Abstract
Interest in elucidating gut-brain-behavior mechanisms and advancing neuropsychiatric disorder treatments has led to a recent proliferation of probiotic trials. Yet, a considerable gap remains in our knowledge of probiotic efficacy across populations and experimental contexts. We conducted a cross-species examination of single- and multi-strain combinations of established probiotics. Forty-eight human (seven infant/child, thirty-six young/middle-aged adult, five older adult) and fifty-eight non-human (twenty-five rat, twenty-seven mouse, five zebrafish, one quail) investigations met the inclusion/exclusion criteria. Heterogeneity of probiotic strains, substrains, and study methodologies limited our ability to conduct meta-analyses.
Human trials detected variations in anxiety, depression, or emotional regulation (single-strain 55.6%; multi-strain 50.0%) and cognition or social functioning post-probiotic intake (single-strain 25.9%; multi-strain 31.5%). For the non-human studies, single- (60.5%) and multi-strain (45.0%) combinations modified stress, anxiety, or depression behaviors in addition to altering social or cognitive performance (single-strain 57.9%; multi-strain 85.0%). Rigorous trials that confirm existing findings, investigate additional probiotic strain/substrain combinations, and test novel experimental paradigms, are necessary to develop future probiotic treatments that successfully target specific neuropsychiatric outcomes.
Keywords: anxiety, depression, cognition, social behavior, stress, human, rat, mouse, zebrafish, quail, gut microbes
1. Introduction
Neuropsychiatric therapies are heterogeneous in their long-term efficacy (Geddes et al., 2000; McEvoy and Nathan, 2007; Serretti and Mandelli, 2010), with some having considerable harmful effects (Correll et al., 2009; De Hert et al., 2012; Lozano et al., 2008). Consequently, the biomedical research community has placed a great emphasis on developing novel treatments that target more objectively quantifiable brain and peripheral biomarkers (Insel, 2014; Niciu et al., 2014; Sanislow et al., 2015). Across organ systems, an increasing number of studies continue to highlight the importance of connections among the brain, mind, and body (Gallagher, 2004; Gold and Charney, 2002; Jones et al., 2006; Muehsam et al., 2017). Therefore, scientists and clinicians are designing studies to expand our knowledge of the bidirectional signaling mechanisms between gut microbes and the brain and their subsequent influence on behavior and mental health for potential neuropsychiatric treatment development (Mayer et al., 2014).
The precise relationship between dysbiosis (i.e. altered gut microbial composition) and neuropsychiatric symptoms in various cohorts is unclear and continues to be investigated. Initial studies in people diagnosed with Major Depressive Disorder (Aizawa et al., 2016; Jiang et al., 2015) indicate a relative increase in Bacteroidetes, Proteobacteria, and Actinobacteria phyla coinciding with a decline in Firmicutes (including Lactobacillus and Bifidobacterium). A relative decrease in Actinobacteria, Lentisphaerae, and Verrucomicrobia phyla has been associated with neuropsychiatric symptom severity in a sample of individuals with Post-Traumatic Stress Disorder (Hemmings et al., 2017). In a preliminary study in people with schizophrenia, a relative reduction in Proteobacteria (Haemophilus, Sutterella, and Clostridium), with a concurrent increase in Firmicutes (Anaerococcus) has been observed (Nguyen et al., 2018). This report also indicates negative symptoms may be uniquely linked to Firmicutes (Ruminococcaceae) colonization whereas current depression could vary by Bacteroidetes (Bacteroides) frequency.
Preliminary animal studies indicate that gut microbial alteration can influence a wide range of neurobehavioral phenotypes across the developmental trajectory (Bruce-Keller et al., 2015; Clarke et al., 2013; Park et al., 2013; Pyndt Jorgensen et al., 2015). One such study demonstrated that ampicillin was successful at restoring phencyclidine-induced gut microbe-cognitive dysregulation (Pyndt Jorgensen et al., 2015). However, there is a heightened awareness of the significant adverse effects antibiotics such as ampicillin can have on gut microbial diversity and physiological function (Dethlefsen et al., 2008; Dethlefsen and Relman, 2011), limiting its neuropsychiatric treatment utility. Moreover, the long-term beneficial versus detrimental effects of antibiotics on gut-brain-behavior interactions have not yet been characterized.
This has accelerated the pursuit of probiotics as a potential neuropsychiatric intervention. Probiotics are live microorganisms that confer health benefits to its host (Hill et al., 2014) with a minimal incidence of adverse effects (Marteau and Shanahan, 2003). Probiotic consumption to maintain gut and overall health has now been implemented in routine medical practice (Gareau et al., 2010). Probiotics have shown significant promise for improving atopic dermatitis, necrotizing enterocolitis, pouchitis, and irritable bowel syndrome - IBS (Sanders et al., 2013), which is postulated to be a gut-brain dysregulation disorder (Blankstein et al., 2010; Kennedy et al., 2014; Kennedy et al., 2012).
However, probiotic trials designed to target stress levels, mood, cognitive, or psychosocial functioning, have only been conducted during the past ten to fifteen years. In addition, the utility of probiotic treatments to improve neuropsychiatric symptoms have not been established. Moreover, while multi-strain probiotic combinations may provide greater health benefits in comparison to single-strains of probiotics for several infections and gastrointestinal disorders (Chapman et al., 2011; Timmerman et al., 2004), this has not yet been evaluated for human or non-human neuropsychiatric outcome trials.
This review examined published probiotic trials conducted across animal species to determine if single- or multi-strain formulations of probiotics have differential effects in modifying neuropsychiatric symptoms or phenotypes.
2. Materials and Methods
The authors of this article are not associated with any of the trials that were examined. This review compared neuropsychiatric outcomes associated with single- and multi-strain probiotic treatments in humans and translational non-human animal models while adhering to systematic review (PRISMA) guidelines (Moher et al., 2009; Shamseer et al., 2015). Studies comprising this article were ascertained with the PubMed Advanced Search Builder http://www.ncbi.nlm.nih.gov/pubmed/advanced and filtered by the English language. Publication dates were unrestricted and ranged from 2006 to 2018. The most recent search was conducted on April 18, 2018. The PubMed searches were supplemented with a collection of original reports from prior systematic review or hypothesis articles obtained from the Cochrane Database of Systematic Reviews http://www.cochranelibrary.com/cochrane-database-of-systematic-reviews/ with the search terms noted below.
Database search keywords were selected to align this review with anxiety, mood, and psychotic disorder phenotypes. Exact search terms included probiotic and (PubMed/Cochrane Database) well-being (32/13), psychological stress (104/10), anxiety (145/30), worry (2/1), depression (171/1), mood (1133/12), bipolar disorder (7/1), mania (8/1), schizophrenia (19/4), psychosis (4/5), post-traumatic stress disorder (1/0), obsessive-compulsive disorder (4/0), negative symptoms (325/27), learning (46/7), memory (82/11), cognition (52/10), motivation (22/3), reward (2/0), social behavior (38/5), social function (16/1), and sickness behavior (16/0), an inflammation-mediated depression phenotype (Brydon et al., 2009).
Most trials selected for review utilized established probiotics belonging to the Bifidobacterium or Lactobacillus genera (Fijan, 2014; Hill et al., 2014). Investigations of Bacillus subtilis, Clostridium butyricum, Enterococcus faecium, Escherichia coli Nissle, Lactococcus lactis, Pediococcus acidilactici, Saccharomyces boulardii, Saccharomyces cerevisiae, and Streptococcus thermophilus, additional species with recognized probiotic properties, were also included in this review. Investigations with Leuconostoc genera were not available with our defined search criteria. Precise substrains varied by trial.
All case reports, retrospective studies, review articles, non-experimental studies (e.g. internet or survey-based), non-randomized or placebo-controlled trials (human), or reports that failed to include a control group (non-human), were eliminated. Since probiotics are defined as live microorganisms (Hill et al., 2014), studies that only investigated heat-killed probiotics were removed (e.g. Shinkai et al. (2013). Additional reports were rejected if the only microbial treatment was classified as a pathogenic or engineered strain (Miyazawa et al., 2015; Shinkai et al., 2013). Infant trials in which probiotic outcomes were assessed in the mothers but not the consuming infants (Mi et al., 2015; Sung et al., 2014) were also excluded.
All trials that failed to assay neuropsychiatric phenotypes were omitted from this review. However, the physiological outcomes from otherwise eligible studies that exposed non-human animals to neuropsychological stress as part of the experimental paradigm have been recorded in Table A.1 (Appendix).
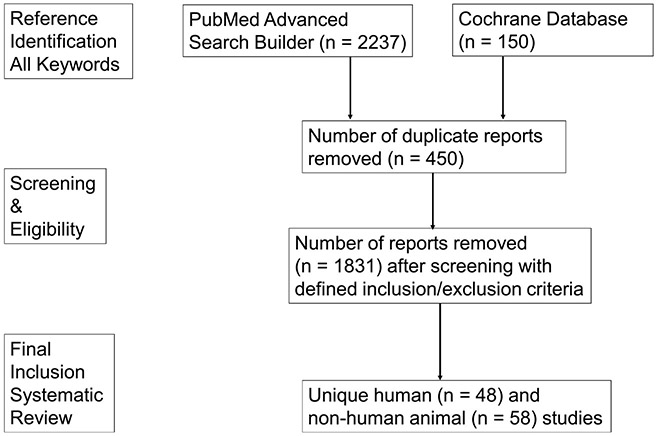
Figure 1 delineates the number of articles from each stage of our literature search strategy to yield the final set of human (Table 1) and non-human (Table 2) trials for review.
Figure 1.
Systematic Review Flow Diagram.
Table 1.
Human Probiotic Trial Designs and Outcomes.
| Probiotic Treatment |
Blinding Status and Study Design |
Sex and Age, years |
Anxiety/ Depression / Emotional Behavior |
Cognition/ Social Function |
Other Probiotic Associated Outcomes |
Reference |
|---|---|---|---|---|---|---|
| Infant/Child | ||||||
| L. rhamnosus GG ATCC531031 × 1010 CFU (n = 46) or placebo (n = 47) Q.D. breastfeeding mothers 14 to 28 days pre-gestation and infants 180 days post-gestation | Double-blind Infants having a mother, father, or sibling with atopic dermatitis, allergic rhinitis, or asthma | Sex not reported Age pre-gestation w/followup at 3, 6, 12, 18 and 24 months | *NSE on fussing/crying | §NA |
#PROT ↓ fecal Clostridia at 6 and 24 months *NSE fecal Bifidobacterium, Bacteroides, Lactobacillus, or Enterococcus |
(Rinne et al., 2006) |
| L. renteri DSM 17938 1 × 108 CFU (n = 238) or placebo (n = 230) drops P.O. Q.D. for 90 days | Double-blind 38-40-week gestation, Apgar >8 at 10 min, normal birth weight Exclusions: antibiotic, antacid, or PPI use |
Male (n = 242) Female (n = 226) Age 0.019 (< 7 days) |
#PROT ↓ fussing or crying | §NA | #PROT ↓ constipation, regurgitation, and healthcare costs | (Indrio et al., 2014) |
| L. reuteri DSM 17938 1 × 106 CFU/g (n = 60) or placebo whey formula (n = 62) Q.D. for 98 days | Double-blind Gestation ≥37 weeks, singleton-birth weight 2500 - 4500g, formula-fed 3 days prior to enrollment. Exclusions: Cow milk allergy, medical disease, hospitalized, IV antibiotic or oral medication (except thrush), #PROT 7 days before enrollment |
Male (n = 78) Female (n = 86) Age 0.038 (14 days) |
#PROT ↑ irritability 14 days post - treatment (*NSE overall) | §NA | *NSE weight gain, sleep, body length, head size, stool | (Cekola et al., 2015) |
| L. renteri DSM 17938 1 × 108 CFU (n = 85) or placebo (n = 82) 5 drops P.O. Q.D. for 30 days | Double-blind Wessel’s colic Exclusions: <2500 g birth weight, medical disease, cow milk allergy, antibiotic or L. reuteri use by infant or mother before enrollment |
Male (n = 85) Female (n = 82) males > Age < 0.249 (91 days) |
*NSE on fussing/cry ing in breastfed infants #PROT ↑ fussing crying in formula fed infants |
§NA | *NSE maternal mental health | (Sung et al., 2014) |
| L. reuteri ATCC55730 (n = 20), B. lactis BB-12 (n = 20) 1 × 107 CFU/g, or placebo (n = 19) in formula Q.D. for 28 days | Double-blind All infants were formula-fed by parental choice prior to enrollment Exclusions: <36 weeks of gestation, chronic disease, congenital abnormalities, <2500 g birth weight, allergies, atopic disease, probiotic exposure within 4 weeks of enrollment |
Female (n = 19) Male (n = 40) Mean Age 0.093 (3-65 days) |
*NSE on fussing/crying | §NA | *NSE crying, night awakening, daily gas, stool effort and consistency | (Weizman and Alsheikh, 2006) |
| L. reuteri Biogaia AB (n = 124) 1 × 108 CFU or placebo (n = 125)5 drops Q.D. from birth until discharge | Double-blind Preterm infants with a gestational age of ≤ 32 weeks, birth weight ≤1500 g, Follow-up analyses of (Oncel et al., 2014) | Male (n = 133) Female (n = 116) Age birth and follow up 18 to 24 months |
§NA | *NSE neurocognition (BSID-II-PDI and MDI) | *NSE visual impairment, hearing impairment | (Akar et al., 2017) |
| S. thermophilus, B. animalis ssp. lactis BB-12, and L. bulgaricus General Mills 5 × 109 CFU/100 mL + 1 g inulin (n =76) yogurt drink or non-synbiotic acidified placebo milk drink (n =73) Q.D. for 112 days | Double-blind Child care attendees Exclusions: premature or low birth weight, allergy, atopic, or medical disease, GI surgery, lactase deficiency or milk intolerance, antibiotic use within 4 or probiotic use within 2 weeks of study enrollment |
Males (n = 74) Females (n = 75) Age 1-4 Mean Age 2.5 |
§NA | #PROT ↑ in social function (Ped-QOL) |
#PROT ↑ in school function (Ped-QOL), watery stool #PROT ↓ fever |
(Ringel-Kulka et al., 2015) |
| Older Adult | ||||||
| L. reuteri DSM 17938 1 × 108 CFU stick pack with rhamnose, galactooligosacch aride B.I.D. (n = 125) or placebo (n = 124) for 84 days | Double-blind Age 65+ Exclusions: GI or IBD, use of GMMS or PPI, participation in other clinical trials within 3 months of enrollment |
Male (n = 97) Female (n = 152) Age 65+ Mean age 72.3 |
*NSE stress, anxiety, or well-being (HADS, PSS, EQ-5D-5L) | §NA | *NSE GSRS | (Ostlund-Lagerstrom et al., 2016) |
| 65 mL milk drink w/ L. casei Shirota 1 × 108 CFU/mL Q.D. or placebo for 21 days | Double-blind Self-reported healthy older adults with stable hypertension for 3 months and diabetes mellitus under dietary or medication control | Male (n = 51) Female (n = 75) Age 48-79 Mean age 61.8 |
#PROT ↓ depression only for sample subset with poor baseline mood (POMS) |
*NSE verbal fluency, episodic memory #PROT ↓ mental clarity |
#PROT ↑ confidence *NSE eating behavior |
(Benton et al., 2007) |
| L. helveticus IDCC3801 four 125mg (n = 10), 250mg (n = 7), 500mg (n = 9) or placebo (n = 10) tablets Q.D. for 84 days | Double-blind Proficiency using computers, education above middle school, ≥ 24 MMSE- Korean, BMI≤ 16 and ≤ 35 Exclusions: Axis I disorder or Axis I treatment within 5 years of enrollment, ≥ 8 GDS-SF, alcohol abuse/dependence within 3 months, GI disease or GI surgery, significant neurological or medical illnesses, supplement or herbal medicine use during the 4 weeks prior to enrollment, compliance < 70% at each study visit |
Male (n = 20) Female (n = 16) Age 60-75 Mean Age 65.0 |
*NSE stress (PSS) and depression (GDS-SF) | #PROT ↑ RVIP | *NSE serum BDNF and whole blood viscosity | (Chung et al., 2014) |
| VSL#3 4.5 × 1011 CFU B.I.D. (n = 10) or placebo (n = 8) for 28 days after surgery | Double-blind Laparoscopic colorectal surgery patients | Male (n = 9) Female (n = 9) Age 70+ Mean Age 72.2 |
§NA | #PROT ↑ social function (SF-36) | #PROT ↓ bowel movement | (Pellino et al., 2013) |
| 200 mL milk drink with L. acidophilus, L. casei, L. fermentum, B. bifidum Tak Gen Zist Pharmaceuticals 2 × 109 CFU/g (n = 30) or placebo (n = 30) for 84 days | Double-blind NINDS-ADRDA diagnosis of Alzheimer’s Disease Exclusions: chronic/metabolic illnesses, probiotic consumption 6 weeks prior to enrollment |
Female (n = 48) Male (n = 12) Age 60-95 Mean Age 79.8 |
§NA | #PROT ↑ cognition (MMSE) |
#PROT ↓ VLDL, QUICKI, MDA, HOMA-B, hs-CRP #PROT ↑ HOMA-IR |
(Akbari et al., 2016) |
| Young/Middle-Aged Adult | ||||||
| Fermented milk L. casei Shirota 1 × 109 CFU/mL Q.D. (n = 24) or placebo drink (n = 23) for 56 days | Double-blind Healthy Japanese Students Exclusions: smokers, age > 30, allergies, mental or medical disease, medication use |
Male (n = 26) Female (n = 21) Age < 30 Mean Age 27.9 |
#PROT ↓ anxiety (STAI) one day before exam | §NA |
#PROT ↑ increase fecal serotonin post-exam #PROT ↓ incidence cold and abdominal illness |
(Kato-Kataoka et al., 2016) |
| Fermented milk with L. casei Shirota 1 × 109 CFU/mL Q.D. (n = 52**) or placebo (n = 41**) for 56 days **new subjects added to study above | Double-blind Healthy Japanese students Exclusions: smokers, age > 30 years, taking medications, mental illness, and score over 60 on SDS |
Male (n = 76) Females (n = 64) Age < 30 Mean Age 22.9 |
*NSE anxiety (STAI) | §NA | #PROT ↓ cold/flu symptoms and cortisol 1 day before exam See Table 2 for Rat Trial Outcomes | (Takada et al., 2016) |
| Fermented milk L. casei Shirota 1 × 109 CFU/mL Q.D. (n = 48**) or placebo drink (n = 46**) for 56 days before exam and 14 days after **pooled sample with above studies | Double-blind 4th grade medical students Exclusions: age > 30 years; physical or mental illness, taking medications; smoker, milk or food allergy |
Male (n = 55) Female (n = 39) Age < 30 Mean Age 22.7 |
#PROT ↑ stress (exam) induced sleep disturbance | #PROT ↑ delta power > 20% immediately prior to exam | §NA | (Takada et al., 2017)Takada et al., 2017) |
| L. casei Shirota 2.4 × 109 CFU (n = 19) or placebo (n = 16) Q.D. for 60 days | Double-blind Stable Chronic Fatigue Syndrome Exclusions: bedridden, meeting criteria for neuropsychiatric disorders except depression or anxiety |
Male (n = 8) Female (n = 27) Age 18-65 Mean Age 41.2 |
#PROT ↓ depression (BDI) | §NA | §NA | (Rao et al., 2009) |
| L. casei Shirota 1 × 1010 CFU Q.I.D. (n = 36) or placebo (n = 36) for 21 days | Double-blind Smokers mean cigarettes/day = 20 Exclusions: other health problems |
Male (n = 72) Age 40-60 Mean Age 50.3 |
*NSE anxiety (STAI) or job stress | §NA | #PROT ↑ NK cell activity | (Reale et al., 2012) |
| L. rhamnosus CGMCC1.37241.6 × 108 CFU/capsule B.I.D. (n = 62) or placebo (n = 63) for 168 days | Double-blind BMI 29 - 41 kg/m2, weight change < 5 kg 3 months before screening Exclusions: pregnancy, breastfeeding, menopause or obesity comorbidities |
Male (n = 45) Female (n = 60) Age 18-55 Mean Age 36.0 |
#PROT ↓ depression (BDI) *NSE PSS, STAI, BES |
#PROT ↑ cognitive restraint | #PROT ↓ body esteem, and ↓ weight, hunger, and disinhibition in female subjects only | (Sanchez et al., 2017) |
| L. helveticus Lafti 2 × 1010 CFU (n = 20) or placebo (n = 19) Q.D. for 98 days | Double-blind National or European/World championship athletes Exclusion: chronic diseases, surgery within 1 year of enrollment, probiotic sensitivity, probiotic or antibiotic use 1 month before enrollment |
Male (n = 29) Female (n = 10) Age 20-26 Mean Age 23.2 |
#PROT ↑ vigor (POMS) | §NA |
*NSE exercise performance #PROT ↑ blood CD4+/CD8+ cells |
(Michalickova et al., 2016)Michalickova et al., 2016) |
| L. helveticus R0052 (n = 145), B. longum ssp. infantis R0033 (n = 147), B. bifidum R0071 (n = 142) capsule 3 × 109 CFU Q.D. or placebo (n = 147) for 42 days | Double-blind Nonsmoking undergraduate students Exclusions: allergies, current cold, antibiotic use 2 months prior to study enrollment |
Male (n = 210) Female (n = 371) Age 18-22 Mean Age 19.9 |
B. bifidum ↓ self-reported stress | §NA |
L. helveticus ↑ diarrhea B. bifidum ↓ diarrhea and incidence cold/flu B. bifidum interaction of stress and sleep *NSE BMI stress interaction |
(Culpepper et al., 2016; Langkamp-Henken et al., 2015) |
| L. rhamnosus HN001 (n = 212) 6 × 109 or placebo (n = 211) for 168 days post gestation and 168 days after birth | Double-blind English-speaking women at 14–16 weeks of gestation, breastfeeding Exclusions: medical problems related to pregnancy, age <16 years, planning to move outside the study center during study, probiotic use, subject or unborn child’s biological father had a history of asthma, hay fever, or eczema requiring medication |
Female (n = 423) Mean Age 33.6 |
#PROT ↓ depression (EPDS) and anxiety (STAI6) | §NA | §NA | (Slykerman et al., 2017) |
| B. animalis ssp. lactis-07 109 CFU (n = 41), B. animalis ssp. lactis-07 2 × 109 CFU + xylooligosaccharide 8g/d (n = 41), xylooligosacchari de 8g/d (n = 41), or maltodextrin placebo (n = 41) Q.D. for 21 days | Double-blind Crossover design Healthy adults, BMI 20–30 kg/m2 Exclusions: physical or mental illness requiring medication or inpatient/outpatient treatment, planned major surgery, history of drug or alcohol abuse, severe allergies or a history of abnormal drug reaction, chronic GI complaints or GI drug use 4 weeks before enrollment, anti-inflammatory or prescription medication use, participation in an experimental drug trial 4 weeks before enrollment, participation in prebiotic or laxative trials within 3 months or use of antibiotics within 6 months of enrollment |
Male (n = 22) Female (n = 22) Age 31–55 Mean Age 43 |
#PROT ↓ self-reported happiness PRET ↑ self-reported vitality and happiness * NSE SYNT |
§NA | *NSE bowel function, plasma HDL, SCFA, Bifidobacterium, Bacteroides/Prevotella, Clostridium, Lactobacillus/Enterococcus/Atopobium | (Childs et al., 2014) |
| S. boulardii Bioflor 2 × 1011 CFU/capsule two capsules B.I.D. (n = 45) or placebo (n = 45) for 28 days | Double-blind Rome II IBS mixed or diarrhea predominant Exclusions: IBS constipation predominant, pregnant/lactating, chronic medical illness, history of abdominal surgery except appendectomy or hernia |
Male (n = 44) Female (n = 46) Age 27-53 Mean Age 41.0 |
*NSE dysphoria (IBS QOL) | #PROT ↑ social functioning (IBS QOL) | #PROT ↓ activity interference (IBS QOL) | (Choi et al., 2011) |
| 1.31 high dose 1-3 × 1010 CFU (n = 28), low dose 3-6 × 109 CFU Q.D.(n = 27), or placebo (n = 29) for 42 days | Double-blind Rome III IBS with diarrhea Exclusions: celiac disease, IBD, or antibiotic/probiotic use 4 weeks prior to enrollment |
Male (n = 31) Female (n = 53) Age 20-70 Mean age 46.8 |
Low and high dose #PROT ↑ mental status (IBS QOL) Low and high dose #PROT ↓ gut specific anxiety |
§NA | *NSE IBS symptoms | (Lorenzo-Zuniga et al., 2014) |
| L. acidophilus NCFM low dose 1 × 109 (n = 112) high dose 1 × 1010 (n = 113) or placebo (n = 115) Q.D. for 84 days | Double-blind Rome III IBS Exclusions: Other GI disease or probiotic use within 3 months of enrollment |
Male (n = 99) Female (n = 292) Age 18-65 Mean Age 47.9 |
Low and high dose #PROT ↓ anxiety (HADS) high dose #PROT ↓ depression (HADS) |
§NA | #PROT ↓ pain for moderate to severe symptom groups | (Lyra et al., 2016) |
| S. cerevisiae CNCM I-3856 8 × 109 CFU/g 1000 mg tablet Q.D. (n = 192) or placebo (n = 187) for 84 days | Double-blind Rome III IBS pain/discomfort ≥ 1 day/week, normal CRP and fecal calprotectin Exclusions: pregnancy, vegetarian, lactose intolerance, gluten sensitivity, antibiotic, antidepressant, opioid, narcotic, or chronic alcohol use |
Male (n = 62) Female (n = 317) Age 18 to 75 Mean Age 45.3 |
*NSE well-being (IBS-QOL) | §NA | #PROT ↓ bloating and pain for IBS constipated subgroup | (Spiller et al., 2016) |
| L. casei ssp. rhamnosus LCR35 2 × 108 CFU T.I.D. (n = 25) or placebo (n = 25) for 28 days | Double-blind Rome III IBS Exclusions: current depression (HAM-D) |
Male (n = 15) Female (n = 35) Age 34-59 Mean Age 47.1 |
*NSE anxiety and depression (HAD) | §NA | #PROT ↓ IBS severity in diarrhea subgroup | (Dapoigny et al., 2012) |
| L. gasseri SBT2055 5 × 108 CFU and B. longum SBT2928 1 × 109 CFU in 100g yogurt with S. thermophilus and L. delbrueckii subsp. Bulgaricus (n = 115) or 100g placebo yogurt with S. thermophilus and L. delbrueckii subsp. bulgaricus (n = 109) Q.D. for 84 days | Double-blind Healthy Japanese volunteers Exclusions: history of significant medical illness, frequent intake of the test yogurt, diarrhea or viral syndrome within the past 30 days, current use of any prescribed medication, use of any other supplements during the trial, pregnancy, smoking > 20 cigarettes/day, drinking > 20 g alcohol/day, history of severe allergic reactions to food and medication |
Male (n = 69) Female (n = 155) Age 32 to 76 Mean Age 53.9 |
#PROT ↓ anxiety (GHQ-28) | §NA |
#PROT ↓ NK cell activity, ACTH, salivary and serum cortisol (males only) *NSE CRP, IgG, IgE |
(Nishihira et al., 2014) |
| L. gasseri CP2305 1 × 1011 CFU T.I.D. (n = 17) or placebo (n = 17) for 28 days | Double-blind Rome III IBS Exclusions: organic GI diseases, severe systemic diseases, pregnant, lactating, history of significant abdominal surgery, severe endometriosis, neurological disorders, or dementia |
Male (n = 15) Female (n = 19) Age 19-82 Mean Age 49.3 |
#PROT ↓ health related worry (IBS-QOL) | §NA | #PROT ↓ Dorea, Enterococcus and Dialister genera | (Nobutani et al., 2017) |
| B. longum NCC3001 1 × 1010 CFU/g Q.D. powder (n = 22) or maltodextrin placebo (n = 22) for 42 days | Double-blind Rome III IBS mixed or diarrhea predominant and HAD score of 8-14 Exclusions: psychiatric disorder except anxiety or depression, antidepressant or anxiolytic use, probiotic use within 1 month or antibiotic use within 3 months of enrollment |
Male (n=20) Female (n=24) Age 26-58 Mean Age 43.0 |
#PROT ↓ depression (HAD-D) *NSE anxiety (HAD-A) |
#PROT ↓ amygdala, frontal, and temporal and ↑ occipital engagement in response to fearful stimuli *NSE SF-36 social function |
#PROT ↑ SF-36 physical *NSE constipation, diarrhea, or pain |
(Pinto-Sanchez et al., 2017) |
| B. longum 2 × 109 CFU (n = 31), 8g psyllium (n = 31), or B. longum + psyllium (n = 32) Bificolon, Nisshin Kyorin Pharmaceuticals Q.D. for 28 days | Double-blind UC mild or remitted w/out UC surgical history Exclusions: UC induction therapy or unstable prednisone or aminosalicylate dose 4 weeks before enrollment |
Male (n = 39) Female (n = 55) Mean Age 36.0 |
#PROT ↑ IBD QOL emotional | SYNT ↑ IBD QOL social | SYNT ↓ CRP SYNT ↑ IBD QOL greater than #PROT or PRET |
(Fujimori et al., 2009) |
| Ecologic® Barrier powder 2.5 × 109 CFU/g Q.D. (n = 20) or placebo (n = 20) for 28 days | Triple-blind Nonsmoking adults Exclusion: Current mood disorder |
Male (n = 8) Female (n = 32) Age 18-23 Mean Age 20.0 |
#PROT ↓ rumination (LEIDS) #PROT ↓ aggressive thoughts *NSE BAI or BDI |
§NA | §NA | (Steenbergen et al., 2015) |
| VSL#3 1.125 × 1011 CFU (n = 57) or placebo (n = 48) Q.D. for 14 days | Double-blind Healthy students subjected to acute psychological stress Exclusions: diagnosed GI disorder, prior probiotic or current antibiotic use |
Male (n = 36) Female (n = 69) Age 18–23 Mean Age 20.2 |
*NSE stress (PASAT) | *NSE (PASAT) | *NSE heart rate, blood pressure (systolic or diastolic) | (Moller et al., 2017) |
| Fermented milk B. animalis ssp. lactis 1.25 × 1010, S. thermophilus 1.2 × 109, L. bulgaricus 1.2 ×109 L. lactis ssp. lactis (n = 12) CFU/cup, non-fermented milk B.I.D. (n = 11), or no probiotic milk drink (n = 13) for 28 days | Double-blind Healthy adults BMI 18-30 kg/m2 Exclusions: GI or psychiatric symptoms |
Female (n = 35) Age 18-55 Mean Age 36.5 |
§NA | #PROT ↓emotion task response in affective, viscerosensory, somatosensory cortices | §NA | (Tillisch et al., 2013) |
| 100g Yogurt with L. acidophilus LA5 and B. lactis BB-12 1 × 107 CFU + one placebo capsule (n = 25); one probiotic capsule L. casei 3 × 103, L. acidophilus 3 × 107, L. rhamnosus 7 × 109, L. bulgaricus 5 × 108, B. breve 2 × 1010, B. longum, S. thermophilus 3 × 108 (CFU/g), and fructooligosaccharide 100mg + conventional yogurt 100g Q.D.(n = 25); or conventional yogurt 100g + one placebo capsule (n = 20) for 42 days | Double-blind Petrochemical workers without chronic illness Exclusions: antibiotic, vitamin, or supplement use |
Male (n = 36) Female (n = 34) Age 20–60 Mean Age 40.0 |
#PROT yogurt and capsule ↓ depression and anxiety (DASS) #PROT yogurt and capsule ↓ GHQ scores |
§NA | *NSE plasma Tryptophan, Cortisol, ACTH or Neuropeptide Y levels | (Mohammadi et al., 2016) |
| L. helveticus R0052 and B. longum R0175 3 × 109 CFU Q.D. (n = 26) or placebo (n = 29) for 30 days | Double-blind Healthy Caucasian adults Exclusions: neurological, psychiatric, renal, hepatic, cardiovascular and respiratory diseases, pregnancy, food allergy, clinical trial participation within two months of study enrollment, psychotropic drug or stimulating nutritional supplements (vitamin C), ginger, guarana, ginseng, dehydroepiandrosterone, melatonin, antioxidants, anxiolyics, antidepressants, selenium, narcotics, replacement hormoes, more than 5 cups of coffee or tea/d, 0.2 liters of cola, 30–40 g of chocolate, three glasses of wine, or two fermented dairy products, or smoking >20 cigarettes |
Male (n = 14) Female (n = 41) Age 30-59 Mean Age 42.8 |
#PROT ↓ depression (HADS-D) and anxiety (HADS-A) #PROT ↓ psychological distress (HSCL-90) #PROT ↓ stress (PSS) in patients with low urinary cortisol |
§NA | ↓ Urinary cortisol See Table 2 for outcomes in rats | (Messaoudi et al., 2011a; Messaoudi et al., 2011b) |
| L. acidophilus Rosell-52 and B. longum Rosell-175 3 × 109 CFU Q.D. (n = 37) or placebo (n = 38) for 21 days | Double-blind Healthy subjects experiencing 2 or more daily stress symptoms (anxiety, nervous, irritable, sleeping problems, GI disturbance) for 30 days prior to enrollment | Male (n = 21) Female (n = 54) Age 27-49 Mean age 38.0 |
*NSE psychological stress #PROT ↓ stress induced abdominal pain and nausea |
§NA | §NA | (Diop et al., 2008) |
| L. helveticus R0052 and B. longum R0175 3 × 109 CFU Q.D. (n = 40) or placebo (n = 39) for 56 days | Double-blind ⩾11 on the QIDS-SR16 or ⩾14 on the DASS-42, age > 16 Exclusions: medical disorder, pregnant or lactating, antidepressant use, suicide or violence risk, probiotic, antibiotic, or psychotropic medication use 4 weeks prior to trial |
Male (n = 17) Female (n = 62) Mean Age 35.0 |
*NSE depression or anxiety (MADRS, QIDS-SR16, DASS) | §NA | *NSE iCGI-SiCGI-I, GAF, CRP, IL-6, TNF-α, IL-1β | (Romijn et al., 2017) |
| L. acidophilus, L. casei, and B. bifidum Tak Gen Zist Pharmaceutical Company (2 × 109 CFU/g Q.D. (n = 20) or placebo (n = 20) for 56 days | Double-blind DSM-IV diagnosis Major Depressive Disorder | Male (n = 6) Female (n = 34) Age 20-55 Mean Age 37.3 |
#PROT ↓ depression (BDI) #PROT ↓ anxiety (BAI) |
§NA |
#PROT ↓ HOMA-IR #PROT ↓ CRP #PROT ↑ glutathione |
(Akkasheh et al., 2016) |
| S. thermophilus SGst01, B. animalis ssp. Lactis SGB06, S. thermophiles, B. bifidum SGB02, L. delbrueckii ssp. Bulgaricus DSM20081, L. acidophilus SGL11, L. plantarum SGL07, L. reuteri SGL01 1.5 × 1010 CFU each in corn maltodextrin, silica, casein, lactose, and gluten (n = 24) or placebo (n = 24) Q.D. for 21 days | Double-blind Crossover design normal weight lean, normal weight obese, pre-obese/obese groups BMI range 19.5 – 30 kg/m2 | Female (n = 48) Age 27-56 Mean Age 34.6 |
#PROT ↓ depression (SCL90R) in normal weight lean #PROT ↓ anxiety (SCL-90R) in pre-obese/obese |
#PROT ↓ avoidance (BUT) *NSE social insecurity, interpersonal distrust, interceptive awareness (EDI-2) |
#PROT ↓ psychotic symptoms (SCL-90R) in normal weight obese and pre-obese/obese | (De Lorenzo et al., 2017) |
| L. acidophilus, L. casei, B. bifidum, and L. fermentum Tak Gen Zist Pharmaceutical Company 2 × 109 CFU/g each Q.D. (n = 30) or placebo (n = 30) for 84 days | Double-blind McDonald RRMS Exclusions: pregnant or lactating, probiotic or prebiotic use prior to trial enrollment |
Male (n = 10) Female (n = 50) Age 18-55 Mean Age 34.1 |
#PROT ↓ depression and anxiety symptoms (BDI, GHQ, DASS) | §NA |
#PROT ↓ insulin, NO, CRP, MDA, HOMA-IR, HOMA-B #PROT ↑ QUICKI, HDL |
(Kouchaki et al., 2016) |
| Lacidofil® 2 × 109 CFU (n = 28) or placebo (n = 32) B.I.D for 84 days | Double-blind Stage 2 or 3 colorectal cancer age > 20, completed treatments between 6 weeks and 2 years prior to enrollment Exclusions: histories of other cancers, colostomies, probiotic consumption, physical or mental disability, chronic diseases, antibiotic use, pregnancy, abnormal liver function, kidney function, or blood cell counts |
Male (n = 35) Female (n = 25) Age 45-67 Mean Age 56.2 |
*NSE emotional well-being (FACT-EWB) #PROT ↓ anxiety and depression (PHQ-9 within group only–NSE between groups) |
*NSE social well-being (FACT-SWB) | #PROT ↓ bowel symptoms, functional well-being, cancer related FACT | (Lee et al., 2014) |
| Bifidobacterium combined with fructooligosaccharide CFU not reported (n = 63) or placebo (n = 62) for 60 days | Double-blind HE - West haven grade 1 or 2 hepatitis B (n = 35), hepatitis C (n = 70), or cryptogenetic cirrhosis (n = 20) venous ammonia >50 mmol/L Exclusions: West haven grade 3+, alcoholism, diabetes mellitus |
Male (n = 62) Female (n = 63) Mean Age 50.1 |
§NA |
#PROT ↓ TMT A and B times, #PROT ↑ Symbol Digit Modalities and Block Design Test |
#PROT ↓ blood ammonia levels | (Malaguarnera et al., 2010) |
| L. rhamnosus GG (n = 14) or placebo (n = 16) 5.5 × 1010 CFU B.I.D. for 56 days | Double-blind Cirrhosis with minimal hepatic encephalopathy Exclusions: alcohol use within 6 months, upper GI bleeding or systemic antibiotics within 6 weeks, current or past treatment for HE, hepatocellular cancer, yogurt/probiotic consumption within 2 weeks, inflammatory bowel disease, history of pancreatitis, psychoactive medication use except anti-depressants, recent absolute neutrophil count <500/mm3 and liver transplant |
Male (n = 25) Female (n = 12) Age 47-65 Mean Age 57.4 |
§NA |
#PROT ↑ SIP social function *NSE digit symbol or block design tests |
#PROT ↓ Enterobacteriaceae abundance #PROT ↓ Clostridiales Incertae Sedis XIV and Lachnospiraceae abundance |
(Bajaj et al., 2014) |
| L. rhamnosus JB-1 1 × 109 CFU (n = 29) or placebo (n = 29) capsules Q.D. for 56 days | Blinding status not reported Crossover design Healthy adult (n = 29) Exclusions: acute or chronic illness, neuropsychiatric disorder (MINI), immunodeficiency, bleeding disorder, color blindness, dyslexia, dyscalculia, experimental drug trial participation, diet, probiotics, antibiotics, antipsychotics, anxiolytics, laxatives, enemas, anti-coagulants NSAIDS, antidepressants or consumption of any psychotropic medication |
Male (n = 29) Age 20-33 Mean Age 24.6 |
*NSE stress, anxiety, depression (PSS, BDI, BAI, STAI, SECPT, Pittsburgh sleep quality index) | *NSE PAL, AST, RVIP, emotional Stroop or recognition task | *NSE IL-1β, IL-6, IL-8, IL-10, TNF-α | (Kelly et al., 2017) |
| C. butyricum 420 mg capsule 1.0 × 107 CFU/g B.I.D. 14 days until one day before surgery (n = 10) or placebo (n = 10) | Blinding status not reported may be single-blind Cancer patients scheduled for laryngectomy | Male (n = 10) Female (n = 10) Age 45-67 Mean 56.075 |
#PROT ↓ anxiety (HAM-A) | §NA | #PROT ↓ serum CRF and heart rate before surgery | (Yang et al., 2016b) |
| L. acidophilus ATCC4356 1.25 × 109 CFU B. longum ATCC15707 1.35 × 109 CFU in vitamin supplement T.I.D. (n = 20) or vitamin supplement without #PROT (n = 20) for 56 days | Blinding Status not reported Irritable Eye Syndrome Exclusions: IBS, systemic, or neuropsychiatric disease |
Groups matched by sex (n = 40) Age 39-53 Mean Age 45.5 |
#PROT ↓ anxiety and depression (HAD) | §NA | #PROT ↓ WBC, monocyte, IL-6, and TNF-α | (Feher et al., 2014) |
| L. rhamnosus GG and B. lactis BB-12 1 × 109 CFU (n = 33) or placebo (n = 32) Q.D. for 98 days | Blinding status not reported DSM-IV schizophrenia or schizoaffective outpatient, PANSS positive ≥ 1 and/or negative ≥ 4 or total ≥ 50, with at least 3 positive or negative items with scores ≥3 at screening, no antipsychotic medication changes within 21 days of enrollment Exclusions: mental retardation, celiac or medical disorder, DSM-IV substance abuse within 3 months, drug trial within 30 days of study enrollment, pregnant or lactating, antibiotic use within 14 days of enrollment |
Male (n = 42) Female (n = 23) Age 18-65 Mean Age 46.3 |
*NSE PANSS general, negative, or positive symptoms | §NA |
#PROT ↓ symptoms in presence of C. albicans antibodies #PROT ↓ constipation |
(Dickerson et al., 2014; Severance et al., 2017; Tomasik et al., 2015) |
Abbreviations:
NA = Not assessed,
NSE = No significant effect of probiotic treatment,
PROT = Probiotic Treatment, ACTH = Adrenocorticotropic hormone, ADHD = Attention Deficit Hyperactivity Disorder, ASD = Autism Spectrum Disorder, AST = Attention Switching Task, ATCC = American Type Culture Collection, BAI = Beck Anxiety Inventory, BDI = Beck Depression Inventory, BDNF = Brain Derived Neurotropic Factor, BES = Binge Eating Scale, B.I.D = Twice a day, BMI = Body Mass Index, BSID-II-MDI = Bayley Scale Infant Development-II-Mental Development Index, BSID-II-PDI = Bayley Scale Infant Development-II-Psychomotor Development Index, BUT = Body Uneasiness Test, CFU = Colony Forming Units, CNCM = Collection Nationale de Cultures de Microorganismes, CRF = Corticotrophin Releasing Factor, CRP = C-Reactive Protein, DASS = Depression and Anxiety Stress Scale, DSM = Deutsche Sammlung von Mikroorganismen, DSM-IV = Diagnostic and Statistical Manual for Mental Disorders – IV, Ecologic® Barrier = B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, and L. lactis W19 and W58, EDI-2 = Eating Disorder Inventory – 2, EPDS = Edinburgh Postnatal Depression Scale, EQ-5D-5L = European Quality of Life, FACT-EWB = Functional Assessment of Cancer Therapy-Emotional Well-being, FACT-SWB = Functional Assessment of Cancer Therapy-Social Well-being, GAF = Global Assessment of Functioning, GDS-SF = Geriatric Depression Scale – Short Form, GHQ = General Health Questionnaire, GI = Gastrointestinal, GMMS = Gastrointestinal motility modulating substances, GSRS = Gastrointestinal Symptom Rating Scale, HADS = Hospital Anxiety and Depression Scale, HAM-A = Hamilton Anxiety Scale, HAM-D = Hamilton Depression Scale, HDL = High Density Lipoprotein, HE = Hepatic encephalopathy, HSCL-90 = Hopkins Symptom Checklist, hs-CRP = high-sensitivity C-reactive protein, HOMA-IR = Homeostatic Insulin Resistance, HOMA-B = Homeostatic Beta cell Function, IBD = Inflammatory Bowel Disease, IBS = Irritable Bowel Syndrome, I.31 = L. plantarum CECT7484 + CECT7485 and P. acidilactici CECT7483, iCGI-I = improved Clinical Global Impression Scale-Improvement, iCGI-S = improved Clinical Global Impression Scale-Severity, IgE = Immunoglobulin E, IgG = Immunoglobulin G, IL-1β = Interleukin-1- beta, IL-6 = Interleukin 6, IL-8 = Interleukin 8, IL-10 = Interleukin 10, IV = Intravenous, Lacidofil® = 95% L. rhamnosus R0011 & 5% L. helveticus R0052, LEIDS = Leiden Index of Depression Sensitivity, MDA = Malondialdehyde, MADRS = Montgomery-Asberg Depression Rating Scale, MINI = Mini-International Neuropsychiatric Interview, NK = Natural Killer, NO = Nitric Oxide, NSAID = Non-Steroidal Anti-Inflammatory Drug, PAL = Paired Associative Learning, PANSS = Positive and Negative Syndrome Scale, PASAT = Paced Auditory Serial Addition Test, Ped = Pediatric, PHQ-9 = Patient Health Questionnaire-9, P.O. = Per Oral, POMS = Profile of Mood States, PPI = Proton Pump Inhibitors, PRET = Prebiotic Treatment, PSS = Perceived Stress Scale, Q.D. = One a day, Q.I.D. = Four times a day, QIDS-16 = Quick Inventory of Depression Symptomatology, QOL = Quality of Life, QUICKI = Quantitative Insulin Sensitivity Check Index, RRMS = Relapse Remitting Multiple Sclerosis, RVIP = Rapid visual information processing, SCFA = Short Chain Fatty Acids, SCL90R = Symptom Checklist Revised, SECPT = Socially Evaluated Cold Pressor Test, SIP = Sickness Impact Profile, SSP = subspecies, STAI = State Trait Anxiety Inventory, SYNT = Synbiotic Treatment, T.I.D. = Three times a day, TMT = Trail Making Test, TNF-α = Tumor Necrosis Factor-alpha, UC = Ulcerative Colitis, VLDL = Very Low-Density Lipoprotein VSL#3 = S. thermophilus DSM 24731, B. longum DSM 24736, B. breve DSM 24732, B. infantis DSM 24737, L. acidophilus DSM 24735, L. plantarum DSM 24730, L. paracasei DSM 24733, L. delbrueckii ssp. bulgaricus DSM 24734, WBC = White Blood Cell
Table 2.
Non-Human Animal Probiotic Trial Designs and Outcomes.
| Probiotic Treatment |
Blinding Status and Study Design |
Sex/Age, days |
Anxiety/ Depression Phenotypes |
Cognition /Social Phenotypes |
Other Probiotic Associated Outcomes |
Reference |
|---|---|---|---|---|---|---|
| Rat (Rattus) | ||||||
| L. plantarum MTCC1325 1.2 × 109 CFU/mL P.O. Q.D. for 60 days | Blinding status not reported Wistar control (n = 6), D-GAL (n = 6), D-GAL + L. plantarum (n = 6), L. plantarum (n = 6) | Male (n = 24) Age 90 days |
#PROT ↑ D-GAL induced ↓ gross behavior | #PROT ↓ D-GAL induced ↑ escape latency (MWM) |
#PROT ↑ D-GAL induced ↓ acetylcholine (HIP, cortex), organ and body weight #PROT ↓ D-GAL induced ↑ acetylcholinesterase |
(Nimgampall e and Kuna, 2017) |
| L. rhamnosus plus B. longum Ningxia Medical University 2 × 109 CFU/mL in 1 × PBS P.O. Q.D. for 12 days | Experimenter blinded behavior SPF Sprague Dawley No treatment (n = 8), 1× PBS control (n = 8), ampicillin (n = 8), ampicillin + PROT (n = 8), ampicillin + clonazepam (n = 8) for 12 days | Male (n = 40) Age 10 days |
#PROT ↓ ampicillin induced ↑ immobility (TST and FST) | #PROT ↓ ampicillin induced ↑ escape latency (MWM) |
#PROT ↑ ampicillin induced ↓ HIP GABA-A receptors *NSE #PROT on ampicillin induced change in gut microbial composition |
(Liang et al., 2017) |
| Ecologic® Barrier 3.8 × 108CFU/mL in water or vehicle Q.D. for 84 days | Experimenter blinded to #PROT and behavior FSL, FRL, & SD FSL CLD + vehicle (n = 11), FSL CLD + #PROT (n= 11), FSL HFD (60%) + #PROT (n= 12), FSL HFD + vehicle (n = 12), FRL CLD + vehicle (n = 12), SD CLD + vehicle (n = 8), SD HFD + vehicle (n = 8) | Male (n = 74) Age 35 days |
#PROT ↓ HFD ↑ depression (FST) in FSL *NSE #PROT depression CLD, locomotion (OFT) |
§NA |
#PROT ↓ CD4/CD8 for CD3+ cells in blood for FSL (*NSE brain) *NSE #PROT body weight, caloric intake, blood glucose, ghrelin, insulin, leptin, MCP, cytokines, WBC |
(Abildgaard et al., 2017a) |
| Ecologic® Barrier 2.5 × 109 CFU/g in water for 35 days during diet (began 35 days prior to #PROT) | Experimenter blinded to #PROT and behavior Sprague-Dawley SD (n= 10), HFD (n = 10), #PROT + SD (n = 10) #PROT + HFD (n = 10) | Male (n = 40) Adult |
#PROT ↓ immobility (FST) *NSE anxiety (OFT) |
*NSE memory (Barnes) | *NSE LPS | (Abildgaard et al., 2017b) |
| L. helveticus R0052 plus B. longum R0175 109 (n = 7) 1010 (n = 8) CFU/day in vehicle with water P.O. Q.D. for 70 days | Experimenter blinded behavior FLR vehicle only (n = 8) FSL (n = 22) 109 #PROT (n = 7), 1010 #PROT (n = 8), or vehicle (n = 7) | Male (n = 30) Adult |
*NSE immobility (FST)*NSE anxiety (OFT) |
*NSE memory (Y-maze, NOR) *NSE social interaction |
#PROT ↓ plasma betaine, NE, DA #PROT ↑ liver SAM *NSE betaine, norepinephrine, dopamine or SAM in PFC or HIP |
(Tillmann et al., 2018) |
| L. helveticus R0052 plus B. longum R0175 3 × 109 CFU P.O. Q.D. for 14 days | Experimenter blinded behavior Wistar #PROT (n = 12), diazepam (n = 12), and placebo (n = 12) | Male (n = 36) Adult |
#PROT and diazepam ↓ burying | §NA | Human trial outcomes are reported in Table 1 | (Messaoudi et al., 2011a) |
| B. infantis 356241 × 1010 CFU/100 mL or vehicle in drinking water Q.D. for 14 days | Experimenter blinded behavior Sprague Dawley #PROT (n = 12) or vehicle (n = 8) | Male (n = 20) Adult |
*NSE FST | §NA |
#PROT ↓ 5-HI A A (frontal cortex), DOPAC (amygdala), body weight #PROT ↓ serum IFN-γ, TNF-α, IL-6 after mitogen stimulation ↓ TNF-α after LPS stimulation #PROT ↓ IFN-γ, IL-6 after CON A stimulation #PROT ↓ plasma tryptophan and kynurenic acid |
(Desbonnet et al., 2008) |
| L. helveticus NS8 109CFU/mL Q.D. for 21 days | Blinding status not reported SPF Sprague Dawley CON (n = 8), chronic RS (n = 8), L. helveticus NS8 during chronic RS (n = 8), citalopram hydrobromide 30 mg/kg during chronic RS (n = 8) | Male (n = 32) Adult |
#PROT ↓anxiety (EPM & OFT) | #PROT ↑ memory (NOR & OPT) |
#PROT ↑ HIP 5-HT, NE, BDNF, IL-10 #PROT ↓ serum CORT, ACTH |
(Liang et al., 2015) |
| VSL#3 (low - 2.5 × 109 CFU or high - 2.5 × 1010 CFU) in maple syrup for 14 days before diet and 26 days during diet modification | Experimenter blinded behavior Sprague Dawley CFD, CFD + VSL#3 low, CFD + VSL#3 high, SD, SD + VSL#3 low, SD + VSL#3 high, (n = 10/group) | Male (n = 60) Adult |
*NSE anxiety (EPM) | VSL#3 high ↑ diet induced memory ↓ (NPR) VSL#3 low and high ↓ memory (NOR) for CFD and SD | CFD + VSL#3 high had ↑ Streptococcus Lactobacillus, Butyrivibrio than CFD alone *NSE overall microbial diversity |
(Beilharz et al., 2017) |
| L. acidophilus ATCC4356, B. lactis DSM 10140, plus L. fermentum ATCC9338 1010 CFU/g B.I.D. for 56 days | Blinding status not reported Wistar diabetic (Streptozotocin 65 mg/kg; n = 10), diabetic + #PROT (n = 10), CON (n= 10), CON + #PROT (n = 10) | Male (n = 40) Age 45 days |
§NA |
#PROT ↑ spatial memory (MWM) in control and diabetic rats #PROT ↑ HIP baseline EPSP and LTP in diabetic rats |
#PROT ↓ serum glucose ↑ insulin and SOD levels in diabetic rats | (Davari et al., 2013) |
| L. plantarum or B. B94 (industrial enzymes company, representative of DSM Company) 1.5 × 108 CFU/mL P.O. Q.D. for 28 days | Blinding status not reported Wistar HIP demyelination model EB (n = 8), EB + L. plantarum (n = 8), EB + B.B94 (n = 8), or saline (n = 8) | Male (n = 32) Age 56-80 days |
§NA | *NSE spatial memory (MWM) | §NA | (Goudarzvand et al., 2016) |
| L. plantarum KY 1032 and L. curvatus HY7601 1 × 1010 CFU Q.D. 48 days (6 days/week) | Blinding status not reported Fischer 344 young, older, older + #PROT, older + rapamycin (n = 6/group) | Male (n = 24) young age not reported older 540 days | §NA | #PROT ↑ memory (Y-maze) | #PROT ↑ HIP doublecortin and ↓ BDNF | (Jeong et al., 2015) |
| ampicillin + L. fermentum NS9 109 CFU/mL (n = 10) for 41 days | Blinding status not reported Sprague Dawley CON (n = 10), ampicillin (n = 10) ampicillin + L. fermenlum NS9 109 CFU/mL (n = 10) | Male (n = 30) Adult |
#PROT ↓ anxiety (EPM) *NSE locomotor activity |
§NA | #PROT ampicillin induced MPO activity | (Wang et al., 2015) |
| VSL#3 1.2 × 1010 CFU/kg in maple syrup Q.D. for 42 days | Blinding status not reported Wistar young, young +#PROT, aged, aged + PROT sample sizes not reported | Male young (90 days) aged (600 – 660 days) | §NA | #PROT ↑ HIP LTP in aged rats ≈ young rats |
#PROT ↓ microglia and ↑ Bacteroidetes in aged rats #PROT up and down regulates cortical gene expression |
(Distrutti et al., 2014) |
| L. paracasei HII01 1 × 108 CFU/mL P.O. in PBS Q.D. for 84 days with diet modification (84 days pre- L. paracasei and 84 days during L. paracasei trial) | Double-blind Wistar (n = 6 per group) Standard diet, HFD, Standard diet +#PROT, HFD +#PROT, Standard diet +#PROT + XOS, HFD + #PROT + XOS, Standard diet + XOS, HFD + XOS | Male (n = 48) Adult |
§NA | #PROT ↑ HFD induced ↓ HIP LTP (fEPSP) | #PROT ↓ HFD induced ↑ in serum LPS, plasma glucose, total cholesterol, LDL cholesterol, serum and brain MDA, insulin, HOMA | (Chunchai et al., 2018) |
| L. helveticus R0052 plus B. longum R0175 1 × 109 CFU in drinking water 7 days pre-MI/sham to 7 days post-MI/sham (14 days) | Experimenter blinded behavior Sprague Dawley MI (n = 9), MI + PROT (n = 9), sham (n = 9), sham + PROT (n = 9) | Male (n = 36) Age 84 days |
#PROT ↓ depression (FST) and anxiety (SDT) in MI not sham | #PROT ↑ social interaction in MI not sham | #PROT ↓ intestinal permeability in MI treated groups | (Arseneault-Breard et al., 2012) |
| L. helveticus NS8 1 × 109 CFU/mL Q.D. in drinking water for 14 days post HA neuroinflammatio n (28 days) | Blinding status not reported SPF Sprague Dawley saline (n = 6), HA (n = 6), HA + #PROT (n = 6) | Male (n = 18) Adult |
#PROT ↓ HA induced anxiety (EPM) | #PROT ↓ HA induced spatial memory deficits (MWM) |
#PROT ↑ 5-HIAA CBM, ↑ 5-HIAA HIP, ↓ 5-HT in HA rats #PROT ↓ HA induced IL-1β, iNOS, and PGE2 in CBM, HIP and PFC |
(Luo et al., 2014) |
| Lacidofil® 1 × 109 CFU/mL for 14 days | Blinding status not reported Sprague Dawley MS model: MS fathers, MS fathers + #PROT (prevention), MS fathers no treatment pups no treatment, MS fathers no treatment pups #PROT (active treatment), MS fathers #PROT (prevention) pups no treatment | Male Female P2-P14 (n = 398 all experiments) | §NA | Preventative and active #PROT ↓ cued fear conditioning and infantile amnesia in pups from MS fathers | §NA | (Callaghan et al., 2016) |
| Lacidofil® 1 × 109 CFU/mL in drinking water for 12 days (P2 to P14) | Double-blind Sprague Dawley #PROT during MS model MS + #PROT (n = 13), MS + vehicle (n = 14), no MS + vehicle (n = 10; n = 9) | Male Pups (n = 37) Age P2-P14 then experiments at P17 Female Mothers (n = 9) |
*NSE MS +#PROT anxiety (EPM) in male pups or female mothers | MS +#PROT ↑ infantile amnesia (7 days post-fear conditioning)*NSE MS + #PROT context dependent freezing in male pups | *NSE MS + #PROT for maternal behavior (pup retrieval) | (Cowan et al., 2016) |
| B. infantis 35624 1 × 1010 CFU/100 mL Q.D. for 45 days | Experimenter blinded behavior Sprague Dawley MS model CON (n = 11), MS (n = 7), MS + citalopram 30 mg/kg (n = 7), and MS + B. infantis (n = 8) | Male (n = 33) Age P2-P14 #PROT P50 to P95 |
#PROT ↓ MS induced depression (immobility, swimming - FST) | §NA |
*NSE stimulated or unstimulated IFN-γ, TNF-α, IL-6, (IL-10 #PROT ↓ MS induced NE and CRF expression in amygdaloid cortex |
(Desbonnet et al., 2010) |
| L. helveticus R0052 and B. longum R0175 109 CFU in 200 mL water Q.D. for 14 days post-MI | Blinding status not reported Sprague Dawley low or high ω-3 PUFA diet w/ or w/out #PROT after MI (n = 16/group) | Male (n = 64) Age 90 days |
#PROT x diet interaction depression (FST) |
#PROT reversed post-MI social interaction deficits in low-PUFA group *NSE post-MI social interaction in high PUFA group #PROT ↑ memory (↓ time and number of trials PAT) |
#PROT ↑ plasma IL-4 in high PUFA group | (Gilbert et al., 2013) |
| B. infantis 35624, B. breve UCC2003, or L. salivcirius UCC118 5 × 109 CFU/mL or vehicle P.O. Q.D. for 14 days | Experimenter blinded behavior Sprague Dawley (n = 40) Wistar-Kyoto (n = 40) PBS, B. infantis, B. breve, L. salivarius (n = 10/group/strain) | Sex not reported (n = 80) Age 63 days |
*NSE anxiety (OFT) | §NA | *NSE plasma corticosterone B. infantis ↓ pain behavior Wistar-Kyoto only | (McKernan et al., 2010) |
| L. fermentum CECT 5716 109 CFU/100g body weight P.O. Q.D. for 3 days pre-MS (age 10 days) or 15 days pre-WAS (age 21 days) | Blinding status not reported Sprague Dawley MS, WAS 8 groups (n = 6/group behavior experiments) | Sex not reported Age 10 and 21 days | *NSE anxiety (OFT) | #PROT ↑ exploratory behavior (OFT) |
#PROT ↓ WAS and MS induced ↑ plasma corticosterone, intestinal permeability #PROT ↑ IFN-γ and ↓ IL-4 after CD3/CD28 stimulation #PROT normalized WAS induced intestinal ZO-1 reorganization |
(Vanhaecke et al., 2017) |
| L. acidophilus (1688FL431-16LA02), L. fermentum (ME3), B. lactis (1195SL609-16BS01) and B. longum (1152SL593-16BL03) 1010 CFU Q.D. in drinking water for 28 days pre- β-amyloid injection and 28 days post-injection | Blinding status not reported Wistar control, control + #PROT, sham surgery, β-amyloid intra-hippocampal injection, β-amyloid intra-hippocampal injection + #PROT (n = 12/group) | Male (n = 60) Age 56 days |
§NA | #PROT ↓ β-amyloid injection induced ↑ in escape latency |
*NSE weight, catalase activity #PROT ↓ β-amyloid injection induced ↑ MDA, SOD, plaques, cell morphology |
(Athari Nik Azm et al., 2018) |
| L. rhamnosus GG 108 CFU/mL in drinking water for approximately 80 days | Blinding status not reported Sprague-Dawley 10 day MS or no MS control diet (n = 5), control diet + #PROT (n = 9), diet + PDX + GOS (n = 5), diet + PDX + GOS +#PROT (n = 9), followed by acute RS | Male only behavior (n = 56) MS Age 2-12 days Behavior Experiments Age 49-100 days |
#PROT + PDX + GOS ↓ MS induced ↑ anxiety (OFT) | #PROT + PDX + GOS ↑ MS induced ↓ spatial memory (MWM) |
#PROT ↓ Nr3c1, Nr3c2, Crhrl in MS group only #PROT + PDX + GOS delayed return of acute stress induced ↑ of corticosterone to baseline |
(McVey Neufeld et al., 2017) |
| Mouse (Mus) | ||||||
| L. fermentum LAB9 109 CFU/200μL or L. casei LABPC 109 CFU/200μL in cow’s milk P.O Q.D. for 28 days pre- LPS induced inflammation (4 days) | Blinding status not reported ICR/HaJ Saline (n = 6) LPS (n = 6) LPS + unfermented milk (n = 6) LPS + L. fermentum, (n = 6) LPS + L. casei (n = 6) |
Male (n = 30) Age 63 days |
§NA | L. fermentum and L. casei ↓ LPS induced spatial memory deficit – escape latency and distance (MWM) |
L. fermentum and L. casei ↑ LPS induced ↓ catalase, SOD, GSH, GPx, MDA, NO, MCP-1 L. fermentum and L. casei ↓ LPS induced ↑ AChE, IL-6, L. fermentum ↓ LPS induced ↑ IL-1β |
(Musa et al., 2017) |
| L. casei DG 109 CFU in saline P.O. for 7 days | Experimenter blinded behavior C57BL/6J (n = 8-9/group) CON, ABX, ABX + #PROT, ABX + saline for 14 days in water | Male (n = 36) Age 64 days |
#PROT ↓ ABX induced immobility (TST, FST) | *NSE social novelty |
*NSE muscle strength, motor coordination #PROT normalized ABX induced changes in BDNF, HIP firing rate, HIP TRN1 phosphorylation, astrocyte and microglia morphology, intestinal 5-HT and OA-5-HT |
(Guida et al., 2017) |
| L. brevis OW38 1 × 109 CFU P.O. for 56 days | Blinding status not reported C57BL/6J young+ vehicle (n = 6), young+ PROT (n = 6), older + vehicle (n = 6), older + PROT (n = 6) | Male (n = 24) Age 120 or 540 days |
§NA | #PROT ↑ memory (Y-maze) in aged mice |
#PROT ↓ age ↑ colonic p-F0X03a, p-mTor, fecal and plasma LPS #PROT ↑ age ↓ HIP BDNF, butyric acid, IL-1β, IL-6, TNF, COX-2, iNOS, NF-κB, claudin-1, ZO-1 |
(Eun et al., 2016) |
| L. plantarum C29 1 × 109 CFU P.O. Q.D. for 5 days post-TNBS induced memory deficit | Blinding status not reported SPF C57BL/6J (n = 6/group) Methods based on (Jeong et al., 2016) | Male (n = 24 - behavior)(n = 42 – colitis) Age 42 days |
§NA | #PROT ↑ TNBS induced ↓ memory (NOR, Y-maze, PAT) |
#PROT ↑ TNBS induced ↓ BDNF #PROT ↑ TNBS induced ↓ Bifidobacteria, Lactobacilli, Clostridia #PROT ↓ TNBS induced ↑ in Enterobacteriacae |
(Lee et al., 2018) |
| L. plantarum MTCC 9510 2 × 1010 CFU/300μl in PBS P.O. for 21 days during sleep deprivation and 28 days during chronic unpredictable stress | Experimenter blinded behavior Swiss Webster LACA chronic unpredictable stress for 28 d (n = 8), chronic unpredictable stress + #PROT (n = 8), naïve (n = 8), naïve +#PROT (n = 8), naïve (n = 6), 72h sleep deprivation (n = 8),72h sleep deprivation + #PROT (n = 8), naïve + #PROT (n = 6) | Male (n = 68) Age not reported |
#PROT ↓ chronic stress and sleep deprivation induced ↓ anxiety and depression behavior (FST, TST, OFT, EPM) | #PROT ↑ chronic stress induced ↓ spatial (MWM) and chronic stress and sleep deprivation induced ↓ working memory (PAT) |
#PROT ↓ stress and sleep deprivation induced ↑ NF-κB, LPS, TNF-α, MAO-A, MAO-B, MDA, GSH, corticosterone #PROT ↑ stress and sleep deprivation induced ↓ HIP BDNF #PROT ↑ cecal Lactobacilli and ↓ Enterobacteriaceae |
(Dhaliwal et al., 2018) |
| E. faceium CFR3003 104 CFU (n = 6), E. faceium CFR3003 108 CFU (n = 6), L. rhanmosus GG MTCC1408 108 CFU (n = 6), or saline (n = 6) P.O. for 28 days | Blinding status not reported CFT-Swiss LPS model of inflammation | Male (n = 24) Age 42 days |
E. faceium - 108 CFU and L. rhanmosus ↓ anxiety (OFT) | §NA |
E. faceium and L. rhanmosus reversed LPS induced ↑ TNF-α and ↓ IL-10 E. faceium -108 CFU and L. rhanmosus ↓ cecum weight and ↑ lactobacilli E. faceium - 104 CFU ↑ GST in HIP ↓ AchE in CTX and STR E. faceium - 108 CFU ↑ cytosolic GABA in CTX, HIP, STR, ↑ cytosolic DA in CTX, ↑ GST in CTX, HIP, STR, ↓ ROS in CTX L. rhanmosus ↓ ROS in HIP, ↑ cytosolic GABA in HIP and DA in STR, ↑ CAT in CTX, HIP ↑ GST in HIP, STR *NSE ROS STR |
(Divyashri et al., 2015) |
| B. fragilis NCTC9343 1 × 1010 CFU in food every other day for 6 days | Blinding status not reported SPF, germ-free and conventional C57BL/6J MIA model pregnant (E12.5) mice injected with saline or 20 mg/kg poly I:C n animals varied ranging from 10-75/group | Male Female Treatment Age approximately 28 days Behavior testing 42 days |
#PROT ↓ anxiety (OFT, burying) in MIA |
#PROT ↑ sensorimoto r gating (PPI) in MIA mice *NSE social interaction |
§NA | (Hsiao et al., 2013) |
| L. rhamnosus JB-1 1.6 × 109 CFU/200ul or saline P.O. Q.D. for 28 days | Blinding status not reported C57BL/6J Chronic social defeat stress began at day 18 of #PROT vehicle (n = 10),#PROT (n = 8), stress (n = 15), stress + #PROT (n= 10) | Male (n = 43) Age 63 days |
#PROT ↓ stress induced anxiety (OFT, light/dark) | #PROT prevented ↓ social interaction with conspecifics |
#PROT ↑ stress induced ↓ in fecal tyramine #PROT ↓ stress induced ↑ in MHCII+, CD11c+, CD80, CD86, #PROT ↓ IL-10+ Treg *NSE stress induced kynurenine, 4- hydroxybutyrate, or 1-methylnicoinamide |
(Bharwani et al., 2017) |
| Lacidofil® 1× 1010 CFU/mL P.O. Q.D. 7 days pre-DSS and 8 days during DSS | Blinding status not reported SPF C57BL/6J DSS model control, #PROT, DSS, DSS + #PROT (n = 9-12/group) | Male (n = 40) Female (n = 40) Age 42-56 days |
#PROT ↓ anxiety (light/dark box) | #PROT ↑ memory in DSS (NOR) |
#PROT ↑ DSS induced loss of cFos in CA1 HIP #PROT ↓ DSS induced dysbiosis |
(Emge et al., 2016) |
| L. rhamnosus NC4007 1010 CFU/100uL (n = 10), B. longum NCC3001 1010 CFU/100uL (n = 16), etanercept, budesonide, or placebo (n = 16) for 10 days after 30 days T. minis infection | Experimenter blinded behavior SPF BALB/c and AKR/J Vagotomy (n = 24) or sham vagotomy (n = 15) before infection | Male (n = 39) Age 42-56 days |
B. longum ↓ T. muris induced anxiety (light/dark box and SDT) *NSE L. rhamnosus on T. muris induced anxiety |
§NA |
B. longum ↑ T. muris induced ↓ brain BDNF *NSE B. longum circulating TNF-alpha *NSE B. longum kynurenine |
(Bercik et al., 2010) |
| B. longum NCC3001 1 × 1010 CFU/mL or vehicle P.O. Q.D. for 14 days (7 days during DSS and 7 days post-DSS) | Experimenter blinded histology SPF AKR/J naïve (n = 13), B. longum (n = 6), DSS (n = 12), sham surgery (n = 11), vagotomy (n = 15), Vagotomy + B. longum (n = 14), vagotomy + DSS (n = 15), vagotomy + DSS + B. longum (n = 15), sham surgery + B. longum (n = 9), B. longum + DSS (n = 11) | Male (n = 151) Age 42-56 days |
#PROT ↓ anxiety (SDT) in control and DSS w/out vagotomy | §NA |
#PROT ↓ excitability of enteric neurons and colonized intestine for sham and vagotomy, *NSE BDNF expression SH-SY5Y cells or colon histology |
(Bercik et al., 2011) |
| B. longum 1714 or B. breve 1205 1 × 109 CFU/mL P.O. Q.D. for 77 days | Experimenter blinded behavior BALB/c B. longum (n = 12), B. breve (n = 12), or vehicle (n = 12) | Male (n = 48) Age 49-56 days |
B. breve ↓ locomotion *NSE B. longum |
B. breve and B. longum ↑ memory (NOR) B. longum ↑ memory (Barnes, cued, and contextual fear conditioning) | *NSE CORT, body weight, colorectal distention | (Savignac et al., 2015) |
| VSL#3 1 × 107 CFU P.O. Q.D. for 10 days | Experimenter blinded behavior C57BL/6J SPF naïve (n = 10), SPF + #PROT (n = 10), SPF + exercise (n = 10), ABX (n = 10), ABX + #PROT (n = 10), ABX + exercise (n = 10), ABX + SPF fecal transplant (n = 10) | Female (n = 70) Age 42-56 days |
§NA | #PROT or exercise ↑ ABX induced memory deficit (NOR) | #PROT or exercise ↑ ABX induced brain monocyte or HIP neurogenesis reduction | (Mohle et al., 2016) |
| Lacidofil® 6 × 109 CFU/mL in water Q.D. for 7 days pre- C. rodentium infection P.O. and 7 days post-infection | Experimenter blinded immunohistochemistry C57BL/6J and Swiss-Webster SPF, germ-free, and conventional #PROT with and without C. rodentium infection or WAS (n = 10-14/group) | Female Age 35-42 days | §NA | #PROT ↑ working memory (T-maze) in C. rodentium infected and WAS mice |
#PROT ↓ serum CORT in C. rodentium infected and WAS mice #PROT ↓ IFNγ in C. rodentium not WAS mice *NSE on TNF-α in C. rodentium or WAS mice |
(Gareau et al., 2011) |
| Lacidofil® 6 × 109 CFU/day or placebo in water for 28 days before WAS | Experimenter blinded immunohistochemistr y C57BL/6JRcigl−/− (n = 4-6/group behavior experiments) no WAS, WAS 1hr for 1 day | Male Female Age 42 to 56 days |
#PROT ↓ anxiety (light/dark) in Rag−/− WAS and no WAS |
#PROT ↓ memory (NOR) in Rag1−/− WAS mice #PROT ↑ memory (NOR) in Rag1−/− naïve mice |
*NSE corticosterone #PROT normalized intestinal ion absorption in no WAS mice only #PROT ↓ abundance of Bacteroides, Enterobacteriaceae, Firmicutes, Lactobacilli |
(Smith et al., 2014) |
| L. pentosus ssp. plantarum C29 1 × 1010 CFU/mouse, P.O. Q.D. for 35 days during D-GAL (D-GAL induced aging 35 days pre-#PROT and 35 days during #PROT) | Blinding status not reported C57BL/6J Naïve (n = 6), D-GAL (n = 6), #PROT (n = 6) | Male (n = 18) Age 140 days (D-GAL) Age 182 days (#PROT) |
§NA | #PROT reversed D-GAL induced ↓ in memory (MWM, Y-maze, PAT) | #PROT reversed D-GAL induced ↓ in BDNF, DCX, and CREB | (Woo et al., 2014) |
| L. johnsonii ATCC33200 or L. renteri MM4-1A ATCC-PTA-6475 1 × 108 CFU in water for 28 days | Double-blind Germ-free C57BL/6J MRD (13.4% FAT, 30% PRO, and 57% CARB), MHFD (60% FAT, 20% PRO, 20% CARB), live #PROT, heat killed #PROT | Male (behavior) Age 49-84 days |
*NSE anxiety (OFT) | L. reuteri ↑ LTP in VTA DA neurons for MHFD L. reuteri ↑ sociability, social novelty, reciprocal social interaction in MHFD | §NA | (Buffington et al., 2016) |
| VSL#3: 1.7 × 1010 CFU P.O. Q.D. 10 days pre- and 10 days post-surgery | Blinding status not reported C57BL/6 SPF BDL model liver inflammation + #PROT (n = 10), BDL no #PROT (n = 10), sham + #PROT (n = 10), sham no #PROT (n = 10) | Males (n = 40) Age 42-56 days |
#PROT ↓ depression (immobility) in BDL model | #PROT ↑ social behavior in BDL model |
#PROT ↓ monocyte infiltration to brain in BDL model #PROT ↓ microglial activation in brain in BDL model #PROT ↓ TNF-α in BDL model *NSE gut permeability or liver injury |
(D'Mello et al., 2015) |
| B. longum 1714 or B. breve 1205 1 × 1010 CFU/mL Q.D. for 42 days | Experimenters blinded behavior BALB/c SIH vehicle (n ≈ 20), escitalopram 20mg/kg (n ≈ 20), B. longum (n ≈ 20), B. breve (n ≈ 20) | Male (n ≈ 80) Age 49 days |
B. longum ↓ SIH escitalopram, B. longum and B. breve ↓ marble burying B. breve ↓ anxiety (EPM) B. longum ↓ anxiety (OFT) B. longum ↓ depression TST *NSE depression (FST) |
§NA |
B. breve ↓ bodyweight gain B. breve ↑ spleen weight *NSE corticosterone |
(Savignac et al., 2014) |
| L. rhanmosus JB-1 109 CFU/mL or control broth P.O. Q.D. for 28 days | Blinding status not reported BALB/c CON (n = 20) CON naïve (n = 8), CON + SIH (n = 8), #PROT (n = 8), or #PROT with stress (n = 8) Vagotomy (n = not reported), vagotomy + #PROT (n = not reported) | Male Age 70-77 days |
#PROT ↓ depression (FST) #PROT ↓ anxiety (EPM, OFT) |
#PROT ↑ cued and contextual memory fear conditioning |
#PROT ↓ stress-induced CORT levels #PROT ↑ GABAB1b mRNA in cingulate of sham not vagotomized mice *NSE on FST or OFT after vagotomy *NSE on SIH |
(Bravo et al., 2011) |
| L. rhanmosus GG 1 × 109 CFU P.O. Q.D. for 14 days | Single-blind behavior BALB/c 5-HT1A/1B receptor agonist model of OCD (RU 24969) primed by social experience and then pretreatment with saline (n = 6), fluoxetine 10 mg/kg for 28 days (n = 12) or #PROT for 14 days (n = 12) before inducing OCD model | Male (n = 36) Age 56 days |
#PROT pretreatment ↓ anxiety (burying) fluoxetine had greater effect than #PROT #PROT and fluoxetine ↓ locomotion (OFT) *NSE aggression |
§NA | §NA | (Kantak et al., 2014) |
| L. casei-01 109 CFU/kg P.O. Q.D. for 20 days | Blinding status not reported Kunming (n = 10/ group) control, SCOP (3mg/kg), SCOP + piracetam (400mg/kg), SCOP + #PROT, SCOP + LSPC (60mg/kg), SCOP + LSPC (90mg/kg), SCOP + #PROT + LSPC (60mg/kg), SCOP + #PROT + LSPC (90mg/kg) | Male (n = 80) Age not reported |
§NA |
#PROT ↑ memory (Y-maze) in SCOP induced amnesia #PROT enhances effect of LSPC on SCOP induced amnesia at both dosages |
#PROT enhances LSPC ability to reduce MDA and increase antioxidant levels *NSE on brain NOS levels |
(Xiao et al., 2014) |
| L. plantarum CCFM639 5 × 109 CFU/mL P.O. Q.D. in milk for 84 days after 28 days aluminum toxicity | Experimenter blinded histology SPF C57BL6/J (n = 10/group) control, aluminum toxicity, aluminum toxicity + live #PROT, aluminum toxicity + heat killed #PROT | Male (n = 40) Age 42 days |
§NA | Live and heat killed #PROT ↑ aluminum induced ↓ spatial memory (MWM) |
Live #PROT ↓ aluminum induced ↑ brain Aβ1-40 & Aβ1-42 Live and heat killed #PROT ↓ aluminum induced ↑ ALT, AST, CRE, BUN, and live aluminum Live and heat killed #PROT ↑ fecal aluminum (day 14 #PROT) Live and heat killed #PROT ↓ aluminum induced ↓ SOD, CAT, GPx, GSH, MDA in liver and brain *NSE brain aluminum |
(Tian et al., 2017) |
| L. helveticus R0052 109 CFU P.O. or no treatment Q.D. for 21 days | Experimenter blinded histology SPF 129/SvEv wild type and IL-10 −/− fed SD (29% PRO, 55% CARB,13% FAT) (n = 5-6), SD + #PROT (n = 5-6), Western-style diet (28% PRO, 49% refined CARB, 33% FAT) (n = 5-6), Western diet plus #PROT (n = 5-6) | Sex not reported Age PD29 |
#PROT ↓ anxiety with western diet wild type and IL-10 −/− *NSE anxiety for IL-10 −/− fed standard chow |
#PROT ↓ memory that ↓ with western diet in IL-10 −/− *NSE anxiety for IL-10 −/− fed standard chow |
#PROT ↑ brain corticosterone, fecal corticosterone, Firmicutes/Bacteroidete s, IL-1B in IL-10 −/− mice | (Ohland et al., 2013) |
| L. plantarum PS128 5 × 109 CFU/mL P.O. Q.D. for 16 days | Blinding status not reported germ-free C57BL/6J live #PROT (n = 10), heat -killed #PROT (n = 10) or pre-warmed saline (n = 10) for 16 days | Male (n = 30) Age 42 days |
#PROT ↓ anxiety (EPM) Live #PROT ↑ locomotion | §NA | Live #PROT ↓ cecum weight Live #PROT ↓ DA, HVA, 5-HT, 5-HIAA in striatum *NSE #PROT in PFC or HIP *NSE #PROT organ histology and serum CORT |
(Liu et al., 2016a) |
| L. plantarum PS128 5 × 109 CFU P.O. Q.D. for 28 days | Blinding status not reported SPF C57BL/6J MS (n = 12), MS + #PROT (n = 10) from PD29, naïve adult mice (n = 10), and naïve adult + #PROT (n = not reported) from 8 weeks | Male Age PD29 and 56 days |
#PROT restores sucrose preference and FST in MS mice but *NSE #PROT for naïve mice #PROT ↓ anxiety (OFT, EPM) naïve mice *NSE anxiety in MS mice #PROT ↑ locomotor activities in MS and naïve mice |
§NA |
#PROT reverses MS ↑ IL-6 and ↓ IL-10 #PROT ↑ PFC dopamine in MS and naïve mice #PROT ↑ 5-HT in naïve #PROT ↓ serum CORT during basal & stressed states for MS not naïve mice #PROT ↑ levels of 5-HT in PFC in MS |
(Liu et al., 2016b) |
| C. butyricum WZMC1016 (CGMCC9831)1 × 108 CFU/200μl saline P.O. Q.D. for 42 days | Blinding status not reported SPF C57BL/6J Streptozotocin sham operation (n = 12), cerebral I/R injury (n = 12), cerebral I/R injury + C. butyricum (n = 12) | Male (n = 36) Age 87 days |
§NA | #PROT ↑ cerebral injury induced ↓ in spatial memory (MWM) | #PROT ↓ cerebral injury induced ↓ p-Akt | (Sun et al., 2016) |
| Zebrafish (Danio rerio) | ||||||
| L. plantarum (USDA-ARS) 2 × 107 CFU/mL single exposure for 2 days | Blinding status not reported Wild-type CV, CV + stress, CR, CR + stress, CR + stress + #PROT, GF, GF + stress, GF + stress + #PROT | Sex not reported Age 4 days post fertilization (#PROT) |
#PROT ↓ anxiety (thigmotaxis) *NSE locomotor activity |
§NA | #PROT ↓ stress induced cortisol level in CR not GF | (Davis et al., 2016a) |
| L. plantarum (USDA-ARS) 1 × 106 CFU/mL B.I.D for 30 days | Blinding status not reported Wild-type chronic unpredictable stress #PROT (n = 5-7), CON (n = 5-7) | Male Female Adult |
#PROT ↓ anxiety (novel tank diving) | §NA |
#PROT restores stress induced dysbiosis *NSE cortisol, lymphocytes, monocytes, neutrophils, eosinophils |
(Davis et al., 2016b) |
| L. rhanmosus GG in feed (CFU not reported) for 14 days | Blinding status not reported Wild-type #PROT (n= 15), #PROT + 0.5% EtOH (n = 15), 0.5% EtOH (n = 15), CON (n = 15) | Male Female Adult |
*NSE anxiety (novel tank diving) for #PROT or #PROT + ethanol groups |
§NA | §NA | (Schneider et al., 2016) |
| L. rhanmosus IMC501 1 × 106 CFU B.I.D. for 28 days | Blinding status not reported Wild-type - heterozygous #PROT (n = 12), CON (n = 12) | Male Female Age 120-180 days |
§NA | #PROT ↑ social (shoaling) and exploratory behavior |
#PROT ↓ Bacteroidetes ↑ BDNF and serotonergic gene expression in brain *NSE gut BDNF |
(Borrelli et al., 2016) |
| P. acidilactici JN039350 and L. plantarum JN039358 109 CFU/g in control and HCD feed (3% of total body weight) B.I.D. for 49 days | Blinding status not reported Wild-type control diet group (n = 6), HCD (n = 6), HCD + P. acidilactici JN039350 (n = 8) and the HCD + L. plantarum JN039358 (n = 8) | Male (n = 28) Adult |
§NA | P. acidilactici JN039350 or L. plantarum JN039358 ↑ HCD ↓ in spatial memory |
#PROT ↑ diet induced ↑ increase in cholesterol #PROT ↓ brain abba, liver abcal and ↓ liver and intestine npc111 expression |
(Lim et al., 2017) |
| Ouail (Coturnix japonica) | ||||||
| P. acidilactici R001 (MA 18/5M) ~ 1.9 × 107 CFU/day for 36 days with unpredictable stress during day 17-21 | Blinding status not reported #PROT (LTI n = 18 and STI n = 16) Control (LTI n = 19 and STI n = 16) |
Female #PROT Age 0-36 days Age 6-7 days for STI & LTI |
*NSE anxiety (OFT) #PROT ↓ emotional reactivity (STI & LTI) |
#PROT ↑ memory day 2 and 3 | *NSE emotional reactivity and memory interaction | (Parois et al., 2017) |
Abbreviations:
NA = Not Assessed,
NSE = No Significant Effect,
PROT = Probiotic Treatment, 5-HT = 5-hydroxytryptamine or Serotonin, abcal = ATP-binding cassette family transporter group Al, appa = amyloid precursor protein type a, ABX = Antibiotics, AchE = Acetylcholinesterase, ACTH = Adrenocorticotropic Hormone, ALT = Alanine Transaminase, AST = Aspartate Transaminase, ATCC = American Tissue Culture Collection, B.I.D. = twice a day, BDL = Bile Duct Ligation, BDNF = Brain Derived Neurotrophic Factor, BNR = Blinding Not Reported, BUN = Blood Urea Nitrogen, CAT = Catalase, CFD = Cafeteria Diet, CLD = Control Diet, CON = Control, CON-A = Concanavalin A, CORT = Corticosterone, CR = Conventionally Raised, CRE = Creatinine, RS = Restraint Stress, CTX = Cortex, CV = Conventionalized, D-GAL = D-galactose, DNBS = Dinitro-Benzene Sulfonic Acid, DSS = Dextran Sodium Sulfate, EB = Ethidium Bromide, Ecologic® Barrier = B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, be. Lactis W19, be. Lactis W58, EPM = Elevated Plus Maze, EPSP = Excitatory Post Synaptic Potentials, EtOH = Ethanol, FRL = Flinders Resistant Line, FSL = Flinders Sensitive Line, FST = Forced Swim Test, GF = Raised in Germ Free Environment, GPx = glutathione peroxidase, GSH = Glutathione, HA = Hyperammonemia (hepatic encephalopathy model), HCD = High Cholesterol Diet, HFD = High Fat Diet, HIP = Hippocampus, HYP = Hypothalamus, LACA = Laboratory Animal Center Albino, Lacidofil® = 95% b. rhamnosus R0011 & 5% b. helveticus R0052, Lc. = Lactococcus, LPS = Lipopolysaccharide, LSPC = Lotus Seedpod Proanthocyanidins, LTI = Long Tonic Immobility, LTP = Long Term Potentiation, MDA = Maiondialdehyde, MHFD = Maternal High Fat Diet, MI = Myocardial Infarction, MI = Myocardial Infarction, MIA = Maternal Immune Activation, ML-7 = myosin light chain kinase inhibitor, MS = Maternal Separation, MTCC = Microbial Type Culture Collection, MWM = Morris Water Maze, NE = Norepinephrine, and npclll = Neimann-Pick Cl-like 1, NOR = Novel Object Recognition, NOS = Nitric Oxide Synthase, OCD = Obsessive-Compulsive Disorder, OFT = Open Field Test, OPT = Object Placement Test, P.O. = PAT = Passive Avoidance Test, Per Oral, PD = Postnatal Day, PFC = Prefrontal Cortex, PGE2 = prostaglandin E2, PPI = Prepulse Inhibition, PRO = Protein, PUFA = Polyunsaturated Fatty Acids, PVN = Paraventricular Nucleus, Q.D. = Once a day, ROS = Reactive Oxygen Species, SAM = S-adenosylmethionine, SCOP = Scopolamine, SD = Standard Diet, STI, SDT = Step Down Test, SIH = Stress Induced Hyperthermia, SOD = Super Oxide Dismutase, SPF = Specific Pathogen Free, = Short Tonic Immobility, STR = Striatum, TNBS = 2,4,6-trinitrobenzenesulfonic acid, TST = Tail Suspension Test, VSL#3 = S. salivarius ssp. thermophilus, B. breve, B. infantis, B. longum, L. acidophilus, b. plantarum, b. casei, b. delbrueckii subsp. Bulgarians, VTA = Ventral Tegmental Area, WAS = Water Avoidance Stress, XOS = Xylooligosaccharide, ZO-1 = Zonula Occludens-1
Due to profound differences in experimental designs, neuropsychiatric outcome assessments, physiological indices, probiotic strains, and substrains reported by the trials ascertained for review (see Tables 1 and 2), meta-analyses were not conducted. Most trials indicating significant differences associated with probiotic treatment reported beneficial effects on neuropsychiatric outcomes. If a probiotic treatment led to a decline in neuropsychiatric performance or functioning, these findings have been highlighted in the Results.
Both review authors assessed the study quality and risk of bias for all trials in Tables 1 and 2 with 100% consensus. Human study quality and risk for bias were evaluated with the PEDro scale (Maher et al., 2003), Quality Index (Downs and Black, 1998), and the Cochrane Collaboration Tool (Higgins et al., 2011). Individual item scores, mean total scores, and interquartile ranges for these assessments are noted in Tables A.2-A.4 (Appendix) for each study. Study quality and risk of bias for the non-human animal trials were estimated with modified criteria from Macleod et al. (2004). The amended scale items, individual item scores for each study, total mean scores, and interquartile range can be examined in Table A.5 (Appendix). Specific-pathogen-free or germ-free conditions were not taken into consideration when scoring the non-human animal study environment. If such conditions were reported, they are noted in Table 2 and summarized in the Results.
For all study quality and bias scales except the Cochrane Collaboration Tool, total scores were normally distributed and higher scores corresponded with greater study quality or lower risk for bias. Each item of the Cochrane Collaboration Tool was dichotomized as having a high or low risk for bias, except when the item criteria could not be obtained from the study report. If this condition was met, the item was recorded as having an unclear risk for bias.
Because the validity and reliability of excluding investigations for systematic review based on these types of assessments continues to be actively debated in the extant literature (Ilgen et al., 2015; Juni et al., 2001), our global assessment of human and non-human trial methodology suggested excessive heterogeneity, and since we were unable to obtain meaningful minimum scores with the quality and risk of bias assessments, these scores were not considered when evaluating studies for final inclusion in Tables 1 and 2. However, to maximize scientific rigor and minimize bias, we excluded reports noted in Table 1 from Tables 4-6 and the Results below if we could not confirm at minimum double-blinding. Notably, the standardized scores for the Quality Index and PEDro Scale were within 2.5 deviations of the mean (i.e. Z-Score Range −2.5 to +2.5). Due to the varied and limited reporting of blinding status for the non-human trials (43.1%), we did not employ the double-blind exclusion strategy for these investigations.
Table 4.
Double- or Triple-Blinded Human Trial Characteristics.
| Lifespan Stage |
n Studies Reportin g/n Total |
M | SD | Range | % Total |
|---|---|---|---|---|---|
| Age, years | |||||
| Infant/Child | 7/7 | 0.414 | .923 | 0-4 | - |
| Young/Middle-Aged Adult | 32/32 | 38.2 | 11.1 | 18-65 | - |
| Older Adult | 5/5 | 70.2 | 7.0 | 48-95 | - |
| Subjects in probiotic treatment arm, n | |||||
| Infant/Child | 7/7 | 92.7 | 71.8 | 20-238 | - |
| Young/Middle-Aged Adult | 32/32 | 51.5 | 50.1 | 12-212 | - |
| Older Adult | 4/5 | 43.8 | 55.0 | 30-125 | - |
| Probiotic treatment duration, days | |||||
| Infant/Child | 6/7 | 93.2 | 63.6 | 28-201 | - |
| Young/Middle-Aged Adult | 32/32 | 61.2 | 59.3 | 14-336 | - |
| Older Adult | 5/5 | 54.3 | 34.5 | 21-84 | - |
| Probiotic dosage, CFU | |||||
| Infant/Child | 7/7 | 7.7 × 108 | 1.9 × 109 | 1 × 106 – 1 × 1010 | - |
| Young/Middle-Aged Adult | 31/32 | 3.4 × 1010 | 8.8 × 1010 | 1 × 107 – 3 × 1011 | - |
| Older Adult | 5/5 | 2.3 × 1011 | 4.5 × 1011 | 1 × 108 – 4.5 × 1011 | - |
| Sex, % males | |||||
| Infant/Child | 6/7 | - | - | - | 51.9 |
| Young/Middle-Aged Adult | 32/32 | - | - | - | 31.5 |
| Older Adult | 5/5 | - | - | - | 38.7 |
Table 6.
Cross-Species Overview of Single- Versus Multi-Strain Probiotic Treatments in Association with Neuropsychiatric Outcomes.
| Trials | N/% single- strain |
N/% multi- strain |
N/% assessed anxiety, depression, or emotional behavior |
N/% assessed cognition or social behavior |
N/% single- strain change in stress, anxiety, or depression |
N/% of single-strain change in cognition or social behavior |
N/% of multi-strain change in stress, anxiety, or depression |
N/% of multi-strain change in cognition or social behavior |
|---|---|---|---|---|---|---|---|---|
| Human – 44 | 27/61.4 | 16/36.4 | 37/84.1 | 17/38.6 | 15/55.6 | 7/25.9 | 8/50.0 | 5/31.5 |
| Infant/Child – 7 | 6/85.7 | 1/14.3 | 5/71.4 | 2/28.6 | 1/16.7 | 0/0 | 0/0 | 1/100.0 |
| Y oung/Middle-Aged Adult – 32* | 18/56.3 | 13/40.6 | 29/90.6 | 11/34.4 | 13/72.2 | 6/33.3 | 8/61.5 | 2/15.4 |
| Older Adult – 5 | 3/60.0 | 2/40.0 | 3/60.0 | 4/80.0 | 1/33.3 | 1/33.3 | 0/0 | 2/100.0 |
| Non-Human – 58 | 38/65.5 | 20/35.5 | 40/69.0 | 42/72.4 | 23/60.5 | 22/57.9 | 9/45.0 | 17/85.0 |
| Rat – 25 | 11/44.0 | 14/56.0 | 18/72.0 | 19/76.0 | 6/54.5 | 6/54.5 | 6/42.9 | 10/71.4 |
| Mouse – 27 | 22/81.5 | 5/18.5 | 18/66.7 | 20/74.1 | 14/63.6 | 14/63.6 | 3/60.0 | 5/100.0 |
| Zebrafish – 5 | 4/80.0 | 1/20.0 | 3/60.0 | 2/40.0 | 2/50.0 | 1/25.0 | NA | 1/100.0 |
| Quail – 1 | 1/100.0 | 0/0.0 | 1/100.0 | 1/100.0 | 1/100.0 | 1/100.0 | NA | NA |
Abbreviations: NA = Not Applicable
One of the double-blind probiotic trials observing significant changes in cognitive function did not report whether the Bifidobacterium formulation tested was a single- or multi-strain combination. This study was excluded from calculations noted in this table.
Table 3 is a human vs. non-human comparison of the multiple neuropsychiatric scales and behavioral measures utilized as indices for stress, anxiety, depression, cognition, and social functioning.
Table 3.
Neuropsychiatric Paradigms and Assessments Utilized in Human and Non-Human Animal Trials.
3. Results
3.1. Human Trial Characteristics
Study characteristics for the human trials are summarized by lifespan stage in Table 4.
Across trials, the most frequent single- and multi-strain combinations were L. casei (seven of forty-eight – 14.6% – six utilized substrain Shirota and one investigated ssp. rhamnosus) and VSL#3 (two of forty-eight – 4.2%) which is comprised of S. thermophilus DSM 24731, B. longum DSM 24736, B. breve DSM 24732, B. infantis DSM 24737, L. acidophilus DSM 24735, L. plantarum DSM 24730, L. paracasei DSM 24733, and L. delbrueckii ssp. bulgaricus DSM 24734, respectively.
Participants varied greatly by age range, physiological conditions, and psychiatric history. The number of investigations that verified medical or neuropsychiatric diagnoses, or lack thereof, with hospital or outpatient records could not be determined. Treatment and placebo group sample sizes were reported for all human studies. Within each trial, treatment and placebo groups were comparable in sex and age range distribution. Notable exceptions:
one infant trial - a greater proportion of males were randomized to the placebo group, and
one older adult investigation - a greater proportion of males were randomized to one of the L. helveticus IDCC3801 treatment conditions.
3.1.1. Infant/Child
Subjects in four out of the seven (57.1%) infant/child studies were defined as healthy. The three remaining trials enrolled infants considered to be premature, diagnosed with Wessel’s colic, or having a first-degree relative with dermatitis, atopic disease, or asthma.
Five of the seven trials (71.4%) reported outcomes associated with L. reuteri (substrains DSM 17938 or ATCC5573). The sixth single-strain study determined the effects of L. rhamnosus GG ATCC53103 consumption. One (14.3%) multi-strain investigation was also conducted with S. thermophilus, B. animalis ssp. lactis BB-12, plus L. bulgaricus.
3.1.2. Older Adult
Three of the five (60%) older adult studies enrolled individuals described as healthy. The two remaining reports conducted probiotic trials for post-surgical colorectal cancer patients or people diagnosed with Alzheimer’s disease.
Probiotic strains tested varied for each of the older adult trials (see Table 1). Three (60%) single- and two (40%) multi-strain formulations were evaluated.
3.1.3. Young/Middle-Aged Adult
Thirty-six young/middle-aged adult trials have been included in Table 1. Thirty-two trials reported double- or triple-blinding. The last four investigations had indistinguishable blinding status and have been excluded from discussion (see Methods).
Eleven (34.4%) of these trials defined study subjects as generally healthy, of which one (9.1%) only enrolled female subjects. Seven (21.9%) investigations enrolled patients diagnosed with Rome II or III IBS. Two (6.3%) studies investigated probiotic treatment in the context of obese or overweight status. Two separate trials (6.3%) enrolled people diagnosed with hepatic encephalopathy. The twelve remaining reports recruited and investigated unique study samples. Precise characteristics have been noted in Table 1.
It is unclear whether an investigation of Bifidobacterium in hepatic encephalopathy patients should be characterized as a single- or multi-strain probiotic treatment. Eighteen out of thirty-two (56.3%) trials were conducted with single strains of probiotics. Six (33.3%) of the eighteen utilized L. casei (substrains - five of six Shirota and one ssp. rhamnosus). Three (16.7%) of the eighteen investigated L. rhamnosus (substrains GG, HN001, or CGMCC1.3724). B. longum (substrains NCC3001 or ssp. infantis R0033) was tested three (16.7%) times. Two studies (11.1%) evaluated L. helveticus (substrains R0052 or Lafti L10). All other single-strain trials were conducted once as noted in Table 1.
Fourteen of the thirty-two (43.8%) young/middle-aged adult studies were multi-strain probiotic investigations. Two out of fourteen (14.3%) analyzed the effects of L. helveticus R0052 plus B. longum R0175. All other multi-strain formulations were tested once (see Table 1).
3.2. Non-Human Trial Characteristics
Major characteristics by animal species are summarized in Table 5.
Table 5.
Non-Human Trial Characteristics*.
| Species |
n Studies Reporting/n Total |
M | SD | Range | % Total |
|---|---|---|---|---|---|
| Age, days | |||||
| Rat | 16/25 | 109.7 | 162.0 | 2-660 | - |
| Mouse | 25/27 | 67.5 | 62.0 | 29-540 | - |
| Zebrafish | 2/5 | 77 | 103.2 | 4-180 | - |
| Quail | 1/1 | 6.5 | NA | NA | - |
| Animals in probiotic treatment arms, n | |||||
| Rat | 23/25 | 18.9 | 12.9 | 6-60 | - |
| Mouse | 21/27 | 20.2 | 12.0 | 6-49 | - |
| Zebrafish | 4/5 | 12.3 | 4.5 | 6-16 | - |
| Quail | 1/1 | 17 | NA | NA | - |
| Probiotic treatment duration, days | |||||
| Rat | 25/25 | 40.2 | 35.1 | 12-168 | - |
| Mouse | 27/27 | 28.3 | 21.2 | 5-84 | - |
| Zebrafish | 4/5 | 27.3 | 19.3 | 2-49 | - |
| Quail | 1/1 | 36 | NA | NA | - |
| Probiotic dosage, CFU | |||||
| Rat | 25/25 | 4.5 × 109 | 5.4 × 109 | 1.0 × 108 – 1.2 × 1010 | - |
| Mouse | 27/27 | 1.1 × 1010 | 2.2 × 1010 | 1.0 × 104 – 1.0 × 1011 | - |
| Zebrafish | 4/5 | 7.3 × 106 | 1.1 × 107 | 1.0 × 106 – 1.0 × 109 | - |
| Quail | 1/1 | 1.9 × 107 | NA | NA | - |
| Sex, % males | |||||
| Rat | 22/25 | - | - | - | 78.7 |
| Mouse | 20/27 | - | - | - | 87.0 |
| Zebrafish | 1/5 | - | - | - | 62.5§ |
| Quail | 1/1 | - | - | - | 0 |
Computed from studies reporting exact probiotic treatment group size, age, or numbers of animals by sex.
One out of five studies reported a total sample of 28 males. Three out of five studies reported using both males and females. One out of five studies did not report whether males or females were utilized.
The most common single-strain probiotics tested were L. rhamnosus (substrains GG, JB-1, NC4007, and IMC501) and L. plantarum (substrains MTCC1325, MTCC9510, KY1032, PS128, and USDA-ARS; eight of fifty-eight trials each – 13.8%). Lacidofil® – 95% L. rhamnosus R0011 and 5% L. helveticus R0052 – was the most frequently investigated multi-strain formulation (five of fifty-eight – 8.6%).
Approximate animal age during probiotic treatment was reported for sixteen out of twenty-five (64.0%) rat, twenty-five out of twenty-seven (92.6%) mouse, and two out of five (40.0%) zebrafish studies. The age of most animals tested neurodevelopmentally coincided with human middle-adulthood. When reported, animal ages for treatment and control groups were comparable. Forty-two out of fifty-eight (72.4%) non-human studies utilized males. Three (5.2%) trials investigated females. Eight (13.8%) investigations observed both male and female animals. Numbers of animals by sex were not reported for five (8.6%) trials.
Blinding status was indicated for twenty-five of the fifty-eight (43.1%) reports. Fifty-one (87.9%) trials administered probiotic in drinking water or provided control animals with an identical vehicle. In addition to the varying experimental paradigms further described by species below, three out of twenty-five (12.0%) rat, thirteen out of twenty-seven (48.1%) mouse, and one out of five (20.0%) zebrafish trials reported rearing animals in germ- or specific-pathogen-free environments concurrent to probiotic treatment.
3.2.1. Rat
Multiple rat strains were utilized across trials. Fifteen of the twenty-five (60.0%) utilized Sprague-Dawley, eight (32.0%) Wistar, two (8.0%) Flinders Sensitive, and one (4.0%) Fischer 344.
Paradigms varied among studies with some including multiple experimental manipulations: 1) eight models of stress (32.0%) – five maternal separation, two restraint, and one water avoidance; 2) six (24.0%) different diet modifications; 3) three (12.0%) aging/neurodegenerative models; 4) two (6.0%) myocardial infarction; and 5) two (6.0%) probiotic rescue of antibiotic-induced dysbiosis. All other designs were utilized once (see Table 2).
Eleven out of the twenty-five (44%) rat studies employed single-strain probiotic therapy. Three out of eleven (27.3%) utilized B. infantis 35624. Three (27.3%) studies determined the effects of L. helveticus (substrains - two NS8 and one MTCC1325). Two (18.2%) trials each of L. plantarum (substrains MTCC1325 and unknown) and L. fermentum (substrains NS9 and CECT5716) were conducted. Additional single strain probiotic treatments were investigated once as noted in Table 2.
Fourteen (66%) rat studies were multi-strain formulation trials. Four out of fourteen (28.6%) trials investigated the effects of L. helveticus R0052 plus B. longum R0175. Two (11.8%) trials each of Ecologic® Barrier, Lacidofil®, and VSL#3 were conducted. The four remaining multi-strain combinations were tested once (see Table 2).
3.2.2. Mouse
The most frequently utilized mouse strain was C57BL6/J (sixteen of twenty-seven - 59.3%) followed by BALB/c (five of twenty-seven - 18.5%). Two (7.4%) trials tested probiotic effects in AKR/J mice. All other strains were investigated once each as noted in Table 2. Two (7.4%) of the twenty-seven studies utilized knockout (−/−) mice: Rag1 on C57BL6/J and IL-10 on 129/SvEv.
The most common experimental manipulation was stress exposure (seven of twenty-seven – 25.9%). Exposure types varied by trial and are noted in Table 2. Aging, colitis, and vagotomy models were investigated three (11.1%) times each. Pathogenic infection, diet modification, antibiotic-induced dysbiosis, lipopolysaccharide (LPS) induced inflammation, antidepressant, or heat-killed probiotic comparisons were employed (7.4%) twice each. All other paradigms were evaluated once (see Table 2).
Twenty-two out of twenty-seven (81.5%) reports can be stratified as single-strain probiotic trials. L. plantarum (substrains C29, CCFM639, MTCC9510, PS128) and L. rhamnosus (substrains GG, JB-1, NC4007) effects were evaluated five times (22.7%) each. Studies with B. longum (substrains NCC3001, 1714) were conducted four (18.2%) times. Three (13.6%) trials utilized L. casei (substrains 01, LABPC, DG) while two (9.1%) separate investigations determined the effects of B. breve 1205. All other single-strain probiotics were tested once (see Table 2).
Five out of twenty-seven (18.5%) trials investigated multi-strain combinations of probiotics. Three of the five (60%) studies utilized Lacidofil® while the two (40%) remaining analyzed VSL#3 intake effects.
3.2.3. Zebrafish
All zebrafish studies were conducted with wild-type animals. One of five (20.0%) reports also noted wild-type as “heterozygous”. Besides probiotic treatment, experimental designs included 1) stress, germ-free, and conventional environments; 2) high cholesterol diet; 3) ethanol exposure; and 4) chronic unpredictable stress.
Probiotic strains tested included two (40%) L. rhamnosus (substrains GG and IMC501), two (40%) L. plantarum USDA-ARS, and one (20%) multi-strain formulation of P. acidilactici JN039350 plus L. plantarum JN039358.
3.2.4. Quail
The single female Japanese quail study investigated the effects of P. acidilactici R001 (MA 18/5M).
3.3. Neuropsychiatric Outcomes Associated with Single- and Multi-Strain Treatments
Table 6 provides a cross-species comparison of single- and multi-strain probiotic trials in association with key neuropsychiatric outcome groupings.
3.3.1. Stress/Anxiety – Human
3.3.1.1. Infant/Child
None of the trials measured anxiety-based phenotypes.
3.3.1.2. Older Adult
Two of five (40.0%) trials assessed probiotic effects on anxiety with L. reuteri DSM 17938 or L. helveticus IDCC3801. No significant variations in anxiety symptoms were reported after 84 days of probiotic consumption.
3.3.1.3. Young/Middle-Aged Adult
Twenty-four out of thirty-two (75.0%) trials determined anxiety outcomes associated with probiotic treatment. Twelve (50.0%) of the twenty-four were single-strain trials including five (38.5%) L. casei (substrains - four Shirota and one ssp. rhamnosus), and two (15.4%) each of L. rhamnosus (HN001 and CGMCC1.3724) and B. longum (ssp. infantis R0033 and NCC3001). Other single-strains were tested once as noted in Table 1. Beneficial effects were observed with L. casei Shirota, L. gasseri CP2305, L. acidophilus NCFM, L. rhamnosus HN001, or B. bifidum R0071.
The twelve (50.0%) multi-strain trials included two (18.2%) L. helveticus R0052 plus B. longum R0175 investigations. All other formulations were tested once (9.1%) as noted in Table 1. Seven (58.3%) of these trials reported improved anxiety symptoms with 1) I.31 (L. plantarum CECT7484 and CECT7485 plus P. acidilactici CECT7483); 2) L. gasseri SBT2055 plus B. longum SBT2928 in 100g yogurt containing S. thermophilus and L. delbrueckii subsp. Bulgaricus; 3) L. helveticus R0052 plus B. longum R0175; 4) L. acidophilus LA5 plus B. lactis BB-12 in yogurt containing L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, and S. thermophilus; 5) L. acidophilus plus L. casei and B. bifidum; 6) S. thermophilus SGst01, B. animalis ssp. Lactis SGB06, S. thermophiles, B. bifidum SGB02, L. delbrueckii ssp. Bulgaricus DSM 20081, L. acidophilus SGL11, L. plantarum SGL07, L. reuteri SGL01; or 7) L. acidophilus, plus L. casei, B. bifidum, and L. fermentum intake. Of note, Lacidofil® associated anxiety reduction was a within-group effect. No significant differences were observed between treatment groups.
3.3.2. Stress/Anxiety – Non-Human
3.3.2.1. Rat
Thirteen out of twenty-five (52.0%) trials assessed anxiety behaviors. Six (46.2%) were single-strain trials that included two (33.3%) L. fermentum (substrains CECT5716 and NS9), two (33.3%) L. helveticus NS8, and the last two tested each of the following probiotics once: L. rhamnosus GG, B. breve UCC2003, B. infantis 35624, and L. salivarius UCC118. Four (66.7%) of the six studies reported significant improvements with L. helveticus NS8, L. rhamnosus GG or L. fermentum NS9 intake.
Multi-strain (seven out of thirteen – 53.8%) evaluation of anxiety symptoms included three (42.9%) L. helveticus R0052 plus B. longum R0175, two (28.6%) Ecologic® Barrier, one Lacidofil®, and one VSL#3 trial. Two of the seven (28.6%) investigations noted a reduction in anxiety-based phenotypes with L. helveticus R0052 plus B. longum R0175 consumption. Notably, one of these studies indicated the beneficial effects of L. helveticus R0052 plus B. longum R0175 for anxiety symptom reduction was specific to the myocardial infarction (MI) model.
3.3.2.2. Mouse
Sixteen out of 27 (59.3%) trials assessed anxiety with L. rhamnosus (substrains NC4007, GG, and JB-1), B. longum (substrains NCC3001 and 1714), L. plantarum (substrains – two PS128 and one MTCC9510), B. breve 1205, L. reuteri MM4-1A ATCC-PTA-6475, L. johnsonii ATCC33200, E. faecium CFR3003, B. fragilis NCTC9343, L. helveticus R0052, or Lacidofil®.
Thirteen out of fourteen (92.9%) single-strain studies reported a reduction in anxiety-based outcomes (see Table 2). One of the L. plantarum PS128 trials observed improvement in anxiety-like phenotypes specifically in mice naïve to maternal separation stress. Although one trial observed anxiety behavior reduction after B. longum NCC3001 intake (10 days), L. rhamnosus NC4007 consumption did not confer the same benefits. A concurrent investigation of B. longum 1714 and B. breve 1205 demonstrated that anxiety reduction was specific to B. breve 1205 treatment.
The two Lacidofil® trials also indicated a significant improvement in anxiety behavior scores post-treatment.
3.3.2.3. Zebrafish
Three out of five (60%) trials investigated probiotic treatment effects on thigmotaxis and novel tank diving using L. plantarum USDA-ARS or L. rhamnosus GG. Both of the L. plantarum USDA-ARS studies (66.7%) reported decreased anxiety-based behaviors in conventionally raised zebrafish after two or thirty days of treatment. However, L. plantarum USDA-ARS was unable to modify anxiety-like phenotypes in a germ-free environment.
3.3.2.4. Quail
P. acidilactici R001 (MA 18/5M) did not have a significant effect on anxiety-based behaviors.
3.3.3. Depression – Human
3.3.3.1. Infant/Child
Five out of seven (71.4%) trials assessed depression behaviors with single-strain probiotics including four (80%) L. reuteri (substrains DSM 17938, ATCC55730, or unknown) and one (20%) L. rhamnosus GG trial. The L. reuteri ATCC55730 trial conducted a parallel investigation with B. lactis BB-12. One of the five (20%) studies reported improvement in fussiness/crying after 90 days of L. reuteri DSM17938 treatment. Two (40%) reported negative effects, i.e. increased crying and irritability, with L. reuteri DSM 17938 intake. The negative findings were not replicated during post-treatment assessment and specific to formula-fed infants for one of the two trials.
3.3.3.2. Older Adult
Two out of five (40%) trials investigated the effects of L. casei Shirota or L. helveticus IDCC3801 on depression outcomes. Although both studies observed no significant differences as a whole, L. casei Shirota consumption for twenty-one days improved depression symptoms for a subset of individuals experiencing poor baseline mood.
3.3.3.3. Young/Middle-Aged Adult
Nineteen of the thirty-two (59.4%) trials assessed depression phenotypes. Ten (52.6%) of the nineteen were single-strain investigations that included two (20.0%) each of B. longum (substrains NCC3001 or unknown), L. casei (substrains ssp. rhamnosus LCR35 or Shirota), L. rhamnosus (CGMCC1.3724 or HN001) and one (10.0%) each of B. animalis ssp. lactis-07, S. boulardii, L. acidophilus NCFM, and L. helveticus Lafti L10. S. boulardii and L. casei ssp. rhamnosus LCR35 had no significant effect (20.0%) on depression outcomes. The eight remaining studies (80.0%) reported a reduction in depression symptoms after probiotic intake.
Nine (47.4%) of the nineteen young/middle-aged adult studies were multi-strain trials. Two investigated the effects of L. helveticus R0052 plus B. longum R0175. All other combinations were tested once as noted in Table 2. The Lacidofil® and one L. helveticus R0052 plus B. longum R0175 trial did not observe significant differences in depression outcomes after probiotic intake. Of note, the Lacidofil® trial reported significant within- but no between-group differences after 84 days of probiotic consumption. The seven (77.8%) remaining studies reported improvements in depression symptoms.
3.3.4. Depression – Non-Human
3.3.4.1. Rat
Nine out of twenty-five (36%) trials investigated three (33.3%) single-strain – two B. infantis 35624 and one L. plantarum MTCC1325 – and six (66.7%) multi-strain – three (50.0%) L. helveticus R0052 and B. longum R0175, two (33.3%) Ecologic® Barrier, and one (16.7%) L. rhamnosus plus B. longum – combinations of probiotics on depression-based outcomes. Two of three (66.7%) single- and five of six (83.3%) multi-strain trials reported significant beneficial changes in depression-like phenotypes post-probiotic intake. One B. infantis 35624 and one L. helveticus R0052 plus B. longum R0175 trial did not modify depression behaviors.
3.3.4.2. Mouse
Seven out of twenty-seven (25.9%) trials analyzed probiotic effects on depression-associated outcomes. Six (85.7%) were single-strain trials – two (33.3%) L. rhamnosus (substrains JB-1 or GG), two (33.3%) L. plantarum (substrains PS128 or MTCC9510), one (16.7%) L. casei DG, and one (16.7%) comparison of B. longum 1714 and B. breve 1205. Single-strain treatments (83.3%) improved depression-like phenotypes with the exceptions of L. rhamnosus GG and B. longum 1714. A VSL#3 (14.3%) trial also indicated a significant reduction in depression-based behaviors.
3.3.4.3. Zebrafish
None of the trials assessed depression-like phenotypes.
3.3.4.4. Quail
P. acidilactici R001 (MA 18/5M) improved emotional reactivity after thirty-six days.
3.3.5. Social Function – Human
3.3.5.1. Infant/Child
One of seven (14.3%) trials investigated a combination of S. thermophilus, B. animalis ssp. lactis BB-12, and L. bulgaricus with 1 g inulin on pediatric Quality of Life (QOL) social functioning. This study reported improved social behavior after one hundred twelve days of probiotic consumption.
3.3.5.2. Older Adult
One of five (20%) trials investigated VSL#3 with significant improvements in SF-36 social functioning at post-treatment assessment (twenty-eight days).
3.3.5.3. Young/Middle-Aged Adult
Six out of thirty-two (18.8%) trials assessed the effects of probiotics on social ability. Four of the six (66.7%) utilized single probiotic strains that included B. longum (substrains NCC3001 or unknown), L. rhamnosus GG, or S. boulardii. Three of the four (75.0%) noted improvement in IBS, IBD, or SIP social function after twenty-eight, twenty-eight, or fifty-six days of probiotic intake, respectively. The two (42.9%) multi-strain trials were Lacidofil® or a combination of S. thermophilus SGst01, plus B. animalis ssp. Lactis SGB06, S. thermophiles, B. bifidum SGB02, L. delbrueckii ssp. Bulgaricus DSM20081, L. acidophilus SGL11, L. plantarum SGL07, and L. reuteri SGL01. Both multi-strain formulations were not effective at modifying social behaviors.
3.3.6. Social Function – Non-Human
3.3.6.1. Rat
Three out of twenty-five (12.0%) rat trials evaluated L. helveticus R0052 plus B. longum R0175 in relation to social interactions. Two of the three (66.7%) indicated improved social function with probiotic consumption for fourteen days post-MI induction or seven days pre- and seven days post-MI.
3.3.6.2. Mouse
Five out of twenty-seven (18.5%) trials assessed the effects of probiotics on social ability. Two of the four (50%) single-strain investigations observed increased social interactions after twenty-eight days of L. rhamnosus JB-1 or L. reuteri MM4-1A-ATCC-PTA-6475 consumption. B. fragilis NCTC9343, L. casei DG, or L. johnsonii ATCC33200 intake did not alter social behaviors. A twenty-day trial of VSL#3 had positive effects on social interactions.
3.3.6.3. Zebrafish
One out of five (20.0%) trials investigated and observed increased shoaling after twenty-eight days of L. rhamnosus IMC501 exposure.
3.3.6.4. Quail
No social functioning phenotypes were assessed.
3.3.7. Cognition – Human
3.3.7.1. Infant/Child
One out of seven (14.3%) trials investigated psychomotor development effects associated with L. reuteri treatment. No significant changes were detected.
3.3.7.2. Older Adult
Three of the five (60%) trials determined the effects of L. casei Shirota, L. helveticus IDCC3801, or a multi-strain combination of L. acidophilus, L. casei, L. fermentum, plus B. bifidum. The L. helveticus IDCC3801 and L. acidophilus, L. casei, L. fermentum, plus B. bifidum trials observed modifications in Rapid Visual Information Processing (RVIP) and Mini Mental Status Exam (MMSE) performance post-probiotic consumption.
3.3.7.3. Young/Middle-Aged Adult
Eight out of thirty-two (25.0%) trials evaluated the effects of probiotic treatment on cognitive indices. Four of the eight (50.0%) were single-strains investigations of L. rhamnosus (substrains JB-1 or CGMCC1.3724), L. casei Shirota, or B. longum NCC3001. Multi-strain combinations included VSL#3, S. thermophilus SGst01, B. animalis ssp. Lactis SGB06, S. thermophiles, B. bifidum SGB02, L. delbrueckii ssp. Bulgaricus DSM20081, L. acidophilus SGL11, L. plantarum SGL07, L. reuteri SGL01, or combination of B. animalis ssp. lactis, plus L. lactis ssp. lactis, S. thermophilus, and L. bulgaricus. Except for one multi- and one single-strain trial, all of the young/middle-aged adult reports noted improved cognitive performance with probiotic consumption. One trial investigated the effects of Bifidobacterium and noted greater block design ability and shorter Trail Making A & B completion times after sixty days of probiotic intake. However, it is unclear if a single- or multi-strain formulation of Bifidobacterium was tested.
3.3.8. Cognition – Non-Human
3.3.8.1. Rat
Seventeen out of twenty-five (68.0%) trials assessed cognitive functioning. Six (35.3%) trials were single-strain investigations that included two (33.3%) L. plantarum (substrains MTCC1325 or unknown), two (33.3%) L. helveticus NS8, and one (16.7%) each of L. paracasei HII01, L. rhamnosus, and B. B94. Five out of the six (83.3%) studies noted cognitive improvement with probiotic treatment. Twenty-eight days of L. plantarum or B. B94 consumption did not modify spatial ability.
The eleven (64.7%) multi-strain combinations were two each of (18.2%) VSL#3, Lacidofil®, and L. helveticus R0052 plus B. longum R0175. All other multi-strain combinations were tested once (see Table 2). Two (18.2%) of the multi-strain trials did not report significant differences in cognitive performance after Ecologic® Barrier or L. helveticus R0052 plus B. longum R0175 consumption. One (9.1%) study reported a decline in memory with Lacidofil® intake in rats exposed to maternal stress. The eight (72.7%) remaining trials observed improvements in cognitive indices post-probiotic treatment.
3.3.8.2. Mouse
Sixteen out of twenty-seven (59.3%) trials reported probiotic effects on cognition. All sixteen studies observed improved cognitive task performance with probiotic treatment. Four out of sixteen (25%) trials were multi-strain designs - three Lacidofil® and one VSL#3. One of the Lacidofil® trials observed beneficial effects on memory in Rag −/− mice naïve to water avoidance stress (WAS). Probiotic treatment reduced memory ability in WAS exposed Rag −/− mice.
3.3.8.3. Zebrafish
One out of five (20.0%) trials investigated cognition-based outcomes. Forty-nine days of P. acidilactici JN039350 plus L. plantarum JN039358 consumption improved high cholesterol diet-induced decline in spatial memory.
3.3.8.4. Quail
P. acidilactici R001 (MA 18/5M) improved memory performance during treatment days two and three.
4. Discussion
4.1. Summary
Across all species, L. rhamnosus (substrains GG, JB-1, NC4007, IMC501) was the most common single-strain probiotic treatment investigated (12.3%). VSL#3 and Lacidofil® were the most frequently tested multi-strain formulations (5.7% each). A gross difference between the human and non-human trials was the greater enrollment of human female subjects (Table 4), whereas non-human studies conducted most experiments with male animals (Table 5).
The cross-species overview (Table 6) indicates that both single- and multi-strain combinations of probiotics may influence cognition, social function, anxiety, depression, or other emotional behaviors with similar efficacy in humans. For the non-human studies, multistrain combinations were more likely to modify cognition and social behavior, whereas single- and multi-strain combinations may be comparable in ability to regulate anxiety, depression, or emotional behaviors. Because variation in probiotic combinations and experimental designs among the human and non-human trials hindered our ability to conduct meaningful meta-analyses (DerSimonian and Kacker, 2007), these and the other findings reported in this review should be considered crude and preliminary estimations.
4.2. Limitations
There are profound gaps in our understanding of probiotic treatments that should be addressed in forthcoming human and non-human translational trials. Non-neuropsychiatric outcomes assayed in conjunction with each probiotic treatment varied widely and included neural, immune, anthropometric, gastrointestinal, neuroendocrine, or metabolic markers. Since these biomarkers are likely to be critical components in our mechanistic understanding of gut-brain interactions, thoughtful incorporation and replication of precise biomarkers is germane to the success of future probiotic investigations.
Specific to the human reports, several healthy adult trials noted in this review did not observe significant probiotic treatment effects on neuropsychiatric outcomes. Although healthy subject investigations minimize illness-related study confounds, these trials are unlikely to capture a sufficient range of neuropsychiatric phenotypes. This coincides with findings from a recent meta-analysis of probiotic trials in relation to depression symptoms (Ng et al., 2018). Additional neuropsychiatric case-control trials with multiple treatment outcome and compliance measures are necessary to confirm prior reports and conduct more rigorous meta-analyses of probiotics in relation to specific symptoms.
The total number of studies conducted with quail, zebrafish, and specific rat and mouse stains are very limited for a comprehensive review. The reporting of the experimental microbial environment (i.e. specific-pathogen-free, germ-free, etc.) for animal trials was inconsistent and should be improved. Significant limitations common to all probiotic trials include reporting bias, sample size, confirmation of probiotic activity and administration vehicle, precise probiotic dosage and treatment duration, differential neuropsychiatric assessments, and an insufficient number of trials for early and late neurodevelopmental stages across the lifespan.
Assessment of study quality and bias had some effectiveness for capturing dropout rates and quality variation. Approximately 59% percent of the human trials reported less than 15% study subject dropout. Intent-to-treat analyses were reported for 45.8% of the human trials. Measures of probiotic treatment adherence (i.e. intake of 75% or greater doses) and exclusion criteria for poor compliance were reported for 29.2% of the human trials. Specific blinding status was reported for 93.1% and 43.1% human and non-human studies, respectively. Although we aimed to minimize bias when evaluating all non-human trials, excessive positive finding reporting for animal studies has been acknowledged (Sena et al., 2010; Tsilidis et al., 2013) and is not easily illuminated with existing study quality and bias assessments. These are critical factors that need to be addressed to improve study quality and develop successful treatments.
The sample sizes for approximately 50% of the human and 90% of the non-human trials were fewer than thirty and fifteen subjects per group, respectively. In addition, studies reporting a priori designations of primary and secondary outcomes customary for rigorous clinical trial designs or sample size estimations to reflect sufficient study power were limited across species. This implies most of the trials evaluated would be considered exploratory investigations. Therefore, larger, adequately powered, replication studies are required to confirm observations from individual trials and those compiled in this systematic review.
Verification of probiotic strain/substrain activity (i.e. in vitro culture) prior to conducting the investigation or post-probiotic treatment fecal sample sequencing was inconsistent across trials. In addition, probiotic intake vehicle varied across trials (i.e. capsule, in yogurt, in water, per oral, etc.). The combination of these factors can lead to significant experimental confounds and thereby influence the validity and reliability of our systematic review observations. Although the exact mechanisms by which probiotics proliferate within the intestinal tract are unclear, administration route may influence successful probiotic colonization. Therefore, future studies may consider confirmatory procedures such as fecal (human and non-human) or intestinal biopsy (non-human) sequencing.
Three human, two rat, and one mouse trial investigated varying “doses” (i.e. colony forming units-CFU) for the same probiotic strain/substrain in relation to neuropsychiatric outcomes. However, more extensive dose-finding experiments will need to be conducted across species, probiotic strains/substrains, and study populations prior to large-scale implementation of probiotics for gut-brain-behavior based outcomes. Treatment duration for most probiotic trials reviewed was less than sixty days with a limited number of trials assessing long-term neuropsychiatric outcomes. A pilot adjunctive probiotic trial in treatment-resistant depressed patients reported the efficacy of an L. acidophilus, B. bifidum, S. thermophiles (2 × 1010 CFU) plus 1600 mg magnesium orotate therapeutic and demonstrated symptom reemergence upon treatment cessation (Bambling et al., 2017). While the study was ineligible for inclusion in this report based on our systematic review criteria; their observations highlight the need for developing longitudinal probiotic investigations and utilizing probiotic treatments as a long-term health and wellness lifestyle modification, rather than a short-term intervention.
Thus far, two double-blind investigations have been conducted with patients diagnosed with significant anxiety or depression symptoms (Akkasheh et al., 2016; Romijn et al., 2017). Investigations of probiotics in patients with prominent mania or psychoses are also limited (Dickerson et al., 2018; Dickerson et al., 2014). While we aimed to distinguish neuropsychiatric outcomes by specific symptoms in the Results section of this review, the sizeable overlap in neuropsychiatric phenotypes, especially anxiety and depression (Sartorius et al., 1996), are well-recognized. Most of the human and non-human probiotic studies utilized varying stress or depression-based assessments. In addition, limited assessments or assays in relation to neuropsychiatric symptoms were conducted within most trials. Standardized neurobehavioral measures and novel symptom models should be developed and incorporated into future trials. To differentiate subsyndromes, future trials could employ multiple experimental strategies (e.g. psychophysical task plus anxiety assessment interview in human studies).
Across species, trials were primarily conducted to coincide with the middle-age adult stage of the lifespan. While this review aimed to highlight the limited studies that enrolled older adults, young/middle-aged adults, and children; early- (12-14 years) and middle- (15-17 years) adolescent stage human or non-human animal trials were not reported in the neuropsychiatric literature. Gut-brain-pathways are highly likely to be modified by gut microbes and probiotics during these critical periods of the lifespan (McVey Neufeld et al., 2016) which should be underscored for future probiotic and gut-brain-behavior studies.
Although neuropsychiatric illnesses are largely characterized by significant cognitive and behavioral alterations; sociodemographic and lifestyle factors are prominent and may modify the effects of probiotics on anxiety, mood, and psychotic disorders. There are preliminary indications that probiotic treatment efficacy may vary by participant sex (Sanchez et al., 2017). Other sociodemographic differences were not reported by the studies reviewed. Non-probiotic neuropsychiatric trials have reported differential treatment response by race or ethnicity (Ellis et al., 2015). Therefore, future studies may need to consider variation in probiotic treatment efficacy by subject race/ethnicity or animal strain.
4.3. Conclusions
Our review indicates that several human and non-human probiotic treatment trials have been conducted with direct or indirect intent to target gut-brain-behavior interactions. However, the large variance in experimental design, sample characteristics, and assessment methodology hinders true comparison and computation of effect sizes for specific phenotypes and probiotic strain or substrain combinations. Although a wide-range of mediating biomarkers and neuropsychiatric assessments can have significant utility as secondary or tertiary outcome analyses, it is important for future human and non-human investigations to improve study power and minimize risk of bias by clearly delineating all post hoc analyses and/or incorporating as many of these variables a priori when computing sample size estimates. Improving the reporting of trial methodology with these crucial details will substantially improve the quality of subsequent systematic reviews and meta-analyses.
Future studies will need to replicate existing findings and develop additional randomized, placebo-controlled, double-blinded, and case-controlled, or cohort probiotic trials. Since probiotic trials are unable to clarify all gut-microbe based treatment mechanisms, large-scale normative data obtained from various human cohorts and non-human animal species and strains are necessary to obtain a more veridical representation of gut microbial composition and variation across the lifespan. Concurrent investigations with special emphasis on neurodevelopmental and neuropsychiatric illness trajectory will be especially valuable. Data obtained from these combined sources will better inform future probiotic treatment study design and hypothesized neuropsychiatric targets.
In brief, the ability to 1) elucidate gut microbe-brain-behavior pathway mechanisms; 2) disentangle unique effects of single- and multi-strain formulations of probiotics, and; 3) implement novel probiotic treatments to target precise neuropsychiatric phenotypes, will require comprehensive review and meta-analyses of future probiotic trial outcomes.
Highlights.
A cross-species review of probiotic effects on neuropsychiatric outcomes was conducted.
Probiotic substrain and experimental design variation hindered formal meta-analyses.
Single and multi-strain formulations of probiotics can modify neuropsychiatric phenotypes.
Rigorous follow-up studies are necessary to confirm initial findings.
Acknowledgments
The authors thank Hayley Palmer for her assistance with the initial literature search. This work was supported the UC San Diego Frontiers Innovation Scholars Program (PI: Depp, C.A.), NIMH T32MH019934 (PI: Jeste, D.V.), and NIMH T32MH018399 (PI: Kelsoe, J.R.) to JMJ.
Appendix
Table A.1.
Probiotic Trials Employing Psychological Stress Paradigms Without Neuropsychiatric Outcome Assessment.
| Probiotic Treatment |
Study Design |
Sex/ Age, days |
Probiotic Associated Outcomes |
References |
|---|---|---|---|---|
| Rat (Rattus) | ||||
| L. farciminis 1011 CFU/day or saline P.O. QD for 15 days | Wistar PRS or sham stress | Female Age Adult |
#PROT ↓ stress-induced visceral hypersensitivity, p-MLC, and colonic permeability | (Ait-Belgnaoui et al., 2006) |
| L. farciminis 1011 CFU/day or saline P.O. QD for 14 days prior to PRS | Wistar sham + saline (n = 8), sham + #PROT (n = 8), sham + saline (n = 8), sham + CRD + #PROT (n = 8), PRS + CRD + saline (n = 8), PRS + #PROT (n = 8), PRS + CRD + saline (n = 8), PRS + CRD + #PROT (n = 8) | Female Age Adult |
#PROT ↓ PRS + CRD induced ↑ in Fos positive spinal cord, PVN, and MeA cells | (Ait-Belgnaoui et al., 2009) |
| L. farciminis 1011 CFU/day, ML-7, saline, or antibiotic P.O. for 14 days | Wistar PRS or sham stress Pathogen Free | Female Age Adult |
#PROT ↓ PRS induced colonic permeability, endotoxemia, PVN CRF expression, circulating LPS, and pro-inflammatory cytokine expression in HYP | (Ait-Belgnaoui et al., 2012) |
| L. farciminis or vehicle 1011 CFU/mL P.O. for 10 days pre-WAS/sham and 4 days with WAS/sham | Wistar WAS or sham stress 12 groups (n = 8 or 14/group) | Male Age Adult |
#PROT ↓ WAS induced visceral sensitivity, intestinal permeability, colonic mucus flattening, and colonic mucin O-glycosylation | (Da Silva et al., 2014) |
| L. farciminis or vehicle 1011 CFU/mL P.O. for 10 days pre-WAS/sham and 4 days with WAS/sham | Wistar WAS or sham stress 4 groups (n = 8/group) | Male Age 63 days |
#PROT bound to colonic mucus in WAS and sham stress #PROT abundance in ileum and colon ↓ after WAS only |
(Da Silva et al., 2015) |
| L. casei Shirota YIT 9029 3 × 109 CFU for 14 days | Fischer 344 Naïve (n = 11), WAS (n = 14), WAS + #PROT (n = 14) | Male (n = 41) Age 56-63 days |
#PROT ↓ WAS induced corticosterone and CRF in the PVN See Table 3 for human trial outcomes |
(Takada et al., 2016) |
| L. rhamnosus Lcr35 (Lcr Lenio® 4.5 × 109 CFU/g or Lcr Restituo®1 × 104, 1.8 × 109, 6.8 × 1010CFU/g for 8 days post-TNBS or sham injection surgery and pre-colonic distention | Sprague Dawley Wistar TNBS, PRS, U50 or naïve 12 groups (n = 9 -10/group) | Male (Sprague Dawley) Female (Wistar) Age Adult |
#PROT ↓ TNBS and PRS induced visceral hypersensitivity #PROT ↓ TNBS induced increase in IL-12p70, TNF-α in colonic tissue |
(Darbaky et al., 2017) |
| B. subtilis CH201 1.5 × 108 CFU/mL QD in drinking water for 44 days with chronic stress beginning day 14, and PD beginning day 30 | Wistar Control, stress, and PD with and w/out #PROT 8 groups (n = 8/group) | Male Age 42 days |
#PROT ↓ inflammatory cells in periodontal tissue, C-terminal telopeptide, and alveolar bone loss in unstressed animals *NSE in stressed animals |
(Foureaux Rde et al., 2014) |
| B. animalis ssp. lactis- BB-12 3 × 109 CFU/mL, P. jenenii 8 × 108 CFU/mL, or vehicle in maternal drinking water for 32 days (10 days before mating) | Wistar control (n = 40), MS (n = 40), adult stress (n = 40), MS + adult stress (n = 40) w/ and w/out #PROT | Male (n = 80) Female (n = 80) Wistar Age 2-14 days (MS) Age 83-85 days adult stress |
#PROT ↓ MS and adults stress induced ↑ IFN-γ (males and females) & IL-6 (males) #PROT ↓ haptoglobin in control, MS and adult stress #PROT ↓ ileal MUC2 expression in MS males and control females |
(Barouei et al., 2015) |
| L. paracasei NCC2461 4 × 1011 CFU/kg diet with arachidonic and docosahexaenoic acids, galactooligosaccharides and fructooligoccharides or control diet for 20 days after 12 days MS | Long Evans MS (n = 84 all experiments) | Male Age 15 and 35 days |
#PROT ↓ stress induced ↑ intestinal permeability and villi changes #PROT ↑ concentration of Enterococci and Bacteroides in MS and no MS |
(Garcia-Rodenas et al., 2006) |
| E. coli Nissle 101 CFU/mL, 104 CFU/mL, 108 CFU/mL or vehicle single dose | WRS (60 min after #PROT), pretreatment with indomethacin 10 groups (n = 6/group) | Sex and Age unknown | #PROT ↓ WRS induced gastric lesions, gastric mucosa IL1-ß mRNA, #PROT ↑ WRS ↓ ghrelin | (Konturek et al., 2009) |
| L. plantarum 8P-A3 and L. fermentum 90T-C4 4 × 108 CFU P.O. for 21 days during chronic social stress | Wistar unstressed, Two types of social stress, canamycin, #PROT 7 groups (n=10/group) | Female (n = 70) Age 180 days |
#PROT ↑ social stress induced ↓ Xbp1 in lymphoblasts and small lymphocytes | (Topol et al., 2014) |
| B. lactis CNCM 1-2494 106, 107, 108, 109, 1010 CFU doses (n = 10/dose), FM with B. lactis CNCM I-2494 Lc. lactis CNCM I-1631 and two yogurt starters: L. bulgaricus and S. thermophilus (n = 10), NFM (n = 10), or saline (n = 10) P.O. QD for 15 days | Wistar w/ (n = 10) or w/out (n = 10) PRS for all #PROT | Female Age Adult |
FM ↓ PRS induced abdominal cramps FM ↓ PRS induced ↑ blood LPS FM prevented stress induced ↑ intestinal permeability *NSE visceral sensitivity response |
(Agostini et al., 2012) |
| L. farciminis 1011 CFU/day P.O. QD for 10 days during WAS | Specific-pathogen-firee Wistar no-stress (n = 8), WAS (n = 12), and WAS + #PROT (n = 12) | Male (n = 16) Female (n = 16) Age 49 days |
*NSE WAS induced ↑ fecal pellet output #PROT ↓ WAS-induced ↑ distal colon IL-6, PRSS1, mucosal mast cell count, and visceromotor response to colorectal distention in WAS exposed rats (females only) #PROT ↓ WAS-induced ↑ distal colon IL-1β (males only) |
(Lee et al., 2017) |
| L. helveticus R0052 and L. rhamnosus R0011 109 CFU/mL in drinking water 7 days pre-WAS or sham stress and 10 days during WAS | Brown Norway SPF: WAS (n = 6), WAS + #PROT (n = 4), sham stress (n = 4), or sham stress + #PROT (n = 4) | Male Age unknown |
#PROT ↓ bacterial translocation into lymph nodes for WAS group | (Zareie et al., 2006) |
| Lacidofil® 108 CFU P.O. and P.R. B.I.D. for 16 days beginning day 4 of MS | Sprague–Dawley MS (16 days) then WAS (10 days age 60-70) (n = 7-15/group) | Female and Male pups Age 4 and 70 days |
#PROT ↓ MS induced ↑ in intestinal permeability, serum corticosterone, and bacterial adhesion to colonic mucosa #PROT ↑ MS induced ↓ in Lactobacilli *NSE intestinal permeability, serum corticosterone in unstressed pups |
(Gareau et al., 2007) |
| Mouse (Mus) | ||||
| L. casei CRL 431 1 × 108CFU/mL QD for 11 days | BALB/c 11 days of food and restraint stress stress + #PROT (n = 9), stress (n = 9), + control (n = 9), control + #PROT (n = 9) | Male Age 35 days |
#PROT reserved stress induced intestinal villi and IgA changes *NSE CORT |
(Palomar et al., 2014) |
| L. casei Shirota YIT9029 5 × 109 CFU/mL or placebo for 84 days during repeated WAS | C57BL/6JTcra +/+, +/−, and −/− repeated WAS 5 days/week for 84 days (n = 5-12/group) | Female Age 56 days |
#PROT ↓ microbial changes induced by repeated WAS inTcra +/+ and ↓ loss of microbial diversity Tcra −/− *NSE repeated WAS induced ↑ corticosterone, colonic IgG, or IgA |
(Arase et al., 2016) |
| L. reuteri ATCC 23272 1 × 108 CFU or vehicle P.O. for 6 days during social stress | C57BL6J Social stress 2hr/day for 6 daysC. rodentium infection during social stress (n =9/group) | Male Age 42 to 56 days |
*NSE social stress induced ↑ C. rodentium #PROT ↓ C. rodentium induced ↑ colonic: mass, TNF-α, iNOS, F4/80+ macrophages, epithelial cell gene expression, CD11b+Ly6ChiCCR2+ cells (blood also) #PROT ↓ social stress induced ↑ C. rodentium translocation to spleen |
(Mackos et al., 2016) |
| B. bifidum, L. acidophilus, S. faecalis 1 × 107 CFU/mL for 10 days with WAS | C57BL6J Unstressed, WAS 1hr/day for 10 days, WAS + #PROT (n = 5/group) | Female Age 35 to 42 days |
#PROT ↓ WAS induced ↑ intestinal inflammation, permeability, proportion of Firmicutes, Bacteroidetes | (Sun et al., 2013) |
Abbreviations:
PROT = Probiotic Treatment, CRD = Colorectal Distention, FM = Fermented Milk, HIP = Hippocampus, LA = Lateral Amygdala, LPS = Lipopolysaccharide, MeA = Medial Nucleus Amygdala, MI = Myocardial Infarction, NAA = N-acetylaspartate, NFM = Non-Fermented Milk, PD = Periodontal Disease, p-MLC = phosphorylated-Myosin Light Chain, P.O. = Per Oral, P.R. = Per Rectal, PRS = Partial Restraint Stress, PRSS = Mucosal Serine Protease Gene, PVN = Paraventricular Nucleus (Hypothalamus), TNBS = 2,4,6-trinitrobenzenesulfonic acid, QD = Daily, WAS = Water Avoidance Stress, WRS = Water Immersion Restraint Stress
Table A.2.
Assessment of Human Trial Quality: PEDro Scale*.
| Reference | 1. Eligibility criteria specified |
2. Random allocation |
3. Concealed allocation |
4. Groups similar at baseline |
5. Subject blindig |
6. Therapist blinding |
7. Assess or blinding |
8. Less than 15% dropouts |
9. Intention -to- treat analysis |
10. Between- group statistical comparisons |
11. Point measures and variability data |
Totala |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infant/Child | ||||||||||||
| (Rinne et al., 2006) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 8 |
| (Indrio et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
| (Cekola et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 10 |
| (Sung et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 10 |
| (Weizman and Alsheikh, 2006) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 10 |
| (Akar et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
| (Ringel-Kulka et al., 2015) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 8 |
| Older Adult | ||||||||||||
| (Ostlund-Lagerstrom et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| (Benton et al., 2007) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Chung et al., 2014) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| (Pellino et al., 2013) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 10 |
| (Akbari et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Young/Middle-Aged Adult | ||||||||||||
| (Kato-Kataoka et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Takada et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Takada et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Rao et al., 2009) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 8 |
| (Reale et al., 2012) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Bajaj et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 10 |
| (Kelly et al., 2017) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 7 |
| (Sanchez et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 10 |
| (Michalickova et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
| (Culpepper et al., 2016; Langkamp-Henken et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| (Slykerman et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| (Childs et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Choi et al., 2011) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
| (Lorenzo-Zuniga et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 10 |
| (Lyra et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| (Spiller et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 10 |
| (Dapoigny et al., 2012) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| (Nishihira et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Nobutani et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Pinto-Sanchez et al., 2017) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| (Yang et al., 2016b) | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| (Fujimori et al., 2009) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
| (Steenberg en et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Moller et al., 2017) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 8 |
| (Tillisch et al., 2013) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
| (Mohammadi et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Messaoudi et al., 2011a; Messaoudi et al., 2011b) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 8 |
| (Diop et al., 2008) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| (Romijn et al., 2017) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| (Akkasheh et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| (Dickerson et al., 2014; Severance et al., 2017; Tomasik et al., 2015) | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 7 |
| (De Lorenzo et al., 2017) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| (Kouchaki et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| (Feher et al., 2014) | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 5 |
| (Lee et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| (Malaguarnera et al., 2010) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
As reported by Maher et al. (2003).
Mean total score = 9.5 (SD 1.2). Interquartile range 9-11.
Table A.3.
Assessment of Human Trial Quality: Quality Index*.
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | Totala |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infant/Child | |||||||||||||||||||||||||||
| (Rinne et al., 2006) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 16 |
| (Indrio et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Cekola et al., 2015) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 22 |
| (Sung et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 24 |
| (Weizman and Alsheikh, 2006) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 22 |
| (Akar et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 22 |
| (Ringel-Kulka et al., 2015) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| Older Adult | |||||||||||||||||||||||||||
| (Ostlund-Lagerstrom et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 24 |
| (Benton et al., 2007) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Chung et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Pellino et al., 2013) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 21 |
| (Akbari et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 22 |
| Young/Middle-Aged Adult | |||||||||||||||||||||||||||
| (Kato-Kataoka et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 18 |
| (Takada et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Takada et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 17 |
| (Rao et al., 2009) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 18 |
| (Reale et al., 2012) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Bajaj et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| (Kelly et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 17 |
| (Sanchez et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 19 |
| (Michalickova et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 22 |
| (Culpepper et al., 2016; Langkamp-Henken et al., 2015) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 24 |
| (Slykerman et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| (Childs et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 23 |
| (Choi et al., 2011) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 21 |
| (Lorenzo-Zuniga et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| (Lyra et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| (Spiller et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 24 |
| (Dapoigny et al., 2012) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 24 |
| (Nishihira et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Nobutani et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 19 |
| (Pinto-Sanchez et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| (Yang et al., 2016b) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 15 |
| (Fujimori et al., 2009) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 21 |
| (Steenbergen et al., 2015) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Moller et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 18 |
| (Tillisch et al., 2013) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 21 |
| (Mohammadi et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 21 |
| (Messaoudi et al., 2011a; Messaoudi et al., 2011b) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 21 |
| (Diop et al., 2008) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 21 |
| (Romijn et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| (Akkasheh et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 23 |
| (Dickerson et al., 2014; Severance et al., 2017; Tomasik et al., 2015) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 20 |
| (De Lorenzo et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 21 |
| (Kouchaki et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 22 |
| (Feher et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 17 |
| (Lee et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 20 |
| (Malaguarnera et al., 2010) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 19 |
As reported by Downs and Black (1998).
Mean total score = 20.8 (SD 2.3). Interquartile range 18-24.
Table A.4.
Risk of Bias* Evaluation: Human Probiotic Trials.
Key: + = Low Risk for Bias, − = High Risk for Bias, ? = Unclear Risk for Bias
As reported by Higgins et al. (2011).
Table A.5.
Non-Human Study Quality and Risk of Bias Assessment*.
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total Scorea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat (Rattus) | |||||||||||||
| (Nimgampalle and Kuna, 2017) | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| (Liang et al., 2017) | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 6 |
| (Abildgaard et al., 2017a) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 9 |
| (Abildgaard et al., 2017b) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 9 |
| (Tillmann et al., 2018) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| (Messaoudi et al., 2011a) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | NA | 0 | 0 | 1 | 9 |
| (Desbonnet et al., 2008) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 7 |
| (Liang et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Beilharz et al., 2017) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 7 |
| (Davari et al., 2013) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 0 | 5 |
| (Goudarzvand et al., 2016) | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 0 | 5 |
| (Jeong et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Wang et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Distrutti et al., 2014) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| (Arseneault-Breard et al., 2012) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | NA | 0 | 0 | 1 | 8 |
| (Luo et al., 2014) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 6 |
| (Callaghan et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Cowan et al., 2016) | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 6 |
| (Desbonnet et al., 2010) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 9 |
| (Gilbert et al., 2013) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | NA | 0 | 0 | 0 | 6 |
| (McKernan et al., 2010) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| (Vanhaecke et al., 2017) | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | NA | 0 | 0 | 0 | 5 |
| (Athari Nik Azm et al., 2018) | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 1 | 5 |
| (McVey Neufeld et al., 2017) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 1 | 6 |
| Mouse (Mus) | |||||||||||||
| (Musa et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 0 | 6 |
| (Guida et al., 2017) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 7 |
| (Jeong et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Dhaliwal et al., 2018) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 7 |
| (Divyashri et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 8 |
| (Hsiao et al., 2013) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Bharwani et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Emge et al., 2016) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
| (Bercik et al., 2010) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 8 |
| (Bercik et al., 2011) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 8 |
| (Savignac et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 7 |
| (Mohle et al., 2016) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 7 |
| (Gareau et al., 2011) | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 6 |
| (Smith et al., 2014) | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 |
| (Woo et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 7 |
| (Buffington et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (D'Mello et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 8 |
| (Savignac et al., 2014) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 0 | 5 |
| (Bravo et al., 2011) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 7 |
| (Kantak et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 8 |
| (Xiao et al., 2014) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 8 |
| (Tian et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 0 | 6 |
| (Ohland et al., 2013) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Liu et al., 2016a) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
| (Liu et al., 2016b) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| (Sun et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 7 |
| Zebrafish (Danio rerio) | |||||||||||||
| (Davis et al., 2016a) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| (Davis et al., 2016b) | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| (Schneider et al., 2016) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 7 |
| (Borrelli et al., 2016) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 6 |
| (Lim et al., 2017) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | NA | 0 | 0 | 0 | 6 |
| Quail (Coturnix japonica) | |||||||||||||
| (Parois et al., 2017) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
Abbreviations: NA = Not Applicable
Mean total score = 6.5 (SD 1.3). Interquartile range 5.5-7.5.
Study quality was assessed with a modified scale reported by Macleod et al. (2004). Our customized scale was operationalized as 1 = Yes, 0 = No or Unsure, NA = Not Applicable for each item as follows: 1) compliance with animal welfare regulations; 2) comprehensive statement of environmental control (temperature, handling, etc.); 3) confirmation of probiotic activity prior to administration; 4) exact animal strain and experimental paradigm noted; 5) exact probiotic treatment (including substrain) noted; 6) control group received probiotic vehicle; 7) individuals administering probiotic were blind to treatment groups; 8) behavioral assays conducted blind to treatment; 9) animals tested in a random order when conducting multiple behavioral assays; 10) a priori power analyses for treatment group sample sizes; 11) a priori primary and secondary outcomes; and 12) acknowledged conflicts of interest or lack thereof.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that this research was conducted in the absence of commercial or financial relationships that could be construed as a potential conflict of interest as noted in the journal’s authorship and disclosure agreements.
References
- Abildgaard A, Elfving B, Hokland M, Lund S, Wegener G, 2017a. Probiotic treatment protects against the pro-depressant-like effect of high-fat diet in Flinders Sensitive Line rats. Brain, behavior, and immunity 65, 33–42. [DOI] [PubMed] [Google Scholar]
- Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S, 2017b. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 79, 40–48. [DOI] [PubMed] [Google Scholar]
- Agostini S, Goubern M, Tondereau V, Salvador-Cartier C, Bezirard V, Leveque M, Keranen H, Theodorou V, Bourdu-Naturel S, Goupil-Feuillerat N, Legrain-Raspaud S, Eutamene H, 2012. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 24, 376–e172. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V, 2012. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37, 1885–1895. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Eutamene H, Houdeau E, Bueno L, Fioramonti J, Theodorou V, 2009. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 21, 567–573, e518–569. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, Theodorou V, 2006. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 55, 1090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H, 2016. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 202, 254–257. [DOI] [PubMed] [Google Scholar]
- Akar M, Eras Z, Oncel MY, Arayici S, Guzoglu N, Canpolat FE, Uras N, Oguz SS, 2017. Impact of oral probiotics on neurodevelopmental outcomes in preterm infants. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 30, 411–415. [DOI] [PubMed] [Google Scholar]
- Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA, Salami M, 2016. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer's Disease: A Randomized, Double-Blind and Controlled Trial. Front Aging Neurosci 8, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A, 2016. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition (Burbank, Los Angeles County, Calif.) 32, 315–320. [DOI] [PubMed] [Google Scholar]
- Arase S, Watanabe Y, Setoyama H, Nagaoka N, Kawai M, Matsumoto S, 2016. Disturbance in the Mucosa-Associated Commensal Bacteria Is Associated with the Exacerbation of Chronic Colitis by Repeated Psychological Stress; Is That the New Target of Probiotics? PloS one 11, e0160736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault-Breard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R, Rousseau G, 2012. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. The British journal of nutrition 107, 1793–1799. [DOI] [PubMed] [Google Scholar]
- Athari Nik Azm S, Djazayeri A, Safa M, Azami K, Ahmadvand B, Sabbaghziarani F, Sharifzadeh M, Vafa M, 2018. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1-42) injected rats. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 43, 718–726. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM, 2014. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Alimentary pharmacology & therapeutics 39, 1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambling M, Edwards SC, Hall S, Vitetta L, 2017. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology 25, 271–274. [DOI] [PubMed] [Google Scholar]
- Barouei J, Moussavi M, Hodgson DM, 2015. Perinatal maternal probiotic intervention impacts immune responses and ileal mucin gene expression in a rat model of irritable bowel syndrome. Beneficial microbes 6, 83–95. [DOI] [PubMed] [Google Scholar]
- Beilharz JE, Kaakoush NO, Maniam J, Morris MJ, 2017. Cafeteria diet and probiotic therapy: cross talk among memory, neuroplasticity, serotonin receptors and gut microbiota in the rat. Molecular psychiatry 23, 351–361. [DOI] [PubMed] [Google Scholar]
- Benton D, Williams C, Brown A, 2007. Impact of consuming a milk drink containing a probiotic on mood and cognition. European journal of clinical nutrition 61, 355–361. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF, 2011. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 23, 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM, 2010. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139, 2102–2112.e2101. [DOI] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P, 2017. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC medicine 15, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankstein U, Chen J, Diamant NE, Davis KD, 2010. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 138, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Borrelli L, Aceto S, Agnisola C, De Paolo S, Dipineto L, Stilling RM, Dinan TG, Cryan JF, Menna LF, Fioretti A, 2016. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Scientific reports 6, 30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF, 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America 108, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, Taylor CM, Welsh DA, Berthoud H-R, 2015. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biological psychiatry 77, 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak A, Whitehead D, Okamura H, Yajima J, Tsuda A, Steptoe A, 2009. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain, behavior, and immunity 23, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M, 2016. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 165, 1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Cowan CS, Richardson R, 2016. Treating Generational Stress: Effect of Paternal Stress on Development of Memory and Extinction in Offspring Is Reversed by Probiotic Treatment. Psychol Sci 27, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Cekola PL, Czerkies LA, Storm HM, Wang MH, Roberts J, Saavedra JM, 2015. Growth and Tolerance of Term Infants Fed Formula With Probiotic Lactobacillus reuteri. Clinical pediatrics 54, 1175–1184. [DOI] [PubMed] [Google Scholar]
- Chapman C, Gibson GR, Rowland I, 2011. Health benefits of probiotics: are mixtures more effective than single strains? European journal of nutrition 50, 1–17. [DOI] [PubMed] [Google Scholar]
- Childs CE, Roytio H, Alhoniemi E, Fekete AA, Forssten SD, Hudjec N, Lim YN, Steger CJ, Yaqoob P, Tuohy KM, Rastall RA, Ouwehand AC, Gibson GR, 2014. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. The British journal of nutrition, 1–12. [DOI] [PubMed] [Google Scholar]
- Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ, 2011. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol 45, 679–683. [DOI] [PubMed] [Google Scholar]
- Chunchai T, Thunapong W, Yasom S, Wanchai K, Eaimworawuthikul S, Metzler G, Lungkaphin A, Pongchaidecha A, Sirilun S, Chaiyasut C, Pratchayasakul W, Thiennimitr P, Chattipakorn N, Chattipakorn SC, 2018. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. Journal of neuroinflammation 15, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y-C, Jin H-M, Cui Y, Kim DS, Jung JM, Park J-I, Jung E-S, Choi E-K, Chae S-W, 2014. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. Journal of Functional Foods 10, 465–474. [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney R, Shanahan F, Dinan T, Cryan J, 2013. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry 18, 666. [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK, 2009. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA : the journal of the American Medical Association 302, 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CS, Callaghan BL, Richardson R, 2016. The effects of a probiotic formulation (Lactobacillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl Psychiatry 6, e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpepper T, Christman MC, Nieves C Jr., Specht GJ, Rowe CC, Spaiser SJ, Ford AL, Dahl WJ, Girard SA, Langkamp-Henken B, 2016. Bifidobacterium bifidum R0071 decreases stress-associated diarrhoea-related symptoms and self-reported stress: a secondary analysis of a randomised trial. Beneficial microbes 7, 327–336. [DOI] [PubMed] [Google Scholar]
- D'Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, Surrette MG, Swain MG, 2015. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 10821–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva S, Robbe-Masselot C, Ait-Belgnaoui A, Mancuso A, Mercade-Loubiere M, Salvador-Cartier C, Gillet M, Ferrier L, Loubiere P, Dague E, Theodorou V, Mercier-Bonin M, 2014. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. American journal of physiology. Gastrointestinal and liver physiology 307, G420–429. [DOI] [PubMed] [Google Scholar]
- Da Silva S, Robbe-Masselot C, Raymond A, Mercade-Loubiere M, Salvador-Cartier C, Ringot B, Leonard R, Fourquaux I, Ait-Belgnaoui A, Loubiere P, Theodorou V, Mercier-Bonin M, 2015. Spatial Localization and Binding of the Probiotic Lactobacillus farciminis to the Rat Intestinal Mucosa: Influence of Chronic Stress. PloS one 10, e0136048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A, 2012. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World journal of gastroenterology 18, 2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbaky Y, Evrard B, Patrier S, Falenta J, Garcin S, Tridon A, Dapoigny M, Silberberg C, Nivoliez A, Diop L, 2017. Oral probiotic treatment of Lactobacillus rhamnosus Lcr35(R) prevents visceral hypersensitivity to a colonic inflammation and an acute psychological stress. Journal of applied microbiology 122, 188–200. [DOI] [PubMed] [Google Scholar]
- Davari S, Talaei SA, Alaei H, Salami M, 2013. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 240, 287–296. [DOI] [PubMed] [Google Scholar]
- Davis DJ, Bryda EC, Gillespie CH, Ericsson AC, 2016a. Microbial modulation of behavior and stress responses in zebrafish larvae. Behavioural brain research 311, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DJ, Doerr HM, Grzelak AK, Busi SB, Jasarevic E, Ericsson AC, Bryda EC, 2016b. Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Scientific reports 6, 33726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Detraux J, Van Winkel R, Yu W, Correll CU, 2012. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nature Reviews Endocrinology 8, 114–126. [DOI] [PubMed] [Google Scholar]
- De Lorenzo A, Costacurta M, Merra G, Gualtieri P, Cioccoloni G, Marchetti M, Varvaras D, Docimo R, Di Renzo L, 2017. Can psychobiotics intake modulate psychological profile and body composition of women affected by normal weight obese syndrome and obesity? A double blind randomized clinical trial. Journal of translational medicine 15, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Kacker R, 2007. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials 28, 105–114. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG, 2008. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of psychiatric research 43, 164–174. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG, 2010. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin ML, Relman DA, 2008. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS biol 6, e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Relman DA, 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl 1, 4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal J, Singh DP, Singh S, Kumar PA, Boparai RK, Bishnoi M, Kondepudi KK, Chopra K, 2018. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation induced behaviour, biochemical and selected gut microbial aberrations in mice. Journal of applied microbiology. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, Schweinfurth L, Stallings C, Sweeney K, Goga J, Yolken RH, 2018. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar disorders. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, Goga J, Khushalani S, Yolken RH, 2014. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. The primary care companion for CNS disorders 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop L, Guillou S, Durand H, 2008. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutrition research (New York, N.Y.) 28, 1–5. [DOI] [PubMed] [Google Scholar]
- Distrutti E, O'Reilly JA, McDonald C, Cipriani S, Renga B, Lynch MA, Fiorucci S, 2014. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PloS one 9, e106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divyashri G, Krishna G, Muralidhara, Prapulla SG, 2015. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: in vitro and in vivo evidence. Journal of medical microbiology 64, 1527–1540. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N, 1998. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology & Community Health 52, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EM, Orom H, Giovino GA, Kiviniemi MT, 2015. Relations between negative affect and health behaviors by race/ethnicity: Differential effects for symptoms of depression and anxiety. Health Psychol. 34, 4. [DOI] [PubMed] [Google Scholar]
- Emge JR, Huynh K, Miller EN, Kaur M, Reardon C, Barrett KE, Gareau MG, 2016. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. American journal of physiology. Gastrointestinal and liver physiology 310, G989–998. [DOI] [PubMed] [Google Scholar]
- Eun SH, Lim SM, Jang SE, Han MJ, Kim DH, 2016. Lactobacillus sakei K17, an inducer of IL-10 expression in antigen-presenting cells, attenuates TNBS-induced colitis in mice. Immunopharmacology and immunotoxicology, 1–8. [DOI] [PubMed] [Google Scholar]
- Feher J, Pinter E, Kovacs I, Helyes Z, Kemeny A, Markovics A, Plateroti R, Librando A, Cruciani F, 2014. Irritable eye syndrome: neuroimmune mechanisms and benefits of selected nutrients. The ocular surface 12, 134–145. [DOI] [PubMed] [Google Scholar]
- Fijan S, 2014. Microorganisms with claimed probiotic properties: an overview of recent literature. International journal of environmental research and public health 11, 4745–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foureaux Rde C, Messora MR, de Oliveira LF, Napimoga MH, Pereira AN, Ferreira MS, Pereira LJ, 2014. Effects of probiotic therapy on metabolic and inflammatory parameters of rats with ligature-induced periodontitis associated with restraint stress. Journal of periodontology 85, 975–983. [DOI] [PubMed] [Google Scholar]
- Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C, 2009. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition (Burbank, Los Angeles County, Calif.) 25, 520–525. [DOI] [PubMed] [Google Scholar]
- Gallagher RM, 2004. Biopsychosocial pain medicine and mind-brain-body science. Physical medicine and rehabilitation clinics of North America 15, 855–882, vii. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, Ornstein K, Rochat F, Corthesy-Theulaz I, 2006. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. Journal of pediatric gastroenterology and nutrition 43, 16–24. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH, 2007. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56, 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Sherman PM, Walker WA, 2010. Probiotics and the gut microbiota in intestinal health and disease. Nature Reviews Gastroenterology and Hepatology 7, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM, 2011. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. [DOI] [PubMed] [Google Scholar]
- Geddes J, Freemantle N, Harrison P, Bebbington P, 2000. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. Bmj 321, 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K, Arseneault-Breard J, Flores Monaco F, Beaudoin A, Bah TM, Tompkins TA, Godbout R, Rousseau G, 2013. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. The British journal of nutrition 109, 50–56. [DOI] [PubMed] [Google Scholar]
- Gold PW, Charney DS, 2002. Diseases of the mind and brain: depression: a disease of the mind, brain, and body. American Journal of Psychiatry 159, 1826–1826. [DOI] [PubMed] [Google Scholar]
- Goudarzvand M, Rasouli Koohi S, Khodaii Z, Soleymanzadeh Moghadam S, 2016. Probiotics Lactobacillus plantarum and bifidobacterium B94: cognitive function in demyelinated model. Medical journal of the Islamic Republic of Iran 30, 391. [PMC free article] [PubMed] [Google Scholar]
- Guida F, Turco F, Iannotta M, De Gregorio D, Palumbo I, Sarnelli G, Furiano A, Napolitano F, Boccella S, Luongo L, Mazzitelli M, Usiello A, De Filippis F, Iannotti FA, Piscitelli F, Ercolini D, de Novellis V, Di Marzo V, Cuomo R, Maione S, 2017. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain, behavior, and immunity 67, 230–245. [DOI] [PubMed] [Google Scholar]
- Hemmings SMJ, Malan-Muller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, Marotz CA, Siebler PH, Braspenning M, Van Criekinge W, Hoisington AJ, Brenner LA, Postolache TT, McQueen MB, Krauter KS, Knight R, Seedat S, Lowry CA, 2017. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls: An Exploratory Study. Psychosomatic medicine 79, 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, 2011. The Cochrane Collaboration tool for assessing risk of bias in randomised trials. Bmj 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME, 2014. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature reviews. Gastroenterology & hepatology 11, 506–514. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK, 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen JS, Ma IW, Hatala R, Cook DA, 2015. A systematic review of validity evidence for checklists versus global rating scales in simulation-based assessment. Medical education 49, 161–173. [DOI] [PubMed] [Google Scholar]
- Indrio F, Di Mauro A, Riezzo G, Civardi E, Intini C, Corvaglia L, Ballardini E, Bisceglia M, Cinquetti M, Brazzoduro E, Del Vecchio A, Tafuri S, Francavilla R, 2014. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: a randomized clinical trial. JAMA pediatrics 168, 228–233. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2014. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. The American journal of psychiatry 171, 395–397. [DOI] [PubMed] [Google Scholar]
- Jeong JJ, Kim KA, Ahn YT, Sim JH, Woo JY, Huh CS, Kim DH, 2015. Probiotic Mixture KF Attenuates Age-Dependent Memory Deficit and Lipidemia in Fischer 344 Rats. Journal of microbiology and biotechnology 25, 1532–1536. [DOI] [PubMed] [Google Scholar]
- Jeong JJ, Kim KA, Hwang YJ, Han MJ, Kim DH, 2016. Anti-inflammaging effects of Lactobacillus brevis OW38 in aged mice. Beneficial microbes 7, 707–718. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B, 2015. Altered fecal microbiota composition in patients with major depressive disorder. Brain, behavior, and immunity 48, 186–194. [DOI] [PubMed] [Google Scholar]
- Jones MP, Dilley JB, Drossman D, Crowell MD, 2006. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 18, 91–103. [DOI] [PubMed] [Google Scholar]
- Juni P, Altman DG, Egger M, 2001. Assessing the quality of controlled clinical trials. Bmj 323, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak PA, Bobrow DN, Nyby JG, 2014. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behavioural pharmacology 25, 71–79. [DOI] [PubMed] [Google Scholar]
- Kato-Kataoka A, Nishida K, Takada M, Suda K, Kawai M, Shimizu K, Kushiro A, Hoshi R, Watanabe O, Igarashi T, Miyazaki K, Kuwano Y, Rokutan K, 2016. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Beneficial microbes 7, 153–156. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF, Clarke G, Dinan TG, 2017. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain, behavior, and immunity 61, 50–59. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Clarke G, O'Neill A, Groeger JA, Quigley EM, Shanahan F, Cryan JF, Dinan TG, 2014. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychological medicine 44, 1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Clarke G, Quigley EM, Groeger JA, Dinan TG, Cryan JF, 2012. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav Rev 36, 310–340. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Sliwowski Z, Koziel J, Ptak-Belowska A, Burnat G, Brzozowski T, Konturek SJ, 2009. Probiotic bacteria Escherichia coli strain Nissle 1917 attenuates acute gastric lesions induced by stress. J Physiol Pharmacol 60 Suppl 6, 41–48. [PubMed] [Google Scholar]
- Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, Tajabadi-Ebrahimi M, Jafari P, Asemi Z, 2016. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clinical nutrition (Edinburgh, Scotland). [DOI] [PubMed] [Google Scholar]
- Langkamp-Henken B, Rowe CC, Ford AL, Christman MC, Nieves C Jr., Khouri L, Specht GJ, Girard SA, Spaiser SJ, Dahl WJ, 2015. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: a randomised, double-blind, placebo-controlled study. The British journal of nutrition 113, 426–434. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Jeong JJ, Han MJ, Kim DH, 2018. Lactobacillus plantarum C29 Alleviates TNBS-Induced Memory Impairment in Mice. Journal of microbiology and biotechnology 28, 175–179. [DOI] [PubMed] [Google Scholar]
- Lee JY, Chu SH, Jeon JY, Lee MK, Park JH, Lee DC, Lee JW, Kim NK, 2014. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 46, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim N, Nam RH, Sohn SH, Lee SM, Choi D, Yoon H, Kim YS, Lee HS, Lee DH, 2017. Probiotics reduce repeated water avoidance stress-induced colonic microinflammation in Wistar rats in a sex-specific manner. PloS one 12, e0188992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Zhou H, Zhang S, Yuan J, Wu H, 2017. Effects of gut microbiota disturbance induced in early life on the expression of extrasynaptic GABA-A receptor alpha5 and delta subunits in the hippocampus of adult rats. Brain research bulletin 135, 113–119. [DOI] [PubMed] [Google Scholar]
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F, 2015. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. [DOI] [PubMed] [Google Scholar]
- Lim FT, Lim SM, Ramasamy K, 2017. Cholesterol lowering by Pediococcus acidilactici LAB4 and Lactobacillus plantarum LAB12 in adult zebrafish is associated with improved memory and involves an interplay between npc1l1 and abca1. Food & function 8, 2817–2828. [DOI] [PubMed] [Google Scholar]
- Liu WH, Chuang HL, Huang YT, Wu CC, Chou GT, Wang S, Tsai YC, 2016a. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behavioural brain research 298, 202–209. [DOI] [PubMed] [Google Scholar]
- Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, Wang S, Tsai YC, 2016b. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain research 1631, 1–12. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Zuniga V, Llop E, Suarez C, Alvarez B, Abreu L, Espadaler J, Serra J, 2014. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World journal of gastroenterology 20, 8709–8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH, 2008. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological psychiatry 64, 461–467. [DOI] [PubMed] [Google Scholar]
- Luo J, Wang T, Liang S, Hu X, Li W, Jin F, 2014. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Science China. Life sciences 57, 327–335. [DOI] [PubMed] [Google Scholar]
- Lyra A, Hillila M, Huttunen T, Mannikko S, Taalikka M, Tennila J, Tarpila A, Lahtinen S, Ouwehand AC, Veijola L, 2016. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World journal of gastroenterology 22, 10631–10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackos AR, Galley JD, Eubank TD, Easterling RS, Parry NM, Fox JG, Lyte M, Bailey MT, 2016. Social stress-enhanced severity of Citrobacter rodentium-induced colitis is CCL2-dependent and attenuated by probiotic Lactobacillus reuteri. Mucosal immunology 9, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod M, Collins T, Howells DW, Donnan GA, 2004. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke; a journal of cerebral circulation 35, 1203–1208. [DOI] [PubMed] [Google Scholar]
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M, 2003. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical therapy 83, 713–721. [PubMed] [Google Scholar]
- Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, Pennisi G, Li Volti G, Galvano F, 2010. Bifidobacterium combined with fructooligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. European journal of gastroenterology & hepatology 22, 199–206. [DOI] [PubMed] [Google Scholar]
- Marteau P, Shanahan F, 2003. Basic aspects and pharmacology of probiotics: an overview of pharmacokinetics, mechanisms of action and side-effects. Best Practice Research Clinical Gastroenterology 17, 725–740. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K, 2014. Gut microbes and the brain: paradigm shift in neuroscience. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy PM, Nathan P, 2007. Effectiveness of cognitive behavior therapy for diagnostically heterogeneous groups: A benchmarking study. Journal of Consulting and Clinical Psychology 75, 344. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Fitzgerald P, Dinan TG, Cryan JF, 2010. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 22, 1029–1035, e1268. [DOI] [PubMed] [Google Scholar]
- McVey Neufeld KA, Luczynski P, Seira Oriach C, Dinan TG, Cryan JF, 2016. What's bugging your teen?-The microbiota and adolescent mental health. Neurosci Biobehav Rev 70, 13. [DOI] [PubMed] [Google Scholar]
- McVey Neufeld KA, O'Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, Cryan JF, 2017. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutritional neuroscience, 1–10. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM, 2011a. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition 105, 755–764. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C, 2011b. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut microbes 2, 256–261. [DOI] [PubMed] [Google Scholar]
- Mi GL, Zhao L, Qiao DD, Kang WQ, Tang MQ, Xu JK, 2015. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: a prospective single blind randomized trial. Antonie van Leeuwenhoek 107, 1547–1553. [DOI] [PubMed] [Google Scholar]
- Michalickova D, Minic R, Dikic N, Andjelkovic M, Kostic-Vucicevic M, Stojmenovic T, Nikolic I, Djordjevic B, 2016. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 41, 782–789. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Kawase M, Kubota A, Yoda K, Harata G, Hosoda M, He F, 2015. Heat-killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Beneficial microbes 6, 441–449. [DOI] [PubMed] [Google Scholar]
- Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Adab Z, Djalali M, Tehrani-Doost M, Hosseini M, Eghtesadi S, 2016. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutritional neuroscience 19, 387–395. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151, 264–269, w264. [DOI] [PubMed] [Google Scholar]
- Mohle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA, 2016. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell reports 15, 1945–1956. [DOI] [PubMed] [Google Scholar]
- Moller CM, Olsa EJA, Ginty AT, Rapelje AL, Tindall CL, Holesh LA, Petersen KL, Conklin SM, 2017. Influence of acute multi-species and multi-strain probiotic supplementation on cardiovascular function and reactivity to psychological stress in young adults: a double-blind, randomized, placebo-controlled trial. Psychosomatic medicine. [DOI] [PubMed] [Google Scholar]
- Muehsam D, Lutgendorf S, Mills PJ, Rickhi B, Chevalier G, Bat N, Chopra D, Gurfein B, 2017. The embodied mind: a review on functional genomic and neurological correlates of mind-body therapies. Neuroscience & Biobehavioral Reviews 73, 165–181. [DOI] [PubMed] [Google Scholar]
- Musa NH, Mani V, Lim SM, Vidyadaran S, Abdul Majeed AB, Ramasamy K, 2017. Lactobacilli-fermented cow's milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. The Journal of dairy research 84, 488–495. [DOI] [PubMed] [Google Scholar]
- Ng QX, Peters C, Ho CYX, Lim DY, Yeo W-S, 2018. A meta-analysis of the use of probiotics to alleviate depressive symptoms. Journal of affective disorders 228, 13–19. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, Knight R, Jeste DV, 2018. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophrenia research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Mathews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, Machado-Vieira R, Zarate CA Jr, 2014. Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depression and anxiety 31, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgampalle M, Kuna Y, 2017. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer's Disease induced Albino Rats. Journal of clinical and diagnostic research : JCDR 11, Kc01–kc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihira J, Kagami-Katsuyama H, Tanaka A, Nishimura M, Kobayashi T, Kawasaki Y, 2014. Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. Journal of Functional Foods 11, 261–268. [Google Scholar]
- Nobutani K, Sawada D, Fujiwara S, Kuwano Y, Nishida K, Nakayama J, Kutsumi H, Azuma T, Rokutan K, 2017. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. Journal of applied microbiology 122, 212–224. [DOI] [PubMed] [Google Scholar]
- Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, Madsen KL, 2013. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology 38, 1738–1747. [DOI] [PubMed] [Google Scholar]
- Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, Oguz SS, Dilmen U, 2014. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Archives of disease in childhood. Fetal and neonatal edition 99, F110–115. [DOI] [PubMed] [Google Scholar]
- Ostlund-Lagerstrom L, Kihlgren A, Repsilber D, Bjorksten B, Brummer RJ, Schoultz I, 2016. Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutrition journal 15, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomar MM, Maldonado Galdeano C, Perdigon G, 2014. Influence of a probiotic lactobacillus strain on the intestinal ecosystem in a stress model mouse. Brain, behavior, and immunity 35, 77–85. [DOI] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM, 2013. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 25, 733–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parois S, Calandreau L, Kraimi N, Gabriel I, Leterrier C, 2017. The influence of a probiotic supplementation on memory in quail suggests a role of gut microbiota on cognitive abilities in birds. Behavioural brain research 331, 47–53. [DOI] [PubMed] [Google Scholar]
- Partty A, Kalliomaki M, Wacklin P, Salminen S, Isolauri E, 2015. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatric research 77, 823–828. [DOI] [PubMed] [Google Scholar]
- Pellino G, Sciaudone G, Candilio G, Camerlingo A, Marcellinaro R, De Fatico S, Rocco F, Canonico S, Riegler G, Selvaggi F, 2013. Early postoperative administration of probiotics versus placebo in elderly patients undergoing elective colorectal surgery: a double-blind randomized controlled trial. BMC surgery 13 Suppl 2, S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, De Palma G, Pigrau M, Ford AC, Macri J, Berner B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P, 2017. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: a Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. [DOI] [PubMed] [Google Scholar]
- Pyndt Jorgensen B, Krych L, Pedersen TB, Plath N, Redrobe JP, Hansen AK, Nielsen DS, Pedersen CS, Larsen C, Sorensen DB, 2015. Investigating the long-term effect of subchronic phencyclidine-treatment on novel object recognition and the association between the gut microbiota and behavior in the animal model of schizophrenia. Physiology & behavior 141, 32–39. [DOI] [PubMed] [Google Scholar]
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC, 2009. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut pathogens 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Boscolo P, Bellante V, Tarantelli C, Di Nicola M, Forcella L, Li Q, Morimoto K, Muraro R, 2012. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. The British journal of nutrition 108, 308–314. [DOI] [PubMed] [Google Scholar]
- Ringel-Kulka T, Kotch JB, Jensen ET, Savage E, Weber DJ, 2015. Randomized, double-blind, placebo-controlled study of synbiotic yogurt effect on the health of children. The Journal of pediatrics 166, 1475–1481.e1471–1473. [DOI] [PubMed] [Google Scholar]
- Rinne M, Kalliomaki M, Salminen S, Isolauri E, 2006. Probiotic intervention in the first months of life: short-term effects on gastrointestinal symptoms and long-term effects on gut microbiota. Journal of pediatric gastroenterology and nutrition 43, 200–205. [DOI] [PubMed] [Google Scholar]
- Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C, 2017. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. The Australian and New Zealand journal of psychiatry, 4867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, Dore J, Tremblay A, 2017. Effects of a Diet-Based Weight-Reducing Program with Probiotic Supplementation on Satiety Efficiency, Eating Behaviour Traits, and Psychosocial Behaviours in Obese Individuals. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, Sherman PM, Mayer EA, 2013. An update on the use and investigation of probiotics in health and disease. Gut 62, 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, Quinn KJ, Sypher I, 2015. NIMH research domain criteria (RDoC). [Google Scholar]
- Sartorius N, Ustun TB, Lecrubier Y, Wittchen H-U, 1996. Depression comorbid with anxiety: Results from the WHO study on "Psychological disorders in primary health care.". The British journal of psychiatry. [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG, Cryan JF, 2014. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 26, 1615–1627. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF, 2015. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behavioural brain research 287, 59–72. [DOI] [PubMed] [Google Scholar]
- Schneider AC, Rico EP, de Oliveira DL, Rosemberg DB, Guizzo R, Meurer F, da Silveira TR, 2016. Lactobacillus rhamnosus GG Effect on Behavior of Zebrafish During Chronic Ethanol Exposure. BioResearch open access 5, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena ES, Van Der Worp HB, Bath PM, Howells DW, Macleod MR, 2010. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS biology 8, e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Mandelli L, 2010. Antidepressants and body weight: a comprehensive review and meta-analysis. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Dickerson FB, Yolken RH, 2017. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain, behavior, and immunity 62, 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 349, g7647. [DOI] [PubMed] [Google Scholar]
- Shinkai S, Toba M, Saito T, Sato I, Tsubouchi M, Taira K, Kakumoto K, Inamatsu T, Yoshida H, Fujiwara Y, Fukaya T, Matsumoto T, Tateda K, Yamaguchi K, Kohda N, Kohno S, 2013. Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: a randomised, double-blind, placebo-controlled trial. The British journal of nutrition 109, 1856–1865. [DOI] [PubMed] [Google Scholar]
- Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, Kang J, Rowden J, Stone P, Crane J, Stanley T, Abels P, Purdie G, Maude R, Mitchell EA, 2017. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Emge JR, Berzins K, Lung L, Khamishon R, Shah P, Rodrigues DM, Sousa AJ, Reardon C, Sherman PM, Barrett KE, Gareau MG, 2014. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. American journal of physiology. Gastrointestinal and liver physiology 307, G793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller R, Pelerin F, Cayzeele Decherf A, Maudet C, Housez B, Cazaubiel M, Justen P, 2016. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United European gastroenterology journal 4, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, Hemert S, Bosch J, Colzato L, 2015. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood, Brain, behavior, and immunity, pp. 258–264. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, Liu J, 2016. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain research 1642, 180–188. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang M, Chen CC, Gillilland M 3rd, Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, Chang YM, Gumucio DL, Owyang C, Kao JY, 2013. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology 144, 1478–1487, 1487.e1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung V, Hiscock H, Tang ML, Mensah FK, Nation ML, Satzke C, Heine RG, Stock A, Barr RG, Wake M, 2014. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. Bmj 348, g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Nishida K, Gondo Y, Kikuchi-Hayakawa H, Ishikawa H, Suda K, Kawai M, Hoshi R, Kuwano Y, Miyazaki K, Rokutan K, 2017. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: a double-blind, randomised, placebo-controlled trial. Beneficial microbes 8, 153–162. [DOI] [PubMed] [Google Scholar]
- Takada M, Nishida K, Kataoka-Kato A, Gondo Y, Ishikawa H, Suda K, Kawai M, Hoshi R, Watanabe O, Igarashi T, 2016. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterology & Motility. [DOI] [PubMed] [Google Scholar]
- Tian F, Yu L, Zhai Q, Xiao Y, Shi Y, Jiang J, Liu X, Zhao J, Zhang H, Chen W, 2017. The therapeutic protection of a living and dead Lactobacillus strain against aluminum-induced brain and liver injuries in C57BL/6 mice. PloS one 12, e0175398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA, 2013. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144, 1394–1401, 1401.e1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann S, Awwad HM, Eskelund AR, Treccani G, Geisel J, Wegener G, Obeid R, 2018. Probiotics Affect One-Carbon Metabolites and Catecholamines in a Genetic Rat Model of Depression. Molecular nutrition & food research 62, e1701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman H, Koning C, Mulder L, Rombouts F, Beynen A, 2004. Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. International journal of food microbiology 96, 219–233. [DOI] [PubMed] [Google Scholar]
- Tomasik J, Yolken RH, Bahn S, Dickerson FB, 2015. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomarker insights 10, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol IA, Kamyshny AM, Abramov AV, Kolesnik YM, 2014. Expression of XBP1 in lymphocytes of the small intestine in rats under chronic social stress and modulation of intestinal microflora composition. Fiziolohichnyi zhurnal (Kiev, Ukraine : 1994) 60, 38–44. [PubMed] [Google Scholar]
- Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, Salman RA-S, Macleod MR, Ioannidis JP, 2013. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS biology 11, e1001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaecke T, Aubert P, Grohard PA, Durand T, Hulin P, Paul-Gilloteaux P, Fournier A, Docagne F, Ligneul A, Fressange-Mazda C, Naveilhan P, Boudin H, Le Ruyet P, Neunlist M, 2017. L. fermentum CECT 5716 prevents stress-induced intestinal barrier dysfunction in newborn rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 29. [DOI] [PubMed] [Google Scholar]
- Wang T, Hu X, Liang S, Li W, Wu X, Wang L, Jin F, 2015. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Beneficial microbes 6, 707–717. [DOI] [PubMed] [Google Scholar]
- Weizman Z, Alsheikh A, 2006. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study. Journal of the American College of Nutrition 25, 415–419. [DOI] [PubMed] [Google Scholar]
- Woo JY, Gu W, Kim KA, Jang SE, Han MJ, Kim DH, 2014. Lactobacillus pentosus var. plantarum C29 ameliorates memory impairment and inflammaging in a D-galactose-induced accelerated aging mouse model. Anaerobe 27, 22–26. [DOI] [PubMed] [Google Scholar]
- Xiao J, Li S, Sui Y, Wu Q, Li X, Xie B, Zhang M, Sun Z, 2014. Lactobacillus casei-01 facilitates the ameliorative effects of proanthocyanidins extracted from lotus seedpod on learning and memory impairment in scopolamine-induced amnesia mice. PloS one 9, e112773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Tang S, Huang H, Zhao X, Ning Z, Fu X, Zhang C, 2016a. Probiotics reduce psychological stress in patients before laryngeal cancer surgery, Asia-Pacific journal of clinical oncology, pp. e92–e96. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao X, Tang S, Huang H, Zhao X, Ning Z, Fu X, Zhang C, 2016b. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia Pac J Clin Oncol 12, e92–96. [DOI] [PubMed] [Google Scholar]
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM, 2006. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55, 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]