemical reduction route. Superlattices Microstruct. 2016;89:68–74. [Google Scholar]
- Jose M., Martin Britto Dhas S.A., Daisy A.D., Jerome S., Das Synthesis and characterization of nano spheres decorated silver bromide nanorods using a two step chemical reduction route. Optik. 2016;127:8019–8023. [Google Scholar]
- Kavitha S.R., Umadevi M., Janani S.R., Balakrishnan T., Ramanibai R. Fluorescence quenching and photocatalytic degradation of textile dyeing waste water by silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;127:115–121. doi: 10.1016/j.saa.2014.02.076. [DOI] [PubMed] [Google Scholar]
- Korshed P., Li L., Liu Z., Wang T. The molecular mechanisms of the antibacterial effect of picosecond laser generated silver nanoparticles and their toxicity to human cells. PLoS One. 2018;13:e0203636. doi: 10.1371/journal.pone.0203636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D.A., Palanichamy V., Roopan S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;127:168–171. doi: 10.1016/j.saa.2014.02.058. [DOI] [PubMed] [Google Scholar]
- Levenson A.C., Jordan V.C. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–3078. [PubMed] [Google Scholar]
- Li S., Shen Y., Xie A., Yu X., Qiu L., Zhang L. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9:852–858. [Google Scholar]
- Mahassni S.H., Al-Reemi R.M. Apoptosis and necrosis of human breast cancer cells by an aqueous extract of garden cress (Lepidium sativum) seeds. Saudi J. Biol. Sci. 2013;20:131–139. doi: 10.1016/j.sjbs.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moukette B.M., Pieme A.C., Biapa P.C., Njimou J.R., Stoller M., Bravi M., Yonkeu Ngogang J. In-Vitro ion chelating, antioxidative mechanism of extracts from fruits and barks of Tetrapleura tetraptera and their protective effects against Fenton mediated toxicity of metal ions on liver homogenates. Evid Based Complement Alternat. Med. 2015;2015:e423689. doi: 10.1155/2015/423689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickavar B., Esbati N. Evaluation of the antioxidant capacity and phenolic content of three Thymus species. J. Acupunct. Meridian Stud. 2012;5:119–125. doi: 10.1016/j.jams.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Raj S., Chand Mali S., Trivedi R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun. 2018;503:2814–2819. doi: 10.1016/j.bbrc.2018.08.045. [DOI] [PubMed] [Google Scholar]
- Ramamurthy C.H., Padma M., samadanam I.D., Mareeswaran R., Suyavaran A., Kumar M.S., Premkumar K., Thirunavukkarasu C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf. B. Biointerfaces. 2013;102:808–815. doi: 10.1016/j.colsurfb.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Raveendran P., Fu J., Wallen S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006;8:34–38. [Google Scholar]
- Roy P., Das B., Mohanty A., Mohapatra S. Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Appl Nanosci. 2017;8:843–850. [Google Scholar]
- Sahoo S.K., Parveen S., Panda J.J. The present and future of nanotechnology in human health care. Nanomedicine. 2007;3:20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Sarkar J., Ray S., Chattopadhyay D., Laskar A., Acharya K. Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternate. Bioprocess Biosyst. Eng. 2012;35:637–643. doi: 10.1007/s00449-011-0646-4. [DOI] [PubMed] [Google Scholar]
- Sen I.K., Mandal A.K., Chakraborti S., Dey B., Chakraborty R., Islam S.S. Green synthesis of silver nanoparticles using glucan from mushroom and study of antibacterial activity. Int. J. Biol. Macromol. 2013;62:439–449. doi: 10.1016/j.ijbiomac.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Senthilkumar S., Siva E., Rajendran A. Characterization and antimicrobial activity of eco-friendly biosynthesis of silver nano particles using an aqueous leaf extract of catharanthus roseus. IOSR-JAP. 2017;71:71–75. [Google Scholar]
- Singh S., Saikia J.P., Buragohain A.K. A novel “green” synthesis of colloidal silver nanoparticles (SNP) using Dillenia indica fruit extract. Colloids Surf. B Biointerfaces. 2013;102:83–85. doi: 10.1016/j.colsurfb.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Sungkaworn T., Triampo W., Nalakarn P., Triampo D., Tang I.M., Lenbury Y.P. The effects of TiO2 nanoparticles on tumor cell colonies : fractal dimension and morphological properties. Int. J. Biomed. Sci. 2008;2(2008):67–74. [Google Scholar]
- Varghese R., Almalki M.A., Ilavenil S., Rebecca J., Choi K.C. Silver nanopaticles synthesized using the seed extract of Trigonella foenum-graecum L. and their antimicrobial mechanism and anticancer properties. Saudi J. Biol. Sci. 2019;26:148–154. doi: 10.1016/j.sjbs.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena S., Devasena T., AnselVishal L., Sathak S.S.M., Arokiyaraj S. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J. Biol. Sci. 2019;26:455–459. doi: 10.1016/j.sjbs.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal K., Rather H.A., Rajagopal K., Shanthi M.P., Sheriff K., Illiyas M., Rather R.A., Manikandan E., Ovarian S., Bhaskar M., Maaza M. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B. 2017;167:282–289. doi: 10.1016/j.jphotobiol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Vivek R., Thangam R., Muthuchelian K., Gunasekaran P., Kaveri K., Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47:2405–2410. [Google Scholar]
- Wong C.C., Bin-Li H., Cheng L.W., Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. [Google Scholar]
- Zaheer Z. Biogenic synthesis, optical, catalytic, and in vitro antimicrobial potential of Ag-nanoparticles prepared using Palm date fruit extract. J Photochem. Photobiol. B. 2018;178:584–592. doi: 10.1016/j.jphotobiol.2017.12.002. [DOI] [PubMed] [Google Scholar]