Abstract
Background
Left ventricular assist devices (LVAD) are increasingly used in patients with advanced heart failure, many of whom have been or will be implanted with an implantable cardioverter defibrillator (ICD). Interaction between both devices is a matter of concern. Subcutaneous ICD (S-ICD) obtains its signals through subcutaneous vectors, which poses special challenges with regards to adequate performance following LVAD implantation.
Case summary
We describe the case of a 24-year-old man implanted with an S-ICD because of idiopathic dilated cardiomyopathy, severe biventricular dysfunction, and self-limiting sustained ventricular tachycardias. After the implantation of a HeartMate 3™ (Left Ventricular Assist System, Abbott) several months later, the S-ICD became useless because of inappropriate sensing due to electromagnetic interference and attenuation of QRS voltage.
Discussion
We reviewed the reported cases in PubMed about the concomitant use of S-ICD and LVAD. Seven case reports about the performance of S-ICD in patients with an LVAD were identified, with discordant results. From these articles, we analyse the potential causes for these differing results. Pump location and operating rates in LVAD, as well as changes in the subcutaneous-electrocardiogram detected by the S-ICD after LVAD implantation are related to sensing disturbances when used in the same patient.
Keywords: Left ventricular assist devices, Subcutaneous implantable cardioverter defibrillator, Electromagnetic interferences, Undersensing, Case report
Learning points
Subcutaneous ICD (S-ICD) sensing may be compromised after left ventricular assist device (LVAD) implantation due to electromagnetic interference and changes in QRS voltage.
Subcutaneous ICD implantation in LVAD potential candidates should be avoided until further data can prove its safety and efficacy.
When an LVAD is implanted in an S-ICD recipient, the defibrillator should be deactivated until a new evaluation of sensing vectors demonstrates appropriate detection.
Introduction
Left ventricular assist devices (LVAD) are being increasingly used in patients with end-stage heart failure, both as bridge to transplantation and destination therapy. Many of these patients are or will be recipients of an implantable cardioverter defibrillator (ICD). The interaction between both cardiac electronic devices represents a new source of potential pitfalls in the management of this growing population. Recent reviews have addressed this issue,1,2 but most available data relate to transvenous ICD.
The entirely subcutaneous ICD (S-ICD) has emerged as an alternative to transvenous ICD aimed to avoid lead-related complications.3,4 As a consequence, recent Guidelines for Sudden Death Prevention5 recommend the use of S-ICD in patients in whom pacing for bradycardia or ventricular tachycardia termination is neither needed nor anticipated especially in patients with a high risk of infection or unsuitable vascular access. However, no limitations regarding S-ICD indication in patients with an implanted LVAD or in candidates to receive these devices are mentioned.
We describe a case of S-ICD sensing dysfunction after the implantation of an LVAD. Moreover, we conducted a systematic search in PubMed and identified seven case reports dealing with S-ICD behaviour in patients with an LVAD.6–12
Timeline
| Date | Event |
| May 2014 | Heart failure, idiopatic dilated cardiomyopathy diagnosis |
| January 2017 (Hospital inpatient) | Heart failure, sustained self-limiting ventricular tachycardia, right and left ventricular thrombus. Negative gene study. |
| February 2017 | EMBLEM MRI S-ICD™ implantation |
| January 2018 | Refractory heart failure, inotropic dependence. |
| March 2018 (Hospital inpatient) | HeartMate 3™ ventricular assist implantation. Subcutaneous implantable cardioverter defibrillator (S-ICD) deactivated before surgery. After surgery S-ICD checked showing inappropriate sensing |
| June 2018 (Hospital discharge) | Pre-discharge S-ICD checked again, showing inappropriate sensing. Remained deactivated |
| September 2018 | Heart transplant. S-ICD explantation |
Case presentation
A 20-year-old man was diagnosed with congestive heart failure due to idiopathic dilated cardiomyopathy in 2014. He remained stable under medical treatment with enalapril, bisoprolol, and eplerenone until January 2017, when he was admitted because of worsening of heart failure, severe biventricular dysfunction and self-limiting sustained ventricular tachycardias. He was referred in February 2017 for ICD implantation and included on the cardiac transplant waiting list due to advanced heart failure status, in spite of treatment with furosemide, sacubitril/valsartan, bisoprolol, eplerenone, and chlorthalidone.
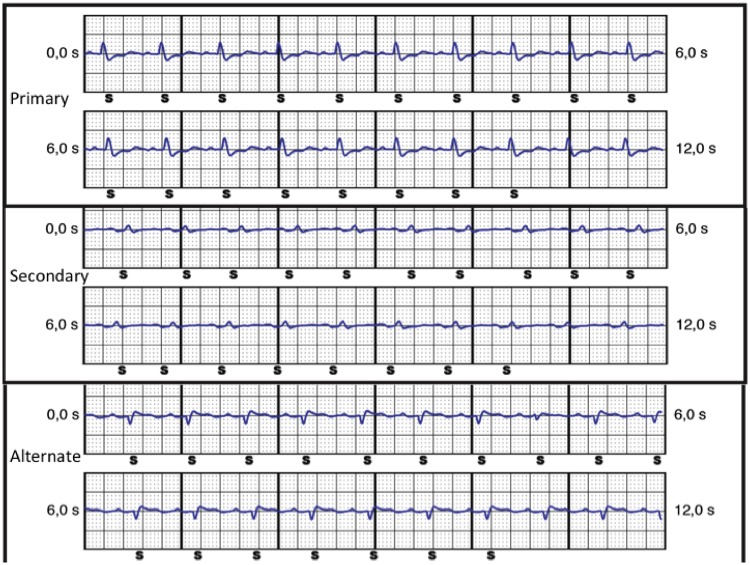
An EMBLEM MRI S-ICD™ (Boston Scientific) was implanted due to the presence of a thrombus in the right ventricle, after successful surface electrocardiogram (ECG) screening. At implant, ventricular detection test showed appropriate sensing in primary and alternate vectors. Secondary vector showed P wave—instead of QRS—detection due to low QRS amplitude (Figure 1). Post-operative course was uneventful, and the patient was discharged from the hospital the following day.
Figure 1.
Electrograms recorded by the subcutaneous implantable cardioverter defibrillator at implant, showing appropriate QRS sensing (S) in primary and alternate vectors, but P-wave oversensing (S) in the secondary vector. Automatic setup selected the primary vector for detection.
The patient was admitted to hospital again in January 2018 because of congestive heart failure exacerbation. Physical examination showed undetectable blood pressure, jugular venous pressure of 10 cm, painful hepatomegaly of 2 cm, and a light oedema in both ankles. The heart rate was 98 b.p.m. without murmurs or extra cardiac sounds. He was treated with dobutamine and then with a 24-h cycle of levosimendan, with a good response, and discharged after 7 days. He was then closely followed in the outpatient clinic but after several ambulatory intermittent levosimendan cycles, a new admission was needed 20 days later because of clinical and analytical worsening (weight gain, oedemas, hypotension, exertional epigastralgia, and serum creatinine 2.3 mg/dL). After a careful echocardiographic and haemodynamic evaluation under low dose of dobutamine due to inotropic dependence, a HeartMate 3™ (Left Ventricular Assist System, Abbott) device was indicated as bridge to transplantation.
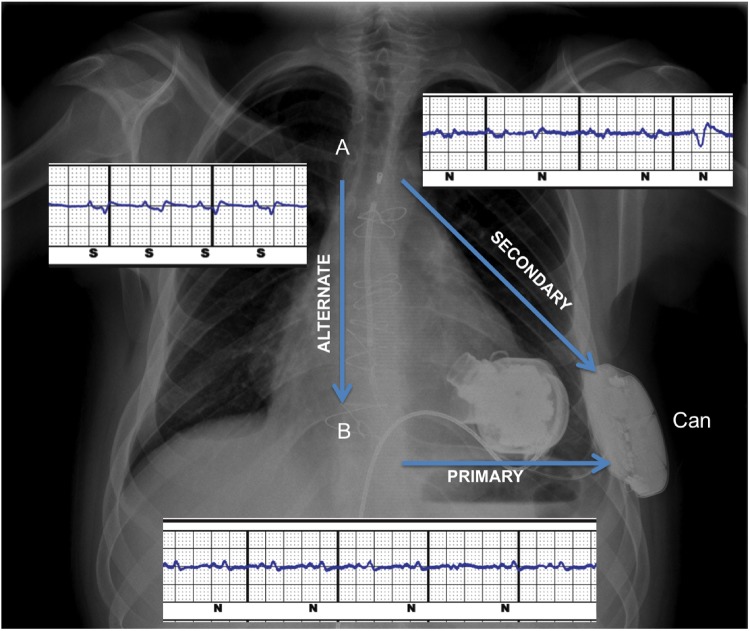
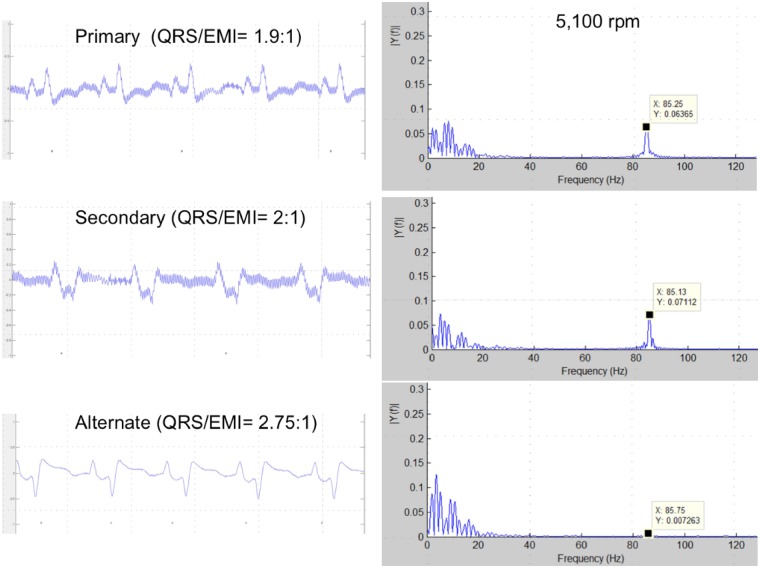
The S-ICD was deactivated before surgery and checked post-implantation, showing failure to appropriately sense QRS signals in all three vectors (Figure 2). Primary and secondary vectors showed an artefact due to electromagnetic interference (EMI) from the LVAD. Furthermore, low-voltage QRS and, intermittently, P waves were classified as noise (‘N’). The alternate vector did not show any apparent EMI, but the device classified P wave as sensed ventricular activity (‘S’) while QRS were undersensed (Figure 2). A MATLAB software-generated Fast-Fourier Transform plot confirmed the detection of a peak of frequency around 90 Hz correlated to HeartMate 3™ operational rate (5100 rpm) (Figure 3).
Figure 2.
Post-operative chest X-ray, showing HeartMate 3™ and subcutaneous implantable cardioverter defibrillator in place. The subcutaneous implantable cardioverter defibrillator lead is neither displaced nor disrupted. Electrograms recorded in the three sensing vectors (arrows) following left ventricular assist device implantation are displayed. An electromagnetic interference can be observed in primary and secondary vectors, as well as a decrease in QRS voltage in the primary vector.
Figure 3.
Left: high-resolution capture displaying electromagnetic interference in the electrograms recorded from the three vectors. The ratio QRS/electromagnetic interference amplitude is higher in the alternate vector, making electromagnetic interference detection less likely. Right: frequency distribution of the same signals in a Fast-Fourier transform plot. Cardiac signal fundamental frequency and its harmonics are in the 0–20 Hz range. Plots displaying the distribution of detected frequencies in primary and secondary vectors show also a peak of frequency around 85 Hz generated by pump activity at 5100 rpm, detectable despite band-pass filter. In the alternate vector plot, an 85 Hz peak frequency is also present, but with lower amplitude.
Both, EMI detection and QRS signal attenuation overrided an appropriate cardiac rhythm sensing, rendering the S-ICD useless. As a good tolerance of ventricular arrhythmias has been reported in LVAD recipients,13 the patient was discharged with the S-ICD deactivated after an exhaustive information. Three months later, the patient received a heart transplant with an uneventful post-operative course.
Discussion
Two cases of HeartWare® (HVAD, HeartWare International) implantation6,11 have been reported in patients with previously implanted S-ICD. In both, EMI were visible in primary and secondary vectors and QRS was classified as noise, while the alternate vector did not display EMI, similarly to our case.
The two published reports of coexisting S-ICD and HeartMate II™ (Left Ventricular Assist System, Abbott),7,8 describe appropriate sensing in all three vectors, with no EMI.
A single case of S-ICD in a patient with a Jarvik 2000® Ventricular Assist Device (Jarvik Heart)10 reported appropriate sensing in all three vectors.
Electromagnetic interference oversensing triggering multiple shocks after HeartMate 3™ implant has been described in two reports.9,12 In both cases, EMI was not observed in the alternate vector, but QRS voltage had decreased and become undetectable in all vectors. Table 1 summarizes the main data drawn from previous reports and from our case.
Table 1.
Summarizes the main data drawn from previous reports and from our case
| S-ICD indication | LVAD type | Operating rate (rpm) | Cycles/s (Hz) | Pump placement | EMI | Usefulness S-ICD | |
|---|---|---|---|---|---|---|---|
| Saeed et al.6 | Before LVAD NIDCM | HeartWare HVAD® | 2500 | 41.66 | Thorax | Yes | No |
| Ahmed et al.11 | Before LVAD NIDCM | HeartWare HVAD® | 2800 | 46.67 | Thorax | Yes | No |
| Gupta et al.7 | After LVAD T-ICD extraction | HeartMate II™ | 9800 | 163.33 | Abdomen | No | Yes |
| Raman et al.8 | After LVAD NIDCM NSVT | HeartMate II™ | 6000–10 000a | 100–166.66 | Abdomen | No | Yes |
| Migliore et al.10 | Before LVAD T-ICD extraction | Jarvik 2000® | NA (7000–13 000)a | 116.66–216.66 | Thorax | No | Yes |
| Pfeffer et al.9 | Before LVAD NIDCM | HeartMate 3™ | NA (2000–5500)a | 33.33–91.66 | Thorax | Yes | No |
| Saini et al.12 | Before LVAD NIDCM | HeartMate 3™ | NA (2000–5500)a | 33.33–91.66 | Thorax | Yes | No |
| López-Gil et al. (this manuscript) | Before LVAD NIDC NSVT | HeartMate 3™ | 5100 | 85 | Thorax | Yes | No |
Parenthesis values are operating rate range of the specific LVAD when not specified in the report.
After LVAD, the S-ICD was implanted after the LVAD; Before LVAD, the S-ICD was implanted before the LVAD; EMI, electromagnetic interference; Hz, rpm/60; LVAD, left ventricular assist device; NA, non-available; NIDCM, non-ischaemic dilated cardiomyopathy; NSVT, non-sustained ventricular tachycardia; S-ICD, subcutaneous implantable cardiac defibrillator; T-ICD, transvenous ICD.
The data of these reported cases provide interesting information about the interaction between S-ICD and the different types of LVAD, suggesting two factors that may compromise adequate S-ICD performance following LVAD implantation: EMI detection and changes in the voltage of cardiac signals.
Electromagnetic interference were not observed in patients with HeartMate II™ and Jarvik 2000®7,8,10 as opposed to patients with HeartWare®6,11 and HeartMate 3™.9,12 S-ICD algorithms for EMI discrimination have not been modified in the three generations of this device, suggesting that the occurrence of disturbances in S-ICD sensing depends on the type of LVAD implanted.
The S-ICD detection system incorporates three possible sensing vectors (Figure 2). An automatic or manual setup selects the best sensing vector for detection, storing a reference subcutaneous-ECG (S-ECG) that will be used for the S-ICD system detection algorithm.3
The device detects all signal events and makes dynamic adjustments in sensitivity in order to detect both, normal cardiac rhythm and ventricular fibrillation. A band-pass filter allows signals between 3 and 40 Hz to pass on for QRS detection, attenuating potential non-cardiac signals. A digital notch filter at 50 or 60 Hz (depending on time zone selected) is also implemented, filtering out the typical frequency of the electrical current. Non-cardiac signals overimposed upon the S-ECG are labelled as noise (N). When a signal is classified as noise, it will not be taking into account in the detection process (Figure 2).14
The S-ICD band-pass and the notch filters attenuate non-cardiac signals, though they do not eliminate them completely, especially those having relatively high amplitude (Figure 3). Therefore, the occurrence of EMI detection from the LVAD by an S-ICD depends on the frequency (Hz) and signal amplitude. HeartMate II™ and Jarvik 2000® work at high rates while HeartWare® and HeartMate 3™ operate at lower rates, generating a signal with a main frequency bordering band-pass filters (Table 1).
The amplitude of the signal detected by S-ICD generated from the LVAD depends on the proximity of the source to the detection vectors. All cases with EMI detection occurred in the primary and secondary vectors, involving the S-ICD generator, but never in the alternate (Figure 2).
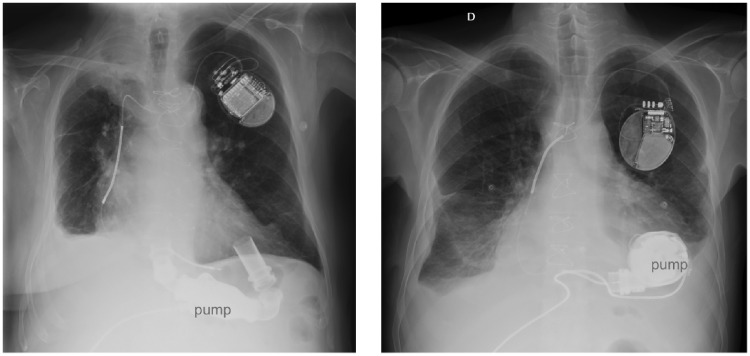
All reported patients with EMI detection had thoracic LVAD pump location,6,9,11,12 while two out of three cases with a correct cardiac signal detection had a Heart Mate II™, which pump is contained in a subdiaphragmatic pocket (Figure 4).7,8 A patient with a Jarvik 2000®,10 with the pump located in the thorax, did sense correctly. Noteworthy, this latter device runs at a very high rate, which makes sensing of EMI less likely.
Figure 4.
Chest X-ray in two patients with left ventricular assist device and conventional implantable cardioverter defibrillator. Left: a Heart Mate II™ device, with the pump in abdominal position. Right: a HeartMate 3™, with the pump in a thoracic pocket.
In the two published cases of inappropriate shocks due to EMI detection,9,12 it is remarkable that the QRS was totally missing9 or very diminished12 in the selected sensing vector. In the absence of a detectable cardiac signal, the S-ICD automatic gain control makes detection of EMI possible despite its very low voltage.
Finally, a QRS signal attenuation in sensing vectors after LVAD implantation is a common finding.6,9,11,12 Two recent studies describe a decrease in QRS voltage in patients with a HeartMate II™,15,16 and HeartWare®,16 drawing attention to the impact that this changes could have on S-ICD detection.
Conclusion
S-ICD sensing can be adversely affected by EMI detection and QRS signal attenuation following HeartWare® or HeartMate 3™ implantation. Jarvik 2000® and HeartMate II™ devices seem to be compatible with an adequate S-ICD performance, although ECG changes described after HeartMate II™ implantation could also compromise S-ICD sensing.
S-ICD implantation should be avoided in LVAD potential candidates until further data can prove its safety and efficacy. If an LVAD is indicated in a patient with an S-ICD already implanted, the defibrillator should be deactivated until a new evaluation of sensing vectors demonstrates appropriate detection in at least one vector in several postures.
Future changes in S-ICD detection algorithms and improvements in their programmability are desirable and could help to overcome current limitations.
Lead author biography

Dr María López-Gil is currently developing her professional career as head of the Arrhythmia Unit (Cardiology Department) in the University Hospital “12 de octubre” (Madrid, Spain). She has worked in the field of Cardiac Electrophysiology and Pacing since 1990. Her area of interest includes catheter ablation of atrial and ventricular arrhythmias, as well as ablation of arrhythmias in paediatric patients. She has also expertise in Cardiac Implantable Devices, especially in Cardiac Resynchronization Therapy.
Supplementary Material
Acknowledgements
The authors acknowledge the technical support provided by Sergio Sáenz (Boston Scientific Corporation) and the involvement and valuable input in clinical case of María Dolores García-Cosío M.D. (Heart Failure and Heart Transplantation Program) and Enrique Pérez de la Sota M.D. (Cardiac Surgery Department) 12 de Octubre University Hospital.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: M.L.-G. and A.F. report consultant fee honoraria from Boston Scientific. All other authors declared no conflict of interest.
References
- 1. Parikh V, Sauer A, Friedman P, Sheldon SH.. Management of cardiac implantable electronic devices in the presence of left ventricular assist devices. Heart Rhythm 2018;15:1089–1096. [DOI] [PubMed] [Google Scholar]
- 2. Berg DD, Vaduganathan M, Upadhyay GA, Singh JP, Mehra MR, Stewart GC.. Cardiac implantable electronic devices in patients with left ventricular assist systems. J Am Coll Cardiol 2018;71:1483–1493. [DOI] [PubMed] [Google Scholar]
- 3. Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT, Fynn SP, Murgatroyd FD, Sperzel J, Neuzner J, Spitzer SG, Ardashev AV, Oduro A, Boersma L, Maass AH, Van Gelder IC, Wilde AA, van Dessel PF, Knops RE, Barr CS, Lupo P, Cappato R, Grace AA.. An entirely subcutaneous implantable cardioverter–defibrillator. N Engl J Med 2010;363:36–44. [DOI] [PubMed] [Google Scholar]
- 4. Bettin M, Larbig R, Rath B, Fischer A, Frommeyer G, Reinke F, Köbe J, Eckardt L.. Long-term experience with the subcutaneous implantable cardioverter-defibrillator in teenagers and young adults. JACC Clin Electrophysiol 2017;3:1499–1506. [DOI] [PubMed] [Google Scholar]
- 5. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL.. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Heart Rhythm 2018;15:e73–e189. [DOI] [PubMed] [Google Scholar]
- 6. Saeed D, Albert A, Westenfeld R, Maxhera B, Gramsch-Zabel H, O’Connor S, Lichtenberg A, Winter J.. Left ventricular assist device in a patient with a concomitant subcutaneous implantable cardioverter defibrillator. Circ Arrhythmia Electrophysiol 2013;6:e32–e33. [DOI] [PubMed] [Google Scholar]
- 7. Gupta A, Subzposh F, Hankins SR, Kutalek SP.. Subcutaneous implantable cardioverter-defibrillator implantation in a patient with a left ventricular assist device already in place. Tex Heart Inst J 2015;42:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raman AS, Shabari FR, Kar B, Loyalka P, Hariharan R.. No electromagnetic interference occurred in a patient with a HeartMate II left ventricular assist system and a subcutaneous implantable cardioverter-defibrillator. Tex Heart Inst J 2016;43:183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfeffer TJ, König T, Duncker D, Michalski R, Hohmann S, Oswald H, Schmitto JD, Veltmann C.. Subcutaneous implantable cardioverter-defibrillator shocks after left ventricular assist device implantation. Circ Arrhythm Electrophysiol 2016;9:1–3. [DOI] [PubMed] [Google Scholar]
- 10. Migliore F, Leoni L, Bottio T, Siciliano M, Ferretto S, Gerosa G, Iliceto S, Bertaglia E.. Subcutaneous implantable cardioverter-defibrillator and left ventricular assist device: a safe and effective approach for refractory heart failure. JACC Clin Electrophysiol 2016;2:246–247. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed AS, Patel PJ, Bagga S, Gilge JL, Schleeter T, Lakhani BA, Ravichandran AK, Donnelley S, Allavatam V, Prystowsky EN, Padanilam BJ.. Troubleshooting electromagnetic interference in a patient with centrifugal flow left ventricular assist device and subcutaneous implantable cardioverter defibrillator. J Cardiovasc Electrophysiol 2018;29:477–481. [DOI] [PubMed] [Google Scholar]
- 12. Saini H, Saini A, Leffler J, Eddy S, Ellenbogen KA.. Subcutaneous implantable cardioverter defibrillator (S-ICD) shocks in a patient with a left ventricular assist device. Pacing Clin Electrophysiol 2018;41:309–311. [DOI] [PubMed] [Google Scholar]
- 13. Vakil K, Kazmirczak F, Sathnur N, Adabag S, Cantillon DJ, Kiehl EL, Koene R, Cogswell R, Anand I, Roukoz H.. Implantable cardioverter-defibrillator use in patients with left ventricular assist devices: a systematic review and meta-analysis. Journal Am Coll Cardiol HF 2016;4:772–779. [DOI] [PubMed] [Google Scholar]
- 14. Karnik AA, Helm RH, Monahan KM.. Mechanisms and management of inappropriate therapy in subcutaneous implantable cardioverter defibrillators. J Cardiovasc Electrophysiol 2019;30:402–409. [DOI] [PubMed] [Google Scholar]
- 15. Martinez SC, Fansler D, Lau J, Novak EL, Joseph SM, Kleiger RE.. Characteristics of the electrocardiogram in patients with continuous-flow left ventricular assist devices. Ann Noninvasive Electrocardiol 2015;20:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zormpas C, Mueller-Leisse J, Koenig T, Schmitto JD, Veltmann C, Duncker D.. Electrocardiographic changes after implantation of a left ventricular assist device—potential implications for subcutaneous defibrillator therapy. J Electrocardiol 2019;52:29–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.