OBJECTIVES:
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) and autoimmune pancreatitis (AIP) are characterized by an abundance of circulating and tissue IgG4-positive plasma cells. T-follicular helper (Tfh) cells are necessary for B-cell differentiation into plasma cells. We aimed at elucidating the presence and phenotype of Tfh cells and their relationship with disease activity in IgG4-SC/AIP.
METHODS:
Circulating Tfh-cell subsets were characterized by multiparametric flow cytometry in IgG4-SC/AIP (n = 18), disease controls with primary sclerosing cholangitis (n = 8), and healthy controls (HCs, n = 9). Tissue Tfh cells were characterized in IgG4-SC/AIP (n = 12) and disease control (n = 10) specimens. Activated PD1+ Tfh cells were cocultured with CD27+ memory B cells to assess their capacity to support B-cell differentiation. Disease activity was assessed using the IgG4–responder index and clinical parameters.
RESULTS:
Activated circulating PD-1+CXCR5+ Tfh cells were expanded in active vs inactive IgG4-SC/AIP, primary sclerosing cholangitis, and HC (P < 0.01), with enhanced PD-1 expression on all Tfh-cell subsets (Tfh1, P = 0.003; Tfh2, P = 0.0006; Th17, P = 0.003). Expansion of CD27+CD38+CD19lo plasmablasts in active disease vs HC (P = 0.01) correlated with the PD-1+ Tfh2 subset (r = 0.69, P = 0.03). Increased IL-4 and IL-21 cytokine production from stimulated cells of IgG4-SC/AIP, important in IgG4 class switch and proliferation, correlated with PD-1+ Tfh2 (r = 0.89, P = 0.02) and PD-1+ Tfh17 (r = 0.83, P = 0.03) subsets. Coculture of PD1+ Tfh with CD27+ B cells induced higher IgG4 expression than with PD1− Tfh (P = 0.008). PD-1+ Tfh2 cells were strongly associated with clinical markers of disease activity: sIgG4 (r = 0.70, P = 0.002), sIgE (r = 0.66, P = 0.006), and IgG4–responder index (r = 0.60, P = 0.006). Activated CXCR5+ Tfh cells homed to lymphoid follicles in IgG4-SC/AIP tissues.
CONCLUSIONS:
Circulating and tissue-activated Tfh cells are expanded in IgG4-SC/AIP, correlate with disease activity, and can drive class switch and proliferation of IgG4-committed B cells. PD1+ Tfh2 cells may be a biomarker of active disease and a potential target for immunotherapy.
INTRODUCTION
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) and autoimmune pancreatitis (AIP) are the biliary and pancreatic manifestations of a systemic fibroinflammatory condition, IgG4-related disease (IgG4-RD), characterized by an abundance of IgG4-positive plasma cells and CD4+ T cells in involved tissue (1). IgG4-SC and AIP are no longer considered benign diseases, with high rates of disease relapse, organ dysfunction and failure, with associated mortality (2). Elevated serum IgG4 and immunoglobulin E (IgE) titers have been described in most patients but are not sensitive or specific enough for diagnosis, monitoring of disease activity, or outcome (3,4). Increased numbers of circulating plasmablasts have been suggested to coincide with both active disease and disease relapse (5). Plasmablast expansion seems to be generated by a T cell–dependent immune response, suggested by enhanced somatic mutation and the re-emergence of new plasmablast clones after B-cell depletion with rituximab (6). CD4+ T cells are necessary for support and coordination of IgG-switched B-cell responses, but their role in IgG4-SC/AIP pathogenesis remains poorly understood.
T-follicular helper (Tfh) cells are an important subset of CD4+ T cells, necessary for B-cell differentiation and class switch in germinal centers (GCs) (7). Tfh cells primarily localize in lymphoid organs, but they are also found in peripheral blood and lesions of diseases (8). Circulating Tfh cells can be identified as Tfh1, Tfh2, and Tfh17 subsets, with corresponding cytokine profiles and differing abilities to provide B-cell support (8). Programmed cell death protein 1 (PD-1) is a marker of cell activation in Tfh cells, is essential for B-cell selection and survival in the GCs, and for maturation of B cells into antibody-secreting cells (8). Expanded PD1+ Tfh cells have been demonstrated in autoimmune diseases, such as Sjogren syndrome (8–10). In this setting, they have been suggested to be a valuable biomarker for the monitoring of dysregulated antibody responses and disease activity (9,11). Expansion of Tfh-cell subsets have recently been demonstrated in patients with systemic IgG4-RD (12–14).
In this study, we sought to evaluate the presence and phenotype of Tfh cells in the circulation and involved organs of patients with IgG4-SC and AIP in a UK cohort.
MATERIALS AND METHODS
Patient recruitment
Patients with IgG4-RD (n = 18 with biliary and/or pancreatic disease), disease controls (DCs) with primary sclerosing cholangitis (PSC) (n = 8), and healthy controls (HCs) (n = 9) were recruited from the John Radcliffe Hospital, Oxford, UK, a tertiary referral center for IgG4-RD and PSC. Ethical approval for the study was obtained from the Research Ethics Committee Oxfordshire (10/H0604/51) and conducted in accordance with the study protocol and the principles of the Declaration of Helsinki (2008) and the International Conference on Harmonization Good Clinical Practices standards. Enrolled study participants provided written informed consent. The study was registered on the National Institute for Health Research (NIHR) UK portfolio (10776).
Diagnostic criteria
The diagnosis of IgG4-SC and AIP was made using the Histology, Imaging, Serology, Other organ involvement and Response to therapy (HISORt) criteria and the International Consensus Diagnostic Criteria (15,16). The Boston Consensus Histopathological Criteria were applied (17). PSC was diagnosed in accordance with the European Association for the Study of the Liver (EASL) guidelines for cholestatic liver disease (18). HCs had no known immune or inflammatory disease.
IgG4–responder index
Disease activity, organ damage, and response to treatment were assessed using the IgG4–responder index (IgG4-RI) (19). This assessment tool has recently been validated in an international multispeciality study for assessment of disease activity and longitudinally for treatment response (20). Active disease was defined as the presence of new or recurrent disease activity in at least 1 organ.
Serological testing for immunoglobulins
Serum immunoglobulins were measured at diagnosis within the IgG4-SC/AIP and PSC cohort. Total serum IgG, IgG1, and IgG4 subclasses were measured by nephelometry (BNII analyzer; Siemens, UK). Total IgE was measured by the automated ImmunoCAP method (Phadia 250; UK).
Circulating peripheral blood mononuclear cell surface staining and flow cytometry
Whole blood was collected from patients with IgG4-SC/AIP (n = 18), patients with PSC (n = 8), and HCs (n = 9). Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation (Axis Shield, Oslo, Norway). For phenotypic analysis of Tfh, Th1, Th2, and Th17 cells, freshly isolated PBMCs were stained and analyzed using flow cytometry (see Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A16).
Memory B-cell and plasmablast culture
Fresh PBMCs were cultured for 6 days in the presence of a nonspecific B-cell stimulant containing 83 ng/mL of pokeweed mitogen extract (Sigma-Aldrich), 2.5 μg/mL of fully methylated CpG (Invitrogen), and a 1:5,000 dilution of fixed Staphylococcusaureus, Cowan (SAC, Calbiochem), which stimulates polyclonal B memory cells to proliferate and differentiate into antibody-secreting cells. After culture, supernatants were preserved at −80 °C for further cytokine analysis (Cytokine immunoassays, see Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A16).
Tfh-cell and B-cell isolation and coculture assay
B cells (3 × 104 cells/well) were cocultured with PD1+ or PD1− Tfh cells (3 × 104 cells/well) in a 1:1 ratio in AIM-V medium (Life Technologies, Carlsbad, CA). Cells were stimulated with staphylococcal enterotoxin B (SEB, 100 ng/mL; Sigma-Aldrich, St. Louis, MO). After 7 days, supernatants were removed and frozen. Cocultured cells were surface stained for antibody-secreting cells and proliferation. Supernatants were collected for detection of IgG4 (by IgG4 ELISA) and cytokines (cytokine immunoassays) further detailed in Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A16.
Histological specimens from patients and controls
Paraffin-embedded tissue specimens were obtained from the Department of Cellular Pathology, John Radcliffe Hospital, Oxford, and Southampton General Hospital, Southampton, UK. Specimens were taken from patients with IgG4-SC/AIP (n = 12: 4 biopsy and 8 resection) and DCs (n = 10: 2 biopsy and 8 resection) with inflammatory (n = 5) and noninflammatory (n = 5) diseases. Tissues were stained with Tfh-cell markers (see Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A16).
RESULTS
Cohort characteristics
Patients with IgG4-SC/AIP (n = 18; 12 men (66.7%), median age 65 years (range 40–80 years)), patients with PSC (n = 8; 6 men (75%), median age 60 years (range 41–70 years)), and HC cohort (n = 9; 4 men (44.4%), median age 49 years (range 44–63 years)) were well matched (P = 0.41). The demographics and clinical characteristics of IgG4-SC/AIP patients with active and inactive disease are detailed in Table 1, with further details provided in Table S1, see Supplementary Digital Content 3, http://links.lww.com/CTG/A18. Overall, 14 of 18 patients with IgG4-SC/AIP (77.8%) had multiorgan involvement with a median disease duration of 2 years (range 0.5–15 years). The cohort with active IgG4-SC/AIP (n = 12) had higher serum IgG4 levels (P = 0.02) and IgG4-RI disease activity scores (P = 0.0001) compared with those with inactive treated disease (n = 6) (Table 1). Overall, an elevated serum IgG4 level correlated with total serum IgG levels (r = 0.83, P < 0.0001), serum IgE levels (r = 0.56, P = 0.04), and eosinophil count (r = 0.64, P = 0.004), but not serum IgG1 (P = 0.50) (Figure S1A-D, see Supplementary Digital Content 2, http://links.lww.com/CTG/A17).
Table 1.
Demographics and characteristics of the IgG4-SC/AIP cohort
Circulating Tfh cells in patients with active IgG4-SC/AIP
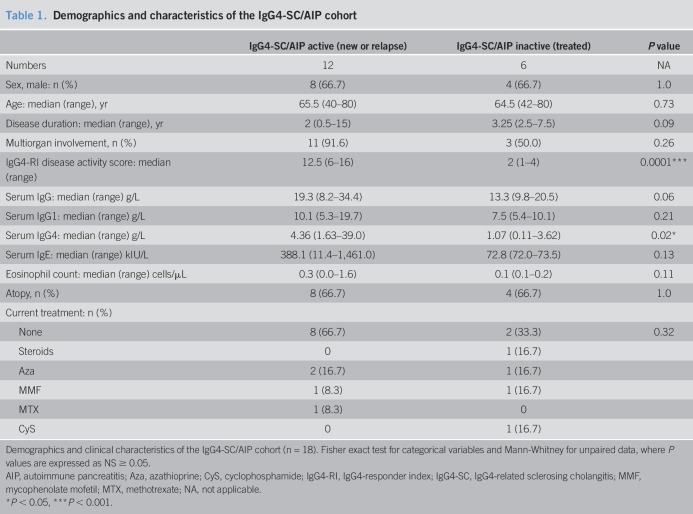
First, we sought to investigate the presence of circulating Tfh cells in patients with active IgG4-SC/AIP (n = 12). Circulating CXCR5+ Tfh cells (representative staining shown in Figure 1a) comprised a lower percentage of the total CD4+ CD45RA− memory T-cell population in patients with active IgG4-SC/AIP and PSC compared with HCs (median IgG4-SC/AIP 10% vs PSC 11% vs HC 21%, P = 0.009) (Figure 1b). There was no difference in the frequency of the Tfh subsets Tfh1 (CXCR3+CCR6−), Tfh2 (CXCR3−CCR6−), or Tfh17 (CXCR3+CCR6+) within the CXCR5+ compartment between IgG4-SC/AIP and HC. However, patients with PSC had a lower percentage of Tfh1 cells compared with either IgG4-SC/AIP or HC group (median IgG4-SC/AIP 28% vs PSC 13% vs HC 31%, P = 0.001) (Figure 1c).
Figure 1.
Circulating Tfh cells have an activated phenotype in IgG4-SC/AIP. Circulating Tfh-cell phenotype was analyzed by flow cytometry. (a) Contour plot showing CXCR3 and CCR6 expression on CD45RA-CD4+CXCR5+ Tfh cells from a representative donor. Three populations within the Tfh cells were identified: CXCR3+CCR6− Tfh1 cells, CXCR3−CCR6− Tfh2 cells, and CXCR3−CCR6+ Tfh17 cells. Gating for PD1 high cells shown. (b) Dot plot showing the percentage of CD4+CXCR5+ cells in active IgG4-SC/AIP, inactive IgG4-SC/AIP, disease controls with PSC, and healthy donors (HCs). (c) Dot plot showing the percentage of Tfh1, Tfh2, and Tfh17 subsets of blood Tfh cells in the 4 groups. (d) Dot plot showing the percentage of Tfh1, Tfh2, and Tfh17 cells expressing PD-1 in the 4 groups. (e) Dot plot showing absolute numbers of Tfh1, Tfh2, and Tfh17 cells expressing PD-1 in active and inactive IgG4-SC/AIP and HC groups. Median shown for all plots, Mann-Whitney and ANOVA with multiple comparisons, P values: NS P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. AIP, autoimmune pancreatitis; HC, healthy control; IgG4-SC, IgG4-related sclerosing cholangitis; PD-1, programmed cell death protein 1; PSC, primary sclerosing cholangitis; Tfh, T-follicular helper.
Circulating Tfh-cell subsets have an activated phenotype in IgG4-SC/AIP
Next, we sought to determine the phenotype of circulating Tfh cells. The surface expression of PD-1 on CXCR5+ Tfh cells (representative staining shown in Figure 1a) was enhanced in active IgG4-SC/AIP and PSC compared with HCs (median IgG4-SC/AIP 18% vs PSC 12% vs HC 9%, P = 0.003) (Figure 1d). PD-1 expression was enhanced on all Tfh-cell subsets in active IgG4-SC/AIP compared with both PSC and HC: Tfh1 (IgG4-SC/AIP 23% vs PSC 14% vs HC 12%, P = 0.04), Tfh2 (IgG4-SC/AIP 16% vs PSC 10% vs HC 5%, P = 0.0002), and Th17 (IgG4-SC/AIP 14% vs PSC 9% vs HC 4%, P = 0.005) cells (Figure 1d). Absolute numbers of PD-1+ Tfh2 cells, but not PD1+ Tfh1 or Tfh17 cells, were also increased (active IgG4-SC/AIP 290 cells vs HC 172 cells per million PBMCs, P = 0.006) (Figure 1e).
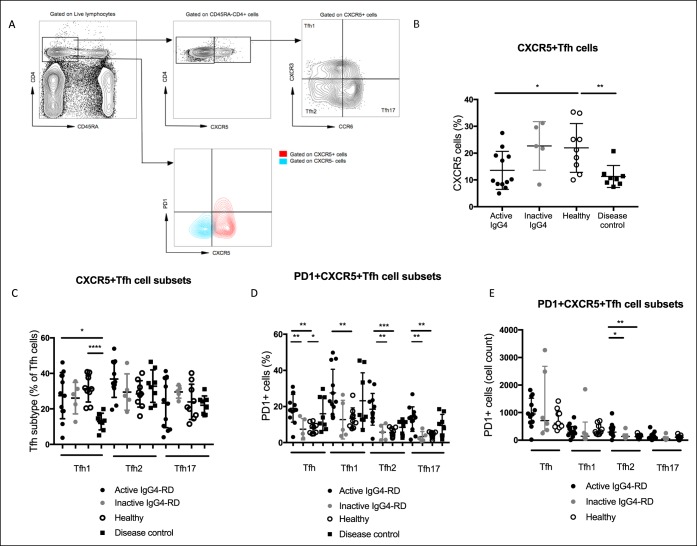
Circulating PD-1+ Tfh2 cells correlate with plasmablasts in active IgG4-SC/AIP
As Tfh cells are essential for B-cell selection and maturation into plasmablasts, we examined the relationship of Tfh-cell subsets with plasmablasts in patients with active IgG4-AIP/SC. Circulating CD27+CD38+CD19lowCD20− plasmablasts (representative staining Figure 2a) were expanded in patients with active IgG4-AIP/SC compared with HCs (8.4% vs 2.4%, P = 0.006) (Figure 2b). PD-1+ Tfh cells, specifically the PD-1 Tfh2-cell subset, correlated with circulating plasmablast number (r = 0.69, P = 0.03) (Figures 2c,d). There was no association with the other Tfh-cell subsets (PD-1 Tfh1 P = 0.10; PD-1 Tfh17 P = 0.33) (data not shown).
Figure 2.
Circulating PD-1+ Tfh2 cells correlate with plasmablasts in active IgG4-SC/AIP. Circulating plasmablasts and Tfh cells were analyzed by flow cytometry. (a) Contour plot showing CD38+ CD20− on CD27+ CD19+(low) cells identifying a plasmablast population from a representative donor. (b) Dot plot showing the percentage of CD27++CD38++CD20−CD19low plasmablasts in active IgG4-SC/AIP and HC. Median values and Mann-Whitney P values as in Figure 1. Correlation plots showing (c) PD-1+ Tfh cells and (d) PD-1+ Tfh2 cells plotted against the percentage of plasmablasts. Spearman rank correlation (r) and P values are expressed as NS ≥ 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001. AIP, autoimmune pancreatitis; HC, healthy control; IgG4-SC, IgG4-related sclerosing cholangitis; PD-1, programmed cell death protein 1; Tfh, T-follicular helper.
Circulating PD1+ Tfh cells and PD-1+ Tfh2 and Tfh17 subsets correlate with IL4 and IL21 cytokine production in IgG4-SC/AIP
Cytokines are important in the class switch and proliferation of IgG4 (and IgE) antibodies. We therefore investigated the relationship of Tfh cells and cytokine production in patients with IgG4-SC/AIP. Cytokines were measured in the supernatants of cultured PBMCs, using nonspecific stimuli to enhance B-cell memory and plasma cell production. Circulating PD1+ Tfh cells correlated with the production of IL-4 (r = 0.89, P = 0.02) and IL-21 (r = 0.83, P = 0.03) and to a lesser extent with IL-13 (P = 0.05) and IL-5 (P = 0.06) (see Table S2, Supplementary Digital Content 3, http://links.lww.com/CTG/A18). Specifically, PD-1+ Tfh2 cells correlated with IL-4 (r = 0.83, P = 0.03) and to a lesser extent with IL-10 (P = 0.05) and IL-21 (P = 0.06) production (Figure S2A-C, see Supplementary Digital Content 2, http://links.lww.com/CTG/A17), whereas PD-1+ Tfh17 cells correlated with IL-21 (r = 1.0, P = 0.001) and to a lesser extent with IL-13 (P = 0.05) and IL-5 (P = 0.05) production (Figure S2D-F, see Supplementary Digital Content, http://links.lww.com/CTG/A17).
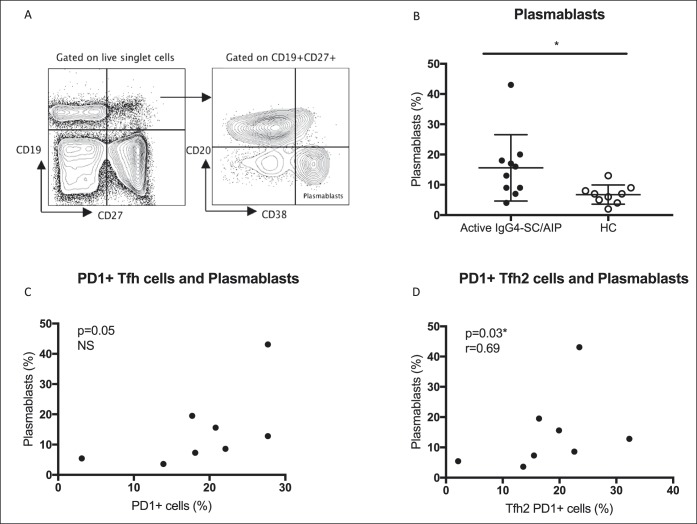
Circulating PD1+ Tfh cells preferentially help B cells to produce IgG4 antibodies
To establish whether activated PD-1+ Tfh cells have the capacity to support B-cell differentiation to produce IgG4 antibodies, we next isolated circulating CD27+ memory B cells from healthy donors and cocultured them with PD1+ or PD1− CD4+CXCR5+ Tfh cells (Figure S3, see Supplementary Digital Content, http://links.lww.com/CTG/A17). IgG4 levels were increased in cell supernatants harvested at day 7 from cultures containing PD1+ compared with PD1− Tfh cells (15.5 vs 2.62 ng/mL, P = 0.008; Figure 3a), despite no difference in the number of CD19loCD27+CD38hi antibody–secreting plasma cells (Figure 3b). Furthermore, levels of IL-4, IL-21, IL-10, and IL-17A cytokines were increased in supernatants from cocultures containing PD1+ compared with PD1− Tfh cells (Figure 3c).
Figure 3.
Circulating PD1+ Tfh cells preferentially help B cells to produce IgG4. Circulating CD27+ memory B cells and PD-1+ or PD-1− Tfh cells from healthy donors were isolated and cocultured in the presence of staphylococcal enterotoxin B (SEB). (a) IgG4 concentration (log10 ng/mL) detected in cell culture supernatant after day 7 of coculture by IgG4 ELISA (3 values are not shown on the logarithmic scale). Data from 9 donors. (b) CD27+ CD38hi antibody–secreting cells as a log10 percentage of CD19lo cells after days 6 and 7 of the coculture. (c) IL-10, IL-21, IL-17A, and IL-4 cytokine concentration in pg/mL detected in cell culture supernatant after days 6 and 7 of the coculture. **Median values and Mann Whitney P values < 0.01. Data from 10 donors. PD-1, programmed cell death protein 1; Tfh, T-follicular helper.
Circulating PD-1+ Tfh cells and PD-1+ Tfh1 and Tfh2 subsets correlate with serum IgG4 and IgE levels in IgG4-SC/AIP
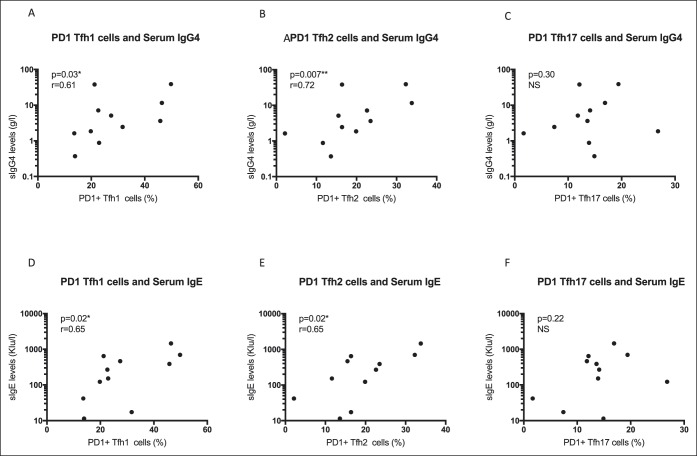
Given the elevated serum IgG4 and IgE titers found in most patients with IgG4-SC/AIP, we next examined the relationship of Tfh cells with serum immunoglobulin levels. Circulating PD-1+ Tfh cells correlated with the serum IgG4 level (r = 0.67, P = 0.01) and IgE level (r = 0.70, P = 0.01) in IgG4-SC/AIP, but not IgG (P = 0.47) or IgG1 (P = 0.09) (Figure S4A-D, see Supplementary Digital Content, http://links.lww.com/CTG/A17). Both PD-1+ Tfh1- and Tfh2-cell subsets correlated with serum IgG4 (Tfh1 r = 0.61, P = 0.03; Tfh2 r = 0.72, P = 0.007) and IgE (Tfh1 r = 0.65, P = 0.02; Tfh2 r = 0.66, P = 0.02) levels (Figure 4a,b,d,e), but not Tfh17 cells (IgG4 P = 0.30; IgE P = 0.22) (Figure 4c,f).
Figure 4.
Circulating PD-1+ Tfh1 and Tfh2 subsets correlate with serum IgG4 and IgE levels in IgG4-SC/AIP. Serum IgG4 and IgE levels were measured by nephelometry and automated ImmunoCAP, respectively. Correlation plots showing serum IgG4 (g/L) plotted against (a) PD-1+ Tfh1 cells, (b) PD1+ Tfh2 cells, and (c) PD1+ Tfh17 cells, and serum IgE (kIU/L) plotted against (d) PD-1+ Tfh1 cells, (e) PD1+ Tfh2 cells, and (f) PD1+ Tfh17 cells. Spearman rank correlation and *P < 0.05, **P < 0.01. AIP, autoimmune pancreatitis; IgG4-SC, IgG4-related sclerosing cholangitis; PD-1, programmed cell death protein 1; Tfh, T-follicular helper.
Circulating PD-1+ Tfh cells and PD-1+ Tfh2 and Tfh17 subsets negatively correlate with the eosinophil count in IgG4-SC/AIP
Peripheral eosinophilia and a clinical history of allergy/atopy have been described in a proportion of patients with IgG4-SC/AIP (3). We thus looked for a relationship of Tfh cells with eosinophils and a history of atopy. There was a negative correlation of PD1+ Tfh cells with the peripheral eosinophil count (r = −0.61, P = 0.02) (Figure S5A, see Supplementary Digital Content 2, http://links.lww.com/CTG/A17), specifically the PD-1+ Tfh2 (r = −0.65, P = 0.01) and Tfh17 (r = −0.60, P = 0.02) subsets (Figure S5B-C, see Supplementary Digital Content 2, Figure S5B-C, http://links.lww.com/CTG/A17). However, there was no relationship of the eosinophil count with PD-1 Tfh1 cells (P = 0.12) (Figure S5D, see Supplementary Digital Content 2, http://links.lww.com/CTG/A17) and no association of PD-1+ Tfh cells or Tfh2 cells with a clinical history of atopy/allergy (Figure S5E-F, see Supplementary Digital Content 2, 2, http://links.lww.com/CTG/A17).
Circulating PD-1+ Tfh cells and PD-1+ Tfh2 and Tfh17 subsets correlate with disease activity in IgG4-SC/AIP
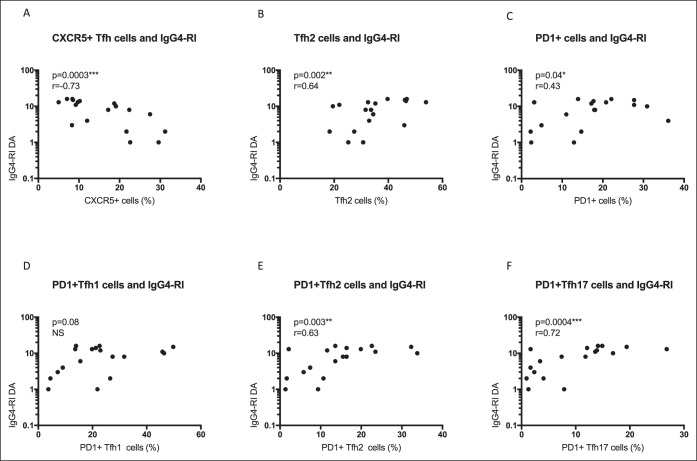
Disease activity, as measured by the IgG4-RI, is an important clinical outcome measure in IgG4-SC/AIP. In patients with IgG4-SC/AIP (n = 18), circulating CD4+CXCR5+ Tfh cells (r = −0.73, P = 0.0003) negatively correlated with the IgG4-RI (Figure 5a). However, Tfh2 cells (r = 0.64, P = 0.002), PD-1+ Tfh cells (r = 0.43, P = 0.04), and specifically PD-1+ Tfh2 (r = 0.63, P = 0.003) and PD-1+ Tfh17 (r = 0.72, P = 0.0004) cell subsets correlated with the IgG4-RI disease activity score (Figure 5b–f). There was no association with organ damage (data not shown).
Figure 5.
Circulating PD-1+ Tfh2 and Tfh17 subsets correlate with disease activity in IgG4-SC/AIP. Disease activity was measured using the IgG4-RI. Correlation plots showing disease activity (DA) (log 10) plotted against the percentage of (a) CXCR5+ Tfh cells, (b) Tfh2 cells, (c) PD-1+ Tfh cells, (d) PD1+ Tfh1 cells, (e) PD1+ Tfh2 cells, and (f) PD1+ Tfh17 cells. Spearman rank correlation and P values as per Figure 2. AIP, autoimmune pancreatitis; IgG4-RI, IgG4-responder index; IgG4-SC, IgG4-related sclerosing cholangitis; PD-1, programmed cell death protein 1; Tfh, T-follicular helper.
Circulating PD1+ Tfh2 cells compared with other biomarkers of disease activity in IgG4-SC/AIP
Clinical markers of active disease include measurements of serum IgG4 and IgE levels, the IgG4-RI disease activity score, and expansion of plasmablasts in peripheral blood. We therefore performed nonlinear multiple regression analysis to compare these biomarkers with PD1+ Tfh2 cells in our IgG4-SC/AIP cohort. The model fit for PD1+ Tfh2 cells, serum IgG4 and IgG4-RI score (R2 = 0.22) was superior to that of plasmablasts, serum IgG4 and IgG4-RI score (R2 = 0.11). Similarly, the model fit for PD1+ Tfh2 cells, serum IgG4 and IgE levels (R2 = 0.56) were superior to that of plasmablasts, serum IgG4 and IgE levels (R2 = 0.24). Lastly, the combination of PD1+ Tfh2 cells, plasmablasts, and serum IgG4 (R2 = 0.36) was superior to PD1+ Tfh2 cells, plasmablasts, and IgG4-RI score (R2 = 0.24).
Circulating PD-1+ Tfh cells and plasmablasts decrease after treatment of IgG4-SC/AIP
To assess the effect of treatment on Tfh-cell subsets, we compared patients with IgG4-SC/AIP with active (n = 12) and inactive treated (n = 6) disease. Serum IgG4 (P = 0.02) and the IgG4-RI (P = 0.0001) were lower in those with treated disease (Table 1). The proportion of PD1+ Tfh cells and plasmablasts decreased after treatment in paired samples pre- and post-treatment (n = 6, P = 0.03) (Figure S6, see , Supplementary Digital Content 4, http://links.lww.com/CTG/A19). There was lower expression of PD-1+ Tfh cells (18.0% vs 4.9%, P = 0.009) and specifically PD-1+ Tfh2-cell (16.4% vs 5.8%, P = 0.002) and Tfh17-cell (Tfh17 13.8% vs 2.4%, P = 0.009) subsets in inactive treated compared with active IgG4-SC/AIP (Figure 1d).
Tissue CD4+CXCR5+ Tfh cells in active IgG4-RD
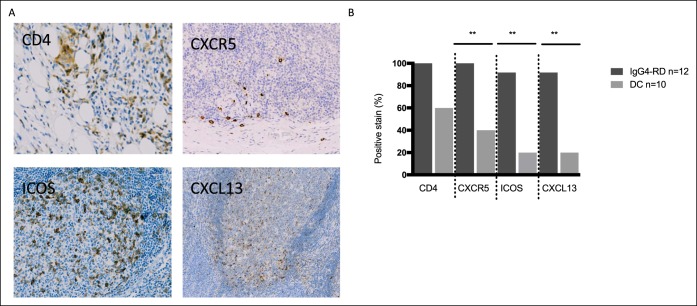
To confirm whether Tfh cells were also present in the tissue, we assessed Tfh-cell markers in involved lesions of patients with active IgG4-SC/AIP (n = 12). Representative staining is shown in Figure 6a, top. CD4+CXCR5+ Tfh cells were present in all (100%) IgG4-RD tissue specimens compared with 4 of 10 DC specimens (40%) from other pancreatic and biliary diseases (P = 0.003) (see Table S3, see Supplementary Digital Content 3, http://links.lww.com/CTG/A18). CXCR5+ staining was defined as “moderate” or “abundant” in 5 of 12 IgG4-RD specimens (41.7%) compared with 2 of 10 DC specimens (20%) (see Table S4, Supplementary Digital Content 3, http://links.lww.com/CTG/A18).
Figure 6.
CD4+ T cells and Tfh cells are present in IgG4-RD tissue specimens and home to lymphoid follicles. Paraffin-embedded tissue specimens were stained for Tfh-cell markers. (a) Representative panels from patients with IgG4-SC/AIP stained for CD4 (liver, top left panel), CXCR5 (pancreas, top right panel), ICOS (pancreas, bottom left panel), and CXCL13 (pancreas, bottom right panel). Positively staining cells stain DAP-positive (brown). (b) Histogram shows the percentage of positively staining specimens for CD4+, CXCR5+, CXCR5+ICOS+, and CXCL13+ cells. Data from 12 patients with IgG4-SC/AIP (red bars) and 10 disease controls (gray bars). **P values as in Figure 2. AIP, autoimmune pancreatitis; DC, disease control; IgG4-RD, IgG4-related disease; IgG4-RI, IgG4–responder index; IgG4-SC, IgG4-related sclerosing cholangitis.
Activated CXCR5+ Tfh cells home to lymphoid follicles in IgG4-RD
To evaluate the phenotype and localization of the CXCR5+ Tfh cells, we next stained tissues with the Tfh-cell activation marker inducable T cell costimulator (ICOS) and the lymphoid homing chemokine CXCL13. Representative staining is shown in Figure 6a, bottom. ICOS+ Tfh cells were present in 11 of 12 IgG4-RD tissue specimens (92%) compared with 2 of 10 DC specimens (20%) (P = 0.002) (see Table S3, Supplementary Digital Content 3, http://links.lww.com/CTG/A18). ICOS+ staining was defined as “moderate” or “abundant” in 6 of 12 IgG4-RD specimens (50%) compared with 2 of 10 DC specimens (20%) (see Table S4, Supplementary Digital Content 3, http://links.lww.com/CTG/A18). CXCL13+ cells were observed in 11 of 12 IgG4-RD specimens (92%) and 2 of 10 DC specimens (20%) (P = 0.002) (see Table S3, Supplementary Digital Content 3, http://links.lww.com/CTG/A18), and were more abundant in number in IgG4-RD than DC (median 17 cells/high power field vs 4 cells/high power field; P = 0.008). The most pronounced CXCR5+ICOS+ staining occurred in lymphoid aggregates, predominantly in salivary glands and lymph nodes, and to a lesser extent in the liver and pancreatic specimens of patients with IgG4-SC/AIP.
DISCUSSION
In this study, we observe Tfh cells in both the circulation and inflammatory lesions in patients with IgG4-SC/AIP. Tfh cells play an important role in T cell–dependent immune responses and are necessary for B-cell differentiation and class switch in GCs (7). The activation marker PD-1 is essential for B-cell selection, survival, and maturation (8). Our data show that patients with IgG4-SC/AIP with active, but not inactive disease, have expanded populations of circulating PD1+ Tfh cells, with expansion of all PD1+ Tfh1-, Tfh2-, and Tfh17-cell subsets. Furthermore, we show that the PD-1+ Tfh2-cell subset was the most prominent, supported by in vitro studies showing the Tfh2 subtype as the most effective in providing B-cell help through direct interaction and cytokine production (8). Our data support observations of expanded activated Tfh cells in patients with IgG4-related dacryoadenitis and sialadenitis (12,21) (IgG4-DS) and in systemic IgG4-RD (22).
The identification of appropriate biomarkers that are linked to disease activity in IgG4-SC/AIP is important. Elevated serum IgG4 and IgE levels are not sufficiently sensitive or specific for this purpose (3,4); however, increased numbers of circulating plasmablasts seem to coincide with both active disease and disease relapse (5). Furthermore, dominant IgG4+ BCR clones have been identified in patients with IgG4-SC/AIP, and a quantitative PCR protocol analyzing the IgG4/IgG RNA ratio in blood may be helpful in monitoring response to corticosteroid therapy (23). Here, we show an increase in the proportion of plasmablasts in active IgG4-SC/AIP and demonstrate a correlation between the PD-1+ Tfh2-cell subset and plasmablast population. Furthermore, we show that circulating PD-1+ Tfh2 cells correlate with serum levels of IgG4 and IgE, the IgG4-RI disease activity score, and are reduced after treatment in line with clinical remission of disease. Indeed, PD1+ Tfh2 cells provide added value to the other clinical markers of active disease and perform better than circulating plasmablast expansion on multiple regression analysis in our cohort. In support of this, Tfh2 cells have been reported to best mirror disease activity in IgG4-DS (14,24). This supports a role for activated circulating PD-1+ Tfh2 cells as a biomarker of disease activity in IgG4-SC/AIP, complementary to other clinical markers, and highlights a role for the Tfh2-plasmablast axis in active IgG4-RD.
The involvement of Th2 and regulatory cells in IgG4-SC/AIP has been the topic of much debate (25–27), with evidence suggesting that circulating Th2 memory cells are restricted to a defined subset of patients with atopy (28). Importantly, Th2-derived cytokines IL-4, IL-5, and IL-13, believed to drive IgG4 (and IgE) class switch, can also be produced by mast cells and Tfh-cell subsets (3,12,14,29,30). Here, we show increased production of IL-4 and IL-21 cytokines and to a lesser extent IL-13, IL-5, and IL-10 from stimulated cells of patients with active IgG4-SC/AIP. The concentrations of IL-4 and IL-21 correlated with PD-1+ Tfh2 (r = 0.89, P = 0.02) and PD-1+ Tfh17 (r = 0.83, P = 0.03) subsets, respectively. IL4+ Tfh cells have been shown to be expanded in salivary gland lesions and draining lymph nodes of patients with IgG4-RD and have been linked to IgG4 class switching in vivo, transcriptionally distinct from Th2 and IL4− Tfh cells (12).
In this study, we demonstrate ex vivo that PD-1+ Tfh cells can support B cells to produce IgG4 antibody, supporting the hypothesis that the expanded population of circulating PD1+ Tfh cells support B-cell differentiation toward IgG4+ plasma cells. Tfh cells have been shown to be more efficient in inducing autologous naive B cells to differentiate into plasmablasts with increased IgG4 secretion, facilitating B-cell proliferation, and inhibiting B-cell apoptosis in patients with IgG4-DS compared with HCs, suggesting a functional role of these cells in the disease (21,31).
We have previously shown that elevated serum IgE levels, peripheral eosinophilia, and a clinical history of allergy and/or atopy are prominent in a subset of patients with IgG4-SC/AIP (3). Here, we show for the first time, a correlation of activated PD-1 Tfh2 subsets with serum IgE levels (positive) and peripheral eosinophilia (negative), independent of a history of allergy and/or atopy. IL-5 is a key cytokine for the development, recruitment to inflammatory sites, and activation of eosinophils (32). Local inflammation can cause recruitment of eosinophils with inflammatory features (33) and fibrotic potential (34). It is plausible that eosinophils migrate into the involved tissue in patients with IgG4-SC/AIP, as evidenced by the presence of abundant tissue eosinophils in disease lesions (3,35), similar to eosinophilic airway diseases (33,34). However, the exact role of these cells in the disease is yet to be defined.
Ectopic GCs are frequently observed in tissue lesions of patients with systemic IgG4-RD, indicating aberrant activation of humoral immune responses (6). Tfh cells are essential for GC formation in lymphoid follicles, B-cell maturation and differentiation, and immunoglobulin class switch. Here, we show the presence of CD4+CXCR5+ Tfh cells, which were localized in lymphoid follicles in GC of disease lesions. The presence of an activated phenotype (ICOS+) in inflammatory lesions supports the theory that these cells orchestrate local B-cell differentiation into IgG4 plasma cells. Indeed, CXCR5+ cells characterized by expression of BCL6 and ICOS infiltrated submandibular glands in IgG4-DS and had a higher capacity than controls to help B cells produce IgG4 (31). CXCL13, produced in abundance by follicular stromal cells within B-cell follicles, is necessary for Tfh-cell migration to the center of follicles to establish GC, and circulating Tfh cells respond to CLCX13 and migrate back to GC (8,36). We demonstrated a reduction in circulating CXCR5+ Tfh cells in active IgG4-SC/AIP and an abundance of tissue CXCL13 in follicles, suggesting the migration of these cells to sites of tissue inflammation in disease. It is plausible that chronic antigenic stimulation, such as repeated occupational or environmental exposures (37,38), may polarize and activate CD4+ Tfh2 cells, leading to the development of GC within lymphoid follicles of affected organs, and the subsequent production and proliferation of IgG4-switched cells. Our findings agree with recent evidence that IL-4+ Tfh cells orchestrate IgG4 class switch in IgG4− lesions (12).
Therapeutic application of our findings requires careful consideration. Treatment of patients with IgG4-RD with rituximab (CD20 B-cell depletion) leads to temporary resolution of disease as in other immune-mediated conditions. Rituximab depletes somatically hypermutated plasmablasts, followed by the reappearance of clonally divergent oligoclonal plasmablast populations. If activated Tfh cells are pathogenic; they may engage with remaining and/or recovering B cells and reactivate the disease. In theory, anti–PD-1 could control Tfh-cell activation, and these agents are approved for the treatment of solid tumors (39). However, conversely, blockade of the PD-1 pathway has been shown to expand Tfh cells and boost the humoral immune response (40,41). Given the importance of IL-21 production by Tfh cells in the formation of GCs, both IL-21 receptor antagonists and IL-21 blocking antibodies have the potential to modulate the Tfh-mediated immune response (42). The efficiency and safety of this approach is unknown. Targeting CXCL13 to inhibit the homing and trafficking of Tfh cells might be a more efficient strategy to prevent accumulation of Tfh in disease lesions (36). Suppression of both Tfh-GC formation and Tfh cell migration using a combined approach (e.g., IL-21 and CXCL13 inhibition) could provide a more robust control of humoral immunity.
Although this is the largest study to date to explore Tfh-cell subsets in patients with pancreatic and biliary IgG4-RD, the numbers in this study are relatively small. Our observations should be confirmed in larger cohorts, such as in the EASL-funded European Repository of patients with IgG4-RD with clinical data linked to biobanked samples, to further explore disease biomarkers and understand pathogenesis. Furthermore, correlations do not necessarily reflect disease causation, and expansions of circulating, lesional or lymph node Tfh cells that reflect the prominent class switching to IgG4 may not necessarily be linked to the process of pathogenesis. Prospective studies that evaluate the sequence in which specific IgG4-RD biomarkers evolve, in addition to further insights into Tfh biology, will help to progress our understanding of the impact on Tfh phenotype on IgG4-RD pathogenesis.
In conclusion, this study reports the expansion of circulating and tissue-infiltrating activated Tfh-cell subsets and correlation of these with markers of disease activity. It also demonstrates that PD-1+ Tfh cells were more efficient at inducing IgG4 production and related cytokines than PD-1− cells. Together, these findings suggest that Tfh cells are important in orchestrating the proliferation and differentiation of IgG4-committed B cells in IgG-SC/AIP. This is the first study to phenotype Tfh subsets specifically in patients with biliary and pancreatic manifestations of IgG4-RD, and all patients had biopsy-proven disease and were part of a prospectively recruited cohort. Circulating PD1+ Tfh2 cells can be considered as a biomarker of disease activity. Further studies to determine the specificity of these Tfh cells and drivers of Tfh-cell activity in IgG4-RD are warranted for the purpose of therapeutic application.
CONFLICTS OF INTEREST
Guarantor of the article: Emma L. Culver, PhD.
Specific author contributions: Eleanor Barnes, PhD, and Emma L. Culver, PhD, contributed equally to this work. T.C. recruited patients, collected samples, assessed disease activity, performed the Tfh-cell flow cytometry and cocultures, analyzed data, and drafted the manuscript. M.M. and L.L. provided experimental consult and support for the Tfh and plasmablast assays. R.S. provided assistance with and analyzed the Luminex and serological data. R.P. recruited patients, collected blood samples, and assessed disease activity within the IgG4-MDT. M.v.H., P.K. and T.R. provided specialist consult on B-cell and Tfh-cell biology and experimental assays. A.B. performed histological specimen analysis and interpretation. E.B. and E.L.C. had the original concept for this study. E.B. is the principal investigator for the IgG4-RD EASL registry study. E.L.C. recruited patients, performed flow assays and histological assessment, assessed disease activity and treatment response, and performed data analysis/interpretation. All authors edited and approved the final manuscript.
Financial support: The study is registered on the UK National Institute for Health Research (NIHR) portfolio as study number 10776. This study was supported by the National Institute of Health Research (NIHR) Biomedical Research Centre, based at Oxford University Hospitals Trust and Oxfordshire Health Service Research Committee (OHSRC) as part of Oxford Hospitals Charity, Oxford. TC received funding from a CORE/Falk bursary, the NIHR and a Wellcome Trust Training Fellowship for Clinicians [211042/Z/18/Z]. R.P. is supported by the the Oxford NIHR BRC. L.L. is supported by a Sanquin Grant [PPOC10-008]. P.K. is supported by a Wellcome Senior Fellowship [091663MA]. E.B. is supported by the MRC as an MRC Senior Clinical Fellow and the Oxford NIHR BRC. E.L.C. is supported by an NIHR Academic Clinical Lectureship and a Wellcome Trust Research Fellowship [095160/Z10/Z]. P.K. is supported by a Wellcome Senior Fellowship [091663MA].
Potential competing interests: The authors have no financial or commercial conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ Activated circulating Tfh cells have been reported in patients with IgG4-DS.
WHAT IS NEW HERE
✓ This is the first study to phenotype Tfh subsets in the peripheral blood and tissue, specifically in patients with biliary and pancreatic IgG4-RD.
✓ Expansion of circulating and tissue-infiltrating activated Tfh-cell subsets is found in active IgG4-SC/AIP, but not inactive disease.
✓ Circulating activated PD1+ Tfh2-cell subset correlates with markers of disease activity including expansion of plasmablasts, serum IgG4 and IgE titers, and the IgG4-RI in IgG4-SC/AIP, and reduces after treatment in line with clinical remission of disease.
✓ Activated CXCR5+ Tfh cells homed to lymphoid follicles in IgG4-SC/AIP tissues.
✓ PD-1+ Tfh cells can support B cells to produce IgG4 antibody and type 2 cytokines ex vivo, supporting a role of Tfh cells in disease pathogeneisis.
TRANSLATIONAL IMPACT
✓ We show that circulating and lesional Tfh cells are activated in IgG4-AIP and IgG4-SC. Circulating PD1+ Tfh2 cells could be a biomarker of disease activity and a potential target for immunotherapy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the CRN nurses, patients with IgG4-RD and PSC, and healthy volunteers who contributed to this project. We acknowledge Jonathan Lau (IgG4 project manager, John Radcliffe Hospital, Oxford, UK) and Dr. Charis Manganis (Academic Fellow, John Radcliffe Hospital, Oxford, UK) for patient recruitment to the study and Abigail Breach and Tony Mellows (Histopathology Department, Southampton General Hospital, Southampton, UK) for antibody titration and staining of tissue specimens.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A16, http://links.lww.com/CTG/A17, http://links.lww.com/CTG/A18, http://links.lww.com/CTG/A19
REFERENCES
- 1.Culver EL, Chapman RW. IgG4-related hepatobiliary disease: An overview. Nat Rev Gastroenterol Hepatol 2016;13:601–12. [DOI] [PubMed] [Google Scholar]
- 2.Huggett MT, Culver EL, Kumar M, et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol 2014;109:1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culver EL, Sadler R, Bateman AC, et al. Increases in IgE, eosinophils, and mast cells can be used in diagnosis and to predict relapse of IgG4-related disease. Clin Gastroenterol Hepatol 2017;15:1444–52.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culver EL, Sadler R, Simpson D, et al. Elevated serum IgG4 levels in diagnosis, treatment response, organ involvement, and relapse in a prospective IgG4-related disease UK cohort. Am J Gastroenterol 2016;111:733–43. [DOI] [PubMed] [Google Scholar]
- 5.Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015;74:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattoo H, Mahajan VS, Della-Torre E, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014;134:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 2000;192:1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morita R, Schmitt N, Bentebibel S, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2012;34:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010;62:234–44. [DOI] [PubMed] [Google Scholar]
- 10.Le Coz C, Joublin A, Pasquali JL, et al. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One 2013;8:e75319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotty ST. Follicular helper cell differentiation, function, and roles in disease. Immunity 2014;41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maehara T, Mattoo H, Mahajan VS, et al. The expansion in lymphoid organs of IL-4+ BATF+ T follicular helper cells is linked to IgG4 class switching in vivo. Life Sci Alliance 2018;1:e201800050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamekura R, Takano K, Yamamoto M, et al. Cutting edge: A critical role of lesional T follicular helper cells in the pathogenesis of IgG4-related disease. J Immunol 2017;199:2624–9. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama M, Suzuki K, Yamaoka K, et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol (Hoboken, N.J.) 2015;67:2476–81. [DOI] [PubMed] [Google Scholar]
- 15.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience. Clin Gastroenterol Hepatol 2006;4:1010–6. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki K, Chari ST, Frulloni L, et al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology 2017;17:1–6. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–92. [DOI] [PubMed] [Google Scholar]
- 18.Beuers U, Boberg KM, Chapman RW. EASL clinical practice guidelines: Management of cholestatic liver diseases. J Hepatol 2009;51:237–67. [DOI] [PubMed] [Google Scholar]
- 19.Carruthers MN, Stone JH, Deshpande V, et al. Development of an IgG4-RD responder index. Int J Rheumatol 2012;2012:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace ZS, Khosroshahi A, Carruthers MD, et al. An International, Multi-Specialty Validation Study of the IgG4-related disease responder index. Arthritis Care Res (Hoboken) 2018;70:1671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama M, Yasuoka H, Yamaoka K, et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther 2016;18:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grados A, Ebbo M, Piperoglou C, et al. T cell polarization toward TH2/TFH2 and TH17/TFH17 in patients with IgG4-related disease. Front Immunol 2017;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doorenspleet ME, Hubers LM, Culver EL, et al. Immunoglobulin G4+ B-cell receptor clones distinguish immunoglobulin G 4-related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology 2016;64:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Kubo S, Nakayamada S, Zhao J, et al. Correlation of T follicular helper cells and plasmablasts with the development of organ involvement in patients with IgG4-related disease. Rheumatology 2018;57:514–24. [DOI] [PubMed] [Google Scholar]
- 25.Zen Y, Fujii T, Harada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007;45:1538–46. [DOI] [PubMed] [Google Scholar]
- 26.Koyabu M, Uchida K, Miyoshi H, et al. Analysis of regulatory T cells and IgG4-positive plasma cells among patients of IgG4-related sclerosing cholangitis and autoimmune liver diseases. J Gastroenterol 2010;45:732–41. [DOI] [PubMed] [Google Scholar]
- 27.Zen Y, Liberal R, Nakanuma Y, et al. Possible involvement of CCL1-CCR8 interaction in lymphocytic recruitment in IgG4-related sclerosing cholangitis. J Hepatol 2013;59:1059–64. [DOI] [PubMed] [Google Scholar]
- 28.Mattoo H, Della-Torre E, Mahajan VS, et al. Circulating Th2 memory cells in IgG4-related disease are restricted to a defined subset of subjects with atopy. Allergy 2014;69:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi M, Ohno K, Takata K, et al. Interleukin 13-positive mast cells are increased in immunoglobulin G4-related sialadenitis. Sci Rep 2015;5:7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi M, Sato Y, Ohno K, et al. T helper 2 and regulatory T-cell cytokine production by mast cells: A key factor in the pathogenesis of IgG4-related disease. Mod Pathol 2014;27:1126–36. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Lin W, Yang H, et al. Aberrant expansion and function of T follicular helper cell subsets in IgG4-related disease. Arthritis Rheumatol (Hoboken, N.J.) 2018;70:1853–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: Changing perspectives in health and disease. Nat Rev Immunol 2013;13:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 2016;126:3279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morimoto Y, Hirahara K, Kiuchi M, et al. Amphiregulin-producing pathogenic Memory T helper 2 cells instruct Eosinophils to secrete Osteopontin and facilitate airway Fibrosis. Immunity 2018;49:134–50.e6. [DOI] [PubMed] [Google Scholar]
- 35.Kamisawa T, Anjiki H, Egawa N, et al. Allergic manifestations in autoimmune pancreatitis. Eur J Gastroenterol Hepatol 2009;21:1136–9. [DOI] [PubMed] [Google Scholar]
- 36.Lee HT, Shiao YM, Wu TH, et al. Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol 2010;37:45–52. [DOI] [PubMed] [Google Scholar]
- 37.Culver EL, Vermeulen E, Makuch M, et al. Increased IgG4 responses to multiple food and animal antigens indicate a polyclonal expansion and differentiation of pre-existing B cells in IgG4-related disease. Ann Rheum Dis 2015;74:944–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Buy Wenniger LJ, Culver EL, Beuers U. Exposure to occupational antigens might predispose to IgG4-related disease. Hepatology 2014;60:1453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat Rev 2017;59:71–8. [DOI] [PubMed] [Google Scholar]
- 40.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009;458:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hams E, McCarron MJ, Amu S, et al. Blockade of B7-H1 (programmed death Ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol 2011;186:5648–55. [DOI] [PubMed] [Google Scholar]
- 42.Choi JY, Seth A, Kashgarian M, et al. Disruption of pathogenic cellular networks by IL-21 blockade leads to disease amelioration in murine lupus. J Immunol 2017;198:2578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.