Natural killer (NK) cells play a key role in antiviral and tumour immunity. In the setting of HIV infection, accumulating evidence implicates NK cells as critical contributors to immune control of HIV. In particular, indirect NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) has been linked to vaccine-induced protective immunity against HIV infection and phenotypes of viral control [1]. With emerging knowledge of specialised subpopulations and increased understanding of NK cell memory and immunoregulatory properties, new prospects have emerged to address remaining challenges in the field of HIV; namely control of persistent inflammation and comorbidity in treated infection, development of an effective vaccine and safe and widely available strategies for a ‘functional cure’.
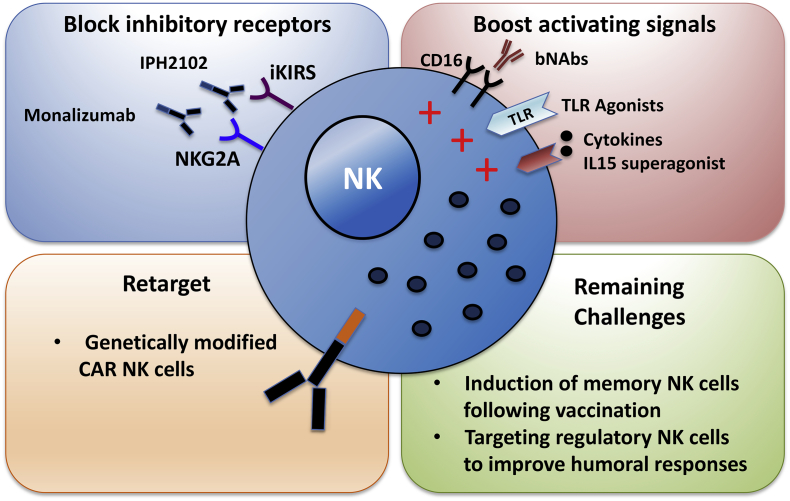
NK cells recognise stress signals, cancer transformation or infection with immediate effector function achieved via expression of a wide array of receptors and integration of finely attuned signals. In addition to cytotoxic elimination of target cells, NK cells are potent producers of cytokines and chemokines and can promote or suppress adaptive and innate immune responses. NK cell pleiotropic functions and remarkable effector agility makes them ideal candidates for immunotherapeutic interventions. Several approaches, including the development of chimeric antigen receptor (CAR) NK cells, show substantial promise in cancer trials and can be translated to direct and augment NK cell responses to improve HIV control. Some examples include activation of NK cell through blocking inhibitory signalling though NKG2A and inhibitory KIRS or activation through TLR agonists and IL15 to increase NK cell antiviral responses [2] (Fig. 1). In addition to strategies boosting NK cell effector capability, the adoptive transfer of haploidentical NK cell infusion following IL-2 stimulation is being evaluated for HIV treatment (clinical trial NCT03346499).
Fig. 1.
Therapeutic strategies to enhance NK cell function in HIV infection and remaining challenges.
Although traditionally considered as innate effector cells, the recognition that NK cell subsets can clonally expand and form long-lasting pools of memory-like cells in response to viral infection and/or immunisation represents a major advance in the field of NK cell research. In humans adaptive NK cells have been described in the context of CMV infection, leading to a substantial and long-lasting increase in NKG2C+ NK cells displaying preferential binding to some HLA-E presented CMV peptides [3]. Further subpopulations of adaptive NK cells are characterised by epigenetic changes, stochastic loss of expression of key proximal signalling molecules, that expand in response to antibody-opsonised targets or immune complexes and are imbued with an enhanced capacity for ADCC [4]. Adaptive NK cells have been described in HIV infection with retained ADCC activity [5,6], indicating that selective NK cell subsets have unique immunologic features. These populations can be potentially targeted for developing antiviral approaches and designing therapeutic vaccines aimed at generating ADCC-promoting antibody responses or in combination with broadly neutralizing antibodies (bNAbs) (clinical trial NCT02018510). Interestingly, NK cell antigen specificity has been reported in non-human primates for SIV-derived antigens and following adenovirus 26 (Ad26) based SIV immunisation [7]. Although direct evidence of HIV-specific NK cells in humans is currently lacking, human NK cells isolated from the liver of humanised mice previously vaccinated with HIV-encoded envelope protein displayed robust antigen-specific recall responses in vitro [8]. However, the precise mechanisms employed by NK cells to recognise and distinguish different antigens remain incompletely understood. Nonetheless, the generation of memory NK cells represents a novel goal of innovative vaccination approaches through more targeted adjuvants and/or specific cytokine signatures. NK cells can also acquire a memory-like phenotype induced as a result of more generalised signals. Cytokine-induced memory NK cells are currently being used in cancer immunotherapy and their enhanced potency could be exploited in HIV infection to achieve eradication [9].
The critical role of NK cells in editing adaptive immune responses has been recently brought into focus by the study of NK cells in HIV-infected individuals who develop bnAbs. A novel pathway involving the recycling endosome effector protein RAB11Fip5 that impacts on NK cell functionality and immunoregulatory role was found to be associated with the induction of bNAbs [10]. This work has increased our understanding of NK cell regulation of humoral responses in humans and highlighted the need for careful dissection of the regulatory functions of NK cells that may need to be tempered in order to elicit robust humoral responses.
Exploiting NK cells and subpopulations with adaptive features is emerging as an exciting field in augmenting therapeutic modalities against chronic viral infection (Fig. 1). Further exploration and understanding of the unique properties of specialised and memory NK cells subsets and the precise mechanisms NK cells employ to regulate adaptive responses remain, however, crucial in better targeting and optimising NK cell responses to improve HIV control and potentially effect a ‘functional cure’.
Disclosure
The author declared no conflicts of interest.
References
- 1.Scully E., Alter G. NK cells in HIV disease. Curr HIV/AIDS Rep. 2016;13(2):85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scully E, Alter G. NK Cells in HIV Disease. Current HIV/AIDS reports. 2016;13(2):85-94. [DOI] [PMC free article] [PubMed]
- 2.Ram D.R., Manickam C., Lucar O., Shah S.V., Reeves R.K. Adaptive NK cell responses in HIV/SIV infections: a roadmap to cell-based therapeutics? J Leukoc Biol. 2019:1–7. doi: 10.1002/JLB.MR0718-303R. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ram DR, Manickam C, Lucar O, Shah SV, Reeves RK. Adaptive NK cell responses in HIV/SIV infections: A roadmap to cell-based therapeutics? Journal of leukocyte biology. 2019. [DOI] [PMC free article] [PubMed]
- 3.Hammer Q., Ruckert T., Borst E.M., Dunst J., Haubner A., Durek P. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018;19(5):453–463. doi: 10.1038/s41590-018-0082-6. [DOI] [PubMed] [Google Scholar]; Hammer Q, Ruckert T, Borst EM, Dunst J, Haubner A, Durek P, et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nature immunology. 2018;19(5):453-63. [DOI] [PubMed]
- 4.Schlums H., Cichocki F., Tesi B., Theorell J., Beziat V., Holmes T.D. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443-56. [DOI] [PMC free article] [PubMed]
- 5.Peppa D., Pedroza-Pacheco I., Pellegrino P., Williams I., Maini M.K., Borrow P. Adaptive reconfiguration of natural killer cells in HIV-1 infection. Front Immunol. 2018;9:474. doi: 10.3389/fimmu.2018.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]; Peppa D, Pedroza-Pacheco I, Pellegrino P, Williams I, Maini MK, Borrow P. Adaptive Reconfiguration of Natural Killer Cells in HIV-1 Infection. Frontiers in immunology. 2018;9:474. [DOI] [PMC free article] [PubMed]
- 6.Zhou J., Amran F.S., Kramski M., Angelovich T.A., Elliott J., Hearps A.C. An NK cell population lacking FcRgamma is expanded in chronically infected HIV patients. J Immunol. 2015;194(10):4688–4697. doi: 10.4049/jimmunol.1402448. [DOI] [PubMed] [Google Scholar]; Zhou J, Amran FS, Kramski M, Angelovich TA, Elliott J, Hearps AC, et al. An NK Cell Population Lacking FcRgamma Is Expanded in Chronically Infected HIV Patients. J Immunol. 2015;194(10):4688-97. [DOI] [PubMed]
- 7.Reeves R.K., Li H., Jost S., Blass E., Li H., Schafer J.L. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16(9):927–932. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, et al. Antigen-specific NK cell memory in rhesus macaques. Nature immunology. 2015;16(9):927-32. [DOI] [PMC free article] [PubMed]
- 8.Nikzad R., Angelo L.S., Aviles-Padilla K., Le D.T., Singh V.K., Bimler L. Human natural killer cells mediate adaptive immunity to viral antigens. Sci Immunol. 2019;4(35) doi: 10.1126/sciimmunol.aat8116. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, et al. Human natural killer cells mediate adaptive immunity to viral antigens. Science immunology. 2019;4(35). [DOI] [PMC free article] [PubMed]
- 9.Garrido C., Abad-Fernandez M., Tuyishime M., Pollara J.J., Ferrari G., Soriano-Sarabia N. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol. 2018;92(12) doi: 10.1128/JVI.00235-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Garrido C, Abad-Fernandez M, Tuyishime M, Pollara JJ, Ferrari G, Soriano-Sarabia N, et al. Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. Journal of virology. 2018;92(12). [DOI] [PMC free article] [PubMed]
- 10.Bradley T., Peppa D., Pedroza-Pacheco I., Li D., Cain D.W., Henao R. RAB11FIP5 expression and altered natural killer cell function are associated with induction of HIV broadly neutralizing antibody responses. Cell. 2018;175(2):387–99 e17. doi: 10.1016/j.cell.2018.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bradley T, Peppa D, Pedroza-Pacheco I, Li D, Cain DW, Henao R, et al. RAB11FIP5 Expression and Altered Natural Killer Cell Function Are Associated with Induction of HIV Broadly Neutralizing Antibody Responses. Cell. 2018;175(2):387-99 e17. [DOI] [PMC free article] [PubMed]