Abstract
Sound encoding is mediated by Ca2+ influx-evoked release of glutamate at the ribbon synapse of inner hair cells. Here we studied the role of ATP in this process focusing on Ca2+ current through CaV1.3 channels and Ca2+ homeostasis in mouse inner hair cells. Patch-clamp recordings and Ca2+ imaging demonstrate that hydrolyzable ATP is essential to maintain synaptic Ca2+ influx in inner hair cells via fueling Ca2+-ATPases to avoid an increase in cytosolic [Ca2+] and subsequent Ca2+/calmodulin-dependent inactivation of CaV1.3 channels.
Keywords: calcium, calmodulin, channel, hair cell, inactivation, ribbon synapse
Introduction
Neurotransmission at the inner hair cell (IHC) synapse is driven by Ca2+ influx (ICa) through CaV1.3 channels (Platzer et al., 2000; Brandt et al., 2003; Dou et al., 2004) that cluster at the active zones (AZs) (Brandt et al., 2005). Within IHCs, this L-type channel activates at low voltage and displays only weak Ca2+ dependent inactivation (CDI) (Yang et al., 2006; Cui et al., 2007). At least two mechanisms of inhibiting CDI (Lee et al., 1999; Peterson et al., 1999) of CaV1.3 in IHCs are currently considered: (1) autoregulation involving the distal and proximal C-terminal domains (Singh et al., 2008) and (2) competition of Ca2+ binding proteins (CaBPs) with calmodulin (Yang et al., 2006; Cui et al., 2007; Schrauwen et al., 2012). However, a unifying picture of CDI regulation during physiological signaling in IHCs has yet to be established.
Here we studied the role of ATP in Ca2+ signaling and CaV1.3 channel regulation in IHCs. Decay (“rundown”) of ICa is commonly observed in whole-cell patch-clamp recordings, suggesting a failure of the channel regulation and/or function upon washout of cell constituents. Adding ATP to the pipette can partially prevent ICa rundown (Chad and Eckert, 1986; Armstrong and Eckert, 1987). Besides being used by kinases and phosphatases, ATP supports the function of ATP-driven pumps and is therefore required for cellular Ca2+ homeostasis and low basal [Ca2+]i (for review, see Mammano et al., 2007). Elevations in basal [Ca2+]i could affect the Ca2+ channel behavior (e.g., via CDI). The regulation of [Ca2+] at ribbon synapses involves Ca2+ buffering and diffusion (Roberts, 1993; Tucker and Fettiplace, 1995; Issa and Hudspeth, 1996; Frank et al., 2009) as well as Ca2+ clearance via Ca2+ ATPase (PMCA) and Na+/Ca2+ exchange (Zenisek and Matthews, 2000; Kennedy, 2002).
Here, we combined patch-clamp recordings and Ca2+ imaging of IHCs during dialysis with different [ATP] or the poorly hydrolyzable analog ATP-γ-S to probe the requirement of ATP hydrolysis for Ca2+ homeostasis and CaV1.3 channel regulation. We demonstrate that interference with ATP hydrolysis dramatically increases [Ca2+]i because of failure of PMCA-mediated Ca2+ clearance and consequently decreases the presynaptic ICa via Ca2+/calmodulin-mediated CDI.
Materials and Methods
Electrophysiology.
IHCs from the apical coil of organs of Corti from C57 Bl/6 mice of either sex (postnatal day 14 [P14] to P16) were patch-clamped (at 20°C–25°C) as described previously (Moser and Beutner, 2000). The pipette solution contained the following (in mm): 134–140 Cs-gluconate, 10 tetraethylammonium-Cl (TEA-Cl), 10 4-AP, 10 CsOH-HEPES, 1 MgCl2, 0.3 NaGTP, 0.5 or 10 EGTA or 10 BAPTA and 0–4 MgATP or 2 Li4-ATP-γ-S, pH 7.2, osmolarity: 295 mOsm/L. CaMKII 290–309, H-89 (both Merck), carboxyeosin, trifluorocarbonylcyanide phenylhydrazone (FCCP) (both Sigma-Aldrich), and fura-2 and Fluo-4FF (both Invitrogen) were dissolved in H2O. KN-93, CaMKII inhibitor XII, KT5720 (all Merck), CGS-9343B (Sigma-Aldrich), and berbamine E6 (Santa Cruz Biotechnology) were dissolved in DMSO. The extracellular solution contained the following (in mm): 110 NaCl, 35 TEA-Cl, 10 HEPES, 1 CsCl, 1 MgCl2, 2 CaCl2, 11.1 glucose, pH 7.2, osmolarity: 300 mOsm/L. BayK 8644 (Biotrend) was added to the extracellular solution. The liquid junction potential was numerically estimated as 14 mV and subtracted. Leak currents were subtracted using the P/10 protocol. The series resistance was typically <15 mΩ.
Camera-based Ca2+ imaging.
IHCs were loaded with 100 μm fura-2 and imaged alternately at 340 and 380 nm using a polychrome IV light source and an Imago VGA CCD operated by Tillvision software (all, Tillphotonics-FEI). Fura-2 measurements were calibrated in vivo and in vitro (Neher, 2013), and [Ca2+] was calculated according to the following equation (Grynkiewicz et al., 1985):
In our experiments, calibration coefficients determined in vivo were Rmin = 0.24, Rmax = 5.15, and Rmed = 1 (yielding Keff of 2457 nm). The KD for fura-2 was found to be 243.5 nm.
Confocal Ca2+ imaging.
Confocal Ca2+ imaging was performed using an Olympus FV300 confocal microscope essentially as described previously (Frank et al., 2009) using 400 μm Fluo-4FF in the pipette solution described above. In brief, carboxytetramethyl-rhodamine-conjugated RIBEYE-binding dimer peptide (10 μm) (Francis et al., 2011) was used to identify synaptic ribbons, and changes in Fluo-4FF (400 μm) fluorescence were repeatedly observed with line scans through the center of the same ribbon during (20 ms) depolarizations to −7 mV.
Data analysis and statistics.
Data analysis and statistics were done in IgorPro and MATLAB. Wilcoxon rank test was used to compare data (with non-normal distribution and/or unequal variances). Correlation was tested using Pearson's correlation. Regression lines were compared among each other by one-way analysis of covariance (ANCOVA) test. Data are presented as mean ± SEM.
Results
IHC CaV1.3 channels require hydrolyzable ATP for proper function
To determine the requirement of ATP for Ca2+ influx, IHCs were dialyzed with 2 mm ATP, ATP-γ-S, or pipette solution lacking ATP. We first assessed the ICa properties (current–voltage relationship, IV) 1–2 min after break-in (Fig. 1A) and thereafter ran a series of depolarization pulses (P1–P3, 20 ms; P4, 100 ms) to the peak ICa potential. Taking into account the molecular weight (MW), series resistance RS, and the estimated cell volume of 2.2 pl, the diffusion time constant for ATP (MW = 507.18 g/mol, RS = 12 mΩ) and ATP-γ-S (MW = 546.98 g/mol, RS = 10 mΩ) was calculated as 67 and 57 s, respectively (Pusch and Neher, 1988). Based on these calculations, the diffusional exchange should have been complete after 3–4 min, when P1 was applied. The cells infused with ATP-γ-S or 0 ATP may not have been completely devoid of ATP because of further ATP supply by oxidative metabolism, glycolysis, or phosphocreatine.
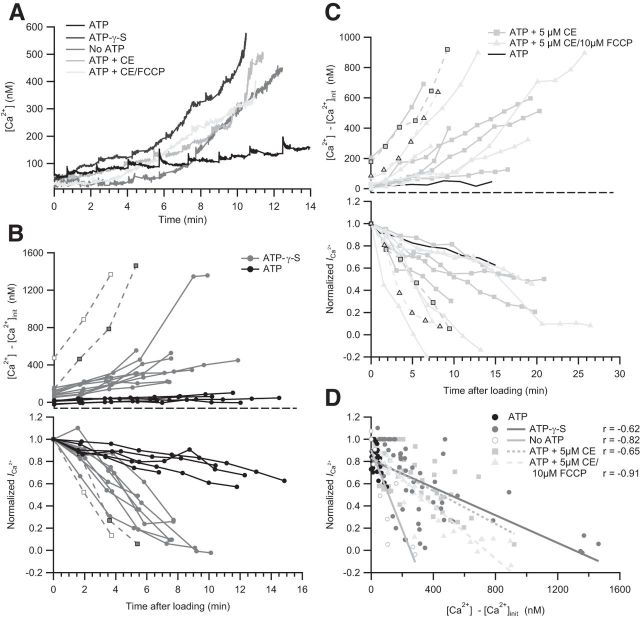
Figure 1.
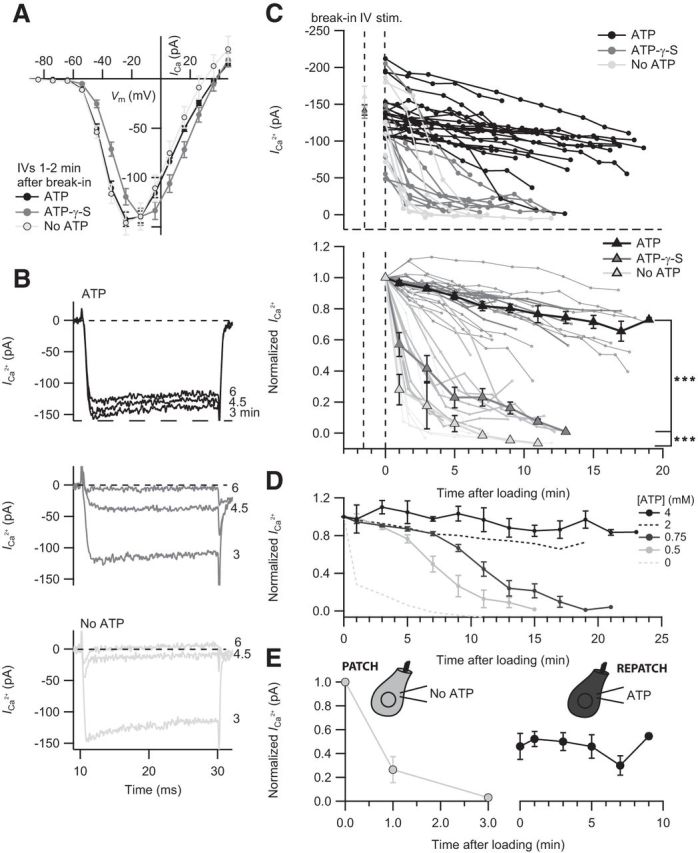
ATP hydrolysis is required for maintaining IHC ICa. A, Unaltered amplitude of ICa revealed by the current–voltage relationship (IV) of IHCs dialyzed with 2 mm ATP (n = 26), 2 mm ATP-γ-S (n = 15), or without exogenous ATP (n = 8) at 1–2 min after break-in. B, Representative ICa in response to 20 ms depolarization to peak ICa potential after 3, 4.5, and 6 min dialysis with ATP, ATP-γ-S, or without ATP. C, Time course of the ICa reduction upon ATP manipulation. Top, The ICa of individual IHCs dialyzed with ATP (n = 21), ATP-γ-S (n = 13), or without ATP (n = 8). Bottom, ICa normalized to the first 20 ms depolarization of each cell. Mean normalized values with SEM displayed as overlay that were binned by time, with a bin size of 120 s. Statistical comparison was performed between 1 min and 11 min after loading. D, The concentration-dependent effect of ATP on Ca2+ channels. Normalized ICa values of IHCs dialyzed with 0.5 (n = 4), 0.75 (n = 5), or 4 mm ATP (n = 5) over time. For comparison, the mean normalized ICa values of IHCs dialyzed with 2 mm (black dashed line) and without ATP (gray dashed line) are displayed. E, The ICa rundown is partially reversible. IHCs initially infused without ATP (n = 3) were repatched with a solution containing 2 mm ATP after 8–13 min. ***p < 0.001.
The initial IVs revealed comparable ICa amplitudes, reflecting the largely unaltered physiological state of the IHCs briefly after break-in. IHCs dialyzed with ATP-γ-S displayed a significantly faster rundown of ICa compared with controls with 2 mm ATP in the pipette (p < 0.001; Fig. 1B,C). For better comparison, ICa values were normalized to the response upon P1 (Fig. 1C). Without exogenous ATP or ATP-γ-S, the ICa rundown was even more pronounced (p < 0.001 for comparison to ATP-γ-S; Fig. 1B,C). Interestingly, 4 mm ATP in the pipette prevented the mild rundown observed with 2 mm ATP (Fig. 1D). On the contrary, lowering [ATP] below 2 mm caused the onset of a fast rundown after a few minutes (Fig. 1D).
We then tested whether the effects of the lack of hydrolyzable ATP on Ca2+ channels is reversible. IHCs were initially dialyzed with a solution lacking ATP, the pipette was gently pulled off enabling resealing of the IHC membrane, and the cell was then repatched with an internal solution containing 2 mm ATP after 8–13 min (Fig. 1E). As observed, ICa could partially be restored and remained stable over another 10 min. Similar results were obtained in IHCs initially dialyzed with ATP-γ-S (data not shown).
Finally, we examined the effects of DHP agonist BayK 8644 on the ICa of ATP-γ-S-treated IHCs because BayK 8644 has been shown to partially overcome inhibition of ICa by lack of hydrolyzable ATP in pituitary GH3 and smooth muscle cells (Armstrong and Eckert, 1987; Ohya and Sperelakis, 1989). We observed a twofold increase in the maximum steady-state ICa amplitude of the initial IV (Fig. 2A) consistent with the augmenting effects of BayK 8644 on IHC CaV1.3 channels (Brandt et al., 2005). However, regardless of BayK 8644, ATP-γ-S still caused a significant rundown of ICa (p = 0.43, compared with 2 mm ATP-γ-S without BayK 8644; Fig. 2B).
Figure 2.
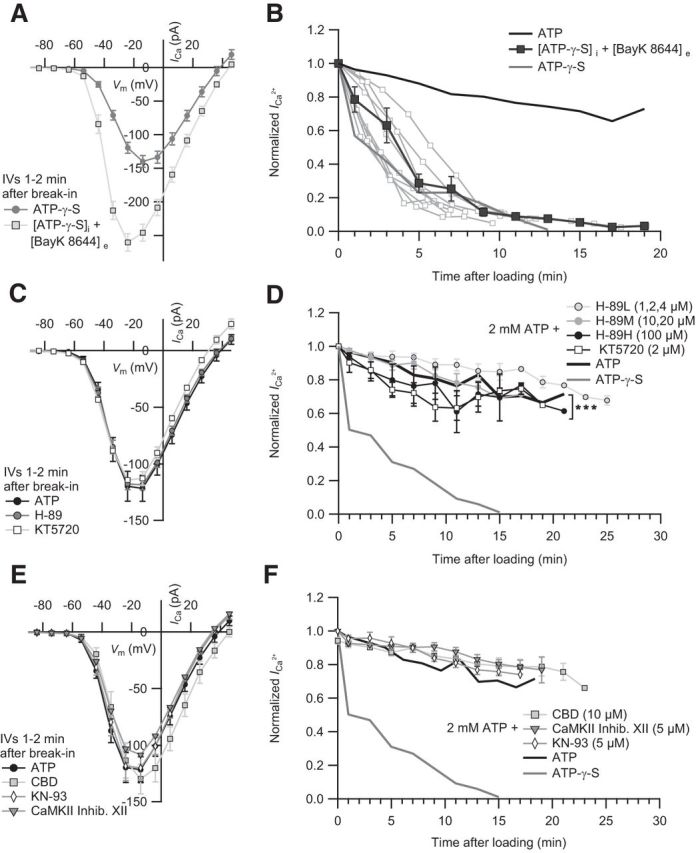
Probing the requirement of IHC ICa for phosphorylation by PKA and CaMKII. A, The IV of IHCs dialyzed with ATP-γ-S in the absence (n = 15) and presence of 5 μm extracellular BayK 8644 (n = 15). B, BayK 8644 did not significantly prevent the ICa rundown (p = 0.43, statistical comparison was performed between 1 min and 13 min after loading). Mean normalized ICa values of IHCs dialyzed with ATP-γ-S (n = 13) in the presence of BayK 8644 (n = 9). For comparison, we display the mean normalized ICa of IHCs dialyzed with ATP. C, The effect of PKA inhibition on IHC ICa. The IVs of IHCs dialyzed with ATP in the absence (n = 5) or presence of intracellular H-89 (n = 14) or KT5720 (n = 5) are comparable. D, The normalized mean ICa values of IHCs dialyzed with low (1, 2, and 4 μm, n = 6), middle (10 and 20 μm, n = 5), or high (100 μm, n = 3) concentration of H-89 or KT5720 (2 μm, n = 6) over time. Statistical comparison was performed between 3 min and 19 min after loading. E, CaMKII inhibition shows no effect on IHC ICa. The IVs of IHCs dialyzed with ATP in the absence (n = 5) or presence of intracellular CBD (n = 4), CaMKII Inhibitor XII (n = 3), or KN-93 (n = 4). F, The rundown of the mean normalized ICa in IHCs dialyzed with CBD (n = 4), inhibitor XII (n = 4), and KN-93 (n = 3) is similar to controls. In comparison, the mean normalized ICa of IHCs dialyzed with ATP or ATP-γ-S is displayed. ***p < 0.001.
Probing for a role of phosphorylation in the regulation of IHC CaV1.3 channels
Because ATP requirement may reflect phosphorylation events relevant to Ca2+ channel function, we tested for effects of the protein kinase A (PKA) inhibitors H-89 (Chijiwa et al., 1990) and KT5720 (Okada et al., 1995), and the calmodulin-dependent kinase II inhibitors CamKII 290–309 (calmodulin binding domain [CBD]) (Basavappa et al., 1999), CaMKII Inhibitor XII (Asano et al., 2010), and KN-93 (Sumi et al., 1991) applied via the pipette that also contained 2 mm ATP. Micromolar concentrations of drugs (see figure legends; Ki or IC50 values in nm range) were chosen after observing no effects at submicromolar levels. None of the tested PKA or CaMKII inhibitors had an effect on the IV (Fig. 2C,E). Of all the kinase inhibitors (Fig. 2D,F), only KT5720 had an effect on the ICa measurements compared with control (p < 0.001).
Correlation between the rise of basal [Ca2+]i and Ca2+ current rundown
Next, we considered the possibility that the lack of hydrolyzable ATP disables Ca2+ pumping and thereby Ca2+ clearance. Using simultaneous fura-2 imaging of [Ca2+]i and whole-cell ICa recordings in IHCs dialyzed with ATP-γ-S (and 0.5 mm EGTA), we found that the ICa reduction coincided with a rise of basal [Ca2+]i (Fig. 3A,B). Furthermore, IHCs that displayed a fast and pronounced elevation of basal [Ca2+]i also displayed the most severe ICa rundown (Fig. 3B, dashed lines), also reflecting in the observed negative correlation between the rise of basal [Ca2+]i and the ICa (Fig. 3D).
Figure 3.
IHC ICa requires intact Ca2+ homeostasis. A, Representative examples of IHC bulk [Ca2+]i obtained by fura-2 imaging under different conditions: with 2 mm ATP, 2 mm ATP-γ-S, no ATP, 2 mm ATP + 10 μm CE, or 2 mm ATP + 5 μm CE + 10 μm FCCP. In each case, the pipette contained 0.5 mm EGTA. B, Simultaneous measurements of [Ca2+] and the normalized ICa of single IHCs dialyzed with 2 mm ATP (n = 5) or 2 mm ATP-γ-S (n = 11). The initial [Ca2+] ([Ca2+]init) measured after sufficient loading of fura-2 was subtracted from subsequent [Ca2+] measurements. Dotted lines indicate two ATP-γ-S-cells with corresponding [Ca2+]i values and ICa. C, Simultaneous measurements of [Ca2+] and the normalized ICa of single IHCs dialyzed with 2 mm ATP + 5 μm CE (n = 6) or with 2 mm ATP + 5 μm CE + 10 μm FCCP (n = 6). For comparison, the mean [Ca2+]i of control IHCs (no drug) is shown (n = 5). D, [Ca2+]i and ICa rundown are correlated for IHCs dialyzed with ATP-γ-S, no ATP (n = 3), ATP + CE, or ATP + CE + FCCP. Pearson correlation coefficient (r) and regression lines are displayed. There are different slopes for no ATP and ATP-γ-S.
We then tested whether the PMCAs are the main mechanism of ATP-dependent Ca2+ clearance and required for maintaining Ca2+ influx in IHCs. Application of the PMCA inhibitor carboxyeosin (CE) caused a rise of the basal [Ca2+]i and a parallel decrease of ICa (Fig. 3A,C,D). Once again, the IHCs with the fastest and largest elevation of basal [Ca2+]i displayed the most severe ICa rundown. We conclude that failure of PMCA-mediated Ca2+ clearance explains most of the ICa reduction observed in the presence of ATP-γ-S. The slope of the regression line of cells dialyzed without ATP was steeper than of the cells containing ATP-γ-S (p = 0.0012), suggesting the contribution of an additional ATP-dependent mechanism to maintenance of ICa that can use ATP-γ-S.
To test for a direct role of mitochondria in Ca2+ homeostasis and regulation of Ca2+ influx, the uncoupling agent FCCP was used. The combined application of FCCP and CE resulted in an elevation of [Ca2+] and corresponding Ca2+ current rundown similar to the one observed with CE alone. However, the slope of the regression line of ICa and basal [Ca2+]i was significantly steeper in the IHCs treated with CE and FCCP compared with either CE or ATP-γ-S (p = 0.0007 for ATP-γ-S, p = 0.002 for CE vs ATP + CE + FCCP, ANCOVA), suggesting that application of FCCP enhances Ca2+ channel inactivation potentially via disruption of mitochondrial ATP generation and/or Ca2+ uptake affecting the clearance of synaptic Ca2+.
Ca2+/calmodulin-mediated CDI of Ca2+ channels underlies the ICa rundown in the absence of hydrolyzable ATP
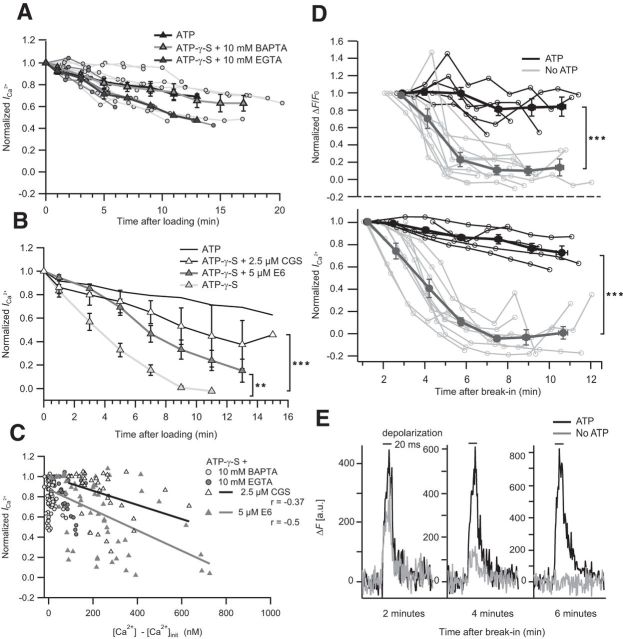
The most parsimonious interpretation of the ICa reduction caused by ATP-γ-S or CE is an increased CDI of Ca2+ channels resulting from the elevated resting [Ca2+]i. To test this hypothesis, we studied the effect of adding a high concentration (10 mm) of the Ca2+ chelators EGTA or BAPTA to the ATP-γ-S-containing pipette solution. In both cases, the pronounced global rise in [Ca2+]i as well as the ICa reduction were prevented (Fig. 4A,C).
Figure 4.
Ca2+ chelators and CaM inhibitors prevent ICa rundown; synaptic Ca2+ signaling requires ATP. A, Normalized ICa of single IHCs dialyzed with 2 mm ATP-γ-S and either 10 mm EGTA (n = 6) or 10 mm BAPTA (n = 9). Normalized mean ICa overlaid. For comparison, the mean normalized ICa of control IHCs is displayed (black solid line). B, The mean normalized ICa of IHCs dialyzed with 2 mm ATP-γ-S and 0.5 mm EGTA in the absence (n = 10) or presence of berbamine E6 (n = 9) or CGS-9343B (n = 8) as well as control IHCs (2 mm ATP, n = 5). Statistical comparison was performed between 3 and 9 min after loading. C, Correlation between ICa and basal [Ca2+]i of ATP-γ-S-infused IHCs is weaker in the presence of CaM inhibitors (compare with Fig. 3D) and absent with high buffer concentrations. D, Rundown of synaptic Ca2+ signals at IHC AZs in the absence of ATP. Normalized ΔF/F0 (top) of Fluo-4FF at an AZ and normalized ICa (bottom) of the same IHCs in the presence (n = 5) and absence (n = 10) of 2 mm ATP in the pipette solution. Thick lines indicate mean ± SEM (bin size, 100 s); thin lines indicate individual traces. Statistical comparison was performed between 4 min and 12 min after break-in. The initial data points of ΔF/F0 were omitted because of noise introduced by low F0. E, Decreasing amplitude of AZ Ca2+ signal in the absence of ATP. Temporal profiles of Fluo-4FF fluorescence at two exemplary AZs in the presence (black) and absence (gray) of 2 mm ATP during 20 ms depolarization (black bars) at 2, 4, and 6 min after break-in. **p < 0.01. ***p < 0.001.
We further tested the hypothesis of an increased Ca2+/calmodulin-mediated CDI by applying the calmodulin inhibitors E6 berbamine (Grant and Fuchs, 2008) and CGS-9343B (Norman et al., 1987). They significantly slowed down ICa rundown despite a comparable increase of the resting [Ca2+] (Fig. 4B,C), further supporting our notion that the lack of hydrolyzable ATP reduces the IHC ICa via a rise in resting [Ca2+] and consecutive CDI of the Ca2+ channels.
Finally, to test how the lack of ATP affects synaptic Ca2+ signals, we combined patch-clamp and confocal Ca2+ imaging that allows spatiotemporal characterization of submicrometer-sized Ca2+ domains at the fluorescently tagged ribbon-type AZs (Frank et al., 2009). We observed that the synaptic Ca2+ domains rapidly disappeared in the absence of ATP from the pipette solution, correlating in time with the rundown of whole-cell ICa (Fig. 4D,E).
Discussion
This study shows that ATP is required for maintaining operational CaV1.3 Ca2+ influx in IHCs via efficient Ca2+ clearance to secure sufficiently low basal [Ca2+]i and avoid steady-state CDI.
ATP dependence of IHC CaV1.3
The role of ATP in the regulation of L-type Ca2+ channels varies among cells of different tissues. First, phosphorylation/dephosphorylation have been shown to regulate channel gating (Xu et al., 2004). Second, ATP may alter Ca2+-dependent proteases, which directly interact with the Ca2+ channel (Altier et al., 2011). Finally, lack of ATP leads to failure of ATP-dependent Ca2+ pumps and to cytosolic Ca2+ accumulation that may trigger CDI of Ca2+ channels (Belles et al., 1988; von Gersdorff and Matthews, 1996).
Based on the present work using potent kinase inhibitors, a modulation of the IHC CaV1.3 channel by phosphorylation via CaMKII is unlikely. The majority of protein kinases (including CaMKII and PKA) can use ATP-γ-S, although it is a poorer substrate than ATP (Palvimo et al., 1985; Ishida et al., 1996), further arguing against an implication of CaMKII- and PKA-mediated phosphorylation in the ATP-γ-S-induced ICa reduction. Interestingly, it has been suggested that ATP-γ-S supports the normal function of CaV1.4 channels in synaptic terminals of bipolar cells by serving kinases as a substrate for thiophosphorylation (Heidelberger et al., 2002). In this context, the better maintained ICa in recordings with ATP-γ-S compared with those without exogenous ATP together with the mild effect on ICa of the PKA inhibitor KT5720 may indicate a modest positive effect of PKA-mediated phosphorylation of CaV1.3 on IHC ICa.
Steady-state CDI of CaV1.3 channels by elevated resting [Ca2+] in IHCs
Parallel measurements of IHC [Ca2+] and ICa revealed a coincident and correlated increase of resting [Ca2+] and ICa inactivation in the absence of hydrolyzable ATP or pharmacological block of PMCAs in IHCs. PMCAs are the major source of Ca2+ extrusion from IHCs (Kennedy, 2002) and other cells with ribbon synapses (Zenisek and Matthews, 2000). Lack of ATP or its hydrolysis (ATP-γ-S) (Eckstein, 1985) disables their pumping activity; indeed, the ATP-γ-S-mediated global Ca2+ increase observed in IHCs could be mimicked by the PMCA inhibitor CE. These findings emphasize the essential role of PMCA-mediated Ca2+ clearance for synaptic transmission. In addition to PMCA endogenous immobile and mobile Ca2+ buffers, the latter estimated at 0.5–1 mm Ca2+ binding sites (Hackney et al., 2005; Johnson and Marcotti, 2008) have been proposed to shape synaptic Ca2+ signals in IHCs (Frank et al., 2009). Our results suggest that, in conditions of metabolic stress that lowers the cytosolic ATP levels (likely 1–2 mm in hair cells) (Puschner and Schacht, 1997; Shin et al., 2007), IHCs may fail to maintain low resting [Ca]i and normal ICa, which would then impede sensory signaling during prolonged stimulation.
Steady-state CDI driven by enhanced basal cytosolic Ca2+ has been documented for CaV channels of cardiomyocytes and retinal bipolar cells (Belles et al., 1988; von Gersdorff and Matthews, 1996). In the latter, the dialysis with elevated Ca2+ led to a block of ICa. In the present study, we corroborated our hypothesis that the absence of hydrolyzable ATP triggers steady-state CDI in IHCs via increased basal cytosolic Ca2+ by showing that EGTA, BAPTA, and the calmodulin inhibitors antagonize the ICa rundown. Work on the molecular mechanism of Ca2+/calmodulin modulation of the C terminus of the CaV channels indicates that the N-terminal lobe of CaM might respond preferentially to the global accumulation of Ca2+ (Dick et al., 2008). IHCs use several mechanisms to counteract CDI. An increase of steady-state inactivation of CaV1.3 channels in IHCs, as found here upon manipulation of the ATP supply, is expected to reduce the rate of transmitter release and, consequently, of spiking in the postsynaptic spiral ganglion neurons. Interestingly, a human mutation in the gene coding for Ca2+ binding protein 2 that antagonizes CDI impairs hearing (Schrauwen et al., 2012) potentially because of increased steady-state inactivation. It is conceivable that IHC Ca2+ influx, synaptic sound coding, and hearing can be compromised also by other mechanisms that lead to enhanced CDI, and the metabolic state of the IHC may couple to sound encoding via the mechanism described in this study.
Footnotes
This work was supported by the German Research Foundation through the priority program 1608 to T.M. and the collaborative research center 889 project A2 to T.M. We thank S. Gerke and C. Senger-Freitag for expert technical assistance and Lars Maier for providing CBD and KN-93.
The authors declare no competing financial interests.
References
- Altier C, Garcia-Caballero A, Simms B, You H, Chen L, Walcher J, Tedford HW, Hermosilla T, Zamponi GW. The Cavβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- Armstrong D, Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987;84:2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano S, Komiya M, Koike N, Koga E, Nakatani S, Isobe Y. 5,6,7,8-Tetrahydropyrido[4,3-d]pyrimidines as novel class of potent and highly selective CaMKII inhibitors. Bioorg Med Chem Lett. 2010;20:6696–6698. doi: 10.1016/j.bmcl.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Basavappa S, Mangel AW, Scott L, Liddle RA. Activation of calcium channels by cAMP in STC-1 cells is dependent upon Ca2+ calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1999;254:699–702. doi: 10.1006/bbrc.1998.9997. [DOI] [PubMed] [Google Scholar]
- Belles B, Malécot CO, Hescheler J, Trautwein W. “Run-down” of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflugers Arch. 1988;411:353–360. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad JE, Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Cui G, Meyer AC, Calin-Jageman I, Neef J, Haeseleer F, Moser T, Lee A. Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 channels in mouse auditory hair cells. J Physiol. 2007;585:791–803. doi: 10.1113/jphysiol.2007.142307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick IE, Tadross MR, Liang H, Tay LH, Yang W, Yue DT. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–834. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Vazquez AE, Namkung Y, Chu H, Cardell EL, Nie L, Parson S, Shin HS, Yamoah EN. Null mutation of alpha1D Ca2+ channel gene results in deafness but no vestibular defect in mice. J Assoc Res Otolaryngol. 2004;5:215–226. doi: 10.1007/s10162-003-4020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Francis AA, Mehta B, Zenisek D. Development of new peptide-based tools for studying synaptic ribbon function. J Neurophysiol. 2011;106:1028–1037. doi: 10.1152/jn.00255.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Khimich D, Neef A, Moser T. Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci U S A. 2009;106:4483–4488. doi: 10.1073/pnas.0813213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Fuchs P. Calcium- and calmodulin-dependent inactivation of calcium channels in inner hair cells of the rat cochlea. J Neurophysiol. 2008;99:2183–2193. doi: 10.1152/jn.01174.2007. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci. 2005;25:7867–7875. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Sterling P, Matthews G. Roles of ATP in depletion and replenishment of the releasable pool of synaptic vesicles. J Neurophysiol. 2002;88:98–106. doi: 10.1152/jn.2002.88.1.98. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kitani T, Fujisawa H. Evidence that autophosphorylation at Thr-286/Thr-287 is required for full activation of calmodulin-dependent protein kinase II. Biochim Biophys Acta. 1996;1311:211–217. doi: 10.1016/0167-4889(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. The entry and clearance of Ca2+ at individual presynaptic active zones of hair cells from the bullfrog's sacculus. Proc Natl Acad Sci U S A. 1996;93:9527–9532. doi: 10.1073/pnas.93.18.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W. Biophysical properties of CaV1.3 calcium channels in gerbil inner hair cells. J Physiol. 2008;586:1029–1042. doi: 10.1113/jphysiol.2007.145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ. Intracellular calcium regulation in inner hair cells from neonatal mice. Cell Calcium. 2002;31:127–136. doi: 10.1054/ceca.2001.0267. [DOI] [PubMed] [Google Scholar]
- Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- Mammano F, Bortolozzi M, Ortolano S, Anselmi F. Ca2+ signaling in the inner ear. Physiology (Bethesda) 2007;22:131–144. doi: 10.1152/physiol.00040.2006. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Measurement of calibration constants for quantitative calcium fluorimetry. Cold Spring Harb Protoc. 2013;2013:947–948. doi: 10.1101/pdb.prot078212. [DOI] [PubMed] [Google Scholar]
- Norman JA, Ansell J, Stone GA, Wennogle LP, Wasley JW. CGS 9343B, a novel, potent, and selective inhibitor of calmodulin activity. Mol Pharmacol. 1987;31:535–540. [PubMed] [Google Scholar]
- Ohya Y, Sperelakis N. Modulation of single slow (L-type) calcium channels by intracellular ATP in vascular smooth muscle cells. Pflugers Arch. 1989;414:257–264. doi: 10.1007/BF00584624. [DOI] [PubMed] [Google Scholar]
- Okada Y, Sato-Yoshitake R, Hirokawa N. The activation of protein kinase A pathway selectively inhibits anterograde axonal transport of vesicles but not mitochondria transport or retrograde transport in vivo. J Neurosci. 1995;15:3053–3064. doi: 10.1523/JNEUROSCI.15-04-03053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palvimo J, Linnala-Kankkunen A, Mäenpää PH. Thiophosphorylation and phosphorylation of chromatin proteins from calf thymus in vitro. Biochem Biophys Res Commun. 1985;126:103–108. doi: 10.1016/0006-291X(85)90577-7. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/S0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/S0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Puschner B, Schacht J. Energy metabolism in cochlear outer hair cells in vitro. Hear Res. 1997;114:102–106. doi: 10.1016/S0378-5955(97)00163-9. [DOI] [PubMed] [Google Scholar]
- Roberts WM. Spatial calcium buffering in saccular hair cells. Nature. 1993;363:74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Schrauwen I, Helfmann S, Inagaki A, Predoehl F, Tabatabaiefar MA, Picher MM, Sommen M, Seco CZ, Oostrik J, Kremer H, Dheedene A, Claes C, Fransen E, Chaleshtori MH, Coucke P, Lee A, Moser T, Van Camp G. A mutation in CABP2, expressed in cochlear hair cells, causes autosomal-recessive hearing impairment. Am J Hum Genet. 2012;91:636–645. doi: 10.1016/j.ajhg.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CE, Wallimann T, Gillespie PG. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem. 2008;283:20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291X(91)92031-E. [DOI] [PubMed] [Google Scholar]
- Tucker T, Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995;15:1323–1335. doi: 10.1016/0896-6273(95)90011-X. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J Neurosci. 1996;16:115–122. doi: 10.1523/JNEUROSCI.16-01-00115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JJ, Hao LY, Kameyama A, Kameyama M. Calmodulin reverses rundown of L-type Ca(2+) channels in guinea pig ventricular myocytes. Am J Physiol Cell Physiol. 2004;287:C1717–C1724. doi: 10.1152/ajpcell.00105.2004. [DOI] [PubMed] [Google Scholar]
- Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, Fuchs PA, Yue DT. Switching of Ca2+-dependent inactivation of Ca(v)1.3 channels by calcium binding proteins of auditory hair cells. J Neurosci. 2006;26:10677–10689. doi: 10.1523/JNEUROSCI.3236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/S0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]