Abstract
Vibrio species are marine bacteria that occur in estuaries worldwide; many are virulent human pathogens with high levels of antibiotic resistance. The average annual incidence of all Vibrio infections has increased by 41% between 1996 and 2005. V. vulnificus (Vv), a species associated with shellfish and occurring in the US Southeast, has ranges of temperature (16–33 °C) and salinity (5–20 ppt) dependencies for optimal growth. Increased water temperatures caused by atmospheric warming and increased salinity gradients caused by sea level rise raise concerns for the effect of climate change on the geographic range of Vv and the potential for increased exposure risk. This research combined monthly field sampling, laboratory analysis, and modeling to identify the current occurrence of Vv in the Winyah Bay estuary (South Carolina, USA) and assess the possible effects of climate change on future geographic range and exposure risk in the estuary. Vv concentrations ranged from 0 to 58 colony forming units (CFU)/mL, salinities ranged from 0 to 28 ppt, and temperature from 18 to 31 °C. A significant empirical relationship was found between Vv concentration and salinity and temperature that fit well with published optimal ranges for growth for these environmental parameters. These results, when coupled with an existing model of future specific conductance, indicated that sea level rise has a greater impact on exposure risk than temperature increases in the estuary. Risk increased by as much as four times compared to current conditions with the largest temporally widespread increase at the most upriver site where currently there is minimal risk.
Keywords: Vibrio vulnificus, Climate change, Risk, Salinity, Temperature, Model
Introduction
Vibrio species are heterotrophic bacteria that occur naturally in estuaries worldwide. Vibrio have been a focus of study because they can be virulent human pathogens, one of the only pathogens with increasing rates of illness in the USA (Prevention 2016). The average annual incidence of all Vibrio infections (foodborne and wound infections) has increased by > 80% from 1996 and 2001 (Ho and Cunha 2009) while foodborne illness alone increased by 41% between 1996 and 2005 (Prevention 2006). The main species of Vibrio that are studied and monitored are Vibrio cholera, V vulnificus, and V parahaemolyticus. They are commonly known as foodborne pathogens that cause illness through seafood consumption as well as wound infections. For example, V. vulnificus can cause serious and life-threatening injuries through open wounds in contaminated water (Oliver 2005). The Centers for Disease Control and Prevention (CDC) estimates 8028 Vibrio infections and 57 deaths occur annually in the USA (Mead et al. 1999). V vulnificus is the leading cause of death from raw shellfish consumption, accounting for 50% of all deaths in the USA due to seafood consumption.
V vulnificus, a species associated with shellfish and occurring in coastal regions globally including the US Southeast, has ranges of temperature (16–33 °C) and salinity (5–20 ppt; 9–32 mS/cm conductivity) for optimal growth (Oliver 2015; Randa et al. 2004; Wetz et al. 2014). They are frequently found in estuaries, which are susceptible to a variety of changes driven by climate and anthropogenic stresses. Increased temperature and sea level rise are expected to alter the geographic range of most estuarine habitats (increase, decrease, and/or shift) and alter the ecological range of many organisms, including pathogenic bacteria. It is likely that the geographic range of V vulnificus will increase as water temperature increases and saline water extends further into formerly freshwater reaches of coastal rivers. The growing season also may be extended (Baker-Austin et al. 2010) which could have negative public health and economic impacts due to the potential increase in the risk of disease from shellfish consumption and, in the case of V. vulnificus, wound infections (Oliver 2005).
The two most important environmental factors that control Vibrio dynamics in estuaries are temperature and salinity (Oliver 2015; Wetz et al. 2014). Vibrio abundance is typically higher during the summer than in the winter when Vibrio levels are generally below detectable concentrations. This is reflected in the environmental conditions for Vibrio occurrence, with temperature and salinity ranges for optimum growth, noted above. The salinity characteristics of estuaries are expected to change in the future due to more extreme weather events as well as sea level rise which will cause spatial and temporal changes to the salinity gradient and other physicochemical characteristics in coastal rivers (Baker-Austin et al. 2017; Dhillon and Inamdar 2014). This has both ecological and human use impli-cations and has received the attention of researchers and water managers (Conrads et al. 2013).
In the Southeastern USA, there has been a mean increase of 2 °F since the 1970s, with the greatest increases in the winter months (Karl et al. 2009). The timing is significant because this is when shellfish are harvested. Since the mid-1970s, cli-mate records show that the number of freezing days in the Southeast decreased by 4 to 7 days per year (Karl et al. 2009). Climate model projections of warming rates for the Southeast are more than double the rates since the mid-1970s. By 2080, the average temperatures in the region are projected to increase by 4.5 or 9 °F under low emission or high emission scenarios, respectively (Karl et al. 2009).
The increase and spread of Vibrio worldwide and domestically is thought to be correlated with climate change variations and new oceanic patterns that introduce warmer waters into colder regions and alter the salinity profile along the longitudinal axis of coastal rivers (Baker-Austin et al. 2016). The poleward movement of Vibrio was evident in both the Northern and Southern hemispheres; recently, Vibrios have been detected in areas where they were previously absent or rarely reported (Baker-Austin et al. 2010). The spread of Vibrios will increase the potential exposure of shellfish consumers throughout the coastal regions in the USA including the Southeast.
The warming temperature effect on the range expansion of Vibrios has been the focus of most of the attention of the actual and potential impact of climate change on the increased occurrence of Vibrios at the continental scale (Baker-Austin et al. 2017; Baker-Austin et al. 2016; Vezzulli et al. 2016). Much less attention has been applied to the effect of changing salinity characteristics at the estuarine scale (Randa et al. 2004; Jacobs et al. 2010). Salinity profiles in coastal rivers are im-pacted by both freshwater inflow and sea level (tidal, seasonal storms, eustatic). To assess the potential for increased inci-dence of Vibrios in estuaries in the Southeast USA, Winyah Bay estuary in South Carolina was chosen as a representative estuary to monitor the occurrence and concentrations of Vibrio with respect to the trends of salinity variation, both now and into the future. Field sampling was used to determine the relationship of V. vulnificus with environmental parameters in the estuary, then the results were coupled with a model of future conductivity trends (Conrads et al. 2013) and tempera-ture increases to assess the potential for range expansion and increased exposure risk.
Materials and Methods
Study Site Description
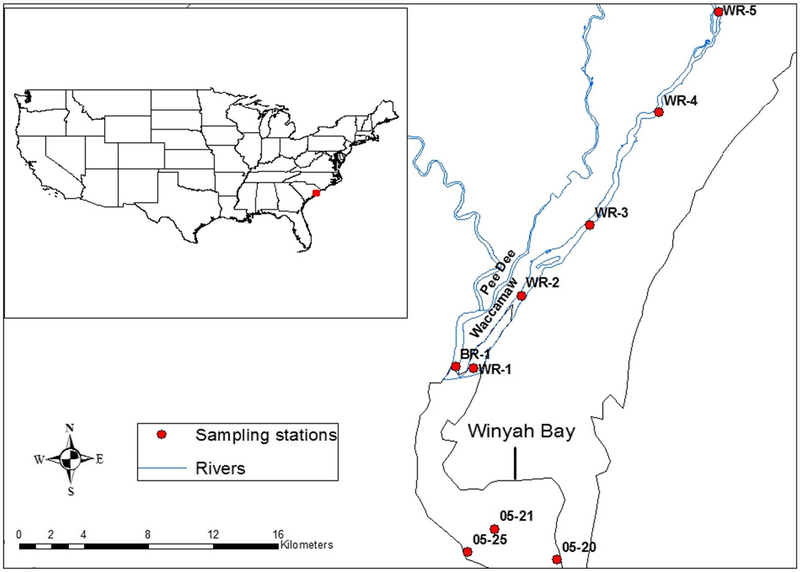
Winyah Bay is an estuary formed at the confluence of the Waccamaw, Pee Dee, Sampit, and Black Rivers in Georgetown County (Fig. 1). It is part of the North InletWinyah Bay National Estuarine Research Reserve system. The Reserve covers 4988.56 ha of natural area and is located 80.5 km north of Charleston, SC (https://coast.noaa.gov/nerrs/reserves/north-inlet-winyah-bay.html). The Winyah Bay estuary drains a watershed of 47,000 km2, the third largest on the US east coast. Annual mean fresh water discharge from the four tributary rivers is 450 m3 s−1, with a maximum of 7800 m3 s−1 (Blood and Vernberg 1992). The salinity differences between surface and bottom water can be more than 20 ppt.
Fig. 1.
The Winyah Bay estuary and sampling sites in the Bay and the Waccamaw River. Water samples were collected and water quality measurements were made at all nine sites. The predictive modeling and risk analysis focused on WR-3, WR-4, and WR-5
Field Sampling
The study monitored nine sampling sites in the Winyah Bay estuary (Fig. 1). Surface and bottom water samples were col-lected in pre-sterilized 2 L polypropylene containers every month between April and October 2012. A subsequent sam-pling occurred October 29 after Hurricane Sandy passed off the coast of South Carolina. Smaller containers of surface and bottom waters were collected during each sampling event for turbidity measurement, which was done using a Hach 2100P turbidimeter (turbidity was not measured for the July and August sampling events). This was used to represent the total suspended solids (Fries et al. 2007). Water samples were transported in coolers with frozen gel packs to the microbiology lab at the National Oceanic and Atmospheric Administration’s Center for Coastal Environmental Health and Biomolecular Research (CCEHBR) in Charleston, S.C. and analyzed within 6–8 h of collection. A YSI 6920 multi-parameter sonde with YSI 650 data recorder was used to mea-sure field parameters including water temperature, specific conductance, salinity, pH, and dissolved oxygen. The sonde was calibrated before each sampling event.
Bacterial Identification
For the first sampling event, we used a wide range of water volumes and dilutions to ensure countable plates (containing 20–200 colonies) for each sample/location. For the rest of the study we used the volumes or dilutions appropriate for a given site. Various volumes of surface and bottom water samples and dilutions of water samples in phosphate-buffered saline (PBS) were filtered through sterile nitrocellulose 0.45 μm, 47 mm filters on a sterile vacuum manifold to determine bacterial plate counts on CHROMagar Vibrio (CAV) media. Plates were incubated overnight (16–24 h) at 37 °C and colonies were counted as presumptive V vulnificus colony forming units (CFU/mL).
V vulnificus colonies grown on CHROMagar formed turquoise or light blue colonies (Williams et al. 2011). Vibrio cholera can also produce blue colonies on CAV media, so quantitative real-time polymerase chain reaction (QPCR) was used to confirm presumptive colonies of V. vulnificus (Baker-Austin et al. 2008). To determine whether the colonies that were randomly selected from the CAV plates were actual V vulnificus, TaqMan-based real-time multiplex PCR assay was used. The Vv hemolysisn A gene (vvhA) was used as a marker (Panicker and Bej 2005). Our approach to measure Vibrio vulnificus (using CHROMagar) per se was not uniquely specific as some colonies measured were other Vibrio species. Due to the potential of inaccuracies of identification and counting colonies by color and morphology to determine bacterial species, presumptive CFUs counted on CAV plates were adjusted by determining the percent of presumptive colonies that were true Vibrio through QPCR. QPCR analysis revealed that for V. vulnificus, the correct rate of identification ranged from 0 to 100%, averaging 61% of initial CFU enumeration (False Positive Rate of 39%). The percent of positive colonies from each site as determined by QPCR was then multiplied by the original presumptive colony counts to determine the adjusted CFU/mL counts and thus provide accurate bacterial abundances V. vulnificus.
Statistical Analysis
This study analyzed the relationship of V. vulnificus (hereafter Vv) concentration with temperature, turbidity, and specific conductance. Natural log-transformed Vv counts (to get a normal distribution) from surface and bottom water samples were plotted as a function of the environmental variables. The plot with specific conductance indicated a quadratic relationship so a squared term was derived. A multiple linear regression model was then developed to evaluate the combined relationship of the environmental variables with Vv. Turbidity was not included in the regression model because estimates of values under future climate scenarios were not available. The regression model was developed using the results from the seven scheduled monthly sampling trips. Two versions of the model were developed, one with a temperature term and one without. This was done to accommodate two types of risk analysis, described below. The regression model was tested using the data from the October 29 special sampling trip following Hurricane Sandy, as the increased flooding from streamflow and storm surge was used as a potential indicator of increased sea level rise. All statistical analysis was done using the Statistical Analysis System (SAS) v9.4 (SAS Institute, Cary, NC).
Predictive Tool
Predictions of Vv concentration in the mid-twenty-first century were made using projections of specific conductance and temperature. Results from the statistical analyses were coupled with projected estimates of specific conductance calculated by the Pee Dee River and Atlantic Intracoastal Waterway Salinity Intrusion Model Decision Support System (PRISM2 DSS) (Conrads et al. 2013). The model was developed using historic streamflow time series from US Geological Survey (USGS) gages for the rivers that flow into Winyah Bay and specific conductance time series for three gage locations in the lower Waccamaw River. Future (2055–2069) specific conductance at the gage locations was predicted using projected streamflow in the rivers and sea level rise along with tidal forcing. Future specific conductance estimates following the current conditions and 0.3048, 0.6096, and 0.9144 m (1.0, 2.0, and 3.0 ft) sea level rise scenarios were run for WR-5, WR-4, and WR-3 stations which correspond to USGS gages 02110809, 021108125, and 02110815, respectively, used for PRISM2.
The PRISM2 projections of specific conductance at the three stations were used with the regression model to predict Vv concentration in the future. PRISM2 projects specific conductance throughout the year. We reduced this to April–October to coincide with the date range of field sampling during this project.
Temperature predictions for the US Southeast also were included in the predictions of future Vv concentrations at the three sites. Water temperature increases of 1, 2, and 3 °C were used, which are within the range of 4–8 °F increase in air temperature often reported for the end of the twenty-first century (USGCRP 2014). Current evidence suggests that winter seawater temperatures in North Inlet have already increased by 2.6 °C from 1981 to 2003 (Allen et al. 2008).
Risk Analysis
The predictive models were coupled with the PRISM2 results to develop two sets of risk analyses. For one analysis, two metrics were derived from the predicted future specific conductance in 0.3048, 0.6096, and 0.9144 m sea level rise scenarios at the three locations (WR-3, WR-4, WR-5). The first metric was the maximum predicted specific conductance. The second metric was the number of days the predicted specific conductance was within the range for optimal growth for Vv.
For the second analysis, the water temperatures measured during the field work phase of this project were used as point estimates of current conditions at each location. Monthly Vv concentrations were projected using the projected specific conductance from the PRISM2 model and assuming 1, 2, and 3 °C increases in water temperature.
For both analyses, risk is a unitless ratio defined as the proportion change in the predicted value of the metric versus the current, or baseline, value. So for example, if the risk metric is 1.4 for the maximum Vv concentration in a given scenario that means the predicted maximum Vv concentration is 40% larger than the baseline. A risk metric of 2.4 means 140% larger than the baseline.
Results
Field Data Results
Vv counts ranged between 0 and 58 CFU/mL with an average of 10.7 CFU/mL (Table 1). Values tended to be higher in bottom water than surface water although the variability was larger also. The same trend occurred for salinity, specific conductance, and turbidity. The higher salinity in bottom water is typical in tidal rivers due to the greater density of saline water; higher turbidity in the bottom water likely is due to a combination of sediment resuspension caused by flow reversals during the tidal cycle and settling of particles from higher in the water column. Temperature ranged from 18 to 31 °C and was relatively uniform throughout the water column. Almost all values were within the optimal temperature range for Vv.
Table 1.
Summary of measured field parameters and V. vulnificus (Vv) concentration in the Winyah Bay estuary
| Vv (CFU/mL) | Temperature (°C) | Salinity (ppt) | Specific conductance (mS/cm) | Turbidity (NTU) | ||
|---|---|---|---|---|---|---|
| Overall | Mean | 10.7 | 24.3 | 8.2 | 13.4 | 40.8 |
| Median | 6.0 | 25.4 | 5.2 | 9.4 | 26.3 | |
| Min | 0 | 18 | 0 | 0 | 0 | |
| Max | 58 | 31 | 28 | 43 | 262 | |
| Standard deviation | 11.6 | 3.9 | 8.7 | 13.8 | 44.7 | |
| Number of samples | 136 | 136 | 136 | 136 | 100 | |
| Surface | Mean | 7.5 | 24.4 | 7.4 | 12.2 | 21.6 |
| Median | 5.0 | 25.5 | 4.2 | 7.1 | 18.9 | |
| Min | 0 | 18 | 0 | 0 | 0 | |
| Max | 40 | 31 | 26 | 40 | 58 | |
| Standard deviation | 8.0 | 4.0 | 8.1 | 13.1 | 12.4 | |
| Number of samples | 68 | 68 | 68 | 68 | 50 | |
| Bottom | Mean | 13.8 | 24.2 | 9.1 | 14.7 | 60.0 |
| Median | 8.5 | 25.3 | 6.0 | 10.6 | 36 | |
| Min | 0 | 18 | 0 | 0 | 8 | |
| Max | 58 | 30 | 28 | 43 | 262 | |
| Standard deviation | 13.6 | 3.9 | 9.2 | 14.6 | 56.0 | |
| Number of samples | 68 | 68 | 68 | 68 | 50 |
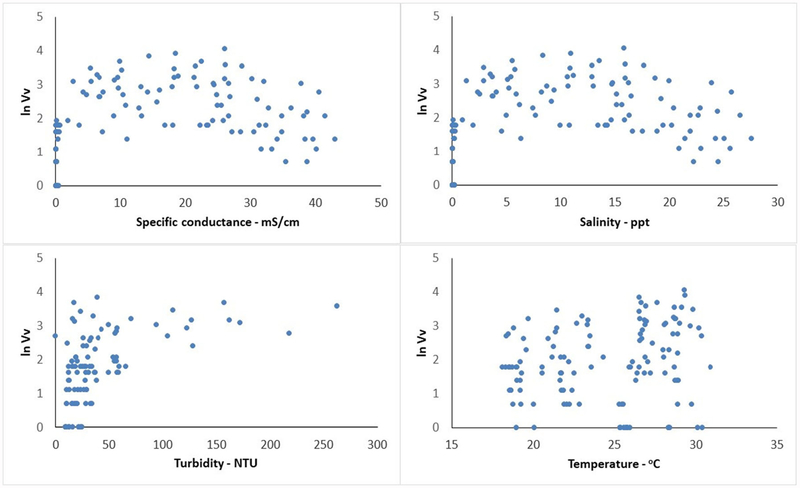
Vv was found in a wide range of salinity conditions, ranging from 0.05 to 27.6 ppt. Counts were the highest when salinity was between 5 and 20 ppt (Fig. 2) specific conductance were linearly related (R2 = 0.999) so for consistency with PRISM2 and the predictive model, specific conductance was used hereafter.) There was not a clear relationship between temperature and Vv count (Fig. 2). All water temperatures during field collection were within or very close to the optimal range for Vv so this result was expected. All of the low Vv counts occurred in water samples with turbidity less than 70 NTU. This suggests a relationship between turbidity and Vv although the nature of the relationship was not clear from these data; high Vv counts also occurred in water with low turbidity. All turbidity values > 66 NTU were from bottom water, which may be a factor in the relationship between turbidity and Vv. Understanding that relationship was not an objective of this research.
Fig. 2.
Scatter plots of natural log-transformed V vulnificus (Vv) concentration with measured field parameters
The scatter plot revealed that the relation between Vv and specific conductance was not linear and followed a quadratic polynomial relation (Fig. 2). In order to incorporate this rela-tionship into the prediction model, a quadratic term was de-rived, specific conductance2, to assess its effect, similar to Johnson et al. (2010).
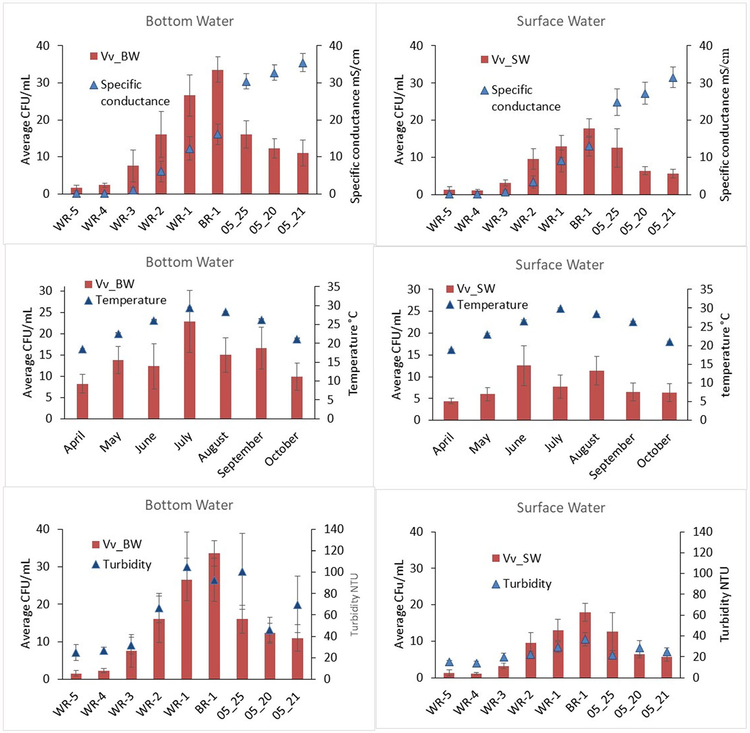
The sampling plan allowed analysis of the data along gra-dients of specific conductance and temperature as well as surface versus bottom water. The Vv concentration increased as specific conductance increased up to a peak then it decreased again (Fig. 3a). This is consistent with the range of tolerance concept for many species with respect to abiotic characteristics of their environment. There also was seasonality associated with water temperature (Fig. 3b) and a relationship with turbidity that was most clearly seen in the bottom water sam-ples (Fig. 3c). Comparisons of Vv levels in surface and bottom water in association with these water quality variables showed similar temporal and spatial patterns, with slightly higher Vv levels in bottom waters.
Fig. 3.
Plots of V vulnificus (Vv) concentration versus water quality parameters (top row, specific conductance; middle row, temperature; bottom row, turbidity) stratified by month and/or sampling station
Prediction Model Results
A multiple regression model was developed to predict ln(Vv) from temperature, specific conductance, and squared specific conductance. The model had an R2 = 0.8817 and all three terms were significant (p <0.0001). A reduced model also was developed without the temperature term. That model had an R2 = 0.7942 and both terms were significant (Table 2).
Table 2.
Results and parameters of the two multiple regression models. One model includes a temperature term and one does not. Their use is explained in the text
| Source | F value | Pr > R | |
|---|---|---|---|
| Model w/ temp | 288.06 | <.0001 | |
| Model w/out temp | 225.79 | <.0001 | |
| R-Square w/ temp | 0.8817 | ||
| R-Square w/out temp | 0.7942 | ||
| Partial R-Sq for temp | 0.0875 | ||
| Parameter estimates w/ temp | |||
| Variable | Param est | Pr > t | Std error |
| Temperature | 0.04276 | <.0001 | 0.00462 |
| Specific conductance | 0.19131 | <.0001 | 0.01803 |
| (Specific conductance)2 | −0.00469 | <.0001 | 0.00049 |
| Parameter estimates w/out temp | |||
| Variable | Param est | Pr > t | Std error |
| Specific conductance | 0.29321 | <.0001 | 0.01876 |
| (Specific conductance)2 | −0.00669 | <.0001 | 0.00058 |
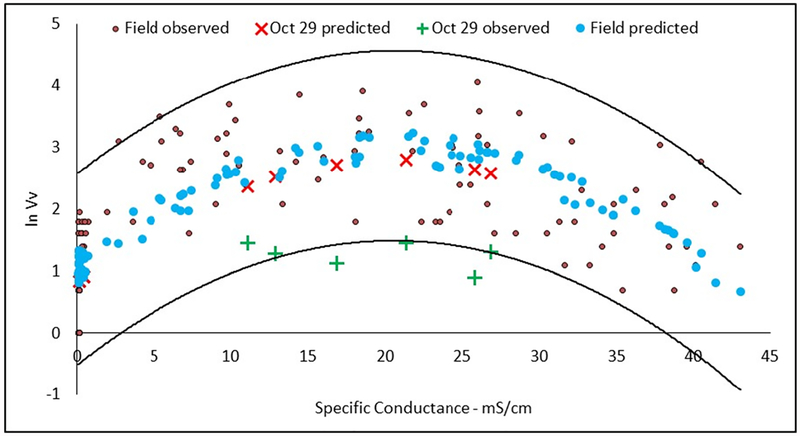
Given the significant opportunity to collect information on the impact of extreme events, the full model was used to predict the Vv counts from the October 29 sampling after Hurricane Sandy. All model predictions of the observed data during the regular monthly sampling are well within the 95% prediction interval. The model tended to over-predict values from the October 29 sampling after Hurricane Sandy except at low specific conductance (Fig. 4). This suggests the storm surge and increased precipitation caused by the hurricane caused conditions in the river that were quite different from the normal conditions during the regular monthly sampling. For example, dilution due to excess precipitation, though not studied here, has been seen in other locations of high streamflow due to hurricanes (Dhillon and Inamdar 2014). Another study that looked at Vv was less conclusive (Shaw et al. 2014). While developing a strong understanding of that relationship was not a focal purpose of this research, it is valuable to note this observation of the influence of an extreme event on Vv distribution because future climate scenarios will not only include increased sea level rise as well as increased tropical storm frequency and intensity as was seen recently with Hurricane Harvey (Emanuel 2017).
Fig. 4.
Natural log-transformed observed V vulnificus (Vv) during the seven regular monthly sampling trips in the Winyah Bay es-tuary, predicted Vv using the multiple regression model developed using data from the seven regular sampling trips, and observed and predicted Vv values from the October 29 special sampling. The solid lines are the 95% confidence interval
Risk Analysis
The models indicate that the impact of future sea level rise may vary over a wide range among the sampling sites (Table 3). The upriver site, WR-5, was projected to have a 10% increase in the maximum Vv concentration but the specific conductance remains below the optimal range for all three SLR scenarios. The middle site, WR-4, had projections of an almost 26 times increase in the number of days with the specific conductance in the optimal range and a 10% increase in the maximum concentration. At the downriver site, WR-3, projections suggested a significant increase in the number days the specific conductance was in the optimal range but a small decrease in the maximum concentration of Vv. The explanation for this apparent contraction is that the maximum specific conductance predicted by PRISM2 increased to 28, 28, and 29 mS/cm in the three SLR scenarios versus 25 mS/cm currently. The larger specific conductance values were in the descending leg of the prediction curve for Vv concentration (Fig. 4).
Table 3.
Change in potential for exposure to V vulnifucus (Vv) based on changes in future specific conductance under sea level rise scenarios. The “Current” column in the upper table is the number of days in April-October during the 15-year simulation period the projected specific conductance is within the optimal range for Vv growth. The current column in the lower table is the maximum projected concentration of Vv in CFU/mL, derived from projections of specific conductance using the reduced regression model discussed in the text. The remaining numbers in both tables are the proportion change from current values. The n/a indicates no value
| Days in optimal range | ||||
|---|---|---|---|---|
| Proportion change from current | ||||
| Station | Current | .3048 m | .6096 m | .9144m |
| WR-5 | 0 | n/a | n/a | n/a |
| WR-4 | 10 | 6.1 | 13.3 | 25.7 |
| WR-3 | 92 | 1.6 | 2.9 | 4.6 |
| Maximum concentration (CFU/mL) | ||||
| Proportion change from current | ||||
| Station | Current | .3048 m | .6096 m | .9144m |
| WR-5 | 2.5 | 1.1 | 1.1 | 1.1 |
| WR-4 | 11.7 | 1.1 | 1.1 | 1.1 |
| WR-3 | 23.4 | 0.8 | 0.8 | 0.8 |
The risk situation was more complex when predicted water temperature increases were factored in and the analysis was stratified by month (Figs. 5, 6, and 7). (The reported percent model error for the underlying PRISM model was 5.5% at WR-3, 3.4% at WR-4, and 1.9% at WR-5 (Conrads et al. 2013). Including these ranges in the figures would provide minimal additional insight into the model results.) There was a seasonal signal, irrespective ofprojected increases in water temperature, in which the risk rises from a low in April to a peak in July and back to a low in October. This signal was seen at all three stations although the magnitude was much larger at WR-4, the middle site. This seasonal pattern was similar to the annual analysis above and also was due to predicted specific conductance closer to the peak in the optimal range. These predicted periods of increased risks in the future at these upriver sites correspond to current periods of maximum risks (July-August) of observed Vv infections today. Also the models do not suggest extended risks to subsequent months beyond which current Vibrio infections occur today (Baker-Austin and Oliver 2018).
Fig. 5.
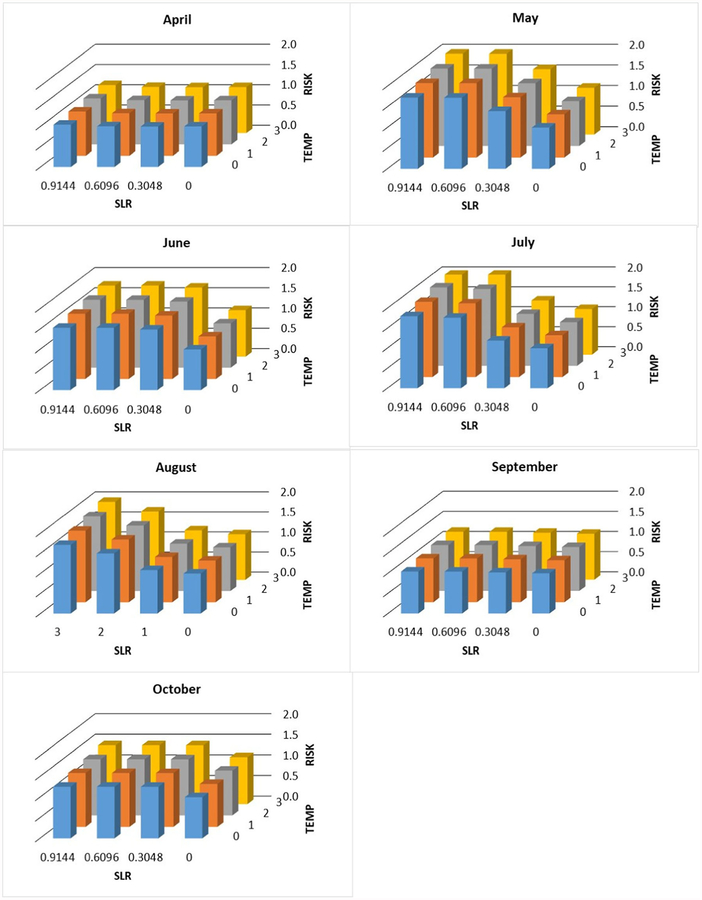
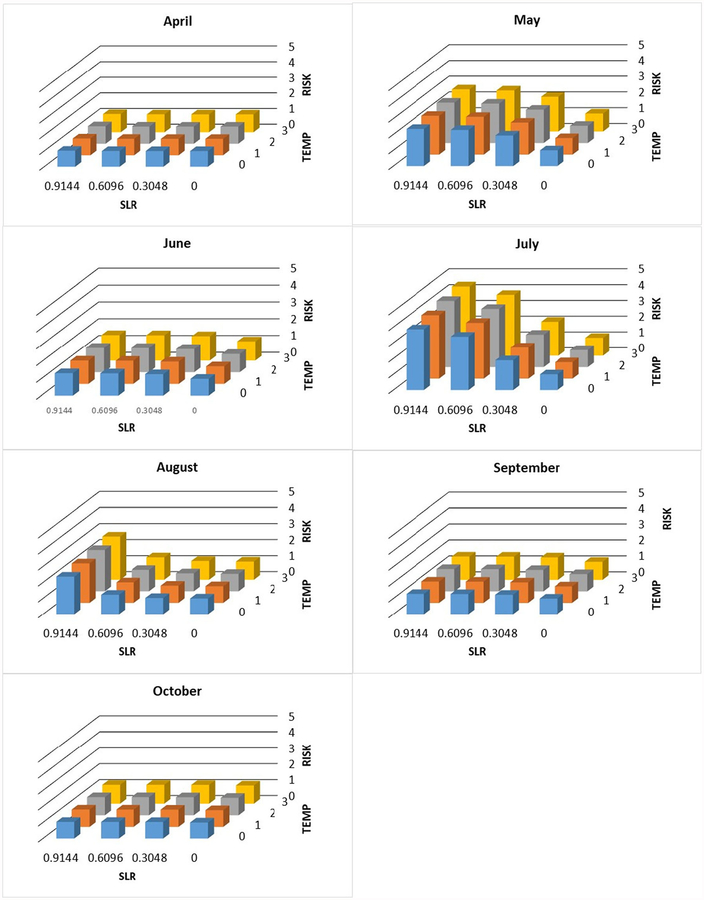
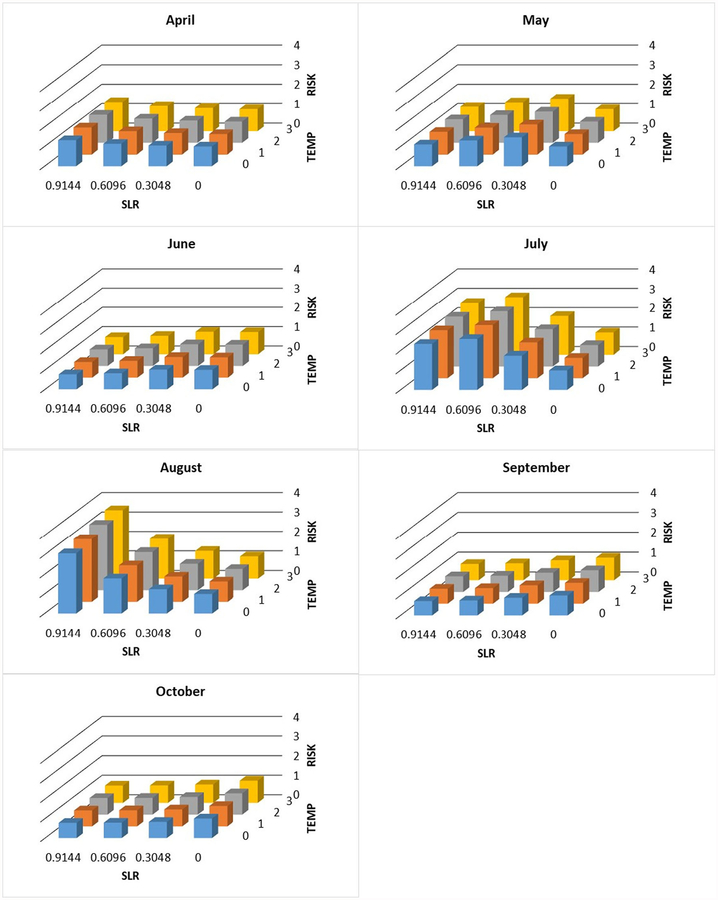
Individual and joint change in exposure risk (unitless proportion, see text) with increasing sea level (SLR, m) and temperature (°C) by month at station WR-5
Fig. 6.
Individual and joint change in exposure risk (unitless proportion, see text) with increasing sea level (SLR, m) and temperature (°C) by month at station WR-4
Fig. 7.
Individual and joint change in exposure risk (unitless proportion, s text) with increasing sea level (SLR, m) and temperature (°C) by month at station WR-3
Increased risk was most often predicted at the upriver site, WR-5. During the study, this site was always freshwater except under the most extreme conditions. During every month covered in this study, except April and September, there was increased risk under all three sea level rise scenarios (Table 4). By contrast, at both WR-3 and WR-4, the increased risk occurred in fewer months and sea level rise scenarios.
Table 4.
Predicted increased V vulnificus (Vv) risk under future climate and sea level rise scenarios in the most upriver portions of the Waccamaw River in the Winyah Bay estuary. Numbers indicate the sea level rise (m) in which the increased risk was projected
| Month/station | WR-3 | WR-4 | WR-5 |
|---|---|---|---|
| May | .3048 | .3048-.9144 | .3048-.9144 |
| June | .3048-.9144 | ||
| July | .3048-.9144 | .3048-.9144 | .3048-.9144 |
| August | .6096,.9144 | .9144 | .6096,.9144 |
| October | .3048-.9144 |
The projected effect of increasing water temperature was not as large as for sea level rise (consistent with the partial R2 of the temperature term in the regression model (Table 2)) but still substantial, especially during the warm season. For example, in July at station WR-3, the risk increased 230% with a 0.9144-m rise in sea level and 270% when 3 °C increase in water temperature was included. At station WR-4, the comparable values are 390 and 440%. At station WR-5, the numbers are 180 and 200%. These numbers are the overall maxima at each station. During most months and with less sea level rise and temperature increase, the values were often considerably less (Figs. 5, 6, and 7).
Discussion
Vv total counts were relatively low in comparison to the counts retrieved from studies in the Gulf Coast where counts reached to more than 100 CFU/mL (Wetz et al. 2008). However, the ecological study of Vv in eastern North Carolina observed a monthly average range of 0.01 to 23 CFU/mL, with the highest concentrations detected during the warm-weather months (Pfeffer et al. 2003). Thus, the counts observed in our study were consistent with other work in the Southeastern US waters. Tidal range differences be-tween the SE Atlantic and Gulf Coast may range from 3 to 4-fold, suggesting that tidal dilution may play a role in ob-served Vv levels. Scott (2016) reported similar tidal dilution effects on rates of E. coli antibiotic resistance in comparisons between the Gulf, Mid Atlantic, and SE Atlantic coasts.
The Vv results in our sampling correspond mostly to its optimal salinity range of between 5 and 20 ppt (Wetz et al. 2014). The quadratic relationship we found (squared specific conductance) throughout the salinity range was consistent with the range of tolerance often observed for the effect of abiotic factors on biological systems. This may be a partial explanation for results from other studies. Kaspar and Tamplin (1993) showed that the occurrence of Vv was highly affected by salinity levels. Other studies, like Hoi et al. (1998), did not reveal significant correlations between Vv abundance and salinity. Wright et al. (1996) showed that salinity was inversely correlated with Vv in their field survey in the Chesapeake Bay. While studies conducted in the Gulf of Mexico by Motes et al. (1998) and Lipp et al. (2001) showed that when salinity was below 15 ppt, Vv was positively correlated to salinity, and was negatively correlated to salinity when salinity levels were elevated above 15 ppt. Factors other than the range of salinity tolerance may be involved. The different studies were at locations with various tidal ranges, salinity gradients, and dynamics, which underscores the importance and complexity of developing site-specific models. Interaction with temperature (Kaspar and Tamplin 1993) and turbidity (Pfeffer et al. 2003; Johnson et al. 2010) also may be influencing Vv abundance.
The highest counts of Vv were found when the salinity ranged between 5 and 19 ppt. This range falls in the optimal range of Vv growth. Notably, in some cases at salinities as low as 2 and 3 ppt, Vv abundances were high. These salinity concentrations fall out of the published optimum range of Vv. The presence of Vv at WR-5, where the lowest specific conductivities were recorded, was surprising and implies the influence of other factors that transport Vv to that location such as storm surge or a complex upriver hydrography that reconnects it to estuarine water. In addition, WR-5 had the greatest increase in predicted Vv risks for a longer temporal period and under a wider range of sea level rise scenarios (0.3048–0.6096 m) versus 0.6096–0.9144 m at more downriver sites (WR-3 and WR-4).
Turbidity in this study ranged between 0 and 262 NTU. Correlation with turbidity was found in several other studies (Pfeffer et al. 2003; Johnson et al. 2010). The studies by Oliver et al. (1983) and Pfeffer et al. (2003) identified a positive correlation among Vibrio spp., other estuarine bacteria, and turbidity. The field data obtained in this study indicated that Vv and turbidity were associated in a positive manner, where the log count of Vv tended to increase with the increase of turbidity. Like many aquatic bacteria, Vibrio are frequently attached to the sediments (Cooksey and Wigglesworth-Cooksey 1995). This correlation between Vibrio and turbidity may be of great importance as other water quality indicator bacteria, such as Enterococcus, have been highly correlated with turbidity (Fries et al. 2006, 2008).
Despite finding a relationship between Vv concentration and turbidity, we could not include turbidity in our prediction model because future projections of turbidity were not available. We collected turbidity measurements opportunistically and include the analysis because of the importance of turbidity in life cycle and dynamics of Vv as mentioned above. The positive relationship between increasing Vibrio concentration and turbidity in our study should be further examined with current long-term weather and water quality data so that future climate change models could include these effects in the future, as many chemical contaminants, microbes, and nutrients are adsorbed to suspended sediments.
Typically, Vv population dynamics have been strongly correlated with temperature. Studies have shown that water temperature is a major variable influencing Vv seasonal fluctuation in estuarine waters (Randa et al. 2004; Thompson et al. 2004). Significant growth of Vv has been repeatedly associated with elevated sea surface temperatures along the Atlantic, Gulf, and Pacific Coasts of the USA (Kelly 1982; Kaysner et al. 1987; Oneill et al. 1992; Kaspar and Tamplin 1993; Lipp et al. 2001; Pfeffer et al. 2003; Randa et al. 2004). A relationship with temperature is not obvious in our data, probably due to limiting the field sampling to late spring through early autumn. Nonetheless temperature was a significant term in the multiple regression model. Our results suggest that although temperature had a significant influence, it was less than specific conductance. Additional sampling throughout the year over multiple years could better clarify this relationship.
These results suggest that maximum Vv concentration was not expected to increase very much but there may be a substantial increase in the occurrence of specific conductance within the optimal range for Vv growth (Table 3). Combined with a recent analysis of frequency and duration of specific conductance within the optimal range, there likely will be a much greater risk of exposure to Vv during recreational activities (the salinities are too low for shellfish growth) in the Waccamaw River (Deeb 2013) because Vv will occur more frequently throughout the year. More than 45% of the current Vv illnesses are caused by wound infections contracted during contact recreational activities and unlike seafood illnesses which occur primarily in immune-compromised individuals, wound infections occur frequently in healthy individuals (Oliver 2005). The risk of exposure increased with increasing water temperature (Figs. 5, 6, and 7). This finding also is consistent with the literature of Vv growth and highlights that future climate may cause increase Vv populations from several changes to abiotic drivers. Of particular interest is the clear range shift indicated by substantial increase in Vv prevalence at WR-4 and the decrease in maximum concentration at WR-3 related to the specific conductance increase into the descending leg of the growth curve.
Trtanj et al. (2016) reported that Vibrio growth rates may be highly responsive to rising sea surface temperatures, particularly in coastal waters, which generally have high levels of the dissolved organic carbon required for Vibrio growth. Salinity may also affect the distribution of Vibrio species. Salinity was affected directly by sea level rise and by changes in delivery of freshwater to coastal waters caused by flooding and drought. Species such as V parahaeomolyticus and V alginolyticus favor higher salinities while V. vulnificus favors more moderate salinities (Trtanj et al. 2016).
A number of different models have been developed to predict levels of vibrios in surface waters (Baker-Austin et al. 2016; de Magny et al. 2009; Froelich et al. 2013; Griffitt and Grimes 2013; Lipp et al. 2001; Vezzulli et al. 2012) and almost all have found that salinity and temperature were the most highly correlated variables for predicting Vibrio levels. Lipp et al. (2001) found that temperature and salinity were the best variables that predicted V. vulnificus levels in Charlotte Harbor, FL. Similarly, Constantin de Magny et al. (2009) used a multivariate empirical habitat model estimating the probability of V cholerae within a range of temperatures and salinities in the Chesapeake Bay, with hydrodynamically generated predictions of ambient temperature and salinity. Baker-Austin et al. (2016) examined the associations between epidemiological data on the emergence and dynamics of Vibrio disease in the Baltic Sea and long-term surface salinity and temperature (SST) records and recent satellite-derived data. They found that temperature along with salinity was highly predictive of increased levels of Vibrio illness there. Griffitt and Grimes (2013) assessed a major influx of freshwater to the Mississippi Sound following the opening of the Bonnet Carre Spillway in Mississippi, which greatly reduced salinity, with results indicating a reduction in densities of V. parahaemolyticus and V. vulnificus with a concurrent increase of V cholerae abundances, with V cholerae becoming the only Vibrio detected once salinity readings dropped to 6 ppt. Froelich et al. (2013) developed a five-parameter mechanistic model based on environmental processes including hydrodynamics, growth, and death rates of Vibrio bacteria to predict total Vibrio abundance in the Neuse River Estuary of eastern North Carolina. The addition of temperature and salinity greatly improved the accuracy of their statistical model. Wetz et al. (2014) also measured V vulnificus levels in surface and bottom waters of the Neuse River Estuary, in NC, using QPCR assays and developed a multivariate regression model that found that V vulnificus levels were positively correlated with temperature and salinity but was negatively correlated with chlorophyll-A and turbidity. V vulnificus was not detected when water temperatures were < 20 °C and 93 % of positive samples were measured when salinities ranged from 10 to 20 psu. Our results were similar to these earlier studies as temperature and salinity were the primary water quality variables useful in predicting V vulnificus levels in Winyah Bay, SC. Surprisingly levels of V vulnificus were measured at very low salinities (< 5 psu).
The risk analysis would be more helpful from a public health planning and prevention perspective if, instead of raw plate counts, the concentration of virulent strains most often observed in clinical cases were used. Using the percentage of virulent strains would provide a better understanding of the potential actual human health risk of changing salinities and water temperature along the Waccamaw River in the future. Future modeling efforts should include data on virulent strains in the model to accurately predict health risks of developing illness.
Jones and Oliver (2009) reported that the molecular basis responsible for virulence in V. vulnificus includes a number of mechanisms such as acid neutralization pathways, expression of capsular polysaccharides, use of iron-acquisition systems, cytotoxicity, motility, and expression of proteins involved in attachment and adhesion. In addition, underlying individuals with compromised immune systems are more prone to infections making the assessment of virulence more difficult to ascribe in terms of human sensitivity. Conversely, virulence is very important in V. vulnificus infections as illnesses may occur with very low levels in surface waters due to high levels of virulence (Martinez-Urtaza et al. 2013). The addition of these Vibrio virulence factors in future models will likely improve public health risk predictions under future climate conditions.
Conclusion
The ability to predict Vv is invaluable and offers many potential benefits, given the public health significance of these bacteria in shellfish illnesses and wound infections. The reported relationship between Vv and environmental parameters has been inconsistent among the different geographic locations (Atlantic, Pacific, Gulf of Mexico, and Black Sea). This represents the complexity of Vibrio spp. distribution, and indicates the possibility that there are interacting factors that control Vibrio occurrence and abundance in a given location.
Studying the interaction between the environmental param-eters, especially water temperature and salinity (the two dominant predictors of Vv), will enhance the ability to predict Vv concentrations. The results of this study clearly suggest the large, perhaps dominant, role of salinity in the prevalence of Vv. There may be a significant range expansion in the Waccamaw River due to upriver increases in salinity. This expansion may cause an exposure increase of as much as 290% (Fig. 6, in July at 0 °C temperature increase and 0.9144 m sea level rise the proportion is 390 which corresponds to a 290% increase).
Even with the limited temporal duration of sampling for this research, a significant temperature effect was found. Thus, sampling throughout the year and for more than 1 year could be useful to investigate whether there is a combined effect between temperature and salinity that could not be seen with these limited data, and perhaps other unidentified parameters. Determining interactions between temperature and salinity may explain some of Vibrio distribution variability, since optimal salinity was influenced by temperature under experimental conditions (Chase and Harwood 2011). Soto et al. (2009) noted that significant differences in growth rates of Vibrio due to salinity existed only when temperatures were low.
A common concern with environmental risk management is how species of concern are able to colonize new locations. For coastal rivers in areas that are subject to tropical storms, the tidal surge associated with them is a typical mechanism for upriver transport of both biotic and abiotic conditions. The predictive model developed here was very good with the samples collected during normal conditions but less so with samples collected after Hurricane Sandy passed the South Carolina coast. This suggests the situation in the aftermath of tropical storms is more complex than simply the relationship of Vv concentration with specific conductance and temperature. Given the ongoing concerns with public health and coastal climate adaptation, it would be beneficial to better understand these dynamics.
Acknowledgements This research was funded by a grant from the National Oceanic and Atmospheric Administration (NOAA) Climate Program Office (grant NA11OAR4310148). The authors wish to acknowledge essential assistance from several staff at the NOAA Center for Coastal Environmental Health and Biomolecular Research, especially Mike Fulton and Marie DeLorenzo who helped direct portions of the microbial research analysis as well as James Daugomah and Blaine West who provided exceptional assistance in the collection of field data. Alison Pierce, a graduate student at the University of South Carolina, also assisted with the laboratory analysis of the Vibrio isolates. Paul Conrads (US Geological Survey) provided assistance with integrating this research with the PRISM2 model. This article is dedicated to the memory of Paul Conrads (d. December 2, 2017), our frequent collaborator and friend.
References
- Allen DM, Ogburn-Matthews V, Buck T, and Smith EM. 2008. Mesozooplankton responses to climate change and variability in a southeastern US estuary (1981–2003). Journal of Coastal Research 10055: 95–110. 10.2112/si55-004.1. [DOI] [Google Scholar]
- Baker-Austin C, and Oliver JD. 2018. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environmental Microbiology 20 (2): 423–430. 10.1111/1462-2920.13955. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C, McArthur JV, Tuckfield RC, Najarro M, Lindell AH, Gooch J, and Stepanauskas R. 2008. Antibiotic resistance in the shellfish pathogen Vibrio parahaemolyticus isolated from the coastal water and sediment of Georgia and South Carolina, USA. Journal of Food Protection 71 (12): 2552–2558. 10.4315/0362-028x-71.12.2552. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C, Stockley L, Rangdale R, and Martinez-Urtaza J. 2010. Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: a European perspective. Environmental Microbiology Reports 2 (1): 7–18. 10.1111/j.1758-2229.2009.00096.x. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C, Trinanes JA, Taylor NGH, Hartnell R, Siitonen A, and Martinez-Urtaza J. 2016. Emerging Vibrio risk at high latitudes in response to ocean warming (vol 3, pg 73, 2013). Nature Climate Change 6 (8): 1 10.1038/nclimate1628. [DOI] [Google Scholar]
- Baker-Austin C, Trinanes J, Gonzalez-Escalona N, and Martinez-Urtaza J. 2017. Non-cholera Vibrios: the microbial barometer of cli-mate change. Trends in Microbiology 25 (1): 76–84. 10.1016/j.tim.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Blood ER, and Vernberg FJ. 1992. Characterization of the physical, chemical, and biological conditions and trends in three South Carolina Estuaries: 1970–1985 Volume II - Winyah Bay and North Inlet Estuaries. Charleston, SC. [Google Scholar]
- CDC. 2016. Foodborne Diseases Active Surveillance Network (FoodNet). https://www.cdc.gov/foodnet/reports/data/incidence-trends.html. Accessed 04/04/2018 2018. [DOI] [PMC free article] [PubMed]
- CDC (Centers for Disease Control and Prevention). 2006. Preliminary FoodNet data on the incidence ofinfection with pathogens transmitted commonly through food - 10 states, United States, 2005. Morbidity and Mortality Weekly Report 55 (14):392–395. [PubMed] [Google Scholar]
- Chase E, and Harwood VJ. 2011. Comparison of the effects of environmental parameters on growth rates of Vibrio vulnificus biotypes I, II, and III by culture and quantitative PCR analysis. Applied and Environmental Microbiology 77 (12): 4200–4207. 10.1128/aem.00135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrads PA, Roehl EA Jr., Daamen RC, and Cook JB. 2013. Simulation of salinity intrusion along the Georgia and South Carolina coasts using climate-change scenarios: U.S. Geological Survey. [Google Scholar]
- Cooksey KE, and Wigglesworth-Cooksey B. 1995. Adhesion of bac-teria and diatoms to surfaces in the sea—a review. Aquatic Microbial Ecology 9 (1): 87–96. 10.3354/ame009087. [DOI] [Google Scholar]
- Deeb Reem. 2013. Climate change effects on Vibrio bacteria in the Winyah Bay estuary and the projected spread of Vibrio under future climatic scenarios. University of South Carolina, Columbia, SC. [Google Scholar]
- Dhillon GS, and Inamdar S. 2014. Storm event patterns of particulate organic carbon (POC) for large storms and differences with dis-solved organic carbon (DOC). Biogeochemistry 118 (1–3): 61–81. 10.1007/s10533-013-9905-6. [DOI] [Google Scholar]
- Emanuel K 2017. Assessing the present and future probability of hurri-cane Harvey’s rainfall. Proceedings of the National Academy of Sciences of the United States of America 114 (48): 12681–12684. 10.1073/pnas.1716222114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JS, Characklis GW, and Noble RT. 2006. Attachment of fecal indicator bacteria to particles in the Neuse River estuary, NC. Journal of Environmental Engineering-ASCE 132 (10): 1338–1345. 10.1061/(asce)0733-9372(2006)132:10(1338). [DOI] [Google Scholar]
- Fries JS, Noble RT, Kelly GM, and Hsieh JL. 2007. Storm impacts on potential pathogens in estuaries. Eos, Transactions of the American Geophysical Union 88 (8): 93–95. [Google Scholar]
- Fries JS, Characklis GW, and Noble RT. 2008. Sediment-water ex-change of Vibrio sp and fecal indicator bacteria: Implications for persistence and transport in the Neuse River estuary, North Carolina, USA. Water Research 42 (4–5): 941–950. 10.1016/j.watres.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Froelich B, Bowen J, Gonzalez R, Snedeker A, and Noble R. 2013. Mechanistic and statistical models of total Vibrio abundance in the Neuse River estuary. Water Research 47 (15): 5783–5793. 10.1016/j.watres.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Griffitt KJ, and Grimes DJ. 2013. Abundance and distribution of Vibrio cholerae, V parahaemolyticus, and V. vulnificus following a major freshwater intrusion into the Mississippi sound. Microbial Ecology 65 (3): 578–583. 10.1007/s00248-013-0203-6. [DOI] [PubMed] [Google Scholar]
- Ho Hoi, and Cunha Burke A.. 2009. Vibrio infections. Medscape; 12p. [Google Scholar]
- Hoi L, Larsen JL, Dalsgaard I, and Dalsgaard A. 1998. Occurrence of Vibrio vulnificus biotypes in Danish marine environments. Applied and Environmental Microbiology 64 (1): 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Rhodes MR, Brown CW, Hood RR, Leight AK, Long W, and Wood R. 2010. Predicting the distribution of Vibrio vulnificus in Chesapeake Bay. Oxford, MD: NOAA National Centers for Coastal Ocean Science, Center for Coastal Environmental Health and Biomolecular Research, Cooperative Oxford Laboratory. [Google Scholar]
- Johnson CN, Flowers AR, Noriea NF, Zimmerman AM, Bowers JC, DePaola A, and Grimes DJ. 2010. Relationships between environmental factors and pathogenic Vibrios in the northern Gulf of Mexico. Applied and Environmental Microbiology 76 (21): 7076–7084. 10.1128/aem.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, and Oliver JD. 2009. Vibrio vulnificus:P disease and patho-genesis. Infection and Immunity 77 (5): 1723–1733. 10.1128/iai.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl Thomas R., Melillo Jerry M., and Peterson Thomas C.. 2009. Global climate change impacts in the United States: Cambridge University Press. [Google Scholar]
- Kaspar CW, and Tamplin ML. 1993. Effects of temperature and salin-ity on the survival of Vibrio-vulnificus in seawater and shellfish. Applied and Environmental Microbiology 59 (8): 2425–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysner CA, Abeyta C, Wekell MM, Depaola A, Stott RF, and Leitch JM. 1987. Virulent-strains of Vibrio-vulnificus isolated from estuaries of the United-States west coast. Applied and Environmental Microbiology 53 (6): 1349–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MT 1982. Effect of temperature and salinity on Vibrio (beneckea) vulnificus occurance in a gulf-coast environment. Applied and Environmental Microbiology 44 (4): 820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp EK, Rodriguez-Palacios C, and Rose JB. 2001. Occurrence and distribution of the human pathogen Vibrio vulnificus in a subtropical Gulf of Mexico estuary. Hydrobiologia 460 (1/3): 165–173. 10.1023/a:1013127517860. [DOI] [Google Scholar]
- de Magny GC, Long W, Brown CW, Hood RR, Huq A, Murtugudde R, and Colwell RR. 2009. Predicting the distribution of Vibrio spp. in the Chesapeake Bay: a Vibrio cholerae case study. Ecohealth 6 (3): 378–389. 10.1007/s10393-009-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Urtaza J, Baker-Austin C, Jones JL, Newton AE, Gonzalez-Aviles GD, and DePaola A. 2013. Spread of Pacific north-west Vibrio parahaemolyticus strain. New England Journal of Medicine 369 (16): 1573–1574. 10.1056/NEJMc1305535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, and Tauxe RV. 1999. Food-related illness and death in the United States. Emerging Infectious Diseases 5 (5): 607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motes ML, DePaola A, Cook DW, Veazey JE, Hunsucker JC, Garthright WE, Blodgett RJ, and Chirtel SJ. 1998. Influence of water temperature and salinity on Vibrio vulnificus in northern gulf and Atlantic Coast oysters (Crassostrea virginica). Applied and Environmental Microbiology 64 (4): 1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD 2005. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiology and Infection 133 (3): 383–391. 10.1017/s0950368805003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD 2015. The biology of Vibrio vulnificus. Microbiology Spectrum 3 (3): 10 10.1128/microbiolspec.VE-0001-2014. [DOI] [PubMed] [Google Scholar]
- Oliver JD, Warner RA, and Cleland DR. 1983. Distribution of Vibrio-vulnificus and other lactose-fermenting vibrios in the marine-envi-ronment. Applied and Environmental Microbiology 45 (3): 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oneill KR, Jones SH, and Grimes DJ. 1992. Seasonal incidence of Vibrio-vulnificus in the Great Bay estuary of New-Hampshire and Maine. Applied and Environmental Microbiology 58 (10): 3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker G, and Bej AK. 2005. Real-time PCR detection of Vibrio vulnificus in oysters: comparison of oligonucleotide primers and probes targeting vvhA. Applied and Environmental Microbiology 71 (10): 5702–5709. 10.1128/aem.71.10.5702-5709.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CS, Hite MF, and Oliver JD. 2003. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Applied and Environmental Microbiology 69 (6): 3526–3531. 10.1128/aem.69.6.3526-3531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randa MA, Polz MF, and Lim E. 2004. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Applied and Environmental Microbiology 70 (9): 5469–5476. 10.1128/aem.70.9.5469-5476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Geoffrey I., Porter Dwayne E., Norman R. Sean, Scott C. Hart, Uyaguari-Diaz Miguel I., Maruya Keith A., Weisberg Steve B. et al. 2016. Antibiotics as CECs: an overview of the hazards posed by antibiotics and antibiotic resistance. Frontiers in Marine Science 3 (24). doi: 10.3389/fmars.2016.00024. [DOI] [Google Scholar]
- Shaw KS, Jacobs JM, and Crump BC. 2014. Impact of hurricane Irene on Vibrio vulnificus and Vibrio parahaemolyticus concentra-tions in surface water, sediment, and cultured oysters in the Chesapeake Bay, MD, USA. Frontiers in Microbiology 5: 10 10.3389/fmicb.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto W, Gutierrez J, Remmenga MD, and Nishiguchi MK. 2009. Salinity and temperature effects on physiological responses of Vibrio fischeri from diverse ecological niches. Microbial Ecology 57 (1): 140–150. 10.1007/s00248-008-9412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, and Polz MF. 2004. Diversity and dynamics of a North Atlantic coastal Vibrio community. Applied and Environmental Microbiology 70 (7): 4103–4110. 10.1128/aem.70.7.4103-4110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trtanj Juli, Jantarasami Lesley, Brunkard Joan, Collier Tracy, Jacobs John, Lipp Erin, McLellan Sandra et al. 2016. Ch. 6: Climate Impacts on Water-Related Illness In The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment, 157–188. Washington, DC: U.S. Global Change Research Program. [Google Scholar]
- USGCRP. 2014. Southeast and the Caribbean. Climate Change Impacts in the United States: The Third National Climate Assessment: Global Change Research Program. [Google Scholar]
- Vezzulli L, Brettar I, Pezzati E, Reid PC, Colwell RR, Hofle MG, and Pruzzo C. 2012. Long-term effects of ocean warming on the prokaryotic community: evidence from the vibrios. ISME Journal 6 (1): 21–30. 10.1038/ismej.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli L, Grande C, Reid PC, Helaouet P, Edwards M, Hofle MG, Brettar I, Colwell RR, and Pruzzo C. 2016. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proceedings of the National Academy of Sciences of the United States of America 113 (34): E5062–E5071. 10.1073/pnas.1609157113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetz JJ, Blackwood AD, Fries JS, Williams ZF, and Noble RT. 2008. Trends in total Vibrio spp. and Vibrio vulnificus concentrations in the eutrophic Neuse River estuary, North Carolina, during storm events. Aquatic Microbial Ecology 53 (1): 141–149. 10.3354/ame01223. [DOI] [Google Scholar]
- Wetz JJ, Blackwood AD, Fries JS, Williams ZF, and Noble RT. 2014. Quantification of Vibrio vulnificus in an estuarine environ-ment: a multi-year analysis using QPCR. Estuaries and Coasts 37 (2): 421–435. 10.1007/s12237-013-9682-4. [DOI] [Google Scholar]
- Williams TC, Froelich BA, and Oliver JD. 2011. Comparison of two selective and differential media for the isolation of Vibrio vulnificus from the environment.
- Wright AC, Hill RT, Johnson JA, Roghman MC, Colwell RR, and Morris JG. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Applied and Environmental Microbiology 62 (2): 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]