Endogenous immunoprecipitation was used to investigate protein interactions of SETD1A complex subunits. An unexpected interaction with the DNA damage protein RAD18 was confirmed for SETD1A but not for other subunits. SETD1A and RAD18 evoked a DNA damage repair phenotype and influenced each other's mRNA and protein expression.
Keywords: Protein-Protein Interactions*, Methylation*, Epigenetics, Macromolecular complex analysis, Proliferation*, DNA damage repair, SET/MLL Complex
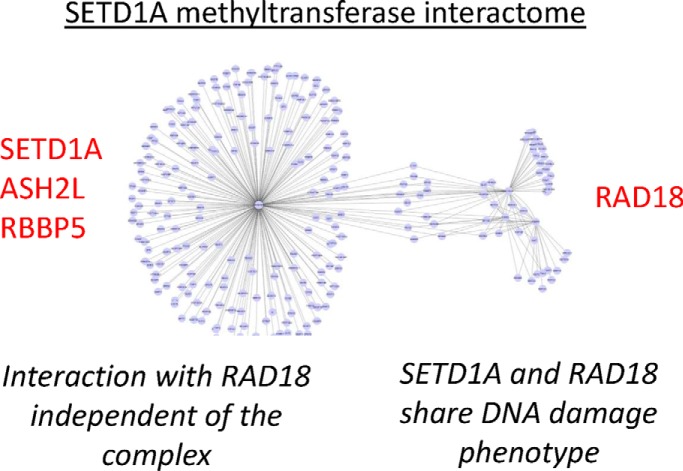
Graphical Abstract
Highlights
Protein interaction screen of SETD1A/COMPASS complex subunits.
Unexpected interaction with DNA damage protein RAD18 was confirmed for SETD1A, but not for other subunits.
SETD1A and/or RAD18 influence each other's mRNA and protein expression levels, and disruption of either gene elicits a similar DNA damage sensitivity phenotype.
Abstract
SETD1A is a SET domain-containing methyltransferase involved in epigenetic regulation of transcription. It is the main catalytic component of a multiprotein complex that methylates lysine 4 of histone H3, a histone mark associated with gene activation. In humans, six related protein complexes with partly nonredundant cellular functions share several protein subunits but are distinguished by unique catalytic SET-domain proteins. We surveyed physical interactions of the SETD1A-complex using endogenous immunoprecipitation followed by label-free quantitative proteomics on three subunits: SETD1A, RBBP5, and ASH2L. Surprisingly, SETD1A, but not RBBP5 or ASH2L, was found to interact with the DNA damage repair protein RAD18. Reciprocal RAD18 immunoprecipitation experiments confirmed the interaction with SETD1A, whereas size exclusion and protein network analysis suggested an interaction independent of the main SETD1A complex. We found evidence of SETD1A and RAD18 influence on mutual gene expression levels. Further, knockdown of the genes individually showed a DNA damage repair phenotype, whereas simultaneous knockdown resulted in an epistatic effect. This adds to a growing body of work linking epigenetic enzymes to processes involved in genome stability.
Dynamic post-translational modification (PTM)1 of histones is a central mechanism in epigenetic pathway regulation. It is involved in control of gene expression, replication of DNA, and the repair of damaged DNA (1). In particular, the precise deposition and removal of PTMs (notably acetylation and methylation) at specific histone residues is typically catalyzed by heteromeric protein complexes. Multiprotein complexes can confer selectivity and the ability to integrate multiple signals to the histone modification reaction (2). Although progress has been made in cataloging the composition of these complexes, much remains to be learned about how they coordinate diverse manipulations of chromatin, both in time and in space. Distinct combinations of PTMs may be present on a single histone molecule, confering a signal to the transcription machinery located near the associated DNA. For example, trimethylation of histone H3 at lysine 4 (H3K4me3) correlates with the presence of RNA polymerase II at promoters, with dimethylation (H3K4me2) and monomethylation (H3K4me1) occurring further downsteam (3). H3K4me1, when found in combination with an acetyl group on lysine 27 (H3K27ac), is associated with gene enhancer activity (4). Other proteins in turn can recognize and bind these PTMs and alternatively inhibit or promote the recruitment of additional activities that alter epigenetic output, for example recruitment of the promoter remodeling complex NURF to H3K4me3 via PHD domains (5, 6).
At least 20 protein complexes are known to catalyze histone methylation or demethylation reactions. Many histone modifying complexes share homologous subunits whose unique or overlapping functions are poorly understood (7). A single methyltransferase complex is responsible for H3K4 methylation in yeast, but in humans six homologs of the main catalytic SET-domain-containing enzyme (SET1A, SET1B, MLL1, MLL2, MLL3, MLL4) define at least six distinct complexes. All six associate with a conserved core module of four proteins (termed WRAD: WRD5, RBBP5, ASH2L, DPY30), but also with proteins specific to individual complexes. These complexes have related but distinct biochemical roles: SET1A- and SET1B-containing complexes are involved in trimethylation of H3K4 at many general promoters, whereas MLL1- and MLL2-containing complexes are responsible for regulation of genes specific to embryo development (8, 9). MLL3 and MLL4 are linked to monomethylation of histones at DNA enhancer elements (10, 11).
The presence of distinct complexes strongly suggests that they are not fully redundant in function, and indeed nonoverlapping phenotypes are observed when the SET-domain-containing proteins are disrupted in animal models (12). Recent proteomics surveys have mapped the physical interactions of SET1/MLL complex subunits. Mak and coworders (2010) investigated protein interactions in a wide range of human chromatin modifying complexes, including the SET1/MLL complex subunits ASH2L, RBBP5, and WDR5 (13). These proteins were expressed with affinity tags following lentivirus delivery to cultured human embryonic kidney (HEK293) cells. Another proteomics study focused on the SET1/MLL family using stable expression of GFP-tagged bait proteins (14). Both studies recapitulated earlier findings whereas the latter identified important discriminating proteins that were uniquely associated with individual complexes.
Here we surveyed the physical interactions of SETD1A in cultured (HEK2923) cells using an immunoprecipitation approach that more faithfully reproduces native cell molecular stoichiometries than overexpression studies. Our work recapitulated the interactions of SETD1A with previously described members of the methyltransferase complex, but unexpectedly we found a physical interaction with the DNA damage repair protein RAD18. Disruption of either SETD1A or RAD18 expression in HEK293 cells resulted in defects in recovery from DNA damage, suggesting that the physical association between these protein reflects functional interplay between them.
EXPERIMENTAL PROCEDURES
Cell Culture
HEK293T cells (CRL-1573, ATCC) were cultured in 10 cm Nunclon tissue culture dishes (Fisher Scientific) in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (Hyclone), 100 U/ml penicillin and 100 U/ml streptomycin (Gibco). Cells were passaged by trypsinizing with 0.25% Trypsin-EDTA (Invitrogen) and plated at a ratio of 1:10.
Isolation of Nuclei
Harvested HEK293T cells were washed in PBS and resuspended in Lysis3T buffer (25 mm Tris·HCl pH 7.6, 150 mm NaCl, 1% NP40, 1% sodium deoxycholate, 0.1% SDS, 2 μg/ml Aprotinin, 1 μg/ml, Leupeptin, 10 mm PMSF). The lysates were incubated for 15 min on ice and cell membranes disrupted mechanically by syringing 5 times with 21G narrow gauge needle and sonicating 3 Å∼ for 2 s at high power. Lysates were incubated on ice for another 15 min and cleared by centrifugation at 14,000 rpm 4 °C 30 min. To harvest the nuclear fraction, lysates were resuspended in an equal volume of Nuclear Buffer (20 mm HEPES pH 7.9, 0.2 mm EDTA, 1.5 mm MgCl2, 20% glycerol, 420 mm NaCl, 2 μg/ml Aprotinin, 1 μg/ml Leupeptin, 10 mm PMSF) and dounced 20 times with tight pestle type B. Lysates were incubated for 45 min rotating to dissociated chromatin-bound proteins and pre-cleared by centrifugation at 14,000 rpm 4C for 30 min.
Immunoprecipitation
60 μl of packed Protein A-Sepharose (Sigma P9424) beads was coupled with 10 μg antibody and incubated in 1 ml of PBS-T (0.1% Tween-20) at 4 °C rotating overnight. Next day, beads were collected by centrifugation at 3000 rpm for 2 min and washed twice in 1 ml of Sodium Borate (0.2 m, pH9.0). Antibodies were crosslinked to beads by incubation in 1 ml of 20 mm dimethyl pimelimidate dihydrochloride (prepare in Sodium Borate) at room temperature rotating for 30 min. Reaction was stopped by washing beads once in 1 ml of 0.2 m Ethanolamine pH 8.0 and incubating for 2 h at room temperature in 1 ml of 0.2 m Ethanolamine pH 8.0. Beads washed once with 1 ml of PBS-T (0.1% Tween-20) and blocked for 2 h in Buffer A (0.2 mg/ml chicken egg albumin (Sigma A5503), 0.1 mg/ml Insulin (Sigma, I9278), 1% fish skin gelatin (Sigma G7041). Crosslinked beads were incubated with nuclear lysate, which contains 250 U/ml Benzonase nuclease, at 4 °C rotating for 2 h. The beads were washed four times in Buffer B (20 mm HEPES ph7.6, 120 mm NaCl, 20% glycerol, 0.02%NP-40, 1.5 mm MgCl2, complete mini tablet, and 0.5 mm DTT). Two washes in Buffer B minus detergent followed. Finally, the beads were treated with 2XSDS-PAGE buffer (Sigma) and heated at 95 °C with shaking at 300 rpm for 5 min. Eluted proteins were centrifuged at 10,000 rpm 4 °C for 5 min in a benchtop microfuge, separated by SDS-PAGE, and analyzed by Western blotting and liquid chromatography mass spectrometry.
Western Blot Analysis
HEK293T cells were resuspended in RIPA buffer (25 mm Tris·HCl pH 7.6, 150 mm NaCl, 1% NP40, 1% sodium deoxycholate, 0.1% SDS, 2 μg/ml Aprotinin, 1 μg/ml Leupeptin, 10 mm PMSF). Lysates were incubated for 15 min on ice and cells membrane were interrupted by syringing 5 times with 23G narrow gauge needle and sonicating 3 × 2 s at high power. Incubate lysates for another 15 min on ice and centrifuged at 14,000 rpm for 30 min at 4 °C. Nuclear pellets were resuspended in Buffer C (20 mm HEPES pH 7.9, 0.2 mm EDTA, 1.5 mm MgCl2, 20% glycerol, 420 mm NaCl, 2 μg/ml Aprotinin, 1 μg/ml Leupeptin, 10 mm PMSF) and dounced 20 times with tight pestle type B (Tight) on ice. Lysates were incubated for 30 min rotating at 4 °C and centrifuged at 14,000 rpm 4 °C for 30 min. Immunoblotting was performed using antibodies mentioned in supplemental Table S1.
Gel Filtration Column Chromatography
The SuperoseTM 6 10/300 GL gel filtration column (GE Healthcare) was equilibrated with one column volume of running buffer (20 mm Tris pH 8.0, 10% Glycerol, 175 mm NaCl, 0.5 mm DTT, 1 mm PMSF). 300–500 μg of total nuclear protein was injected and run through column at 0.35 ml/min. One milliliter fractions were collected and protein was concentrated by incubation with 4 μ L StrataClean resin (Agilent Technologies) for 1 h at room temperature. Resin was collected by centrifugation at 5000 rpm for 3 min and protein was eluted by boiling in 20 μ L 2X SDS sample buffer for 5 min shaking at 1400 rpm. Eluted protein analyzed by SDS-PAGE and immunoblotting.
In-solution Trypsin Digest and Mass Spectrometry
Proteins were treated with trypsin as described (15). Peptide samples were introduced Q Exactive mass spectrometer via an EASY-nLC 1000 UHPLC system (Thermo Fisher) coupled to an in-house packed C18 column (New Objective). Parent ion spectra (MS1) were measured at resolution 70,000, AGC target 3e6. Tandem mass spectra (MS2; up to 10 scans per duty cycle) were obtained at resolution 17,500, AGC target 5e4, collision energy of 25. Data were processed using MaxQuant version 1.5.5.1 using the human UniProt database (release 2016_06; 70,317 entries). The following search parameters were used: Fixed Mod: carbamidomethylation; Variable Mods: methionine oxidation; Trypsin/P digest enzyme; 1 missed cleavage; Precursor mass tolerances 6 ppm; Fragment ion mass tolerances 20 ppm; Peptide FDR 1%; Protein FDR 1%. Data analysis. Raw data, including results files and software needed for viewing spectra is provided via the PRIDE database (16). Stoichiometry and volcano plots of LC-MS/MS data of SETD1A, RAD18, RBBP5, and ASH2L immunoprecipitations in HEK293T cells were performed using Perseus software, version 1.5.5.3 (17) and msVolcano (18) (supplemental Table S9). msVolcano fills the missing intensity values with noise that simulates the detection limit of the machine. For this, the properties of the distribution of measured values are calculated, which are roughly normally distributed (in log space). Then random values are drawn from a population that is centered on themean-1.8*S.D. (Shift parameter), and 0.3*S.D. (Shrink parameter) wide as compared with the original distribution. All output from MaxQuant was also analyzed using the SAINT program (Spectral Count mode), and high scoring interactions (>0.99) were visualized using Cytoscape (version 3.7.1).
siRNA Transfections
siRNAs were purchased from Applied Biosystems®, and all siRNA transfections were performed with 20 μm of siRNA using Lipofectamine® RNAiMAX (BioSciences). See supplemental Table S1 for siRNA reagent details.
Real-time Quantitative PCR
RNA was extracted by NucleoSpin® RNA Plus kit (MACHEREY-NAGEL) from siRNA-transfected HEK293T cells. cDNA was generated by reverse transcriptase PCR using RevertAid First Strand cDNA Synthesis kit (Thermo). Level of relative mRNA expression were determined using the SYBR Green I detection chemistry on LightCycler 480II Real-Time PCR System (Roche). GAPDH was used as normalizing gene. Primers are listed in supplemental Table S1.
Cell Survival Assay
siRNA-transfected HEK293T cells were plated at very low density and was treated with different concentrations of MMC (Sigma Aldrich) as previously described (19).
Experimental Design and Statistical Rationale
In each IP experiment, three biological replicates were compared. The Perseus program was used to carry out statistics (17). Briefly, the LFQ values were transformed (log2) and missing values imputed to a normal distribution (width = 0.3; shift = 1.8), and a two-tailed t test applied with correction for multiple testing (Benjamini). Volcano plots were constructed using the permutation-based approach of Tusher and coworkers (2001) (20), to implement an FDR of 0.05 as described in (17).
RESULTS
A Screen for Physical Interactions of the SETD1A Methyltransferase Complex Under Endogenous Conditions
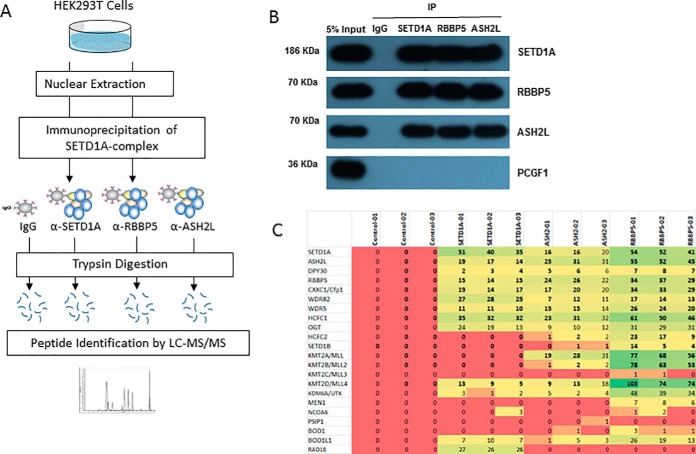
We used commercial antibodies to develop immunoprecipitation protocols capable of purifying SETD1A, RBBP5, ASH2L, and their associated proteins from cultured HEK293 cells (Fig. 1A; supplemental Table S1). The three subunit-specific antibodies, as well as a nonspecific control antibody, were covalently coupled to Protein A beads, and parallel immunoprecipitations were performed in triplicate. Following washing steps, the proteins were removed from the beads by trypsinization, identified using Q Exactive mass spectrometry, and statistically analyzed to establish the set of proteins that reproducibly interacted with each bait. A fraction of the immunoprecipitated sample was retained for Western blotting (co-immunoprecipitation) experiments. The western blots confirmed that all three baits reciprocally precipitated each other as prey (Fig. 1B). As expected, all three baits failed to co-immunoprecipitate with PCGF1, which served as a negative control. PCGF1 is a component of another chromatin-located ubiquitin ligase complex (PRC1, Polycomb Repressor Complex 1). These experiments confirmed the specificity of the immunoprecipitation procedure. Further, no peptides specific to SET1B or other MLL proteins were found in our SET1A immunoprecipitation experiments, indicating a lack of cross-reactivity by our antibody other SET-domain containing homologs (Fig. 1C).
Fig. 1.
A proteomics screen for physical interactors of SET1A-complex subunits under endogenous conditions. A, SETD1A-complex members were immunoprecipitated from nuclear extracts of HEK293T cells. Physically associated proteins were trypsinized on beads, and the resulting peptides identified and quantified using Q Exactive mass spectrometry. B, Input nuclear lysate and samples immunoprecipitated using control- (IgG), SETD1A-, RBBP5-, and ASH2L-specific antibodies were analyzed by Western blotting for reactivity using the same antibodies. C, Spectral counts (the frequency of identification quality MS2 events per 60min LCMS run) are plotted for each immunoprecipitation experiment (carried out in triplicate).
The SETD1A, ASH2L, and RBBP5 Interactome Includes Components of the SETD1A Methyltransferase Complex, As Well As RAD18
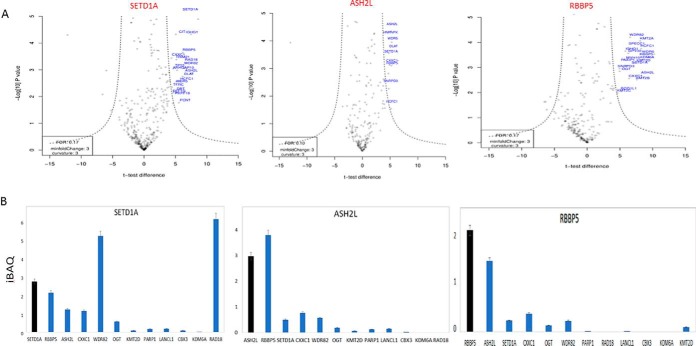
All three bait proteins co-precipitated with previously known members of the SETD1A methyltransferase complex, including the WRAD module proteins and other proteins identified in large-scale screens such as OGT, HCFC1/2, CXXC1, and BOD1/BOD1L1 (13, 14) (Fig. 1C) (supplemental Table S2). As expected, immunoprecipitation by shared SET1/MLL complex components ASH2L and RBBP5 led to detection of SET-domain proteins additional to SET1A (SET1B, MLL1–4). For each set of immunoprecipitation experiments, we plotted the enrichment relative to control (t test difference) against the statistical significance (-log t test p values) for the detected protein (Fig. 2A). Interestingly, the DNA damage repair and E3 ubiquitin ligase protein RAD18, to our knowledge not previously associated with SETD1A, was prominent in the immunoprecipitation volcano plot (Fig. 2A). However, it was undetected by mass spectrometry in the RBBP5 and ASH2L immunoprecipitations, suggesting a specific association with SETD1A that is independent of the SETD1A complex. Stoichiometry plots (based on the iBAQ measure of normalized protein abundance) (21) supported this conclusion (Fig. 2B).
Fig. 2.
Physical interactions of SETD1A-complex subunits. A, Volcano plot projects differences in t test scores (between specific and control immunoprecipitations), against log-transformed t test p values for the SETD1A, RBBP5, and ASH2L experiments using the msVolcano program. The data is based on the MaxQuant LFQ score. Previously described SETD1A-complex component proteins are labeled in red. B, LFQ scores were adjusted using the iBAQ algorithm to plot relative stoichiometry data for previously-described SETD1A-interacting proteins, and for RAD18.
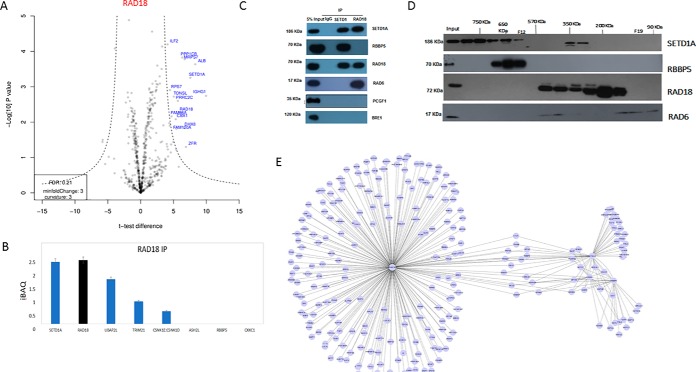
The DNA Damage Repair Protein RAD18 Interacts with SETD1A, But Not with ASH2L or RBBP5
We sought to confirm the interaction between SETD1A and RAD18 by carrying out a similar physical interaction screen using a RAD18-specific antibody (Fig. 3A). Analysis of approximate stoichiometry in the RAD18 interactome purification shows that SETD1A is among the most abundant and statistically significant interactors (Fig. 3A). Notably, other co-purifying SETD1A complex components (RBBP5, ASH2L etc.) were undetected or detected at negligible levels (Fig. 3B; supplemental Table S5). This suggests that a large proportion of the RAD18 present in cultured HEK293 cells associates with SETD1A at ∼1:1 molar ratio. We sought to confirm this independently by co-immunoprecipitation (Fig. 3C). As expected, both SETD1A and RAD18 reciprocally co-precipitated each other, but only SETD1A was reactive with the SET1 complex member RBBP5. RAD6, an E2 ubiquitin ligase known to interact with RAD18, was found to interact with RAD18 but not SETD1A. Importantly, neither protein was found to interact with the chromatin associated PRC1 complex (PCGF1), nor with BRE1, a component of another histone-modifying ubiquitin ligase complex (RNF20/40 E3 complex) that also contains RAD6.
Fig. 3.
Analysis of RAD18 physical interactions under endogenous conditions. A, Volcano plot projections for RAD18 immunoprecipitation LCMS experiment using the msVolcano program. B, iBAQ relative quantitation of RAD18 interactors. C, Co-immunoprecipitation analysis of SETD1A and RAD18. RBBP5 (SETD1A interactor) and RAD6 (RAD18 interactor) serve as positive controls. PCGF1 and BRE1 serves as negative controls. D, Western blot analysis of size exclusion fractions for SETD1A-interacting proteins and controls. E, Network projection of SETD1A-, RBBP5-, ASH2L-, and RAD18-interacting proteins identified using the SAINT algorithm.
We used size exclusion fractionation of HEK293 cell nuclear lysate to further investigate the interaction between SETD1A and RAD18. Probing of the fractions by Western blotting using antibodies specific for SETD1A and RBBP5 protein revealed a high mass distribution (∼600–700 KDa) consistent with a large complex such as the SETD1A/COMPASS complex that contains both proteins (Fig. 3D). The SETD1A antibody was also reactive with a lower mass distribution (∼250–350 KDa) that overlaps with RAD18 antibody reactivity in the same experiment (∼100–500 KDa). This is consistent with a complex containing one molecule each of SETD1A and RAD18 (∼258 KDa), although additional biophysical experiments will be needed to confirm that this is indeed the case. RAD18 is known to form a complex with RAD6 in a 2:1 molar ratio. RAD6 antibody reactivity in the same experiment is consistent with such a complex, and importantly, the elution pattern of RAD6 in the size exclusion experiment is distinct from that of SETD1A.
Finally, to explore the physical interactions of SETD1A, RAD18, and the SETD1A/COMPASS complex in a network context, we used the SAINT (Significance Analysis of INTeractome) (22) algorithm to evaluate the protein interaction data from all four immunoprecipitation screens together. High confidence interactions (MaxP > 0.99) identified by this analysis were projected onto a Network using Cytoscape (23) (supplemental Table S8). The resulting network shows two separate clusters of interactions, one comprising members of the SETD1A complex, and the other centered on RAD18.
Taken together, these experiments indicate that the interaction between SETD1A and RAD18 is specific, independent of the SETD1A-complex, and does not arise from a generalized or extended chromatin interactome.
Disruption of SETD1A or RAD18 Expression Leads to Mutual Downregulation of mRNA and Protein, But Not of Global Histone H3K4me3 Levels
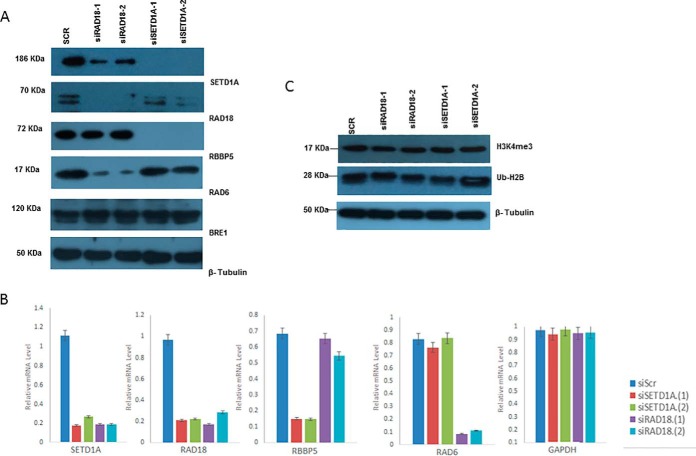
Because SETD1A and RAD18 interact physically, at least in cultured HEK293 cells, the question of functional significance arises. Specifically, we wondered if either protein might influence the levels or activity of the other. Knockdown of SETD1A or RAD18 genes using siRNA led to elimination of detectable protein levels for the cognate protein (Fig. 4A). Surprisingly however, disruption of either gene also leads to reduced protein levels of the other. Further, disruption of SETD1A results in undetectable levels of RBBP5, whereas disruption of RAD18 results in reduced RAD6 protein levels. Thus, we observe a general trend whereby proteins that physically interact also influence expression of each other. Protein levels of BRE1 and β-tubulin were unaffected by any of the siRNA reagents. When we assessed levels of the corresponding mRNAs (SETD1A, RAD18, RBBP5, RAD6), the protein results were mimicked (Fig. 4B), suggesting that any mutual regulation of these proteins operates at the level of transcription. A natural question is whether reduced expression of SETD1A or RAD18 might influence the histone methylation phenotype. However, previous work by others found that knock-down of SETD1A levels in HeLa and tumor cell models failed to show reduced global levels of the SETD1A product H3K4me3, perhaps because of functional redundancy with other methyltransferases (19, 24). We confirmed this in the HEK293 model where knock down of SETD1A or RAD18 resulted in no detectable effect on the H3K4me3 levels, nor on ubiquitinated histone H2B (Fig. 4C).
Fig. 4.
SETD1A and RAD18 mutually influence mRNA and protein expression. A, Protein expression of SETD1A, RAD18, and control proteins following disruption of SETD1A and RAD18 using siRNA. B, Expression of trimethylated histone H3 lysine 4, ubiquitinated histone H2B, and β-tubulin following disruption of SETD1A and RAD18 using siRNA. C, mRNA expression for genes encoding SETD1A, RAD18, and controls following disruption of SETD1A and RAD18 expression.
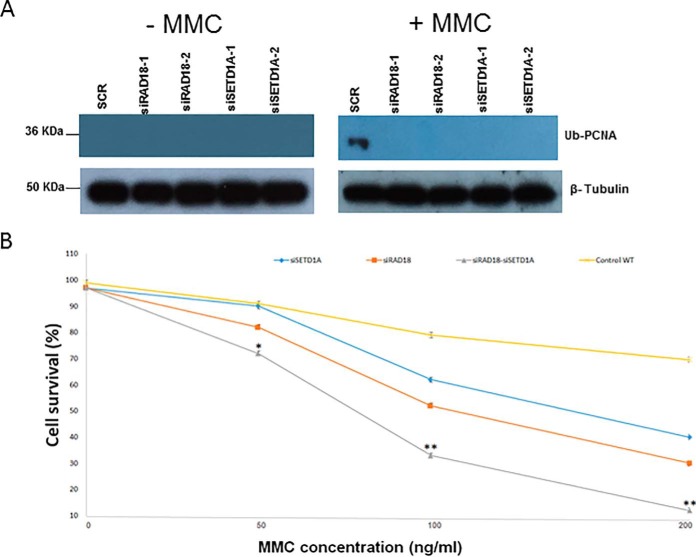
Disruption of SETD1A Results in Impaired DNA Damage Repair
Because the SETD1A-RAD18 interaction appears to occur outside the context of the SETD1A-complex, we next asked if SETD1A contributed to RAD18 function during DNA damage repair. We found that loss of either gene eliminated production of ubiquitinated PCNA following induction of DNA damage using mitomycin C, an inducer of interstrand DNA crosslinking (Fig. 5A). This suggests that both proteins participate in damage repair pathways. Therefore, we asked if loss of SETD1A or RAD18 influenced the proliferation rate of cells treated with mitomycin C. Cell survival studies confirmed the negative effect of loss of SETD1A or RAD18 on cell proliferation (Fig. 5B). Moreover, an enhanced reduction in cell survival levels was observed when both genes were knocked down simultaneously, suggesting an cooperative epistatic effect whereby each gene is at least partially dependent on the other for repair of mitomycin C-induced DNA damage. We confirmed these observations in an independent cell line (NTERA-2 cells), suggesting that the effect may be general to multiple cell types (supplemental Fig. S9).
Fig. 5.
SETD1A and RAD18 share a DNA damage repair phenotype. A, Levels of ubiquitinated PCNA and β-tubulin were determined by Western blotting following disruption of SETD1A and RAD18, with or without treatment of the cells with mitocycin C (MMC). B, Cell survival levels were plotted for cells pre-treated with SETD1A- and/or RAD18-specific siRNAs following addition of difference concentrations of mitomycin C (MMC).
DISCUSSION
The minimal SETD1A complex required to catalyze methylation of histone H3K4 has been defined biochemically using in vitro reconstitution experiments as containing SETD1A, ASH2L, RBBP5, WDR5 and DPY30 (25, 26). However, physical interaction screens using SET1 have found additional proteins, not essential for the histone methyltransferase activity (13, 14). Our work here substantially recapitulates these earlier studies in terms of SETD1A-complex composition, and we also investigate the significance of a novel interaction with the DNA damage repair protein RAD18.
The six human SET/MLL family complexes exhibit partially nonredundant cellular functions. For example, different complexes favor different stages of embryo development (27) or preferentially modify histones located at different target genes (28). Further, biochemical experiments have found distinct dimethylation and trimethylation activities among different in vitro reconstituted complexes (29). The specialization of SET complexes applies not only to the core SET-domain containing proteins, but to the shared (WDR5-RBBP5-ASH2L-DPY30) subunits. Consistent with this, several reports suggest that the methylation activity of SET/MLL complexes may be context-dependent (24, 30).
Considering these observations, our finding of a physical and function connection between SETD1A and RAD18 is intriguing. RAD18 is a ubiquitin ligase important for cell survival following different types of DNA damage including ionizing and UV damage. In combination with the E2 ubiquitin ligase RAD6, RAD18-dependent ubiquitination of the replication clamp protein PCNA facilitates recruitment of various repair enzymes. This permits cells to progress through the steps of DNA replication despite the presence of damaged DNA. RAD18 is also involved in other DNA damage pathways including the homologous recombination (HR) and nonhomologous end joining (NHEJ) pathways. Although RAD18 has so far not been linked to epigenetic modification, DNA repair events are associated with extensive histone modification (e.g. phosphorylation and ubiquitination of H2AX).
Chromatin modifying enzymes, including SET-domain that contain lysine methyltransferases such as EZH2 and KMT2C/2D, have previously been reported to play roles in the response to DNA replication stress (31, 32, 33). We found here that knockdown of SETD1A or RAD18 leads to deficiencies in DNA damage repair. Although a DNA damage phenotype for a chromatin modifying enzyme is at first glance surprising, recent work has highlighted the role of SETD1A in DNA damage repair pathways. H3K4 methylation was shown to play a role in genome stability by preventing damage to stalled replication forks (19). The activity was linked to histone chaperone activity by FANCD2, a component of the Fanconi anemia and homologous recombination pathway. The same group demonstrated a previously unknown role for the SET1-interacting protein BOD1L in protecting replication forks from nuclease degradation (34). Another study linked SETD1A with the DNA damage response in acute myeloid leukemia cells, where a region of the SETD1A protein independent of the SET domain was found to generate a recruitment site for cyclin K during DNA repair (35). Overall, it is clear that epigenetic enzyme complexes including SETD1A play a fundamental role in cross-talk with genome stability and DNA replication pathways, most likely by regulating recruitment and activity at specific loci.
Interestingly, we also found evidence of mutual regulation among SETD1A-complex components and RAD18. This was not restricted to autoregulation at transcriptional level of the proteins themselves, but the levels of SETD1A mRNA were found to impact RAD18 protein levels, whereas conversely, RAD18 mRNA levels impacted SETD1A protein. We noticed similar behavior in an previous study of another chromatin regulatory complex component. Knockdown of the RING finger ubiquitinating enzyme PCGF1 (a component of Polycomb Repressor Complex 1) led to reduced expression of an interacting protein, DPPA4, that was apparently involved in a distinct biological pathway (36). Studies in yeast have found that protein levels of SET1 are highly regulated and influenced by several factors. These include histone H3K4 methylation levels, transcriptional blocking, and recruitment of the protein to target genes (37). In the case of SET1, the feedback regulation was mapped to an autoinhibitory domain at the N terminus of SET1, and it remains to be seen if a similar mechanism operates in mammalian SET1/MLL complexes.
An outstanding question arising from our study concerns the nature of the SETD1A and RAD18 interaction. Size exclusion, co-immunoprecipitation experiments, and statistical network analysis suggested (but did not prove) that SETD1A and RAD18 assemble independently of the SETD1A/COMPASS complex. It is unclear if newly described roles for SET1 in DNA damage repair (34, 35). In vitro reconstitution experiments or cross-link mass spectrometry may be productive in future studies. In conclusion, our work establishes a physical link underlying a functional connection between two distinct cellular processes, the epigenetic regulation of transcription, and DNA damage repair.
DATA AVAILABILITY
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD012549. Annotated MS/MS spectra can be accessed through MS-Viewer (38) with the search keys: a0vbclfehx and pl5impwjdo.
Supplementary Material
Acknowledgments
We acknowledge the help of the UCD Conway Proteomics and Genomics Facilities.
Footnotes
* MA was supported by a PhD Fellowship from the Government of Saudi Arabia. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD012549.
 This article contains supplemental Figures and Tables. The authors declare no competing financial interests.
This article contains supplemental Figures and Tables. The authors declare no competing financial interests.
1 The abbreviations used are:
- PTM
- post-translational modification
- SAINT
- significance analysis of INTeractome.
REFERENCES
- 1. Allis C. D., Jenuwein T. (2016) The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500 [DOI] [PubMed] [Google Scholar]
- 2. Ricketts M. D., Han J., Szurgot M., Marmorstein R. (2018) Molecular basis for chromatin assembly and modification by multi-protein complexes. Protein Sci. doi: 10.1002/pro.3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernstein B. E., Kamal M., Lindblad-Toh K., Bekiranov S., Bailey D. K., Huebert D. J., McMahon S., Karlsson E. K., Kulbokas E. J. 3rd, Gingeras T. R., Schreiber S. L., Lander E. S. (2005) Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 [DOI] [PubMed] [Google Scholar]
- 4. Heintzman N. D., Hon G. C., Hawkins R. D., Kheradpour P., Stark A., Harp L. F., Ye Z., Lee Stuart L. K. R. K., Ching C. W., Ching K. A., Antosiewicz-Bourget J. E., Liu H., Zhang X., Green R. D., Lobanenkov V. V., Stewart R., Thomson J. A., Crawford G. E., Kellis M., Ren B. (2009) Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459, 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vermeulen M., Mulder K. W., Denissov S., Pijnappel W. W., van Schaik F. M., Varier R. A., Baltissen M. P., Stunnenberg H. G., Mann M., Timmers H. T. (2007) Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell 131, 58–69 [DOI] [PubMed] [Google Scholar]
- 6. Wysocka J., Swigut T., Xiao H., Milne T. A., Kwon S. Y., Landry J., Kauer M., Tackett A. J., Chait B. T., Badenhorst P., Wu C., Allis C. D. (2006) A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodeling. Nature 442, 86–90 [DOI] [PubMed] [Google Scholar]
- 7. Herz H. M., Garruss A., Shilatifard A. (2013) SET for life: biochemical activities and biological functions of SET domain-containing proteins. Trends Biochem. Sci. 38, 621–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu M., Wang P.F., Lee J.S., Martin-Brown S., Florens L., Washburn M., Shilatifard A. (2008) Molecular regulation of H3K4 trimethylation by Wdr82, a component of human Set1/COMPASS. Mol. Cell. Biol. 28, 7337–7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu D., Gao X., Cao K., Morgan M. A., Mas G., Smith E. R., Volk A. G., Bartom E. T., Crispino J. D., Di Croce L., Shilatifard A. (2017) Not all H3K4 methylations are created equal: Mll2/COMPASS dependency in primordial germ cell specification. Mol. Cell. 65, 460–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herz H. M., Mohan M., Garruss A. S., Liang K., Takahashi Y. H., Mickey K., Voets O., Verrijzer C. P., Shilatifard A. (2012) Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 26, 2604–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu D., Gao X., Morgan M. A., Herz H. M., Smith E. R., Shilatifard A. (2013) The MLL3/MLL4 branches of the COMPASS family function as major histone H3K4 monomethylases at enhancers. Mol. Cell. Biol. 33, 4745–4754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eissenberg J. C., Shilatifard A. (2010) Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev. Biol. 339, 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mak A. B., Ni Z., Hewel J. A., Chen G. I., Zhong G., Karamboulas K., Blakely K., Smiley S., Marcon E., Roudeva D., Li J., Olsen J. B., Wan C., Punna T., Isserlin R., Chetyrkin S., Gingras A. C., Emili A., Greenblatt J., Moffat J. (2010) A lentiviral functional proteomics approach identifies chromatin remodeling complexes important for the induction of pluripotency. Mol. Cell. Proteomics 9, 811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Nuland R., Smits A. H., Pallaki P., Jansen P. W., Vermeulen M., Timmers H. T. (2013) Quantitative dissection and stoichiometry determination of the human SET1/MLL histone methyltransferase complexes. Mol. Cell. Biol. 33, 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turriziani B., Garcia-Munoz A., Pilkington R., Raso C., Kolch W., and von Kriegsheim A. (2014) On-beads digestion in conjunction with data-dependent mass spectrometry: a shortcut to quantitative and dynamic interaction proteomics. Biology 3, 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vizcaíno J. A., Csordas A., del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., Xu Q.-W., Wang R., and Hermjakob H. (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M. Y., Geiger T., Mann M., and Cox J. (2016) The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 [DOI] [PubMed] [Google Scholar]
- 18. Singh S., Hein M. Y., and Stewart A. F. (2016), msVolcano: A flexible web application for visualizing quantitative proteomics data. Proteomics, 16, 2491–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgs M. R., Sato K., Reynolds J. J., Begum S., Bayley R., Goula A., Vernet A., Paquin K. L., Skalnik D. G., Kobayashi W., Takata M., Howlett N. G., Kurumizaka H., Kimura H., Stewart G. S. (2018) Histone methylation by SETD1A protects nascent DNA through the nucleosome chaperone activity of FANCD2. Mol. Cell. 71, 25–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tusher V. G., Tibshirani R., and Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U.S.A. 98, 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., and Selbach M. (2011) Global quantification of mammalian gene expression control. Nature 473, 337–342 [DOI] [PubMed] [Google Scholar]
- 22. Teo G., Liu G., Zhang J. P., Nesvizhskii A. I., Gingras A.-C., and Choi H. (2013) SAINTexpress: improvements and additional features in Significance Analysis of INTeractome for AP-MS data. J. Proteomics, 100, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shannon P., Markiel A., Ozier O., et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tajima K., Yae T., Javaid S., Tam O., Comaills V., Morris R., Wittner B. S., Liu M., Engstrom A., Takahashi F., Black J. C., Ramaswamy S., Shioda T., Hammell M., Haber D. A., Whetstine J. R., Maheswaran S. (2015) SETD1A modulates cell cycle progression through a miRNA network that regulates p53 target genes. Nat. Commun. 6, 8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel A., Dharmarajan V., Vought V. E., Cosgrove M. S. (2009) On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex. J. Biol. Chem. 284, 24242–24256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takahashi Y. H., Westfield G. H., Oleskie A. N., Trievel R. C., Shilatifard A., Skiniotis G. (2011) Structural analysis of the core COMPASS family of histone H3K4 methylases from yeast to human. Proc. Natl. Acad. Sci. U.S.A. 108, 20526–20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bledau A. S., Schmidt K., Neumann K., Hill U., Ciotta G., Gupta A., Torres D. C., Fu J., Kranz A., Stewart A. F., Anastassiadis K. (2014) The H3K4 methyltransferase Setd1a is first required at the epiblast stage, whereas Setd1b becomes essential after gastrulation. Development 141, 1022–1035 [DOI] [PubMed] [Google Scholar]
- 28. Duncan E. M., Chitsazan A. D., Seidel C. W., Alvarado A. S. (2016) Set1 and MLL1/2 target distinct sets of functionally different genomic loci in vivo. Cell Rep. 17, 930. [DOI] [PubMed] [Google Scholar]
- 29. Shinsky S. A., Monteith K. E., Viggiano S., Cosgrove M. S. (2015) Biochemical reconstitution and phylogenetic comparison of human SET1 family core complexes involved in histone methylation. J. Biol. Chem. 290, 6361–6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thornton J. L., Westfield G. H., Takahashi Y. H., Cook M., Gao X., Woodfin A. R., Lee J. S., Morgan M. A., Jackson J., Smith E. R., Couture J. F., Skiniotis G., Shilatifard A. (2014) Context dependency of Set1/COMPASS-mediated histone H3 Lys4 trimethylation. Genes Dev. 28, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faucher D., Wellinger R. J. (2010) Methylated H3K4, a transcription-associated histone modification, is involved in the DNA damage response pathway. PLoS Genet 6, pii: e1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ray Chaudhuri A., Callen E., Ding X., Gogola E., Duarte A. A., Lee J. E., Wong Lafarga N. V., Calvo J. A., Panzarino N. J., John S., Day A., Crespo A. V., Shen B., Starnes L. M., de Ruiter J. R., Daniel J. A., Konstantinopoulos P. A., Cortez D., Cantor S. B., Fernandez-Capetillo O., Ge K., Jonkers J., Rottenberg S., Sharan S. K., Nussenzweig A. (2016) Replication fork stability confers chemoresistance in BRCA-deficient cells, Nature 535, 382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rondinelli B., Gogola E., Yücel H., Duarte A. A., van de Ven M., van der Sluijs R., Konstantinopoulos P. A., Jonkers J., Ceccaldi R., Rottenberg S., D'Andrea A. D. (2017) EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat. Cell Biol. 19, 1371–1378 [DOI] [PubMed] [Google Scholar]
- 34. Higgs M. R., Reynolds J. J., Winczura A., Blackford A. N., Borel V., Miller E. S., Zlatanou A., Nieminuszczy J., Ryan E. L., Davies N. J., Stankovic T., Boulton S. J., Niedzwiedz W., Stewart G. S. (2015) BOD1L is required to suppress deleterious resection of stressed replication forks. Mol. Cell. 59, 462–477 [DOI] [PubMed] [Google Scholar]
- 35. Hoshii T., Cifani P., Feng Z., Huang C. H., Koche R., Chen C. W., Delaney C. D., Lowe S. W., Kentsis A., Armstrong S. A. (2018) A non-catalytic function of SETD1A regulates cyclin K and the DNA damage response. Cell 172, 1007–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oliviero G., Munawar N., Watson A., Streubel G., Manning G., Bardwell V., Bracken A. P., Cagney G. (2015) The variant polycomb repressor complex 1 component PCGF1 interacts with a pluripotency sub-network that includes DPPA4, a regulator of embryogenesis. Sci Rep. 5, 18388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soares L. M., Radman-Livaja M., Lin S. G., Rando O. J., Buratowski S. (2014) Feedback control of Set1 protein levels is important for proper H3K4 methylation patterns. Cell Rep. 6, 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD012549. Annotated MS/MS spectra can be accessed through MS-Viewer (38) with the search keys: a0vbclfehx and pl5impwjdo.