Abstract
Background
Walking speed is central to emerging consensus definitions of sarcopenia and frailty as well as being a major predictor of future health outcomes in its own right. However, measurement is not always feasible in clinical settings. We hypothesised that self-reported walking speed might be a good marker of objectively measured walking speed for use in this context.
Methods
We investigated the relationship between self-reported and measured walking speed and their associations with clinical characteristics and mortality using data from 730 men and 999 women, aged 61 - 73 years, who participated in the Hertfordshire Cohort Study. Walking speed was measured over 3 metres. Participants rated their walking speed as: “unable to walk”; “very slow”; “stroll at an easy pace”; “normal speed”; fairly brisk” or “fast”.
Results
Self-reported walking speed was strongly associated with measured walking speed among men and women (p<0.001). Average walking speeds ranged from 0.78m/s (95%CI: 0.73, 0.83) among men with “very slow” self-reported walking speed to 0.98m/s (95%CI: 0.93, 1.03) among “fast” walkers (corresponding figures for women were 0.72m/s [95%CI: 0.68, 0.75] and 1.01m/s [95%CI: 0.98, 1.05]). Self-reported and measured walking speeds were similarly associated with clinical characteristics and mortality; among men and women, slower self-reported and measured walking speeds were associated (p<0.05) with increased likelihood of poor physical function, having more systems medicated and with increased mortality risk, with and without adjustment for socio-demographic and lifestyle factors (hazard ratios for mortality per slower band of self-reported walking speed, adjusted for socio-demographic and lifestyle characteristics: men 1.44 [95%CI: 1.11, 1.87]; women 1.35 [95%CI: 1.02, 1.81]).
Conclusion and Implications
Self-reported walking speed is a good marker of measured walking speed and could serve as a useful marker of physical performance in consensus definitions of sarcopenia and frailty when direct measurement of walking speed is not feasible.
Keywords: walking speed, gait speed, sarcopenia, physical performance, self-reported, mortality
Introduction
Walking speed is now widely measured in research settings and increasingly of interest in the clinical setting. Moreover, it now features in emerging consensus definitions of sarcopenia and frailty1–3. Slower customary walking speed among community-dwelling older men and women is a risk factor for adverse outcomes4 including disability in activities of daily living (ADL)5, falls and institutionalisation4, fracture and cognitive decline6 and mortality7.
Guralnik first outlined a protocol for measurement of customary, or usual, walking speed in 1994 as part of a short physical performance battery (SPPB) developed for the assessment of lower extremity function among community-dwelling men and women aged 71 years and older who participated in the EPESE (Epidemiologic Studies of the Elderly) study in the United States8. The SPPB comprised tests of balance, rising from a chair, and walking at usual pace across an 8-foot walking course; poorer (lower) overall summary physical performance (PP) scores were strongly associated with increased self-reported levels of disability in activities of daily living (ADL), such as walking half a mile and climbing stairs, and identified individuals at increased risk of nursing home admission or mortality8.
Since Guralnik’s early paper, direct measurement of physical performance has become commonplace in epidemiological studies and walking speed has been proposed as an appealing way of screening the functional status of older people in research and clinical settings9. In 2009, an International Academy on Nutrition and Ageing (IANA) Taskforce concluded that measured walking speed “is a quick, safe, inexpensive and highly reliable” single-item assessment tool which identifies community-dwelling people at risk of adverse outcomes4.
However, measurement of walking speed requires training of observers; the implementation of a strict measurement protocol if reliable and comparable measures are to be obtained in different research studies and clinical settings; and takes longer than simply asking a person to self-report their customary walking speed. Moreover, not all research studies involve face-to-face contact with study participants (e.g. large postal surveys) and not all research and clinical settings have the space to set up a walking course. In addition, an older person may temporarily lack the ability to complete a walking assessment if they are currently acutely unwell, injured or hospitalised. An alternative approach to characterising customary walking speed would therefore be of value in settings where direct measurement is not feasible.
Guralnik suggested that “performance measures can validly characterise older persons across a broad spectrum of lower extremity function” but emphasised that measurement and self-report approaches complement each other to provide a full assessment of an older person’s functional status8; Sainio10 and Sakari-Rantalal11 support this argument. On this basis we propose that a simple screening question which asks an individual to select the option which best describes their usual walking speed may be useful in epidemiological and clinical settings where direct measurement of walking speed is not achievable.
We conducted a search of OVID MEDLINE(R) for articles in the literature which describe the association between self-reported and objectively measured walking (or gait) speed. Several articles demonstrated associations between measured walking speed and self-reports of level of function, limitations or disability in walking or mobility ADLs10–14, but no articles were identified which investigated whether self-reported walking speed is a good marker of measured walking speed.
We have therefore evaluated the association between self-reported and directly measured walking speed among the community-dwelling older men and women who participated in the Hertfordshire Cohort Study (HCS), UK15. We investigated whether self-reported and measured walking speeds demonstrate similar patterns of association with a range of socio-demographic, lifestyle and clinical characteristics and mortality outcome. Finally, we determined the impact of using self-reported rather than measured walking speed in the European Working Group on Sarcopenia (EWGSOP) consensus algorithm for the diagnosis of sarcopenia16.
Methods
Study population
The Hertfordshire Cohort Study comprises a group of men and women born in that county between 1931 and 1939 whose birth, infancy and early childhood were documented by Health Visitors. 1579 men and 1418 women aged 59-73 years who still lived in Hertfordshire between the end of 1998 and 2004 were interviewed at home by a trained research nurse and subsequently attended clinics for detailed physiological investigations (herein referred to as the HCS baseline interview and clinic). The study has been described in detail previously15.
Self-reported walking speed was ascertained at the HCS baseline interview by asking the participant: “Which of the following best describes your walking speed?”. Participants selected one of the following response options: “unable to walk”; “very slow”; “stroll at an easy pace”; “normal speed”; “fairly brisk”; or “fast”. The baseline interview also ascertained social history (including age left full time education, own current or most recent full time occupation and husband’s current or most recent full time occupation for ever-married women), lifestyle factors (smoking habit and alcohol intake), self-assessed health related quality of life (using the short-form 36 [SF-36] questionnaire17) and medical history (comprising fracture history, previous diagnosis of high blood pressure, stroke/transient ischaemic attack, diabetes [out of pregnancy], symptoms of bronchitis, typical angina [according to the Rose chest pain questionnaire], history of coronary artery bypass graft or angioplasty and details of all currently prescribed or over the counter medications, coded to the British National Formulary).
Investigations conducted at the HCS baseline clinic included measurement of height (to the nearest 0.1cm using a Harpenden pocket stadiometer, Chasmors Ltd, London, UK) and weight (to the nearest 0.1kg on a SECA floor scale, Chasmors Ltd, London, UK). Skinfold thickness (SFT) was measured with Harpenden skinfold calipers in triplicate at the triceps, biceps, sub-scapular and supra-iliac sites on the non-dominant side. A 2 hour fasted oral glucose tolerance test (OGTT) was performed using 75g anhydrous glucose and diabetes mellitus classified according to W.H.O. criteria18. Resting blood pressure was recorded as the mean of three measurements on a Dinamap Model 8101 (GE Medical Systems, Slough, UK). An electrocardiogram (ECG) was performed and graded to the Minnesota protocol19. Measurement of physical performance using Guralnik’s short physical performance battery8 was introduced part way through the HCS fieldwork; time taken to walk 3 metres at a customary pace was recorded to the nearest 1/100th of a second for 767 men and 1,031 women but only 730 men and 999 women completed the test according to protocol without the use of a walking aid and were deemed eligible for inclusion in the analysis sample for this manuscript.
Intra- and inter-observer studies were carried out during the fieldwork. The study had ethical approval from the Hertfordshire and Bedfordshire Local Research Ethics Committee and all participants gave written informed consent.
Statistical methods
Registrar General’s social class was coded from the 1990 Standard Occupational Classification (SOC90) unit group for occupation20 using computer assisted standard occupational coding21. Current social class was coded from own current or most recent full-time occupation for men and never-married women, and from husband’s occupation for ever-married women22. Number of systems medicated was coded according to the British National Formulary. SF-36 data were mapped to eight domain scores, including physical function (PF)17. PF scores were negatively skewed (lower scores implied poorer status) and were dichotomised for analysis: participants with scores in the lowest sex-specific fifth of the distribution (≤75 for men, ≤60 for women) were classified as having “poor” PF. Body mass index in kg/m2 was calculated as weight divided by the square of height. Height and weight were highly correlated (r=0.45, P<0.001 for men; r=0.29, P<0.001 for women); to avoid multi-collinearity problems a sex-specific standardised residual of weight-adjusted-for-height was calculated for inclusion with height in regression models. Averaged skinfold thickness measurements were used to derive body fat percentage according to the Durnin and Womersley equations23. Fat mass was derived by multiplying body weight by percentage body fat. Fat free mass (FFM), a proxy for lean muscle mass, was estimated by subtracting fat mass from body weight. Measured customary walking speed in metres per second (m/s) was calculated by dividing 3 by the time taken to walk 3 metres in the physical performance test. As previously described24, the EWGSOP definition of sarcopenia was implemented for HCS participants on the basis of: low muscle strength (<30kg for men, <20kg for women25); low muscle mass (skin-fold-based fat-free mass in the bottom third of the HCS sex-specific distribution i.e. ≤55.4kg for men, ≤39.7kg for women); and poor physical performance (measured walking speed ≤0.8 m/s16). We also implemented the EWGSOP algorithm for sarcopenia by using a slower than “normal” self-reported walking speed, rather than slow measured walking speed, to identify poor physical performance.
Data were described using means and standard deviations (SD), medians and inter-quartile ranges (IQR) and frequency and percentage distributions. Histograms were used to visually inspect the distributions of variables; measured customary walking speed followed a normal distribution. The cross-sectional association between self-reported and measured walking speed was analysed using analysis of variance (ANOVA). Cross-sectional associations between self-reported and measured walking speed and socio-demographic, lifestyle and clinical characteristics of HCS participants were analysed using univariate and multivariate linear, logistic and poisson regression models. Cox’s proportional hazards model was used to analyse the associations between self-reported and measured walking speed and all-cause mortality from HCS baseline clinic to 31st December 2010. Sensitivity and specificity statistics were calculated for sarcopenia identified via implementation of the EWGSOP algorithm on the basis of slower than “normal” self-reported walking speed, compared with a “gold standard” implementation based on slow measured walking speed (≤0.8m/s). All analyses were carried out for men and women separately using Stata, release 13 26.
Results
The characteristics of the 730 men and 999 women who were included in the analysis sample are illustrated in Table 1. Only 28 (3.8%) men and 50 (5%) women had a self-reported walking speed of “very slow” and 29 (4%) men and 53 (5.3%) women had a self-reported walking speed of “fast”. In total, 287 (39.3%) men and 469 (46.9%) women described their walking speed as “normal”. Mean (SD) measured walking speed was 0.95 (0.14) m/s for men and 0.92 (0.15) m/s for women.
Table 1. Participant characteristics.
| Mean (SD) | Men (n=730) | Women (n=999) |
|---|---|---|
| Age (yrs) | 67.0 (2.6) | 67.1 (2.6) |
| Height(cm) | 174.0 (6.3) | 160.8 (5.8) |
| Weight (kg) | 82.3 (12.9) | 71.3 (13.2) |
| BMI (kg/m2) | 27.1 (3.8) | 27.6 (4.9) |
| Age left education* (<15 yrs) | 118 (16.2%) | 165 (16.5%) |
| Ever smoked* | 475 (65.1%) | 364 (36.4%) |
| High alcohol intake* (≥22M; ≥15F units per week) | 139 (19.1%) | 57 (5.7%) |
| Hypertension* | 319 (43.7%) | 412 (41.4%) |
| Ischaemic heart disease* | 116 (16.1%) | 85 (8.7%) |
| Fracture since 45 yrs age* | 42 (5.8%) | 186 (18.6%) |
| Diabetes* | 117 (16.2%) | 142 (14.5%) |
| Bronchitis* | 30 (4.1%) | 43 (4.3%) |
| Low† SF-36 physical functioning score* | 153 (21.0%) | 206 (20.6%) |
| Number of systems medicated‡ | 1.0 (0.0, 2.0) | 1.0 (1.0, 2.0) |
| Walking speed (self-reported)*:Very slow | 28 (3.8%) | 50 (5.0%) |
| Stroll | 173 (23.7%) | 201 (20.1%) |
| Normal | 287 (39.3%) | 469 (46.9%) |
| Brisk | 213 (29.2%) | 226 (22.6%) |
| Fast | 29 (4.0%) | 53 (5.3%) |
| Measured walking speed (m/s) | 0.95 (0.14) | 0.92 (0.15) |
SD: standard deviation; yrs: years; M: male; F: female; m/s: metres per second
n(%)
Bottom fifth of the sex-specific SF-36 physical functioning score (≤75 for men, ≤60 for women)
Median (lower quartile, upper quartile)
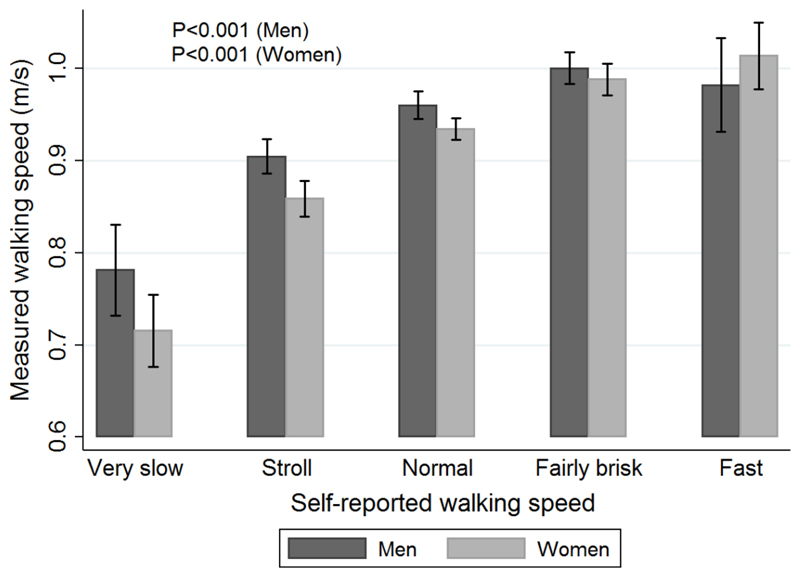
Self-reported walking speed was strongly associated with measured walking speed among men and women (Figure 1). Men and women who had a self-reported walking speed of “very slow” had a mean measured walking speed of 0.78m/s (95%CI: 0.73, 0.83) and 0.72m/s (95%CI: 0.68, 0.75) respectively. In contrast, men and women who had a self-reported walking speed of “fast” had a mean measured walking speed of 0.98m/s (95%CI: 0.93, 1.03) and 1.01m/s (95%CI: 0.98, 1.05) respectively.
Figure 1. Average measured walking speed (95% confidence intervals) according to self-reported walking speed.
P-values from regression analyses testing for trend in measured walking speed across categories of self-reported walking speed.
Table 2 shows the relationships between self-reported and measured walking speed and various socio-demographic, lifestyle and clinical characteristics. Among men, self-reported and measured walking speed were similarly associated with age, weight, BMI, age left education, smoking history and alcohol consumption, whereas height was more strongly associated with measured walking speed than self-reported walking speed. Among women, self-reported and measured walking speed were similarly associated with age, weight, BMI and smoking history, whereas height was more strongly associated with measured walking speed and alcohol consumption was more strongly associated with self-reported walking speed.
Table 2. Associations between measured and self-reported walking speed and socio-demographic, lifestyle and clinical characteristics of Hertfordshire Cohort Study participants.
| Association between walking speed and each characteristic ‡ | Measured walking speed* | Self-reported walking speed† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | ||||||
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | ||
| Age (yrs) | 0.42 (0.23,0.61) | <0.001 | 0.50 (0.34,0.66) | <0.001 | 0.26 (0.06,0.47) | 0.012 | 0.24 (0.06,0.42) | 0.008 | |
| Height(cm) | -0.77 (-1.23,-0.32) | 0.001 | -0.56 (-0.92,-0.21) | 0.002 | -0.22 (-0.72,0.28) | 0.393 | -0.29 (-0.68,0.10) | 0.140 | |
| Weight (kg) | 0.98 (0.05,1.92) | 0.040 | 2.88 (2.08,3.67) | <0.001 | 2.61 (1.60,3.62) | <0.001 | 5.02 (4.19,5.86) | <0.001 | |
| BMI (kg/m2) | 0.57 (0.29,0.84) | <0.001 | 1.32 (1.03,1.61) | <0.001 | 0.92 (0.62,1.21) | <0.001 | 2.03 (1.73,2.34) | <0.001 | |
| Age left education (<15 yrs) | 1.34 (1.09,1.65) | 0.005 | 1.35 (1.14,1.60) | <0.001 | 1.27 (1.02,1.58) | 0.030 | 1.09 (0.91,1.31) | 0.347 | |
| Ever smoked | 1.32 (1.13,1.55) | <0.001 | 1.10 (0.96,1.25) | 0.164 | 1.24 (1.05,1.46) | 0.013 | 1.04 (0.91,1.20) | 0.552 | |
| High alcohol intake § | 1.07 (0.89,1.29) | 0.481 | 0.83 (0.64,1.09) | 0.180 | 1.13 (0.93,1.39) | 0.224 | 0.66 (0.49,0.89) | 0.007 | |
| Hypertension | Unadj | 1.28 (1.10,1.48) | 0.001 | 1.38 (1.21,1.57) | <0.001 | 1.57 (1.32,1.85) | <0.001 | 1.27 (1.10,1.45) | 0.001 |
| Adj | 1.17 (1.00,1.37) | 0.056 | 1.18 (1.02,1.35) | 0.024 | 1.43 (1.20,1.70) | <0.001 | 1.00 (0.86,1.17) | 0.973 | |
| Ischaemic heart disease | Unadj | 1.21 (0.99,1.48) | 0.067 | 1.26 (1.01,1.58) | 0.039 | 1.52 (1.22,1.90) | <0.001 | 1.72 (1.34,2.21) | <0.001 |
| Adj | 1.17 (0.94,1.44) | 0.161 | 1.11 (0.88,1.42) | 0.374 | 1.53 (1.21,1.94) | <0.001 | 1.57 (1.20,2.06) | 0.001 | |
| Fracture since 45 yrs age | Unadj | 1.11 (0.81,1.52) | 0.517 | 0.87 (0.74,1.02) | 0.080 | 0.95 (0.68,1.34) | 0.783 | 0.92 (0.78,1.10) | 0.373 |
| Adj | 1.19 (0.85,1.66) | 0.307 | 0.85 (0.71,1.00) | 0.055 | 1.02 (0.71,1.45) | 0.929 | 0.96 (0.79,1.16) | 0.649 | |
| Diabetes | Unadj | 1.51 (1.22,1.87) | <0.001 | 1.09 (0.91,1.30) | 0.345 | 1.61 (1.29,2.01) | <0.001 | 1.35 (1.11,1.65) | 0.003 |
| Adj | 1.29 (1.03,1.62) | 0.026 | 0.91 (0.75,1.10) | 0.321 | 1.35 (1.06,1.71) | 0.013 | 1.04 (0.83,1.29) | 0.756 | |
| Bronchitis | Unadj | 1.20 (0.83,1.74) | 0.337 | 0.91 (0.67,1.24) | 0.555 | 1.70 (1.13,2.54) | 0.010 | 1.20 (0.86,1.68) | 0.281 |
| Adj | 1.10 (0.74,1.61) | 0.645 | 0.91 (0.66,1.26) | 0.587 | 1.57 (1.04,2.37) | 0.031 | 1.26 (0.88,1.80) | 0.206 | |
| Low SF-36 PF ‖ | Unadj | 2.22 (1.79,2.75) | <0.001 | 2.71 (2.24,3.27) | <0.001 | 3.48 (2.70,4.47) | <0.001 | 4.78 (3.74,6.10) | <0.001 |
| Adj | 2.04 (1.63,2.55) | <0.001 | 2.50 (2.04,3.07) | <0.001 | 3.32 (2.56,4.32) | <0.001 | 4.32 (3.34,5.57) | <0.001 | |
| Systems medicated | Unadj | 1.20 (1.12,1.28) | <0.001 | 1.20 (1.15,1.26) | <0.001 | 1.31 (1.22,1.40) | <0.001 | 1.24 (1.18,1.31) | <0.001 |
| Adj | 1.15 (1.07,1.23) | <0.001 | 1.15 (1.09,1.21) | <0.001 | 1.27 (1.18,1.36) | <0.001 | 1.18 (1.12,1.26) | <0.001 | |
Estimates of association per standard deviation slower measured walking speed
Estimates of association per slower band of self-reported walking speed
Estimates of association are regression coefficients (95% CI) from linear regression models with walking speed as the predictor variable and each of age, height, weight and BMI as the outcome variable in turn. Estimates corresponding to systems medicated illustrate the multiplicative increase (95% CI) in this characteristic, obtained from log-linear regression models. For the remaining characteristics, the estimates are odds ratios (95% CI) for presence versus absence of the attribute as estimated from logistic regression models.
High weekly alcohol intake of ≥22 units per week among men and ≥15 units per week among women
Sex-specific score of ≤75 for men and ≤60 for women on the SF-36 physical functioning (PF) domain
Unadj and Adj indicate whether or not models were adjusted for potential confounding variables. Adjusted models accounted for age, height, weight for height residual, age left education, smoking status and weekly alcohol intake.
In men and women, slower self-reported and measured walking speed were strongly associated with a higher likelihood of having low physical function and having a higher number of systems medicated in both unadjusted and adjusted analyses (Table 2). Among men, self-reported and measured walking speed had the same pattern of association with hypertension, fracture and diabetes whereas ischaemic heart disease and bronchitis had a stronger association with self-reported walking speed than measured walking speed. Among women, self-reported walking speed and measured walking speed had the same pattern of association with hypertension, ischaemic heart disease, fracture and bronchitis, whereas diabetes had a stronger association with self-reported walking speed than measured walking speed.
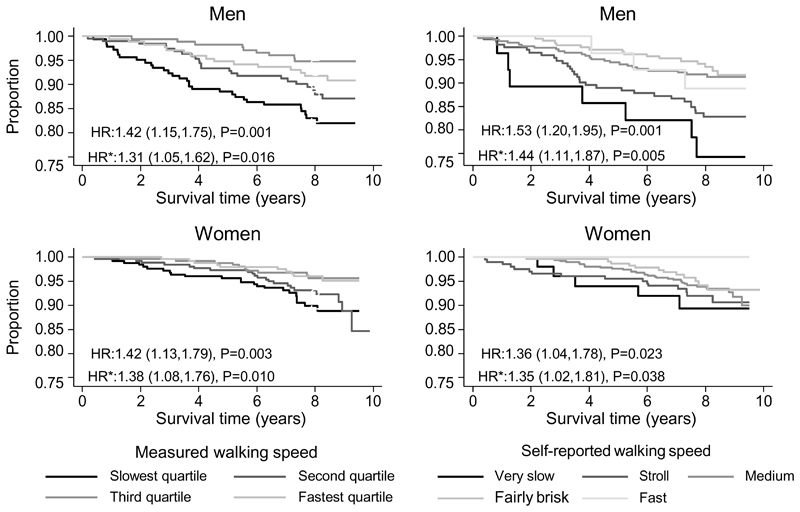
Figure 2 illustrates that slower self-reported and measured walking speed were each also associated with an increased risk of mortality and these relationships remained significant after adjustments for socio-demographic and lifestyle factors. Overall, the results in Table 2 and Figure 2 show that self-reported and measured walking speed were similarly associated with a range of markers of socio-demographic, lifestyle and clinical characteristics as well as mortality.
Figure 2. Survival curves according to measured and self-reported walking speed.
HR: unadjusted hazard ratios from Cox proportional hazard models per slower band of self-reported walking speed or per slower quartile of measured walking speed.
* Hazard ratios adjusted for age, height, weight for height residual, age left education, smoking status, weekly alcohol intake and number of systems medicated.
The prevalence of EWGSOP sarcopenia based on slow measured walking speed, weak grip strength, and low skin-fold based fat-free mass was 4.5% among men and 7.7% among women; these overall prevalences were little altered if slower than “normal” self-reported walking speed was used in place of slow measured walking speed (8.2% among men and 8.6% among women). The two approaches did not identify exactly the same men and women as having sarcopenia but sensitivity and specificity statistics for sarcopenia implemented via self-reported walking speed in comparison with a “gold-standard” implementation via measured walking speed suggested reasonable to good agreement: sensitivities and specificities were 78.8% and 95.1% among men, and 76.6% and 97.2% among women respectively.
Discussion
We have shown that self-reported walking speed is strongly associated with measured walking speed among community-dwelling men and women (59 to 73 years of age) who participated in the Hertfordshire Cohort Study. Moreover, self-reported and measured walking speeds were similarly associated with clinical characteristics and mortality among men and women, with and without adjustment for socio-demographic and lifestyle factors. Finally, we have demonstrated reasonable to good agreement between EWGSOP sarcopenia identified on the basis of self-reported walking speed in comparison with a “gold-standard” implementation based on measured walking speed.
To our knowledge, this is the first study to investigate whether self-reported walking speed is a useful marker of measured walking speed among community-dwelling older men and women. We have identified a strong gradient in measured walking speed according to self-reported walking speed among HCS men and women; average walking speeds among men and women who self-reported “very slow” walking speeds were 0.78m/s (95%CI: 0.73, 0.83) and 0.72m/s (95%CI: 0.68, 0.75) respectively, in contrast with average measured walking speeds of 0.98m/s (95%CI: 0.93, 1.03) and 1.01m/s (95%CI: 0.98, 1.05) among men and women who reported “fast” walking speeds. These magnitudes of difference are substantial given that a change of 0.1m/s in timed customary walking speed has previously been established as representative of a meaningful change27 28. In addition, we have shown that self-reported and measured walking speeds are similarly associated with clinical characteristics and mortality; this is an important criterion to fulfil if self-reported walking speed is to serve as a useful marker of measured walking speed.
Our results have implications for the assessment of walking speed in research studies and clinical settings. We do not dispute the IANA Task Force’s conclusion that measured walking speed is a “quick, safe, inexpensive and highly reliable” single assessment tool9 but we emphasise that it does have its disadvantages. In particular, measurement of walking speed requires training of observers, the implementation of a strict measurement protocol and takes longer to ascertain than self-reported walking speed. Moreover, not all research studies involve face-to-face contact with study participants and not all research and clinical facilities have the space for a walking course. Finally, an older person may temporarily be unable to complete a walking assessment if they are currently acutely unwell, injured or hospitalised. In all these instances, it would be frustrating to be unable to characterise a person’s customary walking speed for use in its own right and to enable operationalization of current definitions of phenotypes such as frailty and sarcopenia; our results suggest that self-reported walking speed might serve as a useful marker of directly measured walking speed among community-dwelling older men and women and could serve as a useful marker of physical performance in consensus definitions of sarcopenia and frailty when direct measurement of walking speed is not feasible. For example, we have demonstrated reasonable to good agreement between EWGSOP sarcopenia identified on the basis of self-reported walking speed in comparison with a “gold-standard” implementation via measured walking speed (sensitivities and specificities were 78.8% and 95.1% among men, and 76.6% and 97.2% among women respectively).
Our study had some limitations. First, we have previously shown that a healthy participant effect is, unsurprisingly, evident in HCS. A healthy participant effect has the potential to bias the associations that we have described between self-reported and measured walking speed, and between these markers of walking speed and other clinical characteristics. However, substantial bias would only be introduced if the associations of interest differed markedly between men and women who participated in HCS in comparison with those who were invited to participate in HCS but chose not to; this seems unlikely. Moreover, we suggest that the extent of any healthy participant effect in HCS is modest with respect to measured walking speed. Average (SD) walking speeds among HCS community-dwelling men and women, aged 61-73 years, were 0.95 (0.14) m/s and 0.92 (0.15) m/s respectively. These are comparable with an average (SD) walking speed of 0.92 (0.27) m/s for 34,485 community-dwelling older adults aged 65 years and older who were included in a recent meta-analysis of the association between gait speed and survival 28, but are unsurprisingly faster than estimated average walking speeds of 0.46m/s (95%CI: 0.34, 0.57) and 0.74m/s (95%CI: 0.65, 0.83) for 7,000 geriatric patients aged 70 years and older in acute care and outpatient settings, respectively29.
Second, we made the a priori decision to exclude from our principal analysis sample the small number of HCS men (n=37) and women (n=32) who did not complete the 3 metre walk test according to protocol without the use of an assistive device (e.g. walking stick); the IANA Task Force4 similarly focused on community-dwelling “autonomous” and “well-functioning” older people in their review of gait speed as a predictor of adverse outcomes. Further descriptive analyses suggested that the 37 men and 32 women excluded from the analysis sample for this paper were on average heavier, left education at an earlier age, were more likely to have ever smoked, had a higher prevalence of co-morbidity, rated their walking speed more slowly, and recorded slower walking times in comparison with the 730 men and 999 women who completed the 3 metre walking test without the use of an assistive device (data not shown). In spite of these average differences in characteristics, the association between measured and self-reported walking speed was as strong and graded among the excluded sample (data not shown) as it was among those who completed the test without the use of an assistive device. However, our sample size was insufficient to permit exploration of the associations between measured and self-reported walking speed and clinical characteristics or mortality outcome among the small number of HCS men and women who used an assistive device to complete the 3 metre walking test.
Third, we acknowledge that our results require replication among groups of men and women in whom the burden of pre-existing walking limitations, and the use of assistive devices, is greater than among the HCS participants e.g. community-dwelling men and women of much older ages than HCS participants, or men and women who live in dependent settings such as warden assisted housing or nursing homes. Published algorithms for the identification of sarcopenia such as that proposed by the European Working Group16 might perhaps also consider stating more clearly whether “slow” measured walking speeds should be identified on the basis of a walking speed that has been achieved without, or with, the use of an assistive device and whether a common cut-point for identification of slow walking speed (such as ≤0.8m/s in the EWGSOP sarcopenia definition) is appropriate irrespective of the use of assistive devices.
Our study also had many strengths. First, we have examined the association between self-reported and measured walking speed using data from a large and well characterised cohort of community-dwelling older men and women. Second, the data were rigorously collected according to strict protocols by trained research nurses and doctors15 30. Third, we were able to investigate the potential impact of a range of socio-demographic, lifestyle and clinical characteristics on the association between measured and self-reported walking speed. Finally, participants in the Hertfordshire Cohort Study have previously been shown to be broadly comparable with participants in the nationally representative Health Survey for England which suggests that the results of the current study are generalisable to the wider population of community-dwelling older people in England15.
In conclusion, self-reported walking speed is a good marker of measured walking speed and could serve as a useful marker of physical performance in consensus definitions of sarcopenia and frailty when direct measurement of walking speed is not feasible.
Funding and Acknowledgements
This work was supported by the Medical Research Council & University of Southampton UK. This study had ethical approval from the Hertfordshire and Bedfordshire Local Research Ethics Committee and all participants gave written informed consent.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Contributor Information
Leo D Westbury, Email: lw@mrc.soton.ac.uk.
Cyrus Cooper, Email: cc@mrc.soton.ac.uk.
References
- 1.Sayer AA, Robinson SM, Patel HP, et al. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing. 2013;42(2):145–50. doi: 10.1093/ageing/afs191. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. Journal of the American Medical Directors Association. 2011;12(6):403–9. doi: 10.1016/j.jamda.2011.04.014. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging. 2009;13(10):881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen J, Neyens JC, van Rossum E, et al. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC geriatrics. 2011;11:33. doi: 10.1186/1471-2318-11-33. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. doi: 10.1093/ageing/afq117. afq117 [pii]; [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 9.Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. The journal of nutrition, health & aging. 2008;12(1):29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 10.Sainio P, Koskinen S, Heliovaara M, et al. Self-reported and test-based mobility limitations in a representative sample of Finns aged 30+ Scandinavian Journal of Public Health. 2006;34(4):378–86. doi: 10.1080/14034940500489859. [DOI] [PubMed] [Google Scholar]
- 11.Sakari-Rantala R, Avlund K, Frandin K, et al. The incidence of mobility restrictions among elderly people in two Nordic localities. A five-year follow-up. Aging-Clinical & Experimental Research. 2002;14(3 Suppl):47–55. [PubMed] [Google Scholar]
- 12.Verghese J, Wang C, Holtzer R. Relationship of clinic-based gait speed measurement to limitations in community-based activities in older adults. Archives of Physical Medicine & Rehabilitation. 2011;92(5):844–6. doi: 10.1016/j.apmr.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Young Y, Rubin G, et al. Self-reported preclinical disability identifies older women with early declines in performance and early disease. Journal of Clinical Epidemiology. 2001;54(9):889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim MJ, Yabushita N, Tanaka K. Exploring effective items of physical function in slow walking speed and self-reported mobility limitation in community-dwelling older adults. Geriatrics & gerontology international. 2012;12(1):50–8. doi: 10.1111/j.1447-0594.2011.00726.x. [DOI] [PubMed] [Google Scholar]
- 15.Syddall HE, Aihie SA, Dennison EM, et al. Cohort profile: the Hertfordshire cohort study. IntJEpidemiol. 2005;34(6):1234–42. doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. afq034 [pii]; [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 18.W.H.O. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: 1999. [Google Scholar]
- 19.Prineas RJ, Crow RS, Blackburn H. The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. Boston: 1982. [Google Scholar]
- 20.OPCS. Standard occupational classification, Vol 1 Structure and definition of major, minor and unit groups. London: 1990. [Google Scholar]
- 21.Elias P, Halstead K, Prandy K. Computer assisted standard occupational coding. London: 1993. [Google Scholar]
- 22.Arber S, Ginn J. Gender and inequalities in health in later life. SocSciMed. 1993;36(1):33–46. doi: 10.1016/0277-9536(93)90303-l. [DOI] [PubMed] [Google Scholar]
- 23.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. The British journal of nutrition. 1974;32(1):77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 24.Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013;42(3):378–84. doi: 10.1093/ageing/afs197. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. JApplPhysiol. 2003;95(5):1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 26.Stata Statistical Software: Release 13 [program] College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 27.Hardy SE, Perera S, Roumani YF, et al. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727–34. doi: 10.1111/j.1532-5415.2007.01413.x. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 28.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(1):39–46. doi: 10.1093/gerona/gls174. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 30.Martin HJ, Syddall HE, Dennison EM, et al. Relationship between customary physical activity, muscle strength and physical performance in older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2008;37(5):589–93. doi: 10.1093/ageing/afn148. [DOI] [PubMed] [Google Scholar]