Glucosylglycerate accumulates to high levels in nitrogen-starved mycobacteria. Here, a thorough structural and biochemical characterization unveils the molecular determinants of the substrate specificity and catalytic mechanism of a mycobacterial glucosylglycerate hydrolase, a highly conserved enzyme involved in the fast recovery of rapidly growing mycobacteria from nitrogen starvation.
Keywords: MhGgH, GH63, glycoside hydrolase, Mycolicibacterium hassiacum, protein structure, molecular recognition, X-ray crystallography, enzyme mechanism, solution scattering
Abstract
Bacteria are challenged to adapt to environmental variations in order to survive. Under nutritional stress, several bacteria are able to slow down their metabolism into a nonreplicating state and wait for favourable conditions. It is almost universal that bacteria accumulate carbon stores to survive during this nonreplicating state and to fuel rapid proliferation when the growth-limiting stress disappears. Mycobacteria are exceedingly successful in their ability to become dormant under harsh circumstances and to be able to resume growth when conditions are favourable. Rapidly growing mycobacteria accumulate glucosylglycerate under nitrogen-limiting conditions and quickly mobilize it when nitrogen availability is restored. The depletion of intracellular glucosylglycerate levels in Mycolicibacterium hassiacum (basonym Mycobacterium hassiacum) was associated with the up-regulation of the gene coding for glucosylglycerate hydrolase (GgH), an enzyme that is able to hydrolyse glucosylglycerate to glycerate and glucose, a source of readily available energy. Highly conserved among unrelated phyla, GgH is likely to be involved in bacterial reactivation following nitrogen starvation, which in addition to other factors driving mycobacterial recovery may also provide an opportunity for therapeutic intervention, especially in the serious infections caused by some emerging opportunistic pathogens of this group, such as Mycobacteroides abscessus (basonym Mycobacterium abscessus). Using a combination of biochemical methods and hybrid structural approaches, the oligomeric organization of M. hassiacum GgH was determined and molecular determinants of its substrate binding and specificity were unveiled.
1. Introduction
In a changing environment, the basic requirements for bacterial growth are not always available. In order to accomplish one single goal, survival, bacteria have evolved different strategies (Rittershaus et al., 2013 ▸). When exposed to a growth-limiting stress, such as desiccation, temperature and pH variations, oxidative stress, hypoxia, antibiotics or nutrient limitation, bacterial populations balance between cell death and decreased growth rates (Finkel, 2006 ▸; Lipworth et al., 2016 ▸; Eoh et al., 2017 ▸). Some of the surviving cells can slow down or suspend their growth to a viable nonreplicating state and persist for months or years (Lewis, 2007 ▸). This process, which is known as dormancy, allows a viable population size to be maintained during the period of stress (Jones & Lennon, 2010 ▸). Despite being genetically identical to replicating bacteria, dormant cells are more tolerant to external stress (Balázsi et al., 2011 ▸; Eldar & Elowitz, 2010 ▸; Dhar & McKinney, 2007 ▸). The dormancy state requires several structural modifications to maintain cell viability. Dormant cells seem to display a more compact and stable chromosome (Summers et al., 2012 ▸; Nair & Finkel, 2004 ▸), a lower transcription rate and more stable messenger RNA (Rustad et al., 2013 ▸), a lower but steady level of ATP (Gengenbacher et al., 2010 ▸; Rao et al., 2008 ▸), increased peptidoglycan mass in the cell wall accompanied by changes in the number and type of cross-links (Lavollay et al., 2008 ▸; Zhou & Cegelski, 2012 ▸), and an accumulation of carbon stores (Bourassa & Camilli, 2009 ▸). Although the type of carbon store varies, the main common goal seems to be guaranteeing a rapidly mobilizable energy source that is able to promote fast cell proliferation when the environmental conditions improve (Shi et al., 2010 ▸), which is an advantage at this moment in outcompeting neighbouring organisms.
In mycobacteria, the dormant state seems to be associated with the accumulation of triacylglycerol (Daniel et al., 2004 ▸) and wax esters (Sirakova et al., 2012 ▸). However, it has been demonstrated that rapidly growing mycobacteria accumulate glucosylglycerate (GG) under severe nitrogen-limiting conditions (Alarico et al., 2014 ▸; Behrends et al., 2012 ▸), which are able to induce dormancy (Anuchin et al., 2009 ▸; Shleeva et al., 2004 ▸). In vitro, GG prevented loss of activity in a number of enzymes (Sawangwan et al., 2010 ▸), suggesting that it is also likely to contribute to protein stability in vivo during a slowly growing or nonreplicating phase. In Mycolicibacterium smegmatis and M. hassiacum, intracellular GG accumulated during nitrogen starvation is quickly depleted when nitrogen availability is restored (Alarico et al., 2014 ▸; Behrends et al., 2012 ▸). In M. hassiacum, GG depletion was associated with the up-regulation of a gene (ggH) coding for a glucosylglycerate hydrolase (GgH; Alarico et al., 2014 ▸). Data on the role of GgH in bacterial survival, dormancy and infection are still lacking. However, the high degree of conservation of GgH among rapidly growing mycobacteria, which based on comparative genomic analyses were recently included in four new genera and separated from the Mycobacterium genus, where only the major human pathogens remain (Gupta et al., 2018 ▸), and in other unrelated phyla suggests that the ability to produce this hydrolase is an evolutionary advantage. Moreover, since ggH expression is up-regulated upon relief of the growth-limiting stress, GgH is likely to participate in the reactivation of growth by hydrolysing GG to glycerate and glucose (Alarico et al., 2014 ▸), which can be quickly used for energy production and for the synthesis of structural molecules (Mendes et al., 2012 ▸) that are necessary for rapid bacterial proliferation. While most mycobacteria are environmental saprophytes, some species have increasingly been detected in oligotrophic drinking-water distribution systems, to which mycobacteria adapt and where they survive for long periods, namely in showerhead biofilms, from which they may access and infect humans (Gebert et al., 2018 ▸). Understanding the mechanisms that allow the reactivation of growth following long periods under nutrient limitation may therefore be important in trying to halt the reactivation process and prevent infection.
M. hassiacum GgH (MhGgH) belongs to the large CAZy family GH63 of glycoside hydrolases (http://www.cazy.org). Only four of the more than 2000 assigned members of family GH63, displaying α-glucosidase (EC 3.2.1.20), α-1,3-glucosidase (EC 3.2.1.84), processing α-glucosidase (EC 3.2.1.106) and mannosylglycerate hydrolase (EC 3.2.1.170) activities, have been structurally characterized (Kurakata et al., 2008 ▸; Barker & Rose, 2013 ▸; Miyazaki et al., 2015 ▸). In order to unveil its molecular mechanism of action, a thorough structural and functional characterization of MhGgH was performed, elucidating its quaternary architecture and providing an atomic detail view of the determinants of substrate specificity, thus providing new insights into a potentially crucial enzyme underlying the metabolic reactivation of rapidly growing mycobacteria following severe nutrient starvation and expanding the options for therapeutic intervention.
2. Experimental procedures
2.1. Site-directed mutagenesis
The point mutations in M. hassiacum ggH were obtained by site-directed mutagenesis using pETM11-MhGgH (Cereija et al., 2017 ▸) as a template and the primers 5′-CACATGTGGAGTTGGGCCGCCGCGTTC-3′ and 5′-GAACGCGGCGGCCCAACTCCACATGTG-3′ (producing pETM11-D43A), 5′-GAGTCCGGGATGGCCAACTCG-3′ and 5′-CGAGTTGGCCATCCCGGACTC-3′ (producing pETM11-D182A), and 5′-TCGTTCGCCGCGTACTACGAA-3′ and 5′-TTCGTAGTACGCGGCGAACGA-3′ (producing pETM11-E419A).
2.2. Expression and purification of MhGgH variants
Escherichia coli BL21 (DE3) cells transformed with the pET30a-MhGgH (Alarico et al., 2014 ▸), pETM11-MhGgH (Cereija et al., 2017 ▸), pETM11-D43A, pETM11-D182A or pETM11-E419A plasmids were used for protein expression as described previously (Cereija et al., 2017 ▸; Alarico et al., 2014 ▸). All proteins were purified using a combination of immobilized metal-affinity and size-exclusion chromatography (Cereija et al., 2017 ▸). The affinity tag of those proteins obtained from pETM11-based constructs was removed by cleavage with TEV protease. The concentration of the purified proteins (in 20 mM Tris–HCl pH 8.0, 400 mM NaCl; storage buffer) was estimated by measuring their absorbance at 280 nm prior to flash-freezing in liquid nitrogen and storage at −80°C until needed.
2.3. Analytical size-exclusion chromatography
Analytical size-exclusion chromatography was performed on a Superdex 200 Increase 5/150 GL column (GE Healthcare) pre-equilibrated with storage buffer. Blue dextran (2000 kDa), ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), ovalbumin (43 kDa) and ribonuclease A (13.7 kDa) were used as standards for column calibration. The K av versus log molecular weight was calculated using the equation K av = (V e − V 0)/(V t − V 0), where V e is the elution volume of the protein, V 0 is the void volume of the column and V t is the column bed volume.
2.4. Dynamic light-scattering analysis
Dynamic light-scattering (DLS) analysis was performed on a Zetasizer Nano ZS DLS system (Malvern Instruments). Protein samples were centrifuged at 13 000g and 4°C for 20 min, loaded onto a ZEN2112 cuvette and three independent measurements were recorded at 20°C. All data were analysed using the Zetasizer software v.7.11 (Malvern Instruments).
2.5. Differential scanning fluorimetry
The melting temperatures of the MhGgH variants were determined using a thermal shift (Thermofluor) assay. Each protein sample (0.5 mg ml−1 final concentration) was centrifuged at 13 000g and 4°C for 15 min, mixed with 5× SYPRO Orange (Life Technologies) in storage buffer and loaded into white 96-well PCR plates (Bio-Rad) sealed with Optical Quality Sealing Tape (Bio-Rad). The plate was heated from 25 to 95°C in 0.5°C steps with 30 s hold time per step on an iCycler iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad) and the fluorescence was followed using a Cy3 dye filter (545 nm excitation/585 nm emission). Each experiment was performed in triplicate. The melting curves were analysed using the CFX Manager software (Bio-Rad) and the melting temperature was determined as the inflection point of the melting curve.
2.6. Chemical syntheses of glucosylglycerate, mannosylglycerate and glucosylglycolate
The chemical syntheses of glucosylglycerate [GG; 2-O-(α-d-glucopyranosyl)-d-glycerate], mannosylglycerate [MG; 2-O-(α-d-mannopyranosyl)-d-glycerate] and glucosylglycolate [GGlycolate; 2-(1-O-α-d-glucopyranosyl)acetic acid] were performed as described previously (Faria et al., 2008 ▸; Lourenço et al., 2009 ▸; Lourenço & Ventura, 2011 ▸).
2.7. Substrate specificity of MhGgH
The catalytic activity of MhGgH was evaluated in a 50 µl reaction mixture consisting of 2.75 µM enzyme, 20 mM GG, 25 mM sodium phosphate pH 6.0, 5 mM MgCl2, 100 mM KCl (standard reaction). The hydrolysis of GG was detected by thin-layer chromatography (TLC; Silica Gel 60, Merck) using a solvent system composed of chloroform/methanol/acetic acid/water [30:50:8:4(v:v:v:v)] and the products were stained as described previously (Alarico et al., 2014 ▸). Enzyme specificity was also probed with 5 and 20 mM MG, GGlycolate, glucosylglycerol [GGlycerol; 2-O-(α-d-glucopyranosyl)-d-glycerol; Bitop] or α-1,4-mannobiose (Carbosynth) as potential substrates. All reactions were performed at 42 and 50°C for 1 h, 3 h and overnight. Control reactions without enzyme were also performed. The reaction products were analysed by TLC as described above for GG, except for those from reactions containing GGlycerol, for which the solvent system used was chloroform/methanol/25% ammonia [30:50:25(v:v:v)].
2.8. Biochemical analysis and kinetic parameters of MhGgH
The effect of temperature and pH on the catalytic activity of MhGgH was evaluated by quantifying the glucose released upon hydrolysis of GG using the Glucose Oxidase Assay Kit (Sigma–Aldrich) as described previously (Alarico et al., 2014 ▸). The temperature profile of MhGgH was traced from 20 to 60°C using the standard reaction conditions (see Section 2.7). The effect of pH on the activity of MhGgH was determined at 55°C using 20 mM sodium acetate (pH 4.0–5.5) or 20 mM sodium phosphate (pH 5.8–7.0) buffer.
Kinetic parameters for the MhGgH-catalysed hydrolysis of GG and MG were determined by quantifying the release of glucose (as described above) or mannose (using the K-MANGL 01/05 assay kit; Megazyme), respectively. A constant enzyme concentration (2.75 µM) was incubated with increasing concentrations of GG (0–35 mM) or MG (0–150 mM) in 20 mM sodium phosphate pH 6.0, 100 mM KCl, 5 mM MgCl2 at 50°C. The maximum velocity (V max) and half constant (K 0.5) were calculated with Prism 5.0 (GraphPad Software) using the allosteric sigmoidal equation. Experiments using GG were performed in triplicate, while those using MG as substrate were performed in duplicate.
2.9. Catalytic activity of MhGgH variants
The catalytic activity of MhGgH variants was evaluated in 50 µl reactions consisting of 2.75 µM enzyme and 10 or 20 mM GG or MG in 25 mM buffer (sodium phosphate pH 6.0, sodium acetate pH 4.5 or 5.0, bis-Tris propane pH 7.0 or Tris–HCl pH 8.0) with 5 or 10 mM MgCl2 in the presence or absence of 100 mM KCl. Reactions were performed at 37, 42 and 50°C for 1 h and overnight. Reaction mixtures with and without wild-type MhGgH were used as controls. The hydrolysis of GG was evaluated by TLC as described in Section 2.7.
2.10. Crystallization of MhGgH variants
Crystals of MhGgH were obtained as described previously (Cereija et al., 2017 ▸). MhGgH crystals growing from less than 30% GOL_P4K (glycerol, PEG 4000) were transferred into a solution containing at least 30% precipitant prior to flash-cooling in liquid nitrogen. The D43A, D182A and E419A MhGgH variants were crystallized in the same conditions, although wild-type MhGgH macro-seeds were employed to promote crystal growth. Complexes of the MhGgH D182A variant with GG, MG and GGlycolate were obtained by soaking the crystals in mother liquor supplemented with 100 mM ligand for 2 h (GG and MG) or 25 min (GGlycolate) before flash-cooling in liquid nitrogen. Complexes of the MhGgH E419A variant with GG, MG, GGlycolate and GGlycerol were obtained by soaking the crystals in mother liquor supplemented with 100 mM ligand for 5 min (GG), 50 min (MG), 40 min (GGlycolate) or 10 min (GGlycerol). An additional crystallization condition was identified in-house at 293 K, yielding hexagonal crystals within a day from drops consisting of equal volumes (1 µl) of protein (9.5 mg ml−1 in storage buffer) and precipitant solution equilibrated against solution No. 22 (0.1 M ADA pH 6.5, 1.0 M ammonium sulfate) from the MembFac sparse-matrix crystallization screen (Hampton Research). These crystals were cryoprotected with Perfluoropolyether PFO-X175/08 (Hampton Research) prior to flash-cooling in liquid nitrogen.
2.11. Data collection and processing
Diffraction data were collected from cryocooled (100 K) single crystals on beamlines ID23-2 (Flot et al., 2010 ▸), ID29 (de Sanctis et al., 2012 ▸), ID30A-1 (Bowler et al., 2015 ▸; Svensson et al., 2015 ▸), ID30A-3 (Theveneau et al., 2013 ▸) and ID30B (Mueller-Dieckmann et al., 2015 ▸) of the European Synchrotron Radiation Facility (ESRF), Grenoble, France and PROXIMA-2A of the French National Synchrotron Source (SOLEIL), Gif-sur-Yvette, France. All data sets were automatically processed using the GrenADES (Grenoble Automatic Data procEssing System) pipeline (Monaco et al., 2013 ▸), except for those collected on the ID30A-3 and PROXIMA-2A beamlines, which were processed with XDS (Kabsch, 2010 ▸) and reduced with utilities from the CCP4 program suite (Winn et al., 2011 ▸). X-ray diffraction data-collection and processing statistics are summarized in Table 1 ▸. The X-ray diffraction images have been deposited in the SBGrid Data Bank (Meyer et al., 2016 ▸).
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the outermost shell.
| Crystal | SeMet MhGgH | MhGgH–Ser–GOL | MhGgH without serine | Apo MhGgH | D43A–Ser–GOL | D182A–Ser–GOL | E419A–Ser–GOL |
|---|---|---|---|---|---|---|---|
| Data collection | |||||||
| Synchrotron-radiation facility | ESRF | ESRF | ESRF | ESRF | ESRF | ESRF | ESRF |
| Beamline | ID29 | ID30B | ID30A-3 | ID29 | ID30A-1 | ID30A-3 | ID30A-1 |
| Detector | PILATUS3 6M, Dectris | PILATUS 6M, Dectris | EIGER X 4M, Dectris | PILATUS3 6M, Dectris | PILATUS3 2M, Dectris | EIGER X 4M, Dectris | PILATUS3 2M, Dectris |
| Wavelength (Å) | 0.97909 | 0.97265 | 0.96770 | 0.96863 | 0.96598 | 0.96770 | 0.96600 |
| Reflections (measured/unique) | 428683/151619 | 595791/135623 | 390265/84736 | 474316/66272 | 460425/114314 | 581967/119738 | 338529/73877 |
| Space group | P21 | P21212 | P21212 | P6222 | P21212 | P21212 | P21212 |
| a, b, c (Å) | 90.8, 86.1, 159.7 | 86.0, 158.8, 87.8 | 85.9, 159.3, 91.2 | 167.0, 167.0, 243.3 | 85.9, 159.1, 88.2 | 86.3, 158.1, 87.7 | 86.2, 159.4, 88.4 |
| α, β, γ (°) | 90.0, 93.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 120.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å) | 57.4–2.04 (2.07–2.04) | 48.6–1.68 (1.74–1.68) | 40.3–2.00 (2.04–2.00) | 49.9–2.54 (2.63–2.54) | 48.7–1.78 (1.85–1.78) | 42.2–1.75 (1.78–1.75) | 48.8–2.07 (2.14–2.07) |
| R merge | 0.105 (0.637) | 0.047 (0.860) | 0.084 (1.276) | 0.109 (1.165) | 0.057 (0.824) | 0.070 (0.987) | 0.094 (1.032) |
| 〈I/σ(I)〉 | 7.6 (1.6) | 16.4 (1.7) | 12.1 (1.2) | 11.9 (1.8) | 13.0 (1.7) | 11.6 (1.6) | 10.0 (1.3) |
| Completeness (%) | 96.5 (82.1) | 99.0 (98.4) | 99.6 (99.8) | 99.8 (99.7) | 98.9 (93.5) | 98.8 (99.3) | 98.9 (92.6) |
| Multiplicity | 2.8 (2.8) | 4.4 (4.5) | 4.6 (4.8) | 7.2 (7.3) | 4.0 (4.0) | 4.9 (5.0) | 4.6 (4.0) |
| Refinement | |||||||
| Resolution (Å) | 57.4–2.04 | 48.6–1.68 | 40.3–2.00 | 49.9–2.54 | 45.4–1.78 | 40.0–1.75 | 48.8–2.07 |
| R work/R free (%) | 19.9/24.5 | 14.6/17.2 | 16.7/21.0 | 16.6/20.4 | 14.9/17.9 | 14.6/17.2 | 16.7/20.8 |
| No. of reflections | |||||||
| Working set | 255146 | 135565 | 84685 | 66183 | 114235 | 119673 | 73714 |
| Test set | 12657 | 6808 | 4330 | 3359 | 5735 | 5953 | 3718 |
| Total No. of atoms | 15955 | 8595 | 8115 | 7635 | 8365 | 8421 | 7767 |
| Ligands at active site | SER, GOL | SER, GOL | GOL | SER, GOL | SER, GOL | SER, GOL | |
| No. of water molecules | 1289 | 838 | 705 | 379 | 761 | 776 | 420 |
| Wilson B factor (Å2) | 28.4 | 25.1 | 33.4 | 53.0 | 29.3 | 26.0 | 39.9 |
| R.m.s. deviations | |||||||
| Bond lengths (Å) | 0.010 | 0.010 | 0.010 | 0.009 | 0.010 | 0.010 | 0.010 |
| Bond angles (°) | 1.099 | 1.031 | 0.993 | 1.058 | 1.009 | 1.019 | 1.004 |
| Ramachandran plot | |||||||
| Favoured (%) | 95.6 | 96.8 | 96.7 | 96.0 | 97.0 | 97.4 | 96.3 |
| Allowed (%) | 4.3 | 3.2 | 3.3 | 3.6 | 3.0 | 2.6 | 3.6 |
| Outliers (%) | 0.1 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.1 |
| Molecules in asymmetric unit | 4 | 2 | 2 | 2 | 2 | 2 | 2 |
| PDB code | 5ohz | 5oi0 | 5ohc | 6q5t | 5oiv | 5oi1 | 5oie |
| SBGrid code | 465 | 467 | 464 | 641 | 471 | 468 | 470 |
| Crystal | D182A–GG | D182A–MG | D182A–GGlycolate | E419A–GG | E419A–MG | E419A–GGlycolate | E419A–GGlycerol |
|---|---|---|---|---|---|---|---|
| Data collection | |||||||
| Synchrotron-radiation facility | ESRF | ESRF | ESRF | ESRF | ESRF | SOLEIL | ESRF |
| Beamline | ID30A-1 | ID30A-1 | ID23-2 | ID30A-1 | ID30B | PROXIMA-2A | ID30A-3 |
| Detector | PILATUS3 2M, Dectris | PILATUS3 2M, Dectris | PILATUS3 2M, Dectris | PILATUS3 2M, Dectris | PILATUS 6M, Dectris | EIGER X 9M, Dectris | EIGER X 4M, Dectris |
| Wavelength (Å) | 0.96600 | 0.96600 | 0.87290 | 0.96599 | 0.97625 | 0.98011 | 0.96770 |
| Reflections (measured/unique) | 801298/133666 | 780685/117039 | 547439/91224 | 301215/64817 | 475955/74395 | 500266/75133 | 322372/76309 |
| Space group | P21212 | P21212 | P21212 | P21212 | P21212 | P21212 | P21212 |
| a, b, c (Å) | 85.3, 159.6, 91.0 | 85.3, 159.6, 91.2 | 86.2, 158.9, 88.0 | 86.1, 159.1, 88.4 | 87.8, 158.2, 87.6 | 86.9, 157.7, 87.6 | 86.9, 158.8, 87.7 |
| α, β, γ (°) | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å) | 49.1–1.71 (1.77–1.71) | 49.1–1.79 (1.85–1.79) | 48.7–1.93 (2.00–1.93) | 48.7–2.17 (2.25–2.17) | 48.8–2.06 (2.13–2.06) | 45.1–2.06 (2.10–2.06) | 45.3–2.05 (2.09–2.05) |
| R merge | 0.070 (1.081) | 0.074 (1.052) | 0.126 (1.227) | 0.091 (0.790) | 0.086 (0.979) | 0.124 (1.410) | 0.091 (0.901) |
| 〈I/σ(I)〉 | 13.8 (1.8) | 16.5 (1.7) | 8.8 (1.5) | 11.3 (1.8) | 12.1 (1.5) | 8.2 (1.3) | 9.4 (1.6) |
| Completeness (%) | 99.5 (99.0) | 99.4 (95.9) | 99.8 (98.7) | 99.8 (99.5) | 98.0 (89.3) | 100.0 (100.0) | 99.4 (100.0) |
| Multiplicity | 6.0 (6.1) | 6.7 (6.5) | 6.0 (6.2) | 4.6 (4.5) | 6.4 (6.0) | 6.7 (6.9) | 4.2 (4.4) |
| Refinement | |||||||
| Resolution (Å) | 45.9–1.71 | 49.1–1.79 | 48.7–1.93 | 45.5–2.17 | 48.8–2.06 | 41.9–2.06 | 45.3–2.05 |
| R work/R free (%) | 14.6/16.8 | 14.4/17.3 | 14.9/18.5 | 15.6/19.8 | 15.5/19.4 | 16.2/20.2 | 15.4/19.5 |
| No. of reflections | |||||||
| Working set | 133615 | 116974 | 91157 | 64760 | 74327 | 75046 | 76246 |
| Test set | 6717 | 5861 | 4566 | 3281 | 3733 | 3625 | 3909 |
| Total No. of atoms | 8376 | 8421 | 8283 | 7817 | 7840 | 7868 | 7944 |
| Ligands at active site | 9WN | 2M8 | GOL, SER, 9YW | 9WN | GOL, SER, 2M8 | GOL, SER, 9YW | A0K |
| No. of water molecules | 847 | 846 | 677 | 419 | 463 | 426 | 566 |
| Wilson B factor (Å2) | 24.9 | 25.6 | 28.3 | 35.7 | 39.5 | 39.7 | 33.7 |
| R.m.s. deviations | |||||||
| Bond lengths (Å) | 0.010 | 0.010 | 0.010 | 0.009 | 0.009 | 0.010 | 0.010 |
| Bond angles (°) | 1.025 | 0.986 | 0.984 | 1.005 | 0.985 | 1.030 | 1.011 |
| Ramachandran plot | |||||||
| Favoured (%) | 97.2 | 96.5 | 97.0 | 96.6 | 97.5 | 96.5 | 96.4 |
| Allowed (%) | 2.8 | 3.5 | 3.0 | 3.3 | 2.4 | 3.4 | 3.1 |
| Outliers (%) | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.5 |
| Molecules in asymmetric unit | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| PDB code | 5oiw | 5oj4 | 5onz | 5oju | 5ojv | 5oo2 | 5ont |
| SBGrid code | 472 | 473 | 482 | 474 | 475 | 483 | 481 |
2.12. Structure determination, model building and refinement
The structure of MhGgH was solved by multi-wavelength anomalous diffraction as reported previously (Cereija et al., 2017 ▸). The refined coordinates were used as a search model to solve the structures of all of the other MhGgH variants and complexes by molecular replacement using Phaser (McCoy et al., 2007 ▸). Alternating cycles of model building with Coot (Emsley et al., 2010 ▸) and refinement with PHENIX (Adams et al., 2010 ▸) were performed until model completion. All crystallographic software was supported by SBGrid (Morin et al., 2013 ▸). Refined coordinates and structure factors were deposited in the Protein Data Bank (Berman et al., 2000 ▸). Refinement statistics are summarized in Table 1 ▸.
2.13. Analysis of crystallographic structures
The crystallographic models were superposed with SUPERPOSE (Krissinel & Henrick, 2004 ▸) and the secondary-structure elements were identified with DSSP (Kabsch & Sander, 1983 ▸; Touw et al., 2015 ▸). The interface area between the monomers was determined using PISA (Krissinel & Henrick, 2007 ▸). The surface electrostatic potential was calculated with APBS (Baker et al., 2001 ▸) using the AMBER force field (Cornell et al., 1995 ▸). Figures depicting molecular models were created with PyMOL (Schrödinger).
2.14. Small-angle X-ray scattering measurements and analysis
Small-angle X-ray scattering (SAXS) measurements were recorded on beamline BM29 (Pernot et al., 2013 ▸) of the ESRF, Grenoble, France with radiation of 0.9919 Å wavelength using a PILATUS 1M detector (Dectris). The protein sample was loaded onto a Superdex 200 3.2/300 GL column (GE Healthcare) and eluted with storage buffer. Measurements (1 Hz data-collection rate) were performed on the column eluate at 4°C over a scattering-vector (s = 4πsinθ/λ) range of 0.033–4.933 nm−1. Data were processed and analysed with the ATSAS package (Petoukhov et al., 2012 ▸; Franke et al., 2017 ▸). A Guinier plot was calculated using PRIMUS/qt (Konarev et al., 2003 ▸). The theoretical scattering curve from the crystallographic model was fitted to the experimental scattering curve with CRYSOL (Svergun et al., 1995 ▸).
3. Results
3.1. Catalytic activity of MhGgH
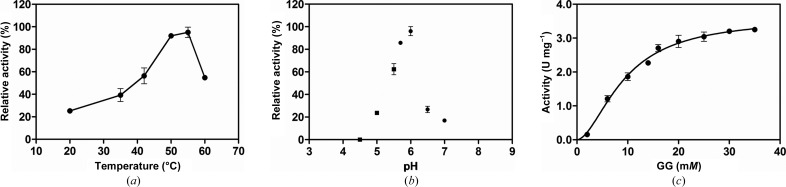
Recombinant M. hassiacum GgH (MhGgH) containing only an additional Gly-Ala dipeptide at the N-terminus (Cereija et al., 2017 ▸) was produced in E. coli and purified to homogeneity. In vitro, MhGgH was able to hydrolyse both GG and MG, although with higher efficiency for the former. Under the conditions tested, this tag-less MhGgH variant displayed maximum activity between 50 and 55°C, which was significantly higher than that reported for the C-terminally tagged variant (MhGgH-His6, 42°C; Alarico et al., 2014 ▸) and was in agreement with the optimal temperature of growth of M. hassiacum (50°C; Tiago et al., 2012 ▸) [Fig. 1 ▸(a)].
Figure 1.
Biochemical characterization of MhGgH. (a) Temperature profile of MhGgH, highlighting its maximal activity at 50–55°C. (b) The effect of pH on the activity of MhGgH assessed in 20 mM sodium acetate (squares) or 20 mM sodium phosphate (circles). (c) Kinetic curve using GG as the substrate. The sigmoidal shape of the experimental curve suggests the existence of a cooperative effect. Error bars correspond to standard deviations.
At its optimal temperature, tag-less MhGgH displayed maximum activity at pH 6.0 [Fig. 1 ▸(b)].
The kinetic parameters for MhGgH were determined at 50°C. The experimental data were best fitted to an allosteric sigmoidal curve for both tag-less and tagged MhGgH, with a Hill coefficient above 1, which suggests positive cooperativity [Fig. 1 ▸(c), Table 2 ▸]. The determined kinetic values for the hydrolysis of GG and MG by tag-less MhGgH reflect an almost tenfold higher hydrolysis efficiency for GG (Table 2 ▸). Under the experimental conditions used it was not possible to obtain a complete kinetic curve for MG, resulting in a rough estimation of the value of K 0.5 that nevertheless suggests higher affinity for GG.
Table 2. Kinetic parameters for hydrolysis of GG and MG.
Experimental data were analysed using the allosteric kinetic model. A lower affinity for MG is expected owing to the higher estimated K 0.5 value.
| Kinetic parameters | GG | MG |
|---|---|---|
| V max (µmol min−1 per milligram of protein) | 3.60 ± 0.18 | 3.09 ± 0.66 |
| K 0.5 (mM) | 9.36 ± 0.69 | 84.18 ± 30.27 |
| h | 1.77 ± 0.20 | 1.29 ± 0.23 |
| R 2 | 0.974 | 0.990 |
3.2. Overall structure of MhGgH
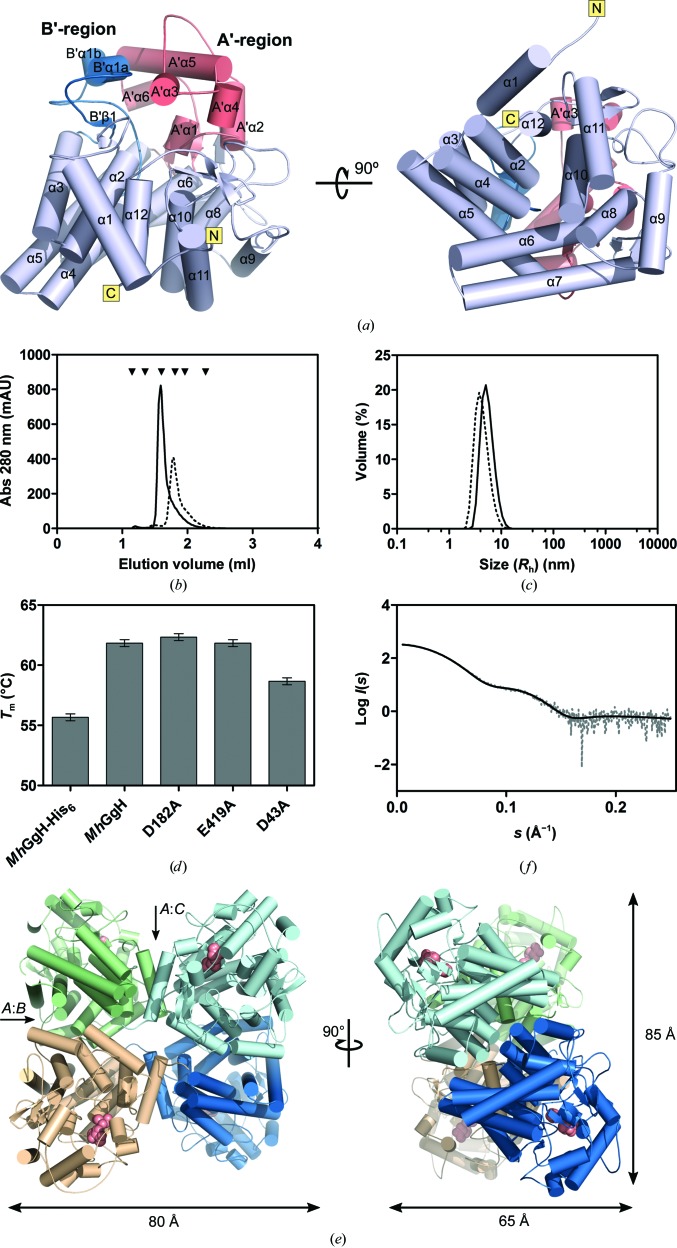
Although both tagged and tag-less MhGgH variants were used in crystallization experiments, only the tag-less variant yielded three-dimensional crystals. Orthorhombic crystals belonging to space group P21212 that diffracted X-rays to beyond 1.7 Å resolution at a synchrotron source were obtained. Despite crystallizing in the same conditions, selenomethionine-substituted MhGgH produced monoclinic crystals (space group P21) that were used for structure solution by multi-wavelength anomalous diffraction at the K absorption edge of selenium as described previously (Cereija et al., 2017 ▸). The orthorhombic crystals contained two MhGgH molecules (here termed A and B) in the asymmetric unit, which were modelled from residues Pro2 to Gly446.
The overall globular MhGgH monomer [Fig. 2 ▸(a)] is composed of an (α/α)6-barrel domain that encompasses helices α2, α4, α6, α8, α10 and α12 in the inner layer and α1, α3, α5, α7, α9 and α11 in the outer layer, and a more flexible cap domain that constrains access to the active site of the enzyme and can in turn be divided into two subdomains termed the A′-region (residues 163–252) and the B′-region (residues 68–118). Five mobile loops are also noteworthy: loop A (between α1 and β1; residues 23–38), loop B (between B′β1 and B′α1a; residues 81–91), loop C (between A′α2b and A′α3; residues 193–205), loop D (between α9 and α10; residues 346–381) and loop E (between β6 and α12; residues 430–434) [Fig. 2 ▸(a), Supplementary Fig. S1].
Figure 2.
Structural and biophysical characterization of MhGgH. (a) Cartoon representation of the overall structure of the MhGgH monomer. The (α/α)6 domain is coloured mauve, and the A′- and B′-regions are coloured salmon and blue, respectively. The N- and C-termini are indicated in yellow boxes. The views in the left and right panels are related by a 90° rotation around x. (b) Analytical size-exclusion chromatogram of tagged (dotted line) and tag-less (solid line) MhGgH variants. The standards used for column calibration (see Section 2) are indicated as inverted black triangles. (c) Analysis of tagged (dotted line) and tag-less (solid line) MhGgH variants by DLS. The tag-less variant displayed a larger hydrodynamic radius (R h = 7.34 nm) and a lower polydispersity index (PdI = 0.092) than the tagged MhGgH variant (R h = 5.75 nm; PdI = 0.201). (d) Melting temperatures of MhGgH variants determined by differential scanning fluorimetry, highlighting the lower stability of the tagged MhGgH variant. Error bars correspond to standard deviations. (e) Quaternary structure of MhGgH. Monomers are coloured green (molecule A), wheat (molecule B), cyan (molecule C) and blue (molecule D). The A:B and A:C interfaces are indicated. The glycerol and serine molecules found in the active-site region are represented by salmon spheres. The approximate dimensions of the homotetramer are indicated. The views on the left and right are related by a 90° rotation around y. (f) Superposition of the experimental SAXS data (dotted grey line) and the theoretical SAXS curve calculated from the tetrameric crystallographic model of MhGgH (solid black line).
M. hassiacum GgH displays 68% secondary-structure identity to the single structurally characterized mannosylglycerate hydrolase Thermus thermophilus HB8 MgH (Tt8MgH; PDB entry 4wva; Miyazaki et al., 2015 ▸), despite the much lower amino-acid sequence identity of 36% (calculated with PDBeFold; Krissinel & Henrick, 2004 ▸). To date, only three other MgH orthologues have been characterized biochemically: the MgH enzymes from Selaginella moellendorffii (Nobre et al., 2013 ▸), T. thermophilus HB27 (99% amino-acid sequence identity to Tt8MgH) and Rubrobacter radiotolerans (Alarico et al., 2013 ▸). Despite relatively low overall amino-acid sequence conservation, some regions are strictly conserved in MhGgH and in all characterized MgH enzymes, including the putative catalytic Asp182 and Glu419 and most of the substrate-interacting residues (Tyr36, Trp40, Trp42, Asp43, Tyr88, Gln115, Gly180, Arg216, Tyr222, Tyr375 and Trp376) (Miyazaki et al., 2015 ▸; Supplementary Fig. S2).
3.3. Quaternary structure of MhGgH
The apparent molecular weights of the MhGgH variants in solution were evaluated by size-exclusion chromatography and DLS [Figs. 2 ▸(b) and 2 ▸(c)]. Both tagged and tag-less MhGgH variants were analysed. While the MhGgH-His6 variant displayed an apparent molecular weight of 92.6 kDa, corresponding to 1.8 times the expected mass of the monomer (50.9 kDa) and compatible with a dimeric arrangement, the apparent molecular weight of tag-less MhGgH was 178.6 kDa, which is 3.5 times greater than the molecular weight of the monomer and is compatible with a trimeric or a tetrameric organization [Fig. 2 ▸(b)]. DLS analysis of the tagged and tag-less MhGgH variants revealed an increase in the hydration radius (from 5.75 nm for tagged MhGgH to 7.34 nm for the tag-less variant), which is in agreement with a higher oligomeric arrangement for tag-less MhGgH, accompanied by a lower polydispersity index, which is indicative of higher homogeneity [Fig. 2 ▸(c)]. In agreement, tag-less MhGgH displayed a significantly higher thermal stability (T m = 62°C) than the tagged variant (T m = 56°C) [Fig. 2 ▸(d)], explaining its higher optimal temperature of activity, and suggesting that the introduction of a hexahistidine tag at the C-terminus of MhGgH affected protein stability by impairing quaternary-structure formation.
In the crystals, MhGgH is arranged as a dimer of dimers with approximate dimensions of 85 × 80 × 65 Å [Fig. 2 ▸(e)]. The total surface area of each monomer is ∼17 300 Å2, of which ∼1600 Å2 is buried in intermonomer contacts. The largest interface area (∼900 Å2) occurs between molecules A and C (interface A:C) and molecules B and D, and involves 14 (A:C) or 12 (B:D) hydrogen bonds. With approximately half of the size (∼480 Å), the interface between dimers A:B and C:D is stabilized by four salt bridges. The smallest interface occurs between molecules A and D (and molecules B and C), with a buried surface of ∼260 Å2 and a single hydrogen bond (Supplementary Table S1). The C-terminus of each MhGgH monomer is in the close vicinity of the A:C (or B:D) interface and the addition of the C-terminal affinity tag is likely to disrupt dimer–dimer association and impact the quaternary organization of the enzyme, which is in line with the observed lower maximum temperature of activity and decreased stability of the MhGgH-His6 variant.
The oligomeric arrangement of MhGgH in solution was also assessed by small-angle X-ray scattering (SAXS). The SAXS data are compatible with a tetrameric arrangement of the enzyme, and superposition of the experimental SAXS curve with that calculated from the crystallographic tetrameric model of MhGgH reveals good agreement, further supporting that the crystallographic oligomer represents the quaternary architecture of the enzyme in solution [Fig. 2 ▸(f)].
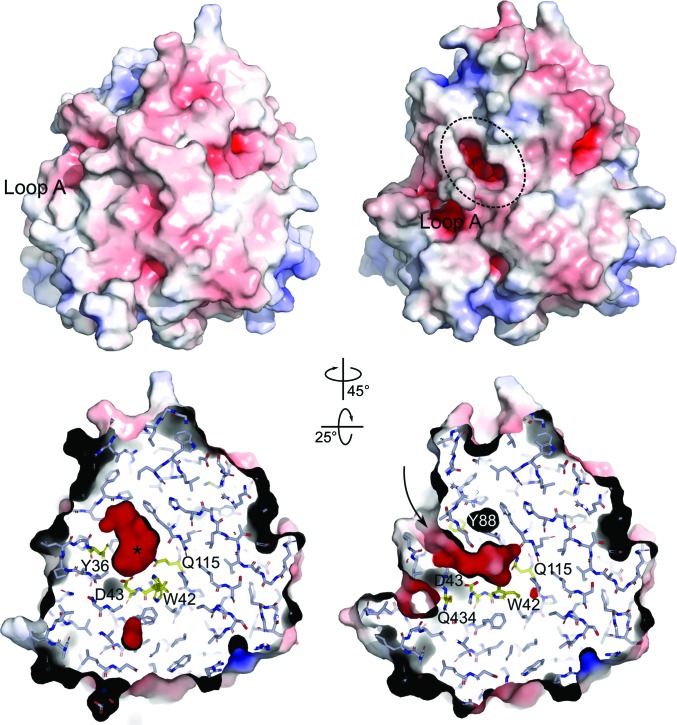
3.4. Open and closed: mobility as an essential feature for substrate binding and hydrolysis
In the orthorhombic crystals, the MhGgH molecules adopt a closed conformation concomitant with the presence of two ligands, a molecule of glycerol and a molecule of serine, which are components of the crystallization buffer, at the active site (MhGgH–Ser–GOL). The glycerol molecule occupies subsite −1 and serine is found at subsite +1 of the active site, inducing a closed state of MhGgH that renders them inaccessible to the solvent (Fig. 3 ▸). These ligands are stabilized mainly by polar contacts, and the putative catalytic residues, Asp182 and Glu419, are facing the lumen of the active-site cavity.
Figure 3.
Closed and open conformations of MhGgH. Solid-surface representation coloured according to electrostatic potential [contoured from −8 kT/e (red) to 8 kT/e (blue)] (upper panel) and cross-section (lower panel) of MhGgH in closed (left) and open (right) conformations. In the closed state (left), the active-site cavity (marked with an asterisk) becomes inaccessible to the solvent. In the open state (right), an opening leading to an acidic cavity is observed (dashed ellipse; upper panel); a negatively charged tunnel (arrow) connects the active-site cavity to the exterior of the molecule (lower panel). Substrate-binding residues are highlighted in yellow. The left and right poses in each panel are related by 25° and 45° rotation around x and y, respectively.
In an alternative crystallization condition (space group P6222), two MhGgH protomers are present in the asymmetric unit, corresponding to molecules A and C of the tetramer observed in the orthorhombic crystals, which were modelled from Pro5 (molecule A) or Ala0 (molecule C) to Gly446. The active sites of both molecules contain only solvent (apo MhGgH) and adopt an open conformation (Fig. 3 ▸). In the open conformation, the active site is accessible to the exterior through a negatively charged tunnel lined by the side chains of Trp40, Asp43, Tyr88, Gln115, Asp212, Ser214, Gln215, Met432, Gln433 and the carbonyl groups of Trp177 and Gly180 (Fig. 3 ▸). In contrast to the closed conformation, the putative catalytic residues point away from the active-site cavity: Asp182 is stabilized by polar contacts with the side chains of Tyr191 and Tyr225 and with the carbonyl group of Arg216 through a water molecule, whereas the side chain of Glu419 is hydrogen-bonded to the side chain of Ser435 and the amide N atoms of Met432 and Thr437 (Supplementary Fig. S3).
The two structures of MhGgH, apo MhGgH and MhGgH–Ser–GOL, corresponding to its open and closed conformations, reveal the structural modifications that occur upon substrate binding. The active site of MhGgH is surrounded by mobile loops that disclose the active site, exposing a polar surface for substrate binding. Indeed, several residues involved in substrate binding are present in these loops, including Tyr36 (loop A), His78, Tyr88 (loop B), Tyr375, Trp376 (loop D) and Gln434 (loop E). Access to the active site is additionally restricted by a flexible cap that also contains important residues for substrate binding (Gly180, Arg216 and Tyr222), as well as the putative catalytic residue Asp182. Substrate binding contributes to the formation of an additional helix in this cap (residues 206–209) that is absent in the open conformation (Supplementary Fig. S4).
A particularly significant modification is observed in the segment Arg21–Ala31 (loop A). In the closed conformation, this loop interacts with the segment Ser431–Ser435 (with polar interactions between the side chain of Asn23 and Gln433 and Ser435) that contains the substrate-interacting residue Gln434, which faces the active site and binds to the substrate. Moreover, upon substrate binding loop A is stabilized by polar contacts with the substrate via Tyr36 and with helices α3 and α12. In the open conformation, Gln433 and Gln434 move outwards (with displacements of their side chains of ∼11 and ∼8 Å, respectively), leading to a concerted rearrangement of loop E and loop A. Loop A (Arg21–Ala31) becomes mostly disordered, with difficult-to-interpret density that only allowed the modelling of two conformations for the segment Leu20–Leu25. In this short stretch, the contribution of Asp24-mediated interactions (with Arg21 or Arg58 of the neighbouring molecule) appears to be central to the overall conformation of this region. Moreover, given their spatial proximity, the conformation adopted by one subunit determines the position of the equivalent region of the neighbouring molecule, potentially impacting enzyme activity, which is in good agreement with the cooperative behaviour observed in the kinetics experiments [Fig. 1 ▸(c)].
3.5. Binding of substrates and substrate analogues to MhGgH
3.5.1. Inactive variants of MhGgH
In order to understand the molecular determinants of substrate binding and specificity, MhGgH was also crystallized in the presence of its substrates (GG and MG), substrate analogues (GGlycerol and GGlycolate) and reaction products (glucose, mannose and glycerate), and wild-type crystals were soaked in buffers containing these compounds. However, none of these approaches yielded crystals of the intended complexes.
To avoid substrate hydrolysis during crystallization or soaking, three catalytically inactive variants of MhGgH were produced. The sequence variations were identified by homology to other characterized MgH enzymes (Supplementary Fig. S2) and analysis of the MhGgH–Ser–GOL ternary-complex structure. Two putative catalytic residues (Asp182 and Glu419) and one substrate-interacting residue (Asp43) were identified and replaced by alanine to produce the D43A, D182A and E419A variants. The thermal stability of the D182A and E419A variants was comparable to that of wild-type MhGgH, while that of the D43A variant was slightly lowered (3°C) [Fig. 2 ▸(d)]. None of the three variants displayed a detectable catalytic activity towards GG or MG (Supplementary Fig. S5).
3.5.2. Inactive variants in complex with substrates
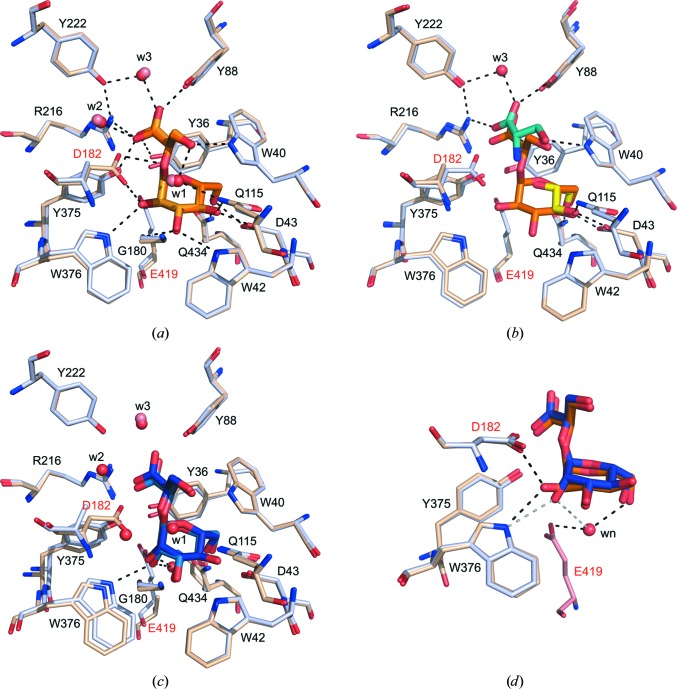
Binary complexes of MhGgH with GG or MG were obtained by soaking orthorhombic crystals of the inactive variants in mother liquor containing these compounds. There is residual electron density compatible with presence of the substrates in the structures of the D182A and E419A variants, but not in that of the D43A variant, independent of the soaking time, highlighting the contribution of Asp43 to substrate binding. For the D182A and E419A MhGgH variants, the glucose/mannose moiety of each substrate occupies subsite −1 and the glycerate moiety occupies subsite +1 of the active site [Figs. 4 ▸(a) and 4 ▸(c)].
Figure 4.
The active site of MhGgH variants in complex with substrates. (a) Superposition of the active-site region of MhGgH D182A and E419A variants in complex with GG. The position of GG (dark or light orange for the D182A or E419A variants, respectively) in the active site of the D182A (light blue) and E419A (wheat) variants is stabilized mainly by direct hydrogen bonds or by water (w)-mediated contacts (dashed lines) with the labelled residues. Water molecules in the D182A and E419A variants are represented by red and salmon spheres, respectively. The catalytic residues (Asp182 and Glu419) are highlighted in red. (b) Superposition of the active-site region of the D182A–GG complex (light blue with the ligand in orange) with that of the MhGgH–Ser–GOL ternary complex (wheat). Hydrogen bonds between serine (cyan) or glycerol (yellow) and the residues of the active site are represented by dashed lines. (c) Superposition of the active-site regions of the MhGgH D182A and E419A variants in complex with MG. The hydrogen-bonding network stabilizing MG at the active site is similar to that observed for GG [interacting residues are shown as in (a)]. The newly established contacts are represented by dashed lines. Water molecules (w) are coloured as in (a). (d) Superposition of the active-site region of the E419A variant of MhGgH in complex with GG and MG and of the D182A variant in complex with MG (Glu419 in salmon). The hydrogen bonds between MhGgH and GG (orange) or MG (blue) are represented by black or grey dashed lines, respectively. The nucleophilic water (wn) is also indicated.
The active sites of MhGgH in the D182A–GG and E419A–GG complexes are virtually identical (r.m.s.d. of 0.24 Å for 14 Cα pairs). The glucose moiety adopts a 4 C 1 chair conformation with an α-anomeric configuration stabilized mainly by hydrogen bonds to the side chains of residues Trp42, Asp43, Gln115, Asp182, Tyr375, Trp376 and Gln434 and to the carbonyl group of Gly180, as well as by a water-mediated contact with Glu419 [Fig. 4 ▸(a)]. The glucose moiety is also oriented by hydrophobic contacts with Tyr36, Trp40, Trp376, Trp381 and Trp436. The glycerate moiety of the substrate is stabilized by polar contacts with Tyr36 (elongated in the D182A variant), Trp40, Tyr88, Arg216 and Tyr375. Water-mediated contacts with Phe89, Gln115, Trp177, Asp182 and Tyr222 also contribute to substrate binding, as do hydrophobic contacts with Trp177. One (D182A) or two (E419A) solvent molecules occupy the space and mimic the interactions of the missing side chains.
The substrate-interacting residues in the complexes between MhGgH variants and GG are organized in a very similar way to that of MhGgH–Ser–GOL [r.m.s.d.s of 0.19 Å (D182A) and 0.22 Å (E419A) for 14 Cα pairs]. The serine and glycerol molecules present in the active site of the MhGgH–Ser–GOL structure partially mimic the substrate, with serine superposing nicely with the glycerate moiety and with glycerol establishing contacts with Asp43, a substrate placeholder that stabilizes the sugar moiety [Fig. 4 ▸(b)]. Taken together, these results suggest that the MhGgH–Ser–GOL and variant–GG complexes are likely to reflect the substrate-binding mode of GG to wild-type MhGgH.
The active site of both variants is also very similar when MG is bound (r.m.s.d. of 0.47 Å for 14 aligned Cα atoms) [Fig. 4 ▸(c)]. A similar hydrogen-bonding network stabilizes both substrates, with the most significant difference being the hydroxyl group at position C2 of the glucose/mannose moiety [Fig. 4 ▸(d)]. While the substrate O15 is hydrogen-bonded to Asp182 and Trp376 in the E419A–GG complex, in the E419A–MG complex only an elongated (3.5 Å) hydrogen bond to Trp376 is preserved, together with a novel water-mediated contact with Gln434.
3.5.3. Mechanism of reaction
At the active site of MhGgH, the glycosidic O atom of both substrates is within hydrogen-bonding distance of the catalytic residue Asp182, while Glu419 establishes a water-mediated contact with both ligands. The distance between the catalytic residues and the substrate, as well as the presence of a single water molecule mediating the Glu419–substrate interaction, suggest that MhGgH hydrolyses GG and MG through a classic inverting mechanism (Supplementary Fig. S6), with Asp182, Glu419 and a water molecule acting as the acid, base and nucleophile, respectively. During hydrolysis, the negatively charged Glu419 is likely to activate the water molecule that performs a nucleophilic attack on the anomeric C atom, while Asp182 donates a proton to the leaving glycerate. Owing to the positions of Glu419 and the water molecule, inversion of the anomeric configuration of the glucose/mannose upon hydrolysis is expected, in agreement with that previously observed for the MgH enzymes from R. radiotolerans and T. thermophilus HB27 (Alarico et al., 2013 ▸).
3.5.4. Inactive variants in complex with substrate analogues
MhGgH was unable to hydrolyse GGlycerol and GGlycolate, despite their considerable similarity to GG (Supplementary Fig. S7). The structures of the E419A variant in complex with both compounds and that of the D182A variant in complex with GGlycolate were determined and help in understanding this behaviour. The most significant differences from the complexes with GG/MG are observed at subsite +1. In the E419A–GGlycerol complex the glycerol moiety of the ligand is stabilized by a direct polar contact with Trp40 and by water-mediated contacts with Tyr88, Gln115, Trp177, Asp182 and Tyr222.
In the case of GGlycolate, the glycolate moiety is found in a single position in the E419A variant but adopts two different conformations in the D182A variant. In the E419A variant, this portion of the substrate analogue is stabilized by direct contacts with Trp40 and Tyr88 and by water-mediated interactions with Gln115, Trp177, His78 and Asp182. In the D182A variant, one of the glycolate conformations is close to that found in the E419A variant (direct contacts with Trp40 and Tyr88 and water-mediated interactions with Gln115 and Trp177), while the second conformation of the ligand is stabilized by hydrogen bonds to Tyr88, Arg216 and Tyr375 and by solvent-mediated contacts with Phe89, Trp177 and Tyr222.
Both substrate analogues establish fewer interactions with the active-site region of MhGgH than bona fide substrates, which certainly impacts on the affinity of the enzyme for these compounds. In particular, interactions with Tyr36, a key residue for active-site closure and organization, are always absent, while different subsets of interactions are observed for GGlycerol and GGlycolate. It is therefore clear that substrate binding in MhGgH is a well coordinated and fine-tuned event involving the concerted movement of flexible loops and interactions with highly conserved residues. Any deviation from these strict interaction patterns will result in a decreased affinity for and highly reduced activity towards the compound, as observed for GGlycerol and GGlycolate.
4. Discussion
Mycobacteria encompass a large number of species, from the well known pathogen M. tuberculosis, which is able to cause tuberculosis in humans and in animals and is still the leading cause of death from a single infectious agent worldwide (World Health Organization, 2018 ▸), to the ubiquitous and opportunistic M. abscessus and the environmental and thermophilic M. hassiacum, which were recently included in the newly created genera Mycobacteroides and Mycolicibacterium, respectively (Gupta et al., 2018 ▸). Although most of the known mycobacteria are considered to be nonpathogenic, an increasing number of infections by opportunistic nontuberculous mycobacteria (NTM) have been reported over the last decade, which is likely to be a result of improved imaging techniques and molecular-sequencing methods that facilitate their identification (Alcaide et al., 2017 ▸). On the other hand, ineffective sanitary control of water-distribution systems, as well as a number of host susceptibility factors, including ageing populations and an increased incidence of chronic diseases, may also be contributing factors to the increased rate of NTM infections detected worldwide (López-Varela et al., 2015 ▸). Nontuberculous mycobacteria display high resilience against stress conditions, including an intrinsically high resistance to disinfectants and antibiotics; for this reason, NTM infections are a considerable clinical challenge for which therapeutic solutions are scarce (Falkinham, 2010 ▸). No significant advances in the treatment of NTM infections in general have recently been achieved, and the lengthy and toxic therapeutic plans in current use are often ineffective, which reinforces the need for more active drug development (Nessar et al., 2012 ▸).
Although scarce, there are reports pointing to the accumulation of GG by environmental mycobacteria during nitrogen-limiting growth (Behrends et al., 2012 ▸; Alarico et al., 2014 ▸), a condition that is able to induce dormancy (Shleeva et al., 2004 ▸; Anuchin et al., 2009 ▸). As a compatible solute, GG can be accumulated intracellularly to high concentrations, and is a potential source of carbon and energy (Nunes-Costa et al., 2017 ▸). Indeed, accumulated GG is quickly depleted upon exposure to an assimilable source of nitrogen, potentially fuelling bacterial growth. A glucosylglycerate hydrolase (GgH) identified in M. hassiacum and found to be highly conserved among rapidly growing mycobacteria is likely to be responsible for the rapid mobilization of GG accumulated during nitrogen starvation by hydrolysing it to glucose and glycerate (Alarico et al., 2014 ▸). A recombinant form of this enzyme containing a C-terminal hexahistidine tag has been characterized biochemically (Alarico et al., 2014 ▸), but failed to form three-dimensional crystals suitable for structural studies. An alternative construct containing a cleavable N-terminal hexahistidine tag was recently generated (Cereija et al., 2017 ▸) and removal of the affinity tag yielded an MhGgH variant with an additional N-terminal Gly-Ala dipeptide, which readily crystallized in two different conditions and diffracted X-rays to 1.7 Å resolution. The crystallographic structure of MhGgH revealed a homotetrameric architecture, which is compatible with the oligomeric organization of the enzyme in solution as assessed by SAXS. The C-terminus of MhGgH was found to be involved in monomer–monomer association, which was likely to be impaired by the C-terminal placement of the affinity tag in the original construct, also explaining the lower stability of this variant.
The MhGgH monomer displays an (α/α)6-barrel domain typical of glycoside hydrolase family 63 (GH63), to which MhGgH belongs. While the active site of GH63 members acting on larger substrates is located in an open, solvent-accessible cleft, those of MhGgH and of MgH from T. thermophilus are covered by a cap domain (subdivided into A′- and B′-regions) with constrained access through a narrow negatively charged tunnel. Upon substrate binding, the cap domain closes, establishing contacts necessary to stabilize and orient the small substrate and to prevent access of bulk solvent to the active site. The open and closed states of MhGgH are determined by the well coordinated movement of several mobile loops that contain some of the substrate-interacting residues.
A kinetic study of MhGgH revealed a cooperative effect between the units of the tetramer, which may result from intersubunit interactions mediated by the mobile loop A. Indeed, in the open conformation loop A regions from adjacent monomers interact, potentially impacting on the enzymatic activity. Substrate binding by one subunit leads to the stabilization of its loop A, which is likely to facilitate access to the active site of the neighbouring subunit.
MhGgH was able to hydrolyse GG more efficiently than MG in vitro, in contrast to the similar efficiency for both substrates displayed by the MgH orthologues from T. thermophilus and S. moellendorfii (Nobre et al., 2013 ▸; Alarico et al., 2013 ▸). This behaviour of MhGgH could be explained by its distinct binding affinities for the two compounds. The α-d-glucose and α-d-mannose moieties of GG and MG, respectively, differ in the orientation of the C2 hydroxyl group, which is equatorial in α-d-glucose and axial in α-d-mannose. As a consequence, the C2 hydroxyl group of the glucose moiety of GG establishes polar contacts with Asp182 and Trp376, while that of the mannose moiety of MG establishes a single contact with Trp376. The larger number of interactions between GG and MhGgH are likely to translate into a higher affinity of binding and to explain the preference of the enzyme for this substrate.
The contribution of subsite +1 to substrate recognition was also evaluated using the substrate analogues GGlycerol and GGlycolate, which differ from GG in the aglycone moiety. In vitro, MhGgH was unable to hydrolyse either compound. The structures of catalytically inactive variants of MhGgH in complex with these substrate analogues were determined, revealing the important contribution of the reducing end of the substrate to active-site closure and the ensuing enzymatic processing.
Substrate binding to MhGgH is a well coordinated event involving highly conserved residues and a complex network of polar contacts. During evolution, the active site of MhGgH has been optimized to harbour specific substrates, such as GG. The presence of MG in M. hassiacum cells has not so far been reported. Assuming that M. hassiacum is unable to produce MG, the ability of MhGgH to hydrolyse this compound may reflect a vestigial function from an ancestor enzyme. On the other hand, since cells are able to exchange molecules with the environment using different strategies, from passive diffusion to active transport, it is also possible that M. hassiacum possesses adequate machinery for scavenging MG released by other organisms from the environment through active export or cell death (Sampaio et al., 2004 ▸) as a potential source of carbon and energy mobilized through hydrolysis by MhGgH.
The antibiotics currently in use mainly counter DNA replication or RNA, protein or cell-wall synthesis, which are indispensable functions for cell growth (Kohanski et al., 2010 ▸). Since these processes are almost suppressed in dormant cells, they are more likely to survive treatment with antibiotics. Indeed, dormant cells have been associated with post-treatment relapse and the development of genetic resistance (Levin & Rozen, 2006 ▸; Gomez & McKinney, 2004 ▸). As a likely intervenient in cell recovery upon nitrogen stress relief, GgH may be viewed as a potential target for the development of new more efficient antimycobacterial drugs. Its comprehensive structural characterization contributes to clarification of the molecular determinants of substrate binding and specificity and provides a detailed molecular scaffold for the rational design of specific inhibitors.
5. Related literature
The following references are cited in the Supporting Information for this article: Bond & Schüttelkopf (2009 ▸) and Sievers et al. (2011 ▸).
Supplementary Material
PDB reference: MhGgH, apo, 6q5t
PDB reference: without serine, 5ohc
PDB reference: SeMet, 5ohz
PDB reference: MhGgH–Ser–GOL, 5oi0
PDB reference: D182A–GG, 5oiw
PDB reference: D43A–Ser–GOL, 5oiv
PDB reference: E419A–GG, 5oju
PDB reference: D182A–MG, 5oj4
PDB reference: E419A–Ser–GOL, 5oie
PDB reference: D182A–Ser–GOL, 5oi1
PDB reference: E419A–MG, 5ojv
PDB reference: E419A–GGycerol, 5ont
PDB reference: D182A–GGlycolate, 5onz
PDB reference: E419A–GGlycolate, 5oo2
Supporting tables and figures. DOI: 10.1107/S2052252519005372/jt5034sup1.pdf
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - Se-Met derivative, source of 5OHZ structure: https://doi.org/10.15785/SBGRID/465
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase, source of 5OI0 structure: https://doi.org/10.15785/SBGRID/467
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase, source of 5OHC structure: https://doi.org/10.15785/SBGRID/464
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase, source of 6Q5T structure: https://doi.org/10.15785/SBGRID/641
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D43A mutant, source of 5OIV structure: https://doi.org/10.15785/SBGRID/471
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant, source of 5OI1 structure: https://doi.org/10.15785/SBGRID/468
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant, source of 5OIE structure: https://doi.org/10.15785/SBGRID/470
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant in complex with glucosylglycerate, source of 5OIW structure: https://doi.org/10.15785/SBGRID/472
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant in complex with mannosylglycerate, source of 5OJ4 structure: https://doi.org/10.15785/SBGRID/473
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant in complex with glucosylglycolate, source of 5ONZ structure: https://doi.org/10.15785/SBGRID/482
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with glucosylglycerate, source of 5OJU structure: https://doi.org/10.15785/SBGRID/474
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with mannosylglycerate, source of 5OJV structure: https://doi.org/10.15785/SBGRID/475
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with glucosylglycolate, source of 5OO2 structure: https://doi.org/10.15785/SBGRID/483
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with glucosylglycerol, source of 5ONT structure: https://doi.org/10.15785/SBGRID/481
Acknowledgments
We acknowledge the European Synchrotron Radiation Facility and the French National Synchrotron Source for the provision of synchrotron-radiation facilities and thank their staff for help with data collection. The support of the X-ray Crystallography, Biointerfaces and Nanotechnology and Cell Culture and Genotyping scientific platforms of i3S (Porto, Portugal) are also acknowledged. We also acknowledge CERMAX of ITQB-NOVA, Oeiras, Portugal for the collection of NMR data.
Funding Statement
This work was funded by Fundação para a Ciência e a Tecnologia grants SFRH/BD/92955/2013 and SFRH/BPD/108299/2015. Programa Operacional Temático Factores de Competitividade grants POCI-01-0145-FEDER-007274, POCI-01-0145-FEDER-029221 (PTDC/BTM-TEC/29221/2017), UID/NEU/04539/2019, and LISBOA-01-0145-FEDER-007660. Programa Operacional Regional Do Norte grant Norte-01-0145-FEDER-000012.
References
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. [DOI] [PMC free article] [PubMed]
- Alarico, S., Costa, M., Sousa, M. S., Maranha, A., Lourenço, E. C., Faria, T. Q., Ventura, M. R. & Empadinhas, N. (2014). Sci. Rep. 4, 6766. [DOI] [PMC free article] [PubMed]
- Alarico, S., Empadinhas, N. & da Costa, M. S. (2013). Enzyme Microb. Technol. 52, 77–83. [DOI] [PubMed]
- Alcaide, F., Peña, M. J., Pérez-Risco, D., Camprubi, D., Gonzalez-Luquero, L., Grijota-Camino, M. D., Dorca, J. & Santin, M. (2017). Eur. J. Clin. Microbiol. Infect. Dis. 36, 1425–1432. [DOI] [PubMed]
- Anuchin, A. M., Mulyukin, A. L., Suzina, N. E., Duda, V. I., El-Registan, G. I. & Kaprelyants, A. S. (2009). Microbiology, 155, 1071–1079. [DOI] [PubMed]
- Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. (2001). Proc. Natl Acad. Sci. USA, 98, 10037–10041. [DOI] [PMC free article] [PubMed]
- Balázsi, G., van Oudenaarden, A. & Collins, J. J. (2011). Cell, 144, 910–925. [DOI] [PMC free article] [PubMed]
- Barker, M. K. & Rose, D. R. (2013). J. Biol. Chem. 288, 13563–13574. [DOI] [PMC free article] [PubMed]
- Behrends, V., Williams, K. J., Jenkins, V. A., Robertson, B. D. & Bundy, J. G. (2012). J. Proteome Res. 11, 3888–3896. [DOI] [PubMed]
- Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N. & Bourne, P. E. (2000). Nucleic Acids Res. 28, 235–242. [DOI] [PMC free article] [PubMed]
- Bond, C. S. & Schüttelkopf, A. W. (2009). Acta Cryst. D65, 510–512. [DOI] [PubMed]
- Bourassa, L. & Camilli, A. (2009). Mol. Microbiol. 72, 124–138. [DOI] [PMC free article] [PubMed]
- Bowler, M. W., Nurizzo, D., Barrett, R., Beteva, A., Bodin, M., Caserotto, H., Delagenière, S., Dobias, F., Flot, D., Giraud, T., Guichard, N., Guijarro, M., Lentini, M., Leonard, G. A., McSweeney, S., Oskarsson, M., Schmidt, W., Snigirev, A., von Stetten, D., Surr, J., Svensson, O., Theveneau, P. & Mueller-Dieckmann, C. (2015). J. Synchrotron Rad. 22, 1540–1547. [DOI] [PMC free article] [PubMed]
- Cereija, T. B., Alarico, S., Empadinhas, N. & Pereira, P. J. B. (2017). Acta Cryst. F73, 536–540. [DOI] [PMC free article] [PubMed]
- Cornell, W. D., Cieplak, P., Bayly, C. I., Gould, I. R., Merz, K. M., Ferguson, D. M., Spellmeyer, D. C., Fox, T., Caldwell, J. W. & Kollman, P. A. (1995). J. Am. Chem. Soc. 117, 5179–5197.
- Daniel, J., Deb, C., Dubey, V. S., Sirakova, T. D., Abomoelak, B., Morbidoni, H. R. & Kolattukudy, P. E. (2004). J. Bacteriol. 186, 5017–5030. [DOI] [PMC free article] [PubMed]
- Dhar, N. & McKinney, J. D. (2007). Curr. Opin. Microbiol. 10, 30–38. [DOI] [PubMed]
- Eldar, A. & Elowitz, M. B. (2010). Nature (London), 467, 167–173. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Eoh, H., Wang, Z., Layre, E., Rath, P., Morris, R., Moody, D. B. & Rhee, K. Y. (2017). Nat. Microbiol. 2, 17084. [DOI] [PMC free article] [PubMed]
- Falkinham, J. O. (2010). Future Microbiol. 5, 951–960. [DOI] [PubMed]
- Faria, T. Q., Mingote, A., Siopa, F., Ventura, R., Maycock, C. & Santos, H. (2008). Carbohydr. Res. 343, 3025–3033. [DOI] [PubMed]
- Finkel, S. E. (2006). Nat. Rev. Microbiol. 4, 113–120. [DOI] [PubMed]
- Flot, D., Mairs, T., Giraud, T., Guijarro, M., Lesourd, M., Rey, V., van Brussel, D., Morawe, C., Borel, C., Hignette, O., Chavanne, J., Nurizzo, D., McSweeney, S. & Mitchell, E. (2010). J. Synchrotron Rad. 17, 107–118. [DOI] [PMC free article] [PubMed]
- Franke, D., Petoukhov, M. V., Konarev, P. V., Panjkovich, A., Tuukkanen, A., Mertens, H. D. T., Kikhney, A. G., Hajizadeh, N. R., Franklin, J. M., Jeffries, C. M. & Svergun, D. I. (2017). J. Appl. Cryst. 50, 1212–1225. [DOI] [PMC free article] [PubMed]
- Gebert, M. J., Delgado-Baquerizo, M., Oliverio, A. M., Webster, T. M., Nichols, L. M., Honda, J. R., Chan, E. D., Adjemian, J., Dunn, R. R. & Fierer, N. (2018). MBio, 9, e01614-18. [DOI] [PMC free article] [PubMed]
- Gengenbacher, M., Rao, S. P., Pethe, K. & Dick, T. (2010). Microbiology, 156, 81–87. [DOI] [PubMed]
- Gomez, J. E. & McKinney, J. D. (2004). Tuberculosis, 84, 29–44. [DOI] [PubMed]
- Gupta, R. S., Lo, B. & Son, J. (2018). Front. Microbiol. 9, 67. [DOI] [PMC free article] [PubMed]
- Jones, S. E. & Lennon, J. T. (2010). Proc. Natl Acad. Sci. USA, 107, 5881–5886. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kabsch, W. & Sander, C. (1983). Biopolymers, 22, 2577–2637. [DOI] [PubMed]
- Kohanski, M. A., Dwyer, D. J. & Collins, J. J. (2010). Nat. Rev. Microbiol. 8, 423–435. [DOI] [PMC free article] [PubMed]
- Konarev, P. V., Volkov, V. V., Sokolova, A. V., Koch, M. H. J. & Svergun, D. I. (2003). J. Appl. Cryst. 36, 1277–1282.
- Krissinel, E. & Henrick, K. (2004). Acta Cryst. D60, 2256–2268. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Kurakata, Y., Uechi, A., Yoshida, H., Kamitori, S., Sakano, Y., Nishikawa, A. & Tonozuka, T. (2008). J. Mol. Biol. 381, 116–128. [DOI] [PubMed]
- Lavollay, M., Arthur, M., Fourgeaud, M., Dubost, L., Marie, A., Veziris, N., Blanot, D., Gutmann, L. & Mainardi, J. L. (2008). J. Bacteriol. 190, 4360–4366. [DOI] [PMC free article] [PubMed]
- Levin, B. R. & Rozen, D. E. (2006). Nat. Rev. Microbiol. 4, 556–562. [DOI] [PubMed]
- Lewis, K. (2007). Nat. Rev. Microbiol. 5, 48–56. [DOI] [PubMed]
- Lipworth, S., Hammond, R. J., Baron, V. O., Hu, Y., Coates, A. & Gillespie, S. H. (2016). Tuberculosis, 99, 131–142. [DOI] [PubMed]
- López-Varela, E., García-Basteiro, A. L., Santiago, B., Wagner, D., van Ingen, J. & Kampmann, B. (2015). Lancet Respir. Med. 3, 244–256. [DOI] [PubMed]
- Lourenço, E. C., Maycock, C. D. & Ventura, M. R. (2009). Carbohydr. Res. 344, 2073–2078. [DOI] [PubMed]
- Lourenço, E. C. & Ventura, M. R. (2011). Carbohydr. Res. 346, 163–168. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Mendes, V., Maranha, A., Alarico, S. & Empadinhas, N. (2012). Nat. Prod. Rep. 29, 834–844. [DOI] [PubMed]
- Meyer, P. A., Socias, S., Key, J., Ransey, E., Tjon, E. C., Buschiazzo, A., Lei, M., Botka, C., Withrow, J., Neau, D., Rajashankar, K., Anderson, K. S., Baxter, R. H., Blacklow, S. C., Boggon, T. J., Bonvin, A. M. J. J., Borek, D., Brett, T. J., Caflisch, A., Chang, C.-I., Chazin, W. J., Corbett, K. D., Cosgrove, M. S., Crosson, S., Dhe-Paganon, S., Di Cera, E., Drennan, C. L., Eck, M. J., Eichman, B. F., Fan, Q. R., Ferré-D’Amaré, A. R., Christopher Fromme, J., Garcia, K. C., Gaudet, R., Gong, P., Harrison, S. C., Heldwein, E. E., Jia, Z., Keenan, R. J., Kruse, A. C., Kvansakul, M., McLellan, J. S., Modis, Y., Nam, Y., Otwinowski, Z., Pai, E. F., Pereira, P. J. B., Petosa, C., Raman, C. S., Rapoport, T. A., Roll-Mecak, A., Rosen, M. K., Rudenko, G., Schlessinger, J., Schwartz, T. U., Shamoo, Y., Sondermann, H., Tao, Y. J., Tolia, N. H., Tsodikov, O. V., Westover, K. D., Wu, H., Foster, I., Fraser, J. S., Maia, F. R. N. C., Gonen, T., Kirchhausen, T., Diederichs, K., Crosas, M. & Sliz, P. (2016). Nat. Commun. 7, 10882.
- Miyazaki, T., Ichikawa, M., Iino, H., Nishikawa, A. & Tonozuka, T. (2015). J. Struct. Biol. 190, 21–30. [DOI] [PubMed]
- Monaco, S., Gordon, E., Bowler, M. W., Delagenière, S., Guijarro, M., Spruce, D., Svensson, O., McSweeney, S. M., McCarthy, A. A., Leonard, G. & Nanao, M. H. (2013). J. Appl. Cryst. 46, 804–810. [DOI] [PMC free article] [PubMed]
- Morin, A., Eisenbraun, B., Key, J., Sanschagrin, P. C., Timony, M. A., Ottaviano, M. & Sliz, P. (2013). Elife, 2, e01456. [DOI] [PMC free article] [PubMed]
- Mueller-Dieckmann, C., Bowler, M. W., Carpentier, P., Flot, D., McCarthy, A. A., Nanao, M. H., Nurizzo, D., Pernot, P., Popov, A., Round, A., Royant, A., de Sanctis, D., von Stetten, D. & Leonard, G. A. (2015). Eur. Phys. J. Plus, 130, 70.
- Nair, S. & Finkel, S. E. (2004). J. Bacteriol. 186, 4192–4198. [DOI] [PMC free article] [PubMed]
- Nessar, R., Cambau, E., Reyrat, J. M., Murray, A. & Gicquel, B. (2012). J. Antimicrob. Chemother. 67, 810–818. [DOI] [PubMed]
- Nobre, A., Empadinhas, N., Nobre, M. F., Lourenço, E. C., Maycock, C., Ventura, M. R., Mingote, A. & da Costa, M. S. (2013). Planta, 237, 891–901. [DOI] [PubMed]
- Nunes-Costa, D., Maranha, A., Costa, M., Alarico, S. & Empadinhas, N. (2017). Glycobiology, 27, 213–227. [DOI] [PubMed]
- Pernot, P., Round, A., Barrett, R., De Maria Antolinos, A., Gobbo, A., Gordon, E., Huet, J., Kieffer, J., Lentini, M., Mattenet, M., Morawe, C., Mueller-Dieckmann, C., Ohlsson, S., Schmid, W., Surr, J., Theveneau, P., Zerrad, L. & McSweeney, S. (2013). J. Synchrotron Rad. 20, 660–664. [DOI] [PMC free article] [PubMed]
- Petoukhov, M. V., Franke, D., Shkumatov, A. V., Tria, G., Kikhney, A. G., Gajda, M., Gorba, C., Mertens, H. D. T., Konarev, P. V. & Svergun, D. I. (2012). J. Appl. Cryst. 45, 342–350. [DOI] [PMC free article] [PubMed]
- Rao, S. P., Alonso, S., Rand, L., Dick, T. & Pethe, K. (2008). Proc. Natl Acad. Sci. USA, 105, 11945–11950. [DOI] [PMC free article] [PubMed]
- Rittershaus, E. S., Baek, S. H. & Sassetti, C. M. (2013). Cell Host Microbe, 13, 643–651. [DOI] [PMC free article] [PubMed]
- Rustad, T. R., Minch, K. J., Brabant, W., Winkler, J. K., Reiss, D. J., Baliga, N. S. & Sherman, D. R. (2013). Nucleic Acids Res. 41, 509–517. [DOI] [PMC free article] [PubMed]
- Sampaio, M. M., Chevance, F., Dippel, R., Eppler, T., Schlegel, A., Boos, W., Lu, Y.-J. & Rock, C. O. (2004). J. Biol. Chem. 279, 5537–5548. [DOI] [PubMed]
- Sanctis, D. de, Beteva, A., Caserotto, H., Dobias, F., Gabadinho, J., Giraud, T., Gobbo, A., Guijarro, M., Lentini, M., Lavault, B., Mairs, T., McSweeney, S., Petitdemange, S., Rey-Bakaikoa, V., Surr, J., Theveneau, P., Leonard, G. A. & Mueller-Dieckmann, C. (2012). J. Synchrotron Rad. 19, 455–461. [DOI] [PubMed]
- Sawangwan, T., Goedl, C. & Nidetzky, B. (2010). Biotechnol. J. 5, 187–191. [DOI] [PubMed]
- Shi, L., Sutter, B. M., Ye, X. & Tu, B. P. (2010). Mol. Biol. Cell, 21, 1982–1990. [DOI] [PMC free article] [PubMed]
- Shleeva, M., Mukamolova, G. V., Young, M., Williams, H. D. & Kaprelyants, A. S. (2004). Microbiology, 150, 1687–1697. [DOI] [PubMed]
- Sievers, F., Wilm, A., Dineen, D., Gibson, T. J., Karplus, K., Li, W., Lopez, R., McWilliam, H., Remmert, M., Söding, J., Thompson, J. D. & Higgins, D. G. (2011). Mol. Syst. Biol. 7, 539. [DOI] [PMC free article] [PubMed]
- Sirakova, T. D., Deb, C., Daniel, J., Singh, H. D., Maamar, H., Dubey, V. S. & Kolattukudy, P. E. (2012). PLoS One, 7, e51641. [DOI] [PMC free article] [PubMed]
- Summers, E. L., Meindl, K., Usón, I., Mitra, A. K., Radjainia, M., Colangeli, R., Alland, D. & Arcus, V. L. (2012). PLoS One, 7, e38542. [DOI] [PMC free article] [PubMed]
- Svensson, O., Malbet-Monaco, S., Popov, A., Nurizzo, D. & Bowler, M. W. (2015). Acta Cryst. D71, 1757–1767. [DOI] [PMC free article] [PubMed]
- Svergun, D., Barberato, C. & Koch, M. H. J. (1995). J. Appl. Cryst. 28, 768–773.
- Theveneau, P., Baker, R., Barrett, R., Beteva, A., Bowler, M. W., Carpentier, P., Caserotto, H., Sanctis, D. d., Dobias, F., Flot, D., Guijarro, M., Giraud, T., Lentini, M., Leonard, G. A., Mattenet, M., McCarthy, A. A., McSweeney, S. M., Morawe, C., Nanao, M., Nurizzo, D., Ohlsson, S., Pernot, P., Popov, A. N., Round, A., Royant, A., Schmid, W., Snigirev, A., Surr, J. & Mueller-Dieckmann, C. (2013). J. Phys. Conf. Ser. 425, 012001.
- Tiago, I., Maranha, A., Mendes, V., Alarico, S., Moynihan, P. J., Clarke, A. J., Macedo-Ribeiro, S., Pereira, P. J. & Empadinhas, N. (2012). J. Bacteriol. 194, 7010–7011. [DOI] [PMC free article] [PubMed]
- Touw, W. G., Baakman, C., Black, J., te Beek, T. A., Krieger, E., Joosten, R. P. & Vriend, G. (2015). Nucleic Acids Res. 43, D364–D368. [DOI] [PMC free article] [PubMed]
- World Health Organization (2018). Global Tuberculosis Report 2018. Geneva: World Health Organization. https://www.who.int/tb/publications/global_report/en/
- Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., Keegan, R. M., Krissinel, E. B., Leslie, A. G. W., McCoy, A., McNicholas, S. J., Murshudov, G. N., Pannu, N. S., Potterton, E. A., Powell, H. R., Read, R. J., Vagin, A. & Wilson, K. S. (2011). Acta Cryst. D67, 235–242. [DOI] [PMC free article] [PubMed]
- Zhou, X. & Cegelski, L. (2012). Biochemistry, 51, 8143–8153. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: MhGgH, apo, 6q5t
PDB reference: without serine, 5ohc
PDB reference: SeMet, 5ohz
PDB reference: MhGgH–Ser–GOL, 5oi0
PDB reference: D182A–GG, 5oiw
PDB reference: D43A–Ser–GOL, 5oiv
PDB reference: E419A–GG, 5oju
PDB reference: D182A–MG, 5oj4
PDB reference: E419A–Ser–GOL, 5oie
PDB reference: D182A–Ser–GOL, 5oi1
PDB reference: E419A–MG, 5ojv
PDB reference: E419A–GGycerol, 5ont
PDB reference: D182A–GGlycolate, 5onz
PDB reference: E419A–GGlycolate, 5oo2
Supporting tables and figures. DOI: 10.1107/S2052252519005372/jt5034sup1.pdf
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - Se-Met derivative, source of 5OHZ structure: https://doi.org/10.15785/SBGRID/465
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase, source of 5OI0 structure: https://doi.org/10.15785/SBGRID/467
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase, source of 5OHC structure: https://doi.org/10.15785/SBGRID/464
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase, source of 6Q5T structure: https://doi.org/10.15785/SBGRID/641
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D43A mutant, source of 5OIV structure: https://doi.org/10.15785/SBGRID/471
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant, source of 5OI1 structure: https://doi.org/10.15785/SBGRID/468
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant, source of 5OIE structure: https://doi.org/10.15785/SBGRID/470
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant in complex with glucosylglycerate, source of 5OIW structure: https://doi.org/10.15785/SBGRID/472
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant in complex with mannosylglycerate, source of 5OJ4 structure: https://doi.org/10.15785/SBGRID/473
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - D182A mutant in complex with glucosylglycolate, source of 5ONZ structure: https://doi.org/10.15785/SBGRID/482
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with glucosylglycerate, source of 5OJU structure: https://doi.org/10.15785/SBGRID/474
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with mannosylglycerate, source of 5OJV structure: https://doi.org/10.15785/SBGRID/475
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with glucosylglycolate, source of 5OO2 structure: https://doi.org/10.15785/SBGRID/483
X-ray diffraction data from Mycobacterial glucosylglycerate hydrolase - E419A mutant in complex with glucosylglycerol, source of 5ONT structure: https://doi.org/10.15785/SBGRID/481