Abstract
FMT has gained enormous momentum in the treatment of acute inflammatory and infectious diseases. Despite an encouraging safety profile, FMT has been met with caution in the oncological setting due to perceived infectious risks in immunocompromised patients. Theoretical risks aside, the application of FMT in oncology may stand to benefit patients, via modulation of treatment efficacy and the mitigation of treatment complications. Here, we summarize most recent safety data of FMT in immunocompromised cohorts, including people with cancer, highlighting that FMT may actually provide protection against bacterial translocation via introduction of a diverse microbiome and restoration of epithelial defenses. We also discuss the emerging translational applications of FMT within supportive oncology, including the prevention and treatment of graft vs. host disease and sepsis, treatment of immunotherapy-induced colitis and restoration of the gut microbiome in survivors of childhood cancer.
Keywords: Fecal microbiota transplantation (FMT), Supportive oncology, Immunocompromised, Safety, Emerging applications
Highlights
-
•
Application of FMT in immunocompromised patients has been limited by perceived risks of translocation and sepsis
-
•
FMT may offer benefits to immunocompromised patients by promoting colonization and restoring intestinal defenses
-
•
New applications of FMT in oncology include modulation of treatment efficacy and mitigation of treatment complications
1. Introduction
Fecal microbiota transplantation (FMT) involves the administration of a fecal suspension into the digestive tract of an individual, with the aim of treating or preventing disease via manipulation of the microbiome [1]. FMT was first reported as a novel treatment of pseudomembranous enterocolitis in 1958. Since that time, the efficacy of FMT for recurrent or refractory Clostridium difficile infection (CDI) has been established in multiple randomized controlled trials and its use in this setting is supported by society guidelines [[1], [2], [3]]. FMT successfully treats recurrent or refractory CDI in >90% of cases, compared with cure rates of 26–30% with the previous standard of care, vancomycin [4,5]. A burgeoning understanding of the gut microbiome coupled with the success of FMT in treating CDI has garnered widespread enthusiasm for the use of FMT across of range of diseases, with several trials demonstrating the efficacy of FMT for the induction of remission of ulcerative colitis [6]. However, a novel application of FMT resides within oncology, with experimental FMT currently under investigation for its ability to modulate treatment efficacy and mitigate serious complications of treatment including sepsis and graft versus host disease (GvHD).
Despite a growing scientific rationale and emerging clinical potential for FMT use in oncology, it has been met with caution due to the perceived risk of bacterial translocation and infection in potentially immunocompromised individuals. This parallels current investigation of FMT for the treatment of CDI, with most clinical trials excluding immunocompromised patients. These recommendations are also echoed in clinical practice guidelines developed for the treatment of CDI in children with hematological cancers [7], and parallel recent recommendations from the Food and Drug Administration/Center for Biologics Evaluation and Research cautioning against FMT in immunocompromised cohorts [8]. While these recommendations are intended to avoid harm, they are based primarily on expert opinion without supportive evidence. The limited evidence currently available suggests that FMT holds no additional risk in immunocompromised individuals [8] and as such, coordinated and interdisciplinary efforts to investigate its safety and efficacy in various indications within oncology are warranted.
This review aims to provide an overview of the current mechanistic understanding of FMT and the emerging applications in supportive oncology with particular emphasis on the adverse events or safety issues regarding reported for experimental FMT in immunocompromised individuals.
2. Proposed mechanism(s) of action of FMT in CDI
To date, the mechanisms of FMT have been primarily investigated in the setting of CDI. Fundamental research into the underlying mechanism(s) for FMT in this setting identify two possible avenues by which FMT is effective: 1) direct interaction with the gut microbiota and 2) modulation of the host's intestinal physiology and immune capacity [9,10].
2.1. FMT and the gut microbiota
CDI is typically induced by exposure to antibiotics, which deplete the indigenous gut flora and leaves an ecological void into which Clostridium difficile may proliferate. Disruption of the host's microbiota allows opportunistic and resistant microbes greater access to intestinal nutrients. This is nicely illustrated in the biosynthesis of sialic acid, a carbon-backboned monosaccharide ubiquitously expressed within glycoproteins and glycolipids [11]. Certain commensal bacteria, including Bacteroides thetaiotaomicron, are able to liberate sialic acid from mucin glycoproteins, but are unable to break it down completely [10]. In contrast, Clostridium difficile possesses the ability to catabolize sialic acid, utilizing it as an energy source. Similarly, microorganisms also liberate monomeric glucose and N-acetylglucosamine, which then become more accessible to Clostridium difficile when indigenous microbiota are absent or suppressed. As such, a potential factor in the efficacy of FMT for CDI is its ability to reintroduce microbial competition, recolonizing the microbial niches that Clostridium difficile would otherwise exploit.
Depletion of microbial diversity also reduces the host's natural defenses against pathogenic bacteria including antimicrobial peptides and bile acids, both of which effectively control Clostridium difficile expansion and spore formation. Bactericidal peptides such as thuricin CD, produced by Bacillus thuringiensis, and nisin, produced by Gram-positive bacteria, both have high potency against certain Gram-positive microbes including Clostridium difficile.10 Similarly, secondary bile salts, generated via bacterial-dependent deconjugation of taurine and glycine, are almost absent in people with recurrent CDI as bacteria with this functional capacity are commonly reduced by antibiotics [12]. Of particular relevance is the loss of Clostridium scindens, a 7α-dehydroxylating gut microbe, which encodes for the bai operon and is responsible conversion of primary bile acids into secondary bile acids [13]. Importantly, these secondary bile acids produce deoxycholic and lithocholic acids, both of which have shown to inhibit germination of Clostridium difficile spores [14]. In line with this understanding, it has been recently demonstrated that successful FMT for recurrent CDI is associated with increased signaling in the bile acid-farneoid X receptor-fibroblast growth factor pathway [15].
2.2. FMT and host immunity
It is well demonstrated that a bidirectional, symbiotic relationship exists between the host and its resident microbes, with continuous signaling from the indigenous microbiota required to maintain a healthy gut barrier function and balanced mucosal immunity. Numerous factors including mucus, tight junctions and antimicrobial peptides, maintain appropriate compartmentalization of the microbiota in the intestinal lumen, regulating their interaction with the underlying mucosal immune system. Antibiotics are well demonstrated to disrupt almost all aspects of this defense, weakening the intestinal barrier and disrupting homeostatic control of paracellular permeability [16]. Once in contact with the epithelia, Clostridium difficile toxins disrupt intestinal tight junctions and promote apoptotic death of colonocytes [17], resulting in increased permeability of the epithelial barrier and interaction of toxins and Clostridium difficile pathogen-associated molecular patterns (PAMPs) with resident mucosal immune cells via toll-like receptors (TLRs). Whilst excessive activation of certain TLRs, such as TLR4, is associated with aberrant immune signaling, activation of other TLR subtypes is associated with dampened immune responses. For example, stimulation of TLR2 via binding of cell wall components of primarily gram-positive microbes, increases interleukin-10 (IL-10) production and is associated with enhanced intestinal barrier function observed in vitro and in vivo [18,19].As such, the efficacy of FMT in treating CDI is also likely to reflect its ability to restore TLR signaling, thus repairing intestinal barrier defenses and controlling exuberant immune responses. Comparable immunomodulatory effects have also been proposed to underpin the emerging efficacy of FMT in IBD, equilibrating aberrant immune signaling towards the indigenous microbiota [20].
3. General safety considerations of FMT
FMT has demonstrated an excellent overall safety profile, with few and primarily mild adverse events reported in the short term. However, the majority of FMT studies that have been reported in the literature have occurred in the last 5–10 years and as such, long-term safety data is lacking. Despite the encouraging safety record of FMT, there is risk of disease transmission from the donor to recipient via stool, including infection, as well as immunological and metabolic disorders [21]. Transmission of an obese phenotype has been demonstrated in animal studies and the possible transmission of a microbiome that drives obesity has been reported in a single human case report [22]. However, it remains difficult to dissect causation in this case due to a variety of other factors that may have explained the weight gain seen in this patient. Rigorous and standardized donor FMT screening protocols are required, which diminish but do not entirely eliminate risk of disease transmission. Although the risks associated with autologous FMT are lower (where pre-emptive collection and storage of a patient's own stool is undertaken so that it may be readministered at a time of need), appropriate safety assessments, processing and storage is essential.
In a large systematic review of adverse events (AEs) for FMT (N = 1089 patients), it was reported that the majority of AEs were gastrointestinal in nature, including abdominal discomfort, acute diarrhea, transient fever, nausea, vomiting and constipation [23]. The overall incidence of AEs was 28·5%. Authors attributed AEs to a variety of causal factors, with only five categories of AEs definitely linked with FMT. AEs definitely linked to FMT were a sore throat, rhinorrhea (both following upper GI administration), a minor mucosal tear and a bowel perforation (both from colonoscopically administered FMT). Thirty-eight kinds of AEs, probably related to FMT, were reported in 35 articles and were considered the result of temporary systemic immune responses to the transplanted bacteria.
Authors reported that the mode of delivery was a key risk factor for FMT-related AEs, with endoscopic manipulation associated with nasal stuffiness, a sore throat, rhinorrhea and upper gastrointestinal hemorrhage. Abdominal discomfort was reported to be more frequent with upper gastrointestinal delivery. Serious adverse events (SAEs) were reported in 9·2% of patients including infection, disease relapse and CDI, with a mortality rate of 3·5%. In both cases of FMT-related mortality, death resulted from aspiration pneumonia in immunocompromised individuals (outlined below).
4. Safety profile of FMT for CDI in immunocompromised recipients
FMT for the treatment of immunocompromised patients has been met with caution due to the perceived risk of bacterial translocation and sepsis. However, systematic review data suggest that FMT for the treatment of CDI in immunocompromised patients is feasible and safe, describing similar rates of serious adverse events to immunocompetent patients [24,25]. A systematic review identified 44 articles in which FMT had been performed for CDI in 303 immunosuppressed patients. A patient was defined as immunocompromised if receiving immunosuppressive agents (including but not limited to mTOR inhibitors, calcineurin inhibitors, anti-TNF agents, other biologic agents, high dose steroids >20 mg/day or ≥ 1 mg/kg for >14 days), or diagnosed with HIV infection (regardless of CD4 count), acquired immune deficiency syndrome (AIDS), inherited or primary immunodeficiency syndromes, hematologic malignancy or solid tumor (active with treatment in past 3 months or in remission for <5 years), solid organ transplant, and/or bone marrow transplant. FMT was primarily delivered via colonoscopy (76%), with the remaining delivered via ingestible capsule/nasal tube/endoscopy or retention enema. Reported efficacy for CDI was 87·7% from a single FMT, which increased to 93% after repeated FMTs, paralleling efficacy rates reported in immunocompetent cohorts.
Two deaths were reported in the immunocompromised FMT cohort, both related to aspiration pneumonia. The first patient aspirated during sedated endoscopic FMT delivery to the duodenum [26] and the other aspirated during the anesthetic for colonoscopic FMT [8]. Other reported adverse events include 2 colectomies due to worsening CDI, 5 episodes of bacteremia or infection, 10 subsequent hospitalizations, 7 otherwise unspecified life-threatening complications, and 7 flares of IBD, as well as generalized abdominal discomfort. The rate of reported complications, as well as the nature of AEs, were comparable to those of immunocompetent cohorts, suggesting that FMT confers no additional risk in immunocompromised patients. However, it must be noted that limited long-term data exists for both immunocompetent and immunocompromised cohorts, and the possibility of late complications remains unclear. There is an observation trial currently underway aiming to collect long-term data for 10 years post FMT (NCT03325855), and FMT registries are planned.
4.1. Shifting the perception of risk in immunocompromised cohorts
Despite the growing body of evidence demonstrating comparable safety profiles for FMT in immunocompetent and immunocompromised cohorts, there remains a perception that FMT may be unsafe in these patients. While larger cohorts of patients are needed to establish whether FMT is safe in immunocompromised patients, there are theoretical reasons why FMT may actually reduce the risk of bacterial translocation and ensuing infection in immunocompromised individuals.
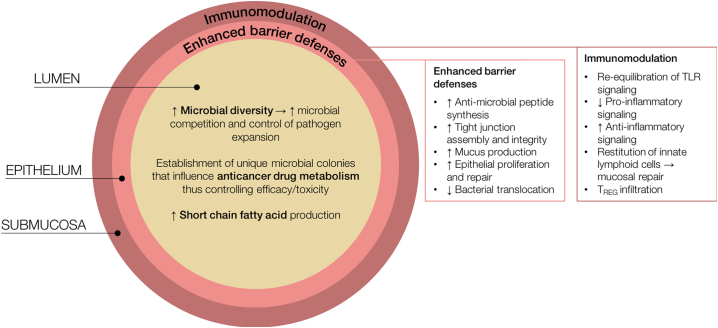
A complex and diverse microbiota is able to resist colonization by pathogenic organisms, through regulation of the intestinal barrier, promotion of epithelial repair and proliferation, mucus production and the generation of antimicrobial peptides (Fig. 1). It has been demonstrated that disruption of the microbiota, induced by antibiotics or other microbial insults, is a key risk factor for infectious complications [27]. Administration of antibiotics prior to pathogen exposure results in a marked increase in colonization and systemic uptake of these pathogens [28]; a phenomenon clearly underpinning the pathobiology of CDI. Similarly, it has been demonstrated that fluoroquinolone administration in allogeneic-stem cell transplant (allo-SCT) recipients, results in domination of the microbiota by aerobic gram-negative bacteria, which is associated with subsequent gram-negative bacteremia [29]. Most recently, dominant microbial phyla in allo-SCT recipients have been shown to predict subsequent bacteremia, suggesting that dominant bacteria are more likely to translocate and drive infectious complications [30]. Similar associations have been demonstrated in the longitudinal analysis of the microbiome in hematopoietic-SCT (HSCT) recipients, in whom a reduction in obligate anaerobes is associated with expansion and domination of pathogenic microbes (including viridans-group streptococci and vancomycin-resistant enterococcus) [31]. As such, delivering a diverse microbiome to immunocompromised patients may in fact decrease the risk of infectious complications and efforts to better characterize the safety of FMT in these cohorts are therefore warranted, albeit with caution.
Fig. 1.
Potential mechanisms by which FMT may be beneficial to those undergoing cancer therapy. Delivering a diverse microbiome serves to promote microbial competition, thus controlling pathogen expansion and reducing the risk of infectious complications. Similarly, FMT has been shown to restore short chain fatty acid profiles, thus aiding epithelial repair and intestinal homeostasis. FMT may also be used to promote a unique microbial phenotype known to induce preferable treatment responses; an approach increasingly investigated for immunotherapy-colitis and efficacy. Promoting microbial diversity via FMT is also likely to enhance natural barrier defenses, including anti-microbial peptides, tight junction assembly/integrity, mucus production and epithelial proliferation. These hold great promise in promoting recovery from acute mucosal injury and preventing bacterial translocation; each of which are key initiating factors in GvHD development. FMT efficacy is also considered to occur via immunomodulating, promoting TLR equilibrium and dampening aberrant inflammatory signaling.
5. Emerging applications of FMT in supportive oncology
People undergoing cancer therapy, many of whom are immunocompromised, are at risk of microbiome disruption due to anti-cancer therapy and antibiotics. It is well demonstrated that numerous anti-cancer agents significantly disrupt the intestinal microbiota with decreases in microbial diversity and a shift towards a gram-negative enterotype [32]. Dominance of opportunistic gram-negative microbes, combined with the direct cytotoxic damage to the intestinal lining (mucositis), predispose patients to secondary infections and various complications, including infection and, in the case of allo-HSCT, GvHD. As such, the potential applications of microbial intervention in supportive oncology can be broadly categorized into the prevention/management of 1) acute toxicities, and 2) secondary complications (Table 1).
Table 1.
Potential applications for FMT in supportive oncology.
| Treatment toxicity management | ||
|---|---|---|
| Indication | Rationale | Approach |
| Gastrointestinal mucositis | GI toxicity is associated with microbial disruption characterized by a loss of overall species diversity and shift towards a gram-negative enterotype. Although causal relationship remains unclear, interventions targeting the microbiota have proven efficacious in various preclinical models, with varying translational success. |
Personalized donor or synthetic FMT to prophylactically enhance outcomes; requires characterization of optimal microbial phenotypes associated with treatment outcome in unique oncological settings. Therapeutic FMT (autologous or donor) to 1) manage acute diarrhea, 2) promote recovery of microbiota and thus mitigate toxicity in subsequent cycles, and 3) restore/maintain microbial diversity to prevent secondary complications. |
| Immunotherapy-induced colitis | A growing evidence base shows stark differences in the microbial composition of patients who develop colitis compared to those that do not. Inconsistencies lie in the microbial phenotype linked with optimal outcomes. | Prophylactic FMT to prevent colitis by modulating microbiota to a composition associated with optimal toxicity profiles. Therapeutic FMT to treat chronic immunotherapy-induced colitis. |
| Cognitive impairment | A growing body of data now implicates the gut-brain axis in neurocognitive function, with anecdotal evidence suggesting the microbiome is critical in chemotherapy-induced neuroinflammation. GI complications often occur in comparable patient cohorts as neurocognitive impairment, indicating common underlying mechanisms. Probiotics and dietary interventions aimed at modulating the microbiota have been shown to be effective in mitigating cognitive impairment in other indications. |
Assuming a ‘healthy’ microbiome at baseline, autologous FMT to maintain individual's indigenous microbes and prevent neuroinflammation via modulation of the gut-brain axis. In cases where baseline microbiome composition is compromised, donor FMT may be used prophylactically or therapeutically. |
| Secondary complication prevention Infection | Pathogen dominance, bacterial translocation and blood stream infection are more prevalent when the microbiota is compromised. Antibiotics and GI toxicity are both risk factor for blood stream infections in oncology cohorts with the microbiome composition able to predict subsequent infections. A diverse microbiome enhances natural defenses against bacterial translocation and pathogen expansion. |
Autologous or donor FMT to maintain microbial diversity throughout treatment to prevent infectious complications via enhancing defenses (intestinal barrier function, mucus production, antimicrobial peptides, bile acid metabolism) and promoting colonization resistance. |
| Graft versus host disease | Species diversity following conditioning chemotherapy predicts GvDH in allo-SCT recipients. | Prophylactic FMT to maintain or restore microbial diversity thus preventing mucosal injury and bacterial translocation; both of which are critical in initiation of GvHD. Therapeutic FMT for the treatment of refractory GvHD. |
| Pediatric oncology | ||
| Late effects (e.g. metabolic disease) | Chronic deficits are observed in the microbiota of survivors of childhood cancer. Many of the late effect experienced by survivors have been linked with the microbiome (e.g. metabolic syndrome). |
Autologous FMT to restore individual's baseline microbiota composition and prevent late effects associated with chronic microbial disruption. |
5.1. Gastrointestinal toxicity/mucositis
Gastrointestinal (GI) toxicity, manifesting as diarrhea, remains one of the most common and clinically significant complications of all anti-cancer therapies. Prior to the widespread availability of genomic technology, GI toxicity was understood to incorporate both direct cytotoxic and indirect inflammatory-based mechanisms leading to epithelial injury and gut dysfunction. Bacterial colonization at the injured sites was documented and linked with secondary complications such as bacteremia and sepsis. Technological advances have facilitated a clearer mechanistic appreciation of the contribution of gut microbiota to GI toxicity in anti-cancer therapy [33,34], however dissecting the causative role for the microbiome in GI toxicity remains challenging. Current evidence suggests that microbial disruption observed in GI toxicity exacerbates the pathobiology of GI toxicity [34] via direct effects on drug metabolism, modulation of the host immune system and disruption of the intestinal barrier. Furthermore, it is becoming increasingly clear that an individual's unique microbiome is critical in shaping their response to treatment [35]. As such, efforts to modulate an individual's pre-treatment microbiome or preserve microbial diversity after treatment are explored.
The identification of cancer therapy-induced microbiome disruption prompted the investigation of probiotic therapies to prevent and treat the symptoms of GI toxicity. Despite strong preclinical support in animal models [[36], [37], [38]], the efficacy of probiotic formulations in humans has been underwhelming. In fact, a recent meta-analysis reported no benefit for probiotics in GI toxicity prevention [39]. Currently available probiotics lack the density and complexity to compete with indigenous microbiota and are not rationally designed to fulfil specific therapeutic roles. Lack of probiotic efficacy is multifactorial, and reflective of variable probiotic formulations and doses, variations in cancer treatment schedules and the lack of diversity in currently available probiotic preparations. As such, FMT represents a far more attractive alternative to probiotics, given its ability to deliver a diverse microbiome with greater bacterial load, as well as the capacity to collect, bank and readminister autologous fecal samples.
The only study to investigate FMT in GI toxicity management was performed in mice following 5-fluorouracil (5-FU) treatment (Table 2) [40]. Le Bastard et al., (2018) reported that autologous FMT (200 μL suspension at 5 g feces/1 mL PBS, delivered by oral gavage for 3 days) was able to restore microbial diversity following exposure to 5-FU and antibiotics. However functional endpoints (e.g. diarrhea/weight loss) were not reported and as such, the translational relevance of this study is limited. These results do however illustrate some important practical points regarding FMT in supportive cancer care. Firstly, this study demonstrates that chemotherapy exposure does not influence FMT durability in mice, with taxonomic order restored following FMT. This was despite FMT being delivered the day after cessation of antibiotics and 5-FU, and coinciding with the time period where cytotoxicity, mucosal injury and diarrhea are likely to be highest. These results echo the findings from Suez et al., (2018) showing a single autologous FMT in humans was sufficient to restore microbial diversity after antibiotic exposure [41]. In fact, authors reported superior efficacy to probiotics, providing an excellent rationale to further investigate FMT as an adjunctive supportive care measure.
Table 2.
Preclinical and clinical studies of FMT in supportive oncology.
| Indication | Study type and description | FMT source/screening | Preparation/dose | Route of administration | Timing/frequency of delivery | Outcome measure(s) | Key finding(s) | Reference |
|---|---|---|---|---|---|---|---|---|
| Gastrointestinal toxicity | Preclinical; N = 18 Male C57BL/6 mice; Ampicillin antibiotics (1 g/L days 1–7) and 5-FU chemotherapy (150 mg/kg day 8) used to mimic oncology setting |
Pellets from untreated mice | Pellets resuspended in PBS (1 pellet/1 mL PBS); pellets were pooled from multiple mice | Oral gavage (200 uL/day) | Daily for 3 days beginning on day after 5-FU (day 9–11) | Microbial diversity (alpha and beta) | 5-FU and ampicillin caused a decrease in microbial diversity, remaining significant for 1 week; mice that received FMT showed no detectable change in diversity | Le Bastard et al. [40] |
| Immunotherapy colitis | Case series; N = 2 Patient 1: 50-year old, female with high grade metaststic urothelial carcinoma (refractory to chemotherapy); treated with combined CTLA-4 and PD-1 blockade; developed steroid-resistant grade II colitis Patient 2: 78-year old male with prostate cancer refractory to chemotherapy and hormonal therapy; received two doses of ipilimumab; developed steroid-resistant grade II colitis |
Single, healthy, unrelated donor; stool collected at 3 separate time points and pooled Stringent donor screening based on exclusion of blood- and stool-based pathogens and relevant medical conditions. Clear summary of exclusion criteria can be found in the supplementary files of the original publication. |
150 g stool, processed within 4 h of passage; diluted 1:10 in 0.85% NaCl; prepared using Stomacher80 Master and filtered through gauze; stored at -80oC and used within 6 months of storage. | Colonoscopically administered (250 mL) | Patient 1: Once Patient 2: Twice |
Endoscopic evaluation, CD4/CD8/FoxP3 immunohistochemistry, 16S microbiome analysis | Patient 1: complete resolution, return of solid daily bowel movement without bleeding; steroids were gradually removed. Endoscopic evaluation showed marked improvement, indicated by a reduction in CD+ T cells and concomitant increase in CD4+ FOXp3+. Patient 2: Partial improvement with persistent ulcers and recurrent abdominal pain. Complete resolution after second FMT. In both patients, the number of OTUs increased following FMT, with principal coordinates analyses of unweighted UniFrac distances demonstrating the microbiome composition was similar to those of the donor. |
Wang et al. [45] |
| Infection | Retrospective clinical study; N = 10 Data was retrospectively analysed from consecutive adult patients diagnosed with hematological malignancy who received FMT during allo-SCT for multi-drug resistant bacteria colonization. All patients were on immunosuppressive medication. |
Healthy related or unrelated donors aged between 18 and 65 with no digestive disorders within 3 months of donation, no chronic disease or treatments, no antibiotics in the past 3 months. Donors were also excluded if they lived in the tropics, or had been hospitalised abroad for >24 h in the year leading up to donation. Complete biological and microbiological assessment was also performed. | Minimum of 50 g of stool was prepared within 6 h of collection. 50–100 g stool mixed with 300 mL 0.9% glycerol/saline, filtered through gauze. Stored at -80oC. In some cases (N = 2), fresh stool was used with preparation occurring within 6 h of donation. |
Enema (N = 8) or nasogastric (N = 2) delivery. | Once; N = 4 received before allo-SCT, N = 6 received after allo-SCT. | Degree of decolonization - Major: 3 consecutive negative microbial cultures) - Persistent: one negative microbial culture at last follow-up |
Major decolonization was achieved in 7/10 patients; persistent colonization was achieved in 6/10 patients Failure occurred in three patients: - Unable to cease antibiotics - Difficulties positioning nasogastroric tube, and unable to retain enema - Insufficient stool load (43 g) N = 2 deaths reported: - Uncontrolled GvHD - Disease progression |
Battipaglia et al. [49] |
| GvHD | Case series; N = 4 Adult patients with steroid resistant (N = 3) or steroid-dependent (N = 1) gastrointestinal acute GvHD All patients on immunosuppressive medication. |
Related donor aged 20–64 years; no tattoos/piercings, no sexual intercourse with a new partner for 3 months, no blood transfusion for 3 months, no travel to tropical areas in 3 months, no antibiotics in last month, no history of malignancy or inflammatory bowel disease, no abdominal symptomology on day of donation. All samples were screened for transmissible diseases. | Fresh, collected on day of donation; store day 4oC under anaerobic conditions until preparation. 200–300 mL sterile saline was added to fecal sample (weight unknown); fecal slurry was filtered through sterile metal sieve and leached through gauze. Sample was prepared under ambient conditions. |
Nasogastric tube | Once, however patients without newly developed adverse events were offered a second FMT between 4 and 14 days after the first at the discretion of the physician. | Efficacy: gut GvHD grade (assessed using validated criteria) - Complete response: resolution of all intestinal signs and symptoms - Partial response: decrease in severity of gut GvHD by minimum of one stage - Progression: worsened gut GvHD symptoms - No change: no significant change in gut GvHD FMT was considered effective if the patient achieved CR or PR in steroid-resistant cases, or a reduction of ≥40% in the dose of steroid in steroid-dependent cases. Safety: Adverse events that first occurred or progressed within 1 week of infusion. |
FMT was effective in all patients, with complete response in 3/4 patients and partial response in 1/4 patients. Adverse events were largely mild and transient; the possibility of an association between FMT and some adverse events could not be completely ruled out. |
Kakihana et al. [50,69] |
| Case report; N = 1 21-year old female with acute, stage III, steroid refractory gastrointestinal GvHD, |
Related donor (sister); no tattoos/piercings, no sexual intercourse with a new partner for 3 months, no blood transfusion for 3 months, no travel to tropical areas in 3 months, no antibiotics in last month, no history of malignancy or inflammatory bowel disease, no abdominal symptomology on day of donation. All samples were screened for transmissible diseases. | 71–144 g donated feces were homogenised with sterile saline; fecal suspension as passed through a metal sieve and filtered through sterile gauze; sample was centrifuged to isolate microbial pellet which was resuspended in 10% glycerol/saline. 450 uL concentrate was encapsulated into a #1 hypomellose capsule and rapidly frozen in liquid nitrogen; stored at -80oC. |
Capsules | 15 capsules per day on days 125, 130, 133 and 144 after transplantation, followed by a second round of FMT on days 173, 181 and 189. | Colonization and uptake of donor microbiome determined by 16S sequencing. | Recipients microbiome composition rapidly reflected that of the donor, increasing in diversity with a high abundance of Lactobacillus. Enterococcus re-emerged at 4 weeks, and the participant was offered another FMT. Second FMT resulted in partial resolution of symptoms, decreasing to grade I GvHD. |
Kaito et al. [51] | |
| Feasibility study; N = 8 Adult patients with steroid refractory gastrointestinal GvHD All patients on immunosuppressive medication. |
Donor FMT prepared from N = 2 healthy female donors, aged 23. No information regarding screening. |
40–50 mL of frozen fecal microbiota resuspended in 200 mL of warm saline. No further information provided on preparation. |
Nasogastric tube | Once | Efficacy: severity of GvHD symptoms (abdominal pain, diarrhea, presence of bloody purulent stools); clinical remission defined if diarrhea and intestinal spasms and/or bleeding disappeared, or stool volume decreased by 500 mL in 3 days. Safety: presentation of adverse events during FMT procedure or at follow up. Durability: 16S sequencing of recipient microbiota. |
All patients achieved symptomatic remission after the FMT. At second follow up, 4/8 patients maintained full cure and 1/8 remained in remission. Improvements in GvHD symptoms seen in 1/8 patients. 2/8 patients relapsed at follow-up. No adverse events were reported. Progression free survival was enhanced in FMT recipients compared to historical controls (no FMT). |
Qi et al. [52] |
5.2. Immunotherapy colitis
A relatively new problem in supportive oncology is the management and prevention of severe colitis induced by newly developed immunotherapies. Treatments with immunotherapies targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1) are associated with increased T-cell activation and effective antitumor immune responses in a subset of ill-defined patients [42]. However, these agents carry a risk of severe colitis. Certain bacterial signatures have been shown to associate with differential responses to immunotherapies [42,43], both in terms of treatment efficacy and toxicity. Additionally, modulation of the gut microbiota via FMT from patients has been shown to alter antitumor immunity and response to immunotherapy in gnotobiotic mice (Table 2) [44]. These findings have now prompted investigation of FMT as a therapeutic option for refractory immunotherapy-induced colitis [45].
In 2018, the first case series of immune checkpoint inhibitor-associated colitis successfully treated with FMT was published [45]. Data, from the treatment of two individuals, highlight the ability of donor FMT (50 g, delivered by colonoscopy) to restore microbial diversity and induce complete resolution of clinical symptoms. Following FMT, there was a substantial reduction in CD8+ T-cell density with a concomitant increase in mucosal CD4+ FoxP3+ in one of the participants, offering a potential mechanism through which FMT could abrogate immunotherapy-associated toxicity (Table 2). A phase 1 trial is currently underway to investigate the efficacy of donor FMT as a prophylactic, adjunct supportive care measure (NCT03772899).
5.3. Graft versus host disease and blood stream infections
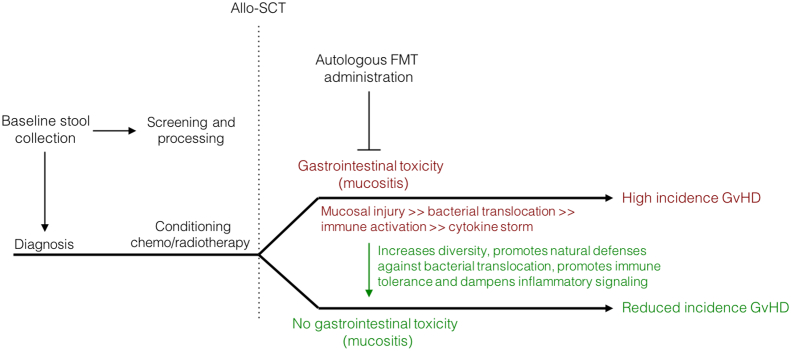
In addition to the direct benefits FMT may yield for acute GI injury (mucositis and colitis), restoring the injured microbiota after the cessation of active cancer therapy may also offer a novel method of preventing secondary complications (Fig. 2). It is clear that acute microbial disruption during cancer therapy predisposes to a number of secondary complications. Of particular importance is its potential application in the prevention of blood stream infection [46] and GvHD [47]; both of which remain significant causes of morbidity and mortality in people undergoing cancer therapy. Whilst FMT for the prevention and management of these complications lacks rigorous and controlled clinical investigation, there is a growing body of data to support its potential use. For example, intraduodenal donor FMT, performed in 20 patients (40% of whom were neutropenic), has been shown to be safe, whilst also aiding in the eradication of antibiotic-resistant bacteria in people with blood disorders (Table 2) [48]. Similarly, donor FMT (administered by rectal enema or nasogastric tube) was shown to safely eradicate multidrug-resistant bacteria in allo-SCT recipients, with 70% of patients showing full decolonization. Whilst encouraging, conclusions regarding the safety and efficacy of FMT in this study must be done so with caution due to the low sample size (N = 10) and inconsistencies in the timing and delivery of FMT [49].
Fig. 2.
Proposed mechanistic framework for autologous FMT in the therapeutic management of acute gastrointestinal toxicity and paralleled prophylaxis of GvHD. FMT delivered therapeutically following allo-SCT may enhance microbial diversity, thus serving to enhance natural defenses to bacterial translocation and mucosal injury. Restoring an injured microbiome may also promote immune tolerance and dampen inflammatory signaling, thus mitigating GvHD development. Whilst autologous FMT is preferential due to the lower risk of transmissible diseases, implementation of appropriate criteria may be warranted to ensure suitable response. Alternatively, donor FMT prepared from a healthy relative or a superdonor may be warranted.
Similarly, a recent case study has shown donor FMT is able to treat acute intestinal steroid-refractory and -dependent GvHD, with 3 of 4 patients showing complete resolution (Table 2) [50]. The same group has also reported the feasibility of delivering encapsulated FMT for GvHD treatment in a single case report [51]. This was further supported in another pilot study (N = 8) in which donor FMT (delivered via nasogastric tube) induced a reparative effect on microbiota composition, increasing diversity and elevating the abundance of Bacteroidetes, Bacteroidaceae, Ruminococcaeae and Desulfovibrionaceae. This was accompanied by a decrease in stool volume, reductions in abdominal pain and clinical resolution (termed “cure” in this study) in 50% of patients [52]. Of the remaining 4 patients, one showed an improvement in symptoms, another was considered remissive and two relapsed. Whilst these results are encouraging, these studies are underpowered, aiming to simply investigate feasibility in small patient cohorts. Randomized control trials are now warranted, and are underway (NCT02269150; NCT03359980; NCT03492502; NCT03549676; NCT03720392).
5.4. Promoting health in survivors of childhood cancer
The plausible benefits of FMT in adjunctive oncology are similar for children as adults and should, in cases where it permits, be investigated in parallel. Guidelines for the management of CDI in children currently recommend against the use of FMT due to perceived infection risks [7]. This largely reflects the exclusion of children from FMT trials, and a consequent paucity of data. However, data from case studies do support the use of FMT in the treatment of refractory CDI in children with IBD [1,53]. Large, randomized control trials are now required to confirm safety and efficacy and refine FMT protocols in children.
Beyond the use of FMT in treating CDI and in an adjunctive oncological setting, there remains a clear opportunity for FMT in pediatric oncology that is yet to be exploited. Survivors of childhood cancer are at an increased risk of developing late effects and chronic disease [[54], [55], [56], [57]]. This is becoming an increasingly clear public health problem, with the large majority (>80%) of children now surviving their diagnosis. Recent evidence now indicates that the microbiota of these survivors of childhood cancer (SCC) is not only disrupted acutely during treatment [58], but also chronically with altered microbiome compositions detected months to years after the cessation of treatment [59]. It has therefore been hypothesized that residual microbial deficiencies may drive the heightened risk of chronic disease, and as such, efforts to restore microbial health after treatment may act to mitigate longer-term microbiome-related diseases.
6. Adjunctive FMT in supportive oncology: how should it look?
6.1. Practical considerations
As outlined, there are a number of relevant applications for FMT in supportive oncology, with several currently under investigation across various institutions. Synonymous with the FMT field is the degree of variation in study protocols and trial designs, begging the question of how should we approach FMT as a supportive adjunct to traditional anti-cancer therapy?
When designing FMT trials in supportive oncology, there are a number of important considerations. Stringent donor selection and screening is important to prevent disease transmission and the FMT delivery methods may need to be adapted to suit the patient population. These measures are of particular importance to patient groups who are heavily immunosuppressed or have comorbidities or treatment-related complications.
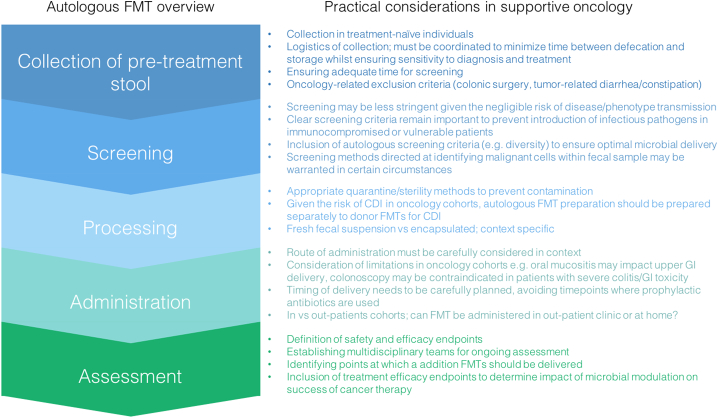
Autologous FMT presents as an attractive option in the adjunctive oncological setting, however processes around stool collection and delivery need to be refined and optimised in the clinical trial setting (Fig. 3). Whilst autologous FMT may be preferable due to a lower risk of disease transmission, there may be risks associated with reintroduction of malignancy (in the case of colorectal cancers) or transmission of a microbiome that drives carcinogenesis. Feasibility of autologous FMT must also be carefully considered ensuring there is sufficient time to collect, prepare and screen the patient's stool between diagnosis and administration of anticancer therapy. Risks associated with route of administration are also important to consider in the context of supportive oncology, with bowel preparation and colonoscopy contraindicated in patients with a friable bowel. Upper GI delivery is also challenging in oncology patients where oral mucositis may compromise oral mobility and the risk of upper GI injury. As such, rectal enemas or oral capsules represent as sensible options, providing greater flexibility to outpatients. Whilst the large majority of FMT trials continue to administer fecal slurries via colonoscopy or upper GI delivery, the efficacy of oral capsules is increasingly demonstrated. Of particular relevance was the landmark study demonstrating that route of delivery (enema vs oral capsule containing lyophilized fecal microbiota from 100 to 200 g) did not influence adverse experiences from FMT and showed comparable efficacy in treating CDI in preliminary investigations [60]. Similarly, third-party FMT prepared as oral capsules was shown to be feasible, safe and associated with an expansion of recipient microbiome diversity in allo-SCT recipients (N = 27) [61]. These studies highlight the theoretical merit of deliverying FMT via oral capsule, an approach which has great potential in the field of supportive oncology which is often complicated by numerous factors. Oral capsules provide flexibility for out-patients and overcome contraindications in many oncology cohorts. However, it must be noted that the number of capsules required to achieve an appropriate microbial load is substantial, with both studies requiring participants to take over 30 capsules for one FMT dose. Within supportive oncology where the goal may be to maintain microbial diversity over a long time period, rather than restore or change its composition, encapsulate FMT may be undermined by issues relating to compliance and the amount of stool required to prepare the appropriate number of capsules per patient.
Fig. 3.
Practical considerations for implementing autologous stool banking and FMT into supportive oncology practices.
The delivery of FMT within supportive oncology also remains a challenge given the lack of gastroenterology expertise amongst clinical care teams supporting people with cancer. FMT in the oncological setting would be best implemented in a centre equipped with the necessary expertise and facilities [1]. FMT for oncology patients should ideally be discussed within a multidisciplinary team (MDT) to guide patient selection and preparation as well as appropriate monitoring and follow-up. An FMT MDT should include oncologists, gastroenterologists, and infectious disease physicians, along with involved nursing and allied health staff [62]. As suggested by Grover et al., (2016), this team would also likely benefit from inclusion of a specialised oncogastroenterologist, with supportive care expertise [63].
6.2. Regulatory considerations
Currently, there is no global common standpoint regarding the regulatory status of FMT, with one important problem being how FMT is defined. The difficulty in characterising fecal material means it has alternatively been described as a ‘drug’, ‘biologic’ or ‘transplant’ [64]. A working group comprised via the National Institutes of Allergies and Infectious Diseases proposed the following definition for regulatory purposes in 2017: “a microbiota transplantation is the transfer of biologic material containing a minimally manipulated community of microorganisms from a human donor to a human recipient (including autologous use) with the intent of affecting the microbiota of the recipient” [65].
Since the landmark clinical FMT trial for recurrent CDI [4], regulations surrounding FMT have undergone many changes worldwide (Table 3), particularly reflected in the USA. The US Food and Drug Administration (FDA), began by announcing that FMT would be classified as an investigational drug, requiring an Investigational New Drug (IND) application to be submitted for each FMT use [66]. The IND process is effort and resource intensive and as such, there was an immediate outcry suggesting that this regulation would severely restrict access to a highly effective treatment [64]. Additionally, there was concern about the unintended side-effect of increasing risks from at-home FMT procedures [66]. The FDA responded shortly after by announcing they would exercise enforcement discretion so IND applications would not be required for CDI that had not responded to standard treatments, but would still be required for any other use of FMT [67].
Table 3.
Current and potential international regulatory frameworks for the administration of FMT.
| Region | Current Framework | Future directions | Reference |
|---|---|---|---|
| USA | The US Food and Drug Administration (FDA) classified FMT as an investigational drug, requiring an Investigational New Drug (IND) application for each FMT use. Outcry due to resource intensive IND process. The FDA responded shortly after by announcing they would exercise enforcement discretion so IND applications would not be required for CDI that had not responded to standard treatments, but would still be required for any other use of FMT. | Draft guidance under public consultation: stool banks would require IND to obtain and distribute stool, but not required for physicians performing the procedure or for hospital laboratories. Classification of FMT as a drug (a live biotherapeutic product) requires thorough characterization and composition consistency for approval- infeasible for FMT. | Verbeke et al. [66] FDA guidance documents [67,70] |
| UK | Must be manufactured in accordance with Medicines and Healthcare products Regulatory Agency (MHRA) guidance for human medicines regulation. Pharmacy exemption possible for single organisation use. FMT not currently recommended for anything apart from CDI (citing insufficient evidence). | Consensus for FMT multidisciplinary team to be formed. Recommend that FMT should be “offered with caution to immunosuppressed patients, in whom FMT appears efficacious without significant additional adverse effects” |
Mullish et al. [71] |
| Europe | Framework varies between countries. European Commission determined fecal transplant does not constitute a cell/tissue transplant. Subsequently argued that individual European Union Member States are free to regulate fecal microbiota transplantation on a national level. | Consensus document suggest Appropriate FMT registries should be implemented, in order to collect data concerning indications, procedure, effectiveness and safety profiles., Specific national rules for the classification of FMT should be followed to implement an FMT centre. |
Cammarota et al. [1] |
| Australia | FMT in local care setting can be delivered - no agreed standards. FMT material meets the definition of a biological (contains human cells/tissues) - prohibits distribution unless Good Manufacturing Practice (GMP) certified. | Public consultation currently occurring. Potential transition period to allow manufacturers and suppliers of FMT to GMP licensing requirements. Need for unique regulatory standards to allow stool banks to operate. | Therapeutic Goods Administration briefing paper [72] Costello and Bryant [73] |
| China | Ten hospitals conducting FMT research and administration. Currently, no standardisation of FMT process and implementation. Consensus on standardized biobanking of samples has been recently reached. | FMT consensus expert group existing since 2016. Draft consensus for screening and ethics to soon be published. Recommendation for a unified FMT registration system. | Shi et al. [74] |
The current regulation of FMT as a drug or live biotherapeutic product has had a significant impact on the ability to deploy this intervention in oncological studies in the USA due to the long and difficult IND process. Additionally, the majority of regulatory frameworks in place focus on recurrent CDI, with little consideration for other indications of FMT.
Finally, there is a need for any regulations to be flexible and graded to cater for future developments in the field such as purified or live biotherapeutic products. There are a vast range of new products currently in development and in clinical trials, and include selected bacterial strains cultured and administered as a capsule, cryopreserved filtered stool products and lyophilised powders that can be reconstituted [65]. These products have a different risk profile and could be produced at industry scales. A proposed regulation framework recently released is careful to be broad enough to include these products [68].
7. Conclusions
FMT is a highly effective therapy for recurrent and refractory CDI. Limited available evidence supports the notion that it is a safe therapy in immunocompetent individuals, however further studies are required. There are a number of possible applications of FMT in supportive oncology, with the potential to manage acute treatment-toxicities and prevent secondary complications of therapy. Efforts should now be directed at determining the feasibility and safety of FMT as an adjunctive supportive care intervention in both pediatric and adult patients undergoing cancer therapy.
8. Search strategy and selection criteria
Data for this review were identified by searches on PubMed and Web of Science. We reviewed the current evidence for FMT in oncology cohorts with particular focus on its use in immunocompromised cohorts. (Fecal microbi* transplant*) AND (immunocompromised host [MeSH]) AND (safety OR adverse event) were used to identify key, English-language publications, with no restrictions on publication dates. Meta-analyses and clinical guidelines were priortised. All levels of evidence were considered, including case studies, with appropriate acknowledgement of study limitations highlighted throughout the review.
9. Outstanding questions
There is a clear scientific and clinical rationale for FMT in the prevention and management of complications of cancer therapy, as well as the modulation of treatment efficacy. Despite the most recent data indicating FMT is safe in immunocompromised individuals, there remains a paucity of data for those that are severely immunocompromised, and as such, more robust data sets are now required to draw appropriate conclusions.
Implementation of FMT within oncology requires feasible FMT protocols to be integrated into the complex treatment schedules of people undergoing cancer therapy, particularly for applications in which autologous FMT is preferential. Furthermore, the durability of FMT during cytotoxic treatment is unclear, particularly given the widespread use of prophylactic antibiotics in many oncological cohorts. Future research should focus on refining FMT protocols for applications in oncology, with particular focus on the collection, screening and preparation of patient fecal material. Mode of delivery must also be appropriately refined in this patient population given complicating factors including oral mucositis (limiting oral uptake and mobility for FMT capsules), inability to perform bowel preparations for colonoscopy and the challenges surrounding FMT delivery in the out-patient setting. In cases where FMT may be used to modulate response rates to immunotherapies, future research should focus on defining the microbial composition that promotes optimal treatment response. Potential risks associated with transfer of circulating malignant cells, or microbial phenotypes that drive carcinogenesis, should also be identified/eliminated.
Acknowledgements
Dr Hannah Wardill is the recipient of an NHMRC CJ Martin Biomedical Research Fellowship (2018-2022, APP1140992).
References
- 1.Cammarota G., Ianiro G., Tilg H. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trubiano J.A., Cheng A.C., Korman T.M. Australasian Society of Infectious Diseases updated guidelines for the management of Clostridium difficile infection in adults and children in Australia and New Zealand. Intern Med J. 2016;46:479–493. doi: 10.1111/imj.13027. [DOI] [PubMed] [Google Scholar]
- 3.Quraishi M.N., Widlak M., Bhala N. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46:479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 4.van Nood E., Vrieze A., Nieuwdorp M. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 5.Cammarota G., Masucci L., Ianiro G. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835–843. doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 6.Costello S.P., Soo W., Bryant R.V., Jairath V., Hart A.L., Andrews J.M. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther. 2017;46:213–224. doi: 10.1111/apt.14173. [DOI] [PubMed] [Google Scholar]
- 7.Diorio C., Robinson P.D., Ammann R.A. Guideline for the management of clostridium difficile infection in children and adolescents with cancer and pediatric hematopoietic stem-cell transplantation recipients. J Clin Oncol. November 1 2018;36(31):3162–3171. doi: 10.1200/JCO.18.00407. [JCO1800407] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly C.R., Ihunnah C., Fischer M. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly C.R., Kahn S., Kashyap P. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149:223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baktash A., Terveer E.M., Zwittink R.D. Mechanistic insights in the success of fecal microbiota transplants for the treatment of Clostridium difficile infections. Front Microbiol. 2018;9:1242. doi: 10.3389/fmicb.2018.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson L.S., Lewis W.G., Lewis A.L. The sialate O-acetylesterase EstA from gut Bacteroidetes species enables sialidase-mediated cross-species foraging of 9-O-acetylated sialoglycans. J Biol Chem. 2017;292:11861–11872. doi: 10.1074/jbc.M116.769232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seekatz A.M., Theriot C.M., Rao K. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe. 2018;53:64–73. doi: 10.1016/j.anaerobe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang D.J., Ridlon J.M., Moore D.R., II, Barnes S., Hylemon P.B. Clostridium scindens baiCD and baiH genes encode stereo-specific 7alpha/7beta-hydroxy-3-oxo-delta4-cholenoic acid oxidoreductases. Biochim Biophys Acta. 2008;1781:16–25. doi: 10.1016/j.bbalip.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanissery R., Winston J.A., Theriot C.M. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. Difficile strains by gut microbiota derived secondary bile acids. Anaerobe. 2017;45:86–100. doi: 10.1016/j.anaerobe.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monaghan T., Mullish B.H., Patterson J. Effective fecal microbiota transplantation for recurrent Clostridioides difficile infection in humans is associated with increased signalling in the bile acid-farnesoid X receptor-fibroblast growth factor pathway. Gut Microbes. 2018:1–7. doi: 10.1080/19490976.2018.1506667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y., Leng Y., Yao G. Effects of antibiotics on intestinal microflora and intestinal mucosal barrier function and its mechanisms. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:1047–1051. doi: 10.3760/cma.j.issn.2095-4352.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Nusrat A., von Eichel-Streiber C., Turner J.R., Verkade P., Madara J.L., Parkos C.A. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cario E. Barrier-protective function of intestinal epithelial toll-like receptor 2. Mucosal Immunol. 2008;1(Suppl. 1):S62–S66. doi: 10.1038/mi.2008.47. [DOI] [PubMed] [Google Scholar]
- 19.Cario E., Gerken G., Podolsky D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 20.Khoruts A., Sadowsky M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508–516. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridaura V.K., Faith J.J., Rey F.E. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341 doi: 10.1126/science.1241214. (1241214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alang N.K.C. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2 doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S., Xu M., Wang W. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shogbesan O., Poudel D.R., Victor S. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol. 2018;2018 doi: 10.1155/2018/1394379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin S.C., Alonso C.D., Moss A.C. Fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with solid organ transplants: an institutional experience and review of the literature. Transpl Infect Dis. 2018;20 doi: 10.1111/tid.12967. [DOI] [PubMed] [Google Scholar]
- 26.Baxter M., Ahmad T., Colville A., Sheridan R. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis. 2015;61:136–137. doi: 10.1093/cid/civ247. [DOI] [PubMed] [Google Scholar]
- 27.Bohnhoff M., Drake B.L., Miller C.P. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med. 1954;86:132–137. doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- 28.van der Waaij D., Berghuis-de Vries J.M., L-V Lekkerkerk. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971;69:405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taur Y., Xavier J.B., Lipuma L. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montassier E., Al-Ghalith G.A., Ward T. Erratum to: pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 2016;8:61. doi: 10.1186/s13073-016-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffe D., Jakubowski A., Sepkowitz K. Prevention of peritransplantation viridans streptococcal bacteremia with early vancomycin administration: a single-center observational cohort study. Clin Infect Dis. 2004;39:1625–1632. doi: 10.1086/425612. [DOI] [PubMed] [Google Scholar]
- 32.Montassier E., Gastinne T., Vangay P. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015;42:515–528. doi: 10.1111/apt.13302. [DOI] [PubMed] [Google Scholar]
- 33.Alexander J.L., Wilson I.D., Teare J., Marchesi J.R., Nicholson J.K., Kinross J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356–365. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- 34.Secombe K.R., Coller J.K., Gibson R.J., Wardill H.R., Bowen J.M. The bidirectional interaction of the gut microbiome and the innate immune system: implications for chemotherapy-induced gastrointestinal toxicity. Int J Cancer. 2018;144(10):2365–2376. doi: 10.1002/ijc.31836. [DOI] [PubMed] [Google Scholar]
- 35.Wardill H.R., Tissing W.J.E. Determining risk of severe gastrointestinal toxicity based on pretreatment gut microbial community in patients receiving cancer treatment: a new predictive strategy in the quest for personalized cancer medicine. Curr Opin Support Palliat Care. 2017;11:125–132. doi: 10.1097/SPC.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 36.Bowen J.M., Stringer A.M., Gibson R.J., Yeoh A.S., Hannam S., Keefe D.M. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther. 2007;6:1449–1454. doi: 10.4161/cbt.6.9.4622. [DOI] [PubMed] [Google Scholar]
- 37.Mi H., Dong Y., Zhang B. Bifidobacterium infantis ameliorates chemotherapy-induced intestinal mucositis via regulating T cell immunity in colorectal Cancer rats. Cell Physiol Biochem. 2017;42:2330–2341. doi: 10.1159/000480005. [DOI] [PubMed] [Google Scholar]
- 38.Yeung C.Y., Chan W.T., Jiang C.B. Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wardill H.R., Van Sebille Y.Z.A., Ciorba M.A., Bowen J.M. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Curr Opin Support Palliat Care. 2018;12:187–197. doi: 10.1097/SPC.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 40.Le Bastard Q., Ward T., Sidiropoulos D. Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci Rep. 2018;8:6219. doi: 10.1038/s41598-018-24342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suez J., Zmora N., Zilberman-Schapira G. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174 doi: 10.1016/j.cell.2018.08.047. [1406-23 e16] [DOI] [PubMed] [Google Scholar]
- 42.Dubin K., Callahan M.K., Ren B. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7 doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaput N., Lepage P., Coutzac C. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 44.Vetizou M., Pitt J.M., Daillere R. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Wiesnoski D.H., Helmink B.A. Author correction: fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24(12):1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montassier E., Al-Ghalith G.A., Ward T. Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 2016;8:49. doi: 10.1186/s13073-016-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staffas A., Burgos da Silva M., van den Brink M.R. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017;129:927–933. doi: 10.1182/blood-2016-09-691394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bilinski J., Grzesiowski P., Sorensen N. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant Bacteria: results of a prospective, single-center study. Clin Infect Dis. 2017;65:364–370. doi: 10.1093/cid/cix252. [DOI] [PubMed] [Google Scholar]
- 49.Battipaglia G., Malard F., Rubio M.T. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematological malignancies carrying multidrug-resistance bacteria. Haematologica. 2019 doi: 10.3324/haematol.2018.198549. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kakihana K., Fujioka Y., Suda W. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128:2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaito S., Toya T., Yoshifuji K. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv. 2018;2:3097–3101. doi: 10.1182/bloodadvances.2018024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi X., Li X., Zhao Y. Treating steroid refractory intestinal acute graft-vs.-host disease with fecal microbiota transplantation: a pilot study. Front Immunol. 2018;9:2195. doi: 10.3389/fimmu.2018.02195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fareed S., Sarode N., Stewart F.J. Applying fecal microbiota transplantation (FMT) to treat recurrent Clostridium difficile infections (rCDI) in children. PeerJ. 2018;6 doi: 10.7717/peerj.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Genschaft M., Huebner T., Plessow F. Impact of chemotherapy for childhood leukemia on brain morphology and function. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz C.L. Health status of adult long-term survivors of childhood cancer:a report from the childhood Cancer survivor study. J Pediatr. 2004;144:407–408. [PubMed] [Google Scholar]
- 56.Schwartz C.L. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45–54. [PubMed] [Google Scholar]
- 57.Welch J.J.G., Kenney L.B., Hirway P. Understanding predictors of continued long-term pediatric cancer care across the region: a report from the consortium for New England childhood cancer survivors. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26564. [DOI] [PubMed] [Google Scholar]
- 58.van Vliet M.J., Tissing W.J., Dun C.A. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009;49:262–270. doi: 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 59.Chua L.L., Rajasuriar R., Azanan M.S. Reduced microbial diversity in adult survivors of childhood acute lymphoblastic leukemia and microbial associations with increased immune activation. Microbiome. 2017;5:35. doi: 10.1186/s40168-017-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang Z.D., Jenq R.R., Ajami N.J. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeFilipp Z., Peled J.U., Li S. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2:745–753. doi: 10.1182/bloodadvances.2018017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullish B.H., McDonald J.A.K., Thursz M.R., Marchesi J.R. Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology. 2018;68:1205. doi: 10.1002/hep.30090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grover S., Lim R.M., Blumberg R.S., Syngal S. Oncogastroenterology. J Clin Oncol. 2016;34:1154–1155. doi: 10.1200/JCO.2015.65.7973. [DOI] [PubMed] [Google Scholar]
- 64.Khoruts A. Is fecal microbiota transplantation a temporary patch for treatment of Clostridium difficile infection or a new frontier of therapeutics? Expert Rev Gastroenterol Hepatol. 2018;12:435–438. doi: 10.1080/17474124.2018.1465818. [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann D.E., Palumbo F.B., Ravel J., Rowthorn V., von Rosenvinge E. A proposed definition of microbiota transplantation for regulatory purposes. Gut Microbes. 2017;8:208–213. doi: 10.1080/19490976.2017.1293223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verbeke F., Janssens Y., Wynendaele E., De Spiegeleer B. Faecal microbiota transplantation: a regulatory hurdle? BMC Gastroenterol. 2017;17:128. doi: 10.1186/s12876-017-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung C.H., Sun X., Kanwar J.R., Bai J.Z., Cheng L., Krissansen G.W. Correction: a cell-permeable dominant-negative survivin protein induces apoptosis and sensitizes prostate cancer cells to TNF-alpha therapy. Cancer Cell Int. 2010;10:43. doi: 10.1186/1475-2867-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann D., Palumbo F., Ravel J., Roghmann M.C., Rowthorn V., von Rosenvinge E. Improving regulation of microbiota transplants. Science. 2017;358:1390–1391. doi: 10.1126/science.aaq0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kakihana K. Fecal microbiota transplantation for acute graft-versus-host disease of the gut. Rinsho Ketsueki. 2017;58:499–505. doi: 10.11406/rinketsu.58.499. [DOI] [PubMed] [Google Scholar]
- 70.Cheung C.H., Sun X., Kanwar J.R., Bai J.Z., Cheng L., Krissansen G.W. A cell-permeable dominant-negative survivin protein induces apoptosis and sensitizes prostate cancer cells to TNF-alpha therapy. Cancer Cell Int. 2010;10:36. doi: 10.1186/1475-2867-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullish B.H., Quraishi M.N., Segal J.P. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67:1920–1941. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 72.Regulation of Faecal microbiota transplants: Briefing paper. 2018. [Google Scholar]
- 73.Costello S.P., Bryant R.V. Faecal microbiota transplantation in Australia: bogged down in regulatory uncertainty. Intern Med J. 2019;49:148–151. doi: 10.1111/imj.14212. [DOI] [PubMed] [Google Scholar]
- 74.Shi Y.C., Yang Y.S. Fecal microbiota transplantation: current status and challenges in China. JGH Open. 2018;2:114–116. doi: 10.1002/jgh3.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]