Abstract
Background:
It has been hypothesized that quadriceps muscle weakness is directly associated with the onset and progression of posttraumatic osteoarthritis after anterior cruciate ligament (ACL) injury and reconstruction (ACLR). This relationship, however, has not been studied with a prospective approach that includes the use of tibiofemoral joint space width difference (JSW-D) measurements to characterize the onset of posttraumatic osteoarthritis before the clinical manifestation of the disease.
Purpose:
To assess the relationship between thigh muscle strength and JSW-D at presurgery baseline and at 1- and 4-year follow-up after ACLR compared with healthy, noninjured participants of similar sex, age, body mass index, and activity level.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A total of 39 unilateral ACL-injured patients and 32 healthy controls were followed prospectively. During each follow-up, JSW, isokinetic knee strength, single-legged hop, and clinical- and patient-oriented outcomes were assessed. At final follow- up, ACL-injured participants who had JSW-D values (considered as the injured minus normal knee) that were less than the 95% confidence interval of controls were considered to be in the ACLR-narrow group, while those with JSW-D values that fell within the confidence intervals were in the ACLR-normal group. Relationships were evaluated between ACLR groups and controls via multilevel regression, as well as Kruskal-Wallis tests for between-group comparisons at 4-year follow-up.
Results:
At 4-year follow-up, 30 participants (79%) were in the ACLR-normal group and 8 (21%) were in the ACLR-narrow group. At baseline, the extension, flexion, and extension/flexion ratio strength values for both ACLR groups were significantly lower than those of controls (P ≤ .05), while the ACLR-narrow group had significantly lower extension strength at 60 and 180 deg/s (P = .04 and .03, respectively), as well as extension/flexion ratio at 60 deg/s (P = .04) in comparison with the ACL-normal group. At 4-year follow-up, 60 deg/s extension strength deficits persisted in the ACLR-narrow group compared with controls and ACLR-normal participants (P = .01 and .04, respectively). Flexion strength at 180 and 300 deg/s was also significantly lower in the ACLR-narrow group compared with ACLR-normal (P = .02 and .04, respectively), as was single-legged hop distance (P = .04).
Conclusion:
Strength deficits present within months after ACL injury and persist through 4 years after ACLR in participants with significantly narrowed JSW-D, compared with ACLR participants with normal JSW-D and controls. This study revealed a significant relationship between quadriceps strength loss that occurred soon after injury and JSW narrowing.
Keywords: anterior cruciate ligament, posttraumatic, osteoarthritis, strength, risk, joint space width
Little is known regarding the initial onset and early progression of posttraumatic osteoarthritis (PTOA) after severe knee trauma, such as that associated with anterior cruciate ligament (ACL) disruption. The clinical signs and symptoms of osteoarthritis after ACL rupture may appear 12 to 15 years after injury.28,41 By the time that patients with these injuries become symptomatic, irreversible damage to the articular cartilage and periarticular tissues has likely occurred.32
The reported incidence of PTOA at 10 to 20 years after ACL injury (regardless of surgical or nonsurgical intervention) is greatly varied in the literature, ranging from 10% to 90%.28 There is some evidence that ACL reconstruction (ACLR) results in more normal anterior-posterior (AP) laxity, improved patient-and functional-oriented outcomes, and a lower rate of PTOA progression at long-term follow-up compared with nonsurgically treated patients.30 It is clear, however, that surgery does not necessarily protect the knee from the development of PTOA after ACL injury46 and that long-term quadriceps strength deficits persist after injury and continue after patients have returned to unrestricted activity.36
Quadriceps and hamstring muscle group weakness after ACL rupture has been well described in the literature.11,16,24–26,33,36,40 It has been theorized that muscular weakness after acute ACL trauma may result from atrophy after prolonged periods of disuse and immobilization18,21,27 or from arthrogenic muscle inhibition.11,17,21,24,34,38 Arthrogenic inhibition is defined as the diminished ability of a muscle to fully contract after acute joint injury as a result of alterations in centrally mediated (afferent or efferent) pathways even though the muscle itself was not injured.19 This phenomenon is believed to be caused by alterations of innate neurologic pathways secondary to intra-articular swelling or disruption of ACL-, capsular-, or thigh muscle–specific mechanoreceptors.21,34,38 Regardless of the pathways by which strength loss or altered functional capacity occurs, these clinical impairments are theorized to be associated with the development of PTOA by decreasing the ability of the quadriceps to attenuate shock34 and maintain a normal distribution of forces and moments across the tibiofemoral joint.2,7 These alterations in function may then influence the articular cartilage mechanical environment within the knee, consequently altering the balance between synthesis and cleavage of cartilage matrix components and initiating the onset of osteoarthritis.2,3,7
Associations between quadriceps strength and primary osteoarthritis have been described.14,31,35,43,44 However, this relationship has yet to be established with strength deficits that occur after acute ACL trauma and tibiofemoral joint space width difference (JSW-D)48 as a measurement of PTOA onset. The JSW-D is a measurement technique that is sensitive to articular cartilage changes before the clinical manifestation of the disease (ie, appreciable joint space loss, subchondral bone sclerosis, obvious osteophyte formation and malalignment of the knee). It is important to determine risk factors associated with the onset and progression of PTOA during the earliest stages of the disease process (before appreciable loss of articular cartilage), when therapeutic intervention would most likely be beneficial at altering its progression.1,8 Consequently, the primary focus of this study was to examine the relationship between isokinetic thigh strength and JSW-D at presurgical baseline and at 1 and 4 years after ACLR compared with a group of healthy, noninjured participants of similar sex, age, body mass index (BMI), and activity level. We hypothesized that ACLR participants in the narrowed JSW-D group would have significantly lower isokinetic knee extension strength at 60 deg/s than would those in the normal JSW-D group and controls at final follow-up. Additional clinical- and patient-oriented outcomes were also assessed between ACLR and control group participants at baseline and final follow-up intervals to describe study groups.
MATERIALS AND METHODS
Study Design and Participants
Data for this investigation were collected during a longitudinal cohort study that was designed to evaluate relationships between biochemical markers of type II collagen metabolism and the progression of PTOA.48,49 In the present study, isokinetic knee strength, single-legged hop (SLH) performance, and patient-oriented outcomes were assessed in 39 participants (20 women) recruited from our orthopaedic clinic at the time of surgical reconstruction for an acute, first-time ACL tear. Patient-, functional-, and clinical-oriented outcomes as well as tibiofemoral JSW were evaluated at baseline (before ACLR) and at 1-year and 4-year follow-up (mean ± standard deviation [SD], 46 ± 9.5 months). Control participants (n = 32, 18 women) of similar sex, age, BMI, and activity level as ACLR participants were recruited from the surrounding community and evaluated at similar time intervals (baseline and 1-year and 3-year follow-up; mean ± SD, 33 ± 6.6 months). The mean time interval between the index injury and surgery date was 70.1 days (range, 18–155 days), and the baseline visit for ACL-injured participants occurred within 3 weeks of surgical reconstruction. The institutional review board of the University of Vermont approved this protocol before participant enrollment, and all participants provided written informed consent before participation in the study.
Entry Criteria
Entry criteria for ACL group participants included age between 14 and 55 years, body mass index between 18.5 and 30, and being moderately active (Tegner activity score ≥ 5). Clinical examination of these individuals revealed no history of previous surgeries to any joint or knee injections, no abnormal laxity of the posterior cruciate ligament or collateral ligaments, no radiographic evidence of fracture or osteoarthritis, normal lower limb alignment (as defined by the 2000 International Knee Documentation Committee [IKDC] Knee Examination form), less than 2/3 meniscectomy in either meniscus, and grade 3A or less articular cartilage lesions based on the International Cartilage Repair Society criteria. After ACLR, all patients participated in a standardized, accelerated postsurgical rehabilitation program.5 Similar entry criteria were employed for control group participants with the following exceptions: These patients reported no knee pain or physical dysfunction as determined by the Knee Injury and Osteoarthritis Outcome Score (KOOS)42 and subjective IKDC form22; they had no history of significant trauma to any joint (defined as that requiring physician referral and/or more than 3 days of modified activities of daily living); and there were no abnormal findings with clinical knee examination (IKDC).
In addition, control participants (at baseline and final follow-up) and ACLR participants (at 4-year follow-up) underwent 3T magnetic resonance imaging (MRI) (Philips Achieva, Philips Healthcare, Best, Netherlands) examinations of both knees to elucidate existing pathologic abnormalities in their uninjured knee at our university research MRI facility. Follow-up MRI was evaluated by an experienced sports medicine fellowship–trained orthopaedic surgeon. These measurements were obtained to ensure that control participant knees as well as the noninjured knee of ACLR patients had no observable ligament, meniscus, or cartilage injuries or disease to be included in these analyses.
Surgical Procedure
The ACLRs were performed by 1 of 2 experienced sports medicine fellowship–trained orthopaedic surgeons. Of 39 ACLRs, 35 (90%) were performed with autologous bone–patellar tendon–bone autografts; 3 (8%) were bone–patellar tendon–bone allografts; and 1 (2%) was reconstructed with a semitendinosus-gracilis 4-strand autograft. In all cases, the graft was tensioned to reestablish the AP laxity of the contralateral/normal knee (±1 mm), which was also evaluated while the patient was under anesthesia immediately before surgery with the KT-1000/S arthrometer (MEDmetric Corporation, San Diego, California).49
Assessment of JSW
A thorough description of the JSW measurement and analysis technique and excellent intraevaluator test-retest reliability have been described.48 Briefly, bilateral posterioranterior view knee radiographs were obtained on all participants at baseline and follow-up visits with a fluoroscopy-assisted semiflexed, metatarsal phalangeal view technique.12 At the completion of all visits, radiographs were scanned, and computer-assisted evaluation of JSWs was performed with a previously validated “midpoint” technique12 (accurate within 0.13 mm; measurement resolution of 0.17 mm) by the same investigator with excellent reliability.48 ACL-injured participants were considered to have significantly narrowed JSW-D values if the difference between their ACL-injured knee and contralateral/healthy knee was more than 1.96 standard deviations below the mean side-to-side JSW-D value of controls (ie, below the lower 95% confidence interval [CI] of the control group). The same investigator (T.W.T.) acquired all radiographs and performed all JSW measurements.
Subjective and Objective Outcomes
Patient-oriented assessments of pain, symptoms, function in sports and recreational activities, function during daily living, and knee-related quality of life were evaluated with the KOOS.42 In addition, the IKDC subjective score,22 Tegner activity scale,47 and Marx activity rating scale29 were utilized. Objective outcomes included evaluation of the SLH test,4 isokinetic strength testing of knee extensor and flexor musculature, instrumented AP knee laxity assessed with the KT-1000 arthrometer,10 and IKDC objective knee evaluation. All outcomes were obtained at baseline and each follow-up visit, with the exception of the SLH test, which was obtained on the uninjured limb at baseline and bilaterally at each follow-up. All measurements were administered by the same investigator (T.W.T.).
Lower extremity physical function was assessed with the SLH test.23 After completing a standardized warmup, submaximal and maximal practice efforts, and a rest period, participants were asked to jump as far as possible while standing on 1 leg (starting position) and landing on the same leg only. They were allotted 3 consecutive jumps on each leg (starting with the uninjured leg first), and the longest jump was used as the maximal distance score for each leg.
Concentric strength about the knee was evaluated with a Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, New York). After a warm-up and familiarization period of submaximal trial repetitions, knee flexion and extension strength were measured at 60, 180, and 300 deg/s through 0° to 90° of flexion with participants seated. Five repetitions were performed bilaterally, starting with the noninjured leg for ACL-injured patients and with body weight–normalized peak torque recorded for each motion. Strong verbal encouragement was provided for all participants during testing to help ensure that maximal effort was being generated and measured.
Bilateral assessment of range of motion, presence of effusion, ligamentous and/or rotatory knee laxity, and orthopaedic physical examination findings were evaluated and scored with the IKDC objective knee examination for all participants. This objective assessment tool compares findings bilaterally (injured vs noninjured knee) and results in a score consisting of a single-letter evaluation grade (A = normal, D = severely abnormal). For the purposes of statistical analyses, letter grades were assigned numerical scores (4–1 for grades A-D, respectively).
Instrumented AP knee laxity was assessed for all participants with a KT-1000 arthrometer per manufacturer guidelines (based on 90-N posterior-directed force and 133-N anterior force).10 Measurements were repeated until 3 identical AP displacement results were obtained with a short “rest” interval between each trial. Measurements were made before any physical function or strength assessments to prevent potential exercise-induced laxity changes.39
Although there does not appear to be a consensus in the literature regarding the definition or presence of leg “dominance” or laterality, to control the potential for limb-side bias effects, a “matched knee” was assigned to control participants to represent the corresponding left- or right-injured knees in the ACLR participant group (ACLR group’s right knee injuries = 42% of total; controls’ “matched” right knees = 43% of total).
Statistical Analyses
Descriptive statistics, including age, BMI, and presurgical activity level (Tegner score), were calculated for each participant group. Isokinetic extension and flexion strength and SLH test values were calculated for ACL-injured group participants (expressed as percentage of injured versus noninjured side) and compared with control side-to-side values (percentage of “injury-matched” control knee vs contralateral control knee). Extension/flexion strength ratio values were calculated for the injured knee of cases and injury-matched knee of controls. Multilevel linear regression was then used to determine baseline differences in isokinetic strength and patient- and clinically oriented outcomes according to all available baseline data for all participants. Multilevel regression was also used to calculate strength change over time from baseline through last available follow-up date among 3 groups of participants: (1) control group (side-to-side JSW difference), (2) ACL-reconstructed participants with normal JSW difference compared with the control group at final follow-up (ACLR-normal), and (3) ACL-reconstructed participants with narrowed JSW difference at final follow-up compared with controls (ACLR-narrow). Between-group comparisons at final follow-up were evaluated with the Kruskal-Wallis tests. Potential confounding variables—including age, sex, BMI, activity level, time between surgery and baseline evaluation, and time between surgery and follow-up—were evaluated with preliminary analyses according to regression models and Kruskal-Wallis tests. Adjusting for these variables within the ACLR participant groups did not influence test results, and consequently, the final statistical analyses were not adjusted for covariates. In addition, since strength changes during the initial period after ACLR may not be linear, the use of piecewise regression was preliminarily explored to evaluate strength change over time in ACLR participants. These results were not different from linear regression analyses, which are consequently reported for ease of interpretation. The alpha level was set a priori at 0.05 for all statistical analyses.
RESULTS
One ACLR participant was lost to follow-up, resulting in data from 38 ACLR patients available for analyses. The ACLR-normal group was composed of 30 patients (79% of total), and the ACLR-narrow group was composed of 8 participants (21% of total) who had narrowed JSW at 4-year follow-up compared with controls (Table 1). The mean 6 SD side-to-side JSW-D (expressed as injured minus contralateral limb) for each group was as follows: for controls, –0.06 6 0.47 mm in the medial and –0.13 6 0.41 mm in the lateral compartments; for ACLR-normal, 0.27 6 0.51 mm medially and –0.10 6 0.40 mm laterally; and ACLR-narrow, –0.55 6 0.78 mm in the medial and –1.21 6 0.57 mm in the lateral compartments. As was described previously,48 some ACLR patients had significantly increased JSW-D compared with controls; however, subjective and objective outcomes in these participants did not differ significantly from ACLR patients falling within the 95% CI of control JSW. Consequently, individuals with increased JSW were included in the ACLR-normal group for all statistical analyses performed herein.
TABLE 1.
Patient Anthropometric Dataa
| Group | Baseline Age, y | Baseline BMI | Preinjury Tegner Score |
|---|---|---|---|
| Control (n = 32) | 27 ± 7 | 23.8 ± 3.3 | 6.2 ± 1.4 |
| ACLR-normal (n = 30) | 28 ± 12 | 25 ± 4 | 7.9 ± 1.5 |
| ACLR-narrow (n = 8) | 28 ± 12 | 25 ± 3 | 6.6 ± 1.2 |
Values are expressed as mean ± standard deviation. ACLR, anterior cruciate ligament reconstruction; BMI, body mass index.
Baseline Comparisons
At baseline, isokinetic strength values were significantly different between controls and both ACL-injured participant groups at all 3 testing speeds for extension (P < .001), flexion (P ≤ .02), and injured knee extension to flexion ratios (P ≤ .05) (Table 2). Between-group differences in the ACL-injured groups were also observed at baseline, with the narrow group participants having significantly lower extension strength values at 60 and 180 deg/s (P = .04 and .03, respectively), as well as with extension/flexion ratio at 60 deg/s (P = .04) (Table 2). The SLH results (calculated per 1- and 4-year follow-up data) also revealed significant differences between controls and normal and narrow patient groups (P = .03 and .002, respectively) but no differences between the normal and narrow groups (P = .10). Mean strength values at baseline as well as between-group effect sizes are presented in Appendix Table A1 (http://ajsm.sagepub.com/supplemental).
TABLE 2.
Results of Between-Group Multilevel Regression Comparisons (P Values)a
| Between-Group Baseline Strength Comparison |
Between-Group Strength Change Over Time (Slope) Comparison |
|||||
|---|---|---|---|---|---|---|
| Control vs ACLR-Normal |
Control vs ACLR-Narrow |
ACLR-Normal vs ACLR-Narrow |
Control vs ACLR-Normal |
Control vs ACLR-Narrow |
ACLR-Normal vs ACLR-Narrow |
|
| Isokinetic testing (direction and velocity) | ||||||
| Extension | ||||||
| 60° | <.0001 | <.0001 | .04 | <.0001 | .0008 | .76 |
| 180° | <.0001 | <.0001 | .03 | .004 | .0005 | .20 |
| 300° | <.0001 | <.0001 | .38 | .05 | .09 | .88 |
| Flexion | ||||||
| 60° | <.0001 | .0003 | .91 | <.0001 | .004 | .18 |
| 180° | <.0001 | <.0001 | .10 | .0003 | .01 | .76 |
| 300° | .005 | .02 | .59 | .003 | .21 | .26 |
| Extension/flexion | ||||||
| 60° | .01 | .0003 | .04 | .37 | .88 | .37 |
| 180° | .03 | .02 | .29 | .88 | .72 | .60 |
| 300° | .01 | .05 | .74 | .84 | .91 | .73 |
| Single-legged hop test (injured/uninjured knee) | .03 | .002 | .10 | .09 | .23 | .75 |
Statistically significant differences are in bold. ACLR, anterior cruciate ligament reconstruction.
As expected, significantly lower clinical- and patient-oriented outcome scores were observed for both the normal and narrow groups compared with controls at baseline (P ≤ .003) (Tables 3 and 4 and Appendix Table A2, available online). No between-group differences were found for ACL-injured participants at baseline for any of these outcomes; however, scores on the KOOS quality of life subscale trended toward significance (P = .07), with narrow group patients reporting lower scores than the normal group (Table 4).
TABLE 3.
Between-Group Comparisons of Patient- and Clinically Oriented Outcomesa
| Baseline |
Final Follow-up |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (Range) |
P |
Median (Range) |
P |
|||||||||
| Outcomes Measure |
Control | ACLR- Normal |
ACLR- Narrow |
Control vs Normal |
Control vs Narrow |
Normal vs Narrow |
Control | ACLR- Normal |
ACLR- Narrow |
Control vs Normal |
Control vs Narrow |
Normal vs Narrow |
| IKDC | ||||||||||||
| Objective | 4.0 (1.0) | 2.0 (2.0) | 2.0 (2.0) | <.0001 | .0003 | .80 | 4.0 (1.0) | 3.0 (2.0) | 3.0 (2.0) | <.0001 | .0004 | .56 |
| Subjective | 100.0 (6.9) | 57.5 (39.1) | 54.0 (25.3) | <.0001 | <.0001 | .19 | 100.0 (12.6) | 92.0 (35.6) | 85.1 (18.4) | <.0001 | <.0001 | .02 |
| KT-1000b | −0.5 (7.5) | −4.0 (9.0) | −6.25 (11.0) | <.0001 | .0002 | .12 | 0 (7.5) | −3.0 (9.5) | −3.25 (8.5) | .0001 | .002 | .38 |
| Marx | 11.0 (16.0) | 0 (14.0) | 0 (8.0) | <.0001 | .0003 | .85 | 10.0 (11.0) | 11.5 (16.0) | 4.0 (11.0) | .34 | .04 | .03 |
| Tegner | 6.0 (4.0) | 3.0 (8.0) | 3.0 (1.0) | <.0001 | .0001 | .46 | 5.5 (6.0) | 7.0 (5.0) | 5.0 (4.0) | .03 | .22 | .01 |
Statistically significant differences are in bold. ACLR, anterior cruciate ligament reconstruction; IKDC, International Knee Documentation Committee.
KT-1000 arthrometer (uninjured – injured knee).
TABLE 4.
Between-Group Comparisons of KOOS Scoresa
| Baseline |
Final Follow-up |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD |
P |
Mean ± SD |
P |
|||||||||
| KOOS Subscale |
Control | ACLR- Normal |
ACLR- Narrow |
Control vs Normal |
Control vs Narrow |
Normal vs Narrow |
Control | ACLR- Normal |
ACLR- Narrow |
Control vs Normal |
Control vs Narrow |
Normal vs Narrow |
| Pain | 99 ± 1.7 | 75 ± 12.6 | 69 ± 6.1 | <.0001 | <.0001 | .20 | 100 ± 1.0 | 94 ± 10.3 | 91 ± 5.9 | .001 | < .0001 | .12 |
| Quality of life | 99 ± 2.5 | 39 ± 19.2 | 26 ± 12.7 | <.0001 | <.0001 | .07 | 98 ± 3.9 | 88 ± 12.9 | 74 ± 15.5 | .0001 | < .0001 | .03 |
| Activities of daily living | 100 ± 0.6 | 86 ± 12.2 | 86 ± 9.9 | <.0001 | <.0001 | .70 | 100 ± 0.6 | 98 ± 4.2 | 99 ± 2.2 | .004 | .008 | .87 |
| Symptoms | 98 ± 3.7 | 67 ± 13.7 | 65 ± 13.7 | <.0001 | <.0001 | .73 | 98 ± 4.2 | 87 ± 13.3 | 86 ± 12.0 | <.0001 | .0007 | .66 |
| Sports | 100 ± 1.5 | 48 ± 25.4 | 39 ± 26.0 | <.0001 | <.0001 | .45 | 99 ± 2.4 | 91 ± 11.5 | 83 ± 15.0 | .001 | < .0001 | .13 |
Statistically significant differences are in bold. ACLR, anterior cruciate ligament reconstruction; KOOS, Knee Injury and Osteoarthritis Outcome Score; SD, standard deviation.
Strength Change Over Time
The slope values obtained from the multilevel linear regression analysis of baseline and final follow-up data were compared among groups to evaluate differences in change in strength over time (Table 2). Control group slope values did not change; however, comparisons of the slopes between controls and both ACLR groups were significantly different for all extension strength tests (P ≤ .05) with the exception of differences between the control group and ACLR-narrow group at 300 deg/s, which were not significant (P = .09). A similar finding was observed between controls and both ACLR groups for all isokinetic flexion strength change over time analyses (P ≤ .01), with the exception of differences between the control group and ACLR-narrow group at 300 deg/sec, which were not statistically significant (P = .21). No significant differences were found between controls and either ACLR group regarding slope values for extension/flexion strength ratio or SLH test (Table 2).
Comparison of regression slope values between the ACLR-normal and ACLR-narrow groups revealed no significant differences for any strength test, indicating that both groups regained strength in their injured legs at a similar rate over time. Extension strength values for the ACLR-narrow group, however, remained lower than those of the ACLR-normal group at final follow-up, indicating that participants in the ACLR-narrow group had significantly lower strength at the presurgical baseline time point, with this significant discrepancy persisting over the 4-year follow-up interval (Figure 1).
Figure 1.
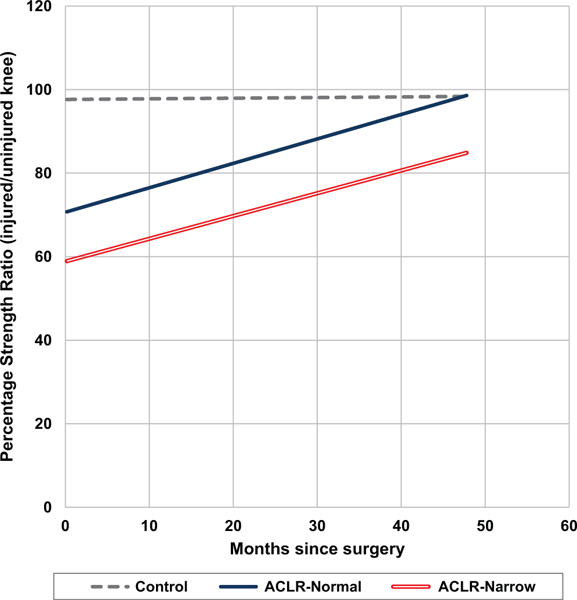
Extension, 60 deg/s: group regression models. ACLR, anterior cruciate ligament reconstruction.
Four-Year Follow-up Comparisons
At 4-year follow-up, extension strength evaluated at 60 deg/s for the ACLR-narrow group was significantly less than that of the control group (P = .01) and the ACLR- normal group (P = .04; Kruskal-Wallis test) (Table 5). Extension strength at 180 and 300 deg/s was also significantly different between controls and ACLR-normal participants but not between controls and ACLR-narrow group or between ACLR subgroups (Table 5). Strength of the knee flexors evaluated at 60 deg/s produced values that were significantly different between the control and ACLR-normal groups, with ACLR-normal participants having higher injured-versus-uninjured percentage strength values compared with controls at 4-year follow-up (P = .009). The ACLR-narrow group developed significantly lower flexion strength values at both 180 and 300 deg/s in comparison with the ACLR-normal group (P = .02 and .04, respectively). Injured knee extension/flexion strength ratios were also significantly lower in both ACLR groups compared with controls (ACLR-normal, P = .005; ACLR-narrow, P = .04); however, no significant differences were found between ACLR groups (Table 5). Injured versus normal knee SLH distance was significantly lower in ACLR-narrow group participants compared with controls (P = .03), as well as between ACLR groups, with the ACLR-narrow group having lower values at 4-year follow-up (P = .04) (Table 5).
TABLE 5.
Strength Comparisons by JSW Group at Final Follow-upa
| Side-to-Side Percentage Strength, Mean ± SD |
Between-Group Comparison, P (Effect Size) |
|||||
|---|---|---|---|---|---|---|
| Isokinetic testing, deg/s | Control | ACLR-Normal | ACLR-Narrow | Control vs Normal | Control vs Narrow | Normal vs Narrow |
| Extension | ||||||
| 60° | 99 ± 11.6 | 95 ± 10.3 | 83 ± 23.1 | .5 (0.28) | .01 (1.14) | .04 (0.86) |
| 180° | 102 ± 12.6 | 94 ± 10.7 | 93 ± 19.1 | .005 (0.62) | .06 (0.71) | .39 (0.09) |
| 300° | 100 ± 9.4 | 92 ± 12.6 | 89 ± 19.9 | .003 (0.67) | .09 (0.86) | .68 (0.19) |
| Flexion | ||||||
| 60° | 95 ± 12.8 | 102 ± 10.7 | 93 ± 15.3 | .009 (0.55) | .77 (0.12) | .08 (0.68) |
| 180° | 98 ± 9.4 | 100 ± 8.1 | 90 ± 12.0 | .29 (0.23) | .11 (0.81) | .02 (1.05) |
| 300° | 97 ± 11.1 | 100 ± 12.5 | 89 ± 10.3 | .41 (0.23) | .08 (0.64) | .04 (0.87) |
| Injured Knee Extension/Flexion Strength Ratio, Mean ± SD |
Between-Group Comparison, P (Effect Size) |
|||||
| Control | ACLR-Normal | ACLR-Narrow | Control vs Normal | Control vs Narrow | Normal vs Narrow | |
| Extension/flexion | ||||||
| 60° | 1.7 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.3 | .005 (0.75) | .04 (0.99) | .78 (0.25) |
| 180° | 1.4 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.2 | .12 (0.50) | .15 (0.46) | .91 (0.04) |
| 300° | 1.3 ± 0.3 | 1.1 ± 0.2 | 1.3 ± 0.2 | .06 (0.57) | .31 (0.46) | .84 (0.11) |
| Single-legged hop test | 99 ± 6.7 | 99 ± 7.1 | 90 ± 11.6 | .83 (0.01) | .03 (1.10) | .04 (1.09) |
Statistically significant differences are in bold. ACLR, anterior cruciate ligament reconstruction; JSW, joint space width; SD, standard deviation.
At 4-year follow-up, comparisons between ACLR groups and controls demonstrated that IKDC objective and subjective scores and AP laxity continued to be significantly lower in ACLR-normal and ACLR-narrow groups (Table 3). The Marx score, however, was highest in ACLR-normal participants and significantly higher in controls and ACLR-normal participants compared with the ACLR-narrow group (Table 3). Tegner activity scores were also significantly higher in ACLR-normal participants compared with controls and those in the ACLR-narrow group (P = .03 and .01, respectively) (Table 3).
At 4-year follow-up, all 5 KOOS subscale scores remained significantly lower in both ACLR groups compared with controls (P ≤ .004 for all comparisons) (Table 4); however, only the quality of life score was able to discriminate between ACLR-normal and ACLR-narrow groups, with the ACLR-narrow group having significantly lower scores at final follow-up (P = .03) (Table 4).
DISCUSSION
To our knowledge, this is the first longitudinal cohort study of acute ACL-injured study participants and healthy matched controls to examine the relationship between thigh muscle strength and tibiofemoral JSW-D from presurgical baseline through 4-year follow-up after ACLR. In this investigation, we found that participants with significantly narrowed JSW-D at 4 years after ACLR had significant strength deficits soon after the injury that persisted over time compared with ACLR participants with normal JSW-D and controls. The JSW measurement approach was used in this investigation because it allowed us to detect small changes in JSW that precede those observed with clinical grading scales (ie, Kellgren-Lawrence [KL], Altman, or IKDC grade). This investigation is also novel in that we examined the relationship between the strength losses that occur soon after ACL injury in 2 subsets of participants (those with and without normal JSW-D) and persisted during the earliest stages of the PTOA disease process. In addition, these analyses occurred before the presentation of substantial pain, loss of function, and gross radiographic abnormalities (ie, KL grade ≥2), which are common to the clinical manifestation of the disease.
Our results coincide with those reported by Keays et al25 in their investigations of risk factors for PTOA in individuals who originally suffered from chronic ACL insufficiency 6 years after surgical reconstruction took place. The findings of their discriminate analysis study of 10 potential risk factors for PTOA at 6-year follow-up revealed that the use of strength variables alone (considered as injured-to-noninjured limb comparisons of quadriceps and hamstring muscle strength and quadriceps/hamstring ratio) were able to discriminate between participants with moderate osteoarthritis (KL grade 2) and those with less advanced disease (KL grades 0 and 1). Oiestad and colleagues33 have also described an association between quadriceps strength loss that occurred between 2 and 10 to 15 years after ACLR follow-ups with moderate to severe knee osteoarthritis (defined as KL grade ≥2). Although both these studies provide strong evidence for the involvement of strength about the knee after ACLR with development of PTOA at long-term follow-up, these investigations used KL grade for osteoarthritis classification of participants. It may be that the use of a more sensitive measure of PTOA (quantified radiographic JSW, MRI- based measures, etc) would have yielded stronger associations between strength and the onset of PTOA at earlier time points. This is likely an essential consideration in the evaluation of interventions designed to prevent or treat the progression of PTOA.
As an exploratory investigation, this study is not without its limitations. It is important for us to point out that of the 8 participants in the ACLR-narrow group, 5 presented with significantly narrowed JSW-D (fell below the 95% CI of controls) at their baseline visit, with 3 additional participants falling into this range during the follow-up period, as was reported.48 It may be that the trauma produced at the time of the index injury was great enough to cause permanent deformation of the tibiofemoral articular cartilage and underlying subchondral bone in these participants. Although not specifically assessed in this investigation, this may have led to significant joint effusion and/or disruption of the involved tissue mechanoreceptors, resulting in a level of disuse atrophy that was never fully recovered during the ACLR rehabilitation process. In addition, only 4 (50%) ACLR-narrow participants would have been considered “high risk” (those treated with any amount of meniscectomy and/or presence of IKDC grade 3 or greater articular cartilage lesions at the time of surgery) as defined by previous investigations.20,45 Of these 4 “high-risk” participants, 3 were classified as KL grade 1 and only 1 as KL grade 2 at 4-year follow-up. The remaining 4 participants (50%) in the ACLR-narrow group had intact menisci and articular cartilage lesions that were less than grade 3 (if any) noted at the time of surgery; all had KL grade 0 radiographs at 4-year follow-up. This lends support to the hypothesis that substantial concomitant articular and meniscal cartilage disruption may not have been the mechanism responsible for the decreased strength and narrowed JSW that occurred soon after injury and persists over 4 years after ACLR in these patients. This also demonstrates that commonly utilized clinical grading scales such as KL grade are not adequately sensitive to detect subtle JSW changes for the purpose of examining knee PTOA risk factors and detecting the onset and/or progression of the disease during its earliest stages.
This study was designed to examine biochemical markers of type II collagen metabolism as they relate to JSW difference,49 and as such, it is limited in that it may not be adequately powered to fully examine differences in isokinetic strength and the objective and subjective outcomes evaluated between our ACLR groups. A post hoc power analysis of our primary outcome (isokinetic knee extension strength evaluated at 60 deg/s) revealed that had we not found a statistically significant difference in this variable among groups at final follow-up, we would have needed 12 study participants per group to have the necessary power to detect a 20% difference in this outcome and be confidant that we were not erroneously accepting the null hypothesis and committing a type II error. Since our analyses did, in fact, reveal that a statistically significant difference existed among group means, which was further substantiated by the calculated effect size values, we are confident that the relationship we are describing is valid. Because of the fact that the analyses performed were exploratory and that for each analysis the relatively few pairwise comparisons were preplanned, it was decided not to adjust for multiple comparisons in these cases. Upon examination of the data, the results found to be statistically significant did seem to be indicative of clinically relevant effects, which were found despite limited power. However, as with all initial, exploratory investigations that provide “hypothesis generating” information to help advance the science, these findings need to be confirmed with prospective investigations designed to thoroughly evaluate muscular strength and function as it relates to PTOA after ACL injury and surgical reconstruction. Additional limitations of this study include the inclusion of allograft (n = 3) and double-bundle semitendinosus-gracilis autograft (n = 1) tissues, in addition to the bone–patellar tendon–bone autografts (n = 34).
Note that the ACLR individuals in this study were fully functioning at 4-year follow-up and reported relatively little pain compared with primary osteoarthritis patients seeking care.49 Because of the fact that these patients were evaluated in a relatively short time frame after ACL injury and subsequent surgical reconstruction, it is not possible to say if they were truly experiencing the initial effects of progressive PTOA at the 4-year follow-up interval; however, the changes in JSW that we observed were approximately 5 times greater than the resolution of the JSW measurement technique that was used (0.17 mm). We hope to follow this cohort longitudinally to further categorize individuals in each risk group.
Although our results appear to be the first to demonstrate a direct association between decreased JSW and strength deficits that present soon after ACL injury and persist throughout the initial 4 years after surgical reconstruction, these findings should be substantiated with additional prospective investigations in a larger cohort. In addition, numerous directly related variables and their interactions need to be evaluated to understand the onset and progression of the PTOA disease process.3 Consequently, recommendations for studies of acutely ACL-injured participants include the evaluation of arthrogenic inhibition21,34 as it relates to localized biochemical markers of inflammation (ie, synovial fluid cytokine analysis)6,13 and strength detriments at presurgical baseline and additional follow-up intervals. Biomechanical gait assessment and evaluation of knee joint proprioception44 should be performed in ACLR participants with decreased strength and narrowed JSW at follow-up compared with ACLR participants with normal JSW and healthy controls to evaluate relationships between strength loss and alterations in gait parameters in this population. Validated MRI- based methods should be used to quantify knee joint effusion and synovitis at baseline and follow-up visits as well as articular cartilage-specific assessments of type II collagen fiber orientation (MRI T2 mapping) and aggrecan content (MRI T1-rho).9,37
Of specific importance is the assessment of muscle structure and function at the cellular level (eg, force velocity and power) as they relate to changes in whole-muscle and whole-body function assessment outcomes. The notion behind such measurements is that they would reflect changes in myofilament structure and function that occurred shortly after the index injury and were recalcitrant to correction, thereby leading to persistent functional deficits. These PTOA-specific outcomes could then be assessed in a multifactorial fashion to examine relationships among strength loss, arthrogenic inhibition, localized biochemical markers of inflammation, and biomechanical gait abnormalities as they relate to articular cartilage structure and composition during the earliest stages of PTOA after ACL rupture.
Through future clinical and translational studies designed to prospectively evaluate these physiologic characteristics and how they relate, we will be able to elucidate specific underlying mechanisms of strength loss at the molecular and cellular levels, as well as their interaction with the “whole organ” and whole-body PTOA disease processes. With this information in hand, we will then be able to assess how potential therapeutic interventions delivered during the early phases of disuse (or “bridge therapies”), such as neuromuscular electrical stimulation and various modes and doses of therapeutic exercise, may decrease or prevent strength losses associated with JSW narrowing and posttraumatic osteoarthritis after significant knee trauma.14–16,18
In conclusion, this study demonstrated an association between isokinetic knee extension and flexion strength and SLH performance and narrowed JSW-D after ACL injury. Specifically, strength deficits are seen within months after ACL injury and persist through 4 years after ACLR in participants with significantly narrowed JSW-D, compared with ACLR participants with normal JSW-D and controls.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases for funding this investigation (R01 AR051477-01) and Mike Desarno, MS, for his assistance with statistical analyses.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for this investigation was provided by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant (R01 AR051477-01).
REFERENCES
- 1.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteo-arthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18(5):514–518. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. [DOI] [PubMed] [Google Scholar]
- 4.Barber SD, Noyes FR, Mangine RE, McCloskey JW, Hartman W. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin Orthop Relat Res. 1990;255:204–214. [PubMed] [Google Scholar]
- 5.Beynnon BD, Johnson RJ, Naud S, et al. Accelerated versus nonac-celerated rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind investigation evaluating knee joint laxity using roentgen stereophotogrammetric analysis. Am J Sports Med. 2011;39(12):2536–2548. [DOI] [PubMed] [Google Scholar]
- 6.Bigoni M, Sacerdote P, Turati M, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2013;31(2):315–321. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40(2):215–222. [DOI] [PubMed] [Google Scholar]
- 8.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14(3):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crema MD, Roemer FW, Marra MD, Guermazi A. MR imaging of intra- and periarticular soft tissues and subchondral bone in knee osteoarthritis. Radiol Clin North Am. 2009;47(4):687–701. [DOI] [PubMed] [Google Scholar]
- 10.Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401–407. [DOI] [PubMed] [Google Scholar]
- 11.Drechsler WI, Cramp MC, Scott OM. Changes in muscle strength and EMG median frequency after anterior cruciate ligament reconstruction. Eur J Appl Physiol. 2006;98(6):613–623. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis DE, Beynnon BD, Richard MJ, Novotny JE, Skelly JM, Cooper SM. Precision and accuracy of joint space width measurements of the medial compartment of the knee using standardized MTP semi-flexed radiographs. Osteoarthritis Cartilage. 2003;11(10):716–724. [DOI] [PubMed] [Google Scholar]
- 13.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farr JN, Going SB, McKnight PE, Kasle S, Cussler EC, Cornett M. Progressive resistance training improves overall physical activity levels in patients with early osteoarthritis of the knee: a randomized controlled trial. Phys Ther. 2010;90(3):356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feil S, Newell J, Minogue C, Paessler HH. The effectiveness of supplementing a standard rehabilitation program with superimposed neuromuscular electrical stimulation after anterior cruciate ligament reconstruction: a prospective, randomized, single-blind study. Am J Sports Med. 2011;39(6):1238–1247. [DOI] [PubMed] [Google Scholar]
- 16.Gerber JP, Marcus RL, Dibble LE, Greis PE, Burks RT, LaStayo PC. Effects of early progressive eccentric exercise on muscle size and function after anterior cruciate ligament reconstruction: a 1-year follow-up study of a randomized clinical trial. Phys Ther. 2009;89(1):51–59. [DOI] [PubMed] [Google Scholar]
- 17.Hart JM, Turman KA, Diduch DR, Hart JA, Miller MD. Quadriceps muscle activation and radiographic osteoarthritis following ACL revision. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):634–640. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa S, Kobayashi M, Arai R, Tamaki A, Nakamura T, Moritani T. Effect of early implementation of electrical muscle stimulation to prevent muscle atrophy and weakness in patients after anterior cruciate ligament reconstruction. J Electromyogr Kinesiol. 2011;21(4): 622–630. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. [Google Scholar]
- 20.Ichiba A, Kishimoto I. Effects of articular cartilage and meniscus injuries at the time of surgery on osteoarthritic changes after anterior cruciate ligament reconstruction in patients under 40 years old. Arch Orthop Trauma Surg. 2009;129(3):409–415. [DOI] [PubMed] [Google Scholar]
- 21.Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med. 2008;27(3):383–404. [DOI] [PubMed] [Google Scholar]
- 22.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 23.Jarvela T, Kannus P, Latvala K, Jarvinen M. Simple measurements in assessing muscle performance after an ACL reconstruction. Int J Sports Med. 2002;23(3):196–201. [DOI] [PubMed] [Google Scholar]
- 24.Jensen K, Graf BK. The effects of knee effusion on quadriceps strength and knee intraarticular pressure. Arthroscopy. 1993;9(1):52–56. [DOI] [PubMed] [Google Scholar]
- 25.Keays SL, Newcombe PA, Bullock-Saxton JE, Bullock MI, Keays AC. Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med. 2010;38(3):455–463. [DOI] [PubMed] [Google Scholar]
- 26.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. [DOI] [PubMed] [Google Scholar]
- 27.Lindstrom M, Strandberg S, Wredmark T, Fellander-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction: a prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431–442. [DOI] [PubMed] [Google Scholar]
- 28.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. [DOI] [PubMed] [Google Scholar]
- 29.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. [DOI] [PubMed] [Google Scholar]
- 30.Mihelic R, Jurdana H, Jotanovic Z, Madjarevic T, Tudor A . Long-term results of anterior cruciate ligament reconstruction: a comparison with non-operative treatment with a follow-up of 17–20 years. Int Orthop. 2011;35(7):1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikesky AE, Mazzuca SA, Brandt KD, Perkins SM, Damush T, Lane KA. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum. 2006;55(5):690–699. [DOI] [PubMed] [Google Scholar]
- 32.Mouritzen U, Christgau S, Lehmann HJ, Tanko LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62(4):332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oiestad BE, Holm I, Gunderson R, Myklebust G, Risberg MA. Quadriceps muscle weakness after anterior cruciate ligament reconstruction: a risk factor for knee osteoarthritis? Arthritis Care Res (Hoboken). 2010;62(12):1706–1714. [DOI] [PubMed] [Google Scholar]
- 34.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89(7): 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3): 405–424. [DOI] [PubMed] [Google Scholar]
- 37.Peterfy CG, Gold G, Eckstein F, Cicuttini F, Dardzinski B, Stevens R. MRI protocols for whole-organ assessment of the knee in osteoarthritis. Osteoarthritis Cartilage. 2006;14(suppl A):A95–A111. [DOI] [PubMed] [Google Scholar]
- 38.Pietrosimone BG, McLeod MM, Lepley AS. A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health. 2012;4(1):31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollard CD, Braun B, Hamill J. Influence of gender, estrogen and exercise on anterior knee laxity. Clin Biomech (Bristol, Avon). 2006;21(10):1060–1066. [DOI] [PubMed] [Google Scholar]
- 40.Reinke EK, Spindler KP, Lorring D, et al. Hop tests correlate with IKDC and KOOS at minimum of 2 years after primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17(2):195–200. [DOI] [PubMed] [Google Scholar]
- 42.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 43.Scopaz KA, Piva SR, Gil AB, Woollard JD, Oddis CV, Fitzgerald GK. Effect of baseline quadriceps activation on changes in quadriceps strength after exercise therapy in subjects with knee osteoarthritis. Arthritis Rheum. 2009;61(7):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segal NA, Glass NA, Felson DT, et al. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sports Exerc. 2010;42(11):2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery: five-to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446–452. [DOI] [PubMed] [Google Scholar]
- 46.Streich NA, Zimmermann D, Bode G, Schmitt H. Reconstructive versus non-reconstructive treatment of anterior cruciate ligament insufficiency: a retrospective matched-pair long-term follow-up. Int Orthop. 2011;35(4):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 48.Tourville TW, Johnson RJ, Slauterbeck JR, Naud S, Beynnon BD. Assessment of early tibiofemoral joint space width changes after anterior cruciate ligament injury and reconstruction: a matched case-control study. Am J Sports Med. 2013;41(4):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tourville TW, Johnson RJ, Slauterbeck JR, Naud S, Beynnon BD. Relationship between markers of type II collagen metabolism and tibiofemoral joint space width changes after ACL injury and reconstruction. Am J Sports Med. 2013;41(4):779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


