Abstract
Background
Undernutrition is a serious global problem that contributes to increased child morbidity and mortality, impaired neurocognitive development, and decreased educational and economic attainment. Current interventions are only marginally effective, and identification of associated metabolic pathways can offer new strategies for intervention.
Methods
Plasma samples were collected at 9 and 36 months from a subset of the PROVIDE child cohort (n = 130). Targeted metabolomics was performed on bile acids, acylcarnitines, amino acids, phosphatidylcholines, and sphingomyelins. Metabolic associations with linear growth and neurocognitive outcomes at four years were evaluated using correlation and penalized-linear regression analysis as well as conditional random forest modeling.
Findings
Different metabolites were associated with growth and neurocognitive outcomes. Improved growth outcomes were associated with higher concentrations of hydroxy-sphingomyelin and essential amino acids and lower levels of acylcarnitines and bile acid conjugation. Neurocognitive scores were largely associated with phosphatidylcholine species and early metabolic indicators of inflammation. All metabolites identified explain ~45% of growth and neurocognitive variation.
Interpretation
Growth outcomes were predominantly associated with metabolites measured early in life (9 months), many of which were biomarkers of insufficient diet, environmental enteric dysfunction, and microbiome disruption. Hydroxy-sphingomyelin was a significant predictor of improved growth. Neurocognitive outcome was predominantly associated with 36 month phosphatidylcholines and inflammatory metabolites, which may serve as important biomarkers of optimal neurodevelopment. The distinct sets of metabolites associated with growth and neurocognition suggest that intervention may require targeted approaches towards distinct metabolic pathways.
Fund
Bill & Melinda Gates Foundation (OP1173478); National Institutes of Health (AI043596, CA044579).
Keywords: Metabolomics, Stunting, Neurocognition, Childhood, Phosphatidylcholine, Sphingomyelin
Research in context.
Evidence before this study
Undernutrition is a global health problem with long-term consequences on mortality, neurodevelopment, and economic achievement. Previous studies have found that nutritional interventions have only a modest effect on growth recovery and optimal neurodevelopment. Multiple causes have been identified for stunting, including insufficient diet and environmental enteric dysfunction, a disease of malabsorption and chronic intestinal inflammation. Subsequent metabolomic studies have identified metabolic pathways associated with disease, but these studies have primarily focused on growth with only limited investigation into the role of metabolic pathways on neurocognition.
Added value of this study
The current study utilized targeted metabolomics to compare plasma metabolites to outcomes of four year growth and neurodevelopment in an undernourished child cohort. Both penalized linear regression and conditional random forest methods were used to select predictors for each outcome. Distinct metabolic pathways were shown to influence growth and neurocognition. Plasma hydroxy-sphingomyelin species were identified as significant predictors of linear growth at four years as well predictors of neurocognitive development to a lesser extent. Neurocognitive outcome at four years was predominantly associated with phosphatidylcholine species from three year plasma.
Implications of all available evidence
The differences between metabolites identified with growth and neurocognitive outcomes suggest that separate, more targeted interventions may be required for these outcomes. Growth outcomes were associated with early time point metabolites, particularly metabolic markers associated with poor diet and environmental enteric dysfunction. Hydroxy-sphingomyelin species are also potential biomarkers of poor growth. Neurocognition was predominantly associated with phosphatidylcholines measured later in development and metabolic indicators of inflammation, implying that environmental stressors or current health status may be the most pressing targets for intervention into this outcome.
Alt-text: Unlabelled Box
1. Introduction
Undernutrition is a severe global health problem, contributing to 45% of deaths in children under the age of five [1]. The most striking manifestation of undernutrition is linear growth stunting: a UNICEF-WHO-World Bank Joint study estimates that 165 million children under the age of five are stunted, with stunting most prevalent in Africa and Asia [2]. While stunting is defined as a height-for-age Z (HAZ) score of less than −2.0 (two standard deviations below the population median), a sizeable number of children that do not meet this threshold still fail to reach proper growth milestones. In addition to increased mortality, stunting in early life can have lifelong consequences, leading to impaired neurocognitive development [3,4] and decreased educational and economic attainment [[5], [6], [7]]. This chronic, lifelong stunting can be passed from mother to neonate before birth [8], reflecting a cyclical “stunting syndrome” that affects multiple generations [9]. Several drivers of stunting have been identified, including insufficient diet [10], frequent infections [11], and environmental enteric dysfunction (EED), a condition of chronic, low level intestinal inflammation associated with poor sanitation [12,13]. Infections and EED have also been linked to poor neurodevelopment [14], although interactions between inflammation, stunting, and neurodevelopment make it difficult to tease apart the contributions of each to disease.
While several causative factors for stunting and impaired neurodevelopment have been identified, current known interventions have been ineffective at alleviating disease. Post-natal nutrient supplementation trials have shown only modest efficacy at correcting linear growth defects [15,16] and proved ineffective at reversing impaired neurodevelopment [5,17] in early life. Previous estimates predict that applying all known interventions at 99% efficiency would result in only a 33% decrease in stunting and a 25% decrease in childhood mortality by three years of life [18]. This finding suggests that there is still a gap in knowledge with regard to what biological pathways can be targeted to intervene in childhood stunting. Several studies have identified metabolic differences associated with poor linear growth or EED [[19], [20], [21], [22], [23]], but these results must be replicated in additional populations to control for cultural or dietary differences. In addition, there have been limited studies investigating how metabolic differences may influence neurocognition within the first years of life, making this a critical area of investigation.
In the current study, targeted metabolomics was performed on plasma samples collected from a 130 child subset of the PROVIDE Bangladeshi growth cohort. Previous analyses of the data collected in this cohort have found associations between maternal and EED biomarkers each with stunting and vaccine failure [24] as well as associations between inflammation and neurocognitive outcomes [25,26]. The random forest method was also used to assess the importance of these factors to predict linear growth and neurodevelopment at two years, determining that maternal and birth anthropometry were the greatest predictors of linear growth outcomes, while inflammatory biomarkers were the most commonly associated predictors with neurodevelopment [27]. However, the underlying metabolic pathways that influence these outcomes have not been investigated.
The objective of this study was to identify metabolic associations with growth and neurocognitive outcomes. Several classes of metabolites were selected for analysis based on previous associations with EED and growth stunting, including bile acids [28], amino acids [20,23], and acylcarnitines [22]. Plasma samples were measured at 9 and 36 months, allowing for identification of the timing of when specific metabolic pathways are important. The growth and neurocognitive outcomes analyzed include height-for-age Z (HAZ) score as a measure of growth stunting, ∆HAZ (the difference between HAZ at enrollment and at four years) as a measure of the trajectory of stunting, and full-scale intelligence quotient (IQ) scores derived from the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III) as a measure of neurocognitive development. Identifying the relationships between metabolites and these outcomes will elucidate pathways associated with growth and neurocognitive impairment that may serve as biomarkers of disease or targets for potential interventions.
2. Materials and methods
2.1. Study population
The Performance of Rotavirus and Oral Polio Vaccines in Developing Countries (PROVIDE) study is a longitudinal study designed to examine oral vaccine efficacy in an undernourished population in Bangladesh. The study design and population of this study has been previously described [29]. Briefly, 700 children were enrolled within the first week of life and followed up to two years while collecting anthropometric growth data as well as stool and serum samples. For the current study, a smaller subset (130) of children from the PROVIDE cohort was followed further beyond two years with anthropometry and neurocognitive assessment using the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) at 4 years of age. HAZ and ΔHAZ growth measurements were taken throughout the first two years of life and then at 6 month intervals up to five years of age. On average, HAZ declined in both the full PROVIDE cohort and the 130 child subset to two years of age, at which point HAZ plateaued.
Neurocognitive assessment with the WPPSI-III in Bangladesh has previously been described [30]. Briefly, the WPPSI-III was culturally adapted for use with Bangladeshi children and assessments were administered at the nearest health clinic. Local staff were trained to administer the test to children and adequate inter-observer and test-retest reliability were achieved. The WPPSI-III assesses performance IQ (using Block Design, Matrix Reasoning, and Picture Completion subtests) and verbal IQ (using Information, Vocabulary, and Comprehension subtests), and these scaled scores are summed to calculate the full-scale IQ score.
2.2. Sample preparation
Plasma samples were collected within seven days of the exact date for the 9 month and 36 month time points for each child. Samples were stored at 4 °C until they were brought to the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), where they were frozen at −80 °C within four hours of collection. Plasma samples taken at 9 and 36 months of life were shipped from icddr,b to the University of Virginia, where they were stored at −80 °C until use. Before use in metabolomics assays, plasma was distributed into aliquots for use in multiple metabolomic assays.
2.3. Metabolite measurement and data validation
Plasma metabolites were analyzed using the AbsoluteIDQ p180 and Bile Acids kits (Biocrates Life Sciences AG, Innsbruck, Austria). The p180 kit is a targeted metabolomics approach for identifying 185 metabolites, including acylcarnitines, amino acids, biogenic amines, phosphatidylcholines, and sphingomyelins, while the Bile Acids kit detects 20 bile acids. Before use in metabolomics assays, plasma samples were thawed, briefly vortexed, then spun at 2800 xg for five minutes. Supernatant (10 μl) was then added to filter plates and samples were processed according to manufacturer's protocol. All reagents used during sample preparation were UHPLC-MS grade. Samples were analyzed by LC-MS/MS on a Waters (Milford, MA) I-Class Acquity chromatography system in-line with a Waters TQ-S mass spectrometer. Compounds were analyzed using TargetLynx XS software with the results subsequently imported into MetIDQ software (Biocrates) for quality control (QC) validation.
Raw metabolite concentrations (μM) were normalized using identical QC samples throughout all plates to control for plate to plate variation. QC samples were proprietary samples spiked with metabolites measured during targeted metabolomics. Normalized metabolite concentrations were exported for further analysis and screened for inclusion in the data set based on two criteria. First, metabolites must be within the valid range in at least 66% of quality control samples run on a plate to be included in analysis. Second, metabolites must be above the limit of detection in at least 60% of all measurements to be included in the valid data set. The QC screen eliminated 4 metabolites from analysis, while the limit of detection screen eliminated 84 metabolites from analysis. Data set validation was performed using R software version 3.4.3 (https://cran.r-project.org).
2.4. Metabolite nomenclature
All metabolites are described using the nomenclature described by Biocrates. Acylcarnitines in this kit are marked by their carbon chain length (C0-C18:2). All metabolites marked with PC are phosphatidylcholine species. The aa or ae designations indicate whether the fatty acid chain is bound by an ester (aa) or ether (ae) bond. The numbers (x:y) represent the total carbon chain length (x) followed by the number of double bonds (y) in the fatty acid chains. All metabolites marked with SM are sphingomyelin species, with SM[OH] representing hydroxy-sphingomyelin species. All metabolites in a class were summed to provide a “total” measurement which was also compared to outcomes of interest. In addition, the ratio of conjugated to unconjugated bile acids and the ratio of kynurenine to tryptophan were also calculated from valid individual measurements taken by the kit.
2.5. Statistical analysis
Initial screening of data sets was performed using Pearson correlation between all valid metabolites and the three outcomes of interest: HAZ, ∆HAZ, and WPPSI-III full-scale IQ score at four years. Associations were defined as a Pearson correlation coefficient of r > 0.2 or r < −0.2. All statistical analyses were performed using R software version 3.4.3 (https://cran.r-project.org). Pearson correlations were calculated using the ‘Hmisc’ package (version 4.1–1) and further adjusted using the False Discovery Rate (FDR) method to account for multiple comparisons for all metabolites measured at a given measurement time. SCAD-penalized linear regression was performed using the ‘grpreg’ package (version 3.2–0). Conditional random forest was performed using the ‘party’ package (version 1.3–1). Figures were generated using Prism 8 software (Graphpad) and R, using the ‘tidyverse’ (version 1.2.1) and ‘ggrepel’ (version 0.8.0) functions to generate volcano plots.
2.6. Ethics statement
The PROVIDE study was approved by the Research Review and Ethics Review Committees at The International Centre for Diarrheal Disease Research, Bangladesh and by the Institutional Review Boards at the University of Virginia, the University of Vermont, and Harvard University. Informed consent was obtained from parents before child participation in the study, and all data was anonymized prior to analysis.
3. Results
3.1. Study population characteristics
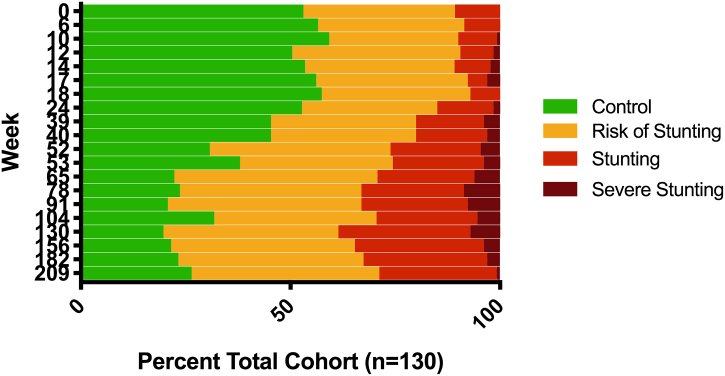
Demographic characteristics for both the full PROVIDE cohort and the 130 children analyzed in the current study are presented in Table 1. The smaller study cohort was representative of the full PROVIDE cohort in regards to age, sex, maternal education, monthly income, and enrollment HAZ, while there were significant differences between HAZ and ∆HAZ at two years as well as the number of days of exclusive breastfeeding. The significant differences in growth between PROVIDE and the smaller subset are likely due to changes in the population as a whole over time, as the subset was composed of children enrolled in the last six months of the original study and both HAZ and ∆HAZ values increased over the course of PROVIDE enrollment (Supplemental Fig. 1). Comparisons between the full cohort and the study cohort are primarily taken at two years, as data was not collected on the larger PROVIDE cohort after this point. Progression of linear growth stunting in the study cohort over the first four years of life is illustrated in Fig. 1. The prevalence of stunting in this subset was 10.8% at enrollment and steadily increased to a rate of 29.6% at two years, after which it remained fairly stable. In addition to the portion of this cohort that was stunted, a substantial portion was at risk for stunting, as defined by −1 > HAZ > −2. Because of the large number of individuals at risk of stunting, data was not categorically separated by stunting status but instead analyzed using HAZ as a continuous variable. The average ∆HAZ scores at two years was calculated as −0.49 ± 0.90, with lower ∆HAZ scores representing worse growth outcome. Neurocognitive development was assessed at four years of age for this study cohort, with average WPPSI-III scores of 84.6 ± 6.4, 81.8 ± 8.6, and 80.7 ± 8.0 for verbal IQ, performance IQ, and full-scale IQ, respectively.
Table 1.
Descriptive characteristics of total PROVIDE cohort and study subset.
| Characteristic | Total (N=700) | Subset (N=130) | P Valuea |
|---|---|---|---|
| Male sex (%) | 368 (52.6%) | 72 (55.4%) | 0.4773 |
| No maternal education (%) | 202 (28.3%) | 43 (33.1%) | 0.9384 |
| Monthly income, BDT | 12,762 ± 9410 | 12,775 ± 8981 | 0.9869 |
| HAZ, Enrollment | −0.90 ± 0.89 | −0.94 ± 0.81 | 0.5673 |
| HAZ, Two Years | −1.61 ± 1.04 | −1.43 ± 0.95 | 0.0277 |
| Delta HAZ, Two Years | −0.70 ± 1.02 | −0.49 ± 0.90 | 0.007 |
| WIS-C Score, Four Years | |||
| Verbal IQ | – | 84.58 ± 6.38 | – |
| Performance IQ | – | 81.80 ± 8.63 | – |
| Full Scale IQ | – | 80.69 ± 7.96 | – |
| Days Exclusive Breast Feeding | 110 ± 62 | 125 ± 59 | 0.0023 |
| Diarrheal Days, Two Years | 30.3 ± 24.1 | 33.9 ± 26.7 | 0.0559 |
Calculated from two-tailed Student's t-test comparing the metabolic subset with total PROVIDE (excluding the metabolic subset, n = 570). Bolded values are significant (P < 0.05) in statistical tests.
Fig. 1.
Progression of stunting in the PROVIDE neurocognitive subset: HAZ measurements were taken from enrollment through four years of age. HAZ measurements were categorized as control (HAZ > −1.0), risk of stunting (HAZ −1.0 to −2.0), stunting (HAZ −2.0 to −3.0) or severe stunting (HAZ < −3.0). The week of each HAZ measurement is indicated on the y-axis, with total percentage for each category indicated on the x-axis.
3.2. Associations between plasma metabolites and four year outcomes
Targeted metabolite analysis was carried out to quantify metabolites from 9 and 36 month plasma samples. Metabolites were initially screened for associations with four-year growth and neurocognitive outcomes by correlation analysis, with association defined as a Pearson's correlation coefficient of r > 0.2 or r < −0.2. The characteristics of associated metabolites with regard to age of collection and class of metabolite are presented in Table 2. Overall, 34 metabolites were associated with HAZ, 37 with ∆HAZ, and 34 with full-scale IQ. A majority of metabolites associated with linear growth outcomes (HAZ and ∆HAZ) were from 9 month plasma samples, while metabolites associated with full-scale IQ score were disproportionately from 36 month plasma samples. All associated metabolites were significantly correlated before adjustment for multiple comparisons. However, due to the large number of metabolites analyzed, only a limited number of metabolites met a false discovery rate (FDR) threshold for significance. Correlations with and without FDR adjustment for all metabolites are reported in Supplemental Table 1.
Table 2.
Characteristics of metabolites associated with four year outcomes.
| Characteristic | HAZ | ΔHAZ | Full-scale IQ |
|---|---|---|---|
| Total metabolites associated | 34 | 37 | 34 |
| Metabolites by plasma sample date | |||
| 9 Month | 21/34 (61.8%) | 19/37 (51.4%) | 5/34 (14.7%) |
| 36 Month | 13/34 (38.2%) | 18/37 (48.6%) | 29/34 (85.3%) |
| Metabolites by classification | |||
| Acylcarnitine (C) | 7/34 (20.6%) | 4/37 (10.8%) | 1/34 (2.9%) |
| Amino acid/biogenic amines | 5/34 (14.7%) | 6/37 (16.2%) | 4/34 (11.8%) |
| Bile acid | 1/34 (2.9%) | 2/37 (5.4%) | 0/34 (0.0%) |
| Phosphatidylcholine (PC) | 8/34 (23.5%) | 17/37 (45.9%) | 22/34 (64.7%) |
| Sphingomyelin (SM) | 13/34 (38.2%) | 8/37 (21.6%) | 7/34 (20.6%) |
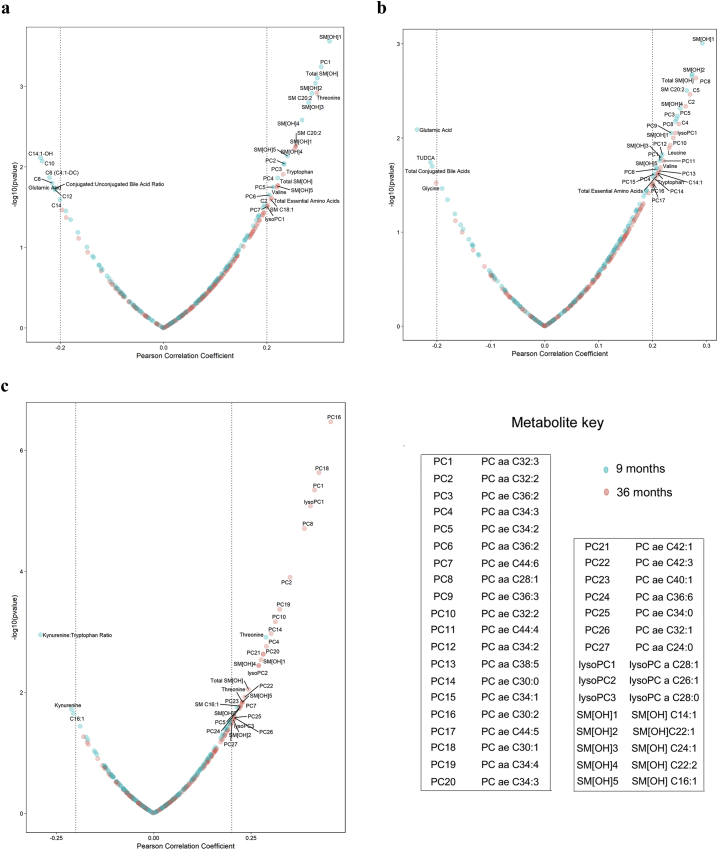
The relationship between individual metabolites and four year outcomes are shown in Fig. 2. The 34 metabolites associated with HAZ were primarily hydroxy-sphingomyelin (SM[OH]) and phosphatidylcholine (PC) species, which were positively associated (Fig. 2A). SM[OH] species were the most prevalent and most strongly associated metabolites with growth, as 80% (8/10) of SM[OH] metabolites measured were positively associated with HAZ. Total SM[OH], which represents the sum of five individual species measurements (SM[OH] C14:1, SM[OH] C16:1, SM[OH] C22:1, SM[OH] C22:2, SM[OH] C24:1), was associated with HAZ at both 9 and 36 months. Essential amino acids at 36 months were also positively associated with HAZ, driven primarily by the amino acids threonine, tryptophan, and valine. Most metabolites were positively associated with HAZ, but negative associations were also observed with several metabolites. While C2 acylcarnitine was positively associated with HAZ, medium to long chain acylcarnitines (C8, C10, C12, C14, C14:1-OH) were negatively associated. In addition to acylcarnitines, poor growth was also associated with a higher ratio of conjugated to unconjugated bile acids as well as the amino acid glutamic acid in 9 month plasma.
Fig. 2.
Associations between plasma metabolites and four year outcomes: Volcano plots of associations between plasma metabolites and (a) HAZ, (b) ∆HAZ, and (c) full-scale IQ score. Pearson correlation coefficient is given on the x-axis, while -log10(pvalue) is given on the y-axis. Dotted vertical lines indicate Pearson correlation coefficient r > 0.2 and r < −0.2, the cutoffs used for association with each outcome. All labeled metabolites met the cutoff for association. The colour of each data point indicates the timepoint at which it was collected, with nine month metabolites shown in blue and 36 month metabolites shown in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Many of the metabolites associated with HAZ were also associated with ∆HAZ. The predominant metabolites associated with ∆HAZ were once again SM[OH] and PCs, although fewer SM[OH] species were associated with ∆HAZ compared to HAZ (Fig. 2B). Essential amino acids at 36 months were also positively associated with ∆HAZ, here driven by leucine, tryptophan, and valine, while glutamic acid was again negatively associated with growth. Bile acid conjugation was once again associated with poor growth, as total bile acid conjugation was negatively associated with ∆HAZ. Medium-to-long chain acylcarnitines, which had been associated with poor HAZ at 4 years, were not associated with ∆HAZ. Rather, short chain acylcarnitines from 36-month plasma were associated with positive ∆HAZ outcomes, including C2, C4, and C5.
The set of metabolites associated with full-scale IQ score was fairly different from those associated with linear growth outcomes. While growth was associated with metabolites from all classes, neurocognitive outcomes were predominantly associated with PC species from 36 month plasma, constituting the eight most highly associated metabolites and 64.7% of all metabolites associated with full-scale IQ score. Several SM[OH] species were also associated with full-scale IQ, and these metabolites were also mostly derived from 36-month plasma. Bile acids, acylcarnitines, and amino acids were generally not associated with neurocognitive outcome. Threonine, the only amino acid associated with full-scale IQ, was positively associated with neurocognitive scoring at both 9 and 36 months. The only metabolites to be negatively associated with neurocognitive outcome were kynurenine and the kynurenine:tryptophan ratio.
3.3. SCAD-penalized linear regression
While a number of metabolic associations were identified, selecting which variables are most important or predictive for outcomes of interest can be difficult when working with large data sets. Penalized linear regression analysis represents an accurate method of identifying predictors from data with a large number of variables or with variables that are highly correlated [31], making it ideal for variable selection from metabolic data sets. Therefore, linear regression with a SCAD penalty was used to identify predictors from metabolites associated with four year outcomes. Before analysis, all metabolites associated within a given outcome were tested for correlations among themselves and single representative metabolites were chosen from any group with Pearson correlation coefficients of r > 0.6. Penalized linear regression was then used on this narrowed down data set to select predictors of four year outcomes.
The metabolites chosen by SCAD as well as the direction of the association between metabolites and outcomes are listed in Table 3. Overall, metabolites selected by SCAD were representative of the metabolites associated with four year outcomes as a whole. Many of the predictors for HAZ were acylcarnitines, with C2 from 36 month plasma being positively associated and C10 and C14:1-OH from 9 month plasma being negatively associated. HAZ was also associated with the conjugated:unconjugated bile acid ratio and total SM[OH] at 9 months as well as threonine at 36 months. Many of the same metabolites or metabolic pathways predictive of HAZ were also selected by SCAD for ∆HAZ. Total SM[OH] at 9 months and C2 at 36 months, which were positive predictors of HAZ, were also positive predictors of ∆HAZ. While the ratio of conjugated:unconjugated bile acids was negatively associated with HAZ, total conjugated bile acids and taurine-conjugated UDCA (TUDCA) in particular were selected for ∆HAZ. There were several metabolites that were distinctly selected as ∆HAZ predictors, including several amino acids (leucine, glycine) and 6 PC species, while only one PC species was predictive of HAZ. Metabolites associated with full-scale IQ were predominantly 36 month PC species (PC aa C32:3, PC ae C30:2, and PC ae C34:3), along with threonine from both 9 and 36 months and 9 month kynurenine. While threonine from 36 month plasma was also selected by SCAD for HAZ, full-scale IQ predictors were largely distinct from those of linear growth.
Table 3.
Metabolomic predictors selected by SCAD-penalized linear regression for four year outcomes.
| Outcome | Metabolite | Time of measurement | Direction of association |
|---|---|---|---|
| HAZ at 4 years | Conjugated:unconjugated bile acid ratio | 9 months | (−) |
| C10 | 9 months | (−) | |
| C14:1-OH | 9 months | (−) | |
| Total SM[OH] | 9 months | (+) | |
| C2 | 36 months | (+) | |
| Threonine | 36 months | (+) | |
| PC ae C44:6 | 36 months | (+) | |
| ∆HAZ from enrollment to 4 years | TUDCA | 9 months | (−) |
| Total conjugated bile acids | 9 months | (−) | |
| Glutamic Acid | 9 months | (−) | |
| PC aa C28:1 | 9 months | (+) | |
| PC aa C34:2 | 9 months | (+) | |
| PC aa C34:3 | 9 months | (−) | |
| Total SM[OH] | 9 months | (+) | |
| C2 | 36 months | (+) | |
| Glycine | 36 months | (−) | |
| Leucine | 36 months | (+) | |
| PC aa C28:1 | 36 months | (+) | |
| PC aa C38:5 | 36 months | (+) | |
| PC ae C44:4 | 36 months | (+) | |
| WPPSI-III full scale IQ score at 4 Years | Threonine | 9 months | (+) |
| Kynurenine | 9 months | (−) | |
| Threonine | 36 months | (+) | |
| PC aa C32:3 | 36 months | (+) | |
| PC ae C30:2 | 36 months | (+) | |
| PC ae C34:3 | 36 months | (+) | |
3.4. Conditional random forest modeling
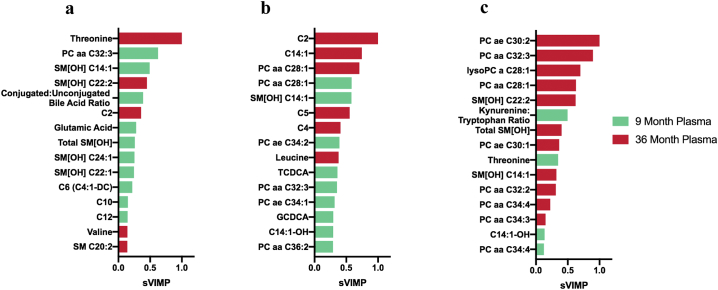
While correlation and linear regression analysis identified metabolites associated with linear growth and neurocognitive outcomes, a separate method was desired to independently validate these findings. Random forest has become a popular machine learning tool for classification and regression of variables from large biomedical data sets [27,32,33]. Therefore, all measured metabolites from 9 and 36 month plasma were analyzed using a conditional random forest algorithm to select and rank metabolites by their importance to each outcome. Conditional variable importance scores were calculated and scaled within each outcome as a proportion of the highest predictor, which was given a score of 1.0. The top 15 predictors for each outcome as ranked by scaled variable importance (sVIMP) scores are presented in Fig. 3, while the raw and scaled variable importance rankings for all metabolites are reported in Supplemental Table 2.
Fig. 3.
Conditional random forest rankings for four year outcomes: Rankings of the top 15 predictors for (a) HAZ, (b) ∆HAZ, and (c) full-scale IQ score as determined by conditional random forest analysis. Bars represent variable importance scores scaled for each outcome. Nine month metabolites are shown in green while 36 month metabolites are shown in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Overall, there was significant overlap between metabolites identified by our initial analysis and those identified by conditional random forest. Metabolites associated by Pearson's correlation for each outcome made up 100% of the top 15 metabolites selected by conditional random forest for HAZ, 80% for ∆HAZ, and 87% for full-scale IQ score. In addition, metabolites selected by SCAD were also selected by conditional random forest, with 71.4% (5/7) of SCAD-selected HAZ metabolites and 66.7% (4/6) of SCAD-selected full-scale IQ metabolites also being ranked in the top 15 metabolites by conditional random forest. While a smaller proportion (4/13, 30.7%) of SCAD-selected ∆HAZ metabolites were chosen by conditional random forest, 3 of the top 5 random forest predictors were selected by SCAD, providing a higher level of confidence for the most important ∆HAZ predictors. These results suggest that the conditional random forest model largely validates our previous analysis.
The strongest predictors of HAZ outcome were threonine at 36 months sVIMP = 1.0), followed by 9 month PC aa C32:3 (sVIMP = 0.62) and SM[OH] species SM[OH] C14:1 (sVIMP = 0.49) and 36 month SM[OH] C22:2 (sVIMP = 0.45). SM[OH] species were highly ranked predictors of HAZ outcome, making up 6 of the top 15 predictors. For ∆HAZ, the strongest predictors were acylcarnitines C2 (sVIMP = 1.0) and C14:1 (sVIMP = 0.75) at 36 months, followed by PC aa C28:1 from 36 month (sVIMP = 0.71) and 9 month (sVIMP = 0.58) plasma. As observed with correlation analysis, several metabolic pathways were associated with both linear growth outcomes. These include bile acid conjugation (for HAZ, conjugated:unconjugated bile acid ratio, sVIMP = 0.39; for ∆HAZ, bile acids TCDCA, sVIMP = 0.36 and GCDCA, sVIMP = 0.29), acylcarnitines (C2 for both HAZ, sVIMP = 0.36 and ∆HAZ, sVIMP = 1.0), essential amino acids (for HAZ, threonine, sVIMP = 1.0, valine, sVIMP = 0.14, and tryptophan, sVIMP = 0.13; for ∆HAZ, leucine, sVIMP = 0.38), and SM[OH] (5 species for HAZ, SM[OH] C14:1, sVIMP = 0.58, for ∆HAZ). The metabolites associated with neurocognitive development were again distinct from those associated with linear growth. The four top most ranked metabolites for full-scale IQ were 36 month phosphatidylcholines: PC ae C30:2 (sVIMP = 1.0), PC aa C32:3 (sVIMP = 0.90), lysoPC a C28:1 (sVIMP = 0.70), and PC aa C28:1 (sVIMP = 0.63). PC species make up 9 of the top 15 predictors of full-scale IQ. Other metabolic pathways selected by SCAD were also observed in the conditional random forest model, including the kynurenine:tryptophan ratio (sVIMP = 0.50) and threonine (sVIMP = 0.35) from 9 month plasma.
The percentage of variation explained was calculated based on the conditional random forest. This analysis estimates that the conditional random forest model explains 44.7% of HAZ variance, 45.6% of ∆HAZ variance, and 43.1% of full-scale IQ score variance at four years.
4. Discussion
The results of this study have implications for the treatment of growth and neurocognitive faltering in undernourished populations. Previous reports suggest that current dietary interventions are insufficient for alleviating growth stunting in children [15,16], potentially due to suboptimal timing or targeting of metabolic pathways. This study has identified key plasma metabolites from both 9 and 36 months that are predictive of four-year growth and neurocognitive outcomes. Of note, several of the most significant 36 month metabolites, including SM[OH] and several PC and acylcarnitine species, were highly correlated between 9 and 36 month timepoints (data not shown). These data indicate that altered levels of these metabolites at early timepoints may predict more pronounced differences later and increase the potential use of these metabolites as biomarkers. Distinct differences were observed between the metabolites associated with growth and those associated with neurocognition, consistent with previous analysis that found different sets of biomarkers to be predictive of these outcomes at two years of age [27]. Differences in metabolites associated with these outcomes suggest that separate metabolic pathways and timings must be targeted for successful intervention.
Growth and neurocognitive outcomes were associated with different classes of metabolites. HAZ and ∆HAZ were represented by all classes measured (bile acids, acylcarnitines, amino acids, phosphatidylcholines, and sphingomyelins) in penalized linear regression and conditional random forest models. The wide range of metabolites associated with growth is consistent with previous studies that found growth outcomes to be associated with multiple factors, including insufficient nutritional intake [10,34], environmental enteric dysfunction (EED) [12,13], and disruption of the intestinal microbiota [35]. Many of the metabolites identified in this study have previously been associated with these disorders [19,22,36]. Poor HAZ and ∆HAZ outcomes were associated with lower concentrations of total essential amino acids in 36 month plasma, particularly threonine, tryptophan, valine, and leucine (Fig. 2A-B), which is likely due to insufficient dietary intake [34]. In addition to being required for protein synthesis, essential amino acids can also inhibit the mTOR pathway, which promotes protein and lipid synthesis [37].Plasma acylcarnitines were also associated with linear growth outcomes, but this association was mixed: short chain acylcarnitines from 36 month plasma were positively associated with growth, while longer chain acylcarnitines from 9 month plasma were negatively associated (Fig. 3A-B). This may be due to differences in function between short and medium-long chain acylcarnitines [38,39] or due to differences in time of measurement. Acylcarnitines are generated as a byproduct of fatty acid oxidation [40], and accumulation of acylcarnitines in plasma is associated with secondary carnitine deficiency, impaired carnitine absorption, or poor diet [41]. EED is known to promote impaired absorption due to tissue damage and villi stunting [42], and acylcarnitine accumulation has been associated with elevated gut permeability [22]. Several other metabolites link growth to impaired absorption through EED, including threonine, a major component of mucus glycoproteins that line the intestines [43] and play a critical role in maintaining intestinal barrier function [44,45] as well as glutamic acid, which was elevated in children with poor growth outcomes (Table 3) and has previously been associated with intestinal permeability [20]. Finally, high levels of bile acid conjugation were associated with poor HAZ and ∆HAZ outcomes (Table 3), which suggests a role for the intestinal microbiota. While bile acid conjugation is a host process that occurs in the liver, deconjugation is performed by intestinal microbes through the activity of bile salt hydrolases (BSH) [46]. BSH-expressing bacteria in the gut have been linked to proper lipid metabolism by influencing host gene expression [47], so disruption of these microbial communities may lead to impaired growth.
While many of these metabolites have previously been associated with growth outcomes, this study found a significant role for both SM[OH] and PC species, which were the most abundant metabolites associated with both HAZ and ∆HAZ (Table 2). PCs are the most abundant phospholipid in mammalian cell membranes [48] and are critical for cell membrane structure and physiology as well as optimal bone growth and development [49,50]. Both total SM[OH] and individual SM[OH] species were strongly associated with growth outcomes by all methods of analysis. An association between higher serum concentrations of some SM[OH] species and improved HAZ was observed in a child cohort in rural Malawi [20], but the authors did not speculate on this finding. The mechanism by which SM[OH] contributes to stunting is not well characterized. No significant associations were observed between four year outcomes and non-hydroxylated sphingomyelins, suggesting that this phenotype is specific to the hydroxylated form of the sphingolipid. Synthesis of hydroxylated sphingomyelin is identical to synthesis of non-hydroxylated sphingomyelin except for the addition of a hydroxyl group to the fatty acid chain by fatty acid-2-hydroxylase (FA2H) before attachment to the ceramide backbone [51]. Most literature regarding sphingolipid hydroxylation pertains to effects on neurocognition, as hydroxyl-sphingolipids are enriched in myelin sheaths [52] and mutation of FA2H has been associated with neurodegeneration [[53], [54], [55]]. These results may explain the observed associations between SM[OH] and neurocognitive outcome (Table 2). FA2H has also been implicated in cAMP signaling [56] and glucose uptake in adipocytes [57], which may explain the growth phenotypes observed.
While metabolic changes have previously been associated with linear growth outcomes, the literature regarding metabolic associations with neurocognition is lacking. Therefore, the current study represents an important overview of metabolic trends that are associated with neurocognitive scoring in an undernourished child cohort. Metabolites associated with full-scale IQ score were disproportionately PC species, which make up 65% of all metabolites associated by Pearson correlation (Table 2) and nine of the top 15 predictors of full-scale IQ by conditional random forest (Fig. 3). Decreased plasma PC concentration has been linked previously to decreased cognitive outcomes in Alzheimer's Disease [58,59], and PC components such as choline [60,61] and specific fatty acids [62,63] have been associated with neurocognitive development. The mechanism by which PCs are associated with neurocognitive development has not been elucidated, but may be due to crosstalk with inflammation. PC is important for maintaining barrier function in the gut [64], and increased intestinal PCs have been associated with decreased intestinal inflammation [[65], [66], [67]]. While much of this data is based on intestinal PCs, plasma PCs may have similar anti-inflammatory effects, which could explain how decreased plasma PC is deleterious to neurocognitive development. While the current study observed an association between phosphatidylcholine species and full-scale IQ score, analysis of lipids was semi-quantitative and lacked the resolution necessary to distinguish which specific fatty acid chains make up each PC species identified (Supplemental Table 3). Further studies are necessary to investigate whether individual fatty acid components are associated with proper neurocognition in undernourished children.
Other metabolites identified in this study emphasize the impact of early inflammation on neurocognition. Non-PC metabolites associated with neurocognitive outcomes include threonine, which was associated with improved outcomes, as well as the biogenic amine kynurenine and the kynurenine:tryptophan ratio (Kyn:Trp), which were associated with poor outcomes (Table 3, Fig. 3). The importance of threonine to proper gut integrity as a mechanism to prevent inflammation has previously been described. Elevated kynurenine and Kyn:Trp ratio are markers of increased indoleamine 2,3-dioxygenase 1 (IDO1) activity, an enzyme that converts tryptophan to kynurenine in response to inflammatory signals [68]. High Kyn:Trp ratio has previously been associated with stunting in other cohorts [69] as well as HIV-associated neurocognitive disorders [70]. This pathway has also been implicated in regulation of inflammation in the central nervous system [71], providing a mechanistic link between gut inflammation and neural development.
In addition to differences in the metabolites associated with each outcome, there were also differences in the times at which these metabolites were measured. Linear growth outcomes were more likely to be associated with 9 month metabolites, which made up 61.8% of associated metabolites for HAZ and 51.4% for ∆HAZ by Pearson correlation analysis (Table 2). Previous studies have shown that maternal and birth anthropometry influence linear growth throughout childhood [[72], [73], [74]]. In contrast, full-scale IQ score was largely associated with 36 month metabolites, which made up 85.3% of metabolites associated by Pearson correlation (Table 2), 64.7% associated by SCAD (Table 3), and 73% of the top 15 metabolites associated by conditional random forest (Fig. 3C). Neurocognition in the PROVIDE cohort has previously been assessed at earlier timepoints using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) [[25], [26], [27]]. When metabolic associations with Bayley-III scores at 18 and 24 months were explored, PC species from 9 month plasma were not significantly associated, but associations with several 24 month PC species were observed (Supplemental Fig. 2). All associations with 24 month plasma metabolites were not assessed because this data was only collected on a smaller subset (75/130) of this cohort, but these results suggest that the association between PCs and neurocognition may represent a later (2+ years post-birth) biological phenomenon. As this is later in childhood and children are no longer exclusively breastfeeding at this point, this may be driven by environmental stressors rather than maternal or birth factors. Childhood neurodevelopment has been previously linked to environmental stressors that may be the predominant influences on metabolic status later in childhood, including the intensity of disease burden [75] or inflammation [76,77].
In this study, plasma metabolites were analyzed for associations with linear growth and neurocognitive development at four years using two independent methods. The sets of metabolites selected using these methods were largely consistent, with over 80% of the top 15 ranked metabolites by conditional random forest associated with outcomes by Pearson correlation. The similarity of metabolite sets identified by these methods reinforces that the metabolites selected represent the strongest predictors of growth and neurocognitive development in this population. Many of the associations observed in the current study are also consistent with previous findings from other groups, including associations for essential amino acids [20,23], bile acid conjugation [28,36], and acylcarnitines [22] with EED and growth outcomes. While the large number of metabolites assayed by the p180 kit resulted in few metabolites meeting a false discovery threshold for significance, the consistency of these results with previous findings instills confidence that these associations are not just artifacts of the large number of metabolites analyzed.
Important questions remain as to the mechanism by which these metabolites are elevated or decreased in the context of undernutrition. While dietary differences may contribute to impaired linear growth or neurocognitive outcomes, members of this cohort live within the same community and likely have similar diets. Plasma metabolites may instead be reflective of environmental stressors such as chronic inflammation or differences in the intestinal microbiota, as changes in microbiome composition can profoundly affect growth [[78], [79], [80]] and neurodevelopmental [81,82] outcomes. Work is currently ongoing to investigate metagenomic data within this cohort for associations with four year outcomes, which may provide a mechanistic link for some of the metabolic associations observed in this study.
While distinct differences were observed between growth and neurocognitive outcomes, there were some similar pathways that may serve as effective targets for intervention. Indicators of inflammation and malabsorption associated with EED were observed at 9 months with both growth (conjugated bile acids, long chain acylcarnitines) and neurocognition (threonine, Kyn:Trp), suggesting that early interventions targeting EED may be effective for both outcomes. In addition, lipid metabolites were associated with both outcomes (Table 2), including several individual SM[OH] and PC species (PC aa C32:3, PC aa C28:1) (Fig. 3). These data indicate that lipid metabolism may be a potential pathway of interest for intervention. There is some evidence that lipid-based interventions may be effective for the treatment of acute malnutrition [83]. In addition, a recent trial in Ecuador found that dietary supplementation with eggs resulted in significant HAZ recovery in children [84], potentially by boosting plasma levels of choline [85]. Future work is necessary to see if this is consistent in other populations, but lipid biosynthesis may represent a novel target for intervention.
The following are the supplementary data related to this article.
List of all metabolites associated with four year outcomes with P and r values
Raw and Scaled Variable Importance Scores for All Metabolites Analyzed by Conditional Random Forest
List of Isobaric and Isomeric Lipid Species Measured by the AbsoluteIDQ p180 Kit® (Adapted from https://www.biocrates.com/images/List-of-Isobaric-and-Isomeric-Lipid-Species_v1_2018.pdf)
Lists of metabolites analyzed and all associations with outcome
Acknowledgments
Acknowledgements
We would like to acknowledge the children and families who participated in the PROVIDE study, without whom this work would not have been possible. We would also like to acknowledge Swapna Kumar and Charles Nelson, who facilitated collection of the neurocognitive assessment data.
Funding sources
Funding for this work was provided by the Bill & Melinda Gates Foundation, Grant ID Number OP1173478, as well as National Institutes of Health Grants AI043596 and CA044579. The funders did not have any role in study design, data analysis, or the writing of this manuscript.
Declarations of interest
Dr. Petri reports grants from Bill & Melinda Gates Foundation and grants from National Institutes of Health during the conduct of the study. The authors declare no other relationships or circumstances that present a potential conflict of interest.
Author contributions
Conceptualization and Study Design (PROVIDE): B Kirkpatrick, WA Petri; Project and Experimental Design: GB Moreau, ER Colgate, G. Ramakrishnan, WA Petri; Direction of the field site: R. Haque; Data Management: U. Nayak; Sample Preparation and Metabolite Measurement: GB Moreau, TE Fox; Data Validation and Statistical Analysis: GB Moreau, HL Cook, JZ Ma; Manuscript Writing and Figures: GB Moreau, G. Ramakrishnan.
References
- 1.Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]; Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. [DOI] [PubMed]
- 2.De Onis M., Brown D., Blossner M., Borghi E. 2012. Levels and trends in child malnutrition. UNICEF-WHO-the World Bank joint child malnutrition estimates. [Google Scholar]; De Onis M, Brown D, Blossner M, Borghi E. Levels and trends in child malnutrition. UNICEF-WHO-The World Bank joint child malnutrition estimates. 2012;
- 3.Berkman D.S., Lescano A.G., Gilman R.H., Lopez S.L., Black M.M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]; Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–71. [DOI] [PubMed]
- 4.Fischer Walker C.L., Lamberti L., Adair L., Guerrant R.L., Lescano A.G., Martorell R. Does childhood diarrhea influence cognition beyond the diarrhea-stunting pathway? PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047908. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fischer Walker CL, Lamberti L, Adair L, Guerrant RL, Lescano AG, Martorell R, et al. Does childhood diarrhea influence cognition beyond the diarrhea-stunting pathway? PLoS One. 2012;7(10):e47908. [DOI] [PMC free article] [PubMed]
- 5.Chang S.M., Walker S.P., Grantham-McGregor S., Powell C.A. Early childhood stunting and later behaviour and school achievement. J Child Psychol Psychiatry. 2002;43(6):775–783. doi: 10.1111/1469-7610.00088. [DOI] [PubMed] [Google Scholar]; Chang SM, Walker SP, Grantham-McGregor S, Powell CA. Early childhood stunting and later behaviour and school achievement. J Child Psychol Psychiatry. 2002;43(6):775–83. [DOI] [PubMed]
- 6.Adair L.S., Popkin B.M., Akin J.S., Guilkey D.K., Gultiano S., Borja J. Cohort profile: the Cebu longitudinal health and nutrition survey. Int J Epidemiol. 2011;40(3):619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, et al. Cohort profile: the Cebu longitudinal health and nutrition survey. Int J Epidemiol. 2011;40(3):619–25. [DOI] [PMC free article] [PubMed]
- 7.Victora C.G., Adair L., Fall C., Hallal P.C., Martorell R., Richter L. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed]
- 8.Victora C.G., de Onis M., Hallal P.C., Blossner M., Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–e480. doi: 10.1542/peds.2009-1519. Mar. [DOI] [PubMed] [Google Scholar]; Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010 Mar;125(3):e473-80. [DOI] [PubMed]
- 9.Prendergast A.J., Humphrey J.H. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34(4):250–265. doi: 10.1179/2046905514Y.0000000158. Internet. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health [Internet]. 2014;34(4):250–65. Available from: 10.1179/2046905514Y.0000000158 [DOI] [PMC free article] [PubMed]
- 10.Rah J.H., Akhter N., Semba R.D., de Pee S., Bloem M.W., Campbell A.A. Low dietary diversity is a predictor of child stunting in rural Bangladesh. Eur J Clin Nutr. 2010;64(12):1393–1398. doi: 10.1038/ejcn.2010.171. [DOI] [PubMed] [Google Scholar]; Rah JH, Akhter N, Semba RD, de Pee S, Bloem MW, Campbell AA, et al. Low dietary diversity is a predictor of child stunting in rural Bangladesh. Eur J Clin Nutr. 2010;64(12):1393–8. [DOI] [PubMed]
- 11.Bloss E., Wainaina F., Bailey R.C. Prevalence and predictors of underweight, stunting, and wasting among children aged 5 and under in western Kenya. J Trop Pediatr. 2004;50(5):260–270. doi: 10.1093/tropej/50.5.260. [DOI] [PubMed] [Google Scholar]; Bloss E, Wainaina F, Bailey RC. Prevalence and predictors of underweight, stunting, and wasting among children aged 5 and under in western Kenya. J Trop Pediatr. 2004;50(5):260–70. [DOI] [PubMed]
- 12.Crane R.J., Jones K.D.J., Berkley J.A. Environmental enteric dysfunction: an overview. Food Nutr Bull. 2015;36(1 0):S76–S87. doi: 10.1177/15648265150361S113. [DOI] [PMC free article] [PubMed] [Google Scholar]; . Crane RJ, Jones KDJ, Berkley JA. Environmental enteric dysfunction: An overview. Food Nutr Bull. 2015;36(1 0):S76–87. [DOI] [PMC free article] [PubMed]
- 13.Merchant A.T., Jones C., Kiure A., Kupka R., Fitzmaurice G., Herrera M.G. Water and sanitation associated with improved child growth. Eur J Clin Nutr. 2003;57(12):1562–1568. doi: 10.1038/sj.ejcn.1601725. [DOI] [PubMed] [Google Scholar]; Merchant AT, Jones C, Kiure A, Kupka R, Fitzmaurice G, Herrera MG, et al. Water and sanitation associated with improved child growth. Eur J Clin Nutr. 2003;57(12):1562–8. [DOI] [PubMed]
- 14.Watanabe K., Petri W.A.J. Environmental enteropathy: elusive but significant subclinical abnormalities in developing countries. EBioMedicine. 2016;10:25–32. doi: 10.1016/j.ebiom.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; Watanabe K, Petri W. A. J. Environmental Enteropathy: Elusive but Significant Subclinical Abnormalities in Developing Countries. EBioMedicine. 2016;10:25–32. [DOI] [PMC free article] [PubMed]
- 15.Dewey K.G., Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4(Suppl. 1):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4 Suppl 1:24–85. [DOI] [PMC free article] [PubMed]
- 16.Prendergast A.J., Humphrey J.H. Stunting persists despite optimal feeding: are toilets part of the solution? Nestle Nutr Inst Work Ser. 2015;81:99–110. doi: 10.1159/000365807. [DOI] [PubMed] [Google Scholar]; Prendergast AJ, Humphrey JH. Stunting Persists despite Optimal Feeding: Are Toilets Part of the Solution? Nestle Nutr Inst Work Ser. 2015;81:99–110. [DOI] [PubMed]
- 17.Murray-Kolb L.E., Khatry S.K., Katz J., Schaefer B.A., Cole P.M., LeClerq S.C. Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch Pediatr Adolesc Med. 2012;166(5):404–410. doi: 10.1001/archpediatrics.2012.37. [DOI] [PubMed] [Google Scholar]; Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC, et al. Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch Pediatr Adolesc Med. 2012;166(5):404–10. [DOI] [PubMed]
- 18.Bhutta Z.A., Ahmed T., Black R.E., Cousens S., Dewey K., Giugliani E. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]; Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–40. [DOI] [PubMed]
- 19.Semba R.D., Shardell M., Trehan I., Moaddel R., Maleta K.M., Ordiz M.I. Metabolic alterations in children with environmental enteric dysfunction. Sci Rep. 2016;6:1–9. doi: 10.1038/srep28009. Internet. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]; Semba RD, Shardell M, Trehan I, Moaddel R, Maleta KM, Ordiz MI, et al. Metabolic alterations in children with environmental enteric dysfunction. Sci Rep [Internet]. 2016;6:1–9. Available from: doi:10.1038/srep28009 [DOI] [PMC free article] [PubMed]
- 20.Semba R.D., Shardell M., Sakr Ashour F.A., Moaddel R., Trehan I., Maleta K.M. Child stunting is associated with low circulating essential amino acids. EBioMedicine [Internet] 2016;6:246–252. doi: 10.1016/j.ebiom.2016.02.030. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]; . Semba RD, Shardell M, Sakr Ashour FA, Moaddel R, Trehan I, Maleta KM, et al. Child Stunting is Associated with Low Circulating Essential Amino Acids. EBioMedicine [Internet]. 2016;6:246–52. Available from: doi:10.1016/j.ebiom.2016.02.030 [DOI] [PMC free article] [PubMed]
- 21.Di Giovanni V., Bourdon C., Wang D.X., Seshadri S., Senga E., Versloot C.J. Metabolomic changes in serum of children with different clinical diagnoses of malnutrition. J Nutr. 2016;146(12):2436–2444. doi: 10.3945/jn.116.239145. Internet. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]; Di Giovanni V, Bourdon C, Wang DX, Seshadri S, Senga E, Versloot CJ, et al. Metabolomic Changes in Serum of Children with Different Clinical Diagnoses of Malnutrition. J Nutr [Internet]. 2016;146(12):2436–44. Available from: 10.3945/jn.116.239145 [DOI] [PMC free article] [PubMed]
- 22.Semba R.D., Trehan I., Li X., Moaddel R., Ordiz M.I., Maleta K.M. Environmental enteric dysfunction is associated with carnitine deficiency and altered fatty acid oxidation. EBioMedicine. 2017;17:57–66. doi: 10.1016/j.ebiom.2017.01.026. Internet. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]; . Semba RD, Trehan I, Li X, Moaddel R, Ordiz MI, Maleta KM, et al. Environmental Enteric Dysfunction is Associated with Carnitine Deficiency and Altered Fatty Acid Oxidation. EBioMedicine [Internet]. 2017;17:57–66. Available from: doi:10.1016/j.ebiom.2017.01.026 [DOI] [PMC free article] [PubMed]
- 23.Kumar M., Ji B., Babaei P., Das P., Lappa D., Ramakrishnan G. Gut microbiota dysbiosis is associated with malnutrition and reduced plasma amino acid levels: lessons from genome-scale metabolic modeling. Metab Eng. 2018;49(May):128–142. doi: 10.1016/j.ymben.2018.07.018. Internet. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]; . Kumar M, Ji B, Babaei P, Das P, Lappa D, Ramakrishnan G, et al. Gut microbiota dysbiosis is associated with malnutrition and reduced plasma amino acid levels: Lessons from genome-scale metabolic modeling. Metab Eng [Internet]. 2018;49(May):128–42. Available from: 10.1016/j.ymben.2018.07.018 [DOI] [PMC free article] [PubMed]
- 24.Naylor C., Lu M., Haque R., Mondal D., Buonomo E., Nayak U. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine [Internet] 2015;2(11):1759–1766. doi: 10.1016/j.ebiom.2015.09.036. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]; . Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, et al. Environmental Enteropathy, Oral Vaccine Failure and Growth Faltering in Infants in Bangladesh. EBioMedicine [Internet]. 2015;2(11):1759–66. Available from: doi:10.1016/j.ebiom.2015.09.036 [DOI] [PMC free article] [PubMed]
- 25.Jiang N.M., Tofail F., Moonah S.N., Scharf R.J., Taniuchi M., Ma J.Z. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr. 2014;14(1):1–9. doi: 10.1186/1471-2431-14-50. Internet. [Available from: BMC Pediatrics] [DOI] [PMC free article] [PubMed] [Google Scholar]; . Jiang NM, Tofail F, Moonah SN, Scharf RJ, Taniuchi M, Ma JZ, et al. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr [Internet]. 2014;14(1):1–9. Available from: BMC Pediatrics [DOI] [PMC free article] [PubMed]
- 26.Jiang N.M., Tofail F., Ma J.Z., Haque R., Kirkpatrick B., Nelson C.A. Early life inflammation and neurodevelopmental outcome in Bangladeshi infants growing up in adversity. Am J Trop Med Hyg. 2017;97(3):974–979. doi: 10.4269/ajtmh.17-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang NM, Tofail F, Ma JZ, Haque R, Kirkpatrick B, Nelson CA, et al. Early life inflammation and neurodevelopmental outcome in Bangladeshi infants growing up in adversity. Am J Trop Med Hyg. 2017;97(3):974–9. [DOI] [PMC free article] [PubMed]
- 27.Donowitz J., Cook H., Alam M., Kabir M., Colgate E., Carmolli M. Role of maternal health and inflammation in infancy in nutritional and neurodevelopmental outcomes of two-year-old Bangladeshi children. PLOS Neglected Trop Dis. 2018:1–20. doi: 10.1371/journal.pntd.0006363. [DOI] [PMC free article] [PubMed] [Google Scholar]; Donowitz J, Cook H, Alam M, Kabir M, Colgate E, Carmolli M, et al. Role of Maternal Health and Inflammation in Infancy in Nutritional and Neurodevelopmental Outcomes of Two-Year-Old Bangladeshi Children. PLOS Neglected Trop Dis. 2018;1–20. [DOI] [PMC free article] [PubMed]
- 28.Semba R.D., Gonzalez-Freire M., Moaddel R., Trehan I., Maleta K.M., Khadeer M. Environmental enteric dysfunction is associated with altered bile acid metabolism. J Pediatr Gastroenterol Nutr. 2017;64(4):536–540. doi: 10.1097/MPG.0000000000001313. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]; Semba RD, Gonzalez-Freire M, Moaddel R, Trehan I, Maleta KM, Khadeer M, et al. Environmental Enteric Dysfunction Is Associated With Altered Bile Acid Metabolism. J Pediatr Gastroenterol Nutr. 2017 Apr;64(4):536–40. [DOI] [PMC free article] [PubMed]
- 29.Kirkpatrick B.D., Colgate E.R., Mychaleckyj J.C., Haque R., Dickson D.M., Carmolli M.P. The “performance of rotavirus and oral polio vaccines in developing countries” (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg. 2015;92(4):744–751. doi: 10.4269/ajtmh.14-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kirkpatrick BD, Colgate ER, Mychaleckyj JC, Haque R, Dickson DM, Carmolli MP, et al. The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study: Description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg. 2015;92(4):744–51. [DOI] [PMC free article] [PubMed]
- 30.Hamadani J.D., Tofail F., Nermell B., Gardner R., Shiraji S., Bottai M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40(6):1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]; Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, et al. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40(6):1593–604. [DOI] [PubMed]
- 31.Lu M., Zhou J., Naylor C., Kirkpatrick B.D., Haque R., Petri W.A. Application of penalized linear regression methods to the selection of environmental enteropathy biomarkers. Biomark Res. 2017;5(1):9. doi: 10.1186/s40364-017-0089-4. Internet. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]; . Lu M, Zhou J, Naylor C, Kirkpatrick BD, Haque R, Petri WA, et al. Application of penalized linear regression methods to the selection of environmental enteropathy biomarkers. Biomark Res [Internet]. 2017;5(1):9. Available from: 10.1186/s40364-017-0089-4 [DOI] [PMC free article] [PubMed]
- 32.Subramanian S., Huq S., Yatsunenko T., Haque R., Mahfuz M., Alam M.A. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature [Internet] 2014;510(7505):417–421. doi: 10.1038/nature13421. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]; . Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature [Internet]. 2014;510(7505):417–21. Available from: doi:10.1038/nature13421 [DOI] [PMC free article] [PubMed]
- 33.Brown E.M., Wlodarska M., Willing B.P., Vonaesch P., Han J., Reynolds L.A. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat Commun. 2015;6:1–16. doi: 10.1038/ncomms8806. Internet. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]; . Brown EM, Wlodarska M, Willing BP, Vonaesch P, Han J, Reynolds LA, et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat Commun [Internet]. 2015;6:1–16. Available from: doi:10.1038/ncomms8806 [DOI] [PMC free article] [PubMed]
- 34.Ghosh S., Suri D., Uauy R. Assessment of protein adequacy in developing countries: quality matters. Br J Nutr. 2012;108(Suppl):S77–S87. doi: 10.1017/S0007114512002577. [DOI] [PubMed] [Google Scholar]; Ghosh S, Suri D, Uauy R. Assessment of protein adequacy in developing countries: quality matters. Br J Nutr. 2012;108 Suppl:S77-87. [DOI] [PubMed]
- 35.Charbonneau M.R., O'Donnell D., Blanton L.V., Totten S.M., Davis J.C., Barratt M.J. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 2016;164(5):859–871. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; . Charbonneau MR, O'’Donnell D, Blanton L V, Totten SM, Davis JC, Barratt MJ, et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell. 2016;164(5):859–71. [DOI] [PMC free article] [PubMed]
- 36.Zhang L., Voskuijl W., Mouzaki M., Groen A.K., Alexander J., Bourdon C. Impaired bile acid homeostasis in children with severe acute malnutrition. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155143. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang L, Voskuijl W, Mouzaki M, Groen AK, Alexander J, Bourdon C, et al. Impaired Bile Acid Homeostasis in Children with Severe Acute Malnutrition. PLoS One. 2016;11(5):e0155143. [DOI] [PMC free article] [PubMed]
- 37.Semba R.D., Trehan I., Gonzalez-Freire M., Kraemer K., Moaddel R., Ordiz M.I. Perspective: the potential role of essential amino acids and the mechanistic target of Rapamycin complex 1 (mTORC1) pathway in the pathogenesis of child stunting. Adv Nutr. 2016 Sep 7;7(5):853–865. doi: 10.3945/an.116.013276. https://www.ncbi.nlm.nih.gov/pubmed/27633102 Internet. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]; . Semba RD, Trehan I, Gonzalez-Freire M, Kraemer K, Moaddel R, Ordiz MI, et al. Perspective: The Potential Role of Essential Amino Acids and the Mechanistic Target of Rapamycin Complex 1 (mTORC1) Pathway in the Pathogenesis of Child Stunting. Adv Nutr [Internet]. 2016 Sep 7;7(5):853–65. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27633102 [DOI] [PMC free article] [PubMed]
- 38.Jones L.L., McDonald D.A., Borum P.R. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49(1):61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]; Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49(1):61–75. [DOI] [PubMed]
- 39.Adams S.H., Hoppel C.L., Lok K.H., Zhao L., Wong S.W., Minkler P.E. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139(6):1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139(6):1073–81. [DOI] [PMC free article] [PubMed]
- 40.Schooneman M.G., Vaz F.M., Houten S.M., Soeters M.R. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62(1):1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62(1):1–8. [DOI] [PMC free article] [PubMed]
- 41.Flanagan J.L., Simmons P.A., Vehige J., Willcox M.D., Garrett Q. Role of carnitine in disease. Nutr Metab. 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]; Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab. 2010;7:30. [DOI] [PMC free article] [PubMed]
- 42.Owino V., Ahmed T., Freemark M., Kelly P., Loy A., Manary M. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics [Internet] 2016;138(6) doi: 10.1542/peds.2016-0641. Available from. [DOI] [PubMed] [Google Scholar]; Owino V, Ahmed T, Freemark M, Kelly P, Loy A, Manary M, et al. Environmental Enteric Dysfunction and Growth Failure/Stunting in Global Child Health. Pediatrics [Internet]. 2016;138(6):e20160641–e20160641. Available from: 10.1542/peds.2016-0641 [DOI] [PubMed]
- 43.Allen A., Pearson J.P. Mucus glycoproteins of the normal gastrointestinal tract. Eur J Gastroenterol Hepatol. 1993;5(4):193–199. [Google Scholar]; Allen A, Pearson JP. Mucus glycoproteins of the normal gastrointestinal tract. Eur J Gastroenterol Hepatol. 1993;5(4):193–9.
- 44.Faure M., Moennoz D., Montigon F., Mettraux C., Breuille D., Ballevre O. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J Nutr. 2005;135(3):486–491. doi: 10.1093/jn/135.3.486. [DOI] [PubMed] [Google Scholar]; Faure M, Moennoz D, Montigon F, Mettraux C, Breuille D, Ballevre O. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J Nutr. 2005;135(3):486–91. [DOI] [PubMed]
- 45.Hamard A., Mazurais D., Boudry G., Le Huerou-Luron I., Seve B., Le Floc'h N. A moderate threonine deficiency affects gene expression profile, paracellular permeability and glucose absorption capacity in the ileum of piglets. J. Nutr Biochem. 2010;21(10):914–921. doi: 10.1016/j.jnutbio.2009.07.004. [DOI] [PubMed] [Google Scholar]; Hamard A, Mazurais D, Boudry G, Le Huerou-Luron I, Seve B, Le Floc'’h N. A moderate threonine deficiency affects gene expression profile, paracellular permeability and glucose absorption capacity in the ileum of piglets. J Nutr Biochem. 2010;21(10):914–21. [DOI] [PubMed]
- 46.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]; Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–59. [DOI] [PubMed]
- 47.Joyce S.A., MacSharry J., Casey P.G., Kinsella M., Murphy E.F., Shanahan F. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A. 2014;111(20):7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]; Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A. 2014;111(20):7421–6. [DOI] [PMC free article] [PubMed]
- 48.Fagone P., Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta. 2013;1831(3):523–532. doi: 10.1016/j.bbalip.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fagone P, Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta. 2013;1831(3):523–32. [DOI] [PMC free article] [PubMed]
- 49.Li Z., Wu G., van der Veen J.N., Hermansson M., Vance D.E. Phosphatidylcholine metabolism and choline kinase in human osteoblasts. Biochim Biophys Acta. 2014;1841(6):859–867. doi: 10.1016/j.bbalip.2014.02.004. [DOI] [PubMed] [Google Scholar]; Li Z, Wu G, van der Veen JN, Hermansson M, Vance DE. Phosphatidylcholine metabolism and choline kinase in human osteoblasts. Biochim Biophys Acta. 2014;1841(6):859–67. [DOI] [PubMed]
- 50.Watkins B.A., Li Y., Lippman H.E., Feng S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essent Fat Acids. 2003;68(6):387–398. doi: 10.1016/s0952-3278(03)00063-2. [DOI] [PubMed] [Google Scholar]; Watkins BA, Li Y, Lippman HE, Feng S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essent Fat Acids. 2003;68(6):387–98. [DOI] [PubMed]
- 51.Kota V., Hama H. 2’-Hydroxy ceramide in membrane homeostasis and cell signaling. Adv Biol Regul. 2014;54:223–230. doi: 10.1016/j.jbior.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kota V, Hama H. 2’-Hydroxy ceramide in membrane homeostasis and cell signaling. Adv Biol Regul. 2014;54:223–30. [DOI] [PMC free article] [PubMed]
- 52.Norton W.T., Cammer W. Myelin. Springer; 1984. Isolation and characterization of myelin; pp. 147–195. [Google Scholar]; Norton WT, Cammer W. Isolation and characterization of myelin. In: Myelin. Springer; 1984. p. 147–95.
- 53.Zoller I., Meixner M., Hartmann D., Bussow H., Meyer R., Gieselmann V. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci. 2008;28(39):9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zoller I, Meixner M, Hartmann D, Bussow H, Meyer R, Gieselmann V, et al. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci. 2008;28(39):9741–54. [DOI] [PMC free article] [PubMed]
- 54.Kruer M.C., Gregory A., Hayflick S.J. GeneReviews. University of Washington; Seattle: 2012. Fatty acid hydroxylase-associated neurodegeneration. Internet. [PubMed] [Google Scholar]; . Kruer MC, Gregory A, Hayflick SJ. Fatty acid hydroxylase-associated neurodegeneration. GeneReviews [Internet]. University of Washington, Seattle; 2012. [PubMed]
- 55.Kirkpatrick R.M., McGue M., Iacono W.G., Miller M.B., Basu S. Results of a “GWAS plus:” general cognitive ability is substantially heritable and massively polygenic. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112390. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kirkpatrick RM, McGue M, Iacono WG, Miller MB, Basu S. Results of a “GWAS plus:” general cognitive ability is substantially heritable and massively polygenic. PLoS One. 2014;9(11):e112390. [DOI] [PMC free article] [PubMed]
- 56.Alderson N.L., Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T schwannoma cells. J Lipid Res. 2009;50(6):1203–1208. doi: 10.1194/jlr.M800666-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alderson NL, Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T schwannoma cells. J Lipid Res. 2009;50(6):1203–8. [DOI] [PMC free article] [PubMed]
- 57.Guo L., Zhang X., Zhou D., Okunade A.L., Su X. Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J Lipid Res. 2012;53(7):1327–1335. doi: 10.1194/jlr.M025742. [DOI] [PMC free article] [PubMed] [Google Scholar]; Guo L, Zhang X, Zhou D, Okunade AL, Su X. Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J Lipid Res. 2012;53(7):1327–35. [DOI] [PMC free article] [PubMed]
- 58.Whiley L., Sen A., Heaton J., Proitsi P., Garcia-Gomez D., Leung R. Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging. 2014;35(2):271–278. doi: 10.1016/j.neurobiolaging.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Whiley L, Sen A, Heaton J, Proitsi P, Garcia-Gomez D, Leung R, et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer'’s disease. Neurobiol Aging. 2014;35(2):271–8. [DOI] [PMC free article] [PubMed]
- 59.Klavins K., Koal T., Dallmann G., Marksteiner J., Kemmler G., Humpel C. The ratio of phosphatidylcholines to lysophosphatidylcholines in plasma differentiates healthy controls from patients with Alzheimer's disease and mild cognitive impairment. Alzheimers Dement (Amst) 2015;1(3):295–302. doi: 10.1016/j.dadm.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Klavins K, Koal T, Dallmann G, Marksteiner J, Kemmler G, Humpel C. The ratio of phosphatidylcholines to lysophosphatidylcholines in plasma differentiates healthy controls from patients with Alzheimer'’s disease and mild cognitive impairment. Alzheimers Dement (Amst). 2015;1(3):295–302. [DOI] [PMC free article] [PubMed]
- 60.Holmes G.L., Yang Y., Liu Z., Cermak J.M., Sarkisian M.R., Stafstrom C.E. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48(1–2):3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]; Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, et al. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48(1–2):3–13. [DOI] [PubMed]
- 61.Meck W.H., Williams C.L. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27(4):385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]; Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27(4):385–99. [DOI] [PubMed]
- 62.Heaton A.E., Meldrum S.J., Foster J.K., Prescott S.L., Simmer K. Does docosahexaenoic acid supplementation in term infants enhance neurocognitive functioning in infancy? Front Hum Neurosci. 2013;7:774. doi: 10.3389/fnhum.2013.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]; Heaton AE, Meldrum SJ, Foster JK, Prescott SL, Simmer K. Does docosahexaenoic acid supplementation in term infants enhance neurocognitive functioning in infancy? Front Hum Neurosci. 2013;7:774. [DOI] [PMC free article] [PubMed]
- 63.Haution-Bitker M., Gilbert T., Vignoles A., Lecardonnel C., Watelet S., Blond E. Associations between plasmatic polyunsaturated fatty acids concentrations and cognitive status and decline in neurocognitive disorders. J Nutr Heal Aging. 2018;22(6):718–725. doi: 10.1007/s12603-018-1010-z. [DOI] [PubMed] [Google Scholar]; Haution-Bitker M, Gilbert T, Vignoles A, Lecardonnel C, Watelet S, Blond E, et al. Associations between Plasmatic Polyunsaturated Fatty Acids Concentrations and Cognitive Status and Decline in Neurocognitive Disorders. J Nutr Heal Aging. 2018;22(6):718–25. [DOI] [PubMed]
- 64.Nervi F. Elsevier; 2000. Significance of Biliary Phospholipids for Maintenance of the Gastrointestinal Mucosal Barrier and Hepatocellular Integrity. [DOI] [PubMed] [Google Scholar]; Nervi F. Significance of biliary phospholipids for maintenance of the gastrointestinal mucosal barrier and hepatocellular integrity. Elsevier; 2000. [DOI] [PubMed]
- 65.Ehehalt R., Wagenblast J., Erben G., Lehmann W.D., Hinz U., Merle U. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand J Gastroenterol. 2004;39(8):737–742. doi: 10.1080/00365520410006233. [DOI] [PubMed] [Google Scholar]; Ehehalt R, Wagenblast J, Erben G, Lehmann WD, Hinz U, Merle U, et al. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand J Gastroenterol. 2004;39(8):737–42. [DOI] [PubMed]
- 66.Mourelle M., Guarner F., Malagelada J.R. Polyunsaturated phosphatidylcholine prevents stricture formation in a rat model of colitis. Gastroenterology. 1996;110(4):1093–1097. doi: 10.1053/gast.1996.v110.pm8612998. [DOI] [PubMed] [Google Scholar]; Mourelle M, Guarner F, Malagelada JR. Polyunsaturated phosphatidylcholine prevents stricture formation in a rat model of colitis. Gastroenterology. 1996;110(4):1093–7. [DOI] [PubMed]
- 67.Stremmel W., Merle U., Zahn A., Autschbach F., Hinz U., Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54(7):966–971. doi: 10.1136/gut.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54(7):966–71. [DOI] [PMC free article] [PubMed]
- 68.Gupta N.K., Thaker A.I., Kanuri N., Riehl T.E., Rowley C.W., Stenson W.F. Serum analysis of tryptophan catabolism pathway: correlation with Crohn's disease activity. Inflamm Bowel Dis. 2012;18(7):1214–1220. doi: 10.1002/ibd.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gupta NK, Thaker AI, Kanuri N, Riehl TE, Rowley CW, Stenson WF, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn'’s disease activity. Inflamm Bowel Dis. 2012;18(7):1214–20. [DOI] [PMC free article] [PubMed]
- 69.Kosek M.N., Mduma E., Kosek P.S., Lee G.O., Svensen E., Pan W.K.Y. Plasma tryptophan and the kynurenine-tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. Am J Trop Med Hyg. 2016;95(4):928–937. doi: 10.4269/ajtmh.16-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kosek MN, Mduma E, Kosek PS, Lee GO, Svensen E, Pan WKY, et al. Plasma tryptophan and the kynurenine-tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. Am J Trop Med Hyg. 2016;95(4):928–37. [DOI] [PMC free article] [PubMed]
- 70.Kandanearatchi A., Brew B.J. The kynurenine pathway and quinolinic acid: pivotal roles in HIV associated neurocognitive disorders. FEBS J. 2012;279(8):1366–1374. doi: 10.1111/j.1742-4658.2012.08500.x. [DOI] [PubMed] [Google Scholar]; Kandanearatchi A, Brew BJ. The kynurenine pathway and quinolinic acid: pivotal roles in HIV associated neurocognitive disorders. Febs j. 2012;279(8):1366–74. [DOI] [PubMed]
- 71.Rothhammer V., Mascanfroni I.D., Bunse L., Takenaka M.C., Kenison J.E., Mayo L. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97. [DOI] [PMC free article] [PubMed]
- 72.Dekker L.H., Mora-Plazas M., Marin C., Baylin A., Villamor E. Stunting associated with poor socioeconomic and maternal nutrition status and respiratory morbidity in Colombian schoolchildren. Food Nutr Bull. 2010;31(2):242–250. doi: 10.1177/156482651003100207. [DOI] [PubMed] [Google Scholar]; Dekker LH, Mora-Plazas M, Marin C, Baylin A, Villamor E. Stunting associated with poor socioeconomic and maternal nutrition status and respiratory morbidity in Colombian schoolchildren. Food Nutr Bull. 2010;31(2):242–50. [DOI] [PubMed]
- 73.Naylor C., Lu M., Haque R., Mondal D., Buonomo E., Nayak U. Environmental Enteropathy, Oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2(11):1759–1766. doi: 10.1016/j.ebiom.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]; Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, et al. Environmental Enteropathy, Oral Vaccine Failure and Growth Faltering in Infants in Bangladesh. EBioMedicine. 2015;2(11):1759–66. [DOI] [PMC free article] [PubMed]
- 74.Donowitz J.R., Alam M., Kabir M., Ma J.Z., Nazib F., Platts-Mills J.A. A prospective longitudinal cohort to investigate the effects of early life giardiasis on growth and all cause diarrhea. Clin Infect Dis. 2016;63(6):792–797. doi: 10.1093/cid/ciw391. [DOI] [PMC free article] [PubMed] [Google Scholar]; Donowitz JR, Alam M, Kabir M, Ma JZ, Nazib F, Platts-Mills JA, et al. A Prospective Longitudinal Cohort to Investigate the Effects of Early Life Giardiasis on Growth and All Cause Diarrhea. Clin Infect Dis. 2016;63(6):792–7. [DOI] [PMC free article] [PubMed]
- 75.Eppig C., Fincher C.L., Thornhill R. Parasite prevalence and the worldwide distribution of cognitive ability. Proc Biol Sci. 2010;277(1701):3801–3808. doi: 10.1098/rspb.2010.0973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; Eppig C, Fincher CL, Thornhill R. Parasite prevalence and the worldwide distribution of cognitive ability. Proc Biol Sci. 2010;277(1701):3801–8. [DOI] [PMC free article] [PubMed] [Retracted]
- 76.Jiang N.M., Tofail F., Moonah S.N., Scharf R.J., Taniuchi M., Ma J.Z. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr. 2014;14:50. doi: 10.1186/1471-2431-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang NM, Tofail F, Moonah SN, Scharf RJ, Taniuchi M, Ma JZ, et al. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr. 2014;14:50. [DOI] [PMC free article] [PubMed]
- 77.Jiang N.M., Tofail F., Ma J.Z., Haque R., Kirkpatrick B., Nelson C.A. Early life inflammation and neurodevelopmental outcome in Bangladeshi infants growing up in adversity. Am J Trop Med Hyg. 2017;97(3):974–979. doi: 10.4269/ajtmh.17-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jiang NM, Tofail F, Ma JZ, Haque R, Kirkpatrick B, Nelson CA, et al. Early Life Inflammation and Neurodevelopmental Outcome in Bangladeshi Infants Growing Up in Adversity. Am J Trop Med Hyg. 2017;97(3):974–9. [DOI] [PMC free article] [PubMed]
- 78.Kane A.V., Dinh D.M., Ward H.D. Childhood malnutrition and the intestinal microbiome. Pediatr Res. 2015;77(1–2):256–262. doi: 10.1038/pr.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kane A V, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatr Res. 2015;77(1–2):256–62. [DOI] [PMC free article] [PubMed]
- 79.Charbonneau M.R., O'Donnell D., Blanton L.V., Totten S.M., Davis J.C.C., Barratt M.J. Sialylated Milk oligosaccharides promote microbiota-dependent growth in models of infant Undernutrition. Cell. 2016;164(5):859–871. doi: 10.1016/j.cell.2016.01.024. Internet. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]; Charbonneau MR, O'’Donnell D, Blanton L V., Totten SM, Davis JCC, Barratt MJ, et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell [Internet]. 2016;164(5):859–71. Available from: 10.1016/j.cell.2016.01.024 [DOI] [PMC free article] [PubMed]
- 80.Hoffman D.J., Campos-Ponce M., Taddei C.R., Doak C.M. Microbiome, growth retardation and metabolism: are they related? Ann Hum Biol. 2017;44(3):201–207. doi: 10.1080/03014460.2016.1267261. [DOI] [PubMed] [Google Scholar]; Hoffman DJ, Campos-Ponce M, Taddei CR, Doak CM. Microbiome, growth retardation and metabolism: are they related? Ann Hum Biol. 2017;44(3):201–7. [DOI] [PubMed]
- 81.Yang I., Corwin E.J., Brennan P.A., Jordan S., Murphy J.R., Dunlop A. The infant microbiome: implications for infant health and neurocognitive development. Nurs Res. 2016;65(1):76–88. doi: 10.1097/NNR.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs Res. 2016;65(1):76–88. [DOI] [PMC free article] [PubMed]
- 82.Warner B.B. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr Res. 2019;85(2):216–224. doi: 10.1038/s41390-018-0191-9. [DOI] [PubMed] [Google Scholar]; Warner BB. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr Res. 2019;85(2):216–24. [DOI] [PubMed]
- 83.Gera T., Pena-Rosas J.P., Boy-Mena E., Sachdev H.S. Lipid based nutrient supplements (LNS) for treatment of children (6 months to 59 months) with moderate acute malnutrition (MAM): a systematic review. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0182096. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gera T, Pena-Rosas JP, Boy-Mena E, Sachdev HS. Lipid based nutrient supplements (LNS) for treatment of children (6 months to 59 months) with moderate acute malnutrition (MAM): A systematic review. PLoS One. 2017;12(9):e0182096. [DOI] [PMC free article] [PubMed]
- 84.Iannotti L.L., Lutter C.K., Stewart C.P., Gallegos Riofrio C.A., Malo C., Reinhart G. Eggs in early complementary feeding and child growth: a randomized controlled trial. Pediatrics. 2017;140(1) doi: 10.1542/peds.2016-3459. [DOI] [PubMed] [Google Scholar]; Iannotti LL, Lutter CK, Stewart CP, Gallegos Riofrio CA, Malo C, Reinhart G, et al. Eggs in Early Complementary Feeding and Child Growth: A Randomized Controlled Trial. Pediatrics. 2017;140(1). [DOI] [PubMed]
- 85.Iannotti L.L., Lutter C.K., Waters W.F., Andres C., Riofr G., Malo C. 2017. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: A randomized controlled trial in Ecuador; pp. 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]; Iannotti LL, Lutter CK, Waters WF, Andres C, Riofr G, Malo C, et al. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: a randomized controlled trial in Ecuador. 2017;1482–9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of all metabolites associated with four year outcomes with P and r values
Raw and Scaled Variable Importance Scores for All Metabolites Analyzed by Conditional Random Forest
List of Isobaric and Isomeric Lipid Species Measured by the AbsoluteIDQ p180 Kit® (Adapted from https://www.biocrates.com/images/List-of-Isobaric-and-Isomeric-Lipid-Species_v1_2018.pdf)
Lists of metabolites analyzed and all associations with outcome