The alternative sigma factor RpoE is essential for the virulence of Vibrio alginolyticus toward marine fish, coral, and other animals in response to sea surface temperature increases. In this study, we characterized another alternative sigma factor, RpoX, which is induced at high temperatures and under low-osmotic-stress conditions. The expression of rpoX is under the tight control of RpoE and RpoX. Although RpoE and RpoX coregulate 105 genes, they are programming different regulatory functions in stress responses and virulence in V. alginolyticus. These findings illuminated the RpoE-RpoX-centered regulatory cascades and their distinct and overlapping regulatory roles in V. alginolyticus, which facilitates unraveling of the mechanisms by which the bacterium causes diseases in various sea animals in response to temperature fluctuations as well as the development of appropriate strategies to tackle infections by this bacterium.
KEYWORDS: ChIP-seq, RNA-seq, RpoE, RpoX, Vibrio alginolyticus, virulence
ABSTRACT
Vibrio alginolyticus is one of the most abundant microorganisms in marine environments and is also an opportunistic pathogen mediating high-mortality vibriosis in marine animals. Alternative sigma factors play essential roles in bacterial pathogens in the adaptation to environmental changes during infection and the adaptation to various niches, but little is known about them for V. alginolyticus. Our previous investigation indicated that the transcript level of the gene rpoX significantly decreased in an RpoE mutant. Here, we found that rpoX was highly expressed in response to high temperature and low osmotic stress and was under the direct control of the alternative sigma factor RpoE and its own product RpoX. Moreover, transcriptome sequencing (RNA-seq) results showed that RpoE and RpoX had different regulons, although they coregulated 105 genes at high temperature (42°C), including genes associated with biofilm formation, motility, virulence, regulatory factors, and the stress response. RNA-seq and chromatin immunoprecipitation sequencing (ChIP-seq) analyses as well as electrophoretic mobility shift assays (EMSAs) revealed the distinct binding motifs of RpoE and RpoX proteins. Furthermore, quantitative real-time reverse transcription-PCR (qRT-PCR) analysis also confirmed that RpoX can upregulate genes associated with flagella, biofilm formation, and hemolytic activities at higher temperatures. rpoX abrogation does not appear to attenuate virulence toward model fish at normal temperature. Collectively, data from this study demonstrated the regulatory cascades of RpoE and an alternative sigma factor, RpoX, in response to heat and osmotic stresses and their distinct and overlapping roles in pathogenesis and stress responses in the marine bacterium V. alginolyticus.
IMPORTANCE The alternative sigma factor RpoE is essential for the virulence of Vibrio alginolyticus toward marine fish, coral, and other animals in response to sea surface temperature increases. In this study, we characterized another alternative sigma factor, RpoX, which is induced at high temperatures and under low-osmotic-stress conditions. The expression of rpoX is under the tight control of RpoE and RpoX. Although RpoE and RpoX coregulate 105 genes, they are programming different regulatory functions in stress responses and virulence in V. alginolyticus. These findings illuminated the RpoE-RpoX-centered regulatory cascades and their distinct and overlapping regulatory roles in V. alginolyticus, which facilitates unraveling of the mechanisms by which the bacterium causes diseases in various sea animals in response to temperature fluctuations as well as the development of appropriate strategies to tackle infections by this bacterium.
INTRODUCTION
The Gram-negative bacterium Vibrio alginolyticus is a halophilic bacterium that is mainly found in marine and estuarine environments, causing high-mortality outbreaks of vibriosis in sea animals; this bacterium is also a notorious foodborne pathogen for humans (1, 2). Similar to other bacteria, the virulence of V. alginolyticus is strictly regulated by environmental factors such as cell density and temperature (3). In our previous study, we reported that the genes involved in the main virulence-associated characteristics of V. alginolyticus, such as biofilm formation, motility, extracellular proteases (Asp, Pep, and MviN), siderophore-dependent iron uptake systems, and type III and VI secretion systems (T3SS and T6SS), are tightly regulated by quorum sensing (QS) (4–10).
Alternative sigma (σ) factors are global regulators that enable the expression of genes associated with stress adaptation and virulence in response to diverse stimuli in both the environment and the host in vibrios (11–13). Our previous investigation demonstrated that the temperature-dependent binding of RpoE to distinct promoters appears to underlie a σE-controlled switch between the expression of virulence genes and adaptation to thermal stress (3). The RpoE protein is essential for the growth of V. alginolyticus, V. cholerae, and V. parahaemolyticus at high temperatures (3, 14–16). RpoS is another well-characterized alternative sigma factor, which controls virulence and cellular responses to reactive oxygen species (ROS), starvation, DNA damage, extreme temperatures, ethanol, and hyperosmolarity (17–19). In V. alginolyticus, RpoS is part of the regulatory networks of virulence and the LuxS quorum-sensing system and responds to high-temperature stress (20). In addition, the alternative sigma factor RpoH activates the transcription of genes involved in the heat shock response in various bacterial species, including vibrios (21). The RpoE protein can directly bind to the promoter of rpoH and activate its expression to respond and adapt to high-temperature conditions in vibrios (3, 22). In V. alginolyticus, the gene rpoX, annotated to encode an RpoS-like alternative sigma factor, was recently implicated as being involved in stress adaptation (23). The detailed regulatory roles associated with rpoX as well as the underlying mechanisms remain unclear.
Our recent transcriptomic analysis of V. alginolyticus identified that rpoX was significantly downregulated in the rpoE mutant (3). In this study, we characterized the roles of the rpoX gene in adaptation to heat stress and the regulation of virulence gene expression. The expression of rpoX was under the strict control of RpoE in response to high temperature and low osmotic stress. In addition, the regulons of RpoE and RpoX were defined with transcriptome sequencing (RNA-seq) and chromatin immunoprecipitation sequencing (ChIP-seq) analyses to illuminate their distinct and overlapping regulatory roles in the stress response, biofilm formation, motility, hemolytic activity, and virulence. These data enriched our understanding of the basis and regulatory networks of vibrio adaptation to osmosis, heat, and other stresses.
RESULTS
RpoX is induced by high temperature and low osmotic stress.
A BLASTP analysis showed that the RpoX protein contains three conserved functional domains: sigma 70 region 1.2 (residues 27 to 59), sigma 70 region 2 (residues 65 to 125), and sigma 70 region 4 (residues 230 to 287) (see Fig. S1A in the supplemental material). In addition, an analysis of the conserved functional domains of the RpoE, RpoH, RpoS, and RpoD proteins from V. alginolyticus indicated that even though the RpoX protein was initially annotated as an RpoS-like sigma factor (23), this protein lacked the featured sigma region 3 of the RpoS protein. In addition, the three conserved domains of RpoX were highly similar (with 27% identity and 45% similarity) to the RpoH sigma factor, which could be directly modulated by the RpoE protein and was responsive to high-temperature stress (Fig. S1A). The BLASTP analysis also indicated that the V. alginolyticus RpoX protein shared 99%, 83%, and 78% identities with the homologous proteins Vp1393 from V. parahaemolyticus, A1Q_0985 from V. harveyi, and ATB83_RS10190 from V. splendidus, respectively (Fig. S1B). These analyses indicated that RpoX is highly conserved in these bacteria and might be an important part of the RpoE regulon.
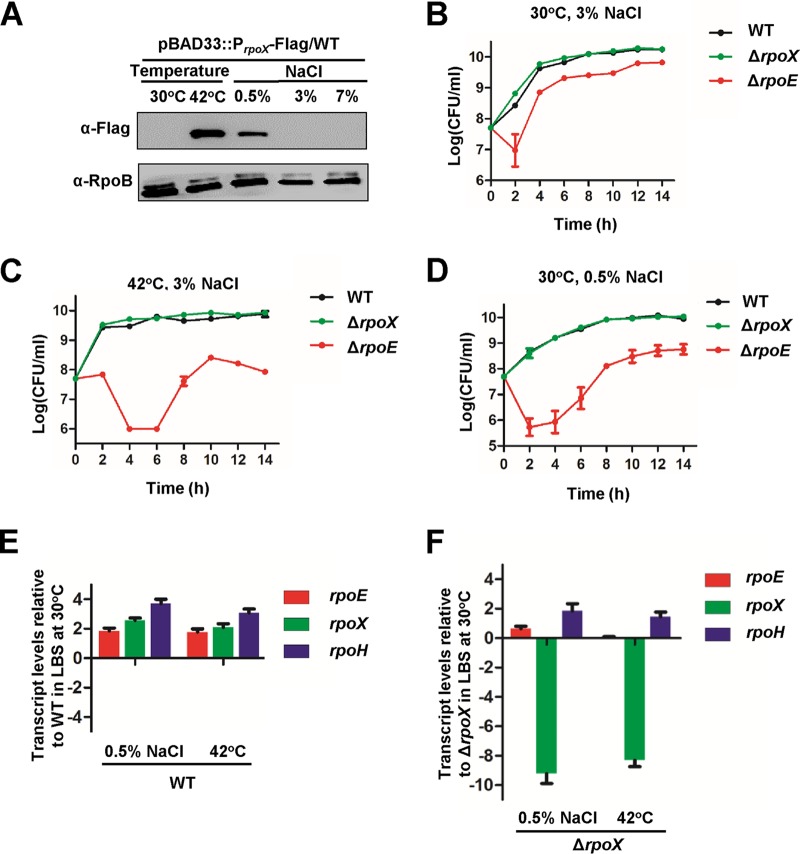
Our previous study indicated that the RpoE protein contributed to different stress responses in V. alginolyticus, such as the responses to high-temperature stress, osmotic stress, and H2O2 (3), and the rpoS mutant strain of V. alginolyticus was also defective in resistance to environmental stresses (20). Although RpoX has been suggested to be involved in stress adaptations, its exact biological roles remain undetermined (23). Therefore, we first examined the expression of RpoX under various stress conditions with a V. alginolyticus strain carrying an rpoX promoter reporter. RpoX protein could be expressed at a high temperature (42°C) and under low-osmotic-stress conditions (0.5% NaCl) (Fig. 1A). The growth of the wild-type (WT), ΔrpoE, and ΔrpoX strains was monitored at 30°C (Fig. 1B) and 42°C (Fig. 1C) in Luria-Bertani (LB) broth containing 3% NaCl and at 30°C in LB broth containing 0.5% NaCl (Fig. 1D), respectively. The severely impaired growth of V. alginolyticus at 42°C in LB broth containing 0.5% NaCl excluded further experiments under these conditions. The ΔrpoE strain had a marked growth reduction under its optimal growth conditions (30°C with 3% NaCl) (Fig. 1B) and had a longer lag phase and reached a drastically lower stationary-phase growth rate than the WT strain at 42°C and in 0.5% NaCl (Fig. 1B to D). However, the ΔrpoX strain did not exhibit a significant difference in growth compared to the WT strain under these conditions (Fig. 1B to D). These investigations indicated that the RpoX protein can be induced at 42°C and in 0.5% NaCl but might not be required for the survival of V. alginolyticus under these conditions.
FIG 1.
Stress-responsive rpoX expression in V. alginolyticus. (A) Western blot assay of RpoX levels in V. alginolyticus strains grown under various temperature or osmotic conditions. A Flag-tag-specific antibody was used to probe WT cells expressing RpoX-Flag driven by the native rpoX promoter. RpoB was used as a loading control. (B to D) Growth curves of WT, ΔrpoX, and ΔrpoE strains in LB medium containing 3% NaCl at 30°C, the normal growth conditions for this halophile (B); 3% NaCl at 42°C (C); and 0.5% NaCl at 30°C (D). Samples were taken and plate counted after serial dilutions with fresh LBS medium. (E and F) qRT-PCR analysis of the transcript levels of rpoX, rpoE, and rpoH in WT (E) and ΔrpoX (F) cells cultured under different stress conditions relative to WT and ΔrpoX cells grown in LBS medium at 30°C, respectively. Total RNA was isolated from the strains after 9 h of growth. The results are presented as the means ± standard deviations (SD) (n = 3).
The RpoE sigma factor can directly regulate the expression of RpoH (3), which shares the same functional domains as the RpoX protein and responds to different types of stress. Therefore, we suspect that the ΔrpoX strain did not exhibit decreased survival at 42°C and in 0.5% NaCl due to induction of the expression of rpoH in the ΔrpoX strain, which might rescue bacterial growth under these stress conditions. After the failure of the trials to generate an rpoH deletion or null mutant to test this hypothesis, we resorted to detection of the expression of rpoE, rpoX, and rpoH in the WT and ΔrpoX strains at 42°C and in 0.5% NaCl. Indeed, the expression of rpoE, rpoX, and rpoH markedly increased in the WT strain cultured in 0.5% NaCl and at 42°C (Fig. 1E). In the rpoX mutant strain, the transcription level of rpoH in 0.5% NaCl and at 42°C was ∼2-fold higher than that under culture conditions of 3% NaCl at 30°C, while the transcription of rpoE exhibited no significant difference between both culture conditions (Fig. 1F), which indicated that the increased expression of rpoH might compensate for the abrogation of RpoX protein to regulate the responses to these stress factors in the ΔrpoX strain. Moreover, these data also suggested that rpoX might be involved in the transcription of rpoE. Taken together, these findings suggested that RpoX is induced by and might be involved in the stress response to 42°C (high temperature) and 0.5% NaCl (low-osmotic-stress conditions).
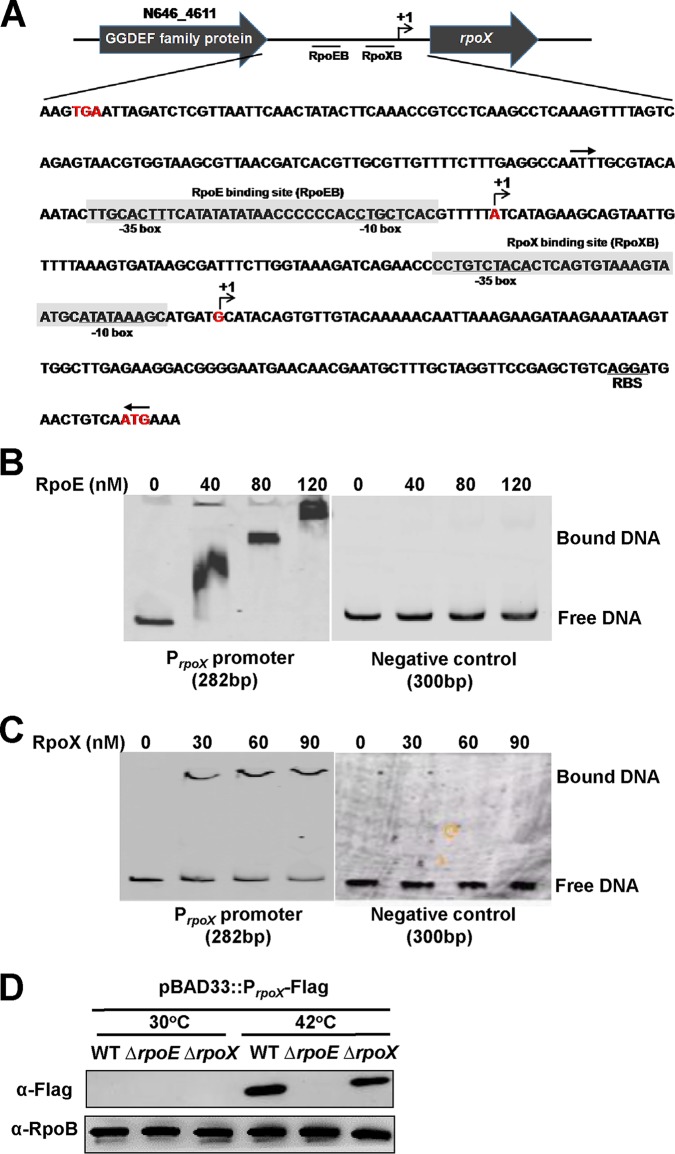
RpoE and RpoX directly bind to the rpoX promoter and activate its transcription.
Based on sequence analysis, the rpoX promoter region contains the conserved −35 motif “GCACTTT,” the −10 motif “TGCTCA” for an RpoE-binding site (Fig. 2A), and the predicted transcriptional start site +1A. However, a 5′ rapid amplification of cDNA ends (RACE) study found another transcriptional start site, +1G (Fig. 2A), which is located 104 bp downstream of the predicted RpoE-binding site, so we suggested that another promoter sequence might exist in the rpoX promoter. In addition, sequence analysis showed another putative sigma factor-binding sequence (TGTCTACA/ATATAAA [−35/−10 motifs]), which was located directly upstream of the transcriptional start site +1G, and we speculate that this site might be the specific binding site of the RpoX protein (Fig. 2A). Overall, the sequence analysis showed that the rpoX promoter contains two conserved binding sites. An electrophoretic mobility shift assay (EMSA) was then used to determine whether the RpoE and RpoX proteins could directly bind to the rpoX promoter. As expected, the RpoE protein bound directly to the rpoX promoter region in a concentration-dependent manner in the presence of high concentrations (10-fold) of a nonspecific poly(dI-dC) competitor, and the RpoE protein could not bind to a negative-control DNA (Fig. 2B). In addition, the EMSA results also showed that the RpoX protein bound directly to its own promoter (Fig. 2C).
FIG 2.
RpoE and RpoX directly bind to the rpoX promoter. (A) Diagram showing the promoter region of the rpoX gene. The RpoE- and RpoX-binding sites and the ribosome-binding site (RBS) are underlined. The regions protected by RpoE/RpoX are shadow boxed. The transcription start sites are labeled as +1 and marked in red. The red letters “TGA” and “ATG” are the stop codon of N646_4611 and the start codon of rpoX, respectively. (B and C) EMSA of RpoE and RpoX specifically binding to the rpoX promoter. Various concentrations of the RpoE and RpoX proteins were added to mixtures of poly(dI-dC) and Cy5-labeled rpoX promoter DNA (a 282-bp fragment of the promoter adjoining the start codon “ATG,” as indicated with the pair of arrows above the sequence). The same reactions were also carried out for a 300-bp fragment of the gyrB promoter region (negative control), which cannot be bound by RpoX and RpoE. (D) Western blotting of RpoX levels in WT, ΔrpoE, and ΔrpoX cells harboring pBAD33::PrpoX-Flag and grown at normal (30°C) or high (42°C) temperatures. RpoB was used as a loading control for the blots.
Given the direct interaction of the RpoE and RpoX proteins with the promoter of rpoX, we next determined whether the RpoE and RpoX proteins could regulate the expression of rpoX in vivo. We transformed the pBAD33::PrpoX-Flag plasmid into WT, ΔrpoE, and ΔrpoX strains, and Western blotting confirmed that the expression of RpoX markedly decreased in the ΔrpoX mutant but was abolished in the ΔrpoE strain at 42°C (Fig. 2D). Taken together, these results show that the expression of rpoX was directly regulated by the sigma factors RpoE and RpoX.
Global analyses of the regulons of RpoE and RpoX in V. alginolyticus.
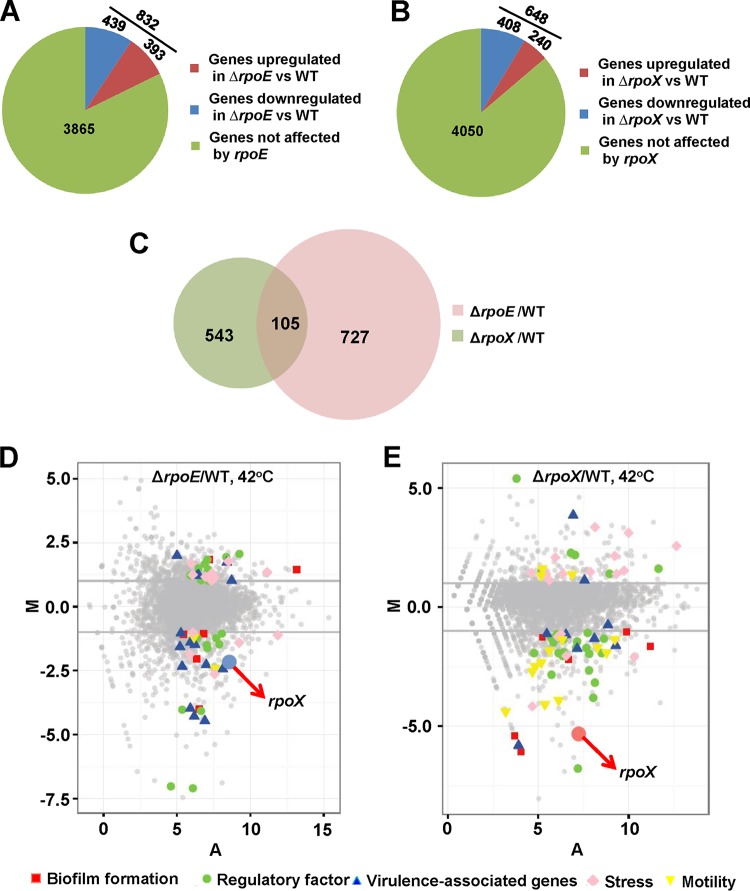
Because RpoE and RpoX were found to be important in the response to high temperatures, we identified genes that are regulated by RpoE and RpoX at high temperatures by comparing the transcriptomes of the WT, ΔrpoX, and ΔrpoE strains at 42°C. The results showed that many genes were regulated by the RpoE and RpoX proteins at high temperatures (the 50 most upregulated and most downregulated genes are listed in Table 1). Comparison of the RNA-seq data for the WT and ΔrpoE strains at 42°C showed that 393 (8.4%) and 440 (9.4%) of the annotated genes were up- and downregulated (log2 fold change [log2FC] ≥ 2 or log2FC ≤ −2; P < 0.001), respectively, in the ΔrpoE strain (Fig. 3A). In addition, comparison of the RNA-seq data for the WT and ΔrpoX strains at 42°C showed that 240 (5.1%) and 408 (8.7%) genes were up- and downregulated (log2FC ≥ 2 or log2FC ≤ −2; P < 0.001), respectively, in the ΔrpoX strain (Fig. 3B). To identify the coregulon of RpoE and RpoX, we compared the RNA-seq data and identified 105 overlapping genes between the regulons of RpoE and RpoX (Fig. 3C), including genes associated with biofilm formation, motility, and stress adaptation. These data suggested that both the RpoE and RpoX proteins are global regulators in V. alginolyticus in response to high-temperature stress.
TABLE 1.
Genes coregulated by RpoE and RpoX at 42°C
| Gene identificationa | Annotationb | Fold change (ΔrpoE/WT) | Fold change (ΔrpoX/WT) | Promoter region bound by RpoX determined by ChIP-seqc | Virulence-associated gened |
|---|---|---|---|---|---|
| N646_4603 | Hypothetical protein | −175.96 | −1,081.83 | Y | |
| N646_4599 | Hypothetical protein | −14.42 | −263.51 | ||
| N646_4604 | Hemolysin D | −21.50 | −119.59 | Y | Y |
| N646_0533 | Anti-anti-sigma regulatory factor | −134.98 | −108.89 | Y | |
| N646_3842 | Hypothetical protein | −2.42 | −104.79 | ||
| N646_4684 | Hypothetical protein | −4.74 | −79.41 | ||
| N646_4605 | Hypothetical protein | −9.17 | −55.37 | ||
| N646_3940 | Hypothetical protein | −5.11 | −47.04 | ||
| N646_0523 | Putative membrane protein of ExoQ family, involved in exopolysaccharide production | −2.49 | −42.23 | Y | |
| N646_4610 | Hypothetical protein, RpoX | −4.44 | −39.73 | Y | Y |
| N646_1050 | Hypothetical protein | −3.20 | −31.18 | ||
| N646_4601 | Hypothetical protein | −44.33 | −29.20 | Y | |
| N646_3768 | Hypothetical protein | −6.06 | −21.91 | ||
| N646_0526 | Putative galactosyltransferase | −5.28 | −19.04 | ||
| N646_4606 | ABC transporter outer membrane component | −2.55 | −17.99 | ||
| N646_1186 | Sodium-type flagellar protein MotY | −2.35 | −16.89 | Y | |
| N646_4602 | Hypothetical protein | −29.71 | −16.78 | ||
| N646_0512 | FMN-dependent NADH-azoreductase | −2.68 | −16.44 | ||
| N646_4598 | Hypothetical protein | −6.38 | −14.22 | ||
| N646_4600 | Hypothetical protein | −31.74 | −11.95 | Y | |
| N646_0722 | Hypothetical protein | −2.15 | −11.33 | ||
| N646_2916 | Hypothetical protein | −20.26 | −10.69 | ||
| N646_2929 | Polar flagellar FlgF | −2.52 | −10.37 | Y | |
| N646_1399 | Hypothetical protein | −1.41 | −10.16 | ||
| N646_4608 | Hypothetical protein | −3.93 | −10.07 | ||
| N646_1161 | Hypothetical protein | −3.12 | −9.17 | ||
| N646_1591 | Hypothetical protein | −2.23 | −8.63 | ||
| N646_0708 | Putative acetyltransferase | −5.03 | −8.55 | ||
| N646_4609 | Hypothetical protein | −4.83 | −8.51 | Y | |
| N646_3939 | Hypothetical protein | −3.08 | −7.66 | ||
| N646_0532 | Hypothetical protein | −32.48 | −7.18 | ||
| N646_0389 | Hypothetical protein | −4.52 | −6.73 | ||
| N646_0390 | Hypothetical protein | −4.98 | −6.44 | ||
| N646_0562 | Hypothetical protein | −4.71 | −6.43 | ||
| N646_0384 | Putative dioxygenase | −2.40 | −6.38 | ||
| N646_3470 | GTP cyclohydrolase II | −2.57 | −6.38 | ||
| N646_0714 | Transcriptional regulator, GntR family protein | −2.75 | −6.24 | ||
| N646_4629 | Cytochrome c oxidase, subunit II | −2.03 | −5.99 | ||
| N646_1446 | 50S ribosomal protein L31 | −0.46 | −5.88 | ||
| N646_0176 | Hypothetical protein | −2.28 | −5.39 | ||
| N646_0385 | 2-Keto-4-pentenoate hydratase/2-oxohepta-3-ene-1,7-dioic acid hydratase | −2.42 | −4.98 | ||
| N646_3844 | Pirin-related protein | −2.69 | −4.98 | ||
| N646_0013 | Hypothetical protein | −111.19 | −4.81 | ||
| N646_4302 | Hypothetical protein | −3.03 | −4.81 | ||
| N646_0530 | Putative capsular polysaccharide biosynthesis | −4.10 | −4.59 | Y | |
| N646_2548 | Glycerol dehydrogenase | −4.40 | −4.31 | ||
| N646_1338 | Flagellar biosynthesis protein FlhF | −5.32 | −3.76 | Y | |
| N646_1844 | Putative fimbrial assembly protein PilM | −2.05 | −3.74 | Y | |
| N646_0713 | Carboxyphosphonoenolpyruvate phosphonomutase | −2.70 | −3.71 | ||
| N646_0456 | Hypothetical protein | −2.36 | −3.61 | ||
| N646_4031 | Hypothetical protein | −7.04 | −3.52 | ||
| N646_4030 | Putative phenylacetate-CoA ligase | −8.34 | −3.41 | ||
| N646_2724 | DNA-binding response regulator PhoB | −2.33 | −3.26 | Y | |
| N646_4032 | Oxidoreductase | −6.58 | −3.26 | ||
| N646_2270 | Hypothetical protein | −3.55 | −3.16 | ||
| N646_3227 | CsuA | −22.07 | −3.06 | Y | |
| N646_4508 | Enoyl-CoA hydratase | −2.04 | −2.99 | ||
| N646_0645 | Amino acid ABC transporter, periplasmic amino-acid-binding protein | −2.64 | −2.89 | ||
| N646_4675 | Hypothetical protein | −2.06 | −2.71 | ||
| N646_2932 | Flagellar P-ring protein FlgI | −3.54 | −2.67 | Y | |
| N646_0301 | Hypothetical protein | −5.28 | −2.67 | ||
| N646_4059 | Hypothetical protein | −2.10 | −2.66 | ||
| N646_2799 | Small protein A | −2.16 | −2.60 | ||
| N646_2936 | Flagellin | −2.28 | −2.57 | Y | |
| N646_3225 | CsuC | −15.77 | −2.49 | Y | |
| N646_4417 | Hypothetical protein | −10.82 | −2.40 | ||
| N646_3072 | Hypothetical protein | −2.52 | −2.36 | ||
| N646_4028 | Hypothetical protein | −14.67 | −2.36 | ||
| N646_0455 | Hypothetical protein | −2.10 | −2.27 | ||
| N646_0799 | Hypothetical protein | −3.72 | −2.27 | ||
| N646_0531 | Periplasmic protein involved in polysaccharide export | −16.16 | −2.27 | Y | |
| N646_0807 | Hypothetical protein | −2.45 | −2.24 | ||
| N646_3224 | CsuD | −5.06 | −2.22 | Y | |
| N646_4029 | Putative high-affinity branched-chain-amino-acid transport ATP-binding protein | −11.73 | −2.21 | ||
| N646_0145 | Tryptophanyl-tRNA synthetase | −2.99 | −2.19 | ||
| N646_3223 | CsuE | −2.97 | −2.17 | Y | |
| N646_0685 | Hypothetical protein | −3.58 | −2.04 | ||
| N646_4419 | Hypothetical protein | −2.18 | −2.03 | ||
| N646_0561 | Formate dehydrogenase accessory protein | −3.67 | −2.00 | ||
| N646_0626 | Hypothetical protein | 5.81 | 2.05 | ||
| N646_2558 | Hypothetical protein | 2.20 | 2.05 | ||
| N646_0313 | Imidazolonepropionase | 2.47 | 2.07 | ||
| N646_2232 | Hypothetical protein | 3.12 | 2.10 | ||
| N646_3280 | Pyruvate formate-lyase | 2.88 | 2.10 | ||
| N646_1747 | Aspartate carbamoyltransferase regulatory subunit | 2.64 | 2.15 | ||
| N646_3214 | Hypothetical protein | 3.47 | 2.15 | ||
| N646_0304 | Outer membrane protein | 2.02 | 2.19 | ||
| N646_0641 | Hypothetical protein | 3.82 | 2.28 | ||
| N646_3434 | Putative ribosomal protein N-acetyltransferase | 2.58 | 2.39 | ||
| N646_2428 | UTP-glucose-1-phosphate uridylyltransferase | 2.36 | 2.47 | ||
| N646_4424 | Hypothetical protein | 2.15 | 2.47 | ||
| N646_4184 | Hypothetical protein | 2.06 | 2.56 | ||
| N646_3925 | Putative acriflavine resistance protein | 2.99 | 2.62 | ||
| N646_3664 | Thermolabile hemolysin | 2.75 | 2.65 | Y | |
| N646_4456 | Putative KHG/KDPG aldolase | 2.44 | 2.81 | ||
| N646_3202 | Putative ABC transporter membrane-spanning permease | 2.03 | 2.96 | ||
| N646_3782 | Hypothetical protein | 3.47 | 4.15 | ||
| N646_3806 | Alcohol dehydrogenase, zinc-binding domain protein | 3.66 | 4.21 | ||
| N646_3556 | Putative hydrolase | 3.26 | 5.04 | ||
| N646_2057 | Hypothetical protein | 2.95 | 5.26 | ||
| N646_4487 | Arginine ABC transporter, periplasmic arginine-binding protein | 11.25 | 5.30 | ||
| N646_3755 | Putative muconate cycloisomerase I | 2.24 | 6.51 | ||
| N646_3685 | Hypothetical protein | 5.67 | 7.51 | ||
| N646_3856 | Hypothetical protein | 3.03 | 7.62 | ||
| N646_3885 | Glyceraldehyde-3-phosphate dehydrogenase | 2.77 | 7.82 | ||
| N646_4611 | Hypothetical protein | 1.02 | −1.27 | Y | |
| N646_1623 | 50S ribosomal protein L19 | 1.09 | 2.37 | Y | |
| N646_1624 | tRNA (guanine-N1)-methyltransferase | 1.03 | 2.67 | Y |
All the genes with differential expression with a P value of <0.001.
FMN, flavin mononucleotide.
Y indicates that the promoter region of the gene was also bound by RpoX, as identified by ChIP-seq analysis.
Y indicates that the gene was annotated as a virulence-associated genes.
FIG 3.
Comparative analyses of the transcriptional responses of V. alginolyticus to rpoE and rpoX abrogation at 42°C. (A and B) Pie charts representing genes differentially transcribed in ΔrpoE (A) and ΔrpoX (B) cells compared to WT cells grown in LBS medium at 42°C. (C) Venn diagrams showing overlapping genes with significantly increased or decreased transcript abundances (FC ≥ 2 or FC ≤ −2; adjusted P value [Padj] of <1 × 10−2) in response to different culture conditions. (D and E) MA plots depicting changes in gene expression between ΔrpoE and WT strains (D) and between ΔrpoX and WT strains (E) in LBS medium at 42°C. The log2 value of the ratios of the abundances of each transcript between the two conditions (M) (y axis) is plotted against the average log2 value of the abundance of that transcript under both conditions (A) (x axis).
The data presented in Fig. 3D and E describe the expression patterns of genes that are potentially associated with virulence (including genes associated with biofilm formation, motility, and virulence), regulatory factors, and stress responses. Our previous study showed that the RpoE protein can regulate motility and virulence (3), and the RNA-seq data confirmed that rpoX transcription could be positively regulated (fold change of −2.4) by RpoE. In addition, the RpoX protein controls the expression of various genes involved in biofilm formation and motility, virulence-associated genes, and regulatory factors. Finally, we also found that the RpoX protein is involved in the stress response (n = 15) via the regulation of heat shock and cold shock proteins, outer membrane proteins, and proteins associated with multidrug resistance. Taken together, our results demonstrated that RpoE and RpoX are important regulatory factors at high temperatures and are responsible for the regulation of virulence-associated genes.
RpoE- and RpoX-binding motifs.
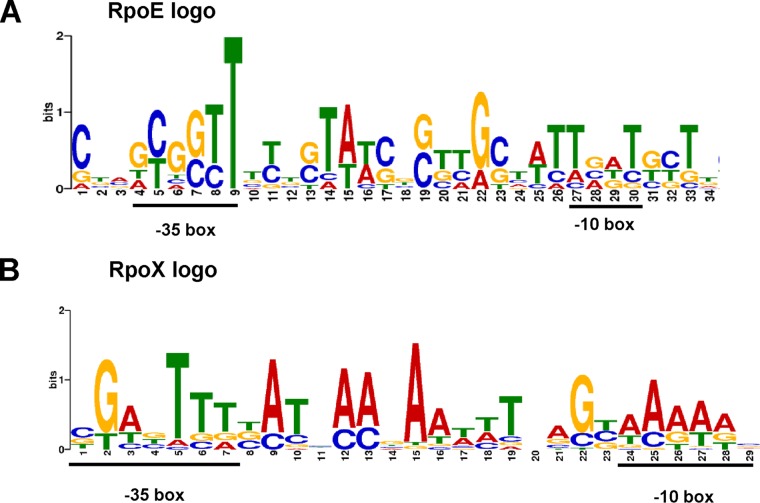
The binding motifs of RpoE and RpoX were generated by the MEME-suite tool (http://meme-suite.org) in search of the regulated genes’ promoter regions in the identified regulon of RpoE and RpoX. As shown in Fig. 4A, the −10 box and −35 box were identified, and the conserved binding site of RpoE was found to be similar to the established RpoE-binding site identified by ChIP-seq in other bacteria (24); the results of our previous study also showed a similar binding site for RpoE in the promoter of luxR (TGACCTT for the −35 region and TCATCA for the −10 region) (3). In addition, based on the RNA-seq data for RpoX at high temperatures, the conserved −35 box and −10 box of the RpoX-binding motif were also revealed (Fig. 4B), and the −35 box and −10 box sequences were similar to the predicted binding sites in the promoter of rpoX (TGTCTACA/ATATAAA) (Fig. 2A). The binding motifs show marked differences between the binding sites of the RpoE and RpoX sigma factors.
FIG 4.
Conserved binding site of RpoE and RpoX generated by RNA-seq data. The most significant RpoE-binding motif (A) or RpoX-binding motif (B) was derived from an RNA-seq binding sequence generated by the MEME-suite tool. The height of each letter represents the relative frequency of each base at different positions in the consensus sequence.
Identification of RpoX-binding regions by ChIP-seq.
We further used ChIP-seq experiments to investigate the possible RpoX-binding loci on the chromosome of V. alginolyticus cultured at a high temperature (42°C) and under low-osmotic-stress conditions (0.5% NaCl). We identified 9 enriched loci (fold change of >2.0; P < 0.01) harboring RpoX-binding peaks at a high temperature (42°C) but only 2 enriched loci exhibiting peaks under low-osmotic-stress conditions (0.5% NaCl), and these 2 peaks were also included in the high-temperature peaks (Table 1). Only 4 of these 9 peaks were located in intergenic regions. We thus chose the 4 related regions for EMSAs. Among these enriched loci, the rpoX promoter was first identified as a binding substrate of RpoX with a fold enrichment of 10.9, and EMSAs also confirmed that the RpoX protein could directly bind to its own promoter (Fig. 2C). In addition, the additional 3 peaks upstream of N646_4603 (7.6-fold), N646_4601 (6.1-fold), and N646_1623 (4.8-fold) were found to be located in distinct promoter regions (Fig. 5A to C). As expected, EMSAs validated that the RpoX protein bound directly to these promoters in the presence of high concentrations (10-fold) of a nonspecific poly(dI-dC) competitor (Fig. 2C and Fig. 5A to C). The gyrB promoter region was used as the negative control, and no peak was found in the promoter region of gyrB during ChIP analysis (Fig. 5D). Overall, four genes containing rpoX-binding sites were identified by EMSAs and ChIP-seq analysis. Among these four ChIP-identified promoter regions, rpoX, N646_4604 (N646_4603 and N646_4604 are in the same operon), and N646_4601 were positively regulated and N646_1623 was negatively regulated by RpoX, as revealed by RNA-seq analysis. Interestingly, the gene N646_4604 encodes RTX-type hemolysin D (HlyD), and HlyD has been reported to be a hemolysin in other bacteria (25), further suggesting a role for RpoX in the pathogenesis of V. alginolyticus.
FIG 5.
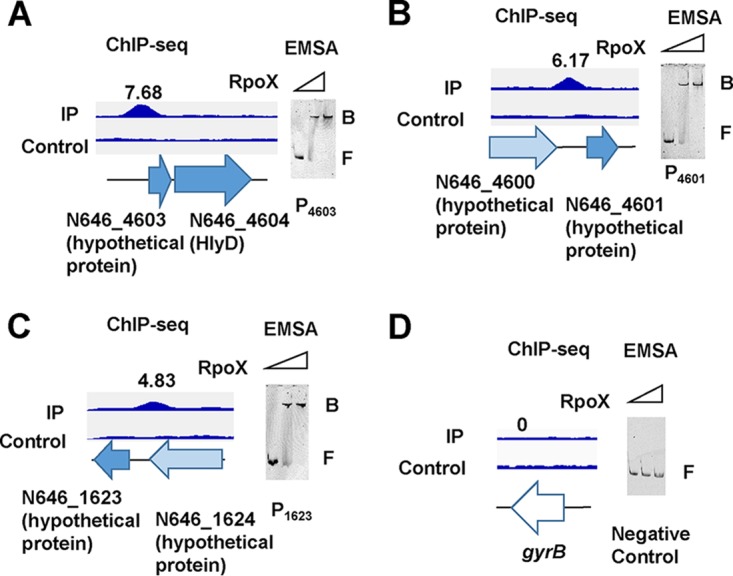
ChIP-seq analysis of genes directly bound and regulated by RpoX. (A to C) N646_4603 (A), N646_4601 (B), and N646_1623 (C) were used for peak comparison of ChIP-seq (left) and EMSA (right) results. The fold enrichment of each of the typical promoters bound by RpoX is shown. (D) A 300-bp fragment of the gyrB promoter region is shown as the negative control, which cannot be bound by RpoX. B, bound DNA; F, free DNA. The numbers above each of the peaks indicate the enrichment fold change relative to the control.
RpoX modulates biofilm formation, motility, and hemolytic activities in V. alginolyticus.
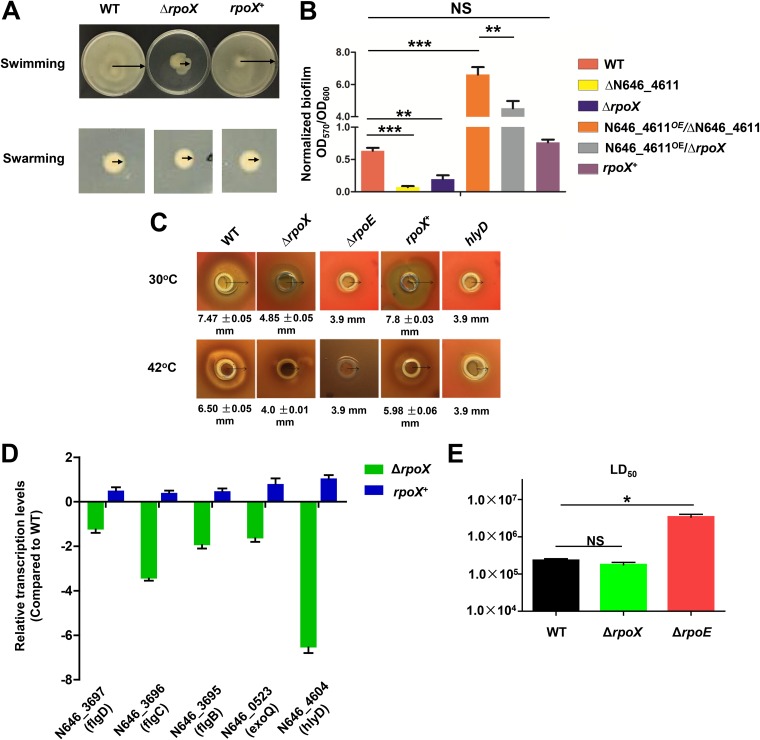
In our RNA-seq analysis, we found that several flagellum-related genes were regulated by the RpoE and RpoX proteins. We thus further investigated the roles of rpoX in the motility of this bacterium. The swimming ability was significantly reduced in the ΔrpoX strain compared with the WT strain, and rpoX complementation restored the swimming ability at high temperatures (Fig. 6A); however, there was no significant difference in swarming abilities between the WT and ΔrpoX strains (Fig. 6A).
FIG 6.
RpoX positively regulates motility, biofilm formation, and hemolytic activities. (A) Motility assays of WT, ΔrpoX, and rpoX+ strains. Diluted cultures were spotted onto swimming and swarming plates (containing 0.3% and 1.5% agar, respectively) and incubated for 48 h or 12 h at 42°C. Three independent cultures were used for each strain, and a representative result is displayed. (B) Assays of biofilm formation by different strains. For WT, ΔN646_4611, ΔrpoX, and rpoX+ strains, biofilm formation in glass tubes containing LBS medium after 48 h of culturing was assayed. N646_4611OE/ΔN646_4611 and N646_4611OE/ΔrpoX strains were cultured in LBS medium with 0.04% l-arabinose for 48 h. The results are presented as the means ± SD (n = 3). **, P < 0.01; ***, P < 0.001; NS, not significant (by t test). (C) Hemolytic activities of WT, ΔrpoX, ΔrpoE, rpoX+, and ΔluxR strains grown on sheep blood agar plates at 30°C (top) and at 42°C (bottom). (D) qRT-PCR analysis of the transcripts of the selected genes. Total RNA was isolated from the ΔrpoX and rpoX+ strains after 12 h of growth in liquid culture. The results are presented as the means ± SD (n = 3). (E) Median lethal dose (LD50) of WT, ΔrpoX, and ΔrpoE strains in zebrafish. Series of dilutions of WT, ΔrpoX, and ΔrpoE strains were intramuscularly inoculated into fish that were acclimated at 30°C for 4 weeks. A total of 30 fish were used for each of the dilutions. The infected fish were cultivated at 30°C and monitored for 7 days. The results are presented as the means ± SD (n = 3). *, P < 0.05 (by t test).
Furthermore, biofilm formation was significantly decreased in the ΔrpoX strain compared with the WT strain, and biofilm formation was restored when the rpoX gene was complemented (Fig. 6B). As shown in Fig. 2A, N646_4611 encodes a GGDEF family protein and is located upstream of the rpoX gene; GGDEF family proteins have been identified as regulators of biofilm formation and motility (24). Therefore, we constructed a ΔN646_4611 mutant strain and an N646_4611-overexpressing strain driven by the pBAD promoter in the ΔN646_4611 mutant strain (N646_4611OE/ΔN646_4611) with the induction of l-arabinose, which showed no apparent influence on V. alginolyticus biofilm formation. Biofilm formation was significantly reduced in the ΔN646_4611 mutant strain and enhanced in the N646_4611OE/ΔN646_4611 strain (Fig. 6B). To determine whether RpoX regulates biofilm formation via N646_4611, we overexpressed the N646_4611 protein in the ΔrpoX strain, N646_4611OE/ΔrpoX, and the level of biofilm formation by the strain was significantly lower than that by the N646_4611OE/ΔN646_4611 strain but higher than that by the N646_4611OE/ΔrpoX strain (Fig. 6B), which suggested that RpoX might not regulate biofilm formation via the N646_4611 protein. RNA-seq data showed that N646_4604, encoding RTX-type hemolysin D, was downregulated in the ΔrpoX strain. ΔrpoX cells showed weaker hemolytic activity than WT cells, and complementation of rpoX restored the activity to the WT level at 30°C and 42°C. The ΔrpoE mutant strain showed drastically reduced hemolytic activity similar to that of the ΔhlyD strain (Fig. 6C).
Quantitative real-time reverse transcription-PCR (qRT-PCR) was then further used to verify the roles of rpoX in the above-mentioned genes’ expression. The results showed that flagellum-related genes (flgD, flgC, and flgB) were downregulated in the ΔrpoX strain compared with the WT strain, and the expression of these genes was restored to WT levels in the rpoX-complemented strain (Fig. 6D). Moreover, the expression of the exopolysaccharide biosynthesis- and biofilm formation-associated gene exoQ and the hemolysin-related gene hlyD was significantly downregulated in the ΔrpoX strain compared to the WT strain, and complementation restored the expression of these genes (Fig. 6D). Taken together, these results demonstrated that the RpoX protein could modulate the expression of flagellum-, biofilm-, and hemolysis-related genes in V. alginolyticus.
Zebrafish were used as a model system to test the impact of RpoE and RpoX on the virulence of V. alginolyticus. The 50% lethal dose (LD50) values for the WT and ΔrpoE strains were 2.5 × 105 and 6.6 × 106 CFU/fish at 30°C, respectively, demonstrating an essential role of rpoE in V. alginolyticus virulence. However, the ΔrpoX strain exhibited an LD50 value of 1.8 × 105 CFU/fish (Fig. 6E), indicating that the deletion of rpoX did not significantly impair virulence toward fish. Collectively, these data illuminated the RpoE-RpoX-centered regulatory cascades and their distinct and overlapping regulatory functions in pathogenesis and in stress responses in V. alginolyticus.
DISCUSSION
Sigma factors can interact with the RNA polymerase (RNAP) core enzyme to generate an RNAP holoenzyme and initiate the transcription of a specific set of genes responsible for the stress response and virulence (26). Here, we identified the rpoX gene, included as part of the regulon of RpoE, and genetic analysis showed that RpoX lacked the region 3 domain that is present in the RpoS protein. We speculate that the RpoX protein might be a paralog of RpoH because they share the same functional domains (see Fig. S1A in the supplemental material) and 45% overall similarity, and both proteins are alternative sigma factors under subhierarchical control by RpoE and are involved in high-temperature and low-osmotic-stress responses (Fig. 1 and 2) (3). Moreover, the high expression level of RpoH seems to be able to rescue the growth defects of the ΔrpoX strain under high-temperature and low-osmotic-stress conditions (Fig. 1F). Further experiments with the rpoH null mutant to compare the regulons of RpoH and RpoX as well as their recognized promoters will validate their homology and functional redundancy in response to stresses.
The dozens of established alternative sigma factors, i.e., rpoH, rpoN, rpoE, and rpoS, etc., are all subject to tight regulation under various specific physiological conditions (12). Interestingly, our data indicated that the expression of rpoX was induced under both low-osmotic-stress conditions and high temperatures (Fig. 1). Although how these conditions exert influences on rpoX expression warrants further investigation, these observations suggested that this alternative sigma factor may be induced in vivo in marine animals or at extremely high sea surface temperatures, orchestrate gene expression in response to these stresses, and thus facilitate Vibrio adaptation under both in vivo and in vitro conditions of the hosts. Indeed, our further transcriptomic and phenotypic investigations indicated that RpoX was involved in the expression of various genes (Table 1 and Fig. 6). We thus unraveled a novel RpoX-involved signal transduction pathway in vibrios to respond to low-osmotic-stress and high-temperature stimuli.
As an alternative sigma factor, RpoE is released via regulated intramembrane proteolysis of the anti-sigma factor RseA triggered by membrane stresses (13) and has been found to be essential for stress adaptation and virulence in response to environmental stimuli in various bacterial pathogens, such as V. parahaemolyticus, V. harveyi, V. cholerae, and Salmonella (15, 16, 27–29). The RpoE protein can directly regulate the expression of the RpoH protein and is responsible for the high-temperature stress response of V. alginolyticus (3). In this study, we found that the RpoE protein can also bind directly to the promoter and control the expression of the RpoX protein (Fig. 2). RNA-seq was used to identify the regulons of the RpoX and RpoE proteins at a high temperature (42°C). The results showed that the regulon of RpoE contains more genes than that of RpoX (Fig. 3A and B), which, in addition to the result that the RpoE protein can directly bind to the promoter of rpoX and trigger the expression of rpoX (Fig. 2B to D), further confirmed that RpoX is at the subhierarchical level in the RpoE regulatory cascade; i.e., RpoE may act upstream of the regulatory cascade of RpoX. ChIP-seq and EMSA results also showed that the rpoX gene can be directly regulated by RpoX (Fig. 2C).
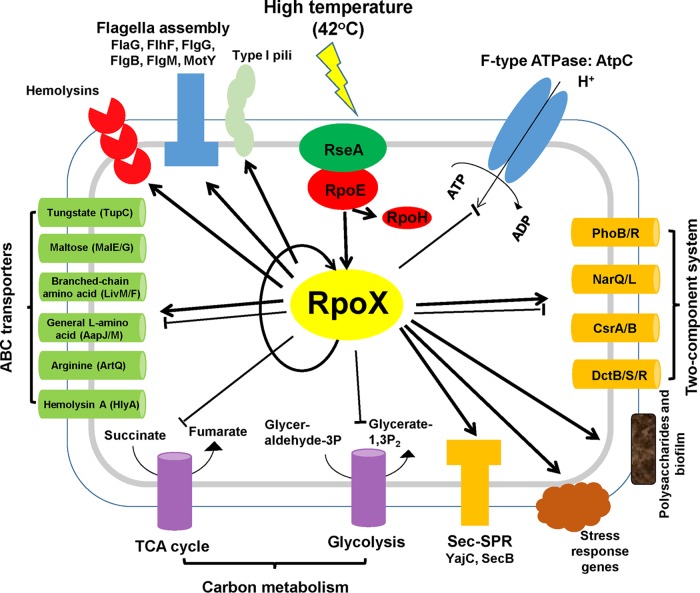
Sigma factors have been reported to be essential regulators of virulence, and we found that many virulence-associated genes, i.e., the genes encoding hemolysin (N646_4604 and N646_3664), exopolysaccharides (N646_0523 and N646_0530), flagella (N646_1186, N646_2929, N646_1338, N646_1844, N646_2932, and N646_2936), and type I pili (N646_3227, N646_3225, N646_3224, and N646_3223) (Fig. 7), were regulated by RpoX or RpoE at high temperatures (Table 1). Accordingly, our study has shown that RpoX was involved in the pathogenesis-related regulation of motility, biofilm formation, and hemolytic activity (Fig. 6). Although the ΔrpoX mutant strain did not show apparent attenuation of lethality to zebrafish (Fig. 6E), it may be related to the pathogenesis process and virulence factor production in an undiscerned manner in response to stresses such as reactive oxygen species (ROS), osmotic changes, and temperatures (23). In V. alginolyticus, swimming ability was increased in the ΔrpoE strain at high temperatures (3), while this ability was significantly decreased in the ΔrpoX strain, suggesting that the regulation of motility by RpoX is independent of RpoE. Taken together, these analyses indicate that RpoX might modulate stress adaptation in an RpoE-dependent manner but regulate motility in an RpoE-independent manner. RNA-seq analyses also support the idea that RpoX might be involved in some RpoE-independent processes and signaling in V. alginolyticus (Table 1).
FIG 7.
Schematic of the regulation network of RpoX in V. alginolyticus. Pathway analysis was performed with the Kobas 3.0 algorithm. The various pathways and their respective cellular locations, as well as the regulatory roles of RpoX, are illustrated with arrows (activation) or bar-ended lines (repression) and are discussed in the text. 3P, 3-phosphate; TCA, tricarboxylic acid.
We thus present a putative scenario where RpoE and RpoX are involved in the heat stress response in V. alginolyticus (Fig. 7). The release of RpoE protein tethered to the inner membrane into the cytoplasm could be a response to high temperatures, triggering the degradation of the anti-sigma factor RseA (3, 16, 27, 28). The RpoE protein can directly bind to the promoter of rpoX and control the expression of this gene. The RpoE sigma factor can directly regulate rpoH and rpoX to mediate the high-temperature response. However, our RNA-seq analysis indicated that, in addition to the high-temperature response, the RpoX protein may be able to regulate many other pathways, such as those associated with virulence processes, ABC transport, flagellar assembly, F-type ATPases, carbon metabolism, type I/II secretion systems, and two-component systems (Fig. 7 and Table 1). In addition, RpoX regulates its own expression, a feature exhibited by many other alternative sigma factors (21, 30). The question remains regarding how RpoE responds to heat or other stresses to orchestrate the expression of rpoX, rpoH, and the gene encoding itself, rpoE, as well as other alternative sigma factors.
In summary, this investigation presented an RpoE-RpoX-centered heat stress response regulatory cascade. These data facilitate an improved understanding of the regulatory networks of various alternative sigma factors contributing to their distinct and overlapping regulatory functions in stress responses and virulence in the pathogen V. alginolyticus.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 2. The V. alginolyticus strains were grown in Luria-Bertani (LB) broth containing 3% sodium chloride (LBS broth) at 30°C as the normal growth conditions. Escherichia coli DH5α (λpir), E. coli SM10 (λpir), and E. coli BL21(DE3) were grown in LB broth at 37°C. When appropriate, the medium was supplemented with carbenicillin (100 μg ml−1), chloramphenicol (25 μg ml−1), kanamycin (50 μg ml−1), or l-arabinose (0.2 mg ml−1).
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH5α λpir | Host for π-requiring plasmids | Laboratory collection |
| SM10 λpir | Host for π-requiring plasmids; conjugal donor; Kmr | 40 |
| BL21(DE3) | Host strain for protein expression | Novagen |
| BL21/pET22b::rpoE | BL21; expression of RpoE; Kmr | This study |
| BL21/pET22b::rpoX | BL21; expression of RpoX; Kmr | This study |
| Vibrio alginolyticus | ||
| EPGS | Wild type; fish isolate; CCTCC strain AB 209306; Carbr | Laboratory collection |
| ΔrpoE | EPGS; in-frame deletion in rpoE; Carbr | 3 |
| ΔrpoX | EPGS; in-frame deletion in rpoX; Carbr | This study |
| ΔluxR | EPGS; in-frame deletion in luxR; Carbr | 3 |
| ΔhlyD | EPGS; hlyD disrupted; Carbr Cmr | This study |
| ΔN646_4611 | EPGS; GGDEF domain deletion in EPGS_03411; Carbr | This study |
| Δasp | EPGS; disrupted in asp; Carbr Cmr | 6 |
| rpoX+ | ΔrpoX; pBAD33 carrying the intact rpoX gene | This study |
| N646_4611OE/ΔN646_4611 | ΔN646_4611; pBAD33 carrying the ORF of N646_4611 | This study |
| N646_4611OE/ΔrpoX | ΔrpoX; pBAD33 carrying the ORF of EPGS_03411; Carbr Cmr | This study |
| WT/pBAD33::Flag | EPGS; pBAD33 carrying the Flag gene; Carbr Cmr | This study |
| WT/pBAD33::PrpoX-Flag | EPGS; pBAD33 carrying the intact rpoX-Flag gene; Carbr Cmr | This study |
| ΔrpoE/pBAD33::PrpoX-Flag | ΔrpoE; pBAD33 carrying the intact rpoX-Flag gene; Carbr Cmr | This study |
| ΔrpoX/pBAD33::PrpoX-Flag | ΔrpoX; pBAD33 carrying the intact rpoX-Flag gene; Carbr Cmr | This study |
| WT/pDM8::PrpoX | EPGS; pDM8 carrying the promoter region of rpoX; Carbr Cmr | This study |
| ΔluxO/pDM8::PrpoX | ΔluxO; pDM8 carrying the promoter region of rpoX; Carbr Cmr | This study |
| ΔluxR/pDM8::PrpoX | ΔluxR; pDM8 carrying the promoter region of rpoX; Carbr Cmr | This study |
| Plasmids | ||
| pDM4 | Suicide vector; pir dependent; R6K; SacBR; Cmr | 41 |
| pDM8 | pSup202 derivative containing promoterless lacZ; Cmr Tcr | 42 |
| pBAD33 | Carrying a mob gene in pBAD33; Cmr | 3 |
| pET28a | Expression vector; Kmr | Novagen |
| pDM4::rpoX | pDM4 with rpoX fragment deleted from nt 4–576; Cmr | This study |
| pDM4::N646_4611 | pDM4 with GGDEF fragment deleted from nt 27–234; Cmr | This study |
| pBAD33::PrpoX-Flag | Plasmid expressing rpoX-Flag driven by PrpoX; Cmr | This study |
| pBAD33::Flag | pBAD33 derivative Flag expression plasmid; Cmr | This study |
| pBAD33::N646_4611 | pBAD33 derivative EPGS_03411 expression plasmid; Cmr | This study |
| pDM8::PrpoX | pDM8 carrying the promoter region of rpoX; Cmr | This study |
| pET22b::rpoE | pET22b carrying the rpoE ORF; Kmr | This study |
| pET22b::rpoX | pET22b carrying the rpoX ORF; Kmr | This study |
nt, nucleotides.
Deletion mutant and complemented strain construction.
In-frame deletion mutants were generated as described in a previous study (10). The fragment was cloned into the XbaI sites of the suicide vector pDM4 (31), and the resulting plasmid was transformed into E. coli DH5α λpir. After sequencing, pDM4 derivatives were transformed into E. coli SM10 λpir. This plasmid was introduced into V. alginolyticus by conjugation. The double-crossover recombinant was selected on LBS agar containing 15% sucrose. The mutation was confirmed by PCR and sequencing. A fragment containing the intact rpoX gene (a 282-bp fragment of the promoter adjoining the start codon “ATG” and the open reading frame [ORF]) and the Flag sequence was cloned into the plasmid pBAD33 to construct a complementation strain (3).
Immunoblot analysis.
For the immunoblot assay, supernatants and bacterial cell pellets were harvested at the same optical density measured at 600 nm (OD600). Next, 15 μl of each sample was loaded onto a 12% denaturing polyacrylamide gel, and proteins were resolved by electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). The membranes were blocked with a 10% skim milk powder solution, incubated with a 1:2,000 dilution of Flag-specific (Sigma-Aldrich, St. Louis, MO) mouse antiserum, and incubated with a 1:2,000 dilution of horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, CA). Finally, the blots were visualized with an enhanced chemiluminescence reagent (Thermo Fisher Scientific Inc., Waltham, MA).
Growth curves.
Bacteria were incubated overnight and then diluted 1:100 in 50 ml of fresh LBS medium. The bacteria were then grown in LBS medium at 30°C or 42°C or in LB broth containing 0.5% NaCl at 30°C, and live bacterial counts were determined at 2, 4, 6, 8, 10, 12, and 24 h. At each time point, 100 μl of fresh culture was serially diluted with phosphate-buffered saline (PBS), and the dilutions were spread onto plates containing solidified LBS medium. The live bacterial count for each plate was obtained after cultivation for 12 h at 30°C.
Total RNA extraction.
Bacteria were incubated overnight and then diluted 1:100 in LBS medium. The bacteria were then grown at 30°C or 42°C and harvested after 9 h. Total RNA was isolated using an RNA extraction kit (Tiangen, Beijing, China). The RNA samples were digested with DNase I (Promega, Madison, WI, USA) to eliminate genomic DNA contamination. Before reverse transcription, regular PCR was routinely performed using the isolated RNA sample as a template to confirm that there was no DNA contamination.
5′ RACE.
We performed 5′ RACE (rapid amplification of cDNA ends) experiments as previously described (3). Six micrograms of total RNA was subjected to dephosphorylation using tobacco acid pyrophosphatase (TAP) (Epicentre) for 60 min at 37°C. The RNA oligonucleotide linker was ligated to total RNA using T4 RNA ligase (New England Biolabs, Beverly, MA, USA) according to the manufacturer’s instructions. cDNA was synthesized using avian myeloblastosis virus (AMV) reverse transcriptase (RT) (TaKaRa) according to the manufacturer’s instructions, using a random primer. First-round PCR amplification was performed using RACE-adapter and the primer rpoX-RACE (Table 3), and second-round PCR amplification was performed using RACE-adapter-nested primers and rpoX-RACE-nested primers (Table 3). The single resulting band was extracted, subcloned, and sequenced.
TABLE 3.
Primers used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| ΔrpoX-up-F | CTAGTGGGGCCCTTCTAGATGCAATTCATTTGAGTATCAGTTTGG |
| ΔrpoX-up-R | GCTAAAAATCTGACAGTTCATCCGTGACAGCTCGG |
| ΔrpoX-down-F | TGAACTGTCAGATTTTTAGCCCCTTATCCTAGCCG |
| ΔrpoX-down-R | CGGGAGAGCTCAGGTTACCCCTGACTTTAACCTTCAACACATCGA |
| ΔrpoX-out-F | TGCTGGAGACCGAGCAATAGTTGCC |
| ΔrpoX-out-R | ACGAGCCATTTAATGGTGCAGAGCA |
| ΔrpoX-in-F | ATGAAAGAATCGTTGTCAGTTGGGA |
| ΔrpoX-in-R | TTAAACCCAACCATCGAATCGGAGA |
| ΔN646_4611-up-F | GAGCTCAGGTTACCCGCATGCAAGATCTATTATGGACTTTGGCGCAGTCATAATG |
| ΔN646_4611-up-R | ATAACTTGGAACGTCATCTCCAGCAATTGCTGATTG |
| ΔN646_4611-down-F | TGGAGATGACGTTCCAAGTTATAACAGACTGTCAGC |
| ΔN646_4611-down-R | CCCTCGAGTACGCGTCACTAGTGGGGCCCTGACAGCTCGGAACCTAGCAAAGCAT |
| ΔN646_4611-out-F | GAGTACGCGGCGAACAACCAAATCG |
| ΔN646_4611-out-R | CGAGACAACAAATCAATGCCCAC |
| ΔN646_4611-in-F | CACCTGATTGAGCAATTGGC |
| ΔN646_4611-in-R | GTAAACTCAGGATAGTAAGC |
| rpoX-Flag-F | CCATACCCGTTTTTTTGGGCTAGCGAATTCAGGCCAATTTGCGTACAAATACTAT |
| rpoX-Flag-R1 | CTTGTCGTCGTCGTCCTTGTAGTCAACCCAACCATCGAATCGGAGACGT |
| Flag-R2 | GGTCAGCATGGGTACCTTTCTCCTCTTTAATTACTTGTCGTCGTCGTCCTTGTAGTC |
| N646_4611-Flag-F | CCATACCCGTTTTTTTGGGCTAGCGAATTCTTCAGCCGTAAAATGAAGCATAGAG |
| N646_4611-Flag-R | CTTGTCGTCGTCGTCCTTGTAGTCATTCACTTGACGAAGCGCAGGTTGA |
| rpoE-pET22b-F | ATCGGATCCGATGAACGAGCAGCTGACCGATC |
| rpoE-pET22b-R | ATATGTCGACGCGTTGCAAAAGAGGTCTGATT |
| rpoX-pET22b-F | ATCGGATCCATGAAAGAATCGTTGTCAGTTGGGA |
| rpoX-pET22b-R | ATATGTCGACAACCCAACCATCGAATCGGAGA |
| pDM8-PrpoX-F | ATCCCGGGAGGCCAATTTGCGTACAAATACTTG |
| pDM8-PrpoX-R | ATCCCGGGTGACAGTTCATCCGTGACAGCTCGG |
| rpoX-RACE-nested | TCACGAGACAACAAATCAATGCCCA |
| rpoX-RACE | CAACATTTCGCGCGCTTCTTCATCA |
| RACE-adapter | GCGCGAATTCCTGTAGA |
| RACE-adapter2 | GCGCGAATTCCTGTAGAACGAAC |
| RNA-Linker | AUAUGCGCGAAUUCCUGUAGAACGAACACUAGAAGAAA |
| PrpoXcy5-F | TGCCTGCAGGTCGACGATCGGCCAATTTGCGTACAAATACTTG |
| PrpoXcy5-R | TGACAGTTCATCCGTGACAGCTCGG |
| PN646_4603cy5-F | TGCCTGCAGGTCGACGATCTCGACTGCTGTTTTTCTTCT |
| PN646_4603cy5-R | ATTTATCATTATCCCACCGCAC |
| PN646_4601cy5-F | TGCCTGCAGGTCGACGATCAATCGACAACAAAGTATACAAACCAAGAC |
| PN646_4601cy5-R | CTCTAGTTCTGACTCCGGCACAT |
| PN646_1623cy5-F | TGCCTGCAGGTCGACGATCAGACTCTTTTGCAAATGGCTTG |
| PN646_1623cy5-R | TTTTTAAATTCCTAGAATAAACTGATACTAAATAAAT |
| gyrB cy5-F | TGCCTGCAGGTCGACGATCGCACTATCAGAGAAAGTTGAGC |
| gyrB cy5-R | CCACCTTCATACATGAAGTGATCA |
| rpoE-qRT-F | TGTTGCTCAAGGGCGTAGAC |
| rpoE-qRT-R | CGATTGCACTGAACACCACC |
| rpoX-qRT-F | TAATGAACAATGGCCGCACG |
| rpoX-qRT-R | GCTCTGCTTTCACCCCTGAG |
| rpoH-qRT-F | GCGAGTTAGGTGTTGAGCCT |
| rpoH-qRT-R | ATAGCATCGGCGCTGTGTAA |
| N646_3697qRTF | GCTCAGTTCTCGCAGGTACA |
| N646_3697qRTR | AACAAGGCCCGCTGTTGATA |
| N646_3696qRTF | AGAGAAGCGTTTTGAGCCGA |
| N646_3696qRTR | ATTCGTTTCGAAGCTGCGTG |
| N646_3695qRTF | GTCCTCGCCAGTAACCTAGC |
| N646_3695qRTR | CACCGGTTTGTTCACACCAC |
| N646_0523qRTF | CGCGTCCCTTCAACCAAATC |
| N646_0523qRTR | ACAGCTCGCACAGATGTCAA |
| N646_4604qRTF | GCAGTACCGAGACTTGGTGG |
| N646_4604qRTR | GTAAACCACGAGGCGATCCA |
| 16S RNA-qRT-F | AAAGCACTTTCAGTCGTGAGGAA |
| 16S RNA-qRT-R | TGCGCTTTACGCCCAGTAAT |
Quantitative real-time reverse transcription-PCR.
Equal amounts of RNA (1 μg) were used to generate cDNA (Toyobo, Tsuruga, Japan) using 6-mer random primers. Three independent qRT-PCR experiments were performed, and each experiment was run in triplicate. The primers for qRT-PCR (Table 3) were designed using the NCBI primer selection tool with predicted product sizes ranging from 100 to 200 bp. The reactions were run on an Applied Biosystems 7500 real-time system (Applied Biosystems), and the transcript levels were normalized to the 16S rRNA levels in each sample by using the ΔΔCT method.
Electrophoretic mobility shift assay.
The purification of 6×His-tagged RpoE and RpoX from E. coli BL21(DE3) with nickel affinity chromatography was performed as previously described (3). For electrophoretic mobility shift assays (EMSAs), purified 6×His-tagged RpoE or RpoX was incubated with different Cy5-labeled DNA probes (Table 2) in 20 μl of loading buffer (10 mM NaCl, 0.1 mM dithiothreitol [DTT], 0.1 mM EDTA, 10 mM Tris [pH 7.4]). After the mixture was incubated at 25°C for 30 min, the samples were resolved using 6% polyacrylamide gel electrophoresis in 0.5× TBE (Tris-boric acid-EDTA) buffer on ice at 100 V for 120 min. Next, the gels were scanned using a Typhoon FLA 9500 instrument (GE Healthcare, Uppsala, Sweden).
RNA-seq analysis.
For RNA-seq analysis of the ΔrpoX or ΔrpoE strain, bacteria were incubated overnight and then diluted 1:100 in LBS medium. The bacteria were then grown at 42°C and harvested after 9 h. The subsequent procedures and statistical analysis were performed as previously described (32).
ChIP-seq analysis.
For the ChIP-seq analysis of RpoX, the pBAD33::PrpoX-Flag and pBAD33::Flag plasmids, encoding RpoX-Flag and the Flag tag alone (control), respectively, were transferred to the ΔrpoX strain. Cultures of each strain grown overnight in LBS medium at 42°C or in LB medium containing 0.5% NaCl at 30°C were diluted (1:100) in 50 ml of fresh LBS medium with 0.04% l-arabinose. After 9 h of growth with shaking, the protein-DNA complexes in the bacterial cells were fixed in vivo with rifampin at a final concentration of 150 μg/ml under the corresponding conditions for 20 min (33) and then cross-linked in vivo with 1% formaldehyde at room temperature for 10 min. Cross-linking was stopped by the addition of 125 mM glycine. The following procedures and statistical analysis were performed as previously described (34). Briefly, bacterial cells were sonicated in SDS lysis buffer, and the DNA was fragmented to 100 to 500 bp and immunoprecipitated (IP) with Flag-labeled beads. IP DNA was collected in elution buffer, followed by reversion of the DNA-protein cross-links and purification of the DNA by phenol-chloroform. DNA fragments were used for library construction with the VAHTS Turbo DNA library prep kit and then sequenced with a MiSeq sequencer (Illumina, San Diego, CA). ChIP-seq reads were mapped to the V. alginolyticus EPGS genome. The enriched peaks were identified using MACS software (35), followed by MEME analysis to generate the RpoX-binding motif (36). KEGG pathway analysis was performed with Kobas 3.0 to illustrate the enriched gene function (37).
Motility, biofilm, and hemolytic activity assays.
The motility assay was performed as previously described (10). Cultures grown overnight were diluted to an OD600 of 1.0 and then spotted onto LBS medium containing 0.3% (swimming) and 1.5% (swarming) agar. After incubation at 30°C for 12 h and 24 h, respectively, bacterial motility was observed. The experiments were performed at least three times, and one representative result is shown.
The biofilm assay was performed as previously described (10). Cultures grown overnight (50 μl) were diluted to 5 ml in LBS medium in glass tubes and incubated at 30°C without shaking for 48 h. A total of 0.04% l-arabinose, which exerts no apparent influence on biofilm formation of the WT, was added to LBS medium to induce the pBAD promoter. The total biofilm was measured by 2% crystal violet staining. The experiments were performed at least three times, and one representative result is shown.
Hemolytic activity assays were performed as previously described (4, 28, 38). V. alginolyticus strains were grown to mid-log phase in LBS medium at 30°C. The bacterial cells were centrifuged at 500 × g, washed three times with PBS, and then resuspended with PBS to a final concentration of 0.5 × 109 CFU/ml. For the blood agar assay, a suspension of 5% defibrinated sheep blood erythrocytes was added to LBS agar medium (45°C to 50°C), mixed gently, and poured into plates. Pellets of 5-μl bacterial suspensions were dropped onto the blood agar plates. The plates were incubated at 30°C or 42°C for 12 h. The experiments were performed at least three times, and one representative result is shown.
LD50 determination.
Median lethal dose (LD50) determination for the WT, ΔrpoX, or ΔrpoE strain in the zebrafish infection model was performed as previously described (39). Healthy fish, each weighing approximately 0.25 g, were obtained from a commercial farm and acclimatized to the laboratory conditions for at least 15 days. Zebrafish were anesthetized with tricaine methanesulfonate (catalog no. MS-222; Sigma-Aldrich) at a concentration of 80 mg/liter. Groups of 10 fish each were injected intramuscularly with bacterial cells adjusted to the required concentrations. Fish mortality was monitored over a period of 7 days postinfection. Fish injected with PBS only served as negative controls. The LD50 values were calculated as described previously (39). The animal work presented here was approved by the Animal Care Committee, East China University of Science and Technology (approval no. 2006272).
Statistical analysis.
GraphPad Prism (version 6) was used to perform the statistical analyses. To compare gene expression or CFU between the groups, a two-tailed Student’s unpaired t test was used. A P value of <0.05 was considered significant.
Data availability.
The sequence reads were deposited in the SRA database under accession no. SRP152034.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grants from the National Natural Science Foundation of China (no. 31772893 to Y.M. and 31772891 to Q.W.), the Ministry of Agriculture of China (CARS-47-G17), and the Science and Technology Commission of Shandong and Shanghai Municipality (2017CXGC0103 and 17391902000).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00234-19.
REFERENCES
- 1.Austin B. 2010. Vibrios as causal agents of zoonoses. Vet Microbiol 140:310–317. doi: 10.1016/j.vetmic.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs Slifka KM, Newton AE, Mahon BE. 2017. Vibrio alginolyticus infections in the USA, 1988–2012. Epidemiol Infect 145:1491–1499. doi: 10.1017/S0950268817000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu D, Guo M, Yang MJ, Zhang YX, Zhou XH, Wang QY. 2016. A σE-mediated temperature gauge controls a switch from LuxR-mediated virulence gene expression to thermal stress adaptation in Vibrio alginolyticus. PLoS Pathog 12:e1005645. doi: 10.1371/journal.ppat.1005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang QY, Liu Q, Ma Y, Rui HP, Zhang YX. 2007. LuxO controls extracellular protease, haemolytic activities and siderophore production in fish pathogen Vibrio alginolyticus. J Appl Microbiol 103:1525–1534. doi: 10.1111/j.1365-2672.2007.03380.x. [DOI] [PubMed] [Google Scholar]
- 5.Rui HP, Liu Q, Ma Y, Wang QY, Zhang YX. 2008. Roles of LuxR in regulating extracellular alkaline serine protease A, extracellular polysaccharide and mobility of Vibrio alginolyticus. FEMS Microbiol Lett 285:155–162. doi: 10.1111/j.1574-6968.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 6.Rui HP, Liu Q, Wang QY, Ma Y, Liu H, Shi CB, Zhang YX. 2009. Role of alkaline serine protease, Asp, in Vibrio alginolyticus virulence and regulation of its expression by LuxO-LuxR regulatory system. J Microbiol Biotechnol 19:431–438. doi: 10.4014/jmb.0807.404. [DOI] [PubMed] [Google Scholar]
- 7.Cao XD, Wang QY, Liu Q, Liu H, He HH, Zhang YX. 2010. Vibrio alginolyticus MviN is a luxO-regulated protein and affects cytotoxicity towards EPC cells. J Microbiol Biotechnol 20:271–280. [PubMed] [Google Scholar]
- 8.Cao XD, Wang QY, Liu Q, Rui HP, Liu H, Zhang YX. 2011. Identification of a luxO-regulated extracellular protein Pep and its roles in motility in Vibrio alginolyticus. Microb Pathog 50:123–131. doi: 10.1016/j.micpath.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z, Zhang LP, Ren CH, Zhao JJ, Chen C, Jiang X, Luo P, Hu CQ. 2011. Autophagy is induced by the type III secretion system of Vibrio alginolyticus in several mammalian cell lines. Arch Microbiol 193:53–61. doi: 10.1007/s00203-010-0646-9. [DOI] [PubMed] [Google Scholar]
- 10.Sheng LL, Lv YZ, Liu Q, Wang QY, Zhang YX. 2013. Connecting type VI secretion, quorum sensing, and c-di-GMP production in fish pathogen Vibrio alginolyticus through phosphatase PppA. Vet Microbiol 162:652–662. doi: 10.1016/j.vetmic.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Bashyam MD, Hasnain SE. 2004. The extra cytoplasmic function sigma factor: role in bacterial pathogenesis. Infect Genet Evol 4:301–308. doi: 10.1016/j.meegid.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Kazmierczak MJ, Wiedmann M, Boor KJ. 2005. Alternative sigma factors and their roles in bacterial virulence. Microbiol Mol Biol Rev 69:527–543. doi: 10.1128/MMBR.69.4.527-543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sineva E, Savkina M, Ades SE. 2017. Themes and variation in gene regulation by extracytoplasmic function (ECF) sigma factors. Curr Opin Microbiol 36:128–137. doi: 10.1016/j.mib.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur J, Davis BM, Waldor MK. 2007. Antimicrobial peptides activate the Vibrio cholerae sigma E regulon through an OmpU-dependent signaling pathway. Mol Microbiol 63:848–858. doi: 10.1111/j.1365-2958.2006.05544.x. [DOI] [PubMed] [Google Scholar]
- 15.Davis BM, Waldor MK. 2009. High-throughput sequencing reveals suppressors of Vibrio cholerae rpoE mutations: one fewer porin is enough. Nucleic Acids Res 37:5757–5767. doi: 10.1093/nar/gkp568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haines-Menges B, Whitaker WB, Boyd EF. 2014. Alternative sigma factor RpoE is important for Vibrio parahaemolyticus cell envelope stress response and intestinal colonization. Infect Immun 82:3667–3677. doi: 10.1128/IAI.01854-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merrikh H, Ferrazzoli AE, Bougdour A, Olivier-Mason A, Lovett ST. 2009. A DNA damage response in Escherichia coli involving the alternative sigma factor, RpoS. Proc Natl Acad Sci U S A 106:611–616. doi: 10.1073/pnas.0803665106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landini P, Egli T, Wolf J, Lacour S. 2014. Sigma S, a major player in the response to environment stresses in Escherichia coli: role, regulation and mechanisms of promoter recognition. Environ Microbiol Rep 6:1–13. doi: 10.1111/1758-2229.12112. [DOI] [PubMed] [Google Scholar]
- 19.Wurm P, Tutz S, Mutsam B, Vorkapic D, Heyne B, Grabner C, Kleewein K, Halscheidt A, Schild S, Reidl J. 2017. Stringent factor and proteolysis control of sigma factor RpoS expression in Vibrio cholerae. Int J Microbiol 307:154–165. doi: 10.1016/j.ijmm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Tian Y, Wang QY, Liu Q, Ma Y, Cao XD, Zhang XY. 2008. Role of RpoS in stress survival, synthesis of extracellular autoinducer 2, and virulence in Vibrio alginolyticus. Arch Microbiol 190:585–594. doi: 10.1007/s00203-008-0410-6. [DOI] [PubMed] [Google Scholar]
- 21.Slamti L, Livny J, Waldor MK. 2007. Global gene expression and phenotypic analysis of a Vibrio cholerae rpoH deletion mutant. J Bacteriol 189:351–362. doi: 10.1128/JB.01297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. 2006. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol 4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao JJ, Chen C, Zhang LP, Hu CQ. 2009. Cloning, identification, and characterization of the rpoS-like sigma factor rpoX from Vibrio alginolyticus. J Biomed Biotechnol 2009:126986. doi: 10.1155/2009/126986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caly DL, Bellini D, Walsh MA, Dow JM, Ryan RP. 2015. Targeting cyclic di-GMP signaling: a strategy to control biofilm formation? Curr Pharm Des 21:12–24. doi: 10.2174/1381612820666140905124701. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Song S, Lee M, Lee S, Lee K, Ha NC. 2016. Crystal structure of a soluble fragment of the membrane fusion protein HlyD in a type I secretion system of Gram-negative bacteria. Structure 24:477–485. doi: 10.1016/j.str.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Mauri M, Klumpp S. 2014. A model for sigma factor competition in bacterial cells. PLoS Comput Biol 10:e1003845. doi: 10.1371/journal.pcbi.1003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacikova G, Skorupski K. 2002. The alternative sigma factor σE plays an important role in intestinal survival and virulence in Vibrio cholerae. Infect Immun 70:5355–5362. doi: 10.1128/IAI.70.10.5355-5362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rattanama P, Thompson JR, Kongkerd N, Srinitiwarawong K, Vuddhakul V, Mekalanos JJ. 2012. Sigma E regulators control hemolytic activity and virulence in a shrimp pathogenic Vibrio harveyi. PLoS One 7:e32523. doi: 10.1371/journal.pone.0032523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Nakayasu ES, Overall CC, Johnson RC, Kidwai AS, McDermott JE, Ansong C, Heffron F, Cambronne ED, Adkins JN. 2015. Global analysis of Salmonella alternative sigma factor E on protein translation. J Proteome Res 14:1716–1726. doi: 10.1021/pr5010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodius VA, Mutalik VK. 2010. Predicting strength and function for promoters of the Escherichia coli alternative sigma factor, σE. Proc Natl Acad Sci U S A 107:2854–2859. doi: 10.1073/pnas.0915066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Zhao LY, Yang MJ, Yin KY, Zhou XH, Leung KY, Liu Q, Zhang YX, Wang QY. 2017. Transcriptomic dissection of the horizontally acquired response regulator EsrB reveals its global regulatory roles in the physiological adaptation and activation of T3SS and the cognate effector repertoire in Edwardsiella piscicida during infection toward turbot. Virulence 8:1355–1377. doi: 10.1080/21505594.2017.1323157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herring CD, Raffaelle M, Allen TE, Kanin EI, Landick R, Ansari AZ, Palsson BØ. 2005. Immobilization of Escherichia coli RNA polymerase and location of binding sites by use of chromatin immunoprecipitation and microarrays. J Bacteriol 187:6166–6174. doi: 10.1128/JB.187.17.6166-6174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu D, Liu H, Yang Z, Zhang Y, Wang Q. 2016. Chromatin immunoprecipitation sequencing technology reveals global regulatory roles of low-cell-density quorum-sensing regulator AphA in the pathogen Vibrio alginolyticus. J Bacteriol 198:2985–2999. doi: 10.1128/JB.00520-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng JX, Liu T, Qin B, Zhang Y, Liu XL. 2012. Identifying ChIP-seq enrichment using MACS. Nat Protoc 7:1728–1740. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machanick P, Bailey TL. 2011. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27:1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie C, Mao XZ, Huang JJ, Ding Y, Wu JM, Dong S, Kong L, Gao G, Li CY, Wei LP. 2011. KOBAS 2.0: a Web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dasgupta N, Ashare A, Hunninghake GW, Yahr TL. 2006. Transcriptional induction of the Pseudomonas aeruginosa type III secretion system by low Ca2+ and host cell contact proceeds through two distinct signaling pathways. Infect Immun 74:3334–3341. doi: 10.1128/IAI.00090-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv Y, Xiao J, Liu Q, Wu H, Zhang Y, Wang Q. 2012. Systematic mutation analysis of two-component signal transduction systems reveals EsrA-EsrB and PhoP-PhoQ as the major virulence regulators in Edwardsiella tarda. Vet Microbiol 157:190–199. doi: 10.1016/j.vetmic.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Liang WL, Wang SX, Yu FG, Zhang LJ, Qi GM, Liu YQ, Gao SY, Kan B. 2003. Construction and evaluation of a safe, live, oral Vibrio cholerae vaccine candidate, IEM108. Infect Immun 71:5498–5504. doi: 10.1128/IAI.71.10.5498-5504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang SY, Lauritz J, Jass J, Milton DL. 2002. A ToxR homolog from Vibrio anguillarum serotype O1 regulates its own production, bile resistance, and biofilm formation. J Bacteriol 184:1630–1639. doi: 10.1128/JB.184.6.1630-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croxatto A, Chalker VJ, Lauritz J, Jass J, Hardman A, Williams P, Camara M, Milton DL. 2002. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J Bacteriol 184:1617–1629. doi: 10.1128/JB.184.6.1617-1629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence reads were deposited in the SRA database under accession no. SRP152034.