Abstract
Background:
Women with a history of hypertensive disorders of pregnancy (HDP) are nearly twice as likely to develop cardiovascular disease (CVD) as women with normotensive pregnancies; however, the emergence of cardiovascular risk factors after a hypertensive pregnancy is less well understood.
Objective:
To identify associations between HDP and maternal CVD risk factors and chart the trajectory of risk factor development after pregnancy.
Design:
Observational cohort study.
Setting:
United States.
Participants:
The study sample comprised 58,671 parous Nurses’ Health Study II participants, free of CVD and risk factors of interest at baseline.
Measurements
Women were followed for self-reported physician-diagnosed chronic hypertension and hypercholesterolemia and confirmed type 2 diabetes mellitus from first birth through 2013—mean follow-up ranged from 25–32 years across the three endpoints. Multivariable Cox proportional hazards models estimated hazard ratios (HR) and 95% confidence intervals (CI), adjusted for pre-pregnancy confounders.
Results:
Women with gestational hypertension (2.9%) or preeclampsia (6.3%) in first pregnancy had an increased rate of chronic hypertension (HR=2.8, CI: 2.6–3.0; HR=2.2, CI: 2.1–2.3), type 2 diabetes mellitus (HR=1.7, CI: 1.4–1.9; HR=1.8, CI: 1.6–1.9), and hypercholesterolemia (HR=1.4, CI: 1.3–1.5; HR=1.3, CI: 1.3–1.4), compared to women with normotensive pregnancies. While these women were more likely to develop CVD risk factors throughout follow-up, the relative risk of chronic hypertension was strongest within five years after first birth. Recurrence of HDP further elevated risks for all endpoints.
Limitations
Nurse participants self-reported exposure.
Conclusions:
Women with hypertensive disorders in first pregnancy had an increased rate of chronic hypertension, type 2 diabetes mellitus, and hypercholesterolemia that persisted for several decades. These women may especially benefit from early screening and lifestyle intervention to reduce lifetime risk of CVD.
Introduction
During reproductive life, approximately 15% of parous women will have at least one pregnancy complicated by a hypertensive disorder, including gestational hypertension and preeclampsia (1). Growing evidence suggests these women are nearly twice as likely to develop cardiovascular disease (CVD) as women with normotensive pregnancies (2–4). Hypertensive disorders of pregnancy (HDP) may reveal subclinical CVD risk under the physiologic “stress test” of pregnancy, providing early insight into CVD risk that might be leveraged to identify high-risk women for targeted prevention from an early age (1, 5). While American Heart Association 2011 guidelines recommend clinicians evaluate CVD risk by screening for a history of HDP, little data exist on which risk factors should be screened as well as the frequency and timing of screening (6).
HDP has been consistently linked to future chronic hypertension, despite the fact that blood pressure returns to normal during postpartum (2, 7–12). Women with a history of HDP demonstrate higher risks of impaired glucose tolerance, insulin resistance, and a three- to four-fold increased risk of type 2 diabetes mellitus (T2DM) (9, 13–22). Women with a history of preeclampsia also have higher levels of total and LDL cholesterol and triglycerides; however, these differences are not consistently statistically significant (16, 18–21, 23–25). Many previous studies are limited by sample size, short follow-up, or incomplete adjustment for potential confounders, such as pre-pregnancy smoking, body mass index (BMI), and family history. Further, while these associations have been observed over variable lengths of follow-up, little is known about the specific timing of risk factor development—knowledge critical to inform screening guidelines.
We examined associations of gestational hypertension and preeclampsia with development of chronic hypertension, T2DM, and hypercholesterolemia. These associations were evaluated in a large longitudinal study with up to 50 years of follow-up from first birth.
Methods
Cohort Description and Selection
The Nurses’ Health Study II (NHSII) is a prospective cohort study of 116,429 female U.S. registered nurses, enrolled at 25–42 years in 1989. Participants are followed biennially via questionnaires, which collect information on lifestyle, health-related behaviors, and incident disease.
Study Sample
Analyses were restricted to respondents to the 2009 questionnaire (n=76,840), which allowed pregnancies to be dated and linked to specific complications. We excluded women who were nulliparous (n=13,253), missing a valid year of first pregnancy (n=12), <18 or >45 years at first birth (n=846), missing gestation length or had a value incompatible with the pregnancy outcome reported (n=292). Women were also excluded if they: 1) reported chronic hypertension, type 1 or type 2 diabetes, hypercholesterolemia, myocardial infarction, fatal coronary heart disease, or stroke before first pregnancy (n=2,470); or 2) were missing date of diagnosis or reported diagnosis of these conditions before 1980 (which precluded dating those events; n=1,210). Finally, since undetected chronic hypertension before pregnancy may be incorrectly captured as incident chronic hypertension directly following pregnancy, we excluded women who reported chronic hypertension within one year after first birth (n=86). This yielded an analytic population of 58,671 women. This analysis was approved by the Institutional Review Board at Brigham and Women’s Hospital.
Hypertensive Disorders of Pregnancy
In 2009, women retrospectively reported their complete pregnancy history. HDP was self-reported as “pregnancy-related high blood pressure” (i.e. gestational hypertension) or “pre-eclampsia/toxemia.” The primary analysis focused on the first pregnancy, as HDP predominantly occurs during first pregnancies (24).
To assess the validity of self-reported preeclampsia, we reviewed medical records of 598 women who reported preeclampsia on biennial questionnaires from 1991–2001 for provider report of preeclampsia or evidence of gestational hypertension (new onset high blood pressure—SBP ≥140mmHg or DBP ≥90mmHg—after 20 weeks gestation) and proteinuria (≥300mg/24h urine, protein-creatinine ratio ≥0.3, or dipstick reading of ≥1+) (26). There were 411 cases of medical record-confirmed preeclampsia for a positive predictive value of 69%. Given the complexity of validating preeclampsia (i.e. confirming normotension before 20 weeks as well as elevated blood pressures and proteinuria after 20 weeks), multiple components of a medical record are required. As 136 medical records had insufficient information available to validate (e.g. missing laboratory data, prenatal and/or labor and delivery records), we excluded these records and obtained a positive predictive value of 89%. If we had complete medical record information for all 598 women, it is likely that the positive predictive value would fall between 69% and 89%.
Recurrent HDP was analyzed in a secondary analysis with follow-up starting at age 40. This analysis was restricted to 45,815 parous women free of CVD events and risk factors at age 40 with no additional pregnancies at age 40 or later.
Cardiovascular Disease Risk Factors
CVD risk factors—chronic hypertension, T2DM, and hypercholesterolemia—were self-reported on biennial questionnaires, beginning in 1989. The 1989 questionnaire retrospectively captured any physician-diagnosed “high blood pressure (excluding during pregnancy),” “diabetes: not during pregnancy,” and “elevated cholesterol” and the year of diagnosis within three categories (“Before 1980”, “1980 to 1984”, “1985 to present”). Women prospectively reported incident CVD risk factor diagnosis on biennial questionnaires beginning in 1991. The midpoint of each date range was assigned as the diagnosis year for chronic hypertension and hypercholesterolemia; for T2DM, the diagnosis year was available through supplemental questionnaire). Previous validation of self-reported high blood pressure in NHSII indicated good agreement with 94% sensitivity and 85% specificity (27). Women who reported a new diagnosis of diabetes received a supplemental questionnaire to report diagnostic test results, symptoms, and treatment. This information was used to classify cases into categories proposed by the National Diabetes Data Group and the American Diabetes Association (28–30). Information on self-reported cholesterol-lowering medication has been collected since 1999. We defined hypercholesterolemia as self-report of hypercholesterolemia or cholesterol-lowering medication use. Self-reported hypercholesterolemia was validated in a similar cohort and demonstrated 86% positive predictive value and 85% negative predictive value (31).
Lifestyle Factors and Medical History
In 1989, participants reported height, current weight, and weight at age 18. Participants updated weight on all biennial questionnaires. BMI (kg/m2) was calculated from reported height and weight information at age 18 and updated every two years from 1989–2013. BMI was derived for ages at which weight was not reported, incorporating data on weight at age 18, weights reported on each questionnaire, and somatograms at ages 20, 30, and 40 (see Appendix). A previous validation study in NHSII demonstrated that recalled weight at age 18 (r=0.87) and self-reported height (r=0.94) were highly correlated with physical examination records (32).
Race/ethnicity, family history of chronic hypertension, and physical activity at age 18 were reported at baseline. History of smoking, alcohol consumption, and oral contraceptive use were also reported in 1989 and updated over follow-up. Biennial questionnaires after 1989 queried family history of diabetes, parental education, and diet. Food frequency questionnaires were used to derive a dietary quality score from the 2010 Alternative Healthy Eating Index (33). Self-reported physician-diagnosed myocardial infarction and stroke were verified through medical record review. Pre-pregnancy information was drawn from the biennial questionnaire immediately before the first pregnancy. As most first births (85%) occurred before baseline, health-related behavior in high school and within varying age ranges from 13 through 42 retrospectively reported in 1989 were used to assign pre-pregnancy values for these women.
Statistical Analysis
We used Cox proportional hazards models to estimate associations between HDP in first pregnancy and chronic hypertension, T2DM, and hypercholesterolemia.28 Women contributed person-time from first birth until development of the CVD risk factor of interest, CVD event (non-fatal myocardial infarction, fatal coronary heart disease, or non-fatal or fatal stroke), death, last returned questionnaire, or 2013. Women were additionally censored at anti-hypertensive medication use for the chronic hypertension analysis and at type 1 diabetes diagnosis for the T2DM analysis. For the 85% of women who delivered their first birth before 1989, the analysis included an average of 9.8 (±5.5) years of follow-up prior to cohort enrollment.
Log-rank tests were used to determine whether the age at and time to risk factor development distributions differed between HDP groups. We calculated multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI), adjusting for variables identified a priori as potential confounders: age at first birth, age at 1989 NHSII baseline, race/ethnicity, parental education, physical activity, family history of chronic hypertension (chronic hypertension models only) or diabetes (T2DM models only), and pre-pregnancy BMI, alcohol consumption, diet, smoking, and oral contraceptive use. We assessed non-linearity of the relationships between age at first birth and age in 1989 with each CVD risk factor using restricted cubic splines with ten knots set at the decile medians (34–36). As tests for non-linearity were significant for age at first birth with chronic hypertension and T2DM and for age in 1989 with hypercholesterolemia (p<0.001 for each test), corresponding spline terms at 26 years for age at first birth and 28 years for age in 1989 were included. To evaluate departures from proportional hazards, we examined the extent to which the effect of HDP on CVD risk factors was modified by years since first birth non-parametrically using restricted cubic splines. The proportional hazards assumption did not hold for any model in Table 2 (p<0.001 for each test); therefore, hazard ratios were also presented within 5-year intervals (Table 3). We investigated the presence of effect modification by preterm delivery (<37 weeks) through a likelihood ratio test, comparing a model with multiplicative interaction terms between 1) gestational hypertension and preterm and 2) preeclampsia and preterm to a model without these terms.
Table 2.
Hazard ratios (95% confidence intervals) for hypertensive disorders in first pregnancy and cardiovascular disease risk factors
| Hypertensive Disorder in First Pregnancy Status | |||
|---|---|---|---|
| Normotensive Pregnancy | Gestational Hypertension | Preeclampsia | |
| Chronic Hypertension | |||
| Cases/Person-years | 16,610/1,459,370 | 979/35,568 | 1,922/84,317 |
| Excess cases per 10,000 person-years | --- | 161 | 122 |
| Median age at development (IQR), years* | 50 (45, 54) | 45 (40, 50) | 46 (40, 51) |
| Model 1 | 1.00 (ref) | 3.18 (2.98, 3.39) | 2.45 (2.34, 2.57) |
| Model 2 | 1.00 (ref) | 2.79 (2.61, 2.97) | 2.21 (2.10, 2.32) |
| Type 2 Diabetes Mellitus | |||
| Cases/Person-years | 3,137/1,691,624 | 187/49,948 | 435/112,344 |
| Excess cases per 10,000 person-years | --- | 19 | 20 |
| Median age at development (IQR), years* | 53 (48, 57) | 52 (47, 56) | 51 (46, 56) |
| Model 1 | 1.00 (ref) | 2.14 (1.84, 2.48) | 2.18 (1.98, 2.42) |
| Model 2 | 1.00 (ref) | 1.65 (1.42, 1.91) | 1.75 (1.58, 1.93) |
| Hypercholesterolemia | |||
| Cases/Person-years | 29,253/1,350,512 | 1,074/38,045 | 2,279/85,378 |
| Excess cases per 10,000 person-years | --- | 66 | 50 |
| Median age at development (IQR), years* | 47 (40, 53) | 46 (40, 52) | 45 (38, 52) |
| Model 1 | 1.00 (ref) | 1.43 (1.34, 1.52) | 1.35 (1.29, 1.40) |
| Model 2 | 1.00 (ref) | 1.36 (1.28, 1.45) | 1.31 (1.25, 1.36) |
IQR = interquartile range. Model 1 is adjusted for age at first birth (years), age in 1989 (years), race/ethnicity (African-American, Latina, Asian, Caucasian (ref), other), and parental education (<9, 9–11, 12, 13–15, ≥16 years (ref)). Model 2 is additionally adjusted for physical activity at ages 18–22 (never, 1–3 (ref), 4–6, 7–9, 10–12 mo/yr), pre-pregnancy smoking (never (ref), past, current), pre-pregnancy BMI (<18.5, 18.5–24.9 (ref), 25–29.9, ≥30 kg/m2), pre-pregnancy alcohol consumption (none (ref), ≤1 drink/week, 2–6/week, ≥1/day), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile (ref) representing the healthiest diet category), pre-pregnancy oral contraceptive use (never (ref), <2, 2-<4, ≥4 years), and family history of chronic hypertension (yes/no; chronic hypertension model only) and family history of type 2 diabetes mellitus (yes/no; type 2 diabetes mellitus model only).
P-value <0.001 from a global test of the difference in the distribution of age at risk factor development between hypertensive disorder in first pregnancy exposure groups
Table 3.
Hazard ratios (95% confidence intervals) for hypertensive disorders in first pregnancy and cardiovascular disease risk factors by years since first birth within five-year intervals, compared to normotensive first pregnancy (reference)
| Hypertensive Disorder in First Pregnancy Status | |||
|---|---|---|---|
| Cases/Person-years | Gestational Hypertension | Preeclampsia | |
| Chronic Hypertension | |||
| ≤5 years | 371/292,788 | 4.29 (3.16, 5.84) | 3.96 (3.07, 5.11) |
| 6–10 years | 1,228/287,252 | 3.62 (3.01, 4.34) | 3.59 (3.11, 4.15) |
| 11–15 years | 2,390/273,710 | 2.92 (2.51, 3.38) | 2.62 (2.33, 2.95) |
| 16–20 years | 3,570/248,421 | 2.87 (2.51, 3.28) | 2.04 (1.83, 2.27) |
| 21–25 years | 4,290/205,778 | 2.18 (1.86, 2.54) | 1.94 (1.75, 2.16) |
| 26–30 years | 3,899/144,421 | 2.08 (1.72, 2.51) | 1.78 (1.57, 2.00) |
| 31–35 years | 2,382/81,124 | 1.81 (1.34, 2.43) | 1.85 (1.57, 2.18) |
| 36–40 years | 1,029/35,295 | 2.04 (1.26, 3.30) | 1.39 (1.03, 1.88) |
| Type 2 Diabetes Mellitus | |||
| ≤5 years | 11/284,750 | *** | *** |
| 6–10 years | 72/287,619 | 2.08 (0.97, 4.49) | 2.64 (1.43, 4.87) |
| 11–15 years | 220/290,780 | 1.29 (0.77, 2.17) | 1.51 (1.01, 2.25) |
| 16–20 years | 472/283,769 | 0.86 (0.54, 1.37) | 2.06 (1.61, 2.65) |
| 21–25 years | 756/260,885 | 1.77 (1.30, 2.41) | 2.03 (1.64, 2.50) |
| 26–30 years | 863/207,632 | 1.93 (1.44, 2.60) | 1.59 (1.27, 1.99) |
| 31–35 years | 754/134,730 | 1.67 (1.13, 2.46) | 1.62 (1.26, 2.07) |
| 36–40 years | 454/67,523 | 2.55 (1.62, 4.00) | 1.85 (1.34, 2.56) |
| Hypercholesterolemia | |||
| ≤5 years | 1,642/291,114 | 1.37 (1.09, 1.72) | 1.44 (1.22, 1.70) |
| 6–10 years | 3,050/279,052 | 1.26 (1.05, 1.51) | 1.53 (1.35, 1.72) |
| 11–15 years | 4,583/259,806 | 1.18 (1.01, 1.38) | 1.30 (1.17, 1.45) |
| 16–20 years | 5,999/229,863 | 1.38 (1.20, 1.57) | 1.21 (1.09, 1.34) |
| 21–25 years | 6,356/185,154 | 1.41 (1.23, 1.62) | 1.30 (1.18, 1.43) |
| 26–30 years | 5,590/125,494 | 1.33 (1.13, 1.57) | 1.16 (1.04, 1.30) |
| 31–35 years | 3,483/67,843 | 1.28 (1.02, 1.62) | 1.29 (1.12, 1.49) |
| 36–40 years | 1,566/27,832 | 1.77 (1.28, 2.45) | 1.13 (0.88, 1.43) |
Models are adjusted for age at first birth (years), age in 1989 (years), race/ethnicity (African-American, Latina, Asian, Caucasian (ref), other), parental education (<9, 9–11, 12, 13–15, ≥16 years (ref)), physical activity at ages 18–22 (never, 1–3 (ref), 4–6, 7–9, 10–12 mo/yr), pre-pregnancy smoking (never (ref), past, current), pre-pregnancy BMI (<18.5, 18.5–24.9 (ref), 25–29.9, ≥30 kg/m2), pre-pregnancy alcohol consumption (none (ref), ≤1 drink/week, 2–6/week, ≥1/day), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile (ref) representing the healthiest diet category), pre-pregnancy oral contraceptive use (never (ref), <2, 2-<4, ≥4 years), and family history of chronic hypertension (yes/no; chronic hypertension model only) and family history of type 2 diabetes mellitus (yes/no; type 2 diabetes mellitus model only). Due to small cell counts, for the type 2 diabetes mellitus model from 6–10 years, women with missing pre-pregnancy smoking (n=352), strenuous physical activity from 18–22 (n=348), and pre-pregnancy alcohol consumption (n=249) were excluded from the analysis.
Small case numbers resulted in unstable estimates for type 2 diabetes mellitus ≤5 years after first birth, as there was n=1 case among women with gestational hypertension and no cases among women with preeclampsia.
Multivariable-adjusted cumulative incidence curves for each risk factor by HDP status in first pregnancy were obtained using the Breslow estimator at the mean and mode values of the continuous and categorical covariates, respectively. To address the violation of the proportional hazards assumption, time-varying multiplicative interaction terms between HDP status in first pregnancy and time since first birth were included. All analyses were conducted using SAS (version 9.4; SAS Institute, Inc., Cary, NC).
Results
First births occurred between 1964 and 2008, at a mean age of 26.8 ± 4.6 years. A total of 5,386 women (9.2%) experienced HDP in their first pregnancy: 2.9% developed gestational hypertension while 6.3% developed preeclampsia. Women with HDP in their first pregnancy were generally similar to women with normotensive pregnancies in demographic and lifestyle characteristics (Table 1). However, women with HDP were more likely to have a family history of chronic hypertension and had fewer children. By the end of follow-up in 2013 (see Appendix), 33.3% of women developed chronic hypertension, 6.4% developed T2DM, and 55.6% developed hypercholesterolemia.
Table 1.
Age-standardized characteristics of Nurses’ Health Study II study participants by hypertensive status in first pregnancy
| Hypertensive Disorder in First Pregnancy Status | |||
|---|---|---|---|
| Normotensive
Pregnancy n=53,285 |
Gestational
Hypertension n=1,699 |
Preeclampsia n=3,687 |
|
| (90.8%) | (2.9%) | (6.3%) | |
| Age at first birth, years, mean (SD)* | 26.8 (4.5) | 27.9 (4.7) | 26.8 (4.6) |
| Age in 1989, years, mean (SD)* | 35.2 (4.6) | 34.5 (4.7) | 34.6 (4.6) |
| White | 93 | 94 | 93 |
| Maternal years of education >12 years | 32 | 32 | 32 |
| Paternal years of education >12 years | 38 | 34 | 37 |
| Strenuous physical activity, 18–22 years | |||
| Never | 29 | 29 | 27 |
| 10–12 months/year | 11 | 11 | 11 |
| Physical activity in 1989, METs per week, mean (SD)† | 26.7 (66.7) | 24.5 (56.4) | 25.9 (59.8) |
| Pre-pregnancy body mass index, kg/m2, mean (SD) | 21.7 (3.5) | 23.1 (4.3) | 22.8 (4.1) |
| Pre-pregnancy Alternative Healthy Eating Index (AHEI) score | |||
| Lowest quintile (unhealthy) | 20 | 22 | 21 |
| Highest quintile (healthy) | 20 | 20 | 19 |
| Pre-pregnancy smoking status | |||
| Never | 68 | 69 | 68 |
| Past | 10 | 9 | 10 |
| Current | 22 | 21 | 22 |
| Pre-pregnancy alcohol intake | |||
| None | 26 | 27 | 28 |
| ≤1 drink/week | 37 | 36 | 36 |
| 2–6 drinks/week | 29 | 29 | 28 |
| ≥1 drink/day | 8 | 8 | 8 |
| Pre-pregnancy oral contraceptive use | |||
| Never | 26 | 25 | 24 |
| <2 years | 24 | 24 | 25 |
| 2-<4 years | 22 | 21 | 21 |
| ≥4 years | 29 | 30 | 30 |
| Family history of chronic hypertension | 51 | 62 | 59 |
| Family history of diabetes | 42 | 46 | 47 |
| Final parity | |||
| 1 birth | 15 | 21 | 21 |
| 2 births | 49 | 48 | 49 |
| 3 births | 26 | 24 | 23 |
| ≥4 births | 10 | 8 | 7 |
MET = metabolic equivalent (of task). Percentages unless otherwise noted. Values of polytomous variables may not sum to 100% due to rounding
Value is not age adjusted
Average metabolic equivalents (METs) per week calculated by frequency and duration of participation in several aerobic activities
In age, race/ethnicity, and parental education-adjusted models, women with HDP in first pregnancy developed chronic hypertension, T2DM, and hypercholesterolemia at higher rates than women with normotensive first pregnancies (Table 2). Hazard ratios were modestly attenuated but remained statistically significant after adjustment for additional pre-pregnancy behaviors and characteristics. Women with gestational hypertension had a 2.8-fold increased rate of chronic hypertension (CI: 2.6–3.0), while women with preeclampsia had a 2.2-fold increased rate (CI: 2.1–2.3). Women with hypertensive first pregnancies had an approximately 70% increased rate of T2DM and 30% increased rate of hypercholesterolemia. Further adjustment for potential mediators, including post-pregnancy smoking, diet, alcohol intake, physical activity, and oral contraceptive use, did not change the results (data not shown). Women with HDP in first pregnancy exhibited elevated rates of CVD risk factor development whether they delivered at term or preterm (Appendix Table 2). However, the elevated rate of hypercholesterolemia associated with HDP history was slightly higher for women who delivered preterm (HR: 1.5, CI: 1.4–1.6) than for those who delivered at term (HR: 1.3, CI: 1.3–1.4; p-value for interaction=0.03).
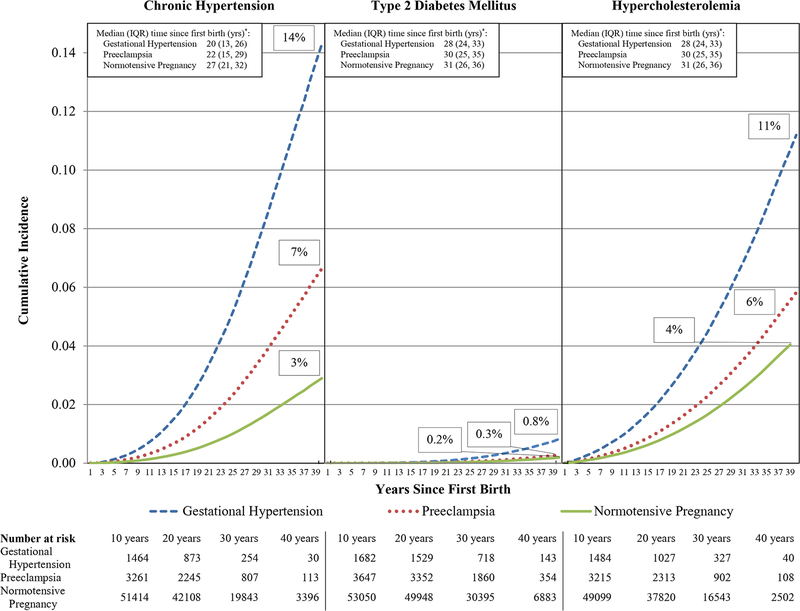
Figure 1 displays the multivariable-adjusted cumulative incidence of chronic hypertension, T2DM, and hypercholesterolemia by HDP status in first pregnancy through 40 years after first birth. Women with gestational hypertension or preeclampsia exhibited a higher cumulative incidence of all CVD risk factors, relative to women with normotensive pregnancies. Women with HDP also developed the CVD risk factors sooner after first pregnancy (Figure 1, p<0.001) and at earlier ages than women with normotensive pregnancies (Table 2, p<0.001).
Figure 1. Multivariable-adjusted cumulative incidence of chronic hypertension, type 2 diabetes mellitus, and hypercholesterolemia by hypertensive disorder in first pregnancy through 40 years since first birth.
Curves are obtained at the mean and mode values for the following continuous and categorical covariates, respectively : age at first birth (27 years), age in 1989 (35 years), race/ethnicity (white), parental education (12 years), physical activity at ages 18–22 (1–3 months/year), pre-pregnancy smoking (never), pre-pregnancy body mass index (normal weight: 18.5–24.9 kg/m2), pre-pregnancy alcohol consumption (≤1 drink/week), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (3rd quintile), pre-pregnancy oral contraceptive use (≥4 years), family history of chronic hypertension (present; chronic hypertension model only) and family history of type 2 diabetes mellitus (absent; type 2 diabetes mellitus model only). * P-value <0.001 from a global test of the difference in the distribution of time to risk factor development between hypertensive disorder in first pregnancy exposure groups. IQR = interquartile range.
Table 3 presents the relationship between HDP in first pregnancy and CVD risk factors, stratified by 5-year intervals through 40 years since first birth. Hazard ratios for chronic hypertension were strongest within the first five years: women with gestational hypertension had a 4.3-fold increased rate (CI: 3.2–5.8), while women with preeclamptic first births had a 4.0-fold increased rate (CI: 3.1–5.1), compared to women with normotensive pregnancies. While women with HDP had elevated rates of chronic hypertension throughout follow-up, the relative rate diminished over time. However, absolute rate differences were lowest in the younger, low incidence years and highest at later time intervals. The statistically significant 1.5 to 2.6-fold increased rate of T2DM among women with preeclampsia appeared as early as 6–10 years after first birth and continued throughout follow-up. Women with gestational hypertension had a statistically significant increased rate of T2DM beginning 21–25 years since first birth, which ranged from 1.7 to 2.6-fold higher than women with normotensive pregnancies. Women with HDP in first pregnancy had statistically significant 1.1 to 1.8-fold increased rates of hypercholesterolemia in the interval immediately after first birth through 35 years for women with preeclampsia and through 40 years after first birth for women with gestational hypertension.
We examined HDP recurrence starting follow-up at age 40 (Appendix Table 1). Women with HDP in first and second or later pregnancies had the highest rates of CVD risk factor development, with hazard ratios ranging from 1.3 for hypercholesterolemia (CI: 1.2–1.5) to 3.5 for chronic hypertension (CI: 3.2–3.9), compared to women with two or more pregnancies and no HDP (Appendix Table 1). Women with any HDP, regardless of whether it occurred in the first or a later pregnancy, exhibited increased rates of CVD risk factor development. Further adjustment for parity made no material difference to these results. Conducting this analysis starting follow-up at age 45, rather than age 40, yielded slightly attenuated hazard ratios but maintained significance and rank across HDP groups (data not shown).
Sensitivity Analyses
The majority of women who developed a CVD risk factor during follow-up developed it before reporting their pregnancy history in 2009 (73% among T2DM cases, 85% among chronic hypertension cases, and 87% among hypercholesterolemia cases). Given this and the retrospective report of HDP exposure in 2009, we conducted a sensitivity analysis with follow-up from 2009–2013. Hazard ratios were attenuated but statistically significant, with the exception of preeclampsia and hypercholesterolemia (HR=1.1, CI: 0.9–1.2). Additional analyses starting follow-up in 1982 for chronic hypertension and hypercholesterolemia (i.e. the first year of diagnosis we could assign for development of these risk factors), excluding women with multiples in first birth (n=819), and utilizing an alternative clinical hypertension endpoint additionally including anti-hypertensive medication use obtained similar results (data not shown). To evaluate the robustness of the results to potential unmeasured confounding, we calculated E-values using the publicly available Online E-Value Calculator (available at: https://www.hsph.harvard.edu/tyler-vanderweele/tools-and-tutorials/) (37–39). E-values for the observed point estimates ranged from 1.7 for preeclampsia and hypercholesterolemia to 3.4 for gestational hypertension and chronic hypertension (Appendix Table 3).
Conclusions
Women with a history of gestational hypertension or preeclampsia in first pregnancy develop chronic hypertension at a rate 2 to 3-fold higher and have 70% and 30% higher rates of T2DM and hypercholesterolemia, respectively, than women with normotensive first pregnancies; these associations held even after accounting for pre-pregnancy confounders, including BMI, smoking, and family history. To our knowledge, this study presents the most complete pre-pregnancy confounding adjustment of the relationship between HDP and CVD risk factors available. Further, this is one of the largest studies with one of the longest lengths of follow-up in the literature. Our ability to describe the trajectory of risk factor development with up to 50 years of follow-up from first birth provides insight into the timing and pathways between HDP and CVD.
This study advances our understanding of the trajectory of CVD risk following a hypertensive pregnancy. Although higher magnitudes of risk have been observed for chronic hypertension and T2DM, those studies were largely unadjusted for pre-pregnancy confounding, followed women for less time, and/or had smaller sample sizes (2, 17, 18). While HDP has been inconsistently related to lipid measures (24) and was not associated with hypercholesterolemia previously (8), we demonstrated associations of gestational hypertension and preeclampsia with incident hypercholesterolemia. Only three previous studies have analyzed preterm or HDP recurrence with any of these CVD risk factors (10, 11, 17). However, one examined recurrence considering only the first two pregnancies (10) and another utilized anti-hypertensive use as a proxy for chronic hypertension (11). Our study demonstrates that recurrent HDP confers the highest risk of chronic hypertension and T2DM, even after adjustment for pre-pregnancy confounding, and is the first to reveal a relationship between recurrent HDP and hypercholesterolemia. Our findings also confirm that the increased rate of CVD risk factors is not restricted to preterm preeclampsia, but occurs across all combinations of HDP and gestation length. Only a few previous studies examined blood pressure (1), chronic hypertension (9, 10, 17, 40, 41), T2DM (9, 14, 17), and lipid values (1) among women with a history of gestational hypertension. Our finding of a higher magnitude of chronic hypertension risk among women with gestational hypertension than preeclampsia is consistent with three previous studies (9, 10, 40) and may suggest different pathologies in these hypertensive disorders. While one previous study calculated ten year cumulative incidence of chronic hypertension among women with and without HDP (10), we expanded our understanding by charting the 40 year cumulative incidences of CVD risk factors in these women.
The primary limitation is the reliance on nurse participants’ self-reported exposure, which was retrospectively reported in 2009 and may induce recall bias. However, maternal self-report of preeclampsia was validated in a subset of NHSII participants and the majority of women had medical record evidence of preeclampsia. Gestational hypertension has not yet been validated but the proportions of first pregnancies complicated by gestational hypertension and preeclampsia are consistent with those seen elsewhere (24). While HDP history was retrospectively reported, length of recall has not been consistently associated with accuracy of maternal recall (42).
Given that 85% of individuals contributed person-time to the analysis that occurred prior to enrollment in NHSII, there may be selection bias. However, based on the retrospective data collected on the 1989 baseline questionnaire, we were able to assign chronic hypertension and hypercholesterolemia onset for cases arising between 1980 and 1989 and for all T2DM cases. As participants were excluded if they developed chronic hypertension or hypercholesterolemia before 1980, we conducted a sensitivity analysis excluding person-time between first birth and 1982 (i.e. the year assigned for women who developed endpoints between “1980 to 1984”) and obtained similar results. Although inclusion in our analysis was dependent on survival to 2009, 98.2% of NHSII participants were alive in 2009. Further, our sensitivity analysis with follow-up from 2009–2013 obtained results consistent with those observed from first birth through 2013.
Information on confounders was also self-reported, which may induce misclassification or residual confounding. However, this study represents the most complete adjustment for pre-pregnancy confounders. Additionally, based on our calculated E-values, an unmeasured confounder would need to be associated with HDP and the CVD risk factor by a magnitude of 1.7 to 3.4-fold, above and beyond the measured confounders, to explain away the observed associations. The only measured confounder of similar magnitude to these E-values was pre-pregnancy obesity, indicating that it is unlikely our associations could be explained away by an unmeasured confounder. As the NHSII cohort includes primarily white nurses, these findings should be generalized to other populations with caution. While our study provided longer follow-up since first birth than many previous studies, it should be noted that NHSII participants were still relatively young at the end of follow-up in 2013. As the cohort ages, the relative risks associated with HDP will likely decline as the absolute risks for CVD risk factors increase.
It is not yet clear whether HDP unmasks pre-existing underlying cardiovascular risk through the “stress test” of pregnancy or whether it induces endothelial or organ damage that alters a woman’s trajectory towards CVD risk factor development. Women who develop preeclampsia tend to have slightly higher blood pressures and a dyslipidemic profile before pregnancy, indicating that preeclampsia may reveal a subclinical trajectory towards development of chronic hypertension and hypercholesterolemia that predates pregnancy (18, 43). Regardless of whether the relationship is correlational or causal, a history of HDP may help identify women at increased risk for CVD risk factors and events.
Women with gestational hypertension or preeclampsia in first pregnancy are at increased risk for chronic hypertension, T2DM, and hypercholesterolemia shortly following pregnancy, compared to women with normotensive pregnancies. This increased risk persists for several decades. These women may benefit from screening and lifestyle interventions to reduce cardiovascular risk and delay disease onset. In the same way guidelines exist to screen for T2DM among women with a history of gestational diabetes (44), our findings may inform similar guidelines for CVD risk factor screening among women with a history of HDP.
Supplementary Material
Acknowledgement:
The authors thank the reviewers and editors for their helpful comments on an earlier draft of this paper.
Funding: This research was funded by the National Institutes of Health grant UM1 CA176726. This work was supported by awards from the American Heart Association (12PRE9110014, 13GRNT17070022). JJS was supported by Training Grant T32HL098048 from the National Heart, Lung, and Blood Institute and by Training Grant T32HD060454 from the National Institute of Child Health and Human Development. LJT was supported by the Ruth L. Kirchstein National Research Service Award (NHLBI F31 HL131222).
Primary Funding Source: National Institutes of Health.
Role of the Funding Source
The National Institutes of Health had no role in the design, conduct, analysis, or reporting of the study.
Appendix Table 1. Hazard ratios (95% confidence intervals) for experience of hypertensive disorders in pregnancy (HDP) before age 40 and cardiovascular disease risk factors after age 40 (n=45,815)
| Pregnancy History at Age 40 | Chronic Hypertension | Type 2 Diabetes Mellitus | Hypercholesterolemia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Pregnancy | Second or Later Pregnancies | Cases | Person- years | HR (95% CI) | Cases | Person- years | HR (95% CI) | Cases | Person- years | HR (95% CI) |
| Normotensive | All Normotensive | 9,504 | 554,504 | 1.00 (Ref) | 1,428 | 659,537 | 1.00 (Ref) | 16,209 | 526,278 | 1.00 (Ref) |
| Normotensive | No further pregnancies | 1,756 | 101,586 | 1.02 (0.97, 1.08) | 289 | 122,653 | 1.01 (0.89, 1.15) | 3,071 | 95,855 | 1.05 (1.01, 1.09) |
| Normotensive | Any HDP | 434 | 11,272 | 2.40 (2.18, 2.64) | 90 | 16,224 | 2.12 (1.71, 2.63) | 481 | 12,353 | 1.30 (1.19, 1.43) |
| HDP | All Normotensive | 896 | 29,288 | 1.85 (1.73, 1.98) | 147 | 38,626 | 1.63 (1.38, 1.94) | 1,071 | 30,384 | 1.21 (1.13, 1.28) |
| HDP | No further pregnancies | 357 | 9,682 | 2.24 (2.01, 2.49) | 72 | 13,609 | 1.83 (1.44, 2.33) | 420 | 10,121 | 1.45 (1.32, 1.60) |
| HDP | Any HDP | 357 | 6,628 | 3.53 (3.17, 3.93) | 65 | 10,545 | 2.17 (1.69, 2.79) | 319 | 8,159 | 1.32 (1.18, 1.48) |
HDP = hypertensive disorders of pregnancy. The six exposure categories included in this table are: 1) Normotension in all pregnancies, 2) Normotension in first pregnancy, no additional pregnancies, 3) Normotension in first pregnancy, HDP (preeclampsia or gestational hypertension) in at least one later pregnancy, 4) HDP in first pregnancy, normotension in all subsequent pregnancies, 5) HDP in first pregnancy, no additional pregnancies, and 6) HDP in first pregnancy with recurrence in at least one later pregnancy. Women free from the endpoint at age 40 and with no births after age 40 contribute person-time to the models above from age 40 on.
Models are adjusted for age at first birth (years), age in 1989 (years), race/ethnicity (African-American, Latina, Asian, Caucasian (ref), other), parental education (<9, 9–11, 12, 13–15, ≥16 years (ref)), physical activity at ages 18–22 (never, 1–3 (ref), 4–6, 7–9, 10–12 mo/yr), pre-pregnancy smoking (never (ref), past, current), pre-pregnancy BMI (<18.5, 18.5–24.9 (ref), 25–29.9, ≥30 kg/m2), pre-pregnancy alcohol consumption (none (ref), ≤1 drink/week, 2–6/week, ≥1/day), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile (ref) representing the healthiest diet category), pre-pregnancy oral contraceptive use (never (ref), <2, 2-<4, ≥4 years), and family history of chronic hypertension (yes/no; chronic hypertension model only) and family history of type 2 diabetes mellitus (yes/no; type 2 diabetes mellitus model only).
Appendix Table 2. Hazard ratios (95% confidence intervals) for hypertensive disorders in first pregnancy and cardiovascular disease risk factors by gestation length at delivery
| Hypertensive Disorder in Pregnancy & Gestation Length at Delivery Status | |||||||
|---|---|---|---|---|---|---|---|
| Term (≥37 weeks) | Preterm (<37 weeks) | ||||||
| Normotensive Pregnancy 49,099 (83.7%) | Gestational Hypertension 1,566 (2.7%) | Preeclampsia 3,112 (5.3%) | Hypertensive Disorder of Pregnancy 4,678 (8.0%) | Gestational Hypertension 133 (0.2%) | Preeclampsia 575 (1.0%) | Hypertensive Disorder of Pregnancy 708 (1.2%) | |
| Chronic Hypertension | |||||||
| Cases/Person-years | 15,234/1,347,395 | 903/32,892 | 1,624/72,261 | 2,527/105,153 | 76/2,676 | 298/12,056 | 374/14,732 |
| Excess cases per 10,000 person-years | --- | 161 | 112 | 127 | 171 | 134 | 141 |
| Model 1 | 1.00 (ref) | 3.19 (2.98, 3.41) | 2.42 (2.30, 2.55) | 2.65 (2.54, 2.76) | 3.36 (2.68, 4.21) | 2.78 (2.48, 3.12) | 2.88 (2.60, 3.19) |
| Model 2 | 1.00 (ref) | 2.82 (2.63, 3.01) | 2.18 (2.07, 2.29) | 2.37 (2.27, 2.47) | 2.70 (2.15, 3.39) | 2.51 (2.24, 2.82) | 2.54 (2.29, 2.82) |
| Type 2 Diabetes Mellitus | |||||||
| Cases/Person-years | 2,836/1,560,189 | 171/46,261 | 379/95,969 | 550/142,230 | 16/3,687 | 56/16,375 | 72/20,062 |
| Excess cases per 10,000 person-years | --- | 19 | 21 | 20 | 25 | 16 | 18 |
| Model 1 | 1.00 (ref) | 2.15 (1.84, 2.51) | 2.26 (2.03, 2.51) | 2.22 (2.03, 2.44) | 2.49 (1.52, 4.07) | 2.03 (1.56, 2.64) | 2.11 (1.67, 2.67) |
| Model 2 | 1.00 (ref) | 1.66 (1.42, 1.94) | 1.80 (1.62, 2.01) | 1.76 (1.60, 1.93) | 1.79 (1.09, 2.93) | 1.60 (1.23, 2.09) | 1.64 (1.29, 2.07) |
| Hypercholesterolemia | |||||||
| Cases/Person-years | 26,877/1,246,593 | 992/35,235 | 1,928/73,179 | 2,920/108,414 | 82/2,810 | 351/12,199 | 433/15,009 |
| Excess cases per 10,000 person-years | --- | 66 | 48 | 54 | 76 | 72 | 73 |
| Model 1 | 1.00 (ref) | 1.43 (1.34, 1.52) | 1.33 (1.27, 1.39) | 1.36 (1.31, 1.41) | 1.52 (1.22, 1.89) | 1.52 (1.37, 1.69) | 1.52 (1.38, 1.67) |
| Model 2 | 1.00 (ref) | 1.36 (1.28, 1.45) | 1.29 (1.23, 1.35) | 1.31 (1.26, 1.36) | 1.46 (1.18, 1.82) | 1.49 (1.34, 1.66) | 1.49 (1.35, 1.63) |
Tests for effect modification by preterm delivery: p=0.08 for chronic hypertension, p=0.68 for type 2 diabetes mellitus, and p=0.03 for hypercholesterolemia.
Model 1 is adjusted for age at first birth (years), age in 1989 (years), race/ethnicity (African-American, Latina, Asian, Caucasian (ref), other), and parental education (<9, 9–11, 12, 13–15, ≥16 years (ref)).
Model 2 is additionally adjusted for physical activity at ages 18–22 (never, 1–3 (ref), 4–6, 7–9, 10–12 mo/yr), pre-pregnancy smoking (never (ref), past, current), pre-pregnancy BMI (<18.5, 18.5–24.9 (ref), 25–29.9, ≥30 kg/m2), pre-pregnancy alcohol consumption (none (ref), ≤1 drink/week, 2–6/week, ≥1/day), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile (ref) representing the healthiest diet category), pre-pregnancy oral contraceptive use (never (ref), <2, 2-<4, ≥4 years), and family history of chronic hypertension (yes/no; chronic hypertension model only) and family history of type 2 diabetes mellitus (yes/no; type 2 diabetes mellitus model only).
These results are drawn from 6 different models—one model with hypertensive disorders in pregnancy and normotensive first pregnancies split out by term and preterm delivery (i.e. 4 exposure categories) and another model with preeclamptic, gestational hypertensive, and normotensive first pregnancies split out by term and preterm deliveries (i.e. 6 exposure categories) for each of the 3 CVD risk factor outcomes; normotensive term served as the reference group for all contrasts. Results for women with normotensive preterm are not shown above but were obtained within the same models: fully-adjusted chronic hypertension HR=1.10 (95% CI: 1.04, 1.16); type 2 diabetes mellitus HR=1.20 (1.06, 1.35); hypercholesterolemia HR=1.08 (1.03, 1.12).
Appendix Table 3. E-values for the observed associations between hypertensive disorders in first pregnancy and cardiovascular disease risk factors
| Hypertensive Disorder in First Pregnancy Status | ||
|---|---|---|
| Gestational Hypertension | Preeclampsia | |
| Chronic Hypertension | ||
| Observed association | 2.79 (2.61, 2.97) | 2.21 (2.10, 2.32) |
| E-value (point estimate) | 3.45 | 2.85 |
| E-value (CI) | 3.27 | 2.72 |
| Type 2 Diabetes Mellitus | ||
| Observed association | 1.65 (1.42, 1.91) | 1.75 (1.58, 1.93) |
| E-value (point estimate) | 2.69 | 2.90 |
| E-value (CI) | 2.19 | 2.54 |
| Hypercholesterolemia | ||
| Observed association | 1.36 (1.28, 1.45) | 1.31 (1.25, 1.36) |
| E-value (point estimate) | 1.78 | 1.70 |
| E-value (CI) | 1.66 | 1.61 |
The observed associations displayed above are the fully adjusted hazard ratios presented in Table 2, shown here as a reference. E-values were calculated using the publicly available Online E-Value Calculator (available at: https://www.hsph.harvard.edu/tyler-vanderweele/tools-and-tutorials/) for chronic hypertension and hypercholesterolemia based on a “hazard ratio (outcome prevalence >15%)” and for type 2 diabetes based on a “hazard ratio (outcome prevalence <15%).” E-values for 1) the point estimate and 2) the limit of the 95% confidence interval (CI) closest to the null (i.e. the lower limit for the above CIs) represent the magnitude of the association that an unmeasured confounder would have to have with both the exposure (hypertensive disorder in first pregnancy) and outcome (cardiovascular disease risk factor), above and beyond measured confounding, to 1) explain away the observed association and 2) render the observed association no longer statistically significant, respectively.
Footnotes
Publisher's Disclaimer: Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Disclosures: There are no author relationships with industry.
Reproducible Research Statement:
Study protocol and data set: Not available.
Statistical code: Available from Dr. Stuart (e-mail: jstuart@mail.harvard.edu)
References
- 1.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–22. [DOI] [PubMed] [Google Scholar]
- 5.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325(7356):157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−−2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28(1):1–19. [DOI] [PubMed] [Google Scholar]
- 8.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, et al. Earlier Age of Onset of Chronic Hypertension and Type 2 Diabetes Mellitus After a Hypertensive Disorder of Pregnancy or Gestational Diabetes Mellitus. Hypertension. 2015;66(6):1116–22. [DOI] [PubMed] [Google Scholar]
- 9.Mannisto T, Mendola P, Vaarasmaki M, Jarvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engeland A, Bjorge T, Klungsoyr K, Skjaerven R, Skurtveit S, Furu K. Preeclampsia in pregnancy and later use of antihypertensive drugs. Eur J Epidemiol. 2015;30(6):501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh NI, Norberg M, Ingelsson E, Cnattingius S, Vasan RS, Domellof M, et al. Association of Pregnancy Complications and Characteristics With Future Risk of Elevated Blood Pressure: The Vasterbotten Intervention Program. Hypertension. 2017;69(3):475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaway LK, Lawlor DA, O’Callaghan M, Williams GM, Najman JM, McIntyre HD. Diabetes mellitus in the 21 years after a pregnancy that was complicated by hypertension: findings from a prospective cohort study. Am J Obstet Gynecol. 2007;197(5):492 e1–7. [DOI] [PubMed] [Google Scholar]
- 14.Feig DS, Shah BR, Lipscombe LL, Wu CF, Ray JG, Lowe J, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. 2013;10(4):e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein FH. Late vascular effects of toxemia of pregnancy. N Engl J Med. 1964;271:391–5. [DOI] [PubMed] [Google Scholar]
- 16.Hyperinsulinemia Laivuori H. 17 years after preeclamptic first pregnancy. J Clin Endocrinol Metab. 1996;81(8):2908–11. [DOI] [PubMed] [Google Scholar]
- 17.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–51. [DOI] [PubMed] [Google Scholar]
- 18.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335(7627):978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manten GT, Sikkema MJ, Voorbij HA, Visser GH, Bruinse HW, Franx A. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy. 2007;26(1):39–50. [DOI] [PubMed] [Google Scholar]
- 20.Smith GN, Walker MC, Liu A, Wen SW, Swansburg M, Ramshaw H, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. 2009;200(1):58e1–8. [DOI] [PubMed] [Google Scholar]
- 21.Wolf M, Hubel CA, Lam C, Sampson M, Ecker JL, Ness RB, et al. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89(12):6239–43. [DOI] [PubMed] [Google Scholar]
- 22.Wu P, Kwok CS, Haththotuwa R, Kotronias RA, Babu A, Fryer AA, et al. Pre-eclampsia is associated with a twofold increase in diabetes: a systematic review and meta-analysis. Diabetologia. 2016;59(12):2518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drost JT, van der Schouw YT, Maas AH, Verschuren WM. Longitudinal analysis of cardiovascular risk parameters in women with a history of hypertensive pregnancy disorders: the Doetinchem Cohort Study. BJOG. 2013;120(11):1333–9. [DOI] [PubMed] [Google Scholar]
- 24.Rich-Edwards JWR, Roberts JM. Epidemiology of Pregnancy-Related Hypertension. In: Chesley’s Hypertensive Disorders in Pregnancy. Chesley’s Hypertensive Disorders in Pregnancy. 4th ed ed. Waltham (MA): Elsevier Inc; ; 2015:37–56. [Google Scholar]
- 25.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42(1):39–42. [DOI] [PubMed] [Google Scholar]
- 26.ACOG Committee on Practice Bulletins--Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–67. [DOI] [PubMed] [Google Scholar]
- 27.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52(5):828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. [DOI] [PubMed] [Google Scholar]
- 29.Group NDD. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–57. [DOI] [PubMed] [Google Scholar]
- 30.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130(4 Pt 1):278–84. [DOI] [PubMed] [Google Scholar]
- 31.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. [DOI] [PubMed] [Google Scholar]
- 32.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570–2. [PubMed] [Google Scholar]
- 33.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 35.Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat. 2009;5(1):Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26(20):3735–52. [DOI] [PubMed] [Google Scholar]
- 37.Localio AR, Stack CB, Griswold ME. Sensitivity Analysis for Unmeasured Confounding: E-Values for Observational Studies. Ann Intern Med. 2017;167(4):285–6. [DOI] [PubMed] [Google Scholar]
- 38.Mathur MBP, Riddell CA, Vanderweele TJ. Website and R package for computing E-values Epidemiology. 2018; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 40.Marin RM, Portal CG, Sánchez M, Sánchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy. 2000;19:199–209. [DOI] [PubMed] [Google Scholar]
- 41.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stuart JJ, Bairey Merz CN, Berga SL, Miller VM, Ouyang P, Shufelt CL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health (Larchmt). 2013;22(1):37–47. [DOI] [PubMed] [Google Scholar]
- 43.Seely EW, Tsigas E, Rich-Edwards JW. Preeclampsia and future cardiovascular disease in women: How good are the data and how can we manage our patients? Semin Perinatol. 2015;39(4):276–83. [DOI] [PubMed] [Google Scholar]
- 44.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.