Platelet-type von Willebrand disease (PT-VWD; MIM177820) is a very rare dominant platelet disorder caused by missense variants in the GP1BA gene that codes for glycoprotein Ibalpha (GPIbα), the receptor for von Willebrand factor (VWF).1 These variants are gain-of-function (GOF) variants that result in enhanced binding of VWF to platelet GPIbα with subsequent removal of high-molecular-weight forms of VWF in plasma.2,3 Though this results in hyper-responsive platelets, PT-VWD patients present with mild mucocutaneous bleeding symptoms and intermittent thrombocytopenia with presence of some larger platelets. For years, it was hypothesized that this thrombocytopenia was the result of enhanced platelet clearance from the circulation due to the presence of the VWF-loaded platelets, but this was actually never shown in vivo. No previous attempts had been made to study megakaryopoiesis in these patients.
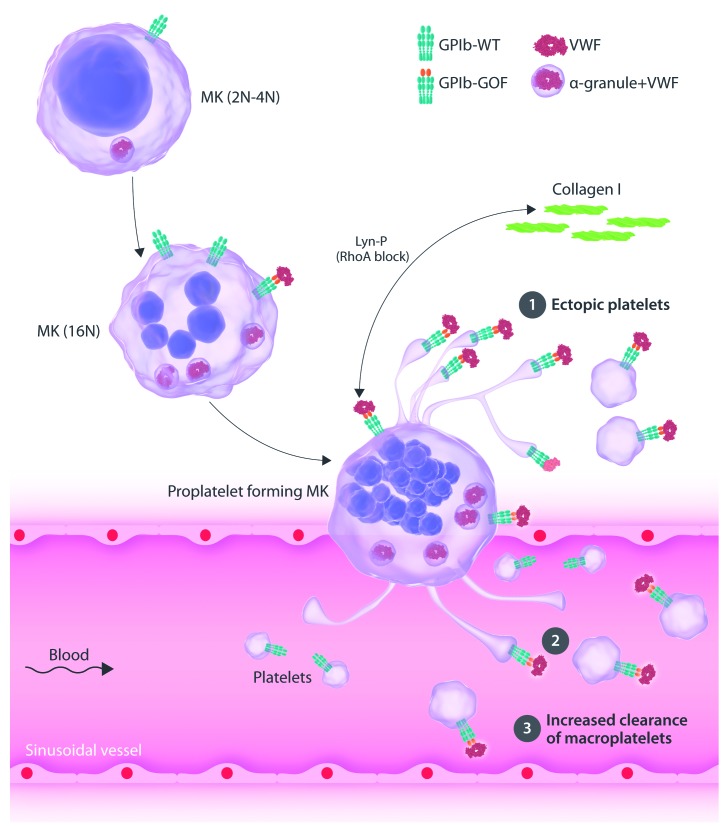
The study by Bury et al., in this issue of the Journal, now provides three rationales that corroborate the clinical and laboratory defects found for PT-VWD by studying megakaryopoiesis and platelet clearance using a human (p.M239V) and mouse (p.G233V) model for this disease (Figure 1).4 They found that: 1) ectopic platelets are released in the bone marrow; 2) PT-VWD megakaryocytes (MK) release larger but less (pro)platelets; and 3) GPIb-VWF positive platelets are more rapidly released from the blood circulation. These mechanisms support a combined defect in platelet formation and clearance to explain thrombocytopenia in PT-VWD. This study has used CD34+ hematopoietic stem cells (HSC) from a PT-VWD patient to study in vitro megakaryopoiesis. Data supported a mild defect in proplatelet formation with a reduction in the number of proplatelet tips that were larger, though a similar percentage from all MK were able to form proplatelets when compared to those from healthy donors. Interestingly, proplatelet formation was enhanced when PT-VWD MK were spread on collagen (but not on VWF or fibrinogen) and signaling studies revealed that this was due to increased enhanced Lyn phosphorylation (Lyn-P) resulting from the spontaneous GPIb-VWF interaction. Lyn-P blocks the normal RhoA-dependent inhibition of proplatelet formation in the presence of collagen I. Such a defect would result in the ectopic release of platelets in the bone marrow, and histological examination of bone marrow sections from the PT-VWD patient showed the presence of slightly more platelets (P<0.05) when compared to slides from three controls and three immune thrombocytopenia patients. Probably more studies in other PT-VWD patients are required to support these findings, though at least mouse studies using a PT-VWD knock-in model confirmed all these findings. The PT-VWD mouse model was also used to study platelet clearance and a significant reduced platelet half-life was observed for platelets with mutant GPIbα that captured VWF. Interestingly, desmopressin (DDAVP) administration to PT-VWD mice to increase their plasma VWF levels further decreased their lower platelet count, and this was associated with a further drop in platelet lifespan. PT-VWD mouse platelets did not expose increased levels of phosphatidylserine, excluding an important role for apoptosis as mediator for platelet clearance.
Figure 1.
Model for platelet-type von Willebrand disease (PT-VWD)-associated thrombocytopenia explains a defect in platelet formation and clearance. Megakaryocytes (MK) mature in the bone marrow and express von Willebrand factor (VWF) that is typically stored in their alpha (α) granules and the glycoprotein Ib (GPIb) receptor. PT-VWD is caused by a gain-of-function (GOF) variant in GPIbα that results in spontaneous binding to VWF. Thrombocytopenia present in PT-VWD seems to result from diverse mechanisms. 1) Ectopic platelets are released in the bone marrow due to enhanced Lyn phosphorylation (Lyn-P) as a result of the GPIb-VWF interaction and Lyn-P blocks the normal RhoA-dependent inhibition of proplatelet formation in the presence of collagen. 2) PT-VWD MK release larger but less (pro)platelets. 3) GPIb-VWF positive platelets are more rapidly released from the blood circulation. WT: wild type.
PT-VWD can be misdiagnosed as type 2B von Willebrand disease (VWD2B; MIM613554), as these two platelet disorders have very similar phenotypic parameters and clinical symptoms.5 VWD2B is caused by dominant GOF variants in the gene coding for von Willebrand factor VWF, generating mutant VWF with enhanced affinity for GPIbα with subsequent removal of high-molecular-weight forms of VWF in plasma. VWD2B patients and mice display mild bleeding and macrothrombocytopenia.6–8 Different studies have already investigated possible mechanisms that could explain the thrombocytopenia present in VWD2B. Briefly, by studying nine unrelated VWD2B patients with different GOF variants,6,7 electron microcopy showed that their platelets are larger, with the presence of giant platelets and platelet agglutinates. In vitro megakaryopoiesis studies using VWD2B HSC showed a reduction in proplatelet formation from enlarged swellings, and MK with a disorganized demarcation membrane system and abnormal granule distribution, pointing to a role of mutant VWF during MK maturation in conditions where the GOF-VWF could only be produced by the MK.6 When HSC from healthy donors are cultured in conditions where exogenous wild-type (WT) or GOF (p.R1306W) VWF is added to the thrombopoietin containing medium, (pro)platelet-formation was enhanced compared to conditions without VWF, but, remarkably, the GOF-VWF had a more pronounced stimulatory effect compared to WT-VWF. This seems to indicate that the GOF-VWF produced by VWD2B MK act differently to when adding exogenous GOF-VWF to normal MK. Interestingly, treatment of VWD2B MK with exogenous WT-VWF did raise the (pro)platelet counts though these remained lower than the numbers obtained from control MK. In vitro megakaryopoiesis studied for PT-VWD was only performed in conditions of endogenous VWF produced by the MK. The role of platelet- versus endothelial cell-derived VWF for in vivo megakaryopoiesis in healthy or diseases models has not yet been studied. A similar MK defect was described later for the VWD2B (p.V1316M) knock-in mouse model.8 HSC from these mice produce less pro-platelet-forming MK (in contrast to the PT-VWD mouse model) with decreased numbers of proplatelets per MK that have a larger size (similar to the PT-VWD mouse model). Signaling studies revealed a strong upregulation of the RhoA/LIMK/cofilin pathway that resulted in F-actin accumulation as likely cause of a (pro)platelet formation defect.8 Interestingly, the proplatelet formation defect present in mouse VWD2B MK could be rescued by treatment with LIMkinase (LIMK) or RhoA kinase (ROCK1) inhibitors.8 Moreover, the defect in platelet count and size could be rescued in VWD2B mice that were treated with a LIMK inhibitor.8 This pathway was not studied in PT-VWD models, and it would be very interesting to see if a similar approach could also rescue the thrombocytopenia in PT-VWD mice. The possibility of ectopic platelet release in VWD2B was not studied in the mouse model, but a bone marrow aspirate in a child with VWD2B revealed the presence of large platelet clumps and megakaryocyte nuclei surrounded by halos of clumped platelets.9 Further studies should be performed to strengthen this initial finding. Finally, enhanced clearance of VWF-platelet complexes has been suggested to occur in VWD2B.10 Studies in VWF2B mice have shown the involvement of macrophages in the removal of such VWF-platelet complexes, and significantly more platelets were found in liver and spleen of VWD2B mice compared with control mice.11 Moreover, a significantly reduced platelet half-life was observed for VWD2B platelets that have captured mutant VWF.11 Similar to observations in PT-VWD, this shorter platelet half-life was not due to differences in the apoptotic properties detected in VWD2B platelets.12 It was recently found that GPIbα upon binding to active VWF (e.g. VWD2B plasma) under physiological shear stress unfolds its mechanosensory domain near the platelet surface. This then triggers intracellular signaling, with exposure of β-galactose on the platelet surface that could favor platelet clearance via its interaction with the Ashwell–Morell receptor.13 A follow-up study did quantify β-galactose, as a marker for sialic acid removal, on platelets from VWD2B patients and mice and confirmed increased levels.14 However, treatment of VWD2B mice with sialidase inhibitors was not associated with the recovery of a normal platelet count.14 Further studies are definitely required to clarify the exact cause of enhance platelet clearance expected for VWD2B and PT-VWD.
A comparison of studies in PT-VWD versus VWD2B that have focused on the cause of thrombocytopenia in these platelet disorders clearly show similarities, but also point to unanswered questions. Platelet formation and clearance defects have been described for both, but it remains unclear if any process is dominant for these diseases. Genetic studies are required to diagnose these disorders.15
Supplementary Material
References
- 1.Miller JL, Cunningham D, Lyle VA, Finch CN. Mutation in the gene encoding the alpha chain of platelet glycoprotein Ib in platelet-type von Willebrand disease. Proc Natl Acad Sci U S A. 1991;88(11):4761–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss HJ, Meyer D, Rabinowitz R, et al. Pseudo-von Willebrand’s disease. An intrinsic platelet defect with aggregation by unmodified human factor VIII/von Willebrand factor and enhanced adsorption of its high-molecular-weight multimers. N Engl J Med. 1982;306(6):326–333. [DOI] [PubMed] [Google Scholar]
- 3.Murata M, Russell SR, Ruggeri ZM, Ware J. Expression of the phenotypic abnormality of platelet-type von Willebrand disease in a recombinant glycoprotein Ib alpha fragment. J Clin Invest. 1993;91(5):2133–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bury L, Malara A, Momi S, Petito E, Balduini A, Gresele P. Mechanisms of thrombocytopenia in platelet-type Von Willebrand Disease. Haematologica. 2019;104(7):1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Othman M. Platelet-type von Willebrand disease: a rare, often misdiagnosed and underdiagnosed bleeding disorder. Semin Thromb Hemost. 2011;37(5):464–469. [DOI] [PubMed] [Google Scholar]
- 6.Nurden P, Debili N, Vainchenker W, et al. Impaired megakaryocytopoiesis in type 2B von Willebrand disease with severe thrombocytopenia. Blood. 2006;108(8):2587–2595. [DOI] [PubMed] [Google Scholar]
- 7.Nurden AT, Federici AB, Nurden P. Altered megakaryocytopoiesis in von Willebrand type 2B disease. J Thromb Haemost. 2009;7(S1):277–281. [DOI] [PubMed] [Google Scholar]
- 8.Kauskot A, Poirault-Chassac S, Adam F, et al. LIM kinase/cofilin dys-regulation promotes macrothrombocytopenia in severe von Willebrand disease-type 2B. JCI Insight. 2016;1(16):e88643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slayton WB, Patel M, Sola-Visner M, et al. Type 2B von Willebrand disease associated with the release of platelet agglutinates from megakaryocytes in the bone marrow. J Pediatr Hematol Oncol. 2008;30(9):708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saba HI, Saba SR, Dent J, Ruggeri ZM, Zimmerman TS. Type IIB Tampa: a variant of von Willebrand disease with chronic thrombocytopenia, circulating platelet aggregates, and spontaneous platelet aggregation. Blood 1985;66(2):282–286. [PubMed] [Google Scholar]
- 11.Casari C, Du V, Wu YP, et al. Accelerated uptake of VWF/platelet complexes in macrophages contributes to VWD type 2B-associated thrombocytopenia. Blood. 2013;122(16):2893–2902. [DOI] [PubMed] [Google Scholar]
- 12.Berrou E, Kauskot A, Adam F, et al. Apoptotic Platelet Events Are Not Observed in Severe von Willebrand Disease-Type 2B Mutation p.V1316M. PLoS One. 2015;10(12):e0143896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng W, Xu Y, Chen W, et al. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nat Commun. 2016;7:12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont A, Soukaseum C, Cheptou M, et al. Relevance of platelet desialylation and thrombocytopenia in type 2B von Willebrand disease: preclinical and clinical evidence. Haematologica. 2019. February 28 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton A, Ozelo M, Leggo J, et al. Frequency of platelet type versus type 2B von Willebrand disease. An international registry-based study. Thromb Haemost. 2011;105(3):501–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.