Abstract
Background:
Although the risks of using central nervous system depressant (CNS-D) medications with alcohol are well-documented, little is known about trends in prescribed use of these medications among individuals who regularly consume alcohol (i.e., trends in “concurrent use”). We examined changes in the prevalence of prescribed CNS-D medications among individuals who drank alcohol on 52 or more occasions in the past year (“regular drinking”). CNS-D medications included sedative-hypnotics (subclassified as anxiolytics or sleep medications) and opioids.
Methods:
We used eight cross-sectional cycles of the National Health and Nutrition Examination Survey (1999-2000 to 2013-2014) from participants age 20 and older (n=37,709). We used log-binomial regression to examine (1) prevalence trends of prescribed CNS-D medication use, (2) trend differences by drinking status, and (3) correlates of CNS-D medication use.
Results:
Among those who drink regularly, the relative annual increase in prevalence of sedative-hypnotic use was 5.3% (95% CI: 2.7-7.9): anxiolytic and sleep medication use increased annually by 3.7% (95% CI: 0.8-6.7) and 11.2% (95% CI: 6.5-16.0) respectively. Opioid use trends among those who drink regularly were not statistically significant but were non-linear. Differences in CNS-D medication trends between those who drink regularly and those who drink infrequently/abstain were not statistically significant. Those who drink regularly were less likely than those who drink infrequently/abstain to use opioids (adjusted relative risk [ARR]: 0.69, 95% CI: 0.60-0.78) and anxiolytics (ARR: 0.71, 95% CI: 0.61-0.81), but not sleep medications (ARR: 1.04, 95% CI: 0.80-1.35). Those age 40 and older were 2-5 times as likely as those age 20-29 to use sedative-hypnotics.
Conclusion:
Among those who drink regularly, the prevalence of prescribed sedative-hypnotic use increased and prescribed opioid use remained common. These trends indicate that a substantial portion of the population is at risk for alcohol-related adverse drug reactions – particularly those age 40 and older.
Keywords: Opioid, Benzodiazepine, Sleep Medication, Alcohol, Trends
Introduction
The United States has undergone sharp increases in morbidity and mortality stemming from separate and combined use of alcohol and prescription drugs. For example, the annual incidence proportion of acute alcohol-related emergency department visits increased by 40% from 2006 to 2014 – approximately 3.5% per year – and the annual incidence proportion of emergency department visits for alcohol-related adverse drug reactions more than doubled over roughly the same period – increasing 10.7% per year (White et al., 2018; Castle et al., 2016). Although the prevalence of current alcohol use and binge drinking has been increasing, changes in drinking patterns are modest in comparison to alcohol-related morbidity and mortality trends. Meta-analyses of data from six national surveys suggest that from 2000 to 2016, the prevalence of any past-year alcohol use increased by 0.3% per year and past-year binge drinking increased by about 0.7% per year among U.S. adults (18+) (Grucza et al., 2018). Notably, though, these increases have been highest among individuals age 50-64, with increases of 0.6% and 2.7% per year for any alcohol use and binge drinking respectively (Grucza et al., 2018). Over roughly the same period, the prevalence of past 30-day prescription medication use among adults increased by approximately 2.5% per year (Kantor et al., 2015). Because the prevalence of prescription drug use increases with age (Kantor et al., 2015), it seems likely that the unique set of risks posed by the interplay of alcohol, prescribed medications, and age may be one factor contributing to the trends in alcohol and alcohol-drug morbidity and mortality noted above.
Co-administration of alcohol and alcohol-interactive medications increases one’s risk of several adverse events such as falls, overdose, or motor vehicle accidents (Moore et al., 2007; Weathermon and Crabb, 1999; Hansen et al., 2015). Central nervous system depressant medications (a subclass of alcohol-interactive medications) warrant particularly close scrutiny due to their disproportionate contribution to the incidence of alcohol-related adverse drug reactions. Approximately 3% to 7% of the U.S. population currently uses prescribed central nervous system depressant medications such as sedative-hypnotics (e.g., anxiolytics such as benzodiazepines, or sleep medication “Z-drugs” such as zolpidem) or opioids (Kantor et al., 2015; Bachhuber et al., 2016; Bertisch et al., 2014; Mojtabai, 2018). Yet these medications were implicated in over 40% of the alcohol-related adverse drug reactions that occurred between 2005 and 2011 (Castle et al., 2016). By contrast, 27% of the population uses cardiovascular agents (Kantor et al., 2015), but these medications are involved in less than 10% of alcohol-related adverse drug reactions (Castle et al., 2016).
The disproportionate contribution that sedative-hypnotics and opioids make to the incidence of alcohol-related adverse drug reactions is not surprising in light of the pharmacodynamic properties of these medications. For example, co-administration of alcohol and sedative-hypnotics potentiates the inhibitory effect of GABA transmission, creating stronger sedating effects and cognitive impairment (Moody, 2012; Langtry and Benfield, 1990; Davies et al., 2000; Hesse et al., 2003; Gudin et al., 2013). Co-administration of alcohol and prescription opioids such as hydrocodone or oxycodone magnifies the risk of respiratory depression via synergistic effects between μ-opioid and GABA receptor activity in the central nervous system (White and Irvine, 1999). Of note, those aged 40 and older are 1.5 to 3 times as likely to be using sedative-hypnotics or opioids as those under the age of 40 (Chong et al., 2013; Kaufmann et al., 2016; Parsells Kelly et al., 2008; Campbell et al., 2010). This is concerning for two reasons: first, as detailed above, more older adults are using alcohol and binge drinking than in prior years (Grucza et al., 2018); second, older adults are uniquely vulnerable to the adverse effects of alcohol and medication co-administration because of metabolic, digestive, and cognitive changes that occur with age (Moore et al., 2007).
Although the risks of using sedative-hypnotics or opioids with alcohol are well-documented and frequently communicated by regulatory bodies (e.g., Food and Drug Administration), little is known about trends in prescribed use of these medications among those who regularly consume alcohol (i.e., trends in “concurrent use”). It is critical that we examine changes in concurrent use given the separate increases in alcohol use and in central nervous system depressant medication use that have been observed. In addition, it is unknown whether those who report regular alcohol consumption are more or less likely to have prescriptions for central nervous system depressant medications relative to those who report infrequent alcohol consumption or abstinence, and whether the magnitude of such differences has changed over time. Understanding these trends and relationships could potentially help explain the documented increases in alcohol-related adverse drug reactions and acute ED admissions. Therefore, the primary goal of the present study was to examine changes in the prevalence of prescribed sedative-hypnotic and opioid medication use among those who do and do not regularly consume alcohol, from the years 1999 to 2014. Specifically, we sought to (1) compare how the prevalence of prescribed sedative-hypnotic and opioid use has changed among those who drink regularly and those who drink infrequently or abstain; (2) determine whether the magnitude of change in the prevalence of prescribed sedative-hypnotic and opioid use among those who drink regularly was proportional to the magnitude of change among those who drink infrequently or abstain; and (3) determine which groups are most likely to use prescribed sedative-hypnotics or opioids (i.e., those who drink regularly vs. those who drink infrequently/abstain, male vs. female, white vs. minority, etc).
Materials and Methods
Data Source.
We utilized data from the National Health and Nutrition Examination Survey (NHANES), which has been conducted continuously since 1999 and is overseen by the Centers for Disease Control and Prevention (CDC). NHANES uses multistage area probability sampling to generate a nationally representative sample of approximately 5,000 domiciled civilian residents, and data are released as two-year survey cycles (e.g., 2000-2001, 2002-2003, etc). Participants are interviewed by dietary and health interviewers, physicians, and medical technicians (Zipf et al., 2013). Questionnaires are administered face-to-face in participants’ homes (in-home interview), and subsequent physical examinations are conducted in Mobile Examination Centers (MEC). The net response rate is typically about 75% for the in-home interview and 70% for the examination. The presence and effect of nonresponse biases on estimates produced using NHANES data have been judged to be minimal (Centers for Disease Control, 2018).
Measures analyzed in this study included sociodemographics and prescription drug use, both of which were assessed during the in-home interview. Alcohol use is assessed in conjunction with physical exams conducted in the MECs using audio-computer assisted self-interview. Thus, analyses utilized data from participants who participated in both the in-home interview and MEC portions of the NHANES. Analyses were also limited to ages 20 and over because alcohol data were not available for individuals under the age of 20 for all years. Additional details about NHANES procedures are available elsewhere (Grucza et al., 2018).
Alcohol and Prescription Drug Measures.
Frequency of past-year alcohol use was assessed with the item “In the past 12 months, how often did you drink any type of alcoholic beverage?” Respondents are provided with a small text box and instructed to type the frequency of their alcohol consumption (as numeric characters) as well the unit in which they are reporting that frequency (per week, per month, or per year). Using this item, we defined “regular” alcohol use as consuming alcohol on average one or more times per week in the past year, (i.e., at least 52 times in the past year). Drinking less than 52 times in the past year was classified as “infrequent drinking or abstention.” In supplemental analyses, we examined binge drinking and average drinks per drinking day among those who reported regular or infrequent alcohol consumption to verify our assumption that excess risk is likely to be concentrated among those who report regular alcohol consumption. As an additional robustness check, we separated those who abstain from those who drink infrequently and examined whether medication use differed between these groups.
Participants who reported having taken any prescription medications in the past 30 days were asked to show their medication containers to the interviewer, who entered the product name in the computer. NHANES interviewers are instructed to encourage reluctant participants to show their medication containers by explaining that collecting accurate medication information is critical for monitoring the health of the United States. Participants who refuse to provide physical containers are asked to verbally report their medication use instead (16.7% of respondents). Entered medication names were matched to a prescription drug database, Lexicon Plus® by Cerner Multum, Inc., which is used to classify the medications by therapeutic drug categories. Prescription drug data are stored in an event-level database, (i.e., each participant may have multiple prescriptions). Records involving sedative-hypnotics or opioids were extracted according to the classification terms described in Box 1. Certain drugs with low abuse potential were excluded, such as antihistamines and supplements. Sedative-hypnotics were sub-classified by indication as anxiolytics and sleep medications. Anxiolytics were primarily benzodiazepines, and sleep medicines were predominantly zolpidem, eszopiclone, zaleplon, and ramelteon. To exclude medications used short-term for acute medical problems, we extracted only records for which medications were prescribed for 30 days or more.
Box 1:
Drug classification inclusion and exclusion criteria
| Sedative-Hypnotics |
| Include: “benzodiazepine” “barbiturate” “sedative” “anxiolytic” |
| Exclude: “narcotic” “nutraceutical” “antihistamine” “respiratory agent” “buspirone” “doxepin” “Acetaminophen; dichloralphenazone; isometheptene” |
| Anxiolytics |
| Include “benzodiazepine” “barbiturate” “anxiolytics” |
| Sleep Medications |
| All others |
| Opioids |
| Include “narcotic” |
| Exclude: none. |
During the years under study, there were n=43,793 respondents age 20 or over who participated in the in-home interview. Of these, n=41,659 participated in the MEC portion, but n=3,853 did not provide any alcohol data. Of the remaining n=37,806 individuals, n=24 refused to answer or were missing on prescription data and n=73 did not provide enough alcohol data to calculate regular drinking status – resulting in a final sample size of n=37,709.
Sociodemographic Characteristics.
Characteristics used for this study included sex, age (20-29 years, 30-39 years, 40-49 years, 50-64 years, and ≥65 years), race/ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, and non-Hispanic other race - including multi-racial), and highest education level attained (less than high school, high school graduate/GED or equivalent, some college or associate’s degree, and college graduate or above).
Statistical Analysis.
For a descriptive summary of the sample, we classified participants into one of four categories: (1) no regular drinking or prescribed sedative-hypnotics or opioids, (2) no regular drinking but prescribed sedative-hypnotics or opioids, (3) regular drinking but no prescribed sedative-hypnotics or opioids, (4) regular drinking and prescribed sedative-hypnotics or opioids. We calculated the proportion of participants in each of the four categories within strata of sociodemographic variables as well as by year of data collection.
Biennial prevalence of sedative-hypnotic and opioid use was estimated for the entire sample and separately for those who reported regular drinking and those who reported infrequent drinking or abstention. Log-binomial regression models with exponentiated coefficients were used to model the prevalence of prescription drug use as a function of year. The resulting estimates of the relative risk (RR) per year were converted to the annual percent change (APC) in prevalence (relative to the prior year), calculated as 100*(RR-1). These regression models were used to estimate the prevalence for each medication category at the beginning and end of the observation period (i.e., for years 1999-2000 and for years 2013-2014). We also tested whether the magnitude of change in the prevalence of prescription medication use differed between those who drink regularly and those who drink infrequently or abstain by testing for interactions between year and drinking status (i.e., medication prevalence = year, drinking status, year*drinking status).
Based on earlier studies showing non-linear opioid prescribing trends during the years under study (Guy et al., 2017), we conducted supplemental analyses to test for non-linearity. Following earlier studies (Jones and McAninch, 2015) we utilized the Joinpoint Statistical Software to identify the presence of year-specific inflection points in overall trends of opioids or sedative-hypnotic use (National Cancer Institute, 2018; Kim et al., 2000). If an inflection point was detected, we then created a piecewise log-binomial regression model with a knot placed at the identified inflection year. We then analyzed APC trends in medication use before and after the knot, among the full population, among those who reported regular drinking, and among those who reported infrequent drinking or abstention.
Finally, prescribed opioid and sedative-hypnotic use was modeled as a function of regular drinking status while adjusting for time and sociodemographic covariates described above using log-binomial regression models. We used this model to determine whether regular drinking status and other covariates were associated with medication use.
All analyses were conducted using Stata version 14.2 for Windows (StataCorp, 2015) taking into account the complex sample design of the NHANES (sampling weights, strata, and primary sampling units) for variance estimation using Taylor Series Linearization (Woodruff, 1971).
Results
Sample Characteristics
Table 1 describes the prevalence of regular drinking status (regular vs. infrequent/abstain) with and without sedative-hypnotic or opioid use in the full population and among sociodemographic subgroups. Over the entire study period, an estimated 2.9% of the population reported regular alcohol consumption and use of sedative-hypnotics or opioids for 30 days or longer. Two-tailed Rao–Scott chi-square tests indicated that over the entire study period, the prevalence of the four drinking/medication status groups was unequally distributed across strata for each demographic variable (all p<0.001; Table 1). Regular alcohol consumption combined with use of sedative-hypnotics or opioids for 30 days or longer was more common among men compared to women (3.3% vs. 2.5%) and among Whites compared to minorities (3.4% vs. < 2%). It was also more common for older age groups than younger and was most common in the 40-49 and 50-64 year age groups (3.3% and 3.7% respectively). Combination use was lowest among those without a high-school diploma (1.9%) but varied little across other educational attainment groups. Finally, there was a trend toward greater use of sedative-hypnotics or opioids in combination with regular drinking over time (FRao–Scott=1.89, p=0.02), starting with a prevalence of 1.8% in 1999-2000, increasing to 3.2% in 2013-14. The proportion of the sample that reported sedative-hypnotic or opioid use without regular drinking also increased from 5.7% in 1999-2000 to 9.6% in 2013-14, and the proportion who reported regular drinking without sedative-hypnotic or opioid use increased from 31.7% in 1999-2000 to 34.8% in 2011-12 but dropped to 32.0% in 2013-14. The overall prevalence of regular drinking in the population – with or without concurrent CNS-D medication use – increased from 33.5% (95% CI: 29.4, 37.8) in 1999-2000, to 35.2% (95% CI: 31.9, 38.7) in 2013-14 – representing a 0.9% (95% CI: 0.1, 1.8) relative annual increase (not presented in tables).
Table 1:
Prevalence of combinations of regular drinking status and sedative-hypnotic/opioid use status within each sociodemographic stratum (all years combined) and within each survey cycle yeara
| Group: Infrequent Drinking/Abstain | Group: Regular Drinking | ||||
|---|---|---|---|---|---|
| No sedative-hypnotic or opioid use, % (SE) | Either sedative-hypnotic or opioid use, % (SE) | No sedative-hypnotic or opioid use, % (SE) | Either sedative-hypnotic or opioid use, % (SE) | ||
| N | (N=23,612) | (N=2,999) | (N=10,212) | (N=886) | |
| Full Sample | 37,709 | 57.4 (0.6) | 7.8 (0.3) | 32.0 (0.7) | 2.9 (0.1) |
| Sexb | |||||
| Women | 19,317 | 64.7 (0.6) | 9.8 (0.3) | 23.1 (0.7) | 2.5 (0.1) |
| Men | 18,392 | 49.8 (0.7) | 5.6 (0.3) | 41.3 (0.8) | 3.3 (0.2) |
| Age (years)b | |||||
| 20-29 | 6,680 | 59.1 (1.1) | 3.7 (0.3) | 35.0 (1.1) | 2.1 (0.2) |
| 30-39 | 6,263 | 58.7 (0.9) | 5.7 (0.4) | 33.5 (0.9) | 2.1 (0.2) |
| 40-49 | 6,316 | 54.5 (1.0) | 7.7 (0.5) | 34.4 (1.0) | 3.3 (0.3) |
| 50-64 | 9,004 | 54.8 (0.9) | 9.6 (0.5) | 31.9 (0.9) | 3.7 (0.3) |
| 65+ | 9,446 | 61.2 (0.8) | 11.8 (0.5) | 24.1 (0.8) | 2.8 (0.2) |
| Race/Ethnicityb | |||||
| White | 17,997 | 53.0 (0.8) | 8.6 (0.4) | 35.0 (0.9) | 3.4 (0.2) |
| Black | 7,669 | 66.0 (0.8) | 6.7 (0.4) | 25.4 (0.6) | 1.9 (0.2) |
| Hispanic | 9,537 | 68.9 (0.7) | 5.0 (0.3) | 24.9 (0.6) | 1.3 (0.2) |
| Other | 2,506 | 69.1 (1.2) | 6.0 (0.7) | 23.0 (1.1) | 1.9 (0.4) |
| Educationb | |||||
| No HS Diploma | 10,548 | 65.3 (0.8) | 10.4 (0.6) | 22.4 (0.7) | 1.9 (0.2) |
| HS Only | 8,772 | 59.9 (0.8) | 9.3 (0.5) | 27.9 (0.9) | 2.9 (0.3) |
| HS+, Some College | 10,462 | 57.5 (0.7) | 8.1 (0.4) | 31.3 (0.8) | 3.1 (0.2) |
| College Degree | 7,884 | 49.8 (1.1) | 4.3 (0.3) | 42.8 (1.1) | 3.2 (0.3) |
| Survey yearc | |||||
| 1999-2000 | 4,151 | 60.8 (1.8) | 5.7 (0.5) | 31.7 (2.2) | 1.8 (0.2) |
| 2001-2002 | 4,613 | 60.6 (2.4) | 6.4 (0.4) | 30.7 (2.2) | 2.3 (0.3) |
| 2003-2004 | 4,299 | 59.0 (1.4) | 9.1 (1.1) | 29.5 (1.8) | 2.5 (0.3) |
| 2005-2006 | 4,342 | 57.6 (1.5) | 7.6 (0.7) | 31.7 (1.6) | 3.1 (0.3) |
| 2007-2008 | 5,211 | 58.1 (1.5) | 8.5 (1.1) | 30.4 (1.9) | 3.0 (0.2) |
| 2009-2010 | 5,324 | 54.5 (1.5) | 7.4 (0.6) | 34.6 (1.4) | 3.5 (0.3) |
| 2011-2012 | 4,677 | 54.1 (1.7) | 7.5 (0.6) | 34.8 (2.2) | 3.6 (0.5) |
| 2013-2014 | 5,092 | 55.2 (1.5) | 9.6 (0.8) | 32.0 (1.7) | 3.2 (0.3) |
Regular alcohol consumption defined as consuming alcohol at least 52 times in the past year. Infrequent Drinking/Abstaining defined as using alcohol less than 52 times in the past year. Opioid and sedative-hypnotic use defined as prescribed use of either medication for 30 days or longer.
Two-tailed Rao–Scott chi-square test, p<0.001
Two-tailed Rao–Scott chi-square test, p=0.01
Medication Frequencies and Prevalence.
Table 2 presents the ten most commonly reported prescribed CNS-D medications as well as the prevalence of use of each medication in the U.S. over the entire study period. Hydrocodone (an opioid) was the most commonly endorsed CNS-D prescription (24% of CNS-D prescription medication mentions) and was used by 3.2% of the population. The most commonly reported prescribed benzodiazepine was Alprazolam (9.6% of CNS-D prescription medication mentions) and was used by 1.4% of the population. The most commonly reported prescribed sleep medication was Zolpidem (7.9% of CNS-D prescription medication mentions) and was used by 1.3% of the population.
Table 2.
Top ten most commonly reported prescribed CNS-D medications and prevalence of use in the United States (years 1999-2014 combined)
| Medication Name | Dataset Frequency | Percent of CNS-D Medication Prescriptions | Prevalence of Medication in U.S. Population | Standard Error of Prevalence |
|---|---|---|---|---|
| Hydrocodone | 1639 | 24.1 | 3.2 | 0.16 |
| Alprazolam | 654 | 9.6 | 1.4 | 0.11 |
| Oxycodone | 576 | 8.5 | 1.2 | 0.10 |
| Tramadol | 543 | 8.0 | 1.0 | 0.07 |
| Zolpidem | 537 | 7.9 | 1.3 | 0.07 |
| Clonazepam | 400 | 5.9 | 0.8 | 0.05 |
| Lorazepam | 383 | 5.6 | 0.8 | 0.07 |
| Propoxyphene | 360 | 5.3 | 0.8 | 0.06 |
| Diazepam | 229 | 3.4 | 0.4 | 0.06 |
| Temazepam | 188 | 2.8 | 0.3 | 0.04 |
Modeled Prevalence and APC of Medication Use by Drinking Status
Estimates of the annual prevalence of sedative-hypnotic and opioid use are presented in Figure 1A, with estimates stratified by drinking status presented in Figure 1B. Unadjusted and sociodemographic-adjusted estimates of APC (relative to the prior year) of the prevalence of medication use—overall and by regular drinking status—are reported in Table 3, along with modeled prevalence estimates for the years 1999-2000 and 2013-14. Table 3 also shows the APC and modeled prevalence estimates for the two sub-classes of sedative-hypnotics. Overall, opioid use increased by an average of 2.5% (95% CI: 1.0, 4.0) per year. Modeled prevalence estimates increased from 5.4% in 1999-2000 to 7.6% in 2013-14. Among those who reported regular drinking, there was a non-significant increase in opioid use from 1999-2000 to 2013-14 (APC: 1.4, 95% CI: −1.2, 4.0). The estimated increase in opioid use among those who drank infrequently or abstained was significant (APC: 3.0, 95% CI: 1.5, 4.5). Increases in the prevalence of sedative-hypnotic use among both groups were larger and statistically significant. For example, the modeled prevalence of sedative-hypnotic use among those who reported regular drinking doubled from 2.9% in 1999-2000 to 6.0% in 2013-14. Both anxiolytics and sleep medications contributed to this increase, but increases in sleep medication use were particularly pronounced. Prevalence of sleep medication use was below 0.8% in 1999-2000, but by 2013-14, had risen to 2.8% among those who reported regular alcohol consumption and 2.3% among those who drank infrequently or abstained. Adjustment for sociodemographics generally had minimal impact on the APC estimates. Results of interaction tests for a time by regular drinking status interaction did not suggest statistically significant differences for any drug. One plausible interpretation of these results is that trends (i.e., slopes) in medication use among those who reported regular alcohol consumption were similar to trends in medication use among those who reported infrequent alcohol consumption or abstention. However, confidence interval results provided a range of diverging trend estimates, and thus the possibility of larger trend differences cannot be completely ruled out.
Figure 1A:
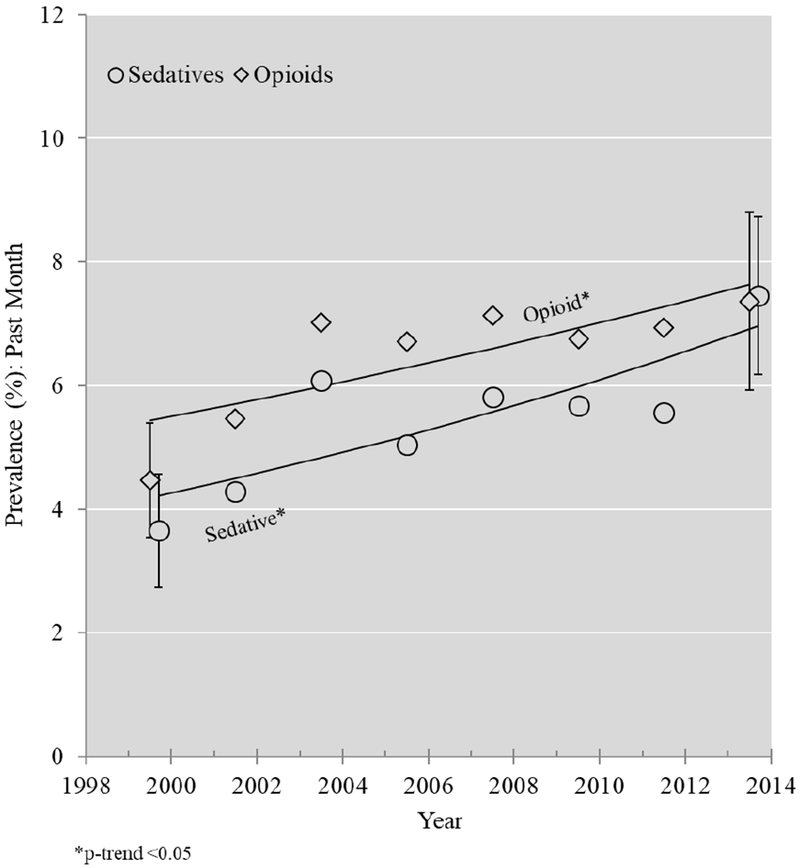
Prevalence estimates and log-binomial modeled annual percent change trends for opioid use and sedative-hypnotic use among the entire sample
Figure 1B:
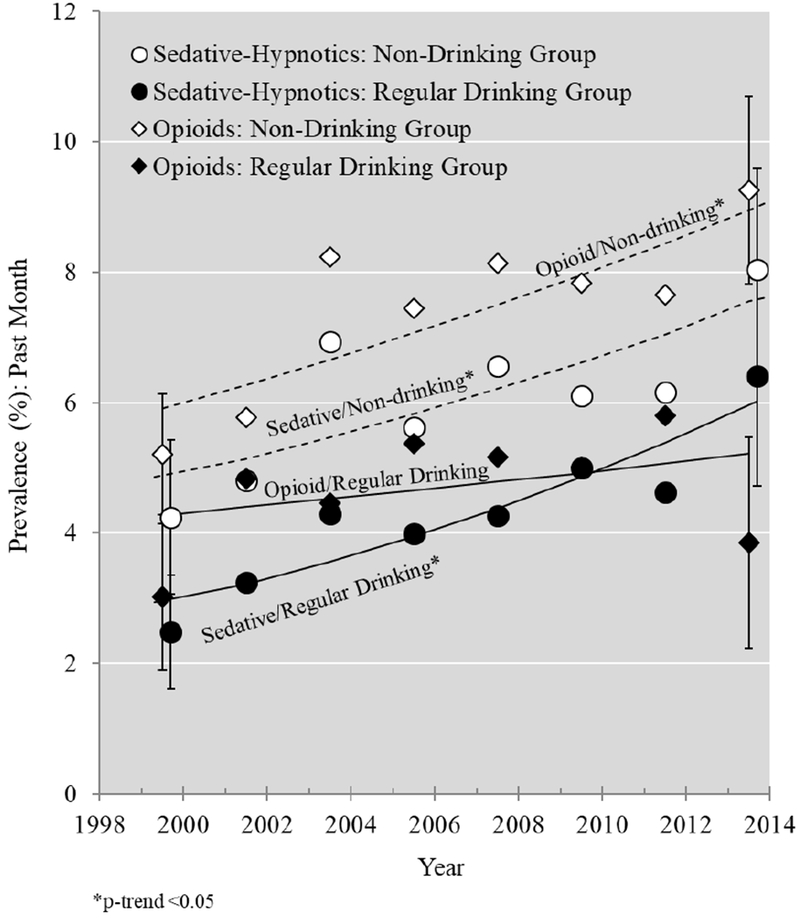
Prevalence estimates and log-binomial modeled annual percent change trends for opioid use and sedative-hypnotic use among respondents who drink regularly and respondents who drink infrequently/abstain
Table 3:
Annual percent change from 1999/2000 to 2013/2014 and modeled prevalence of use of opioids and sedative-hypnotics overall and by regular drinking status.
| Annual Percent Change (95% CI) |
Modeled Prevalence (95% CI) |
|||
|---|---|---|---|---|
| Unadjusted |
Adjusteda |
1999/2000 |
2013/2014 |
|
| Overall | ||||
| Opioids | 2.5 (1.0, 4.0) | 3.0 (1.6, 4.4) | 5.4 (4.8, 6.2) | 7.6 (6.7, 8.7) |
| Sedative-Hypnotic | 3.6 (2.0, 5.3) | 3.8 (2.1, 5.5) | 4.2 (3.6, 4.9) | 6.9 (6.2, 7.7) |
| Anxiolytics | 2.4 (0.5, 4.3) | 2.7 (0.8, 4.7) | 3.6 (3.1, 4.3) | 5.1 (4.4, 5.9) |
| Sleep Meds | 9.6 (7.3, 11.9) | 9.3 (6.9, 11.8) | 0.7 (0.5, 0.9) | 2.5 (2.2, 2.9) |
| Group: Regular Drinking | ||||
| Opioids | 1.4 (−1.2, 4.0)ns | 1.9 (−0.7, 4.5)ns | 4.3 (3.5, 5.2) | 5.2 (4.1, 6.5) |
| Sedative-Hypnotic | 5.3 (2.7, 7.9) | 4.6 (2.0, 7.4) | 2.9 (2.3, 3.6) | 6.0 (4.9, 7.2) |
| Anxiolytics | 3.7 (0.8, 6.7) | 3.4 (0.4, 6.4) | 2.3 (1.8, 3.0) | 3.9 (3.1, 4.9) |
| Sleep Meds | 11.2 (6.5, 16.0) | 9.9 (5.0, 14.9) | 0.6 (0.4, 1.0) | 2.8 (2.1, 3.6) |
| Group: Infrequent Drinking/Abstain | ||||
| Opioids | 3.0 (1.5, 4.5) | 3.5 (2.1, 4.9) | 6.0 (5.2, 6.9) | 9.1 (8.0, 10.3) |
| Sedative-Hypnotic | 3.2 (1.5, 4.9) | 3.6 (1.8, 5.3) | 4.8 (4.1, 5.6) | 7.5 (6.6, 8.5) |
| Anxiolytics | 2.2 (0.2, 4.3) | 2.6 (0.5, 4.8) | 4.3 (3.6, 5.1) | 5.8 (4.9, 6.8) |
| Sleep Meds | 8.7 (5.7, 11.9) | 8.8 (5.8, 12.0) | 0.7 (0.5, 1.0) | 2.3 (1.9, 2.8) |
Models adjusted for sex, race, age, and education.
Notes: (1) regular alcohol drinking defined as consuming alcohol at least 52 times in the past year; (2) infrequent drinking/abstain defined as using alcohol less than 52 times in the past year; (3) opioid and sedative-hypnotic use defined as prescribed use of either medication for 30 days or longer; “ns” indicates not statistically significant with alpha set at 0.05.
Adjusted Associations Between Drinking Status and Prescribed Medication Use.
Analyses of the associations between drinking and medication use adjusted for sociodemographic covariates and time are summarized in Table 4. After adjusting for sociodemographics and time, those who reported regular alcohol consumption were less likely to have used sedative-hypnotics (RR: 0.79, 95% CI: 0.70, 0.89) or opioids (RR: 0.69, 95% CI: 0.60, 0.78) than those who reported drinking infrequently or abstaining. However, when sedative-hypnotics were broken into the two sub-classes, there was no statistically significant difference in risk of sleep medication use (RR: 1.04, 95% CI: 0.80, 1.35), suggesting that those who reported regular drinking were potentially just as likely to use these medications as those who reported infrequent drinking or abstention. Importantly, the wide confidence interval indicates that potential estimates compatible with the observed data ranged from a 20% decrease in risk to a 35% increase in risk. Table 4 also sheds further light on the strength of the relationship between sociodemographic variables and use of each class of medication. For example, Whites were more likely to use both sedative-hypnotic and opioids than non-Whites, and education was protective for most classes of drugs except for sleep medications. Finally, the risk of use of each medication class was higher among those age 30+ compared to those age 20-29, but the risk for those age 40+ was markedly larger than the risk for those age 30-39.
Table 4:
Ratios of the prevalence of medication use across categories of drinking status, sociodemographic characteristics, and over time.
| Model 1: Opioid RR (95% CI) | Model 2: Sedative-Hypnoticsa RR (95% CI) | Model 3: Anxiolytics RR (95% CI) | Model 4: Sleep Medications RR (95% CI) | |
|---|---|---|---|---|
| Regular Drinking (vs. Infrequent drinking/abstinence) | 0.69 (0.60, 0.78) | 0.79 (0.70, 0.89) | 0.71 (0.61, 0.81) | 1.04 (0.80, 1.35) |
| Sex (vs. Male) | ||||
| Female | 0.86 (0.79, 0.94) | 0.67 (0.60, 0.74) | 0.62 (0.56, 0.70) | 0.79 (0.62, 0.99) |
| Race (vs. White) | ||||
| Black | 0.79 (0.69, 0.92) | 0.43 (0.37, 0.50) | 0.41 (0.34, 0.49) | 0.50 (0.38, 0.66) |
| Hispanic | 0.48 (0.40, 0.57) | 0.45 (0.36, 0.55) | 0.44 (0.35, 0.56) | 0.46 (0.33, 0.64) |
| Other | 0.72 (0.57, 0.91) | 0.63 (0.44, 0.90) | 0.56 (0.37, 0.84) | 0.72 (0.44, 1.19) |
| Age (vs. 20-29) | ||||
| 30-39 | 1.36 (1.11, 1.67) | 1.76 (1.33, 2.32) | 1.64 (1.23, 2.19) | 2.96 (1.49, 5.85) |
| 40-49 | 1.79 (1.48, 2.17) | 2.64 (2.04, 3.40) | 2.31 (1.78, 3.00) | 5.57 (3.11, 9.97) |
| 50-64 | 1.89 (1.51, 2.35) | 3.22 (2.48, 4.19) | 2.93 (2.23, 3.86) | 5.93 (3.35, 10.51) |
| 65+ | 1.62 (1.33, 1.98) | 3.32 (2.61, 4.22) | 3.01 (2.33, 3.89) | 5.33 (2.89, 9.81) |
| Educ. (vs. < HS) | ||||
| HS Diploma | 0.81 (0.72, 0.92) | 0.94 (0.81, 1.08) | 0.94 (0.80, 1.11) | 0.89 (0.61, 1.28) |
| >HS, no Degree | 0.76 (0.68, 0.86) | 0.91 (0.79, 1.05) | 0.87 (0.74, 1.03) | 1.15 (0.81, 1.63) |
| College Degree+ | 0.41 (0.34, 0.49) | 0.64 (0.54, 0.77) | 0.54 (0.44, 0.65) | 1.13 (0.82, 1.56) |
| Time (yr) | 1.03 (1.02, 1.05) | 1.04 (1.02, 1.06) | 1.03 (1.01, 1.05) | 1.09 (1.07, 1.12) |
The category of sedative-hypnotics is comprised of anxiolytics and sleep medications.
Notes: (1) relative risks (i.e. “RR”) calculated using log-binomial model; (2) all models contained all variables presented in the table; (3) regular drinking defined as consuming alcohol at least 52 times in the past year; (4) infrequent drinking/abstain defined as using alcohol less than 52 times in the past year; (5) opioid and sedative-hypnotic use defined as prescribed use of either medication for 30 days or longer.
Sensitivity Analyses
Supplemental analysis of additional drinking patterns revealed that among those who reported regular drinking, 46.0% (95% CI: 44.7, 47.4) had binge drank on five or more days in the past year whereas, among those who reported infrequent drinking, 10.8% (95% CI: 10.0, 11.6) had binge drank on five or more days in the past year (Supplemental Table 1). Additionally, on average, those who reported regular drinking had consumed 3.2 (95% CI: 3.1, 3.3) drinks per drinking day in the past year whereas those who reported infrequent drinking had consumed 2.4 (95% CI: 2.4, 2.5) drinks per drinking day (Supplemental Table 2).
In the second set of supplemental analyses, we used Joinpoint to detect year-specific inflection points. We identified non-linearity in opioid use prevalence trends (Supplemental Table 3 and Supplemental Figures 1-3). In the full population, there were large increases in opioid use from 1999-2000 to 2003-04 (APC: 11.3%, 95% CI: 4.9, 18.1) and no notable changes from 2003-04 to 2013-14 (APC: 0.5%, 95% CI: −2.0, 2.9). Joinpoint did not identify non-linearity in sedative-hypnotic trends.
In the last set of supplemental analyses, we checked for differences in medication use between those who abstained and those who drank infrequently. Results from log-binomial models presented in Supplemental Table 4 indicated that those who used alcohol infrequently were less likely than those who abstained to use opioids (RR: 0.77, 95% CI: 0.68, 0.87) and anxiolytics (RR: 0.66, 95% CI: 0.56, 0.78). Second, similar to the primary results presented in Table 3, results from log-binomial models presented in Supplemental Table 5 indicated APC increases in opioid and sleep medication use among those who abstain and those who drank infrequently – suggesting that both groups contributed to the observed increases in medication use. One notable exception was that increases in anxiolytic use were driven by those who drink infrequently (APC: 5.9, 95% CI: 2.2, 9.6) rather than those who abstain (APC: −0.7, 95% CI: −2.60, 1.30).
Discussion
Across a series of nationally representative samples of individuals reporting regular alcohol consumption, the prevalence of prescribed sedative-hypnotic use doubled to 6%, and the prevalence of prescribed opioid use remained high at approximately 4% between 1999 and 2014. Notably, increases in subtypes of sedative-hypnotics were uneven: the increase in use of sleep medication “Z-drugs” such as zolpidem was approximately three times as high as the increase in use of anxiolytics such as benzodiazepines. Furthermore, while those who drank regularly were significantly less likely than those who drank infrequently or abstained to be using anxiolytics, they were just as likely to use sleep medications. Opioid prescription trends were more complex. Primary analyses indicated strong increases in prescription opioid use among the entire population largely driven by increases among those reporting infrequent drinking or abstinence. However, prescription opioid use trends were non-linear. Opioid use increased between 1999 and 2004, after which point, the prevalence of opioid use remained relatively stable. Overall, the results from these analyses indicate that a substantial portion of the population is at risk of adverse consequences due to alcohol-medication interactions and, in the case of sedative-hypnotics, that risk may be increasing.
The rapid increase in the prevalence of sleep medication use warrants comment, particularly given that those who report regular alcohol consumption are just as likely to use these medications as those who drink infrequently or abstain. We found that the overall prevalence of sleep medication use among all U.S. adults increased sharply by nearly 10% annually (albeit the initial prevalence was low). Other studies that did not examine alcohol use have documented similarly large increases (Moloney et al., 2011; Ford et al., 2014). One potential explanation for this trend is that physicians perceive these medications to be lower risk than benzodiazepines and may be more inclined to prescribe them (Siriwardena et al., 2006; Hoffmann, 2013). However, it is not clear that sleep medications are any less risky, given that they are pharmacologically related to benzodiazepines and still place individuals at high risk for several types of injuries and adverse effects (Chung et al., 2013; Bush, 2013).
Individuals age 40 and older were two to three times as likely to use sedative-hypnotics, and about 1.5 to 2 times as likely to use opioids as those age 20-29. These results are consistent with other studies of prescription medication prevalence that did not examine drinking status (Kantor et al., 2015; Bertisch et al., 2014; Campbell et al., 2010; Olfson et al., 2015). These findings are concerning given the marked increase in binge drinking among this population over the past 15 years, (Grucza et al., 2018) and that the physiological changes that occur with age make individuals more susceptible to alcohol-related adverse drug reactions (Moore et al., 2007; Day, 2013). The potential interplay between changing drinking patterns and increasingly common use of these medications in the context of an aging population could be contributing substantially to the rising alcohol- and drug-related emergency room visits and deaths in the U.S (Case and Deaton, 2015; Castle et al., 2016; White et al., 2018), and highlights the need for continued surveillance of adverse events and alcohol-drug interactions, especially among middle-aged and older adults (Breslow et al., 2015).
This study has several limitations. First, our analyses do not account for the quantity of alcohol consumed or for detailed drinking patterns. Many of those who reported regular drinking may drink moderately on drinking days. Notably, however, supplemental analyses indicated that 46% of those who report regular alcohol consumption reported binge drinking on at least five days in the prior year and drank an average of 3.2 drinks on each drinking day. In addition, we limited our analyses to overall classes of medications – changes in drug scheduling and monitoring laws may have led to differences over time in the frequency with which specific types of drugs within a class are prescribed. Furthermore, the present study does not account for medication dose, frequency of medication use, medication adherence, or pharmacokinetic and pharmacodynamic properties of specific medications – all factors that may substantially alter the risk of adverse consequences associated with concurrent alcohol use. For example, individuals may not have taken medications on drinking days or may not have used alcohol and medications in a manner that put them at risk for injury (i.e., concurrent use does not necessarily constitute co-administration). An additional limitation is that this study’s operational definition of concurrent use relies on the assumption that alcohol use patterns remain stable and equally distributed over the entire year. It is possible that several months before their NHANES interview, participants ceased alcohol use and began using a medication. To the extent that this pattern of behavior occurred, the results presented in this analysis would over-estimate the prevalence of concurrent use.
Limitations notwithstanding, it is clear that the number of individuals at risk for adverse alcohol-drug interactions has increased markedly. In addition, should prescription sedative-hypnotic use continue to increase in the general U.S. population, it is reasonable to expect a proportional increase in sedative-hypnotic use among those who drink regularly, in the absence of intervention. Although prescription opioid use seems to be stable, it remains alarmingly common among those who drink regularly. Additionally, certain subpopulations, such as those age 40 and older, continue to be exposed to an unnecessarily high risk of alcohol-related adverse drug reactions and related deleterious outcomes. Taken together, these findings support the notion that alcohol and prescription drug co-use could be playing a significant role in current alcohol-related morbidity and mortality in the United States.
Supplementary Material
Acknowledgments:
We gratefully acknowledge the Centers for Disease Control for making data publicly available.
Support: National Institute on Alcohol Abuse and Alcoholism R21AA025689; National Institute on Drug Abuse R21DA044744. The funding agency and data providers had no role in the design, conduct, collection, management, analysis, or interpretation of data, or the preparation, review, or approval of this paper.
Footnotes
Interests: JTB is a member of the board of directors and treasurer of MySafeRx Inc., a non-profit scientific research organization. He receives no financial compensation from this organization.
LJB is listed as an inventor on Issued U.S. Patent 8,080,371,“Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. Other authors have no interests to declare.
References
- Bachhuber MA, Hennessy S, Cunningham CO & Starrels JL (2016) Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States, 1996-2013. Am J Public Health, 106(4), pp 686–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertisch SM, Herzig SJ, Winkelman JW & Buettner C (2014) National use of prescription medications for insomnia: NHANES 1999-2010. Sleep, 37(2), pp 343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Dong C & White A (2015) Prevalence of alcohol-interactive prescription medication use among current drinkers: United States, 1999 to 2010. Alcohol Clin Exp Res, 39(2), pp 371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DM 2013. Emergency Department Visits Attributed to Overmedication That Involved the Insomnia Medication Zolpidem The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US). [PubMed] [Google Scholar]
- Campbell CI, Weisner C, Leresche L, Ray GT, Saunders K, Sullivan MD, Banta-Green CJ, Merrill JO, Silverberg MJ, Boudreau D, Satre DD & Von Korff M (2010) Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health, 100(12), pp 2541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A & Deaton A (2015) Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A, 112(49), pp 15078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle IJ, Dong C, Haughwout SP & White AM (2016) Emergency Department Visits for Adverse Drug Reactions Involving Alcohol: United States, 2005 to 2011. Alcohol Clin Exp Res, 40(9), pp 1913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control (2018) National Health and Nutrition Examination Survey: Impact of Declining Response Rates on Nonresponse Bias, 2013-2016, Executive Summary. Centers for Disease Control, Atlanta, GA. [Google Scholar]
- Chong Y, Fryer CD & Gu Q (2013) Prescription sleep aid use among adults: United States, 2005-2010. NCHS Data Brief, 127), pp 1–8. [PubMed] [Google Scholar]
- Chung SD, Lin CC, Wang LH, Lin HC & Kang JH (2013) Zolpidem Use and the Risk of Injury: A Population-Based Follow-Up Study. Plos One, 8(6), pp e67459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M, Newell JG, Derry JM, Martin IL & Dunn SM (2000) Characterization of the interaction of zopiclone with gamma-aminobutyric acid type A receptors. Mol Pharmacol, 58(4), pp 756–62. [DOI] [PubMed] [Google Scholar]
- Day C 2013. Benzodiazepines in Combination with Opioid Pain Relievers or Alcohol: Greater Risk of More Serious ED Visit Outcomes The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US). [PubMed] [Google Scholar]
- Ford ES, Wheaton AG, Cunningham TJ, Giles WH, Chapman DP & Croft JB (2014) Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care survey 1999-2010. Sleep, 37(8), pp 1283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Sher KJ, Kerr WC, Krauss MJ, Lui CK, McDowell YE, Hartz S, Virdi G & Bierut LJ (2018) Trends in Adult Alcohol Use and Binge Drinking in the Early 21st-Century United States: A Meta-Analysis of 6 National Survey Series. Alcohol Clin Exp Res, 42(10), pp 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudin JA, Mogali S, Jones JD & Comer SD (2013) Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med, 125(4), pp 115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP Jr., Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB & Dowell D (2017) Vital Signs: Changes in Opioid Prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep, 66(26), pp 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RN, Boudreau DM, Ebel BE, Grossman DC & Sullivan SD (2015) Sedative Hypnotic Medication Use and the Risk of Motor Vehicle Crash. Am J Public Health, 105(8), pp e64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LM, von Moltke LL & Greenblatt DJ (2003) Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs, 17(7), pp 513–32. [DOI] [PubMed] [Google Scholar]
- Hoffmann F (2013) Perceptions of German GPs on benefits and risks of benzodiazepines and Z-drugs. Swiss Med Wkly, 143(w13745. [DOI] [PubMed] [Google Scholar]
- Jones CM & McAninch JK (2015) Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am J Prev Med, 49(4), pp 493–501. [DOI] [PubMed] [Google Scholar]
- Kantor ED, Rehm CD, Haas JS, Chan AT & Giovannucci EL (2015) Trends in Prescription Drug Use Among Adults in the United States From 1999-2012. JAMA, 314(17), pp 1818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann CN, Spira AP, Alexander GC, Rutkow L & Mojtabai R (2016) Trends in prescribing of sedative-hypnotic medications in the USA: 1993-2010. Pharmacoepidemiol Drug Saf, 25(6), pp 637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ & Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med, 19(3), pp 335–51. [DOI] [PubMed] [Google Scholar]
- Langtry HD & Benfield P (1990) Zolpidem. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs, 40(2), pp 291–313. [DOI] [PubMed] [Google Scholar]
- Mojtabai R (2018) National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf, 27(5), pp 526–534. [DOI] [PubMed] [Google Scholar]
- Moloney ME, Konrad TR & Zimmer CR (2011) The medicalization of sleeplessness: a public health concern. Am J Public Health, 101(8), pp 1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DE 2012. Drug Interactions with Benzodiazepines: Epidemiologic Correlates with Other CNS Depressants and In Vitro Correlates with Inhibitors and Inducers of Cytochrome P450 3A4 In: Mozayani A & Raymon L (eds.) Handbook of Drug Interactions. [Google Scholar]
- Moore AA, Whiteman EJ & Ward KT (2007) Risks of combined alcohol/medication use in older adults. Am J Geriatr Pharmacother, 5(1), pp 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2018) Joinpoint Trend Analysis Software.
- Olfson M, King M & Schoenbaum M (2015) Benzodiazepine use in the United States. JAMA Psychiatry, 72(2), pp 136–42. [DOI] [PubMed] [Google Scholar]
- Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L & Mitchell AA (2008) Prevalence and characteristics of opioid use in the US adult population. Pain, 138(3), pp 507–13. [DOI] [PubMed] [Google Scholar]
- Siriwardena AN, Qureshi Z, Gibson S, Collier S & Latham M (2006) GPs’ attitudes to benzodiazepine and ‘Z-drug’ prescribing: a barrier to implementation of evidence and guidance on hypnotics. Br J Gen Pract, 56(533), pp 964–7. [PMC free article] [PubMed] [Google Scholar]
- StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- Weathermon R & Crabb DW (1999) Alcohol and medication interactions. Alcohol Res Health, 23(1), pp 40–54. [PMC free article] [PubMed] [Google Scholar]
- White AM, Slater ME, Ng G, Hingson R & Breslow R (2018) Trends in Alcohol-Related Emergency Department Visits in the United States: Results from the Nationwide Emergency Department Sample, 2006 to 2014. Alcohol Clin Exp Res, 42(2), pp 352–359. [DOI] [PubMed] [Google Scholar]
- White JM & Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction, 94(7), pp 961–72. [PubMed] [Google Scholar]
- Woodruff RS (1971) A Simple Method for Approximating the Variance of a Complicated Estimate. Journal of the American Statistical Association, 66(334), pp 411–414. [Google Scholar]
- Zipf G, Chiappa M, Porter K, Ostchega Y, Lewis BG, Dostal J (2013) National Health and Nutrition Examination Survey: Plan and Operations, 1999-2010. Vital Health Stat 1, 1–37. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


