Abstract
Colorectal cancer (CRC) is one type of tumor with the highest frequency and mortality worldwide. Although current treatments increase patient survival, it is important to detect CRC in early stages; however, most CRC, despite responding favorably to treatment, develop resistance and present recurrence, a situation that will inevitably lead to death. In recent years, it has been shown that the main reason for drug resistance is the presence of colon cancer stem cells (CSC). Pericytes are also capable of tumor homing and are important cellular components of the tumor microenvironment (TME), contributing to the formation of vessels and promoting metastasis; however, they have not been considered very important as a therapeutic target in cancer. In this review, we highlight the contribution of pericytes and cancer stem cells to some classical hallmarks of cancer, namely, tumor angiogenesis, growth, metastasis, and evasion of immune destruction, and discuss therapies targeting pericytes and cancer stem cells in CRC.
Keywords: Cancer stem cells, Pericytes, CRC treatment, Chemoresitance
Background
Colorectal cancer (CRC) is a major cause of morbidity and mortality throughout the world. It is the third most common cancer worldwide and the most common malignant tumor in the lower digestive tract [1]. The populations of cells that make up a cancer are manifestly heterogeneous at the genetic, epigenetic, and phenotypic levels. Predominant cell types include immune cells, fibroblasts, adipocytes, endothelial cells (ECs), mesenchymal stroma/cancer stem cells (CSC) and pericytes [2].
The response to treatment is affected by the complexity and immune diversity within the tumor microenvironment (TME) [3]. Immune cell infiltration is a predictive factor in primary tumors, which correlates with tumor mass reduction and patient survival. There is a great interpersonal variability in the same kind of tumor with infiltrating immune cells, including effector T lymphocytes (CTLs), T-helper (TH) cells, T-regulatory cells (T-reg), B cells, natural killer (NK) cells, dendritic cells (DCs) cells, macrophages, myeloid derived suppressor cells (MDSC), and granulocytes [4]. Also, recent studies in CRC have attributed a good prognosis to infiltration by Th1 cells, M1 macrophages, dendritic cells and NK cells, while the presence of M2 macrophages, MDSCs, Th17 and B cells has been associated with a poor outcome [4].
The main mechanisms that eliminate tumor cells in CRC are gamma IFN and TNF (α and β) producing CD4 + TH1 cells and IL10 secreted by FoxP3+ regulatory T cells by NK or γδ T cells that suppress or downregulate induction and proliferation of effector T cells at the tumor site [5, 6]. Cancer-associated fibroblasts (CAFs) are the dominant cell type within the reactive stroma of many tumor types like CRC. This promotes invasiveness by secreting metalloproteinase as CXCL12, which activates CXCL12/CXCR4 signaling [7]. Growth factors, such as transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF), released by tumor cells, are key mediators of CAF activation and contribute markedly to self-renewal of CSC and the development of chemotherapy drug resistance (by secreting TGF-β1). Adipocytes in obesity can actively secrete multiple adipokines and cytokines such as leptin, adiponectin, IL-6, MCP-1 (monocyte chemoattractant protein 1), and TNF-α which are proinflammatory signals [8]. Over time, chronic inflammation can cause DNA damage and promote cancer growth and metastasis. Macrophages contribute as growth tumor cells by inducing formation of new blood vessels from existing ones; this is called angiogenesis. Tumor angiogenesis not only provides the tumor cells with nutrients and oxygen and allows removal of metabolic wastes, but also presents the metastatic tumor cells with points of entry to the circulatory system. Some proangiogenic factors, such as vascular endothelial growth factor (VEGF) and stromal cell-derived factor 1 (SDF-1) promote the repair of injured vascular endothelial cells and neovascularization. Some studies have shown that CXCL12 promotes the synthesis and secretion of VEGF, and CXCL12 combined with VEGF enhances ischemic angiogenesis [9].
Efforts to profile tumor-infiltrating immune cells often have inherent limitations in sample availability, great interpersonal variability, and technological capability, thus restricting research into the local immune response. Therefore, tumor recurrence and metastasis are two critical survival-influencing factors of CRC [10].
Many researchers have observed that some cancer cells acquire the characteristics of cancer stem cells (CSC) through epithelial–mesenchymal transition (EMT), which is responsible for promoting invasion, metastasis and chemotherapy and radiotherapy resistance [11]. Furthermore, successful development of extravasation depends on pericyte cells and signals from the niche in the TME.
The purpose of this article is to highlight the importance of CSC and pericytes in the TME as principal microRNAs innovative therapeutic strategies that can be used for CRC.
Cancer stem cells
Tumor-initiating cells or cancer stem cells (CSCs) are a subpopulation in tumor tissue that are distinct from non-malignant stem cells. CSCs possess unique characteristics such as self-renewal and diferentiation cloning to lineages inside epithelial tissue, giving them great heterogeneity [12]. This can be reflected in the intra-tumoral histological variability recognized a few years ago. They express detoxifying enzymes or efflux bombs that have high efficacy for drug molecule extrusion outside cells; providing them with resistance mechanisms against chemotherapy and radiotherapy. Aside from their high efficiency to generate tumors, slow growth rate, homing and treatment resistance are main characteristics responsible for recurrence and metastasis [13]. In normal intestinal tissue growth, the signaling pathways, Wingless/Int (WNT), Hedgehog (Hh), and Notch, are considered the most important regulators of stemness maintenance and self-renewal [14]. However, aberrant activation of these pathways serve as signaling pathways for the maintenance and proliferation of CSC in tumorigenesis [15, 16].
For CSC stemness maintenance, WNT promotes transcription of NANOG, OCT4, KLF4, EGFR, and LGR5 (GPR49). A Lgr5+CD44+EpCAM+ subpopulation could generate more colonies than any other subpopulation, indicating a higher tumorigenic potential that can produce metastatic disease and strictly defines as markers CSC in human CRC [17]. Aberrant activation of Notch protects CSCs from apoptosis via inhibition of the cell cycle kinase inhibitor p27 as well as ATOH1, a transcription factor [18]. Fender et al. suggested that Notch-1 can increase expression of the EMT/stemness-associated proteins, CD44, Slug, Smad-3, and induce Jagged-1 (Jag-1) expression by increased migration and increased anchorage independent growth [19]. In colon cancer, Notch activation in cancer cells by adjacent blood vessel cells increases trans-endothelial migration, and therefore, metastasis [20]. The expression of Jag1 by ECs activates Notch signaling in local pericyte precursor cells to induce pericyte differentiation [21]. Also, WNT and Hh signaling frequently operate in unison to control cell growth, development, and tissue homeostasis of normal and neoplastic stem cells by regulating gene transcription of VEGF, cMyc, Nanog, Sox2 and Bmil. The Hh pathway controls expression of ABC transporter proteins such as multi-drug resistance protein-1, leading to chemoresistance of CSCs, which effects survival, EMT, metastasis, and CSC expansion [22]. For a more detailed review of the mechanisms involved in these routes, we recommend works by Zhan et al. for WNT [23], Skoda et al. for Hh [24] and Brzozowa et al. for Notch [25].
The discovery of CSC antigens is not based on the overexpression of typical tumor antigens but on the presence of antigens in populations of cells that have stem cell-like properties. However, it is important to note that variable expression levels of antigens on CSCs and their frequent coexpression on normal stem cells have made CSC antigen distinction difficult (Lgr5, CD44, CD24, CD26, CD29, CD166, CD326, CD133, EpCAM and ALDH). LGR5+ CSCs are required for the maintenance of established liver metastases [26].
Three genes, OCT4, SOX2 and NANOG, play a dominant role in regulating pluripotency and are known to influence stem cell maintenance, tumor growth, invasion, EMT and metastasis. However, SALL4 was recognized recently as a zinc finger transcriptional factor regulating multiple targeted genes (OCT4, SOX2, and KLF4, Bmi-1 and PTEN). SALL4 is capable of stimulating Wnt/β-catenin signaling by directly binding to β-catenin and functioning as an oncogene in diverse tumors (leukemia, liver cancer, breast cancer, gastric and CRC). Previously, SALL4 mRNA levels in the blood were found to be significantly higher in patients with CRC than in control subjects, but lower in patients with a local cancer than in those with invasive CRC [27].
The remarkable complexity that involves cancer from the point of view of colon stem cells can be observed by the large number of markers they have and how their expression is modified depending on the factors that are exposed inside and outside of the TME. CRC develops as a result of serial alterations in oncogenes and tumor suppressor genes (APC, KRAS and TP53) [28]. However, recent studies reported that hypoxia-associated cell-type plasticity and epigenetic alterations can deregulate fundamental signaling pathways controlling self-renewal and differentiation, including Wnt, Notch, Myc and Hh pathways, contributing to this CSC heterogeneity and the potential implications for generating metastasis by EMT [29, 30].
Epithelial–mesenchymal transition (EMT)
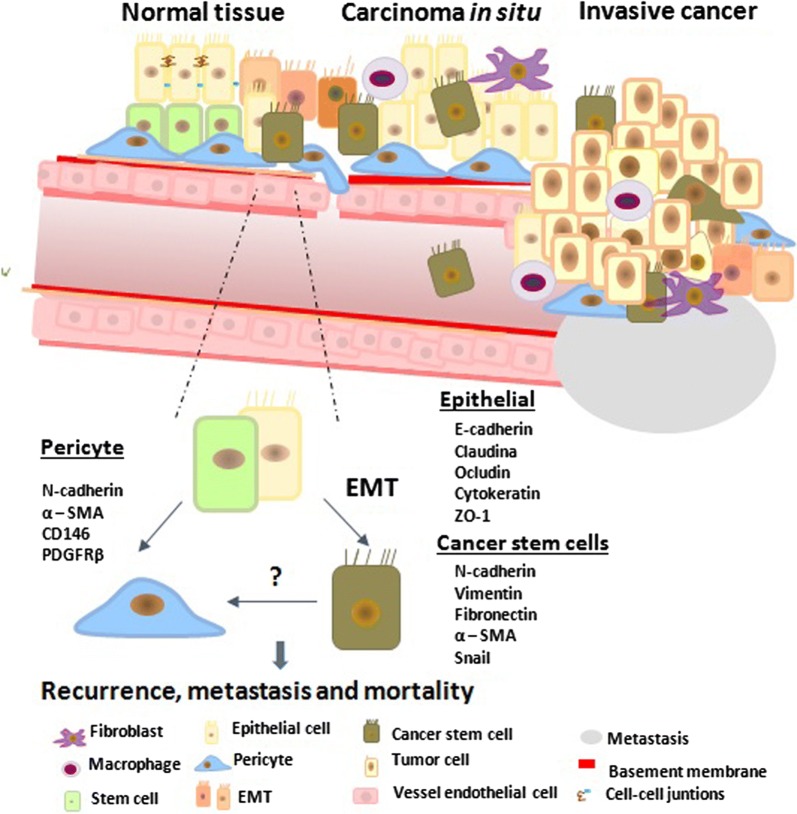
The normal transition of colon or rectum mucosal cells from epithelial to mesenchymal (EMT) cells regulates healthy intestinal architecture and also defines the balance between proliferation and differentiation mediated by the WNT pathway. CRC mutations in the APC gene (present in 80% of sporadic cancers) result in constant activation of the Wnt pathway (β-catenin), promoting the transition to the mesenchymal phenotype [31]. It is considered that during this transition process, a mechanism is activated where tumor (epithelial) cells lose their polarity as well as adhesion mediated by E-cadherin downregulation of other epithelial genes, components of the tight junctions; this includes members of the claudin family and cytokeratins, which produce the reorganization of the cytoskeleton. Also, during this process, the basement membrane and the extracellular matrix are destroyed by secretion of enzymes such as matrix metalloproteinase, which cause cells to pass from an adherent epithelial phenotype to a non-adherent mesenchymal phenotype [32]. Therefore, the phenotype fibroblast-like cell of non-adherent cells into spindle-shaped which characteristically upregulate mesenchymal markers; e.g., vimentin, N-cadherin and fibronectin which are associated with invasion of adjacent tissues and the formation of metastases [33]. Metastases originate because non-adherent cells circulate through the lymphatic and vascular blood systems which, in the final analysis, contribute to the intra- or extravasation of the transformed cells [31, 32].
The EMT process is regulated by TGF-β. This signal induces the expression of other growth factors such as fibroblast specific protein (FSP1), smooth muscle alpha actin (SMAα), vascular endothelial growth factor (VEGF) and the cytokines, IL-6, IL-23 and/or IL-1β (pro-inflammatory) from CD4+ T lymphocytes, which participate in maintaining a microenvironment to promote this complex process. In addition, the activation of transcription factors such as Snail1/2, Slug, Twist1 and Zeb1/2 and pathways such as Wnt, Hedgehog (HH), bone morphogenic protein (BMP), Notch, and platelet-derived growth factor (PDGF), Oct4 and Sox2, are involved in uncontrolled proliferation, regulate downexpression of E-cadherin, and proteases that promote loss of cell adhesion and stemmess phenotype [34, 35].
Recent studies suggest that MSCs induce EMT in colon cancer cells via direct cell-to-cell contact or indirect communication between MSC-derived exosomes which may play an important role in colon cancer metastasis. Also, in human CRC, EMT enhances the migratory and invasive properties of cancer cells which results in invasive lesions and tumor peripheries at the interface between cancer cells and host cells surrounded by ECM [36].
Pericytes
Pericytes are specialized mesenchymal cells present at intervals along the walls of capillaries (and post-capillary venules), which vary greatly in morphology and marker expression in different tissues [37]. Mesenchymal stem cells and pericytes display remarkable similarities in terms of their marker expression, their ability to self-renew, and their potential to differentiate into multiple cell types such as adipocytes, chondrocytes, osteocytes and myocytes in culture.
Furthermore, some pericyte markers are PDGFR-β (platelet-derived growth factor receptor-beta), NG2 (chondroitin sulfate proteoglycan 4), CD13 (alanyl (membrane) aminopeptidase), αSMA (alpha-smooth muscle actin) [38], Desmin and CD146, are not uniquely found on pericytes but are also expressed on other cell types, most notably endothelial and smooth muscle cells, and are often dynamically expressed [39, 40]. Recent studies have shown that CD146 is constitutively expressed in the pericytes of several organs and functions as a component of endothelial junctions to reduce the paracellular permeability of peripheral endothelial cells. CD146 (also known as MCAM, S-endo-1, P1H12, and MUC18) was identified as a novel endothelial biomarker for angiogenesis in the tumor progression of several malignancies. CD146 is a potential marker for the diagnosis of malignancy in cervical and endometrial cancer, including melanoma and lung cancer [41, 42].
Pericytes residing in different tissues have been termed according to their function and morphology, such as hepatic stellate cells in the liver and glomerular mesangial cells in the kidney. The morphology of pericytes can be stellate or spindle-like with finger-like projections surrounding the vessels which are now believed to have a role in regulating blood flow and inflammatory cell trafficking [43]. Under pathological conditions, pericytes may differentiate into myofibroblasts, contributing to kidney fibrosis [44].
Pericytes are involved in the preservation of vascular stability and homeostasis, including regulation of blood flow, structural maintenance of the vasculature, vascular permeability, and remodeling of ECM [45]. Emerging evidence demonstrated that pericytes are an important cellular component in the TME associated with angiogenesis, metastasis, resistance to treatment and patient mortality; however, the mechanisms are poorly understood [44].
Endothelial cells (ECs) that line the inner surface of vessels, directly participate in oxygen delivery, nutrient supply, and removal of waste products. During blood vessel maturation, endothelial cells (ECs) secrete platelet-derived growth factor (PDGF), which chemoattracts pericytes that express PDGFRβ. The ligand binding with the receptor provides vessel stability. VEGF produced by endothelial cells is crucial for normal vascular homeostasis. It is known that during EMT, PDGFR is expressed by stromal cells of mesenchymal origin, such as pericytes, which derive mainly from the cephalic region and the neural crest [46] From EMT, mesothelial cells attach to the pericytes of the intestine, liver, heart and lung. This is very important since during tumor development, some tumor cells after EMT suffer a loss of junctions from neighboring cells, diminishing expression of E-cadherin; also, high levels of PDGFR can begin to express markers similar to pericytes (NG2 and SMA) [47, 48]. This represents the epithelial transition to pericytes (EPT), a process induced by TGF-β, which may also activate the EMT program as well as contribute to the development of both normal and tumor pericytes (Fig. 1). In this way, some tumor cells are recruited or differentiated to pericytes to help vascularize tumor tissue and intratumoral vasculature, promoting metastasis [49]. These malignant pericytes may further acquire properties that promote their mobility and invasiveness during tumor metastasis [50]. Thus, malignant pericytes may be of central importance for both tumor angiogenesis and tumor metastasis [51].
Fig. 1.
Interaction pericytes and cancer stem cells. Tumorigenesis activates EMT-promoting transcription factors (TWIST, SNAIL and ZEB) through pathways known to play critical as WNT, NOTCH, TGF-β and NF-κB cascades and hypoxia. Cancer stem cells were recently found to function as pericyte progenitors thus reciprocal interaction between pericytes and CSC is highly beneficial to tumor development, contributing to tumor angiogenesis and metastasis
Angiogenesis involves the formation of new vessels to supply nutrients to the tumor, promoting cancer survival, growth, and dissemination. This complex process is regulated through ECs and pericytes that express high levels of PDGF and VEGF/VEGFR (receptor tyrosine kinases such as VEGFR1, VEGFR2, and VEGFR3). Factors are involved in stimulating tumor angiogenesis indirectly by inducing VEGF, TGF-α and β, TNF-α, keratinocyte growth factor, insulin-like growth factor I (IGF-I), FGF, PDGF, and cytokines [interleukin (IL)-1α and IL-6 and EGF on tumor cells]. EGF, a key EGFR ligand, is one of the many growth factors that drive VEGF expression. EGFR is one of four members of the HER/erbB family of receptor tyrosine kinases [HER1 (EGFR/erbB1), HER2 (neu, erbB2), HER3 (erbB3), and HER4 (erbB4)] that is present on all epithelial and stromal cells, and on many smooth muscle cells; however, EGFR overexpression and aberrant EGFR expression has been observed in numerous tumor cell correlates with increased proliferative, angiogenic activity, and poor prognosis [52]. Increased proliferation and angiogenesis by EGFR are thought to be caused by the binding ligands TGFα and EGF, which have shown to function as chemoattractants for endothelial cells and promote the expression of VEGF by tumor cells. Many observations indicate that anti-angiogenic therapy may have limited efficacy, and in most patients, the cancers eventually display resistance to this treatment. Previous studies have shown that this resistance mechanism is associated with hypoxia-induced alterations. Tumor cell deprivation of oxygen induces HIF1α which dimerizes with HIF1β and translocates to a nucleus where transcription regulates expression of genes, such as VEGF, PDGF, bFGF, erythropoietin, angiopoietin, and placental growth factor (PIGF) which increase cell proliferation, metabolism, and abnormal tumor blood vessels [53]. Activation of EMT is a molecular pathway that evades therapeutic efficiency and produces resistance to anti-angiogenic therapy. During this process, a few CSCs, using the EPT, give rise to cancer cells that function as pericytes to stabilize blood vessels. Migration of CSC to blood vessels in the primary tumor is a natural part of the intravasation process, which depends on EMT and EPT produced signals that coordinate to generally enable cancer cells to be chemoattracted or associated with ECs, and help stabilize the vasculature or intravasate for metastasis.
The capacity of CSCs to generate vascular pericytes allows active vascularization in CRC to support tumor growth [54]. Therefore, we believe that pericytes may have a crucial role in mediating therapeutic resistance in CRC. Several studies of pericyte and tumor development were mostly focused on angiogenesis, showing that blockage of pericyte recruitment or function leads to reduced tumor growth due to compromised vessel structure and extravasation tumor cells [47]. Also, poor pericyte coverage has also been confirmed to have a correlation with a worst prognosis for patients with cancer that originates leaky vessels that increase intratumoral/interstitial plasma volume and elevate local pressure contributing to the progression and metastasis in the tumor by releasing factors that affect tumor invasion. High vascular density at the CRC invasion front is directly associated with recurrence, metastasis, and patient mortality. Ultimately, pericyte-targeted therapies should be tested in combination with other treatment modalities to address possible synergistic effects avoiding metastatic spread [55]. Hsu et al. [56] recently demonstrated in patients with metastasic CRC with wild-type KRAS exon 2, who had received cetuximab (anti-EGFR) and then bevacizumab (anti-VEGF), and standard chemotherapy, an increased overall survival by reductions in microvasculature density and tumor metastasis. The principle of first blocking EGFR is based on eliminating the vasculature that promotes tumor growth after that the tumor cells become more susceptible to being eliminated by antiangiogenic therapy. Until now the use of antiangiogenic agents is far from being effective in CRC since resistance to these treatments occurs mainly through the EMT and EPT routes. We believe that this additive effect in the treatment of CRC should be addressed not only in CSC but also in pericytes and this is why we review the main therapeutic targets in CRC.
Therapeutic strategy
First-line treatment in patients with CRC is FOLFOX, which includes 5-fluorouracil (5-FU), oxaliplatin, and leucovorin. However, most patients develop resistance to this treatment and die within 1–10 years after its initiation [57]. Angiogenesis is required for invasive tumor growth and metastasis, which is mediated through VEGF and EGFR. Patients with metastatic CRC are currently treated with irinotecan and immunotherapy (bevacizumab, ramucirumab, and Ziv-aflibercept against VEGF and either cetuximab or panitumumab against EGFR) [58] DJ-1 (PARK7/CAP1/RS) is a multifunctional protein that protects neurons from oxidative stress by activating Akt/mTOR, MEK/ERK, NF-κB, and HIFα signaling pathways. Overexpression of DJ-1 in many tumor types correlated with promoting cancer cell survival, proliferation, and metastasis. Results recently suggest that DJ-1 is a potential prognostic and therapeutic target in invasive CRC [59]. More recently, the DART protein MGD007 was designed to co-engage T lymphocytes with CRC cells through the cell surface antigens, CD3 and gpA33, respectively, in order to promote T-cell recruitment and anti-tumor activity [60]. In addition, novel 89Zr-labeled anti-LGR5 mAbs were developed for evaluating the imaging potential of the CSC marker and were useful for stratifying patients that would respond best to an LGR5-targeted ADC therapy, and for monitoring treatment response in CRC [61]. Targeting strategies in self-renewal pathways in CSCs, including their pharmacological antagonists Hh ligand Inhibitors (PTCH1 inhibitor or RU-SKI [62]. GLI Antagonists (TAK-441-trial advanced CRC), SMO Inhibitors, Anti-DLL4/NOTCH Antibodies [63, 64]. (OMP-21M18, REGN421, and MEDI0639 for anti-angiogenesis), γ-secretase inhibitors [65] (PF-03084014 inhibitor is generally safe and well tolerated by oral administration in advanced cancer). Wnt ligand inhibitors such as OMP-54F28 [66] which is a recombinant protein formed by the fusion of the immunoglobulin Fc to the CRD of FZD8 for blocked WNT, are undergoing clinical trials [67] despite being a promising strategy, it still has limitations such as the systemic toxicity of the antibodies used to block any of the pathways involved in the maintenance of CSC.CRISPR/Cas9 has become a powerful tool for changing the genome of many organisms. The open-label phase I study (NCT02793856) using CRISPR for cancer therapy was programmed cell death protein-1 (PD-1) knockout engineered. PD-1, a member of the CD28 superfamily of T-cell regulators expressed in a wide range of immune cells, including peripherally activated T cells, B cells, monocytes, NK cells, and DCs that consist of an Ig-V like extracellular domain, a transmembrane domain, and a cytoplasmic domain that harbors two tyrosine-based signaling motifs, interacts with two ligands [68]. These ligands, PD-L1 (CD274 or B7H1) and PD-L2 (CD273), were found expressed in some tumor cells. PD-L1 is expressed in many cell types such as vascular endothelium, reticular fibroblasts, non-mesenchymal stem cells, islet cells, astrocytes, neuronal cells, and keratinocytes. Interactions between the extracellular domains of PD-L1 and PD-1 attenuate T cell-activating signals and lead to inhibiting proliferation, survival, and production of growth factors such as EGF, TGF-β, and GM-CSF, and cytokines such as INFγ, TNF-α, IL-6 and IL-17. Activation of the PD-1/PD-L1 signaling pathway causes immunosuppression of T cell function, which is considered the main factor responsible for response immune escape [69]. However, cancer stromal cells can contribute to tumor microenvironment upregulates PD-L1 expression, by express GM-CSF and VEGF and promotes immune suppression. This effect is called “adaptive immune resistance”, because the tumor protects itself by inducing PD-L1 in response to IFN-γ produced by activated T cells. T cells ex vivo are evaluated for treating metastatic non-small cell lung cancer that has progressed after all standard treatments. Patients enrolled in the gene-editing trial provided peripheral blood lymphocytes and PD-1 knockout of T-cells by CRISPR/Cas9 performed ex vivo. The edited lymphocytes were selected, expanded and subsequently infused back into the patients. Four other trials applying the same concept of PD-1 knockout for treatment have been registred for other cancer types, including prostate, bladder, esophageal and renal cell cancer [62]. Recent studies propose as a target for colorectal cancer EGFR (overexpressed in 60–80% of aggressive tumors) or CAE as chimeric antigen receptors allow T-cells to recognize tumor cells and quickly destroy them [70]. This strategy is novel with safe and efficient results; mainly in hematological tumors with a lower response in solid tumors. New treatment approaches are still required since these present disadvantages such as side effects after their administration. In addition, it is still necessary to evaluate for prolonged periods if the resident tumor cells that do not evade this treatment by EMT are not able to develop metastasis. A recent report demonstrated that PD-L1 induces ZEB1, which activates OCT4 and Nanog signaling and upregulation of EMT on CSC. These promote chemoresistance and metastasis by increased phosphorylation of AKT and ERK, resulting in activation of the PI3K/AKT and MAPK/ERK pathways and an increase of MDR1 expression. Recently, Nivolumab, an anti-PD-L1 drug was approved for metastatic CRC resistant to fluoropyrimidine, oxaliplatin and irinotecan [68, 71]. This is because the therapeutic targets used are not specific to this cell population (CSC) and the pericytes, as the cells required to ensure the establishment of the metastases have not yet been taken into account.
Circular RNAs (circRNAs) are abundant and important members of the non-coding RNA family which are generally expressed at low levels and exhibit cell-type-specific and tissue-specific patterns, with an average half-life of 19–24 h and whose function remain mostly unknown [72]. There has recently been considerable attention on circRNA as a molecule that regulates or controls miRNA expression; therfore, they play a significant role in many fields of cancer biology. In tumor biology, circRNA emerges as an effective biomarker for the detection of cancers mainly because it allows differenciation between a normal cell and a tumor cell as well as exhibiting dynamic global changes in its expression levels during tumor progression [73]. In addition, because circRNA have normally been detected in saliva and blood, they can help as biomarkers that are able to predict sensitivity, the risk of metastasis or the prognosis of treatment. An example as a predictor of 5FU resistance, Xiong et al. identified three upregulated circRNAs (0007031, hsa_circ_0000504 and hsa_circ_0007006) in CRC by microarray analysis [74, 75]. However, until now they have not been used for therapeutic purposes. Also, the importance that these could have in colon cancer is unknown.
miRNAs are small 22-nucleotide non-coding RNAs that are distributed and abundant in almost all human tissue. They modulate hundreds of genes simultaneously and, therefore, control multiple signaling pathways involved in several processes such as apoptosis, proliferation, differentiation and migration [75]. Gene silencing by microRNAs occurs through imperfect/perfect complementary base pairing between a miRNA guide strand and the 3′ UTR region of the mRNA mainly; however, it has been detected that miRNAs bind to the 5′ UTR coding sequence as well as within promoter regions. The binding of miRNAs to the UTR region leads to translational repression or miRNA degradation [76] while miRNA interaction with the promoter region has been reported to induce transcription.
The dominant pathway by which miRNAs are processed begins with a pri-miRNA gene that is transcribed and processed by microprocessor complex and Drosha in the nucleus to form a pre-miRNA (precursor miRNA). Then pre-miRNA is exported to the cytoplasm via the activity of Exportin5/RanGTP-dependent manner and processed to produce the mature miRNA duplex by Dicer, AGO2 and TRBP, which are necessary components in the formation of the RNA-induced silencing complex (RISC). The RISC is then guided by the biological active strand to messenger RNA (mRNA) targets, which lead to gene silencing via mRNA degradation or translational inhibition [76].
During cancer initiation and progression, the expression levels of multiple miRNAs are aberrantly up or downregulated, resulting in an imbalance of cell pathways that reflect particular disease states associated with the regulatory response to chemotherapy, differentiation, proliferation, and migration in different malignancies which are useful for therapeutic purposes, and as diagnostic and prognostic biomarkers in cancer. Therefore, they may be strong weapons in the fight against chemoresistance in colon CSC. Gene expression studies have identified the clinical importance of miRNAs in pericytes or CSC on CRC. This is summarized in Table 1.
Table 1.
| miRNA | Expression level | Target | Findings | Technique | References |
|---|---|---|---|---|---|
| 21* | ↑ | ITGβ4 | A prognostic tool, proliferation, invasion and metastasis | qPCR | 10.4161/epi.26842 [91] |
| 23a | ↑ | E-cadherin | Induced EMT process associated CRC metastasis | qPCR | 10.1093/carcin/bgt274 [92] |
| ↓ | ZO-1 | Increasing vascular permeability and migration | 10.3892/etm.2017.4972 [93] | ||
| 24* | ↑ | Paxillin | Inhibited the killing effect of NK cells to colorectal cancer cells | qRT-PCR and Western blot | 10.1016/j.biopha.2018.02.024 [94] |
| 34a | ↓ | Inh3 | Increased lymph node infiltration and metastasis in colon cancer patients | miRNA target prediction software miRWalk | 10.1053/j.gastro.2017.04.017 [95] |
| 126 | ↑ | BCL-2 and p53 | Potential tumour suppressor | qRT-PCR | 10.1016/j.yexcr.2015.10.004 [96] |
| 137 | ↓ | TCF4 | Suppresses cell proliferation, migration and invasion in colon cancer cell lines | RT-qPCR | 10.3892/ol.2018.8364 [97] |
| 143* | ↑ | IGF-IR | Inhibited cell proliferation, migration, tumor growth, angiogenesis and increased chemosensitivity to oxaliplatin treatment | qRT-PCR and western blot | 10.4161/cc.24477 [98] |
| 150 | ↑ | c-Myb | Inhibits cell proliferation, induces cell apoptosis and inhibits cell migration and invasion in human CRC cells | qRT-PCR, western blot and DNA constructs and luciferase target assay | 10.1111/jcmm.12398 [99] |
| 200 | ↓ | ZEB1, ETS1 and FLT1 | Increased EMT | TaqMan MicroRNA assays | http://doi.org/10.1136/gutjnl-2011-301846 [100] |
| 203 | ↑ | SOCS3 | Potential for metastasis; promoted the differentiation of monocytes to M2 macrophages |
RT-qPCR and Gene Set Enrichment Analysis (GSEA) TargetScan and miRanda |
10.18632/oncotarget.20009 [101] 10.1177/1947601911425832 [102] |
| 221 | ↑ | RECK, RelA and STAT3 | Migration and invasion in vitro and metastasis in vivo |
qRT-PCR and western blot qRT-PCR and western blot |
10.1016/j.febslet.2013.11.014 [103] 10.1053/j.gastro.2014.06.006 [104] |
| 1246 | ↑ | CCNG2 | Promoted the proliferation, colony formation, invasion and migration, and inhibited the apoptosis | RT-qPCR and Dual luciferase reporter assay | 10.3892/mmr.2015.4557 [105] |
↑ = ; ↓ = *clinical trials
The effectiveness of microRNAS as nucleotide-based molecules has been compromised by inherent characteristics that they possess, such as: (1) stimulation of the innate immune system after induction of interferon responses; (2) inefficient binding due to a mutation in the sequence of the target mRNA; (3) short duration of the silencing effect, which requires high and sustained concentrations of payload in the target tissue. It also has other features such as serum instability due to rapid degradation by endo- and exonucleases in the bloodstream; inefficient cell entry inherent in the negatively charged nature of miRNA molecules, poor pharmacokinetic profile associated with a half-life of about 5 min, and rapid renal clearance due to their low molecular mass (≈ 13 kDa) [76–82] which can be overcome with efficient delivery systems. The properties of vector systems that can modify miRNA expression are briefly presented in Table 2 [83, 84].
Table 2.
Vector systems
| Vectors | Advantages | Disadvantages |
|---|---|---|
| Using VIRUS | ||
| Adenovirus |
↑ Efficiency and vector titers Insert capacity (max 8 Kb) |
No integration Short-term expression ↑Immunogenicity |
| Adeno-associated virus |
↑ Efficiency and vector titers ↓ Toxicity, no pathogenic ↓ Risk of mutagenesis Remains predominantly episomal |
Requires helper virus to replicate Insert capacity (3-5 Kb) |
| Retrovirus |
↓ Immune response in host Insert capacity (8 Kb) Integrates into genome |
↓ Vector titers Incorpotates into dividing cells only Restricted tropism ↑ Risk of insertional mutagenesis |
| Lentivirus |
Uptake in dividing and not dividing cells ↑ Insert capacity (8 Kb) Integrates into genome Next generation is self-inactiving for safe |
↓ Vector titers Restricted tropism Risk of insertional mutagenesis |
| Non VIRAL | ||
| Liposomes |
Protect degradation by nucleasas Dose-dependent toxicity cationic polymers (PEI and PAMAM) ↓ Immune response in host rapid clearance from the bloodstream |
Toxic effects on the liver and the kidney in mice ↓ Circulation half-life (minute–hours) |
| Nanoparticles |
Protect degradation by nucleasas ↑ Circulation half-life (synthetic polymers sustained release over a period of days to several weeks) Dose-dependent toxicity ↑ Penetrability and solubility enhanced drug stability and biocompatibility facile synthesis and easy structural modification targeted drug delivery (specify and inespecify) |
Toxic effects depends on the size and biodistribution |
| DNA nanostructures |
Protect degradation by nucleasas Small size ↑ Precision and flexibility Non-toxic DNA nanostructures with their powerful structural control ↑ Biodistribution, biocompatibility |
Localization and mapping of nanorobots in the human body are difficult using conventional optical microscopy techniques Effect desired require coordination collective nanorobots |
Cationic polymers that are frequently used for intracellular delivery are polyethyleneimine (PEI) and polyamide amine dendrimers (PAMAM)
Encapsulating or protecting the microRNA by a vector with a reporter gene or cell tracking-dye allows evaluation of the activity in an in vivo model. A recent work evaluated an oral delivery system intended for treatment of colon cancer by encapsulating hSET1 antisense and SN38 anticancer in nanoparticles with results effective against HT29 cells. Also, more recently it was proposed against CRC to encapsulate miR-204-5p with poly (d, l-lactide-co-glycolide)/poly (l-lactide)-block-poly (ethylene glycol)-folate polymer to promote apoptosis and inhibit cell proliferation in an in vitro xenograft model with Luc-HT-29 [85–87]. Although it is a very promising area in the treatment against cancer, it still requires further evaluation of the role of different vectors to find the most suitable and safe, efficient and without long-term toxicity for its application in humans.
Conclusions
As mentioned before, the important role that pericytes and tumor stem cells play in treatment resistance of patients with CRC makes these cells ideal candidates to limit tumor progression. Tumor suppressive microRNAs are potent molecules that might cure cancer. Recently, it was reported as advanced strategies for delivery of these microRNAs to the cell DNA-Doxorubicin against to HT-29 cells. Nano-sized DNA structures are of low cost, high stability, and feasible to synthesize. They are biosafe due to their lack of exogenous immune activity. Folic acid-DNA tetra-Dox strategy facilitates the targeted delivery of Doxorrubicin, enhances the anticancer HT-29 colon cancer efficiency of chemotherapy agent on colon cancer cells and provides a promising inspiration and idea for drug design [86, 88]. This delivery system is a very innovative and safe methodology; however, so far they have not been realized as a miRNA delivery system. That is why we believe that this therapeutic strategy could change the landscape of CRC.
Acknowledgements
Special thanks to Orlando Solis Coronado for contributing in part with the bibliographical searches.
Abbreviations
- CRC
colorectal cancer
- ECs
endothelial cells
- TME
tumor microenviroment
- CSC
cancer stem cells
- CTLs
cytotoxic T lymphocytes
- TH
T helper cells
- T-reg
T-regulatory cells
- NK
natural killer
- DCs
dendritic cells
- MDSC
myeloid derived suppressor cells
- IFNs
interferons
- TNF
tumor necrosis factor
- CAFs
cancer-associated fibroblasts
- CXCL12
motif chemokine 12
- CXCR4
chemokine receptor type 4
- TGF- α
transforming growth factor alpha
- TGF- β
transforming growth factor beta
- PDGF
platelet-derived growth factor
- FGF
fibroblast growth factor
- CAF
cancer-associated fibroblast
- IL-6
interleukin 6
- MCP-1
monocyte chemoattractant protein 1
- TNF-α
tumor necrosis factor alfa
- DNA
deoxyribonucleic acid
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptors
- SDF-1
stromall cell-derived factor 1
- EMT
epithelial–mesenchymaltransition
- Hh
Hedgehog
- RNA
ribonucleic acid
- ATOH1
atonal BHLH transcription factor 1
- LGR5+
leucine-rich repeat-containing G-protein coupled receptor 5
- ALDH1
aldehyde dehydrogenase
- SMA
smooth muscle actin
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- CD
cluster of differentiation
- HER
human epidermal growth factor receptor
- KLF4
Kruppel-like factor 4
- Bmi-1
polycomb complex protein
- PTEN
phosphatase and tensin homolog gene
- ECM
extracellular matrix
- MSC
mesenchymal stem cell
- PDGFR- β
platelet-derived growth factor receptor-beta
- EPT
epithelial to pericyte transition
- KRAS
Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- ADC
antibody drug conjugate
- PD-1
programmed cell death protein-1
- PD-L1
programmed death-ligand 1
- PD-L2
programmed death-ligand 2
- ZEB1
Zinc Finger E-Box Binding Homeobox 1
- MDR1
multidrug resistance protein 1
- EBV
Eppstein–Barr virus
- CAR
chimeric antigen receptor
- TRAC
T cell receptor α chain
- CMD
carboxymethyl dextran
- PEI
polyethyleneimine
- PAMAM
polyamide amine dendrimers
Authors’ contributions
ENGT made the literature analysis and wrote, discussed and revised the manuscript of this review. PDG analyzed and corrected the manuscript. CIVS and AMG, made the literature analysis and revised the design of the image. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree with the final version of the manuscript and give their consent for its publication.
Competing interests
The authors declare that they have no compeitng interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elsa N. Garza Treviño, Email: egarza.nancy@gmail.com
Paulina Delgado González, Email: delgadogzz.pau@gmail.com.
Carlos I. Valencia Salgado, Email: carlosvalenciasalgado@gmail.com
Alejandra Martinez Garza, Email: alem.mtz@gmail.com.
References
- 1.Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer. 2018;119(7):785–792. doi: 10.1038/s41416-018-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 2017;6(12):2115–2125. doi: 10.1002/sctm.17-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelands J, Kuppen PJK, Vermeulen L, Maccalli C, Decock J, Wang E, et al. Immunogenomic classification of colorectal cancer and therapeutic implications. Int J Mol Sci. 2017;18(10):2229–2239. doi: 10.3390/ijms18102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Presti E, Pizzolato G, Corsale AM, Caccamo N, Sireci G, Dieli F, et al. γδ T cells and tumor microenvironment: from immunosurveillance to tumor evasion. Front Immunol. 2018;9(06):1–10. doi: 10.3389/fimmu.2018.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetta HF, Elkady A, Mekky MA, Abdelmalek MO, Sayed HI, Bazeed S, et al. Interplay between gut microbiota and T lymphocytes in colorectal cancer. Color Cancer Open Access. 2017;3(2):1–6. doi: 10.21767/2471-9943.100042. [DOI] [Google Scholar]
- 7.Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017;14(3):2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabuso M, Homer-Vanniasinkam S, Adya R, Arasaradnam RP. Role of tissue microenvironment resident adipocytes in colon cancer. World J Gastroenterol. 2017;23(32):5829–5835. doi: 10.3748/wjg.v23.i32.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau EYT, Ho NPY, Lee TKW. Cancer stem cells and their microenvironment: biology and therapeutic implications. Stem Cells Int. 2017;2017:11. doi: 10.1155/2017/3714190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Yuan L, Lu Q, Xu H, He X. Distinctive profiles of tumor-infiltrating immune cells and association with intensity of infiltration in colorectal cancer. Oncol Lett. 2018;15(3):3876–3882. doi: 10.3892/ol.2018.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garza-Treviño EN, Said-Fernández SL, Martínez-Rodríguez HG. Understanding the colon cancer stem cells and perspectives on treatment. Cancer Cell Int. 2015;15(1):1–9. doi: 10.1186/s12935-015-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22(3):457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismaiel NEHS, Sharaf WM, Helmy DO, Zaki MM, Badawi MA, Soliman ASA. Detection of cancer stem cells in colorectal cancer: histopathological and immunohistochemical study. Open access Maced J Med Sci. 2016;4(4):543–547. doi: 10.3889/oamjms.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12(8):445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanuytsel T, Senger S, Fasano A, Shea-Donohue T. Major signaling pathways in intestinal stem cells. Biochim Biophys Acta. 2013;1830(2):2410–2426. doi: 10.1016/j.bbagen.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaemmerer E, Jeon MK, Berndt A, Liedtke C, Gassler N, Kaemmerer E, et al. Targeting Wnt signaling via notch in intestinal carcinogenesis. Cancers. 2019;11(4):555. doi: 10.3390/cancers11040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng Z, Xia Q, Chen J, Li Y, Xu J, Zhao E, et al. Lgr5 + CD44 + EpCAM + strictly defines cancer stem cells in human colorectal cancer. Cell Physiol Biochem. 2018;46(2):860–872. doi: 10.1159/000488743. [DOI] [PubMed] [Google Scholar]
- 18.Vinson KE, George DC, Fender AW, Bertrand FE, Sigounas G. The Notch pathway in colorectal cancer. Int J Cancer. 2016;138(8):1835–1842. doi: 10.1002/ijc.29800. [DOI] [PubMed] [Google Scholar]
- 19.Fender AW, Nutter JM, Fitzgerald TL, Bertrand FE, Sigounas G. Notch-1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer. J Cell Biochem. 2015;116(11):2517–2527. doi: 10.1002/jcb.25196. [DOI] [PubMed] [Google Scholar]
- 20.Suman S, Das TP, Ankem MK, Damodaran C. Targeting Notch signaling in colorectal cancer. Curr Colorectal Cancer Rep. 2014;10(4):411–416. doi: 10.1007/s11888-014-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meurette O, Mehlen P. Notch signaling in the tumor microenvironment. Cancer Cell. 2018;34(4):536–548. doi: 10.1016/j.ccell.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Zhu X, Liu W, Ruan T, Tao K. Hedgehog signaling pathway in colorectal cancer: function, mechanism, and therapy. Onco Targets Ther. 2017;10:3249–3259. doi: 10.2147/OTT.S139639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci. 2018;18(1):8–20. doi: 10.17305/bjbms.2018.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brzozowa-Zasada M, Piecuch A, Dittfeld A, Mielańczyk Ł, Michalski M, Wyrobiec G, et al. Notch signalling pathway as an oncogenic factor involved in cancer development. Contemp Oncol. 2016;20(4):267–272. doi: 10.5114/wo.2016.61845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatano Y, Fukuda S, Hisamatsu K, Hirata A, Hara A, Tomita H. Multifaceted interpretation of colon cancer stem cells. Int J Mol Sci. 2017;18(7):1446–1460. doi: 10.3390/ijms18071446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munro MJ, Wickremesekera SK, Peng L, Tan ST, Itinteang T. Cancer stem cells in colorectal cancer: a review. J Clin Pathol. 2018;71(2):110–116. doi: 10.1136/jclinpath-2017-204739. [DOI] [PubMed] [Google Scholar]
- 28.Hg J, Jenkins G, Williams N, Ap G, Beynon J, Da H. Review of the role of intra-tumour heterogeneity in colorectal cancer. Cancer Res Oncol. 2016;2(2):1–10. [Google Scholar]
- 29.Kim H, Lin Q, Glazer PM, Yun Z. The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Res. 2018;20(1):16. doi: 10.1186/s13058-018-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41. doi: 10.1186/s12943-017-0600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nistico P, Bissell MJ, Radisky DC. Epithelial–mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harbor Perspect Biol. 2012;4(2):1–12. doi: 10.1101/cshperspect.a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diepenbruck M, Christofori G. Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11(4):451–464. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial–mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract. 2015;211(8):557–569. doi: 10.1016/j.prp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Lazennec G, Lam PY. Recent discoveries concerning the tumor—mesenchymal stem cell interactions. Biochim Biophys Acta Rev Cancer. 2016;1866(2):290–299. doi: 10.1016/j.bbcan.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Takigawa H, Kitadai Y, Shinagawa K, Yuge R, Higashi Y, Tanaka S, et al. Mesenchymal stem cells induce epithelial to mesenchymal transition in colon cancer cells through direct cell-to-cell contact. Neoplasia. 2017;19(5):429–438. doi: 10.1016/j.neo.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cathery W, Faulkner A, Maselli D, Madeddu P. Concise review: the regenerative journey of pericytes toward clinical translation. Stem Cells. 2018;36(9):1295–1310. doi: 10.1002/stem.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 40.Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1007/s10544-018-0298-0. [DOI] [PubMed] [Google Scholar]
- 41.Zeng P, Li H, Lu P-H, Zhou L-N, Tang M, Liu C-Y, et al. Prognostic value of CD146 in solid tumor: a systematic review and meta-analysis. Sci Rep. 2017;7(1):4223. doi: 10.1038/s41598-017-01061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Luo Y, Hui H, Cai T, Huang H, Yang F, et al. CD146 coordinates brain endothelial cell-pericyte communication for blood–brain barrier development. Proc Natl Acad Sci USA. 2017;114(36):E7622–E7631. doi: 10.1073/pnas.1710848114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong SP, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther. 2015;151:107–120. doi: 10.1016/j.pharmthera.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci USA. 2016;113(38):E5618–E5627. doi: 10.1073/pnas.1608384113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther. 2017;171:30–42. doi: 10.1016/j.pharmthera.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55(3):261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 47.Shenoy AK, Jin Y, Luo H, Tang M, Pampo C, Shao R, et al. Epithelial-to-mesenchymal transition confers pericyte properties on cancer cells. J Clin Invest. 2016;126(11):4174–4186. doi: 10.1172/JCI86623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenoy AK, Lu J. Relevance of epithelial-to-pericyte transition in cancer. Mol Cell Oncol. 2017 doi: 10.1080/23723556.2016.1260672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Wang Y, Rashid MH, Liu M, Angara K, Mivechi NF, et al. Malignant pericytes expressing GT198 give rise to tumor cells through angiogenesis. Oncotarget. 2017;8(31):51591–51607. doi: 10.18632/oncotarget.18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 52.Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;03:203–220. doi: 10.1158/1541-7786.MCR-06-0404. [DOI] [PubMed] [Google Scholar]
- 53.Mathonnet M, Perraud A, Christou N, Akil H, Melin C, Battu S, et al. Hallmarks in colorectal cancer: angiogenesis and cancer stem-like cells. World J Gastroenterol. 2014;15:4189–4196. doi: 10.3748/wjg.v20.i15.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shangguan W, Fan C, Chen X, Lu R, Liu Y, Li Y, et al. Endothelium originated from colorectal cancer stem cells constitute cancer blood vessels. Cancer Sci. 2017;108(7):1357–1367. doi: 10.1111/cas.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribeiro AL, Okamoto OK. Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 2015;2015:1–8. doi: 10.1155/2015/868475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu H, Liu Y, Wang C, Chou W-C, Hsu Y-J, Chiang J-M, et al. Sequential cetuximab/bevacizumab therapy is associated with improved outcomes in patients with wild-type KRAS exon 2 metastatic colorectal cancer. Cancer Med. 2019 doi: 10.1002/cam4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8(1):57–84. doi: 10.1186/s12935-015-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shinagawa T, Tanaka T, Nozawa H, Emoto S, Murono K, Kaneko M, et al. Comparison of the guidelines for colorectal cancer in Japan, the USA and Europe. Ann Gastroenterol Surg. 2018;2(1):6–12. doi: 10.1002/ags3.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J, Liu H, Zhang L, Liu X, Zhang C, Wang Y, et al. DJ-1 promotes colorectal cancer progression through activating PLAGL2/Wnt/BMP4 axis. Cell Death Dis. 2018;9(9):865. doi: 10.1038/s41419-018-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore PA, Shah K, Yang Y, Alderson R, Roberts P, Long V, et al. Development of MGD007, a gpA33 x CD3-bispecific DART protein for T-Cell immunotherapy of metastatic colorectal cancer. Mol Cancer Ther. 2018;17(8):1761–1772. doi: 10.1158/1535-7163.MCT-17-1086. [DOI] [PubMed] [Google Scholar]
- 61.Azhdarinia A, Voss J, Ghosh SC, Simien JA, Hernandez Vargas S, Cui J, et al. Evaluation of anti-LGR5 antibodies by immunopet for imaging colorectal tumors and development of antibody-drug conjugates. Mol Pharm. 2018;15(6):2448–2454. doi: 10.1021/acs.molpharmaceut.8b00275. [DOI] [PubMed] [Google Scholar]
- 62.Zhan T, Rindtorff N, Betge J, Ebert MP, Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol. 2018;55:106–119. doi: 10.1016/j.semcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Smith DC, Eisenberg PD, Manikhas G, Chugh R, Gubens MA, Stagg RJ, et al. A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (Anti-DLL4) in patients with previously treated solid tumors. Clin Cancer Res. 2014;20(24):6295–6303. doi: 10.1158/1078-0432.CCR-14-1373. [DOI] [PubMed] [Google Scholar]
- 64.Kahlert UD, Mooney SM, Natsumeda M, Steiger HJ, Maciaczyk J. Targeting cancer stem-like cells in glioblastoma and colorectal cancer through metabolic pathways. 2017;140(1):10–22. doi: 10.1002/ijc.30259. [DOI] [PubMed] [Google Scholar]
- 65.Smith DC, Eisenberg P, Stagg R, Manikhas G, Pavlovskiy A, Sikic B, et al. A first-in-human, phase I trial of the anti-DLL4 antibody (OMP-21M18) targeting cancer stem cells in patients with advanced solid tumors. In: 2nd EORTC-NCI-AACR symposium on molecular targets and cancer therapeutics. Berlin, Germany; 2010.
- 66.Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 67.Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. 2018;8:386. doi: 10.3389/fonc.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arasanz H, Gato-Cañas M, Zuazo M, Ibañez-Vea M, Breckpot K, Kochan G, et al. PD1 signal transduction pathways in T cells. Oncotarget. 2017;8(31):51936–51945. doi: 10.18632/oncotarget.17232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJC, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng H-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8(35):59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enuka Y, Lauriola M, Feldman ME, Sas-Chen A, Ulitsky I, Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44(3):1370–1383. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei B, Tian Z, Fan W, Ni B. Circular RNA: a novel biomarker and therapeutic target for human cancers. Int J Med Sci. 2019;16(2):292–301. doi: 10.7150/ijms.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang M, Xin Y. Circular RNAs: a new frontier for cancer diagnosis and therapy. J Hematol Oncol. 2018;11(1):21. doi: 10.1186/s13045-018-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong W, Ai Y-Q, Li Y-F, Ye Q, Chen Z-T, Qin J-Y, et al. Microarray analysis of circular RNA expression profile associated with 5-fluorouracil-based chemoradiation resistance in colorectal cancer cells. Biomed Res Int. 2017 doi: 10.1155/2017/8421614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuninty PR, Schnittert J, Storm G, Prakash J. MicroRNA targeting to modulate tumor microenvironment. Front Oncol. 2016 doi: 10.3389/fonc.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402. doi: 10.3389/fendo.2018.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costa DF, Torchilin VP. Micelle-like nanoparticles as siRNA and miRNA carriers for cancer therapy. Biomed Microdevices. 2018;20(3):59. doi: 10.1007/s10544-018-0298-0. [DOI] [PubMed] [Google Scholar]
- 80.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aaldering LJ, Tayeb H, Krishnan S, Fletcher S, Wilton SD, Veedu RN. Smart functional nucleic acid chimeras: enabling tissue specific RNA targeting therapy. RNA Biol. 2015;12(4):412–425. doi: 10.1080/15476286.2015.1017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conde J, Edelman ER, Artzi N. Target-responsive DNA/RNA nanomaterials for microRNA sensing and inhibition: the jack-of-all-trades in cancer nanotheranostics? Adv Drug Deliv Rev. 2015;81(1):169–183. doi: 10.1016/j.addr.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Navarro G, Pan J, Torchilin VP. Micelle-like nanoparticles as carriers for DNA and siRNA. Mol Pharm. 2015;12(2):301–313. doi: 10.1021/mp5007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar V, Mahato RI. Delivery and targeting of miRNAs for treating liver fibrosis. Pharm Res. 2015;32(2):341–361. doi: 10.1007/s11095-014-1497-x. [DOI] [PubMed] [Google Scholar]
- 85.Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3(7):1–19. doi: 10.1038/S41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Şalva E, Turan SÖ, Eren F, Akbuğa J. The enhancement of gene silencing efficiency with chitosan-coated liposome formulations of siRNAs targeting HIF-1α and VEGF. Int J Pharm. 2015;478(1):147–154. doi: 10.1016/j.ijpharm.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 87.Zheng B, Chen L, Pan C-C, Wang J-Z, Lu G-R, Yang S-X, et al. Targeted delivery of miRNA-204-5p by PEGylated polymer nanoparticles for colon cancer therapy. Nanomedicine. 2018;13(7):769–785. doi: 10.2217/nnm-2017-0345. [DOI] [PubMed] [Google Scholar]
- 88.Zhang G, Zhang Z, Yang J. DNA tetrahedron delivery enhances doxorubicin-induced apoptosis of HT-29 colon cancer cells. Nanoscale Res Lett. 2017;12(1):495–502. doi: 10.1186/s11671-017-2272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ueno K, Hirata H, Hinoda Y, Dahiya R. Frizzled homolog proteins, microRNAs and Wnt signaling in cancer. Int J Cancer. 2013;132(8):1731–1740. doi: 10.1002/ijc.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang K, Liu L, Zhang T, Zhu Y, Qiu F, Wu X, et al. Oxaliplatin-incorporated micelles eliminate both cancer stem-like and bulk cell populations in colorectal cancer. Int J Nanomed. 2011;6:3207–3218. doi: 10.2147/IJN.S26268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferraro A, Kontos CK, Boni T, Bantounas I, Siakouli D, Kosmidou V, et al. Epigenetic regulation of miR-21 in colorectal cancer: iTGB4 as a novel miR-21 target and a three-gene network (miR-21-ITGΒ4-PDCD4) as predictor of metastatic tumor potential. Epigenetics. 2014;9(1):129–141. doi: 10.4161/epi.26842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng H, Li W, Wang Y, Xie T, Cai Y, Wang Z, et al. miR-23a inhibits E-cadherin expression and is regulated by AP-1 and NFAT4 complex during Fas-induced EMT in gastrointestinal cancer. Carcinogenesis. 2014;35(1):173–183. doi: 10.1093/carcin/bgt274. [DOI] [PubMed] [Google Scholar]
- 93.Wan Y, Shen A, Qi F, Chu J, Cai Q, Sferra TJ, et al. Pien Tze Huang inhibits the proliferation of colorectal cancer cells by increasing the expression of miR-34c-5p. Exp Ther Med. 2017;14(4):3901–3907. doi: 10.3892/etm.2017.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L, Zhang L, Shi Y. miR-24 inhibited the killing effect of natural killer cells to colorectal cancer cells by downregulating Paxillin. Biomed Pharmacother. 2018;101:257–263. doi: 10.1016/J.BIOPHA.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 95.Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153(2):505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 96.Ebrahimi F, Gopalan V, Wahab R, Lu C-T, AnthonySmith R, Lam AKY. Deregulation of miR-126 expression in colorectal cancer pathogenesis and its clinical significance. Exp Cell Res. 2015;339(2):333–341. doi: 10.1016/j.yexcr.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Bi W, Xia M, Wang X. miR-137 suppresses proliferation, migration and invasion of colon cancer cell lines by targeting TCF4. Oncol Lett. 2018;15(6):8744–8748. doi: 10.3892/ol.2018.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian X, Yu J, Yin Y, He J, Wang L, Li Q, et al. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12(9):1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng J, Yang Y, Zhang P, Wang F, Ma Y, Qin H, et al. miR-150 functions as a tumour suppressor in human colorectal cancer by targeting c-Myb. J Cell Mol Med. 2014;18(10):2125–2134. doi: 10.1111/jcmm.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62(9):1315–1326. doi: 10.1136/gutjnl-2011-301846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takano Y, Masuda T, Iinuma H, Yamaguchi R, Sato K, Tobo T, et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget. 2017;8(45):78598–78613. doi: 10.18632/oncotarget.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ru P, Steele R, Hsueh EC, Ray RB. Anti-miR-203 upregulates SOCS3 expression in breast cancer cells and enhances cisplatin chemosensitivity. Genes Cancer. 2011;2(7):720–727. doi: 10.1177/1947601911425832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qin J, Luo M. MicroRNA-221 promotes colorectal cancer cell invasion and metastasis by targeting RECK. FEBS Lett. 2014;588(1):99–104. doi: 10.1016/j.febslet.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 104.Liu S, Sun X, Wang M, Hou Y, Zhan Y, Jiang Y, et al. A microRNA 221- and 222-mediated feedback loop maintains constitutive activation of NFκB and STAT3 in colorectal cancer cells. Gastroenterology. 2014;147(4):847–859. doi: 10.1053/j.gastro.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S, Zeng Y, Zhou J-M, Nie S-L, Peng Q, Gong J, et al. MicroRNA-1246 promotes growth and metastasis of colorectal cancer cells involving CCNG2 reduction. Mol Med Rep. 2016;13(1):273–280. doi: 10.2147/IJN.S26268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.