INTRODUCTION:
Muscle mass has been shown to be a prognostic marker in patients with liver cirrhosis. Transversal psoas muscle thickness normalized by height (TPMT/height) obtained by routine computed tomography is a simple surrogate parameter for sarcopenia. TPMT/height, however, is not sex specific, which might play a role in risk stratification. Its association with acute-on-chronic liver failure (ACLF) has not been established yet. ACLF is associated with systemic inflammatory dysregulation. This study aimed at evaluating the role of sarcopenia in ACLF development of patients with decompensated cirrhosis receiving transjugular intrahepatic portosystemic shunt (TIPS) using sex-specific TPMT/height.
METHODS:
One hundred eighty-six patients from the prospective Non-invasive Evaluation Program for TIPS and Follow Up Network cohort (observational, real-world TIPS cohort with structured follow-up) were analyzed. TPMT/height was measured from routine computed tomography. The sex-specific cutoff was determined to classify patients as sarcopenic and nonsarcopenic for 1-year mortality after TIPS. Clinical outcome was compared. Primary end points were ACLF and 1-year mortality after TIPS. Secondary end points were development of decompensations (hepatic encephalopathy and ascites) after TIPS.
RESULTS:
The sex-specific cutoff increases the diagnostic accuracy with regard to primary and secondary end points compared with the unisex cutoff. Sex-specific sarcopenia classification is an independent predictor of 1-year mortality and ACLF development in patients with cirrhosis receiving TIPS. Patients in the sarcopenia group showed significantly higher rates of mortality, ascites, overt hepatic encephalopathy, and ACLF after TIPS compared with the nonsarcopenia group. The Chronic Liver Failure Consortium Acute Decompensation score as a marker of systemic inflammation was significantly higher in sarcopenic patients.
CONCLUSIONS:
This study demonstrates for the first time that sarcopenia is related to ACLF development and systemic inflammation. The prognostic value of TPMT/height can be improved by using sex-specific cutoffs. ClinicalTrials.gov identifier: NCT03584204.
INTRODUCTION
Liver cirrhosis is a growing health care burden, and the management of its complications is challenging, whereas mortality in many other pathologies is declining (1). Sarcopenia is defined as pathological muscle loss in patients with chronic diseases, and its role in the outcome of patients with cirrhosis is widely accepted (2). Different methods have been proposed to diagnose sarcopenia using cross-sectional imaging (2–8). Most methods evaluated computed tomography (CT) and require special software, which may be time consuming and difficult to implement in clinical routine (4,7). A simpler and fast method uses the right transversal psoas muscle thickness normalized by height (TPMT/height), which is an independent predictor of mortality for patients with cirrhosis on the waiting list for liver transplantation (5,9). Similar to sarcopenia, TPMT/height may also be influenced by a number of factors. Sex, in particular, seems to influence the muscle structure and its distribution in the body and thus possibly influences the diagnosis and the pattern of sarcopenia in cirrhosis (10). Cumulative data emphasize the role of sex in the sarcopenia pattern suggesting different cutoffs for female and male patients (2,8).
Patients with cirrhosis with acute decompensations (ADs), in particular, are at a higher risk of death and require more health care resources (11–16). AD can lead to a systemic inflammatory response and progress to acute-on-chronic liver failure (ACLF), a syndrome with high short-term mortality. Systemic inflammation has been associated with age-related sarcopenia and development of ACLF (17,18). Although a number of risk factors for the development of ACLF have been discussed, the relationship of sarcopenia with ACLF and systemic inflammation has not been investigated yet (19–21).
Therefore, we conducted this analysis of the prospective Non-invasive Evaluation Program for TIPS and Follow Up Network (NEPTUN) study to evaluate the role of sarcopenia in ACLF development of patients with cirrhosis receiving transjugular intrahepatic portosystemic shunt (TIPS) using sex-specific TPMT/height.
METHODS
Study population
For this study, we included patients from the NEPTUN study, which prospectively included patients with decompensated cirrhosis who underwent the TIPS procedure in a structured monocentric follow-up program. Noninvasive methods for risk stratification were evaluated. For inclusion in this analysis, CT had to be available. Exclusion criteria were lack of available or poor-quality CT.
The primary end point was 1-year mortality after TIPS. Secondary end points were development of ACLF stratified by fatal and nonfatal at 1 and 2 years and ADs (ascites and overt hepatic encephalopathy (HE)) during follow-up after TIPS. ACLF and overt HE were defined according to the European Association for the Study of the Liver guideline (12,22).
Biochemical blood analyses were performed using standard laboratory tests. The local ethics committee of the University of Bonn approved the study (029/13), and all patients agreed to and signed informed written consent in accordance with the Declaration of Helsinki for the procedures they underwent. This study is part of the NEPTUN cohort registered at ClinicalTrials.gov (identifier: NCT03584204).
Assessment of muscle parameters
For all examinations, commercially available clinical CT imaging systems (Philips Brilliance 64 or Philips Brilliance 256 iCT; both Philips Healthcare, Best, the Netherlands) were used. We analyzed the transversal psoas muscle thickness (TPMT) as previously described in cross-sectional images on the level of the umbilicus (5). Briefly, the maximum transverse diameter of the right psoas muscle was measured in millimeters and normalized for height (in meters) to calculate TPMT/height (see Figure 1b, Supplementary Digital Content 1, http://links.lww.com/CTG/A22). We chose the umbilicus because it is easy to identify in CT and it was used as a landmark in the aforementioned description of the method (5). The umbilicus in this cohort was located at the level of L4 in 70%, L5 in 20%, and L3 in 10% of patients (data not shown). The median time between CT and TIPS was 542 ± 88 days. The assessments were performed by 2 hepatologists (M.P. and C.C.) who had been trained by an expert radiologist (C.M.).
Statistical analysis
We performed descriptive statistics for all variables. Nonparametric testing was used to compare different groups when suitable. Paired nonparametric testing was used to compare data before and after the TIPS procedure of the same patients. For the selection of cutoff values of TPMT/height, receiver operating characteristic (ROC) analysis with 1-year survival as end point was calculated. To examine the impact of muscle indexes on survival and ACLF, we used the Kaplan-Meier curve with the log-rank test. Univariate and multivariate risk factor analyses were performed with Cox regression for 1-year mortality, fatal and nonfatal ACLF, occurrence of ascites, and episodes of HE as end points. Multivariate analysis included all values with P < 0.05 from univariate Cox regression. Dependent variables such as bilirubin and model of end-stage liver disease (MELD) and MELD-sodium (MELD-Na) were included separately in multivariate analysis to avoid collinearity. Concordance of the sarcopenia definitions was expressed by calculating concordance (C-index). Continuous variables are presented as median (range). Categorical variables are presented as absolute cases or percentage. All data were analyzed using SPSS (version 24; IBM, Armonk, NY). Prism (version 5; GraphPad, LaJolla, CA) was used for data plotting.
RESULTS
General patient characteristics
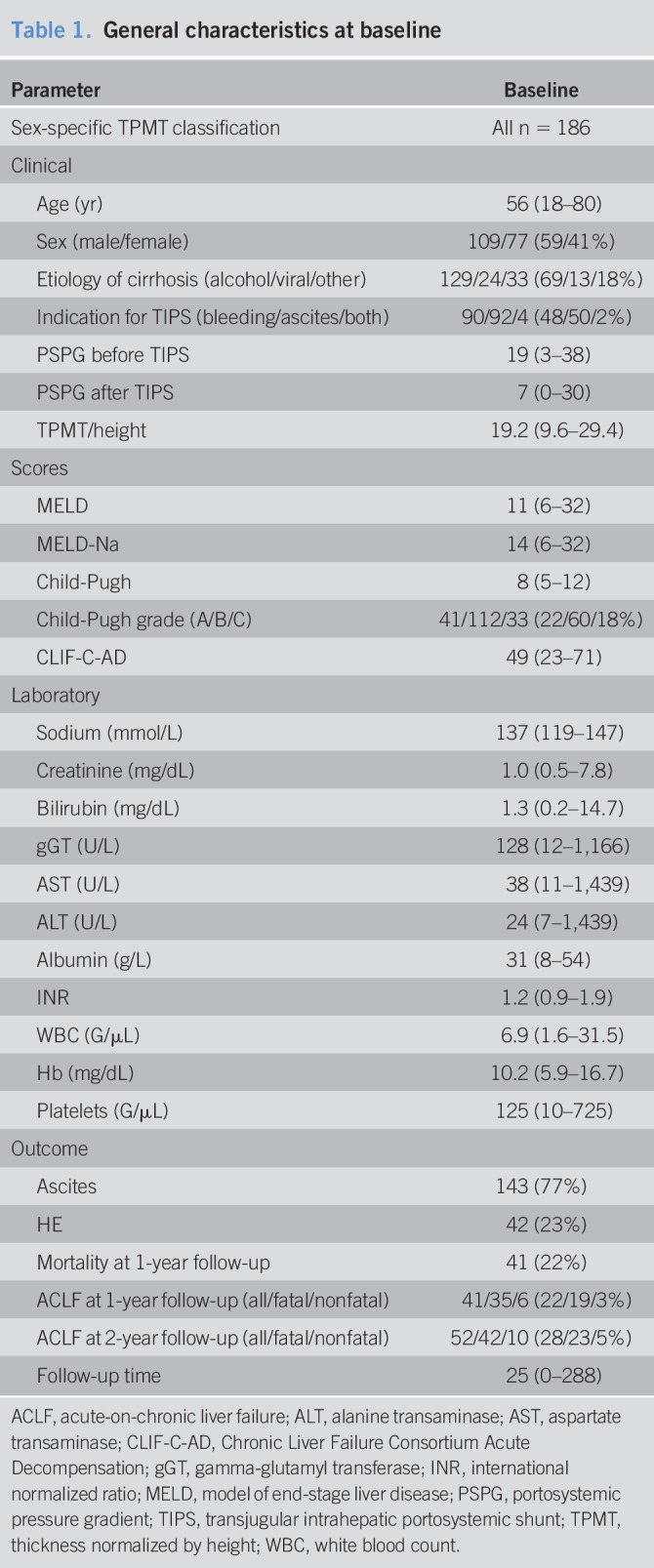
In this study, 186 patients with decompensated cirrhosis from the NEPTUN study were included (see Figure 1a, Supplementary Digital Content 1, http://links.lww.com/CTG/A22). Of these 186 included patients, 109 (59%) were men. The median age at TIPS procedure was 56 (18–80) years. Alcohol was the most common etiology of cirrhosis (129 patients, 69%), whereas 24 patients (13%) had chronic viral hepatitis B and/or C infection and 18% other etiologies. Ninety-six patients (52%) received TIPS for refractory ascites, and 90 patients for variceal bleeding (48%). The median MELD score was 11 (Table 1). The median follow-up period was 2.1 years (0–24 years).
Table 1.
General characteristics at baseline
Sex-specific sarcopenia classification
As TPMT/height has been reported to predict waiting list mortality, we performed ROC analysis of TPMT/height with 1-year survival as the end point. This resulted in an area under the curve (AUC) of 0.732 (confidence interval (CI) 0.648–0.816, P < 0.001), which is in line with previous reports. The reported unisex cutoff of 16.8 mm/m was used to classify sarcopenia (see Figures 1b and c, Supplementary Digital Content 1, http://links.lww.com/CTG/A22) (4,5).
To evaluate whether sex-specific differences and therefore sex-specific cutoff values would improve the method, we performed sex-specific ROC analysis. This revealed an optimal cutoff of 17.8 mm/m (AUC 0.754, CI 0.654–0.854, P < 0.001) for men and 14.0 mm/m (AUC 0.740, CI 0.606–0.874, P < 0.010) for women for 1-year mortality (see Figures 1d and e, Supplementary Digital Content 1, http://links.lww.com/CTG/A22).
With sex-specific classification, misclassification is lower compared with unisex classification. For 1-year mortality, sex-specific classification has a higher C-index and therefore a higher diagnostic accuracy.
Sarcopenia and survival
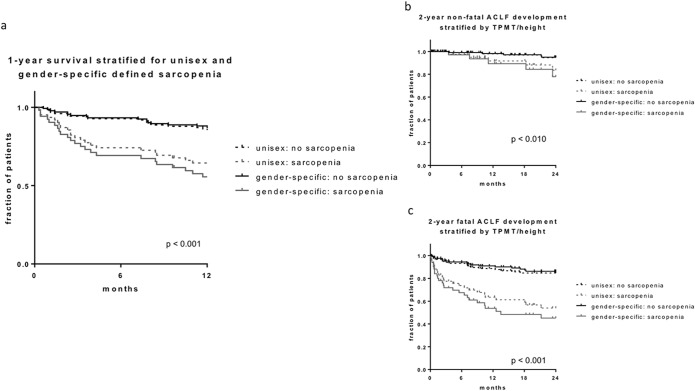
A Kaplan-Meier survival curve for 1-year mortality showed a significantly increased mortality in sarcopenic patients (Figure 1a). Interestingly, sex-specific classification provided superior discrimination compared with unisex classification of sarcopenia (Figure 1a). There was no significant difference regarding the causes of death between the sarcopenia and the nonsarcopenia groups (data not shown).
Figure 1.
(a) Kaplan-Meier curve for 1-year survival stratified by unisex and sex-specific sarcopenia classification. (b) Kaplan-Meier curve for development of nonfatal acute-on-chronic liver failure (ACLF) at 2-year follow-up stratified by unisex and sex-specific sarcopenia classification. (c) Kaplan-Meier curve for development of fatal ACLF at 2-year follow-up stratified by unisex and sex-specific sarcopenia classification. CLIF-C-AD, Chronic Liver Failure Consortium Acute Decompensation; HE, hepatic encephalopathy; MELD, model of end-stage liver disease.
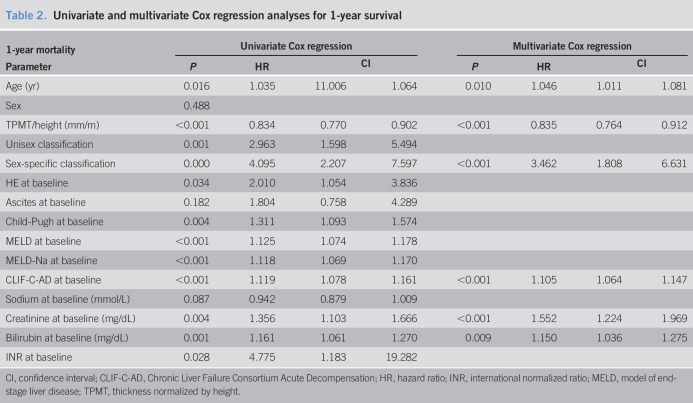
Cox regression analysis for 1-year mortality revealed Child-Pugh, MELD, MELD-Na, Chronic Liver Failure Consortium (CLIF-C) AD score, creatinine, bilirubin, international normalized ratio (INR), and age, as well as TPMT/height and unisex and sex-specific sarcopenia classification as dependent risk factors. Only sex-specific sarcopenia classification was shown to be an independent predictor alongside CLIF-C AD score, age, serum creatinine, and bilirubin (Table 2). Unisex classification did not reach statistical significance.
Table 2.
Univariate and multivariate Cox regression analyses for 1-year survival
Interestingly, sarcopenic patients receiving TIPS for refractory ascites had a higher risk of 1-year mortality compared with those with variceal bleeding as indication for TIPS (hazard ratio 5.296 vs 2.832).
Sarcopenia and development of ACLF
Sarcopenic patients showed significantly higher rates of nonfatal ACLF development compared with nonsarcopenic patients (Figure 1b). Also, ACLF with fatal outcome occurred significantly more often in sarcopenic patients (Figure 1c). With sex-specific classification, misclassification is lower compared with unisex classification for development of ACLF at 1-year follow-up (C-index 0.66 vs 0.73) (see Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A22).
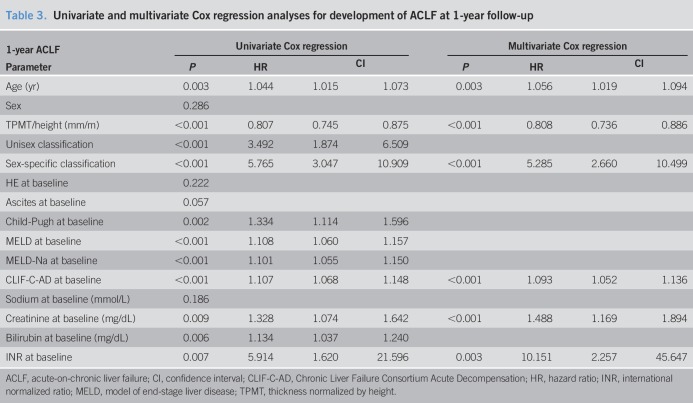
We performed univariate Cox regression to identify risk factors for 1-year ACLF development. Apart from the expected prognostic markers Child-Pugh, MELD, MELD-Na, CLIF-C AD score, creatinine, bilirubin, INR, and age, this also revealed TPMT/height and unisex and sex-specific sarcopenia classification as dependent predictors of ACLF development. In multivariate Cox regression analysis, unisex classification failed. Sex-specific cutoff-defined sarcopenia, CLIF-C AD score, age, serum creatinine, and INR were independent predictors of 1-year ACLF development (Table 3). Stratified for indication for TIPS, sarcopenic patients with refractory ascites had a higher of development of ACLF than sarcopenic patients with variceal bleeding as indication for TIPS (hazard ratio 6.845 vs 3.439).
Table 3.
Univariate and multivariate Cox regression analyses for development of ACLF at 1-year follow-up
Univariate and multivariate Cox regression analyses were performed separately for nonfatal ACLF and fatal ACLF. In multivariate analysis, only sex-specific classification and CLIF-C AD score were found to be significant (see Table 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A22). For nonfatal ACLF, only sex-specific classification and age were independent predictors (see Table 4, Supplementary Digital Content 1, http://links.lww.com/CTG/A22).
Sarcopenia and AD
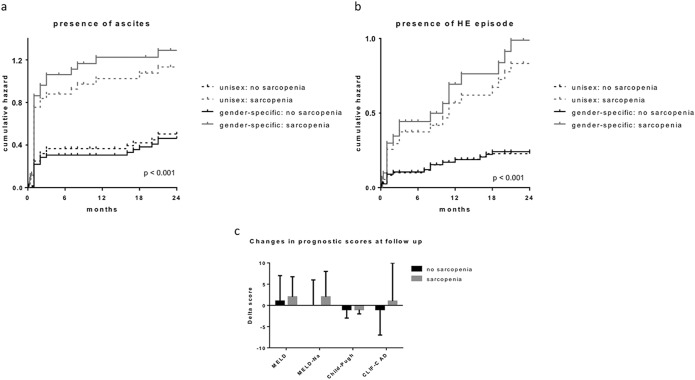
Absence of sarcopenia was associated with faster resolution of ascites after TIPS, whereas sarcopenic patients displayed a longer response to ascites despite patent TIPS (Figure 2a). Furthermore, sarcopenic patients had a significantly higher rate of episodes of HE compared with nonsarcopenic patients (Figure 2b). For the 2 end points, ascites and HE, the sex-specific cutoff showed an improved discrimination compared with the unisex cutoff.
Figure 2.
(a) Cumulative hazard function for the presence of ascites (persistence and/or reoccurrence) after TIPS stratified by unisex and sex-specific sarcopenia classification. (b) Cumulative hazard function for the occurrence of episode of hepatic encephalopathy after TIPS stratified by unisex and sex-specific sarcopenia classification. (c) Diagram showing changes in prognostic scores before and after TIPS. ACLF, acute-on-chronic liver failure; TPMT, thickness normalized by height.
Sarcopenia and scores and systemic inflammation
The sarcopenia group showed a higher fraction of alcoholic etiology and ascites as indication for TIPS compared with the nonsarcopenia group. MELD, MELD-Na, CLIF-C AD, and Child-Pugh score were significantly higher in the sarcopenia group. After TIPS, increase in MELD and MELD-Na score was higher in sarcopenic patients than in nonsarcopenic patients. In fact, the CLIF-C AD score showed a decrease in nonsarcopenic patients, whereas it increased in sarcopenic patients (Figure 2c).
At follow-up, all prognostic scores (MELD, MELD-Na, Child-Pugh, and CLIF-C AD score) were significantly worse in the sarcopenia group. Interestingly, in the nonsarcopenia group, MELD, Child-Pugh, and CLIF-C AD score improved at follow-up, resulting in a significantly lower risk of decompensations in this group (see Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A22).
Apart from the prognostic scores, especially white blood cell count, as a marker of systemic inflammation, levels of INR and hemoglobin were significantly better in the nonsarcopenia group during follow-up (see Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A22).
DISCUSSION
The present study demonstrates that sex-specific TPMT/height-defined sarcopenia in the NEPTUN cohort identifies patients at risk of development of ACLF and risk of death.
In real life, patients with cirrhosis usually seek medical attention, including CT, at the time of decompensating events. In addition, CT is performed for evaluation and planning of interventions, such as TIPS or liver transplant. In these situations, the potential to predict outcome is extremely important to stratify patient care. Using these imaging methods, different measures of muscle mass have been described. TPMT/height is an easy-to-assess measure of muscle mass (5). It was previously reported that TPMT/height could predict survival in patients on the waiting list for liver transplantation (5). Interestingly, in that cohort, the authors elaborated only 1 cutoff for both male and female patients. There is growing evidence of the importance to recognize the sex differences in sarcopenic patients (2,4,23–25). Females tend to have significantly lower muscle indices, which is in line with our results and supports our data (2,8,26). These previous data explain, in part, our findings that the sex-specific cutoff is superior to the unisex cutoff used previously (5). There are several possible explanations for these discrepant findings. First, patients listed for liver transplantation have a higher MELD score than patients receiving TIPS, which was also the case in a previously published cohort with a mean MELD score of 19 (5), whereas in our cohort, the patients were not as advanced with a median MELD score of 11. Second, another key difference in patient characteristics is the rather low proportion of females in the previously published cohort of 21% (5), whereas in our cohort, it is 41%. These differences in characteristics possibly explain the inferior diagnostic accuracy of unisex cutoff compared with the sex-specific cutoff in our cohort. Therefore, sex-specific cutoff values should be applied to minimize misclassification. This is emphasized even more by our results, which identify sex-specific sarcopenia classification as an independent predictor of mortality, whereas the unisex cutoff failed in the multivariate analysis. Further multicentric studies with large patient cohorts are needed to determine comparable sex-specific cutoff values (2,4,5,9,27–29). This is especially important regarding the relatively low negative predictive value of our classification in this cohort. It may well be higher in larger cohorts with lower prevalence of 1-year mortality.
Apart from the prediction of survival in these patients, another example is that TPMT/height-defined sarcopenia is associated with higher rates of overt HE. This has been supported by another study using skeletal muscle index at L3 vertebra (L3-SMI) requiring special software in a smaller cohort (30), underlining the robustness of our study. Moreover, the association of sarcopenia with survival and HE is important because this facilitates the caregiver to select patients who may benefit from additional therapeutic options, such as dietary strategies in sarcopenic patients (31–33).
The limitations of this study are in line with the nature of all retrospective studies, namely that selection bias cannot be excluded despite the cohort showing fairly representative characteristics for patients with cirrhosis in a TIPS program. However, probably not all decompensating events, including nonfatal ACLF, are recorded because we do not have all the information about admissions to other hospitals. This possibly explains the lower accuracy of prediction of nonfatal ACLF compared with fatal ACLF in our cohort. However, we are confident that we have detected all ACLF developments with fatal outcome, which seems to be the more severe clinical entity in our patients.
Although the relationship of sarcopenia with survival has been established on several different occasions, the main novel finding of this study is the association of sarcopenia with development of ACLF. Recently, ACLF has been characterized as a syndrome with very high short-term mortality, which may also develop in outpatients with cirrhosis (34). Interestingly, the presence of sarcopenia predicts—independent of other factors—the development of ACLF in TIPS patients. Further factors known to be associated with the development of ACLF and death were CLIF-C AD score, creatinine, and INR, predicting the development of ACLF in TIPS patients. This stresses the robustness of our data. However, both presence of sarcopenia and TMPT/height outperform the MELD score in the prediction of ACLF, which has been designed to predict mortality after TIPS. This finding might be also due to the chosen time frame as the development of ACLF in TIPS patients might occur at a later stage, while MELD predicts a 3-month mortality. ACLF is a dynamic syndrome, which can reverse rapidly, especially in the earlier stages.
To avoid the overrepresentation of ACLF due to laboratory value fluctuations, progressive ACLF—ending with death—was defined in this study as fatal ACLF and was evaluated separately. Importantly, half of the patients with sarcopenia developed fatal ACLF during the first year after TIPS, whereas less than 10% of the patients without sarcopenia developed ACLF, indicating that sarcopenia might play at least a predisposing and probably also a pathogenic role in the development of ACLF.
Through the secretion of soluble peptides, or myokines, skeletal muscle interacts in metabolic processes with other organs such as the liver. In turn, metabolic cues from these organs are received by skeletal muscle, adapting their response accordingly. Cross-talking between anabolic and catabolic pathways characterizes the signaling. Systemic inflammation evokes a catabolic reaction in skeletal muscle leading to excessive energy expenditure and ultimately sarcopenia (35). Systemic inflammation has also been identified in the pathogenesis of ACLF development (17,18,36). It is conceivable that sarcopenia is therefore a clinical expression of underlying chronic systemic inflammation, which might facilitate development of ACLF. Interestingly, in this cohort, leukocyte count, a surrogate of systemic inflammation, is significantly higher in sarcopenic patients, further underlining the tight association of systemic inflammation with sarcopenia and ACLF. The question of whether sarcopenia predisposes for systemic inflammation and ACLF development or systemic inflammation causes sarcopenia and ACLF is beyond the scope of this study. This needs to be investigated in the future because it could also offer therapeutic or preventive approaches. Nevertheless, these results demonstrate that especially the early identification of sarcopenic patients is of clinical relevance because these patients are at risk of developing ACLF. In these patients, shorter follow-up periods might help to better monitor their progress.
Recently, a plethora of different techniques for estimation of muscle mass and frailty has been published. TPMT/height has been debated for the asymmetrical shape of the psoas muscle and because this method chooses the umbilicus as anatomical landmark, whereas other techniques use, e.g., the psoas muscle area or L3-SMI (4,5). The umbilicus as a landmark shows some variability and has a limitation in patients with giant umbilical hernia. However, in the current literature, there are conflicting data on the different performance of psoas muscle compared with other muscle parameters, where L3-SMI seems to perform better in cohorts with high fraction of hepatocellular carcinoma (4,5,37). In our cohort, hepatocellular carcinoma was excluded before treatment with TIPS. A test battery to perform frailty assessment was not possible in this retrospective study (38). However, despite its limitations, this study shows the value of TPMT/height in predicting ACLF and death. Finally, the requirement of CT for this study represents a selection bias, which led to the exclusion of several patients. The variable time between CT and TIPS might represent a limitation, and CT performed just before the TIPS procedure would have been ideal and should be considered in future prospective studies.
As for the clinical routine, we emphasize the simplicity and convenience of the method, as cross-sectional imaging is routinely performed in cirrhotic patients, e.g., for evaluation for liver transplantation or TIPS procedure. Hence, no additional cost for evaluation of sarcopenia is needed. The measurement itself is fast and reproducible, which is the main limitation for the clinical use of anthropometric parameters, such as mid-arm muscle circumference (8,9).
In conclusion, this study shows that the use of sex-specific cutoffs offers an improvement of prognostic value of TPMT/height. Furthermore, this study, for the first time, demonstrates the association of TPMT/height defined with the development of ACLF.
CONFLICTS OF INTEREST
Guarantor of the article: Jonel Trebicka, MD, PhD.
Specific author contributions: Michael Praktiknjo, MD and Caroline Clees, MD contributed equally as first authors. M.P. and C.C.: acquisition of data, analysis and interpretation of data, drafting of the manuscript, and statistical analysis. A.P., S.F., B.L., and V.K.K.: acquisition of data and analysis and interpretation of data. C.P.S. and M.M.: administrative support. C.M.: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content, and study supervision. J.T.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript regarding important intellectual content, funding recipient, administrative, technical, and material support, and study supervision.
Financial support: The authors were supported by grants from the Deutsche Forschungsgemeinschaft (SFB TRR57), Cellex Foundation and European Union's Horizon 2020 research and innovation program GALAXY study (No. 668031), LIVERHOPE (No. 731875), MICROB-PREDICT (No. 825694) and by Challenge Grant “MicrobLiver” grant number NNF15OC0016692 from the Novo Nordisk Foundation. The funders had no influence on study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential competing interests: None.
Study Highlights.
WHAT IS KNOWN
✓ ACLF is a syndrome with high short-term mortality occurs in decompensated cirrhosis.
✓ Sarcopenia is related to mortality in cirrhosis.
✓ TPMT/height reflects sarcopenia and predicts survival in cirrhosis.
WHAT IS NEW HERE
✓ Sarcopenic patients after TIPS develop more frequently ACLF, especially fatal ACLF.
✓ Sarcopenia is an independent predictor of ACLF and mortality in patients with cirrhosis after TIPS.
✓ TPMT/height performs better in the prediction of ACLF and mortality when sex-specific cutoffs are implemented.
TRANSLATIONAL IMPACT
✓ Sex-specific TPMT/height can be used in stratification of follow up timing.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Gudrun Hack, Silke Bellinghausen, Nadine Köstlmeier, and Kristin Gehrmann for their excellent technical assistance and Sabine Dentler for critical reading.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A22
REFERENCES
- 1.Williams R, Ashton K, Aspinall R, et al. Implementation of the lancet standing commission on liver disease in the UK. Lancet 2015;386:2098–111. [DOI] [PubMed] [Google Scholar]
- 2.Praktiknjo M, Book M, Luetkens J, et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology 2018;67:1014–26. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golse N, Bucur PO, Ciacio O, et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl 2017;23:143–54. [DOI] [PubMed] [Google Scholar]
- 5.Durand F, Buyse S, Francoz C, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 2014;60:1151–7. [DOI] [PubMed] [Google Scholar]
- 6.Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol 2014;20:8061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giusto M, Lattanzi B, Albanese C, et al. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 2015;27:328–34. [DOI] [PubMed] [Google Scholar]
- 9.Huguet A, Latournerie M, Debry PH, et al. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: A retrospective cohort study. Nutrition 2018;51–52:73–9. [DOI] [PubMed] [Google Scholar]
- 10.Moctezuma-Velázquez C, Low G, Mourtzakis M, et al. Association between low testosterone levels and sarcopenia in cirrhosis: A cross-sectional study. Ann Hepatol 2018;17:615–23. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo V, Moreau R, Kamath PS, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers 2016;2:16041. [DOI] [PubMed] [Google Scholar]
- 12.Angeli P, Bernardi M, Villanueva C, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis [Internet]. J Hepatol 2018;69:406–60. [DOI] [PubMed] [Google Scholar]
- 13.Trebicka J. Emergency TIPS in a Child-Pugh B patient: When does the window of opportunity open and close? J Hepatol 2017;66:442–50. [DOI] [PubMed] [Google Scholar]
- 14.Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152:157–63. [DOI] [PubMed] [Google Scholar]
- 15.Salerno F, Cammà C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis of individual patient data. Gastroenterology 2007;133:825–34. [DOI] [PubMed] [Google Scholar]
- 16.Allen AM, Kim WR, Moriarty JP, et al. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology 2016;64:2165–72. [DOI] [PubMed] [Google Scholar]
- 17.Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol 2017;8:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laleman W, Claria J, Van der Merwe S, et al. Systemic inflammation and acute-on-chronic liver failure: Too much, not enough. Can J Gastroenterol Hepatol 2018;2018:1027152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37:1437.e1–9. [DOI] [PubMed] [Google Scholar]
- 20.Gustot T, Fernandez J, Garcia E, et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243–52. [DOI] [PubMed] [Google Scholar]
- 21.Praktiknjo M, Lehmann J, Nielsen MJ, et al. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun 2018;2:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the european association for the study of the liver. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 23.Montano-Loza AJ, Meza-Junco J, Prado CMM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166–73:173.e1. [DOI] [PubMed] [Google Scholar]
- 24.Prado CMM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 25.Peng LN, Lee WJ, Liu LK, et al. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J Cachexia Sarcopenia Muscle 2018;9:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tachi Y, Kozuka A, Hirai T, et al. Impact of myosteatosis on skeletal muscle volume loss in patients with chronic liver disease. J Gastroenterol Hepatol 2018. [Epub ahead of print February 27, 2018.] [DOI] [PubMed] [Google Scholar]
- 27.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010;211:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz RJ, Dew MA, Myaskovsky L, et al. Objective radiologic assessment of body composition in patients with end-stage liver disease: Going beyond the BMI. Transplantation 2013;95:617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl 2012;18:1209–16. [DOI] [PubMed] [Google Scholar]
- 30.Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol 2017;15:934–6. [DOI] [PubMed] [Google Scholar]
- 31.Hiraoka A, Michitaka K, Kiguchi D, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2017;29:1416–23. [DOI] [PubMed] [Google Scholar]
- 32.Yoh K, Nishikawa H, Enomoto H, et al. Effect of exercise therapy on sarcopenia in pancreatic cancer: A study protocol for a randomised controlled trial. BMJ Open Gastroenterol 2018;5:e000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liguori I, Russo G, Aran L, et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin Interv Aging 2018;13:913–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piano S, Tonon M, Vettore E, et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol 2017;67:1177–84. [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Baos S, Prieto-Potin I, Román-Blas JA, et al. Mediators and patterns of muscle loss in chronic systemic inflammation. Front Physiol 2018;9:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebadi M, Wang CW, Lai JC, et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle 2018;9:1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.