Abstract
Background
Identifying and treating patients with transient ischemic attack is an effective means of preventing stroke. However, making this diagnosis can be challenging, and over a third of patients referred to stroke prevention clinic are ultimately found to have alternate diagnoses.
Aims
We performed a systematic review to determine how neurologists diagnose transient ischemic attack.
Summary of review
A systematic literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using MEDLINE, Embase, and the Cochrane Library databases. Publications eligible for inclusion were those that included information on the demographic or clinical features neurologists use to diagnose transient ischemic attacks or transient ischemic attack–mimics. Of 1666 citations, 210 abstracts were selected for full-text screening and 80 publications were ultimately deemed eligible for inclusion. Neurologists were more likely to diagnose transient ischemic attack based on clinical features including negative symptoms or speech deficits. Patients with positive symptoms, altered level of consciousness, or the presence of nonfocal symptoms such as confusion or amnesia were more likely to be diagnosed with transient ischemic attack–mimic. Neurologists commonly include mode of onset (i.e. sudden versus gradual), recurrence of attacks, and localizability of symptoms to a distinct vascular territory in the diagnostic decision-making process. Transient ischemic attack diagnosis was more commonly associated with advanced age, preexisting hypertension, atrial fibrillation, and other vascular risk factors.
Conclusions
Neurologists rely on certain clinical and demographic features to distinguish transient ischemic attacks from mimics, which are not currently reflected in widely used risk scores. Clarifying how neurologists diagnose transient ischemic attack may help frontline clinicians to better select patients for referral to stroke prevention clinics.
Keywords: Transient ischemic attack, stroke, stroke mimic, stroke prevention, decision analysis
Introduction
In about 20% of cases, stroke is preceded by an episode of temporary symptoms called a transient ischemic attack (TIA).1 Studies have shown that identifying and treating patients with TIA is an effective means of preventing stroke.2,3 Since the highest risk for stroke is in the first 48 h following symptom onset,4–6 it is critical that diagnosis and assessment occur rapidly.
Unfortunately, diagnosing TIA can be difficult, as it depends on detailed history-taking; by definition, patients' symptoms have resolved at the time of assessment, and there is no established biomarker for TIA. As a consequence, approximately 30–50% of patients referred to stroke prevention clinics (SPCs) with a provisional diagnosis of TIA are ultimately found not to have had a TIA.7–9 This situation is problematic as high volumes of referrals of patients with TIA–mimics are directly related to delays in the care of TIA patients.3
While a major focus of recent research has been on risk-stratifying patients with TIA in order to decrease wait times for the highest risk patients, the many proposed risk scores10–13 suffer from an important weakness: they are derived from, and applied to, an undifferentiated population of patients with transient neurological symptoms including both patients with TIAs and mimics (e.g. migraine or seizure).8,14 In other words, the risk scores themselves do not differentiate patients with TIA from other clinical syndromes.
Multiple small studies15–17 have looked at TIA diagnosis post hoc using expert panels for adjudication, though none has studied decision-making in vivo and none has sought to describe the diagnostic process, i.e. why a certain diagnosis is made. As such, we performed a systematic review to assess how and why neurologists call a particular clinical event a TIA or a mimic. We chose to study neurologists because they are considered stroke experts in most countries and because expertise is currently the “gold standard” for TIA diagnosis. Ultimately our goal is to make the SPC referral process more efficient by developing a method of selecting patients with TIA as accurately as possible from all those presenting to emergency departments and ambulatory clinics with transient neurological symptoms.18
Methods
A systematic review was performed to address the question: “How do neurologists diagnose TIA?” We adhered to the PRISMA 2009 statement and conformed to its checklist (Supplementary Figure I).19
Search strategy and selection criteria
Keywords were selected and submitted to a librarian who created an initial search strategy. The search strategy was then revised to ensure that key studies were not omitted. Databases searched included MEDLINE, Embase, and the Cochrane Library. Supplementary Figure II details the search strategy that was used for MEDLINE. Similar strategies were utilized for the Embase and the Cochrane Library. The searches were conducted from inception of each database until 23 February 2017 with no language or date restrictions. The reference lists of manuscripts selected for inclusion were hand-searched for any additional potentially relevant citations that were not captured with the electronic search strategy alone.
Manuscripts were included if they explicitly addressed TIA diagnosis or if their inclusion criteria directly informed our study. To reduce the risk of publication bias, both peer-reviewed publications and unpublished studies (e.g. conference abstracts without a subsequent publication) were included.
Both quantitative and qualitative studies were eligible for inclusion. All observational studies, including cohort, case–control, and cross-sectional designs, and all interventional studies with primary or secondary outcomes aimed at answering our research question were included. Studies that did not directly focus on answering our question but indirectly revealed neurologists' diagnostic decision-making by way of key statements or study inclusion criteria were also included. To ensure this systematic review was comprehensive and reflective of expert practice, textbooks and reviews, including nonsystematic approaches such as opinion pieces, commentaries, and literature reviews, were eligible for inclusion if written by a neurologist. Because the diagnosis of TIA depends upon clinical judgment, we included manuscripts containing statements of expert opinion and experience-based reasoning, both of which are often best reflected in nonsystematic reviews.
Manuscripts reflective of a nonneurologist only were excluded, as our goal was to focus on neurologist diagnostic decision-making. These exclusions were classified as “wrong setting.” Unpublished studies were eligible for inclusion but were excluded if there was not enough information in the abstract alone to answer our research question and if a full publication did not follow.
Although the initial search strategy, as well as the title/abstract screening stage did not have language restrictions, during full-text screening, studies were excluded if the full text was in a language other than English as translation services were unavailable. Language restrictions were not imposed earlier to allow us to keep track of the number of articles deemed ineligible simply due to language and therefore to allow us to assess the magnitude of any potential language bias.
Studies of pediatric patients (under the age of 18) were similarly excluded during the full-text screening stage as the objective of our systematic review was to identify clinical features of TIA, and patients in this population may experience different symptoms, may be unable to recognize transient neurological deficits, or may be unable to express their symptoms.
Screening
Search results were compiled using Covidence systematic review software.20 Duplicate references were automatically detected and removed. Two reviewers independently screened each citation based on title and abstract. Pilot screening was performed for the first 25 records to ensure reviewers were consistent and to decrease conflicts. All disagreements were resolved through consensus discussion between the two reviewers with input from a third reviewer.
Two reviewers then independently reviewed the full text of each article included from title and abstract screening, assessing eligibility for inclusion. All disagreements were again resolved through consensus discussion with input from a third reviewer.
Data extraction and synthesis
An abstraction datasheet was created using Microsoft Excel (2010) and two reviewers independently extracted study-level characteristics (e.g. study design, country of conduct) and the relevant data from each publication. The focus was on the identification of factors associated with diagnosis of TIA or TIA–mimic, and the process utilized by neurologists in their diagnostic decision-making. Where available, the rates of diagnosis of TIA and TIA–mimic were also collected.
The results from the two independent extractions were then compared to ensure accuracy and completeness. Any discrepancies were resolved through discussion between the two reviewers. The data were then compiled using QSR's International NVivo 11 qualitative data analysis software.21
We performed a multistep “thematic synthesis,” which began with coding of text in NVivo in a “line-by-line” fashion, ensuring all relevant information was captured by the two reviewers. From the codes that were created, common themes emerged and concepts were grouped into the following categories: (1) symptoms suggestive of TIA, (2) qualitative features suggestive of TIA, (3) symptoms suggestive of TIA–mimic, (4) qualitative features suggestive of TIA–mimic, (5) risk factors and demographic features more common in TIA, and (6) risk factors and demographic features more common in TIA–mimic. Finally, analytical themes were generated from these descriptive themes to answer our initial question—“How do neurologists diagnose TIA?”
The only quantitative information collected, TIA–mimic rate, was analyzed with descriptive statistics.
Critical appraisal
For descriptive purposes, each article that was included was assessed for risk of bias and strength/quality of evidence. Records were critically appraised based on six criteria—clarity of statement of aims, appropriateness of methodology, reliability/validity of data collection tools, reliability/validity of methods of data analysis, clarity of statement of results, and overall relevance to our question. These criteria were adapted from various quality assessment tools available for qualitative research and selected after discussion between three reviewers.22,23 Two reviewers performed the ratings independently and evaluations were compared. Disagreements were settled through consensus-based discussion.
Results
Search results
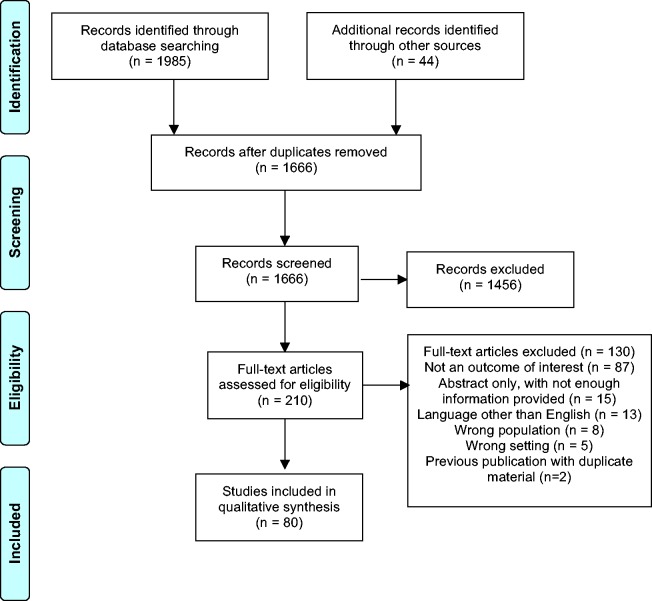
The database search identified 1985 citations (Figure 1). An additional 44 were later identified by hand-searching citation lists. With duplicates removed, there were a total of 1666 references. After title and abstract screening, 1456 records were excluded and the remaining 210 articles were assessed for eligibility with full-text review. Ultimately 80 records were included in this review (see Supplementary References). Reasons for exclusion were categorized as: (1) outcome not of interest; (2) abstract only, without enough information provided; (3) language other than English; (4) wrong population; (5) wrong setting; and (6) previous publication with duplicate material. A reference list of the excluded studies with reasons for exclusion is presented in the Supplementary Methods.
Figure 1.
PRISMA flow diagram.
Study characteristics
Study characteristics are presented in Supplementary Table 1. A total of 21 different countries were represented, with the United States (31 publications), and the United Kingdom (20 publications) being most common. Seven unpublished cohort studies and one unpublished case–control study were included (conference abstracts without subsequent manuscript publications). Publications included were mostly cohort design (49%), followed by literature review (21%) (Table 1).
Table 1.
Comparison of included records by study design
| Study design | Number (%) |
|---|---|
| Cohort study | 39 (49%) |
| Literature review | 17 (21%) |
| Opinion | 8 (10%) |
| Case report | 5 (6%) |
| Case series | 4 (5%) |
| Cross-sectional study | 3 (4%) |
| Case–control | 1 (1%) |
| Systematic review | 1 (1%) |
| Survey | 1 (1%) |
| Textbook | 1 (1%) |
Critical appraisal
Critical appraisal assessments are summarized in Table 2. Critical appraisal of each individual study is presented in Supplementary Table 2. Statement of aims and statement of results were clear in the vast majority of publications (94 and 90%, respectively). Data collection was performed appropriately in 88% of publications. Appraisal questions related to methodology and data analysis were not applicable to certain study designs including opinion pieces and literature reviews. Where applicable, however, most studies performed well on this quality measure. Many of the studies (60%) were ultimately found to be of low relevance, but were included in our analysis because they did contribute content to the data collected.
Table 2.
Summary of the critical appraisal for included studies
| Appraisal question | Yes | No | Unclear | N/A |
|---|---|---|---|---|
| Is there a clear statement of aims or a clearly defined question? | 75 (94%) | 5 (6%) | 0 (0%) | 0 (0%) |
| Was the methodology employed appropriate to the research question? | 47 (59%) | 0 (0%) | 4 (5%) | 29 (36%) |
| Was the data collection performed appropriately? | 70 (88%) | 9 (11%) | 1 (1%) | 0 (0%) |
| Was the data analysis rigorous? | 34 (42%) | 5 (6%) | 6 (8%) | 35 (44%) |
| Was there a clear statement of results? | 72 (90%) | 8 (10%) | 0 (0%) | 0 (0%) |
| Was the overall relevance to our research question high? | 32 (40%) | 48 (60%) | 0 (0%) | 0 (0%) |
N/A: not applicable.
Descriptive themes
The main themes that emerged are presented in the following subsections:
1) Symptoms suggestive of TIA
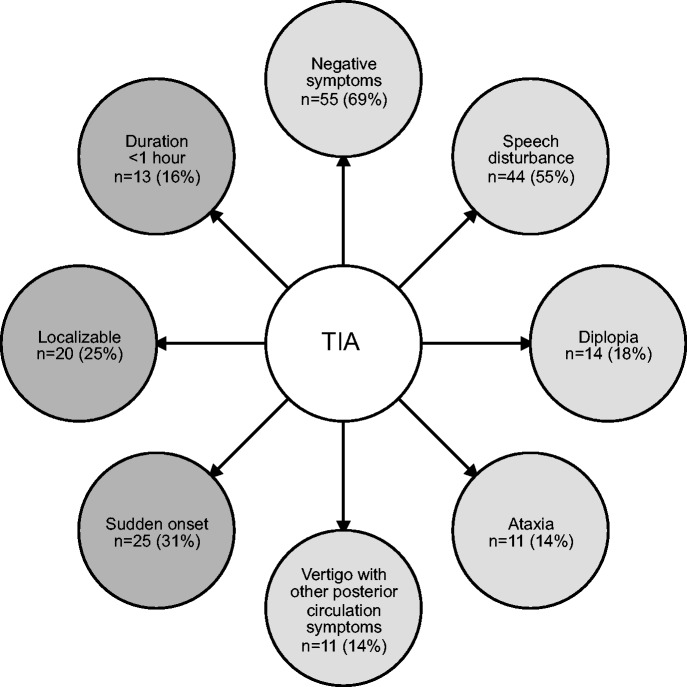
The most common clinical features that neurologists noted were suggestive of TIA rather than a non-TIA diagnosis are presented in Figure 2 including the frequency of each reference.
Figure 2.
Commonly identified clinical features suggestive of TIA. Symptoms are depicted in light gray bubbles and qualitative features are depicted in dark gray bubbles. N = number of publications making reference to each differentiating feature. The percentage of included studies is shown in parentheses. TIA: transient ischemic attack.
Overall, “negative symptoms”—characterized by loss of function—were the most frequently described (69% of included studies). This broad category included motor, sensory, and visual symptoms. Terms utilized to describe negative motor symptoms included “hemiparesis,” “unilateral arm/face/leg weakness,” “loss of motor function,” and “loss of muscle power.” Negative sensory symptoms were described with the terms “sensory loss” and “numbness.” Both monocular and binocular negative visual symptoms were encompassed by the negative visual symptoms category. Terms grouped included “homonymous hemianopsia,” “visual field deficit,” “visual loss,” “cortical blindness,” “monocular blindness,” and “amaurosis fugax.”
The second-most frequently described symptom neurologists noted to be in keeping with TIA was “speech disturbance” (55% of included studies). Although some authors used specific terms such as “aphasia” or “dysarthria,” others used more general terms such as “speech disturbance” or “impaired speech.” These nonspecific terms were difficult to interpret separately and therefore all references to speech and communication were grouped into one category.
Other symptoms commonly considered by neurologists to be supportive of a diagnosis of TIA included “ataxia,” “diplopia,” and “vertigo with other posterior circulation symptoms.” These were described in 14–18% of records. “Dizziness” was not a helpful differentiating feature: while some authors identified it as a symptom suggestive of TIA, a similar number of articles stated that it was a symptom more suggestive of TIA–mimic. When specified as “isolated vertigo” neurologists were also much more likely to diagnose a TIA–mimic.
2) Qualitative features suggestive of TIA
In addition to the clinical symptoms described above, the expert diagnostic process also identified pattern of onset, localizability, and duration as important elements considered in diagnostic decision-making. In almost one-third of the articles (n = 25), neurologists were more likely to diagnose TIA if the onset of symptoms was “sudden,” “maximal at onset,” “nonprogressive,” or “acute.” Another characteristic identified in one quarter of included articles (n = 20) was “localizability” of the symptoms; terms grouped together included “focal,” “corresponding to a vascular territory,” and “consistent with a known stroke syndrome.” The last characteristic that was commonly identified as a TIA feature was symptoms with a “duration less than 1 h.”
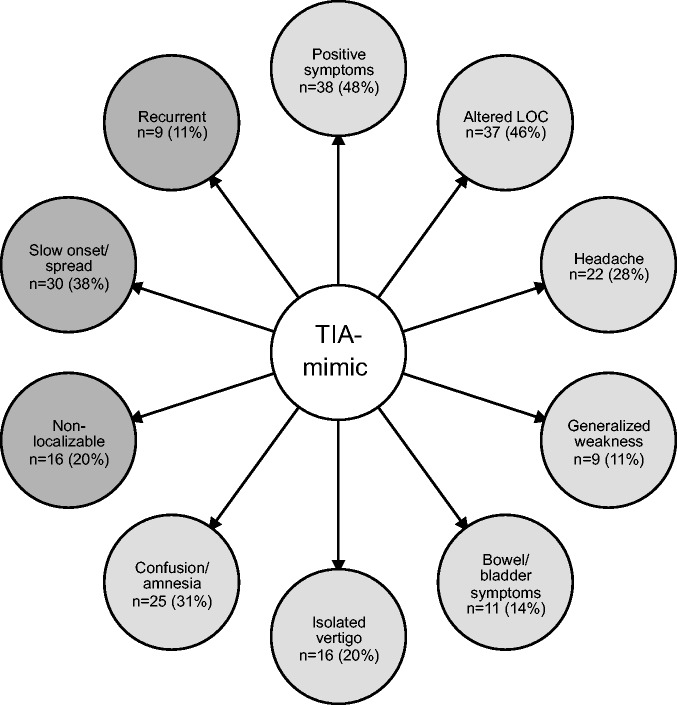
3) Symptoms suggestive of TIA–mimic
Figure 3 displays the features of transient neurological disturbance that neurologists consider to be indicative of a TIA–mimic diagnosis. The most commonly identified symptoms were those that fell under the category of “positive symptoms,” including motor, sensory, or visual phenomena (48% of records). Positive motor symptoms were described with the following terms: “jerking,” “shaking,” “seizure-like activity,” and “involuntary movement.” Terms used to describe positive sensory symptoms included “tingling” and “paresthesias.” “Scintillating scotoma,” “flashing lights,” and “visual aura” were grouped under the positive visual phenomena subsection.
Figure 3.
Commonly identified clinical features suggestive of TIA–mimic. Symptoms are depicted in light gray bubbles and qualitative features are depicted in dark gray bubbles. N = number of publications making reference to each differentiating feature. The percentage of included studies is shown in parentheses. TIA: transient ischemic attack.
The next category of TIA–mimic symptoms identified by neurologists was “altered level of consciousness (LOC)” (46% of included studies). Within this category we grouped any disturbance in consciousness including “presyncope,” “loss of consciousness,” “decreased level of consciousness,” and “impaired consciousness.”
“Confusion” was separated from “altered level of consciousness” and was grouped with “cognitive symptoms” and “amnesia.” The presence of any of these symptoms was frequently considered to be supportive of a TIA–mimic diagnosis (31% of records). Other recurrent TIA–mimic themes were “headache,” “bowel or bladder symptoms,” and “generalized weakness.”
4) Qualitative features suggestive of TIA–mimic
Features of symptoms suggestive of TIA–mimic included the inverse of those seen with TIA, including “nonfocal” and “nonlateralizing.” “Slow onset” of symptoms typically swayed neurologists toward a non-TIA diagnosis, as did, “slow progression,” “slow spread,” or “march” of symptoms (n = 30). The presence of a “Jacksonian march” was specifically identified as a key mimic feature. Lastly, many authors considered TIA–mimic more likely if the patient had had “recurrent” or “stereotyped” episodes (n = 9).
5) Risk factors and demographic features more common in TIA
Risk factors and demographic features associated with the diagnosis of TIA included advanced age, atrial fibrillation, preexisting hypertension, previous stroke/TIA, or other vascular risk factors including dyslipidemia and type II diabetes (Figure 4). These features were mentioned relatively infrequently compared to the clinical characteristics described above.
Figure 4.
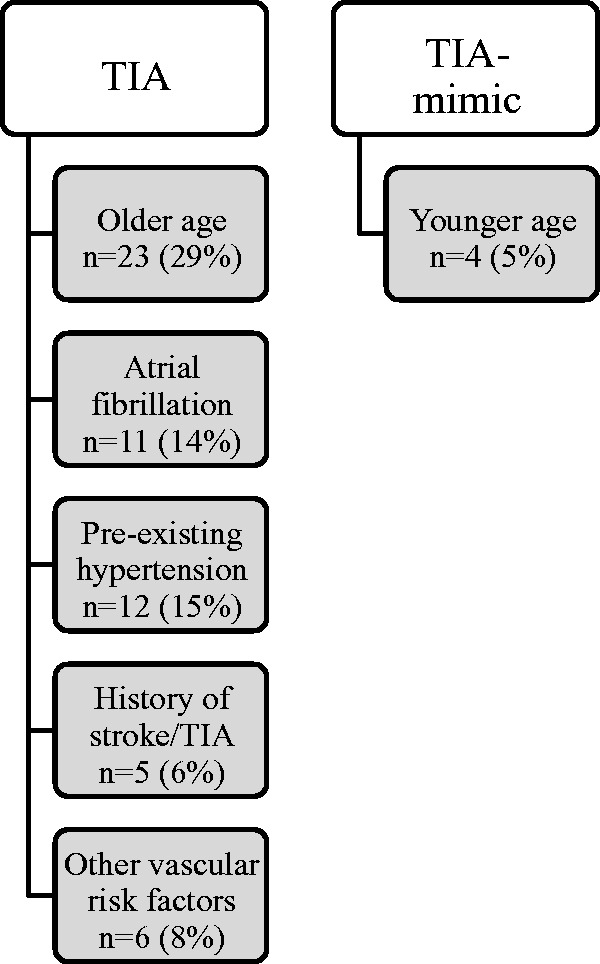
Risk factors and demographic features commonly identified as predictors of TIA diagnosis and TIA–mimic diagnosis. N = number of publications making reference to each risk factor. The percentage of included studies is shown in parentheses. TIA: transient ischemic attack.
6) Risk factors and demographic features more common in TIA–mimic
The only demographic feature that was consistently associated with a diagnosis of TIA–mimic diagnosis was younger age (Figure 4).
7) TIA–mimic rate
Twenty-seven (34%) of the included articles provided a TIA–mimic rate. The mimic rates ranged between 6 and 73%,24,25 with a median mimic rate of 36% (25th, 75th percentiles range: 26%, 50%).
Discussion
Diseases are often defined in relation to blood tests, imaging findings, or some combination thereof. In the absence of such markers, diseases are diagnosed through a process of decision-making by experts, and this is the state of TIA in contemporary medicine. Therefore, we sought to perform a systematic review of all relevant qualitative, quantitative, and mixed methods studies that would inform our understanding of the process by which neurologists diagnose TIA. While in some regions, nonneurologists (i.e. geriatricians) may provide stroke care, we chose to limit the scope of our search to neurologists for the sake of consistency and as a general reflection of practice in most regions. To the best of our knowledge, this is the first qualitative systematic review to assess how neurologists diagnose TIA.
Our study has revealed that according to neurologists, the most consistent predictors for a diagnosis of TIA include negative symptoms (loss of motor, sensory, or visual function) and speech disturbance. The strongest predictors for TIA–mimic are positive symptoms (such as motor jerking, sensory tingling, or visual scotomas) and any alteration of consciousness. Certain characteristics including pattern of onset, localizability of symptoms, and symptom recurrence were also important discriminative diagnostic features. While these findings may appear obvious to those who are experts, that speaks to the accuracy of our results at capturing their decision-making process. Moreover, these findings are not obvious to nonexperts, suggesting the importance of work like this. We recognize that this study is a preliminary step to further characterizing the decision-making process surrounding TIA.
Diffusion-weighted (DWI) MRI is more sensitive than CT for detecting acute ischemia, and up to one-third of patients diagnosed with TIA are found to have an infarct on DWI MRI.26 Consequently, many organizations have moved away from the traditional “time-based” definition of TIA toward a new “tissue-based” definition.27 While MRI can be a very useful tool and certainly reduces the rate of false-negative diagnoses, it still cannot replace expert assessment, especially for those patients who are MRI-negative. Furthermore, MRI is not available in all healthcare settings. For these reasons, we chose to focus our study entirely on the clinical diagnosis of TIA.
In the absence of a reliable tool for the diagnosis of TIA, frontline clinicians frequently apply risk-stratification instruments such as the ABCD2 score for diagnostic purposes.28 The ABCD and later ABCD2 scores were developed from populations of patients with a provisional diagnosis of TIA, many of whom where later given a final diagnosis of TIA–mimic by experts.2 When applied in a blanket fashion to any patient with transient neurological symptoms, these instruments can result in a large number of inappropriate urgent referrals to the SPC since TIA–mimics can very easily generate high ABCD2 scores.29 We believe that a more standardized decisional process should be established for TIA so that the inappropriate use of risk-stratification tools can be avoided.
To address this deficiency, two diagnostic algorithms have previously been developed for TIA—the Dawson Score and the Diagnosis of TIA Score (DOTS).30,31 The Dawson Score is a clinical scoring tool developed in a specialist setting that considers nine predictive variables and was found to be of limited utility in a primary care setting.32 It has been criticized for struggling with posterior circulation32 and retinal31 events. In contrast, the DOTS considers 17 variables, many of which reflected the factors we identified in our systematic review. It had a sensitivity of 89% (CI: 84–93%) and a specificity of 76% (70–81%)31 in an internal cohort, but has not yet been externally validated. Ultimately, these scores are seeking to approximate a diagnostic process that, until now, had not yet been described empirically.
Most of the variables in the DOTS were identified by our systematic review; however, our systematic review has also identified several novel concepts, which are not reflected in any previously developed TIA diagnostic algorithms, including the pattern of onset/spread of symptoms and recurrence/stereotyped nature of episodes. We recognize that in the right clinical context, recurrent or stereotyped symptoms do not exclude vascular etiology altogether (e.g. capsular warning syndrome). This highlights the importance of considering the whole clinical picture rather than making decisions based on isolated features. Another important lesson from our study is that neurologists clearly rely on focal/lateralizable symptoms for the diagnosis of TIA. While we acknowledge that some populations, especially elderly women, may present with “nontraditional” stroke symptoms,33 evidence is conflicting and more research is needed on this subject.
We intend to use the results of our systematic review to inform further in vivo studies on the expert diagnosis of TIA. Our goal is to identify reliable factors that will help frontline clinicians make a provisional diagnosis of TIA with more accuracy. Rather than creating a new TIA score, we hope to focus our efforts on education around the key elements used in the process of TIA diagnosis. The dissemination of knowledge to primary care and emergency room physicians could have a substantial impact on patient care, as it would decrease the number of patients falsely labeled with a TIA event. As such, the quality and volumes of referrals to SPCs could be improved, contributing to enhanced efficiency of stroke prevention interventions. High rates of TIA–mimics referred for assessment contributes to delays in care through bottlenecking. If we are able to improve wait times, particularly for high-risk TIA patients, this could ultimately reduce stroke rates. The implications on health services are also significant, as better referrals would lead to marked cost savings by decreasing the number of unnecessary tests ordered for referred patients, and ultimately reducing the costs associated with preventable strokes.
This systematic review is not without limitations. Given the nature of TIA diagnosis, a variety of qualitative research studies have informed our analysis. Furthermore, we chose to include literature reviews and opinion pieces since expert opinion is often best reflected using these approaches. Since there is no confirmatory test for TIA, we are relying on the assumption that neurologist opinion is the “gold standard.” This naturally introduces potential bias as there will always be an element of subjectivity when it comes to making a diagnosis based on a patient's history alone. Unfortunately, we do not see any way to avoid this since at the present time there are no blood biomarkers or imaging tests available to reliably distinguish TIAs from TIA–mimics. Finally, another limitation of our study is that our literature search was performed as of February 2017.
In conclusion, our systematic review has identified the key clinical characteristics that neurologists consider when differentiating between TIAs and TIA–mimics. We intend to explore this distinction further by studying real-world decision-making for patients referred to our SPC. Educating frontline clinicians on the features identified could have a significant impact on patient care and our healthcare system.
Supplemental Material
Supplemental Material for How do neurologists diagnose transient ischemic attack: A systematic review by Tess Fitzpatrick, Sophia Gocan, Chu Q Wang, Candyce Hamel, Aline Bourgoin, Dar Dowlatshahi, Grant Stotts and Michel Shamy in International Journal of Stroke
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding provided by the Stroke Research Consortium, University of Ottawa Brain and Mind Research Institute.
References
- 1.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000; 284: 2901. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370: 1432–1442. [DOI] [PubMed] [Google Scholar]
- 3.Hall R, Khan F, O'Callaghan C, et al. Ontario stroke evaluation report 2013: spotlight on secondary stroke prevention and care, Toronto: Institute for Clinical Evaluative Sciences, 2013. [Google Scholar]
- 4.Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke 2012; 43: 1013–1017. [DOI] [PubMed] [Google Scholar]
- 5.Gladstone DJ, Kapral MK, Fang J, Laupacis A, Tu JV. Management and outcomes of transient ischemic attacks in Ontario. Can Med Assoc 2004; 170: 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6: 1063–1072. [DOI] [PubMed] [Google Scholar]
- 7.Bradley D, Cronin S, Kinsella JA, et al. Frequent inaccuracies in ABCD2 scoring in non-stroke specialists' referrals to a daily Rapid Access Stroke Prevention service. J Neurol Sci 2013; 332: 30–34. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan OC, Merwick A, Kelly LA, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke 2009; 40: 3449–3454. [DOI] [PubMed] [Google Scholar]
- 9.Martin PJ, Young G, Enevoldson TP, Humphrey PR. Overdiagnosis of TIA and minor stroke: experience at a regional neurovascular clinic. QJM 1997; 90: 759–763. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005; 366: 29–36. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369: 283–292. [DOI] [PubMed] [Google Scholar]
- 12.Merwick Á, Albers GW, Amarenco P, et al. Addition of brain and carotid imaging to the ABCD2 score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol 2010; 9: 1060–1069. [DOI] [PubMed] [Google Scholar]
- 13.Perry JJ, Sharma M, Sivilotti MLA, et al. A prospective cohort study of patients with transient ischemic attack to identify high-risk clinical characteristics. Stroke 2014; 45: 92–100. [DOI] [PubMed] [Google Scholar]
- 14.Josephson SA, Sidney S, Pham TN, Bernstein AL, Johnston SC. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke 2008; 39: 3096–3098. [DOI] [PubMed] [Google Scholar]
- 15.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke 2006; 37: 769–775. [DOI] [PubMed] [Google Scholar]
- 16.Castle J, Mlynash M, Lee K, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke 2010; 41: 1367–1370. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26: 630–635. [DOI] [PubMed] [Google Scholar]
- 18.Coutts SB, Barrett KM. TIA risk stratification: what an event was and why it happened are more important than a score. Neurology 2015; 85: 304–305. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses. Ann Intern Med 2014; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 20.Covidence systematic review software. Melbourne: Veritas Health Innovation, www.covidence.org.
- 21.NVivo qualitative data analysis software. QSR International Pty Ltd. Version 11, 2017.
- 22.Critical Appraisal Skills Programme (CASP). CASP (systematic review) checklist, www.casp-uk.net/ (2017, accessed 10 August 2017).
- 23.Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Díaz-Guzmán J, Sierra Hidalgo F, Calleja Castaño P, Martínez Salio A, Sánchez Sánchez C, Delgado Babiano A, et al. Identification of disorders mimicking acute stroke in a stroke unit: a case–control study. Cerebrovasc Dis 2014; 37(suppl 1): 693. [Google Scholar]
- 25.Lee W, Frayne J. Transient ischaemic attack clinic: an evaluation of diagnoses and clinical decision making. J Clin Neurosci 2015; 22: 645–648. [DOI] [PubMed] [Google Scholar]
- 26.Wardlaw J, Brazzelli M, Miranda H, et al. An assessment of the cost-effectiveness of magnetic resonance, including diffusion-weighted imaging, in patients with transient ischaemic attack and minor stroke: a systematic review, meta-analysis and economic evaluation. Health Technol Assess 2014; 18: 1–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 28.Edwards D, Cohn SR, Mavaddat N, et al. Varying uses of the ABCD2 scoring system in primary and secondary care: a qualitative study. BMJ Open 2012; 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bose P, Wilson A, Mistri A. Diagnosis and management of transient ischemic attacks in primary care: a systematic review. J Prim Health Care 2017; 9: 114–130. [DOI] [PubMed] [Google Scholar]
- 30.Dawson J, Lamb KE, Quinn TJ, et al. A recognition tool for transient ischaemic attack. QJM 2009; 102: 43–49. [DOI] [PubMed] [Google Scholar]
- 31.Dutta D. Diagnosis of TIA (DOT) score – design and validation of a new clinical diagnostic tool for transient ischaemic attack. BMC Neurol 2016; 16: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasserson DS, Mant D, Hobbs FDR, Rothwell PM. Validation of a TIA recognition tool in primary and secondary care: implications for generalizability. Int J Stroke 2015; 10: 692–696. [DOI] [PubMed] [Google Scholar]
- 33.Girijala RL, Sohrabji F, Bush RL. Sex differences in stroke: review of current knowledge and evidence. Vasc Med 2017; 22: 135–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for How do neurologists diagnose transient ischemic attack: A systematic review by Tess Fitzpatrick, Sophia Gocan, Chu Q Wang, Candyce Hamel, Aline Bourgoin, Dar Dowlatshahi, Grant Stotts and Michel Shamy in International Journal of Stroke