Abstract
Stroke has long been regarded as focal disease with circumscribed damage leading to neurological deficits. However, advances in methods for assessing the human brain and in statistics have enabled new tools for the examination of the consequences of stroke on brain structure and function. Thereby, it has become clear that stroke has impact on the entire brain and its network properties and can therefore be considered as a network disease. The present review first gives an overview of current methodological opportunities and pitfalls for assessing stroke-induced changes and reorganization in the human brain. We then summarize principles of plasticity after stroke that have emerged from the assessment of networks. Thereby, it is shown that neurological deficits do not only arise from focal tissue damage but also from local and remote changes in white-matter tracts and in neural interactions among wide-spread networks. Similarly, plasticity and clinical improvements are associated with specific compensatory structural and functional patterns of neural network interactions. Innovative treatment approaches have started to target such network patterns to enhance recovery. Network assessments to predict treatment response and to individualize rehabilitation is a promising way to enhance specific treatment effects and overall outcome after stroke.
Keywords: stroke, plasticity, network, rehabilitation
1. Introduction
Stroke is the leading cause of adult disability in western countries. The resulting neurological deficits have enormous impact on activities of daily living, quality of life, and health costs (Dobkin, 1995, Mayo et al., 2002, Feigin et al., 2016). Rehabilitation requires an in depth understanding of mechanisms underlying neurological deficits and their recovery in order to propose appropriate treatments and to design novel interventional approaches.
Our concepts of the mechanisms underlying stroke deficits have long been influenced by a localizationist view, which has been predominant since Broca's discovery of specific deficits resulting from a focal brain lesion (Broca, 1861). Later, it was reinforced by neuroimaging with assessments of lesion configurations (e.g., Glascher et al., 2009) or functionally specific activations of specialized brain areas in healthy subjects (e.g., Belliveau et al., 1991, Rao et al., 1993). In this view, neurological deficits result from the destruction of circumscribed and functionally specialized brain regions. Similarly, recovery from neurological deficits can be seen in a localizationist tradition as the reorganization of circumscribed preserved brain areas close to the lesion. Pioneering non- human primate studies into brain plasticity after stroke have shown that recovery from neurological deficits can arise from reorganization of preserved perilesional areas to the functions previously assumed by the damaged tissue (Nudo et al., 1996). Functional imaging in humans has shown dynamic changes of task-related activations in nearby perilesional areas after stroke (Feydy et al., 2002, Ward et al., 2003, Saur et al., 2006). In consequence, rehabilitation treatments are designed to enable repetitive and intensive activation of reorganized perilesional areas (Dong et al., 2006, Kleim et al., 2008, Dancause et al., 2011).
However, the brain is a network with extremely dense interconnections. It can therefore be expected that a stroke will not only induce local damage resulting in necrosis of brain tissue, but will also impact the brain network resulting in malfunction in connected areas that are remote from the stroke lesion (Carrera et al., 2014). This argument has already been put forward by opponents of Broca, and later by von Monakow with the influential concept of diaschisis suggesting a loss of excitability at distant brain areas (von Monakow, 1914). In rodent stroke models small focal lesion to the motor cortex caused widespread diaschisis within and across both hemispheres (Buchkremer-Ratzmann et al., 1996, 1997). Yet, no appropriate imaging and statistical methodology was at first available to investigate these predictions in human stroke patients and the clinical importance of the concept has long remained controversial.
More recent methods for brain imaging and statistical analysis have opened new opportunities for looking into network aspects of brain function in general and of brain plasticity in particular. Simulations of neural networks have suggested that stroke lesions induce massive network-wide changes in neural activity (Honey et al., 2008, Alstott et al., 2009). Advances in non-invasive imaging allow us to examine anatomical fibre tracts connecting the brain areas, i.e., structural connectivity, with good precision in human stroke patients. Furthermore, we can examine the impact of stroke lesions on functional collaborations among distant areas using statistical measure of so-called functional connectivity. Finally, we can manipulate neural connections between distant brain areas using non-invasive stimulation of the brain across the skull. Together, these developments have demonstrated that stroke-related neurological deficits as well as recovery depend on network-wide processes.
The present review will first summarize current methodological possibilities and difficulties in the assessment of the brain as a network. We will then line out influential new concepts on stroke plasticity arising from the assessment of network processes. Finally, we will review that a network perspective on stroke has consequences for clinical practice. Most importantly, network-wide changes can become target of new treatment approaches. Prognosis and treatment can be adapted to individual needs of each patient hence contributing to personalized medicine.
As plasticity of motor function has received much more attention and study than cognitive domains, most concepts will be derived first for the motor domain. Evidence for an extensions of these concepts to language, neglect and other cognitive functions will be considered in a second step.
2. Non-invasive assessments of brain networks
2.1. Structural connectivity and diffusion weighted imaging
In the last years, there has been a rise in interest and usage of diffusion weighted imaging (DWI) to study brain networks in basic and clinical neuroscience. DWI provides the unique opportunity of analysing in vivo white matter bundles connecting specific brain areas within highly specialized functional neuronal networks. Determining the structural integrity and organization of these connections adds to the understanding of deficits after a focal brain lesion, like in traditional localizationism and dysconnection syndromes (Catani et al., 2005, Thiebaut De Schotten et al., 2015), but also to the understanding of the mechanisms, course and potential of reorganization and recovery processes. These aspects make the technique a highly valuable information source to study rehabilitation in stroke (Koch et al., 2017). Since the first studies using diffusion tensor imaging (DTI) (Basser et al., 1994) there has been a large effort developing novel acquisition techniques giving rise to more detailed analyses of underlying neuronal structures like diffusion curtosis imaging (Jensen et al., 2005a), HARDI (Tuch et al., 2002), q-space imaging (King et al., 1994, Callaghan, 1996) or Diffusion Spectrum Imaging (Wedeen et al., 2005). These technical improvements enable new insights in clinical neuroscience.
In DWI the MR signal is sensitive to the dispersion of water molecules on a voxel-by-voxel basis, iteratively done in multiple directions estimating the distribution of diffusivity in space, as well as different amounts of diffusion weighting, which allows more detailed analyses of microstructure. The different aspects of acquisition give rise to the two main approaches in DWI: microstructural imaging and tractography.
2.1.1. Microstructural imaging
In microstructural imaging, recent effort has focused on developing higher and more complex models describing integrity of neuronal tissue (for a detailed review please see (Alexander et al., 2017, Assaf et al., 2017). The tensor model introduced in 1994 (Basser et al., 1994) has been and still is most frequently used in clinical neuroscience. Microstructural integrity measurements based on the eigenvalues of the tensor (axial and radial diffusion), composite scores like mean diffusivity (MD), and fractional anisotropy (FA) are sensitive to different degrees of density, orientation and coherence of both axonal and myelin features of white matter tracts (for review (Basser et al., 2002, Beaulieu, 2002). Still, the tensor model seems to be insufficient (Jeurissen et al., 2013) in case of multiple fibre populations or complex fibre architecture like crossing, kissing and fanning fibres (Leergaard et al., 2010), as commonly found in brain regions, like e.g., in the centrum semiovale or corona radiata, in which association, projection, and commissural fibres are co-localized. Additionally, the interpretation of the meaning of alterations in diffusion parameters (e.g., reduced fractional anisotropy) for the tissue structure is not straightforward (Assaf et al., 2017, O'Donnell et al., 2017). A cautious interpretation of change of structural properties after stroke is therefore necessary. This motivated novel developments based on i) higher angular estimation of fibre orientation by constrained spherical deconvolution (Tournier et al., 2004) optimized tractography (see below) and ii) a more complex modelling of microstructure. Latter includes the usage of multi-tensor models (Malcolm et al., 2010, Chu et al., 2015) or diffusion kurtosis imaging (Jensen et al., 2005a), a model free fitting measuring deviation from a Gaussian distributed signal, which has been used for clinical prediction studies evaluating e.g., the corticospinal tract in stroke (Hui et al., 2012, Spampinato et al., 2017). A very promising approach, especially for the usage in clinical research, is the compartment model framework. In this approach, the description of microstructure is not done on a voxel-by-voxel basis, but based on the estimation of different compartments within each voxel. Intra-axonal diffusion is modelled as an impermeable cylinder or fibre shape with restricted diffusion, while a less restricted diffusion model is used for extra-axonal diffusion. Models based on the compartment framework like CHARMED (Assaf et al., 2004, 2005) and NODDI (Zhang et al., 2012) gave rise to novel indices of microstructural integrity like axonal and neurite density (Alexander et al., 2010) and the orientation dispersion index, which found interest in clinical studies on stroke (Adluru et al., 2014) and other neurological pathologies (Winston, 2012, Caverzasi et al., 2016). Compartment modelling of white matter specific diffusion metrics in 44 acute and subacute stroke patients reveals a small increase in fibre density, with a strong decrease in intra-axonal diffusivity indicating axonal swelling in the lesion (Hui et al., 2012). Still, more work is needed to investigate biological fundaments of diffusion signal alterations during the time course after stroke. Future perspectives in microstructure modelling include the estimation of axon diameter distributions (AxCaliber and ActiveAx) (Assaf et al., 2008, Zhang et al., 2011, Benjamini et al.,2016).
2.1.2. Tractography
The diffusion signal is further used to estimate the orientation distribution of fibres in every voxel. By following these indirect measurements of fibre orientation voxel-by-voxel using deterministic or probabilistic tractography algorithms, it is possible to reconstruct long-range white matter pathways in the brain (for detailed methodological review please see (Jeurissen et al., 2017, O'Donnell et al., 2017), which has become an essential part in studying structural brain connectivity. The estimation of fibre orientation can be based on different models of diffusion reaching from tensor based approaches to constrained spherical deconvolution (Tournier et al., 2004, Dell'Acqua et al., 2013). The latter allows higher angular resolution and thus increased accuracy of tractography. Complex fibre architecture can lead to false negative, or - far more relevant - false positive results (invalid bundles) as recently shown by an international tractography challenge (Maier-Hein et al., 2017). This implies the need for validation of different tractography algorithms based on ground truth datasets. Further models of elevating the accuracy of fibre tractography have been recently introduced to e.g., include microstructural information in the processing and reconstruction of fibres – microstructural informed tractography (Daducci et al., 2015, 2016, Girard et al., 2017).
Analysing major white matter bundles found in the human brain have been a key element in clinical neuroscience (Thiebaut De Schotten et al., 2015, Maier-Hein et al., 2017). For example, the corticospinal tract (CST), the arcuate fascicle (AF) or the superior longitudinal fascicle (SLF) have been main targets in studying recovery of motor impairment (for detailed review: Koch et al., 2016, Puig et al., 2017, Ramsey et al., 2017), aphasia (Marchina et al., 2011, Forkel et al., 2014) and neglect (Lunven et al., 2015) after stroke, respectively. Still the SLF for example contains three major subdomains, which include fibre tracts connecting specific areas within a parietofrontal distribution (Makris et al., 2005, Schulz et al., 2015b, Thiebaut De Schotten et al.,2015). Similar, corticospinal pathways include fibres belonging to the pyramidal tract as well as alternate motor fibres and cortico-cerebellar pathways, which can be disentangled and have been analysed separately in stroke recovery (Lindenberg et al., 2010, Lindenberg et al., 2012, Schulz et al., 2015a, Schulz et al., 2017b). Thus, defining precise fibre bundles connecting specific areas of interest by means of tractography is a promising approach deepening the understanding of white matter connectivity in translational research. Furthermore, those specific connections can be modelled in network configurations analysing dependencies of connectivity between certain white matter tracts (Granziera et al., 2012, Schulz et al., 2015b, Schulz et al., 2017a).
Finally, tractography is used in a whole brain approach, reconstructing the entire human structural connectome. Analysing the high dimensional dataset of hubs and connections requires different mathematical approaches to draw conclusions. Hereby, graph theory is used to describe network alterations and configurations by means of, e.g., modular small-worldness or economic features like cost and efficiency and rich club nodes (for further information please see section 2.2.4 and more detailed articles on this topic (Sporns et al., 2005, Hagmann et al., 2008, Bullmore etal., 2009, 2012).
DWI analyses provide the unique opportunity to study adjacent, but also widely distributed alterations of neuronal structures and networks, reorganization and microstructural changes in reaction to focal pathologies, which makes it a key technique for translational research and for studying network alterations in recovery after stroke. Still every result should be interpreted with caution and in regards of the limitation of the technique.
2.2. Assessing functional and effective connectivity
The analysis of functional collaboration between brain areas can in principle be based on any imaging technique capable of measuring brain activity. The assessment of hemodynamic fluctuations with fMRI, and of electromagnetic neural activity with EEG and magnetoencephalography (MEG) have been most influential for the study of network functions in humans.
Traditional neuroimaging statistics have mostly treated each brain area as independent from the rest and quantified local activations. Conversely, network approaches quantify the statistical dependency between two or more recording sites in order to estimate the strength of interregional neural interactions (Varela et al., 2001). Interregional neural communication is thought to be accompanied by a synchronization, or statistical dependency, of oscillations between different brain regions (Fries, 2005). If two or more regions show “similar” or interdependent activity they are considered to be interacting and communicating. In the following, we will summarize key concepts and problems that are relevant for the assessment of brain plasticity after stroke.
2.2.1. Advantages of network imaging
Network approaches to functional imaging provide some practical advantages beyond the possibility of taking into account the network character of the brain.
In traditional clinical neuroimaging, patients have to perform specific tasks, which are designed to activate selected brain regions of interest. Yet, the ability of stroke patients to correctly perform tasks is often limited. This is particularly evident in studies that address questions related to neurological deficits and their recovery. For instance, studies assessing the reorganization of the motor cortex in patients with hemiplegia require the patients to perform repetitive movements, which is precisely the task they cannot accomplish in a sufficiently controlled manner due to their deficit (Weiller et al., 1992, 1993). Conversely, neural communication can be studied not only during tasks, but also during a so-called resting-state without explicit task. Studies using functional magnetic resonance imaging (fMRI) have shown that spontaneous fluctuations of brain activity at rest are highly organized and coherent within specific neuro-anatomical systems (Greicius et al., 2003, Fox et al., 2005, Damoiseaux et al., 2006). Furthermore, the pattern of coherence between brain regions observed at rest often resembles the pattern of brain activation induced by corresponding tasks (Vincent et al., 2007). Thus, a careful analysis of coherence between brain regions gives access to the functional brain organization even for resting-state recordings.
This approach also offers the possibility to study multiple brain networks concomitantly and hence provides a systems perspective on brain function. While classical imaging required a separate task for each network/function to be studied, network imaging allows studying, e.g., motor and language networks in parallel, as well as their interactions.
2.2.2. Types of neural interactions and their assessment
Many different methods for assessing neural interactions have been proposed. In a first approximation, they can be grouped into methods for quantification of statistical dependency (functional connectivity, FC), and methods that explain observed dependencies within a model of causal influence (effective connectivity, EC) (Friston, 2011).
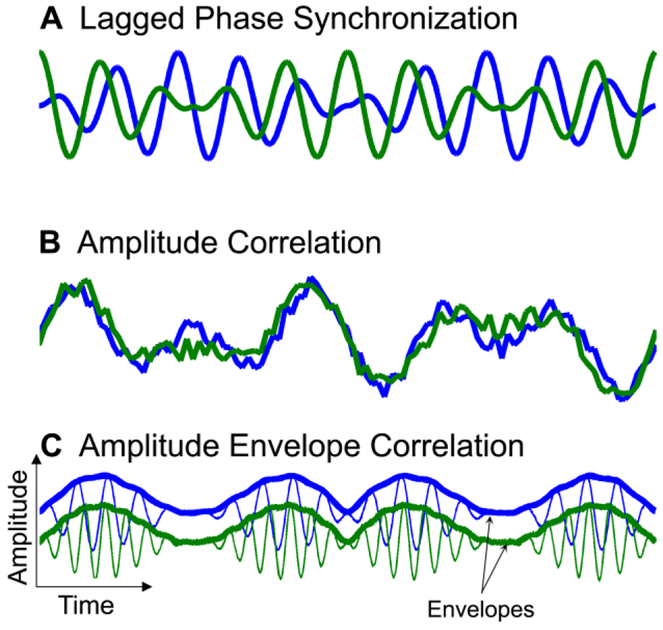
Neural interactions and hence functional connectivity can occur in different forms. Figure 1 shows three coupling types which have been observed in the human brain (Guggisberg et al., 2015). Weak coupling can be associated with synchronization of oscillation phases between nodes while the amplitudes of the network nodes remain uncorrelated (Fig. 1A) (Rosenblum et al., 1996, Osipov et al., 2003). For instance, the human alpha rhythm shows such phase synchronization (Guggisberg et al., 2008, Hillebrand et al., 2012, Marzetti et al., 2013). Increasing the coupling strength between the oscillators leads to complete synchronization with the appearance of amplitude correlation (Fig. 1B) (Rosenblum et al., 1996, Osipov et al., 2003). This can be observed in slow resting-state fluctuations (<0.1 Hz) of the hemodynamic fMRI signal (Greicius et al., 2003), and in delta and infra-delta (<4 Hz) oscillations of local field potentials recorded with electrocorticography (He et al., 2008). Finally, network nodes can synchronize the appearance of bursts of faster rhythms, which leads to a correlation of their amplitude envelopes (Fig. 1C) (Bruns et al., 2000, Gonzalez-Miranda, 2002). Amplitude envelope correlation (AEC) can be observed in human resting-state alpha and beta-band activity (de Pasquale et al., 2010, Brookes et al., 2011a, Brookes et al., 2011b, de Pasquale et al., 2012, Hipp et al., 2012). The relative role of the different coupling types is incompletely understood, but it is likely that different coupling types account for different aspects of network interactions and may provide complementary insights on human brain function and disease (Engel et al., 2013, Guggisberg et al., 2015).
Figure 1. Schematic representation of different synchronization types.
Modified after (Guggisberg et al., 2015), with permission.
In fMRI studies, FC is most frequently quantified with the canonical correlation coefficient (CC) (e.g., Biswal et al., 1995, Greicius et al., 2003, Fox et al., 2005). It quantifies the similarity of amplitudes, i.e., complete synchronization, of hemodynamic fluctuations between pairs of brain regions or voxels. Alternatively, independent component analysis can achieve a full-brain analysis of amplitude similarities which are divided into different spatial components (Damoiseaux et al., 2006). These spatial components happen to correspond to different functional networks, also named resting-state networks (Raichle et al., 2007, Raichle, 2011).
In the case of EEG and MEG, the CC is not well suited for the fast and rich spectral content of typical neural oscillations. This is because the CC is modified by the superposition of several different frequencies, even though the coupling at a given frequency may remain constant. More importantly, time lags between different brain areas, which often occur due to neural transmission, additionally bias the magnitude of the CC despite constant coupling strength. Instead, EEG/MEG studies most frequently quantify FC with measures of phase coupling using indices, such as coherence (Lopes da Silva et al., 1973) or phase locking relationships (Lachaux et al., 1999, Stam et al., 2007). Alternatively, AEC (Brookes et al., 2011b) provides topographies of networks that are more similar to findings in fMRI than it is the case for measures of phase coupling (Brookes et al., 2011a). In addition, there are many other indices (for a comparison, see, e.g., Dauwels et al., 2010).
EC tests hypotheses on the information flow between limited numbers of brain regions. It embeds experimental measures of FC within different models of interactions which can be compared in terms of their statistical evidence (Friston, 2011).
A first approach of EC that is frequently used in EEG/MEG studies is based on autoregressive modelling of time series within Granger’s concept of causality (Kaminski et al., 2001, Astolfi et al., 2005). It consists in modelling signal fluctuations at a given brain area from a mixture of past fluctuations at one or several other areas. Granger causality measures are abundantly used for MEG and EEG recordings. Conversely, its application to fMRI time series is problematic for several reasons, in particular also due to the limited time resolution of fMRI (Friston, 2011).
A second approach, dynamic causal modelling (DCM), is based on a pre-specified model of neuronal sources and their directed influence in time. It then explicitly tests which of several models best represents the observed signal. This allows formalizing scientific hypothesis testing in the context of neural interactions. DCM can be applied to EEG/MEG as well as fMRI data, as the generative model of neural sources can be adapted to each imaging modality (Friston et al., 2003, Friston, 2011).
EC measures have the advantage of being multivariate, i.e., of taking into account indirect neural interactions occurring via an observed third brain area. Furthermore, they enable assessing forward and backward connections between two areas separately. This provides insights on the strength of information flow for each direction. On the other hand, they require some prior knowledge about involved brain areas and likely neural interactions.
2.2.3. Difficulties and potential pitfalls
An important issue in network imaging based on fMRI is the presence of motion and physiological artefacts during the scan, which give appearance to spurious statistical dependencies between brain sites. This is all the more important in the assessment of stroke patients who typically are less able to remain immobile. Several algorithms for correction have been proposed, which are based on temporal band-pass filtering of BOLD signals, removal of movement regressors, independent component analyses, or recordings of physiological signals (Power et al., 2012, Satterthwaite et al., 2013, Power et al., 2014). There is an ongoing debate whether the global signal, i.e., the average time course of the whole brain, the ventricles, or the white matter, should be removed (Saad et al., 2012, Burgess et al., 2016).
Since fMRI measures neural activity only indirectly and given the difficulties with removing nonneural signals from the measurements, it is not always straightforward to interpret observations in fMRI studies of network interactions (Kelly et al., 2012). This is of particular concern in the context of stroke and cerebro-vascular disease, as fMRI signals are influenced by vascular pathology or by changes in the hemodynamic response close to the lesion. Surprisingly few studies have addressed this issue, but there is evidence for abnormal hemodynamic signals in patients with brain lesions which might impact estimates of network interactions (D'Esposito et al., 2003, Murata et al., 2006, de Haan et al., 2013, Bonakdarpour et al., 2015, Agarwal et al.,2016). For instance, de Haan et al. (2013) found decreased hemodynamic responses in structurally intact perilesional brain areas of stroke patients without neurological or neuropsychological deficits. In another study, stroke patients with aphasia had a delayed hemodynamic response compared to patients with aphasia due to a neurodegenerative disorder (Bonakdarpour et al., 2015). Hence, vascular pathology may have an unspecific and rather diffuse impact on fMRI signals, which may confound with the effect of behaviour.
EEG and MEG have therefore an advantage in stroke patients, as they do not depend on the hemodynamic response. On the other hand, they can be subject to problems related to the spread of electromagnetic potentials to the surface sensors. The potential arising in a given grey matter source spreads with light speed throughout the brain and the scalp and is picked up by multiple EEG/MEG sensors. This spread is called volume conduction and is in fact the reason we are able to record EEG and MEG at the scalp surface.
Brain lesions may alter the current spread from the brain to the sensors, which might decrease the reliability of source localization with EEG and MEG. Simulations in traumatic brain injury have indeed demonstrated that the source localization error introduced by lesions with high conductivity (i.e., with oedema or fresh blood) can be substantial in the vicinity of the lesions and reach tens of millimetres (van den Broek et al., 1998, Irimia et al., 2013). However, evidence from epilepsy (Brodbeck et al., 2011, Lascano et al., 2016) and brain tumours (Martino et al., 2011, Lascano et al., 2014) shows that, despite the presence of brain lesions, high-density EEG/MEG provide localization accuracy that is useful for guiding the extent of surgery. This demonstrates that the precision EEG and MEG imaging is mostly sufficient for localizations at the sub-lobar level. Furthermore, head models taking into account lesion and skull configurations are now increasingly available and further reduce the error introduced by lesions (Irimia et al.,2013).
Volume conduction also produces a number of pitfalls in the investigation of network interactions with EEG and MEG. This is the case not only in stroke patients, but needs to be taken into account in all studies. Because of volume conduction, a single source is represented in multiple sensors. If we quantify the FC between such sensors, we obtain artificial similarity of signals, and hence inflated and distorted values of FC between sensors, even if there is in fact no true interaction between the brain areas underneath the sensors (Schoffelen et al., 2009).
We can use inverse solutions to estimate neural oscillations in the brain from EEG/MEG sensor data. This partially inverts volume conduction. However, inverse solutions provide an incomplete removal of volume conduction because even the best available inverse solutions have limited spatial resolution. This means that the current reconstructed at a given brain location arises in fact not only from activity at this source, but is additionally influenced by activity in adjacent locations. This effect is called spatial leakage and entails that neural activity reconstructed at a given location is a linear combination of activity from a region surrounding this location. Moreover, the spatial resolution of inverse solutions is not homogeneous throughout the brain but worse for areas with less sensor coverage such as deeper brain regions. Overall, this leads to an overestimation and distortion of the magnitude of network interactions (Guggisberg et al., 2008, Schoffelen et al., 2009, Sekihara et al., 2011).
These issues can be easily overcome by using appropriate measures of FC. The first such measure that was introduced is the imaginary component of coherence (IC) (Nolte et al., 2004). IC exploits the fact that artificial similarities among time series arising from volume conduction and spatial leakage occur with zero phase lag between sites, while true neural interactions may require phase delays due to neural transmission. Coherence is complex valued with real and imaginary components. The real component represents coupling with near zero time lag while the imaginary component represents lagged coupling. We can therefore omit the real component of coherence and use only interactions occurring with a certain time lag represented by IC (Sekihara et al., 2011). Although this may also remove true coupling occurring with zero phase lag, the remaining lagged interactions usually suffice for most applications. Other corrections are also available (Stam et al., 2007, Brookes et al., 2011a, Ghuman et al., 2011, Pascual-Marqui et al., 2011, Hipp et al., 2012). Furthermore, Granger causal measures of EC inherently ignore zero-lag interactions and hence control for volume conduction. Despite the availability of appropriate solutions, some studies still do not use them and report possibly spurious and artificial interactions.
Most experts recommend combining inverse solutions with corrected measures of FC to reconstruct neural brain interactions as this allows integrating information on head geometry for a more precise localization of interacting areas (Gross et al., 2013). In some cases, where the location of the sources is not important, it is also possible to compute FC directly between EEG or MEG sensors as long as corrected measures of FC are used. In the case of EC, the combination with inverse solutions is more debated as EC measures may be sensitive to phase changes introduced by such preprocessing steps (Kaminski et al., 2014).
2.2.4. Graph theory
Analyses of FC and EC produce multidimensional arrays of results. Graph theory provides a means for dimension reduction. It abstracts from single interactions between pairs of regions and instead derives network properties on a more global level (Bullmore et al., 2009, Stam et al., 2012, De Vico Fallani et al., 2014). These properties can be computed in principle from any kind of measure of FC or EC.
Graph theory distinguishes between nodes and edges of a network. In neuroimaging, nodes correspond to brain areas and edges to neural interactions. It is hence possible to characterize the properties of a given brain area in the network, or to characterize aspects of network interactions (Newman, 2004, Bullmore et al., 2009, Stam et al., 2012). When characterizing nodes in the context of stroke plasticity, the property of node degree or node centrality is of particular interest. It indicates the number of connections or the sum of interactions of a brain area of interest with the rest of the brain network. Although different versions of this measure are available, they all quantify the overall importance of the brain area in the network.
When considering network interactions, it is often investigated whether the brain network has small-world properties, i.e., whether it provides an optimal compromise between local specialization and global integration. To achieve this, it should have high local connectedness among neighbouring brain areas and, at the same time, high long-distance connectedness to more distant brain areas. The healthy human brain was found to show such smallworldedness (Achard et al., 2006).
2.3. Non-invasive brain stimulation
Non-invasive brain stimulation techniques allow us to study the organization and reorganization in the intact and diseased human brain. In this section, we will review how brain network function is assessed using two non-invasive neurophysiological methods such as transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES). For TMS techniques, single pulse TMS, and paired pulse TMS are used while single stimuli are used for TES.
Non- invasive brain stimulation is also used to modulate the excitability of the stimulated neuropil. In the post- stroke recovery period it is used in combination with rehabilitation strategies. One of these strategies is the use of repetitive transcranial magnetic stimulation (rTMS) which has the potential to improve motor recovery after stroke (Khedr et al., 2005, Mansur et al., 2005, Khedr et al., 2010, Buetefisch et al., 2011b). For transcranial electrical stimulation protocols, most evidence is available for transcranial direct current stimulation (tDCS) (Nitsche et al., 2000, 2011). Protocols using alternating current (Antal et al., 2008) or random noise (Terney et al., 2008) will not be reviewed in this article.
In the following sections we will illustrate how TMS with its high temporal and topographical resolution provides means to measure excitability of functional connections within a cortical area (intracortical) and between cortical areas (intercortical) located in the same hemisphere or across hemispheres of the intact and lesioned brain such as stroke. In the second part we will discuss how rTMS and tDCS are used to modulate excitability in the stimulated neuropil and connected brain areas to study their function, their contribution to behaviour or impact on excitability of the probed neuronal network and as a new strategy in rehabilitation of stroke patients.
2.3.1. TMS derived excitability measures of neuronal networks
TMS is an established non-invasive brain stimulation technique, in which the focal application of brief magnetic fields evokes electrical currents in the cerebral cortical neuropil. Depending on the orientation of the coil, the configuration of the pulse and the intensity of the stimulation, the transient current activates the fast conducting pyramidal tract neurons (PTN) trans-synaptically through horizontal connections. A motor evoked potential (MEP) that is recorded with electromyography (EMG) via surface electrodes mounted over the targeted muscle reflects the number and excitability of the activated neurons..
2.3.2. Excitability of primary motor cortex and its corticospinal projections
The TMS or TES evoked MEPs are means to probe the entire pathway from primary motor cortex to alpha motorneurons with their projections to the muscle via the peripheral nerve. The smallest stimulation intensity required to elicit an MEP defines the resting motor threshold (RMT) (Rossini et al., 1994, Mishory et al., 2004). As TMS activates the PTN transyaptically, the motor threshold (MT) depends on the excitability of the synapses of stimulated cortico-cortical axons making contact with the PTN (Amassian et al., 1987). The MT is obtained at the location of the scalp that produces the largest MEP response of the target muscle with the smallest intensity of stimulation, the so-called hot spot. The presence of a measurable MEP indicates functional connectivity along the entire pathway. This is very valuable information in neurological diseases affecting these neuronal structures. In patients after stroke, TMS provides means to measure the functional efficacy of the corticospinal output system early after the infarction. Specifically, within the first 72 hours after stroke, an absent MEP has been related to poor functional recovery (Binkofski et al., 1996, Nardone et al., 2002, Stinear et al., 2012). For localization of the site of excitability changes within the corticospinal system, additional measures are necessary. Other additional measures that help to differentiate the site of changes in excitability include the assessment of spinal excitability by means of F-waves (Mercuri et al., 1996) or the H-reflexes (Fuhr et al., 1991).
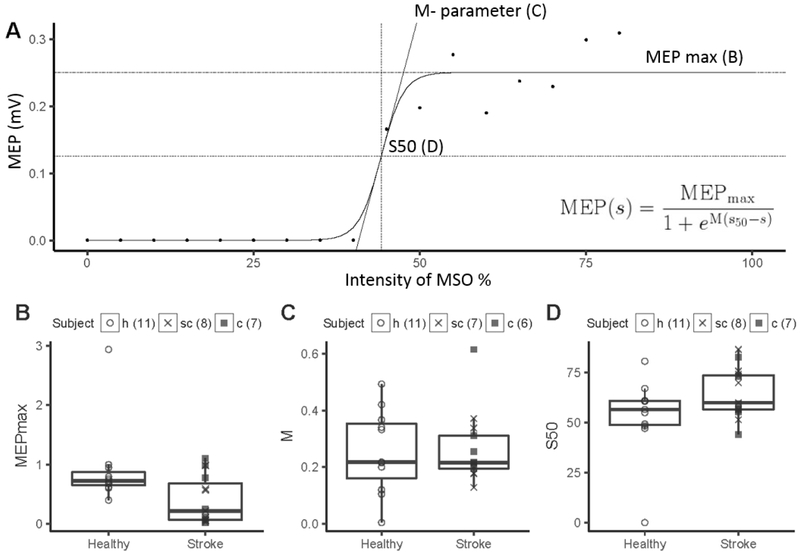
More detailed analysis of M1 excitability is derived from stimulus response curves (SRC) where TMS is applied to a M1 hot spot at increasing intensities and evoked MEP amplitudes are plotted against intensities (Ridding et al., 1997). Increases in MEP amplitude with increasing TMS intensities reflect recruitment of neurons that are either intrinsically less excitable or more distant from the hot spot (Ridding et al., 1997). The curves follow a sigmoid function that is characterized by three curve parameter when modelled with the Boltzman function (slope, maximum MEP amplitude (MEPmax), and intensity of the stimulator output to produce 50% of MEPmax (k)). When SRC is measured at a constant level of motor activity, these 3 curve parameter completely characterize the input- output relationship of the M1 cortiospinal pathway (Capaday, 1997, Devanne et al., 1997, Capaday et al., 1999).
Using a single intensity to study excitability of M1 output is problematic because the location of the MEP on the SRC in not known. Changes in M1 excitability will result in greater increases in MEP amplitude with its location on the slope when compared to its location on the plateau. In stroke patients, evoking an MEP with M1 stimulation of the affected hemisphere requires higher intensities resulting in abnormally high RMT (Boroojerdi et al., 1996, Liepert et al., 2000b, Manganotti et al., 2002, Freundlieb et al.) and SRCs are shifted to the right with less steepness of the slope and smaller MEPmax (Figure 2) (Buetefisch et al., 2018). The calculation of the curve parameter with the Boltzman function is the most comprehensive analysis of the SRC but limited to curves with a defined plateau. In stroke patients this may not be feasible and calculation of the area under the curve or the sum of MEP amplitudes derived from stimulation at increasing intensities can be used.
Figure 2. Ipsilesional M1 excitability measured with TMS.
A) The stimulus response curve (SRC) evoked by transcranial magnetic stimulation of increasing intensities (35% to 80% of maximum stimulator output) were plotted for a single stroke patient. B, C, D). A three-parameter Boltzmann function was fitted to all SRCs that reached a plateau using the Levenberg-Marquard least-squares algorithm (insert) to extract three curve parameters: MEPmax (plateau of SRC), S50 (TMS intensity needed to elicit an MEP of an amplitude corresponding to the inflection point) and M (slope) parameter. The data are plotted for 15 stroke patients with cortical or subcortical location of a stroke affecting their M1 output system and 11 right handed age- matched healthy subjects. The number of subjects for each parameter is indicated in the figure. MEPmax was statistically significant lower in stroke subjects than in healthy subjects (p=0.02). There was no statistically significant difference in M-parameter and S50 between stroke and healthy subjects. This approach povides a more detailed analysis of M1 excitability. When measured at a constant level of motor activity (here, at rest), the three SRC curve parameters (S50, M-parameter, and MEPmax) completely characterize the input-output relationship of the M1 corticospinal output(Devanne et al., 1997). Therefore, a change in one or more parameters indicates a change in the input-output relationship in iM1 and its corticospinal output. The abnormally low MEPmax found here suggests that CST output from iM1 was reduced after stroke (Buetefisch et al., 2018).
2.3.3. Excitability of M1 intracortical circuitry
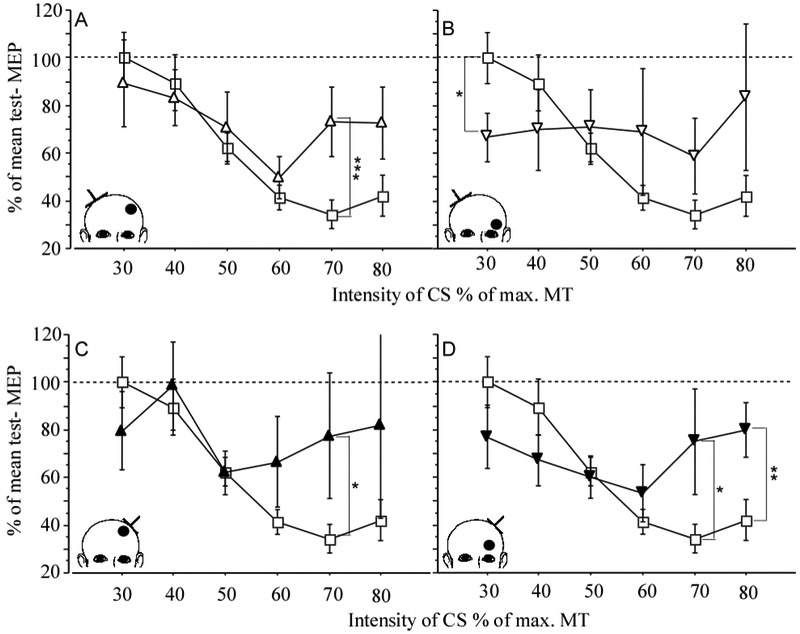
The excitability of M1 intracortical circuitry can be estimated indirectly by means of a paired pulse TMS paradigm. In this paradigm two pulses are delivered through the same coil. A suprathreshold test stimulus (TS) is preceded by a subthreshold conditioning stimulus (CS) at different interstimulus intervals (ISI) (Kujirai et al., 1993). With short ISI of 2-4 ms the CS produce an inhibitory effect of the subsequent TS evoked MEP and is referred to as short interval intracortical inhibition (SICI) (Kujirai et al., 1993). Most investigators use an intensity for TS and CS that is based on the individual’s RMT. For TS the intensity is usually set at 120% RMT and for CS at 80% RMT (Kujirai et al., 1993). Alternatively, investigators set the intensity of the TS to produce an MEP of about 1mV because CS has its maximum inhibitory effect on the test MEP amplitudes of 1- 4 mV (Sanger et al., 2001). MEP amplitudes below 0.2 mV should be avoided as the inhibitory effect of CS reaches a floor effect. While a strong linear relationship between RMT and intensity of CS that produces maximum inhibition can be demonstrated in healthy subjects, there is no relationship between these two parameter when testing M1 excitability in stroke patients (Butefisch et al., 2003, Butefisch et al., 2008). This would question the usefulness of MT as a reference for selecting the appropriate intensity for CS. Because RMT is abnormally high in the affected hemisphere, the CS intensity corresponding to 80% RMT is maybe too high to capture the inhibitory effect of these lower threshold inhibitory interneurons. Instead, it is recommended to test multiple subthreshold CS intensities between 60%-80% MT. As the threshold for inhibitory neuronal circuitry is lower than that for excitatory neuronal circuitry (Schafer et al., 1997, Chen et al., 1998, Butefisch et al., 2003) testing the effects of lower CS intensity on the test MEP allows the separation of the effects mediated by these respective networks in more detail (Schafer et al., 1997, Chen et al., 1998, Fisher et al., 2002, Butefisch et al., 2003) (Figure 3).
Figure 3.
Effect of stroke location along the primary motor output (either cortical or subcortical) on primary motor cortex excitability. Paired pulse TMS was used to measure short interval cortical inhibition (SICI) in 23 chronic stroke patients. The data was compared to 20 healthy age matched controls. Upper panel: CONTROL (square) and contra-lesional M1 of patients with cortical (open triangle, A) and subcortical location of infarction (open inverted triangle, B). Lower panel: CONTROL (squares) and ipsilesional M1 of patients with cortical (black triangle, C) and subcortical location of infarction (black inverted triangle, D). Mean ± SE. * p< .05, ** p< .02, *** p< .01. Inserts illustrate the location of the lesion (black dot) and the site of TMS (inverted T). CS= intensity of conditioning stimulus, MT = motor threshold. (Butefisch et al., 2008).
The CS likely evokes an inhibitory postsynaptic potential through activation of low threshold inhibitory neuronal circuitry (see below) which inhibits the TS related generation of action potential in connected pyramidal tract neurons (Kujirai et al., 1993). This effect is mediated by positive GABAA-receptor modulators (Ziemann et al., 1996) and arises in close proximity to the stimulated area (Di Lazzaro et al., 1998).
In addition to the assessment at rest (termed resting-state SICI), measurement of SICI during the pre-movement period (termed event-related SICI) allows to determine intracortical inhibition during the course of a movement (e.g., (Heise et al., 2010, 2013)). With this approach, one gains information on resting-state levels of intracortical inhibition and additionally about time-locked modulation of intracortial inhibition towards movement onset with high temporal resolution. Typically, in healthy subjects, intial resting-state inhibition turns close to movement onset into dishinbition/facilitation promoting the motor cortex to perform the movement (Heise et al., 2010). In contrast to the healthy situation, chronic stroke patients show an impaired range of modulating inhibition to disinhibition in the pre-movement period closer to the onset of movements. This was associated with residual motor function (Hummel et al., 2009). In a longitudinal study of stroke patients, resting state SICI did not add to the prediction of functional outcome after one year (Liuzzi et al., 2014). However, the patients, who showed disinhibition in event-related SICI in the acute phase post-stroke recovered best within the year. Thus, resting-state SICI and event-telated SICI represent not identical, but differential properties of intracortical inhibition.
At longer ISI of 8-30 ms the effect of the CS on the TS evoked MEP is facilitatory, termed intracortical facilitation (ICF) (Kujirai et al., 1993). The neuronal populations mediating ICF are less well understood but they are distinct from those mediating SICI and appear to be located in cortex (Ziemann et al., 1996, Di Lazzaro et al., 2006). In this paradigm the facilitatory effect of CS is not affected by the strength of the TS (Sanger et al., 2001). After stroke ICF remains usually unchanged (Liepert et al., 2000a, 2000b, Butefisch et al., 2003).
The role of GABA receptor type B-expressing interneurons can be probed by the contralateral cortical silent period protocol, in which a single suprathreshold TMS pulse (i.e. above MT) is delivered during tonic muscle activation of the targeted muscle. This results in the disruption of M1 activity that is reflected in EMG silence following the MEP lasting 40-300 ms (Fuhr et al., 1991, Inghilleri et al., 1993). Other TMS paradigms probing intracortical inhibition are the long-interval intracortical inhibition (LICI) (Wassermann et al., 1996, Inghilleri et al., 2003) and the short- latency afferent inhibition protocols (Mariorenzi et al., 1991).
2.3.4. Excitability of primary motor cortex and its cortico-cortical connections.
The influence of other brain areas projecting to M1 can be assessed by probing the effect of a conditioning stimulus (CS) applied through a coil placed over the brain area of interest followed by a suprathreshold test stimulus (TS) applied through the coil overlaying the M1 hot spot of a target muscle. The paired pulses are intermixed with single TS and single CS pulses applied at random. By measuring the effect of the CS on the MEP amplitude evoked by a TS the excitability of the connection between M1 and the other brain area of interest is determined (Koch et al., 2007, Koch et al., 2008a, Liuzzi et al., 2010, 2014). Because of its excellent temporal resolution, TMS in this design can be used to determine time dependent changes in the excitability of cortical projections to M1 in processes of interest. For example, this approach has been used to examine the connections between M1 and posterior parietal cortex (Koch et al., 2007, 2009b) or premotor cortex (Baumer et al., 2009, Davare et al., 2009) of either side (for review (Koch et al., 2009a)).
2.3.5. Excitability of interhemispheric connections between primary motor cortices.
In addition to the corticospinal projections and cortico-cortico connections within the same hemisphere, the primary motor cortices of the two hemispheres are connected to each other. The majority of these connections are located in the corpus callosum and are primarily excitatory (for detailed review (Dancause et al., 2015). An inhibitory effect from one M1 on the homotopic area of the other M1 can be demonstrated with TMS when the CS is applied to one M1 and the TS to the homotopic area of the other M1, termed interhemispheric inhibition (IHI) (Ferbert et al., 1992). The intensities of the TMS pulses are usually adjusted to produce a MEP of about 1 mV and the ISI is typically 10 ms (Ferbert et al., 1992). In contrast to SICI the inhibitory effect of CS is inversely influenced by the strength of the TS with less inhibitory effect at higher intensities (MEP amplitudes > 1 mV) (Daskalakis et al., 2002). Usually, the paired pulses are intermixed with single TS and single CS pulses applied at random. The amount of IHI from one motor cortex on the other motor cortex is expressed as percentage of the mean MEP amplitude of the single TS pulses (Ferbert et al., 1992). The differential effect of TS intensity on the inhibitory effect of CS would suggest that the neurons mediating IHI have a lower threshold or are located more superficially (Daskalakis et al., 2002).
While resting IHI is measured with the subject at rest, active IHI is measured during movement preparation. In healthy subjects the inhibitory effect of one M1 on the other M1 decreases during the preparatory phase of movement execution (Murase et al., 2004). The extent of IHI depends on the movement kinematics (Duque et al., 2005, Wischnewski et al., 2016). As background EMG activity may increase closer to the onset of movement, quantification of EMG background is necessary to exclude the possibility that EMG background related increases in test MEP amplitude result in less inhibitory effect of the CS (Wischnewski et al., 2016). Resting and active IHI are affected by stroke (Butefisch et al., 2008, Jones et al., 2013, Dancause et al., 2015). See discussion below. Taken together, TMS protocols with their excellent topographical and temporal resolution allow to gain information during rest, but also during the pre-movement period. These two situations represent different properties of cortical processing and provide thus a more complete picture of motorcortical physiology.
2.3.6. Repetitive transcranial magnetic stimulation (rTMS)
When adhered to the published guidelines (Rossi et al., 2009), rTMS can be used safely to modulate excitability in the stimulated M1 (Chen et al., 1997, Maeda et al., 2000, Sommer et al., 2002, Bagnato et al., 2005, Fitzgerald et al., 2006, Di Lazzaro et al., 2008), but also remotely (Strens et al., 2002, Kobayashi et al., 2004, Chung et al., 2015), which in specific settings can produce measurable behavioral effects. In this regard, rTMS when applied to the brain area of interest is a means to probe its contribution to the studied task.
In general, rTMS applied to M1 at high frequency is thought to produce an excitatory effect (Pascual-Leone et al. 1994;Di Lazzaro et al. 2002; Fitzgerald et al. 2006;Daskalakis et al. 2006), while rTMS at low frequency produces an inhibitory effect (Chen et al. 1997; Daskalakis et al. 2006; Fitzgerald et al. 2006). For example when low frequency rTMS of M1 is coupled to the execution of a movement in a strict temporal relationship, the stimulated M1 excitability is increased and behavior improved (Bütefisch et al., 2004, Buetefisch et al., 2014a). However, more recent findings have demonstrated that effects of rTMS are highly variable, such that effects can even be inversed (Hamada et al., 2013, Wiethoff et al., 2014, Hordacre et al., 2015, Li et al., 2015, Nicolo et al., 2015a, Vallence et al., 2015). Moreover, the results of meta- analyses on the effectiveness of rTMS in stroke rehabilitation therapy do not agree on the available evidence to either support or reject it (Adeyemo et al., 2012, Hsu et al., 2012, Elsner et al., 2013, Hao et al., 2013). Better characterization of brain changes induced by rTMS is necessary to understand the potential impact of rTMS on the functional anatomy and plasticity of synaptic networks, to optimize therapeutic rTMS protocols, and to assess their safety.
2.3.7. Repetitive transcranial electric stimulation
Under the label repetitive transcranial electric stimulation, approaches based on transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS) are summarized. In the following, we will focus on tDCS, as the large bulk of studies with tES has been done by applying tDCS. In accordance with current guidelines (Antal et al., 2017) tDCS is a safe and well tolerated technique to modulate cortical excitability, neuronal plasticity and behaviour non-invasively (Hummel et al., 2005c, Nitsche et al., 2008). In tDCS a low current is delivered through the skull via two surface electrodes (Nitsche and Paulus 2000) ranging from approaches with larger, topographically less specific to multiple smaller electrodes with higher topographical resolution (Antal et al., 2017). For motor cortex stimulation, the stimulating electrode is placed over M1 and a reference electrode over the contralateral supraorbital ridge. The application of the weak current over the cerebral cortex induces polarity-specific alteration of stimulated neuropil, which can outlast the stimulation for minutes to hours, an ideal time window for rehabilitative treatment sessions. The primary mechanism of tDCS is a subthreshold alteration of the resting membrane potential, whereas the after-effects seem to resemble synaptic plasticity of glutamatergic connections (Liebetanz et al., 2002, Nitsche et al., 2003, 2004); for review (Stagg et al., 2011). More recently, tDCS related modulation of the local field potential depending on the anodal or cathodal polarity of the applied current were reported for sensory cortex in the awake animal (Marquez-Ruiz et al., 2012), which corresponds to the observed effects on MEP amplitudes when tDCS is applied to M1 (Nitsche et al., 2000). While these are promising results, the authors of a recent consensus paper stated that the “overall, reproducibility remains to be fully tested, effect sizes with present techniques vary over a wide range, and the basis of observed inter-individual variability in tDCS effects is incompletely understood” (Buch et al., 2017).
3. Principles of network plasticity after stroke
3.1. Structural connectivity
In traditional localizationism, the location and size of tissue-loss in grey matter following an occlusion of a cerebral artery in respect to the perfusion territory of anterior, medial or posterior cerebral arteries are related to specific functional deficits of an individual patient. This leads to inference of highly specialized functions of grey matter regions in the direct correlation of area and function. In clinical structural neuroimaging this concept is followed by lesion symptom mapping (e.g., Karnath et al., 2018). Still, group analyses in stroke patients reveal that the majority of lesions are affecting to a large part white matter or a combination of both white and grey matter, whereas isolated grey matter lesions are seen in less than 15% (Kang et al., 2003, Wessels et al., 2006, Corbetta et al., 2015). Following, the lesion - symptom relationship is accompanied by a lesion - network relationship, in which the analyses of white matter networks maintain a crucial role (Fox, 2018). In structural connectivity analyses of recovery after stroke, two main aspects of networks are most relevant and studied: degeneration (see section 3.1.1) and reorganization (see section 3.1.2), which will be the main focus in the following section.
3.1.1. Disconnection and secondary degeneration
The integrity of the CST, as the crucial outflow tract of the motor system, is the most investigated white matter pathway in studies of motor recovery after stroke. CST integrity has been described with the lesion load – the volume overlay of motor fibres and ischemic lesion, the number of connecting fibres (Sterr et al., 2010, Zhu et al., 2010, Sterr et al., 2014, Feng et al., 2015), fractional anisotropy extracted from DTI (Stinear et al., 2007, Buch et al., 2016b, Guggisberg et al., 2017), or with motor evoked potentials after TMS (Byblow et al., 2015).
Greater damage to the CST is robustly associated with more severe motor impairment in acute and chronic phases as well as with less improvement, as shown in multiple convergent studies (Stinear et al., 2007, Kim et al., 2015, Schulz et al., 2015b, Ramsey et al., 2017, Peters et al.,2018). More recent studies have additionally demonstrated that severe damage to the CST seems to be a main factor leading to a particularly poor pattern of motor improvement with severe chronic impairment (Byblow et al., 2015, Feng et al., 2015, Buch et al., 2016b, Guggisberg et al., 2017). Indeed, the evolution of motor function after stroke follows two divergent paths (Prabhakaran et al., 2008, Winters et al., 2015). Patients will either recover about 70% of maximum possible improvement or show little to no improvement. Byblow et al. (2015) observed that absent MEPs in the acute phase after stroke reliably predict which patients will follow this particularly poor pattern of recovery. Similarly, strong asymmetries in FA of the CST predict the poor outcome pattern in patients with severe initial impairment (Buch et al., 2016b, Guggisberg et al., 2017).
The disconnection of association fibres induced by stroke lesions has also a relevant impact on the recovery of other cognitive domains, such as aphasia (Hosomi et al., 2009, Kim et al., 2013, Ramsey et al., 2017), neglect (Lunven et al., 2015, Vaessen et al., 2016), or others (Epelbaum et al., 2008, Ramsey et al., 2017). Hereby, the loss of specific white matter tracts measured with DTI usually leads to specific loss of functions, known as dysconnection syndromes (Catani et al., 2005, Thiebaut de Schotten et al., 2005). This is also seen in pure subcortical stroke lesions (Marebwa et al., 2017). In this regard, patients, in whom stroke lesions affect areas with a high overlap of association fibres are more likely to suffer from deficits in several cognitive domains depending on the affected white matter tracts (Corbetta et al., 2015). This is where ‘lottery’ is entering the lesion-function relationship for stroke patients and emphasizes how important the individual lesion location in relation to white matter tracts might be for prediction of recovery and personalized treatment protocols.
We can thus derive a first principle of network plasticity after stroke stating that stroke lesions to specific white matter tracts are associated with particularly severe clinical deficits and with less recovery. This has been shown most extensively for damage to the CST and motor function, but seems to be valid also for associative tracts and cognitive function.
Several studies have described a secondary reduction of microstructural integrity of the ipsilesional CST (Puig et al., 2010, Lindenberg et al., 2012) (for review see (Koch et al., 2016, Puig et al., 2017)), formally known as Wallerian degeneration. Further, antero- and retrograde degeneration has been directly associated with lesions affecting the CST (Liang et al., 2007) and the secondary loss of integrity as measured by changes in fractional anisotropy (FA) has been found to be a major predictor for motor recovery after stroke (Thomalla et al., 2004, 2005, Ma et al., 2014, Guggisberg et al., 2017). In one study, patients with particularly poor motor recovery showed secondary degradation of white-matter integrity in extensive parts of the affected hemisphere (Guggisberg et al., 2017). Still, there are different suggestions about the time course of degeneration over the recovery process, some showing a progressive degeneration until the subacute phase (Liang et al., 2007, Moller et al., 2007, Yu et al., 2009), some don’t (Radlinska et al., 2010). This might be due to the spatial relationship to the lesion and different time points of evaluation. Further, stroke-related changes are likely the result of both degeneration and regeneration processes, and therefore the mechanisms underlying differences in FA values are likely mixed (Jones et al., 2011). When looking at CST FA and motor recovery, different involvement of fibers from primary motor cortex (M1), premotor cortex, and somatosensory areas are not considered. Considering the fact that only about 40% of the CST is from M1, two large CST lesions revealed with CST FA may have different amounts of spared corticospinal fibers from the M1. This limits the precision of this measure when looking at correlation of CST FA and more specific measures such as hand motor function. In this setting TMS measures of M1 output is a better predictor of spared corticospinal fibers from M1 hand function (Buetefisch et al., 2018).
The association between damage to white matter tracts with the respective degeneration and functional outcome is seen not only for projection fibres. Some tractography studies could show that cortical areas directly connected to subcortical stroke lesions showed a specific reduction in cortical thickness explainable by secondary neuro-axonal degeneration (Cheng et al., 2015, Duering et al., 2015), in which the white matter loss induces cortical disconnection beyond the lesion (Bonilha et al., 2014).
Exploiting the full degree of multidimensionality in structural connectivity whole connectome analyses represents another promising method to understand network alterations in stroke. In this regard, several studies promote the idea that stroke lesions lead to modular fragmentation and clustering resulting in weaker inter-modular integrations and a total decrease of information transfer and communicability especially remotely to the lesion. This has been shown both, in aphasia (Gleichgerrcht et al., 2015, Yourganov et al., 2016, Marebwa et al., 2017) and motor affection (Crofts et al., 2011, Kuceyeski et al., 2014, 2015) after stroke, supporting additionally the idea of secondary degeneration. This indicates a large effect on local and global networks induced by a focal brain lesion (Saenger et al., 2017, Foulon et al., 2018). Furthermore, these analyses might be also interesting for prediction of functional outcome. Especially the multivariate modelling including both white and grey matter information is promising in explaining functional outcome (Barrett et al., 2016, Yourganov et al., 2016), whereas a preservation of global and local network architecture of crucial cortical regions seems important for a sufficient treatment response (Bonilha et al., 2016).
Taken together post-stroke disconnection and secondary degeneration are major factors impacting on the degree of impairment and recovery. This suggests a second principle of network plasticity after stroke. Fibre tracts with stroke damage tend to degenerate during the first weeks after stroke, which leads to atrophy of the corresponding grey matter, less clinical recovery, and worse long-term outcome. This effect is not restricted to local perilesional areas but concerns global brain networks in both hemispheres.
However, other factors are involved in the process of recovery, such as the intrinsic capacity of the brain to regain and relearn lost functions after stroke by mechanisms of neuroplasticity and functional reorganisation (see below).
3.1.2. Reorganization
Neuroplasticity based on long-term potentiation and depression-like effects plays a crucial role for functional recovery after stroke and is mostly related to synaptic alterations. This paragraph will focus on the growing evidence of remodelling capability in white matter pathways detectable by diffusion imaging (for review (Assaf et al., 2017)). Studies suggest an increase of microstructural integrity following intensive training in healthy participants even after five days (Blumenfeld-Katzir et al., 2011) and more prominent in a long-term follow up (Bengtsson et al., 2005, Scholz et al., 2009, Sagi et al., 2012, Zatorre et al., 2012, Thiebaut De Schotten et al.,2014). Animal models suggest, that stroke lesions are accompanied by a strong trend of building new structural (Jones et al., 2011) and functional connections between specific areas (Frost et al., 2003, Dancause et al., 2005). Thus, revealing white matter connections which correlate with residual function or recovery, and their temporal dynamics, could provide insights into recovery processes of the brain in terms of remodelling after focal lesions (Schulz et al., 2015a), thereby bridging the gap between animal models and human neuroscience.
In motor recovery, further white matter tracts besides the already discussed CST have been in the focus of research. It has been shown, that additional projection fibres like alternating motor fibres including the cortico-rubral and cortico-reticular system support the recovery of function (Lindenberg et al., 2010, Rüber et al., 2012, Schulz et al., 2017b). There are hypotheses, based on animal studies, that these polysynaptic pathways, in comparison to the CST, show a higher capacity of remodelling and are therefore most suitable for supporting regain of function (for review (Koch et al., 2017)). It is crucial to take the different key hubs of the motor network into account including the cerebellum. It has been suggested that interactions based on structural connectivity between the cerebellum and the motor cortex impact on functional reorganization, residual motor functions and recovery after stroke (Wessel et al., 2018). One study by Schulz et al. (Schulz et al., 2015a) demonstrated, that both the afferent and efferent connections of the cerebellum with M1 were related to residual motor function and skilled motor control in chronic stroke. Moreover, this relationship was not seen in healthy participants and was independent of the level of damage to the CST, which point towards compensatory mechanisms of reorganization. Determining the functional relevance of the different motor tracts provides additional insights in mechanisms relevant for recovery, however these approaches are limited by the fact that they have been applied independently for each network tract (Koch et al., 2017). To deepen the understanding especially on a network level as basis towards making predictions on an individual level, it is inevitable to analyse the different tracts together and evaluate their interactions, their functional role and differential and synergistic impact for individual recovery. First steps in this direction have been achieved in the motor domain. Structural connectivity analyses could show that secondary motor areas like SMA and PMd and their spinal descending fibres are associated with residual function in chronic stroke patients (Schulz et al., 2012, Peters et al., 2018). Additional to the relevance of the CST, intra-hemispheric parieto-frontal cortico-cortical connections of the lesioned hemisphere between the IPS and the PMv and between PMv and M1 are associated with residual motor function in the chronic stage of recovery (Schulz et al., 2015b). Moreover, the analysis of dependencies within these networks is a very important and promising way to understand reorganization in the individual patients. Hereby, a current study showed that the above discussed impact of structural connectivity between PMv and M1 contributes only relevantly to residual motor function in patients with a severely affected corticospinal tract (Schulz et al., 2017a). Contrarily, the impact of alternating motor fibres on motor recovery was independent of the affection of the CST and seems to be a more general phenomenon of stroke recovery (Schulz et al., 2017b). Still, considering both pathways at the same time increased the accuracy of prediction of motor recovery (Lindenberg et al., 2010).
Less is known about structural reorganization processes in regard to the recovery of cognitive functions. One study in patients with aphasia could present an increase in the integrity of arcuate fascicle following melodic intonation therapy (Schlaug et al., 2009).
In conclusion, assessments of structural network plasticity have suggested a third principle of plasticity, stating that recovery after stroke depends on structural connectivity within distributed networks. Thereby, alternative and/or newly formed connections can compensate for the loss of usual pathways. This has been shown so far mainly within the motor domain and its respective networks.
In this growing field of network alterations after stroke, several questions are still open, challenging and interesting for future research. They will guide the way to individualized prediction and understanding of recovery processes, selection of personalized treatment and predicting the magnitude of therapeutic interventions. To achieve these goals there are several limitations, which have to be overcome. Firstly, there is a lack of longitudinal evaluation of white matter integrity to detect indices for plastic changes and reorganization in white matter tracts. These increases in integrity over time, as reflected in multiple white matter metrics (see section 2.1.1), might show indirectly the capacity of reorganization and structural plasticity of white matter tissue and its underlying histological mechanisms.
Secondly, the correlations between interindividual variance of microstructure and functional outcome and recovery after stroke implies a problem of gaining inferences. Two scenarios seem possible. Is the variance in microstructure a direct result of degeneration and/or regeneration and reorganization processes in consequence to the ischemic lesion or is it explained by a natural variability of structural connectivity pre-existing in healthy populations (Thiebaut de Schotten et al., 2011) (Johansen-Berg et al., 2007), genetically and epigenetically determined, which gains functional importance after the stroke.
Thus, the (genetically determined) structural prerequisites of a patient might significantly contribute to the capacity for functional reorganisation and recovery. Furthermore, the inter-individual variability of the stroke lesion topography and its affection of white matter fibres are not well studied in its relation to motor recovery and reorganization processes. Associated white matter diseases like subcortical arteriosclerotic encephalopathy add even more complexity to the understanding and have been largely neglected so far. Moving towards precision medicine with personalized treatment, the field has to focus on individual prediction of outcome and tailored treatment selection. To achieve these goals, factors like structural and functional connectivity and lesion load have to be evaluated in combined models. One way to account for this is the usage of computational modelling and clustering, which achieved increasing interest in clinical neuroscience. For example, support vector machine learning approaches are used to analyse functional connectivity in stroke and predict individual recovery (Rehme et al., 2015a, 2015b), automatized lesion detection and lesion symptom mapping (Rondina et al., 2017) and the analyses of individual phenotypes in e.g. depression (Drysdale et al., 2017).
More work is needed to deepen our understanding of different phenotypes of reorganization in order to pave the way towards personalized medicine in stroke rehabilitation. For this, structural connectivity analyses especially in a multimodal fashion including quantitative structural imaging, functional imaging and electrophysiological measurement is very promising and will provide deep insights in the systems neuroscience mechanisms of functional recovery.
3.2. Functional network plasticity
A focal stroke lesion leads not only to local dysfunction, but to altered neural communication in directly or indirectly connected brain areas. Evidence for this comes from realistic modelling of the human brain network (Honey et al., 2008, Alstott et al., 2009) and from animal experiments (van Meer et al., 2010). Analyses of functional and effective connectivity provide a non-invasive means for empirical assessments of these network effects in human stroke patients.
Non-invasive imaging has confirmed that stroke lesions lead to large-scale changes in neural interactions across the entire brain, but observations have been quite variable across studies. This variability is not surprising given that the examined patients had variable lesions, network configurations and that different coupling types were studied using different imaging modalities. Overall, network changes were reported in multiple spatial configurations which were not limited to specific networks. However, some reproducible principles can be derived from the different studies. In the following, we will first summarize concepts arising from the disruption of neural interactions and then consider the plastic enhancement of neural interactions.
3.2.1. Disrupted functional connectivity and neurological deficits
The first and most consistent finding of studies on network effects of stroke has concerned reduced inter-hemispheric FC between homologous motor, language, and spatial attention areas. These changes can be observed already in the acute stroke stage, but remain present up to the chronic stage in patients with persisting clinical deficits. Importantly, interhemispheric FC disruptions were linearly associated with corresponding neurological deficits of the patients (He et al., 2007, Warren et al., 2009, Carter et al., 2010, Golestani et al., 2013, Sasaki et al., 2013, Urbin et al., 2014). For instance, Carter et al. (2010) observed reduced interhemispheric FC between the motor cortices of stroke patients, which was associated with motor deficits. FC reductions between parietal brain areas were associated with neglect.
Furthermore, ipsilesional nodes also have reduced interactions with other nodes of a given functional network. This has been shown in particular for the motor network (Sharma et al., 2009). Assessments of EC with dynamic causal modelling have revealed reduced excitatory interactions between premotor and primary motor as well as between supplementary and primary motor areas (Grefkes et al., 2008). These changes were present in the acute stage and tended to normalize in subacute stages in patients with good recovery (Rehme et al., 2011). Again, disrupted EC was associated with motor impairments.
Other studies have demonstrated reduced FC to nodes of other networks of the ipsilesional and contralesional hemisphere (Park et al., 2011, Yin et al., 2012, Wang et al., 2014, Xu et al., 2014), although these changes were more variable and dependent on the time after stroke.
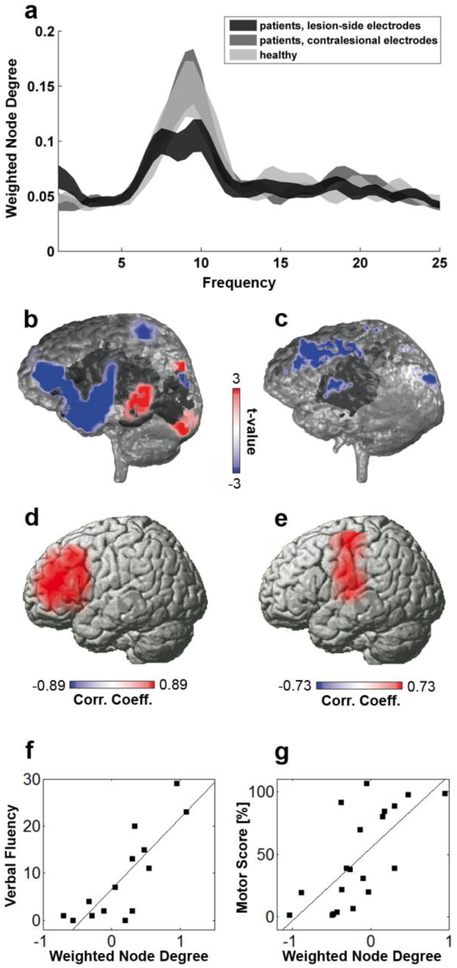
In sum, ipsilesional nodes can show complex disruptions of FC and EC with various other brain areas, both within and across networks, depending on the lesion, the individual network configuration, and the time after stroke. In order to abstract from the individually variable patterns of affected connections, it is useful to quantify the overall FC of a node of interest with the entire brain. This can be achieved with the graph theoretical measure node degree or node centrality (see section 2.2.4). EEG, MEG, and fMRI recordings have consistently found reduced node degree in stroke from the acute to the chronic stage after stroke and this was linearly correlated with the severity of clinical deficits. For instance, network imaging with high-density EEG showed that the more spontaneous neural activity in Broca’s area was coherent with the rest of the brain (i.e., the greater the node degree of Broca's area), the better patients were able to produce words (see Fig. 4). This has been reproduced for motor, language, and spatial attention functions using resting-state recordings (Wang et al., 2010b, Dubovik et al., 2012, Westlake et al., 2012, Guggisberg et al., 2015). Furthermore, the same observation can be made during movement tasks for motor function (Gerloff et al., 2006, De Vico Fallani et al., 2013).
Figure 4. Disruptions of network interactions after stroke are associated with neurological deficits.
The affected hemisphere of stroke patients shows a global reduction of alpha-band coherence with all other brain regions (a). This disconnection concerns brain areas that are clinically dysfunctional. For instance, a patient with Broca aphasia shows reduced global alpha coherence in left front-temporal areas (b, blue color; stroke lesion is marked in dark gray), a patient with motor deficits in precentral areas (c). Local decreases in alpha-band coherence between a given brain area and the rest of the brain are linearly correlated with neurological deficits. In other words, the less a brain region remains coherent with the rest of the brain after a lesion, the worse patients perform in corresponding motor and cognitive functions (d-g). Modified after (Dubovik et al., 2012) with permission.
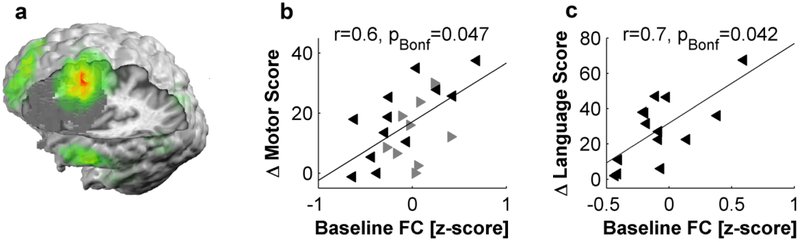
Improvement of neurological deficits during rehabilitation goes in parallel with a proportional normalization of FC and degree of a given node (Wang et al., 2010b, Rehme et al., 2011, Westlake et al., 2012, Golestani et al., 2013, Wu et al., 2015).
We can thus derive a fourth principle of network alterations after stroke stating that any disruption of interactions of a given brain area is associated with proportional deficits in functions depending on the node properties. These disruptions can be observed already during a resting-state condition without explicit tasks, as well as during task execution. This enforces the concept that neurological deficits do not arise only because of local tissue damage, but are also associated with a loss of neural interactions of areas that are not directly affected by the stroke lesion.
Stroke also seems to impact the topographical characteristics of neural interactions, as quantified with graph theory (see 2.2.4). In particular, it reduces local specialization (as indicated by a reduction of clustering coefficients and local efficacy), integration capacity, and small-world properties of the brain network (Wang et al., 2010b, De Vico Fallani et al., 2013, Duncan et al., 2016, Adhikari et al., 2017, Caliandro et al., 2017).
EEG and MEG studies suggested that stroke effects on network interactions take place at preferential frequency bands. At rest, changes are most visible in the alpha and beta frequency bands (Dubovik et al., 2012, Guggisberg et al., 2015, Wu et al., 2015, Caliandro et al., 2017). Furthermore, several different coupling types seem to be concerned including phase synchronization and AEC(Guggisberg et al., 2015). For instance, Guggisberg et al. (2015) found that reduced alpha-band phase synchronization and reduced beta-band AEC in stroke patients were linearly correlated with neurological deficits. During motor tasks, the most consistent changes have been reported in the beta frequency band (Gerloff et al., 2006, De Vico Fallani et al., 2013).
It should be noted that a variation of FC and the associated behavioural impact is not specific to stroke, as similar observations have been made in other focal and diffuse pathologies and even in healthy subjects showing interindividual variations in performance (e.g., Guggisberg et al., 2008, Wang et al., 2010a, Dubovik et al., 2013, Sadaghiani et al., 2015). Hence, the association between variations in FC and behaviour is a normal phenomenon, which can be amplified by stroke or any other pathology leading to disruptions in network interactions.
3.2.2. Network plasticity
There is increasing evidence that interregional neural interactions are involved in brain plasticity. From animal literature, we know that cortical remapping and axonal sprouting is accompanied by coherent neural oscillations between perilesional areas and surrounding tissue (Carmichael et al., 2002, Frost et al., 2003, Buch et al., 2016a). The study of functional connectivity in human stroke patients provides indirect evidence for similar processes in humans. Correlations between network interactions before therapy and clinical improvement during therapy periods have been observed at various time points after stroke. In particular, nodes associated with deficient neurological functions were found to enhance their overall importance in the brain network (i.e., their node degree) by increasing their functional connectivity with other areas. This was predictive of future clinical improvement (Wang et al., 2010b, Buch et al., 2012, Westlake et al., 2012, De Vico Fallani et al., 2013, Nicolo et al., 2015b). For instance, a greater node degree of perilesional motor area (at rest and during motor tasks), as quantified with high-density EEG, during the first weeks after stroke was associated with greater clinical motor recovery observed in subsequent months (Fig. 5). This increase in overall interactions is therefore remarkably reproducible and observable during tasks and at rest. It seems to occur typically during the first weeks after stroke, after an initial hypoconnectivity in the acute stage.
Figure 5. Network plasticity after stroke.
Perilesional areas can show enhanced beta-band coherence with the rest of the brain during the first weeks after stroke. An example is shown in yellow-red in (a), the lesion is marked in dark grey. Enhancement of coherence between ipsilesional M1 and the rest of the brain was associated with better motor improvement in the subsequent months (b). Coherence between Broca’s area and the rest of the brain was associated with language improvement (c). Modified after (Nicolo et al., 2015b) with permission.
This leads to the fifth principle stating that preserved ipsilesional brain areas can enhance their interactions with the rest of the brain and this might contribute to future clinical recovery.
The mechanisms by which network interactions might contribute to plasticity are not well understood and will need to be clarified in the future. Recent work suggests that synchronous network oscillations may be important for axon myelination which is tightly regulated by neuronglia interactions (Fields et al., 2015). FC could thus help preserve and strengthen newly-formed projections. Furthermore, the creation of new synaptic connections might be associated with a transient increase in synchronous oscillations between the involved brain areas.
EEG studies further suggest that beta oscillation frequencies are preferred for recovery-related neural interactions in the first weeks after stroke (Nicolo et al., 2015b). This might reflect distinct molecular environments after unilateral stroke. Animal models of stroke have shown that gammaaminobutyric acid (GABA) and glutamate are the two main synaptic signalling systems implicated in stroke plasticity. These neurotransmitters also modulate the amplitude and phases of EEG rhythms at specific frequencies (Jensen et al., 2005b, Yamawaki et al., 2008, Kohl et al., 2010, Ronnqvist et al., 2013, Li et al., 2014). Furthermore, the local concentration of GABA at motor nodes was found to be inversely correlated with the magnitude of fMRI FC (Stagg et al., 2014). Hence, we can speculate that plastic changes in network interactions may reflect alterations in neurotransmitter concentrations.
However, these hypotheses are so far supported only by correlational evidence which needs to be confirmed in interventional studies achieving an experimental modulation of network interactions. A first step in this direction has recently been provided by a study in 10 patients with chronic stroke. An enhancement of the FC (node degree) of preserved ipsilesional motor areas with neurofeedback led to a significant increase in motor function. This was not the case in a control condition where patients enhanced FC of a brain area not directly implicated in motor function with neurofeedback (Mottaz et al., 2018). Hence an enhancement of network interactions of specific brain nodes seems to be causally related to reduced motor impairment.
3.2.3. Associations with structural connectivity
Disruptions of FC are, at least in part, mediated by structural damage to connecting fibres. For instance, disruptions of interhemispheric FC are associated with the amount of structural damage to transcallosal fibre tracts as measured with DTI (Chen et al., 2013). Similarly, plastic enhancement of FC depends on structural integrity of white matter. This has been shown in particular for motor function, in which damage of the cortico-spinal tract (CST) was associated with lower FC of cortical motor nodes (Carter et al., 2012, Cunningham et al., 2015, Guggisberg et al., 2017). However, multimodal assessments have suggested that a combination of CST integrity and cortical FC provides superior prediction of motor recovery than structural imaging alone (Burke Quinlan et al., 2015, Volz et al., 2015, Wu et al., 2015, Guggisberg et al., 2017). Hence, FC changes are partly constrained by structural damage to white matter, but variations independent of structure seem to occur and be associated with clinical recovery.
3.3. The influence of the contralesional hemisphere
Ipsilesional M1 (iM1) reorganization plays an important role in post-stroke motor recovery, and is a primary target for rehabilitation therapy (Liepert et al., 1998, Wittenberg et al., 2003, Hummel et al., 2005c, Khedr et al., 2005, Khedr et al., 2010, Buetefisch et al., 2011a, Dancause et al., 2011, Zimerman et al., 2012). Yet, reorganization of the contralesional hemisphere may serve as additional source for recovery and could be targeted with rehabilitation therapy (Chollet et al., 1991, Weiller et al., 1992, Cramer et al., 1997, Cao et al., 1998, Dancause et al., 2011). This has been shown mostly for motor function. In this section we will review data derived from studies of stroke patients using different modalities such as MRI, fMRI, EEG, and TMS to available from animal stroke models to discuss the structural and functional changes in the contralesional hemisphere and in the interaction between the two hemispheres.
Currently the role of the contralesional M1 (cM1) in motor recovery after stroke and its potential as new target for rehabilitation efforts is topic of intense discussions and research efforts in humans and animal stroke models (Hummel et al., 2008, Jones et al., 2013, Dancause et al.,2015). In task-based fMRI studies cM1 (corresponding to ipsilateral M1 with respect to the moving hand) activation is consistently reported when patients move their affected hand (Calautti et al., 2003, Butefisch et al., 2005, Rehme et al., 2012). In cross-sectional studies of patients in the subacute phase after stroke a shift from an initially abnormal bilateral activation of motor area (Chollet et al., 1991, Weiller et al., 1992, Cramer et al., 1997, Cao et al., 1998, Johansen-Berg et al., 2002, Small et al., 2002, Ward et al., 2003, Butefisch et al., 2005, Nair et al., 2007) towards a more normal unilateral activation pattern of ipsilesional motor areas (corresponding to contralateral motor areas with respect to the moving hand) in chronic phase after stroke (Ward et al., 2003) were described. These findings were still present when kinematics of the movements were matched (Ward et al., 2007) and execution of strictly uni-manual movements were confirmed by electromyography (Butefisch et al., 2005). Further, abnormally increased cM1 excitability was demonstrated in multiple studies when explored with the paired pulse TMS technique (Figure 3, SICI, IHI, see above) (Boroojerdi et al., 1996, Shimizu et al., 2002, Butefisch et al., 2003, Murase et al., 2004, Butefisch et al., 2008).
The interpretation of these findings is complicated by the fact that the role of iM1 in the control of uni-manual hand movements of healthy subjects is not well understood. Demand on motor task dependent activity of iM1 is seen (Winstein et al., 1997, Hummel et al., 2003, Seidler et al., 2004, Verstynen et al., 2005, Talelli et al., 2008, Buetefisch et al., 2014b) that tends to increase as a function of age (Talelli et al., 2008, Zimerman et al., 2014) but does not seem to mediate control through uncrossed ipsilateral corticospinal projections (Soteropoulos et al., 2011). In the context of an incomplete understanding of the role of iM1 in motor control, the interpretation of findings pertaining to the role of cM1 in motor recovery after stroke remains controversial (for review see e.g. (Hummel et al., 2008)).
From a structural perspective, there is growing evidence of structural connectivity within the contra-lesional hemisphere being involved in network reorganization. One longitudinal study using diffusion spectrum magnetic resonance imaging could show that intra- and interhemispheric structural connectivity of the contra-lesional motor network showed changes in microstructural integrity, whereas the change in intra-hemispheric motor network correlated strongly with clinical measurements of performance (Granziera et al., 2012). This idea was strengthened by additional magnetization transfer ratio measurement combined with generalized fractional anisotropy showing both axonal and myelin remodelling in these contra-lesional motor pathways (Lin et al., 2015). Also contra-lesional projection fibres seem to be involved in process of reorganization, in which decreases as well as increases of structural integrity was reported in the contra-lesional CST (Schaechter et al., 2009, Borich et al., 2011).
There is also evidence for alterations of neural interactions in the contralesional hemisphere after stroke, both from fMRI and EEG analyses (Park et al., 2011, Dubovik et al., 201 2). One EEG study has observed pathologically enhanced neural coherence of contralesional nodes in patients 3 months after stroke. This hyperconnectivity was correlated with more severe neurological deficits in motor and cognitive functions relying on the concerned nodes, hence mimicking observations with hyperactivity (Dubovik et al., 2012). Similar observations have recently been made with fMRI (Guo et al., 2019).
The factors that influence the extent and outcome of contralesional motor area reorganization are not known. As already indicated for task-based fMRI studies of stroke recovery, time since stroke is one variable that is consistently reported as impacting measure of M1 reorganization and behavior, while the report of the effect of lesion volume on these processes are more inconsistent. Schaechter and Perdue (2008) demonstrated in chronic stroke patients a linear relationship between abnormally increased affected hand movement related cM1 activity and extend of CST damage. Further, the observed differential effect of stroke on cM1 excitability and the relationship between cM1 excitability and IHI (see below for deails) (Butefisch et al., 2008) supports the notion that location of the stroke seems to impact reorganizational processes. A further factor may be age. Most studies of the healthy brain were done in young adults. In older subjects, inhibition of the contralesional motor cortex might lead to an impairment of motor functions and learning (Zimerman et al., 2014) supporting the view that activity in cM1 during unilateral hand movements might have differential functional role in old or neurological patients.
In rodent stroke models, functional and structural reorganizational changes in cM1 are seen (for detailed review (Jones et al., 2013, Dancause et al., 2015)) that depend on the lesion size (Kim et al., 2010). On a synaptic level, reorganizational changes in cM1 include long-lasting down-regulation of GABAA-receptor function (Buchkremer-Ratzmann et al., 1996, Neumann-Haefelin et al., 2000) and up-regulation of NMDA-receptor function (Qu et al., 1998, Witte, 1998), both mechanisms operating in increases of synaptic efficacy such as long-term potentiation (LTP). Structural changes included an increase in neuropil volume (Hsu et al., 2005), use-dependent dendritic growth followed by dendritic pruning, synapse formation and changes in the specific structure of synaptic connections have been described (Jones et al., 1994, Jones et al., 1996, Hsu et al., 2005).
Despite intense research efforts, the influence of the reorganized cM1 on recovery after stroke is currently not known. In the chronic phase of post-stroke recovery in humans, cM1 seems to interfere with motor function of the paretic limb in a subset of patients as decreasing cM1 excitability by cortical stimulation results in improved performance of the paretic limb (Mansur et al., 2005, Fregni et al., 2006, Zimerman et al., 2012). The proposed mechanisms underlying this “maladaptive” response (Cicinelli et al., 1997, Traversa et al., 1998) is thought to include an abnormally increased inhibition of lesioned M1 (ipsilesional M1 (ilM1) by the cM1 (Murase et al., 2004, Duque et al., 2005, Hummel et al., 2005c) (termed “interhemispheric inhibition model” (Murase et al., 2004)). The assessments of effective connectivity of motor networks after stroke with fMRI found excessive inhibition from cM1 to the affected M1 which correlated with worse motor function after stroke (Grefkes et al., 2008). Finally, there is some evidence that inhibitory rTMS protocols applied over the the cM1 can revert this maladaptive influence and improve motor function (Grefkes et al., 2010, Hsu et al., 2012).
In contrast to the notion of a negative influence of the contralesional hemisphere, there is emerging evidence to suggest a potentially supportive role of contralesional motor areas such as dorsal premotor cortex (cPMd) (Johansen-Berg et al., 2002, Lotze et al., 2006, Ward et al., 2007) and cM1 (Butefisch et al., 2003, Butefisch et al., 2005, Gerloff et al., 2006, Lotze et al., 2006, Nair et al., 2007, Butefisch et al., 2008). After infarction of M1 or its corticospinal projections, there is abnormally increased excitatory neural activity and activation in cM1 (Liepert et al., 2000a, Shimizu et al., 2002, Butefisch et al., 2003, Lotze et al., 2006, Butefisch et al., 2008) that correlates with favorable motor recovery (Butefisch et al., 2003, Butefisch et al., 2005, Lotze et al., 2006, Butefisch et al., 2008). Decreasing cM1 (Lotze et al., 2006) or cPMd (Johansen-Berg et al., 2002, Lotze et al., 2006) activity in these patients may result in deterioration of paretic limb performance. Similarly, inhibiting the contralesional hemisphere in rats that recovered from large ischemic infarcts generates more behavioral deficits of the impaired forelimb in comparison to control animals (Biernaskie et al., 2005).
Very few studies have examined the relationship between increased cM1 excitability and IHI to address the question, whether an abnormally increased cM1 excitability results in excessive inhibition of the iM1 (Butefisch et al., 2008). In subacute stroke patients with infarctions involving M1 or its corticospinal projections, cM1 SICI was abnormally low indicating increased cM1 excitability. Importantly, SICI was normal at low CS intensities but abnormally decreased at higher CS intensities indicating as shift towards an increase of excitatory activity in these neuronal circuits (Butefisch et al., 2008). Further, abnormally increased cM1 excitability occurred in the presence of both, normal and abnormally reduced resting IHI from iM1 on cM1 and resting IHI from cM1 on iM1 was normal (Butefisch et al., 2008). These findings suggest that the increased cM1 excitability can only partially be explained by loss of IHI from the lesioned on non-lesioned hemisphere. As decreased SICI of cM1 did not result in excessive IHI from the non-lesioned on lesioned hemisphere with subsequent suppression of iM1 excitability and all patients showed excellent recovery of motor function, decreased SICI of cM1 may represent an adaptive process supporting recovery (Witte, 1998, Nudo, 1999).
More recent findings have also cast doubts on the validity of the interhemispheric inhibition model for unselected groups of stroke patients. TMS studies and meta-analyses in larger stroke populations have not been able to reproduce an over-excitability of cM1 (Stinear et al., 2015, McDonnell et al., 2017). Reducing inter-hemispheric inhibition in stroke patients later than 2 weeks after stroke did not lead to improvements in motor recovery in recent studies (Volz et al., 2017, Nicolo et al., 2018b). Hence, many of the observations underlying the interhemispheric inhibition model could not be reproduced in subsequent trials.
Taken together, there is evidence from human and animal studies that activity in cM1 will impact motor function of the paretic limb but this may differ for subgroups of patients. The precise factors that specifcally influence the role of cM1 in the recovery process are not known. Identifying subgroups, which share specfic functional roles of areas of the contralesional hemisphere may help move forward towards personalized treatment strategies to improve the outcome post stroke.
Variants of the interhemispheric inhibition model have also been proposed for recovery from aphasia. Some models of aphasia recovery posit that an overactivity of right language nodes may be deleterious for recovery (reviewed in: (Hamilton et al., 2011)). Some evidence for this comes from studies in healthy participants suggesting that activation of a bilateral network including right temporal and right inferior frontal areas leads to less efficient learning than exclusive activation of left areas (Wong et al., 2007, Mei et al., 2008). Furthermore, activity in the left hemisphere seems to be more critical for naming performance than activity in the right hemisphere in stroke patients (Cao et al., 1999, Winhuisen et al., 2005). However, studies using NIBS to inhibit right language nodes in healthy volonteers did not observe improvements in language performance or verbal learning (Mottaghy et al., 1999, Nicolo et al., 2016).
Interestingly, the best evidence for a pathological role of contralesional hyperactivity comes from studies on spatial neglect (Muri et al., 2013). Decreased activity of the right posterior parietal cortex, as measured with functional MRI is accompanied by a significant increase of activity in the left posterior parietal cortex (Corbetta et al., 2005). Furthermore, dual-pulse TMS studies have suggested that interactions between the left posterior parietal cortex and the left (contralesional) motor cortex are enhanced in neglect patients (Koch et al., 2008b). Most importantly, several trials have confirmed that inhibitory rTMS applied over the left (contralesional) posterior parietal cortex reduces signs of neglect as compared to a sham stimulation group, both in neuropsychological tests and everyday living (Nyffeler et al., 2009, Cazzoli et al., 2012, Koch et al., 2012, Hopfner et al., 2015).
Finally, there is evidence that pathways in the contralesional hemisphere contribute to cognitive function after stroke. Structural readouts of the contralesional arcuate fasciculus was related to language performance (Dacosta-Aguayo et al., 2014) and recovery of aphasia (Forkel et al., 2014), the ventral and dorsal attention networks to spatial attention (Umarova et al., 2014).
In sum, these observations lead to a sixth priniciple of network plasticity. The contralesional hemisphere shows prominent structural and functional changes after stroke, but their precise role for recovery is insufficiently understood. Depending on factors such as lesion configuration and time after stroke, the contralesional hemisphere can play a supportive role for recovery, or it may participate in maladaptive plasticity.
4. Therapeutic implications
4.1. Modulation of structural connectivity, impact of structural connectivity on therapeutic interventions based on brain stimulation.
Despite the improvements of acute therapy in stroke with area-wide coverage with stroke units, thrombolysis and mechanical recanalization, there is still a large amount of patients suffering from long-lasting deficits after stroke (> 75%) in multiple domains (motor function, language/speech, cognition) impacting seriously the patients quality of life, independence, social and professional integration. Thus, there is a strong need of further improvement in neurorehabilitation treatment strategies. The prediction of the natural degree of recovery is an evolving field using functional scores as well as multimodal imaging. Markers like initial impairment, imaging of the CST, and TMS-evoked potentials have been suggested to be suitable for prediction of motor recovery (Stinear et al., 2012, 2017). The predictive value of ipsilesional M1 TMS evoked MEP is high with respect to its sensitivity and specificity when obtained within the first week after stroke. The Odds ratios varied between 5.49 and 13.50 for functional recovery in the presence of an MEP (Hendricks et al., 2002). An increase of the acutely (within 3 days of stroke) reduced MEP amplitudes at 1 month follow up (Binkofski et al., 1996) and greater MEP amplitude in the chronic stage (> 6 month since stroke) were positively correlated with recovered hand motor function (Buetefisch et al., 2018). While there are a few patients with excellent recovery of motor function despite an absent MEP or late MEP reappearance (Traversa et al., 1997), TMS measures of MT and SRC curves are sensitive and specific measures of M1 corticospinal circuitry and can be used in cross sectional or longitudinal studies of motor recovery after stroke.
Still, there remains a large unexplained variability in recovery, especially in patients with a moderate-severe initial impairment level. Predictive markers determined from e.g., imaging data being associated with treatment responses could serve further in selecting highly susceptible patients to certain therapies in the acute, subacute or chronic phase of recovery.
Taken together structural imaging especially when evaluated longitudinally in larger, heterogeneous cohorts of stroke patients will pave the way to use this technique for prediction of pattern of recovery, degree of recovery, magnitude of treatment effects and thus selection of individualized treatment strategies to maximize the effect of neuro-rehabilitative treatments. Bringing this information together with data form functional imaging and cognitive, functional abilities might even be more powerful to define phenotypes of patients at an early stage to provide the optimal treatment at each stage of the recovery process, especially promising for techniques like brain stimulation with a large heterogeneity in the treatment response.
In addition, white matter tracts, which are crucial for neurological function (see section 3.1.1) are becoming a target for therapy. There is first evidence that NIBS might be able to enhance integrity of white matter tracts (Kim et al., 2010, Zheng et al., 2015, Guo et al., 2016). For instance, tDCS reduced neuronal axon degradation at the internal capsule in rats (Kim et al., 2010). In human stroke patients, ipsilesional high-frequency rTMS over motor cortex improved integrity of the cortico-spinal tract, as reflected by higher fractional anisotropy (FA) (Guo et al.,2016). Similarly, cathodal tDCS over the contralesional M1 reduced secondary white-matter degradation of patients with severe motor impairment (Nicolo et al., 2018a).
4.2. Modulation of ipsilesional network interactions
The observation that network interactions are correlated with current and future neurological function directly leads to the question whether their modulation through therapy might be feasible and clinically useful. We will first consider traditional behavioural treatment approaches and then explore newer concepts.
It is important to note that current therapy approaches with intensive exercise already are associated, among other neural effects, with concomitant enhancements of FC and EC (Rehme et al., 2011, Westlake et al., 2012, Golestani et al., 2013, Wu et al., 2015). The causal relevance of network effects of traditional therapies is currently unknown. Nevertheless, given the limited efficacy of exercise in some patients, there is an interest in new treatment approaches which might further enhance network interactions and clinical recovery.
Several reports have demonstrated that non-invasive cortical stimulation can enhance motor cortical excitability, functional reorganization, and the beneficial effects of motor training on performance in the healthy brain (Muellbacher et al., 2000, Bütefisch et al., 2004, Hummel et al., 2005a, 2005b, Khedr et al., 2005, Reis et al., 2009, Hummel et al., 2010, Buetefisch et al., 2011a, Dancause et al., 2011, Zimerman et al., 2013, Buetefisch et al., 2014a, Zimerman et al., 2014) and stroke affected brain (Hummel et al., 2005a, 2008, Khedr et al., 2010, Buetefisch et al., 2011a, Zimerman et al., 2012). M1 in intact brain or M1 in the injured brain are frequent targets of these interventional approaches.
Studies in healthy subjects as well as stroke patients have demonstrated that non-invasive brain stimulation (NIBS) can modulate clinically relevant patterns of FC and EC. The two most frequently used stimulation methods, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) can influence interhemispheric FC between homologous motor areas (Strens et al., 2002, Amadi et al., 2014), EC between motor nodes (Grefkes et al., 2010, Volz et al., 2016), and node degree of parietal and motor nodes (Rizk et al., 2013, Nicolo et al., 2018b). Importantly, network modulations induced by rTMS and tDCS were correlated with proportional clinical improvements in functions depending on the stimulated node. It remains currently insufficiently understood how the stimulation modality and parameters influence the network effects. Generally, inhibitory protocols tend to reduce the overall FC of the stimulated node (Grefkes et al., 2010, Rizk et al., 2013), while excitatory protocols tend to increase them (Volz et al., 2016), but see next paragraph. Network imaging can be used to explore mechanisms underlying the clinical effects of brain stimulation and to identify optimal setups for different patient populations.
One of the main difficulties with NIBS consists in an individually variable behavioural response, which limits the overall effect size. One main reason for this variability is that the expected inhibition or excitation does in fact not occur in all patients and can be even be paradoxically inverted (Hamada et al., 2013, Wiethoff et al., 2014, Hordacre et al., 2015, Li et al., 2015, Nicolo et al., 2015a, Vallence et al., 2015). This applies also to changes in FC, such that disruptions of FC can occur instead of the expected enhancements or vice versa (Rizk et al., 2013, Nicolo et al.,2016). Imaging of network interactions may help predict which subject and patient will show the expected canonical vs. a paradoxical response. Several studies in healthy subjects have demonstrated that the observed response pattern depends on the intrinsic network state of the subject at the time of stimulation. In the case of inhibitory rTMS, studies targeting the right parietal area or the right Broca homogue showed that high node degree at the stimulated area before stimulation was associated with the expected reduction of local activation and FC, and with a canonical behavioural effect on naming and visuospatial exploration. Conversely, patients with low pre-stimulation node degree showed no or even the opposite effect (Rizk et al., 2013, Nicolo et al., 2016). In the case of excitatory rTMS and anodal tDCS, the expected excitatory effect could be observed preferentially in patients with high pre-stimulation FC and EC of motor nodes (Cardenas-Morales et al., 2014, Hordacre et al., 2017). In sum, only nodes with large prestimulation interactions show the expected response to NIBS, while no or even a paradoxical response can occur in nodes with low connectivity. It remains to be demonstrated whether these findings also apply to stroke patients.
A second promising treatment approach for modulating FC and EC is based on the technology of brain-computer interfaces (BCI) which enables the monitoring of brain activity and the generation of a real-time output about specific changes in activity patterns. The recorded subject receives a feedback about the neural activity associated his/her efforts and can thus learn to voluntarily modulate brain function (Kamiya, 1969). This has been shown in particular for activity in the sensorimotor cortex in alpha and beta frequencies (~8-30 Hz), which is suppressed by real or imagined movements (Arroyo et al., 1993, Pfurtscheller et al., 2006). Neurofeedback training of a suppression of this rhythm is used with some success for motor rehabilitation (Buch et al., 2008, Ramos-Murguialday et al., 2013). Neurofeedback training of sensorimotor rhythms improves motor cortex FC as a marker of recovery (Várkuti et al., 2013, Sugata et al., 2014, Vukelic et al., 2015, Biasiucci et al., 2018). More recently, it has been shown that healthy subjects and stroke patients can learn to voluntarily modulate specific patterns of FC (Sacchet et al., 2012, Koush et al., 2013, Liew et al., 2016). In particular, neurofeedback training of the node degree at the ipsilesional primary motor cortex was associated with improved motor performance of stroke patients (Mottaz et al., 2015, 2018).
Sensory stimulations in the tactile (Freyer et al., 2012) or auditory domains (Solca et al., 2016) have been reported to modulate FC in healthy volunteers. Recent evidence suggests that this might also be the case in stroke patients and be associated with improved recovery (Lai et al., 2016, Hakon et al., 2018, Pan et al., 2018, Sharififar et al., 2018)
4.3. Modulation of contralesional influences
As discussed in the previous sections, the extent to which cM1 contributes to motor recovery after stroke is not known but many currently employed rTMS and tDCS protocols are designed with the assumption that following stroke, ipsilesional M1 is hypoactive while cM1 is hyperactive and should be inhibited (Hummel et al., 2008, Dancause et al., 2011). Accordingly, low frequency rTMS (Takeuchi et al., 2005, Fregni et al., 2006, Dafotakis et al., 2008, Nowak et al., 2008, Takeuchi et al., 2009) or cathodal stimulation (Hesse et al., 2007, Nair et al., 2011, Fusco et al., 2014) of cM1 has been used to inhibit its hyperactivity while excitatory higher frequency rTMS (Kim et al., 2006, Takeuchi et al., 2009), task- locked rTMS (Buetefisch et al., 2010) or anodal stimulation (Hummel et al., 2005a, Hesse et al., 2007, Celnik et al., 2009, Geroin et al., 2011) have been used to increase ipsilesional hypoactive M1.
In healthy subjects 1 Hz rTMS applied to M1 of one hemisphere results in increased corticomotor excitability in the opposite M1 (Plewnia et al., 2003, Schambra et al., 2003), and improved performance in the corresponding hand (Kobayashi et al., 2004, Buetefisch et al., 2011b) depending on the level of motor demand (Buetefisch et al., 2011b).
Meta- analyses on the effectiveness of rTMS or tDCS in stroke rehabilitation therapy don’t agree on the available evidence to either support or reject it (Adeyemo et al., 2012, Hsu et al., 2012, Elsner et al., 2013, Hao et al., 2013, Kang et al., 2016). Specifically, Hsu et al included 18 trials of rTMS treatment in motor rehabilitation of stroke and reported a significant effect size of 0.55 for motor outcome (95% CI, 0.37– 0.72) (Hsu et al., 2012). Additional subgroup analysis revealed a tendency for superior effects in patients with subcortical stroke using low frequency rTMS of cM1. In contrast, the review by Hao et al of 19 randomized sham or no treatment controlled rTMS treatment trials for motor rehabilitation after stoke revealed no significant effect on clinical motor outcome measures (Hao et al., 2013). Elsner et al. reviewed the effects of tDCS on improvement of ADL (Barthel index) in stroke rehabilitation treatment (Elsner et al., 2013). A total of 6 (326 patients) randomised controlled trials were included (including the results of the first phase of cross- over studies) for analysis of this main outcome measure. They report no significant effect immediately after therapy and a modest effect at follow up (median difference in the Barthel Index of 11.13 points (95% CI 2.89 -19.37). In the most recent meta- analysis of 17 tDCS trials in motor rehabilitation after stroke a positive effect of tDCS on retention of motor training related improvements were reported (Kang et al., 2016). While the results seem encouraging, the lack of specificity for the timing of the tDCS treatment in relation to the motor training or the montage of the stimulating electrodes are disturbing.
NIBS has also been used to inhibit the contralesional hemisphere to improve spatial neglect. Inhibitory rTMS applied over the contralesional posterior parietal cortex reliably reduced neglect in different studies with significant and durable improvements also in activities of daily living (Cazzoli et al., 2012, Koch et al., 2012, Hopfner et al., 2015). Current evidence therefore suggests that neglect reduction is the most promising application of contralesional inhibition, but large scale studies are still lacking.
A meta-analysis studying the effects of inhibitory NIBS over right-hemispheric homologous language areas concluded on a moderate beneficial effect on naming performance, but the number of treated patients is still small (Otal et al., 2015).
Additional information is necessary to understand which patients benefit from the different available techniques. One approach towards addressing the gap in our knowledge on the role of the contralesional hemisphere could consist of undertaking high quality, large multi-center clinical trials which is the conclusion that authors of these recent meta- analysis arrived at. This would allow the identification of subgroups that are particular responsive to the treatment. However, a more mechanistic approach where an effective motor training is combined with rTMS and tDCS protocols that are prescribed according to their known effects on the stimulated neuropil and the characteristics of the patients with respect to the location of the lesion, time since stroke, individual functional and structural connectivity, and clinical deficit may provide more information towards improved understanding to specific effects of the prescribed intervention.
5. Conclusions
Techniques for non-invasive assessment of brain networks in stroke patients have opened new opportunities and perspectives for the understanding of the consequences of stroke and its impact on recovery. In particular, they have enabled a systems neuroscience approach revealing the global repercussions of stroke and recovery on brain networks. Yet, they require specific care to avoid confounds. The consequences of the new network perspective start to become visible in interventional strategies and clinical care. New treatments have emerged, such as non-invasive brain stimulation or neurofeedback, which directly target network consequences of stroke. Pioneering studies with these new approaches have had moderate success. More recent developments of network imaging allow investigating the neural origins of variability in response to treatment and to provide models for selecting optimal treatment strategies for individual patients based not only on their clinical evaluation, but also on their individual network state. For instance, in the case of non-invasive brain stimulation, this leads to new clinical trials, in which the stimulation effect is tested in specific subgroups of patients. Stratification of patients can be based on network parameters such as structural lesion load to specific tracts, structural connectivity and dysconnectivty, or network states of functional neural interactions, e.g. intrahemispheric or interhemispheric.
Highlights.
Assessment of network consequences of stroke opens new perspectives, but beware of pitfalls.
Insights into network plasticity after stroke have suggested novel targets for therapy.
Network-states may help identify individualized treatment approaches for patients.
Acknowledgments
This work was supported by the Swiss National Science Foundation (320030-129679 and CRSII5-170985) to AGG; the Defitech Foundation (Morges, Switzerland) to FCH, the Wyss Foundation (WCP024A and WCP024B, Wyss Center for Bio and Neuroengineering, Geneve, Switzerland) to FCH, the Strategic Focal Area “Personalized Health and Related Technologies (PHRT)” of the ETH Domain (2017-205) to FCH, and National Institutes of Neurological Diseases and Stroke and National Institutes of Child Development and Health at the National Institutes of Health, Bethesda, MD, USA (R21HD067906, R01NS090677) to CB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemo BO, Simis M, Macea DD, Fregni F. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Front Psychiatry. 2012;3:88. doi: 10.3389/fpsyt.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari MH, Hacker CD, Siegel JS, Griffa A, Hagmann P, Deco G, et al. Decreased integration and information capacity in stroke measured by whole brain models of resting state activity. Brain. 2017;140:1068–85. doi: 10.1093/brain/awx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adluru G, Gur Y, Anderson JS, Richards LG, Adluru N, DiBella EV. Assessment of white matter microstructure in stroke patients using NODDI. Conf Proc IEEE Eng Med Biol Soc 2014;2014:742–5. doi: 10.1109/EMBC.2014.6943697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sair HI, Pillai JJ. The Resting-State Functional Magnetic Resonance Imaging Regional Homogeneity Metrics-Kendall's Coefficient of Concordance-Regional Homogeneity and Coherence-Regional Homogeneity-Are Valid Indicators of Tumor-Related Neurovascular Uncoupling. Brain Connect. 2017;7:228–35. doi: 10.1089/brain.2016.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Dyrby TB, Nilsson M, Zhang H. Imaging brain microstructure with diffusion MRI: practicality and applications. NMR Biomed. 2017:1–26. doi: 10.1002/nbm.3841. [DOI] [PubMed] [Google Scholar]
- Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJ, et al. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage. 2010;52:1374–89. doi: 10.1016/j.neuroimage.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. Modeling the impact of lesions in the human brain. PLoS Comput Biol. 2009;5:e1000408. doi: 10.1371/journal.pcbi.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi U, Ilie A, Johansen-Berg H, Stagg CJ. Polarity-specific effects of motor transcranial direct current stimulation on fMRI resting state networks. Neuroimage. 2014;88:155–61. doi: 10.1016/j.neuroimage.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol. 1990;77:390–401. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Antal A, Alekseichuk I, Bikson M, Brockmoller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. 2017;128:1774–809. doi: 10.1016/j.clinph.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1:97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Arroyo S, Lesser RP, Gordon B, Uematsu S, Jackson D, Webber R. Functional significance of the mu rhythm of human cortex: an electrophysiologic study with subdural electrodes. Electroencephalogr Clin Neurophysiol. 1993;87:76–87. doi: 10.1016/0013-4694(93)90114-b. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage. 2005;27:48–58. doi: 10.1016/j.neuroimage.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59:1347–54. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Freidlin RZ, Rohde GK, Basser PJ. New modeling and experimental framework to characterize hindered and restricted water diffusion in brain white matter. Magn Reson Med. 2004;52:965–78. doi: 10.1002/mrm.20274. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Johansen-Berg H, Thiebaut de Schotten M. The role of diffusion MRI in neuroscience. NMR Biomed. 2017:1–16. doi: 10.1002/nbm.3762. [DOI] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, Babiloni C, Carducci F, Basilisco A, et al. Assessing cortical functional connectivity by linear inverse estimation and directed transfer function: simulations and application to real data. Clin Neurophysiol. 2005;116:920–32. [DOI] [PubMed] [Google Scholar]
- Bagnato S, Curra A, Modugno N, Gilio F, Quartarone A, Rizzo V, et al. One-hertz subthreshold rTMS increases the threshold for evoking inhibition in the human motor cortex. Exp Brain Res. 2005;160:368–74. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Hamilton RH. Drawing on the right brain for aphasia recovery. Neurology 2016;86:1566–7. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: Theory, experimental design and data analysis - A technical review. NMR Biomed. 2002;15:456–67. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. [DOI] [PubMed] [Google Scholar]
- Baumer T, Schippling S, Kroeger J, Zittel S, Koch G, Thomalla G, et al. Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest--a bifocal TMS study. Clin Neurophysiol. 2009;120:1724–31. doi: 10.1016/j.clinph.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Beaulieu C The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN Jr., McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–9. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–50. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Benjamini D, Komlosh ME, Holtzclaw LA, Nevo U, Basser PJ. White matter microstructure from nonparametric axon diameter distribution mapping. Neuroimage. 2016;135:333–44. doi: 10.1016/j.neuroimage.2016.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9:2421. doi: 10.1038/s41467-018-04673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–99. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metbolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1996;39:460–70. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. [DOI] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One. 2011;6:e20678–e. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Beeson PM, DeMarco AT, Rapcsak SZ. Variability in blood oxygen level dependent (BOLD) signal in patients with stroke-induced and primary progressive aphasia. Neuroimage Clin. 2015;8:87–94. doi: 10.1016/j.nicl.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of Anomia Treatment in Aphasia Is Associated With Preserved Architecture of Global and Left Temporal Lobe Structural Networks. Neurorehabil Neural Repair. 2016;30:266–79. doi: 10.1177/1545968315593808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke. 2014;45:988–93. doi: 10.1161/STROKEAHA.113.004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich MR, Randhawa BK, Wadden KP, Boyd LA. Contralesional corticospinal tract integrity is predictive of motor function after stroke. Stroke. 2011;42 [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–70. [DOI] [PubMed] [Google Scholar]
- Broca P Remarks on the Seat of the Faculty of Articulated Language, Following an Observation of Aphemia (Loss of Speech). Bulletin de la Société Anatomique. 1861;6:330–57. [Google Scholar]
- Brodbeck V, Spinelli L, Lascano AM, Wissmeier M, Vargas MI, Vulliemoz S, et al. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain. 2011;134:2887–97. doi: 10.1093/brain/awr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Hale JR, Zumer JM, Stevenson CM, Francis ST, Barnes GR, et al. Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage. 2011a;56:1082–104. doi: 10.1016/j.neuroimage.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, et al. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci U S A. 2011b;108:16783–8. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns A, Eckhorn R, Jokeit H, Ebner A. Amplitude envelope correlation detects coupling among incoherent brain signals. Neuroreport. 2000;11:1509–14. [PubMed] [Google Scholar]
- Buch E, Weber C, Cohen LG, Braun C, Dimyan MA, Ard T, et al. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910–7. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Liew SL, Cohen LG. Plasticity of Sensorimotor Networks: Multiple Overlapping Mechanisms. Neuroscientist. 2016a. doi: 10.1177/1073858416638641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Modir Shanechi A, Fourkas AD, Weber C, Birbaumer N, Cohen LG. Parietofrontal integrity determines neural modulation associated with grasping imagery after stroke. Brain. 2012;135:596–614. doi: 10.1093/brain/awr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Rizk S, Nicolo P, Cohen LG, Schnider A, Guggisberg AG. Predicting motor improvement after stroke with clinical assessment and diffusion tensor imaging. Neurology. 2016b;86:1924–5. doi: 10.1212/WNL.0000000000002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin Neurophysiol. 2017;128:589–603. doi: 10.1016/j.clinph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain. Stroke. 1996;27:1105–9; discussion 9-11. [DOI] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, Witte OW. Extended brain disinhibition following small photothrombotic lesions in rat frontal cortex. Neuroreport. 1997;8:519–22. [DOI] [PubMed] [Google Scholar]
- Buetefisch C, Heger R, Schicks W, Seitz R, Netz J. Hebbian-type stimulation during robot-assisted training in patients with stroke. Neurorehabil Neural Repair. 2011a;25:645–55. doi: 10.1177/1545968311402507. [DOI] [PubMed] [Google Scholar]
- Buetefisch CM, Hines B, Shuster L, Pergami P, Mathes A. Motor demand-dependent improvement in accuracy following low-frequency transcranial magnetic stimulation of left motor cortex. J Neurophysiol. 2011b; 106:1614–21. doi: 10.1152/jn.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetefisch CM, Howard C, Korb C, Haut MW, Shuster L, Pergami P, et al. Conditions for enhancing the encoding of an elementary motor memory by rTMS. Clin Neurophysiol. 2014a. doi: 10.1016/j.clinph.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetefisch CM, Howard C, Pergami P, Korb C, Hobbs G. Hebbian-type stimulation modulates training induced motor cortex plasticity Society of Neuroscience. San Diego: 2010. [Google Scholar]
- Buetefisch CM, Pirog Revill K, Haut MW, Kowalski GM, Wischnewski M, Pifer M, et al. Abnormally reduced primary motor cortex output is related to impaired hand function in chronic stroke. J Neurophysiol. 2018. doi: 10.1152/jn.00715.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetefisch CM, Revill KP, Shuster L, Hines B, Parsons M. Motor demand-dependent activation of ipsilateral motor cortex. J Neurophysiol. 2014b;112:999–1009. doi: 10.1152/jn.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–49. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Kandala S, Nolan D, Laumann TO, Power JD, Adeyemo B, et al. Evaluation of Denoising Strategies to Address Motion-Correlated Artifacts in Resting-State Functional Magnetic Resonance Imaging Data from the Human Connectome Project. Brain Connect. 2016;6:669–80. doi: 10.1089/brain.2016.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77:132–45. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–6. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Kleiser R, Korber B, Muller K, Wittsack HJ, Homberg V, et al. Recruitment of contralesional motor cortex in stroke patients with recovery of hand function. Neurology. 2005;64:1067–9. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–81. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22:4–21. [DOI] [PubMed] [Google Scholar]
- Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol. 2015;78:848–59. doi: 10.1002/ana.24472. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–66. [DOI] [PubMed] [Google Scholar]
- Caliandro P, Vecchio F, Miraglia F, Reale G, Della Marca G, La Torre G, et al. Small-World Characteristics of Cortical Connectivity Changes in Acute Stroke. Neurorehabil Neural Repair. 2017;31:81–94. doi: 10.1177/1545968316662525. [DOI] [PubMed] [Google Scholar]
- Callaghan PT. NMR imaging, NMR diffraction and applications of pulsed gradient spin echoes in porous media. Magn Reson Imaging. 1996;14:701–9. doi: 10.1016/S0730-725X(96)00152-X. [DOI] [PubMed] [Google Scholar]
- Cao Y, D'Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–22. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KM. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30:2331–40. [DOI] [PubMed] [Google Scholar]
- Capaday C Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods. 1997;74:201–18. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–39. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Cardenas-Morales L, Volz LJ, Michely J, Rehme AK, Pool EM, Nettekoven C, et al. Network connectivity and individual responses to brain stimulation in the human motor system. Cereb Cortex. 2014;24:1697–707. doi: 10.1093/cercor/bht023. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–70. doi: 20026605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137:2408–22. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012;26:7–19. doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–39. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Caverzasi E, Papinutto N, Castellano A, Zhu AH, Scifo P, Riva M, et al. Neurite Orientation Dispersion and Density Imaging Color Maps to Characterize Brain Diffusion in Neurologic Disorders. J Neuroimaging. 2016;26:494–8. doi: 10.1111/jon.12359. [DOI] [PubMed] [Google Scholar]
- Cazzoli D, Muri RM, Schumacher R, von Arx S, Chaves S, Gutbrod K, et al. Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain. 2012;135:3426–39. doi: 10.1093/brain/aws182. [DOI] [PubMed] [Google Scholar]
- Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40:1764–71. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front Neurol. 2013;4:178. doi: 10.3389/fneur.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–403. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–81. [DOI] [PubMed] [Google Scholar]
- Cheng B, Schulz R, Bonstrup M, Hummel FC, Sedlacik J, Fiehler J, et al. Structural plasticity of remote cortical brain regions is determined by connectivity to the primary lesion in subcortical stroke. J Cereb Blood Flow Metab. 2015;35:1507–14. doi: 10.1038/jcbfm.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. [DOI] [PubMed] [Google Scholar]
- Chu CY, Huang JP, Sun CY, Zhang YL, Liu WY, Zhu YM. Estimating intravoxel fiber architecture using constrained compressed sensing combined with multitensor adaptive smoothing. Int J Imaging Syst Technol. 2015;25:285–96. doi: 10.1002/ima.22148. [DOI] [Google Scholar]
- Chung SW, Rogasch NC, Hoy KE, Fitzgerald PB. Measuring Brain Stimulation Induced Changes in Cortical Properties Using TMS-EEG. Brain Stimul. 2015;8:1010–20. doi: 10.1016/j.brs.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2-4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–50. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–10. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85:927–41. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–27. [DOI] [PubMed] [Google Scholar]
- Crofts JJ, Higham DJ, Bosnell R, Jbabdi S, Matthews PM, Behrens TEJ, et al. Network analysis detects changes in the contralesional hemisphere following stroke. NeuroImage. 2011;54:161–9. doi: 10.1016/j.neuroimage.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham DA, Machado A, Janini D, Varnerin N, Bonnett C, Yue G, et al. Assessment of inter-hemispheric imbalance using imaging and noninvasive brain stimulation in patients with chronic stroke. Arch Phys Med Rehabil. 2015;96:S94–103. doi: 10.1016/j.apmr.2014.07.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dacosta-Aguayo R, Grana M, Fernandez-Andujar M, Lopez-Cancio E, Caceres C, Bargallo N, et al. Structural integrity of the contralesional hemisphere predicts cognitive impairment in ischemic stroke at three months. PLoS One. 2014;9:e86119. doi: 10.1371/journal.pone.0086119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci A, Dal Palu A, Descoteaux M, Thiran JP. Microstructure Informed Tractography: Pitfalls and Open Challenges. Front Neurosci. 2016;10:247. doi: 10.3389/fnins.2016.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci A, Dal Palu A, Lemkaddem A, Thiran JP. COMMIT: Convex optimization modeling for microstructure informed tractography. IEEE Trans Med Imaging. 2015;34:246–57. doi: 10.1109/TMI.2014.2352414. [DOI] [PubMed] [Google Scholar]
- Dafotakis M, Grefkes C, Eickhoff SB, Karbe H, Fink GR, Nowak DA. Effects of rTMS on grip force control following subcortical stroke. Exp Neurol. 2008;211:407–12. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–79. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–95. doi: 10.1016/b978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Touvykine B, Mansoori BK. Inhibition of the contralesional hemisphere after stroke: reviewing a few of the building blocks with a focus on animal models. Prog Brain Res. 2015;218:361–87. doi: 10.1016/bs.pbr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Musha T, Cichocki A. A comparative study of synchrony measures for the early diagnosis of Alzheimer's disease based on EEG. Neuroimage. 2010;49:668–93. [DOI] [PubMed] [Google Scholar]
- Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–7. doi: 10.1016/j.cortex.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Thompson PD, Dick JP, Nakashima K, Marsden CD. Different sites of action of electrical and magnetic stimulation of the human brain. Neurosci Lett. 1987;75:101–6. [DOI] [PubMed] [Google Scholar]
- de Haan B, Rorden C, Karnath HO. Abnormal perilesional BOLD signal is not correlated with stroke patients' behavior. Front Hum Neurosci. 2013;7:669. doi: 10.3389/fnhum.2013.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, et al. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci U S A. 2010;107:6040–5. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, et al. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron. 2012;74:753–64. doi: 10.1016/j.neuron.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vico Fallani F, Pichiorri F, Morone G, Molinari M, Babiloni F, Cincotti F, et al. Multiscale topological properties of functional brain networks during motor imagery after stroke. Neuroimage. 2013;83:438–49. doi: 10.1016/j.neuroimage.2013.06.039. [DOI] [PubMed] [Google Scholar]
- De Vico Fallani F, Richiardi J, Chavez M, Achard S. Graph analysis of functional brain networks: practical issues in translational neuroscience. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua F, Bodi I, Slater D, Catani M, Modo M. MR diffusion histology and micro-tractography reveal mesoscale features of the human cerebellum. Cerebellum. 2013;12:923–31. doi: 10.1007/s12311-013-0503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–38. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–66. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, et al. Low-frequency repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2008;586:4481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–71. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–8. [DOI] [PubMed] [Google Scholar]
- Dobkin B The economic impact of stroke. Neurology. 1995;45:S6–9. [PubMed] [Google Scholar]
- Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–5. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovik S, Bouzerda-Wahlen A, Nahum L, Gold G, Schnider A, Guggisberg AG. Adaptive reorganization of cortical networks in Alzheimer's disease. Clin Neurophysiol. 2013;124:35–43. doi: 10.1016/j.clinph.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Dubovik S, Pignat JM, Ptak R, Aboulafia T, Allet L, Gillabert N, et al. The behavioral significance of coherent resting-state oscillations after stroke. Neuroimage. 2012;61:249–57. doi: 10.1016/j.neuroimage.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology. 2015;84:1685–92. doi: 10.1212/WNL.0000000000001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan ES, Small SL. Increased Modularity of Resting State Networks Supports Improved Narrative Production in Aphasia Recovery. Brain Connect. 2016;6:524–9. doi: 10.1089/brain.2016.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–6. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013;11:CD009645. doi: 10.1002/14651858.CD009645.pub2. [DOI] [PubMed] [Google Scholar]
- Engel AK, Gerloff C, Hilgetag CC, Nolte G. Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron. 2013;80:867–86. doi: 10.1016/j.neuron.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, et al. Pure alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex. 2008;44:962–74. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–24. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78:860–70. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–7. [DOI] [PubMed] [Google Scholar]
- Fields RD, Woo DH, Basser PJ. Glial Regulation of the Neuronal Connectome through Local and Long-Distant Communication. Neuron. 2015;86:374–86. doi: 10.1016/j.neuron.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, De Schotten MT, Dell'Acqua F, Kalra L, Murphy DGM, Williams SCR, et al. Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain. 2014;137:2027–39. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnehun S, Levy R, Rosso C, Urbanski M, et al. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience. 2018;7:1–17. doi: 10.1093/gigascience/giy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med. 2018;379:2237–45. doi: 10.1056/NEJMra1706158. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–22. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Freundlieb N, Philipp S, Drabik A, Gerloff C, Forkert ND, Hummel FC. Ipsilesional motor area size correlates with functional recovery after stroke: a 6-month follow-up longitudinal TMS motor mapping study. Restor Neurol Neurosci. 2015;33:221–31. doi: 10.3233/RNN-140454. [DOI] [PubMed] [Google Scholar]
- Freyer F, Reinacher M, Nolte G, Dinse HR, Ritter P. Repetitive tactile stimulation changes resting-state functional connectivity-implications for treatment of sensorimotor decline. Front Hum Neurosci. 2012;6:144. doi: 10.3389/fnhum.2012.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–80. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–14. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–62. [DOI] [PubMed] [Google Scholar]
- Fusco A, Assenza F, Iosa M, Izzo S, Altavilla R, Paolucci S, et al. The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: an experimental trial. Biomed Res Int. 2014;2014:547290. doi: 10.1155/2014/547290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. [DOI] [PubMed] [Google Scholar]
- Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, Smania N. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabil. 2011;25:537–48. doi: 10.1177/0269215510389497. [DOI] [PubMed] [Google Scholar]
- Ghuman AS, McDaniel JR, Martin A. A wavelet-based method for measuring the oscillatory dynamics of resting-state functional connectivity in MEG. Neuroimage. 2011;56:69–77. doi: 10.1016/j.neuroimage.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard G, Daducci A, Petit L, Thiran JP, Whittingstall K, Deriche R, et al. AxTract: Toward microstructure informed tractography. Hum Brain Mapp. 2017;38:5485–5500. doi: 10.1002/hbm.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, et al. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–91. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Kocher M, Nesland T, Rorden C, Fridriksson J, Bonilha L. Preservation of structural brain network hubs is associated with less severe post-stroke aphasia. Restor Neurol Neurosci. 2015;34:19–28. doi: 10.3233/RNN-150511. [DOI] [PubMed] [Google Scholar]
- Golestani AM, Tymchuk S, Demchuk A, Goodyear BG. Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair. 2013;27:153–63. doi: 10.1177/1545968312457827. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Miranda JM. Amplitude envelope synchronization in coupled chaotic oscillators. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;65:036232. [DOI] [PubMed] [Google Scholar]
- Granziera C, Daducci A, Meskaldji DE, Roche A, Maeder P, Michel P, et al. A new early and automated MRI-based predictor of motor improvement after stroke. Neurology. 2012;79:39–46. doi: 10.1212/WNL.0b013e31825f25e7. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–46. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50:233–42. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Baillet S, Barnes GR, Henson RN, Hillebrand A, Jensen O, et al. Good practice for conducting and reporting MEG research. Neuroimage. 2013;65:349–63. doi: 10.1016/j.neuroimage.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Honma SM, Findlay AM, Dalal SS, Kirsch HE, Berger MS, et al. Mapping functional connectivity in patients with brain lesions. Ann Neurol. 2008;63:193–203. doi: 10.1002/ana.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Nicolo P, Cohen LG, Schnider A, Buch ER. Longitudinal Structural and Functional Differences Between Proportional and Poor Motor Recovery After Stroke. Neurorehabil Neural Repair. 2017;31:1029–41. doi: 10.1177/1545968317740634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Rizk S, Ptak R, Di Pietro M, Saj A, Lazeyras F, et al. Two Intrinsic Coupling Types for Resting-State Integration in the Human Brain. Brain Topogr. 2015;28:318–29. doi: 10.1007/s10548-014-0394-2. [DOI] [PubMed] [Google Scholar]
- Guo J, Yang M, Biswal BB, Yang P, Liao W, Chen H. Abnormal Functional Connectivity Density in Post-Stroke Aphasia. Brain Topogr. 2019;32:271–82. doi: 10.1007/s10548-018-0681-4. [DOI] [PubMed] [Google Scholar]
- Guo Z, Jin Y, Peng H, Xing G, Liao X, Wang Y, et al. Ipsilesional High Frequency Repetitive Transcranial Magnetic Stimulation Add-On Therapy Improved Diffusion Parameters of Stroke Patients with Motor Dysfunction: A Preliminary DTI Study. Neural Plast. 2016;2016:6238575. doi: 10.1155/2016/6238575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakon J, Quattromani MJ, Sjolund C, Tomasevic G, Carey L, Lee JM, et al. Multisensory stimulation improves functional recovery and resting-state functional connectivity in the mouse brain after stroke. Neuroimage Clin. 2018;17:717–30. doi: 10.1016/j.nicl.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011;118:40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev. 2013;5:CD008862. doi: 10.1002/14651858.CD008862.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105:16039–44. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise KF, Steven B, Liuzzi G, Thomalla G, Jonas M, Muller-Vahl K, et al. Altered modulation of intracortical excitability during movement preparation in Gilles de la Tourette syndrome. Brain. 2010;133:580–90. doi: 10.1093/brain/awp299. [DOI] [PubMed] [Google Scholar]
- Heise KF, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J Neurosci. 2013;33:9039–49. doi: 10.1523/jneurosci.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks HT, Zwarts MJ, Plat EF, van Limbeek J. Systematic review for the early prediction of motor and functional outcome after stroke by using motor-evoked potentials. Arch Phys Med Rehabil. 2002;83:1303–8. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25:9–15. [PubMed] [Google Scholar]
- Hillebrand A, Barnes GR, Bosboom JL, Berendse HW, Stam CJ. Frequency-dependent functional connectivity within resting-state networks: an atlas-based MEG beamformer solution. Neuroimage. 2012;59:3909–21. doi: 10.1016/j.neuroimage.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci. 2012;15:884–90. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O. Dynamical consequences of lesions in cortical networks. Hum Brain Mapp. 2008;29:802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfner S, Cazzoli D, Muri RM, Nef T, Mosimann UP, Bohlhalter S, et al. Enhancing treatment effects by combining continuous theta burst stimulation with smooth pursuit training. Neuropsychologia. 2015;74:145–51. doi: 10.1016/j.neuropsychologia.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Hordacre B, Moezzi B, Goldsworthy MR, Rogasch NC, Graetz LJ, Ridding MC. Resting state functional connectivity measures correlate with the response to anodal transcranial direct current stimulation. Eur J Neurosci. 2017;45:837–45. doi: 10.1111/ejn.13508. [DOI] [PubMed] [Google Scholar]
- Hordacre B, Ridding MC, Goldsworthy MR. Response variability to non-invasive brain stimulation protocols. Clin Neurophysiol. 2015;126:2249–50. doi: 10.1016/j.clinph.2015.04.052. [DOI] [PubMed] [Google Scholar]
- Hosomi A, Nagakane Y, Yamada K, Kuriyama N, Mizuno T, Nishimura T, et al. Assessment of arcuate fasciculus with diffusion-tensor tractography may predict the prognosis of aphasia in patients with left middle cerebral artery infarcts. Neuroradiology. 2009;51:549–55. doi: 10.1007/s00234-009-0534-7. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur J Neurosci. 2005;22:2069–80. doi: 10.1111/j.1460-9568.2005.04370.x. [DOI] [PubMed] [Google Scholar]
- Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–57. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- Hui ES, Fieremans E, Jensen JH, Tabesh A, Feng W, Bonilha L, et al. Stroke assessment with diffusional kurtosis imaging. Stroke. 2012;43:2968–73. doi: 10.1161/STROKEAHA.112.657742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005a;128:490–9. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005b;19:14–9. [DOI] [PubMed] [Google Scholar]
- Hummel F, Kirsammer R, Gerloff C. Ipsilateral cortical activation during finger sequences of increasing complexity: representation of movement difficulty or memory load? Clin Neurophysiol. 2003;114:605–13. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Celnik P, Pascual-Leone A, Fregni F, Byblow WD, Buetefisch CM, et al. Controversy: Noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1:370–82. doi: 10.1016/j.brs.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol. 2005c;18:667–74. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Heise K, Celnik P, Floel A, Gerloff C, Cohen LG. Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiol Aging. 2010;31:2160–8. doi: 10.1016/j.neurobiolaging.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Steven B, Hoppe J, Heise K, Thomalla G, Cohen LG, et al. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009;72:1766–72. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–34. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Lorenzano C, Conte A, Frasca V, Manfredi M, Berardelli A. Effects of transcranial magnetic stimulation on the H reflex and F wave in the hand muscles. Clin Neurophysiol. 2003;114:1096–101. doi: S1388245703000567 [pii]. [DOI] [PubMed] [Google Scholar]
- Irimia A, Goh SY, Torgerson CM, Chambers MC, Kikinis R, Van Horn JD. Forward and inverse electroencephalographic modeling in health and in acute traumatic brain injury. Clin Neurophysiol. 2013;124:2129–45. doi: 10.1016/j.clinph.2013.04.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn ResonMed. 2005a;53:1432–40. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005b;26:347–55. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Descoteaux M, Mori S, Leemans A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2017:e3785–e. doi: 10.1002/nbm.3785. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34:2747–66. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TEJ, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36 doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Allred RP, Jefferson SC, Kerr AL, Woodie DA, Cheng SY, et al. Motor system plasticity in stroke models: intrinsically use-dependent, unreliably useful. Stroke. 2013;44:S104–6. doi: 10.1161/STROKEAHA.111.000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jefferson SC. Reflections of experience-expectant development in repair of the adult damaged brain. Dev Psychobiol. 2011;53:466–75. doi: 10.1002/dev.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Kleim JA, Greenough WT. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Res. 1996;733:142–8. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994; 14:2140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M, Blinowska KJ. Directed Transfer Function is not influenced by volume conduction-inexpedient pre-processing should be avoided. Front Comput Neurosci. 2014;8:61. doi: 10.3389/fncom.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M, Ding M, Truccolo WA, Bressler SL. Evaluating causal relations in neural systems: granger causality, directed transfer function and statistical assessment of significance. Biol Cybern. 2001;85:145–57. [DOI] [PubMed] [Google Scholar]
- Kamiya J Operant control of the EEG alpha rhythm and some of its reported effects on consciousness In: Tart C, editor. Altered states of consciousness. New York: Wiley; 1969. p. 489–501. [Google Scholar]
- Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol. 2003;60:1730–4. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- Kang N, Summers JJ, Cauraugh JH. Non-invasive Brain Stimulation Improves Paretic Limb Force Production: A Systematic Review and Meta-Analysis. Brain Stimul. 2016;9:662–70. doi: 10.1016/j.brs.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Sperber C, Rorden C. Mapping human brain lesions and their functional consequences. NeuroImage. 2018;165:180–9. doi: 10.1016/j.neuroimage.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn Sci. 2012;16:181–8. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–8. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AA. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121:30–7. doi: 10.1111/j.1600-0404.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kim YH, Kim MS, Park CH, Lee A, Chang WH. Prediction of Motor Recovery Using Diffusion Tensor Tractography in Supratentorial Stroke Patients With Severe Motor Involvement. Ann Rehabil Med. 2015;39:570–6. doi: 10.5535/arm.2015.39.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Jang SH. Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. AJNR Am J Neuroradiol. 2013;34:785–90. doi: 10.3174/ajnr.A3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Jones TA. Lesion size-dependent synaptic and astrocytic responses in cortex contralateral to infarcts in middle-aged rats. Synapse. 2010;64:659–71. doi: 10.1002/syn.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–6. [DOI] [PubMed] [Google Scholar]
- King MD, Houseman J, Roussel SA, van Bruggen N, Williams SR, Gadian DG. q-Space imaging of the brain. Magn Reson Med. 1994;32:707–13. doi: 10.1002/mrm.1910320605. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–39. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–8. [DOI] [PubMed] [Google Scholar]
- Koch G, Bonni S, Giacobbe V, Bucchi G, Basile B, Lupo F, et al. theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology. 2012;78:24–30. doi: 10.1212/WNL.0b013e31823ed08f. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–22. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, et al. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci. 2008a;28:5944–53. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, et al. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008b;131:3147–55. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behav Brain Res. 2009a;202:147–52. doi: 10.1016/j.bbr.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, et al. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol. 2009b;587:4281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P, Schulz R, Hummel FC. Structural connectivity analyses in motor recovery research after stroke. Ann Clin Transl Neurol. 2016;3:233–44. doi: 10.1002/acn3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Hummel FC. Toward precision medicine: tailoring interventional strategies based on noninvasive brain stimulation for motor recovery after stroke. Curr Opin Neurol. 2017;30:388–97. doi: 10.1097/WCO.0000000000000462. [DOI] [PubMed] [Google Scholar]
- Kohl MM, Paulsen O. The roles of GAbAb receptors in cortical network activity. Adv Pharmacol. 2010;58:205–29. doi: 10.1016/s1054-3589(10)58009-8. [DOI] [PubMed] [Google Scholar]
- Koush Y, Rosa MJ, Robineau F, Heinen K, S WR, Weiskopf N, et al. Connectivity-based neurofeedback: dynamic causal modeling for real-time fMRI. Neuroimage. 2013;81:422–30. doi: 10.1016/j.neuroimage.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A, Kamel H, Navi BB, Raj A, Iadecola C. Predicting future brain tissue loss from white matter connectivity disruption in ischemic stroke. Stroke. 2014;45:717–22. doi: 10.1161/STROKEAHA.113.003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A, Navi BB, Kamel H, Relkin N, Villanueva M, Raj A, et al. Exploring the brain's structural connectome: A quantitative stroke lesion-dysfunction mapping study. Hum Brain Mapp. 2015;36:2147–60. doi: 10.1002/hbm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MI, Pan LL, Tsai MW, Shih YF, Wei SH, Chou LW. Investigating the Effects of Peripheral Electrical Stimulation on Corticomuscular Functional Connectivity Stroke Survivors. Top Stroke Rehabil. 2016;23:154–62. doi: 10.1080/10749357.2015.1122264. [DOI] [PubMed] [Google Scholar]
- Lascano AM, Grouiller F, Genetti M, Spinelli L, Seeck M, Schaller K, et al. Surgically relevant localization of the central sulcus with high-density somatosensory-evoked potentials compared with functional magnetic resonance imaging. Neurosurgery. 2014;74:517–26. doi: 10.1227/NEU.0000000000000298. [DOI] [PubMed] [Google Scholar]
- Lascano AM, Perneger T, Vulliemoz S, Spinelli L, Garibotto V, Korff CM, et al. Yield of MRI, high-density electric source imaging (HD-ESI), SPECT and PeT in epilepsy surgery candidates. Clin Neurophysiol. 2016;127:150–5. doi: 10.1016/j.clinph.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Leergaard TB, White NS, De Crespigny A, Bolstad I, Arceuil H, et al. Quantitative histological validation of diffusion MRI fiber orientation distributions in the rat brain. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0008595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Guo Z, Wang Y, Li X, Henderson Z, Lu CB. A model of synaptic plasticity: activation of mGluR I induced long-term theta oscillations in medial septal diagonal band of rat brain slice. Neurol Sci. 2014;35:551–7. doi: 10.1007/s10072-013-1543-1. [DOI] [PubMed] [Google Scholar]
- Li LM, Uehara K, Hanakawa T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci. 2015;9:181. doi: 10.3389/fncel.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Zeng J, Liu S, Ling X, Xu A, Yu J, et al. A prospective study of secondary degeneration following subcortical infarction using diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2007;78:581–6. doi: 10.1136/jnnp.2006.099077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–47. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000a;23:1761–3. [DOI] [PubMed] [Google Scholar]
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000b;111:671–6. [DOI] [PubMed] [Google Scholar]
- Liew SL, Rana M, Cornelsen S, Fortunato de Barros Filho M, Birbaumer N, Sitaram R, et al. Improving Motor Corticothalamic Communication After Stroke Using Real-Time fMRI Connectivity-Based Neurofeedback. Neurorehabil Neural Repair. 2016;30:671–5. doi: 10.1177/1545968315619699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Daducci A, Meskaldji DE, Thiran JP, Michel P, Meuli R, et al. Quantitative Analysis of Myelin and Axonal Remodeling in the Uninjured Motor Network After Stroke. Brain Connect. 2015;5:401–12. doi: 10.1089/brain.2014.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–7. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp. 2012;33:1040–51. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi G, Horniss V, Hoppe J, Heise K, Zimerman M, Gerloff C, et al. Distinct temporospatial interhemispheric interactions in the human primary and premotor cortex during movement preparation. Cereb Cortex. 2010;20:1323–31. doi: 10.1093/cercor/bhp196. [DOI] [PubMed] [Google Scholar]
- Liuzzi G, Horniss V, Lechner P, Hoppe J, Heise K, Zimerman M, et al. Development of movement-related intracortical inhibition in acute to chronic subcortical stroke. Neurology. 2014;82:198–205. doi: 10.1212/wnl.0000000000000028. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH, van Lierop TH, Schrijer CF, van Leeuwen WS. Organization of thalamic and cortical alpha rhythms: spectra and coherences. Electroencephalogr Clin Neurophysiol. 1973;35:627–39. [DOI] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunven M, Thiebaut De Schotten M, Bourlon C, Duret C, Migliaccio R, Rode G, et al. White matter lesional predictors of chronic visual neglect: a longitudinal study. Brain. 2015;138:746–60. doi: 10.1093/brain/awu389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Liu A, Li Z, Zhou X, Zhou S. Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. J Clin Neurosci. 2014;21:1388–92. doi: 10.1016/j.jocn.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–5. [DOI] [PubMed] [Google Scholar]
- Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 2017;8:1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr., et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Malcolm JG, Shenton ME, Rathi Y. Filtered multitensor tractography. IEEE Trans Med Imaging. 2010;29:1664–75. doi: 10.1109/TMI.2010.2048121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–43. [DOI] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–4. [DOI] [PubMed] [Google Scholar]
- Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke. 2011;42:2251–6. doi: 10.1161/strokeaha.110.606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marebwa BK, Fridriksson J, Yourganov G, Feenaughty L, Rorden C, Bonilha L. Chronic post-stroke aphasia severity is determined by fragmentation of residual white matter networks. Sci Rep. 2017;7:8188. doi: 10.1038/s41598-017-07607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariorenzi R, Zarola F, Caramia MD, Paradiso C, Rossini PM. Non-invasive evaluation of central motor tract excitability changes following peripheral nerve stimulation in healthy humans. Electroencephalogr Clin Neurophysiol. 1991;81:90–101. [DOI] [PubMed] [Google Scholar]
- Marquez-Ruiz J, Leal-Campanario R, Sanchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci U S A. 2012;109:6710–5. doi: 10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, Honma SM, Findlay AM, Guggisberg AG, Owen JP, Kirsch HE, et al. Resting functional connectivity in patients with brain tumors in eloquent areas. Ann Neurol. 2011;69:521–32. doi: 10.1002/ana.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti L, Della Penna S, Snyder AZ, Pizzella V, Nolte G, de Pasquale F, et al. Frequency specific interactions of MEG resting state activity within and across brain networks as revealed by the multivariate interaction measure. Neuroimage. 2013;79:172–83. doi: 10.1016/j.neuroimage.2013.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83:1035–42. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017;10:721–34. doi: 10.1016/j.brs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Mei L, Chen C, Xue G, He Q, Li T, Xue F, et al. Neural predictors of auditory word learning. Neuroreport. 2008;19:215–9. doi: 10.1097/WNR.0b013e3282f46ea9. [DOI] [PubMed] [Google Scholar]
- Mercuri B, Wassermann EM, Manganotti P, Ikoma K, Samii A, Hallett M. Cortical modulation of spinal excitability: an F-wave study. Electroencephalogr Clin Neurophysiol. 1996;101:16–24. doi: 0013469495001646 [pii]. [DOI] [PubMed] [Google Scholar]
- Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT. 2004;20:160–5. doi: 00124509-200409000-00007 [pii]. [DOI] [PubMed] [Google Scholar]
- Moller M, Frandsen J, Andersen G, Gjedde A, Vestergaard-Poulsen P, Ostergaard L. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. J Neurol Neurosurg Psychiatry. 2007;78:587–92. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Hungs M, Brugmann M, Sparing R, Boroojerdi B, Foltys H, et al. Facilitation of picture naming after repetitive transcranial magnetic stimulation. Neurology. 1999;53:1806–12. [DOI] [PubMed] [Google Scholar]
- Mottaz A, Corbet T, Doganci N, Magnin C, Nicolo P, Schnider A, et al. Modulating functional connectivity after stroke with neurofeedback: Effect on motor deficits in a controlled cross-over study. Neuroimage Clin. 2018;20:336–46. doi: 10.1016/j.nicl.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaz A, Solca M, Magnin C, Corbet T, Schnider A, Guggisberg AG. Neurofeedback training of alpha-band coherence enhances motor performance. Clin Neurophysiol. 2015;126:1754–60. doi: 10.1016/j.clinph.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–7. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Murata Y, Sakatani K, Hoshino T, Fujiwara N, Kano T, Nakamura S, et al. Effects of cerebral ischemia on evoked cerebral blood oxygenation responses and BOLD contrast functional MRI in stroke patients. Stroke. 2006;37:2514–20. doi: 10.1161/01.STR.0000239698.50656.3b. [DOI] [PubMed] [Google Scholar]
- Muri RM, Cazzoli D, Nef T, Mosimann UP, Hopfner S, Nyffeler T. Non-invasive brain stimulation in neglect rehabilitation: an update. Front Hum Neurosci. 2013;7:248. doi: 10.3389/fnhum.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage. 2007;34:253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29:411–20. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Tezzon F. Inhibitory and excitatory circuits of cerebral cortex after ischaemic stroke: prognostic value of the transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 2002;42:131–6. [PubMed] [Google Scholar]
- Neumann-Haefelin T, Witte OW. Periinfarct and remote excitability changes after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:45–52. [DOI] [PubMed] [Google Scholar]
- Newman ME. Analysis of weighted networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:056131. [DOI] [PubMed] [Google Scholar]
- Nicolo P, Fargier R, Laganaro M, Guggisberg AG. Neurobiological Correlates of Inhibition of the Right Broca Homolog during New-Word Learning. Front Hum Neurosci. 2016;10:371. doi: 10.3389/fnhum.2016.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolo P, Magnin C, Pedrazzini E, Nguyen-Danse A, Guggisberg AG. Transcranial direct current stimulation reduces secondary white-matter degradation after stroke. Brain Stimul. 2018a;11:1417–9. doi: 10.1016/j.brs.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Nicolo P, Magnin C, Pedrazzini E, Plomp G, Mottaz A, Schnider A, et al. Comparison of Neuroplastic Responses to Cathodal Transcranial Direct Current Stimulation and Continuous Theta Burst Stimulation in Subacute Stroke. Arch Phys Med Rehabil. 2018b;99:862–72 e1. doi: 10.1016/j.apmr.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Nicolo P, Ptak R, Guggisberg AG. Variability of behavioural responses to transcranial magnetic stimulation: Origins and predictors. Neuropsychologia. 2015a;74:137–44. doi: 10.1016/j.neuropsychologia.2015.01.033. [DOI] [PubMed] [Google Scholar]
- Nicolo P, Rizk S, Magnin C, Pietro MD, Schnider A, Guggisberg AG. Coherent neural oscillations predict future motor and language improvement after stroke. Brain. 2015b;138:3048–60. doi: 10.1093/brain/awv200. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex. 2004;14:1240–5. doi: 10.1093/cercor/bhh085. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011;29:463–92. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115:2292–307. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, Karbe H, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741–7. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol. 1999;9:740–7. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–4. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Cazzoli D, Hess CW, Muri RM. One session of repeated parietal theta burst stimulation trains induces long-lasting improvement of visual neglect. Stroke. 2009;40:2791–6. doi: 10.1161/STROKEAHA.109.552323. [DOI] [PubMed] [Google Scholar]
- O'Donnell LJ, Daducci A, Wassermann D, Lenglet C. Advances in computational and statistical diffusion MRI. NMR Biomed. 2017:e3805–e. doi: 10.1002/nbm.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipov GV, Hu B, Zhou C, Ivanchenko MV, Kurths J. Three types of transitions to phase synchronization in coupled chaotic oscillators. Phys Rev Lett. 2003;91:024101. [DOI] [PubMed] [Google Scholar]
- Otal B, Olma MC, Floel A, Wellwood I. Inhibitory non-invasive brain stimulation to homologous language regions as an adjunct to speech and language therapy in post-stroke aphasia: a meta-analysis. Front Hum Neurosci. 2015;9:236. doi: 10.3389/fnhum.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LH, Yang WW, Kao CL, Tsai MW, Wei SH, Fregni F, et al. Effects of 8-week sensory electrical stimulation combined with motor training on EEG-EMG coherence and motor function in individuals with stroke. Sci Rep. 2018;8:9217. doi: 10.1038/s41598-018-27553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42:1357–62. doi: 10.1161/strokeaha.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koukkou M, Kochi K, Anderer P, Saletu B, et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans A Math Phys Eng Sci. 2011;369:3768–84. doi: 10.1098/rsta.2011.0081. [DOI] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–63. [DOI] [PubMed] [Google Scholar]
- Peters DM, Fridriksson J, Stewart JC, Richardson JD, Rorden C, Bonilha L, et al. Cortical disconnection of the ipsilesional primary motor cortex is associated with gait speed and upper extremity motor impairment in chronic left hemispheric stroke. Hum Brain Mapp. 2018;39:120–32. doi: 10.1002/hbm.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlögl A, Lopes da Silva FH. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage. 2006;31:153–9. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport. 2003;14:609–12. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–41. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- Puig J, Blasco G, Schlaug G, Stinear CM, Daunis-i-Estadella P, Biarnes C, et al. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology. 2017;59:343–51. doi: 10.1007/s00234-017-1816-0. [DOI] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Blasco G, Daunis IEJ, Prats A, Prados F, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010;31:1324–30. doi: 10.3174/ajnr.A2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Res. 1998;813:374–80. [DOI] [PubMed] [Google Scholar]
- Radlinska B, Ghinani S, Leppert IR, Minuk J, Pike GB, Thiel A. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology. 2010;75:1048–54. doi: 10.1212/WNL.0b013e3181f39aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain Connect. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90; discussion 97-9. [DOI] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Broetz D, Rea M, Laer L, Yilmaz O, Brasil FL, et al. Brain-machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol. 2013;74:100–8. doi: 10.1002/ana.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey LE, Siegel JS, Lang CE, Strube M, Shulman GL, Corbetta M. Behavioural clusters and predictors of performance during recovery from stroke. Nat Hum Behav. 2017;1 pii: 0038. doi: 10.1038/s41562-016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–8. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage. 2012;59:2771–82. doi: 10.1016/j.neuroimage.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage. 2011;55:1147–58. doi: 10.1016/j.neuroimage.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Volz LJ, Feis DL, Bornilcar-Focke I, Liebig T, Eickhoff SB, et al. Identifying neuroimaging markers of motor disability in acute stroke by machine learning techniques. Cereb Cortex. 2015a;25:3046–56. doi: 10.1093/cercor/bhu100. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Volz LJ, Feis DL, Eickhoff SB, Fink GR, Grefkes C. Individual prediction of chronic motor outcome in the acute post-stroke stage: Behavioral parameters versus functional imaging. Hum Brain Mapp. 2015b;36:4553–65. doi: 10.1002/hbm.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–4. [DOI] [PubMed] [Google Scholar]
- Rizk S, Ptak R, Nyffeler T, Schnider A, Guggisberg AG. Network mechanisms of responsiveness to continuous theta-burst stimulation. Eur J Neurosci. 2013;38:3230–8. doi: 10.1111/ejn.12334. [DOI] [PubMed] [Google Scholar]
- Rondina JM, Park CH, Ward NS. Brain regions important for recovery after severe post-stroke upper limb paresis. J Neurol Neurosurg Psychiatry. 2017;88:737–43. doi: 10.1136/jnnp-2016-315030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnqvist KC, McAllister CJ, Woodhall GL, Stanford IM, Hall SD. A multimodal perspective on the composition of cortical oscillations. Front Hum Neurosci. 2013;7:132. doi: 10.3389/fnhum.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum MG, Pikovsky AS, Kurths J. Phase synchronization of chaotic oscillators. Phys Rev Lett. 1996;76:1804–7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Physiological studies of electric and magnetic stimulation of the human brain. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:29–35. [PubMed] [Google Scholar]
- Rüber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012;79:515–22. doi: 10.1212/WNL.0b013e31826356e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet MD, Mellinger J, Sitaram R, Braun C, Birbaumer N, Fetz E. Volitional control of neuromagnetic coherence. Front Neurosci. 2012;6:189. doi: 10.3389/fnins.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Poline JB, Kleinschmidt A, D'Esposito M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc Natl Acad Sci U S A. 2015;112:8463–8. doi: 10.1073/pnas.1420687112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenger VM, Ponce-Alvarez A, Adhikari M, Hagmann P, Deco G, Corbetta M. Linking Entropy at Rest with the Underlying Structural Connectivity in the Healthy and Lesioned Brain. Cereb Cortex. 2017:1–11. doi: 10.1093/cercor/bhx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the Fast Lane: New Insights into Neuroplasticity. Neuron. 2012;73:1195–203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Abe M, Okumura E, Okada T, Kondo K, Sekihara K, et al. Disturbed resting functional inter-hemispherical connectivity of the ventral attentional network in alpha band is associated with unilateral spatial neglect. PLoS One. 2013;8:e73416. doi: 10.1371/journal.pone.0073416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–84. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30:3461–74. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex. 2008;18:638–47. [DOI] [PubMed] [Google Scholar]
- Schafer M, Biesecker JC, Schulze-Bonhage A, Ferbert A. Transcranial magnetic double stimulation: influence of the intensity of the conditioning stimulus. Electroencephalogr Clin Neurophysiol. 1997;105:462–9. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–3. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009;1169:385–94. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen JM, Gross J. Source connectivity analysis with MEG and EEG. Hum Brain Mapp. 2009;30:1857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Frey BM, Koch P, Zimerman M, Bönstrup M, Feldheim J, et al. Cortico-Cerebellar Structural Connectivity Is Related to Residual Motor Output in Chronic Stroke. Cereb Cortex. 2015a:bhv251–bhv. doi: 10.1093/cercor/bhv251. [DOI] [PubMed] [Google Scholar]
- Schulz R, Koch P, Zimerman M, Wessel M, Bonstrup M, Thomalla G, et al. Parietofrontal motor pathways and their association with motor function after stroke. Brain. 2015b;138:1949–60. doi: 10.1093/brain/awv100. [DOI] [PubMed] [Google Scholar]
- Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke. 2012;43:2248–51. doi: 10.1161/sTrOKEAHA.112.662619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Park E, Lee J, Chang WH, Lee A, Kim YH, et al. Interactions Between the Corticospinal Tract and Premotor-Motor Pathways for Residual Motor Output After Stroke. Stroke. 2017a;48:2805–11. doi: 10.1161/STROKEAHA.117.016834. [DOI] [PubMed] [Google Scholar]
- Schulz R, Park E, Lee J, Chang WH, Lee A, Kim YH, et al. Synergistic but independent: The role of corticospinal and alternate motor fibers for residual motor output after stroke. NeuroImage: Clinical. 2017b; 15:118–24. doi: 10.1016/j.nicl.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. Neuroimage. 2004;22:1775–83. [DOI] [PubMed] [Google Scholar]
- Sekihara K, Owen JP, Trisno S, Nagarajan SS. Removal of spurious coherence in MEG source-space coherence analysis. IEEE Trans Biomed Eng. 2011;58:3121–9. doi: 10.1109/TBME.2011.2162514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharififar S, Shuster JJ, Bishop MD. Adding electrical stimulation during standard rehabilitation after stroke to improve motor function. A systematic review and meta-analysis. Ann Phys Rehabil Med. 2018. doi: 10.1016/j.rehab.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol. 2009;66:604–16. doi: 10.1002/ana.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–907. [DOI] [PubMed] [Google Scholar]
- Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–57. [DOI] [PubMed] [Google Scholar]
- Solca M, Mottaz A, Guggisberg AG. Binaural beats increase interhemispheric alpha-band coherence between auditory cortices. Hear Res. 2016;332:233–7. doi: 10.1016/j.heares.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Sommer M, Lang N, Tergau F, Paulus W. Neuronal tissue polarization induced by repetitive transcranial magnetic stimulation? Neuroreport. 2002;13:809–11. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Edgley SA, Baker SN. Lack of evidence for direct corticospinal contributions to control of the ipsilateral forelimb in monkey. J Neurosci. 2011;31:11208–19. doi: 10.1523/JNEUROSCI.0257-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato MV, Chan C, Jensen JH, Helpern JA, Bonilha L, Kautz SA, et al. Diffusional Kurtosis Imaging and Motor Outcome in Acute Ischemic Stroke. AJNR Am J Neuroradiol. 2017;38:1328–34. doi: 10.3174/ajnr.A5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Amadi U, Gudberg CA, Ilie AS, Sampaio-Baptista C, et al. Local GABA concentration is related to network-level resting functional connectivity. Elife. 2014;3:e01465. doi: 10.7554/eLife.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007;28:1178–93. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, van Straaten EC. The organization of physiological brain networks. Clin Neurophysiol. 2012;123:1067–87. doi: 10.1016/j.clinph.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Sterr A, Dean PJ, Szameitat AJ, Conforto AB, Shen S. Corticospinal tract integrity and lesion volume play different roles in chronic hemiparesis and its improvement through motor practice. Neurorehabil Neural Repair. 2014;28:335–43. doi: 10.1177/1545968313510972. [DOI] [PubMed] [Google Scholar]
- Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair. 2010;24:413–9. doi: 10.1177/1545968309348310. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;135:2527–35. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–80. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Ackerley SJ, Smith MC, Borges VM, Barber PA. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol. 2017;4:811–20. doi: 10.1002/acn3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Petoe MA, Byblow WD. Primary Motor Cortex Excitability During Recovery After Stroke: Implications for Neuromodulation. Brain Stimul. 2015;8:1183–90. doi: 10.1016/j.brs.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin Neurophysiol. 2002;113:1279–85. [DOI] [PubMed] [Google Scholar]
- Sugata H, Hirata M, Yanagisawa T, Shayne M, Matsushita K, Goto T, et al. Alpha band functional connectivity correlates with the performance of brain-machine interfaces to decode real and imagined movements. Front Hum Neurosci. 2014;8:620. doi: 10.3389/fnhum.2014.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–6. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med. 2009;41:1049–54. doi: 10.2340/16501977-0454. [DOI] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage. 2008;40:1772–81. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–55. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut De Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex. 2014;24:989–95. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- Thiebaut De Schotten M, Dell'Acqua F, Ratiu P, Leslie A, Howells H, Cabanis E, et al. From phineas gage and monsieur leborgne to H.M.: Revisiting disconnection syndromes. Cereb Cortex. 2015;25:4812–27. doi: 10.1093/cercor/bhv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, et al. Atlasing location, asymmetry and intersubject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage. 2011;54:49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, et al. Direct Evidence for a Parietal- Frontal Pathway Subserving Spatial Awareness in Humans. Science. 2005;309:2226–8. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Röther J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage. 2004;22:1767–74. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Weiller C, Rother J. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2005;76:266–8. doi: 10.1136/jnnp.2004.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage. 2004;23:1176–85. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–7. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the 'affected' and 'unaffected’ hemispheres in human stroke. Brain Res. 1998;803:1–8. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Van Wedeen J. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–82. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Umarova RM, Reisert M, Beier TU, Kiselev VG, Kloppel S, Kaller CP, et al. Attention-network specific alterations of structural connectivity in the undamaged white matter in acute neglect. Hum Brain Mapp. 2014;35:4678–92. doi: 10.1002/hbm.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbin MA, Hong X, Lang CE, Carter AR. Resting-state functional connectivity and its association with multiple domains of upper-extremity function in chronic stroke. Neurorehabil Neural Repair. 2014;28:761–9. doi: 10.1177/1545968314522349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen MJ, Saj A, Lovblad KO, Gschwind M, Vuilleumier P. Structural white-matter connections mediating distinct behavioral components of spatial neglect in right brain-damaged patients. Cortex. 2016;77:54–68. doi: 10.1016/j.cortex.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Vallence AM, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience. 2015;304:266–78. doi: 10.1016/j.neuroscience.2015.07.043. [DOI] [PubMed] [Google Scholar]
- van den Broek SP, Reinders F, Donderwinkel M, Peters MJ. Volume conduction effects in EEG and MEG. Electroencephalogr Clin Neurophysiol. 1998;106:522–34. [DOI] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–72. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Várkuti B, Guan C, Pan Y, Phua KS, Ang KK, Kuah CWK, et al. Resting State Changes in Functional Connectivity Correlate With Movement Recovery for BCI and Robot-Assisted Upper-Extremity Training After Stroke. Neurorehabil Neural Repair. 2013;27:53–62. doi: 10.1177/1545968312445910. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–22. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Rehme AK, Michely J, Nettekoven C, Eickhoff SB, Fink GR, et al. Shaping Early Reorganization of Neural Networks Promotes Motor Function after Stroke. Cereb Cortex. 2016;26:2882–94. doi: 10.1093/cercor/bhw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz LJ, Sarfeld AS, Diekhoff S, Rehme AK, Pool EM, Eickhoff SB, et al. Motor cortex excitability and connectivity in chronic stroke: a multimodal model of functional reorganization. Brain Struct Funct. 2015;220:1093–107. doi: 10.1007/s00429-013-0702-8. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Vollmer M, Michely J, Fink GR, Rothwell JC, Grefkes C. Time-dependent functional role of the contralesional motor cortex after stroke. Neuroimage Clin. 2017;16:165–74. doi: 10.1016/j.nicl.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Monakow C Localization im Grosshirn und der Abbau der Funktion durch korticale Herde. Wiesbaden: JF Bergmann; 1914. [Google Scholar]
- Vukelic M, Gharabaghi A. Self-regulation of circumscribed brain activity modulates spatially selective and frequency specific connectivity of distributed resting state networks. Front Behav Neurosci. 2015;9:181. doi: 10.3389/fnbeh.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Qin W, Zhang J, Tian T, Li Y, Meng L, et al. Altered functional organization within and between resting-state networks in chronic subcortical infarction. J Cereb Blood Flow Metab. 2014;34:597–605. doi: 10.1038/jcbfm.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Negreira A, LaViolette P, Bakkour A, Sperling RA, Dickerson BC. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010a;20:345–51. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010b;133:1224–38. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–96. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–73. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–42. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, et al. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–63. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–86. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. AnnNeurol. 1992;31:463–72. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–9. [DOI] [PubMed] [Google Scholar]
- Wessel MJ, Hummel FC. Non-invasive Cerebellar Stimulation: a Promising Approach for Stroke Recovery? Cerebellum. 2018;17:359–71. doi: 10.1007/s12311-017-0906-1. [DOI] [PubMed] [Google Scholar]
- Wessels T, Wessels C, Ellsiepen A, Reuter I, Trittmacher S, Stolz E, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol. 2006;27:35–9. [PMC free article] [PubMed] [Google Scholar]
- Westlake KP, Hinkley LB, Bucci M, Guggisberg AG, Byl N, Findlay AM, et al. Resting state alpha-band functional connectivity and recovery after stroke. Exp Neurol. 2012;237:160–9. doi: 10.1016/j.expneurol.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014;7:468–75. doi: 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–63. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol. 1997;77:1581–94. [DOI] [PubMed] [Google Scholar]
- Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg. 2012;2:254–65. doi: 10.3978/j.issn.2223-4292.2012.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the Proportional Recovery Model for the Upper Extremity After an Ischemic Stroke. Neurorehabil Neural Repair. 2015;29:614–22. doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Kowalski GM, Rink F, Belagaje SR, Haut MW, Hobbs G, et al. Demand on skillfulness modulates interhemispheric inhibition of motor cortices. J Neurophysiol. 2016;115:2803–13. doi: 10.1152/jn.01076.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte OW. Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol. 1998;11:655–62. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. [DOI] [PubMed] [Google Scholar]
- Wong PC, Perrachione TK, Parrish TB. Neural characteristics of successful and less successful speech and word learning in adults. Hum Brain Mapp. 2007;28:995–1006. doi: 10.1002/hbm.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138:2359–69. doi: 10.1093/brain/awv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin W, Chen H, Jiang L, Li K, Yu C. Contribution of the resting-state functional connectivity of the contralesional primary sensorimotor cortex to motor recovery after subcortical stroke. PLoS One. 2014;9:e84729. doi: 10.1371/journal.pone.0084729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Stanford IM, Hall SD, Woodhall GL. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151:386–95. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Yin D, Song F, Xu D, Peterson BS, Sun L, Men W, et al. Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLoS One. 2012;7:e52727. doi: 10.1371/journal.pone.0052727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L. Multivariate Connectome-Based Symptom Mapping in Post-Stroke Patients: Networks Supporting Language and Speech. J Neurosci. 2016;36:6668–79. doi: 10.1523/JNEUROSCI.4396-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Zhu C, Zhang Y, Chen H, Qin W, Wang M, et al. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. NeuroImage. 2009;47:451–8. doi: 10.1016/j.neuroimage.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–36. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hubbard PL, Parker GJM, Alexander DC. Axon diameter mapping in the presence of orientation dispersion with diffusion MRI. NeuroImage. 2011;56:1301–15. doi: 10.1016/j.neuroimage.2011.01.084. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-kingshott CA, Alexander DC. NeuroImage NODDI : Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61:1000–16. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front Hum Neurosci. 2015;9:229. doi: 10.3389/fnhum.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–5. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–35. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. [DOI] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Gerloff C, Cohen LG, Hummel FC. Disrupting the ipsilateral motor cortex interferes with training of a complex motor task in older adults. Cereb Cortex. 2014;24:1030–6. doi: 10.1093/cercor/bhs385. [DOI] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43:2185–91. doi: 10.1161/strokeaha.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Ann Neurol. 2013;73:10–5. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]