INTRODUCTION:
Prostate-specific membrane antigen (PSMA) was originally found to be specifically expressed in normal prostate, and its expression was upregulated in almost all stages of prostate cancer. In recent years, PSMA was also found to be expressed in tumor-associated vasculature in many nonprostatic solid tumors. However, the expression pattern of PSMA in hepatocellular carcinoma (HCC) is not well studied.
METHODS:
In this study, we examined PSMA expression in 103 HCC tissues using immunohistochemical staining and analyzed the association between PSMA expression and other clinicopathological features and prognosis.
RESULTS:
Among the 103 cases, 27 cases (26%) showed PSMA expression in more than 50% of tumor-associated vasculature, 49 cases (48%) showed PSMA expression in less than 50% of vasculature, and 27 cases (26%) did not have detectable PSMA expression. Vascular PSMA expression was associated with several clinicopathological features, such as tumor stage, tumor differentiation, lymph node metastasis, and Ki-67 index. Furthermore, high vascular PSMA expression was also associated with poor prognosis in patients with HCC. Univariate and multivariate analyses showed that high vascular PSMA expression can be used as an independent prognostic marker for HCC.
DISCUSSION:
Our study provides the evidence that PSMA is specifically expressed in tumor-associated vasculature of HCC, and vascular PSMA expression may be used as a novel prognostic marker and a vascular therapeutic target for HCC.
INTRODUCTION
Tumor angiogenesis is a common feature of solid tumors. Tumor-associated vasculature forms the pathological basis for the growth, invasion, and metastasis of solid tumors. Specific inhibition on vascularization in solid tumors has been proven to be an effective strategy for cancer treatment (1). The inhibition on tumor neovascularization can be achieved through interfering with angiogenic growth factors or directly targeting the molecules that are specifically expressed in tumor-associated vasculature. However, the angiogenic growth factors and molecular markers on tumor blood vessels are also shared by nonmalignant conditions (2). Thus, it is of vital importance to identify molecular markers that are specifically expressed in tumor-associated vasculature, which will definitely promote more accurate targeted therapy for solid tumors.
Prostate-specific membrane antigen (PSMA) is a type II transmembrane protein, which contains a large extracellular domain, a transmembrane domain, and a short intracellular domain. PSMA was originally found to be specifically expressed in the epithelial cells of normal prostate. Later on, many studies reported that PSMA expression was upregulated in almost all stages of prostate cancer (PCa), and its expression is much higher in poorly differentiated, metastatic, and hormone-refractory cases (3–7). Thus, PSMA has been considered to be an ideal target for PCa therapy (8–10). Recent years, more and more literatures reported that PSMA was also expressed in the vasculature of many cancer types, such as breast cancer, lung cancer, gastric cancer, colorectal cancer, pancreatic cancer, renal cell carcinoma, and bladder cancer, but not in normal vascular endothelial cells (11–25). Thus, PSMA has also been considered to be an effective target for the cancer types with vascular PSMA expression (8,10). However, its expression pattern in hepatocellular carcinoma (HCC) is not well studied.
In this study, we examined PSMA expression in 103 HCC samples by immunohistochemistry (IHC) staining and analyzed the association between its expression and other clinicopathological features and prognosis. We found that PSMA is specifically expressed in tumor-associated vasculature in a subset of HCC samples. We also found that vascular PSMA expression is correlated with other clinicopathological features and poor prognosis. Our results indicated that vascular PSMA expression may be used as a novel prognostic marker and a therapeutic target for HCC.
METHODS
Patients
This study was approved by the Ethics Committee of Fourth Military Medical University, and all participating patients have given their written informed consent. In this study, 103 HCC tissue samples were obtained from patients who underwent surgery at Xijing Hospital from 2010 to 2017. Formalin-fixed paraffin-embedded tumor blocks were obtained from the Department of Pathology of Xijing Hospital. Patients were followed up from the date of surgery, with an average follow-up period of 50 months (1–116 months). Detailed pathology diagnosis was provided by experienced pathologists according to the seventh edition of the American Joint Committee on Cancer staging manual. Clinical information was derived from the electronic medical record.
IHC staining
A 4-μm thick tissue piece was cut from a representative wax block and placed on a glass slide, dewaxed by xylene, and dehydrated with gradient alcohol; then, antigen retrieval was performed at high temperature and pressure in 10 nM, pH 6.0 citrate buffer. After endogenous peroxidase was inactivated, sections were blocked with nonimmune serum and incubated with primary antibody in a humidified box for overnight at 4 °C. After being washed 3 times with phosphate buffered saline, sections were incubated with horseradish peroxidase-labeled secondary antibody at room temperature for 30 minutes, followed by 3 times wash with phosphate buffered saline again. Diaminobenzidine was used as a chromogen substrate. Sections were then counterstained with hematoxylin, raised in water, dehydrated in ascending concentrations of ethanol, followed by clearance with xylene, and cover slipped permanently for light microscopy observation. Anti-PSMA antibody (#12815) and Anti-CD31 antibody (#3528) were purchased from Cell Signaling Technology. The IHC staining kit was purchased from Fuzhou Maixin Biotech. All procedures were performed according to the manufacturer's instructions.
Evaluation of IHC staining
CD31 staining in serial sections was used to identify tumor-associated vasculature. For the evaluation of vascular PSMA expression, first, we randomly selected 5 fields under low magnification (×100) and counted the number of CD31+ vascular structures, then we chose the fields with microvessel density greater than 40 as hot spot area, and last, we examined PSMA expression in 3 fields under high magnification (×200). Vascular PSMA expression was assessed in a semiquantitative manner. Lesions with no detectable PSMA expression were scored as “0”; lesions with PSMA staining in 1%–50% of CD31+ vasculatures were scored as “1”; and lesions with PSMA staining in >50% of CD31+ vasculatures were scored as “2.” For statistical analysis, samples with a staining score of 0 and 1 were grouped as “low PSMA expression,” and samples with a staining score of 2 were grouped as “high PSMA expression.”
Statistical analysis
All statistical analysis was performed using IBM SPSS statistical software (version 23). Descriptive statistics, such as median, range, and absolute and relative frequencies, were calculated to define the study cohort characterizations. The χ2 test was used to assess the association between PSMA expression and various clinicopathological features. Survival time is defined from the day of surgery until death. Survival curve was generated using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios with corresponding 95% confidence intervals were estimated using Cox proportional hazards models. P values < 0.05 were considered to be statistically significant.
RESULTS
PSMA is specifically expressed in tumor-associated vasculature in HCC
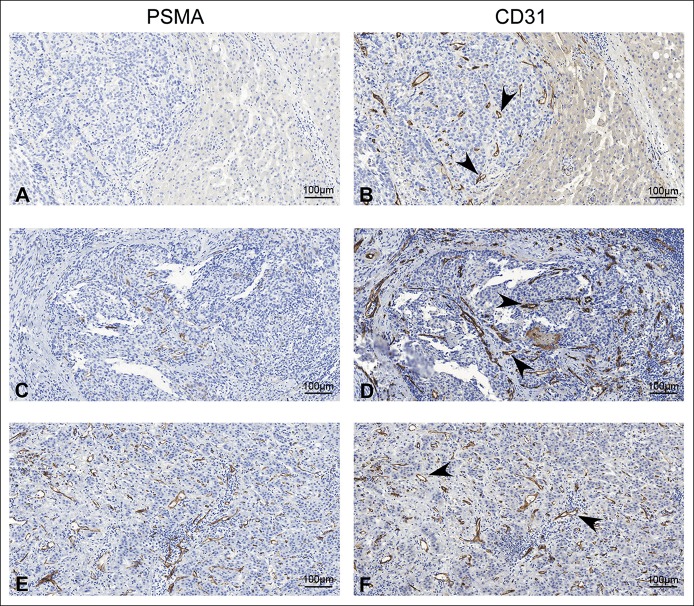
Among the 103 HCC samples, 27 samples (26%) did not show detectable PSMA expression (score 0), 49 samples (48%) showed PSMA expression in ≤50% of tumor-associated vasculature (score 1), and 27 samples (26%) showed PSMA expression in >50% of tumor-associated vasculature (score 2). PSMA expression was not observed in HCC tumor cells in all the samples we examined. In addition, we also examined PSMA expression in 5 normal liver tissues; PSMA was also not observed in all these normal liver tissues (data not shown). Representative IHC staining results in HCC with different PSMA expression was shown in Figure 1a,c, and e. Vascular structure was also confirmed by the staining of CD31, a well-established endothelial cell marker (Figure 1b,d, and f). These results demonstrate that PSMA was specifically expressed in tumor-associated vasculature in HCC.
Figure 1.
Representative IHC staining results to show PSMA expression in tumor-associated vasculature of HCC. (a, c, and e) Representative pictures to show different PSMA expression levels in HCC. (a) No PSMA expression (score = 0); (c) positive PSMA expression in ≤50% of tumor-associated vasculature (score = 1); (e) positive PSMA expression in >50% of tumor-associated vasculature (score = 2) (scale bar = 100 μm). (b, d, and f) Representative pictures to show CD31 expression in serial sections; arrowheads indicate the microvascular structure (scale bar = 100 μm). HCC, hepatocellular carcinoma; IHC, immunohistochemistry; PSMA, prostate-specific membrane antigen.
Vascular PSMA expression is associated with other clinicopathological features in HCC
All clinicopathological features of the patients were summarized in Table 1. The association between vascular PSMA expression and other clinicopathological features was presented in Table 2. Patients were divided into 2 groups based on PSMA expression in tumor-associated vasculature. No significant association was found between vascular PSMA expression and age or sex. However, patients with moderate/poorly differentiated tumors were more likely than patients with well-differentiated tumors to have high PSMA expression (P = 0.0005). Patients with high tumor stage and lymph node metastasis were also prone to have high PSMA expression (P = 0.004; P = 0.023). Tumor-node-metastasis (TNM) stage was also found to be associated with PSMA expression, and patients with TNM stage III/IV were more likely than patients with TNM stage I/II to have high PSMA expression (P = 0.007). We also analyzed the association between PSMA expression and Ki-67 proliferation index. All the samples were divided into 2 groups according to Ki-67 index, 1 group with Ki-67 index >10% and the other with Ki-67 index ≤10%, since Ki-67 index of normal liver tissue was less than 10% (26). We found that patients with high Ki-67 index were tended to have high PSMA expression than patients with low Ki-67 index. These results indicate that vascular PSMA expression is associated with other clinicopathological features, such as tumor differentiation, tumor stage, lymph node metastasis, TNM stage, and Ki-67 index in HCC.
Table 1.
Clinicopathological features of 103 patients with hepatocellular carcinoma
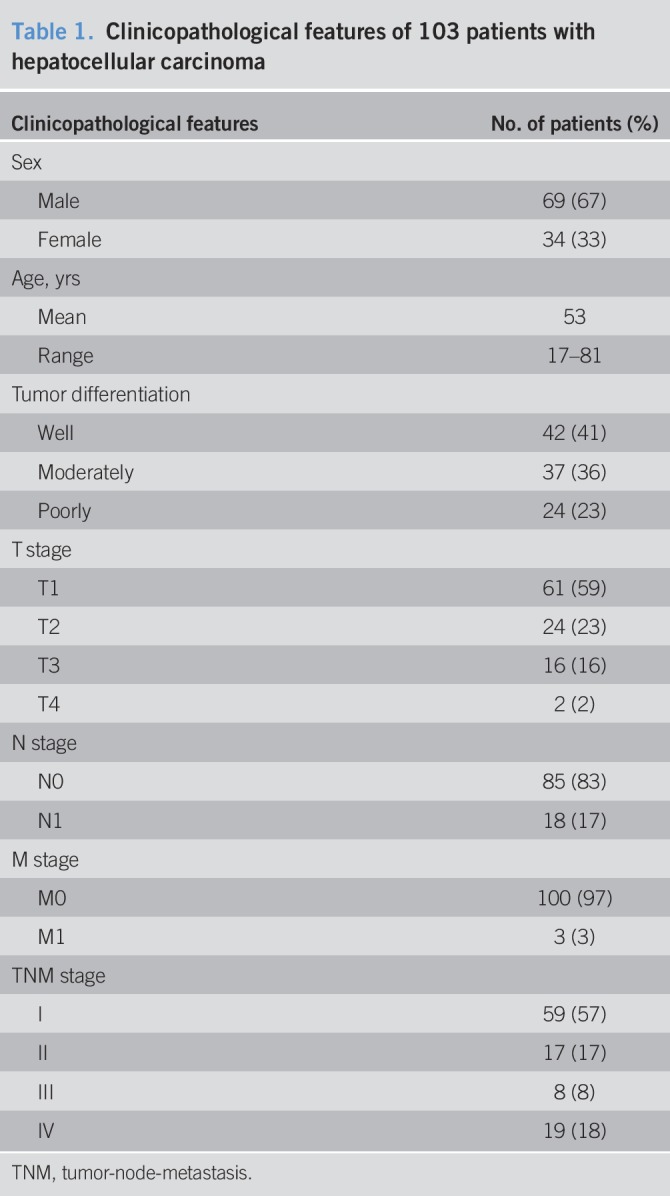
Table 2.
Correlation between vascular PSMA expression and clinicopathological features in patients with hepatocellular carcinoma (n = 103)
Vascular PSMA expression predicts poor prognosis in HCC
To further evaluate the prognostic value of vascular PSMA expression in HCC, we analyzed the relationship between PSMA expression and overall survival (OS). Survival curve of patients with different PSMA expression was generated using Kaplan-Meier analysis and a log-rank test. As shown in Figure 2, significant difference in OS was found between patients with high PSMA expression and low PSMA expression (P < 0.001). Patients with high PSMA expression tended to have an increased risk of death than patients with low PSMA expression. We also analyzed prognostic factors for OS using Cox regression analysis (Table 3). Univariate analysis showed that vascular PSMA expression (P < 0.001), tumor stage (P < 0.001), lymph node metastasis (P < 0.001), distant metastasis (P = 0.032), TNM stage (P < 0.001), tumor differentiation (P = 0.002), and Ki-67 (P = 0.001) were all associated with prognosis of HCC. Multivariate analysis revealed that vascular PSMA expression (P = 0.013) and TNM stage (P = 0.007) were associated with OS. These results indicate that vascular PSMA expression could be used as an independent prognostic marker in HCC.
Figure 2.
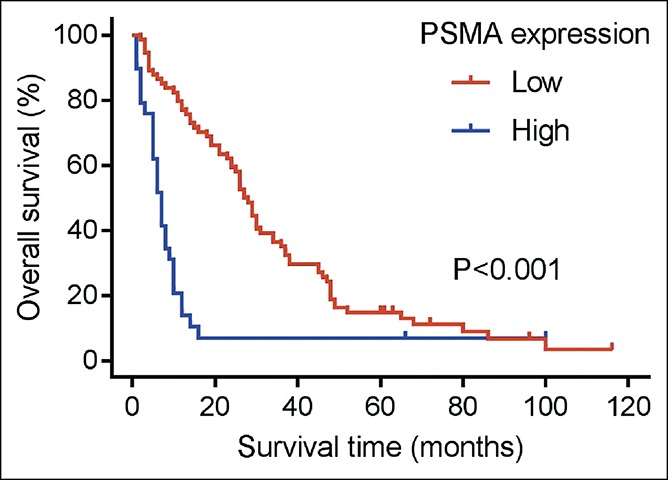
Kaplan-Meier survival curve to show the correlation between vascular PSMA expression and overall survival in patients with HCC. HCC, hepatocellular carcinoma; PSMA, prostate-specific membrane antigen.
Table 3.
Cox regression analysis of prognostic factors for overall survival in patients with hepatocellular carcinoma (n = 103)
DISCUSSION
The growth of solid tumor is critically dependent on their capacity to acquire blood supply, and it has been demonstrated that targeted destruction of tumor vasculature is an effective strategy for solid tumor treatment. Angiogenesis inhibitors have been shown to have effective antitumor activity in a broad spectrum of cancer types (1,27). However, novel molecular targets that are specifically expressed in tumor-associated vasculature are urgently needed.
PSMA is a transmembrane protein, which was originally found to be expressed in normal prostate tissue. Later, its expression was found to be upregulated in almost all stages of PCa tissues, making it an ideal target for the therapy of PCa (8–10). In recent years, researchers have found that PSMA is also selectively expressed in tumor-associated vasculature in a variety of solid tumors (11–25). Thus, PSMA has also been considered to be an effective vascular target for these cancer types (8,10).
Vascular PSMA expression has been shown to be correlated with other clinicopathological features and may predict prognosis in certain cancer types. For example, in oral squamous cell carcinoma, significant difference in OS was observed between high/low vascular PSMA expression, and high PSMA expression can be used as an independent marker for poor prognosis (11). In breast cancer, vascular PSMA expression has also been shown to be correlated with other clinicopathological features, such as tumor size, Ki-67 proliferation index, and 10-year OS (13).
Until now, the expression pattern of PSMA in HCC has not been systemically studied. Recently, Kesler et al. reported that 68Ga-PSMA PET-CT (positron emission tomography-computed tomography) can be used for the imaging of HCC, and 68Ga-PSMA was mainly taken up by tumor microvessels in HCC lesions. Based on these findings, they performed IHC staining of PSMA in 5 HCC samples and found that PSMA was intensely expressed in tumor microvessel in these samples (28).
In our study, we evaluated PSMA expression in 103 patients with HCC using IHC staining and confirmed that PSMA is specifically expressed in the vasculature of HCC, and its vascular expression is significantly correlated with other clinicopathological features such as tumor stage, tumor differentiation, lymph node metastasis, TNM stage, and Ki-67 index. We also found that patients with high vascular PSMA expression tended to have shorter OS than patients with low vascular PSMA expression. In addition, using Cox regression analysis, we found that high vascular PSMA expression is closely correlated with poor prognosis of patients with HCC and can be used as an independent prognostic marker in HCC. Because the sample number in this study is still not big enough, we believe that more studies with increased sample numbers are still needed to confirm the prognostic value of vascular PSMA expression in HCC.
Previously, PSMA has been shown to play an important role in angiogenesis. Conway et al. found that angiogenesis is severely impaired in PSMA-null animals, and PSMA may promote endothelial cell invasion and angiogenesis through an autoregulatory loop involving laminin-specific integrin and PAK1 (p21-activated kinase 1) (29). They also found that matrix metalloproteinase 2 and PSMA sequentially digest laminin into small peptides, which can enhance endothelial cell adhesion and migration through integrin α6β1 and focal adhesion kinase, thus promoting angiogenesis (30,31). However, more studies are still needed to clarify the detailed mechanism how PSMA regulates angiogenesis.
PSMA has a large extracellular domain, which can be recognized by antibodies, peptides, RNA aptamers, and small molecules, making it an ideal molecule for targeted therapy (32–34). PSMA-targeted therapy has been well studied in metastatic PCa (35–37). Because of its specific vascular expression in nonprostatic solid tumors, PSMA has also been examined as a vascular target for the therapy of these tumors. For example, phase I trials of 111In-labeled monoclonal antibody J591 against PSMA were performed in multiple solid tumor types including kidney, bladder, lung, breast, colorectal and pancreatic cancers, and melanoma. Results confirmed that tumor vasculature of these solid tumors can be selectively and safely targeted using this antibody (38,39). Because PSMA was also specifically expressed in the vasculature of HCC, these PSMA targeting strategies may also be applied for HCC treatment.
In summary, the current findings confirmed that vascular PSMA expression exists in a subset of HCC samples, and vascular PSMA expression is correlated with other clinicopathological features and can be used as an independent prognostic marker for HCC. Thus, we conclude that PSMA can be used as a novel prognostic marker and a therapeutic vascular target in HCC.
CONFLICTS OF INTEREST
Guarantor of the article: The corresponding authors (Weihong Wen, PhD, He Wang, PhD, and Weijun Qin, PhD) are accepting full responsibility for the conduct of the study. They have had access to the data and have control of the decision to publish.
Specific author contributions: Dian Jiao and Yu Li contributed equally to this work. D.J. contributed in conducting the study, collecting and interpreting data, and drafting the manuscript. Y.L. and F.Y. contributed in performing the experiments and collecting and interpreting data. D.H., J.W., S.S., F.T. contributed in data analysis and constructive discussions. Z.G., W.X., and G.L. contributed in reagents and materials preparation. A.Z. and A.-G.Y. contributed in technical discussion and suggestions. W.Q., H.W., and W.W. contributed in planning the study and drafting the manuscript. All authors have approved the final draft submitted.
Financial support: This work was supported by grants from the National Natural Science Foundation of China (Nos. 81372225, 81372771, 81472392, 81772734, and 81630069), the open project program of the State Key Laboratory of Cancer Biology (No. CBSKL201705, Fourth Military Medical University), and the Key research and development program of Shaanxi Province (2018SF-215).
Potential competing interests: The authors declare that they have no conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ PSMA exists in normal prostate, and its expression is upregulated in prostate cancer.
✓ PSMA is also expressed in tumor-associated vasculature in some nonprostatic solid tumors.
WHAT IS NEW HERE
✓ PSMA is specifically expressed in tumor-associated vasculature in a subset of HCC.
✓ Vascular PSMA expression is associated with clinicopathological features and predicts poor prognosis.
TRANSLATIONAL IMPACT
✓ Vascular PSMA can be used as a prognostic marker and a therapeutic vascular target for HCC.
ACKNOWLEDGEMENTS
We thank Mrs. Honglei Li (Department of Pathology of Xijing Hospital, Fourth Military Medical University, China) for detailed pathology diagnosis. We thank Mrs. Lijun Wang (Department of Pathology, Fourth Military Medical University, China) for IHC staining analysis. We also thank Dr. Hongxiang Bao (Department of Statistics, Fourth Military Medical University, China) for statistical analysis.
REFERENCES
- 1.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer 2005;5:436–46. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer 2002;2:83–90. [DOI] [PubMed] [Google Scholar]
- 3.Chang SS. Overview of prostate-specific membrane antigen. Rev Urol 2004;6(Suppl 10):S13–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res 2003;9:6357–62. [PubMed] [Google Scholar]
- 5.Sweat SD, Pacelli A, Murphy GP. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998;52:637–40. [DOI] [PubMed] [Google Scholar]
- 6.Bostwick DG, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intraepithelialneoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998;82:2256–61. [DOI] [PubMed] [Google Scholar]
- 7.Wright GL, Jr, Haley C, Beckett ML, et al. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol 1995;1:18–28. [DOI] [PubMed] [Google Scholar]
- 8.Elsasser-Beile U, Buhler P, Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr Drug Targets 2009;10:118–25. [DOI] [PubMed] [Google Scholar]
- 9.Bühler P, Wolf P, Elsässer-Beile U. Targeting the prostate-specific membrane antigen for prostate cancer therapy. Immunotherapy 2009;1:471–81. [DOI] [PubMed] [Google Scholar]
- 10.Slovin SF. Targeting novel antigens for prostate cancer treatment: Focus on prostate-specific membrane antigen. Expert Opin Ther Targets 2005;9:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haffner MC, Laimer J, Chaux A, et al. High expression of prostate-specific membrane antigen in the tumor-associated neo-vasculature is associated with worse prognosis in squamous cell carcinoma of the oral cavity. Mod Pathol 2012;25:1079–85. [DOI] [PubMed] [Google Scholar]
- 12.Chang SS, Reuter VE, Heston WD, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res 1999;59:3192–8. [PubMed] [Google Scholar]
- 13.Wernicke AG, Varma S, Greenwood EA, et al. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS 2014;122:482–9. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Moy P, Kim S, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res 1997;57:3629–34. [PubMed] [Google Scholar]
- 15.Baccala A, Sercia L, Li J, et al. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology 2007;70:385–90. [DOI] [PubMed] [Google Scholar]
- 16.Chang SS, Reuter VE, Heston WD, et al. Metastatic renal cell carcinoma neovasculature expresses prostate-specificmembrane antigen. Urology 2001;57:801–5. [DOI] [PubMed] [Google Scholar]
- 17.Samplaski MK, Heston W, Elson P, et al. Folate hydrolase (prostate-specific membrane [corrected] antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Mod Pathol 2011;24:1521–9. [DOI] [PubMed] [Google Scholar]
- 18.Haffner MC, Kronberger IE, Ross JS, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol 2009;40:1754–61. [DOI] [PubMed] [Google Scholar]
- 19.Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997;3:81–5. [PubMed] [Google Scholar]
- 20.Wang HL, Wang SS, Song WH, et al. Expression of prostate-specific membrane antigen in lung cancer cells and tumorneovasculature endothelial cells and its clinical significance. PLoS One 2015;10:e0125924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wernicke AG, Edgar MA, Lavi E, et al. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Arch Pathol Lab Med 2011;135:1486–9. [DOI] [PubMed] [Google Scholar]
- 22.Chang SS, O'Keefe DS, Bacich DJ, et al. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res 1999;5:2674–81. [PubMed] [Google Scholar]
- 23.Kasoha M, Unger C, Solomayer EF, et al. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin Exp Metastasis 2017;34:479–90. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Hadi M, Ismail Y, Younis L. Prostate-specific membrane antigen (PSMA) immunoexpression in the neovasculature of colorectal carcinoma in Egyptian patients. Pathol Res Pract 2014;210:759–63. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt LH, Heitkötter B, Schulze AB, et al. Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PLoS One 2017;12:e0186280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King KL, Hwang JJ, Chau GY, et al. Ki-67 expression as a prognostic marker in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 1998;13:273–9. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J. Angiogenesis. Annu Rev Med 2006;57:1–18. [DOI] [PubMed] [Google Scholar]
- 28.Kesler M, Levine C, Hershkovitz D, et al. (68)Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: A prospective pilot study. J Nucl Med 2018. [Epub ahead of print July 12, 2018.] [DOI] [PubMed] [Google Scholar]
- 29.Conway RE, Petrovic N, Li Z, et al. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol 2006;26:5310–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway RE, Joiner K, Patterson A, et al. Prostate specific membrane antigen produces pro-angiogenic laminin peptides downstream of matrix metalloprotease-2. Angiogenesis 2013;16:847–60. [DOI] [PubMed] [Google Scholar]
- 31.Conway RE, Rojas C, Alt J, et al. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis 2016;19:487–500. [DOI] [PubMed] [Google Scholar]
- 32.Lupold SE, Hicke BJ, Lin Y. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res 2002;62:4029–33. [PubMed] [Google Scholar]
- 33.Coffey S, Singh P, Topaloglu O, et al. A dimeric peptide that binds selectively to prostate-specific membrane antigen and inhibits its enzymatic activity. Cancer Res 2006;66:9171–7. [DOI] [PubMed] [Google Scholar]
- 34.Benesova M, Schafer M, Bauder-Wust U, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med 2015;56:914–20. [DOI] [PubMed] [Google Scholar]
- 35.Milowsky MI, Nanus DM, Kostakoglu L, et al. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol 2004;22:2522–31. [DOI] [PubMed] [Google Scholar]
- 36.Bander NH, Nanus DM, Milowsky MI, et al. Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate-specific membrane antigen. Semin Oncol 2003;30:667–76. [DOI] [PubMed] [Google Scholar]
- 37.Galsky MD, Eisenberger M, Moore-Cooper S, et al. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol 2008;26:2147–54. [DOI] [PubMed] [Google Scholar]
- 38.Milowsky MI, Nanus DM, Kostakoglu L, et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advranced solid tumors. J Clin Oncol 2007;25:540–7. [DOI] [PubMed] [Google Scholar]
- 39.Morris MJ, Pandit-Taskar N, Divgi CR, et al. Phase I evaluation of J591 as a vascular targeting agent in progressive solid tumors. Clin Cancer Res 2007;13:2707–13. [DOI] [PubMed] [Google Scholar]