Significance
The fossil record reveals evidence of dramatic distributional shifts through time for many groups of organisms. One striking example is the early fossil record of modern birds, which shows that many bird groups currently restricted to the tropics were formerly found at high latitudes in North America and Europe. Tracking potentially suitable habitat for these clades over the last 56 million years reveals that cooling trends throughout this period may have largely dictated the geographic distributions of these “tropical” groups, complicating our understanding of where on Earth many of these lineages originated.
Keywords: climate change, niche conservatism, latitudinal diversity gradient, ecological niche modeling, historical biogeography
Abstract
Many higher level avian clades are restricted to Earth’s lower latitudes, leading to historical biogeographic reconstructions favoring a Gondwanan origin of crown birds and numerous deep subclades. However, several such “tropical-restricted” clades (TRCs) are represented by stem-lineage fossils well outside the ranges of their closest living relatives, often on northern continents. To assess the drivers of these geographic disjunctions, we combined ecological niche modeling, paleoclimate models, and the early Cenozoic fossil record to examine the influence of climatic change on avian geographic distributions over the last ∼56 million years. By modeling the distribution of suitable habitable area through time, we illustrate that most Paleogene fossil-bearing localities would have been suitable for occupancy by extant TRC representatives when their stem-lineage fossils were deposited. Potentially suitable habitat for these TRCs is inferred to have become progressively restricted toward the tropics throughout the Cenozoic, culminating in relatively narrow circumtropical distributions in the present day. Our results are consistent with coarse-scale niche conservatism at the clade level and support a scenario whereby climate change over geological timescales has largely dictated the geographic distributions of many major avian clades. The distinctive modern bias toward high avian diversity at tropical latitudes for most hierarchical taxonomic levels may therefore represent a relatively recent phenomenon, overprinting a complex biogeographic history of dramatic geographic range shifts driven by Earth’s changing climate, variable persistence, and intercontinental dispersal. Earth’s current climatic trajectory portends a return to a megathermal state, which may dramatically influence the geographic distributions of many range-restricted extant clades.
Extant avian biodiversity is represented by nearly 11,000 living species, which inhabit virtually every conceivable subaerial environment from the poles to the equator (1). However, despite the ubiquity of birds and their penchant for dispersal, extant birdlife is unequally distributed across the Earth. In particular, avian diversity—in terms of both species numbers and higher taxonomic groups—is skewed toward tropical environments on the southern continents (i.e., those that formerly composed the Mesozoic supercontinent of Gondwana).
This pattern led earlier avian historical biogeographic investigations to conclude that vicariance driven by Gondwanan breakup, which was largely completed by the end of the Mesozoic, played a predominant role in triggering deep phylogenetic and geographic divergences within crown birds (e.g., ref. 2). However, recent phylogenetic divergence time studies suggest that most deep divergences within crown birds took place after the Cretaceous–Paleogene (K–Pg) mass extinction (3–7), roughly 66.02 million years ago (8). Although Australia, Antarctica, and South America maintained connectivity into the Paleogene (9, 10), Mesozoic Gondwanan vicariance appears to have played no role in either the diversification or geographic expansion of the avian crown group. Nonetheless, analytical reconstructions of higher order avian historical biogeography invariably recover strong evidence for an origin of most modern diversity on southern landmasses (2, 6, 11).
The crown bird fossil record has unique potential to reveal where different groups of birds were formerly distributed in deep time. Fossil evidence, for example, has long indicated that total-group representatives of clades restricted to relatively narrow geographic regions today were formerly found in different parts of the world (12–23). In particular, the Paleogene record of fossil birds has yielded abundant evidence that many extant clades restricted to southern landmasses had fossil stem-group representatives in the Northern Hemisphere (e.g., refs. 11–13, 15, 19, 21, and 24–38). Collectively, such biogeographic disjunctions between early stem-group representatives and extant taxa cloud our ability to infer ancestral ranges for the deepest crown bird subclades. The general sparseness and Northern Hemisphere bias of the avian fossil record, however, has limited attempts to incorporate bird fossils into large-scale hypotheses of avian biogeographic evolution. Even studies that have integrated phylogenetically constrained avian fossils into analytical reconstructions of ancestral biogeography (6) have been criticized for effectively “swamping out” information from the fossil record by virtue of the limited amount of fossil data compared with extant data in such analyses (39). Thus, ancestral biogeographic reconstructions may have limited potential to reveal whether modern geographic distributions of avian higher clades are truly reflective of their areas of origin or, instead, obscure a history of profound biogeographic shifts throughout their evolutionary history.
The avian fossil record reveals information on where early representatives of various lineages were found and, just as importantly, when in Earth history these birds lived. Paleontological evidence for major fluctuations in avian historical biogeographic patterns therefore raises questions about the extent to which historical factors, such as Cenozoic climatic change, may have been responsible for driving historical shifts in avian geographic range, as has been demonstrated for ectothermic clades, such as turtles (40), and dismissed as a factor influencing the demise of nonavian dinosaurs in the Late Cretaceous (41). Here, we integrate both past and present avian distributional data and climate characterizations to model how habitable regions for 10 neornithine higher level clades (Fig. 1) have changed throughout the Cenozoic. We test whether we can predict the presence of high-latitude Paleogene fossil occurrences of these 10 clades, which are currently restricted to tropical and subtropical latitudes, assuming climatic niche conservatism and given estimates of paleoclimate. On the basis of our analyses, we suggest that climatic changes have played a major role in forcing range contractions for all of these major “tropical” clades toward their present-day geographic distributions. Our results have important implications for the study of avian historical biogeography in deep time and that of other vagile, climatically sensitive clades.
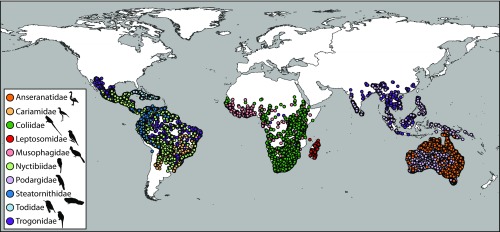
Fig. 1.
Present-day occurrences for the 10 neornithine clades studied. Geographic ranges are circumtropical and predominantly restricted to vestiges of Gondwana (Africa, South America, and Australasia).
Results
We modeled suitable habitat for 10 neornithine higher level clades using Maxent (42). The number of environmentally unique occurrences used in model calibration ranged from 103 (Leptosomidae) to 9,545 (Trogonidae) (SI Appendix, Table S1). Model verification exercises suggest that Maxent models of clade tolerances were statistically significant (P value < 0.05) (SI Appendix, Table S1). The discriminatory capacity of the model was evaluated using area under the curve scores; all scores were high and ranged from 0.73 (Trogonidae) to 0.97 (Leptosomidae and Steatornithidae; SI Appendix, Table S1).
Suitable conditions were modeled for each clade in the present; these models were then transferred (projected) onto estimates of past climate for four Paleogene time periods with avian fossil records: Ypresian (∼56 to 47.8 Ma), Priabonian (∼38 to 33.9 Ma), Rupelian (∼33.9 to 28.1 Ma), and Chattian (∼28.1 to 23.03 Ma) (SI Appendix, Figs. S1–S20). We then evaluated whether these paleo-projections correctly predicted penecontemporaneous fossil occurrences for each total clade (Fig. 2 and SI Appendix, Figs. S21–S30). Of 19 Paleogene clade/locality occurrences investigated, only 4 were not predicted as highly suitable by our ecological models (Fig. 2 and SI Appendix, Figs. S21–S30 and Table S2). Virtually all Ypresian-aged fossil localities were predicted as suitable, but more recent (Priabonian to Chattian) fossil occurrences were predicted with less fidelity (SI Appendix, Table S2). Even so, all younger fossil occurrences aside from one (Todidae) were predicted as suitable under at least one paleo-plate and threshold model, and, when suitable habitat was not predicted by our ecological models, it was usually (∼60%) found within only 150 km of a clade-specific Paleogene fossil locality—potentially within levels of paleo-plate reconstruction uncertainty (SI Appendix, Table S2).
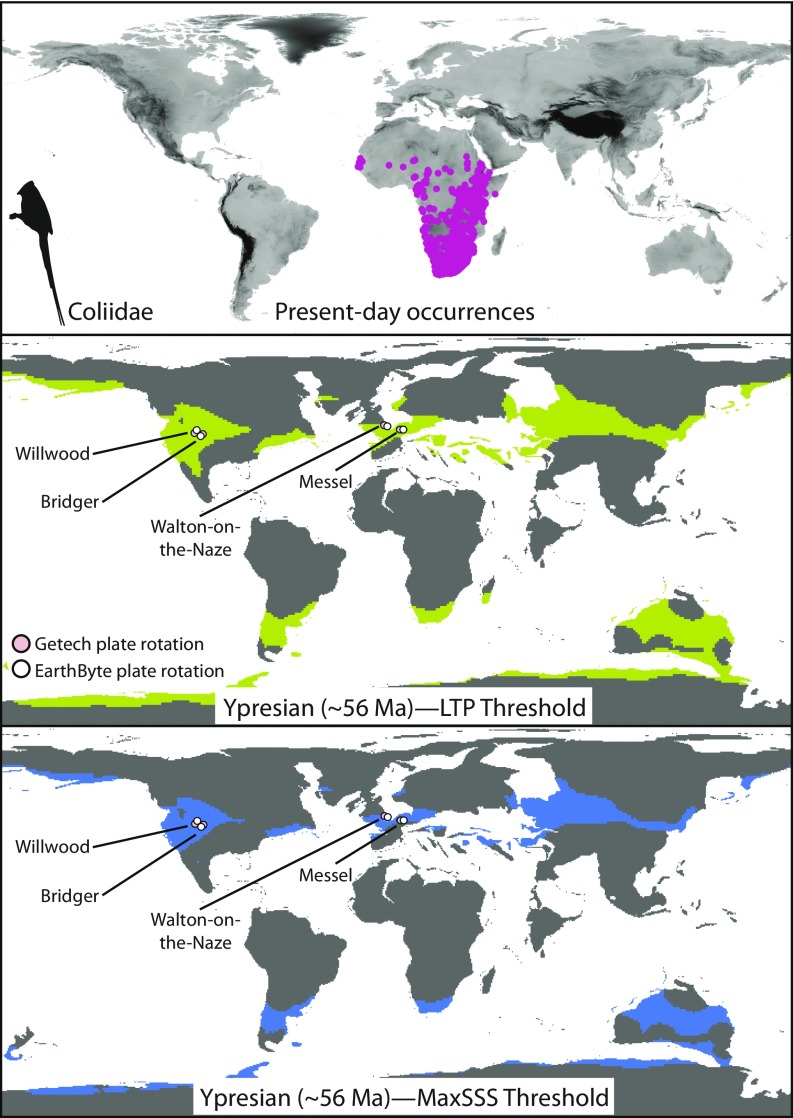
Fig. 2.
Present-day occurrences (Top) for Coliidae as derived from GBIF. Using these occurrences, models of abiotic tolerances for this clade were projected onto estimates of past climate conditions at the Ypresian, an interval when numerous fossil representatives of this group were deposited. Note the accurate correspondence between fossil localities (shown on map) and model predictions. Maps are shown for both the least training presence (LTP; green) (Middle) and MaxSSS (blue) (Bottom) threshold methods; pink and white occurrences represent Getech and EarthByte paleo-plate rotational models, respectively (see Methods for details). For other clades, see SI Appendix, Figs. S21–S30.
Our ability to predict fossil occurrences was not dependent on the geographic extent of estimated suitable habitat for a given time slice and clade. That is, predicted suitable habitat ranged from only 3.75% [Todidae, maximizing the sum of sensitivity and specificity (MaxSSS) threshold] to 23.61% [Podargidae, least training presence (LTP) threshold] of terrestrial areas globally in fossil-bearing time periods (SI Appendix, Table S2). We did not predict suitable conditions for any of these clades at fossil localities in the present day, with the exception of two of the five Coliidae localities (Messel and Walton-on-the-Naze). The relatively broad climatic tolerances of Coliidae may help explain their comparatively late persistence into the Miocene of Europe (22), suggesting that they may have been less susceptible to climate-driven range contraction than the other clades examined (43).
Ecological models were significantly better at predicting fossil occurrences than random expectations based on binomial tests (P < 0.05), regardless of threshold or paleo-plate model choice, except for Leptosomidae (significant at α = 0.096 for LTP threshold, and α = 0.078 for MaxSSS threshold) and Todidae, for which models failed to predict fossil occurrences. Estimates of suitable habitat were more restricted when using the MaxSSS threshold (versus LTP threshold), resulting in fewer predicted occurrences for this threshold method (SI Appendix, Table S2). The restricted geographic distributions predicted for Leptosomidae and Todidae in the Paleogene are likely a result of their especially narrow present-day distributions, which may complicate ecological modeling (44): Leptosomidae are found only in Madagascar, Mayotte, and the neighboring Comoro Islands while Todidae are endemic to the Greater Antilles and small adjacent islands (45).
Novel environmental combinations can be encountered when projecting ecological models to different regions and/or time periods (46). In these instances, it is difficult to determine, using correlative approaches, whether these unique climatic conditions would be suitable for occupancy by species and higher clades. Although areas predicted as suitable in our paleo-projections were unaffected by novel climate combinations, novel climates were estimated at low latitudes (from approximately ±23° latitude) from the Ypresian to the Chattian. These novel combinations derived primarily from warmer minimum and maximum monthly temperatures estimated for the Paleogene than exist today. Therefore, whether tropical latitudes would have been suitable for these clades in deep time, especially given estimates of extreme Eocene warmth, remains an open question (47). Discoveries of Paleogene fossil birds from low latitudes will be of major importance for clarifying the composition of tropical avian communities in deep time (12).
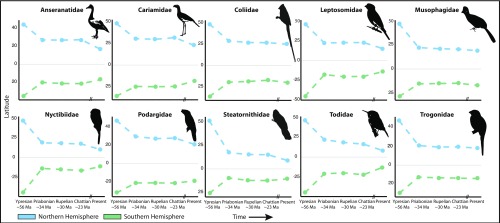
Most suitable area in the Ypresian was inferred at fairly high latitudes (∼40° to 50°), with the bulk of suitable habitat shifting equatorward in a stepwise manner toward the present (Fig. 3). This pattern is evident across all examined clades, with the sharpest contractions of habitable distributions coinciding with the Eocene–Oligocene transition (48, 49), and the next sharpest occurring in the Neogene (Fig. 3 and SI Appendix, Fig. S31). As a result, the latitudinal centroid of estimated suitable habitat for each clade moved equatorward through time (Fig. 3 and SI Appendix, Fig. S31).
Fig. 3.
Temporal shifts in the centroid of suitable habitat for each clade. Binary suitability maps were converted to polygons and used to find the “center of mass” for areas presenting suitable conditions in each time slice; Northern (blue) and Southern (green) Hemispheres were calculated separately. Results are shown for the least training presence (LTP) threshold; see SI Appendix, Fig. S31 for MaxSSS threshold results.
Discussion
Models of suitable conditions for neornithine clades calibrated using modern-day distributional and climate data were able to accurately predict the distributions of these clades’ fossil stem-group representatives through the Paleogene, a critical interval in avian evolutionary history, during which many of the deepest neornithine phylogenetic divergences are inferred to have taken place (4–6). This predictive ability was not predicated on broad estimates of the paleo-distributions for studied clades, but rather on narrow bands of suitable habitat estimated at latitudes higher than those occupied by these clades today. Indeed, across our 10 focal clades, we recovered an average difference of ∼20° latitude between centroids of suitable habitat predicted for the Eocene (Ypresian) and those predicted for the present day (Fig. 3 and SI Appendix, Fig. S31).
The importance of our results is twofold. First, our methods assumed that niche models conditioned on the modern geographic distributions of avian clades accurately encompassed the climatic tolerances of their early stem-group representatives. Although an important source of uncertainty, evidence supporting conservation of clade tolerances over evolutionary timescales has been reported in a variety of clades (50–59). It is striking that we were able to use present-day models of clade tolerances to accurately predict paleo-distributions for these clades’ stem-group relatives under conditions estimated to have occurred up to ∼56 million years ago, especially in light of the varied ecological habits and geographic distributions of the clades studied (Fig. 2). As such, our results provide first-order support for conservatism of the coarse-scale manifestations of species temperature and precipitation tolerances over geological timescales.
Second, we corroborate climate as a major long-term driver of crown neornithine biogeographic patterns (43, 60). Niche models calibrated on extant clade-level data and projected onto Ypresian to Chattian paleoclimatic reconstructions predicted shifts through time in the centroid of estimated habitable areas of more than 20° latitude for all examined clades. These analyses suggest that Cenozoic climatic change may have been predominantly responsible for driving dramatic shifts in the geographic distributions of these avian clades.
Both Cenozoic paleobiogeography (11, 12, 14, 19, 39, 61) and the timing of the extant neornithine radiation (4–7) cast doubt on Mesozoic Gondwanan vicariance as the mechanism underlying the extreme modern asymmetry of endemic higher order neornithine diversity in the Southern and Northern Hemispheres, raising questions about the origin of pervasive trans-Antarctic avian distributional patterns (2, 62).
A detailed stratigraphic analysis of the European fossil bird record suggests that the assembly of modern avian biogeographic distributions biased toward equatorial latitudes may be the product of two independent waves of geographic range contraction by avian clades throughout the Cenozoic (43). That study noted that a comparatively recent Miocene wave of European extinctions seems to have overwhelmingly affected representatives of clades that are now found in the modern Afrotropical zoogeographic zone [e.g., mousebirds (Coliiformes)] or which currently exhibit pantropical distributions [e.g., parrots (Psittaciformes) and trogons (Trogoniformes)]. In contrast, earlier Paleogene extinctions seem to have eliminated taxa whose crown-group representatives are now found in the neotropics, Madagascar, and Australasia (43). Mayr (12, 43) argued that extirpation of “tropical” European taxa during the Paleogene cannot be attributed reliably to climatic cooling during this period (contra ref. 63) since many “tropical” taxa persisted into the cooler Oligocene and even into the early Neogene. However, the extirpation of “tropical” neornithine taxa in the Paleogene of North America (e.g., ref. 11) may be more reliably attributed to ecosystem changes related to cooling throughout the Eocene (43). Our inferred patterns of long-term contraction of habitable distributions throughout the Neogene (Fig. 3) are consistent with further Miocene climatic cooling driving extirpations and equatorward range contractions (43).
“Paratropical” forests indicative of well-watered, warmer, and more equable climates, such as those persisting at lower latitudes today (64–66), were widespread across North America during the Eocene and coincided with the presence of stem taxa whose crown group representatives are now restricted to lower latitudes. The distribution of these megathermal climates (sensu ref. 67) and associated forests in North America declined substantially toward the end of the Eocene, which had a profound effect on the diversity and composition of North American mammalian and squamate communities (68–72). If many taxa comprising the Paleogene North American avifauna were adapted to megathermal conditions, the extirpation of these taxa from North America may reflect the elimination of these warmer habitats at higher latitudes, resulting in a sharpening of the latitudinal biodiversity gradient (56, 73). Under a model of phylogenetic niche conservatism, habitat tracking may result in broad-scale range constriction, across multiple clades, in response to climate change (71). Indeed, investigations of early Eocene squamate faunas from North America suggest that taxa once common at midlatitudes may have contributed substantially to populating lower latitude biotas in the present day (71). The neornithine fossil record from North America during this interval appears to corroborate this pattern, emphasizing the critical relevance of paleontological data to our understanding of the historical biogeography of extant clades (e.g., refs. 60 and 74).
Estimating the areas of origin of major extant bird clades has emerged as an especially controversial topic in contemporary bird systematics (e.g., refs. 39 and 75), despite ever-improving historical biogeographic models (e.g., refs. 76 and 77) and large-scale avian molecular phylogenies (4–6, 78, 79). We suggest that a primary focus on inferring deep-time areas of origin for major bird clades, which may not be unambiguously discernible given our present knowledge of the avian fossil record (11, 71), overlooks a more achievable goal: discerning the mechanisms that have driven avian range evolution throughout the Cenozoic. This would result in a clearer picture of how and when major avian subclades are likely to have acquired their present-day distributions. We provide quantitative evidence that protracted environmental change throughout the Cenozoic has forced the long-term, equatorward contraction of avian geographic distributions. Delineating between competing biogeographic models whereby major extant clades presently restricted to the tropics originated at low latitudes, or simply became restricted to these areas over the course of the Cenozoic, will rely on renewed focus on Cenozoic fossil avifaunas from Gondwanan continents (12, 75, 80, 81). However, such work has already recovered evidence of extant lineages with restricted distributions, such as total-clade Opisthocomiformes (represented today only by the Amazonian endemic Opisthocomus hoazin) occurring on additional Gondwanan and Laurasian continents throughout the Neogene (82, 83).
Although the early Cenozoic avian fossil record is rich (e.g., ref. 12), important temporal and geographical gaps remain. Considering our evidence for apparent avian niche conservatism and habitat tracking over geological timescales, we suggest that the application of ecological modeling tools may provide a first approximation of regions likely to have been inhabited by various bird groups through time, which may aid in guiding paleontological exploration.
Predicting the influence of human-induced climatic change on short- and long-term organismal distributions is an urgent goal in contemporary biology, and projections of major geographic range shifts in the face of Earth’s current climatic trajectory are becoming ever more common (e.g., refs. 84–86). As arguably the most vagile of the major groups of living vertebrates, birds may be more likely than others to undergo dramatic saltational shifts in their geographic distributions, evidenced by historical transoceanic colonization of new continents by extant bird species within historical memory (e.g., refs. 87 and 88). Marginalized across geographic timescales, the frequency of such stochastic dispersal events may explain the apparent habitat-tracking success of birds through the Cenozoic, provided that newly colonized areas are suitable for long-term occupancy by the pioneering species.
While explicit predictions are beyond the scope of the present work, our conclusions would seem to suggest that climatic changes over the coming decades and centuries may induce major distributional changes across the avian tree of life, as has been suggested recently for corals in the marine realm (86). The extremely rapid pace of anthropogenic climate change, however, may instead make it more likely that major groups with restricted distributions are driven to extinction in situ. Unraveling the relative likelihood of these outcomes will be an important goal of future work in avian biogeography and macroecology.
Methods
Full details of our methods are presented in SI Appendix, including details of clade selection and model caveats. Supporting data, including environmental layers used to calibrate present-day ecological niche models and median ecological niche models generated from Maxent, are archived open access at Zenodo (89).
Ecological Model Inputs.
Distributional data for each extant species within our focal clades were drawn from the Global Biodiversity Information Facility (www.gbif.org) (Fig. 1 and SI Appendix, Figs. S21–S30). To characterize present-day climatic landscapes for ecological modeling, we used four environmental variables at 5′ spatial resolution from the WorldClim bioclimatic dataset (90): maximum temperature of warmest month, minimum temperature of coldest month, precipitation of the wettest month, and precipitation of the driest month. Estimates of past climates were simulated for four time periods: Ypresian (∼56 to 47.8 Ma), Priabonian (∼37.8 to 33.9 Ma), Rupelian (∼33.9 to 28.1 Ma), and Chattian (∼28.1 to 23.03 Ma). Data were derived from Paleogene simulations produced by two general circulation models (GCMs): FAMOUS (91) and HadCM3L (92, 93).
Ecological Modeling.
Clade tolerances were quantified using Maxent v.3.3.3k, a maximum entropy algorithm that estimates suitable environmental combinations for species under a null expectation that suitability is proportional to availability (42). We used present-day environmental conditions to constrain clade tolerances, and resulting models were then projected onto Eocene and Oligocene climatic conditions to estimate the geographic regions that would have been suitable for these clades from the Ypresian through the Chattian. Resulting ecological models produced estimates of suitable abiotic conditions for clades based on present-day climatic characterizations, without consideration of dispersal or biotic constraints.
Postmodeling Analyses.
We assessed the ability of paleo-projections of suitable habitat to correctly predict fossil occurrence localities. The correspondence between fossil sites and paleo-projections was analyzed as follows: Fossil sites were transformed (paleo-rotated) so that they reflected their geographical position during the period in which they were deposited. Two paleo-plate models were used for transformations: Getech (93) and EarthByte via the PaleoGIS extension for ArcGIS (94). Localities were accorded a buffer of 25 km using the “gBuffer” function in the “rgeos” package for R (95). Localities were buffered to account for uncertainty in both paleo-plate rotations and georeferencing and to reflect the minimum likely area the fossil would have occupied when extant. These buffered localities were then intersected with the suitable area predicted for the time period corresponding to the age of the fossil site, using a custom script written in R.
We assessed the probability of randomly predicting fossil occurrences for each clade in each time slice using binomial tests (96). Analyses were performed for each clade characterized by more than one occurrence in a given time slice, using the following parameters: n = the number of successfully predicted occurrences, K = the total number of occurrences, and P = the probability of successfully predicting an occurrence, defined by the percentage of predicted suitable terrestrial area globally.
Temporal shifts in the centroid of suitable habitat predicted for each clade were calculated using the “gCentroid” function in the “rgeos” package for R (95). The binary suitability maps were converted to polygons, and these polygons were used to find the “center of mass” (also known as “true centroid”) of the areas presenting suitable conditions for each time slice; Northern and Southern Hemispheres were calculated separately (Fig. 3 and SI Appendix, Fig. S31).
Supplementary Material
Acknowledgments
We thank Michelle Casey (Towson University) for advice on paleo-plate rotation models. E.E.S. was supported by a Division of Earth Sciences National Science Foundation Postdoctoral Fellowship and Leverhulme Grant DGR01020. A.F. and D.J.L. were funded by United Kingdom National Environmental Research Council Grants NE/K014757/1, NE/I005722/1, NE/I005714/1, and NE/P013805/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data from this study have been deposited on the online data archive Zenodo (DOI: 10.5281/zenodo.2658119).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903866116/-/DCSupplemental.
References
- 1.Gill F., Donsker D., Eds., IOC World Bird List (Version 9.1, International Ornithological Congress, 2019), 10.14344/IOC.ML.9.1. [Google Scholar]
- 2.Cracraft J., Avian evolution, Gondwana biogeography and the Cretaceous-Tertiary mass extinction event. Proc. Biol. Sci. 268, 459–469 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ericson P. G. P., et al. , Diversification of neoaves: Integration of molecular sequence data and fossils. Biol. Lett. 2, 543–547 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis E. D., et al. , Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346, 1320–1331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prum R. O., et al. , A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Claramunt S., Cracraft J., A new time tree reveals Earth history’s imprint on the evolution of modern birds. Sci. Adv. 1, e1501005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berv J. S., Field D. J., Genomic signature of an Avian Lilliput effect across the K-Pg extinction. Syst. Biol. 67, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clyde W. C., Ramezani J., Johnson K. R., Bowring S. A., Jones M. M., Direct high-precision U–Pb geochronology of the end-Cretaceous extinction and calibration of Paleocene astronomical timescales. Earth Planet. Sci. Lett. 452, 272–280 (2016). [Google Scholar]
- 9.Scher H. D., Martin E. E., Timing and climatic consequences of the opening of Drake passage. Science 312, 428–430 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Livermore R., Hillenbrand C. D., Meredith M., Eagles G., Drake passage and Cenozoic climate: An open and shut case? Geochem. Geophys. Geosyst. 8, Q01005 (2007). [Google Scholar]
- 11.Field D. J., Hsiang A. Y., A North American stem turaco, and the complex biogeographic history of modern birds. BMC Evol. Biol. 18, 102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayr G., Paleogene Fossil Birds (Springer, Berlin, 2009), p. 262. [Google Scholar]
- 13.Mourer-Chauviré C., Les oiseaux fossiles des phosphorites du Quercy (Éocène supérieur a Oligocène supérieur): Implications paléobiogéographiques. Geobios 15 (suppl. 1), 413–426 (1982). [Google Scholar]
- 14.Olson S. L., “Aspects of global avifaunal dynamics during the Cenozoic” in Acta XIX Congressus Internationalis Ornithologici, Ouellet H., Ed. (University of Ottawa Press, Ottawa, 1989), vol. 2, pp. 2023–2029. [Google Scholar]
- 15.Mayr G., “Avian remains from the Middle Eocene of the Geiseltal (Sachsen-Anhalt, Germany)” in Proceedings of the 5th symposium of the Society of Avian Paleontology and Evolution: Beijing, 1–4 June 2000, Zhou Z., Zhang F., Eds. (Science Press, Beijing, 2002), pp. 77–96. [Google Scholar]
- 16.Mayr G., New or previously unrecorded avian taxa from the Middle Eocene of Messel (Hessen, Germany). Mitt Mus Naturkd Berl Geowiss Reihe 3, 207–219 (2000). [Google Scholar]
- 17.Peters D. S., “Idiornis tuberculata n. spec., ein weiterer ungewöhnlicher Vogel aus der Grube Messel (Aves: Gruiformes: Cariamidae: Idiornithinae)” in Acta Palaeornithologica, Peters D. S., Ed. (Cour Forsch Inst Senckenberg, Frankfurt, 1995), vol. 181, pp. 107–119. [Google Scholar]
- 18.Mourer-Chauviré C., Les Gruiformes (Aves) des Phosphorites du Quercy (France). 1. Sous-ordre Cariamae (Cariamidae et Phorusrhacidae). Palaeovertebrata 13, 83–143 (1983). [Google Scholar]
- 19.Mourer-Chauviré C., Les relations entre les avifaunes du Tertiaire inférieur d’Europe et d’Amérique du Sud. Bull. Soc. Geol. Fr. 170, 85–90 (1999). [Google Scholar]
- 20.Mourer-Chauviré C., The avifauna of the Eocene and Oligocene Phosphorites du Quercy (France): An updated list. Strata 13, 135–149 (2006). [Google Scholar]
- 21.Ksepka D. T., Clarke J. A., Affinities of Palaeospiza bella and the phylogeny and biogeography of mousebirds (Coliiformes). Auk 126, 245–259 (2009). [Google Scholar]
- 22.Mayr G., “Birds–The most species-rich vertebrate group in Messel” in MESSEL–An Ancient Greenhouse Ecosystem, Smith K. T., Schaal S. F., Habersetzer J., Eds. (Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main, 2018), pp. 169–214. [Google Scholar]
- 23.Mayr G., The early Eocene birds of the Messel fossil site: A 48 million-year-old bird community adds a temporal perspective to the evolution of tropical avifaunas. Biol. Rev. Camb. Philos. Soc. 92, 1174–1188 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Mayr G, Peters D (1998) The mousebirds (Aves: Coliiformes) from the Middle Eocene of Grube Messel (Hessen, Germany). Senckenbergiana Lethaea 78, 179–197. [Google Scholar]
- 25.Mayr G., A new species of Plesiocathartes (Aves? Leptosomidae) from the middle Eocene of Messel, Germany. PaleoBios 22, 10–20 (2002). [Google Scholar]
- 26.Olson S. L., The anseriform relationships of Anatalavis Olson and Parris (Anseranatidae), with a new species from the lower Eocene London Clay. Smithson. Contrib. Paleobiology 89, 231–243 (1999). [Google Scholar]
- 27.Olson S. L., An early Eocene oilbird from the green river formation of Wyoming (Caprimulgiformes: Steatornithidae). Documents des Laboratoires de Géologie de Lyon 99, 57–69 (1987). [Google Scholar]
- 28.Mayr G., The Palaeogene Old world potoo Paraprefica Mayr, 1999 (Aves, Nyctibiidae): Its osteology and affinities to the new world Preficinae Olson, 1987. J. Syst. Palaeontology 3, 359–370 (2005). [Google Scholar]
- 29.Nesbitt S. J., Ksepka D. T., Clarke J. A., Podargiform affinities of the enigmatic Fluvioviridavis platyrhamphus and the early diversification of Strisores (“Caprimulgiformes” + Apodiformes). PLoS One 6, e26350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayr G., Comments on the osteology of Masillapodargus longipes Mayr 1999 and Paraprefica major Mayr 1999, caprimulgiform birds from the middle Eocene of Messel (Hessen, Germany). Neues Jahrb. Geol. Palaontol. Monatsh., 65–76 (2001). [Google Scholar]
- 31.Mourer-Chauviré C., “Les Caprimulgiformes et les Coraciiformes de l’Eocène et de l'Oligocène des Phosphorites du Quercy et description de deux genres nouveaux de Podargidae et Nyctibiidae” in Acta XIX Congressus Internationalis Ornithologici (University of Ottawa Press, Ottawa, 1989), pp. 2047–2055. [Google Scholar]
- 32.Mayr G., Knopf C., A tody (Alcediniformes, Todidae) from the early Oligocene of Germany. Auk 124, 1294–1304 (2007). [Google Scholar]
- 33.Mourer-Chauviré C., Les Todidae (Aves, Coraciiformes) des Phosphorites du Quercy (France). Proc. K. Ned. Akad. Wet. Ser. B 88, 407–414 (1985). [Google Scholar]
- 34.Olson S. L., Oligocene fossils bearing on the origins of the Todidae and the Momotidae (Aves: Coraciiformes). Smithson. Contrib. Paleobiology 27, 111–119 (1976). [Google Scholar]
- 35.Mayr G., A second skeleton of the early Oligocene trogon Primotrogon wintersteini Mayr 1999 (Aves: Trogoniformes: Trogonidae) in an unusual state of preservation. Senckenbergiana Lethaea 81, 335–338 (2001). [Google Scholar]
- 36.Mayr G., An archaeotrogon (Aves: Archaeotrogonidae) from the middle Eocene of the Grube Messel (Hessen, Germany)? J. Ornithol. 139, 121–129 (1998). [Google Scholar]
- 37.Mourer-Chauviré C., The Archaeotrogonidae of the Eocene and Oligocene phosphorites du Quercy (France). Contrib. Sci. 330, 17–31 (1980). [Google Scholar]
- 38.Ksepka D. T., Stidham T. A., Williamson T. E., Early Paleocene landbird supports rapid phylogenetic and morphological diversification of crown birds after the K-Pg mass extinction. Proc. Natl. Acad. Sci. U.S.A. 114, 8047–8052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayr G., Avian higher level biogeography: Southern Hemispheric origins or Southern Hemispheric relicts? J. Biogeogr. 44, 956–958 (2017). [Google Scholar]
- 40.Waterson A. M., et al. , Modelling the climatic niche of turtles: a deep-time perspective. Proc. R. Soc. B 283, 20161408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiarenza A. A., et al. , Ecological niche modelling does not support climatically-driven dinosaur diversity decline before the Cretaceous/Paleogene mass extinction. Nat. Commun. 10, 1091 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips S. J., Anderson R. P., Schapire R. E., Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259 (2006). [Google Scholar]
- 43.Mayr G., Two-phase extinction of “Southern Hemispheric” birds in the Cenozoic of Europe and the origin of the neotropic avifauna. Palaeobiodivers. Palaeoenviron. 91, 325–333 (2011). [Google Scholar]
- 44.Saupe E., et al. , Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecol. Modell. 237, 11–22 (2012). [Google Scholar]
- 45.Del Hoyo J., Elliot A., Sargatal J., Handbook of the Birds of the World (Lynx Editions, Barcelona, 1992). [Google Scholar]
- 46.Owens H. L., et al. , Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. J. Ecol. Modell. 263, 10–18 (2013). [Google Scholar]
- 47.Inglis G. N., et al. , Mid-latitude continental temperatures through the early Eocene in western Europe. Earth Planet. Sci. Lett. 460, 86–96 (2017). [Google Scholar]
- 48.Stehlin H. G., Remarques sur les faunules de mammifères des couches éocènes et oligocènes du Bassin de Paris. Bull. Soc. Geol. Fr. 19, 488–520 (1909). [Google Scholar]
- 49.Meng J., McKenna M. C. J. N., Faunal turnovers of Palaeogene mammals from the Mongolian plateau. Nature 394, 364 (1998). [Google Scholar]
- 50.Strubbe D., Beauchard O., Matthysen E., Niche conservatism among non‐native vertebrates in Europe and North America. Ecography 38, 321–329 (2015). [Google Scholar]
- 51.Feldman A., Sabath N., Pyron R. A., Mayrose I., Meiri S., Body sizes and diversification rates of lizards, snakes, amphisbaenians and the tuatara. Global Ecol. Biogeography 25, 187–197 (2016). [Google Scholar]
- 52.Cooper N., Freckleton R. P., Jetz W., Phylogenetic conservatism of environmental niches in mammals. Proc. R. Soc. B 278, 2384–2391 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson A. T., Soberón J., Sánchez-Cordero V., Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Saupe E., et al. , Macroevolutionary consequences of profound climate change on niche evolution in marine molluscs over the past three million years. Proc. R. Soc. B 281, 20141995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prinzing A., Durka W., Klotz S., Brandl R., The niche of higher plants: Evidence for phylogenetic conservatism. Proc. Biol. Sci. 268, 2383–2389 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawkins B. A., Diniz‐Filho J. A. F., Jaramillo C. A., Soeller S. A., Post‐Eocene climate change, niche conservatism, and the latitudinal diversity gradient of New World birds. J. Biogeogr. 33, 770–780 (2006). [Google Scholar]
- 57.Crisp M. D., et al. , Phylogenetic biome conservatism on a global scale. Nature 458, 754–756 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues J. F. M., Villalobos F., Iverson J. B., Diniz-Filho J. A. F., Climatic niche evolution in turtles is characterized by phylogenetic conservatism for both aquatic and terrestrial species. J. Evol. Biol. 32, 66–75 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Wiens J. J., Graham C. H., Moen D. S., Smith S. A., Reeder T. W., Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: Treefrog trees unearth the roots of high tropical diversity. Am. Nat. 168, 579–596 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Blondel J., Mourer-Chauviré C., Evolution and history of the western Palaearctic avifauna. Trends Ecol. Evol. (Amst.) 13, 488–492 (1998). [DOI] [PubMed] [Google Scholar]
- 61.James H. F., Paleogene fossils and the radiation of modern birds. Auk 122, 1049–1054 (2005). [Google Scholar]
- 62.Cracraft J., Continental drift, paleoclimatology, and the evolution and biogeography of birds. J. Zool. 169, 455–543 (1973). [Google Scholar]
- 63.Lindow B. E. K., Dyke G. J., Bird evolution in the Eocene: Climate change in Europe and a Danish fossil fauna. Biol. Rev. Camb. Philos. Soc. 81, 483–499 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Grande L., The Lost World of Fossil Lake: Snapshots from Deep Time (University of Chicago Press, Chicago, Illinois, 2013). [Google Scholar]
- 65.MacGinitie H. D., The Eocene Green flora of northwestern Colorado and northeastern Utah. Univ. Calif. Publ. Geol. Sci. 83, 1–149 (1969). [Google Scholar]
- 66.Grande L., Studies of paleoenvironments and historical biogeography in the Fossil Butte and Laney Members of the Green River Formation. Rocky Mountain Geology 30, 15–32 (1994). [Google Scholar]
- 67.van Steenis C. G. G. J., The land-bridge theory in botany with particular reference to tropical plants. Blumea 11, 235–372 (1962). [Google Scholar]
- 68.Webb S. D., A history of savanna vertebrates in the new world. Part I: North America and the Great interchange. Annu. Rev. Ecol. Syst. 8, 355–380 (1977). [Google Scholar]
- 69.Janis C. M., Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annu. Rev. Ecol. Evol. 24, 467–500 (1993). [Google Scholar]
- 70.Cox C. B., Plate tectonics, seaways and climate in the historical biogeography of mammals. Mem. Inst. Oswaldo Cruz 95, 509–516 (2000). [DOI] [PubMed] [Google Scholar]
- 71.Smith K. T., A new lizard assemblage from the earliest Eocene (Zone Wa0) of the Bighorn Basin, Wyoming, USA: Biogeography during the warmest interval of the cenozoic. J. Syst. Palaeontology 7, 299–358 (2009). [Google Scholar]
- 72.Gauthier J. A., Fossil xenosaurid and anguid lizards from the early Eocene Wasatch formation, southeast Wyoming, and a revision of the Anguioidea. Rocky Mountain Geol. 21, 7–54 (1982). [Google Scholar]
- 73.Mannion P. D., Upchurch P., Benson R. B. J., Goswami A., The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. (Amst.) 29, 42–50 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Ksepka D. T., Thomas D. B., Multiple cenozoic invasions of Africa by penguins (Aves, sphenisciformes). Proc. Biol. Sci. 279, 1027–1032 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cracraft J., Claramunt S., Conceptual and analytical worldviews shape differences about global avian biogeography. J. Biogeogr. 44, 958–960 (2017). [Google Scholar]
- 76.Yu Y., Harris A. J., Blair C., He X., RASP (Reconstruct Ancestral state in phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 87, 46–49 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Landis M. J., Matzke N. J., Moore B. R., Huelsenbeck J. P., Bayesian analysis of biogeography when the number of areas is large. Syst. Biol. 62, 789–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. O., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Oliveros C. H., et al. , Earth history and the passerine superradiation. Proc. Natl. Acad. Sci. U.S.A. 116, 7916–7925 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Field D. J., Preliminary paleoecological insights from the Pliocene avifauna of Kanapoi, Kenya: Implications for the ecology of Australopithecus anamensis. J. Hum. Evol., S0047-2484(17)30349-4 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Tambussi C. D., Degrange F. J., South American and Antarctic Continental Cenozoic Birds: Paleobiogeographic Affinities and Disparities (Springer, La Plata, Argentina, 2013). [Google Scholar]
- 82.Mayr G., De Pietri V. L., Earliest and first Northern Hemispheric hoatzin fossils substantiate Old World origin of a “Neotropic endemic”. Naturwissenschaften 101, 143–148 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Mayr G., Alvarenga H., Mourer-Chauviré C., Out of Africa: Fossils shed light on the origin of the hoatzin, an iconic Neotropic bird. Naturwissenschaften 98, 961–966 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Bonebrake T. C., et al. , Managing consequences of climate-driven species redistribution requires integration of ecology, conservation and social science. Biol. Rev. Camb. Philos. Soc. 93, 284–305 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Pecl G. T., et al. , Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355, eaai9214 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Jones L. A., et al. , Coupling of palaeontological and neontological reef coral data improves forecasts of biodiversity responses under global climatic change. R. Soc. Open Sci. 6, 182111 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crosby G. T., Spread of the cattle egret in the western Hemisphere. Bird-Banding 43, 205–212 (1972). [Google Scholar]
- 88.Oswald J. A., et al. , Evolutionary dynamics of hybridization and introgression following the recent colonization of Glossy ibis (Aves: Plegadis falcinellus) into the new world. Mol. Ecol. 28, 1675–1691 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Saupe E. E., et al. , Data from "Climatic shifts drove major contractions in avian latitudinal distributions throughout the Cenozoic." Zenodo. https://zenodo.org/record/2658119#.XOQXTFJKhpg. Deposited May 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 91.Sagoo N., Valdes P., Flecker R., Gregoire L. J., The early Eocene equable climate problem: Can perturbations of climate model parameters identify possible solutions? Philos. Trans. R. Soc. A 371, 20130123 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Kennedy A. T., Farnsworth A., Lunt D. J., Lear C. H., Markwick P. J., Atmospheric and oceanic impacts of Antarctic glaciation across the Eocene-Oligocene transition. Philos Trans A Math Phys Eng Sci 373, 20140419 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Lunt D. J., et al. , Palaeogeographic controls on climate and proxy interpretation. Clim. Past Discuss. 12, 1181–1198 (2016). [Google Scholar]
- 94.Seton M., et al. , Global continental and ocean basin reconstructions since 200Ma. Earth Sci. Rev. 113, 212–270 (2012). [Google Scholar]
- 95.Bivand R., Rundel C., rgeos: Interface to Geometry Engine–Open Source (GEOS) (R package version 0.3-19, 2016). https://rdrr.io/cran/rgeos/. Accessed 12 September 2017.
- 96.Anderson R. P., Gómez‐Laverde M., Peterson A. T., Geographical distributions of spiny pocket mice in South America: Insights from predictive models. Glob. Ecol. Biogeogr. 11, 131–141 (2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.