Abstract
As we age, there is an increased risk for the development of tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) infection. Few studies consider that age-associated changes in the alveolar lining fluid (ALF) may increase susceptibility by altering soluble mediators of innate immunity. We assessed the impact of adult or elderly human ALF during Mtb infection in vitro and in vivo. We identified amplification of pro-oxidative and proinflammatory pathways in elderly ALF and decreased binding capability of surfactant-associated surfactant protein A (SP-A) and surfactant protein D (SP-D) to Mtb. Human macrophages infected with elderly ALF–exposed Mtb had reduced control and fewer phagosome–lysosome fusion events, which was reversed when elderly ALF was replenished with functional SP-A/SP-D. In vivo, exposure to elderly ALF exacerbated Mtb infection in young mice. Our studies demonstrate how the pulmonary environment changes as we age and suggest that Mtb may benefit from declining host defenses in the lung mucosa of the elderly.
Keywords: aging, tuberculosis, alveolar lining fluid, mycobacteria, oxidation, surfactant
Tuberculosis (TB) currently causes more death across the globe than any other single pathogen, and is due to the bacterium Mycobacterium tuberculosis (Mtb). The global number of TB cases and mortality is higher in populations >50 years of age [1], with the elderly population (≥65 years old) in the highest risk group of developing TB independent of race, ethnicity, and gender [2]. In developed countries, the elderly population has TB rates 30% higher than the adult population [3]. Although most cases of elderly TB manifest as reactivation of a latent Mtb infection, evidence supports that elderly individuals may be more susceptible to primary Mtb infection [4].
Advancing age contributes to systemic immune dysfunction. As we age, systemic “inflammaging,” a basal chronic inflammation that comes with natural aging, has been suggested as a direct contributor to the decline of innate immune responses in the elderly [5]. Inflammaging is also reported at local levels in the lungs potentially related to an increased level of oxidation of the alveolar lining fluid (ALF; lung mucosa hypophase containing innate immunomodulators such as surfactant protein A [SP-A] and surfactant protein D [SP-D], hydrolytic enzymes [hydrolases], complement cascade, mannose-binding lectin, antimicrobial peptides, etc) [6]. Associations between inflammaging, decreased functional pulmonary immunity, and susceptibility to respiratory infections have raised questions as to whether inflammaging could be a mechanism exploited by Mtb during infection in the elderly. Few studies have considered whether changes induced by inflammaging to innate soluble ALF components can impact lung function sufficiently to increase susceptibility to infection. Accumulated evidence indicates an importance of ALF components in directly mediating cellular innate immune responses to Mtb [7–13].
In this study, we examined the status and influence of adult ALF (A-ALF) or elderly ALF (E-ALF) on Mtb infection in vitro and in vivo. Proteomic analyses of human A-ALF and E-ALF revealed the existence of a pro-oxidative lung environment in elderly individuals. We further determined that this pro-oxidative environment hinders innate immune responses, particularly within antimicrobial proteins that serve as the first host defense to infection. Indeed, in E-ALF, SP-A and SP-D had decreased binding to Mtb. When Mtb is exposed to E-ALF, macrophages were unable to control the intracellular growth of Mtb, and this inability was directly related to SP-A and SP-D dysfunction in elderly ALF as replenishment of E-ALF with functional SP-A or SP-D restored the ability of human macrophages to control Mtb intracellular growth. In vivo experiments with mice showed that exposure of Mtb to E-ALF accelerated Mtb virulence and pathogenicity. These findings provide new insights into the importance of the baseline immunologic state within the lung alveolus in old age and how it could drive susceptibility of the elderly to pulmonary diseases.
MATERIALS AND METHODS
Human Subjects and Ethics Statement
All experimental procedures with animals were approved by The Ohio State University (OSU) Institutional Animal Care and Use Committee (IACUC) number 2012A00000132 and Texas Biomedical Research Institute (IACUC number 1611-MU). Human subject studies were carried out in strict accordance with the US Code of Federal and Local Regulations (OSU institutional review board numbers 2012H0135, 2007H0262, and 2008H0119 and Texas Biomedical Research Institute HSC20170667H). Blood (from healthy adults, aged 18–45 years) and bronchoalveolar lavage fluid (BALF, from adults [4 women and 4 men; age range, 25–42 years] and elderly persons [3 women and 4 men; age range, 71–81 years]), immediately prior to partial lung resection, were collected from both sexes without discrimination of race or ethnicity after written consent as we described previously [6]. For all donors, their preoperative diagnosis was lung nodule, lung nodule with mass, or abnormal parathyroid and thymus, without clinical features. Bronchoalveolar lavage (BAL) was performed on the healthy lobe of each donor lung. Human donors with the following comorbidities were excluded: smokers; injection/noninjection drug users; excessive alcohol users; or those with acute pneumonia, upper/lower respiratory tract infections, any kind of acute illness/chronic condition, heart disease, diabetes, asthma, chronic sinusitis/bronchitis, chronic obstructive pulmonary disease, renal failure, liver failure, hepatitis, thyroid disease, rheumatoid arthritis, immunosuppression or taking nonsteroidal anti-inflammatory agents, human immunodeficiency virus (HIV)/AIDS, cancer requiring chemotherapy, leukemia/lymphoma, seizure history, blood disorders, lidocaine allergies (used during the BAL), pregnancy, nontuberculous mycobacterial infections, and TB. Each “n” value represents a different human BALF donor and/or a different blood donor as specified in the figure legends.
Collection of BALF and ALF
BALF was collected and concentrated to obtain the ALF physiological concentration present within the lung (at 1 mg/mL of phospholipid) as previously described [7, 14–17].
Proteomic Analyses
Trichloroacetic acid was added to ALF aliquots containing 50 µg of protein. Precipitated proteins were acetone washed, dried, reduced/alkylated (iodoacetamide), digested with trypsin, and analyzed by 2D liquid chromatography electrospray ionization–tandem mass spectrometry (2D LC-MS/MS) on a Thermo Fisher Orbitrap Fusion mass spectrometer. Proteins were identified by searching the human subset of the SwissProt database by means of Mascot (Matrix Science). Determination of probabilities of protein and peptide assignments (1% false discovery rate) was accomplished with Scaffold (Proteome Software). Scaffold was also used to output the results as total ion current (TIC; the sum of the TIC values of all tandem [MS2] spectra assigned to a protein) as an estimate the relative abundance of each protein in each sample. Fold change (log2) is reported as E-ALF relative to A-ALF.
Complement and Surfactant Protein Binding Assays
Mtb Erdman green fluorescent protein (GFP) (provided by Dr Horwitz, University of California, Los Angeles) [18] single bacterial suspensions were obtained and further exposed to A-ALF or E-ALF for 12 hours at 37°C as described previously [7]. ALF-exposed Mtb bacteria were washed, suspended in 0.9% sodium chloride (NaCl), and plated (1.0 × 107) onto a medium-binding 96-well plate [19]. Monoclonal antibodies directed against SP-A, SP-D, and complement component 3 (CO3), and horseradish peroxidase–conjugated secondary antibodies and 3,3',5,5'-Tetramethylbenzidine substrate were used to quantify the amount of these proteins bound to the Mtb cell wall surface after exposure to A-ALF or E-ALF by colorimetric measurement at optical density 450.
In Vitro Infections Using Human Macrophages
Peripheral blood mononuclear cells from healthy adults were collected and monocyte-derived macrophages (MDMs) and human alveolar macrophages (hAMs) were obtained as described previously [7]. MDM monolayers were infected with A-ALF– or E-ALF–exposed Mtb at a multiplicity of infection (MOI) of 1:1 for 2 hours [7, 14–17], washed, and maintained for up to 96 hours. MDMs were lysed and colony-forming units (CFUs) enumerated as described previously [7]. Alternatively, hAMs were collected from healthy adults by BAL, washed twice in an excess volume of cold 0.9% NaCl, resuspended in RPMI medium containing 20% autologous serum and 1000 U/mL of penicillin, allowed to adhere for 3 hours at 37°C with 5% carbon dioxide, and washed to remove penicillin prior to infection under the same conditions as described above for MDMs. At each time point, CFUs were enumerated as indicated above. For SP-A and SP-D replenishment studies, E-ALF was replenished with a physiological concentration of SP-A (10 µg, in-house purified) [11, 20] or SP-D (1 µg, commercial [BD Biosciences]), during the 12-hour exposure of Mtb to ALF [7, 21–23].
Immunocytochemistry and Confocal Microscopy
Macrophage monolayers on glass coverslips were infected for 2 hours with A-ALF– or E-ALF–exposed Mtb at MOI 10:1 and processed as previously described [7, 15–17] (see Supplementary Materials).
Mice
Specific-pathogen-free, female C57BL/6 mice were purchased from Charles River Laboratories at 3 months of age and maintained in microisolator cages, with sterilized water and chow ad libitum, in a standard vivarium or Biosafety Level 3 for Mtb infections. Mice were acclimatized for at least 1 week prior to studies.
Mtb Infections of Mice
C57BL/6 mice were infected with 0.9% NaCl, A-ALF– or E-ALF–exposed Mtb via aerosol using an inhalation exposure system calibrated to deliver 40–100 CFU per mouse [14]. At predetermined days postinfection (DPI), mice were euthanized; organs were aseptically removed and homogenized; and Mtb burden was calculated as previously described [14].
Cytokine Quantification by Enzyme-Linked Immunosorbent Assay
Cytokines concentrations in mouse organ homogenates were assessed by enzyme-linked immunosorbent assay as described previously [14, 24] (see Supplementary Materials for details).
Histopathology
Mouse lung lobes were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin to visualize lung morphology as described previously [14, 24] (see Supplementary Materials for details).
Statistical Analysis
Statistical significance was determined using Prism 4 software (GraphPad Software). In these studies, “n” values represent the number of times that an experiment was performed using both different cell donors and different human ALFs. The unpaired, 2-tailed Student t test was used for 2-group comparisons.
RESULTS
The Pulmonary Environment Shifts Toward a Proinflammatory, Pro-oxidative State in E-ALF, Decreasing Soluble Innate Immune Protein Function
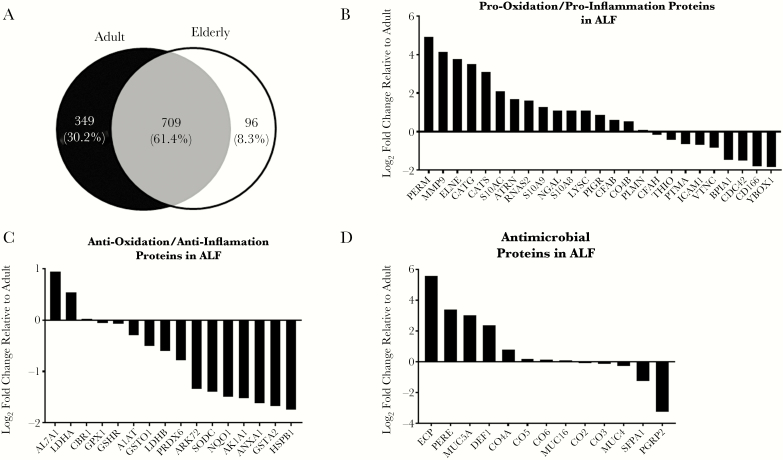
Based on our previous observations [6, 25], we hypothesized that the aging human lung environment exists in a proinflammatory and pro-oxidative state. Thus, we expected that proteins associated with inflammatory and oxidative signatures would be elevated in the ALF of elderly humans. We performed a proteomic assessment of A-ALF and E-ALF by 2D LC-MS/MS. We identified a total of 1154 unique proteins across 8 samples (4 adults and 4 elderly), with adults having 349 (30.2%) unique proteins, elderly 96 (8.3%), and 709 (61.4%) shared across both groups (Figure 1A). For proteins of interest (anti/pro-oxidative, anti/proinflammatory, and antimicrobial), we calculated their fold change in the E-ALF relative to adult based on the total TIC (for representative plots, see Supplementary Figures 1 and 2). In general, E-ALF contained higher levels of pro-oxidative/proinflammatory proteins (Figure 1B) and lower levels of anti-oxidative/anti-inflammatory proteins (Figure 1C). The relative levels of some antimicrobial proteins did not follow this pattern (Figure 1D).
Figure 1.
Aging is associated with increased proinflammatory and pro-oxidative signatures in lung alveolar lining fluid (ALF). A, Number of proteins shared between and found uniquely in adult or elderly ALF (A-ALF and E-ALF, respectively) identified by 2D liquid chromatography electrospray ionization–tandem mass spectrometry. B, Fold change relative to A-ALF based on total (total ion current [TIC]; the sum of the TIC values of all tandem [MS2] spectra assigned to a protein) for proteins classified as pro-oxidative or proinflammatory found in human ALF. C, Fold change relative to A-ALF based on total TIC values for proteins classified as anti-oxidative or anti-inflammatory found in human ALF. D, Fold change relative to A-ALF based on total TIC values for proteins classified as antimicrobial found in human ALF. Values in B–D correspond to log2 (median total TIC E-ALF / median total TIC A-ALF) for n = 4 in each group. Abbreviations: A1AT, α-1-antitrypsin; AK1A1, alcohol dehydrogenase; AL7A1, α-aminoadipic semialdehyde dehydrogenase; ANXA1, annexin A1; ARK72, aflatoxin B1 aldehyde reductase member 2; ATRN, attractin; BPIA1, BPI fold-containing family A member 1; CATG, cathepsin G; CATS, cathepsin S; CBR1, carbonyl reductase; CD166, CD166 antigen; CDC42, cell division control protein 42 homolog; CFAB, complement factor B; CO2, complement component 2; CO3, complement component 3; CO4A, complement component 4a; CO4B, complement component 4b; CO5, complement component 5; CO6, complement component 6; DEF1, neutrophil defensin 1; ECP, eosinophil cationic protein; ELNE, neutrophil elastase; FHR1, complement factor H–related protein 1; GPX1, glutathione peroxidase 1; GSHR, glutathione reductase, mitochondrial; GSTA1, glutathione S-transferase A1; GSTA2, glutathione S-transferase A2; HSPB1, heat shock protein β-1; ICAM1, intercellular adhesion molecule 1; LDHA, l-lactate dehydrogenase A chain; LDHB, l-lactate dehydrogenase B chain; LYSC, lysozyme C; MMP9, matrix metalloproteinase 9; MUC4, mucin 4; MUC5A, mucin 5AC; MUC16, mucin 16; NQO1, NAD(P)H dehydrogenase [quinone] 1; NGAL, neutrophil gelatinase-associated lipocalin; PERE, eosinophil peroxidase; PERM, myeloperoxidase; PIGR, polymeric immunoglobulin receptor; PLMN, plasminogen; PGRP2, N-acetylmuramoyl-l-alanine amidase (in humans); PRDX6, peroxiredoxin 6; PTMA, prothymosin-α; RNAS2, nonsecretory ribonuclease; S10A8, protein S100-A8; S10A9, protein S100-A9; S10AC, protein S100-A12; SFPA2, pulmonary surfactant-associated protein A2; SODC, superoxide dismutase [Cu-Zn]; THIO, thioredoxin; VTNC, vitronectin; YBOX1, nuclease-sensitive element-binding protein 1.
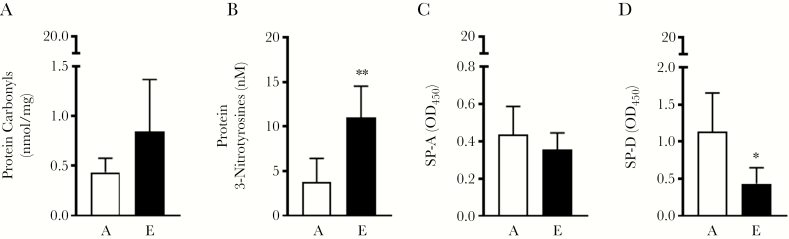
We further confirmed that E-ALF exists in a heightened level of oxidation by measuring carbonyl and 3-nitrotyrosine residues in proteins, which are indicators of damage from reactive oxygen species and reactive nitrogen intermediates, respectively. We observed a trend for increased protein carbonyls (Figure 2A), whereas 3-nitrotyrosine residues were significantly increased (Figure 2B), providing evidence that proteins in E-ALF have a higher degree of oxidation compared to proteins in A-ALF.
Figure 2.
Aging is associated with increased protein oxidation and reduced protein function. A, Protein carbonyls detected by enzyme-linked immunosorbent assay (ELISA) in adult or elderly alveolar lining fluid (A-ALF and E-ALF, respectively). B, Protein 3-nitrotyrosines detected by ELISA in A-ALF or E-ALF. C and D, Mycobacterium tuberculosis (Mtb) was incubated in physiological concentrations and conditions of A-ALF (“A,” white bars) or elderly ALF (“E,” black bars). Exposed bacteria were immobilized onto a cell culture plate and probed with monoclonal antibodies directed against surfactant protein A (SP-A) and surfactant protein D (SP-D). Relative quantities of bound protein were measured by standard ELISA by measuring the absorbance at optical density 450 (OD450). Experiments for n = 4 in each group are shown, mean ± standard error of the mean; Student t test of human A-ALF vs E-ALF. *P < .05, **P < .005, ns, not significant.
Previous results led us to hypothesize that increased levels of oxidation could negatively impact the function of soluble innate immune proteins that interact with Mtb and macrophages. Thus, we focused on 2 alveolus-associated collectins, SP-A and SP-D, shown to be important modulators of host immunity to Mtb by influencing initial host–pathogen interactions [9, 26, 27]. We assessed the ability of SP-A and SP-D to bind to the Mtb cell wall, finding that both SP-A and SP-D in elderly human ALF had reduced binding, especially SP-D (Figure 2C and 2D). This observation of ALF protein dysfunction was also observed for another important ALF component implicated in Mtb pathogenesis, C3 (Supplementary Figure 3). Overall, these results support the concept that heightened oxidation/inflammation in E-ALF is associated with decreased innate immune function.
Prior Exposure of Mtb to E-ALF Provides a Bacterial Growth Advantage in Human Macrophages
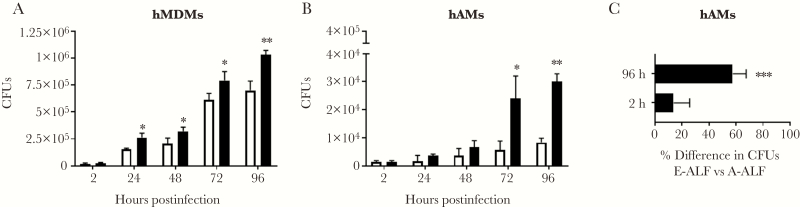
We determined if prior exposure of Mtb to E-ALF impacts the capacity of human macrophages to control Mtb infection. As opposed to bacteria exposed to A-ALF, Mtb exposed to E-ALF had a significant growth advantage in both human primary MDMs (Figure 3A) and hAMs (Figure 3B), which became more apparent over time. In hAMs, the difference in CFUs between A-ALF– and E-ALF–exposed Mtb was 13.7% at 2 hours postinfection, which increased to 57.5% at 96 hours postinfection (Figure 3C).
Figure 3.
Exposure of Mycobacterium tuberculosis (Mtb) to elderly alveolar lining fluid (E-ALF) is associated with increased bacterial intracellular growth in macrophages in vitro. Human monocyte-derived macrophage (hMDM, A) monolayers (shown is a representative experiment in triplicate of n = 8, mean ± standard deviation [SD], using 4 different adult alveolar lining fluids [A-ALFs] and 4 different E-ALFs) and human alveolar macrophages (hAMs, B; shown is a representative experiment in triplicate of n = 3 [mean ± SD], using 3 different A-ALFs and 3 different E-ALFs) were infected with human A-ALF– or E-ALF–exposed Mtb at a multiplicity of infection 1:1. Monolayers were lysed at the indicated time points to assess bacterial burden by colony-forming unit (CFU) counts. Despite similar numbers of bacteria at 2 hours postinfection, E-ALF–exposed Mtb had a significant growth advantage in macrophages at later time points. C, Percentage of E-ALF–exposed Mtb at 2 hours and 96 hours postinfection compared with A-ALF–exposed Mtb. A difference of 57.5% was observed at the 96-hour time point (overall data from n = 3 in triplicate, mean ± standard error of the mean). Student t test of human A-ALF vs E-ALF at each time point. *P < .05, **P < .005, ***P < .0005.
Prior Exposure of Mtb to E-ALF Reduces Phagosome-Lysosome Fusion in Mtb-Infected Macrophages
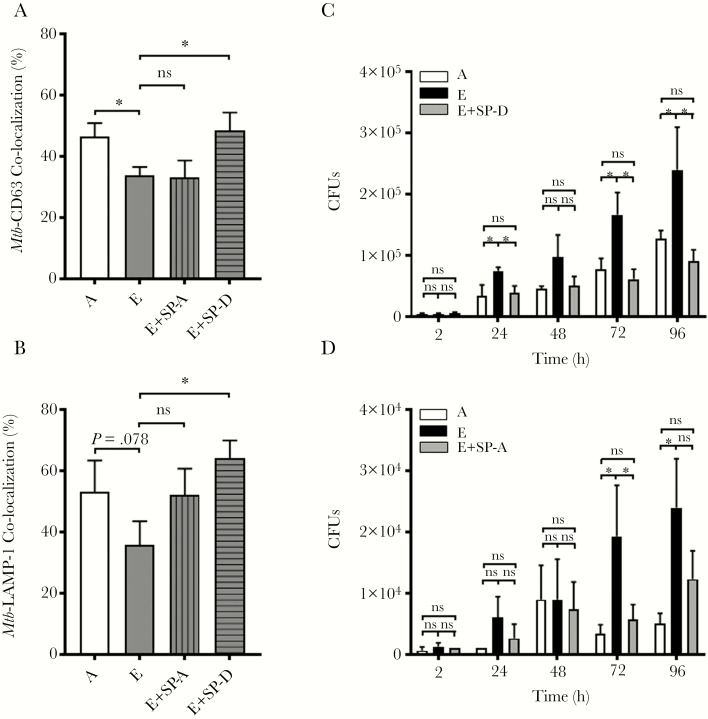
We next explored whether the increased intracellular growth of Mtb exposed to E-ALF was due to altered macrophage killing mechanisms. Our results (Figure 4A and 4B; Supplementary Figure 4A) indicate that upon 2 hours’ infection, E-ALF–exposed GFP Mtb had a decreased percentage of co-localization events with both CD63 and LAMP-1–positive endosomal/lysosomal compartments when compared to A-ALF–exposed GFP Mtb; however, acidification of these compartments was not different (Supplementary Figure 4B). Moreover, no differences were observed in terms of E-ALF– vs A-ALF–exposed GFP Mtb co-localization with LC3-II compartments (autophagy) (Supplementary Figure 4C). Together, our data suggest that changes in ALF components and their functional status with increased age associate with decreased phagocyte capacity to control Mtb infection in vitro.
Figure 4.
The enhanced growth of elderly alveolar lining fluid (E-ALF, “E”)–exposed Mycobacterium tuberculosis (Mtb) in human macrophages is associated with decreased phagosome–lysosome fusion and supplementation of E-ALF with functional surfactant protein A (SP-A) and surfactant protein D (SP-D) restores control and trafficking of Mtb. Human macrophages were adhered to glass coverslips and infected with adult alveolar lining fluid (A-ALF, “A”)– or E-ALF–exposed green fluorescent protein-Mtb (multiplicity of infection 10:1) for 2 hours. Cell monolayers were fixed, permeabilized, and stained with antihuman CD63 (A) or lysosomal-associated membrane protein (LAMP-1) (B). phagosome-lysosome (P-L) fusion events were visualized with confocal microscopy and enumerated by counting at least 50 independent events. Original magnification at ×600. Experiments from n = 8 (CD63, using 8 different monocyte-derived macrophage [MDM] human donors and 4 different A-ALFs and 4 different E-ALFs) and n = 3 (LAMP-1, using 3 different MDM human donors and 3 different A-ALFs and 3 different E-ALFs) performed in duplicate or triplicate, mean ± standard error of the mean; Student t test A-ALF vs E-ALF. *P < .05. C and D, Macrophages were infected (colony-forming units [CFUs]) as described above with A-ALF– or E-ALF–exposed Mtb, or E-ALF–exposed Mtb that had been replenished with functional SP-D or SP-A. Replenishment of E-ALF with SP-D led to significant decreases in bacterial burden and restored P-L fusion, whereas replenishment with SP-A had similar, albeit smaller effects. Representative experiment of n = 3 using 3 different human A-ALFs and 3 different E-ALFs performed in duplicate or triplicate, mean ± standard deviation; Student t test A-ALF vs E-ALF at each time point. *P < .05, **P < .005, ns, not significant.
Replenishment of E-ALF With SP-D Restores the Macrophage Capacity to Control Mtb Infection
To test our hypothesis that the loss of control observed in E-ALF–exposed Mtb-infected macrophages is due to innate soluble ALF protein dysfunction, we replenished E-ALF with functional SP-A or SP-D. Mtb bacilli were exposed to the replenished ALFs prior to infection and washed. In all groups studied, replenishment with SP-A or SP-D did not impact the CFUs at 2 hours postinfection (Figure 4C and 4D); however, at later time points, replenishment of E-ALF with SP-D allowed macrophages to control the exposed Mtb at levels equivalent to macrophages infected with A-ALF–exposed Mtb (Figure 4C). Furthermore, we observed that SP-D replenishment of E-ALF–exposed Mtb increased phagosome-lysosome (P-L) fusion events in macrophages to levels equal to those observed by macrophages infected with A-ALF–exposed Mtb (a 15% increase in P-L fusion) (Figure 4A and 4B). Results using E-ALF replenished with SP-A showed similar trends (Figure 4A, 4B, and 4D). Overall, these data provide evidence for the impact of changes in SP-A and, especially, SP-D function during aging in altering macrophage susceptibility to Mtb infection.
Exposure to E-ALF Increases Mtb Virulence and Pathogenicity In Vivo
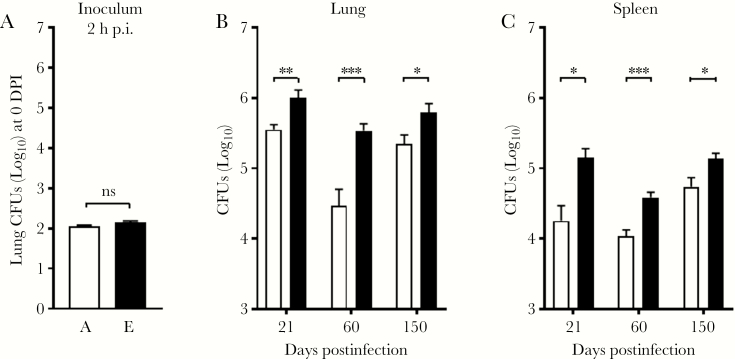
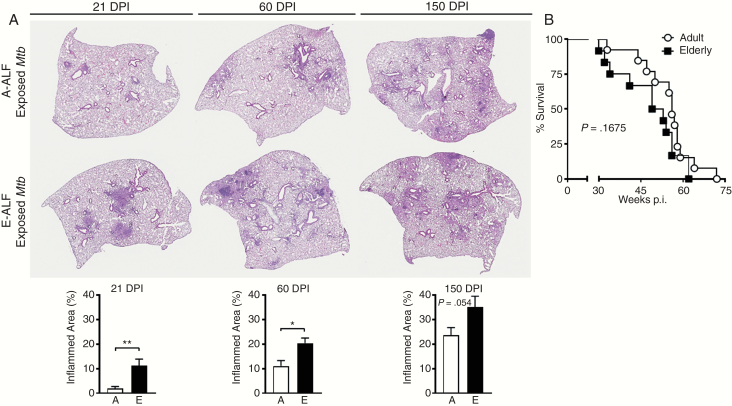
To test whether the failure to control infection with E-ALF–exposed Mtb is also observed in vivo, C57BL/6 mice were infected with a low-dose aerosol of Mtb previously exposed to A-ALF or E-ALF. Despite equal uptake into the lung (Figure 5A), mice infected with E-ALF–exposed Mtb displayed significantly higher bacterial burden in the lung at 21 DPI (Figure 5B), harboring approximately 0.5 log10 more Mtb bacilli than mice infected with A-ALF–exposed Mtb. Bacterial burden in mice infected with E-ALF–exposed Mtb remained significantly higher at later time points (60 and 150 DPI). Differences in Mtb growth in the spleen followed a similar pattern as in the lung, albeit with a lower overall burden (Figure 5C). When measuring the relative amount of inflammation in the lung over time (Figure 6A), mice infected with E-ALF–exposed Mtb developed significantly larger areas of inflammation at 21 DPI (11.31 vs 1.97), 60 DPI (20.23 vs 11.05), and 150 DPI (35.10 vs 23.57). We did not observe a statistically significant change between groups in overall mouse survival, although there was a trend to lower survival in mice infected with E-ALF–exposed Mtb (Figure 6B) (median survival, 51.00 vs 56.00 weeks).
Figure 5.
Elderly alveolar lining fluid (E-ALF, “E”)–exposed Mycobacterium tuberculosis (Mtb) demonstrates increased virulence in vivo. C57BL/6J mice were infected with a low-dose aerosol of Mtb strain Erdman previously exposed to adult alveolar lining fluid (A-ALF, “A”) or E-ALF and washed. At the indicated days postinfection (p.i.), mice were euthanized to determine bacterial burden (colony-forming units [CFUs]) in lungs and spleen. Individual organs were homogenized in 0.9% sodium chloride, and serial dilutions were plated. A, Mice received equal inoculums at 2 hours postinfection. Mice infected with E-ALF–exposed Mtb have a higher bacterial burden in both the lung (B) and the spleen (C). Pooled results from n = 2 with 4–5 mice/group, using 2 A-ALFs and 2 E-ALFs, a set of different A-ALF vs E-ALF in each experiment, mean ± standard error of the mean; Student t test for A-ALF vs E-ALF at each time point. *P < .05, **P < .005, ***P < .0005, ns, not significant.
Figure 6.
A, Elderly alveolar lining fluid (E-ALF, “E”)–exposed Mycobacterium tuberculosis (Mtb) demonstrates increased Mtb-induced immunopathology in the lung. C57BL/6J mice were infected with a low-dose aerosol of Mtb strain Erdman previously exposed to adult alveolar lining fluid (A-ALF, “A”) or E-ALF and washed. At 21, 60, and 150 days postinfection (DPI), mice were euthanized and lungs fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin to visualize tissue morphology. Areas of cell aggregation and infiltration (inflammation) were quantified using Aperio Imagescope by calculating the area of inflammatory foci (ie, involvement) divided by the total area of the lung. Quantification for 21, 60, and 150 DPI are shown. Representative images at a final magnification of ×20. Pooled results from n = 2 with 4–5 mice/group, using 2 A-ALFs and 2-E-ALFs, a set of different A-ALF vs E-ALF in each experiment, mean ± standard error of the mean; Student t test. *P < .05; **P < .005. B, Survival was monitored across a period of 80 weeks postinfection (p.i.). Mice were euthanized when they met the exclusion criteria documented in animal care and use protocols, and the date was documented. Pooled experiment from n = 2 with 10–20 mice; using 2 A-ALFs and 2-E-ALFs, a set of different A-ALF vs E-ALF in each experiment. Log-rank test.
When we further compared 0.9% NaCl-exposed (buffer used during the BAL procedure to obtain ALF) vs A-ALF–exposed vs E-ALF–exposed Mtb infection in vivo, our results indicate that A-ALF–exposed Mtb growth was controlled better as the infection progressed; conversely, E-ALF–exposed Mtb grew faster (studies up to 60 DPI; Supplementary Figure 5A). In this regard, E-ALF–exposed Mtb induced proinflammation in the lung environment marked by significantly higher levels of interleukin (IL) 12p40 and interferon-γ (the latter was not sustained as infection progressed), and significantly lower levels of IL-4, IL-10, IL-6, and tumor necrosis factor (Supplementary Figure 5B).
DISCUSSION
Previous mechanistic studies support an important role for soluble innate immune components in the lung in the early host response to Mtb infection (reviewed in [28]). Here we show that exposing Mtb to E-ALF results in significant alterations in macrophages’ ability to control Mtb growth. Our findings support a critical role for ALF innate soluble antimicrobial components in the overall cellular immune response to Mtb. Poor innate immune control early during infection in the elderly may lead to poor adaptive immune responses, increasing bacterial burden and immunopathology during the course of infection and disease. Although we did not observe a significant change in overall mouse survival in the current study (5-week difference), the trend toward decreased survival combined with increased immunopathology may decrease overall quality of life when translated to humans.
Global proteomic analysis of human ALF uncovered substantial differences between adults and elderly individuals, with 349 unique proteins identified in A-ALF and only 96 in E-ALF. Although both samples shared 709 proteins, a nonsignificant trend for fewer unique proteins in E-ALF was observed. This result is consistent with transcriptional/translational attrition [29, 30]. We observed a shift from an anti-inflammatory/antioxidative state in adults to a more proinflammatory/pro-oxidative state in the ALF of elderly individuals. This was confirmed by the increased presence of protein carbonyls and 3-nitrotyrosine residues within E-ALF proteins. Furthermore, mass spectrometry analysis revealed that prolines, tryptophans, and cysteines of proteins in E-ALF are oxidized (data not shown) [31]. Importantly, oxidation of amino acids can induce spatial conformational changes of proteins, resulting in the formation of protein–protein cross-linkages leading to protein fragmentation, which could inhibit their function [32, 33].
In addition to decreased numbers of unique proteins, we also observed a decreased binding of SP-A, SP-D, and C3 to the cell wall of Mtb after incubation of bacteria with E-ALF. This observation further supports our hypothesis that soluble innate proteins in ALF lose function with increasing age. SP-A and SP-D are important host soluble ALF proteins that help mediate macrophage responses to pathogens including Mtb [34, 35]. Whereas SP-D can enhance phagolysosomal fusion in macrophages, leading to the increased killing of Mtb [10], the outcome of SP-A–mediated phagocytosis is unclear [36]. SP-A supports the phagocytosis of Mtb by increasing mannose receptor expression on the macrophage surface and, thus, may indirectly promote survival of Mtb within nonacidified phagosomes [37, 38]. Overall, studies suggest a host neutral or detrimental role for SP-A and a host beneficial role for SP-D in connection with Mtb infection. In the context of aging, a study of young (18–23 years) vs middle-aged/elderly (37–77 years) individuals found increasing levels of SP-A with age, while the levels of SP-D remained unchanged [39]. This difference could be sufficient to tip the balance, favoring the negative properties associated with SP-A and diluting the beneficial properties of SP-D, predisposing elderly individuals to Mtb infection. Relative to previously published studies on SP-A/SP-D and Mtb, it is clear that exposure of the bacteria to ALF components alters the cell wall of Mtb in ways that impact its binding to innate immune molecules, including opsonins, and Mtb–macrophage interactions.
Our data provide evidence that SP-A and SP-D are dysfunctional in E-ALF, and their replenishment, more so SP-D than SP-A, helps macrophages control the infection. In this context, in vitro studies revealed that human lung ALF components are directly involved in Mtb survival within the host [9–11], and human population studies reinforce these findings with the identification of polymorphisms for several ALF components (eg, SP-A/-D, mannose binding lectin) [40] that are associated with host susceptibility to TB [41–43]. Similarly, our intracellular trafficking studies revealed that macrophages infected with human E-ALF–exposed Mtb display fewer Mtb-CD63/LAMP-1 co-localization events compared with macrophages infected with A-ALF–exposed Mtb. Because we used macrophages from adult donors for our study, these findings implicate ALF components directly as the reason why macrophages are less capable of killing Mtb. Although we focused on known ALF components implicated in Mtb pathogenesis (ie, SP-A/SP-D, complement), other antimicrobial components in ALF (eg, mannose-binding lectin, defensins, lysozyme, α-1-antitrypsin [44], as well as low molecular antioxidants [ascorbate, urate, glutathione]) [45, 46], could play a role during Mtb infection.
In vivo, C57BL/6 mice infected with E-ALF–exposed Mtb displayed significantly higher bacterial burden in the lung and spleen at 21, 60, and 150 DPI compared with mice infected with A-ALF–exposed Mtb. A similar trend was observed in the pulmonary immunopathology. At every time point studied, mice infected with E–ALF–exposed Mtb exhibited more widespread inflammation in their lungs compared to mice infected with A-ALF–exposed Mtb. These differences in inflammation were maintained over time. Interestingly, this increase in bacterial number in the lung and spleen, as well as the increase in inflammation, did not translate to significantly poorer mouse survival, but it did reduce median survival by approximately 10%. Thus, in the context of an in vivo infection, our data suggest that the antimicrobial properties of ALF may play a role early during infection, setting a new CFU level in mice, which progresses with infection but does not prevent the establishment of the infection or inhibit Mtb growth in the long term in the mouse model. Alternatively, the increase in inflammation observed during E-ALF–exposed Mtb infection may drive a stronger adaptive immune response as the infection moves forward. Nonetheless, significant changes in inflammation in the mouse model may provide insight that is relevant to human infection and disease.
Finally, our data provide additional support to the theory of inflammaging within the lung. Here we provide evidence that human ALF is biologically important in the context of infection in vivo. In particular, loss of function of innate immune proteins in human ALF may be an important contributing factor to the increased susceptibility of elderly individuals to infectious diseases, including Mtb. Altogether, our data reveal a critical antimicrobial and homeostatic effect of human ALF that may gradually wane as we age, rendering us susceptible to respiratory infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr Patsy Skabla for assisting with patient enrollment; the Campus Chemical Instrument Center, the Proteomics and Mass Spectrometry facility, the University Laboratory Animal Resources, and the Comparative Pathology and Mouse Phenotyping Shared Resource (P30 CA016058) in the Department of Veterinary Biosciences at The Ohio State University (OSU) for their technical support; and the facilities and programmatic support of the OSU Biosafety Level 3 Program.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health (NIH), the Department of Health and Human Services, or the United States government.
Financial support. This work was supported by the National Institute on Aging (NIA), NIH (grant number P01 AG-051428 to J. T., J. B. T., L. S. S., W. P. L., and S. H. W.), J. B. T. was supported by Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation. J. I. M. was partially supported by the National Institute of General Medical Sciences of the NIH (grant number T32 GM068412), by a supplement by the National Institute of Allergy and Infectious Diseases of the NIH (grant number R01 AI093570-S1), and by a supplement by the NIA (grant number P01 AG-051428-S1). R. A. was supported by the OSU Dean’s Distinguished University Fellowship.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Keystone Symposia on Aging, Inflammation and Immunity, Austin, Texas, 27 February 2018.
References
- 1. World Health Organization (WHO). Global tuberculosis report 2018. Geneva, Switzerland: WHO, 2018; 1–277. [Google Scholar]
- 2. Murray CJ, Ortblad KF, Guinovart C, et al. . Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pratt RH, Winston CA, Kammerer JS, Armstrong LR. Tuberculosis in older adults in the United States, 1993-2008. J Am Geriatr Soc 2011; 59:851–7. [DOI] [PubMed] [Google Scholar]
- 4. Stead WW. Tuberculosis among elderly persons: an outbreak in a nursing home. Ann Intern Med 1981; 94:606–10. [DOI] [PubMed] [Google Scholar]
- 5. Piergallini TJ, Turner J. Tuberculosis in the elderly: why inflammation matters. Exp Gerontol 2018; 105:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moliva JI, Rajaram MV, Sidiki S, et al. . Molecular composition of the alveolar lining fluid in the aging lung. Age (Dordr) 2014; 36:9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis–macrophage interactions and the capacity to control infection. J Immunol 2011; 187:372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlesinger LS, Horwitz MA. Complement receptors and complement component C3 mediate phagocytosis of Mycobacterium tuberculosis and Mycobacterium leprae. Int J Lep 1990; 58:200–1. [PubMed] [Google Scholar]
- 9. Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol 1999; 163:312–21. [PubMed] [Google Scholar]
- 10. Ferguson JS, Martin JL, Azad AK, et al. . Surfactant protein D increases fusion of Mycobacterium tuberculosis–containing phagosomes with lysosomes in human macrophages. Infect Immun 2006;74:7005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol 1995; 155:5343–51. [PubMed] [Google Scholar]
- 12. Ferguson JS, Weis JJ, Martin JL, Schlesinger LS. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun 2004; 72:2564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scordo JM, Olmo-Fontanez AM, Kelley HV, et al. . The human lung mucosa drives differential Mycobacterium tuberculosis infection outcome in the alveolar epithelium. Mucosal Immunol 2019. doi: 10.1038/s41385-019-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moliva JI, Hossfeld AP, Canan CH, et al. . Exposure to human alveolar lining fluid enhances Mycobacterium bovis BCG vaccine efficacy against Mycobacterium tuberculosis infection in a CD8(+) T-cell-dependent manner. Mucosal Immunol 2018; 11:968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arcos J, Diangelo L, Scordo J, et al. . Lung mucosa lining fluid modifies Mycobacterium tuberculosis to reprogram human neutrophil killing mechanisms. J Infect Dis 2015; 212:948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arcos J, Sasindran SJ, Moliva JI, et al. . Mycobacterium tuberculosis cell wall released fragments by the action of the human lung mucosa modulate macrophages to control infection in an IL-10-dependent manner. Mucosal Immunol 2017; 10:1248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scordo JM, Arcos J, Kelley HV, et al. . Mycobacterium tuberculosis cell wall fragments released upon bacterial contact with the human lung mucosa alter the neutrophil response to infection. Front Immunol 2017; 8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tullius MV, Harth G, Horwitz MA. High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism. Infect Immun 2001; 69:6348–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlesinger LS, Horwitz MA. A role for natural antibody in the pathogenesis of leprosy: antibody in nonimmune serum mediates C3 fixation to the Mycobacterium leprae surface and hence phagocytosis by human mononuclear phagocytes. Infect Immun 1994; 62:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCormack FX, Calvert HM, Watson PA, Smith DL, Mason RJ, Voelker DR. The structure and function of surfactant protein A. Hydroxyproline- and carbohydrate-deficient mutant proteins. J Biol Chem 1994; 269:5833–41. [PubMed] [Google Scholar]
- 21. Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol 2002; 169:3565–73. [DOI] [PubMed] [Google Scholar]
- 22. Mason RJ, Greene K, Voelker DR. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol 1998; 275:L1–13. [DOI] [PubMed] [Google Scholar]
- 23. Nishikiori H, Chiba H, Ariki S, et al. . Distinct compartmentalization of SP-A and SP-D in the vasculature and lungs of patients with idiopathic pulmonary fibrosis. BMC Pulm Med 2014; 14:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moliva JI, Hossfeld AP, Sidiki S, et al. . Selective delipidation of Mycobacterium bovis BCG enables direct pulmonary vaccination and enhances protection against Mycobacterium tuberculosis. Mucosal Immunol 2019. doi: 10.1038/s41385-019-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canan CH, Gokhale NS, Carruthers B, et al. . Characterization of lung inflammation and its impact on macrophage function in aging. J Leukoc Biol 2014; 96:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasula R, Downing JF, Wright JR, Kachel DL, Davis TE Jr, Martin WJ 2nd. Surfactant protein A (SP-A) mediates attachment of Mycobacterium tuberculosis to murine alveolar macrophages. Am J Respir Cell Mol Biol 1997; 17:209–17. [DOI] [PubMed] [Google Scholar]
- 27. Sidobre S, Nigou J, Puzo G, Riviere M. Lipoglycans are putative ligands for the human pulmonary surfactant protein A attachment to mycobacteria. J Biol Chem 2000; 275:2415–22. [DOI] [PubMed] [Google Scholar]
- 28. Torrelles JB, Schlesinger LS. Integrating lung physiology, immunology, and tuberculosis. Trends Microbiol 2017; 25:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rattan SI. Synthesis, modification and turnover of proteins during aging. Adv Exp Med Biol 2010; 694:1–13. [DOI] [PubMed] [Google Scholar]
- 30. Southworth LK, Owen AB, Kim SK. Aging mice show a decreasing correlation of gene expression within genetic modules. PLoS Genet 2009; 5:e1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell 2004; 3:161–7. [DOI] [PubMed] [Google Scholar]
- 32. Pereira LF, de Souza AP, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech Ageing Dev 2011; 132:187–94. [DOI] [PubMed] [Google Scholar]
- 33. Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol 2008; 181:7977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. Role of C-type lectins in mycobacterial infections. Curr Drug Targets 2008; 9:102–12. [DOI] [PubMed] [Google Scholar]
- 35. Carlson TK, Brooks M, Meyer D, et al. . Pulmonary innnate immunity: soluble and cellular host defenses of the lung. In: Marsh C, Tridandapani S, Piper M, eds. Regulation of innate immune function. Kerala: Transworld Research Network, 2010:165–211. [Google Scholar]
- 36. Gaynor CD, Schlesinger LS. Pulmonary surfactant protein A enhances adherence of Mycobacterium tuberculosis by human macrophages: evidence for a direct SP-A macrophage interaction. Clin Res 1994; 42:301A-A. [Google Scholar]
- 37. Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol 2002; 169:3565–73. [DOI] [PubMed] [Google Scholar]
- 38. Beharka AA, Crowther JE, McCormack FX, et al. . Pulmonary surfactant protein a activates a phosphatidylinositol 3-kinase/calcium signal transduction pathway in human macrophages: participation in the up-regulation of mannose receptor activity. J Immunol 2005; 175:2227–36. [DOI] [PubMed] [Google Scholar]
- 39. Ilumets H, Mazur W, Toljamo T, et al. . Ageing and smoking contribute to plasma surfactant proteins and protease imbalance with correlations to airway obstruction. BMC Pulm Med 2011; 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun 2012; 80:3343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Azad AK, Curtis A, Papp A, Webb A, Knoell D, Sadee W, et al. . Allelic mRNA expression imbalance in C-type lectins reveals a frequent regulatory SNP in the human surfactant protein A (SP-A) gene. Genes Immun 2013; 14:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haataja R, Hallman M. Surfactant proteins as genetic determinants of multifactorial pulmonary diseases. Ann Med 2002; 34:324–33. [DOI] [PubMed] [Google Scholar]
- 43. Malik S, Greenwood CM, Eguale T, Kifle A, Beyene J, Habte A, et al. . Variants of the SFTPA1 and SFTPA2 genes and susceptibility to tuberculosis in Ethiopia. Hum Genet 2006; 118:752–9. [DOI] [PubMed] [Google Scholar]
- 44. Notter RH. Lung surfactants: basic science and clinical applications. New York: Marcel Dekker, 2000:1–444. [Google Scholar]
- 45. Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987; 63:152–7. [DOI] [PubMed] [Google Scholar]
- 46. Madebo T, Lindtjørn B, Aukrust P, Berge RK. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. Am J Clin Nutr 2003; 78:117–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.