Abstract
Drosophila light-dependent channels, TRP and TRPL, reside in the light-sensitive microvilli of the photoreceptor's rhabdomere. Phospholipase C mediates TRP/TRPL opening, but the gating process remains unknown. Controversial evidence has suggested diacylglycerol (DAG), polyunsaturated fatty acids (PUFAs, a DAG metabolite), phosphatidylinositol bisphosphate (PIP2), and H+ as possible channel activators. We tested each of them directly in inside-out TRP-expressing patches excised from the rhabdomere, making use of mutants and pharmacology. When patches were excised in darkness TRP remained closed, while when excised under illumination it stayed constitutively active. TRP was opened by DAG and silenced by ATP, suggesting DAG-kinase (DGK) involvement. The ATP effect was abolished by inhibiting DGK and in the rdgA mutant, lacking functional DGK, implicating DGK. DAG activated TRP even in the presence of a DAG-lipase inhibitor, inconsistent with a requirement of PUFAs in opening TRP. PIP2 had no effect and acidification, pH 6.4, activated TRP irreversibly, unlike the endogenous activator. Complementary liquid-chromatography/mass-spectrometry determinations of DAG and PUFAs in membranes enriched in rhabdomere obtained from light- and dark-adapted eyes showed light-dependent increment in six DAG species and no changes in PUFAs. The results strongly support DAG as the endogenous TRP agonist, as some of its vertebrate TRPC homologs of the same channel family.
Keywords: diacylglycerol, Drosophila, microvilli, photoreceptors, phototransduction, TRP
Introduction
Drosophila phototransduction has served as a model system for sensory and lipid signaling. It takes place in a single column formed by ∼50,000 tightly packed light-sensitive microvilli disposed along the photoreceptor soma, termed rhabdomere. Photoisomerized rhodopsin activates phospholipase Cβ (PLC), which hydrolyzes phosphatidylinositol bisphosphate (PIP2) into 1,4,5-inositol trisphosphate (IP3), diacylglycerol (DAG), and a proton. This leads to the opening the two light-dependent ion channels, the transient receptor potential (TRP) and the TRP-like (TRPL), by an as yet undetermined mechanism. DAG, its polyunsaturated fatty acids metabolites (PUFAs; Hardie and Postma, 2008) and protons (Huang et al., 2010), and reduction in PIP2 have been implicated in channel gating, while IP3 and Ca2+ have been ruled out; however, the overall evidence is controversial.
Extracellular PUFAs applications to voltage-clamped photoreceptors activate the light-dependent current (Chyb et al., 1999). PUFAs and DAG open TRP and TRPL from the cytoplasmic side of excised rhabdomeral membrane patches (Delgado and Bacigalupo, 2009). DAG and PUFA applications to heterologously expressed TRPL have yielded conflicting results, since its basal activity and responsiveness to these lipids varies with the expression system (Lev et al., 2012a). Evidence for PIP2 is also contradictory. Protons activate the channels in photoreceptors and excised patches from cells heterologously expressing TRPL (Huang et al., 2010).
Efforts to identify the endogenous TRP and TRPL activator have failed for various reasons: (1) difficulties in interpreting whole-cell recordings from photoreceptors to understand the complex transduction events occurring in highly confined subcellular compartments (microvilli); (2) the microvillus size (∼1 × 0.05 μm) makes direct electrophysiological measurements daunting; (3) heterologous expression of the main transduction channel TRP (Reuss et al., 1997) has been intractable thus far (Vaca et al., 1994; Gillo et al., 1996; Xu et al., 1997); (4) TRPL behavior is inconsistent among the cells where it has been expressed (Lev et al., 2012a); and (5) biochemical techniques are limited by the insignificant rhabdomeral material offered by Drosophila eyes.
To obviate these difficulties, we assessed the study of TRP agonist in inside-out patches excised directly from the rhabdomere with extremely small patch-clamp pipettes, with the advantage over photoreceptor recordings of being a less complex preparation and over heterologous expression of having the channels in their native membrane. As TRP carries ∼95% of the phototransduction current (Reuss et al., 1997), we restricted our study to mutants expressing only this channel for a more precise interpretation of the recordings. Our approach allowed direct application of substances from the cytoplasmic side, mimicking the physiological situation. Further, the finding that the patches preserved the DAG-kinase (DGK) enzymatic activity allowed powerful in situ biochemical experiments.
In complimentary experiments using a novel rhabdomeral membrane preparation (Muñoz et al., 2013), we performed a detailed high-resolution study of rhabdomeral DAGs and PUFAs that have been implicated in phototransduction, obtained from light and dark-adapted eyes. Illumination elevates DAG levels but has no effect on PUFAs. Together, our findings strongly support that DAG serves as endogenous agonist of Drosophila light-dependent channels and that PUFAs, PIP2, and protons have no relevant role.
Materials and Methods
Flies.
Drosophila melanogaster flies were raised under dark and dim light cycles at 18°C. We used the following D. melanogaster strains: wild-type (Oregon-R), trpl302, rdgAPC-38, norpAP24, ninaE and Kir2.1R228. The flies were kindly provided by Drs. Roger Hardie (Cambridge University, UK) and Craig Montell (Johns Hopkins University, Baltimore, MD). The mutant ninaE;rdgA trpl was obtained by crossing the strain ninaE;trpl with rdgAPC-38.
Ommatidia with exposed rhabdomeres.
Eyes were removed from adult flies of either sex and their retinae mechanically disrupted with a sharp tungsten wire under low divalent cation Drosophila Ringer's solution containing the following (in mm): 120 NaCl, 4 KCl, 1 EGTA, 0.7 mm CaCl2, 10 HEPES, 2.5 l-proline, and 25 sucrose, pH 7.15. Ommatidia with partly disaggregated photoreceptors resulting from this procedure were chosen for the experiments, as the terminal segments of their rhabdomeres became exposed and were clearly identifiable by their distinctive brightness (Delgado and Bacigalupo, 2009). The cells were viewed with an Olympus IX70 inverted microscope, using either DIC optics (60× objective) or phase contrast (100× objective, NA 1.25).
Electrophysiology.
Single-channel currents were recorded with an Axopatch 200B patch-clamp amplifier (Molecular Devices). Recording pipettes of ≥50 MΩ resistance were made of borosilicate glass capillaries with internal filament with a horizontal puller (Sutter Instruments). In all experiments the pipettes were filled with low divalent cation Ringer's solution and the same solution was used externally. A multibarreled puffer pipette (∼0.5 μm each tip) connected to a computer-controlled Picospritzer (custom made) was used to apply the desired solutions to the membrane patches.
Electrophysiological data were digitized by a Digidata 1440 interface (Molecular Devices) and stored in a PC. pClamp 10 software (Molecular Devices) was used for acquisition and analysis. The recordings were sampled at 10,000 Hz and low-pass filtered at 1000 Hz for analysis. All voltages are expressed as pipette potentials (Vp). The recordings were corrected for the shift of the baseline current with the pClamp correction routine.
Rhabdomeral membrane preparation.
The rhabdomeral membrane fraction was prepared as previously described (Muñoz et al., 2013). Briefly, for each sample, 250 eyes were dissected out from adult flies previously anesthetized with CO2 and collected in cold divalent cation-free Drosophila Ringer's solution. Following this, the eyes were exposed for 10 min to light (light-adapted rhabdomeres; white light source) or to darkness (dark-adapted rhabdomeres) at room temperature, and then frozen in liquid nitrogen. The frozen eyes were mechanically disrupted by agitation in a Mini-bead beater (model 3110BXEUR; BioSpec Products). The homogenate was centrifuged in a tabletop centrifuge at 10 × g for 10 min at 4°C. The supernatant was recentrifuged at 2370 × g for 5 min at 4°C and the resulting pellet was used for the measurements. This fraction was identified by Western blotting using TRP as rhabdomere marker.
DAG and fatty acid quantification.
Total lipids were extracted as previously described (Matyash et al., 2008). 1,2-Diheptadecanoyl-sn-glycero-3-phosphocholine was used as general inner standard and 1,3-stearoyl-2-OH-sn-glycerol d5 (5 μg each) was used as DAG inner standard. Dried lipids were stored at −80°C and measured as previously described (Hummel et al., 2011). Lipids extracts were resuspended in 200 μl buffer B and transferred to a glass vial. Two microliters of this sample were injected to a C8 reversed phase column (100 mm × 2.1 mm × 1.7 μm particles; Waters Acquity UPLC system). The mass spectra were obtained with a mass spectrometer (MS; Exactive; Thermo Fisher). To build calibration curves we used four commercial DAG standards–1,2-dipalmitoyl-sn-glycerol, 1-palmitoyl-2-oleoyl-sn-glycerol, 1-stearoyl-2-palmitoyl-rac-glycerol, and 1,2-dioctadecanoyl-rac-glycerol–at four different concentrations (0.5, 5, 35, and 100 ng/ml), fitted by a lineal regression. Using ultra-performance liquid chromatography-reverse phase (UPLC-RP), the DAG standards were separated and detected as [M + Na]+ adduct ions in positive ion mode and free fatty acids in a negative ion mode. The limits of detection and quantification were 0.01–0.05 pg/μl and 0.1–0.5 pg/μl, respectively. The same conditions were used to analyze DAG and fatty acids from enriched-rhabdomeral samples of light- and dark-exposed wild-type flies.
Chemicals.
1-Oleoyl-2-acetyl-sn-glycerol (a soluble DAG analog; Sigma Products) was prepared every day from a stock solution in DMSO to 5 μm final concentration in divalent cation-free Drosophila Ringer's solution. The lipase inhibitor RHC-80267 (Sutherland and Amin, 1982; Biomol Research Laboratories) stock solution was prepared in ethanol to a final concentration of 50 mm. The specific DGK inhibitor R59022 (de Chaffoy de Courcelles et al., 1985; Enzo Life Sciences) was prepared in DMSO to a final concentration of 5 μm. PIP2 (Echelon Biosciences) was prepared in Ringer's solution at 2.7 mm. Chloroform and methanol (2:1, HPLC grade; Merck) was used to extract the lipids from the biological matrix. 1,2-di-palmitoyl-sn-glycerol, 1-palmitoyl-2-oleoyl-sn-glycerol; 1-stearoyl-2-palmitoyl-rac-glycerol; 1,2-distearoyl-rac-glicerol, 1,3-distearoyl-2-hydroxy-sn-glycerol-d5, and 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids). 1,2-di-octanoyl-3-phospho-(1′-myo-inositol-4′,5′-biphosphate; Echelon Biosciences).
Results
DAG opens channels in quiescent excised patches
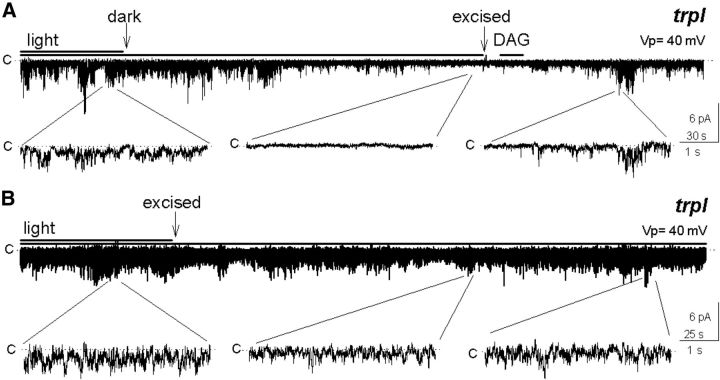
To explore the identity of native agonists of the TRP channels, we tested the efficacy of candidate agonists in opening TRP in excised patches. Cell-attached rhabdomeral membrane patches exhibited high TRP activity under illumination, but when light was turned off the channel gradually closed. When these patches were subsequently excised in the dark, channel activity remained quiescent, independently of the subsequent light condition. We then applied exogenous DAG after which the channels opened (Fig. 1A; n = 5), with a comparable response delay to those found in studies involving activation of channels by exogenous lipids (Chyb et al., 1999; Perozo et al., 2002). This result supports a role for DAG as channel agonist.
Figure 1.
Multi-TRP channel activity of excised inside-out rhabdomeral membrane patches depends on the light condition previous to excision. A, The high activity of the channels observed under illumination ceased after turning off the light and stayed off after excision (arrow), and was restored by a subsequent application of 5 μm DAG (n = 5). B, The channels were open in the attached patch under illumination and the activity did not diminish after excision (n = 5).
If DAG in fact serves as TRP agonist, manipulations of the endogenous biochemical regulation of DAG should influence channel activity in predictable ways. Two lines of evidence address this issue, indicating (1) DGK activity, which reduces DAG levels in the presence of ATP, is retained in excised patches and (2) modifying DGK activity influenced TRP in a fashion consistent with a role of DAG as channel agonist.
ATP lowers channel activity in active patches
We found that channel activity in rhabdomeral patches depended on the light condition preceding excision. In sharp contrast to the closed state of the channel in the patches excised in darkness (Fig. 1A), those excised in the light exhibited a pronounced constitutive TRP activity (Fig. 1B; n >100; Delgado and Bacigalupo, 2009). These observations suggest that the channel remained indefinitely in the mode it was in at the time of excision, open or closed. A reasonable interpretation is that the levels of the channel agonist in the excised patches were no longer modified by the transduction machinery, which stopped functioning after excision. Our subsequent experiments, however, suggest that DGK activity, which reduces DAG levels in the presence of ATP, is retained in excised patches in a functional state.
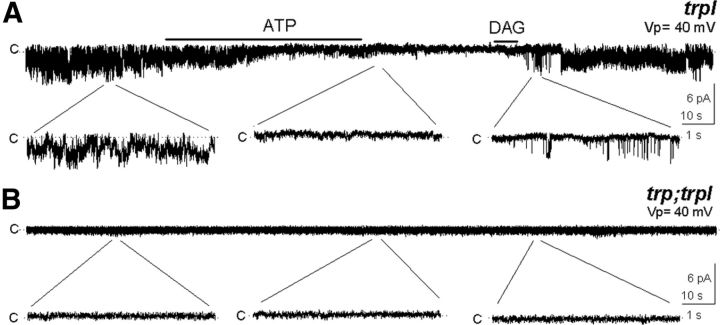
We previously observed that adding millimolar ATP to the solution bathing the cytoplasmic side of the patch drastically decreased TRP and TRPL activity in excised patches (Delgado and Bacigalupo, 2009). This closely resembles the physiological situation, where PLC generates DAG, which is subsequently phosphorylated to phosphatidic acid by DGK. This is illustrated by the experiment in Figure 2A, where ATP ended the constitutive activity of TRP; a subsequent DAG application reopened the channel. As a negative control we tested DAG in the double mutant lacking both channels, trp;trpl. As expected these patches were silent when excised in the light (Delgado and Bacigalupo, 2009), even in the presence of DAG (Fig. 2B; n = 6).
Figure 2.
Membrane patches retain DGK enzymatic activity. A, TRP activity was reduced by 5 mm ATP and recovered by addition of DAG (n = 6), revealing the presence of DGK. B, No channel activity was detected in excised patches from trp;trpl in the presence of DAG (n = 6).
These results strongly support that DAG opens the channels and suggest that DGK remains in the excised patches in a functional condition.
Excised membrane patches retained a functional DGK
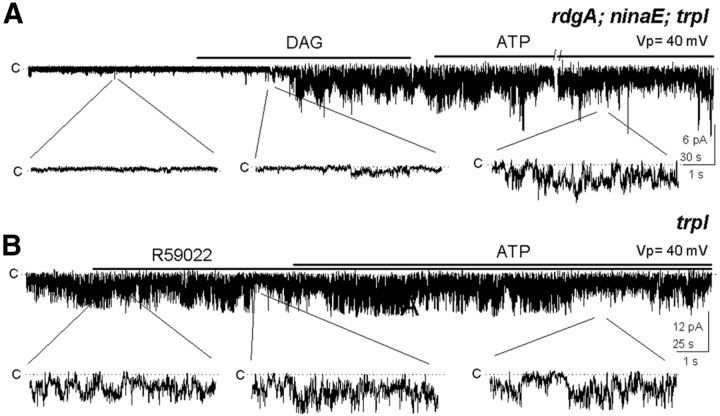
A previous immunochemical study detected DGK predominantly in the cytoplasmic subrhabdomeral cisternae, a cytoplasmic membrane system located at close distance from the microvilli (Masai et al., 1997); however, because of the limited resolution of such methodology, the presence of this kinase in the microvilli cannot be ruled out. To determine whether DGK was responsible for silencing TRP, we undertook two different strategies. In the first one we used a mutant defective in the gene encoding DGK, rdgA. For clarity and simplicity, we chose a mutant fly deficient in rhodopsin (ninaE) and TRPL, rdgA;ninaE trpl, because its photoreceptors are unresponsive to light and is virtually devoid of DGK. As we expected, those patches were silent when excised under illumination. Application of DAG activated TRP, although in this case it could not be silenced with ATP, consistent with the lack of DGK (Fig. 3A; n = 6) and contrary with the participation of other kinases.
Figure 3.
The effect of ATP requires DGK. A, Patch from rdgA;ninaE;trpl flies, lacking the DGK (rdgA) and rhodopsin (ninaE) and expressing TRP was silent, but was activated by DAG and could not be shut off by ATP (n = 6). B, Addition of ATP to a trpl patch in the presence of the DGK inhibitor R59022 did not affect the high TRP activity (n = 7).
In the second approach we made use of the specific pharmacological DGK inhibitor, R59022 (5 μm). We puffed Ringer's solution supplemented with the drug to a constitutively active patch and in its presence we added ATP; the ATP effect was completely abolished (Fig. 3B; n = 7), as in the DGK mutant. The fact that mutating the gene encoding DGK and inhibiting the enzyme gave identical results makes it unlikely that the effect of the mutation may have been caused by an alteration in the molecular composition of the mutant rhabdomere. This result implies that DGK is retained in the excised patches and that TRP closes as a result of DAG phosphorylation by DGK.
PUFAs do not participate in TRP activation in the excised patches
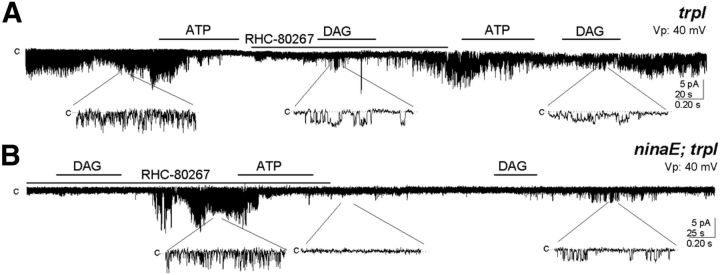
We explored the possibility that endogenous PUFAs activate TRP in the rhabdomeral membranes. To this goal we used the DAG lipase (DGL) inhibitor RHC-80267 (250 μm) on excised rhabdomeral patches from trpl and from ninaE;trpl flies. After closing the channel with a brief application of ATP to a trpl patch, we added the inhibitor and, in its presence, we applied DAG. This lipid was able to open the channel in the presumed absence of DGL activity. To check the possibility that RHC-80267 was ineffective in blocking DGL and DAG was turned into PUFAs, and PUFAs opened TRP rather than DAG, we applied ATP again. Contrary to the expectation if PUFA were the activator, ATP closed the channel, in agreement with the notion that DAG opens TRP. Furthermore, a new addition of DAG restored the channel activity (Fig. 4A; n = 3).
Figure 4.
DGL is dispensable for TRP activation by DAG. A, Following reduction of the constitutive TRP activity by ATP in a trpl patch, the lipase inhibitor RHC-80276 (250 μm) was added and in its presence we applied DAG, which activated the channel, and responded to a subsequent ATP addition (n = 4). B, In the ninaE;trpl double mutant, DAG activates a silent TRP patch despite the presence of the lipase inhibitor, and ATP turned it off (n = 3).
We performed an additional experiment with the same aim, this time on a ninaE;trpl fly. DAG activated TRP in the presence of RHC-80267 in an initially silent excised patch, and ATP closed it. A second addition of DAG reopened the channel (Fig. 4B; n = 3), showing that DGL was unnecessary.
Altogether, these results are in conflict with a role of PUFAs as the activator of TRP in the rhabdomere. Conversely, they strongly support a major role of DAG in gating the light-dependent channels in the microvilli.
PIP2 is not involved in TRP activity of excised patches
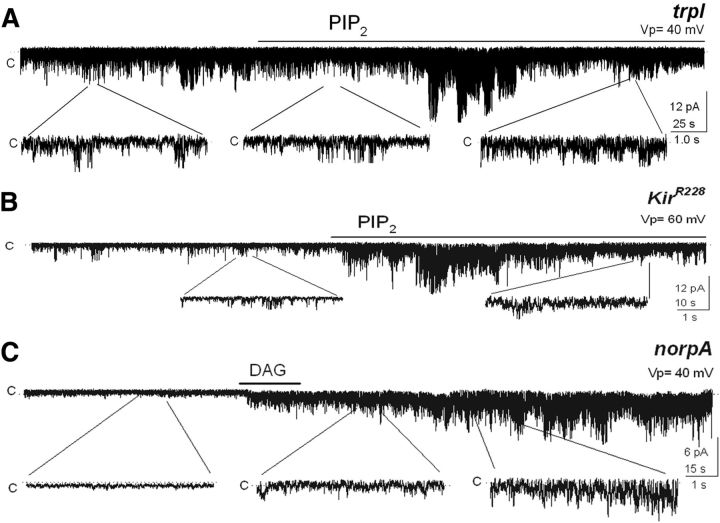
The drop in PIP2 that occurs in the membrane during phototransduction as a result of PLC activation has also been evaluated as a possible means of TRP and TRPL opening (Estacion et al., 2001; Parnas et al., 2009). According to this view, an application of PIP2 to a patch containing a constitutively active channel would be expected to reduce such activity, as this would mimic the dark-adapted condition of the photoreceptor. In contrast, we found no effect of this lipid on the channel, arguing against a possible role of PIP2 in TRP activation (Fig. 5A; n = 7).
Figure 5.
PIP2 has no effect on channel activity. A, PIP2 (PI(4,5)P2 diC8; 25 μm) was without effect on constitutive TRP activity (n = 7). B, PIP2 applied to a patch from a fly expressing the Kir2.1 inward rectifying K+ channel on a wt background opened this channel. TRP and TRPL were kept silent by the presence of ATP plus the TRP blocker La3+ (40 μm). The basal activity corresponds to Kir2.1 opening events. (n = 7). C, Patch from the mutant norpA (lacks PLC) presumably having high PIP2 levels was silent and became activated by DAG (n = 4).
The lack of effect of PIP2 may be explained by a failure to incorporate the added lipid into the membrane. We tested this on photoreceptors ectopically expressing the inward rectifying K+ channel Kir2.1, a channel that requires PIP2 and a membrane potential more negative than −40 mV to open (Hardie et al., 2001). Since the transgenic fly that we had available expressed the two light-dependent channels, we conducted the experiments under conditions in which both of them were closed. We achieved this by supplementing the internal solution with ATP (5 mm) and La3+ (40 μm), an effective TRP blocker (Hochstrate, 1989; Hardie and Minke, 1992; Delgado and Bacigalupo, 2009). As illustrated by Figure 5B, the patch presented a low background activity in this solution at Vp = 60 mV, most likely reflecting occasional Kir 2.1 events. The addition of PIP2 produced a robust activation of Kir2.1 (∼25 pS; Fig. 5B; n = 5). At a pipette potential of −40 mV the channel was closed, as expected (data not shown). Thus, these observations validate the PIP2 experiment and lead to the conclusion that PIP2 depletion in the rhabdomere does not mediate the opening of TRP.
We also examined whether DAG had an effect on patches with high levels of PIP2. To achieve this condition, we used a mutant hypomorphic for PLC, termed norpA. Here the patch was silent, as expected from the absence of this key component of phototransduction, presumably having high PIP2 levels; addition of DAG opened TRP (Fig. 5C; n = 4), resembling what happened upon applying DAG to a trpl patch excised in the dark (Fig. 1A). Thus, a decrement in PIP2 does not seem to be a requirement for opening the channel.
According to a recent report and contrary to the evidence of the literature described above, addition of PIP2 to excised patches from Sf9 expression cell system-enhanced TRPL activity (Huang et al., 2010). Such evidence is not supported by our experiments in the excised rhabdomeral membranes. Altogether these results argue against an involvement of PIP2 in the activation of TRP in the rhabdomere.
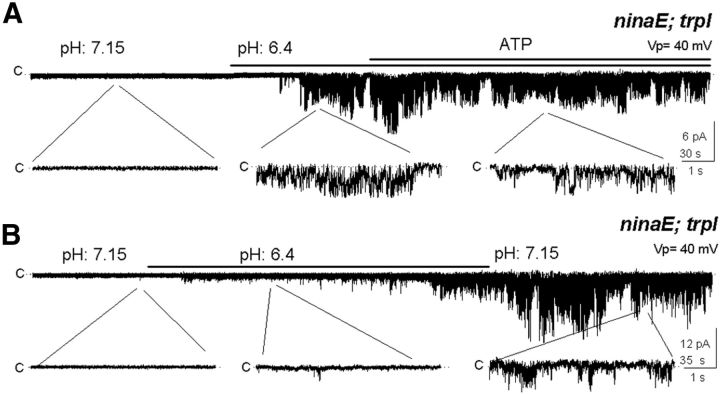
Protons irreversibly open TRP
Another mechanism proposed for TRP activation involves the proton released when PLC hydrolyzes PIP2 in combination with the resulting drop in pH (Huang et al., 2010). We investigated this possibility in ninaE;trpl patches, which were initially silent as in darkness and therefore, presumably containing high PIP2 levels. Remarkably, lowering pH from 7.15 (control) to 6.4 irreversibly activated TRP, remaining open after restoring pH to 7.15 (Fig. 6B; n = 5).
Figure 6.
Acidification opens TRP irreversibly. A, A shift from pH 7.15 to 6.4 opens TRP in the double mutant inaE;trpl, staying open after shifting back to pH 7.15 (n = 8). B, TRP opened by acidification could not be closed by ATP (n = 5).
In the same ninaE;trpl mutant, ATP was ineffective in closing channels opened by acidification. After the channel was activated by lowering pH to 6.4, TRP remained open in the presence of added ATP. The ineffectiveness of ATP contrasts with its effect of closing the DAG-activated TRP (Fig. 6A; n = 3).
These results are inconsistent with the possibility that reversible protonation determines the open/close state of the channel in the native rhabdomeral membrane during the light response.
Light increments DAG but does not change PUFA levels in the rhabdomere
If DAG indeed serves as TRP agonist, we would predict that DAG levels in the rhabdomere should increase in response to light stimuli. To test this idea, we measured DAG levels using ion chromatography in our membrane preparation enriched in rhabdomere membranes (Muñoz et al., 2013), comparing DAG levels in light conditions with those in dark conditions. Here, TRP and actin were used as rhabdomeral markers and lamin Dmo and superoxide dismutase as nuclei and mitochondria markers to detect contamination with their respective membranes (Muñoz et al., 2013). Although contamination with nuclei was considerable, it was unlikely that their lipid composition was light dependent, therefore it should not have affected our light/dark data. Mitochondria contamination was lower, but it should not affect the measurements as well.
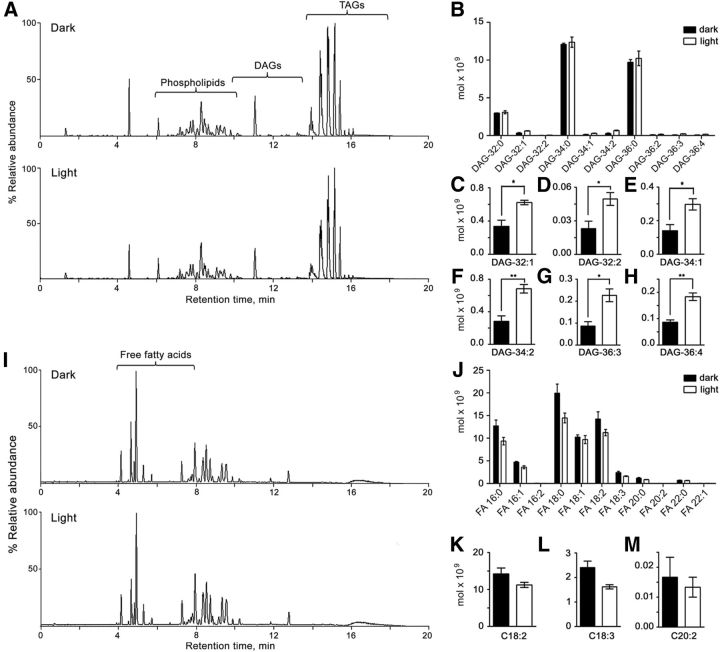
Figure 7A shows a total ion chromatogram (TIC) of the rhabdomeral membranes, where the different lipids of the sample were separated in positive ion mode. We extracted the corresponding peaks by isolating each single mass associated to a specific DAG, generating an extracted ion chromatogram (XIC) and quantified the relative sodium adduct intensity from each chromatogram. We identified a total of 10 DAG species, which eluted within the 9.1–12.6 min interval (Fig. 7A,B). DAG abundances were extremely low in comparison with the other lipids of the rhabdomeral membranes, like phospholipids and triacylglycerols, both in light- and dark-adapted eyes (Fig. 7A). All unsaturated DAGs underwent a significant increase in response to light, except DAG-36:2 (Fig. 7B–H). In contrast, the most abundant DAGs were saturated (DAG-32:0, DAG-34:0, DAG-36:0; Fig. 7B) and were not altered by illumination.
Figure 7.
Light increments unsaturated DAGs but not PUFAs in rhabdomeral membranes. A, Total lipid separation from a membrane fraction obtained by means of UPLC-MS (see Materials and Methods) from eyes pre-exposed to darkness (top) and illumination (bottom). TIC of mass spectra recorded in positive ion mode. B, Quantification of DAG species in light and dark conditions. C–H, Quantification of unsaturated DAGs, in further detail; *p < 0.05, **p < 0.01. I, TIC recorded in negative ion mode. J, Quantitative analysis of total free fatty acids. K–M, Quantitative analysis of PUFAs in further detail. There were no statistical differences in PUFAs (p > 0.05). Mean ± SEM (n = 3).
Similarly, if PUFAs serve as TRP agonists, contrary to our conclusion based on electrophysiological results, we would predict that PUFAs levels in the rhabdomere would be light dependent. To test this, we measured PUFA levels in rhabdomeral membranes obtained from dark- and light-adapted eyes, and found no difference (Fig. 7I–M). Figure 7I shows a TIC of the rhabdomeral membrane recorded in negative mode. We extracted the corresponding peaks by isolating each single mass associated to a specific fatty acid, generated an XIC chromatogram, and quantified the relative sodium adduct intensity. We identified a total of 11 fatty acids, which eluted within the 3.4–7.1 min interval (Fig. 7I,J). The longest fatty acid was C22 and the only PUFAs of the sample were hexadecadienoic (16:2), linoleic (C18:2), α-linolenic (C18:3), and eicosadienoic (C20:2) acids, none of which significantly changed with light (Fig. 7K–M). These measurements seem to rule out a role of PUFAs in the activation of TRP in the rhabdomere.
Our lipid measurements are consistent with the notion that DAG serves as the endogenous activator of the light-dependent channels and that PUFAs do not play a direct role, consistent with our electrophysiological evidence.
Discussion
We addressed the long-standing question of the identity of the endogenous activator of the light-dependent channels in Drosophila photoreceptors with complementary electrophysiological and biochemical approaches. Our electrophysiological approach offers several major advantages over previous studies: (1) the light-dependent channels could be examined in their native microvilli membrane and in the absence of any other channels, unlike expression systems (Delgado and Bacigalupo, 2009), avoiding ambiguities in the origin of whole-cell currents; (2) the substances of interest (putative agonists, inhibitors) could be applied directly to the cytoplasmic side of the channels; (3) the TRP channels could be investigated in isolation in trpl flies; and (4) retention of DGK in excised rhabdomeral patches provided a unique and powerful tool to evaluate the different putative activators of the channels. The biochemical approach allowed us to carry out precise measurements of microvillar levels of DAG and PUFAs and evaluate how they are influenced by light. The two separate lines of evidence strongly support DAG as the physiological agonist of Drosophila TRP and militate against PUFAs.
TRP and TRPL belong to the TRPC subfamily, whose mammalian members (TRPMC1–7) are their homologs. TRPC2, 3, 6, and 7 are opened by DAG in a PKC-independent manner (Hofmann et al., 1999; Okada et al., 1999; Lucas et al., 2003; Trebak et al., 2003; Estacion et al., 2006). TRPC2 is involved in pheromone transduction and their activation is not mediated by PUFAs (Lucas et al., 2003).
TRP and TRPL channels in rhabdomeral-attached patches can be activated by light and in excised-patches by DAG and PUFAs (arachidonic and linolenic acids), but not by monounsaturated fatty acids (oleic and elaidic acids; Chyb et al., 1999; Delgado and Bacigalupo, 2009), similarly to voltage-clamped photoreceptors (Chyb et al., 1999). DAG and PUFA activate ectopic TRPL in Sf9 insect cells (Estacion et al., 2001), in agreement with our results. Nevertheless, further investigations of DAG and PUFAs on TRPL in different expression systems yielded conflicting results (Lev et al., 2012a). Therefore, it was not possible to establish on the basis of all these data whether DAG and PUFA serve as endogenous channel activators. Here we provide strong new evidence bearing on these questions.
Four observations are key to this study: (1) TRP activity in the excised patches depended on whether they were excised from dark- or light-adapted rhabdomeres, suggesting that the channel agonist levels stayed as at the moment of excision; (2) ATP drastically suppressed TRP activity; (3) DAG opened TRP; and (4) light-induced rhabdomeral DAG increment but no change in PUFAs. These observations point to DAG as channel agonist and argue against a central role for PUFAs.
Further evidence supporting DAG in channel gating is the residence of two enzymes involved in DAG turnover in the microvilli: PLC in the transducisome complex (Tsunoda et al., 1997; Montell, 1999) and DGK, as shown here. Although no estimates are available for microvillar DGK levels, they are likely to be quite low, considering estimates of only ∼1000 rhodopsins, ∼50 G-proteins, ∼100 PLCs, ∼25 TRPs, and ∼2 TRPL per microvillus (Hardie and Postma, 2008). DGK was specifically required for ATP-induced inhibition of DAG-dependent TRP activity, independently of other kinases, including PKC (Tsunoda et al., 1997), rhodopsin kinase (Lee et al., 2004), or kinases of the phosphoinositide metabolism (Hardie and Postma, 2008). DAG-activated TRP could not be silenced by ATP when DGK activity was abolished either mutationally or pharmacologically. This is in agreement with a functional study in photoreceptors of the double mutant rdgA;trp, with a milder retinal degeneration than rdgA, resulting in a sluggish photoresponse deactivation. This phenotype presumably reflects an abnormally slow DAG decay, prolonging TRPL activity (Raghu et al., 2000).
DAGs were previously measured in Drosophila rhabdomeres (Muñoz et al., 2013), although the specific DAG species involved in phototransduction had not been described. Using a novel UPLC-MS, we found significant and consistent light-dependent increments in six of 10 DAG species of the rhabdomere (Fig. 7). Their low abundance and minimal light-dependent increments are consistent with a role as channel activators, allowing the tight regulation of the DAG concentration needed to comply with the fast photoresponse kinetics.
Several lines of evidence rule out direct roles for DGL (which generates PUFAs from DAG) in the light response: (1) in DGL mutants photoresponses persist, only diminished with repetitive stimulation (Leung et al., 2008); (2) DAG-induced excitation still proceeds when abolishing DGL pharmacologically (Fig. 4); (3) rhabdomeral PUFAs do not increase with light (Fig. 7); (4) DGL is apparently localized in the cytoplasm and not in the microvilli (Leung et al., 2008); and (5) PUFA diffusion from the cytoplasm to the TRP channels in the microvilli seems too slow to account for the ∼20 ms photoresponse latency. To our knowledge translational diffusion of PUFAs in a lipid bilayer has not been determined, but diffusion coefficients (D) for phospholipids are available; for example, it can be calculated that dilauroylphosphatidylcholine (DLPC; D = 3 × 10−8 cm2/s) in a plain DLPC bilayer (Almeida et al., 2005) takes 166 ms to diffuse 1 μm, the approximate length of a microvillus. Protein translocation between microvilli and cytoplasm normally occurs (e.g., TRPL, Gαq, and arrestin), although in a timescale of minutes to hours (Hardie and Postma, 2008).
Our results are at odds with PIP2 as a TRP agonist. PIP2 was proposed to function as such based on the observations that PLC added to excised patches from TRPL-expressing, Sf9 insect cell-incremented channel activity, presumably by decreasing PIP2, while PIP2 application reversed this effect (Estacion et al., 2001), resembling the situation in a stimulated photoreceptor. Although interesting, aside from the dependence of TRPL behavior in the expression cell system, neither PLC enzymatic activity nor incorporation of PIP2 into the membrane was directly demonstrated. A possible role of PIP2 in TRPL activation is complicated by conflicting observations, including an inhibitory effect of PIP2 (Estacion et al., 2001; Parnas et al., 2009; Huang et al., 2010), an excitatory effect of PIP2 (Huang et al., 2010), and no effect of PIP2 (Lev et al., 2012b) on channel activity. Our results show that neither increments of PIP2 levels, monitored in photoreceptors expressing the PIP2-sensitive Kir2.1 channel, nor low levels of this lipid presumably existing in the native patches excised under illumination, influenced TRP behavior in the native rhabdomeral membrane patches, contrary to a role of PIP2 as TRP activator.
Our results are in conflict with a light-dependent role for pH changes in determining the open/close states of the channels in the photoreceptors. Recently, the idea that protons released during PLC-catalyzed PIP2 hydrolysis could activate TRPL in the fly was proposed (Huang et al., 2010). Fluorescence measurements showed a modest light-induced pH decrement (0.1 U). Prolonged exposure to acidified extracellular solution, pH 6.4, supplemented with protonophore was necessary for activating only a small inward current, and decreased internal pH by 0.28 U. Intriguingly, while depletion of PIP2 using bright light (Hardie et al., 2001) drastically sensitized the cell to low pH, a rather high spontaneous TRPL activity was reversibly potentiated by lowering pH by 0.75 U in inside-out patches from TRPL-expressing Drosophila S2 cells. In such patches PIP2 activated TRPL at both high and low pH, unlike in Sf9 insect cell patches, where PIP2 had the opposite effect (Estacion et al., 2001), and in native rhabdomeral membranes, where the lipid had no effect (Fig. 5). We observed in excised rhabdomeral patches that a similar pH reduction (7.15–6.4) than tested in S2 cell patches also activated TRP, although in this case the channel stayed permanently open either after returning to high pH or applying ATP, contrary to the expectation for the physiological agonist of TRP.
The latency of channel activation by DAG is long compared with that of light responses, for unclear reasons. This is a common feature in channel activation by lipophilic agents (Chyb et al., 1999; Perozo et al., 2002). The long latencies in the lysophosphatidylcholine activation of a mechanosensitive channel reconstituted in liposomes recorded in excised patches with regular patch pipettes (2–4 MΩ) were attributed to a slow incorporation of the lipid into the bilayer (Perozo et al., 2002). Other possibilities may be a slow access of applied lipids to the patch membrane through the tips of the high-resistance pipettes (>50 MΩ) used in this study and a physical distortion of the microvillar structure produced upon sealing the pipette to the microvilli. The observed slow TRP activation is consistent with the seconds long latencies preceding the light-induced opening of Drosophila light-dependent channels in rhabdomeral-attached patches (Delgado and Bacigalupo, 2009). Interestingly, latencies of ∼7 s were recorded in cell-attached rhabdomeral patches of Limulus (Bacigalupo and Lisman, 1983) and ∼0.5 s in scallop photoreceptors (Nasi and Gomez, 1992), also substantially longer than their respective photoresponses.
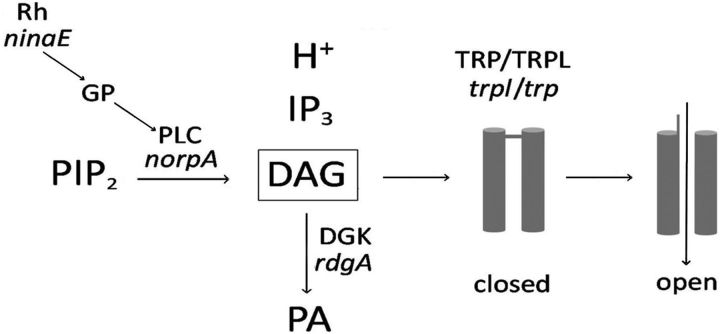
Together, our findings provide strong support for DAG as the main agonist of the light-dependent channels of Drosophila retinal photoreceptors and rule out a direct role for PUFAs in channel activation. Furthermore, neither light-dependent changes in PIP2 or pH appear to underlie TRP activation (Fig. 8).
Figure 8.
Model for Drosophila phototransduction. Light-activated rhodopsin (Rh) couples to a G-protein (GP) activating PLC, which cleaves PIP2 into IP3, DAG, and a proton. DAG opens TRP and TRPL, allowing an inward cationic current generating a depolarizing receptor potential, while PIP2 and H+ have no relevant role in this process. The genes encoding for the corresponding proteins are denoted in italics.
Footnotes
Supported by MIDEPLAN ICM-P05-001 (J.B.), FONDECYT 1100730 (R.D., J.B.), and CONICYT MSSCI Fellowship 22110957 (Y.M.). We thank Dr. Peter O'Day for invaluable suggestions.
The authors declare no competing financial interests.
References
- Almeida PF, Vaz WL, Thompson TE. Lipid diffusion, free area, and molecular dynamics simulations. Biophys J. 2005;88:4434–4438. doi: 10.1529/biophysj.105.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo J, Lisman JE. Single-channel currents activated by light in Limulus ventral photoreceptors. Nature. 1983;304:268–270. doi: 10.1038/304268a0. [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles DC, Roevens P, Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985;260:15762–15770. [PubMed] [Google Scholar]
- Delgado R, Bacigalupo J. Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: activation by light and lipids. J Neurophysiol. 2009;101:2372–2379. doi: 10.1152/jn.90578.2008. [DOI] [PubMed] [Google Scholar]
- Estacion M, Sinkins WG, Schilling WP. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J Physiol. 2001;530:1–19. doi: 10.1111/j.1469-7793.2001.0001m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estacion M, Sinkins WG, Jones SW, Applegate MA, Schilling WP. Human TRPC6 expressed in HEK 293 cells forms non-selective cation channels with limited Ca2+ permeability. J Physiol. 2006;572:359–377. doi: 10.1113/jphysiol.2005.103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillo B, Chorna I, Cohen H, Cook B, Manistersky I, Chorev M, Arnon A, Pollock JA, Selinger Z, Minke B. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry. Proc Natl Acad Sci U S A. 1996;93:14146–14151. doi: 10.1073/pnas.93.24.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-S. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Postma M. The senses: a comprehensive reference. Vol. 1. San Diego, CA: Academic; 2008. Phototransduction in microvilliar photoreceptors of Drosophila and other invertebrates. [Google Scholar]
- Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 2001;30:149–159. doi: 10.1016/S0896-6273(01)00269-0. [DOI] [PubMed] [Google Scholar]
- Hochstrate P. Lanthanum mimicks the trp photoreceptor mutant of Drosophila in the blowfly Calliphora. J Comp Physiol A. 1989;166:179–187. doi: 10.1007/BF00193462. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20:189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Hummel J, Segu S, Li Y, Irgang S, Jueppner J, Giavalisco P. Ultra performance liquid chromatography and high resolution mass spectrometry for the analysis of plant lipids. Front Plant Sci. 2011;2:54. doi: 10.3389/fpls.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Xu H, Montell C. Rhodopsin kinase activity modulates the amplitude of the visual response in Drosophila. Proc Natl Acad Sci U S A. 2004;101:11874–11879. doi: 10.1073/pnas.0402205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron. 2008;58:884–896. doi: 10.1016/j.neuron.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Katz B, Minke B. The activity of the TRP-like channel depends on its expression system. Channels. 2012a;6:86–93. doi: 10.4161/chan.19946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Katz B, Tzarfaty V, Minke B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J Biol Chem. 2012b;287:1436–1447. doi: 10.1074/jbc.M111.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/S0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- Masai I, Suzuki E, Yoon CS, Kohyama A, Hotta Y. Immunolocalization of Drosophila eye-specific diacylglycerol kinase, rdgA, which is essential for the maintenance of the photoreceptor. J Neurobiol. 1997;32:695–706. doi: 10.1002/(SICI)1097-4695(19970620)32:7<695::AID-NEU5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–268. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- Muñoz Y, Fuenzalida K, Bronfman M, Gatica A, Sepúlveda M, Bacigalupo J, Roth AD, Delgado R. Fatty acid composition of Drosophila photoreceptor light-sensitive microvilli. Biol Res. 2013;46:289–294. doi: 10.4067/S0716-97602013000300010. [DOI] [PubMed] [Google Scholar]
- Nasi E, Gomez MP. Light-activated ion channels in solitary photoreceptors of the scallop Pecten irradians. J Gen Physiol. 1992;99:747–769. doi: 10.1085/jgp.99.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Parnas M, Katz B, Lev S, Tzarfaty V, Dadon D, Gordon-Shaag A, Metzner H, Yaka R, Minke B. Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J Neurosci. 2009;29:2371–2383. doi: 10.1523/JNEUROSCI.4280-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Raghu P, Usher K, Jonas S, Chyb S, Polyanovsky A, Hardie RC. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron. 2000;26:169–179. doi: 10.1016/S0896-6273(00)81147-2. [DOI] [PubMed] [Google Scholar]
- Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/S0896-6273(00)80416-X. [DOI] [PubMed] [Google Scholar]
- Sutherland CA, Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982;257:14006–14010. [PubMed] [Google Scholar]
- Trebak M, St J Bird G, McKay RR, Birnbaumer L, Putney JW., Jr Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem. 2003;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;267:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/S0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]