Abstract
The hippocampus (HPC) is known to play an important role in learning, a process dependent on synaptic plasticity; however, the molecular mechanisms underlying this are poorly understood. ΔFosB is a transcription factor that is induced throughout the brain by chronic exposure to drugs, stress, and variety of other stimuli and regulates synaptic plasticity and behavior in other brain regions, including the nucleus accumbens. We show here that ΔFosB is also induced in HPC CA1 and DG subfields by spatial learning and novel environmental exposure. The goal of the current study was to examine the role of ΔFosB in hippocampal-dependent learning and memory and the structural plasticity of HPC synapses. Using viral-mediated gene transfer to silence ΔFosB transcriptional activity by expressing ΔJunD (a negative modulator of ΔFosB transcriptional function) or to overexpress ΔFosB, we demonstrate that HPC ΔFosB regulates learning and memory. Specifically, ΔJunD expression in HPC impaired learning and memory on a battery of hippocampal-dependent tasks in mice. Similarly, general ΔFosB overexpression also impaired learning. ΔJunD expression in HPC did not affect anxiety or natural reward, but ΔFosB overexpression induced anxiogenic behaviors, suggesting that ΔFosB may mediate attentional gating in addition to learning. Finally, we found that overexpression of ΔFosB increases immature dendritic spines on CA1 pyramidal cells, whereas ΔJunD reduced the number of immature and mature spine types, indicating that ΔFosB may exert its behavioral effects through modulation of HPC synaptic function. Together, these results suggest collectively that ΔFosB plays a significant role in HPC cellular morphology and HPC-dependent learning and memory.
SIGNIFICANCE STATEMENT Consolidation of our explicit memories occurs within the hippocampus, and it is in this brain region that the molecular and cellular processes of learning have been most closely studied. We know that connections between hippocampal neurons are formed, eliminated, enhanced, and weakened during learning, and we know that some stages of this process involve alterations in the transcription of specific genes. However, the specific transcription factors involved in this process are not fully understood. Here, we demonstrate that the transcription factor ΔFosB is induced in the hippocampus by learning, regulates the shape of hippocampal synapses, and is required for memory formation, opening up a host of new possibilities for hippocampal transcriptional regulation.
Keywords: ΔFosB, dendritic spines, hippocampus, learning, transcription
Introduction
Hippocampal synaptic plasticity is critical for memory consolidation, and many immediate early genes (IEGs) engender epigenetic, transcriptional, and direct effector changes that regulate neuroplasticity and memory consolidation, including Zif268, Arc, c-Fos, and FosB (Guzowski, 2002; Shepherd and Bear, 2011; Alberini and Kandel, 2015). IEGs are driven by neural activity and are induced robustly in a variety of regions in response to stress (Cullinan et al., 1995; Kovács, 2008), drugs (Hope et al., 1992; Larson et al., 2010; Guez-Barber et al., 2011), and other environmental stimuli, such as experiential events (Lanahan and Worley, 1998; Guzowski et al., 2001; Stack et al., 2010). ΔFosB is a transcription factor within the Fos family that is known to regulate synaptic plasticity in brain reward regions, such as the nucleus accumbens (NAc), prefrontal cortex, and ventral tegmental area (VTA; Nestler et al., 1999; Robison and Nestler, 2011). It is unique among the IEGs in that it is stably induced by chronic stimuli, such as repeated stress (Nikulina et al., 2008; Nestler, 2015), chronic antidepressant treatment (Vialou et al., 2010; Thibault et al., 2014), and repeated administration to drugs of abuse (Nestler et al., 1999; Perrotti et al., 2008; Robison and Nestler, 2011). Whereas the induction of most Fos family proteins is transient, ΔFosB has a half-life of ∼8 d in vivo (Carle et al., 2007; Ulery-Reynolds et al., 2009), making it an ideal candidate mechanism for long-term changes in gene expression resulting from chronic stimuli.
In the NAc, ΔFosB is known to regulate gene transcription of key target molecules associated with cellular growth and synaptic plasticity, including GluA2 receptor (Kelz et al., 1999), calcium/calmodulin-dependent kinase II (CaMKII; Robison et al., 2013), nuclear factor-κB (Ang et al., 2001), and cyclin-dependent protein kinase 5 (Cdk5; Bibb et al., 2001). Considering the nature of these targets, it is not surprising that ΔFosB regulates the number and morphology of NAc dendritic spines (Maze et al., 2010; Grueter et al., 2013), as well as NAc synaptic strength and the apparent number of silent synapses (Grueter et al., 2013). Although many of these gene targets are also critical to hippocampal synaptic plasticity and morphology, as well as spatial learning, the role of ΔFosB in these processes remains unknown.
Previous evidence indicates that ΔFosB is induced robustly in the hippocampus by drugs of abuse (Perrotti et al., 2008), and we reported recently that it is also induced in the hippocampus by antidepressants (Vialou et al., 2015). Importantly, ΔFosB induction in the NAc is dependent on the function of the transcription factors cAMP response element-binding protein (CREB) and serum response factor (SRF; Vialou et al., 2012), both of which are essential for hippocampal function and are induced by spatial learning (Mizuno et al., 2002; Porte et al., 2008; Alberini and Kandel, 2015). Moreover, genetic knock-out of the FosB gene causes malformation of the hippocampus and impairment in spatial memory (Solecki et al., 2008; Yutsudo et al., 2013). However, because FosB is an IEG and important for development (Redemann-Fibi et al., 1991), the effects of hippocampal ΔFosB on learning and memory are impossible to ascertain using FosB null mutants, because the genetic deletion of FosB impairs early hippocampal development and adult neurogenesis (Yutsudo et al., 2013). Therefore, we sought to use temporally and spatially discrete viral-mediated manipulation of ΔFosB expression and activity to assess the role of ΔFosB in the adult hippocampus. Thus, the goal of the present study is to characterize the induction of hippocampal ΔFosB by learning and to identify any role of ΔFosB in hippocampal cell morphology or learning and memory.
Materials and Methods
Animals.
The study followed guidelines described in the Guide for the Care and Use of Laboratory Animals, eighth edition (Institute of Laboratory Animal Resources, 2011). Before any testing, all experimental procedures were approved by the Institutional Animal Care and Use Committee at Michigan State University or at the Mount Sinai School of Medicine. After arrival to the facility, male mice (n = 4 per cage) or rats (n = 2 per cage) were group housed for at least 3 d before experimentation in a 12 h light/dark cycle with ad libidum food and water. Animals were weighed and briefly handled daily before and throughout the study.
Surgery.
Stereotaxic surgery was performed to inject viral vectors into the hippocampus of adult male mice. Thirty gauge needles (Hamilton Company) were bilaterally placed at the following coordinates: 10° angle; −2.2 mm anteroposterior (AP); ±2.5 mm mediolateral. Purified high-titer virus (0.6 μl) was infused separately (0.3 μl/infusion) over 3 min periods at two sites: −2.1 mm dorsoventral (DV) and −1.9 mm DV. After both infusions, the needles remained at the injection site for a 5 min after infusion to allow diffusion of the viral particles. Previously validated (Robison et al., 2013) viral vectors included the following: adeno-associated virus (AAV2) expressing GFP alone (AAV–GFP), AAV expressing GFP and ΔFosB (AAV–ΔFosB), AAV expressing GFP and ΔJunD (AAV–ΔJunD), herpes simplex virus (HSV) expressing GFP alone (HSV–GFP), HSV expressing GFP and ΔFosB (HSV–ΔFosB), or HSV expressing GFP and ΔJunD (HSV–ΔJunD). HSVs were obtained from the Massachusetts Institute of Technology Viral Core Facility, and AAVs were obtained from the University of North Carolina Vector Core.
Open-field activity.
The open-field (OF) apparatus consisted of a custom-made, square white polyvinylchloride foam box (38 × 38 × 35 cm). Animals were tested for 30 min, and activity was recorded with a digital CCD camera connected to a computer running an automated video tracking software package (Clever Sys).
Novel object recognition.
Novel object recognition (NOR) was assessed using a 3 d paradigm including habituation, training, and testing. Mice were first habituated to the OF for 1 h, and OF activity was recorded at this time. Twenty-four hours later, two similar objects were placed near the corners of the square apparatus, and animals were allowed to explore the apparatus for 30 min. Objects pairs consisted of metal and plastic objects, including Lego blocks, miniature action figures, spark plugs, and metal knobs. Mice did not show consistent biases for any one object over another. The following day, mice were tested for NOR. One object was removed and replaced with another dissimilar object, and mice were allowed to freely explore the apparatus for 5 min. Behavior was video recorded, and time spent in the 7 cm square corners around the objects was assessed.
Contextual fear conditioning.
Contextual fear conditioning (FC) consisted of a 2 d procedure: conditioning and testing. For conditioning, mice were placed into an operant chamber equipped with a house light, metal grid floor, and footshock stimulus generator (Coulbourn Instruments) for 180 s. This was followed by the delivery of three mild electric footshocks (0.8 mA, 1 s duration), each separated by a 60 s intershock interval in which no shock was delivered. Mice remained in the chamber for another 60 s after the last delivery. Twenty-four hours after conditioning, mice were placed back into the conditioning chamber for 8 min. The percentage of time spent freezing was video scored by a blind, independent observer. Freezing was defined as the lack of skeletal movement for periods >1 s.
Temporally dissociated passive avoidance learning paradigm.
Temporally dissociated passive avoidance (TDPA) was assessed across 5 d and was performed as described previously (Zhang et al., 2008) with slight modifications. During each daily session, the mouse was placed into the lit half of a light–dark passive avoidance chamber (Coulbourn Instruments) and allowed to explore for 1 min before a door was opened to allow entry into the dark half of the chamber. After entry into the dark half, the door was closed and a 5 min dissociation period was allowed to pass before delivery of a mild electric footshock (0.8 mA, 2 s duration). The animal was returned to its home cage 30 s after the footshock. The crossover latency (in seconds) to enter the dark half after door opening was scored across daily sessions as an index of learning. Maximal latency was set at 600 s.
Elevated plus maze.
Elevated plus maze (EPM) behavior was measured using a custom-built apparatus (aluminum base with polyvinylchloride arms) based on plans from ANY-maze (www.anymaze.com; Stoelting). The maze was laid out in the shape of a plus sign as viewed from above. It consisted of four gray-colored, interconnected runways (5 × 35 cm) with two open and two closed arms, elevated 45 cm from the ground. Closed arms were enclosed on the long sides of the runway by two 15 cm high gray walls. Animals were placed into the center of the maze facing an open arm, and behavior was video recorded as described above for 5 min. The percentage of time spent in the open arms and the percentage of entries into the open arms were quantified as assessments of anxiety-like behavior.
Two-bottle choice for sucrose preference.
A standard two-bottle choice procedure was assessed across 4 d. Singly housed mice were first given access to two bottles of drinking water on the top of their home cage for 3 d to assess baseline drinking and to acclimate the mice to the bottles. Then, mice were given two-bottle choice, in which one of the bottles was replaced with a 1% sucrose solution. Sucrose solution consumed per day as a percentage of total liquid consumption was measured as an index of anhedonia.
Morris water maze.
The rat Morris water maze (MWM) was performed essentially as described previously (Morris et al., 1982). The pool of water (∼1.3 m diameter, 26°C) was made opaque with nontoxic white tempera paint, and platforms (8 cm diameter) were placed 0.3 m from the wall either 1 cm below the surface (hidden) or 2 cm above the surface (visible). Rats were given six training sessions per day for 5 consecutive days, and a probe trial was performed on day 6. If a rat failed to find the hidden platform in 60 s, it was placed onto the platform. Rats remained on the platform for 15 s to allow spatial orientation at the end of each trial. A computerized tracking system (Datawave Technologies) recorded and analyzed swim-path data, including latency to reach the platform (in seconds), time in each quadrant, and the average swim speed.
Mice were assayed in the MWM as described previously (Zhang and Wang, 2013). Briefly, behavior was measured using an automated video-tracking system (WaterMaze; Coulbourn Instruments) in a water-filled pool with the surface covered with white polypropylene beads and a platform. Procedures included visible platform training (six trials, day 1), hidden platform training (four trials per day; days 2–6), and a probe test (day 7). During visible platform training, a flag was attached to the platform and placed randomly at different locations for each trial. During hidden platform training, the flag was removed and the platform was always in the same location across trials. Trials lasted 60 s or when the mouse reached the platform, whichever came first. Intertrial intervals lasted 60 min. Trial latencies and swim speed (centimeters per second) were recorded during training. During the probe test, the platform was removed and mice were allowed to freely explore the maze for 60 s. The time spent in each quadrant was recorded.
Novel environment exposure.
Mice were removed from their home cage and placed in a novel enriched environment along with their cage mates (five mice per cage) for 60 min/d for 10 consecutive days. The novel environment consisted of a round polycarbonate chamber 48 inches across with 8-inch walls containing four equal connected subdivisions, each of which contained different novel objects similar to those used for the NOR test (described above). Low lighting was used, and the experimenter stayed outside of the room to minimize stress.
Western blotting for ΔFosB.
Brains were extracted rapidly on cold ice and then sliced into 1 mm sections, and the dorsal hippocampus was removed with a 12 gauge punch and frozen immediately on dry ice. Samples were then processed for SDS-PAGE and transferred to PVDF membranes for Western blotting with chemiluminescence. Blots were probed for FosB (5G4; 1:500; Cell Signaling Technology) and assayed for total protein using Swift Membrane Stain (G Biosciences). Protein was quantified using NIH ImageJ software.
Immunohistochemical assay for FosB.
Animals were perfused transcardially with ice-cold PBS, followed by 10% Formalin. Brains were postfixed 24 h in 10% Formalin, cryopreserved in 30% sucrose, and then sliced into 35 μm sections. Immunohistochemistry was performed using anti-FosB primary antibody (sc-48; 1:500; Santa Cruz Biotechnology) and visualized by 3,3′-diaminobenzidine (DAB) staining (Vector Laboratories).
Immunofluorescence for FosB and JunD.
Animals were perfused and brains sliced as above. Immunofluorescence was performed using mouse anti-FosB (ab11959; 1:500; Abcam) or mouse anti-JunD (ab28837; 1:500; Abcam) and a goat anti-GFP (ab5450; 1:1000; Abcam) primary antibody and corresponding secondary antibodies (705-545-147 and 711-165-152; 1:200; Jackson ImmunoResearch). Fluorescent images were visualized on an Olympus FluoView 1000 filter-based laser scanning confocal microscope.
Spine analysis.
Animals were perfused as above, and brains were sliced into 100 μm sections. Sections were mounted on slides using DPX mounting medium (Sigma-Aldrich), and GFP fluorescence was visualized using an Olympus FluoView 1000 filter-based laser scanning confocal microscope. Spines were analyzed essentially as described previously (Christoffel et al., 2011). Briefly, dendritic segments 50–150 μm away from the soma were chosen randomly from HSV-infected cells that express GFP. Images were acquired on a confocal LSM 710 (Carl Zeiss) for morphological analysis using NeuronStudio with the rayburst algorithm. NeuronStudio classifies spines as thin, mushroom, or stubby based on the following values: (1) aspect ratio; (2) head/neck ratio; and (3) head diameter. Spines with a neck can be classified as either thin or mushroom, and those without a significant neck are classified as stubby. Spines with a neck are labeled as thin or mushroom based on head diameter.
Statistical analysis.
All statistical analyses were performed using Prism software (GraphPad Software). Anxiety phenotypic behavior (EPM and OF activity), MWM probe test performance (in mice), contextual FC freezing, protein quantification, and dendritic spine number were analyzed using Student's t tests. MWM training performance (in rats) was analyzed using one-way repeated-measures (RM) ANOVA, followed by Holm–Sidak corrected post hoc comparisons for simple effects. Sucrose preference (percentage), NOR, MWM training performance (mice), TDPA latency, and body mass were analyzed using two-way RM ANOVAs. This was followed by Holm–Sidak-corrected post hoc comparisons for simple effects. Alpha criterion of 0.05 was set for all analyses.
Results
Induction of hippocampal ΔFosB by novel environmental exposure and spatial learning
A variety of stimuli have been reported to induce ΔFosB in many brain regions, including the hippocampus (Nikulina et al., 2008; Robison and Nestler, 2011; Nestler, 2015), but it is unknown whether exposure to complex environments and spatial learning, the primary function of the rodent hippocampus, induces ΔFosB in this region. Therefore, we exposed mice to a novel, enriched environment for 60 min/d for 10 d and compared the expression of ΔFosB in the hippocampus with that of animals remaining in their home cages. As expected, exposure to the novel environment significantly induced ΔFosB protein in the dorsal hippocampus (Fig. 1A; t(15) = 2.90, p = 0.011).
Figure 1.
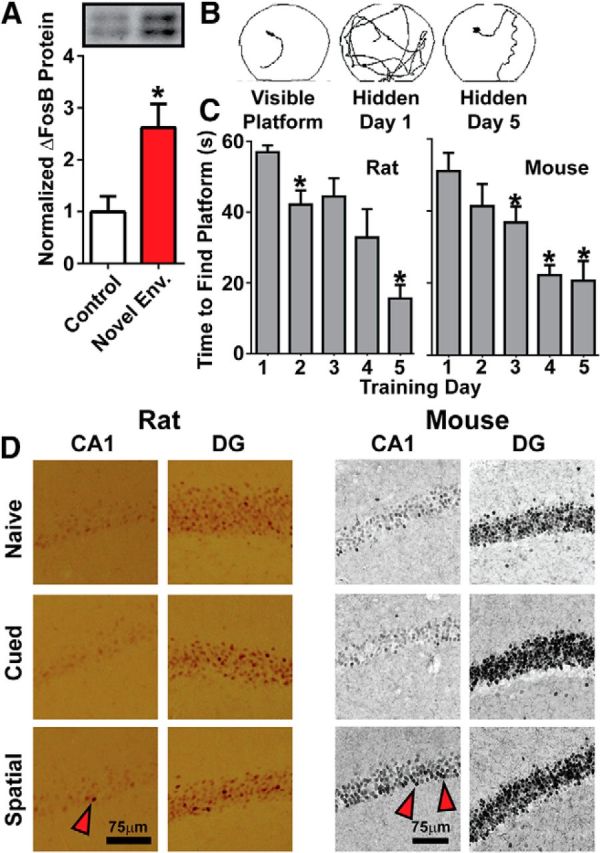
Induction of ΔFosB in the hippocampus by novelty exposure and spatial learning. A, Quantitation and representative Western blots (inset) of ΔFosB in the hippocampus show significant increases after novel environmental exposure (red; n = 9) compared with mice left in their home cage (white; n = 8; *p < 0.05). B, Representative paths of rats seeking a visible (cued) or hidden (spatial) platform in the MWM test. C, Escape latency from the MWM in rats (left) and mice (right) using a hidden platform significantly decreased across training days (n = 6–8; *p < 0.05 compared to day 1). D, Representative 40× images of coronal sections stained for FosB gene products from naive, cued learning, and spatial learning rats (left) and mice (right). Red arrowheads indicate FosB+ pyramidal cells in CA1.
We also aimed to ascertain whether specific spatial learning could induce ΔFosB and whether this effect was species specific. Rats or mice were trained on a standard MWM paradigm in cued learning using a visible platform, which does not require the dorsal hippocampus, or in spatial learning using a nonvisible platform, which requires the use of the hippocampus to learn orientation based on spatial cues around the maze (Vorhees and Williams, 2006). Animals readily acquired the ability to locate the hidden platform across days, showing a decrease in escape latency each day (Fig. 1B, rats: F(2.122,10.61) = 7.864, p = 0.0075; significant on days 2 and 5 by RM ANOVA, followed by Holm–Sidak multiple comparison test; Fig. 1C, mice: F(4.000,28.00) = 13.92, p < 0.0001; significant on days 3–5). Immunohistochemical analysis for FosB-positive (FosB+) expressing (FosB/ΔFosB) cells in the hippocampus (Fig. 1D) revealed induction of FosB+ cells in the CA1 of spatial learning rats compared with naive animals and cued learning animals (Table 1). Because tissue was extracted 1 d after the last training, it is likely that the FosB+ cells represent the presence of ΔFosB, because full-length FosB expression is transient, i.e., <24 h (Carle et al., 2007). We also noted that the pattern of FosB+ cells in the CA1 region of spatial learning animals was sparse, with only a few very dark cells (Fig. 1D, red arrow), possibly consistent with the encoding of individual memories during learning. We also observed that both spatial and cued learning increased FosB+ cells in the dentate gyrus (DG) of both mice and rats (Table 1), perhaps indicating that the stress of exposure to the MWM or the novel environment of the testing chamber is sufficient to induce ΔFosB in this region. This is unsurprising, because we have reported previously that chronic stress can induce ΔFosB in the DG (Vialou et al., 2014, 2015), and we saw a similar induction of ΔFosB in the rat NAc (Table 1), a region in which ΔFosB is also induced by stress (Vialou et al., 2010, 2014, 2015). Induction in the CA3 region was inconsistent between species and not correlated with swimming stress (Table 1), indicating that ΔFosB in CA3 may be unrelated to learning or stress. These findings collectively suggest that exposure to a novel environment and the process of spatial learning robustly induce ΔFosB in the hippocampus.
Table 1.
Induction of ΔFosB by spatial and cued learning
| Brain region | Control cells/mm2 (mean ± SEM) (n) | Cued cells/mm2 (mean ± SEM) (n) | Spatial cells/mm2 (mean ± SEM) (n) | F (one-way ANOVA) | p (one-way ANOVA) |
|---|---|---|---|---|---|
| Rats | |||||
| CA1 | 2.10 ± 0.66 (5) | 2.88 ± 0.43 (4) | 5.10 ± 1.00 (5)* | F(2,11) = 4.316 | 0.0413 |
| CA3 | 3.50 ± 0.67 (5) | 6.00 ± 1.02 (4) | 4.90 ± 0.91 (5) | F(2,11) = 2.038 | 0.176 |
| DG | 17.70 ± 0.75 (5) | 30.88 ± 4.04 (4)* | 40.10 ± 4.05 (5)* | F(2,11) = 13.02 | 0.0013 |
| NAc | 58.17 ± 7.15 (6) | 93.42 ± 4.17 (6)* | 89.83 ± 7.95 (6)* | F(2,15) = 8.582 | 0.0033 |
| Mice | |||||
| CA1 | 69.15 ± 11.0 (8) | 92.64 ± 12.7 (8) | 143.9 ± 19.5 (7)* | F(2,20) = 6.752 | 0.0057 |
| CA3 | 533.7 ± 60.2 (8) | 844.9 ± 70.9 (8)* | 736.1 ± 56.3 (7) | F(2,20) = 6.448 | 0.0069 |
| DG | 1222 ± 67.4 (8) | 1893 ± 83.8 (8)* | 1586 ± 131.9 (7)* | F(2,20) = 13.08 | 0.0002 |
FosB+ cells as visualized by DAB staining (Fig. 1) in control, cued learning, and spatial learning mice and rats by brain region. Statistics represent one-way ANOVA with Tukey's multiple comparison post hoc test used to compare conditions.
*p < 0.05, significant difference from the control group.
Transcriptional silencing of hippocampal ΔFosB impairs learning and memory
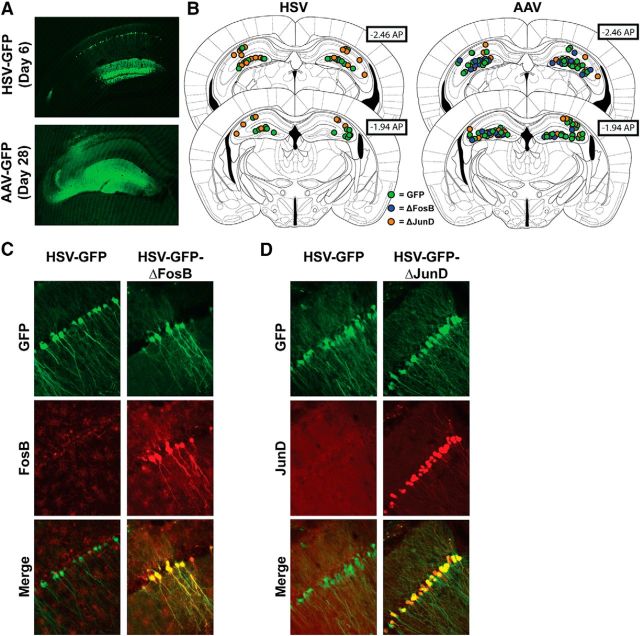
Because learning was found to induce ΔFosB, we hypothesized that silencing ΔFosB-mediated activator protein-1 transcriptional activity in the hippocampus would produce spatial learning and memory impairment. We used viral vector-mediated (AAV2 or HSV) expression of GFP and ΔFosB, GFP and the ΔFosB inhibitor ΔJunD, or GFP alone (as control), targeted to the dorsal hippocampus of mice, and examined behavior using a battery of tests that are associated with hippocampal function. Using AAV, gene transfer of GFP was detected in both pyramidal and nonpyramidal cells in CA3 and CA1 and granule and nongranule cells in DG subregions of the hippocampus (Fig. 2A), whereas HSV expression was more clearly restricted to pyramidal and granule cells (Fig. 2A,C,D), as has been reported previously (Burger et al., 2004). We validated the specific overexpression of ΔFosB in transduced neurons by immunofluorescence (Fig. 2C) and noted that basal expression of FosB was present in pyramidal neurons and in microglia likely activated by the injection-related tissue damage, as suggested by recent findings (Nomaru et al., 2014). We also validated the viral overexpression of ΔFosB by quantitative PCR of tissue punches from injected hippocampus and found a 271.2 ± 74.5-fold increase in ΔFosB message in tissue transduced with HSV–GFP–ΔFosB over tissue transduced with HSV–GFP alone (t(13) = 3.9, p = 0.0018), which is not unexpected when using the cytomegalovirus promoter (Papadakis et al., 2004).
Figure 2.
Viral-mediated gene transfer in the hippocampus. A, Representative fluorescent coronal images of the hippocampus (approximately −2.0 mm AP) 6 d after injection of HSV–GFP or 28 d after injection of AAV–GFP. B, Coronal figures (−1.94 and −2.46 AP; Paxinos and Franklin, 2012) showing representative center fluorescence distribution from GFP (green circles), GFP–ΔJunD (orange circles), or GFP–ΔFosB (blue circles) infusions. Immunofluorescence of CA1 neurons close to the injection site of HSV–GFP and HSV–GFP–ΔFosB (C) or HSV–GFP–ΔJunD (D) reveals specific transgene expression only in cells induced with virus.
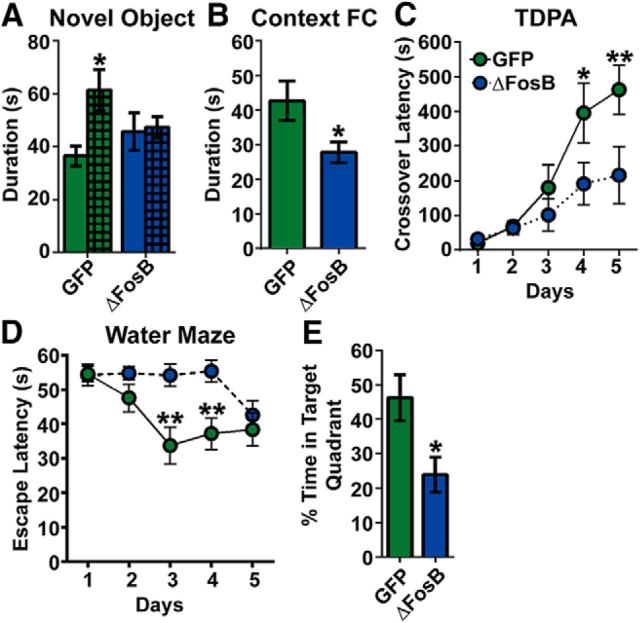
We specifically examined learning and memory behavior across a battery of tests including: NOR memory, contextual FC freezing behavior, TDPA learning, and spatial learning in the MWM. In the NOR task (Fig. 3A), as expected, control mice (AAV–GFP) spent more time with a novel object than a familiar one (t(7) = 2.54, p = 0.038). However, mice that received AAV–GFP–ΔJunD spent equal time with both objects, indicating impairment in recognition memory (p > 0.05). We next assessed memory for contextual fear in the same group of mice (Fig. 3B). We found that mice expressing ΔJunD spent less time freezing compared with GFP controls (t(14) = 2.41, p = 0.030), indicating impairment in memory for contextual fear.
Figure 3.
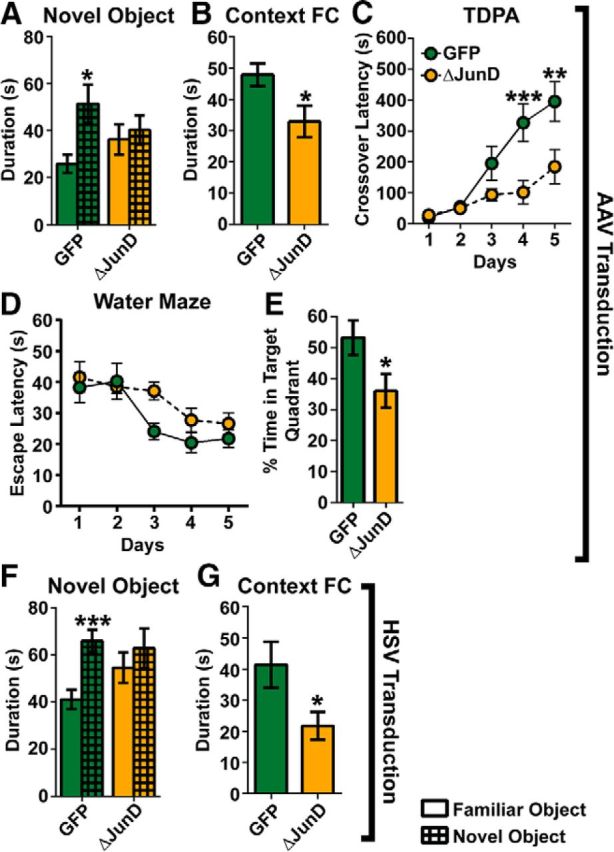
Transcriptional silencing of hippocampal ΔFosB impairs learning and memory. A, NOR in mice that received stereotaxic hippocampal injections of AAV–GFP (GFP, green; n = 8) and AAV–GFP–ΔJunD (ΔJunD, orange; n = 8). The duration of time spent with a familiar object (blank bars) and a novel object (crosshatched bars) was measured. GFP mice spent significantly more time with the novel object than the familiar object (*p < 0.05 compared with familiar), whereas the ΔJunD mice spent an equal amount of time with both objects. B, Contextually fear conditioned (Context FC) freezing (seconds) in AAV–GFP (n = 8) and AAV–GFP–ΔJunD (n = 8) mice. ΔJunD significantly decreased contextual freezing (*p < 0.05 compared with GFP). C, TDPA performance was measured in mice that received AAV–GFP (n = 16) or AAV–GFP–ΔJunD (n = 16). Latency (seconds) to crossover to the shock-paired dark side of the box was measured. ΔJunD mice had significantly lower crossover latency at days 4–5 (**p < 0.01, ***p < 0.001, respectively, compared with GFP). D, Spatial learning in the MWM was measured after AAV–GFP (n = 8) or AAV–GFP–ΔJunD (n = 8). Escape latency (seconds) did not differ between groups across training days. E, The probe test revealed that ΔJunD mice spent significantly less time in the target quadrant (*p < 0.05 compared with GFP). F, Mice received stereotaxic hippocampal infusions of HSV–GFP (n = 16) or HSV–GFP–ΔJunD (n = 15). GFP mice spent significantly more time with the novel object than the familiar object (*p < 0.001 compared with familiar), whereas the ΔJunD mice spent an equal amount of time with both objects. G, Context FC freezing after HSV–GFP (n = 8) and HSV–GFP–ΔJunD (n = 8). ΔJunD mice spent significantly less time freezing (*p < 0.05 compared with HSV–GFP). Error bars indicate mean ± SEM.
TDPA learning (Fig. 3C) was assessed in a separate cohort of mice (n = 16 per group) to test context-associative fear learning using a paradigm that has been demonstrated previously to be impaired by hippocampal lesions (Zhang et al., 2008). We observed that, although both GFP and ΔJunD groups increased their latency to cross over to the footshock-associated “dark” side over time (Fday(4,120) = 21.24, p < 0.001), ΔJunD mice were significantly impaired (Fgroup(1,30) = 8.58, p = 0.007; Fday × group(4,120) = 5.22, p = 0.001) in the formation of this association between the dark side of the apparatus and the pain of the footshock. Specifically, ΔJunD mice had significantly attenuated crossover latency at days 4 and 5 (p = 0.001 for both days), indicating that hippocampal ΔFosB transcriptional activity is required for passive avoidance learning.
Spatial learning was assessed in another cohort by performance in the MWM. Escape latencies decreased across days of hidden platform training for both groups (Fig. 3D; F(4,56) = 8.44, p < 0.001), but only nonsignificant differences were observed between groups (p > 0.05). Swim speed also decreased across days for both groups (data not shown; F(4,56) = 12.3, p < 0.001), but no group differences were observed (p > 0.05). A probe test was then conducted with the platform removed. When measured in the probe test, ΔJunD-expressing mice exhibited longer latency to find the target area where the platform had been (t(14) = 3.56, p = 0.003) and spent significantly less time in the target quadrant compared with GFP control mice (Fig. 3E; t(14) = 2.21, p = 0.044). In addition, ΔJunD mice had fewer crossings over the (previous) location of the hidden platform (data not shown; t(14) = 2.23, p = 0.043). These data suggest that ΔFosB transcriptional activity in the hippocampus is required for spatial learning and reference memory.
Because AAV-mediated gene transfer requires up to 2 weeks to reach peak expression and thus experiments are performed 3 weeks after surgery, it is possible that the effects of AAV-mediated silencing of ΔFosB transcriptional activity are attributable to homeostatic adaptations to long-term changes in ΔFosB-mediated gene expression (Janson et al., 2001). To determine the acute effects of silencing ΔFosB transcriptional activity, we used HSV-mediated gene transfer, which begins within hours of surgery and lasts 5–7 d, allowing behavioral assessment within 2–5 d of surgery (Fig. 2). Using a cohort of mice that received hippocampal injections of HSV–GFP or HSV–GFP–ΔJunD we found that, in the NOR test, control GFP mice spent more time with a novel object (Fig. 3F; t(15) = 4.36, p = 0.001), whereas HSV–GFP–ΔJunD mice did not (p > 0.05). Similarly, when we tested context FC behavior, we found that HSV–GFP–ΔJunD mice spent less time freezing compared with GFP mice (Fig. 3G; t(14) = 2.28, p = 0.039). Thus, these data were consistent with AAV results and suggest that memory formation requires acute ΔFosB-mediated changes in hippocampal gene expression.
Overexpression of ΔFosB in hippocampus also induces impairments in learning and memory
Spatial memories must be distinct to be recalled without interference, and recent studies indicate that discrete hippocampal networks allow for storage of large quantities of uncorrelated spatial information (Moser et al., 2015). In fact, in rodents, a spatial location or memory can be linked directly to the plasticity of single cells or small groups of cells within the hippocampal–entorhinal circuit (Moser et al., 2015), suggesting that changes in gene expression mediating that plasticity must be equally discrete. Therefore, we examined how indiscriminate viral overexpression of ΔFosB in hippocampus would affect learning and memory. We again used AAV to overexpress ΔFosB (or GFP as control) in the hippocampus of mice, and mice were tested across the same battery of learning tasks. As expected, control GFP mice expressing only GFP spent more time with a novel object (Fig. 4A; t(26) = 2.89, p = 0.008), but mice overexpressing ΔFosB were significantly impaired in recognition of the novel object (p > 0.05). In context FC, ΔFosB-overexpressing mice spent significantly less time freezing than GFP control mice (Fig. 4B; t(14) = 2.32, p = 0.036). These results indicate that overexpression of ΔFosB, similar to silencing ΔFosB, impairs recognition and contextual fear memory.
Figure 4.
Overexpression of ΔFosB in the hippocampus impairs learning and memory. A, NOR in mice that received stereotaxic hippocampal injections of AAV–GFP (GFP, green; n = 14) or AAV–GFP–ΔFosB (ΔFosB, blue; n = 16). GFP mice spent significantly more time with the novel object than the familiar object (*p < 0.05 compared with familiar), whereas the ΔFosB mice spent an equal amount of time with both objects. B, Contextually fear conditioned (Context FC) freezing (seconds) in AAV–GFP (n = 8) and AAV–GFP–ΔFosB (n = 8) mice. ΔFosB mice had significantly greater contextual freezing (*p < 0.05 compared with GFP). C, TDPA performance was measured in mice that received AAV–GFP (n = 15) or AAV–GFP–ΔFosB (n = 16). ΔFosB mice had significantly lower crossover latency at days 4–5 (*p < 0.05, **p < 0.01, respectively, compared with GFP). D, Spatial learning in the MWM was measured after AAV–GFP (n = 7) or AAV–GFP–ΔFosB (n = 7). Escape latency (seconds) was significantly decreased by ΔFosB at days 3–4 (**p < 0.01 for both days compared with GFP). E, Probe test quadrant time revealed that ΔFosB mice spent significantly less time in the target quadrant (*p < 0.05 compared with GFP). Error bars indicate mean ± SEM.
In TDPA, we replicated findings from the ΔJunD experiments (Fig. 4C). Both GFP control and ΔFosB-overexpressing mice increased their crossover latency (Fday(4,84) = 17.27, p < 0.001). However, ΔFosB overexpression produced impairment, i.e., reduction, in crossover latency (Fgroup(1,21) = 4.41, p = 0.048; Fday × group(4,84) = 3.06, p = 0.021), specifically at days 4 (p = 0.038) and 5 (p = 0.010). The effects of hippocampal ΔFosB overexpression on spatial learning were assessed in a separate cohort in the MWM. Escape latency (Fig. 4D) decreased in the GFP control group (Fgroup(1,12) = 7.00, p = 0.021), with significant differences between GFP controls and ΔFosB overexpressing mice at days 3 and 4 (Fday × group(4,48) = 4.39, p = 0.004; p = 0.002 for day 3; p = 0.005 for day 4). Additionally, although trial speed decreased across days for both groups (data not shown; Fday(4,48) = 22.31, p < 0.001), ΔFosB-overexpressing mice were significantly slower (Fgroup(1,12) = 7.53, p = 0.018), specifically at days 2–4 compared with GFP controls (Fday × group(4,48) = 4.05, p = 0.007; p < 0.05 for days 2–4). During the probe test, no differences were observed in latency to target (p > 0.05), but ΔFosB-overexpressing mice spent significantly less time in the target quadrant (Fig. 4E; t(12) = 2.67, p = 0.021). No differences were observed between groups in the number of crossings over the (previous) location of the hidden platform (data not shown; p > 0.05). These findings, along with the results from NOR and contextual fear memory, collectively indicate that nonselective overexpression of ΔFosB in the hippocampus impairs learning and memory. This further suggests that discrete expression of ΔFosB in cells directly encoding a given memory and only in those cells is required for proper learning.
Overexpression, but not silencing, of ΔFosB in hippocampus induces anxiety-like behavior
ΔFosB transcriptional activity in the NAc is linked to mood and anxiety (Vialou et al., 2010), and the hippocampus is associated directly with maladaptive stress responses, leading to depression and/or anxiety disorders (Sapolsky, 2001; Campbell and Macqueen, 2004; Shin and Liberzon, 2010). Therefore, we assessed whether silencing ΔFosB transcriptional activity in the hippocampus would lead to anxiety and depressive phenotypes. Mice that received AAV–GFP, AAV–GFP–ΔJunD, or AAV–GFP–ΔFosB were assayed across a battery of tests to examine anxiety-like and depressive phenotypes, including OF behavior, EPM, sucrose preference, and body weight. Thigmotaxis (e.g., avoidance of the center) behavior in the OF was found to be different between groups. ΔFosB overexpression, but not ΔJunD expression, decreased the time spent in the center of the OF (Fig. 5A; F(2,29) = 9.12, p < 0.001). No differences were observed in center entries (Fig. 5B; p > 0.05). Activity, e.g., distance moved, was also unaffected by ΔFosB overexpression or silencing (Fig. 5C; p > 0.05), indicating that locomotor activity is unaltered by hippocampal ΔFosB. Similar to the OF test, behavior in the EPM showed an anxiogenic phenotype after ΔFosB overexpression. Specifically, ΔFosB overexpression, but not ΔJunD expression, decreased both the time spent in the open arms (Fig. 5D; F(2,29) = 3.59, p = 0.040; GFP vs ΔFosB, p = 0.025) and entries into the open arms (Fig. 5E; F(2,29) = 6.64, p = 0.004; GFP vs ΔFosB, p = 0.004). No differences were observed in activity, e.g., distance moved, in the EPM (Fig. 5F; p > 0.05). These results suggest collectively that indiscriminate hippocampal ΔFosB overexpression, but not silencing of ΔFosB transcriptional activity, induces anxiety-like behavior.
Figure 5.
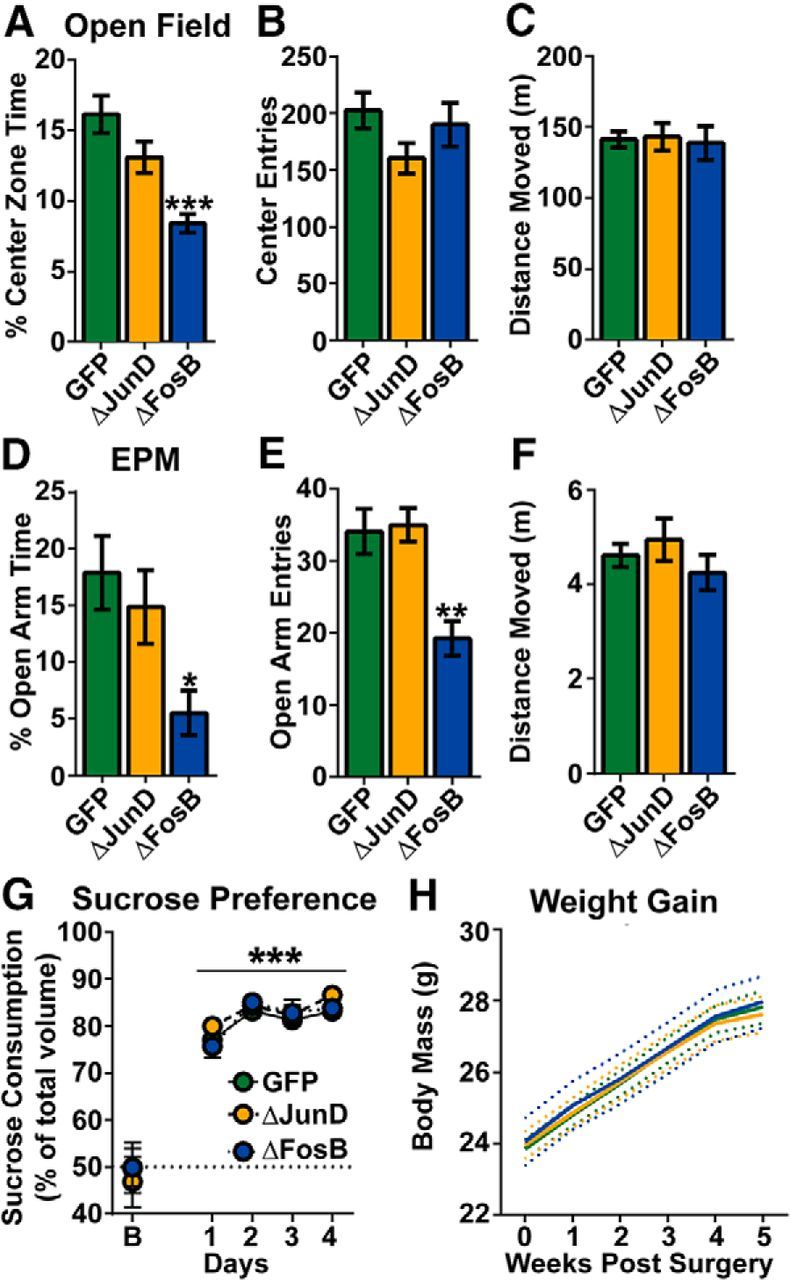
Overexpression, but not transcriptional silencing, of ΔFosB in hippocampus produces anxiety-like behavior. A, OF behavior was assessed in AAV–GFP (green; n = 16), AAV–GFP–ΔJunD (orange; n = 8), and AAV–GFP–ΔFosB (blue, n = 8) mice. Center zone time (percentage) was reduced significantly by ΔFosB (*p < 0.001 compared with GFP), but no differences were observed between GFP and ΔJunD. B, Center zone entries were not different between groups. C, Locomotor activity in an OF, measured by distance moved (meters), was not different between groups. D, Open arm time (percentage) in an EPM was decreased in mice that received ΔFosB (*p < 0.05 compared with GFP). E, Open arm entries were also reduced in mice that received ΔFosB (**p < 0.01 compared with GFP). F, Distance moved (meters) in the EPM was not different between groups. G, Preference for sucrose (percentage) increased in all groups compared with a sucrose-free baseline (***p < 0.001 compared with baseline; B). No group differences were observed in sucrose preference. H, Body weight (grams) was measured across weeks after AAV surgery (n = 15–16 per group). Although mice steadily increased total body weight, no differences were observed between groups (dotted lines represent 95% confidence interval). Error bars indicate mean ± SEM.
Anhedonia, such as decreased preference for natural rewards, and perturbations in body weight are hallmarks of clinical depression and can be modeled in mice (Nestler and Hyman, 2010). Thus, another cohort of mice was assayed for sucrose preference (percentage) using the two-bottle choice procedure to assess anhedonia. All mice (AAV–GFP, AAV–GFP–ΔJunD, and AAV–GFP–ΔFosB) increased preference for the bottle containing the 1% sucrose solution compared with baseline (Fig. 5G; Fday(4,120) = 54.81, p < 0.001), and no differences between groups (p > 0.05) were observed in baseline drinking or preference for sucrose solution. Finally, mice were assayed for changes in body weight, and no group differences were observed (Fig. 5H; p > 0.05). Thus, neither silencing nor overexpression of hippocampal ΔFosB induces a depressive (anhedonic) phenotype, nor does it produce body weight perturbations.
Hippocampal ΔFosB controls dendritic spine morphology
ΔFosB induces changes in cellular physiology and morphology in NAc D1 medium spiny neurons (Grueter et al., 2013). In particular, NAc ΔFosB induction increases the number of silent synapses along with a corresponding increase in immature dendritic spines. Therefore, we used HSV-mediated overexpression of GFP, GFP–ΔFosB, or GFP–ΔJunD in the mouse hippocampus to determine the effects of ΔFosB on hippocampal dendritic spine morphology. We found that overexpression of ΔFosB caused an increase in total spines in CA1 hippocampal neurons (Fig. 6A,B; t(38) = 8.26, p < 0.001). Furthermore, we found that, although ΔFosB did not increase the number of mushroom spines (p > 0.05), it did increase the number of thin (t(38) = 4.97, p < 0.001) and stubby (t(38) = 7.20, p < 0.001) spines, indicating that ΔFosB overexpression induces immature spines in hippocampal neurons. In contrast, expression of ΔJunD caused a decrease in total spines (Fig. 6A,B; t(39) = 4.09, p < 0.001) that was driven by decreases in thin (t(39) = 4.18, p < 0.001) and mushroom (t(39) = 3.09, p = 0.004) spines but did not affect stubby spines (p > 0.05). These data indicate that ΔFosB regulates basal spine formation and stabilization in CA1, although levels of ΔFosB in CA1 neurons in the absence of learning were quite low (Fig. 1, Table 1). Combined with the effects of ΔFosB overexpression, this indicates that ΔFosB modulates the number and function of hippocampal synapses, a possible mechanism for its effects on learning and memory.
Figure 6.
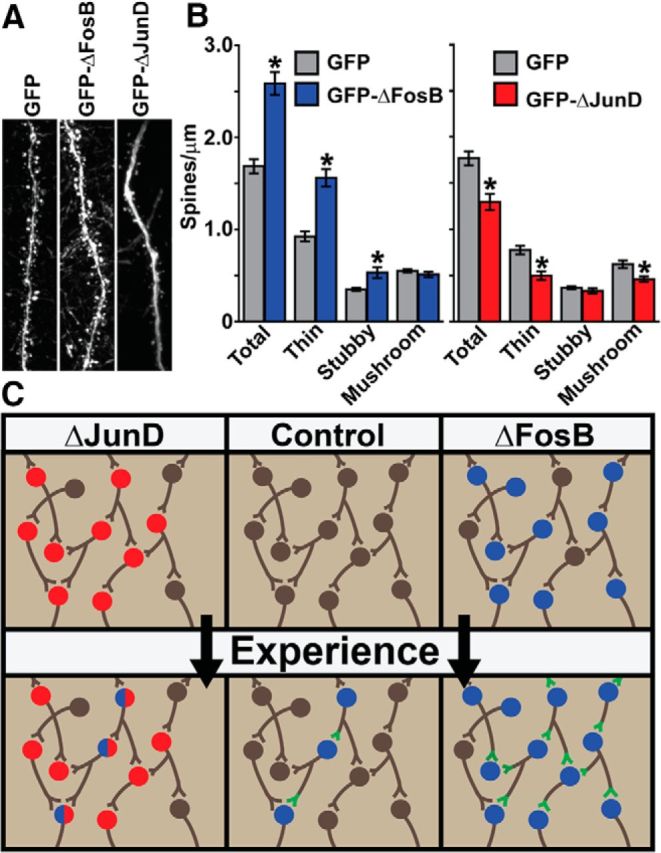
ΔFosB regulates hippocampal spine formation. Mice received hippocampal infusions of HSV–GFP, HSV–GFP–ΔFosB, or HSV–GFP–ΔJunD and dendritic spine analysis was conducted on hippocampal CA1 pyramidal neurons. A, Representative dendrites showing spines from GFP+ hippocampal CA1 neurons. B, Quantitation of spines in CA1 neurons shows ΔFosB (blue; n = 30) had significantly more total spines, thin spines, and stubby spines compared with GFP alone (gray; n = 30; *p < 0.001), with no significant difference in mushroom spines. ΔJunD (red; n = 19) had significantly fewer total spines, thin spines, and mushroom spines compared with GFP alone (gray; n = 22; *p < 0.001), with no significant difference in stubby spines. Error bars indicate mean ± SEM. C, Model depicting experience-dependent changes in hippocampal cells under control conditions or when virally transduced with ΔJunD (red) or ΔFosB (blue). Green indicates changes in synaptic structure/function resulting from ΔFosB expression within a circuit that may underlie learning.
Discussion
We demonstrate for the first time that the transcription factor ΔFosB is important for hippocampal-dependent learning and memory. Specifically, we observed that silencing the transcriptional activity of hippocampal ΔFosB impaired learning and memory across a battery of hippocampal-dependent memory tasks. It is likely that, during learning and exposure to novel environments, groups of hippocampal cells encoding an engram for a particular association express ΔFosB and that the expression of ΔFosB mediates downstream signaling events that prepare the cell for synaptic morphological and physiological changes associated with memory consolidation. This hypothesis is further bolstered by the fact that ΔFosB in the NAc is known to directly regulate the expression of target genes that are essential for hippocampal synaptic plasticity and learning (Guzman-Karlsson et al., 2014), such as CDK5 (Kumar et al., 2005), CaMKII, and GluA receptors (Robison and Nestler, 2011).
These candidate targets of hippocampal ΔFosB have been studied extensively in the hippocampus. For example, CDK5 regulates neural development and neurogenesis (Lagace et al., 2008; Su and Tsai, 2011), glutamatergic neurotransmission (Morabito et al., 2004), and synaptic plasticity (Bibb, 2003; Dhariwala and Rajadhyaksha, 2008; Su and Tsai, 2011). CaMKII has long been known to be a molecular substrate of synaptic plasticity and memory in the hippocampus (Shonesy et al., 2014), and its activity is necessary for long-term potentiation induction (Malinow et al., 1989), AMPA receptor trafficking and function (Malinow and Malenka, 2002), and structural remodeling of the synapse (Okamoto et al., 2007). In addition, these activities, and interactions with NMDA receptors and other postsynaptic proteins (Robison et al., 2005a; Robison et al., 2005b), may contribute to a variety of neuropsychiatric diseases (Robison, 2014). Specifically in D1 medium spiny neurons of the NAc, ΔFosB regulates the induction of CaMKII gene expression by cocaine and that CaMKII provides a feedforward loop via its direct phosphorylation of a stability site on ΔFosB (Robison et al., 2013), which suggests a close relationship between these two molecules in addiction. ΔFosB overexpression in inducible bitransgenic mice has also been shown previously to increase GluA2 levels (Kelz et al., 1999), and ΔFosB overexpression increases the number of AMPA-receptor-lacking silent synapses in D1 medium spiny neurons of the NAc (Grueter et al., 2013). This is significant in that AMPA receptor activity and expression is related directly to the hippocampal synaptic activity and plasticity critical for memory formation (Guzman-Karlsson et al., 2014).
Despite this evidence, a gap in knowledge exists for the specific contribution of hippocampal ΔFosB to memory formation. Early reports suggested the possibility of such a contribution, because ΔFosB is expressed highly within the hippocampus and electrical stimulation of this region induces ΔFosB mRNA expression (Cole et al., 1990; Nakabeppu and Nathans, 1991). In addition, FosB knock-out mice display malformation of the hippocampus, reduced hippocampal neurogenesis, and spatial learning deficits (Yutsudo et al., 2013). These data indicate that FosB is important for development of the hippocampus and perhaps adult neurogenesis but, because of the nature of the knock-out model, do not test the acute role of FosB in learning. The current finding that ΔFosB regulates spine formation in CA1 hippocampal neurons suggests that ΔFosB plays an important role in synaptic plasticity, the first evidence that hippocampal ΔFosB plays a role in the function of fully differentiated hippocampal neurons. It will now be important to test whether experience-dependent (e.g., learning-induced) changes in hippocampal spine morphology require ΔFosB.
The specific mechanisms for the induction of ΔFosB by learning or novel environmental exposure are currently unknown, but again, previous studies from the NAc provide a model. For example, CREB and SRF mediate the induction by cocaine of ΔFosB in NAc (Vialou et al., 2012) and are essential in hippocampal function and learning (Knöll and Nordheim, 2009; Alberini and Kandel, 2015). Furthermore, the induction of ΔFosB may be regulated by dopamine from the VTA, which provides a signal to hippocampal neurons relating the salience and novelty of events (Lisman and Grace, 2005). Dopamine has been shown to enhance plasticity in CA1 pyramidal neurons (Li et al., 2003) and, more recently, to mediate network-level activity and memory persistence (McNamara et al., 2014). Indeed, this may explain why drugs of abuse, such as cocaine, are able to produce strong induction of ΔFosB in hippocampus (Perrotti et al., 2008) and may also explain the strong associative memories of drug effects and drug-taking contexts.
Interestingly, overexpression of ΔFosB in the hippocampus produced impairments in learning and memory similar to those observed with inhibition of FosB transcriptional activity. Although it may be hypothesized that increased ΔFosB expression is associated with memory consolidation and learning, the current finding may be explained by an overall decrease in the signal-to-noise ratio. We hypothesize that FosB gene products accumulate specifically in the relatively few cells of the hippocampus encoding a particular memory, i.e., the engram (Mayford, 2014; Fig. 1 and model in Fig. 6). Thus, non-specific overexpression of ΔFosB throughout entire regions of the hippocampus masks the specific effects of ΔFosB in the engram neurons, losing their signal in the “noise” created by overexpression. The new memory circuits are not consolidated because they cannot be distinguished from the other nonrelevant circuits artificially expressing ΔFosB. New evidence is beginning to emerge that synchronous neural networks within the hippocampus may underlie memory consolidation (Patel, 2015), consistent with the view that the hippocampus encodes memories via specific networks (Abraham, 2008; Neves et al., 2008), supporting this signal-to-noise hypothesis. Alternatively, the impairment may be attributable to aberrant hippocampal network reorganization, because seizures, which are known to induce both ΔFosB (Chen et al., 1995) and its target CDK5 (Chen et al., 2000), also induce reorganization of granule cells in the DG (Parent et al., 1997). Finally, it may be that global overexpression of ΔFosB interferes with homeostatic mechanisms characteristic of hippocampal networks (Pozo and Goda, 2010). However, all of these possibilities hint that non-selective ΔFosB overexpression may be a detriment to intrahippocampal network-level activity and plasticity, which may impinge on memory consolidation.
ΔFosB overexpression also caused an increase in immature dendritic spines in the hippocampus, as has been reported previously with overexpression in the NAc (Grueter et al., 2013; Robison et al., 2013). This may be indicative of a priming effect, in which ΔFosB accumulation causes the neuron to produce immature synapses that are ready to be consolidated rapidly into mature mushroom synapses after addition neuronal activation, i.e., new learning. This may also indicate a mechanism by which circuits that have been activated repeatedly by particularly salient stimuli may remain poised for recall, for instance, those encoding associations between drugs of abuse and the context of drug exposure that appear to underlie relapse to addiction behaviors (Crombag et al., 2008; Lasseter et al., 2010). However, it is important to note that general overexpression of ΔFosB in the hippocampus does not promote learning but rather impairs it. Thus, if ΔFosB primes synapses for rapid learning, it must require additional cell-specific signaling events to be effective.
We also found increased anxiety as a result of global ΔFosB overexpression in the hippocampus. The current findings are in line with the theory of Gray and McNaughton (2000) for hippocampal involvement in anxiety, with new optogenetic evidence indicating an important contribution of the hippocampus (for review, see Fournier and Duman, 2013). Global ΔFosB overexpression may impair attentional gating, specifically the ability to attribute salience to aversive cues. In addition, animals that attribute too much salience to nonpredictive environmental cues tend to also place high negative valence on aversive cues (Morrow et al., 2011). A clinical example of this may be observed in posttraumatic stress disorder (PTSD), in which afflicted individuals are unable to differentiate safety signals from threatening stimuli (Jovanovic et al., 2012). Safety signal encoding and contextual conditioning are regulated by the amygdala and hippocampus (Wiltgen et al., 2006; Alvarez et al., 2008; Marschner et al., 2008; Sangha et al., 2013). Therefore, the lack of safety signal discrimination in clinical PTSD may be attributed to hippocampal dysfunction and overactive amygdala activity (Pitman et al., 2012). Similarly, in the current study, ΔFosB overexpression throughout the hippocampus may prevent proper hippocampal regulation of amygdalar activity, leading to the observed increase in anxiety-like behaviors. It is interesting to note that chronic social defeat stress, one model of PTSD and anxiety, also promotes ΔFosB expression throughout hippocampus (Vialou et al., 2015). However, this hippocampal ΔFosB is unlikely to be the sole cause of anxiety in the social defeat model, because hippocampal ΔFosB also accumulates with chronic antidepressant treatment (Vialou et al., 2015), a regimen that does not produce anxiety.
The current findings indicate that learning induces ΔFosB in specific cells encoding memories in the hippocampus and suggest that this expression is essential for learning. This highlights the need for large-scale candidate gene and omics approaches to identify hippocampal ΔFosB gene targets to uncover the mechanism by which ΔFosB regulates hippocampal cell and synaptic function. This novel role for ΔFosB is particularly relevant, because it suggests that, by impairing the functions of hippocampal cells active during memory formation, we might impair a specific memory. Thus, ΔFosB and its target genes could be therapeutic targets for neuropsychiatric diseases associated with impaired memory, such as depression, PTSD, and addiction.
Footnotes
This research was supported by Whitehall Foundation Grant 2013-08-43 (A.J.R.), Multidisciplinary Training in Environmental Toxicology Training Grant T32-ES007255 (A.L.E.), and a 2014 National Alliance for Research on Schizophrenia and Depression Young Investigator Award from the Brain and Behavior Research Foundation (A.L.E.). We thank Kenneth Moon for outstanding technical support.
The authors declare no competing financial interests.
References
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021741. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, Nestler EJ. Induction of nuclear factor-κB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79:221–224. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- Bibb JA. Role of Cdk5 in neuronal signaling, plasticity, and drug abuse. Neurosignals. 2003;12:191–199. doi: 10.1159/000074620. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, Nestler EJ. Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur J Neurosci. 2007;25:3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Nye HE, Kelz MB, Hiroi N, Nakabeppu Y, Hope BT, Nestler EJ. Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- Chen J, Zhang Y, Kelz MB, Steffen C, Ang ES, Zeng L, Nestler EJ. Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: role of ΔFosB. J Neurosci. 2000;20:8965–8971. doi: 10.1523/JNEUROSCI.20-24-08965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AJ, Abu-Shakra S, Saffen DW, Baraban JM, Worley PF. Rapid rise in transcription factor mRNAs in rat brain after electroshock-induced seizures. J Neurochem. 1990;55:1920–1927. doi: 10.1111/j.1471-4159.1990.tb05777.x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dhariwala FA, Rajadhyaksha MS. An unusual member of the cdk family: Cdk5. Cell Mol Neurobiol. 2008;28:351–369. doi: 10.1007/s10571-007-9242-1. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Duman RS. Illuminating hippocampal control of fear memory and anxiety. Neuron. 2013;77:803–806. doi: 10.1016/j.neuron.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. Ed 2. New York: Oxford UP; 2000. [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, Fanous S, Golden SA, Schrama R, Koya E, Stern AL, Bossert JM, Harvey BK, Picciotto MR, Hope BT. FACS identifies unique cocaine-induced gene regulation in selectively activated adult striatal neurons. J Neurosci. 2011;31:4251–4259. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Karlsson MC, Meadows JP, Gavin CF, Hablitz JJ, Sweatt JD. Transcriptional and epigenetic regulation of Hebbian and non-Hebbian plasticity. Neuropharmacology. 2014;80:3–17. doi: 10.1016/j.neuropharm.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Ed 8. Washington, DC: National Academy of Sciences; 2011. [Google Scholar]
- Janson CG, McPhee SW, Leone P, Freese A, During MJ. Viral-based gene transfer to the mammalian CNS for functional genomic studies. Trends Neurosci. 2001;24:706–712. doi: 10.1016/S0166-2236(00)01954-8. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Knöll B, Nordheim A. Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci. 2009;32:432–442. doi: 10.1016/j.tins.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kovács KJ. Measurement of immediate-early gene activation: c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW. Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. J Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M. The search for a hippocampal engram. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130161. doi: 10.1098/rstb.2013.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res. 2002;133:135–141. doi: 10.1016/S0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–243. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Rowland DC, Moser EI. Place cells, grid cells, and memory. Cold Spring Harb Perspect Biol. 2015;7:a021808. doi: 10.1101/cshperspect.a021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-R. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. FosB: a transcriptional regulator of stress and antidepressant responses. Eur J Pharmacol. 2015;753:66–72. doi: 10.1016/j.ejphar.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J. ΔFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res. 1999;835:10–17. doi: 10.1016/S0006-8993(98)01191-3. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP., Jr Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of μ-opioid receptor mRNA and FosB/ΔFosB immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomaru H, Sakumi K, Katogi A, Ohnishi YN, Kajitani K, Tsuchimoto D, Nestler EJ, Nakabeppu Y. Fosb gene products contribute to excitotoxic microglial activation by regulating the expression of complement C5a receptors in microglia. Glia. 2014;62:1284–1298. doi: 10.1002/glia.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. Network mechanisms underlying the initiation and generation of sharp-wave-associated ripple oscillations. J Neurosci. 2015;35:2323–2325. doi: 10.1523/JNEUROSCI.4215-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. Ed 4. San Diego: Academic; 2012. [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of ΔFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte Y, Buhot MC, Mons NE. Spatial memory in the Morris water maze and activation of cyclic AMP response element-binding (CREB) protein within the mouse hippocampus. Learn Mem. 2008;15:885–894. doi: 10.1101/lm.1094208. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redemann-Fibi B, Schuermann M, Müller R. Stage and tissue-specific expression of fosB during mouse development. Differentiation. 1991;46:43–49. doi: 10.1111/j.1432-0436.1991.tb00864.x. [DOI] [PubMed] [Google Scholar]
- Robison AJ. Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci. 2014;37:653–662. doi: 10.1016/j.tins.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bartlett RK, Bass MA, Colbran RJ. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-d-aspartate receptor NR2B subunits and alpha-actinin. J Biol Chem. 2005a;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, MacMillan LB, Carmody LC, Bartlett RK, Colbran RJ. Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and alpha-actinin-2. J Biol Chem. 2005b;280:35329–35336. doi: 10.1074/jbc.M502191200. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, Wee S, Koob G, Turecki G, Neve R, Thomas M, Nestler EJ. Behavioral and structural responses to chronic cocaine require a feedforward loop involving ΔFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, Janak PH. Safety encoding in the basal amygdala. J Neurosci. 2013;33:3744–3751. doi: 10.1523/JNEUROSCI.3302-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci U S A. 2001;98:12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Jalan-Sakrikar N, Cavener VS, Colbran RJ. CaMKII: a molecular substrate for synaptic plasticity and memory, Chap 3. In: Zafar UK, Muly EC, editors. Progress in molecular biology and translational science. San Diego: Academic; 2014. pp. 61–87. [DOI] [PubMed] [Google Scholar]
- Solecki W, Krowka T, Kubik J, Kaczmarek L, Przewlocki R. Role of fosB in behaviours related to morphine reward and spatial memory. Behav Brain Res. 2008;190:212–217. doi: 10.1016/j.bbr.2008.02.040. [DOI] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Tsai LH. Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- Thibault MA, Eagle AL, Kaska S, Nestler EJ, Mazei-Robison MS, Vialou V, Robison AJ. Region-specific induction of FosB isoforms in mouse brain after stress or chronic fluoxetine exposure. Soc Neurosci Abstr. 2014;40:426.13. [Google Scholar]
- Ulery-Reynolds PG, Castillo MA, Vialou V, Russo SJ, Nestler EJ. Phosphorylation of DeltaFosB mediates its stability in vivo. Neuroscience. 2009;158:369–372. doi: 10.1016/j.neuroscience.2008.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iñiguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolaños CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Feng J, Robison AJ, Ku SM, Ferguson D, Scobie KN, Mazei-Robison MS, Mouzon E, Nestler EJ. Serum response factor and cAMP response element binding protein are both required for cocaine induction of ΔFosB. J Neurosci. 2012;32:7577–7584. doi: 10.1523/JNEUROSCI.1381-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Thibault M, Kaska S, Cooper S, Gajewski P, Eagle A, Mazei-Robison M, Nestler EJ, Robison AJ. Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology. 2015;99:28–37. doi: 10.1016/j.neuropharm.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutsudo N, Kamada T, Kajitani K, Nomaru H, Katogi A, Ohnishi YH, Ohnishi YN, Takase K, Sakumi K, Shigeto H, Nakabeppu Y. fosB-null mice display impaired adult hippocampal neurogenesis and spontaneous epilepsy with depressive behavior. Neuropsychopharmacology. 2013;38:895–906. doi: 10.1038/npp.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang H. Mice overexpressing type 1 adenylyl cyclase show enhanced spatial memory flexibility in the absence of intact synaptic long-term depression. Learn Mem. 2013;20:352–357. doi: 10.1101/lm.030114.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Moon C, Chan GC, Yang L, Zheng F, Conti AC, Muglia L, Muglia LJ, Storm DR, Wang H. Ca-stimulated type 8 adenylyl cyclase is required for rapid acquisition of novel spatial information and for working/episodic-like memory. J Neurosci. 2008;28:4736–4744. doi: 10.1523/JNEUROSCI.1177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]