Abstract
Energy metabolism is one of the most recognized targets of thyroid hormone action, which indeed plays a critical role in modulating energy expenditure in all of its components. This is because thyroid hormone receptors are ubiquitous, and thyroid hormones interact and influence most metabolic pathways in virtually all systems throughout the entire life of the organism. The pleiotropic actions of thyroid hormone are the results of interaction between the local availability of T3 and the signal transduction machinery, which confer in physiologic conditions time and tissue specificity of the hormonal signal despite negligible variations in circulating levels. Historically, the measurement of energy expenditure has been used as the gold standard for the clinical assessment of the hormonal action until the advent of the immunoassays for TSH and thyroid hormone, which have since been used as proxy for measurement of thyroid hormone action. Although the clinical correlates between thyroid hormone action and energy expenditure in cases of extreme dysfunction (florid hyperthyroidism or hypothyroidism) are well recognized, there is still controversy on the effects of moderate, subclinical thyroid dysfunction on energy expenditure and, ultimately, on body weight trajectory. Moreover, little information is available on the effects of thyroid hormone replacement therapy on energy expenditure. This mini review is aimed to define the clinical relevance of thyroid hormone action in normal physiology and functional disorders, as well the effects of thyroid hormone therapy on energy expenditure and the effects of changes in energy status on the thyroid hormone axis.
Keywords: thyroid hormone, energy expenditure, energy metabolism, indirect calorimetry, thyroid 11 dysfunction
The effects of thyroid hormone (TH) are pervasive throughout the various tissues [1] and across the development of the organism [2], yet the most recognized TH action is the regulation of energy expenditure (EE). Historically, basal metabolic rate (BMR) measurement by indirect calorimetry has represented the gold standard for the assessment of TH action [3] until the advent of immunoassay methods for the direct measurement of TH and TSH [4]. Although the dramatic effects of severe dysfunction, either hyperthyroidism or hypothyroidism, on EE are obvious and well described [5, 6], the molecular underpinnings of the effects of TH on EE are not completely understood, and the effects of subtle dysfunction of the thyroid axis on EE have not been clearly defined as yet. Despite this uncertainty, the belief that minimal changes in TH signaling cause considerable perturbation in EE, symptoms and clinical indices of euthyroidism are widespread, even in the absence of abnormalities in the TSH or TH levels [7, 8]. Furthermore, the prominent effects of TH on energy metabolism have prompted intense research toward the therapeutic use of TH supplementation or the development of TH analogs to promote energy dissipation and ultimately weight loss [9]. The aim of this mini review is to describe the pleiotropic actions of TH on EE in both physiology and pathologic states as well to review the effects of TH replacement or supplementation on EE.
1. Energy Expenditure: Definition and Components
In sedentary individuals, resting EE (REE) accounts for the majority (60% to 80%) of total EE (TEE) [10]. The term REE is often used interchangeably with BMR, and it is defined as the minimum amount of energy necessary to maintain the individual alive awake in a steady state of energy balance. Of interest, in dynamic states of energy balance such as prolonged fast, REE can decrease substantially [10]. Substrate oxidation (respiration) is required to maintain the basic functions of the organism, which include delivery of substrate and oxygen to the tissues, cellular structural integrity and functions, among them the maintenance of gradients between intracellular and extracellular compartments.
Voluntary physical activity, also called exercise activity–related thermogenesis (EAT), and non–exercise activity–related thermogenesis (NEAT), spontaneous fidgeting, are likely the most important modifiable variables in the TEE in most people and can vary significantly between individuals [11] and within the same subject on a day-to-day basis in relation to the physical activity [11].
An additional lesser-known component of EE is the thermic effect of food (alternatively defined as postprandial thermogenesis), which represents the energy loss following food intake above and beyond the requirements for absorption, storage, and digestion. The thermic effect of food is considered an energy metabolism homeostatic short loop directed to dissipate acute calorie loads, that generally ranges between 8% and 15% of energy intake, and the variance has been associated with nutrient composition and energy content of consumed foods [12].
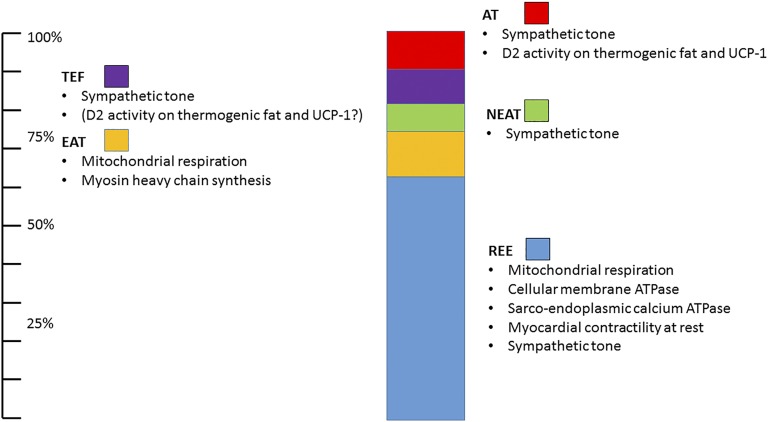
Finally, adaptive thermogenesis is defined as the energy dissipation required to maintain the core temperature when individuals are exposed to environmental temperatures below the thermoneutral zone, which is estimated to be 28°C in humans [13]. Adaptive thermogenesis is also subdivided in shivering thermogenesis, due to muscle fasciculation and involuntary contraction, and nonshivering thermogenesis, which results from direct conversion of chemical energy into heat, mostly by the shunting of the proton gradient in the mitochondrial membrane by the uncoupling protein-1 (UCP-1) predominantly expressed in brown and beige fat [14]. Adaptive thermogenesis was thought to be relevant only in small and hibernating mammals, but over the last decade, observational and intervention studies have demonstrated that adaptive thermogenesis can account for a substantial fraction of human TEE, up to 15% via nonshivering, and greater during intense shivering thermogenesis [15]. The components of EE and their relative contribution to TEE are shown in Fig. 1.
Figure 1.
Schematic representation of TEE components. The greater determinant of TEE in sedentary humans is REE (light blue). EAT accounts for ∼15% of TEE, but it is the most variable component of TEE. Additional components include NEAT, thermic effect of food (TEF), and adaptive thermogenesis (AT). All TEE components are directly or indirectly modulated by the TH action (see text for details).
Remarkably, all of the components of TEE are modulated by TH (reviewed in detail by Vaitkus et al. [16]). Briefly, TH exerts a pervasive role on REE by directly regulating (and thus promoting ATP utilization) metabolic cycles such as the lipolysis/lipogenesis, glycogenolysis/gluconeogenesis, phosphofructokinase/fructose 1,6-diphosphatase, hexokinase/glucose-6-phosphatase, and protein synthesis and catabolism [17]. Additionally, TH action accelerates ion leaks across the cell membrane, most notably via the Na+/K+ ATPase, and sarcoendoplasmic calcium ATPase, requiring additional ATP consumption to maintain the ion gradients [18].
Moreover, the inotropic and chronotropic actions of TH on the myocardium are well described and account for a demonstrable change in oxygen consumption as a result of changes in the TH status, both in vitro and in vivo [19].
TH action also has a remarkable modulatory effect on mitochondrial respiration by promoting mitochondrial biogenesis directly [20] and by stimulating the transcription of PGC-1α [21], which is considered the master regulator of aerobic respiration. Additionally, TH action has the ability of shunting the proton gradient in the inner membrane of the mitochondria, effectively diverting chemical energy into heat. Aside from UCP-1, TH decreases the efficiency of oxidative phosphorylation of the mitochondrial respiration by activating the mitochondrial permeability transition pore and by modulating the ADP/ATP translocase [22].
The exquisite local, tissue-specific modulation of the TH action resulting from the interaction of circulating levels of TH with local conversion of T4 into T3, its transport within the target cell, and the interaction with the receptor isoforms and transcription apparatus enables a remarkable variability of the TH signaling (and as a result a modulation of oxygen consumption) at a cellular level [23]. This is exemplified by the effects of cold exposure on brown adipose tissue (BAT) by which the activation of the β3-adrenergic pathway promotes a transient state of “cellular hyperthyroidism” driven by the action of the type 2 deiodinase, which in turn results in a local stimulation of transcription of UCP-1, and mitochondrial membrane permeability, ultimately promoting a dissociation between mitochondrial respiration and ATP production, generating an extremely efficient and timely generation of heat to maintain the core temperature [24]. At the cellular level, TH action is modulated by a group of enzymes containing selenoproteins. Among these three enzymes, type 1 and 2 deiodinase convert T4 (inactive or prohormone) to active TH T3, allowing local concentration changes and regulation of hormone action in certain tissues without showing considerable variation on serum levels. Type 2 deiodinase has a high affinity for T4 and is primarily found in brain, pituitary gland, thyroid, muscle, and brown fat and particularly important in the time- and tissue-specific regulation of TH action in thermogenesis.
Collectively, TH action plays a critical role in the modulation of EE in vertebrates, and it has a unique role in the maintenance of core temperature in warm-blooded animals. These effects are mostly permissive, and in physiologic states, the local modulation of the hormonal signaling rather than changes in circulating levels of TH is responsible for the cellular specificity of TH on respiration and ultimately on EE.
2. Measurement of Energy Expenditure
The most reliable method for the measurement of EE and its components is the indirect calorimetry which is based on the Weir’s equation in which EE = [3.94 × volume of oxygen uptake (VO2)] + [1.1 × carbon dioxide output (VCO2)] [25]; hence, by measuring the differential in O2 and CO2 between gas inhaled and exhaled, one can calculate the calorie consumption, on the assumption the individual is in a steady nutritional state and is not engaging in substantial physical activity. Historically, this method has represented the gold standard for the office-based assessment of thyroid function as BMR [26]. Additionally, by measuring the differential in CO2 production versus O2 consumption, one can generate the respiratory quotient, which in turn allows the estimation of substrate utilization, in which 1 represents all carbohydrate, whereas 0.7 represents all fat oxidation [27].
The vast majority of indirect calorimeter EE measurements are performed by the ventilated hood method, which relies on a portable gas analyzer apparatus connected to a small dome hood that surrounds the study subject [28]. Ventilated hood methods are “open” by which the apparatus measures exclusively expired air, whereas the inhaled air is “estimated” by measuring a reference air sample. The advantages of this method reside in the portability of the apparatus and the relative low cost. Conversely, the recordings using the ventilated hood method are limited in time by the necessity of keeping the subject immobile, awake, and calm. Moreover, the sensitivity and accuracy of the system are greatly reduced by the fact that the inhaled air is estimated/calculated rather than measured [29]. Hence, changes in humidity or O2 or CO2 concentration (e.g., recording performed in small rooms with more than one observer) may negatively affect the accuracy of the recordings. Conversely, by fitting a portable indirect calorimeter with a mask, one can record gas exchange during exercise.
The whole-room calorimeter “metabolic chamber” method is based on the continuous measurement of air entering and exiting a sealed room with continuous mixing of the air (turbulent flow) in which the study subject can reside for prolonged period of time, up to a few days, and perform normal daily activities, including exercise [30, 31]. This method is currently considered the gold standard for the measurement of EE, because its accuracy is greater than the ventilated hood [32], and it allows the recording of all of the EE components in near free-living conditions [10]. Major limitations of the whole-room calorimeter method are represented by the cost of the apparatus and by the inability of measuring real-life conditions.
An alternative method for the estimation of EE is based on the administration of water doubly labeled with stable isotopes (2H218O) and the measurement of the differential disappearance of hydrogen vs oxygen molecules [33] as measured by mass spectroscopy. This method allows the estimation of average TEE over a long period of time (usually 1 month), and its major advantage resides in the recording of EE in free-living conditions [33] without the risk associated with the exposure to ionizing radiations. The disadvantages are related to its cost, the inability of measuring the EE components, and the fact that the measurement is based on a series of assumptions such as stable weight and average diet.
Accelerometers represent an attractive alternative for the estimation of EE [34]. The technology is inexpensive and noninvasive, but the measurement is based on multiple assumptions, resulting in a low accuracy [35].
Collectively, multiple methods are available for the estimation of EE and its components, and at the present time, the whole-room indirect calorimeter represents the most accurate tool, able to measure the entire spectrum of EE and its components, but its costs and requirement of highly specialized personnel severely limit its applications (Table 1).
Table 1.
Comparison of Major Methods Used to Measure EE in Humans
| Methods | EE Components | Advantages | Limitations | |||||
|---|---|---|---|---|---|---|---|---|
| REE | TEF | EAT | NEAT | AT | TEE | |||
| Whole-room calorimeter | Yes | Yes | Yesa,b | Yesc | Yes | Yes | Accurate (∼5% error), specific parts of TEE can only be measured with this method | Expensive, requires specialized team; not representing free-living conditions |
| Hood/canopy (open-circuit) | Yes | Yesb | Yes | No | Yesb | No | Portable, easy to use, available, cheaper | Good accuracy for REE, dependent subject mobility, confined subject |
| Doubly labeled water | No | No | No | No | No | Yes | Accurate (<5% error), able to apply long-term free-living conditions | Very expensive, requires expertise, can measure only TEE |
| Wearable devices (accelerometers, heart monitors) | No | No | Yes | Yes | No | No | Inexpensive, able to apply long-term free-living conditions | Inaccurate |
| Personal logs | No | No | Yesb | No | No | No | Inexpensive, able to apply long- term free-living conditions | Highly inaccurate, subject to recorder bias |
Abbreviations: AT, adaptive thermogenesis; TEF, thermic effect of food.
Chen et al. [31].
Technically challenging.
Requires the use of wearable devices.
3. Clinical Correlates of Thyroid Disorders on EE
A. Hyperthyroidism and EE
Florid hyperthyroidism exemplifies the pervasive effects of TH on EE, and indeed, the measurement of BMR was used to assess the severity and course of the disease [3]. Aside the direct effects of TH on cellular respiration and ion channels (see above), the energetic costs of hyperthyroidism include its effect on the cardiovascular system [19] and, via the synergistic effects on the sympathetic nervous system, the fine tremors and increase in spontaneous movements, leading to a notable increase in NEAT [36]. Additionally, there is experimental evidence that a maladaptive activation of the nonshivering thermogenesis in BAT provides additional contribution to the increase in EE associated with hyperthyroid states [37, 38]. This is likely mediated by the positive transcriptional effects of T3 on UCP-1 [39]. In this case, T3 is not generated locally within the brown adipocyte by the action of type 2 deiodinase, which is inhibited by T3 [38], but rather deriving from the excess of circulating hormone.
Experimentally, Kim et al. [40] studied six Korean women with Graves disease during the course of a year after diagnosis and measured EE by indirect calorimetry at baseline, then at 4, 8, 12, 26, and 52 weeks after treatment with methimazole. At baseline, REE was 140% of predicted REE, which reduced to 113% at week 52. Free T3, total T3, and free T4 were significantly correlated with REE as well as peripheral deiodinase activity [40]. In another study, metabolic changes in REE were measured (by indirect calorimetry) during the treatment course of hyperthyroidism in 21 Chinese women. EE was found to be significantly reduced (28.7 ± 4.0 kcal/kg to 21.5 ± 4.1 kcal/kg; P < 0.001), and respiratory quotient was increased (0.76 to 0.81; P = 0.037), indicating a shift in substrate utilization from fat to carbohydrate oxidation [41]. Of note, changes in the REE were correlated with FT3 levels; however, leptin levels did not change significantly.
Although the changes in EE during clinical hyperthyroidism have been clearly demonstrated in clinical studies, the literature on the effects of subclinical hyperthyroidism is very limited and inconclusive [42, 43].
B. Hypothyroidism and EE
The dramatic increase in EE observed in hyperthyroidism is mirrored by a decrease in EE of similar entity during hypothyroidism, although its contribution to weight gain and obesity is comparatively reduced [44, 45]. The possibility of fine-tuning the thyroid homeostasis (at least as it relates to TSH levels) by varying the levothyroxine (LT4) dose in patients who are deficient of, or completely devoid of, endogenous TH secretion has provided the opportunity of studying the correlation between hypothyroidism and EE.
Al-Adsani et al. [46] measured REE by ventilated hood in a small group (nine patients) of patients with hypothyroid at different intervals by adjusting LT4 dose to achieve a normal, slightly reduced and a slightly elevated TSH level. The authors demonstrated an inverse correlation between TSH and REE with a change of 15% for a TSH ranging from 0.1 to 10 μIU/mL. Of interest, free T4 remained within the normal range in all of the study volunteers. Nonetheless, the changes in REE with different LT4 doses were demonstrated in every patient [46].
A small study comparing subjects with frankly hypothyroid and subclinical hypothyroid and healthy control subjects did not observe any difference in their sleeping EE when measured by accelerometers and metabolic equivalent of task (MET) at baseline and after achieving euthyroidism [47]. This is not surprising because the frank hypokinesia is observed only in extremely severe forms of hypothyroidism; hence, the limited sensitivity of the accelerometer technique likely contributed to decreasing the ability of estimating small differences in EE.
Recently, Samuels et al. [48] have performed several clinical observations to investigate the effect of TH treatment on EE, body composition and symptoms of thyroid dysfunction like cognition, mood, or life quality measures in patients with hypothyroid.
In one cross-sectional study, the authors compared EE and body composition in patients who were receiving suppressive doses of LT4, chronic replacement dose, and euthyroid control subjects. The results of the study demonstrated that the REE of patients treated with replacement LT4, compared with individuals treated with suppressive LT4 dose, was 6% lower. Of interest, when the comparison was made with control subjects devoid of thyroid pathology, the REE was still significantly lower (4%), suggesting that LT4 therapy does not completely normalize EE. In the same group, the group free T3 correlated with REE and was significantly lower in the replacement therapy group compared with the other two groups [48]. Although the study population was heterogeneous, including patients with hypothyroid with different etiologies, and the EE recording was limited to REE, it suggests that normalization of TSH may not equate to a state of euthyroidism as it relates to energy metabolism and that a state of tissue hypothyroidism may contribute to a decrease in EE.
Following this observation, the same group conducted a cross-sectional analysis to assess the association among 140 patients with EE and TH replacement hypothyroid treated with LT4. In this study population, REE did not differ significantly between patients achieving low-normal (TSH ≤ 2.5 μIU/mL) vs high normal TSH (TSH >2.5 μIU/mL). Conversely, free T3 level showed a direct correlation with EE, but also with indices of adiposity including body mass index (BMI), body composition, and fat free mass [49]. This latter observation is consistent with other cross-sectional studies that have clearly defined the positive association between circulating levels of T3 and adiposity [50, 51].
Most recently, in a double-blind intervention study, Samuels et al. [52] investigated the effects of LT4 therapy adjustments in 138 patients with hypothyroid. The authors enrolled patients with hypothyroidism treated with LT4 (baseline TSH 2.21 ± 0.13 μIU/mL) and modified the LT4 dose to different therapeutic goals (0.34 to 2.50, 2.51 to 5.60, and 5.61 to 12.0 μIU/mL, respectively) for 6 months. This study failed to demonstrate a significant difference in EE at the end of treatment [52]. In contrast, a secondary analysis of the data demonstrated that increases in REE/lean body mass correlated directly with increases in free T4 and free T3 levels and inversely with TSH levels.
This last study was originally designed to capture REE by ventilated hood and total EE by doubly labeled water, but unfortunately, only a minority of the study participants were assessed with the latter. Additionally, physical activity was measured by accelerometers. The use of multiple complementary methods to record EE enabled the investigators to obtain a comprehensive assessment of the components of EE in patients with hypothyroid. The results of the study, though, did not show considerable differences in EE components, BMI, or body composition among the three arms. Conversely, the authors observed a trend between REE corrected for lean body mass and changes in free T3 because of the therapy, with a point estimate of 0.11 kcal/kg/day increase in corrected REE for each 10 pg/dL increase in free T3. This trend did not reach statistical significance after correction for multiple comparisons. Of interest, consistent with other studies, TSH levels were inversely correlated with fat oxidation, and a nonsignificant direct correlation trend was observed between fat oxidation and free T3. These results indicate changes in LT4 dosage within a relative wide range of target TSH have only a limited effect on EE [52]. Importantly, the limitations inherent to the sensitivity of the techniques used to assess EE, and its components have likely contributed to reduce the statistical power of the study and thus to underestimate the relation between TH replacement dose and changes in EE and its components.
Collectively, the data indicate that changes in LT4 therapy within the range commonly used in clinic are not sufficient to generate a measurable change in EE recordings, whereas there is a good correlation between circulating levels of T3 and EE. Of interest, in a trial [53] in which we substituted pharmacoequivalent doses of liothyronine for LT4 (as indicated by a TSH within a 0.5 to 1.5 μIU/mL), we observed a substantial increase in circulating T3 levels and a robust weight loss. In contrast, no remarkable difference was observed in EE (measured by ventilated hood method). This apparent paradox can be explained by the relative lack of sensitivity of the instrument and by its inability to capture the entire spectrum of the EE components.
C. Syndromes of Resistance to TH and Energy Metabolism
The syndromes of resistance to TH (RTH) [54] due to inactivating mutations of the alpha or beta TH receptor isoforms are “experiments of nature” that illustrate the effects of selective inhibition of TH signaling. Although the RTH syndrome due to mutations of the beta receptor gene has been described more than half a century ago [55], the RTH syndrome due to mutations in the alpha receptor gene was discovered only recently [56], presumably because of the very subtle changes in TH and TSH in patients with syndromic features, which did not point investigators to assess the molecular pathology of the TH axis. The differential relative expression of the two receptors helps in explaining the dramatic differences in phenotype and in the effects of energy metabolism. The selective prominent expression of the beta TH receptor in the pituitary and liver explains the paradoxical effects of mutations by which the phenotype is commonly associated with a state of increased metabolism due to a shift of the TSH-TH correlation, with consequent overexposure of the other tissues to TH signaling. Of note, this is inconsistent with the original case described by Refetoff et al. [55], which was severely hypothyroid and hypometabolic. This apparent paradox is explained by the fact that the patient was homozygous for TH receptor beta mutations. Nonetheless, the overall correlation genotype/phenotype in RTH beta is still not completely defined, and patients sharing the same mutations can have dramatically different clinical presentations [57], ranging from asymptomatic, to mild resistance, to florid hyperthyroidism with severe hypermetabolic state, as observed in the selective pituitary RTH [58] (Weintraub syndrome) [59]. The relative expression of the mutant receptor in tissues due to the interaction with transcription factors and enhancer sequences explains in part the variability in the dominant-negative effect of the mutant allele, leading to the different phenotype [60].
Mitchell et al. [61] showed that REE was substantially higher (∼18%) in patients with RTH beta compared with euthyroid controls. In this study, taking the skeletal muscle as a major determinant of EE, the author performed 13C/31P-magnetic resonance spectroscopy in a subset of these patients to assess in vivo resting mitochondrial energy metabolism, which showed a substantial increase (∼60%) in mitochondrial energy uncoupling. Thyroid function, as reflected by T4 levels, was twofold increased, and REE was increased by ∼15% in these individuals, as compared with age- and BMI-matched control subjects. Rates of substrate oxidation via the tricarboxylic acid cycle were 75% higher in the muscle of RTH individuals compared with control subjects [120.7 ± 17.2 vs 68.9 ± 5.1 nmol/(g × min)]. Resting rates of muscle ATP synthesis, as assessed by 31P-magnetic resonance spectroscopy, tended to be slightly lower in RTH patients compared with control subjects, resulting in a significant increase (∼60%) in mitochondrial energy uncoupling, as assessed as the ratio between tricarboxylic acid cycle flux and rates of ATP synthase flux in the RTH group as compared with the control subjects. Collectively, RTH beta is associated with a considerable increase in EE, whereas the RTH alpha is associated with a hypometabolic state due to the near ubiquitous distribution of the alpha receptor with minimal effects on the hypothalamus-pituitary-thyroid axis [62].
Aside from the RTH syndromes, pseudohypoparathyroidism type 1A [63], which is caused by a maternally inherited loss of function of the GNAS gene encoding for the stimulatory subunit alpha of the G-protein signal transduction complex, is associated with mild resistance to TSH and therefore with a relative state of hypothyroidism, which in turn leads to decreased EE, contributing to their obese phenotype. Multiple other mechanisms and associated conditions are also described and likely contribute to the final clinical outcome, including decreased thermic effect of food [64] and insulin resistance.
Interestingly, the generalized decrease in deiodinase activity due to mutations of the SBP2 gene causes a mild form of hypothyroidism, myopathy, and generalized reduction in the metabolism of reactive oxidative species [65, 66] is associated with failure to thrive [67] rather than, as one could expect, from the relative decrease in T3 levels due to reduction in T4 to T3 conversion. To the best of our knowledge, no formal assessment of EE has been performed in these patients, and it is possible that the complex multiorgan manifestation of the syndrome trumps the predicted reduction in EE.
D. Functional Adaptation of TH Axis to Energy Status
Although the TH axis role as a major driver of EE is well defined and recognized, mounting evidence indicates that the overall energy status of the organism affects the TH axis in a sort of afferent loop. In this context, the TH axis can be expanded to an adipose tissue-hypothalamus-pituitary-thyroid axis in which the secretion of leptin from adipocytes stimulates the release of TRH, which in turn promotes the secretion of TSH, ultimately promoting the secretion of TH and peripheral conversion of T4 into T3. The clinical correlate of these observations is the association between adiposity and serum T3 levels [51], and several cross-sectional studies that indicate a noteworthy association between T3 levels and EE (see above). Importantly, this correlation is lost following weight reduction interventions, and the decrease in T3 (and EE) observed in post–weight loss individuals [68, 69] is thought to be a major contributor to the “metabolic memory” [70], which in turn is a major driver in weight regain. Redman et al. [71] showed that after 15% calorie restriction and weight loss, a metabolic chamber measured REE reduced ∼7% after corrected for weight loss and also associated with reduced TH axis. Similar observations were made in post–bariatric surgery patients after weight loss [72]. These changes are not limited to drastic weight loss interventions; in fact, we have documented a reduction in reduced total T3 and in the peripheral conversion of T4 into T3 after moderate weight loss over a period of 1 year [50]. Conversely, experimental overnutrition increases T3 levels and EE, likely as a short-term compensatory mechanism [73]. It has to be noted that this study was performed in prisoners [74], and thus, the validity of the informed consent process in this research project is questionable.
The replacement of T3 during weight loss interventions or its therapeutic use at supraphysiologic doses aimed to maintain or to increase EE to facilitate weight loss and postintervention weight maintenance have been attempted [75], and dangerously high doses of THs are occasionally found in nutritional supplements [76], the narrow therapeutic index of liothyronine, and its association with cardiovascular side effects [77] has prevented this application. In contrast, a more recent analysis of 29 common weight loss supplements demonstrated a much lesser amount of T3 or T4 in these products, possibly because of the enhanced awareness [78]. Nonetheless, the use of tissue- or receptor-specific thyromimetics [79] could overcome these shortcomings.
Aside from its direct action on energy-dissipating organs, TH has a key regulatory role in the central nervous system. Recent studies show that TH action in the hypothalamus regulates the metabolism in liver and BAT via the sympathetic and parasympathetic branches. T3 within the paraventricular nucleus controls glucose production and hepatic insulin sensitivity via sympathetic liver innervation. Conversely, T3 within the ventromedial nucleus induces sympathetic nervous system–mediated BAT activation. Timing and the route of administration, as well as acute vs chronic use of T3, were also found to be contributing factors to the effects of TH action in the central nervous system [80].
4. Conclusions
The effects of TH on energy metabolism are pervasive and well recognized in clinical evident thyroid dysfunction. The relative paucity of data on the effects of mild thyroid dysfunction on EE and energy metabolism is likely due to the relative insensitivity of the tools used to assess EE and its components in humans. Modulation of the TH axis to exploit its actions on EE remains an area of active research and promising drug development.
Acknowledgments
Disclosure Summary: F.S.C. has received consultant fees from Akrimax Pharmaceuticals, Institut Biochimique SA, and Acella Pharmaceuticals. The Division of Endocrinology, Diabetes and Metabolism of Virginia Commonwealth University has received an unrestricted grant from Institut Biochimique SA. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- BMR
basal metabolic rate
- EAT
exercise activity–related thermogenesis
- EE
energy expenditure
- LT4
levothyroxine
- NEAT
non–exercise activity–related thermogenesis
- REE
resting energy expenditure
- TEE
total energy expenditure
- TH
thyroid hormone
- UCP-1
uncoupling protein-1
References and Notes
- 1. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol. 2014;221(3):R87–R103. [DOI] [PubMed] [Google Scholar]
- 3. Anderson AB. Hyperthyroidism: relation of the basal metabolism to the clinical signs. BMJ. 1941;2(4203):117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Utiger RD. Radioimmunoassay of human plasma thyrotropin. J Clin Invest. 1965;44(8):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwen KA, Schröder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2(2):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, Vasan RS. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168(6):587–592. [DOI] [PubMed] [Google Scholar]
- 7. Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98(9):3562–3571. [DOI] [PubMed] [Google Scholar]
- 8. Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab. 2016;101(12):4964–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Senese R, de Lange P, Petito G, Moreno M, Goglia F, Lanni A. 3,5-Diiodothyronine: a novel thyroid hormone metabolite and potent modulator of energy metabolism. Front Endocrinol (Lausanne). 2018;9:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine JA. Measurement of energy expenditure. Public Health Nutr. 2005;8(7A):1123–1132. [DOI] [PubMed] [Google Scholar]
- 11. Levine JA. Nonexercise activity thermogenesis--liberating the life-force. J Intern Med. 2007;262(3):273–287. [DOI] [PubMed] [Google Scholar]
- 12. Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond). 2004;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–660. [DOI] [PubMed] [Google Scholar]
- 14. Oelkrug R, Polymeropoulos ET, Jastroch M. Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol B. 2015;185(6):587–606. [DOI] [PubMed] [Google Scholar]
- 15. Cohen P, Spiegelman BM. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes. 2015;64(7):2346–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaitkus JA, Farrar JS, Celi FS. Thyroid hormone mediated modulation of energy expenditure. Int J Mol Sci. 2015;16(7):16158–16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oppenheimer JH, Schwartz HL, Lane JT, Thompson MP. Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Invest. 1991;87(1):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freake HC, Oppenheimer JH. Thermogenesis and thyroid function. Annu Rev Nutr. 1995;15(1):263–291. [DOI] [PubMed] [Google Scholar]
- 19. Vargas-Uricoechea H, Bonelo-Perdomo A, Sierra-Torres CH. Effects of thyroid hormones on the heart. Clin Investig Arterioscler. 2014;26(6):296–309. [DOI] [PubMed] [Google Scholar]
- 20. Weitzel JM, Iwen KA. Coordination of mitochondrial biogenesis by thyroid hormone. Mol Cell Endocrinol. 2011;342(1-2):1–7. [DOI] [PubMed] [Google Scholar]
- 21. Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213(1):1–11. [DOI] [PubMed] [Google Scholar]
- 22. Yehuda-Shnaidman E, Kalderon B, Azazmeh N, Bar-Tana J. Gating of the mitochondrial permeability transition pore by thyroid hormone. FASEB J. 2010;24(1):93–104. [DOI] [PubMed] [Google Scholar]
- 23. Verhoeven AJ, Kamer P, Groen AK, Tager JM. Effects of thyroid hormone on mitochondrial oxidative phosphorylation. Biochem J. 1985;226(1):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem. 2006;281(42):31894–31908. [DOI] [PubMed] [Google Scholar]
- 25. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1-2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawin C. The heritage of the thyroid: a brief history. In: Braverman LE, Cooper DS, eds. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text. 8th edPhiladelphia, PA: JB Lippincott Company; 2000:1–4. [Google Scholar]
- 27. Lam YY, Ravussin E. Indirect calorimetry: an indispensable tool to understand and predict obesity. Eur J Clin Nutr. 2017;71(3):318–322. [DOI] [PubMed] [Google Scholar]
- 28. Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22(4):377–388. [DOI] [PubMed] [Google Scholar]
- 29. Galgani JE, Castro-Sepulveda M, Pérez-Luco C, Fernández-Verdejo R. Validity of predictive equations for resting metabolic rate in healthy humans. Clin Sci (Lond). 2018;132(16):1741–1751. [DOI] [PubMed] [Google Scholar]
- 30. Kumahara H, Tanaka H, Schutz Y. Physical activity under confinement and free-living conditions. Physiol Behav. 2010;100(4):350–356. [DOI] [PubMed] [Google Scholar]
- 31. Chen S, Wohlers E, Ruud E, Moon J, Ni B, Celi FS. Improving temporal accuracy of human metabolic chambers for dynamic metabolic studies. PLoS One. 2018;13(4):e0193467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rising R, Whyte K, Albu J, Pi-Sunyer X. Evaluation of a new whole room indirect calorimeter specific for measurement of resting metabolic rate. Nutr Metab (Lond). 2015;12(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerterp KR. Doubly labelled water assessment of energy expenditure: principle, practice, and promise. Eur J Appl Physiol. 2017;117(7):1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plasqui G. Smart approaches for assessing free-living energy expenditure following identification of types of physical activity. Obes Rev. 2017;18(Suppl 1):50–55. [DOI] [PubMed] [Google Scholar]
- 35. Jeran S, Steinbrecher A, Pischon T. Prediction of activity-related energy expenditure using accelerometer-derived physical activity under free-living conditions: a systematic review. Int J Obes. 2016;40(8):1187–1197. [DOI] [PubMed] [Google Scholar]
- 36. Levine JA, Nygren J, Short KR, Nair KS. Effect of hyperthyroidism on spontaneous physical activity and energy expenditure in rats. J Appl Physiol (1985). 2003;94(1):165–170. [DOI] [PubMed] [Google Scholar]
- 37. Lahesmaa M, Orava J, Schalin-Jäntti C, Soinio M, Hannukainen JC, Noponen T, Kirjavainen A, Iida H, Kudomi N, Enerbäck S, Virtanen KA, Nuutila P. Hyperthyroidism increases brown fat metabolism in humans. J Clin Endocrinol Metab. 2014;99(1):E28–E35. [DOI] [PubMed] [Google Scholar]
- 38. Arrojo E Drigo R, Fonseca TL, Werneck-de-Castro JP, Bianco AC. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim Biophys Acta. 2013;1830(7):3956–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guerra C, Roncero C, Porras A, Fernández M, Benito M. Triiodothyronine induces the transcription of the uncoupling protein gene and stabilizes its mRNA in fetal rat brown adipocyte primary cultures. J Biol Chem. 1996;271(4):2076–2081. [DOI] [PubMed] [Google Scholar]
- 40. Kim MJ, Cho SW, Choi S, Ju DL, Park DJ, Park YJ. Changes in body compositions and basal metabolic rates during treatment of Graves’ disease. Int J Endocrinol. 2018;2018:9863050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chng CL, Lim AY, Tan HC, Kovalik JP, Tham KW, Bee YM, Lim W, Acharyya S, Lai OF, Chong MF, Yen PM. Physiological and metabolic changes during the transition from hyperthyroidism to euthyroidism in Graves’ disease. Thyroid. 2016;26(10):1422–1430. [DOI] [PubMed] [Google Scholar]
- 42. Kvetny J. Subclinical hyperthyroidism in patients with nodular goiter represents a hypermetabolic state. Exp Clin Endocrinol Diabetes. 2005;113(2):122–126. [DOI] [PubMed] [Google Scholar]
- 43. Toubro S, Sørensen TI, Rønn B, Christensen NJ, Astrup A. Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. J Clin Endocrinol Metab. 1996;81(7):2670–2674. [DOI] [PubMed] [Google Scholar]
- 44. McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. 2014;1311(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johansen K, Hansen JM, Skovsted L. The preferential role of triiodothyronine in the regulation of basal metabolic rate in hyper- and hypothyroidism. Acta Med Scand. 1978;204(5):357–359. [DOI] [PubMed] [Google Scholar]
- 46. al-Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82(4):1118–1125. [DOI] [PubMed] [Google Scholar]
- 47. Ulas T, Buyukhatipoglu H, Eren MA, Dal MS, Torun A, Aydogan T, Demir ME, Turan MN. Evaluation of sleeping energy expenditure using the SenseWear Armband in patients with overt and subclinical hypothyroidism. Clin Invest Med. 2012;35(3):E126–E131. [DOI] [PubMed] [Google Scholar]
- 48. Samuels MH, Kolobova I, Smeraglio A, Peters D, Purnell JQ, Schuff KG. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid. 2016;26(3):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Samuels MH, Kolobova I, Antosik M, Niederhausen M, Purnell JQ, Schuff KG. Thyroid function variation in the normal range, energy expenditure, and body composition in L-T4-treated subjects. J Clin Endocrinol Metab. 2017;102(7):2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agnihothri RV, Courville AB, Linderman JD, Smith S, Brychta R, Remaley A, Chen KY, Simchowitz L, Celi FS. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le TN, Celi FS, Wickham EP III. Thyrotropin levels are associated with cardiometabolic risk factors in euthyroid adolescents. Thyroid. 2016;26(10):1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Samuels MH, Kolobova I, Niederhausen M, Purnell JQ, Schuff KG. Effects of altering levothyroxine dose on energy expenditure and body composition in subjects treated with LT4. J Clin Endocrinol Metab. 2018;103(11):4163–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, Skarulis MC, Kozlosky M, Csako G, Costello R, Pucino F. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96(11):3466–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta. 2013;1830(7):3987–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Refetoff S, DeWind LT, DeGroot LJ. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967;27(2):279–294. [DOI] [PubMed] [Google Scholar]
- 56. Moran C, Chatterjee K. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract Res Clin Endocrinol Metab. 2015;29(4):647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brucker-Davis F, Skarulis MC, Grace MB, Benichou J, Hauser P, Wiggs E, Weintraub BD; The National Institutes of Health Prospective Study. Genetic and clinical features of 42 kindreds with resistance to thyroid hormone. Ann Intern Med. 1995;123(8):572–583. [DOI] [PubMed] [Google Scholar]
- 58. Gershengorn MC, Weintraub BD. Thyrotropin-induced hyperthyroidism caused by selective pituitary resistance to thyroid hormone. A new syndrome of “inappropriate secretion of TSH”. J Clin Invest. 1975;56(3):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pappa T, Refetoff S. Human genetics of thyroid hormone receptor beta: resistance to thyroid hormone beta (RTHβ). Methods Mol Biol. 2018;1801:225–240. [DOI] [PubMed] [Google Scholar]
- 60. Alberobello AT, Congedo V, Liu H, Cochran C, Skarulis MC, Forrest D, Celi FS. An intronic SNP in the thyroid hormone receptor β gene is associated with pituitary cell-specific over-expression of a mutant thyroid hormone receptor β2 (R338W) in the index case of pituitary-selective resistance to thyroid hormone. J Transl Med. 2011;9(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mitchell CS, Savage DB, Dufour S, Schoenmakers N, Murgatroyd P, Befroy D, Halsall D, Northcott S, Raymond-Barker P, Curran S, Henning E, Keogh J, Owen P, Lazarus J, Rothman DL, Farooqi IS, Shulman GI, Chatterjee K, Petersen KF. Resistance to thyroid hormone is associated with raised energy expenditure, muscle mitochondrial uncoupling, and hyperphagia. J Clin Invest. 2010;120(4):1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Singh BK, Yen PM. A clinician’s guide to understanding resistance to thyroid hormone due to receptor mutations in the TRα and TRβ isoforms. Clin Diabetes Endocrinol. 2017;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roizen JD, Danzig J, Groleau V, McCormack S, Casella A, Harrington J, Sochett E, Tershakovec A, Zemel BS, Stallings VA, Levine MA. Resting energy expenditure is decreased in pseudohypoparathyroidism type 1A. J Clin Endocrinol Metab. 2016;101(3):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shoemaker AH, Lomenick JP, Saville BR, Wang W, Buchowski MS, Cone RD. Energy expenditure in obese children with pseudohypoparathyroidism type 1a. Int J Obes. 2013;37(8):1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37(11):1247–1252. [DOI] [PubMed] [Google Scholar]
- 66. Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, Luan J, Montano S, Lu J, Castanet M, Clemons N, Groeneveld M, Castets P, Karbaschi M, Aitken S, Dixon A, Williams J, Campi I, Blount M, Burton H, Muntoni F, O’Donovan D, Dean A, Warren A, Brierley C, Baguley D, Guicheney P, Fitzgerald R, Coles A, Gaston H, Todd P, Holmgren A, Khanna KK, Cooke M, Semple R, Halsall D, Wareham N, Schwabe J, Grasso L, Beck-Peccoz P, Ogunko A, Dattani M, Gurnell M, Chatterjee K. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120(12):4220–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hamajima T, Mushimoto Y, Kobayashi H, Saito Y, Onigata K. Novel compound heterozygous mutations in the SBP2 gene: characteristic clinical manifestations and the implications of GH and triiodothyronine in longitudinal bone growth and maturation. Eur J Endocrinol. 2012;166(4):757–764. [DOI] [PubMed] [Google Scholar]
- 68. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87(5):2391–2394. [DOI] [PubMed] [Google Scholar]
- 69. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. [DOI] [PubMed] [Google Scholar]
- 70. Dall’Asta C, Paganelli M, Morabito A, Vedani P, Barbieri M, Paolisso G, Folli F, Pontiroli AE. Weight loss through gastric banding: effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity (Silver Spring). 2010;18(4):854–857. [DOI] [PubMed] [Google Scholar]
- 71. Redman LM, Smith SR, Burton JH, Martin CK, Il'yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018;27(4):805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang X, You T, Lenchik L, Nicklas BJ. Resting energy expenditure changes with weight loss: racial differences. Obesity (Silver Spring). 2010;18(1):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Danforth E Jr, Horton ES, O’Connell M, Sims EA, Burger AG, Ingbar SH, Braverman L, Vagenakis AG. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest. 1979;64(5):1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sims EA, Danforth E Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. [DOI] [PubMed] [Google Scholar]
- 75. Hollingsworth DR, Amatruda TT Jr, Scheig R. Quantitative and qualitative effects of L-triiodothyronine in massive obesity. Metabolism. 1970;19(11):934–945. [DOI] [PubMed] [Google Scholar]
- 76. Kang GY, Parks JR, Fileta B, Chang A, Abdel-Rahim MM, Burch HB, Bernet VJ. Thyroxine and triiodothyronine content in commercially available thyroid health supplements. Thyroid. 2013;23(10):1233–1237. [DOI] [PubMed] [Google Scholar]
- 77. Cohen PA, Goday A, Swann JP. The return of rainbow diet pills. Am J Public Health. 2012;102(9):1676–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Seger CD, Xuemei H, Braverman LE, Yeh MW, Bernet VJ, Singh RJ, Rhee CM, Leunget AM. Negligible thyroid hormone content present in nonprescription U.S. weight loss products. Thyroid. 2017;27(2):300–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mondal S, Mugesh G. Novel thyroid hormone analogues, enzyme inhibitors and mimetics, and their action. Mol Cell Endocrinol. 2017;458:91–104. [DOI] [PubMed] [Google Scholar]
- 80. Zhang Z, Boelen A, Bisschop PH, Kalsbeek A, Fliers E. Hypothalamic effects of thyroid hormone. Mol Cell Endocrinol. 2017;458:143–148. [DOI] [PubMed] [Google Scholar]