Abstract
The ζ-inhibitory peptide (ZIP) is considered a candidate inhibitor of the atypical protein kinase Mζ (PKMζ). ZIP has been shown to reverse established LTP and disrupt several forms of long-term memory. However, recent studies have challenged the specificity of ZIP, as it was reported to exert its effect also in PKMζ knock-out mice. These results raise the question of what are the targets of ZIP that may underlie its effect on LTP and memory. Here we report that ZIP as well as its inactive analog, scrambled ZIP, induced a dose-dependent increase in spontaneous activity of neurons in dissociated cultures of rat hippocampus. This was followed by a sustained elevation of intracellular calcium concentration ([Ca2+]i) which could not be blocked by conventional channel blockers. Furthermore, ZIP caused an increase in frequency of mEPSCs followed by an increase in membrane noise in patch-clamped neurons both in culture and in acute brain slices. Finally, at 5–10 μm, ZIP-induced excitotoxic death of the cultured neurons. Together, our results suggest that the potential contribution of cellular toxicity should be taken into account in interpretation of ZIP's effects on neuronal and behavioral plasticity.
SIGNIFICANCE STATEMENT The ζ-inhibitory peptide (ZIP) is considered a candidate inhibitor of the atypical protein kinase Mζ (PKMζ). ZIP has been shown to reverse established LTP and disrupt several forms of long-term memory. Here we report that ZIP as well as its inactive analog, scrambled ZIP, induced a dose-dependent increase in spontaneous activity of neurons in dissociated cultures and brain slices of rat hippocampus. Furthermore, ZIP caused a dose- and time-dependent neuronal death in the dissociated cultures. These findings impact on the assumption that ZIP erases memory due to specific inhibition of PKMz.
Keywords: calcium, culture, hippocampus, PKM, toxicity, ZIP
Introduction
The atypical protein kinase Cζ (PKCζ) consists of a C-terminal catalytic domain linked by a hinge region to an N-terminal regulatory domain, which includes an autoinhibitory pseudosubstrate sequence that can block the active site (Nishizuka, 1995). PKMζ is a brain-specific isoform of PKCζ that lacks the regulatory domain, and is hence an “autonomously active” catalytic unit, capable of phosphorylating its substrates once activated by phosphoinositide-dependent protein kinase-1 (PDK1; Sacktor et al., 1993; Hernandez et al., 2003). The myristoylated ζ-inhibitory peptide (ZIP) is a synthetic cell-permeable inhibitor of PKMζ, originally designed based on the amino acid sequence of the endogenous PKCζ pseudosubstrate region (Laudanna et al., 1998). Applying ZIP onto hippocampal slices has been shown to reduce established LTP (Sajikumar et al., 2005; Serrano et al., 2005), suggested to occur through the specific inhibition of PKMζ-mediated increase in postsynaptic AMPARs (Ling et al., 2006). Moreover, infusing ZIP into distinct brain regions such as the hippocampus, amygdala, insular cortex and sensorimotor cortex was reported to erase several forms of long-term memory, including spatial memory (Pastalkova et al., 2006; Serrano et al., 2008; Hardt et al., 2010), fear conditioning (Serrano et al., 2008; Kwapis et al., 2012), conditioned taste aversion (Shema et al., 2007, 2009), and place avoidance (von Kraus et al., 2010). PKMζ has also been directly implicated in memory persistence by demonstrating that alteration of its level, either by protein infusion or by overexpression, markedly affects long-term memory either proactively or retroactively (Drier et al., 2002; Shema et al., 2011; Xue et al., 2015). The specificity of ZIP, however, has been questioned (Lisman, 2012; Wu-Zhang et al., 2012; but see Sacktor and Fenton, 2012). Volk et al. (2013) and Lee et al. (2013) reported that both constitutive and conditional PKCζ/PKMζ knock-out (KO) mice display normal hippocampal LTP and are able to form memories (Lee et al., 2013; Volk et al., 2013). Furthermore, ZIP was reported to impair LTP and memory in PKCζ/PKMζ KO animals, suggesting a yet unknown mechanism of action, unrelated to PKMζ inhibition.
In the present study, we investigated the effects of ZIP on spontaneous activity and viability of cultured rat hippocampal neurons and acute slices, using confocal [Ca2+]i imaging and patch-clamp recording. Our results suggest that myristoylated ZIP can destabilize the cell membrane in a dose-dependent manner, allowing calcium to flow into cells and ultimately lead to their excitotoxic death.
Materials and Methods
Primary brain cultures.
Experiments were approved by institutional animal care and use committee. Primary hippocampal neurons were prepared as previously detailed (Goldin et al., 2001). Cells were grown in 5% CO2, 37°C incubator, with 10% HS in enriched MEM. Cultures were used for experiments at the age of 13–20 d in vitro (DIV).
Calcium Imaging.
Cells were loaded with 2 μm Fluo-4AM (Invitrogen) for 1 h in standard extracellular medium (in mm: 129 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10.5 glucose, 10 HEPES) and imaged on a stage of a Zeiss LSM 510 laser scanning confocal microscope, with a 40× oil-immersion objective (1.3 NA). Imaging the cultures was performed with 488 nm light at an image rate of 2–5 frames per second. Sample recordings are presented as ΔF/F units (ΔF/F = F(t)−F(0)/F(0)). The following chemicals were diluted into standard extracellular medium or DMSO: ZIP, Scr-ZIP, APV (10 μm), DNQX (5 μm), NiCl (50 μm; Su et al., 2002), bicuculline (10 μm),TTX (1 μm), chelerythrine (1 μm), and staurosporine (100 nm) just before use. ZIP was obtained from three different suppliers, AnaSpec, QCB, and GL Biochem, all yielding similar results. It was prepared fresh from powder just before use, was easily soluble, and was not stored beyond the day of experiment.
Viability assay.
Cell death of ZIP-treated primary neuronal cell cultures was quantified using Calcein-AM/propidium iodide (PI) staining assay. Cultures were initially loaded with 2 μm Calcein-AM (Sigma-Aldrich) for 30 min, and imaged in 1.8 ml of standard medium in the presence of PI (2.5 μm, Sigma-Aldrich). Two-channel images were acquired, where green (488 nm) and red (545 nm) fluorescent cells represent live and dead cells, respectively. Cultures were imaged for 90 min in 4 min intervals. Survival probability was calculated as [[no. live cells (at time t)/no. live cells (t = 0)] × 100].
Electrophysiology in culture.
Neurons in hippocampal cultures were recorded at 13–14 DIV as detailed previously (Goldin et al., 2001). Neurons were recorded with patch pipettes. Spontaneous miniature EPSCs (mEPSCs) were recorded in medium containing 0.5 μm TTX and 10 μm bicuculline. Signals were amplified with MultiClamp 700B, recorded with pClamp9 (Molecular Devices) and analyzed with Minianalysis software (Synaptosoft). In the analysis the threshold for inclusion of mEPSCs was 7 pA, where noise RMS was ∼1–2 pA. Events with a long rise or decay time (>10 m/s) were excluded. mEPSCs were not included when the membrane noise level increased so as to interfere with the detection. Thus, the number of mEPSCs is a conservative estimate of the total number of events.
Hippocampal slices.
Slices were prepared from male Wistar rats (2–3 weeks of age) as detailed previously (Maggio and Segal, 2009). Slices were incubated for 1 h in carbogenated (5% CO2–95% O2) artificial CSF (aCSF) at room temperature. The medium contained the following (in mm): 124 NaCl, 2 KCl, 26 NaHCO3, 1.24 KH2PO4, 2.5 CaCl2, 2 MgSO4, and 10 glucose, pH 7.4. CA1 pyramidal cells were recorded with patch pipettes containing the following (in mm): 136 K-gluconate, 10 KCl, 5 NaCl, 10 HEPES, 0.1 EGTA, 0.3 Na-GTP, 1 Mg-ATP, and 5 phosphocreatine, pH 7.2 (with a resistance of 5–10 MΩ). Signals were amplified with Axopatch 200B and recorded with PClamp-10 (Molecular Devices). Data were analyzed using Microsoft Excel 2010, Mini Analysis 6.0.3 and MATLAB R2013b.
Data analysis.
Data were expressed as mean ± SEM, and analyzed using Microsoft Excel 2010 and MATLAB R2013b. Statistical comparisons were made using t tests or ANOVA. Significance was set at *p < 0.05, **p < 0.01, and ***p < 0.005.
Results
ZIP induces an increase in rate of calcium transients followed by a sustained elevation of intracellular calcium in cultured neurons
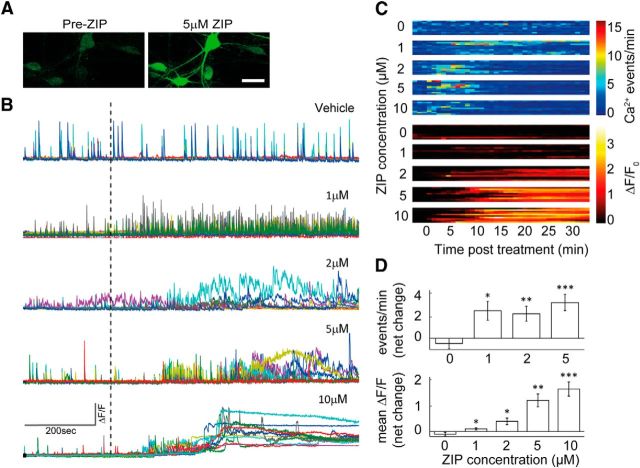
Neurons in culture express spontaneous network bursts that can be consistently monitored using calcium imaging methods. Previous slice and primary culture experiments used ZIP at a range of 1–5 μm, whereas in vivo, ZIP has been infused into the brain at 0.5–1 μl volume, reaching a concentration of 10 mm. We therefore applied ZIP at a range of 1–10 μm to spontaneously active cultures, monitored for several minutes before drug application. At 1 μm, ZIP caused a significant increase in spontaneous activity (Fig. 1B–D; from 1.6 ± 0.33 to 4.3 ± 0.78 events/min, paired t test, *p < 0.05). Concentrations >1 μm produced a second phase, where the increase in spontaneous activity was replaced by a sustained elevation of [Ca2+]i. (Fig. 1A,D, bottom). At this point, [Ca2+]i reached levels at which fluo-2 was saturated (Fig. 1B; 5 and 10 μm net change in mean ΔF/F 1.33 ± 0.27 and 1.8 ± 0.3, paired t test, **p < 0.01, ***p < 0.005, respectively), with no apparent recovery. To separate these two phases, for each cell trace, high-frequency calcium transients and low-frequency calcium waves were calculated for 1 min epochs (Fig. 1C). When averaged across all cultures (n = 50 dishes, 560 cells), 1–2 μm is the concentration above which spontaneous calcium transients are replaced by sustained elevation of [Ca2+]i (Fig. 1D). This suggests that ZIP causes destabilization of the membrane, resulting in massive, irreversible rise of [Ca2+]i, possibly leading to excitotoxic damage.
Figure 1.
ZIP induces an increase in spontaneous activity and sustained elevation of [Ca2+]i. A, Sample field-of-view of cultured neurons loaded with Fluo2-AM, before and after exposure to 5 μm ZIP treatment. Scale bar, 20 μm. B, Calcium imaging sample traces of hippocampal cultures. Cells were initially imaged for baseline spontaneous activity, and then treated with vehicle (0 μm)/1 μm/2 μm/5 μm/10 μm ZIP; dashed line marks point of application. C, Plots depict each ZIP concentration and its separate effect on calcium events frequency and baseline [Ca2+]i. D, Bar graphs summarize total net change in event frequency (top) or mean ΔF/F (bottom) of cultured neurons (n = 50 coverslips, 560 cells, values are mean ± SEM, post-treatment vs baseline paired t test, *p < 0.05, **p < 0.01, ***p < 0.005).
ZIP effect on calcium influx is mimicked by scr-ZIP and not affected by calcium channel blockers
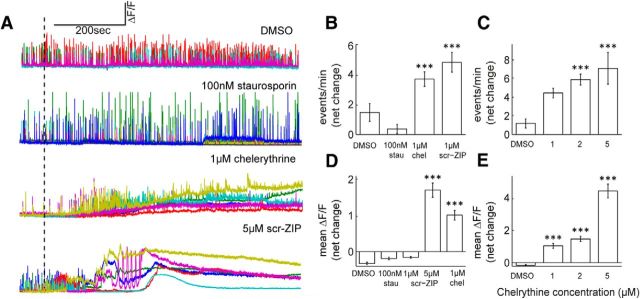
Scrambled ZIP (Scr-ZIP) is reported to have approximately an order of magnitude lower affinity for PKMζ compared with ZIP, and has been used as a control peptide (Pastalkova et al., 2006; Shema et al., 2007). The effects of Scr-ZIP, as well as those of the nonselective PKC inhibitors chelerythrine and staurosporine, were measured in the cultures. At 1 μm, Scr-ZIP produced a larger effect compared with ZIP on spontaneous activity (Fig. 2A,B,D; from 5.42 ± 0.6 to 10.2 ± 0.99 events/min, ***p < 0.005) and on [Ca2+]i at 5 μm (net change in mean ΔF/F 1.69 ± 0.19, paired t test, ***p < 0.005). Chelerythrine, previously shown to mimic the effect of ZIP on LTP and behavior at 1 μm (Ling et al., 2002; Serrano et al., 2008) and to compromise membrane integrity of cultured cardiac myocytes (Yamamoto et al., 2001), produced a similar increase in spontaneous activity and [Ca2+]i (Fig. 2A,C,F from 3.07 ± 0.47 to 6.74 ± 0.65 events/min and net change in mean ΔF/F 1.01 ± 0.14, ***p < 0.005). Finally, staurosporine, previously shown to have no effect either on late-LTP or behavior at 100 mm compared with ZIP (Ling et al., 2002; Pastalkova et al., 2006), was ineffective with respect to spontaneous activity or [Ca2+]i (Fig. 2A,B,D).
Figure 2.
ZIPs effect can be mimicked by scr-ZIP and chelerythrine but not by staurosporine. A, Calcium imaging sample traces of hippocampal cultures. Cells were initially imaged for baseline spontaneous activity, and then treated with vehicle (DMSO)/100 nm staurosporine/1 μm Chelerythrine/5 μm scr-ZIP; dashed line marks point of application. B, D, Bar graphs summarize total net change in event frequency (B) or mean ΔF/F (D) of cultured neurons (n = 14 coverslips, 134 cells, post-treatment vs baseline paired t test, ***p < 0.005). C, E, Chelerythrine increases event frequency and mean [Ca2+]i in a dose-dependent manner. Bar graphs summarize total net change in event frequency (C) or mean ΔF/F (E) of cultured neurons (n = 11 coverslips, 93 cells, post-treatment vs baseline paired t test, ***p < 0.005).
Calcium ions can enter neurons through multiple types of channels and receptors (Higley and Sabatini, 2008). To determine the involvement of calcium channels in the observed aberrant [Ca2+]i, a mixture of channel blockers, including TTX, Ni2+, APV, bicuculline, and DNQX was applied before ZIP application. These channel antagonists could not prevent [Ca2+]i elevation, indicating that none of the blocked channels or receptors are involved in calcium entry (Fig. 3A,C,E,F; net change in mean ΔF/F 1.84 ± 0.36, paired t test, ***p < 0.005), and implying a different membrane-dependent mechanism through which ZIP exerts its action. To evaluate the involvement of [Ca2+]i stores in calcium elevation, cultures were placed in calcium free medium and in the presence of the calcium chelator EGTA. Under these conditions synaptic activity is blocked and [Ca2+]i elevation does not occur after treatment with 5 μm ZIP, ruling out an intracellular source of calcium. However, restoring the culture to a standard external media, was immediately followed by a sharp elevation in [Ca2+]i in ZIP-treated cells (Fig. 3B, bottom; net change in mean ΔF/F 1.32 ± 0.26, paired t test, ***p < 0.005), indicative of a destabilized membrane. Activity of vehicle-treated cultures was restored and even elevated due to possible homeostatic mechanisms (Fig. 3B, top, D).
Figure 3.
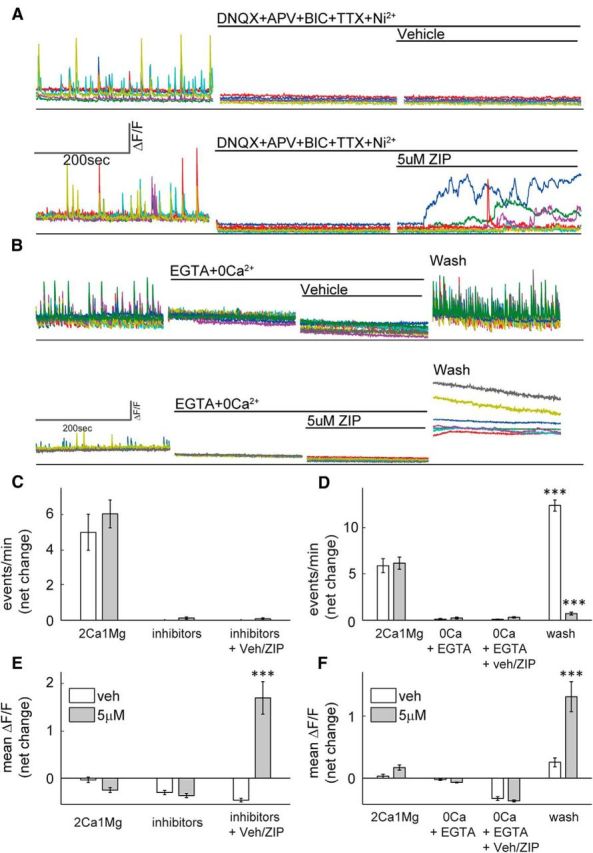
ZIP-induced elevation in [Ca2+]i is not prevented by channel blockers and originates from extracellular sources. A, B, Calcium imaging sample traces of hippocampal cultures. A, Cells were initially imaged in standard medium to confirm spontaneous activity, then treated with a mixture of channel blockers (DNQX+APV+BIC+TTX+Ni2+) and finally treated with vehicle (top) or 5 μm ZIP (bottom). B, Cells were initially imaged in standard medium to confirm spontaneous activity, and then switched to a Ca2+-free medium added with EGTA (EGTA+0Ca2+), then treated with vehicle (top) or 5 μm ZIP (bottom) and finally washed and restored to standard medium (wash). C, E, Bar graphs summarize total net change in event frequency (C) or mean ΔF/F (E) of cultured neurons [n = 4 coverslips, 25 cells, post-treatment (inhibitors + Veh/ZIP) vs baseline (2Ca1 Mg), paired t test, ***p < 0.005]. D, F, Bar graphs summarize total net change in event frequency (D) or mean ΔF/F (F) of cultured neurons [n = 6 coverslips, 48 cells, post-treatment (wash) vs baseline (2Ca1 Mg), paired t test, ***p < 0.005].
ZIP increases spontaneous activity and membrane noise
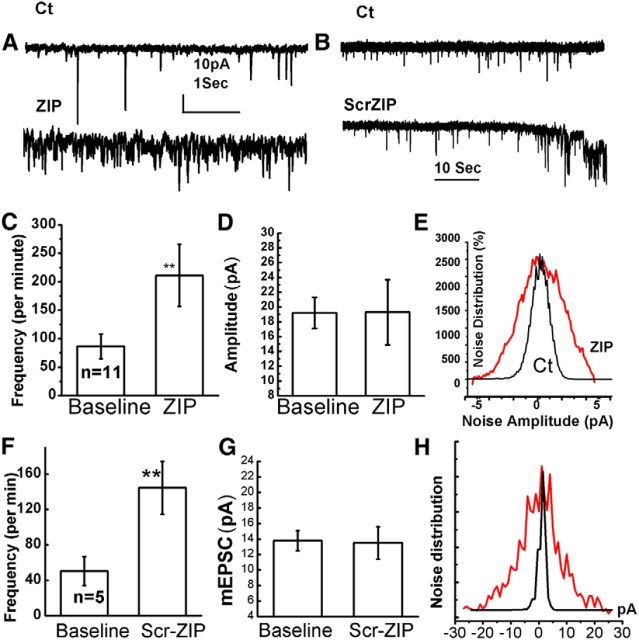
To further validate the imaging findings, the effects of ZIP (2–5 μm) were tested in 11 voltage-clamped cultured neurons, one cell per dish. Initially, the cell was clamped at −60 mV, in presence of TTX in the medium so as to record spontaneous mEPSCs. Stable recordings were made for a period of 2 min, after which ZIP was added to the medium (Fig. 4). Recording was continued for up to 12 min or was terminated sooner if the cell was ‘lost’. It is noteworthy that in standard conditions cells can easily be recorded for >12 min with no sign of deterioration of membrane properties. In all the cells tested with ZIP, following a variable delay (between 1 and 6 min), the cell increased production of mEPSCs (Fig. 4C) from 86.4 ± 21 to 211 ± 54 events per minute (t test, paired comparison, p < 0.01), without a change in mean amplitude of these events (Fig. 4D,E). Subsequently, membrane noise increased significantly in all of the recorded neurons (Fig. 3E) to the extent that no clear mEPSC events could be identified. The instability of the membrane continued until the current required for holding the membrane at −60 mV increase above −200 pA, at which time recording was terminated. A similar series of experiments was conducted with five neurons (1 per dish) exposed to 5 μm scr-ZIP (Fig. 4B, F–H). In all of them, a significant increase in spontaneous mEPSCs (p < 0.01), was followed by an increase in membrane noise and loss of stable recording (Fig. 4 B, F–H).
Figure 4.
Electrophysiological effects of ZIP in patch-clamped cultured neurons. A, mEPSCs in control cell (top) and following exposure to ZIP (bottom). B, Similar experiments before (top) and during exposure to Scr-ZIP (bottom). The presence of ZIP (5 μm) in both cases causes an increase in membrane noise, as well as an increase in rate of mEPSCs. C, D, Bar graphs summarizing the rate (C) and amplitudes (D) of spontaneous events recorded from a population of 11 cells, one in each glass, before and after exposure to ZIP. E, Noise distribution in a sample neuron before (black) and after exposure to ZIP (red). F–H, Similar summary diagrams of recording of five neurons exposed to Scr-ZIP. The rate of mEPSCs in both ZIP and Scr-Zip is significantly higher than in baseline conditions.
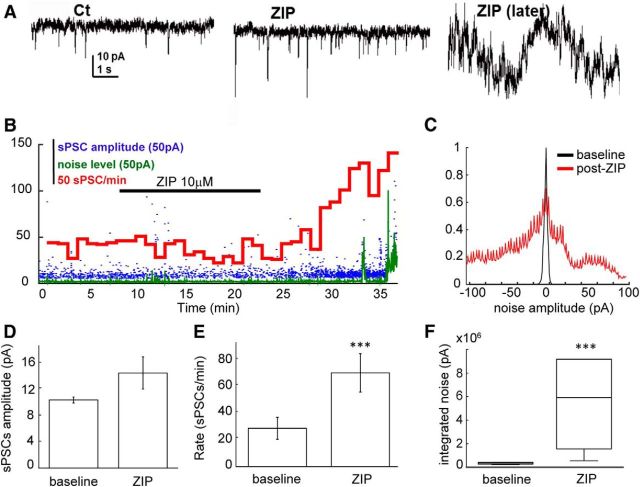
We then investigated the effect of 10 μm ZIP on spontaneous activity in six acute brain slices of 2- to 3-week-old rats, using patch-clamp recordings from CA1 pyramidal neurons. Similar to cultures, ZIP induced an increase in the number of sEPSCs recorded from neurons (Fig. 5E; from 26.7 ± 7.6 to 65.3 ± 13.3 events per minute, paired t test, p < 0.005, n = 5), reflecting an overall rise in network activity. Enhanced frequency was subsequently followed by a dramatic elevation of membrane noise level (Fig. 5C). The amplitude of sEPSCs was unaltered. These results indicate that exposure to ZIP caused the cells to increase their spontaneous activity. This phase was gradually replaced by an overall instability of the membrane, reflected by an increase in membrane noise levels. Together these results corroborate in the slice the data acquired with primary cultures.
Figure 5.
Effects of ZIP on neurons in hippocampal slices. A, Sample traces of the spontaneous activity of CA1 neuron, recorded in voltage-clamp mode, holding potential −70 mV. In the control condition (left), after exposure to ZIP (middle right), and right, a few minutes later (right). B, Example of time course of observed changes: blue, amplitude of sEPSCs; green, noise level (SD for every 1 s window of the recording); red, number of sEPSCs for every 1 min window of the recording. C, Averaged noise histogram (bin size, 1 pA, normalized by dividing by the total amount of events, averaged over n = 6 recordings). D, The change in the averaged amplitude of sPSCs (p = 0.152, paired Student's t test). E, Frequency of sPSCs (number of sPSCs/min, p = 0.002, paired Student's t test). F, Boxplot showing the increase in the noise level [integrated noise: amplitude (pA) multiplied by the number of events, p value: 0.002 Wilcoxon rank sum test].
ZIP induces rapid cell death in a dose-dependent manner
Following the demonstration that ZIP-induces [Ca2+]i elevation and an increase in membrane noise, we hypothesized that this will eventually lead to changes in cell viability. To this end, a viability assay (Slepian et al., 1996) was used. In this assay, the cell-permeant Calcein-AM is converted into its highly fluorescent Calcein form through intracellular esterase activity, indicating that the cell is viable. PI is excluded from intact cell membranes, and only intercalates into the DNA of damaged cells, resulting in a dramatic increase in its fluorescence (Fig. 6A).
Figure 6.
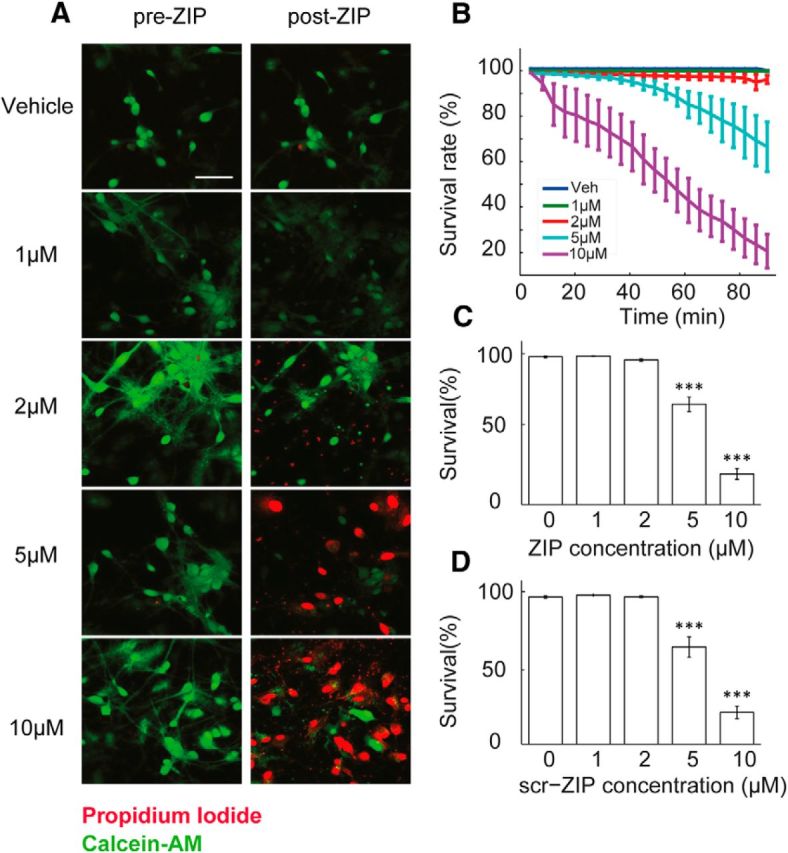
ZIP and scr-ZIP induce rapid cell death of cultured hippocampal neurons in a dose-dependent manner. A, Viability was determined using Calcein-AM (green) and PI (red). Cultures were exposed to different doses of ZIP [Veh (0 μm)/1 μm/2 μm/5 μm/10 μm]. Scale bar, 50 μm. B, Dose–response curve was constructed from time lapse images taken in 4 min intervals to assess the rate of cellular death following treatment with ZIP. C, Bar graph summarizes total change in culture viability (n = 22 coverslips, 3544 cells, values are mean ± SEM,90 min post-ZIP-treatment vs viability pretreatment, paired t test, ***p < 0.005). D, Scr-ZIP induces similar changes in culture viability. Bar graphs summarize total change in culture viability (n = 13 coverslips, 2365 cells, 90 min post-scr-ZIP-treatment vs viability pretreatment, paired t test, ***p < 0.005).
We observed no significant cell death within 85 min posttreatment with either a vehicle or 1–2 μm ZIP, although morphological changes such as cell swelling and dendritic blebbing were evident with 2 μm ZIP, indicative of cellular stress (Trump and Berezesky, 1996). A substantial decrease is culture viability took place within minutes of exposure to 5 μm and even more so with 10 μm ZIP (Fig. 6B,C; 67.38 ± 4.85% and 20.58 ± 3.67% respectively, t test, ***p < 0.005). Thus, ZIP induces cell death in primary hippocampal cultures in a dose-dependent manner >1–2 μm ZIP, subsequent to elevation of [Ca2+]i. Additionally, although not quantified in our experiments, the feeder glial layer present in the culture demonstrated similar cellular stress and damage, further implying a mechanism unrelated to the kinase inhibition, as glia cells do not express PKMζ (Hirai et al., 2003). Similar results were obtained using scr-ZIP (Fig. 6D; 65.17 ± 6.7% and 21.34 ± 4.19%, respectively, paired t test, ***p < 0.005). Together, these results indicate that in addition to an increase in spontaneous activity followed by a sustained elevation of [Ca2+]i, ZIP causes a deterioration of cell membrane integrity leading to extensive death of primary neurons in culture.
Discussion
We demonstrate that spontaneous activity of primary cultures is enhanced by ZIP applied at a concentration equal to or lower than what has been used previously. Furthermore, a dose-dependent elevation of [Ca2+]i was seen following application of ZIP. These effects could not be blocked by conventional channel blockers, and were mimicked by scr-ZIP and a second PKC inhibitor, chelerythrine. Finally, a fast and dose-dependent deterioration of culture viability followed exposure to ZIP.
We hence show that neuronal activity is dramatically increased by ZIP application, and that a second dose-dependent phase, characterized by a sustained elevation of [Ca2+]i quickly follows. We observed a threshold concentration for these effects, 1–2 μm, at which a transition occurs between increased spontaneous activity and sustained elevation of [Ca2+]i (Fig. 1).
The frequency of mEPSCs was also increased by ZIP, followed by elevation of membrane noise to the point where mEPSCs could not be distinguished above noise level (Fig. 4). This transient increase in mEPSCs may result from local changes in excitability of presynaptic or postsynaptic terminals, and reflect an increase in network activity. Additionally, ZIP increased sEPSCs frequency in patched pyramidal neurons in slices, which was followed by a dramatic increase in membrane noise (Fig. 5). Acute slices are closer than primary cultures to the in vivo situation. These results raise the possibility that responses similar to those detected by us in culture and slice may also occur in the brains of behaving animals infused with ZIP.
In addition to demonstrating ZIP-induced LTP reversal on slices produced from PKCζ/PKMζ knock-out mice, Volk et al. (2013) also reported ZIP effect on LTP to be dose-dependent, with 1 μm producing no change in LTP maintenance or baseline transmission, 2–2.5 μm producing effective reversal below baseline in half the cases and 5 μm reversing LTP below baseline in all cases. Together with our results, reversal of LTP (Serrano et al., 2005; Pastalkova et al., 2006; Volk et al., 2013) may result from a depotentiation-like process, where an increase in spontaneous activity, equivalent to stimulation at low rate (a standard means for inducing LTD or depotentiation) can suppress LTP (Mulkey and Malenka, 1992).
Scr-ZIP, reported not to block memory in vivo (Pastalkova et al., 2006; Shema et al., 2007), mimicked the effects of ZIP in our experiments. The PKC inhibitor Chelerythrine produced an increase in frequency, similarly to ZIP. Staurosporine did not produce any of the changes mentioned. These results hence show that ZIP and chelerythrine, two molecules shown to reverse LTP and behavior (Ling et al., 2002; Pastalkova et al., 2006; Serrano et al., 2008), act similarly by increasing spontaneous activity and [Ca2+]i in primary cultures.
Abnormally high concentration of calcium in the cytoplasmic compartment is toxic and may lead to necrosis via activation of catabolic pathways (Kass and Orrenius, 1999; Berridge et al., 2000). Lim et al. (2008) reported a dose-dependent elevation of [Ca2+]i in response to ZIP application onto human mast cells (HMC-1), which quickly led to cell degranulation. Using a viability assay (Fig. 6), we show that ZIP produced a rapid dose-dependent cell death. These events occurred primarily at 5 μm and above. Lower ZIP concentrations produced detectible morphological changes, including cell swelling and dendritic beading, similarly to those expected in excitotoxic stress (Park et al., 1996; Ikegaya et al., 2001).
The concentration of ZIP following diffusion in in vivo experiments has been debated (Lisman, 2012; Sacktor and Fenton, 2012). An injection of 10 mm ZIP solution has been used in most of the behavioral studies, assuming a gradient to a concentration of 5 μm (Sacktor and Fenton, 2012) at the site of action, which however, was estimated to be 100 μm by Lisman, (2012). The larger complexity and internal milieu in situ notwithstanding, our results in culture and in slice suggest that an injection of a 10 mm solution is expected to cause an elevation of firing rate, an increase in membrane noise and [Ca2+]i, and ultimately cause cell death near the injection site, all of which may contribute to memory loss. Since ZIP-induced amnesia was reported to still leave the affected brain system capable of relearning the same task, the possibility cannot be excluded that the long-term memory encoding cells are preferentially targeted by ZIP in vivo, or that only part of the circuit required for encoding and memory is affected, resulting in graceful degradation of the circuit and leaving parts of it capable of relearning.
In attempting to interpret our results, one should consider the molecular structure of ZIP and its similarity to bacterial and synthetic lipopeptides, which consist of four to five positive amino acid residues and are attached to a lipid moiety that compensates for the short amino acid chain and allows them to permeate the plasma membrane (Makovitzki et al., 2006). Also, this may explain why ZIP and Scr-ZIP both induce increased activity and cell death, as only the order, but not the amino acid composition of the peptide is changed. In that respect it is worth noting that another drug used for studying LTP and behavior, chelerythrine, has been shown to have antibacterial properties, and cause cell death (Yamamoto et al., 2001).
ZIP was reported not to alter in vivo the rat-evoked EPSPs of dentate gyrus neurons to perforant path stimulation, although the published records show a complete blockade of population spikes by ZIP (Pastalkova et al., 2006) and CA1 single-cell firing up to a day after injection (Barry et al., 2012). The possibility should be considered that multiple effects of ZIP are favored differentially in different preparations. Furthermore, it is of note that the evidence for the involvement of PKMζ in long-term memory is not based solely on the use of ZIP, though many studies do conclude that PKMζ is involved because of the effect of ZIP. Established long-term memory was enhanced or blocked, respectively, by overexpression of the PKMζ gene or of the dominant-negative version of it at least 1 week after learning was established (Shema et al., 2011). These findings, combined with our current report, call for further investigation of cellular targets of ZIP in situ, and particularly its relevance to PKMζ and memory.
Footnotes
We thank Efrat Biton for the preparation of the cultures, Dr. Eduard Korkotian for help with the imaging, and Noga Cohen and Uri Korisky for help with data analysis.
The authors declare no competing financial interests.
References
- Barry JM, Rivard B, Fox SE, Fenton AA, Sacktor TC, Muller RU. Inhibition of protein kinase Mζ disrupts the stable spatial discharge of hippocampal place cells in a familiar environment. J Neurosci. 2012;32:13753–13762. doi: 10.1523/JNEUROSCI.0319-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- Goldin M, Segal M, Avignone E. Functional plasticity triggers formation and pruning of dendritic spines in cultured hippocampal networks. J Neurosci. 2001;21:186–193. doi: 10.1523/JNEUROSCI.21-01-00186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt O, Migues PV, Hastings M, Wong J, Nader K. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus. 2010;20:691–695. doi: 10.1002/hipo.20708. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain: implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: practical and functional considerations. Neuron. 2008;59:902–913. doi: 10.1016/j.neuron.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Hirai T, Niino YS, Chida K. PKCζII, a small molecule of protein kinase C ζ, specifically expressed in the mouse brain. Neurosci Lett. 2003;348:151–154. doi: 10.1016/S0304-3940(03)00780-8. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Kim JA, Baba M, Iwatsubo T, Nishiyama N, Matsuki N. Rapid and reversible changes in dendrite morphology and synaptic efficacy following NMDA receptor activation: implication for a cellular defense against excitotoxicity. J Cell Sci. 2001;114:4083–4093. doi: 10.1242/jcs.114.22.4083. [DOI] [PubMed] [Google Scholar]
- Kass GE, Orrenius S. Calcium signaling and cytotoxicity. Environ Health Perspect. 1999;107:25–35. doi: 10.2307/3434469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Gilmartin MR, Helmstetter FJ. Intra-amygdala infusion of the protein kinase Mζ inhibitor ZIP disrupts foreground context fear memory. Neurobiol Learn Mem. 2012;98:148–153. doi: 10.1016/j.nlm.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, McMahon T, Dadgar J, Fischbach-Weiss SC, Messing RO. Prkcz null mice show normal learning and memory. Nature. 2013;493:416–419. doi: 10.1038/nature11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Choi JW, Kim HS, Kim YH, Yea K, Heo K, Kim JH, Kim SH, Song M, Kim JI, Ryu SH, Suh PG. A myristoylated pseudosubstrate peptide of PKC-zeta induces degranulation in HMC-1 cells independently of PKC-zeta activity. Life Sci. 2008;82:733–740. doi: 10.1016/j.lfs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mζ enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Lisman J. Memory erasure by very high concentrations of ZIP may not be due to PKM-ζ. Hippocampus. 2012;22:648–649. doi: 10.1002/hipo.20980. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J Neurosci. 2009;29:2857–2866. doi: 10.1523/JNEUROSCI.4399-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovitzki A, Avrahami D, Shai Y. Ultrashort antibacterial and antifungal lipopeptides. Proc Natl Acad Sci U S A. 2006;103:15997–16002. doi: 10.1073/pnas.0606129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-C. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3:215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006a;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Fenton AA. Appropriate application of ZIP for PKMζ inhibition, LTP reversal, and memory erasure. Hippocampus. 2012;22:645–647. doi: 10.1002/hipo.20978. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: the role of protein kinase Mζ in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shema R, Hazvi S, Sacktor TC, Dudai Y. Boundary conditions for the maintenance of memory by PKMzeta in neocortex. Learn Mem. 2009;16:122–128. doi: 10.1101/lm.1183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mζ in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- Slepian MJ, Massia SP, Whitesell L. Pre-conditioning of smooth muscle cells via induction of the heat shock response limits proliferation following mechanical injury. Biochem Biophys Res Commun. 1996;225:600–607. doi: 10.1006/bbrc.1996.1217. [DOI] [PubMed] [Google Scholar]
- Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, Beck H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK. The role of altered [Ca2+]i regulation in apoptosis, oncosis, and necrosis. Biochim Biophys Acta. 1996;1313:173–178. doi: 10.1016/0167-4889(96)00086-9. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-ζ is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–423. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kraus LM, Sacktor TC, Francis JT. Brezina V, editor. Erasing sensorimotor memories via PKMzeta inhibition. PLoS One. 2010;5:e11125. doi: 10.1371/journal.pone.0011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC. Cellular pharmacology of protein kinase Mζ (PKMζ) contrasts with its in vitro profile: implications for PKMζ as a mediator of memory. J Biol Chem. 2012;287:12879–12885. doi: 10.1074/jbc.M112.357244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Zhu ZZ, Han HB, Liu JF, Meng SQ, Chen C, Yang JL, Wu P, Lu L. Overexpression of protein kinase Mζ in the prelimbic cortex enhances the formation of long-term fear memory. Neuropsychopharmacology. 2015;40:2146–2156. doi: 10.1038/npp.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Seta K, Morisco C, Vatner SF, Sadoshima J. Chelerythrine rapidly induces apoptosis through generation of reactive oxygen species in cardiac myocytes. J Mol Cell Cardiol. 2001;33:1829–1848. doi: 10.1006/jmcc.2001.1446. [DOI] [PubMed] [Google Scholar]