Abstract
Background
Our preclinical data showed that the leukotriene A4 hydrolase (LTA4H) pathway plays a role in colorectal cancer (CRC). High expression of LTA4H and leukotriene B4 receptor type 1 (BLT1) were also associated with CRC survival probability. Clinical samples were evaluated to determine whether LTA4H could serve as a therapeutic target and whether leukotriene B4 (LTB4) could be used as a biomarker for evaluating the efficacy of bestatin in CRC.
Methods
Patients with Stage I-III CRC did or did not receive bestatin prior to surgery. Evaluable pairwise CRC patient blood samples were collected to evaluate LTB4 concentration. Tissues were processed by immunohistochemistry to detect the LTA4H pathway and Ki-67 expression. We also determined whether LTA4H or BLT1 was associated with CRC survival probability and explored the mechanism of bestatin action in CRC.
Findings
Samples from 13 CRC patients showed a significant decrease in LTB4, the LTA4H signaling pathway, and Ki-67 in the bestatin-treated group compared with the untreated group. LTA4H and BLT1 are overexpressed in CRC and associated with CRC survival probability. Bestatin effectively inhibited LTB4 and tumorigenesis in the ApcMin/+ and CRC patient-derived xenograft mouse model.
Interpretation
These results demonstrate that LTB4 could serve as a biomarker for evaluating bestatin efficacy in CRC and the antitumor effects of bestatin through its targeting of LTA4H and support further studies focusing on LTA4H inhibition in CRC.
Keywords: Bestatin, LTA4H, LTB4, BLT1, Colorectal cancer
Research in context.
Evidence before this study
The overactivation of the leukotriene A4 hydrolase (LTA4H) pathway plays an important role in many cancers, including colorectal cancer (CRC). Therefore, LTA4H is considered to be a target for cancer chemoprevention and chemotherapy.
Added value of this study
In this study, we showed that the classical LTA4H inhibitor, bestatin, significantly inhibited LTB4 production. LTB4 is catalytic product of LTA4H and might serve as a blood biomarker for bestatin efficacy in CRC patients. Bestatin also significantly down-regulated the LTA4H signaling pathway and reduced proliferation and expression of Ki-67. Furthermore, bestatin effectively decreased the concentration of LTB4 and inhibited colorectal tumorigenesis in the ApcMin/+ mouse model and in a CRC patient-derived xenograft (PDX) SCID mouse model. These animal studies confirmed data obtained from clinical patient samples and support further studies for LTA4H inhibition in CRC.
Implications of all the available evidence
Our studies suggest that LTB4 can serve as a blood biomarker for bestatin efficacy in CRC patients. In addition, our findings suggest that LTA4H is a promising molecular target in CRC.
Alt-text: Unlabelled Box
1. Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer among both men and women in the United States [1]. Genetic factors as well as environmental factors that drive colorectal tumorigenesis have attracted extensive interest [2,3]. Although therapies have improved significantly, CRC still has high mortality rates. The number of deaths in 2017 is estimated to be about 50,260 people in the U.S. [4]. Therefore, CRC is a well-known multifactorial disease and presents an urgent problem that needs to be addressed.
Eicosanoids, including prostaglandins and leukotrienes (LTs), are biologically active lipids that are implicated in various pathological processes, such as inflammation and cancer [5,6]. In the eicosanoid metabolic processes, leukotriene A4 hydrolase (LTA4H) is an epoxide hydrolase that catalyzes conversion of the unstable allelic epoxide leukotriene A4 (LTA4) to leukotriene B4 (LTB4), which is known to have classical biological functions including chemotaxis, endothelial adherence, and activation of leukocytes [[7], [8], [9]]. It exerts its actions through a transmembrane protein receptor, LTB4 receptor type 1 (BLT1) [10]. Previous studies showed that LTB4 is overproduced in various types of human cancer and stimulates cancer cell proliferation [[11], [12], [13]]. Therefore, the LTA4H/LTB4 pathway might play an important role in the process of carcinogenesis. LTA4H is highly expressed in some human cancers, including esophageal adenocarcinoma, lung cancer, and thyroid cancer [14]. Our previous study showed that the LTA4H protein is highly expressed in skin cancer, and its inhibition leads to suppression of cancer development [15]. Thus, LTA4H might be a potential target for suppressing cancer progression. However, the relationship and mechanism of LTA4H and CRC has not been well studied.
Bestatin (ubenimex) is a dipeptide immunomodulator that was discovered in the culture supernatant fraction from Streptomyces olivoreticuli [16,17]. It is a well-characterized inhibitor of LTA4H [18,19] and was shown to attenuate LTB4 synthesis and reduce the burden of esophageal adenocarcinoma in a rat model [20]. In cell-based studies, bestatin inhibited the invasion of human metastatic tumor cells and induced apoptosis in human non-small-cell lung cancer cell lines [21]. In clinical studies, bestatin prolonged the survival of patients with acute adult nonlymphocytic leukemia, who also received chemotherapy [22]. It also has an immunomodulatory effect in patients with lymphoma after autologous bone marrow transplantation [23].
We found that LTA4H and BLT1 are highly expressed in colitis and correlate with colon adenocarcinoma. High expression of LTA4H and BLT1 also negatively correlated with CRC patient survival probability. In this study, bestatin effectively reduced LTB4 expression level and the expression of LTA4H downstream proteins in patient samples. It also significantly inhibited tumor cell proliferation by suppressing Ki-67 protein expression in CRC patients. Knockdown of LTA4H or treatment with bestatin significantly attenuated proliferation and colony formation of CRC cells. Our in vivo study results showed that bestatin could inhibit colorectal tumorigenesis in both an ApcMin/+ mouse model and a patient-derived xenograft (PDX) mouse model. Overall, these results indicate that bestatin targets the LTA4H pathway to efficiently prevent progression of advanced CRC and that LTB4 might be used as a biomarker for evaluating treatment efficacy of bestatin.
2. Patients and methods
2.1. Patient samples and study design
The screening criteria for eligible subjects included age ≥ 18 years, stage I-III diagnostic CRC patients who received bestatin (30 mg/d, p.o.) around 7 days before surgical resection or were untreated. Patients provided pairwise blood samples and tumor specimens of sufficient quality for subsequent analysis. Exclusion criteria included subjects with tumor recurrence or metastasis and existence of other malignancies, uncontrolled hypertension, or serious cardiovascular disease, liver and renal dysfunction, spleen resection or other serious hematopoietic system diseases, uncontrollable infection, or severe peptic ulcer. The patients who received bestatin treatment before surgical resection were placed into a bestatin-treated group. The patients who did not receive treatment with bestatin before surgical resection were put into an untreated group. Pairwise blood samples from the bestatin-treated or untreated group were used to evaluated changes in LTB4 concentration and patient tissues were processed to clarify differences in the LTA4H pathway between the bestatin-treated group and untreated group. Tumor proliferation marker Ki-67 was evaluated. This study was conducted with the approval of the Affiliated Tumor Hospital of Zhengzhou University Research Ethics Committee (Zhengzhou, Henan, China; No. 2018084).
2.2. Reagents and antibodies
Media, gentamicin, penicillin, and l-glutamine were all purchased from Invitrogen (Grand Island, NY, USA) and bestatin was purchased from BOC Sciences (Shirley, NY, USA). Antibodies to detect LTA4H (160250, RRID: AB_10079032) and BLT1 (120114, RRID: AB_327854) were obtained from Cayman Chemicals (Ann Arbor, MI, USA). Antibodies to detect β-actin (sc-47778, RRID: AB_2714189), GAPDH (sc-25778, RRID: AB_10167668), Ki-67 (sc-7846, RRID: AB_2142374) and c-Myc (sc-40, RRID: AB_627268) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies to evaluate p-MEK (Ser217/221) (#9121, RRID: AB_331648), MEK (#9122, RRID: AB_823567), p-ERK1/2 (Thr202/Tyr204) (#9101, RRID: AB_331646), ERK1/2 (#9102, RRID: AB_330744), CD8 (#98941, RRID: AB_2756376), and Ki-67 (#9027, RRID: AB_2636984) were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.3. Cell culture and transfection
Human colonic epithelial cells (HCEC), a normal human colon cell line, was kindly provided by Dr. Jerry. W. Shay (University of Texas Southwestern Medical Center, Dallas, Texas). The cells were isolated from biopsy samples from patients and immortalized by the nononco-genic protein cyclin-dependent kinase 4 (Cdk4) and the catalytic component of human telomerase (hTERT) [24]. The colorectal adenocarcinoma cell lines (DLD-1, HCT-15, HT-29, HCT 116), and 293T cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were cytogenetically tested and authenticated before being frozen. Each vial of frozen cells was thawed and maintained in culture for a maximum of 8 weeks. Following ATCC protocols, all cells were cultured in a 5% CO2 humidified incubator at 37 °C. DLD-1 and HCT-15 cells were cultured with RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 1% antibiotics. HT-29 and HCT 116 cells were cultured with McCoy's 5A medium containing 10% FBS and 1% antibiotics and 293T cells were cultured at 37 °C in a humidified incubator with 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 1% antibiotics.
2.4. Lentiviral infection
The lentiviral expression vector (PLKO.1-shLTA4H) and packaging vectors (pMD2.0G and psPAX) were purchased from OpenBioSystems (Huntsville, AL, USA) and were transfected into 293 T cells using the iMFectin Poly DNA transfection reagent (GenDEPOT) following the manufacturer's instructions. The lentivirus plasmids shLTA4H (#1, TRCN0000050863, CCGGCGGCCCTTATTCA AGGATCTTCTCGAGAAGATCCTTGAATAAGGGCCGTTTTTG; #2, TRCN0000050864, CCGG GCCTCCCATAAAGCCCAATTACTCGAGTAATTGGGCTTTATGGGAGGCTTTTTG; #3, TRCN0000050865, CCGGCCCTGCTACCTGATTGCTTTACTCGAGTAAAGCAATCAGGTAGC AGGGTTTTTG; #4, TRCN0000050866, CCGGCCTTCTGTGAAATTAACCTATCTCGAGATAG GTTAATTTCACAGAAGGTTTTTG; #5, TRCN0000050867, CCGGCGCAATTCCTTTGGCGCT AAACTCGAGTTTAGCGCCAAAGGAATTGCGTTTTTG) were purchased from BMGC RNAi (University of Minnesota, Minneapolis, MN, USA). When cells reached 70–80% confluence, iMFectin-DNA complexes were dropped into the medium of 293 T cells and incubated with cells for 12 h. Then medium was replaced with fresh complete medium containing serum and antibiotics. After collecting the viral supernatant fractions at 24 and 48 h, DLD-1 and HCT-15 cells were infected and screened with puromycin.
2.5. Crystal violet staining assay
Cells (2 × 104 per well) for measuring proliferation and cells (5 × 104 per well) for testing compound cytotoxicity were seeded into 24-well plates. After overnight incubation, cells were treated with different concentrations of bestatin. Cells for measuring cytotoxicity were incubated for 24 or 48 h and cells for measuring proliferation were incubated for 24, 48, or 72 h. After washing 3 times with phosphate buffered saline (PBS), cells from each different time point were fixed with methanol for 10 min and stained with 0.2% (w/v) crystal violet in 2% (v/v) ethanol for 10 min. Then, 0.5% (w/v) sodium dodecyl sulfate in 50% (v/v) ethanol was added to each well. Absorbance was measured at an optical wavelength of 540 nm using the Thermo Multiskan plate-reader (Thermo Fisher Scientific, Waltham, MA, USA).
2.6. Anchorage-independent cell growth assay
Cells (8 × 103 per well) were suspended in 1 ml BME/10% FBS/0.33% agar with different concentrations of bestatin or vehicle and plated on 3 ml of solidified BME/10% FBS/0.5% agar with vehicle or the same concentrations of bestatin in each well of 6-well plates and cultured for 1 to 2 weeks. shMOCK- and shLTA4H-expressing cells (8 × 103 per well) were suspended in 1 ml BME/10% FBS/0.33% agar and plated on 3 ml of solidified BME/10% FBS/0.5% agar and cultured for 1 to 2 weeks. The number of colonies was determined by microscope using the Image-Pro Plus software program (Media Cybernetics, Inc. Rockville, MD, USA).
2.7. Mouse model and drug administration
C57BL/6J-ApcMin/+ mice (ApcMin+ mice, RRID: IMSR_JAX:002020) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were bred and genotyped according to the Jackson Laboratory genotyping protocol (APC-mutant: 5′-TTCTGAGAAAGACAGAAGTTA-3′; APC-common: 5′-TTCCACTTTGGCATAAGGC-3′; APC-wild-type:5′ - GCCATCCCTTCACGT TAG-3′). Mice (5–6 weeks) were divided into 3 groups: 1) control group; 2) bestatin 5 mg/kg group; and 3) bestatin 15 mg/kg group. The bestatin-treated groups were gavaged with bestatin (5 or 15 mg/kg) daily for 8 weeks. Mice were weighed weekly and checked daily for any health problems. At the end of the study, mice were euthanized with CO2 and intestine, colorectal tissues, and blood samples were collected. All animal studies were performed and approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC).
2.8. Patient-derived xenograft (PDX) mouse model
CRC tissue fragments were collected from the 1st Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan, China) and cut into 2–3 mm fragments and implanted into female SCID mice (Vital River Laboratories Co, Ltd., Beijing, China) within 2 h. This study was approved by the Zhengzhou University Institutional Animal Care and Use Committee (Zhengzhou, Henan, China). Once tumor volumes reached approximately 150 mm3, mice were divided into the 3 groups and treated with vehicle (PBS) or bestatin daily. Mice from the control group were treated with PBS, mice from the low dose bestatin group were treated with 5 mg/kg bestatin and, mice from the high dose group of bestatin were treated with 15 mg/kg bestatin. Tumor volume and body weight were recorded every week.
2.9. Immunohistochemical staining
Two human colon disease spectrum tissue microarrays (CO2081 and BC05002b) were obtained from US Biomax, Inc. (Rockville, MD, USA). Another two human colon adenocarcinoma tissue microarrays (HColA180Su14) were obtained from Shanghai Outdo Biotech Co, Ltd. (Shanghai, China). Human CRC tissues and adjacent normal tissues were collected from the Affiliated Tumor Hospital of Zhengzhou University. The colon tissue from ApcMin/+ mice and the colon tumor tissues from the PDX mouse model were embedded in paraffin for subsequent analysis. Slides were incubated with different primary antibodies (BLT1, 1:50; p-ERK1/2, 1:200; p-MEK, 1:50, c-Myc, 1:75; CD8, 1:100; Ki-67, 1:50) overnight at 4 °C. Then the slides were incubated with the secondary antibody from Vector Laboratories (Burlingame, CA, USA; anti-rabbit 1:150, anti-mouse 1:150) for 1 h at room temperature. Slides were stained using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA). The slides were counterstained with hematoxylin after developing with 3,3′-diaminobenzidine and were analyzed using the Image-Pro PLUS (v.6) computer software program (Media Cybernetics, Inc., USA).
2.10. Quantitative real-time PCR
Total RNA from tissues was extracted using the Trizol Reagent (Invitrogen) and 100 ng of total RNA was used for analysis. The real-time primers for BLT1 were: F:5′ CCTGTGTCACTATGT CTGCGGA-3′; and R:5′ ATCGCCTTGGTGCGTAGCTTCT-3′. The real-time primers for c-Myc were: F:5′ CCTGGTGCTCCATGAGGAGAC-3′; and R:5′ CAGACTCTGACCTTTTGCCAGG-3′. The real-time primers for Ki-67 were: F:5′ GAAAGAGTGGCAACCTGCCTTC-3′; and R:5′ GCACCAAGTTTTACTACATCTGCC-3′.
2.11. LTB4 production assay
Whole blood was collected from patients and serum was separated and stored at −80 °C until analysis. Serum from mice was also collected and stored at −80 °C. shMOCK- and shLTA4H-expressing cells were seeded and medium was collected after 48 h and LTB4 concentration was measured using the LTB4 ELISA kit from Cayman Chemicals (Ann Arbor, MI, USA).
2.12. Western blot analysis
To determine protein concentration, a protein assay kit (Bio-Rad Laboratories, Hercules, CA) was used. After subjection to SDS-PAGE, the samples were transferred to polyvinylidene difluoride (PVDF) membranes (EMD Millipore Corporation). Then the membranes were blocked with 5% nonfat milk for 1 h at room temperature and incubated with specific primary antibodies overnight at 4 °C. Finally, the membranes were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Protein bands were visualized with a chemiluminescence reagent (GE Healthcare Biosciences).
2.13. Statistical analysis
All quantitative data are expressed as mean values ± S.E. or S.D. as indicated. A one-way ANOVA was used for statistical analysis. Survival curves were tested by the log-rank test. p < .05 was used as the criterion for statistical significance.
3. Results
3.1. LTA4H and BLT1 are overexpressed in colon adenocarcinoma and colitis and are associated with CRC patient survival probability
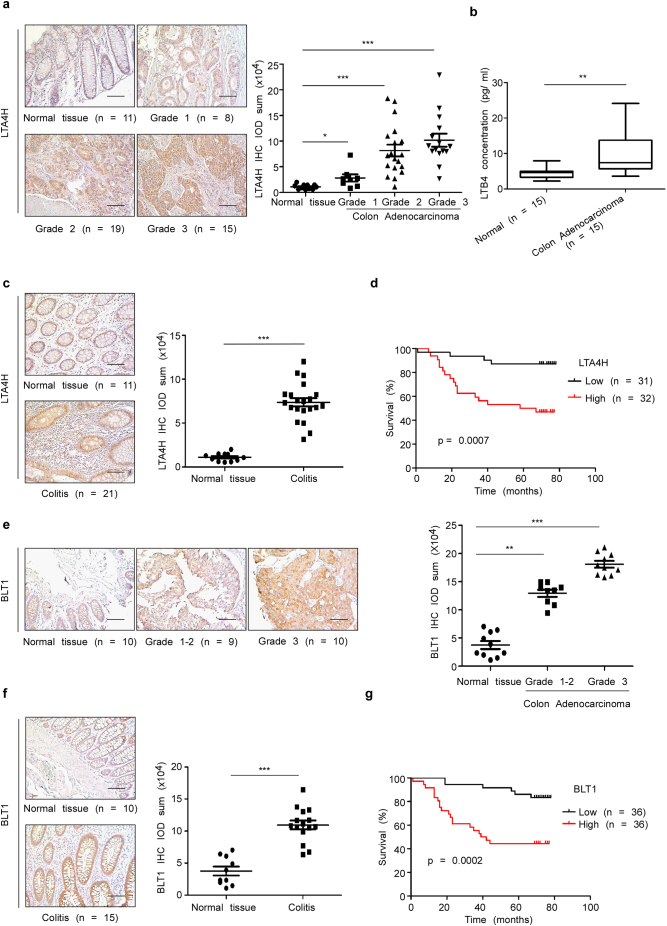
In the present study, we studied the molecular mechanism of LTA4H's action in CRC development. To determine whether LTA4H is associated with CRC progression, protein expression was determined using colon disease spectrum tissue arrays. Overexpression of LTA4H was observed in colon adenocarcinoma tissues compared with normal human colon tissues (Fig. 1a). LTB4, is the catalytic product of LTA4H and showed a higher concentration in CRC patients samples compared with normal adult subjects (Fig. 1b). LTA4H was also highly expressed in colitis tissue (Fig. 1c) (tissue microarray CO2081). We also analyzed the relationship between elevated LTA4H expression and CRC patient survival probability by using the KM plotter (Fig. 1d, tissue microarray HColA180Su14). The result indicated that the survival probability of CRC patients with high LTA4H expression is significantly lower than that of patients with low LTA4H expression. Several CRC cell lines also highly express LTA4H (Fig. s1). LTB4 is produced by LTA4H and can increase BLT1 mRNA and protein levels [25]. Consequently, BLT1, a high affinity receptor of LTB4, also showed higher expression in colon adenocarcinoma compared with normal human colon tissues (Fig. 1e) and is also highly expressed in colitis (Fig. 1f; tissue microarray BC05002b). Interestingly, high expression of BLT1 was also linked with lower survival probability (Fig. 1g, tissue microarray HColA180Su14). These results indicate that LTA4H might be a critical molecule in human colon carcinogenesis and associated with CRC patient survival probability.
Fig. 1.
LTA4H and BLT1 are overexpressed in human colon adenocarcinoma tissues and colitis and are associated with CRC patient survival probability. (a) LTA4H levels in human colon tissue were analyzed by immunohistochemistry and the density score from each sample was determined (right panel). Representative cases are shown (left panels). The asterisk (*, ***) indicates a significant (p < .05, p < .001, respectively) increase in LTA4H expression in colon adenocarcinoma tissue compared with normal tissue (scale bar, 100 μm). (b) LTB4 concentration in human serum was detected by ELISA assay. The asterisks (**) indicate a significant (p < .01) difference in CRC patients compared with normal adult subjects. (c) LTA4H levels in human colitis were analyzed by immunohistochemistry. The asterisks (***) indicate a significant (p < .001) increase in LTA4H expression in colitis tissue compared with normal tissue (scale bar, 100 μm). (d) Kaplan-Meier survival curves showed the life span of CRC patients with LTA4H high expression (n = 32) or LTA4H low expression (n = 31). (e) BLT1 levels in human colon tissue were analyzed by immunohistochemistry. The asterisks (**, ***) indicate a significant (p < .01, p < .001, respectively) increase in BLT1 expression in colon adenocarcinoma tissue compared with normal tissue (scale bar, 100 μm). (f) BLT1 levels in human colitis were analyzed by immunohistochemistry. The asterisks (***) indicate a significant (p < .001) increase in BLT1 expression in colitis tissue compared with normal tissue (scale bar, 100 μm). (g) Kaplan-Meier survival curves relative to BLT1 expression were analyzed for CRC patients.
3.2. Patient and tumor characteristics
After screening, a total of 13 patients were available based on the inclusion criteria (Table 1). In the untreated group (n = 6), the median age was 72.5 years (range, 42–90 years), 33.3% were male, and 33.3% were current smokers. No patients were stage I, 66.7% were stage II, and 33.3% were stage III. In the bestatin-treated group (n = 7), the median age was 60 years (range, 41–72 years), 71.4% were male, and 28.6% were current smokers. The percentage of patients in this group at stage I was 28.6%, stage II 57.1%, and stage III 14.3%.
Table 1.
Demographics and baseline characteristics.
| Characteristic | Untreated (n = 6) | Bestatin-treated (n = 7) |
|---|---|---|
| Median age, years (range) | 72.5 (42–90) | 60 (41–72) |
| Sex, No. (%) | ||
| Male | 2 (33.3) | 5 (71.4) |
| Female | 4 (66.7) | 2 (28.6) |
| Smoking history, No. (%) | ||
| Never smoker | 4 (66.7) | 5 (71.4) |
| Current smoker | 2 (33.3) | 2 (28.6) |
| Tumor stage at screening No. (%) | ||
| I | 0 (0) | 2 (28.6) |
| II | 4 (66.7) | 4 (57.1) |
| III | 2 (33.3) | 1 (14.3) |
| IV | 0 (0) | 0 (0) |
3.3. Bestatin suppresses the LTB4 blood biomarker in CRC patients
Peripheral blood samples from day 0 and day 7 from untreated and bestatin-treated patients were collected. Matched peripheral blood samples were used to evaluate LTB4 concentration as a potential biomarker for CRC by using an ELISA assay. At day 0, the LTB4 concentration in the bestatin-treated and untreated group showed no statistical difference. In a comparison of the LTB4 concentration in matched samples from days 0 and 7, we found that the LTB4 concentration in the bestatin-treated group (5 of 7 patients;71.4%) showed a decreased LTB4 concentration; whereas in the untreated group, only 2 of 6 patients (33.3%) showed decreased LTB4 (Fig. 2a). At the same time, the percentage change in LTB4 at day 7 compared with day 0 was analyzed and shown individually (Fig. 2b). The average change in LTB4 concentration decreased 26.76% in the bestatin-treated group, whereas LTB4 increased 23.74% in the untreated group. The change in LTB4 was significantly different between the bestatin-treated and untreated group (Fig. 2c).
Fig. 2.
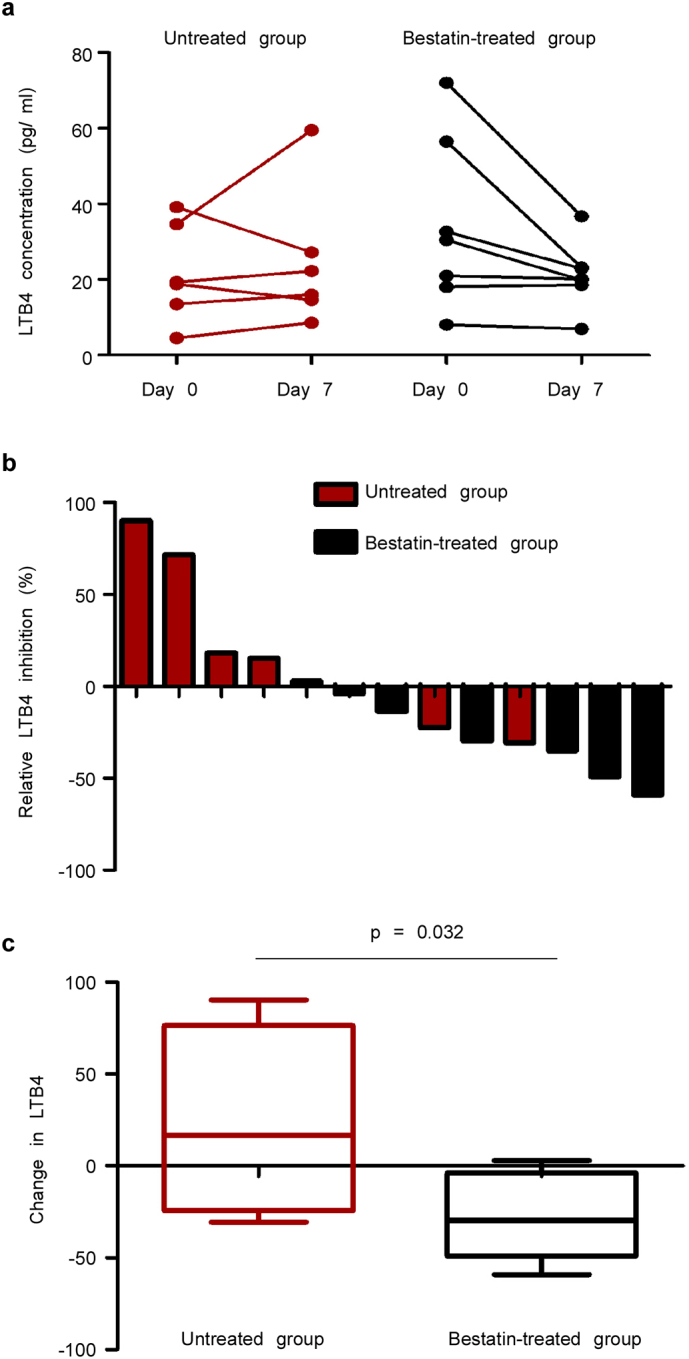
Bestatin suppresses the LTB4 blood biomarker in CRC patients. (a) LTB4 serum concentration was evaluated in pre-treated and post-treated in bestatin-treated patients. LTB4 serum concentration in untreated patients was also measured at 0 and 7 days. Individual changes are show from day 0 to day 7. (b) The percentage of individual relative change in LTB4 from day 0 to day 7 is shown in a waterfall plot. (c) The relative change in LTB4 in the bestatin-treated group was compared with the untreated group. The data are shown as mean values ± S.E. (untreated group, n = 6; bestatin-treated group, n = 7).
3.4. Bestatin suppresses LTA4H pathway protein expression in CRC patients
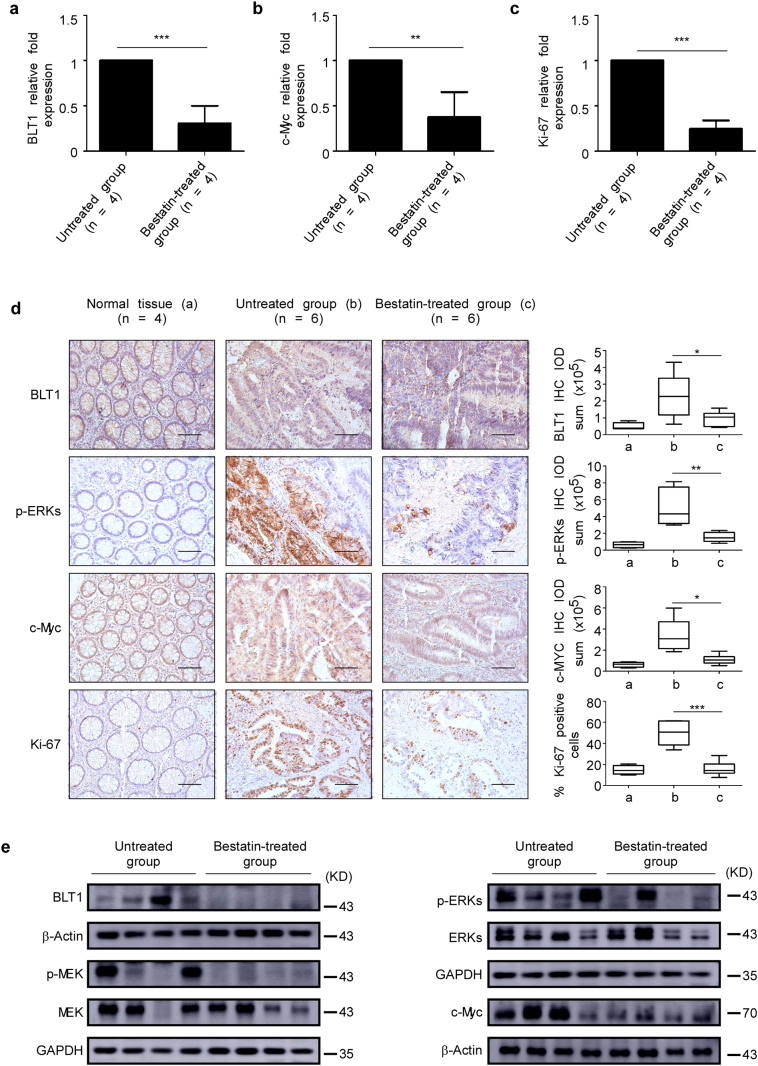
The mRNA levels of BLT1, c-Myc, and Ki-67 are suppressed in the bestatin-treated group compared with the untreated group (Fig. 3a–c). Immunohistochemical analysis of colon tissues from the untreated and the bestatin-treated groups confirmed a reduced protein level of BLT1, p-ERKs, c-Myc, and Ki-67 (Fig. 3d). Western blot results also showed that the expression of BLT1, p-MEK, p-ERK1/2, and c-Myc was reduced in tissues from bestatin-treated group (Fig. 3e). Collectively, these data indicate that bestatin blocked signaling through the LTA4H/BLT1/ERKs oncogenic axis, ultimately resulting in inhibition of CRC.
Fig. 3.
Bestatin suppresses the BLT1-ERK1/2 signaling pathway in CRC patients. (a–c) mRNA levels of BLT1, c-Myc, and Ki-67 in human CRC patient tissues were analyzed by quantitative PCR (qPCR). Relative mRNA levels were normalized against GAPDH. The data are shown as mean values ± S.E. from triplicate experiments and the asterisks (**, ***) indicate a significant (p < .01, p < .001) decrease in tissues from the group treated with bestatin compared with the untreated group. (d) Immunohistochemistry analysis was used to determine the expression of BLT1, p-ERK1/2 (Thr202/Tyr204), c-Myc,and Ki-67 in patients treated with bestatin compared with untreated patients. The asterisks (*, **, ***) indicate a significant (p < .05, p < .01, p < .001, respectively) decrease in protein expression in the bestatin-treated group compared with the untreated group (scale bar, 100 μm). (e) The expression of BLT1, p-MEK (Ser217/221), p-ERK1/2 (Thr202/Tyr204), c-Myc, and Ki-67 was also determined by Western blot.
3.5. Knocking down LTA4H expression blocks colony formation of colon cancer cells
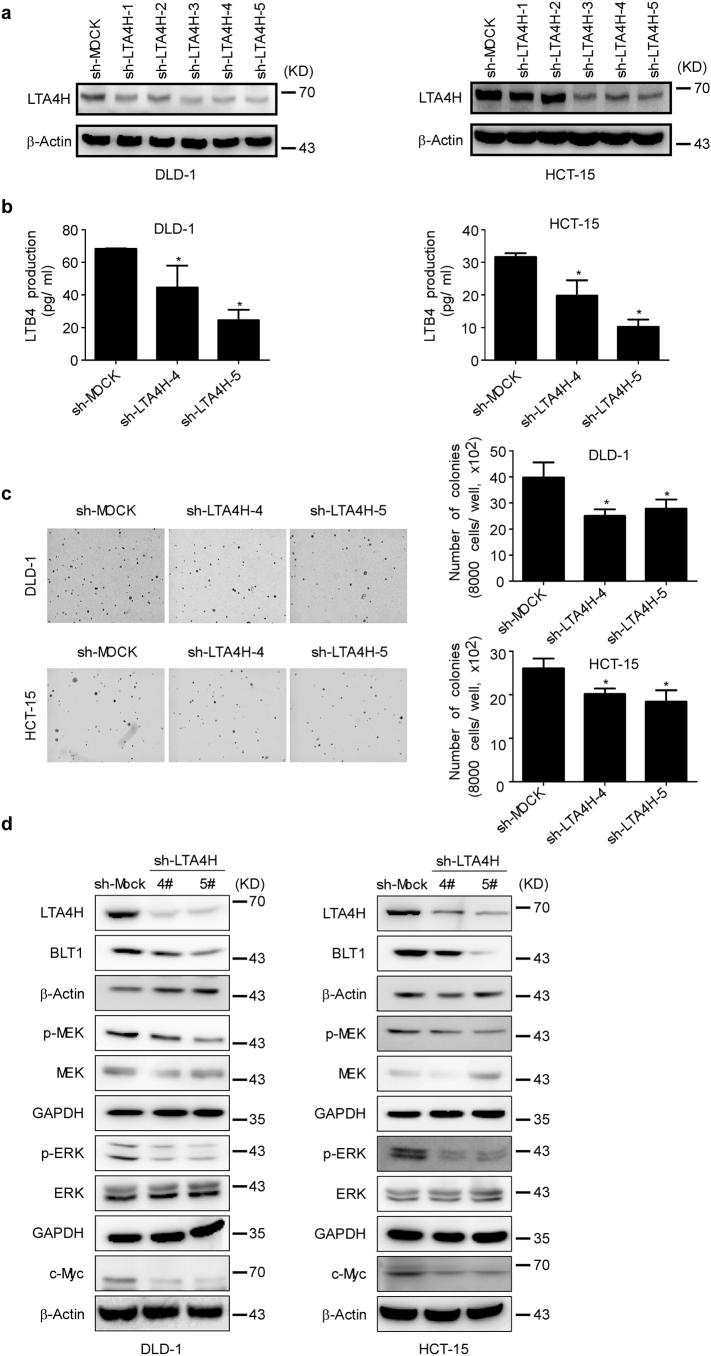
To investigate the role of LTA4H in colon carcinogenesis, we first depleted LTA4H expression in DLD-1 and HCT-15 colon cancer cell lines (Fig. 4a). LTA4H works as a bifunctional zinc enzyme by exhibiting activities of both epoxide hydrolase and aminopeptidase. Depletion of LTA4H significantly suppressed LTB4 production in both DLD-1 and HCT-15 colon cancer cells (Fig. 4b), Depletion of LTA4H also reduced colony growth in both DLD-1 and HCT-15 cells (Fig. 4c). LTB4 can bind to the BLT1 high affinity receptor and promote cell proliferation and survival by activating the BLT1/ERK1/2 and PI3-K/Akt pathways [26,27]. Consistently, depletion of LTA4H inhibited BLT1 expression, thus affecting the downstream MEK/ERK1/2 pathways. The same results were observed in both DLD-1 and HCT-15 colon cancer cells (Fig. 4d). These results confirm that LTA4H plays an important role in colon carcinogenesis and could be regarded as a target for preventing or treating colon cancer.
Fig. 4.
LTA4H mediates cell transformation. (a) Knockdown of LTA4H expression in DLD-1 and HCT-15 colon cancer cell lines. (b) LTB4 production is decreased in DLD-1 and HCT-15 colon cancer cell lines after knockdown of LTA4H. The data are shown as mean values ± S.E. from triplicate experiments. The asterisk (*) indicates a significant (p < .05) decrease in LTB4 production in shLTA4H cells compared to shMOCK cells. (c) DLD-1 or HCT-15 cells were transfected with shLTA4H or shMOCK and grown in soft agar and then colonies were counted. The data are shown as mean values ± S.E. from triplicate experiments. The asterisk (*) indicates a significant (p < .05) decrease in colony formation in shLTA4H cells compared to shMOCK cells. (d) DLD-1 or HCT-15 colon cancer cells were transfected with shLTA4H or shMOCK. Cells were harvested and the expression levels of phosphorylated and total proteins were analyzed by Western blotting.
3.6. Bestatin inhibits colony formation and proliferation of colon cancer cells
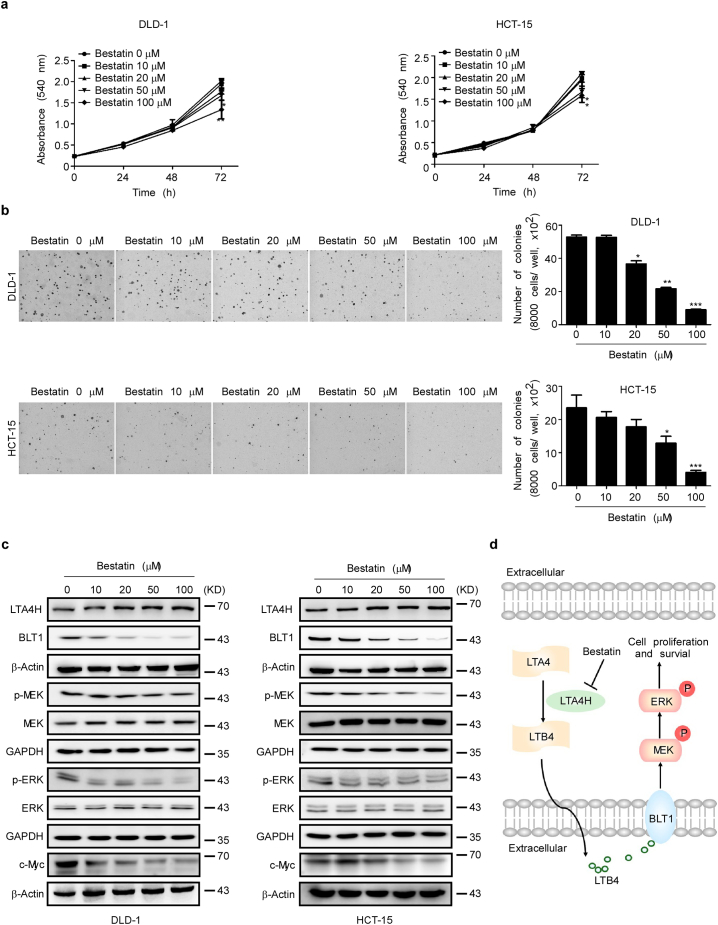
Previous results indicated that LTA4H was highly expressed in human CRC. We next determined whether bestatin (Fig. s2a) could affect proliferation and colony formation of CRC cells. Bestatin- treatment had no cytotoxic effects on normal HCEC cells (Fig. s2b). DLD-1 and HCT-15 cells were treated with different doses of bestatin and the results revealed that bestatin inhibited proliferation of these cells (Fig. 5a). The colony formation of DLD-1 and HCT-15 cells was inhibited by bestatin (Fig. 5b). Notably, bestatin markedly suppressed BLT1 expression and inhibited phosphorylation of MEK and ERK1/2 (Fig. 5c). c-Myc also is regulated by the ubiquitin-mediated proteasomal degradation pathway [28] and is phosphorylated and stabilized by the ERK1/2 pathway [29]. Thus, the ability of bestatin to inhibit colon tumorigenesis might be due to its targeting and inhibition of LTA4H activity leading to attenuation of the BLT1/ERK1/2 pathways. This would maintain c-Myc at lower levels because of increased ubiquitin-mediated proteasomal degradation. Thus, bestatin shows a good inhibitory effect against CRC in cells. To further confirm that bestatin precisely targets LTA4H, we conducted a colony formation assay with shMOCK- and shLTA4H-expressing colon cancer cells. The results showed that bestatin could not inhibit colony formation in LTA4H knockdown cells (Fig. s5a, b). Overall, bestatin targets LTA4H, suppresses LTB4 secretion, inhibits BLT1 and the associated downstream ERK1/2 pathway, and then attenuates colon cancer progression (Fig. 5d).
Fig. 5.
Bestatin inhibits anchorage-independent growth and proliferation of colon cancer cells. (a) DLD-1 or HCT-15 colon cancer cells were treated with different concentrations of bestatin for 0, 24, 48, or 72 h and proliferation was estimated by a crystal violet staining assay. The data are shown as mean values ± S.E. from triplicate experiments. The asterisks (*, **) indicate a significant (p < .05, p < .01, respectively) decrease in proliferation compared to the vehicle-treated control group. (b) DLD-1 or HCT-15 colon cancer cells were treated with increasing doses of bestatin. The data are shown as mean values ± S.E. from triplicate experiments. The asterisks (*, **, ***) indicate a significant (p < .05, p < .01, p < .001, respectively) decrease in colony formation compared to the vehicle-treated control group. (c) DLD-1 or HCT-15 colon cancer cells were treated with different concentrations of bestatin. Cells were harvested and the expression levels of phosphorylated and total proteins were analyzed by Western blotting. (d) The schematic mechanism of bestatin's effects against colon cancer mediated through the LTA4H-BLT1-ERK1/2 pathway is illustrated. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
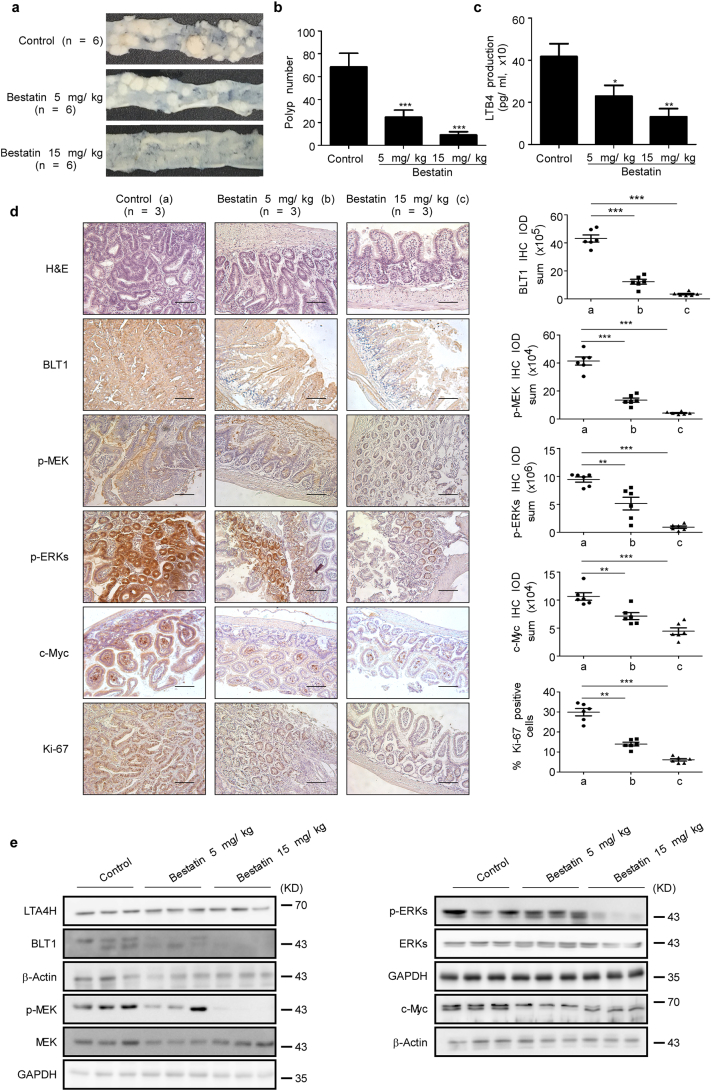
3.7. Bestatin reduces intestinal adenoma formation in ApcMin/+ mice
The adenomatous polyposis coli (apc) gene encodes a key tumor suppressor that plays an important role in colorectal tumorigenesis [30] and we determined whether bestatin could be effective against CRC in vivo. The ApcMin/+ mouse carries a point mutation in the apc gene and these mice develop multiple intestinal neoplasia (Min) after they spontaneously lose the heterozygous wild-type apc allele. We examined polyp formation of control mice and mice treated with different amounts of bestatin by appearance and hematoxylin and eosin (H&E) staining (Fig. 6a, Fig. s3a, b). The mice from the untreated control group developed more polyps throughout the small and large intestines compared with the bestatin-treated groups (Fig. 6b). The untreated control group also exhibited higher concentration of LTB4 in serum compared with the bestatin-treated group (Fig. 6c). The body weight of all animals remained stable after daily treatment with bestatin or vehicle by oral gavage (Fig. s3c). Additionally, immunohistochemical analysis showed that BLT1 and phosphorylation of MEK, ERK1/2, and c-Myc were substantially suppressed in the bestatin-treated group compared with the untreated control group. Bestatin also reduced the expression of Ki-67 (Fig. 6d) and Western blot analysis showed that bestatin attenuated signaling through the BLT1/ERK1/2 pathway (Fig. 6e). These results suggest that bestatin can control colorectal tumorigenesis and the subsequent manifestation of the Min phenotype in ApcMin/+ mice.
Fig. 6.
Bestatin prevents intestinal adenoma formation in ApcMin/+ mice. (a) Representative photographs show polyp formation in ApcMin/+ mice treated or not treated with bestatin. (b) Polyp number was measured in mice. The asterisks (***) indicate a significant (p < .001) decrease in polyp formation in mice treated with bestatin compared with the control group. The data are shown as mean values ± S.E., (n = 6). (c) LTB4 production was measured in these groups. The data are shown as mean values ± S.E. and the asterisks (*, **) indicate a significant (p < .01, p < .001, respectively) decrease in LTB4 production in bestatin-treated mice compared with the control group. (d) Immunohistochemistry analysis was used to determine the level of BLT1, Ki-67, and phosphorylation of ERK1/2 (Thr202/Tyr204) and MEK (Ser217/221) in the control group compared with the bestatin-treated group. Representative photographs for each protein and each group are shown. The integrated optical density (IOD) was evaluated using the Image-Pro Premier software (v.9.0) program. The asterisks (*, **, ***) indicate a significant (p < .05, p < .01, p < .001, respectively) decrease in protein expression compared with the control group (Scale bar, 100 μm). (e) Representative western blot analysis confirmed that bestatin inhibited expression of BLT1 and downstream signaling pathways.
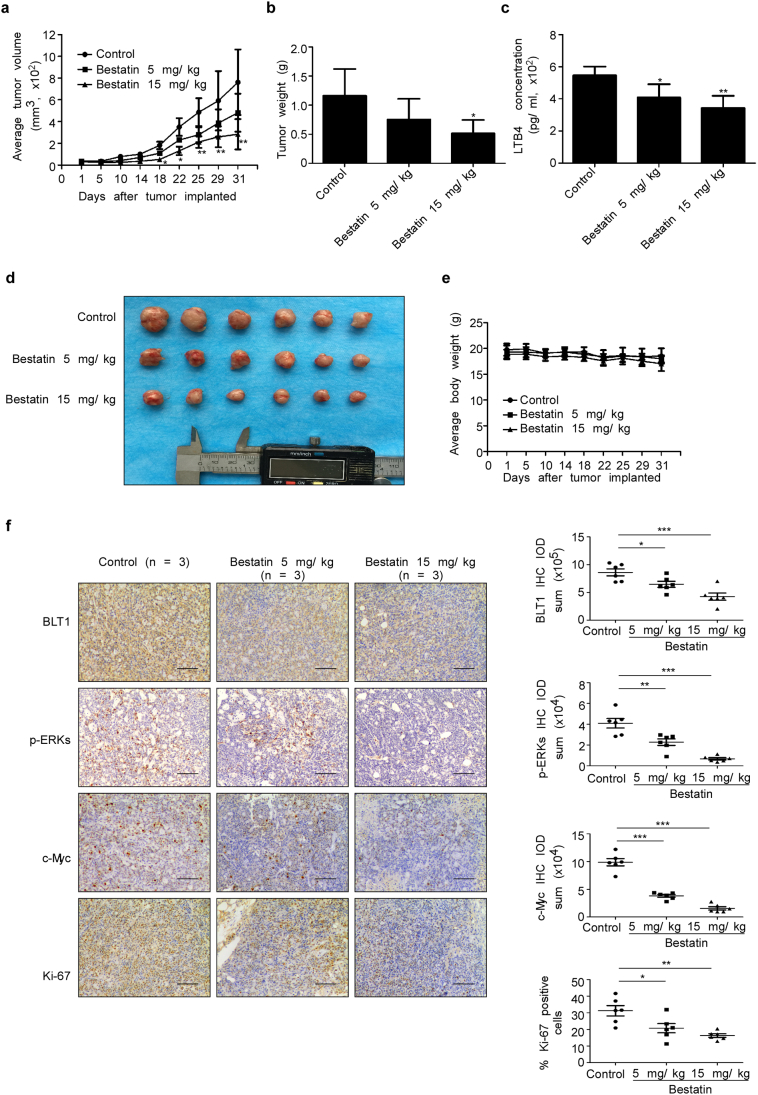
3.8. Bestatin suppresses tumor growth in a PDX mouse model
Patient-derived xenografts (PDXs) have been used more frequently to investigate the effectiveness of drugs because they are believed to retain most of the original molecular characteristics and heterogeneity of the cancer patient [31,32]. To further investigate the clinical effect of bestatin, we established a PDX mouse model that mimics tumors from the human CRC patient. These mice were treated or not treated with bestatin. The results showed that bestatin suppressed tumor growth, in terms of both tumor size (Fig. 7a) and tumor weight (Fig. 7b). Bestatin also reduced LTB4 concentration in mouse serum (Fig. 7c). Photographs showed differences in tumor size in each of the 3 groups (Fig. 7d). Bestatin had no effect on mouse body weight (Fig. 7e). Moreover, immunohistochemical analysis results showed that bestatin inhibited the expression of BLT1, p-ERK1/2, c-Myc, and Ki-67 compared with the untreated control group (Fig. 7f). Overall, these results provide evidence suggesting that bestatin might serve as chemotherapeutic agent against CRC.
Fig. 7.
Bestatin inhibits tumor growth in a PDX mouse model. (a) Mice bearing PDX tumors were treated or not treated with bestatin. The asterisks (*, **) indicate a significant (p < .05, p < .01, respectively) decrease in tumor volume in mice treated with bestatin compared with the control group. (b) Tumor weight was measured at the end of the study. The asterisk (*) indicates a significant (p < .05) decrease in tumor weight in bestatin-treated mice compared with control mice. The data are shown as mean values ± S.E., (n = 6). (c) LTB4 concentration was measured in mouse serum. The asterisks (*, **) indicate a significant (p < .05, p < .01, respectively) decrease in LTB4 concentration in bestatin-treated mice compared with control mice. (d) Photographs of tumors from each group are shown. (e) Bestatin treatment has no effect on the body weight of mice. (f) Immunohistochemistry analysis was used to determine the levels of BLT1, p-ERK1/2 (Thr202/Tyr204), c-Myc, and Ki-67 in bestatin-treated and control mice. Representative photographs for each protein and each group are shown. The integrated optical density (IOD) was evaluated using the Image-Pro Premier software (v.9.0) program (scale bar, 100 μm). The asterisks (*, **) indicate a significant (p < .05, p < .01, respectively) decrease in protein expression compared with the control group.
4. Discussion
Targeted therapy is a type of cancer treatment strategy that targets changes in cancer cells that help them grow, divide, and spread. Additionally, studies focusing on elucidation of the molecular pathology that drives cancer will help to design promising therapies. This study took advantage of clinical samples to clarify the LTA4H pathway as a target for bestatin-treatment in CRC. Several key findings were obtained from our study with the LTA4H inhibitor bestatin in CRC patients. These findings support further study focusing on the function of bestatin in CRC patients. First, a substantial subset (71.4%) of evaluable patients in bestatin-treated group showed decreases in LTB4 concentration. The relative LTB4 change was significantly different in the bestatin-treated group compared with the untreated group. Second, we observed down-regulation of LTA4H pathway-related proteins in the bestatin-treated group compared with the untreated group. Finally, Ki-67 is a prognostic marker in colorectal cancer [33] and it also was significantly reduced in the bestatin-treated group.
In this investigation, we focused on the LTA4H pathway because of its important role in cancer development [14,34,35]. LTA4H is an important target for cancer chemoprevention and chemotherapy [36]. Here, we showed that LTA4H is highly expressed in colitis and colon adenocarcinoma compared with normal colon tissue. BLT1 is an LTB4 receptor and is also highly expressed in colitis and colon adenocarcinoma. Moreover, the survival probabilities of CRC patients with high LTA4H and BLT1 expression were significantly lower compared to patients with low LTA4H and BLT1 expression. Furthermore, LTB4 is a catalytic product of LTA4H and is easy to detect and assess the change in concentration in patient blood samples. Thus, LTB4 might be a blood biomarker useful for evaluating the effect of inhibiting the LTA4H pathways. LTB4 also might be useful as a simple and effective indicator for monitoring sensitivity to LTA4H inhibitors and drug-resistance. Thus, targeting the LTA4H pathway might be an effective therapeutic strategy in CRC.
Pharmaceutical companies have shown substantial interest in targeting LTA4H therapeutically to alleviate LTB4-mediated pathologies. However, despite the generation of several excellent inhibitors, these drugs have failed to demonstrate clinical efficacy or have been withdrawn from trials owing to deleterious side effects [37]. However, bestatin meets the requirements of long-term application for cancer chemoprevention due to its low toxicity [38]. This drug has been used against various human cancers, including leukemia, lymphoma, malignant melanoma, and lung cancer [23,[39], [40], [41]]. Bestatin also can reduce cellular invasion of several different cancers [42,43]. Most importantly, bestatin as a Food and Drug Administration (FDA)-approved drug in China that is used for adjunct therapy against solid tumors in the clinic. However, the efficacy of bestatin against CRC still needs to be further explored. Our results clearly showed that bestatin inhibited LTA4H activity and substantially down-regulated the LTA4H pathway in CRC patients.
The ex vivo results indicated that bestatin directly targeted LTA4H and strongly suppressed proliferation and colony formation of CRC cells by inhibiting LTA4H activity and BLT1 expression. The ApcMin+ mouse model is a genetically engineered mouse model (GEMMs) with a functional immune system [44]. This mouse model was developed in an attempt to recreate changes observed in human colorectal carcinogenesis. Our data (Fig. 6) showed that treatment with bestatin effectively reduces colorectal tumorigenesis in these mice. Indeed, Bodduluri Haribabu, et al. consistently reported that BLT1 depletion decreased effector immune cell infiltration and accelerated tumorigenesis in the TC-1 cervical cancer model [45], the B16 melanoma model [46], and the ApcMin+ mouse model [47]. However, this group also indicated that deletion of BLT1 plays a protective role in lung tumorigenesis [48]. Consistent with our previous discovery BLT1 inhibition in cancer cells could suppress tumor cell proliferation [15,49]. BLT1 obviously has different functions in cancer cells and immune cells. Our data showed that DLD-1 and HCT-15 colon cancer cell proliferation and colony formation were strongly inhibited by bestatin treatment (Fig. 5a, b). Additionally, BLT1 expression was also markedly suppressed by bestatin treatment (Fig. 5c). Furthermore, bestatin effectively reduced colorectal tumorigenesis in the ApcMin+ mouse model and IHC analysis showed that BLT1 expression observed in irregularly-arranged glandular or adenoid structures of colon tissue was suppressed substantially (Fig. 6d). At the same time, BLT1 expression with CD8-positive staining was also suppressed by bestatin treatment (Fig. s4a, b). Importantly, analysis of patient samples further confirmed that in colon irregularly arranged glandular or adenoid structures, BLT1 expression was inhibited by bestatin treatment (Fig. 3d). Thus, we conclude that the antitumor effects of bestatin mediated through the LTA4H/BLT1/ERK1/2 signaling pathway reduced colon cancer progression in both colon cancer cells and immune cells. We also showed that bestatin inhibited tumor growth of a patient-derived xenograft (PDX) mouse model.
However, this study still has several important limitations. First, the number of samples collected in this study is small. Secondly, we collected pairwise blood samples but did not collect pairwise tumor samples and finally, the absence of a clinical endpoint limits interpretation of our mechanistic findings. Based on positive results and the problems, we plan to perform a large-scale clinical trial to clarify the function of bestatin in the LTA4H pathway and CRC. Despite these limitations, we affirmed that bestatin significantly reduced LTB4 concentration in CRC patients after treatment. Betatin also significantly attenuated LTA4H downstream signaling, including expression of the CRC prognostic marker Ki-67. Furthermore, the LTA4H inhibitor suppressed CRC initiation and progression ex vivo and in vivo, further supporting these findings. Overexpression of LTA4H was observed in colon adenocarcinoma samples. LTA4H is an epoxide hydrolase that catalyzes conversion of the unstable allelic epoxide LTA4 to LTB4 and BLT1 is a high affinity receptor for LTB4. The mRNA and protein levels of BLT1 are increased by the induction of LTB4. Furthermore, cell proliferation and survival are promoted by activating the BLT1/MEK/ERK1/2 pathway. Bestatin is a well-characterized inhibitor of LTA4H and was shown to attenuate LTB4 synthesis. Thus, bestatin reduces polyp number through the LTA4H/ BLT1/ERK1/2 signaling pathway. Collectively, these results demonstrate the antitumor effects of bestatin mediated through LTA4H and support further study for LTA4H inhibition in CRC.
Acknowledgments
Acknowledgments
The authors thank Todd Schuster for supporting experiments and Tara Adams for supporting animal experiments.
Financial support
This work was supported by the Hormel Foundation and National Institutes of Health grants CA187027, CA166011, and CA196639. This work was supported by China Scholarship Council.
Declaration of interests
The authors declare no conflicts of interest.
Author's contribution
S.M.Z., Z.M.D., and Z.D. designed the study. S.M.Z., K.Y. and K.D.L. performed, acquired and analyzed the data. Z.P.G., D.L., G.G.J., G.C.W. and R.H.B. provided blood and tissues from colon cancer patients. M.Y.Y. and Q.W. performed some experiments. S.M.Z., K.Y. and A.M.B. wrote paper. S.M.Z., K.Y. and S.H·S revised the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.05.008.
Contributor Information
Ziming Dong, Email: dongzm@zzu.edu.cn.
Zhiping Guo, Email: zhiping74@hotmail.com.
Zigang Dong, Email: dongx004@umn.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Koliaraki V., Pallangyo C.K., Greten F.R., Kollias G. Mesenchymal cells in colon cancer. Gastroenterology. 2017;152(5):964–979. doi: 10.1053/j.gastro.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 3.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 4.Cheng J., Dwyer M., Okolotowicz K.J., Mercola M., Cashman J.R. A novel inhibitor targets both Wnt signaling and ATM/p53 in colorectal cancer. Cancer Res. 2018;78(17):5072–5083. doi: 10.1158/0008-5472.CAN-17-2642. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Dubois R.N. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larre S., Tran N., Fan C., Hamadeh H., Champigneulles J., Azzouzi R. PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins Other Lipid Mediat. 2008;87(1–4):14–19. doi: 10.1016/j.prostaglandins.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Haeggstrom J.Z. Leukotriene biosynthetic enzymes as therapeutic targets. J Clin Invest. 2018;128(7):2680–2690. doi: 10.1172/JCI97945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snelgrove R.J., Jackson P.L., Hardison M.T., Noerager B.D., Kinloch A., Gaggar A. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330(6000):90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh D.Y., Olefsky J.M. G protein-coupled receptors as targets for anti-diabetic therapeutics. Nat Rev Drug Discov. 2016;15(3):161–172. doi: 10.1038/nrd.2015.4. [DOI] [PubMed] [Google Scholar]
- 10.Kato K., Yokomizo T., Izumi T., Shimizu T. Cell-specific transcriptional regulation of human leukotriene B(4) receptor gene. J Exp Med. 2000;192(3):413–420. doi: 10.1084/jem.192.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu N., Li Y., Zhao Y., Wang Q., You J.C., Zhang X.D. A novel positive feedback loop involving FASN/p-ERK1/2/5-LOX/LTB4/FASN sustains high growth of breast cancer cells. Acta Pharmacol Sin. 2011;32(7):921–929. doi: 10.1038/aps.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P., Sun Z., Chan D., Cartwright C.A., Vijjeswarapu M., Ding J. Zyflamend reduces LTB4 formation and prevents oral carcinogenesis in a 7,12-dimethylbenz[alpha]anthracene (DMBA)-induced hamster cheek pouch model. Carcinogenesis. 2008;29(11):2182–2189. doi: 10.1093/carcin/bgn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bortuzzo C., Hanif R., Kashfi K., Staiano-Coico L., Shiff S.J., Rigas B. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim Biophys Acta. 1996;1300(3):240–246. doi: 10.1016/0005-2760(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen X., Wang S., Wu N., Yang C.S. Leukotriene A4 hydrolase as a target for cancer prevention and therapy. Curr Cancer Drug Targets. 2004;4(3):267–283. doi: 10.2174/1568009043333041. [DOI] [PubMed] [Google Scholar]
- 15.Oi N., Yamamoto H., Langfald A., Bai R., Lee M.H., Bode A.M. LTA4H regulates cell cycle and skin carcinogenesis. Carcinogenesis. 2017;38(7):728–737. doi: 10.1093/carcin/bgx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umezawa H., Aoyagi T., Suda H., Hamada M., Takeuchi T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot (Tokyo) 1976;29(1):97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 17.Suda H., Takita T., Aoyagi T., Umezawa H. The structure of bestatin. J Antibiot (Tokyo) 1976;29(1):100–101. doi: 10.7164/antibiotics.29.100. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Wang C., Jia Y., Liu Z., Shu X., Liu K. Resveratrol increases anti-proliferative activity of bestatin through downregulating P-glycoprotein expression via inhibiting PI3K/Akt/mTOR pathway in K562/ADR cells. J Cell Biochem. 2016;117(5):1233–1239. doi: 10.1002/jcb.25407. [DOI] [PubMed] [Google Scholar]
- 19.Muskardin D.T., Voelkel N.F., Fitzpatrick F.A. Modulation of pulmonary leukotriene formation and perfusion pressure by bestatin, an inhibitor of leukotriene A4 hydrolase. Biochem Pharmacol. 1994;48(1):131–137. doi: 10.1016/0006-2952(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Li N., Wang S., Wu N., Hong J., Jiao X. Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. J Natl Cancer Inst. 2003;95(14):1053–1061. doi: 10.1093/jnci/95.14.1053. [DOI] [PubMed] [Google Scholar]
- 21.Ezawa K., Minato K., Dobashi K. Induction of apoptosis by ubenimex (Bestatin) in human non-small-cell lung cancer cell lines. Biomed Pharmacother. 1996;50(6–7):283–289. doi: 10.1016/0753-3322(96)84827-x. [DOI] [PubMed] [Google Scholar]
- 22.Ota K., Kurita S., Yamada K., Masaoka T., Uzuka Y., Ogawa N. Immunotherapy with bestatin for acute nonlymphocytic leukemia in adults. Cancer Immunol Immunother. 1986;23(1):5–10. doi: 10.1007/BF00205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ino K., Bierman P.J., Varney M.L., Heimann D.G., Kuszynski C.A., Walker S.A. Monocyte activation by an oral immunomodulator (bestatin) in lymphoma patients following autologous bone marrow transplantation. Cancer Immunol Immunother. 1996;43(4):206–212. doi: 10.1007/s002620050323. [DOI] [PubMed] [Google Scholar]
- 24.Roig A.I., Eskiocak U., Hight S.K., Kim S.B., Delgado O., Souza R.F. Immortalized epithelial cells derived from human colon biopsies express stem cell markers and differentiate in vitro. Gastroenterology. 2010;138(3) doi: 10.1053/j.gastro.2009.11.052. [1012-21 e1-5] [DOI] [PubMed] [Google Scholar]
- 25.Qiu H., Johansson A.S., Sjostrom M., Wan M., Schroder O., Palmblad J. Differential induction of BLT receptor expression on human endothelial cells by lipopolysaccharide, cytokines, and leukotriene B4. Proc Natl Acad Sci U S A. 2006;103(18):6913–6918. doi: 10.1073/pnas.0602208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ihara A., Wada K., Yoneda M., Fujisawa N., Takahashi H., Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103(1):24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 27.Tong W.G., Ding X.Z., Talamonti M.S., Bell R.H., Adrian T.E. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun. 2005;335(3):949–956. doi: 10.1016/j.bbrc.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.H., Hu L.L., Gonzalez-Navajas J., Seo G.S., Shen C., Brick J. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16(6):665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sears R., Nuckolls F., Haura E., Taya Y., Tamai K., Nevins J.R. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14(19):2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park K.S., Jeon S.H., Kim S.E., Bahk Y.Y., Holmen S.L., Williams B.O. APC inhibits ERK pathway activation and cellular proliferation induced by RAS. J Cell Sci. 2006;119(Pt 5):819–827. doi: 10.1242/jcs.02779. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y., Zhao J., Zhang Y., Li K., Li T., Chen X. Establishment of lung cancer patient-derived xenograft models and primary cell lines for lung cancer study. J Transl Med. 2018;16(1):138. doi: 10.1186/s12967-018-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin S.H., Lim D.Y., Reddy K., Malakhova M., Liu F., Wang T. A small molecule inhibitor of the beta-catenin-TCF4 interaction suppresses colorectal cancer growth in vitro and in vivo. EBioMedicine. 2017;25:22–31. doi: 10.1016/j.ebiom.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oshima C.T., Iriya K., Forones N.M. Ki-67 as a prognostic marker in colorectal cancer but not in gastric cancer. Neoplasma. 2005;52(5):420–424. [PubMed] [Google Scholar]
- 34.Sun Z., Sood S., Li N., Ramji D., Yang P., Newman R.A. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis. 2006;27(9):1902–1908. doi: 10.1093/carcin/bgl039. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y., Wang X., Zhang X., Sun Z., Chen X. Ethanol promotes chemically induced oral cancer in mice through activation of the 5-lipoxygenase pathway of arachidonic acid metabolism. Cancer Prev Res. 2011;4(11):1863–1872. doi: 10.1158/1940-6207.CAPR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vo T.T.L., Jang W.J., Jeong C.H. Leukotriene A4 hydrolase: an emerging target of natural products for cancer chemoprevention and chemotherapy. Ann N Y Acad Sci. 2018;1431(1):3–13. doi: 10.1111/nyas.13929. [DOI] [PubMed] [Google Scholar]
- 37.Low C.M., Akthar S., Patel D.F., Loser S., Wong C.T., Jackson P.L. The development of novel LTA4H modulators to selectively target LTB4 generation. Sci Rep. 2017;7 doi: 10.1038/srep44449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talmadge J.E., Lenz B.F., Pennington R., Long C., Phillips H., Schneider M. Immunomodulatory and therapeutic properties of bestatin in mice. Cancer Res. 1986;46(9):4505–4510. [PubMed] [Google Scholar]
- 39.Kobayashi T., Miyawaki S., Tanimoto M., Kuriyama K., Murakami H., Yoshida M. Randomized trials between behenoyl cytarabine and cytarabine in combination induction and consolidation therapy, and with or without ubenimex after maintenance/intensification therapy in adult acute myeloid leukemia. The Japan Leukemia study group. J Clin Oncol. 1996;14(1):204–213. doi: 10.1200/JCO.1996.14.1.204. [DOI] [PubMed] [Google Scholar]
- 40.Aozuka Y., Koizumi K., Saitoh Y., Ueda Y., Sakurai H., Saiki I. Anti-tumor angiogenesis effect of aminopeptidase inhibitor bestatin against B16-BL6 melanoma cells orthotopically implanted into syngeneic mice. Cancer Lett. 2004;216(1):35–42. doi: 10.1016/j.canlet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 41.Ichinose Y., Genka K., Koike T., Kato H., Watanabe Y., Mori T. Randomized double-blind placebo-controlled trial of bestatin in patients with resected stage I squamous-cell lung carcinoma. J Natl Cancer Inst. 2003;95(8):605–610. doi: 10.1093/jnci/95.8.605. [DOI] [PubMed] [Google Scholar]
- 42.Scornik O.A., Botbol V. Bestatin as an experimental tool in mammals. Curr Drug Metab. 2001;2(1):67–85. doi: 10.2174/1389200013338748. [DOI] [PubMed] [Google Scholar]
- 43.Wang X., Niu Z., Jia Y., Cui M., Han L., Zhang Y. Ubenimex inhibits cell proliferation, migration and invasion by inhibiting the expression of APN and inducing autophagic cell death in prostate cancer cells. Oncol Rep. 2016;35(4):2121–2130. doi: 10.3892/or.2016.4611. [DOI] [PubMed] [Google Scholar]
- 44.Walrath J.C., Hawes J.J., Van Dyke T., Reilly K.M. Genetically engineered mouse models in cancer research. Adv Cancer Res. 2010;106:113–164. doi: 10.1016/S0065-230X(10)06004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma R.K., Chheda Z., Jala V.R., Haribabu B. Expression of leukotriene B(4) receptor-1 on CD8(+) T cells is required for their migration into tumors to elicit effective antitumor immunity. J Immunol. 2013;191(6):3462–3470. doi: 10.4049/jimmunol.1300967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chheda Z.S., Sharma R.K., Jala V.R., Luster A.D., Haribabu B. Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J Immunol. 2016;197(5):2016–2026. doi: 10.4049/jimmunol.1502376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jala V.R., Maturu P., Bodduluri S.R., Krishnan E., Mathis S., Subbarao K. Leukotriene B4-receptor-1 mediated host response shapes gut microbiota and controls colon tumor progression. Oncoimmunology. 2017;6(12) doi: 10.1080/2162402X.2017.1361593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satpathy S.R., Jala V.R., Bodduluri S.R., Krishnan E., Hegde B., Hoyle G.W. Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nat Commun. 2015;6:7064. doi: 10.1038/ncomms8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong W.G., Ding X.Z., Hennig R., Witt R.C., Standop J., Pour P.M. Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2002;8(10):3232–3242. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material