Abstract
Significance: Hypertrophic cardiomyopathy (HCM) is a cardiac genetic disease characterized by left ventricular hypertrophy, diastolic dysfunction, and myocardial disarray. Disease onset occurs between 20 and 50 years of age, thus affecting patients in the prime of their life. HCM is caused by mutations in sarcomere proteins, the contractile building blocks of the heart. Despite increased knowledge of causal mutations, the exact path from genetic defect leading to cardiomyopathy is complex and involves additional disease hits.
Recent Advances: Laboratory-based studies indicate that HCM development not only depends on the primary sarcomere impairment caused by the mutation but also on secondary disease-related alterations in the heart. Here we propose a vicious mutation-induced disease cycle, in which a mutation-induced energy depletion alters cellular metabolism with increased mitochondrial work, which triggers secondary disease modifiers that will worsen disease and ultimately lead to end-stage HCM.
Critical Issues: Evidence shows excessive cellular reactive oxygen species (ROS) in HCM patients and HCM animal models. Oxidative stress markers are increased in the heart (oxidized proteins, DNA, and lipids) and serum of HCM patients. In addition, increased mitochondrial ROS production and changes in endogenous antioxidants are reported in HCM. Mutant sarcomeric protein may drive excessive levels of cardiac ROS via changes in cardiac efficiency and metabolism, mitochondrial activation and/or dysfunction, impaired protein quality control, and microvascular dysfunction.
Future Directions: Interventions restoring metabolism, mitochondrial function, and improved ROS balance may be promising therapeutic approaches. We discuss the effects of current HCM pharmacological therapies and potential future therapies to prevent and reverse HCM.
Keywords: hypertrophic cardiomyopathy, mitochondrion, pathophysiological mechanism, reactive oxygen species, redox state, sarcomeric gene mutation
I. Genotype–Phenotype Relationship in Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) was first described in 1958 by Robert Teare who observed severe unexplained thickening of the septum in a 14-year-old boy who had suddenly collapsed on the school playground (252). It was not until 1989 that an underlying genetic cause was first established with recent observations identifying a disease-causing mutation in ∼63% of all HCM individuals (105, 208). Currently >1400 HCM mutations have been found, of which the majority (∼95%) reside in genes encoding the sarcomere apparatus, the contractile building blocks of the heart muscle (65). Mutations in genes encoding the thick filament proteins β-myosin heavy chain (β-MyHC; MYH7 gene) and cardiac myosin-binding protein-C (cMyBP-C; MYBPC3 gene) account for ∼80% of all identified sarcomere gene mutations (12, 105, 208, 214). Less common are the HCM mutations in the genes encoding thin filament proteins such as cardiac troponin T (cTnT; TNNT2 gene), cardiac troponin I (cTnI; TNNI3 gene), α–tropomyosin (Tm; TPM1 gene), and actin (ACTC1 gene), and the myosin light chains (LC1, essential light chain (MYL3 gene) and LC2, regulatory light chain (MYL2 gene)) (12, 111, 214). Based on these findings, HCM is considered a disease of the sarcomere, although HCM can be caused by other genetic and nongenetic causes (e.g., inborn errors of metabolism and neuromuscular and mitochondrial diseases). The focus in this review is on HCM caused by sarcomeric gene mutations, the most common cause of HCM.
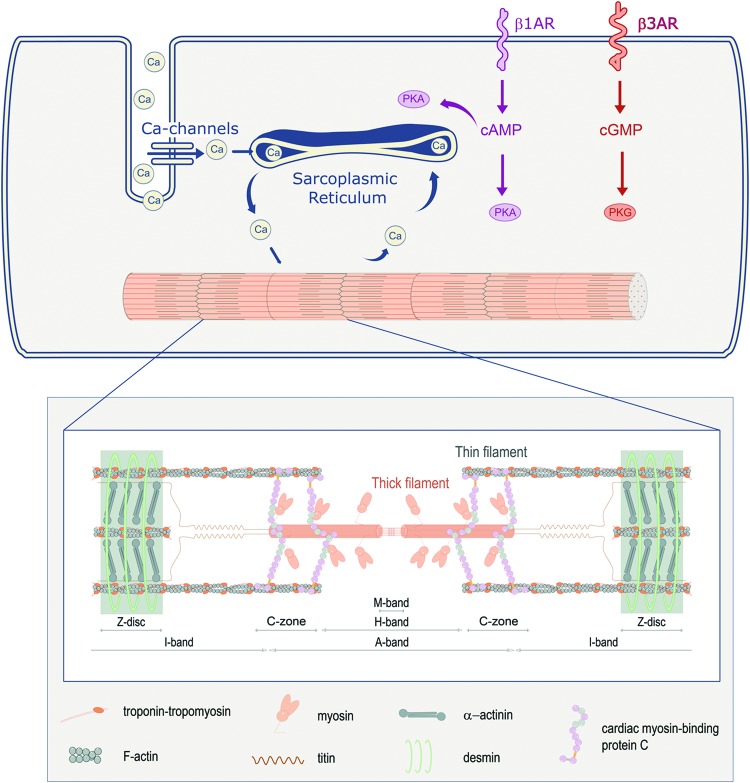
To understand how a sarcomere mutant protein impairs muscle contraction and relaxation, background is provided on the role of sarcomeres (myofilaments) during excitation–contraction coupling in cardiac muscle cells (23). To coordinate contraction of cardiomyocytes during a heartbeat, the heart makes use of the automatic depolarization of sinus node cells. This depolarization wave is spread throughout the myocardium by electrical coupling of cardiomyocytes and the conduction system of the heart. Once a cardiomyocyte depolarizes, Ca2+ enters the cell and triggers a much larger release of Ca2+ from the intracellular Ca2+-storage organelle (Fig. 1), the sarcoplasmic reticulum (SR). This increase in intracellular Ca2+ raises cytosolic [Ca2+] from ∼0.15 μM during diastole to a peak of ∼1.6 μM [Ca2+] during systole, sufficient to activate myofilament force development and shortening (23).
FIG. 1.
Cardiac muscle cell activation and role of sarcomeres. Contraction is initiated on Ca2+ entry in the muscle cell, which activates Ca2+-release from the SR. Ca2+ binds to the myofilaments, which causes contraction. To relax Ca2+ detaches and is pumped back into the SR via the SR-Ca2+-ATPase pump SERCA. During increased cardiac stress (exercise), Ca2+ detachment from the myofilaments is increased via reduced myofilament Ca2+-sensitivity by activation of β1-AR and via cAMP of PKA. Activation of β3-AR increases the activity of PKG via cGMP, which also reduces myofilament Ca2+-sensitivity. The inset shows the composition of the myofilaments, with the most affected HCM sarcomeric proteins cardiac myosin-binding protein C, myosin heavy chain, and troponin T. β1-AR, β1-adrenergic receptors; cAMP, cyclic AMP; cGMP, cyclic GMP; HCM, hypertrophic cardiomyopathy; PKA, protein kinase A; PKG, protein kinase G; SR, sarcoplasmic reticulum. Color images are available online.
Sarcomere proteins are the regulators of contraction and relaxation of the heart. cTnI is the “inhibitor” of the trimeric cardiac troponin (cTn) complex, which together with cardiac troponin C (cTnC) and cTnT controls the position of Tm on the thin actin filament in response to varying [Ca2+] (134). With the rise of cytosolic [Ca2+] and fiber lengthening, Ca2+ binding affinity to cTnC increases (107, 108), which promotes conformational changes of the troponin complex. This results in the release of cTnI's inhibition from actin, allowing Tm's position to shift closer to the inner domain of actin, enabling actin–myosin interactions and myofilament sliding and shortening (144). As muscle fibers are shortened, Ca2+ is actively released from cTnC (107, 108), consistent with reduced actin–myosin interactions, leading to Ca2+ reuptake into the SR with decreases of intracellular [Ca2+] (∼0.15 μM) (23); this results in myocardial relaxation during diastole, required for proper filling of the heart. cMyBP-C also regulates the interaction between actin and myosin, and thereby mediates contraction and relaxation of the myofilaments (Fig. 1) (218). Based on the central role of sarcomeric proteins in cardiomyocyte contraction and relaxation, it is not surprising that defects (mutations) in these proteins perturb cardiomyocyte function (172, 225, 248, 274).
HCM is the most common genetic heart disease with an estimated prevalence ranging from 1:500 to 1:200 and the most frequent cause of sudden cardiac death at young age in the general population in the Western world (163, 165, 221). In an adult, HCM is clinically defined by a wall thickness ≥15 mm in one or more left ventricular (LV) myocardial segments, which is not explained solely by loading conditions. However, genetic and nongenetic disorders can present with lesser degrees of wall thickening (13–14 mm). In these cases, the diagnosis of HCM requires evaluation of other features, including family history, noncardiac symptoms and signs, electrocardiogram abnormalities, laboratory tests, and multimodality cardiac imaging. The clinical diagnosis of HCM in first-degree relatives of HCM patients is based on the presence of otherwise unexplained increased LV wall thickness (≥13 mm) in one or more LV myocardial segments (65).
The onset and disease presentation are extremely diverse, ranging from asymptomatic mutation carriers (genotype-positive, phenotype-negative; G+/Ph−) to symptomatic patients with severe cardiac remodeling, including hypertrophy, cellular disarray, fibrosis, and vascular dysfunction (11, 164). Most patients have the classic form of HCM with asymmetric septal hypertrophy and LV outflow tract (LVOT) obstruction (HOCM) at rest or during exercise (178). A small number of HCM patients (∼4%) progress to an end-stage phase of HCM, characterized by systolic dysfunction (ejection fraction <50%) and adverse LV remodeling evident from regression of hypertrophy and/or cavity enlargement (97). Development of cardiac arrhythmias and sudden cardiac arrest are a major cause for the high mortality of young individuals with sarcomere gene mutations (249, 270). With the advance in genetic screening, more mutation carriers without a phenotype (G+/Ph−) are currently being identified, increasing the number of people who are insecure of their fate as treatment to prevent or cure disease is lacking.
As HCM is an autosomal dominant genetic disorder, most patients are heterozygous for the mutation and carry one mutant and one normal allele. However, apparently carrying a heterozygous sarcomere gene mutation alone cannot fully explain the HCM pathology as a mutation that causes HCM in one individual, while the identical mutation is harmless in a sibling (65). In addition, HCM typically develops at an older age (>20 years), while sarcomere gene mutations are present from birth. This illustrates that the HCM pathophysiological mechanism is more complex than a sarcomere gene mutation alone. Current treatment options for HCM patients are limited and nonspecific, and treatment options for G+/Ph− individuals to prevent HCM development are lacking. This can be explained by the fact that the molecular links between the causal sarcomere gene mutations and the HCM phenotype are still unresolved.
II. Functional Defects Caused by Mutant Proteins Cannot Solely Account for HCM Progression
The existence of mutation hot spots was initially proposed as underlying cause of HCM, where some mutations, either due to the affected mutant gene or the specific gene location, would confer a benign versus malignant phenotype to patients (271, 272). The latter was largely based on in vitro studies with reconstituted HCM mutant proteins, which indirectly showed derailments of myofilament relaxation, evidenced by high filament activation at low [Ca2+]—high filament Ca2+-sensitivity (210, 211). These studies supported the concept that the presence of the poison peptide harbors enough potential to drive pathological features of HCM. Nevertheless, there are several limitations to the in vitro solution approaches, including the fact that they solely contain purified/reconstituted actin, Tm, cTn, and myosin, and thus exclude up- and downstream signaling effectors and all the complex cellular cross talk of cardiomyocytes. In addition, because in vitro studies have 100% expression of the poison peptide they do not resemble the vast majority of heterozygous mutations in HCM.
III. Toxic Dose of Mutant Protein
As indicated above, not all mutation carriers develop HCM. Because the vast majority of HCM patients are heterozygous for the mutation, cardiomyocytes produce mutant protein in addition to the normal protein (Fig. 2). We propose the hypothetical concept that the balance between mutant and normal protein is an important determinant of disease onset (i.e., protein dosage). Disease might become evident when the mutant protein dosage is above a toxic threshold, or the normal protein level is below a certain threshold. The latter is observed in HCM caused by MYBPC3 truncating mutations, which cause haploinsufficiency (i.e., reduced normal protein levels) rather than truncated cMyBP-C (168, 261). We recently provided evidence that haploinsufficiency in engineered heart tissue alters contractile function when cMyBP-C protein levels are equal to or drop below 73% (274). Proof for the mutation dosage effect comes from rare cardiomyopathy cases, in which both alleles are mutated. These homozygous or compound mutations are associated with a reduced life expectancy and heart transplantation at young age (212, 268). Notably, the expression level of the heterozygous mutant protein, that is, protein dosage, has been associated with varying phenotypes in HCM animals in which higher expression levels of mutant proteins coincided with more severe forms of disease (57, 185). In accordance, studies in human showed that the expression levels of mutant proteins are associated with HCM severity (101, 255). We recently established in human patient tissue the link between protein dosage and impaired myofilament function in membrane-permeabilized cardiomyocytes by directly exchanging healthy and/or mutant myofilament proteins (70, 225). The latter experiments showed that the perturbation in myofilament function depends on the level of mutant troponin, while functional deficits were corrected on replacement of mutant protein by normal troponin complex.
FIG. 2.
Decline in the protein quality control system. Due to an age-related decline in the PQC system, mutant protein levels may increase above a toxic threshold, while levels of normal proteins decrease. This imbalance in protein composition may alter cardiac function, which will trigger a secondary disease-related decline in the PQC system further aggravating cardiac dysfunction and remodeling. Left: Cardiac MRI image of a mutation carrier without a phenotype (G+/Ph−). Right: Cardiac MRI image of a symptomatic HCM patient. MRI, magnetic resonance imaging; PQC, protein-quality-control. Color images are available online.
Proper cardiac functioning depends on a healthy balance in protein synthesis, folding, assembly, trafficking, and clearance (174). This homeostasis of cellular proteins is tightly regulated by the physiological protein-quality-control (PQC) system. We hypothesize that the levels of mutant protein are maintained at a low level during infanthood by the PQC system. Hence, the PQC system might prevent the onset of HCM by suppressing the accumulation of mutant protein while keeping the normal protein at sufficient levels in cardiac cells. Due to an age-related decline in the PQC system and the burden of comorbidities (e.g., hypertension (42)), mutant protein levels might increase above a toxic threshold, while levels of normal proteins decrease. As explained in the next paragraphs, allelic imbalance and epigenetics may be other factors (besides a decline in the PQC system and comorbidities) affecting the dosage of toxic protein in HCM patients. Future studies should define if and how much each mechanism is contributing to the changes of the toxic protein dosage during the life span of HCM patients. The imbalance in protein composition will alter cardiac function, which will trigger clinically apparent disease (Fig. 2). The protein dosage effect may explain the large variability of symptoms and severity of human HCM, and conciliates laboratory-based evidence gathered over the last two decades.
A. HCM allelic imbalance
Another mechanism that may contribute to HCM pathology is cell-to-cell differences in expression of mutant protein, resulting in mosaic expression patterns. Expression of genes is a stochastic process, both at transcription and translation levels (206). Gene expression is not a continuous process, but occurs in bursts, which vary in frequency and size (52). These bursts occur randomly, although transcriptional activators can increase burst size, and lead to large cell-to-cell variability of mRNA expression from individual genes (205). In disease states with an autosomal dominant inheritance pattern (such as HCM), this cell-to-cell variability can give rise to mosaic expression of mutant protein, especially when allelic imbalance is taken into account. As the vast majority of HCM cases are caused by heterozygous mutations, expression of the wild-type (WT) and mutant allele can occur simultaneously in cardiomyocytes. If both alleles are always transcribed to a similar degree, and mRNA stability is the same, this would result in a one to one ratio of WT and mutant protein in all cells. However, equal expression from both alleles does not occur; a phenomenon called allelic imbalance. By using single-cell sequencing it was shown that at one time point most cells predominantly transcribe one allele, with the other allele being expressed at low levels or not at all (27).
Even though on tissue level the allelic balance may appear unaltered, there can be a large cell-to-cell variation. Normally this would not be an issue in cells, as both alleles would produce the same protein, but this is not the case in HCM. In HCM, allelic imbalance may lead to cell-to-cell variation in mutant protein expression, as was recently shown in a group of HCM patients carrying different MYH7 mutations (138). Kraft et al. observed that variation in Ca2+ sensitivity of myofilament force production of cardiomyocytes of the same heart was much larger in HCM patients than in controls. They showed that the ratio of MYH7 WT-to-mutant expression varied considerably between cells (138), confirming the allelic imbalance previously shown in other cell types (27, 55). This mosaic expression of mutant protein, with direct functional consequences on the force producing capabilities of the cells, would lead to a loss of homogeneity of contracting myocardium (28). This would produce local strain and would decrease efficiency of contraction, possibly further driving disease progression. As the cell-to-cell variability in gene expression in cardiomyocytes increases with age (15), this could contribute to the age-dependent increase of HCM disease penetrance.
B. Epigenetics
Epigenetic modifications have been identified in heart failure (282) and may affect cellular levels of mutated protein in HCM. Epigenetics is the heritable regulation of gene modulation through modification of chromosomal structural proteins or additions to the nucleic acid without a change in the gene sequence. DNA methylation of CpG dinucleotides is one of several epigenetic mechanisms (e.g., ATP-dependent chromatin remodeling, histone modification) that cells use to control gene expression. Methylation of CpG islands in the promoter region is an important determinant of gene expression, and it has been demonstrated that the methylation of regulatory elements within the 5′ region of the MYH7 gene is inversely related to β-MyHC mRNA steady-state levels (43). Since the β-MyHC isoform increases at the expense of the α-MyHC isoform during pathologic hypertrophy (87), alterations of the level of methylation of the MYH7 gene promoter may underlie the changes in transcription in (mutated) MYH7.
In myectomy samples of HCM patients, activation of the chromatin-remodeling protein BRG1 has been identified and its level correlated with disease severity and MyHC isoform changes. In mouse embryos, Brg1 preserved fetal cardiac differentiation by interacting with the chromatin-modifying factors histone deacetylase and poly (ADP ribose) polymerase to repress α-MyHC and activate β-MyHC (93). Upregulation of the chromatin remodeling factor DPF3a has also been identified in hearts of HOCM patients. Activation of DPF3a on hypertrophic stimuli switched cardiac fetal gene expression from being silenced by the transcriptional repressors HEY to being activated by BRG1 (50). Another epigenetic change in HCM patients is the upregulation of the expression of the histone trimethyl demethylase JMJD2A, a global regulator of chromatin remodeling (292). In mice, it was shown that JMJD2A catalyzed the demethylation of trimethylated H3K9 and activated transcription of prohypertrophic genes under pathologic conditions (292). Interestingly, oxidative stress has been identified in HCM patients (described in section XI) and may affect the epigenetic regulation of genes by changes in the function of histones and DNA modifying enzymes and by oxidative conversion of 5-methylcytosine to 5-hydroxymethylcytosine (180).
Epigenetic modifications may also play a role in the origination of HCM mutations (de novo mutations). Based on the large number of G to A and C to T mutations in MYH7, MYBPC3, TNNI3, and TNNT2 in HCM as well as the relative mutability of the CG dinucleotide, it has been postulated that deamination of methylated CpG dinucleotides within coding regions of these cardiac genes may explain the origination and the large number of HCM mutations in these genes (51, 176, 177). Protein quality control systems and epigenetic modulations also affect each other, and may be involved in the delayed onset and variable expression of HCM phenotypes (14). Taken together, epigenetic modifications may affect the cellular level of mutated protein in HCM patients, either by directly modifying the expression of the mutated genes or indirectly by affecting the expression of fetal gene programs and other hypertrophic genes.
Besides the expression of mutant proteins, many pathological changes in cardiomyocytes have been described in HCM: an increase in myofilament Ca2+-sensitivity, metabolic perturbations such as excessive cellular ATP utilization and elevations of [ADP], mitochondrial dysfunction, a blunted length-dependent activation of myofilaments, cardiomyocyte hypertrophy, perturbed SR Ca2+-ATPase (SERCA) activity, disruption of the β-adrenergic receptor (β-AR) signaling pathway, and disturbances in redox signaling. In this review, we discuss these pathological changes in more detail and how they affect cardiac function in HCM.
IV. Vicious Mutation-Induced Cycle
Based on recent insight, we propose a hypothetical concept in which mutation-induced perturbations initiate a vicious cycle that further impairs the balance between mutant and normal proteins, leading to cardiac muscle and vascular disease (Fig. 3). Myocardial energy depletion (i.e., inefficient ATP regeneration) plays a causal role in the initiation of cardiac dysfunction [reviewed by Ashrafian et al. (11, 18, 49, 112, 133, 219, 259)]. A heart with a sarcomere mutation provokes high myofilament ATPase demand and increases mitochondrial workload. In addition to perturbed energetics, studies showed impaired relaxation (diastolic dysfunction), which occurs even before hypertrophy (81, 104, 179). Our recent study (224) provided evidence that cellular elevations of ADP, resulting from disturbed ATP regeneration, impair myocardial relaxation of the human HCM heart. Based on this intriguing finding, we propose that high levels of ADP occur early in HCM development, resulting from increased sarcomere energetic demands, and represent an important cause of diastolic dysfunction. In addition to changes in ADP levels, the excessive breakdown of AMP due to the overactivity of AMP deaminase may be another link between the energetic perturbations and diastolic dysfunction (193).
FIG. 3.
Proposed mutation-induced cycle in hypertrophic cardiomyopathy. Mutant protein increases energetic costs for sarcomere contraction and thereby impairs efficiency of cardiac performance. High energetic cost of sarcomere contraction may also be caused by increased sensitivity of the sarcomeres to Ca2+ (i.e., increased myofilament Ca2+-sensitivity), which is a hallmark of HCM. The high energetic costs for sarcomere contraction will cause energy depletion and thereby affect other energy-consuming processes in the cell involved in the maintenance of intracellular Ca2+-handling, metabolic substrate levels, and mitochondrial function. Perturbations in intracellular [Ca2+], metabolites, and mitochondrial function will impair diastolic function, which will cause hypoperfusion of the heart. Reduced cardiac perfusion, together with mitochondrial dysfunction, will reduce energy supply to the heart. Reduced energy supply will inhibit the PQC system resulting in an accumulation of mutant relative to normal protein. Disease-related derailments in the PQC system will exacerbate disease by the imbalance in sarcomere protein composition. In addition, hypoperfusion of the heart will trigger cell death and initiate secondary remodeling and dysfunction of endothelial and muscle cells, and will trigger maladaptive processes such as an increased adrenergic drive, post-translation protein modifications, cellular hypertrophy, reduced myofibril density, and fibrosis. Color images are available online.
Olivotto et al., showed reduced cardiac perfusion in HCM patients, which was most severe in patients with a sarcomere mutation (32, 191). The observation of a reduced coronary flow reserve in HCM patients with normal coronary angiograms led to the concept of microvascular (endothelial) dysfunction as secondary pathomechanism in HCM development (190, 197). Blunted coronary flow in response to adenosine (i.e., endothelial dysfunction) has been observed in hypertrophied and nonhypertrophied regions of the heart (190). Vascular endotheliopathy may be widespread, since HCM patients display a reduced peripheral vascular endothelial function (lower flow-mediated dilation) (100). Even before the onset of myocardial hypertrophy, HCM-causing mutations are associated with cardiac diastolic dysfunction and changes in peripheral microvascular function (69). In line with this, adverse changes of serum biomarkers reflect endothelial dysfunction at an early stage of HCM (60, 68).
Coronary and systemic microvascular (endothelial) dysfunction may be induced by both flow-related and biochemical changes. Flow-related changes in HCM may be induced by elevated LV end-diastolic pressure (diastolic dysfunction) and by LVOT obstruction in HOCM. Microvascular (endothelial) dysfunction may also be induced by the following: (i) vasoactive mediators from the pulmonary circulation that may be released in response to increased LV filling pressure; (ii) compensatory mechanisms in the early phase of vascular disease (e.g., upregulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase pathways); and (iii) changes in the autonomic nervous system (68, 69). Microvascular dysfunction in HCM likely contributes to the blunted myocardial blood flow during stress and to ischemia, which is an important complication of HCM (33). These studies suggest that mutation-induced cardiac contractile dysfunction precedes and possibly causes vascular (endothelial) dysfunction, subsequently initiating remodeling (hypertrophy, fibrosis) of the heart.
Overall, previous studies indicate that the mutation-induced myocardial energy depletion triggers metabolic and mitochondrial changes causing diastolic dysfunction and hypoperfusion of the heart before onset of cardiac hypertrophy. The causality and sequence of these muscular and vascular changes need to be defined to develop a targeted therapy. Moreover, as not all mutation carriers develop cardiac disease, knowledge of mutation-induced key pathomechanisms enables to identify people at risk to develop HCM.
V. Direct Mutation Effects
Central in the vicious mutation-induced cycle is that an inefficient ATP utilization during cardiac pump function plays a causal role in the initiation of cardiac dysfunction. It has been proposed that HCM mutations cause excessive cellular ATP utilization that drives the heart into failure, that is, the “myocardial energy depletion” hypothesis (11, 18, 49, 112, 133, 219, 259). The energy depletion theory was based on the observation that the the phosphocreatine (PCr)/ATP ratio, a cardiac marker of energetic state, was reduced by 30% in patients with manifest HCM compared to controls (49). Imaging studies using [11C]-acetate positron emission tomography and cardiovascular magnetic resonance imaging to assess the ratio between myocardial oxygen consumption and external work revealed reduced myocardial efficiency in HCM patients (86). The reduced cardiac efficiency was already evident in asymptomatic mutation carriers without hypertrophy indicative for a direct mutation effect on cardiac energetics (Fig. 4) (254, 280).
FIG. 4.
Impaired efficiency of cardiac contraction at early stage of the disease. Imaging studies in mutation carriers and patients with obstructive HCM revealed reduced efficiency of cardiac performance at an early stage of the disease before onset of hypertrophy. The energy deficiency was larger in carriers of MYH7 mutation compared with MYBPC3 mutation carriers. By use of cardiovascular MRI, left ventricular volumes and mass were defined to calculate myocardial external efficiency, that is, the ratio between external work and myocardial oxygen consumption. Left: Cardiac MRI image of a mutation carrier without a phenotype. Right: Cardiac MRI image of a symptomatic HCM patient. Figure has been adapted from Witjas-Paalberends et al. (280) and Güçlü et al. (86) with permission (Oxford University Press and Wolters Kluwer). MYBPC3, gene encoding cardiac myosin-binding protein-C; MYH7, gene encoding β-myosin heavy chain. Color images are available online.
At the cardiac muscle cell level, several mutation-mediated changes in myofilament function may underlie reduced cardiac efficiency in HCM (Fig. 5). First, the mutation-induced increase in ATP utilization of sarcomeres, which was reported in HCM animal models (75, 99, 237, 244). A study in human tissue harboring the R403Q MYH7 mutation showed faster cross-bridge detachment evident from an increased kinetics of relaxation in single myofibrils compared to controls (20), which indicates increased energetic cost of tension generation caused by mutant myosin. In line with these findings, our studies in cardiac tissue from HCM patients with thick filament (MYH7 and MYBPC3) mutations showed an increased cost of muscle contraction, that is, tension cost (ratio between ATP utilization and generated force) was significantly higher compared to controls (Fig. 5A) (70, 279). A proof-of-concept study showed that replacing mutant with healthy sarcomeric protein rescues the energy deficiency in patient tissue (70).
FIG. 5.
Defects at the myofilament level in hypertrophic cardiomyopathy. At the myofilament level, sarcomere mutations were shown to (A) increase tension cost (i.e., reduce efficiency of sarcomere contraction) and (B) increase myofilament Ca2+-sensitivity (indicated by the arrow). (C) Maximal force-generating capacity (black arrow) and myofilament Ca2+-sensitivity (white arrow) increase on stretching from short (1.8 μm) to long (2.2 μm) sarcomere lengths for the donor. Sarcomere mutations (MYH7mis and TNNI3mis) impair this length-dependent myofilament activation. (A) Adapted from Witjas-Paalberends et al. (280) with permission (Oxford University Press). (B) Adapted from Sequeira et al. (225) with permission. (C) Donor has been adapted from Sequeira et al. (225) with permission. (C) (MYH7mis and TNNI3mis) original data. Color images are available online.
Second, a mutation-related increase in myofilament Ca2+-sensitivity was found in in vitro studies using recombinant mutant proteins (210, 211) and HCM mouse models (18, 74, 186, 200, 219), which will increase both force development and ATP consumption. In accordance with HCM mouse models, our studies in human cardiac tissue revealed high Ca2+-sensitivity in HCM human cardiac tissue (Fig. 5B) (139, 225, 261, 262).
Third, the additional burden of comorbidities, including hypertension (42), likely magnifies the rise in systolic stress caused by the already high myofilament Ca2+-sensitivity in HCM myofilaments, further exacerbating energetic load. During increased workloads (afterload augmentation), the dissociation of the actin–myosin interaction is substantially delayed, consistent with slowing of ADP removal from the myosin nucleotide site, the last step in the myosin-ATPase cycle (189). Increases of ADP levels at the myofilament will feedforward enhancement of mitochondrial workload in HCM in an attempt to maintain ADP levels at a normal level.
A fourth aspect, which may contribute to inefficient contraction of the heart, is a blunted length-dependent activation of myofilaments, the cellular basis of the Frank–Starling mechanism of the heart. The ability of the heart to adjust the force of its contraction in response to the dynamic changes in ventricular filling forms the basis of the Frank–Starling Law. In human HCM, a limited Frank–Starling reserve has been observed (53). This is supported by recent studies in human (81, 104) and mice (74) with HCM that clearly show defects of cardiac relaxation in the absence of left ventricle hypertrophy. The diminished capacity to relax decreases diastolic filling and limits the effects exerted by length increases, that is, the reserve is not used. Indeed a restricted Frank–Starling reserve has been observed in HCM mutation carrier patients and HCM animals with severe LV hypertrophy (81, 116, 230, 234). In human HCM with sarcomeric mutations, we recently reported impairment of cardiomyocyte length-dependent activation (Fig. 5C) (225).
These mutation-induced impairments in the efficiency of muscle contraction (Fig. 5) may underlie reduced efficiency of cardiac contraction at an early stage of the disease (Fig. 4), before secondary remodeling of the heart.
VI. Metabolic Perturbations
The primary energy-producing reaction used by the cardiac muscle to perform work is the hydrolysis of ATP at the myofilaments. Synthesis of new ATP occurs by glycolytic pathways, β-oxidation and the Krebs cycle. However, due to their small and slow rate of synthesis compared to the hydrolysis of ATP, rapid supply is largely dependent on ATP regeneration from ADP resulting from the mitochondrial oxidative phosphorylation pathway and is coupled to phosphotransfer reactions (Fig. 6, right). The major phosphotransferase in cardiomyocytes is creatine kinase (CK). As illustrated in Figure 6 (right panel), because of the relatively slow diffusion of ATP and ADP in cell compartments, CK present at the myofilaments regenerates ADP to ATP using PCr that is regenerated at the mitochondria (84). Under physiological conditions, ATP regeneration is sufficient to maintain normal ATP levels and prevent cellular ADP accumulation (∼10 mM ATP vs. ∼60 μM ADP, respectively) (Fig. 6) (84). As mentioned above, in human HCM, the myocardial energy reserve is diminished evident from the reduction in the PCr/ATP content ratio (49).
FIG. 6.
Excitation/contraction energetics coupling in healthy cardiomyocytes. Left, Ca2+-induced Ca2+-release from the SR increases cytosolic [Ca2±], leading to activation of myofilaments. Ca2+-release from myofilaments allows for myofilament relaxation. Right, Zoomed view of the creatine (Cr) and phosphocreatine (PCr) export pathway in healthy cardiomyocytes. Mitochondrial ATP synthase regenerates ATP from ADP, which via mitochondrial CK the phosphoryl group in ATP is used to generate PCr inside the mitochondria. Muscle CK uses the shuttled PCr to rapidly regenerate ATP from ADP at the myofilaments. CK, creatine kinase; NCX, Na+/Ca2+ exchanger. Color images are available online.
The absolute ATP levels in the heart will, however, never be sufficiently low to solely explain cardiac dysfunction in HCM (6, 45). For instance, measurements of absolute ATP levels in HCM animals showed that the reduction in [ATP] is not rate limiting for cardiomyocyte relaxation irrespective of rest, work, or stress; ATP maximally decreased from 10 to 7 mM (99, 117, 237), while as little as 0.1 mM ATP is sufficient for cardiac relaxation (45). The levels of ATP in HCM are thus sufficiently maintained by the PCr-CK pathway. However, this pathway is unable to buffer elevations of [ADP], as [ADP] increases up to 130 μM in HCM animal models, which were accompanied by significant decreases in PCr content (16–20 mM in health vs. 10–14 mM in disease) (Fig. 7, right) (99, 117, 237).
FIG. 7.
Excitation/contraction energetics coupling in hypertrophic cardiomyopathy cardiomyocytes. Left, HCM-causing mutations lead to higher myofilament ATPase-activity in cardiomyocytes, thereby enhancing cellular [ADP]. Defective CK and mitochondria function are unable to rapidly regenerate ATP. Elevated [ADP] leads to high myofilament Ca2+-sensitivity and Ca2+-buffering (sticky myofilaments, designed as a fishnet trapping Ca2+ at the myofilaments). Right, Zoomed view of the creatine (Cr)/phosphocreatine (PCr) export pathway in HCM cardiomyocytes. Impaired mitochondrial function in concert with reduced CK function and low PCr amount limits the capacity of the Cr-PCr pathway to buffer ATP, leading to ADP accumulation at the myofilaments. Color images are available online.
We recently evaluated the consequences of these elevations of [ADP], as reported in HCM animal models (≥100 μM), on myofilament function (223, 224). High [ADP] increased myofilament Ca2+-sensitivity/tension (Fig. 8) and Ca2+-binding affinity (sticky myofilaments), associated with impaired cardiac relaxation (Fig. 7, left). Accordingly, HCM mouse models with Ca2+-sensitized myofilaments showed marked diastolic dysfunction, which coincided with high Ca2+-buffering and localized energy deprivation at membrane junction sites (18, 112, 219). In addition to the direct Ca2+-sensitizing effect of ADP, elevations of ADP, together with a reduction in ATP, will reduce the free energy released from ATP hydrolysis (ΔGATP) and limit performance of ATP-dependent cardiac ion pumps, including SERCA (6). Perturbed SERCA activity will limit reuptake of Ca2+ into the SR and together with the high Ca2+-buffering potentiated by the sticky HCM myofilaments will limit relaxation of the heart. High [ADP] will thus exert a direct (myofilament Ca2+-sensitization) and indirect (impaired SERCA) detrimental effect in HCM pathology.
FIG. 8.
Effects of pathological ADP levels on cardiomyocyte function. Myofilament Ca2+-tension in HCM and nonfailing donor membrane-permeabilized cardiomyocytes. Ca2+-tension of HCM cardiomyocytes was measured in the presence of pathological ADP levels (100 μM ADP), while ADP was absent in donor cardiomyocytes, mimicking in vivo intracellular milieu. Gray panel depicts the free [Ca2+] range in in vivo cardiomyocytes (i.e., ∼0.15 diastolic to 1.6 μM systolic [Ca2+]). Cardiomyocyte Ca2+-tension is corrected for myofibril density. HCM cardiomyopathy tissue is hypercontractile versus donors at the free [Ca2+] in vivo range. Figure has been adapted from Sequeira et al. (225) and Sequeira et al. (224) with permission. Color images are available online.
VII. Myofilament Ca2+-Sensitization: Direct and Indirect Players
Significant efforts have been made to identify a common pathomechanism in HCM disease that would explain the diverse multitude of intracellular events. Impaired relaxation is a universal finding in humans (24, 81, 98, 104, 160, 183, 273) and HCM animal models (36, 67, 74, 131–133, 184, 185, 219, 250), irrespective of fibrosis and/or hypertrophy. Inefficient myocardial relaxation in HCM can be explained by the elevated myocardial activation at low diastolic [Ca2+]—high myofilament Ca2+-sensitivity—which has the potential of delaying the onset of ventricular relaxation and limiting proper filling. Increased myofilament Ca2+-sensitivity is now a well-accepted and established feature in HCM disease (18, 74, 139, 186, 200, 219, 225, 261, 262), which may be (i) primarily caused by the mutant sarcomeric proteins or (ii) secondarily induced by the mutant sarcomeric proteins (secondary disease-related mechanisms). As mentioned above, the presence of the poison peptide harbors enough potential to drive pathological features of HCM: certain gene mutations, or specific locations on some of the affected genes, increase myofilament Ca2+-sensitivity more compared to more benign mutations. Thus, the direct impact of a mutant protein on myofilament Ca2+-sensitivity seems to depend on the location of a mutation within the affected gene. This was clearly illustrated by studies from the Knollmann group, which showed that the effect on myofilament Ca2+-sensitivity of different TNNT2 mutations is highly variable, ranging from no effect to mice harboring highly Ca2+-sensitized myofilaments associated with increased diastolic [Ca2+] and an increased susceptibility to arrhythmias (219, 230).
Studies in human cardiac samples from patients with obstructive cardiomyopathy revealed, however, that the high myofilament Ca2+-sensitivity was largely explained by reduced phosphorylation of cTnI, indicative for a secondary disease-related disruption of the β-AR signaling pathway (139, 187, 225, 261, 262). Activation of β1-AR desensitizes the myofilaments to Ca2+ and enhances the rates of myocardial relaxation during increased cardiac stress as occurs during exercise. Myofilament Ca2+-sensitivity is reduced via protein kinase A (PKA)-mediated phosphorylation of sarcomeric proteins such as cTnI (233, 275, 276). One of the most reported perturbations is the downregulation and desensitization of β1-AR in heart failure, and subsequent reduction in PKA activity (29, 94). Reduced β-AR signaling has been reported in several HCM mouse models (186, 220).
Not only is PKA activity reduced in HCM but changes in PKA localization have, likewise, been observed as well. In a mouse model carrying Mybpc3 truncating mutation (263), β-adrenergic stimulation led to a normal increase in PKA phosphorylation of phospholamban—a key regulator of Ca2+-reuptake into the SR—but a blunted increase in cTnI phosphorylation (186). This led to contractile dysfunction, despite normal Ca2+ transients. This preferential signaling to the SR over the myofilament is likely mediated by changes in A-kinase anchoring proteins (AKAPs) that mediate subcellular localization of PKA signaling (243).
Apart from reduced sarcomeric protein phosphorylation, reactive oxygen species (ROS)-related post-translational oxidative modifications of sarcomeric protein have been associated with enhanced myofilament Ca2+-sensitivity in HCM mouse model carrying a Tm mutation (277). And finally, as mentioned above, perturbations in cellular metabolites (including high [ADP]) are able to increase the sensitivity of HCM myofilaments to Ca2+ (223, 224). Direct mutation effects and secondary disease mechanisms appear to exert an additive effect on Ca2+-sensitivity of myofilaments (Fig. 9). We propose that as a consequence of ongoing disease, the secondary disease mechanisms enhance the mutation-induced increase in myofilament Ca2+-sensitivity and thereby exacerbate the energetic burden on the heart.
FIG. 9.
Interaction between myofilament Ca2+-sensitivity, mutant protein, and secondary disease effects. (A) Activation of β-adrenergic receptors desensitizes the myofilaments for Ca2+ via PKA-mediated phosphorylation of sarcomeric proteins. (B) A hallmark of HCM is increased myofilament Ca2+-sensitivity, directly caused by mutant protein, reduced PKA-mediated protein phosphorylation, oxidative protein modifications, and increased [ADP]. High Ca2+-sensitivity will increase energetic costs for contraction and limit relaxation of the heart. Schematic figure based on original data from Sequeira et al. (225) and Sequeira et al. (224) and Wilder et al. (277). Color images are available online.
VIII. Decoupling Between the PCr-CK Pathway and Mitochondrial Oxidative Phosphorylation
A major question then arises of how to explain the large relative elevations of [ADP] in HCM. As mentioned above, rapid supply of ATP to cardiomyocytes largely depends on ATP regeneration from ADP resulting from the coupling of the mitochondrial oxidative phosphorylation pathway and the CK-PCr pathway (Fig. 6, right). PCr provides a spatiotemporal shuttle from the mitochondria to sites of high energy consumption—myosin-ATPase at the myofilaments—and thereby overcomes the relatively slow physiological diffusion of ATP and ADP (114). Muscle CK localized at the myofilaments will use this PCr pool to quickly buffer ADP back to ATP (266). Thus, it is expected that HCM-induced alterations in the PCr-CK system can have profound effects on the myocardial energetic reserve, leading to increased levels of cellular ADP. This is supported by studies showing that human HCM patients have low PCr levels, which associated with reduced CK-flux (3, 232). In addition, impaired mitochondrial architecture and function have been reported in HCM animal models (154, 250) and humans (258). The perturbations in the PCr-CK system and mitochondria will exhaust the myocardial energy reserve in HCM and contribute to myocardial diastolic dysfunction.
Inefficient ATP utilization may drive exhaustion of the myocardial reserve in HCM by potentiating the imbalance between the mitochondria and the PCr-CK system because (Fig. 7, left) (i) myofilaments containing mutant proteins utilize higher levels of ATP, via an increase of the detachment rate of myosin and/or increased amount of force-producing cross-bridges (i.e., increase in tension cost), and Ca2+-sensitization of myofilaments and thereby elevate intracellular [ADP]; as a result (ii) sustained mitochondrial ADP-workload occurs, which over time stimulates ADP-stimulated state 3 respiration, which will stimulate commensurate ROS formation (detailed below); (iii) increasing cellular [ADP] exacerbates the mutation-mediated increase in myofilament Ca2+-sensitivity/and Ca2+-buffering, perturbing myocardial relaxation and causing diastolic dysfunction (Fig. 7, left); (iv) since CK is the main target of ROS in myofibrils (175) and high levels of cardiac ROS are found in HCM (discussed below) (38, 39, 41, 61, 135, 150, 188, 222, 277), high levels of ROS may result in oxidative modification of CK and thereby reducing the function. Thus, perturbed CK activity at the myofilaments further disrupts the balance between ATP and ADP.
Deprivations in myocardial bioenergetics may underlie reduced coronary perfusion caused by elevations in myofilament Ca2+-sensitivity and perturbed myocardial relaxation. Adequate coronary perfusion to the heart muscle occurs during diastole; coronary inflow (i.e., oxygen delivery) is slower during systole as a result of narrowing of coronary arteries, but peaks during the diastolic phase (53). Impaired myocardial relaxation can therefore limit coronary flow, in particular during increased stress (e.g., exercise). As mentioned above, improper cardiac perfusion (32, 191) and reduced coronary blood flow reserve have been observed in HCM patients (190, 197). The high Ca2+-sensitized HCM muscle likely accounts for these observations in human HCM as it promotes a basal state of diastolic sarcomeric activation (high systolic stress), responsible for derailing the diastolic phase and restricting coronary blood flow. This vicious cycle of impaired coronary perfusion provides a source of localized energy deprivation, which is observed in HCM animals (Fig. 3) (112). This focal energy deprivation will give rise to additional elevations of [ADP], but also Pi reductions in the PCr pool, and coincident changes in cellular pH (6). In support, myocardial ischemia has been detected in all HCM patients who survived a cardiac arrest episode (59). While the initial mutation-mediated changes in cellular energetics may be reversible, ischemic damage of the heart will cause irreversible changes to the heart muscle, including severe mitochondrial damage, collagen formation, and microvascular rarefaction (35, 85, 191). Finally, afterload augmentation as in recent hypertensive HCM cases (42) shares the potential to magnify the already elevated levels of ADP, resulting from slower rates of ADP removal from myosin due to increased affinity of actin–myosin interactions at high filament loads (189).
IX. Oxidative Stress
Here we discuss the role of altered cardiac redox conditions (reduction–oxidation reactions) in HCM pathology, with a specific focus on HCM caused by sarcomeric gene mutations. A disturbed redox balance on top of a sarcomeric gene mutation may play a central role in HCM pathology and contribute to HCM initiation and progression. Excessive cellular ROS—referred to as oxidative stress—may be an important link between a causal sarcomeric mutation and the HCM phenotype. This is based on data that show (i) increased levels of oxidative stress markers in the heart and blood of HCM patients and animal models of HCM (38, 39, 41, 61, 72, 82, 135, 150, 188, 222, 277); (ii) lowering oxidative stress with antioxidants in HCM animal models prevents and reverses HCM (150, 161, 277); (iii) cardiac oxidative stress in the absence of sarcomeric gene mutations associates with diastolic dysfunction, fibrosis, cardiac hypertrophy, and arrhythmias, all hallmarks of HCM (5, 118, 151, 256, 294). We will provide an overview of studies in which oxidative stress markers and endogenous antioxidant enzymes have been investigated in HCM. The effects of antioxidant treatment of HCM animal models are also outlined. In addition, we discuss potential mechanisms by which sarcomeric gene mutations may lead to a disturbed cardiac redox balance and HCM pathology. Finally, we discuss the effects of current HCM pharmacological therapies and potential future therapies on the redox state of the heart.
X. Redox Signaling, Oxidative Stress, and Antioxidant Defense
Oxygen (O2) utilization is essential to humans as we are dependent on O2 oxidation for ATP regeneration, detoxification, and biosynthesis. Nevertheless, whenever O2 accepts single electrons, it modifies the O2 molecule into, unstable, ROS. As illustrated in Figure 10A, electron transfer to O2 generates superoxide radical anion (O2•−), which progressively forms hydrogen peroxide (H2O2), hydroxyl radical (HO•), and eventually water (H2O) (136, 260). The O2•− anion is extremely reactive and short-lived, which in addition to its poor lipid solubility cannot diffuse far within cell compartments. This contrasts with H2O2, which due to its high lipid solubility, and relatively long-lived properties, can diffuse through membranes and generate HO• at specific sites, including the mitochondria (Fig. 10B) (227, 260). The HO• is considered the most potent form of ROS (260).
FIG. 10.
Cellular reactive oxygen species production and damaging effects. (A) Four (one-electron) steps for oxygen reduction. Electron transfer to oxygen (O2) generates superoxide radical anion (O2•−), which progressively forms hydrogen peroxide (H2O2) and the hydroxyl radical (HO•). (B) ROS-mediated cardiomyocyte injury. O2•− and HO• initiate myofilament and cytosolic protein damage with amino acid residues oxidized and degraded/deactivated. Lipid peroxidation of sarcolemma, SR, mitochondrial and nuclear membranes, disrupts phospholipids, and increases cellular permeability to ions and water. Mitochondrial membrane disruption additionally causes more ROS production. Nuclear and mtDNA can be oxidized, leading to strand breaks and transcription impairment. The O2•− anion is extremely reactive, nevertheless due to its poor lipid solubility cannot diffuse far within cell compartments. This contrasts with H2O2, which due to its high lipid solubility can diffuse through membranes and generate HO• at specific sites. The HO• is considered the most potent form of ROS. (C) DNA oxidative damage. Conversion of guanine to 8-oxodG by the HO• radical is the most frequently observed damage to nucleotides. Addition of HO• to the guanine backbone interferes with polymerases and/or allows nucleotide mispairing. Cellular levels of 8-oxodG are used as a marker to estimate the amount of oxidative damage to DNA in cells. (D) Lipid peroxidation. Lipid peroxidation initiates when hydrogen atoms, closed to the double bond of a (poly)unsaturated fatty acid from phospholipids, are extracted by HO• (i.e., desaturation) forming a very unstable lipid radical. Rearrangements of the single electron change the molecular structure of the lipid radical. In the presence of O2, the lipid radical chain reaction is propagated, forming a lipid peroxyl radical. Removal of hydrogen atoms from other (poly)unsaturated fatty acids forms lipid hydroperoxide. Eventually, lipid degradation occurs with formation of MDA and 4-hydroxyalkenal products. MDA appears in the urine and blood and its levels are used to assess lipid damage. ROS-induced damage to lipid membranes, including the sarcolemma, nucleus, sarcoplasmic reticulum, and the mitochondria, forms lipid radicals and lipid peroxides; lipid peroxidation of cellular membranes alters their structure, disrupts lipid bilayer integrity, and increases susceptibility to permeability of ions and water. 8-oxodG, 8-oxo-7-hydrodeoxyguanosine; MDA, malondialdehyde; mtDNA, mitochondrial DNA; ROS, reactive oxygen species. Color images are available online.
ROS are redox signaling molecules that mediate important post-translational modifications with pronounced effects on multiple cellular functions. ROS levels are tightly balanced through nonenzymatic and enzymatic antioxidant systems; nevertheless, under certain pathological conditions, the levels of ROS exceed the cell's antioxidant capacity due to either a decrease in antioxidant buffering capacity or resulting from an increase of ROS production, or both. Uncontrolled levels of ROS cause damage to proteins, DNA, and lipids (Fig. 10B) (31, 62, 140, 286).
Under physiological conditions, up to 5% of total O2 utilized in cells is converted to ROS with the mitochondrial respiratory chain, the largest ROS producer; at any given moment, the mitochondria consume 85–90% of total cellular O2 (115, 260). Energy retrieved from fuel oxidation (i.e., Krebs cycle), mostly conserved in the reduced forms of NADH and FAD(2H), is able to regenerate ATP from ADP by the process of oxidative phosphorylation at the mitochondria (Fig. 11A). The mitochondrial electron transport chain comprised a series of complex electron transfer proteins that span the inner mitochondrial membrane (complexes I, III, and IV), whereas succinate dehydrogenase (complex II) is rooted in the mitochondrial inner membrane via the integral membrane proteins SDHC and SDHD (Fig. 11A) (16, 137).
FIG. 11.
Mitochondrial electron transport chain. (A) Components of the electron transport chain. NADH dehydrogenase (complex I), cytochrome b–c1 complex (complex III), cytochrome c oxidase (complex IV), and ATP synthase (complex V) span the inner mitochondrial membrane. Reduced forms of NADH (complex I) and FAD(2H) donate electrons (e−) to the transport chain via complex I or complex II, respectively, which are sequentially transferred to electron carriers, including the lipid soluble CoQ, complex III, CytC, and complex IV. Complex IV accepts electrons from the electron transport chain and reduces O2 into H2O. As electrons pass through the electron transfer chain, protons (H+) are pumped across the mitochondrial matrix to the inner mitochondrial space (at complexes I, III, and IV; complex II lacks a proton pumping mechanism), responsible for establishing an electrochemical proton gradient at the inner mitochondrial membrane. The creation of the electrochemical proton gradient forces protons back inside the matrix at the complex V, which uses the proton gradient energy to regenerate ATP from ADP (and Pi). The electron transport chain is coupled to the rate of ATP regeneration by the electrochemical proton gradient-coupled oxidative phosphorylation. Under physiological conditions, approximately up to 5% of O2 in cells is converted to ROS, with complexes I and III the main sites for ROS production. (B) Supercomplex formation in the presence of cardiolipin. Cardiolipin is a unique type of phospholipid only found on mitochondrial membranes with higher enrichment at the inner mitochondrial membrane (∼22%) with minute levels of enrichment at the outer mitochondrial membrane (∼3%). Cardiolipin promotes membrane curvature of the inner mitochondrial membrane and importantly restructures the electron transport chain complexes into supercomplexes to improve electron transfer efficiency. (C) Cardiolipin peroxidation. Due to the high (poly)unsaturated fatty acid content, cardiolipin is particularly vulnerable to lipid peroxidation, which reduces formation of supercomplexes. In addition, affinity of CytC, and potentially CoQ, to the inner mitochondrial membrane is lost. CytC release from the mitochondrial intermembrane compartments into the cytosol activates programmed cell death. (D) SS-31 cardioprotective effects of cardiolipin. SS-31 specifically targets and stabilizes cardiolipin's location at the inner mitochondrial membrane, likely facilitating the formation of mitochondrial supercomplexes, in combination with reductions of electron leakage (and ROS formation) with improved ATP regeneration. CoQ, coenzyme Q; CytC, cytochrome c. Color images are available online.
Using NADH or FAD(2H) as substrates, electrons (e−) are transferred to the electron transport chain via the NADH dehydrogenase (complex I) or complex II, respectively (Fig. 11A); these electrons are then sequentially transferred to electron carriers, including the lipid soluble coenzyme Q (CoQ), the cytochrome b-c1 complex (complex III), cytochrome c (CytC), and ultimately to the cytochrome c oxidase (complex IV). Complex IV contains the binding site for O2, which accepts electrons from the electron transport chain and reduces the O2 molecule into the stable molecule of H2O (Fig. 11A) (16, 137). As electrons pass through the electron transport chain, protons (H+) are pumped across the mitochondrial matrix to the inner mitochondrial space (at complexes I, III, and IV; complex II lacks a proton pumping mechanism), thereby establishing an electrochemical proton gradient at the inner mitochondrial membrane. The creation of this electrochemical proton gradient drives protons back into the matrix at the ATP synthase complex (complex V), which uses this energy to regenerate ATP from ADP (and Pi) (Fig. 11A).
The electron transport chain is tightly coupled to the rate of ATP regeneration by the electrochemical proton gradient coupled oxidative phosphorylation. Formation of ROS is largely dependent on accidental electron leakage throughout the respiratory chain to O2, with complexes I and III of the respiratory chain as the main sites for ROS generation (137). In addition to the mitochondria, NADPH oxidases (NOX) are major sites for ROS production. NOX are a family of protein complexes that catalyze the transfer of electrons from NADPH to O2 resulting in the generation of ROS. Moreover, ROS can also be produced by xanthine oxidase (XO) and uncoupling of nitric oxide synthase (NOS). Monoamine oxidases (MAOs) are flavoenzymes located within the outer mitochondrial membrane and are responsible for the oxidative deamination of neurotransmitters and dietary amines and are also recognized as a major source of ROS in experimental models of heart failure (121). In high concentrations, ROS cause irreversible modifications to proteins, DNA, and lipids (Fig. 10B) (31, 62, 140, 286).
A. Protein modifications
Several amino acids, including methionine, cysteine, arginine, histidine, and proline, are extremely vulnerable to HO• radical damage (22, 293). HO• has no specificity as it reacts with almost every organic molecule with rate constants near the limit of diffusion, whereas H2O2 specifically oxidizes thiols through a two-electron nucleophilic substitution reaction (73). Protein oxidation modifies proteins' chemical and physical environment, such as their solubility, structure, conformation, as well as their function. Oxidative modification of amino residues is known to result in protein fragmentation due to increased susceptibility to proteolysis, but additionally is able to create protein–protein crosslinks, preventing formation of protein aggregates and degradation (22, 293). Importantly, protein oxidation disrupts myofilament protein enzyme activity, including those involved in myofilament contraction, Ca2+-reuptake, ATP regeneration, and others (17, 175). One emerging class of reversible protein redox modifications occurs at the thiol sidechain of cysteine residues, which can produce multiple chemically distinct alterations to proteins (e.g., s-glutathionylation and protein disulfide formation) (31, 269). A class of irreversible redox modifications include protein carbonylation and tyrosine nitration (forms protein nitrotyrosine), which can affect myofilament contractile function (e.g., Ca2+-sensitivity of myofilaments; Fig. 9) (26, 240). Protein oxidation may also affect protein function indirectly by interfering with protein phosphorylation, which by itself is a highly important post-translational modification involved in cellular function (240).
It has been demonstrated that nitroxyl (HNO), the one-electron-reduced form of nitric oxide, exerts a direct effect on myofilament proteins increasing myofilament Ca2+ responsiveness by promoting disulfide bond formation between critical cysteine residues (76). HNO is highly thiophilic, binding directly to thiols and thiol-containing proteins and leading to their oxidation. Evidence suggests that the next-generation HNO donors have therapeutic potential in the management of acute decompensated heart failure, by exerting robust enhancement of LV function and vasodilator effects, together with powerful ROS-suppressing and antiremodeling actions (129). Whether the increase in myofilament Ca2+-sensitivity induced by cardiac oxidative protein modifications is beneficial or detrimental for the heart will depend on the underlying cause and the clinical phenotype of heart disease.
B. DNA damage
DNA is particularly sensitive to oxidative damage by the HO• radical, which is able to cause base alterations in DNA or cause DNA strand breaks (62, 140). In specific, conversion of guanine residues to 8-oxo-7-hydrodeoxyguanosine (8-oxodG) by the HO• radical is frequently observed; the addition of HO• to the guanine backbone interferes with polymerase function during replication and/or transcription (Fig. 10C). Cellular levels of 8-oxodG are used as a marker to estimate the amount of oxidative damage to cells (140). Under (patho)physiological conditions, oxidative damage to mitochondrial DNA (mtDNA) occurs at a higher rate than that of nuclear DNA (227). This is largely explained by the fact that mtDNA lacks the same repair mechanisms available for nuclear DNA, but also mtDNA's close position to mitochondrial ROS production sites makes it a more vulnerable target for ROS (227). Cardiomyocytes are postmitotic and mtDNA mutations or alterations are known to accumulate in nondividing cells during a lifetime, leading to mitochondrial dysfunction and energetic imbalance (251).
C. Lipid damage
ROS-induced damage to lipid membranes, including the sarcolemma, nucleus, SR, and mitochondrial membranes, leads to the formation of lipid radicals and lipid peroxides—lipid peroxidation (Fig. 10D) (286). Malondialdehyde (MDA) and 4-hydroxyalkenal (4-HAE) are generated from the end-degradation products of lipids and are frequently used as blood and urine markers of lipid radical damage (Fig. 10D) (286). In addition, isoprostanes are biomarkers of lipid peroxidation and specific end products of the peroxidation of polyunsaturated fatty acids.
The two most prevalent ROS that can profoundly affect lipids are the HO• and hydroperoxyl (13). Although it has been reported that H2O2 and O2•− are essentially unreactive with lipids (91), this does not mean that production of these ROS species does not play an important role in lipid peroxidation. ROS are unstable, and electron transfer to O2 generates O2•−, which progressively forms H2O2, HO•, and eventually water (H2O) (Fig. 10A). Therefore, although lipids react mainly with HO•, production of H2O2 and O2•− in the cell can eventually lead to lipid peroxidation.
Lipid peroxidation changes the lipid bilayer molecular structure and fluidity, causing its disruption, potentially increasing cardiomyocyte permeability to ions and water (Fig. 10D). Along these lines, lipid peroxidation is able to destabilize the inner mitochondrial membrane integrity, feedforwarding additional electron leakage and ROS formation. A unique type of phospholipid is exclusively found at the mitochondrial membrane of mammals—cardiolipin—comprising up to 20% of the total phospholipid content of the inner mitochondrial membrane with minor enrichment at the outer mitochondrial membrane (∼3%) (44, 110, 192). Cardiolipin is needed to restructure the electron transport chain complexes into supercomplexes to improve electron transfer, and promotes in the process membrane curvature of the inner mitochondrial membrane (Fig. 11B) (198, 245). However due to its high (poly)unsaturated fatty acid content, cardiolipin is highly vulnerable to lipid peroxidation (Fig. 10D), which reduces formation of the supercomplexes, and lowers the affinity of cytochrome C (and potentially also of CoQ) to the inner mitochondrial membrane (Fig. 11C) (245). Cytochrome C release from the mitochondrial intermembrane compartments into the cytosol activates the apoptotic cell death program (102).
Antioxidant enzymes, including catalase (CAT), superoxide dismutases (SODs), and glutathione peroxidase (GPX), scavenge ROS and regulate overall ROS levels to maintain physiological homeostasis. Other defense mechanisms against oxidative stress are reduced glutathione (GSH) and NADH. Both beneficial and harmful effects of ROS largely depend on the intracellular site, type, and amount of ROS production as well as the activity of endogenous antioxidant defense systems.
XI. Oxidative Stress Markers in the Heart and Serum of HCM Patients
Various studies have evaluated markers of cardiac oxidative stress in HCM patients during the last decade. In 2005, Nakamura et al. (188) provided the first evidence for an altered cardiac redox balance in HCM patients. The oxidative stress marker 4-hydroxy-2-nonenal (4-HNE)-modified protein, a marker for lipid peroxidation, was identified via immunohistochemistry in cardiomyocytes in all endomyocardial biopsies from the right ventricular (RV) side of the septum from HCM patients. 4-HNE is an end-degradation product of phospholipid peroxidation (a species of 4-HAE peroxidative products; Fig. 10D), which is able to react and modify proteins forming a 4-HNE-modified protein (236). The level of 4-HNE-modified protein in HCM was significantly higher compared to age-matched controls (Table 1) (188). The levels of 4-HNE-modified protein differed (Table 1) among HCM patient subgroups (nonobstructive, HOCM, and systolic dysfunction). Levels were highest in patients with systolic dysfunction and correlated with LV dilatation, indicating that this oxidative stress marker of lipid peroxidation correlated with disease stage.
Table 1.
Oxidative Stress Markers in Hypertrophic Cardiomyopathy Patients
| R | Marker | Group [sample number]: value (percentage of control) | Tissue, genotype | Age (yrs) of control vs. (all) patient group |
|---|---|---|---|---|
| (188) | HNE-modified protein | Ctrl [10]: 3548 μm2 (100%) | Heart, UK | 55 ± 8 vs. 60 ± 9 |
| All HCM [31]: 16,753 μm2 (472%)* | ||||
| HNCM [12]: 10,825 μm2 (305%)* | ||||
| HOCM [11]: 15,454 μm2 (436%)* | ||||
| HCM-SD [8]: 27,431 μm2 (773%)* | ||||
| (135) | 8-oxodG -positive cardiomyocytes | Ctrl [14]: ∼10% (100%) | Heart, UK | 54 ± 17 vs. 61 ± 11 |
| HCM [21]: ∼21% (210%)* | ||||
| (61) | 8-isoprostaglandin F2α | Ctrl [54]: 29.9 pg/mL (100%) | Serum, UK | 45 ± 13 vs. 47 ± 15 |
| All HCM [54]: 35.4 pg/mL (118%)* | ||||
| HNCM [33]: 31.4 pg/mL (105%) | ||||
| HOCM [21]: 41.6 pg/mL (139%)+ | ||||
| (155) | GSH/GSSG ratio | Ctrl [13]: ∼2.0 (100%) | Heart, 1 homozygous TNNT mutation, others UK | 44 ± 12 vs. 43 ± 14 |
| CM [14]: ∼0.5 (25%)* | ||||
| Carbonyl content | Ctrl [13]: ∼0.15 nmol/mg (100%) | |||
| CM [14]: ∼0.6 nmol/mg (400%)* | ||||
| MDA + HAE content | Ctrl [13]: ∼15 nmol/mg of protein (100%) | |||
| CM [14]: ∼22 nmol/mg of protein (147%)* | ||||
| (201) | HNE-modified protein | Ctrl [5–6]: 100% | Heart, MYBPC3, TNNT2, or TPM1 mutations in 12/22, 7 UK | 59 ± 4 vs. 49 ± 3* |
| HCM [5–6]: ∼132% | ||||
| Protein carbonyls | Ctrl [5–6]: 100% | |||
| HCM [5–6]: ∼140% | ||||
| (199) | MDA concentration | Ctrl [10]: ∼1.6 nmol/mg (100%) | Heart, UK | 43 (17–72) vs. 45 (36–59) |
| HCM [10]: ∼1.5 nmol/mg (94%) | ||||
| Total carbonylated proteins | Ctrl [10]: ∼1.0 (100%) | |||
| HCM [10]: ∼1.2 (120%) | ||||
| (39) | iNOS expression | Ctrl [18]: 1.8 ± 1.2 Pi (100%) | Heart, UK | 48 ± 8 vs. age matched |
| HCM [18]: 33.0 ± 11.1 Pi (1833%)* | ||||
| Nitrotyrosine levels | Ctrl [18]: 1.7 ± 1.3 Pi (100%) | |||
| HCM [18]: 28.7 ± 15.3 Pi (1688%)* |
Oxidative stress markers in the heart or serum of HCM patients and control groups. Significantly different compared to controls (*), hypertrophic obstructive cardiomyopathy (HOCM) significantly different compared to hypertrophic nonobstructive cardiomyopathy (HNCM; +, both groups were not compared to control). A (∼) sign indicates that the value of the marker had to be estimated from the figures. All HCM, average of all subgroups; CM, cardiomyopathy group of seven HCM patients and seven patients with other cardiomyopathies.
8-oxodG, 8-oxo-7-hydrodeoxyguanosine; Ctrl, control; GSH/GSSG, reduced glutathione/oxidized glutathione; HAE, hydroxyalkenals; HCM, hypertrophic cardiomyopathy; HCM-SD, HCM with systolic dysfunction; HNE, hydroxynonenal; iNOS, inducible nitric oxide synthase; MDA, malondialdehyde; Pi, percentage of positive pixels on total number of pixels; R, reference; UK, unknown.
Koda et al. (135) reported a significant increase in the percentage of cardiomyocyte nuclei that contained 8-oxodG, a marker of oxidative stress damage to DNA, in LV endomyocardial biopsies from HCM patients compared to nonhypertrophic controls (Table 1). A strong correlation was found between the incidence of myocytes immunopositive for 8-oxodG and the DNA repair enzymes proliferating cell nuclear antigen and redox factor 1, following immunohistochemistry staining of cardiomyocyte nuclei (135). The latter suggests increased nuclear DNA oxidation in HCM cardiomyocytes.
Oxidative stress in serum of HCM patients with preserved LV systolic function was identified by Dimitrow et al. (61). Serum level of 8-isoprostaglandin F2α (8-iso-PGF2α), a stable marker of oxidative stress produced on nonenzymatic lipid peroxidation, was significantly elevated in HCM compared with controls (Table 1) (61). HOCM patients displayed higher 8-iso-PGF2α levels compared with the non-HOCM subgroup. Anatomic (mitral-septal distance) and hemodynamic (subaortic gradient) indexes of LVOT obstruction correlated with 8-iso-PGF2α levels. This highlights that HCM is also characterized by systemic oxidative stress, with highest levels in HOCM patients.
In 2015, Lynch et al. (155) reported an increase in protein oxidative stress markers in the hearts of cardiomyopathy patients compared with control hearts. The cardiomyopathy group included seven patients with HCM (six HOCM), and seven patients with other nonhypertrophic cardiomyopathies (e.g., severe ischemia, unknown cause, RV heart failure with Uhl's syndrome). The ratio of reduced GSH to oxidized GSH (GSSG), a protein oxidative stress marker, was significantly decreased in the cardiomyopathy group. In addition, the protein carbonyl content, a general indicator of protein oxidation, was significantly elevated in the cardiomyopathy group (Table 1). Finally, the lipid peroxidation markers MDA and 4-HAE (Fig. 10D) were significantly higher in the cardiomyopathy group (Table 1) (155). The same year, Chimenti et al. (39) found a significant increase in iNOS and nitrotyrosine levels in cardiomyocytes from LV endomyocardial biopsies of HCM patients compared with control (Table 1). The elevated iNOS expression may have contributed to the increased cardiac nitrotyrosine levels in HCM as O2•− reacts with increased tissue levels of NO and forms the strong oxidant peroxynitrite, which is a reactive nitrogen species (RNS) that triggers nitrotyrosine formation through nitration of protein tyrosine residues (39).
Not all studies reported a significant increase in cardiac oxidative stress markers in HCM. Although Predmore et al. (201) showed that protein carbonyls and 4-HNE protein levels were ∼30–40% higher in HCM samples from the intraventricular septum compared with nonfailing samples (Table 1), the difference did not reach statistical significance (201). A limitation of this study is the significantly younger age of the HCM patients compared with the control group (Table 1), since aging has been associated with higher levels of oxidative stress markers in different tissues of healthy people [reviewed here (265)]. Likewise, Pisano et al. (199) found no significant differences in cardiac protein carbonylation (although ∼20% higher in HCM) and lipid degradation products in HOCM compared to nonfailing hearts (Table 1) (199). A limitation of the latter study is that 3 out of 10 HCM patients had hypertension as associated disease.
In summary, a significant increase in oxidative stress markers, including damage to proteins, DNA, and lipids, has been revealed in the heart (39, 135, 188) and serum (61) of HCM patients, with a trend to higher levels in more severe HCM phenotypes (Table 1) (61, 188). Contrasting findings between the studies might be explained by differences in group size, source of myocardial samples, and sensitivity of oxidative stress markers, and the heterogeneity of the patients' clinical and genetic background. Many HCM patients in the studies described above were not genetically tested for sarcomeric gene mutations (Table 1) (39, 61, 135, 188, 199). In addition, no comparisons were made to sarcomere mutation-negative samples with respect to oxidative stress markers. Future studies on oxidative stress markers (proteins, DNA, and lipids) are warranted in genetically and phenotypically well-characterized HCM patients and controls, to identify if sarcomeric mutations affect ROS levels in the heart and serum of HCM patients. Moreover, studies on oxidative stress markers in the blood should be extended to G+/Ph− mutation carriers. These studies may identify a disturbed redox balance as an early factor in sarcomeric HCM pathology.
XII. Oxidative Stress Markers in HCM Animal Models
Based on studies of human samples, which are usually obtained at one time point and at an advanced disease stage, it is difficult to establish oxidative stress as a causal factor in HCM pathology. In this section, we discuss the studies that evaluated markers of oxidative stress in animal models of HCM due to sarcomeric mutations.
A. MYH mutations
Effects of MYH mutations on oxidative stress markers have been evaluated in different species. Transgenic rabbits, 18 months old, with cardiac-restricted expression of β-MyHC-R403Q showed a significant increase in LV mass and wall thickness compared with nontransgenic (NTG) rabbits (222). The hearts of the β-MyHC-R403Q rabbits showed a significant increase in lipid peroxidation degradation products (MDA and 4-HAE) and a nonsignificant increase (27%) in oxidized mtDNA (8-oxodG mtDNA) compared with NTG rabbits (Table 2) (222). The levels of 8-oxodG nuclear DNA were not affected in β-MyHC-R403Q rabbits. In a later study, a significant increase was also reported in oxidized GSH to total GSH ratio (protein oxidation) in the heart and blood of 3-year-old β-MyHC-R403Q rabbits (Table 3) (150).
Table 2.
Cardiac Oxidative Stress in Hypertrophic Cardiomyopathy Animal Models
| HCM | Marker | Values (percentage of control) | R |
|---|---|---|---|
| β-MyHC-R403Q rabbits | MDA +4-HAE | NTG: 4.51 ± 0.9 μM/g of protein (100%) | (222) |
| β-MyHC-R403Q: 9.04 ± 6.1 μM/g of protein (200%)* | |||
| 8-oxodG mitochondrial DNA | NTG: 100% | ||
| β-MyHC-R403Q: 127% (p = 0.08) | |||
| 8-oxodG nuclear DNA | NTG: 100% | ||
| β-MyHC-R403Q: ∼83% | |||
| mutant rat α-MyHC mice | ROS/RNS | Wild-type: ∼1.7 nM (100%) | (157) |
| HCM: ∼1.9 nM (112%) | |||
| TBARS | Wild-type: ∼0.68 μM (100%) | ||
| HCM: ∼0.76 μM (112%) | |||
| cMyBP-C -A31P cats (4 of 9 cats) and 1 wild-type negative | Mt H2O2 release, state 3 with CI-LS | Control: ∼0.037 nmol/min/mg Mt protein (100%) | (41) |
| HCM: ∼0.065 nmol/min/mg Mt protein (176%)* | |||
| Mt H2O2 release per O2 consumption, state 3 with CI-LS | Control: ∼0.016% (100%) | ||
| HCM: ∼0.058% (363%)* | |||
| Mt H2O2 release, state 3 with CII-LS | Control: ∼0.125 nmol/min/mg Mt protein (100%) | ||
| HCM: ∼0.120 nmol/min/mg Mt protein (96%) | |||
| TBARS | Control: ∼46 nmol/g tissue (100%) | ||
| HCM: ∼71 nmol/g tissue (154%)* | |||
| cTnT-R92Q mice | MDA (TBARS) | NTG: ∼0.15 nmol/g (100%) | (38) |
| cTnT-R92Q: ∼0.25 nmol/g (167%)* | |||
| 4-HNE protein levels | NTG: ∼0.24 band intensity/GAPDH (100%) | ||
| cTnT-R92Q: ∼0.55 band intensity/GAPDH (229%)* | |||
| NADPH oxidase 4 protein levels | NTG: ∼0.25 band intensity/GAPDH (100%) | ||
| cTnT-R92Q: ∼0.49 band intensity/GAPDH (196%)* | |||
| cMyBP-C KI mice | cMyBP-C S-glutathiolynation | Wild-type: ∼1.0 (100%) | (72) |
| KI-HCM: ∼6.0 (600%)* | |||
| cTnT-I79 N, cTnT-R278C mice | Cytosolic protein carbonylation | Wild-type: 100% | (82) |
| cTnT-I79 N: ∼129%* | |||
| cTnT-R278C: ∼84%* | |||
| Total heart protein carbonylation | Wild-type: 100% | ||
| cTnT-I79 N: ∼123% | |||
| cTnT-R278C: ∼109% |
Cardiac oxidative stress markers in HCM animal models due to sarcomeric gene mutations. Significantly different compared to control (*). A (∼) sign indicates that the value of the marker had to be estimated from the figures.
4-HAE, 4-hydroxyalkenals; 4-HNE, 4-hydroxynonenal; cMyBP-C KI mice, mice carrying a G > A transition on the last nucleotide of exon 6 (c.772G>A); CI-LS, complex I-linked substrates; Mt, mitochondrial; NTG, nontransgenic; R, reference; ROS/RNS, reactive oxygen/nitrogen species; TBARS, thiobarbituric acid-reactive substances.
Table 3.
N-Acetylcysteine Treatment in Hypertrophic Cardiomyopathy Animal Models
| HCM | Marker | Values (percentage of control) | Tissue | R |
|---|---|---|---|---|
| cTnT- R92Q mice | MDA +4-HAE | NTG: 0.21 ± 0.07 nmol/g (100%) | Heart | (161) |
| cTnT-R92Q: 0.22 ± 0.03 nmol/g (105%) | ||||
| cTnT-R92Q NAC: 0.13 ± 0.01 nmol/g (62%)# | ||||
| NTG: 0.049 ± 0.005 nmol/mL (100%) | Plasma | |||
| cTnT-R92Q: 0.048 ± 0.009 nmol/mL (98%) | ||||
| cTnT-R92Q NAC: 0.030 ± 0.014 nmol/mL (61%)# | ||||
| 8-oxodG nuclear/Mt DNA | No values reported: no significant differences between NTG, R92Q, and R92Q NAC mice | Heart | ||
| β-MyHC-R403Q rabbits | GSSG/GSH+GSSG | NTG: 0.05 ± 0.03 (100%) | Heart | (150) |
| β-MyHC-R403Q: 0.17 ± 0.01 (340%)* | ||||
| β-MyHC-R403Q NAC: 0.06 ± 0.02 (120%)# | ||||
| NTG: 0.06 ± 0.02 (100%) | Plasma | |||
| β-MyHC-R403Q: 0.13 ± 0.07 (217%)* | ||||
| β-MyHC-R403Q NAC: 0.05 ± 0.04 (83%)# | ||||
| Relative levels of glutathiolated α-actin | NTG: 1.00 ± 0.00 (100%) | Heart | ||
| β-MyHC-R403Q: 0.66 ± 0.17 (66%)* | ||||
| β-MyHC-R403Q NAC: 0.98 ± 0.17 (98%)# | ||||
| Tm-E180G mice | cMyBP-C S-glutathionylation | NTG: ∼0.1 A.U. (100%) | Heart | (277) |
| Tm-E180G: ∼0.68 A.U. (680%)* | ||||
| Tm-E180G NAC: ∼0.1 A.U. (100%)# | ||||
| Tm S-glutathionylation | NTG: ∼1.2 A.U. (100%) | |||
| Tm-E180G: ∼1.7 A.U. (142%) | ||||
| Tm-E180G NAC: ∼0.7 A.U. (58%)# | ||||
| MyHC carbonylation | NTG: ∼4.8 × 106 A.U. (100%) | |||
| Tm-E180G: ∼1.6 × 107 A.U. (333%)* | ||||
| Tm-E180G NAC: ∼1.1x107A.U. (229%)*,# | ||||
| Actin, cTnT, Tm carbonylation | No significant differences between NTG, Tm-E180G, and Tm-E180G NAC mice |
Effects of N-acetylcysteine (NAC) treatment on oxidative stress markers in the heart or serum of animal models of hypertrophic cardiomyopathy due to sarcomeric gene mutations. Significantly different compared to control (*), significantly different compared to cTnT-R92Q, β-MyHC-R403Q, or Tm-E180G without NAC treatment/placebo (#). A (∼) sign indicates that the value of the marker had to be estimated from the figures. GSSG/GSH+GSSG, relative levels of oxidized to total glutathione.
MyHC, myosin heavy chain; R, reference; Tm, tropomyosin.
Magida and Leinwand (157) investigated the levels of the lipid peroxidation marker thiobarbituric acid-reactive substances (TBARS) in HCM hearts from a mouse model expressing a rat α-MyHC-R403Q mutation in combination with a deletion of 59 amino acids bridged by the addition of 9 nonmyosin amino acids. Male mice, 12 months old, displayed an end-stage HCM phenotype with contractile dysfunction and chamber dilation. No significant differences were found for overall RNS and ROS by TBARS in the LV between 12- and 15-month-old α-MyHC-R403Q and WT mice, although both markers were slightly increased (∼12%, Table 2) in HCM.
B. MYBPC3 mutations
Christiansen et al. (41) studied mitochondrial oxidative phosphorylation capacity and mitochondrial ROS production in cardiac muscle from domestic cats with HCM due to MYBPC3 mutations (Table 2) (41). HCM cats, ∼7 years old, displayed LV hypertrophy and a significantly higher ejection fraction compared with age-matched control cats, resembling the human HCM phenotype. HCM cats showed impaired myocardial mitochondrial oxidative phosphorylation capacity in response to nonfatty and fatty acid substrates. Both mitochondrial ROS release and cardiac TBARS were increased in HCM cats (Table 2) (41). These changes were specific to the heart, since no significant differences were found in soleus skeletal muscle between groups.
As indicated above, increased protein oxidation may impair cardiac function via post-translational protein modifications. Flenner et al. (72) studied the redox modification S-glutathiolation of cMyBP-C in Mybpc3-targeted knockin mouse model (cMyBP-C KI) of HCM. These mice carry a G > A transition on the last nucleotide of exon 6 (c.772G>A), which is associated with a bad prognosis in humans. cMyBP-C KI mice display LV hypertrophy, reduced systolic and diastolic function, and a higher myofilament Ca2+-sensitivity (74, 172, 173). S-glutathionylated cMyBP-C levels (normalized to total cMyBP-C level, which was in cMyBP-C KI ∼17% of the cMyBP-C WT level) were approximately sixfold higher in ventricles from ∼8-month-old cMyBP-C KI than in WT mice. These studies show that mutations in cMyBP-C affect the cardiac redox balance and that an increased mitochondrial ROS production may contribute to the disease progression in HCM.
C. Tnnt2 mutations
Compared with NTG sham mice, a significant increase in myocardial MDA and 4-HNE content and NOX4 (Table 2) was found in 32-week-old sham-operated female cTnT-R92Q transgenic mice with cardiac-restricted expression of a human HCM TNNT2 mutation (R92Q) (38). Sham operations were performed because these groups served as controls for ovariectomized mice (discussed below under estrogen treatment). Impaired diastolic function, higher LV systolic performance, reduced myocardial ATP levels (ADP levels were not quantified), and mitochondrial respiratory function were observed in cTnT-R92Q sham mice compared with NTG sham mice (38). Marian et al. (161) did not find changes in cardiac lipid peroxidation (MDA and 4-HAE) and oxidized nuclear- and mtDNA (Table 3) in 15-month-old cTnT-R92Q transgenic mice, which displayed increased LV systolic performance, cardiac fibrosis, and myocyte disarray. The different observations in cTnT-R92Q mice may be explained by differences in age and gender, control groups (sham-operated versus nonoperated control group), and markers/assays used to determine oxidative stress.
Gilda et al. (82) recently studied the levels of oxidized proteins in two transgenic HCM mouse models with different Tnnt2 mutations (I79 N and R278C). The cTnT-I79 N mutation is known to increase myofilament Ca2+-sensitivity and is associated with a high risk of sudden cardiac death, whereas the cTnT-R278C mutation does not alter myofilament Ca2+-sensitivity and is associated with a milder, late-onset phenotype (103, 181). Major pathways affected in 3-month-old cTnT-I79 N hearts relative to cTnT-R278C and WT hearts were the antioxidant systems, ubiquitin/proteasome system (UPS), and energy production pathways (82). The UPS is the primary cellular selective proteolytic system that targets damaged, misfolded, and mutant proteins for degradation by the ubiquitination machinery and proteolysis by the proteasome. The core subunit of the proteasome (20S) also degrades oxidized proteins (226). Mild or transient oxidative stress upregulates UPS activity, whereas severe or sustained oxidative stress impairs UPS function (226). Levels of oxidized cytosolic protein (protein carbonylation, Table 2) and of ubiquitinated proteins were significantly increased in cTnT-I79 N mice relative to WT and cTnT-R278C mice (82). Despite higher proteasome subunit expression in cTnT-I79 N than cTnT-R278C hearts, both 20S and 26S chymotrypsin-like activities were decreased. This finding was consistent with proteasome impairment in cTnT-I79 N mice relative to WT and cTnT-R278C mice (82). These studies show that the cardiac redox balance is affected in HCM animal models with Tnnt2 mutations and may indicate a link between high myofilament Ca2+-sensitivity and oxidative modifications.
D. Tpm1 mutations
cMyBP-C S-glutathionylation and MyHC carbonylation (Table 3), markers of protein oxidation, were significantly increased in myofilaments of transgenic mice with a Tpm1 mutation (Tm-E180G), which display a phenotype similar to human HCM (277). Tm-E180G mice, 2 months old, show an increased LV mass, diastolic dysfunction, and increased myofilament Ca2+-sensitivity compared with NTG mice (277). Interestingly, the six to seven times higher cMyBP-C S-glutathionylation in Tm-E180G mice (Table 3) is comparable with the increase found in cMyBP-C KI mice (Table 2) (72, 277). No significant difference was found in Tm S-glutathionylation, although levels were ∼42% higher in Tm-E180G than in NTG mice (Table 3). Sasagawa et al. (216) established downregulation of glutathione S-transferase kappa 1 (Gstk1) as a common mechanism underlying HCM, possibly through increasing oxidative stress and increasing the expression of HCM marker genes. This was described as a general hypertrophic response, since it was found not only in mice with mutations in Tpm1 (Tm-E180G mice) and Myh6 but also in mice with transverse aortic constriction (a model of LV pressure overload).
To conclude, oxidative stress markers are increased in rabbit (MYH), cat (MYBPC3), and mouse (Myh, Mybpc3, Tnnt2, Tpm1) models of HCM (38, 41, 72, 82, 150, 222, 277). Levels of lipid peroxidation and degradation products are increased in HCM animal models (38, 41, 222), which might have deleterious effects for the heart (e.g., cellular and organelle membrane disruption, Fig. 10) (124). Oxidative modifications of myofilament proteins are also detected in HCM animal models (72, 277), which may underlie diastolic dysfunction (detailed in section XIII) (277). In contrast to HCM patients (135), no significant increase was found in nuclear DNA oxidation in animal models with HCM (161, 222), in opposition with a trending elevation of mtDNA oxidative damage (222). The latter is likely the resulting increase of mitochondrial ROS formation observed in HCM animal models (38, 41), which may contribute to the disturbed cardiac redox state identified in HCM (38).
XIII. Oxidative Stress as a Target for Therapy in HCM Animal Models
Antioxidant treatment to prevent or reverse HCM due to sarcomeric mutations has been evaluated in three studies, all using the antioxidant N-acetylcysteine (NAC) dissolved in drinking water. Although NAC is rapidly absorbed following an oral dose, only a small percentage of the intact NAC molecule arrives in the plasma and subsequently in tissue (128). An extensive first pass metabolism by the cells of the small intestine and the liver results in the incorporation of NAC into protein peptide chains and the formation of a variety of metabolites of NAC (128). NAC is a thiol, an ROS scavenger, and a precursor of L-cysteine and GSH, the largest intracellular thiol pool (10, 288). NAC has been used in therapeutic practices for several decades as a mucolytic agent (class of drugs that help mucus clearance) and for the treatment of numerous disorders, including paracetamol intoxication (64).
Marian et al. (161) treated cTnT-R92Q mice, ∼9 months old, for 24 weeks with a placebo or NAC. NAC treatment exerted positive effects, while the placebo-treated cTnT-R92Q mice displayed increased LV systolic performance, cardiac fibrosis, and myocyte disarray. Increased cellular ROS can lead to the activation of the mitogen-activated protein kinase (MAPK) family, which consists of the extracellular signal-regulated protein kinases (ERK), the c-Jun N-terminal protein kinases (JNK), and the p38 MAPK (169, 235). A significant increase in MAPK signaling levels of phosphorylated (activated) p44/42, p38, and JNK MAPKs was found in placebo-treated cTnT-R92Q mice compared with NTG mice (161). NAC treatment significantly reduced lipid peroxidation (Table 3) in the heart and plasma, and exerted antifibrotic effects. NAC reduced the expression levels of mRNAs encoding procollagen and the myocardial collagen volume fraction, which is an index for the measurement of interstitial collagen (161). Interestingly, NAC treatment also significantly reduced the levels of phosphorylated (activated) p44/42, p38, and JNK MAPKs, suggesting that NAC restored the cardiac redox balance in cTnT-R92Q mice.
Lombardi et al. (150) studied if NAC treatment could reverse established cardiac fibrosis and hypertrophy in the β-MyHC-R403Q rabbit model. β-MyHC-R403Q rabbits, 2-year old, display cardiac hypertrophy and interstitial fibrosis with preserved global systolic function (150). Two-year-old β-MyHC-R403Q rabbits were treated with NAC or placebo for 12 months. After 1 year, β-MyHC-R403Q rabbits in the placebo group displayed cardiac hypertrophy, fibrosis, systolic dysfunction, and an increase in oxidized-to-total GSH ratio in the heart and blood compared with NTG rabbits (Table 3). In addition, myocardial phosphorylated (active) p38 and dephosphorylated (active) nuclear factor of activated T cells (NFATc1) were significantly higher in placebo-treated β-MyHC-R403Q rabbits compared with NTG rabbits. A recent study identified RNA binding protein human antigen R as a novel mediator of cardiac hypertrophy downstream of the p38 MAPK pathway, and suggested modulation of NFAT activity as a potential mechanism (231). NAC treatment restored oxidized-to-total GSH ratio (Table 3) and reversed cardiac hypertrophy and interstitial fibrosis. NAC also reduced the propensity for ventricular arrhythmias and prevented systolic dysfunction. In addition, NAC reduced myocardial levels of phosphorylated p38 and reduced the prohypertrophic active (dephosphorylated) NFATc1 levels to NTG levels. This study illustrates that NAC treatment even reverses established HCM, most likely via thiol-sensitive mechanisms.
Wilder et al. (277) investigated whether oxidative modifications of sarcomeric proteins are responsible for diastolic dysfunction in a Tm-E180G HCM mouse model. This was based on previous studies in a hypertensive mouse model showing diastolic abnormalities of cardiac myofilaments (i.e., slower actin–myosin cycling kinetics and increased myofilament Ca2+-sensitivity by ROS-induced cMyBP-C S-glutathionylation) without alterations of Ca2+-handling (118, 151, 229). At 2 weeks of age, Tm-E180G mice already exhibit cardiac hypertrophy, interstitial fibrosis, and diastolic dysfunction with preserved systolic function (7). One-month-old Tm-E180G mice and NTG littermates were treated for 30 days with NAC. NAC reversed baseline diastolic dysfunction and hypertrophy in Tm-E180G mice (277). The significantly increased cMyBP-C S-glutathionylation and MyHC carbonylation in Tm-E180G mice were entirely or partly reversed to NTG levels by NAC, respectively (Table 3). NAC also reduced myofilament Ca2+-sensitivity and normalized (reduced) phosphorylation of phospholamban in Tm-E180G mice to nearly NTG levels. Interestingly, phosphorylation (activation) of ERK1/2 was increased in hearts from Tm-E180G mice and returned to NTG levels by NAC treatment. The authors concluded that oxidative myofilament modifications mediated diastolic function, which was evident from the fact that relieving this modification reversed established diastolic dysfunction and hypertrophy in HCM.
These studies show that an altered redox state significantly contributes to the HCM phenotype and that antioxidant treatment appears to be a promising therapeutic approach for targeting the underlying pathophysiology of HCM due to a sarcomeric gene mutation (150, 161, 277). Importantly, MAPK signaling was activated in all HCM animal models in the placebo-treated group, and returned to NTG levels on NAC treatment (150, 161, 277). Since activation of MAPK has been implicated as a mediator of fibrosis and hypertrophy (83, 127, 145, 231, 247, 284), NAC treatment might have prevented or reversed fibrosis and hypertrophy by restoring the cardiac redox balance and lowering MAPK and NFATc1 activation (182, 231). Therefore, NAC might exert its beneficial effects by lowering cardiac ROS levels and preventing excessive levels of sarcomeric protein oxidation (associated with diastolic dysfunction) and MAPK activation (associated with fibrosis and hypertrophy). Because the functional mechanisms of NAC in clinical applications are not fully understood (64), some caution is warranted in interpretations about the mechanisms underlying its beneficial effects in HCM.
Besides the expected effects of NAC to reduce extracellular cystine to cysteine, in vitro and in vivo evidence indicates that NAC is able to enhance the intracellular biosynthesis of GSH (128). GSH effectively scavenges free radicals and other ROS (HO•, lipid peroxyl radical, O2•−, and H2O2) directly and indirectly through enzymatic reactions (9). NAC is a thiol (sulfhydryl containing) compound that also directly scavenges ROS (HO• and H2O2) and hypochlorous acid, whereas NAC is a fairly poor scavenger of O2- (10). NAC also possesses a reducing property through its thiol/disulfide exchange activity involving the direct interaction with target proteins with a cysteine residue or a thiol group (242). It has been shown that NAC, in contrast to other ROS scavengers, directly binds to proteasome inhibitors and inactivates their proteasome inhibitory function (90). Since NAC exerts so many different effects, additional approaches such as overexpression of antioxidant enzymes, stimulating endogenous antioxidants, treatment with other antioxidant compounds (including thiol and nonthiol antioxidants) or with agents that prevent excessive ROS formation should define how NAC exerts its beneficial effects in HCM animal models.
XIV. Antioxidant Enzymes in HCM Pathology
The altered redox state in HCM pathology may be primarily caused by an increase in cardiac ROS production. However, the reduced capacity of endogenous antioxidants to neutralize ROS elevations may secondarily contribute to the impaired redox state. In this section, we outline the studies that reported on the expression and activity of antioxidant enzymes in hearts from HCM patients and HCM animal models due to sarcomeric gene mutations.
In HOCM patients, Pisano et al. (199) found a significantly lower CAT activity compared to nonfailing hearts (Table 4), whereas GPX or mitochondrial Mn-SOD (SOD2) activity was unchanged (Table 4). Interestingly, identical changes in enzyme activity were previously reported in a pig model of naturally occurring HCM compared with control pigs (148). These changes are also in accordance with a 4.5-fold reduction in cardiac CAT mRNA levels, and no change in GPX1 mRNA levels (Table 4) in 18-month-old β-MyHC-R403Q rabbits with cardiac hypertrophy and an increase in cardiac lipid peroxidation (Table 2) compared with NTG rabbits (222). CAT enzyme activity was 10% lower in rabbits carrying the β-MyHC-R403Q mutation compared with NTG rabbits, although this result was not statistically significant (Table 4) (222). A significant reduction in mitochondrial SOD2 protein level (Table 4) was found in 32-week-old cTnT-R92Q mice, which showed impaired diastolic function, increased lipid peroxidation (Table 2), and reduced mitochondrial respiratory function compared with NTG mice (38).
Table 4.
Endogenous Antioxidants in Hypertrophic Cardiomyopathy Heart
| HCM | Antioxidant | Values (percentage of control) | R |
|---|---|---|---|
| Human, no genetic testing | CAT activity | Control: ∼9.7 μmol/min/mg (100%) | (199) |
| HCM: ∼5.0 μmol/min/mg (52%)* | |||
| GPX activity | Control: ∼67.0 nmol/min/mg (100%) | ||
| HCM: ∼78.5 nmol/min/mg (117%) | |||
| SOD2 activity | Control: ∼8.8 U/mg (100%) | ||
| HCM: ∼7.3 U/mg (83%) | |||
| β-MyHC-R403Q Rabbits | CAT mRNA | NTG: onefold change (100%) | (222) |
| β-MyHC-R403Q: −4.5-fold change (22%)* | |||
| CAT protein | No difference between NTG and Q403 | ||
| CAT activity | NTG: 137.6 ± 19.6 nM/μg/min (100%) | ||
| β-MyHC-R403Q: 123.5 ± 17.3 nM/μg/min (90%) | |||
| GPX1, HMOX1 mRNA | No difference between NTG and β-MyHC-R403Q | ||
| cTnT-R92Q mice | SOD2 protein | NTG: ∼0.72 band intensity/GAPDH (100%) | (38) |
| cTnT-R92Q: ∼0.49 band intensity/GAPDH (68%)* | |||
| cTnT-I79 N, cTnT-R278C mice | Antioxidant capacity | Wild-type: ∼0.07 TEU (100%) | (82) |
| cTnT-I79 N: ∼0.14 TEU (200%)* | |||
| cTnT-R278C: ∼0.12 TEU (171%) | |||
| TRX-1 expression | Wild-type: 100% | ||
| cTnT-I79 N: ∼140%# | |||
| cTnT-R278C: ∼85% |
Endogenous antioxidant enzyme expression and activity in HCM hearts. Significantly different compared to control (*), significantly different compared to cTnT-R278C (#). A (∼) sign indicates that the value of the marker had to be estimated from the figures.
CAT, catalase; GPX, glutathione peroxidase; HMOX1, heme oxygenase 1; R, reference; SOD, superoxide dismutase; TEU, trolox equivalent units; TRX-1, thioredoxin-1.
Antioxidant systems were established as one of the major pathways affected in hearts of 3-month-old cTnT-I79 N mice relative to cTnT-R278C and WT mice (82). Total cardiac antioxidant capacity was approximately two times higher in cTnT-I79 N hearts compared with WT hearts (Table 4), together with higher levels of cytosolic carbonylated protein (Table 2). Western blotting showed a significant upregulation of the antioxidant enzyme thioredoxin (TRX)-1 in cTnT-I79 N compared with cTnT-R278C hearts (Table 4). TRX-1 is a mainly cytosolic antioxidant enzyme that reduces oxidized proteins through its ability to reduce cysteine residues, thereby altering the structure of its target proteins (152). This study shows that although the total cardiac antioxidant capacity was upregulated in relatively young mice from this HCM model with a poor prognosis, cTnT-I79 N hearts already displayed higher levels of cardiac oxidative stress.
GSH is a water-soluble tripeptide that directly neutralizes ROS due to the presence of its thiol group and breaks disulfide bridges formed inside and between proteins. GSH is also the reducing substrate for GPX in the removal of H2O2 and maintains the levels of reduced GPX. In its antioxidant actions, GSH forms an intermolecular disulfide end-product (oxidized glutathione, GSSG). GSSG can be exported from the cell or transformed back to GSH by the combined action of GSH reductase and the NADPH cofactor (209). An increased GSSG/GSH ratio (Table 3) was found in the heart and blood from β-MyHC-R403Q rabbits with established HCM (150). As a result, less of the antioxidant capacity of GSH is present in the heart, which may have contributed to the HCM phenotype. NAC is a precursor of GSH, and NAC-treatment of β-MyHC-R403Q rabbits with established HCM replenished GSH stores and reversed cardiac hypertrophy and fibrosis (150). This implies that GSH levels are important for the cardiac redox balance and function.
In summary, changes in antioxidant enzyme activity may contribute to a disturbed cardiac redox balance in HCM pathology. Interestingly, cardiac CAT activity and GSH antioxidant capacity may be reduced in HCM patients and/or HCM animal models (148, 199, 222). SOD converts O2•− into H2O2, which is converted into H2O and O2 by CAT and GPX. A reduced CAT activity and GSH antioxidant capacity may therefore reduce ROS scavenging and sensitize the HCM heart for oxidative stress. Although total cardiac antioxidant capacity may be upregulated in an early stage of HCM, this may not be sufficient to fully prevent cardiac oxidative stress. Since thioredoxin 2 (Trx2) is a major H2O2 buffer in mitochondria of the heart, the status of Trx2 in HCM is an interesting target for future investigation (238). The regulatory mechanisms of antioxidant enzyme genes in HCM remain to be explored and might shed more light on the specificity for HCM pathology. Antioxidant enzyme activity most likely depends on the stage of heart disease, and future studies should verify and extend the findings listed in Table 4 to different disease stages.
XV. How Sarcomeric Mutations and Secondary Disease Mechanisms Contribute to Excessive Levels of ROS and HCM Pathology
A relevant question, also with respect to future therapies, is how do sarcomeric mutations contribute to excessive levels of cardiac ROS and HCM pathology? Multiple (mal)adaptive changes have been identified in the HCM heart that may cause or prevent a disturbed redox balance (Fig. 12). In this section, we discuss the potential relationships between sarcomeric mutations and excessive levels of cardiac ROS in HCM pathology.
FIG. 12.
Sarcomeric mutations contribute to excessive levels of ROS and the HCM phenotype. Mutations in sarcomeric genes may affect the cardiac redox balance via multiple pathways. Sarcomeric mutations alter sarcomere function, which may affect mechanotransduction and increase energetic cost and mitochondrial activity. In addition, expression of mutant proteins may contribute to UPS dysfunction, which might result in ER stress. These processes affect the cardiac redox balance. Other mechanisms that are likely to contribute to cardiac oxidative stress in HCM are microvascular dysfunction causing local ischemia, mitochondrial dysfunction, a strong adrenergic drive, activation of the angiotensin II (Ang II)-dependent pathway, changes in antioxidant capacity, and possibly an increased xanthine oxidase activity. Excessive levels of cardiac ROS in HCM lead to oxidative modifications of sarcomeric proteins, which may contribute to diastolic dysfunction. It may also activate the MAPK family, which has been linked to cardiac fibrosis and hypertrophy. In addition, oxidation of Ca2+/calmodulin kinase II (CaMKII) may contribute to its sustained activation, which slows down L-type Ca2+ current inactivation and increases the late Na+ current amplitude. This contributes to action potential duration prolongation and related arrhythmias. Lipid peroxidation and DNA oxidation may cause cell damage and/or effect cell signaling. This shows that a disturbed cardiac redox state has a central role in HCM pathology. ER, endoplasmic reticulum; ERK, extracellular signal-regulated protein kinases; GSH, reduced glutathione; JNK, c-Jun N-terminal protein kinases; MAPK, mitogen-activated protein kinases; MyHC, myosin heavy chain; NOS, nitric oxide synthase; UA, uric acid; UPS, ubiquitin/proteasome system. Color images are available online.
A. Cardiac mechanical stress
Sarcomeric mutations may increase cardiac mechanical stress and thereby affect the cardiac redox state (Fig. 12). In the heart, cardiomyocytes contract against a mechanical load during each heartbeat, and excessive mechanical stress, in combination with the rise in systolic stress caused by high myofilament Ca2+-sensitivity, can additionally drive cardiomyopathy progression (119). The intrinsic mechanisms that enable the heart to respond and adapt to changes in preload and afterload to maintain adequate cardiac output are described by the Frank–Starling law and the Anrep effect, respectively (37). However, it is not well understood how cardiomyocytes sense the mechanical load and transduce the load information to biochemical reactions to affect cell function, a process called mechano/chemotransduction (37).
Jian et al. (119) explored the effects of mechanical stress in a cell-in-gel system, which imposes an afterload (mechanical load) during single cardiomyocyte contraction. Cardiomyocytes from cTnT-R92Q mice exhibited enhanced mechanotransduction and frequent arrhythmogenic Ca2+ sparks. Selective inhibition of neuronal NOS suppressed afterload-induced spontaneous Ca2+ activities (119). In WT cardiomyocytes, NOS and NOX2 were involved in afterload-associated mechano/chemotransduction during contraction. Increases in Ca2+ transient and sparks were attributable to increased ryanodine receptor (RyR) sensitivity (119). Prosser et al. (202) showed that physiologic stretch rapidly activates NOX2 in heart cells to produce ROS in a process dependent on microtubules. Contraction-induced shortening and relaxation alone may induce NOX2-ROS production (203). Therefore, mutation-induced changes in sarcomere function (e.g., increased Ca2+-sensitivity; Fig. 9) may alter mechano/chemotransduction and increase ROS/RNS production via sustained activation of NOS and NOX (Fig. 12).
B. Mitochondrial activation alters the cardiac redox state
Since sarcomeric mutations predispose the human HCM myocardium into a high Ca2+-sensitivity state with elevated myosin-ATPase activity (224, 225, 280), one can arguably say that the rise in systolic stress drives elevations of cellular [ADP], which is found in HCM animals (99, 117, 237). Myofilament elevations of [ADP] will boost mitochondrial ATP regeneration. Sustained mitochondrial ADP-workload stimulates ADP-stimulated state 3 respiration, which will stimulate commensurate ROS formation (Fig. 11A). In addition, premature electron leakage from the electron transport chain may also cause ROS formation in HCM, and may partly explain the high levels of cardiac ROS in HCM (38, 39, 41, 61, 72, 82, 135, 150, 188, 222, 277). Proteomic and metabolomic data suggest that cardiac energy production pathways are accelerated in cTnT-I79 N HCM mice (82). This would favor ROS formation, since mitochondrial ROS production was found to be proportional to the respiration rate in pig ventricular myocytes (48). A recent report in HCM animals (with Mybpc3 and Tnnt2 mutations) provides validation of the latter idea, where increases of ATP regeneration associated with increases in mitochondrial ROS formation (56). These studies, at least, indicate that an increased myofilament Ca2+-sensitivity with elevated/inefficient myosin-ATPase activity increases mitochondrial ROS formation in HCM (Fig. 12).
C. Mitochondrial damage
Mitochondrial damage, which results in escalating amounts of ROS (16), may also contribute to elevated cardiac ROS production in HCM. Mitochondrial damage has been identified in many HCM studies, and one can speculate the resulting damaging effects of ROS in mitochondrial proteins, DNA, and lipid membranes (Fig. 10). In cTnT-R92Q mice, abnormal mitochondrial morphology, reduced relative mtDNA content, and reduced mitochondrial respiratory function were identified (38). mtDNA encodes several proteins involved in the assembly of mitochondrial complexes I, III, IV, and V (153). mtDNA oxidative damage tends to increase in HCM animals (222), which coincided with impaired cardiac mitochondrial oxidative phosphorylation and enhanced mitochondrial ROS release (41). In addition, since lipid peroxidation and/or lipid degradation products are observed in HCM (41), disrupted mitochondrial morphology and diminished oxidative phosphorylation capacity may also result from altered mitochondrial phospholipid bilayers (see below, Peptide SS-31). Moreover, in cardiomyocytes from HCM patients, the mitochondrial number was increased, but mean cross-sectional area was reduced, together with a loss of cardiac mtDNA content (199). Altogether, this shows that mitochondrial damage is an important feature of HCM pathology. It is well known that impaired mitochondrial function, through inefficient oxidative phosphorylation, produces escalating amounts of ROS (16). Excessive levels of ROS cause progressive mitochondrial damage, which may result in a vicious cycle (16, 41).
To the best of our knowledge, mitochondrial Ca2+ levels in HCM are unknown and most likely depend on the disease stage and type of mutation. It could be hypothesized that prolongation of Ca2+ transients (e.g., in mutant MyHC transgenic mice) may result in an increase in mitochondrial [Ca2+], since mitochondria can sequester large amounts of Ca2+ from the cytosol (154). On the contrary, Coppini et al. showed an enhanced late Na+ current and reported an increased [Na+]i in HCM cardiomyocytes (46, 47). O'Rourke and colleagues have demonstrated that elevated cytosolic Na+ increases the rate of the mitochondrial Na+−Ca2+ exchanger. This would lower mitochondrial [Ca2+], by promoting mitochondrial Ca2+ efflux and decreasing the ability of mitochondria to accumulate Ca2+ during conditions of high demand (149, 156, 259). Vakrou and Abraham (259) proposed that without Ca2+-induced Kreb's cycle stimulation, NADH and NADPH become more oxidized and are unable to recharge antioxidant systems. This may lead to ROS accumulation in the mitochondrial matrix, mtDNA and lipid oxidative damage, with additional ROS diffusion into the cytosol. Altogether, impaired mitochondrial function and mitochondrial damage may contribute to the disturbed redox state and HCM pathology (Fig. 12).
D. UPS dysfunction
Mutations in genes encoding sarcomeric proteins lead to the expression of poison proteins and truncated proteins (168, 261). Mutated and oxidized proteins, in addition to misfolded, damaged, and normal short-lived proteins, are degraded by the UPS (226). The ability to maintain a functional proteome declines during aging (264). Therefore, mutated sarcomeric protein expression coupled with oxidative stress and aging may increase the substrate required by the UPS to process to abnormal levels. In addition, severe or sustained oxidative stress impairs UPS function (226). In accordance, an impaired UPS function was found in HCM patients (34, 201) and in mouse models with sarcomeric mutations (34, 215). UPS dysfunction in the heart may lead to an increase in mutant and oxidized proteins and eventually to accumulation and aggregation of mutant and other misfolded and damaged proteins (217). Since the endoplasmic reticulum (ER) is sensitive to changes in its environment, accumulation of these proteins can affect ER structure, function, and integrity. This may result in the failure of the ER to cope with the excess protein load, which is termed “ER stress” (207). In addition, UPS and mitochondrial systems are tightly coupled to each other, and once dysfunction starts it is difficult to identify which one was the initial trigger (213). Since ER stress and mitochondrial dysfunction both lead to ROS production (16, 159), UPS dysfunction may result in a vicious circle (Fig. 3), leading to more mutant and oxidized proteins and a disturbed redox balance.
E. Microvascular dysfunction
Microvascular dysfunction is another common HCM feature and is likely to disturb the cardiac redox state. Microvascular dysfunction is not a primary component of HCM pathogenesis and may be caused by diastolic dysfunction. As discussed above, inefficient myocardial relaxation in HCM can be explained by the elevated myocardial activation at low diastolic [Ca2+] - high myofilament Ca2+-sensitivity—which can be a primary cause of the mutant sarcomeric protein or a secondary cause of changes in myofilament protein phosphorylation or perturbations in cellular metabolites (including high [ADP]). Other causes of microvascular dysfunction at a later disease stage may be cardiac fibrosis, myocyte disarray, and reduced arteriolar density (i.e., rarefaction) (33, 35, 166, 197, 254). Inadequate myocardial blood perfusion on demand is likely predisposing HCM patients to myocardial ischemia (33, 35, 166, 167, 197, 285). Myocardial ischemia/reperfusion injury leads to an increase in ROS production, caused by ROS derived from O2 that reperfuses previously hypoxic cardiomyocytes (120, 195). Succinate-driven reverse electron transport through complex I could conceivably be a major source of ROS in the context of regional cardiac ischemia (40). Therefore, microvascular dysfunction will result in local ischemia, which contributes to a disturbed redox balance in HCM patients (Fig. 12).
F. Myocardial fibrosis
Myocardial fibrosis is an early hallmark of HCM and is thought to contribute to arrhythmias and LV diastolic and systolic dysfunction (106). Fibrosis (collagen-rich extracellular matrix) is presumed to derive from fibroblast-like cells within the heart. Although the molecular mechanism responsible for fibrosis in HCM has not been completely elucidated, it has been demonstrated that cardiac fibrosis in mice with HCM is mediated by nonmyocyte proliferation and required the profibrotic transforming growth factor beta (TGF-β) (253). In addition, chronic administration of the angiotensin II type 1 receptor antagonist losartan to mutation-positive, hypertrophy-negative mice prevented the emergence of nonmyocyte proliferation and fibrosis (253). Via paracrine and/or autocrine signaling, TGF-β and possibly other factors [angiotensin II, aldosterone (147, 257)] stimulate nonmyocyte proliferation and/or expression of profibrotic molecules. Cardiac oxidative stress also contributes to cardiac fibrosis. Ischemia, infection, toxic agents, or comorbidities that contribute to a systemic inflammatory state, all induce oxidative stress in noncardiomyocytes and/or cardiomyocytes. This reduces myocardial NO bioavailability, which leaves profibrotic action of growth-promoting hormones such as endothelin-1, angiotensin II, and aldosterone unopposed (194).
G. Adrenergic and angiontensin II stimulation
HOCM is characterized by a strong adrenergic drive. It has been demonstrated that both α-AR and β-AR stimulations provoke cardiac oxidative stress and are involved in cardiac remodeling (247, 291). Angiotensin II modifies cardiac hypertrophy in HCM patients and the molecular mechanisms of angiotensin II pathophysiological activity involve the stimulation of NOX and the production of mitochondrial ROS (54, 58). Therefore, an increased adrenergic drive and angiotensin II receptor stimulation in HCM may contribute to an increase in ROS production in the heart (Fig. 12).
In summary, sarcomeric mutations may affect the cardiac redox balance in HCM via different pathways (Fig. 12). Some may already be present in G+/Ph− mutation carriers (increased cardiac mechanical stress and reduced efficiency of contraction), while others present at a later disease stage (mitochondrial, UPS, and microvascular dysfunction). An interesting hypothesis is that sarcomeric mutations in G+/Ph− mutation carriers lead to a modest increase in cellular ROS production, which activates the endogenous antioxidant defense system to maintain the cardiac redox balance and therefore is not directly harmful. When the protection is no longer sufficient because ROS production further increases or antioxidant defense decreases, ROS may start to play a significant role in HCM pathology. More research is needed to establish which mechanisms prevent or contribute to an altered redox state at early and advanced disease stages.
Aging is also related to cellular oxidative stress (71). An elevated ROS production in healthy mutation carriers may already sensitize the heart for additional ROS at an older age/advanced disease stage. The combination of mutant sarcomeric protein, increased Ca2+-sensitivity, and high energetic cost for contraction, and an impaired redox state may explain why the HCM cardiac phenotype occurs later in life while the mutation is present from birth.
The disturbed redox balance may link multiple pathophysiological processes and HCM hallmarks. As discussed above and illustrated in Figure 12, excessive levels of cardiac ROS in HCM may contribute to diastolic dysfunction (via oxidative modifications of sarcomeric proteins) and cardiac hypertrophy and fibrosis (via activation of the MAPK family) (150, 161, 277). In addition, ROS plays a significant role as proarrhythmic substrate (256). How ROS contribute to arrhythmias in HCM is not yet established. One explanation is that a disturbed cardiac redox state leads to interstitial fibrosis, which is an important determinant of arrhythmogenesis and a risk factor for sudden cardiac death (228).
Alternatively, because a disturbed redox state is sufficient to deteriorate mitochondrial oxidative phosphorylation, it may have enough potential to cause myocardial energy deprivation and cause arrhythmic episodes. Indeed, this was shown to be the true for the cTnT-I79 N HCM mice, associating with energy deprivation, arrhythmia susceptibility, and sudden cardiac death (18, 112, 219); a recent report (56) showed that in the cTnT-I79 N HCM animal model, cardiomyocytes exhibited high levels of ROS production, which were, however, corrected with a drug therapy that reduces ROS levels (see below Peptide SS-31), that additionally prevented arrhythmic episodes and death. Together, these findings provide evidence for the link between ROS and arrhythmias in HCM.
In addition, Coppini et al. (47) showed that an enhanced late Na+ current is an important contributor to the electrophysiological and intracellular Ca2+ dynamic abnormalities in cardiomyocytes from HOCM patients. The authors discussed that an increased ROS production combined with intracellular Ca2+ overload may lead to a sustained activation of Ca2+/calmodulin kinase II (CaMKII) in HCM (47, 66). By slowing down L-type Ca2+-current inactivation and increasing late sodium current amplitude, enhanced CaMKII activity contributed to an action potential duration prolongation and related arrhythmias (47). This shows that a disturbed redox balance in HCM might increase the risk for arrhythmias via different routes.
In previous studies it was demonstrated that oxidative modification of SERCA contributes to decreased SERCA activity and impaired Ca2+-handling and impaired cardiomyocyte relaxation in the senescent mouse heart, and mediated decreased SERCA activity in mice with chronic hemodynamic overload (204). Future studies should investigate if ROS also directly affect the Ca2+-handling proteins (SERCA, RyR) and thereby disturbing Ca2+-handling in HCM pathology (146). Overall, a disturbed cardiac redox state in HCM may contribute to diastolic dysfunction, cardiac hypertrophy, interstitial fibrosis, and cardiac arrhythmias (Fig. 12).
XVI. Therapies
Current treatment options for HCM patients are nonspecific and treat the symptoms rather than the underlying cause. Pharmacological therapy is similar to standard heart failure therapy and includes β-blockers, Ca2+ antagonists, diuretics, and angiotensin-converting enzyme (ACE) inhibitors (12). LVOT obstruction can be surgically corrected by septal myectomy or alcohol ablation, while the risk of sudden cardiac death in HCM is managed by the use of implantable cardioverter defibrillators following risk stratification (12).
XVII. Restoring the Redox Balance as a Promising Therapeutic Approach in HCM Patients
Based on previous trials with antioxidants in patients with cardiovascular disease or other diseases, antioxidant treatment in HCM patients would not be expected to be successful. However, there are many potential reasons why antioxidant treatment has failed in the past (63, 196, 241). (i) The wrong antioxidants may have been used, since it is now known that supraphysiological concentrations of vitamins C and E would be required to compete with the reaction of O2•− and NO (63). (ii) Some antioxidants can act as pro-oxidants under deleterious conditions and exert a damaging effect (63, 196). (iii) Treatment with one antioxidant may not be beneficial because many antioxidants are highly dependent on other antioxidants (196). (iv) Treatment might have started too late in high-risk patients at a late stage of disease (63, 241). (v) Treatment may not have resulted in a sufficiently high antioxidant concentration at the cellular target site (63). (vi) ROS are mediators of normal signaling processes and a suboptimal dosage of antioxidants may perturb the delicate redox balance required for normal cell function. (vii) Perhaps the most important, one fails to acknowledge that the likely beneficial effects of antioxidants are masked by the early irreversible induced-damaged effects of ROS on proteins, DNA, and lipids. For instance, because mtDNA encodes several proteins involved in the assembly of mitochondrial complexes I, III, IV, and V (153), and mtDNA oxidative damage tends to increase in HCM (222), formation of the latter complexes is likely hampered (Fig. 11). Along these lines, defaulted and/or degradation of phospholipid bilayers—caused by lipid peroxidation—require constant turnover and/or synthesis of fatty acyl chains, which are partly determined by the available levels of (un)saturated fatty acid content in cells. The latter maintains cellular membrane fluidity, structure, and organization (Figs. 10 and 11).
Alternatively, one must consider that future therapies should have combinatorial targets, not solely restricted to reducing ROS in cells (see below Peptide SS-31, potential effects). Furthermore, the negative trials should not be regarded as evidence against a role for oxidative stress in cardiovascular disease. Early prevention of ROS formation by targeting the ROS sources might be a more effective strategy for restoring the redox balance than scavenging of ROS. Ideally, only the ROS sources at a specific location contributing to pathology should be targeted. In this section, we discuss the potential effects on redox balance of the current pharmacological treatment strategies in HCM patients. In addition, we discuss the effects of potential future therapies.
A. Current HCM therapies
The current pharmacological treatment strategies in HCM patients might already have beneficial effects for the cardiac redox balance. Medical treatment of the HCM patients in the studies listed in table 1 consisted of β-blockers (61, 135, 188, 199, 201), Ca2+ antagonists (61, 135,188), ACE-inhibitors (135, 188), angiotensin receptor blockers (135, 188), diuretics (188), antiarrhythmic drug (135, 188) (amiodarone) (188) and/or anticoagulants (135). Most of these treatment strategies may have direct or indirect antioxidant effects.
There is substantial evidence that β-blockers derive some of their effectiveness in heart failure through direct or indirect antioxidant properties (125, 287). The Ca2+ antagonist verapamil may also have direct and indirect antioxidant effects and reduce lipid peroxidation and oxidative stress (1, 158). Beneficial effects of ACE-inhibitors in diabetic cardiomyopathy may be related to the inhibition of ROS production via an angiotensin II-dependent pathway (283). The diuretic furosemide has been demonstrated to have antioxidant capacity as well (123, 141). Finally, the antiarrhythmic drug amiodarone was shown to protect cardiomyocytes against oxidative stress-mediated injury by directly scavenging ROS (113). These studies show that the current treatment strategies in HCM patients may have beneficial effects for the cardiac redox state. Some of these drugs may reduce cellular ATP usage (and hence reduce ADP elevations), including β-blockers, ACE-inhibitors, and angiotensin receptor blockers, which are traditionally considered energy-sparing therapies and used to improve symptoms and reduce mortality in congestive heart failure patients (171).
B. N-acetylcysteine
The beneficial effects of NAC in HCM animal models with sarcomeric mutations have already been detailed above (150, 161, 277). These studies indicate that NAC or other interventions that restore the cardiac redox balance are promising strategies for targeting the underlying pathophysiology of HCM due to sarcomeric gene mutation. Interestingly, a clinical trial (phase 1 study) is underway, investigating the effect of NAC in HCM patients carrying sarcomeric mutations (clinical trial identifier NCT01537926) (8, 277).
C. Peptide SS-31
SS-31 belongs to a family of small cell-permeable peptides that target and concentrate in the inner mitochondrial membrane by selective binding to cardiolipin. SS-31 scavenges ROS and reduces mitochondrial ROS production and is currently under clinical investigation (NCT02788747) for nongenetic forms of systolic heart failure (245, 246). In a recent study, Kohlhaas et al. (abstract BS58) (56) investigated the effect of SS-31 in HCM mice (cTnT-I79 N), displaying energy deprivation, arrhythmia susceptibility, diastolic dysfunction, and sudden death (18, 112, 219). Administration of SS-31 restored mitochondrial bioenergetics and prevented ventricular electrical conduction abnormalities in cTnT-I79 N mice. In response to isoproterenol in vivo, SS-31 abrogated the reduced QRS widening and diminished arrhythmia episodes and death. The authors concluded that targeting mitochondrial ROS provides a novel therapeutic approach to prevent arrhythmias in HCM patients. The beneficial effects of SS-31 are potentially related to its role in the stabilization of cardiolipin: (245) restructuring of the inner mitochondrial membrane likely facilitates the formation of mitochondrial supercomplexes, hence improving both (i) electron transfer flow with improved ATP regeneration and (ii) reducing electron leakage and ROS formation (Fig. 11D).
D. Coenzyme Q
The effects of CoQ administration in addition to conventional treatment in HCM patients have been evaluated. CoQ is a lipid-soluble compound that is not protein bound in the inner mitochondrial membrane and acts as an electron carrier from complex I to III (Fig. 11A). Langsjoen et al. (142) reported that CoQ treatment of seven HCM patients improved fatigue and dyspnea symptoms and reduced the mean interventricular septal thickness by 24%. Adarsh et al. (4) showed that therapeutic administration of CoQ in 46 HCM patients significantly improved NYHA class, quality of life on 6-min walk test, and diastolic dysfunction. CoQ significantly reduced LVOT gradient in obstructive cases, and reduced mean interventricular septal thickness by 22.4%. In addition, four cases of ventricular tachycardia were reported in the control group, versus 0 cases in the CoQ group.
It is likely that the beneficial effects of CoQ treatment in HCM patients are at least partly related to restoration of the redox balance. Because CoQ is an electron acceptor/carrier (Fig. 11A), it may directly scavenge electrons in the inner mitochondrial membrane thereby preventing accidental ROS formation. Furthermore, because CoQ is not protein bound and hence is free to diffuse in or out of the mitochondrial membrane, one can speculate that CoQ levels potentially drop in HCM in combination to disruptions of the inner mitochondrial membrane (Fig. 11C). Future studies need to confirm the effects and working mechanisms of CoQ administration in HCM.
E. Estrogen
The effects of treatment with estrogen, which may act not only via estrogen receptors but also as an antioxidant and may reduce the risk of cardiovascular disease in women (25), has been tested in HCM animal models. cTnT-R92Q mice were ovariectomized at 20 weeks of age and treated with either placebo or 17β-estradiol for 12 weeks (38). Sham-operated cTnT-R92Q female mice, 32 weeks old, displayed impaired diastolic function, reduced mitochondrial respiratory function, and an increase in oxidative stress markers (Table 2) compared with NTG sham mice. In response to ovariectomy, cardiac function further decreased and myocardial MDA concentrations increased compared with sham cTnT-R92Q mice. Administration of 17β-estradiol in ovariectomized cTnT-R92Q increased SOD2 protein level, improved myocardial function, prevented myocardial energy dysregulation, and reduced the levels of NOX4 and MDA (38). Therefore, estrogen treatment might have exerted some of its beneficial effects by reducing myocardial oxidative stress via changes in SOD2 and NOX4 activity. However, another study demonstrated that estrogenic compounds are not always cardioprotective and can even be lethal in male mice with HCM with a myosin mutation (88). In addition, results from several trials suggested that hormone replacement (estrogen–progestin hormone combination or estrogen alone) therapies in postmenopausal women are not protective with respect to cardiovascular diseases and might even be harmful (122). Therefore, care should be taken with estrogen treatment in HCM patients as a strategy to reduce oxidative stress.
F. Metabolic therapies
Previous studies have indicated that sarcomere mutations increase energetic costs of cardiac contraction, which will increase the energy demand of the heart and may lead to metabolic perturbations (e.g., resulting in reduced cellular [ATP] and elevated [ADP]) (11, 86, 259, 279, 280). Fatty acids are energy-rich substrates and generate more ATP per gram substrate than glucose (239). However, an important advantage of glucose metabolism is that less O2 is used per ATP molecule synthesized. Christiansen et al. (41) hypothesized that the limited myocardial fatty acid metabolism in HCM cats may be a compensatory change as a metabolic adaptation to meet a higher energy demand under O2-limited conditions in the HCM heart, which will be true if O2 delivery is a limitation. In accordance, treatment with perhexiline, which shifts substrate use from fatty acids to glucose, improved cardiac high-energy phosphate metabolism and exercise capacity in HCM patients (2). It has also been demonstrated that perhexiline is a direct NOX2 inhibitor (77, 130). Perhexiline treatment of a cMyBP-C KI mouse model of HCM also increased GSH levels and the production of NADPH, which suggests that metabolic therapies may also reduce cardiac oxidative stress (79).
G. XO inhibitor
Uric acid (UA) is a nitrogenous end-product of purine metabolism and a ubiquitous antioxidant in body fluids, but ROS are produced on UA production. UA is produced by XO, which reduces O2 to O2•−/H2O2. XO expression and activity are increased in cardiomyocytes isolated from failing hearts and inhibition of XO-derived ROS in heart failure may improve abnormal myocardial energy metabolism, endothelial dysfunction, cardiac hypertrophy, and fibrosis (21, 89). In heart failure, the increase in UA production may be caused by the increased abundance and activity of XO, or by an increase in XO substrate resulting from enhanced ATP breakdown to adenosine and hypoxanthine (21). Hyperuricemia is a common finding in chronic heart failure patients and the OPT-CHF study showed that serum UA may serve as a valuable biomarker to target XO inhibition in heart failure (21, 96). Evidence exists against the direct toxicity of UA and that the mechanism of improvement in endothelial function with allopurinol (a potent XO inhibitor) lies in its ability to restore the vascular redox balance, and not in urate reduction (80). Therefore, serum UA levels most likely reflect the degree of XO activity and resultant oxidative stress.
Zhu et al. (295) recently reported that in a study in 588 HCM patients, UA levels are an independent predictor of adverse outcomes. During a follow-up of 5 years, elevated levels of UA were independently associated with an increased risk of cardiovascular death, all-cause mortality, cardiac events, heart failure events, and arrhythmic events in HCM patients. In addition, Zhang et al. (289) showed that serum UA levels were significantly and independently associated with LV mass index in women with HOCM, but not in men. Authors stated that the increase in UA could be a reflection of increased XO activity (Fig. 12), resulting in abnormal energy metabolism and in impairment of coronary microvascular function (95, 295). Future studies need to establish whether XO activity and XO-generated ROS are indeed elevated in HCM, as suggested by increased UA plasma levels in HCM patients (289, 295). If XO activity is increased in HCM pathology, blocking XO-generated ROS may be an alternative and promising strategy to restore the cardiac redox balance in HCM patients.
Although speculative, a disturbed cardiac redox balance may underlie several features of HCM. (i) A local increase in cellular ROS, triggered by local wall stress, mitochondrial or microvascular dysfunction may underlie asymmetric (septal) remodeling of the heart, while the mutation is present in all cardiomyocytes. (ii) Aging is related to an altered cellular redox balance (71), which may underlie the onset of disease at an “older” (20–50 years) age. (iii) A reduced susceptibility to oxidative stress (122) may explain the older age of HCM onset in women compared to men. (iv) Strenuous physical activity is known to trigger ROS production and may underlie increased risk of acute cardiac arrest by ROS-mediated arrhythmias (256). Diet (e.g., antioxidants) and genetic predisposition of mutation carriers may affect the cardiac redox state and whether a mutation carrier develops HCM or not. As an example, Yamada et al. (281) showed that the phenotypic expression of nonfamilial HCM may be influenced by a genetic polymorphism of the PAFAH1B1 gene, encoding plasma platelet-activating factor acetylhydrolase (PAF-AH). PAF-AH acts as a key defense against oxidative stress by hydrolyzing PAF and oxidized phospholipids. It was suggested that the polymorphism in plasma PAFAH1B1 resulted in the loss of plasma PAF-AH activity and exacerbated cardiac damage (e.g., more severe interstitial fibrosis, higher LV end-diastolic pressure), although this polymorphism was unlikely to be the causative factor for the disease (281).
It is important to stress that a disturbed cardiac redox balance is not the only pathological mechanism leading to HCM as illustrated in Figure 12. More research is needed to establish how important the changes in the cardiac redox state are compared to other pathological mechanisms in HCM (e.g., myocardial energy depletion). In addition to sarcomeric mutations, HCM can be caused by inborn errors of metabolism (e.g., Fabry disease), neuromuscular diseases (e.g., Friedreich's ataxia), and mitochondrial diseases (12). Cardiac oxidative stress is also reported in these forms of cardiomyopathy and most likely contributes to pathology (39, 162). A disturbed redox balance can be triggered by many different routes. If a disturbed redox state is indeed an important trigger for the development of the HCM phenotype, this could explain why the same disease (and HCM hallmarks) can be triggered by so many different underlying causes.
It is about a decade ago that the first evidence for cardiac oxidative stress in HCM patients was reported (188). It is interesting that the first trial with an antioxidant (NAC) to restore the cardiac redox balance in HCM patients is now underway, although treatment strategies that scavenge ROS may be less effective than strategies that prevent excessive ROS formation (e.g., SS-31). If sufficient proof is built in future studies, interventions to restore the cardiac redox balance may even be initiated at an early disease stage in asymptomatic mutation carriers to prevent HCM development.
XVIII. Breaking the Vicious Cycle
In summary, during past years, several cellular pathomechanisms have been elucidated as the cause of dysfunction and remodeling of the HCM heart. Based on mutation-induced and secondary disease-related cellular perturbations, multiple treatment strategies have been proposed to prevent dysfunction and remodeling of the heart at early and late stages of HCM [reviewed by Tardiff et al. (249)]. On the basis of the mutation-induced vicious circle of pathologic remodeling in HCM (Fig. 3), we propose additional treatment strategies that may prevent cardiac disease by targeting early cellular changes in HCM (Fig. 13).
FIG. 13.
Breaking the mutation-induced cycle in hypertrophic cardiomyopathy. The vicious cycle may be broken by interventions to target metabolism (metabolic therapy), to lower myofilament Ca2+-sensitivity (e.g., stimulation of β3AR, β3AR agonist) and thereby reduce sarcomeric energy consumption, by interventions targeted at the mitochondria to reduce oxidative stress and improve energy supply, and by components that boost the PQC system and restore protein balance in the cell. HSPs, heat shock proteins. Color images are available online.
First, myocardial energy depletion may be compensated by metabolic therapy. As described above, perhexiline treatment exerted a beneficial effect in symptomatic (advanced) HCM (2). In the healthy heart, energy demand is met by oxidation of fatty acids and carbohydrates. Although fatty acids represent the predominant fuel for the heart, they provide less ATP per O2 molecule in comparison to carbohydrates. Thus, agents that temporarily shift metabolism away from the preferred fatty acids toward carbohydrates (perhexiline, ranolazin) would increase ATP supply and may prevent cardiac disease.
Second, normalizing Ca2+-sensitivity may be beneficial by reducing cellular energy demand and improving diastolic function. Intriguingly, β3-AR activation increases the activity of protein kinase G (PKG) via cyclic GMP (cGMP) (Fig. 1) (78, 92), which has been shown to phosphorylate “PKA” phosphorylation sites on sarcomeric proteins and reduce myofilament Ca2+-sensitivity (143). As such, β3-AR activation provides a novel route to decrease Ca2+-sensitivity, relax the sarcomeres, and reduce energetic costs in HCM (Fig. 13). Activation of β3-AR is mediated via a pathway that is distinct from β1-AR. Moreover, β3-AR is resistant to desensitization and remains operative despite chronic adrenergic drive. Cardiac-specific expression of β3-AR in mice provided protection from hypertrophic remodeling, fibrosis, and dysfunction in various nongenetic disease models (19). Therefore, this pathway provides an attractive novel target for therapeutic interventions to counterbalance detrimental secondary disease-related effects of β1-AR downregulation in HCM.
As described above, there are multiple interventions that target mitochondria and thereby reduce the formation of ROS and improve cellular energetic status. SS-1 (elamipretide, formerly known as bendavia) is currently tested in mitochondrial myopathy and several heart failure trials (ClinicalTrial.govs). Likewise, Khondrion compounds are being studied in patients with mitochondrial diseases (Khenergy trial). These novel drugs may proof beneficial in HCM and warrant further investigation.
The PQC system includes intracellular surveillance by a large and diverse family of proteins termed chaperones, of which the largest group consists of heat shock proteins (HSPs). HSPs assist in the correct folding of nascent or incomplete proteins, preventing them from forming insoluble aggregates (170, 267, 278). HSP-inducing drugs, such as geranylgeranylacetone (commonly known as GGA), prevent derailment of proteostasis and cellular remodeling in cardiomyocytes, Drosophila, and in a dog model with atrial fibrillation (30, 109, 126, 290). Novel compounds to normalize protein homeostasis may represent a likely therapy to restore the sarcomere protein balance (Fig. 2; mutant vs. normal protein) at early and late disease stages. These therapies, already used in clinical practice for other disease entities and target general biological process (e.g., mitochondrial dysfunction, PQC system), may prevent or delay HCM when given at the appropriate (early or advanced) disease stage.
Acknowledgments
This work was supported by the Netherlands Cardiovascular Research Initiative: “An initiative with support of the Dutch Heart Foundation, CVON2014-40 DOSIS” and the National Institute of health (NIH) R01 HL063038 and NIH R01 HL76038.
Abbreviations Used
- β-AR
β-adrenergic receptor
- 4-HAE
4-hydroxyalkenal
- 4-HNE
4-hydroxy-2-nonenal
- 8-iso-PGF2α
8-isoprostaglandin F2α
- 8-oxodG
8-oxo-7-hydrodeoxyguanosine
- ACE
angiotensin-converting enzyme
- CaMKII
Ca2+/calmodulin kinase II
- cAMP
cyclic AMP
- CAT
catalase
- cGMP
cyclic GMP
- CK
creatine kinase
- cMyBP-C
cardiac myosin-binding protein-C
- CoQ
coenzyme Q
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- Cyt C
cytochrome c
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated protein kinases
- G+/Ph−
genotype-positive, phenotype-negative (mutation carriers without a phenotype)
- GPX
glutathione peroxidase
- GSH
reduced glutathione
- GSSG
oxidized GSH
- H2O2
hydrogen peroxide
- HCM
hypertrophic cardiomyopathy
- HNO
nitroxyl
- HO•
hydroxyl radical
- HOCM
obstructive HCM
- HSPs
heat shock proteins
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal protein kinases
- LV
left ventricular
- LVOT
LV outflow tract
- MAPK
mitogen-activated protein kinases
- MDA
malondialdehyde
- mtDNA
mitochondrial DNA
- MYBPC3
gene encoding cardiac myosin-binding protein-C
- MYH7
gene encoding β-myosin heavy chain
- MyHC
myosin heavy chain
- NAC
N-acetylcysteine
- NFATc1
nuclear factor of activated T cells
- NOS
nitric oxide synthase
- NOX
NADPH oxidases
- NTG
nontransgenic
- O2•−
superoxide radical anion
- PAF-AH
platelet-activating factor acetylhydrolase
- PCr
phosphocreatine
- PKA
protein kinase A
- PKG
protein kinase G
- PQC
protein-quality-control
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RV
right ventricular
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- TBARS
thiobarbituric acid-reactive substances
- Tm
tropomyosin
- TGF-β
transforming growth factor-beta
- TNNI3
gene encoding cardiac troponin I
- TNNT2
gene encoding cardiac troponin T
- TPM1
gene encoding α–tropomyosin
- TRX
thioredoxin
- UA
uric acid
- UPS
ubiquitin/proteasome system
- WT
wild type
- XO
xanthine oxidase
References
- 1. Abd Allah ES, Ahmed MA, and Abdel Mola AF. Comparative study of the effect of verapamil and vitamin D on iron overload-induced oxidative stress and cardiac structural changes in adult male rats. Pathophysiology 21: 293–300, 2014 [DOI] [PubMed] [Google Scholar]
- 2. Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, and Frenneaux M. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 122: 1562–1569, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Abraham MR, Bottomley PA, Dimaano VL, Pinheiro A, Steinberg A, Traill TA, Abraham TP, and Weiss RG. Creatine kinase adenosine triphosphate and phosphocreatine energy supply in a single kindred of patients with hypertrophic cardiomyopathy. Am J Cardiol 112: 861–866, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adarsh K, Kaur H, and Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors 32: 145–149, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Afanas'ev I. ROS and RNS signaling in heart disorders: could antioxidant treatment be successful? Oxid Med Cell Longev 2011: 13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen DG. and Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circ Res 60: 153–168, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Alves ML, Dias FA, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, Hinken AC, Warren CM, Utter MS, Davis RT, 3rd, Sadayappan S, Robbins J, Wieczorek DF, Solaro RJ, and Wolska BM. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ Cardiovasc Genet 7: 132–143, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ammirati E, Contri R, Coppini R, Cecchi F, Frigerio M, and Olivotto I. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail 18: 1106–1118, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Aquilano K, Baldelli S, and Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol 5: 196, 1–12, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aruoma OI, Halliwell B, Hoey BM, and Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6: 593–597, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Ashrafian H, Redwood C, Blair E, and Watkins H. Hypertrophic cardiomyopathy:a paradigm for myocardial energy depletion. Trends Genet 19: 263–268, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, and Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35: 2733–2779, 2014 [DOI] [PubMed] [Google Scholar]
- 13. Ayala A, Munoz MF, and Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014: 360438, 1–31, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bach SV. and Hegde AN. The proteasome and epigenetics: zooming in on histone modifications. Biomol Concepts 7: 215–227, 2016 [DOI] [PubMed] [Google Scholar]
- 15. Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, and Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature 441: 1011–1014, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Balaban RS, Nemoto S, and Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Balderas-Villalobos J, Molina-Muñoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gómez-Viquez NL. Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol 305: H1344–H1353, 2013 [DOI] [PubMed] [Google Scholar]
- 18. Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, and Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Inv 118: 3893–3903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belge C, Hammond J, Dubois-Deruy E, Manoury B, Hamelet J, Beauloye C, Markl A, Pouleur AC, Bertrand L, Esfahani H, Jnaoui K, Gotz KR, Nikolaev VO, Vanderper A, Herijgers P, Lobysheva I, Iaccarino G, Hilfiker-Kleiner D, Tavernier G, Langin D, Dessy C, and Balligand JL. Enhanced expression of beta3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 129: 451–462, 2014 [DOI] [PubMed] [Google Scholar]
- 20. Belus A, Piroddi N, Scellini B, Tesi C, D'Amati G, Girolami F, Yacoub M, Cecchi F, Olivotto I, and Poggesi C. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J Physiol 586: 3639–3644, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bergamini C, Cicoira M, Rossi A, and Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail 11: 444–452, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Berlett BS. and Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272: 20313–20316, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Betocchi S, Hess OM, Losi MA, Nonogi H, and Krayenbuehl HP. Regional left ventricular mechanics in hypertrophic cardiomyopathy. Circulation 88: 2206–2214, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Bhavnani BR. and Stanczyk FZ. Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action. J Steroid Biochem Mol Biol 142: 16–29, 2014 [DOI] [PubMed] [Google Scholar]
- 26. Borbely A, Toth A, Edes I, Virag L, Papp JG, Varro A, Paulus WJ, van der Velden J, Stienen GJ, and Papp Z. Peroxynitrite-induced alpha-actinin nitration and contractile alterations in isolated human myocardial cells. Cardiovasc Res 67: 225–233, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Borel C, Ferreira PG, Santoni F, Delaneau O, Fort A, Popadin KY, Garieri M, Falconnet E, Ribaux P, Guipponi M, Padioleau I, Carninci P, Dermitzakis ET, and Antonarakis SE. Biased allelic expression in human primary fibroblast single cells. Am J Hum Genet 96: 70–80, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brenner B, Seebohm B, Tripathi S, Montag J, and Kraft T. Familial hypertrophic cardiomyopathy: functional variance among individual cardiomyocytes as a trigger of FHC-phenotype development. Front Physiol 5: 392, 1–15, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation 101: 558–569, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Brundel BJ, Shiroshita-Takeshita A, Qi X, Yeh YH, Chartier D, van Gelder IC, Henning RH, Kampinga HH, and Nattel S. Induction of heat shock response protects the heart against atrial fibrillation. Circ Res 99: 1394–1402, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Cai Z. and Yan LJ. Protein oxidative modifications: beneficial roles in disease and health. J Biochem Pharmacol Res 1: 15–26, 2013 [PMC free article] [PubMed] [Google Scholar]
- 32. Camici PG, Olivotto I, and Rimoldi OE. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol 52: 857–864, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Cannon RO, 3rd, Rosing DR, Maron BJ, Leon MB, Bonow RO, Watson RM, and Epstein SE. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation 71: 234–243, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Carrier L, Schlossarek S, Willis MS, and Eschenhagen T. The ubiquitin-proteasome system and nonsense-mediated mRNA decay in hypertrophic cardiomyopathy. Cardiovasc Res 85: 330–338, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, and Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 349: 1027–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Chandra M, Rundell VLM, Tardiff JC, Leinwand LA, de Tombe PP, and Solaro RJ. Ca2+ activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol 280: H705–H713, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Chen-Izu Y. and Izu LT. Mechano-chemo-transduction in cardiac myocytes. J Physiol 595, 3949–3958, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, Zhang Z, Hu F, Yang W, Yuan J, Cui J, Hao S, Hu J, Zhou Y, and Qiao S. 17beta-estradiol prevents cardiac diastolic dysfunction by stimulating mitochondrial function: a preclinical study in a mouse model of a human hypertrophic cardiomyopathy mutation. J Steroid Biochem Mol Biol 147: 92–102, 2015 [DOI] [PubMed] [Google Scholar]
- 39. Chimenti C, Scopelliti F, Vulpis E, Tafani M, Villanova L, Verardo R, De Paulis R, Russo MA, and Frustaci A. Increased oxidative stress contributes to cardiomyocyte dysfunction and death in patients with Fabry disease cardiomyopathy. Hum Pathol 46: 1760–1768, 2015 [DOI] [PubMed] [Google Scholar]
- 40. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, and Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Christiansen LB, Dela F, Koch J, Hansen CN, Leifsson PS, and Yokota T. Impaired cardiac mitochondrial oxidative phosphorylation and enhanced mitochondrial oxidative stress in feline hypertrophic cardiomyopathy. Am J Physiol 308: H1237–H1247, 2015 [DOI] [PubMed] [Google Scholar]
- 42. Claes GR, van Tienen FH, Lindsey P, Krapels IP, Helderman-van den Enden AT, Hoos MB, Barrois YE, Janssen JW, Paulussen AD, Sels JW, Kuijpers SH, van Tintelen JP, van den Berg MP, Heesen WF, Garcia-Pavia P, Perrot A, Christiaans I, Salemink S, Marcelis CL, Smeets HJ, Brunner HG, Volders PG, and van den Wijngaard A. Hypertrophic remodelling in cardiac regulatory myosin light chain (MYL2) founder mutation carriers. Eur Heart J 37: 1815–1822, 2016 [DOI] [PubMed] [Google Scholar]
- 43. Clifford CP. and Nunez DJ. Human beta-myosin heavy chain mRNA prevalence is inversely related to the degree of methylation of regulatory elements. Cardiovasc Res 38: 736–743, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Colbeau A, Nachbaur J, and Vignais PM. Enzymac characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta 249: 462–492, 1971 [DOI] [PubMed] [Google Scholar]
- 45. Cooke R. and Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J 28: 241–258, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coppini R, Ferrantini C, Mazzoni L, Sartiani L, Olivotto I, Poggesi C, Cerbai E, and Mugelli A. Regulation of intracellular Na(+) in health and disease: pathophysiological mechanisms and implications for treatment. Glob Cardiol Sci Pract 2013: 222–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coppini R, Ferrantini C, Yao L, Fan P, Del Lungo M, Stillitano F, Sartiani L, Tosi B, Suffredini S, Tesi C, Yacoub M, Olivotto I, Belardinelli L, Poggesi C, Cerbai E, and Mugelli A. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127: 575–584, 2013 [DOI] [PubMed] [Google Scholar]
- 48. Cortassa S, Aon MA, Winslow RL, and O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J 87: 2060–2073, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Ostman-Smith I, Clarke K, and Watkins H. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 41: 1776–1782, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Cui H, Schlesinger J, Schoenhals S, Tonjes M, Dunkel I, Meierhofer D, Cano E, Schulz K, Berger MF, Haack T, Abdelilah-Seyfried S, Bulyk ML, Sauer S, and Sperling SR. Phosphorylation of the chromatin remodeling factor DPF3a induces cardiac hypertrophy through releasing HEY repressors from DNA. Nucleic Acids Res 44: 2538–2553, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. D'Cruz LG, Baboonian C, Phillimore HE, Taylor R, Elliott PM, Varnava A, Davison F, McKenna WJ, and Carter ND. Cytosine methylation confers instability on the cardiac troponin T gene in hypertrophic cardiomyopathy. J Med Genet 37: E18, 1–6, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dar RD, Razooky BS, Singh A, Trimeloni TV, McCollum JM, Cox CD, Simpson ML, and Weinberger LS. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proc Natl Acad Sci U S A 109: 17454–17459, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davies JE, Whinnett ZI, Francis DP, Manisty CH, Aguado-Sierra J, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH, and Mayet J. Evidence of a dominant backward-propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation 113: 1768–1778, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Deinum J, van Gool JM, Kofflard MJ, ten Cate FJ, and Danser AH. Angiotensin II type 2 receptors and cardiac hypertrophy in women with hypertrophic cardiomyopathy. Hypertension 38: 1278–1281, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Deng Q, Ramskold D, Reinius B, and Sandberg R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343: 193–196, 2014 [DOI] [PubMed] [Google Scholar]
- 56. Kohlhaas M, Friederich FW, Sequeira V, Flenner F, Otto O, Venkataraman R, Bay J, Nickel A, Knollmann B, Carrier L, Eschenhagen T, Maack C. BS58 - Reverse-mode transhydrogenase triggers mitochondrial reactive oxygen species emission and arrhythmias in hypertrophic cardiomyopathy. Clin Res Cardiol 105: 1, 2016 [Google Scholar]
- 57. Dhandapany PS, Sadayappan S, Xue Y, Powell GT, Rani DS, Nallari P, Rai TS, Khullar M, Soares P, Bahl A, Tharkan JM, Vaideeswar P, Rathinavel A, Narasimhan C, Ayapati DR, Ayub Q, Mehdi SQ, Oppenheimer S, Richards MB, Price AL, Patterson N, Reich D, Singh L, Tyler-Smith C, and Thangaraj K. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet 41: 187–191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dikalov SI. and Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal 19: 1085–1094, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dilsizian V, Bonow RO, Epstein SE, and Fananapazir L. Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 22: 796–804, 1993 [DOI] [PubMed] [Google Scholar]
- 60. Dimitrow PP, Undas A, Bober M, Tracz W, and Dubiel JS. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep 59: 715–720, 2007 [PubMed] [Google Scholar]
- 61. Dimitrow PP, Undas A, Wolkow P, Tracz W, and Dubiel JS. Enhanced oxidative stress in hypertrophic cardiomyopathy. Pharmacol Rep 61: 491–495, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Dizdaroglu M, Jaruga P, Birincioglu M, and Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32: 1102–1115, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Drummond GR, Selemidis S, Griendling KK, and Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Elbini Dhouib I, Jallouli M, Annabi A, Gharbi N, Elfazaa S, and Lasram MM. A minireview on N-acetylcysteine: an old drug with new approaches. Life Sci 151: 359–363, 2016 [DOI] [PubMed] [Google Scholar]
- 65. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, and Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J 35: 2733–2779, 2014 [DOI] [PubMed] [Google Scholar]
- 66. Erickson JR, He BJ, Grumbach IM, and Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev 91: 889–915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Evans CC, Pena JR, Phillips RM, Muthuchamy M, Wieczorek DF, Solaro RJ, and Wolska BM. Altered hemodynamics in transgenic mice harboring mutant tropomyosin linked to hypertrophic cardiomyopathy. Am J Physiol 279: H2414–H2423, 2000 [DOI] [PubMed] [Google Scholar]
- 68. Fernlund E, Gyllenhammar T, Jablonowski R, Carlsson M, Larsson A, Arnlov J, and Liuba P. Serum biomarkers of myocardial remodeling and coronary dysfunction in early stages of hypertrophic cardiomyopathy in the young. Pediatr Cardiol 38: 853–863, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fernlund E, Schlegel TT, Platonov PG, Carlson J, Carlsson M, and Liuba P. Peripheral microvascular function is altered in young individuals at risk for hypertrophic cardiomyopathy and correlates with myocardial diastolic function. Am J Physiol 308: H1351–H1358, 2015 [DOI] [PubMed] [Google Scholar]
- 70. Ferrara C, Witjas-Paalberends ER, Piroddi N, Scellini B, Tesi C, Sequiera V, dos Remedios C, Schlossarek S, Leung J, Carrier L, Redwood C, Marston S, van der Velden J, and Poggesi C. The HCM-associated cardiac troponin T mutation K280 N increases the energetic cost of tension generation in human cardiac myofibrils. Biophys J 104: 187a, 2013 [Google Scholar]
- 71. Finkel T. and Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Flenner F, Friedrich FW, Ungeheuer N, Christ T, Geertz B, Reischmann S, Wagner S, Stathopoulou K, Sohren KD, Weinberger F, Schwedhelm E, Cuello F, Maier LS, Eschenhagen T, and Carrier L. Ranolazine antagonizes catecholamine-induced dysfunction in isolated cardiomyocytes, but lacks long-term therapeutic effects in vivo in a mouse model of hypertrophic cardiomyopathy. Cardiovasc Res 109: 90–102, 2016 [DOI] [PubMed] [Google Scholar]
- 73. Forman HJ, Maiorino M, and Ursini F. Signaling functions of reactive oxygen species. Biochemistry 49: 835–842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fraysse B, Weinberger F, Bardswell SC, Cuello F, Vignier N, Geertz B, Starbatty J, Krämer E, Coirault C, Eschenhagen T, Kentish JC, Avkiran M, and Carrier L. Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of MYBPC3 mutation in heterozygous knock-in mice. J Mol Cell Cardiol 52: 1299–1307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Frey N, Brixius K, Schwinger RHG, Benis T, Karpowski A, Lorenzen HP, Luedde M, Katus HA, and Franz WM. Alterations of tension-dependent ATP utilization in a transgenic rat model of hypertrophic cardiomyopathy. J Biol Chem 281: 29575–29582, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, and Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function/novelty and significance. Circ Res 111: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gatto GJ, Jr., Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, and Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem 28: 95–104, 2013 [DOI] [PubMed] [Google Scholar]
- 78. Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, and Le Marec H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102: 1377–1384, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gehmlich K, Dodd MS, Allwood JW, Kelly M, Bellahcene M, Lad HV, Stockenhuber A, Hooper C, Ashrafian H, Redwood CS, Carrier L, and Dunn WB. Changes in the cardiac metabolome caused by perhexiline treatment in a mouse model of hypertrophic cardiomyopathy. Mol Biosyst 11: 564–573, 2015 [DOI] [PubMed] [Google Scholar]
- 80. George J, Carr E, Davies J, Belch JJ, and Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114: 2508–2516, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Germans T, Rüssel I, Götte M, Spreeuwenberg M, Doevendans P, Pinto Y, van der Geest R, van der Velden J, Wilde A, and van Rossum A. How do hypertrophic cardiomyopathy mutations affect myocardial function in carriers with normal wall thickness? Assessment with cardiovascular magnetic resonance. J Cardiovas Mag Res 12: 1–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gilda JE, Lai X, Witzmann FA, and Gomes AV. Delineation of molecular pathways involved in cardiomyopathies caused by troponin T mutations. Mol Cell Proteomics 15: 1962–1981, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gonzalez-Teran B, Lopez JA, Rodriguez E, Leiva L, Martinez-Martinez S, Bernal JA, Jimenez-Borreguero LJ, Redondo JM, Vazquez J, and Sabio G. p38gamma and delta promote heart hypertrophy by targeting the mTOR-inhibitory protein DEPTOR for degradation. Nat Commun 7: 10477, 1–16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Greenhaff PL. The creatine-phosphocreatine system: there's more than one song in its repertoire. J Physiol 537: 657, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Güçlü A, Happe C, Eren S, Korkmaz IH, Niessen HW, Klein P, van Slegtenhorst M, Schinkel AF, Michels M, van Rossum AC, Germans T, and van der Velden J. Left ventricular outflow tract gradient is associated with reduced capillary density in hypertrophic cardiomyopathy irrespective of genotype. Eur J Clin Invest 45: 1252–1259, 2015 [DOI] [PubMed] [Google Scholar]
- 86. Güçlü A, Knaapen P, Harms HJ, Parbhudayal RY, Michels M, Lammertsma AA, van Rossum AC, Germans T, and van der Velden J. Disease stage-dependent changes in cardiac contractile performance and oxygen utilization underlie reduced myocardial efficiency in human inherited hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 10, 1–12, 2017 [DOI] [PubMed] [Google Scholar]
- 87. Gupta MP. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J Mol Cell Cardiol 43: 388–403, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Haines CD, Harvey PA, Luczak ED, Barthel KK, Konhilas JP, Watson PA, Stauffer BL, and Leinwand LA. Estrogenic compounds are not always cardioprotective and can be lethal in males with genetic heart disease. Endocrinology 153: 4470–4479, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hajjar RJ. and Leopold JA. Xanthine oxidase inhibition and heart failure: novel therapeutic strategy for ventricular dysfunction? Circ Res 98: 169–171, 2006 [DOI] [PubMed] [Google Scholar]
- 90. Halasi M, Wang M, Chavan TS, Gaponenko V, Hay N, and Gartel AL. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem J 454: 201–208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Halliwell B. and Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142: 231–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hammond J. and Balligand JL. Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: from contractility to remodeling. J Mol Cell Cardiol 52: 330–340, 2012 [DOI] [PubMed] [Google Scholar]
- 93. Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, Zhou B, and Chang CP. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature 466: 62–67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Harding SE, Brown LA, Wynne DG, Davies CH, and Poole-Wilson PA. Mechanisms of beta adrenoceptor desensitisation in the failing human heart. Cardiovasc Res 28: 1451–1460, 1994 [DOI] [PubMed] [Google Scholar]
- 95. Hare JM. and Johnson RJ. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 107: 1951–1953, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Hare JM, Mangal B, Brown J, Fisher C, Jr., Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP, and Investigators O-C. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol 51: 2301–2309, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, and Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 114: 216–225, 2006 [DOI] [PubMed] [Google Scholar]
- 98. Hayashida W, Kumada T, Kohno F, Noda M, Ishikawa N, Kojima J, Himura Y, and Kawai C. Left ventricular regional relaxation and its nonuniformity in hypertrophic nonobstructive cardiomyopathy. Circulation 84: 1496–1504, 1991 [DOI] [PubMed] [Google Scholar]
- 99. He H, Javadpour MM, Latif F, Tardiff JC, and Ingwall JS. R-92 L and R-92 W mutations in cardiac troponin T lead to distinct energetic phenotypes in intact mouse hearts. Biophys J 93: 1834–1844, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Heffernan KS, Napolitano CA, Maron MS, Patvardhan EA, Patel AR, Pandian NG, Karas RH, and Kuvin JT. Peripheral vascular endothelial function in patients with hypertrophic cardiomyopathy. Am J Cardiol 105: 112–115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Helms AS, Davis FM, Coleman D, Bartolone SN, Glazier AA, Pagani F, Yob JM, Sadayappan S, Pedersen E, Lyons R, Westfall MV, Jones R, Russell MW, and Day SM. Sarcomere mutation-specific expression patterns in human hypertrophic cardiomyopathy. Circ Cardiovasc Genet 7: 434–443, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hengartner MO. The biochemistry of apoptosis. Nature 407: 770–776, 2000 [DOI] [PubMed] [Google Scholar]
- 103. Hernandez OM, Szczesna-Cordary D, Knollmann BC, Miller T, Bell M, Zhao J, Sirenko SG, Diaz Z, Guzman G, Xu Y, Wang Y, Kerrick WG, and Potter JD. F110I and R278C troponin T mutations that cause familial hypertrophic cardiomyopathy affect muscle contraction in transgenic mice and reconstituted human cardiac fibers. J Biol Chem 280: 37183–37194, 2005 [DOI] [PubMed] [Google Scholar]
- 104. Ho CY, Carlsen C, Thune JJ, Havndrup O, Bundgaard H, Farrohi F, Rivero J, Cirino AL, Andersen PS, Christiansen M, Maron BJ, Orav EJ, and Køber L. Echocardiographic strain imaging to assess early and late consequences of sarcomere mutations in hypertrophic cardiomyopathy. Circ Cardiovas Gen 2: 314–321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ho CY, Charron P, Richard P, Girolami F, Van Spaendonck-Zwarts KY, and Pinto Y. Genetic advances in sarcomeric cardiomyopathies: state of the art. Cardiovasc Res 105: 397–408, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ho CY, Lopez B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, Gonzalez A, Colan SD, Seidman JG, Diez J, and Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 363: 552–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hofmann PA. and Fuchs F. Evidence for a force-dependent component of calcium binding to cardiac troponin C. Am J Physiol 253: C541–C546, 1987 [DOI] [PubMed] [Google Scholar]
- 108. Hofmann PA. and Fuchs F. Bound calcium and force development in skinned cardiac muscle bundles: effect of sarcomere length. J Mol Cell Cardiol 20: 667–677, 1988 [DOI] [PubMed] [Google Scholar]
- 109. Hoogstra-Berends F, Meijering RA, Zhang D, Heeres A, Loen L, Seerden JP, Kuipers I, Kampinga HH, Henning RH, and Brundel BJ. Heat shock protein-inducing compounds as therapeutics to restore proteostasis in atrial fibrillation. Trends Cardiovasc Med 22: 62–68, 2012 [DOI] [PubMed] [Google Scholar]
- 110. Hovius R, Lambrechts H, Nicolay K, and de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta 1021: 217–226, 1990 [DOI] [PubMed] [Google Scholar]
- 111. Huang W, Kazmierczak K, Zhou Z, Aguiar-Pulido V, Narasimhan G, and Szczesna-Cordary D. Gene expression patterns in transgenic mouse models of hypertrophic cardiomyopathy caused by mutations in myosin regulatory light chain. Arch Biochem Biophys 601: 121–132, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Huke S, Venkataraman R, Faggioni M, Bennuri S, Hwang HS, Baudenbacher F, and Knollmann B. Focal energy deprivation underlies arrhythmia susceptibility in mice with calcium-sensitized myofilaments. Circ Res 112: 1334–1344, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ide T, Tsutsui H, Kinugawa S, Utsumi H, and Takeshita A. Amiodarone protects cardiac myocytes against oxidative injury by its free radical scavenging action. Circulation 100: 690–692, 1999 [DOI] [PubMed] [Google Scholar]
- 114. Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res 81: 412–419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Inoue M, Sato E, Nishikawa M, Park A, Kira Y, Imada I, andUtsum iK. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem 10: 2495–2505, 2003 [DOI] [PubMed] [Google Scholar]
- 116. Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Arteaga GM, John Solaro R, Liggett SB, and Wieczorek DF. Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis. Am J Physiol Heart Circ Physiol 293: H949–H958, 2007 [DOI] [PubMed] [Google Scholar]
- 117. Javadpour MM, Tardiff JC, Pinz I, and Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest 112: 768–775, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jeong EM, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, Wang Q, Greener I, Dudley SC, Jr., and Solaro RJ. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol 56: 44–54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jian Z, Han H, Zhang T, Puglisi J, Izu LT, Shaw JA, Onofiok E, Erickson JR, Chen YJ, Horvath B, Shimkunas R, Xiao W, Li Y, Pan T, Chan J, Banyasz T, Tardiff JC, Chiamvimonvat N, Bers DM, Lam KS, and Chen-Izu Y. Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci Signal 7: ra27, 1–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kalogeris T, Bao Y, and Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2: 702–714, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, and Paolocci N. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal 20: 267–280, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kander MC, Cui Y, and Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med 21: 1024–1032, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kang MY, Tsuchiya M, Packer L, and Manabe M. In vitro study on antioxidant potential of various drugs used in the perioperative period. Acta Anaesthesiol Scand 42: 4–12, 1998 [DOI] [PubMed] [Google Scholar]
- 124. Karimi Galougahi K, Antoniades C, Nicholls SJ, Channon KM, and Figtree GA. Redox biomarkers in cardiovascular medicine. Eur Heart J 36: 1576–1582, 1582a-b, 2015 [DOI] [PubMed] [Google Scholar]
- 125. Kawai K, Qin F, Shite J, Mao W, Fukuoka S, and Liang CS. Importance of antioxidant and antiapoptotic effects of beta-receptor blockers in heart failure therapy. Am J Physiol Heart Circ Physiol 287: H1003–H1012, 2004 [DOI] [PubMed] [Google Scholar]
- 126. Ke L, Meijering RA, Hoogstra-Berends F, Mackovicova K, Vos MJ, Van Gelder IC, Henning RH, Kampinga HH, and Brundel BJ. HSPB1, HSPB6, HSPB7 and HSPB8 protect against RhoA GTPase-induced remodeling in tachypaced atrial myocytes. PLoS One 6: e20395, 1–10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kehat I. and Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 122: 2727–2735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev 3: 114–127, 1998 [PubMed] [Google Scholar]
- 129. Kemp-Harper BK, Horowitz JD, and Ritchie RH. Therapeutic potential of nitroxyl (HNO) donors in the management of acute decompensated heart failure. Drugs 76: 1337–1348, 2016 [DOI] [PubMed] [Google Scholar]
- 130. Kennedy JA, Beck-Oldach K, McFadden-Lewis K, Murphy GA, Wong YW, Zhang Y, and Horowitz JD. Effect of the anti-anginal agent, perhexiline, on neutrophil, valvular and vascular superoxide formation. Eur J Pharmacol 531: 13–19, 2006 [DOI] [PubMed] [Google Scholar]
- 131. Kim S-J, Iizuka K, Kelly RA, Geng Y-J, Bishop SP, Yang G, Kudej A, McConnell BK, Seidman CE, Seidman JG, and Vatner SF. An α-cardiac myosin heavy chain gene mutation impairs contraction and relaxation function of cardiac myocytes. Am J Physiol 276: H1780–H1787, 1999 [DOI] [PubMed] [Google Scholar]
- 132. Knollmann BC, Blatt SA, Horton K, de Freitas F, Miller T, Bell M, Housmans PR, Weissman NJ, Morad M, and Potter JD. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79 N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem 276: 10039–10048, 2001 [DOI] [PubMed] [Google Scholar]
- 133. Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD, and Morad M. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res 92: 428–436, 2003 [DOI] [PubMed] [Google Scholar]
- 134. Kobayashi T. and Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol 67: 39–67, 2005 [DOI] [PubMed] [Google Scholar]
- 135. Koda M, Takemura G, Okada H, Kanoh M, Maruyama R, Esaki M, Li Y, Miyata S, Kanamori H, Li L, Ogino A, Kondo T, Minatoguchi S, Fujiwara T, and Fujiwara H. Nuclear hypertrophy reflects increased biosynthetic activities in myocytes of human hypertrophic hearts. Circ J 70: 710–718, 2006 [DOI] [PubMed] [Google Scholar]
- 136. Koppenol WH, Stanbury DM, and Bounds PL. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic Biol Med 49: 317–322, 2010 [DOI] [PubMed] [Google Scholar]
- 137. Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, and Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 47: 333–343, 2009 [DOI] [PubMed] [Google Scholar]
- 138. Kraft T, Montag J, Radocaj A, and Brenner B. Hypertrophic cardiomyopathy: cell-to-cell imbalance in gene expression and contraction force as trigger for disease phenotype development. Circ Res 119: 992–995, 2016 [DOI] [PubMed] [Google Scholar]
- 139. Kraft T, Witjas-Paalberends ER, Boontje NM, Tripathi S, Brandis A, Montag J, Hodgkinson JL, Francino A, Navarro-Lopez F, Brenner B, Stienen GJM, and van der Velden J. Familial hypertrophic cardiomyopathy: functional effects of myosin mutation R723G in cardiomyocytes. J Mol Cell Cardiol 57: 13–22, 2013 [DOI] [PubMed] [Google Scholar]
- 140. Kroese LJ. and Scheffer PG. 8-hydroxy-2′-deoxyguanosine and cardiovascular disease: a systematic review. Curr Atheroscler Rep 16: 452, 1–8, 2014 [DOI] [PubMed] [Google Scholar]
- 141. Lahet JJ, Lenfant F, Courderot-Masuyer C, Ecarnot-Laubriet E, Vergely C, Durnet-Archeray MJ, Freysz M, and Rochette L. In vivo and in vitro antioxidant properties of furosemide. Life Sci 73: 1075–1082, 2003 [DOI] [PubMed] [Google Scholar]
- 142. Langsjoen PH, Langsjoen A, Willis R, and Folkers K. Treatment of hypertrophic cardiomyopathy with coenzyme Q10. Mol Aspects Med 18 Suppl: S145–S151, 1997 [DOI] [PubMed] [Google Scholar]
- 143. Lee D, Vahebi S, Tocchetti C, Barouch L, Solaro RJ, Takimoto E, and Kass D. PDE5A suppression of acute β-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol 105: 337–347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lehman W. and Craig R. Tropomyosin and the steric mechanism of muscle regulation. Adv Exp Med Biol 644: 95–109, 2008 [DOI] [PubMed] [Google Scholar]
- 145. Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, and Wang Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation 111: 2494–2502, 2005 [DOI] [PubMed] [Google Scholar]
- 146. Li Q, Su D, O'Rourke B, Pogwizd SM, and Zhou L. Mitochondria-derived ROS bursts disturb Ca(2)(+) cycling and induce abnormal automaticity in guinea pig cardiomyocytes: a theoretical study. Am J Physiol Heart Circ Physiol 308: H623–H636, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, and Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103: 789–791, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lin CS, Liu CY, Sun YL, Chang LC, Chiu YT, Huang SY, Lin JH, Yang PC, Chu R, Huang MC, and Mao SJ. Alteration of endogenous antioxidant enzymes in naturally occurring hypertrophic cardiomyopathy. Biochem Mol Biol Int 43: 1253–1263, 1997 [DOI] [PubMed] [Google Scholar]
- 149. Liu T. and O'Rourke B. Regulation of the Na+/Ca2+ exchanger by pyridine nucleotide redox potential in ventricular myocytes. J Biol Chem 288: 31984–31992, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, Willerson JT, Betocchi S, Wickline SA, Efimov IR, and Marian AJ. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation 119: 1398–1407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, and Dudley SC., Jr. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res 110: 841–850, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lu J. and Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med 66: 75–87, 2014 [DOI] [PubMed] [Google Scholar]
- 153. Lu J, Sharma LK, and Bai Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell Res 19: 802–815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lucas DT, Aryal P, Szweda LI, Koch WJ, and Leinwand LA. Alterations in mitochondrial function in a mouse model of hypertrophic cardiomyopathy. Am J Physiol 284: H575–H583, 2003 [DOI] [PubMed] [Google Scholar]
- 155. Lynch TLt, Sivaguru M, Velayutham M, Cardounel AJ, Michels M, Barefield D, Govindan S, dos Remedios C, van der Velden J, and Sadayappan S. Oxidative Stress in Dilated Cardiomyopathy Caused by MYBPC3 Mutation. Oxid Med Cell Longev 2015: 424751, 1–14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, and O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 99: 172–182, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Magida JA. and Leinwand LA. Metabolic crosstalk between the heart and liver impacts familial hypertrophic cardiomyopathy. EMBO Mol Med 6: 482–495, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mak IT. and Weglicki WB. Comparative antioxidant activities of propranolol, nifedipine, verapamil, and diltiazem against sarcolemmal membrane lipid peroxidation. Circ Res 66: 1449–1452, 1990 [DOI] [PubMed] [Google Scholar]
- 159. Malhotra JD. and Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 160. Maras D, Chung R, Duncan A, Li W, Thorp C, Stellan M, Lindqvist P, and Henein MY. Patterns of cardiac dysfunction coinciding with exertional breathlessness in hypertrophic cardiomyopathy. Int J Cardiol 170: 233–238, 2013 [DOI] [PubMed] [Google Scholar]
- 161. Marian AJ, Senthil V, Chen SN, and Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol 47: 827–834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Marmolino D. Friedreich's ataxia: past, present and future. Brain Res Rev 67: 311–330, 2011 [DOI] [PubMed] [Google Scholar]
- 163. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, and Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 92: 785–789, 1995 [DOI] [PubMed] [Google Scholar]
- 164. Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, and Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 102: 858–864, 2000 [DOI] [PubMed] [Google Scholar]
- 165. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, and Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 276: 199–204, 1996 [PubMed] [Google Scholar]
- 166. Maron BJ, Wolfson JK, Epstein SE, and Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol 8: 545–557, 1986 [DOI] [PubMed] [Google Scholar]
- 167. Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, and Camici PG. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 54: 866–875, 2009 [DOI] [PubMed] [Google Scholar]
- 168. Marston S, Copeland O, Jacques A, Livesey K, Tsang V, McKenna WJ, Jalilzadeh S, Carballo S, Redwood C, and Watkins H. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res 105: 219–222, 2009 [DOI] [PubMed] [Google Scholar]
- 169. Matos TJ, Duarte CB, Goncalo M, and Lopes MC. Role of oxidative stress in ERK and p38 MAPK activation induced by the chemical sensitizer DNFB in a fetal skin dendritic cell line. Immunol Cell Biol 83: 607–614, 2005 [DOI] [PubMed] [Google Scholar]
- 170. McLendon PM. and Robbins J. Proteotoxicity and cardiac dysfunction. Circ Res 116: 1863–1882, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. McMurray JJV. and Pfeffer MA. Heart failure. Lancet 365: 1877–1889, 2005 [DOI] [PubMed] [Google Scholar]
- 172. Mearini G, Stimpel D, Geertz B, Weinberger F, Kramer E, Schlossarek S, Mourot-Filiatre J, Stoehr A, Dutsch A, Wijnker PJ, Braren I, Katus HA, Muller OJ, Voit T, Eschenhagen T, and Carrier L. Mybpc3 gene therapy for neonatal cardiomyopathy enables long-term disease prevention in mice. Nat Commun 5: 5515, 2014 [DOI] [PubMed] [Google Scholar]
- 173. Mearini G, Stimpel D, Kramer E, Geertz B, Braren I, Gedicke-Hornung C, Precigout G, Muller OJ, Katus HA, Eschenhagen T, Voit T, Garcia L, Lorain S, and Carrier L. Repair of Mybpc3 mRNA by 5′-trans-splicing in a Mouse Model of Hypertrophic Cardiomyopathy. Mol Ther Nucleic Acids 2: e102, 1–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Meijering RA, Henning RH, and Brundel BJ. Reviving the protein quality control system: therapeutic target for cardiac disease in the elderly. Trends Cardiovasc Med 25: 243–247, 2015 [DOI] [PubMed] [Google Scholar]
- 175. Mekhfi H, Veksler V, Mateo P, Maupoil V, Rochette L, and Ventura-Clapier R. Creatine kinase is the main target of reactive oxygen species in cardiac myofibrils. Circ Res 78: 1016–1027, 1996 [DOI] [PubMed] [Google Scholar]
- 176. Meurs KM. and Kuan M. Differential methylation of CpG sites in two isoforms of myosin binding protein C, an important hypertrophic cardiomyopathy gene. Environ Mol Mutagen 52: 161–164, 2011 [DOI] [PubMed] [Google Scholar]
- 177. Meurs KM. and Mealey KL. Evaluation of the flanking nucleotide sequences of sarcomeric hypertrophic cardiomyopathy substitution mutations. Mutat Res 642: 86–89, 2008 [DOI] [PubMed] [Google Scholar]
- 178. Michels M, Olivotto I, Asselbergs FW, and van der Velden J. Life-long tailoring of management for patients with hypertrophic cardiomyopathy: awareness and decision-making in changing scenarios. Neth Heart J 25: 186–199, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Michels M, Soliman OII, Kofflard MJ, Hoedemaekers YM, Dooijes D, Majoor-Krakauer D, and ten Cate FJ. Diastolic abnormalities as the first feature of hypertrophic cardiomyopathy in Dutch myosin-binding protein C founder mutations. JACC Cardiovasc Imag 2: 58–64, 2009 [DOI] [PubMed] [Google Scholar]
- 180. Mikhed Y, Gorlach A, Knaus UG, and Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol 5: 275–289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Miller T, Szczesna D, Housmans PR, Zhao J, de Freitas F, Gomes AV, Culbreath L, McCue J, Wang Y, Xu Y, Kerrick WG, and Potter JD. Abnormal contractile function in transgenic mice expressing a familial hypertrophic cardiomyopathy-linked troponin T (I79 N) mutation. J Biol Chem 276: 3743–3755, 2001 [DOI] [PubMed] [Google Scholar]
- 182. Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 63: 467–475, 2004 [DOI] [PubMed] [Google Scholar]
- 183. Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, Quiñones MA, Roberts R, and Marian AJ. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 104: 128–130, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Nagueh SF, Kopelen HA, Lim D-S, Zoghbi WA, Quiñones MA, Roberts R, and Marian AJ. Tissue Doppler imaging consistently detects myocardial contraction and relaxation abnormalities, irrespective of cardiac hypertrophy, in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation 102: 1346–1350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Najafi A, Schlossarek S, van Deel ED, van den Heuvel N, Güçlü A, Goebel M, Kuster DWD, Carrier L, and van der Velden J. Sexual dimorphic response to exercise in hypertrophic cardiomyopathy-associated MYBPC3-targeted knock-in mice. Pflugers Arch 467: 1303–1317, 2015 [DOI] [PubMed] [Google Scholar]
- 186. Najafi A, Sequeira V, Helmes M, Bollen IAE, Goebel M, Regan JA, Carrier L, Kuster DWD, and van der Velden J. Selective phosphorylation of PKA targets after β-adrenergic receptor stimulation impairs myofilament function in Mybpc3-targeted HCM mouse model. Cardiovas Res 110: 200–214, 2016 [DOI] [PubMed] [Google Scholar]
- 187. Najafi A, Sequeira V, Kuster DWD, and Van der Velden J. β-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest 46: 362–374, 2016 [DOI] [PubMed] [Google Scholar]
- 188. Nakamura K, Kusano KF, Matsubara H, Nakamura Y, Miura A, Nishii N, Banba K, Nagase S, Miyaji K, Morita H, Saito H, Emori T, and Ohe T. Relationship between oxidative stress and systolic dysfunction in patients with hypertrophic cardiomyopathy. J Card Fail 11: 117–123, 2005 [DOI] [PubMed] [Google Scholar]
- 189. Nyitrai M. and Geeves M. Adenosine diphosphate and strain sensitivity in myosin motors. Philos Trans R Soc Lond B Biol Sci 359: 1867–1877, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Olivotto I, d'Amati G, Basso C, Van Rossum A, Patten M, Emdin M, Pinto Y, Tomberli B, Camici PG, and Michels M. Defining phenotypes and disease progression in sarcomeric cardiomyopathies: contemporary role of clinical investigations. Cardiovasc Res 105: 409–423, 2015 [DOI] [PubMed] [Google Scholar]
- 191. Olivotto I, Girolami F, Sciagrà R, Ackerman MJ, Sotgia B, Bos JM, Nistri S, Sgalambro A, Grifoni C, Torricelli F, Camici PG, and Cecchi F. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J Am Coll Cardiol 58: 839–848, 2011 [DOI] [PubMed] [Google Scholar]
- 192. Osman C, Voelker DR, and Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol 192: 7–16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Paolocci N, Tavazzi B, Biondi R, Gluzband YA, Amorini AM, Tocchetti CG, Hejazi M, Caturegli PM, Kajstura J, Lazzarino G, and Kass DA. Metalloproteinase inhibitor counters high-energy phosphate depletion and AMP deaminase activity enhancing ventricular diastolic compliance in subacute heart failure. J Pharmacol Exp Ther 317: 506–513, 2006 [DOI] [PubMed] [Google Scholar]
- 194. Paulus WJ. and Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013 [DOI] [PubMed] [Google Scholar]
- 195. Pei H, Yang Y, Zhao H, Li X, Yang D, Li D, and Yang Y. The role of mitochondrial functional proteins in ROS production in ischemic heart diseases. Oxid Med Cell Longev 2016: 8, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Persson T, Popescu BO, and Cedazo-Minguez A. Oxidative stress in Alzheimer's disease: why did antioxidant therapy fail? Oxid Med Cell Longev 2014: 427318, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, and Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 115: 2418–2425, 2007 [DOI] [PubMed] [Google Scholar]
- 198. Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, and Schägger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem 278: 52873–52880, 2003 [DOI] [PubMed] [Google Scholar]
- 199. Pisano A, Cerbelli B, Perli E, Pelullo M, Bargelli V, Preziuso C, Mancini M, He L, Bates MG, Lucena JR, Della Monica PL, Familiari G, Petrozza V, Nediani C, Taylor RW, d'Amati G, and Giordano C. Impaired mitochondrial biogenesis is a common feature to myocardial hypertrophy and end-stage ischemic heart failure. Cardiovasc Pathol 25: 103–112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro JR, and Wieczorek DF. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol 33: 1815–1828, 2001 [DOI] [PubMed] [Google Scholar]
- 201. Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, and Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation 121: 997–1004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Prosser BL, Ward CW, and Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011 [DOI] [PubMed] [Google Scholar]
- 203. Prosser BL, Ward CW, and Lederer WJ. X-ROS signalling is enhanced and graded by cyclic cardiomyocyte stretch. Cardiovasc Res 98: 307–314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Qin F, Siwik DA, Lancel S, Zhang J, Kuster GM, Luptak I, Wang L, Tong X, Kang YJ, Cohen RA, and Colucci WS. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc 2: e000184, 1–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Raj A, Peskin CS, Tranchina D, Vargas DY, and Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol 4: e309, 1707–1719, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Raj A. and van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135: 216–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Rao RV. and Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol 16: 653–662, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M, and Project EHF. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107: 2227–2232, 2003 [DOI] [PubMed] [Google Scholar]
- 209. Rizzo AM, Berselli P, Zava S, Montorfano G, Negroni M, Corsetto P, and Berra B. Endogenous antioxidants and radical scavengers. Adv Exp Med Biol 698: 52–67, 2010 [DOI] [PubMed] [Google Scholar]
- 210. Robinson P, Griffiths PJ, Watkins H, and Redwood CS. Dilated and hypertrophic cardiomyopathy mutations in troponin and α-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res 101: 1266–1273, 2007 [DOI] [PubMed] [Google Scholar]
- 211. Robinson P, Mirza M, Knott A, Abdulrazzak H, Willott R, Marston S, Watkins H, and Redwood C. Alterations in thin filament regulation induced by a human cardiac troponin T mutant that causes dilated cardiomyopathy are distinct from those induced by troponin T mutants that cause hypertrophic cardiomyopathy. J Biol Chem 277: 40710–40716, 2002 [DOI] [PubMed] [Google Scholar]
- 212. Roncarati R, Viviani Anselmi C, Krawitz P, Lattanzi G, von Kodolitsch Y, Perrot A, di Pasquale E, Papa L, Portararo P, Columbaro M, Forni A, Faggian G, Condorelli G, and Robinson PN. Doubly heterozygous LMNA and TTN mutations revealed by exome sequencing in a severe form of dilated cardiomyopathy. Eur J Hum Genet 21: 1105–1111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ross JM, Olson L, and Coppotelli G. Mitochondrial and ubiquitin proteasome system dysfunction in ageing and disease: two sides of the same coin? Int J Mol Sci 16: 19458–19476, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Santos S, Marques V, Pires M, Silveira L, Oliveira H, Lanca V, Brito D, Madeira H, Esteves JF, Freitas A, Carreira IM, Gaspar IM, Monteiro C, and Fernandes AR. High resolution melting: improvements in the genetic diagnosis of hypertrophic cardiomyopathy in a Portuguese cohort. BMC Med Genet 13: 17, 1–13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Sarikas A, Carrier L, Schenke C, Doll D, Flavigny J, Lindenberg KS, Eschenhagen T, and Zolk O. Impairment of the ubiquitin-proteasome system by truncated cardiac myosin binding protein C mutants. Cardiovasc Res 66: 33–44, 2005 [DOI] [PubMed] [Google Scholar]
- 216. Sasagawa S, Nishimura Y, Okabe S, Murakami S, Ashikawa Y, Yuge M, Kawaguchi K, Kawase R, Okamoto R, Ito M, and Tanaka T. Downregulation of GSTK1 Is a common mechanism underlying hypertrophic cardiomyopathy. Front Pharmacol 7: 162, 1–13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Schlossarek S, Frey N, and Carrier L. Ubiquitin-proteasome system and hereditary cardiomyopathies. J Mol Cell Cardiol 71: 25–31, 2014 [DOI] [PubMed] [Google Scholar]
- 218. Schlossarek S, Mearini G, and Carrier L. Cardiac myosin-binding protein C in hypertrophic cardiomyopathy: mechanisms and therapeutic opportunities. J Mol Cell Cardiol 50: 613–620, 2011 [DOI] [PubMed] [Google Scholar]
- 219. Schober T, Huke S, Venkataraman R, Gryshchenko O, Kryshtal D, Hwang HS, Baudenbacher FJ, and Knollmann BC. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ Res 111: 170–179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Schulz EM, Wilder T, Chowdhury SAK, Sheikh HN, Wolska BM, Solaro RJ, and Wieczorek DF. Decreasing tropomyosin phosphorylation rescues tropomyosin-induced familial hypertrophic cardiomyopathy. J Biol Chem 288: 28925–28935, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Semsarian C, Ingles J, Maron MS, and Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 65: 1249–1254, 2015 [DOI] [PubMed] [Google Scholar]
- 222. Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, Roberts R, and Marian AJ. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res 97: 285–292, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sequeira V, Najafi A, McConnell M, Fowler ED, Bollen IAE, Wüst RCI, Remedios Cd, Helmes M, White E, Stienen GJM, Tardiff J, Kuster DWD, and van der Velden J. Synergistic role of ADP and Ca2+ in diastolic myocardial stiffness. J Physiol 593: 3899–3916, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Sequeira V, Najafi A, Wijnker P, Remedios Cd, Michels M, Kuster DWD, and van der Velden J. ADP-stimulated contraction: a predictor of thin-filament activation in cardiac disease. Proc Natl Acad Sci USA 112: E7003–E7012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Sequeira V, Wijnker PJ, Nijenkamp LL, Kuster DW, Najafi A, Witjas-Paalberends ER, Regan JA, Boontje N, Ten Cate FJ, Germans T, Carrier L, Sadayappan S, van Slegtenhorst MA, Zaremba R, Foster DB, Murphy AM, Poggesi C, Dos Remedios C, Stienen GJ, Ho CY, Michels M, and van der Velden J. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res 112: 1491–1505, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shang F. and Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med 51: 5–16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Shigenaga MK, Hagen TM, and Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci 91: 10771–10778, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Shirani J, Pick R, Roberts WC, and Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 35: 36–44, 2000 [DOI] [PubMed] [Google Scholar]
- 229. Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, and Dudley SC., Jr. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation 121: 519–528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Sirenko SG, Potter JD, and Knollmann BC. Differential effect of troponin T mutations on the inotropic responsiveness of mouse hearts - role of myofilament Ca2+ sensitivity increase. J Physiol 575: 201–213, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Slone S, Anthony SR, Wu X, Benoit JB, Aube J, Xu L, and Tranter M. Activation of HuR downstream of p38 MAPK promotes cardiomyocyte hypertrophy. Cell Signal 28: 1735–1741, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, and Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 114: 1151–1158, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Solaro RJ, Moir AJG, and Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature 262: 615–617, 1976 [DOI] [PubMed] [Google Scholar]
- 234. Somura F, Izawa H, Iwase M, Takeichi Y, Ishiki R, Nishizawa T, Noda A, Nagata K, Yamada Y, and Yokota M. Reduced myocardial sarcoplasmic reticulum Ca2+-ATPase mRNA expression and biphasic force-frequency relations in patients with hypertrophic cardiomyopathy. Circulation 104: 658–663, 2001 [DOI] [PubMed] [Google Scholar]
- 235. Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, and Pae HO. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: how Can ROS Activate MAPK Pathways? J Signal Transduct 2011: 792639, 1–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Spickett CM. The lipid peroxidation product 4-hydroxy-2-nonenal: advances in chemistry and analysis. Redox Biol 1: 145–152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Spindler M, Saupe KW, Christe ME, Sweeney HL, Seidman CE, Seidman JG, and Ingwall JS. Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest 101: 1775–1783, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Stanley BA, Sivakumaran V, Shi S, McDonald I, Lloyd D, Watson WH, Aon MA, and Paolocci N. Thioredoxin reductase-2 is essential for keeping low levels of H(2)O(2) emission from isolated heart mitochondria. J Biol Chem 286: 33669–33677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Stanley WC, Recchia FA, and Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 240. Stathopoulou K, Wittig I, Heidler J, Piasecki A, Richter F, Diering S, van der Velden J, Buck F, Donzelli S, Schroder E, Wijnker PJ, Voigt N, Dobrev D, Sadayappan S, Eschenhagen T, Carrier L, Eaton P, and Cuello F. S-glutathiolation impairs phosphoregulation and function of cardiac myosin-binding protein C in human heart failure. FASEB J 30: 1849–1864, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol 101: 14D-19D, 2008 [DOI] [PubMed] [Google Scholar]
- 242. Sun SY. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther 9: 109–110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Surdo NC, Berrera M, Koschinski A, Brescia M, Machado MR, Carr C, Wright P, Gorelik J, Morotti S, Grandi E, Bers DM, Pantano S, and Zaccolo M. FRET biosensor uncovers cAMP nano-domains at beta-adrenergic targets that dictate precise tuning of cardiac contractility. Nat Commun 8: 15031, 1–14, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Sweeney HL, Feng HS, Yang Z, and Watkins H. Functional analyses of troponin T mutations that cause hypertrophic cardiomyopathy: insights into disease pathogenesis and troponin function. Proc Natl Acad Sci USA 95: 14406–14410, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171: 2029–2050, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Szeto HH. and Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther 96: 672–683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Takimoto E. and Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49: 241–248, 2007 [DOI] [PubMed] [Google Scholar]
- 248. Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev 10: 237–248, 2005 [DOI] [PubMed] [Google Scholar]
- 249. Tardiff JC, Carrier L, Bers DM, Poggesi C, Ferrantini C, Coppini R, Maier LS, Ashrafian H, Huke S, and van der Velden J. Targets for therapy in sarcomeric cardiomyopathies. Cardiovas Res 105: 457–470, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, Robbins J, and Leinwand LA. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Inv 104: 469–481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Taylor RW. and Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet 6: 389–402, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J 20: 1–8, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, and Seidman JG. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest 120: 3520–3529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Timmer SA, Germans T, Brouwer WP, Lubberink M, van der Velden J, Wilde AA, Christiaans I, Lammertsma AA, Knaapen P, and van Rossum AC. Carriers of the hypertrophic cardiomyopathy MYBPC3 mutation are characterized by reduced myocardial efficiency in the absence of hypertrophy and microvascular dysfunction. Eur J Heart Fail 13: 1283–1289, 2011 [DOI] [PubMed] [Google Scholar]
- 255. Tripathi S, Schultz I, Becker E, Montag J, Borchert B, Francino A, Navarro-Lopez F, Perrot A, Ozcelik C, Osterziel KJ, McKenna WJ, Brenner B, and Kraft T. Unequal allelic expression of wild-type and mutated beta-myosin in familial hypertrophic cardiomyopathy. Basic Res Cardiol 106: 1041–1055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Tse G, Yan BP, Chan YW, Tian XY, and Huang Y. Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Front Physiol 7: 313, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Tsybouleva N, Zhang L, Chen S, Patel R, Lutucuta S, Nemoto S, DeFreitas G, Entman M, Carabello BA, Roberts R, and Marian AJ. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation 109: 1284–1291, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Unno K, Isobe S, Izawa H, Cheng XW, Kobayashi M, Hirashiki A, Yamada T, Harada K, Ohshima S, Noda A, Nagata K, Kato K, Yokota M, and Murohara T. Relation of functional and morphological changes in mitochondria to myocardial contractile and relaxation reserves in asymptomatic to mildly symptomatic patients with hypertrophic cardiomyopathy. Cardiovasc Res 30: 1853–1862, 2009 [DOI] [PubMed] [Google Scholar]
- 259. Vakrou S. and Abraham MR. Hypertrophic cardiomyopathy: a heart in need of an energy bar? Front Physiol 5: 309, 1–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Valko M, Rhodes CJ, Moncol J, Izakovic M, and Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160: 1–40, 2006 [DOI] [PubMed] [Google Scholar]
- 261. van Dijk SJ, Dooijes D, dos Remedios C, Michels M, Lamers JM, Winegrad S, Schlossarek S, Carrier L, ten Cate FJ, Stienen GJ, and van der Velden J. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation 119: 1473–1483, 2009 [DOI] [PubMed] [Google Scholar]
- 262. van Dijk SJ, Paalberends ER, Najafi A, Michels M, Sadayappan S, Carrier L, Boontje NM, Kuster DWD, van Slegtenhorst M, Dooijes D, dos Remedios C, ten Cate FJ, Stienen GJM, and van der Velden J. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail 5: 36–46, 2012 [DOI] [PubMed] [Google Scholar]
- 263. Vignier N, Schlossarek S, Fraysse B, Mearini G, Krämer E, Pointu H, Mougenot N, Guiard J, Reimer R, Hohenberg H, Schwartz K, Vernet M, Eschenhagen T, and Carrier L. Nonsense-mediated mRNA decay and ubiquitin-proteasome system regulate cardiac myosin-binding protein C mutant levels in cardiomyopathic mice. Circ Res 105: 239–248, 2009 [DOI] [PubMed] [Google Scholar]
- 264. Vilchez D, Saez I, and Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun 5: 5659, 1–13, 2014 [DOI] [PubMed] [Google Scholar]
- 265. Voss P. and Siems W. Clinical oxidation parameters of aging. Free Radic Res 40: 1339–1349, 2006 [DOI] [PubMed] [Google Scholar]
- 266. Wallimann T, Wyss M, Brdiczka D, Nicolay K, and Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281: 21–40, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Wang X. and Robbins J. Heart failure and protein quality control. Circ Res 99: 1315–1328, 2006 [DOI] [PubMed] [Google Scholar]
- 268. Wang X. and Robbins J. Proteasomal and lysosomal protein degradation and heart disease. J Mol Cell Cardiol 71: 16–24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Wani R, Nagata A, and Murray BW. Protein redox chemistry: post-translational cysteine modifications that regulate signal transduction and drug pharmacology. Front Pharmacol 5: 224, 1–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Watkins H. Sudden death in hypertrophic cardiomyopathy. N Eng J Med 342: 422–424, 2000 [DOI] [PubMed] [Google Scholar]
- 271. Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, and Seidman CE. Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiomyopathy. N Eng J Med 332: 1058–1065, 1995 [DOI] [PubMed] [Google Scholar]
- 272. Watkins H, Rosenzweig A, Hwang D-S, Levi T, McKenna W, Seidman CE, and Seidman JG. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Eng J Med 326: 1108–1114, 1992 [DOI] [PubMed] [Google Scholar]
- 273. Wigle ED, Rakowski H, Kimball BP, and Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 92: 1680–1692, 1995 [DOI] [PubMed] [Google Scholar]
- 274. Wijnker PJ, Friedrich FW, Dutsch A, Reischmann S, Eder A, Mannhardt I, Mearini G, Eschenhagen T, van der Velden J, and Carrier L. Comparison of the effects of a truncating and a missense MYBPC3 mutation on contractile parameters of engineered heart tissue. J Mol Cell Cardiol 97: 82–92, 2016 [DOI] [PubMed] [Google Scholar]
- 275. Wijnker PJM, Foster DB, Tsao AL, Frazier AH, dos Remedios C, Murphy AM, Stienen GJM, and van der Velden J. Impact of site-specific phosphorylation of the protein kinase A sites ser23 and ser24 of cardiac troponin in human cardiomyocytes. Am J Physiol 304: H260–H268, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Wijnker PJM, Sequeira V, Foster DB, Li Y, dos Remedios CG, Murphy AM, Stienen GJM, and van der Velden J. Length-dependent activation is modulated by cardiac troponin I bisphosphorylation at Ser23 and Ser24 but not by Thr143 phosphorylation. Am J Physiol 306: H1171–H1181, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Wilder T, Ryba DM, Wieczorek DF, Wolska BM, and Solaro RJ. N-acetylcysteine reverses diastolic dysfunction and hypertrophy in familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 309: H1720–H1730, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Willis MS. and Patterson C. Proteotoxicity and cardiac dysfunction. N Engl J Med 368: 1754–1755, 2013 [DOI] [PubMed] [Google Scholar]
- 279. Witjas-Paalberends ER, Ferrara C, Scellini B, Piroddi N, Montag J, Tesi C, Stienen GJ, Michels M, Ho CY, Kraft T, Poggesi C, and van der Velden J. Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation. J Physiol 592: 3257–3272, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Witjas-Paalberends ER, Guclu A, Germans T, Knaapen P, Harms HJ, Vermeer AM, Christiaans I, Wilde AA, Dos Remedios C, Lammertsma AA, van Rossum AC, Stienen GJ, van Slegtenhorst M, Schinkel AF, Michels M, Ho CY, Poggesi C, and van der Velden J. Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc Res 103: 248–257, 2014 [DOI] [PubMed] [Google Scholar]
- 281. Yamada Y, Ichihara S, Izawa H, Tanaka M, and Yokota M. Association of a G994—> T (Val279—> Phe) polymorphism of the plasma platelet-activating factor acetylhydrolase gene with myocardial damage in Japanese patients with nonfamilial hypertrophic cardiomyopathy. J Hum Genet 46: 436–441, 2001 [DOI] [PubMed] [Google Scholar]
- 282. Yang J, Xu WW, and Hu SJ. Heart failure: advanced development in genetics and epigenetics. Biomed Res Int 2015: 352734, 1–11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ye G, Metreveli NS, Ren J, and Epstein PN. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes 52: 777–783, 2003 [DOI] [PubMed] [Google Scholar]
- 284. Yin Q, Lu H, Bai Y, Tian A, Yang Q, Wu J, Yang C, Fan TP, Zhang Y, Zheng X, Zheng X, and Li Z. A metabolite of Danshen formulae attenuates cardiac fibrosis induced by isoprenaline, via a NOX2/ROS/p38 pathway. Br J Pharmacol 172: 5573–5585, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Yoshida N, Ikeda H, Wada T, Matsumoto A, Maki S, Muro A, Shibata A, and Imaizumi T. Exercise-induced abnormal blood pressure responses are related to subendocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 32: 1938–1942, 1998 [DOI] [PubMed] [Google Scholar]
- 286. Young IS. and McEneny J. Lipoprotein oxidation and atherosclerosis. Biochem Soc Trans 29: 358–362, 2001 [DOI] [PubMed] [Google Scholar]
- 287. Yue TL, Lysko PG, Barone FC, Gu JL, Ruffolo RR, Jr., and Feuerstein GZ. Carvedilol, a new antihypertensive drug with unique antioxidant activity: potential role in cerebroprotection. Ann N Y Acad Sci 738: 230–242, 1994 [DOI] [PubMed] [Google Scholar]
- 288. Zafarullah M, Li WQ, Sylvester J, and Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 60: 6–20, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Zhang C, Liu R, Yuan J, Cui J, Hu F, Yang W, Zhang Y, Yang C, and Qiao S. Gender-related differences in the association between serum uric acid and left ventricular mass index in patients with obstructive hypertrophic cardiomyopathy. Biol Sex Differ 7: 22, 1–12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Zhang D, Ke L, Mackovicova K, Van Der Want JJ, Sibon OC, Tanguay RM, Morrow G, Henning RH, Kampinga HH, and Brundel BJ. Effects of different small HSPB members on contractile dysfunction and structural changes in a Drosophila melanogaster model for Atrial Fibrillation. J Mol Cell Cardiol 51: 381–389, 2011 [DOI] [PubMed] [Google Scholar]
- 291. Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A, and Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res 65: 230–238, 2005 [DOI] [PubMed] [Google Scholar]
- 292. Zhang QJ, Chen HZ, Wang L, Liu DP, Hill JA, and Liu ZP. The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J Clin Invest 121: 2447–2456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Zhang W, Xiao S, and Ahn D. Protein oxidation: basic principles and implications for meat quality. Crit Rev Food Sci Nutr 53: 1191–1201, 2013 [DOI] [PubMed] [Google Scholar]
- 294. Zhou S, Sun W, Zhang Z, and Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Longev 2014: 260429, 1–16, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Zhu L, Wang J, Wang Y, Jia L, Sun K, Wang H, Zou Y, Tian T, Liu Y, Zou J, Hui R, Yuan Z, and Song L. Plasma uric acid as a prognostic marker in patients with hypertrophic cardiomyopathy. Can J Cardiol 31: 1252–1258, 2015 [DOI] [PubMed] [Google Scholar]