Abstract
Background:
Mostly published as case reports or series, the role of apheresis in hypertriglyceridemia (HTG)-related acute pancreatitis (AP) remains unclear. We performed a systematic review of available literature on this topic with specific focus on disease severity.
Methods:
A search of electronic databases (PubMed, EMBASE, Cochrane) and gray literature yielded 5020 articles of which 74 met criteria for inclusion (301 unique patients). Relevant data were abstracted from full manuscripts and analyzed.
Results:
Most patients were young (mean age 37.9 ± 10.4 years) and male (71.5%). About two-thirds (69.7%) received apheresis within 48 h and most required only 1 or 2 sessions (84.4%). Apheresis resulted in an average reduction of serum TG by 85.4% (p < 0.001). There was high variability in reporting the presence of and criteria to define severe AP (reported 221/301, 73.4%; present 85/221, 38.5%) or organ failure (reported 104/301, 34.6%; present 52/104, 50.0%). Improvement was reported in the majority of patients (reported 144/301, 47.8%, present 136/144, 94.4%) mainly by clinical symptoms or laboratory tests. Overall mortality was 7.1% (21/294) which increased to 11.8% (10/85) with severe AP and 19.2% (10/52) with organ failure.
Conclusions:
Apheresis effectively reduces serum TG levels. However, due to uncontrolled data, reporting bias and lack of a comparison group, definitive conclusions on the efficacy of apheresis in reducing AP severity cannot be made. We propose which patients may be best suitable for apheresis, type of studies needed and outcome measures to be studied in order to provide empiric data on the role of apheresis in HTG-related AP.
Keywords: Hyperlipidemia, Pancreatitis, Severity, Plasmapheresis, Hypertriglyceridemia, Plasma exchange
Introduction
Hypertriglyceridemia (HTG) is a well-recognized cause of acute pancreatitis (AP). A serum triglyceride (TG) level of ≥1000 mg/dl is believed to initiate an attack of AP in some individuals. Serum TG levels fall rapidly after admission as a result of discontinuation of dietary supply from fasting and a reduction of hepatic very low density lipoprotein output due to infusion of hypocaloric intravenous fluids. Although suggested to be more severe than AP from other etiologies, definitive data on the role of HTG on AP severity is lacking due to small sample sizes and poorly defined outcomes [1].
The severity of AP is related to host factors such as age, truncal obesity, lifestyle habits such as alcohol intake, and, local and systemic response to pancreatic injury [2]. In recent studies, unsaturated fatty acids generated from lipolysis of fat within and surrounding the pancreas by pancreatic release of enzymes in AP, were noted to drive local and systemic complications [3,4]. The amount of both intrapancreatic [3] and visceral fat [6,7] have been positively correlated with obesity, which possibly explains the increased risk of severe AP in obese patients [5,8].
Most patients with HTG-induced pancreatitis receive standard treatment with pancreas rest, analgesia, supportive care for organ failure, and management directed towards local complications. TG themselves are not toxic, but serve as a source of unsaturated fatty acids. Increased production of unsaturated fatty acids from local and systemic activity of pancreatic lipase therefore may contribute to severity of AP. Hence, excess TG from the plasma can be removed with HTG-induced pancreatitis by a variety of techniques (apheresis, plasmapheresis, plasma exchange, low-density lipoprotein [LDL] apheresis) with a goal of reducing disease severity.
Over the years, numerous, mainly retrospective, case reports or small series have reported on the use of apheresis in patients with HTG-related AP. However, due to variability in patient selection and reporting of data on severity and efficacy, the role of removing excess TG from the serum in these patients remains unclear. No guidelines exist on which patients are the optimal candidates for such a treatment, and its beneficial effect on the severity of AP. In its recent guidelines, the American Society of Apheresis gave apheresis a weak recommendation as a treatment modality in AP [9].
The aim of this systematic review was to critically review the available literature on this topic with a specific focus on the role of apheresis in reducing AP severity.
Methods
Terminology
This review uses the term “apheresis” to describe all techniques (apheresis, plasmapheresis, plasma exchange, pheresis, LDL apheresis) used to remove excess TG from the serum.
Literature review and study selection
Two health sciences librarians (AK, RT) in collaboration with study author (BC) searched PubMed (1946-current), EMBASE (1947-current) and the Cochrane Database of Systematic Reviews (1995-current) databases. Gray literature search included BIOSIS Previews (1926-current), NIH RePORTER (2015 fiscal year), OAIster (dates not applicable), WHO International Clinical Trials Registry Platform (2004-current), ClinicalTrials.gov (2000-current) and Pro-Quest Digital Dissertations (1861-current). A PubMed search query was developed combining three concepts: Plasmapheresis, Hyperlipidemia and Pancreatitis (Supplemental Appendix 1). The PubMed query was then adapted for use in the remaining databases. All databases were searched for the time period January 1, 1980–August 29, 2014, with no other limits placed on the searches. Additional articles were identified by examination of reference lists from key articles.
For inclusion in this review, an article should have been published in English language, included patients reported to have confirmed AP, identified HTG as the etiology of AP, and utilized apheresis as a treatment modality. Articles were excluded if apheresis was used as a preventative measure rather than for treatment. If more than one publication from the same authors was identified, the articles were cross-examined for replicated patient data and if an overlap existed, only unique patient-specific data was recorded and the duplicate information was excluded.
Data abstraction
Manuscripts meeting the inclusion criteria were carefully examined and data were systematically extracted by first author (BC) under supervision of the senior author with secondary review and discussion as needed on documents and data to potentially be abstracted and included (DY). Details of each study including first author, year published, country where study was conducted, study design, and number of patients were recorded. Study design was simplified to case reports (single patient) or case series (two or more patients). Patient specific information when available was recorded for age, sex, race, type of HTG (Fredrickson classification) [10], secondary risk factors (body mass index [BMI], alcohol intake, diabetes mellitus [DM], pregnancy, medications), history of prior AP, serum TG and cholesterol levels, intensive care unit [ICU] admission, apheresis details, disease severity, adjunct treatment used (insulin infusion, heparin infusion, or medications such as fibrate, statin, or niacin if started prior to or at the same time as apheresis) and disease related outcomes. Obesity was defined according to World Health Organization criteria of BMI ≥30 kg/m2 and overweight as BMI 25–30 kg/m2.
Data specific to apheresis was recorded for the type of fluid replacement used (albumin [regardless of concentration] or plasma, and in case of concurrent use, that fluid which was used more frequently was recorded), anticoagulant utilized (citrate or heparin, and if both were used, continuous infusion or anticoagulant used for greater period of time was recorded), timing of apheresis initiation, and number of sessions. Information was recorded for the first available serum TG level, total cholesterol level, and their levels after apheresis was completed. Unit conversions for TG and cholesterol levels were performed using an online lipid conversion calculator (http://www.onlineconversion.com/cholesterol.htm).
Outcomes were divided into laboratory test-oriented (lipid levels) and patient-oriented (severity parameters, mortality, length of stay, complications). Severity data included clinical setting (ICU or medical floor); AP severity criteria (Ranson, Acute Physiology and Chronic Health Evaluation [APACHE-II], computed tomography [CT] severity index, Balthazar, Japanese, Glasgow); organ failure: presence, duration (transient <48 h, persistent ≥48 h), and organs affected. Organ failure was recorded if laboratory and clinical information were available by the following definitions: respiratory – PaO2 <60 mmHg or mechanical ventilation requirement; cardiovascular – systolic blood pressure <90 mmHg or diastolic blood pressure <60 mmHg or use of vasopressors; renal – increase in serum creatinine ≥0.3 mg/dl from baseline or ≥50% increase from baseline or <0.5 ml/kg/hour of urine output or requirement of hemodialysis. Other organ failure was dependent on reporting by study authors.
Episodes of AP were recorded as categorically severe if authors specifically mentioned the term “severe.” Severity criteria reported were then used to verify this description when possible by calculating criteria-specific scores. The cutoffs used to define severity included Ranson ≥3, APACHE-II ≥8, CTSI >5, Balthazar D-E, Japanese ≥2, and Glasgow ≥3 [11–15].
Efficacy information comprised improvement in clinical symptoms (e.g. abdominal pain, nausea, vomiting), lipid levels and other laboratory tests, severity index score, organ failure, radiographic abnormalities, length of stay and mortality. Local complications such as necrosis, fluid collections, pseudocysts, and any procedures required for treatment (drainage, laparotomy) when reported were recorded.
Analysis
A descriptive analysis was performed. Available information on individual patients was recorded and summarized as mean ± standard deviation or median and range for continuous variables and as proportions for categorical variables. Univariate comparisons were performed using student’s t-test or Mann Whitney-U test for continuous variables and chi-squared or Fischer’s exact test for categorical variables as appropriate using Stata 13.0 (StataCorp. 2013). All tests were two-sided and significance was considered at α = 0.05. When individual patient data was not available (e.g. TG levels in case series), summary data from the publication is presented.
Results
Study characteristics
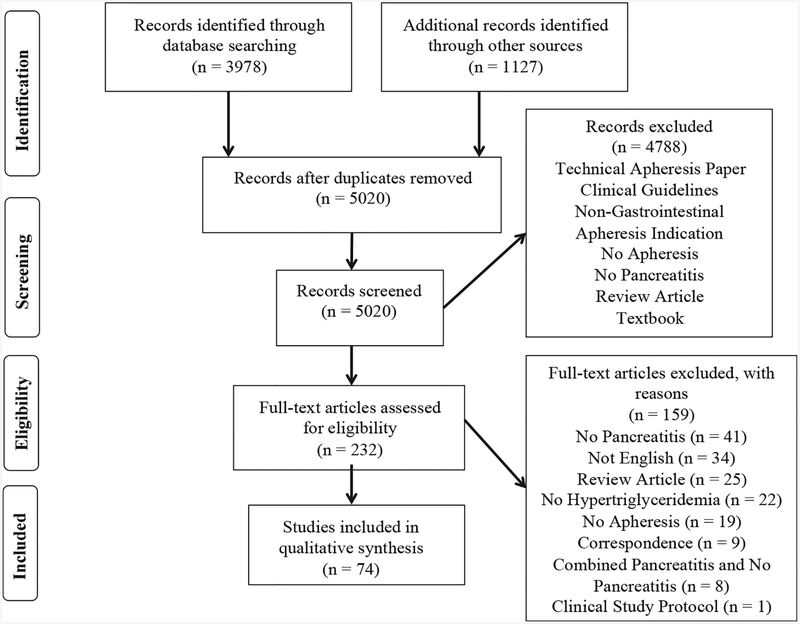
Initial resource searches yielded 5020 manuscripts after removal of duplicate entries. All were screened for inclusion, of which 232 full-text articles were eligible for critical evaluation and ultimately 74 articles (1982–2014) met inclusion criteria (Fig. 1). Of these, 48 were case reports and 26 case series with 2–111 patients for a total of 301 unique patients (Table 1). There were 8 articles with duplicate patients and one with entirely duplicated patients [16]. Of the 301 unique patients, 194 (64.5%) were from Europe, 35 (11.6%) from North America, and 72 (23.9%) from other regions.
Fig. 1.
Publication selection process.
Table 1.
Publications included in the systematic review.
| First author | Year | Journal | Volume (Issue) | Study | Country | No. patients |
|---|---|---|---|---|---|---|
| Gerard | 1982 | Vox Sang | 43 (3) | Case Report | France | 1 |
| Yamauchi | 1986 | Tohoku J Exp Med | 148 (2) | Case Report | Japan | 1 |
| Flynn | 1987 | Ann Internal Med | 107(1) | Case Report | US | 1 |
| Deleplanque | 1988 | Ann Med Interne (Paris) | 139 (S1) | Case Report | France | 1 |
| Achard | 1991 | Intensive Care Med | 17 (4) | Case Report | France | 1 |
| Swoboda | 1993 | Gastroenterology | 104 (5) | Case Report | Austria | 1 |
| Mayan | 1996 | Isr J Med Sci | 32 (9) | Case Report | Israel | 1 |
| Saravanan | 1996 | J Clin Gastroenterol | 22 (4) | Case Report | US | 1 |
| Ford | 1998 | Anaesth Intensive Care | 26 (5) | Case Report | US | 1 |
| Raza | 2001 | Scott Med J | 46 (6) | Case Report | UK | 1 |
| Routy | 2001 | J Clin Apher | 16 (3) | Case Report | Canada | 1 |
| Bildirici | 2002 | Acta Obstet Gynecol Scand | 81 (5) | Case Report | Denmark | 1 |
| Kella | 2002 | Journal of Clin Lipidology | 6 (5) | Case Report | US | 1 |
| Coman | 2003 | J Clin Apher | 18 (3) | Case Report | France | 1 |
| Iskandar | 2004 | Am J Med Sci | 328 (5) | Case Report | US | 1 |
| Kohli | 2006 | Dig Dis Sci | 51 (12) | Case Report | US | 1 |
| Lams | 2006 | Eur J Anaesthesiol | 23 (12) | Case Report | UK | 1 |
| Lersch | 2006 | Transfusion | 46 (11) | Case Report | Switzerland | 1 |
| Bhavsar | 2008 | J Pancreas | 9 (5) | Case Report | US | 1 |
| Kfoury-Baz | 2008 | Transfusion | 48 (6) | Case Report | Lebanon | 1 |
| Martin | 2009 | South Med J | 102 (10) | Case Report | US | 1 |
| Michalakis | 2009 | Cases Journal | 2 (11) | Case Report | Greece | 1 |
| Kayatao | 2010 | Arch Gynecol Obstet | 281 (3) | Case Report | Turkey | 1 |
| Durval | 2011 | Minerva Anestesiol | 77 (10) | Case Report | Italy | 1 |
| Ko | 2011 | Gastroenterol Hepatol | 26 (S5) | Case Report | Taiwan | 1 |
| Pai | 2011 | Transfusion | 51 (9) | Case Report | US | 1 |
| Stefanuttia | 2011 | Transfus Apher Sci | 45 (1) | Case Report | Italy | 1 |
| Bajpai | 2012 | Transfusion | 52 (5) | Case Report | India | 1 |
| Cahalane | 2012 | Case Rep Med | NA | Case Report | Ireland | 1 |
| Castro | 2012 | Rev Bras Ter Intensiva | 24 (3) | Case Report | Brazil | 1 |
| Chan | 2012 | Hong Kong Med J | 18 (6) | Case Report | Hong Kong | 1 |
| Lufti | 2012 | Pediatrics | 129 (1) | Case Report | US | 1 |
| Madhra | 2012 | Diabetic Medicine | 29 (10) | Case Report | UK | 1 |
| Parulekar | 2012 | QJ Med | 105 (9) | Case Report | UK | 1 |
| Safi | 2012 | Chest | 142 (4) | Case Report | US | 1 |
| Serpytis | 2012 | J Pancreas | 13 (6) | Case Report | UK | 1 |
| Sevastru | 2012 | BMJ Case Rep | NA | Case Report | UK | 1 |
| Tampieri | 2012 | Emergency Care Journal | 8 (2) | Case Report | Italy | 1 |
| Atluri | 2013 | Am J Gastroenterol | 108 (S1) | Case Report | US | 1 |
| Bota | 2013 | Am J of Emerg Med | 31 (2) | Case Report | US | 1 |
| Kirk | 2013 | Crit Care Med | 41 (12) | Case Report | US | 1 |
| Rajendran | 2013 | Diabetic Medicine | 30 (8) | Case Report | UK | 1 |
| Salem | 2013 | Am J Gastroenterol | 108 (S1) | Case Report | US | 1 |
| Sharma | 2013 | Crit Care Med | 41 (12) | Case Report | US | 1 |
| Chelu | 2014 | J Gen Intern Med | 29 (S1) | Case Report | US | 1 |
| Gupta | 2014 | Case Rep Obstet Gynecol | NA | Case Report | US | 1 |
| Qiu | 2014 | J Clin Apher | 29 (1) | Case Report | US | 1 |
| Reper | 2014 | Eur J Obstet Gynecol Reprod Biol | 179 | Case Report | Belgium | 1 |
| Richter | 1987 | Ann Intern Med | 106 (5) | Case Series | Germany | 2 |
| Piolet | 1996 | Pancreas | 13 (1) | Case Series | France | 2 |
| Lennertz | 1999 | Therapeutic Apheresis |
3 (3) | Case Series | Germany | 5 |
| Shinar | 2001 | Transfus Apher Sci | 24 (2) | Case Series | Israel | 8 |
| Furuya | 2002 | Therapeutic Apheresis | 6 (6) | Case Series | Japan | 2 |
| Yeha | 2003 | J Clin Apher | 18 (4) | Case Series | Taiwan | 17 |
| Yeha | 2003 | J Clin Apher | 18 (1) | Case Series | Taiwan | 1 |
| Chen | 2004 | World J Gastroenterol | 10 (15) | Case Series | China | 20 |
| Bae | 2005 | Korean J Gastroenterol | 46 (6) | Case Series | Korea | 2 |
| Kyriakidisa | 2005 | Pancreatology | 5 (2–3) | Case Series | Greece | 5 |
| Nikou | 2005 | Ann Gastroenterol | 18(3) | Case Series | Greece | 7 |
| Kyriakidisa | 2006 | Digestion | 73 (4) | Case Series | Greece | 4 |
| Ramdhaney | 2006 | Am J Gastroenterol | 101 (9) | Case Series | US | 2 |
| Al-Humoud | 2008 | Ther Apher Dial | 12 (3) | Case Series | Kuwait | 8 |
| Gubenseka | 2009 | Ther Apher Dial | 13 (4) | Case Series | Slovenia | 0 |
| Salazar Ramirez | 2009 | Intensive Care Med | 35 (S1) | Case Series | Spain | 6 |
| Stefanuttia | 2009 | Artif Organs | 33 (12) | Case Series | Italy | 12 |
| Kadikoylu | 2010 | Transfus Apher Sci | 43 (3) | Case Series | Turkey | 2 |
| Syed | 2010 | J Clin Apher | 25 (4) | Case Series | US | 4 |
| Bayraktaroglu | 2010 | Endocrine Abstracts | 20 | Case Series | - | 7 |
| Altun | 2012 | J Anaesthesiol Clin Pharm | 28 (2) | Case Series | Turkey | 2 |
| Yanardag | 2012 | Transfus Apher Sci | 47 (S1) | Case Series | Turkey | 4 |
| Chiang | 2012 | J Clin Apher | 27 (1) | Case Series | US | 4 |
| Kouba | 2013 | J Clin Apher | 28 (2) | Case Series | US | 5 |
| Ramirez-Bueno | 2014 | Eur J Intern Med | 25 (2) | Case Series | Spain | 11 |
| Gubenseka | 2014 | PLOS ONE | 9 (7) | Case Series | Slovenia | 111 |
US: United States; UK: United Kingdom; NA: not applicable.
Studies that included duplicate patients as prior publications. Number of patients reflects unique patients in article.
Demographics, secondary factors and etiology
While age (269/301, 89.4%) and sex (263/301, 87.4%) was reported in most patients, information on BMI, race, prior episodes of AP, baseline lipid abnormality and secondary risk factors was reported less frequently and in a variable fraction of patients (Table 2).
Table 2.
Demographic data, baseline lipid abnormalities and secondary factors.
| Total patients (n = 301) | n (%) or mean ± SD (of whom information available) |
|
|---|---|---|
| Information available N (% of Total) | ||
| Age (years) | 269 (89.4) | 37.9 ± 10.4 |
| Sex | 263 (87.4) | |
| Male | 188 (71.5) | |
| Female | 74 (28.1) | |
| Race | 18 (6.0) | |
| Caucasian | 7 (38.9) | |
| Black | 3 (16.7) | |
| Hispanic | 5 (27.8) | |
| Asian | 3 (16.7) | |
| BMI (kg/m2) | 44 (14.6) | 26.6 ± 4.9 |
| Prior pancreatitis | 78 (25.9) | |
| Yes | 55 (70.5) | |
| No | 23 (29.5) | |
| Baseline lipid | 117 (38.9) | |
| abnormality (Fredrickson classification) |
||
| I | 1 (0.9) | |
| II | 7 (6.0) | |
| III | 2 (1.7) | |
| IV | 23 (19.7) | |
| V | 20 (17.1) | |
| Othera | 64 (54.7) | |
| Second factor | 149 (49.5) | |
| Diabetes | 75 (50.3) | |
| Alcohol | 57 (38.3) | |
| Pregnancy | 18 (12.1) | |
| Medication | 9 (6.0) | |
| Otherb | 3 (2.0) |
BMI: body mass index.
Other lipid abnormalities described by authors included “dyslipidemia” (n = 61), “familial HTG” (n = 1), type IV or V (n = 1), and peroxisome proliferator-activated receptor gamma genetic mutation (n = 1).
Authors listed coronary artery disease, amyloidosis, and uremia as contributing factors in one case each.
Among patients with reported data, most were young (mean age 37.9 ± 10.4 years, range 10–73) and male (188/263, 71.5%). Obesity was present in 7/25 (28.0%) with an additional 6/25 (24.0%) being overweight. The majority of patients had prior history of AP (55/78, 70.5%). The most prevalent hyperlipidemias were Type IV (23/117, 19.7%) and Type V (20/117, 17.1%). In 61 patients, “primary hyperlipidemia” or “dyslipidemia” without specific Fredrickson classification was reported and one each had “familial HTG”, “Type IV or V”, and a genetic mutation in peroxisome proliferatory-activated receptor gamma. The most common secondary factors included diabetes (75/149, 50.3%), alcohol consumption (57/149, 38.3%) and pregnancy (18/149, 12.1%). Medications were implicated in 9 patients (9/149, 6.0%) and included estrogen-containing oral contraceptive pills in 3, and one each with asparaginase, isoretinoin, exogenous testosterone, entecavir, ritonavir, capecitabine and venlafaxine. One patient was taking contraceptive pills and isoretinoin simultaneously. Other rare contributing factors identified were uremia, amyloidosis and coronary artery disease (one each). Fourteen patients had more than one secondary risk factor (14/149, 9.4%). Of patients identified with primary hyperlipidemia, 24/117(20.5%) were reported to have a concurrent secondary risk factor.
Apheresis
Information on the type of fluid replacement was reported in over half of patients (175/301, 58.1%) (Table 3). Plasma was the predominant fluid more frequently (131/175, 74.9%) than albumin (45/175, 25.7%). One patient received both fluids in equal proportion. During apheresis, anticoagulation with predominantly heparin was more common (101/173, 58.4%) than citrate (73/173, 42.2%). One patient received both heparin and citrate in equal amounts. The majority of patients (51/73, 69.9%) were initiated on apheresis within 48 h of admission and most patients (195/231, 84.4%) required one or two sessions.
Table 3.
Apheresis information and adjunctive therapy.
| Total patients (n = 301) | n (% of whom information available) | |
|---|---|---|
| Information available N (% ofTotal) | ||
| Fluid replacementa | 175 (58.1) | |
| Plasma | 131 (74.9) | |
| Albumin | 45 (25.7) | |
| Peripheresis anticoagulanta |
173 (57.5) | |
| Citrate | 73 (42.2) | |
| Heparin | 101 (58.4) | |
| Timing (Hours)b | 117 (38.9) | |
| <24 | 26 (35.6) | |
| 24–48 | 25 (34.2) | |
| 48–72 | 12 (16.4) | |
| >72 | 10 (13.7) | |
| No. sessions | 231 (76.7) | |
| 1 | 149 (62.2) | |
| 2 | 46 (19.9) | |
| 3 | 18 (7.8) | |
| >3 | 18 (7.8) | |
| Adjunct therapy | 85 (28.2) | |
| IV Insulin | 35 (41.2) | |
| IV Heparin | 13 (15.3) | |
| Fibrate | 28 (32.9) | |
| Niacin | 4 (4.7) | |
| Statin | 3 (3.5) | |
| Nafamostat mesilate |
2 (2.4) |
IV: intravenous.
The predominant fluid or anticoagulant was recorded. One patient received plasma and albumin in equal proportion and one patient received anticoagulation with citrate and heparin in equal amounts.
73 patients had individual timing information available. 44 patients from case series were reported as cumulative timing.
Adjunct systemic medical therapy to decrease serum TG levels was reported in 85 patients (85/301, 28.2%). Intravenous insulin (35/85, 41.2%) was used more frequently than intravenous heparin (13/85, 15.3%) and fibrate (28/85, 32.9%) or other agents, which were used infrequently (Table 3).
Serum lipid levels
Individual patient data for initial TG measurement was available in 135 patients with a mean value 4576 ± 4025 mg/dl (Table 4). Post-apheresis TG levels were reported in 83 cases with mean value 668 ± 695 mg/dl representing an 85.4% mean reduction (p < 0.001). Initial cholesterol levels were reported in 78 patients with a mean value 719 ± 394 mg/dl which decreased to 226 ± 131 mg/dl (n = 31) after apheresis representing a 68.6% reduction (p < 0.001). In the largest case series consisting of 111 patients [17], TG and cholesterol levels decreased 59.1% and 41.1% respectively.
Table 4.
Serum Triglyceride and Cholesterol levels pre- and post-apheresis.
| Initial TG (mg/dl) | Post TG (mg/dl) | Per. reduction | Initial Ch (mg/dl) | Post Ch (mg/dl) | Per. reduction | ||
|---|---|---|---|---|---|---|---|
| Individual patients | No. patients | 135 | 83 | 78 | 31 | ||
| Mean ± SD | 4576 ± 4025 | 668 ± 695 | 85.4 | 719 ± 394 | 226 ± 131 | 68.6 | |
| Range | 532–26651 | 81–3815 | 182–1900 | 89–576 | |||
| Gubensek (n = 111) | Mean ± SD | 3897 ± 2746 | 1594 ±1329 | 59.1 | 657 ± 309 | 387 ±271 | 41.1 |
| Al-Humoud (n = 8) | Mean ± SD | 9767 ± 12966 | 3407 ± 4322 | 65.1 | 517 ±308 | 149 ± 52 | 71.2 |
TG: Triglyceride; Ch: Cholesterol.
Data on representative case series shown. Other case series not depicted due to heterogenous cumulative statistics and representations.
Disease severity
Data on clinical setting for treatment was reported in 145/301 patients (48.2%). ICUs (141/145, 97.2%) were utilized more often than medical floors (3/145, 2.1%) while one patient received apheresis in both the ICU and floor (Table 5). The criteria used to classify severity of AP were reported for 73.4% patients (221/301 overall; 25/48, 52.1% of case reports, 196/253, 77.5% of case series). Nearly one-third of the patients (73/221, 33.0%) were evaluated by more than one criterion. The most commonly used criteria were APACHE-II (154/221, 69.7%) and Ranson (89/221, 40.3%). Imaging-based criteria (CT scan, CT-severity index, Balthazar) were used in 83/221 patients (37.6%). Two patients were classified as severe by authors based on ultrasound findings and undefined “severity score”. More than half of patients evaluated by Ranson criteria qualified for severe AP (48/89, 53.9%) while 53/154 (34.4%) were classified as severe by APACHE-II criteria. Patients measured by Balthazar, Glasgow, and Japanese criteria were nearly all severe (Balthazar: 21/25, 96.0%; Glasgow: 10/10, 100%; Japanese: 2/2, 100%).
Table 5.
Measures of pancreatitis severity.
| Total patients (n = 301) | n (% of whom information available) | |
|---|---|---|
| Information available N (% of Total) | ||
| Setting | 145 (48.2) | |
| ICU | 141 (97.2) | |
| Floor | 3 (2.1) | |
| Both | 1 (0.7) | |
| Severity classification |
221 (73.4) | Severe by criteriaa (n = 85) |
| APACHE-II | 154 (69.7) | 53 (34.4) |
| Ranson | 89 (40.3) | 48 (53.9) |
| CTSI | 12 (5.4) | 2 (16.7) |
| Balthazar | 25 (11.3) | 21 (96.0) |
| Glasgow | 10 (4.5) | 10 (100.0) |
| Japanese | 2 (0.9) | 2 (100.0) |
| Other/Undefined | 8 (3.6) | 7 (87.5) |
| Organ failure | 104 (34.6) | |
| Present | 52 (50.0) | |
| Absent | 52 (50.0) | |
| Transient | - | 10 (19.2) |
| Persistent | 37 (71.2) | |
| Unknown | 5 (9.6) | |
| Single | - | 22 (42.3) |
| Multiple | 20 (38.5) | |
| Unknown | 10 (19.2) | |
| Organs failed | - | 51 (98.1) |
| Respiratory | 35 (68.6) | |
| Renal | 22 (43.1) | |
| Cardiovascular | 16 (31.4) | |
| Otherb | 6 (11.8) | |
| Unknown | 2 (3.9) | |
| SAP or organ failure | 254 (84.4) | |
| Yes | 116 (43.8) | |
| No | 149 (56.2) | |
| Overall mortality | 294 (97.7) | 21c(7.1) |
| Case reports | 48 (100.0) | 2 (4.2) |
| Case series | 246 (97.2) | 19 (7.7) |
ICU: intensive care unit; APACHE: Acute Physiology and Chronic Health Evaluation; CT: computed tomography; CTSI: computed tomography severity index; SAP: severe acute pancreatitis.
Severity defined by Ranson ≥3, APACHE ≥8, CTSI >5, Balthazar grade D-E, Japanese ≥2, or Glasgow ≥3.
Two patients with central nervous system failure, one patient with hepatic, intestinal, and coagulation failure.
Does not include three fetal deaths.
Severe AP by any criteria was noted in 38.5% patients (85/221 overall; 19/25 76.0% in case reports, 66/196 33.6% in case series). Information on organ failure was mentioned only in a third of cases (104/301, 34.6% overall; 40/48, 83.3% of case reports, 64/253, 25.3% of case series) and was present in 52/104 (50.0% overall, 27/40, 67.5% in case reports, 25/64, 39.1% in case series). Persistent organ failure was more common (37/52, 71.2%) than transient (10/52, 19.2%) with unknown duration in 9.6% (5/52) patients. Single organs were affected (22/52, 42.3%) more often than multiple (20/52, 38.5%) while in the remainder (10/52, 19.2%) the number of organs failing was unknown. Respiratory failure was most common (35/51, 68.6%), followed by renal (22/51, 43.1%), and cardiovascular failure (16/51, 31.4%). Other organs reported as failing included central nervous system (2/51, 3.9%), along with combined hepatic, intestinal, and coagulation failure in one patient. Hemodialysis was required in 50% (11/22) patients with renal failure. Information to determine either severe AP or organ failure was available in 84.4% patients (254/301 overall; 43/48, 89.6% in case reports, 211/253, 83.4% in case series) and 43.8% (116/265 overall; 34/43, 79.1% in case reports, 82/211, 38.9% in case series) had either severe AP or organ failure. Length of stay was reported for 56.8% (171/301) patients with a median stay of 15 days (range 3–150).
Information regarding local complications was infrequently reported (50/301, 16.6% patients), most commonly as pancreatic necrosis (26/50, 52.0%), followed by abdominal infection (9/50, 18.0%) and pseudocyst (5/50, 10.0%). Two patients were reported to require percutaneous drains and 6 underwent laparotomy.
Efficacy of apheresis
Overall, clinical status following apheresis was reported in 144/301 patients (47.8%), and nearly all (136/144, 94.4%) were reported to have some form of improvement. When mentioned, improvement was observed in 93.1% (54/58) patients with severe AP, and 88.0% (44/50) with organ failure and 92.2% (71/77) with severe AP or organ failure. The efficacy criteria most commonly included improvement in symptoms such as abdominal pain, nausea, or vomiting (95/144, 66.0%), laboratory values (including TG levels, 89/144, 61.8%), and organ failure (35/144, 24.3%). Other efficacy criteria used were improvement in severity score (10/144, 6.9%) as well as radiographic imaging (6/144, 4.2%).
A subgroup analysis of the 35 patients with organ failure who improved was performed. Information regarding both organ failure improvement and the type of organ(s) that failed was available for 28 (80.0%) patients. Most patients (20/28, 71.4%) had one organ affected while 10.7% (3/28) had two and 17.9% (5/28) had three organs fail. The most common organ system reporting improvement was respiratory (21/28, 75.0%), followed by renal (10/28, 35.7%), cardiovascular (8/28, 28.6%), and central nervous system (2/28, 7.1%). However, these rates may be a reflection of specific organ failure prevalence rather than clinical response to apheresis.
Mortality
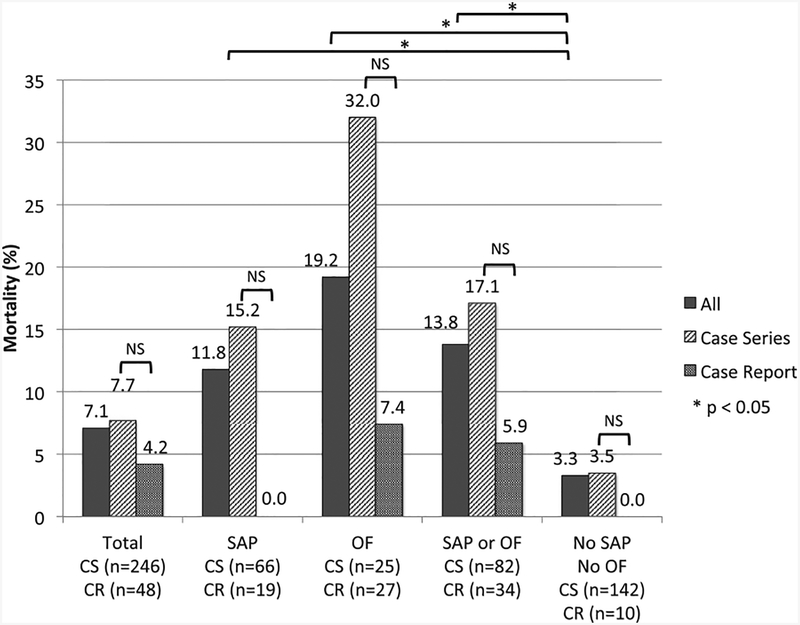
Information on mortality by report type and available information on disease severity and organ failure is shown in Table 5 and Fig. 2. The overall mortality was 7.1% (21/294; case reports 4.2% [2/48], case series 7.7% [19/246]). Three additional fetal deaths were reported (2 in case reports, 1 in case series). As expected, when compared with patients without severe AP or organ failure (3.3%), the risk of death was higher in the presence of severe AP (11.8%, p = 0.022), organ failure (19.2%, p < 0.001) and severe AP or organ failure (13.8% p = 0.002). For each category, the risk of death was lower in case reports when compared with case series, although statistical significance was observed only in patients with organ failure (p = 0.036) likely due to type II error.
Fig. 2. Mortality by study design and presence of severe acute pancreatitis or organ failure.
SAP: severe acute pancreatitis; OF: organ failure; CR: case report; CS: case series.
Discussion
In this systematic review of published literature we noted apheresis to be highly efficacious in reducing serum TG levels. There was high variability in the reporting of severe AP, criteria to define severe AP and rationale for performing apheresis. Data on the efficacy of apheresis was mostly limited to improvement in clinical symptoms (e.g. abdominal pain, nausea, vomiting) and laboratory tests with little information provided on the impact of apheresis on AP severity. The uncontrolled nature of data, reporting bias especially in case reports (higher prevalence of severe disease/organ failure but successful outcome, i.e. low mortality rate), and lack of a comparison group make it difficult to draw definitive conclusions on the efficacy of apheresis in reducing the severity of AP. Our results highlight the need for well designed studies with clearly defined outcome measures to define the role of apheresis in HTG-related AP.
The demographic distribution (mostly young, male, often with a prior history of pancreatitis) and presence of secondary factors (diabetes, alcohol abuse and medication, with the exception of a somewhat higher prevalence of pregnant patients) is consistent with the reported literature suggesting that apheresis was offered equally for subgroups of patients [1]. The prevalence of severe AP using any criteria (28.2% overall, 38.5% when data was reported), organ failure (17.2% overall, 50.0% when data was reported) was higher than would be expected in a community population [18] suggesting that apheresis was preferentially performed in patients with suspected severe or severe AP. However, performance of apheresis was not limited to these subgroups as suggested by ~50% of the 301 who received apheresis having no clear indicators suggestive of severe AP or organ failure.
As expected, apheresis was highly effective in reducing serum TG levels. After one or two sessions, serum TG levels were reduced by more than 60–80%. However, if and how much benefit apheresis imparted on reducing the severity of AP is difficult to determine. Most reports considered improvement of clinical symptoms (e.g. abdominal pain, etc.) and laboratory tests (e.g. TG levels) as a measure of efficacy without providing adequate information to evaluate the impact of apheresis on severity parameters (e.g. organ failure, local complications). If mortality is used to assess efficacy, among patients in whom the presence of severe AP or organ failure could be determined, it ranged from 11.8 to 19.2%. However, interpretation of this data in the absence of a control group (i.e. HTG-related AP patients with similar characteristics who did not receive apheresis) makes it difficult to know if mortality in these patients would have been different if apheresis was not performed.
The severity of AP is generally linked to the magnitude of the inflammatory response, with risk of prolonged stay and poor outcomes linked first to systemic inflammation, measured as the systemic inflammatory response syndrome (SIRS), and then persistent SIRS, which leads to multiorgan dysfunction, with associated morbidity and mortality [19]. A variety of severity scores document the state of systemic dysfunction and development of the severe endpoints [20]. Other than volume resuscitation and aggressive management of organ failure, no specific therapies are currently available to reduce the severity of AP. As mentioned previously, recent studies have linked the generation of unsaturated fatty acids in AP from visceral fat as important drivers of local and systemic complications [3,4]. Availability of excess TG in the serum of patients with HTG provides additional substrate for production of unsaturated fatty acids by the action of pancreatic lipase released during an episode of AP, potentially increasing the risk of severe AP. Therefore, removal of excess TG by apheresis is a biologically plausible and attractive option to reduce the severity of HTG-related AP.
Three questions need consideration. First, which patients with HTG-related AP are most likely to benefit from apheresis. In our opinion (we follow this in our practice), the best candidates would be patients with predicted severe or severe AP based on the Revised Atlanta Classification [21] with severe (≥1000 mg/dl) or very severe (≥2000 mg/dl) HTG who continue to have elevated serum TG after appropriate resuscitation with intravenous fluids and support for organ failure [21]. The timing for initiation of apheresis need to be individualized based on resuscitation achieved and clinical status but within 24 h up to 96 h after the onset of symptoms may represent the best window of opportunity. Intravenous infusion of insulin can be a useful adjunctive therapy to lower serum TG levels especially in patients with associated diabetes, and may eliminate the need for apheresis in borderline situations. This time frame represents the period when the effect of optimized conservative measure (fluid resuscitation and adjunct therapies) can be evaluated and the need for further intervention can be determined [21].
Second, what types of studies are needed to provide empiric data on the role of apheresis in HTG-related AP. The best study design would be randomized controlled trials in patients with suspected severe or severe AP with clearly defined outcome measures. Well-designed observational studies (prospective better than retrospective) using appropriately matched control group may be an alternative approach. A limitation of the latter approach would be selection bias as apheresis may be offered to patients with more severe disease.
Third, what outcome measures should be used to determine efficacy. These should include one or more of the following - mortality, infectious complications (e.g. infected necrosis, bacteremia), presence/duration/improvement in organ failure, SIRS, need for interventions for local complications, length of hospitalization and readmissions. Studies should also document TG and lipase levels at admission, note any pre-existing dyslipidemias and secondary risk factors.
Fluid replacement used in most reports was plasma. Its usefulness over albumin is believed to be due to repletion of α1 anti-protease and α2 macroglobulin [22] to bind and quench free proteases; however, rigorous studies have not been performed and recent studies challenge this convention [23]. We were unable to sufficiently evaluate differences in outcomes based on the type of fluid or anticoagulant used since the majority of reports with both fluid (or anticoagulant) and mortality data were case series with cumulative outcomes making individual patient statistical calculations limited and prone to Type I error. One recent series suggested benefit of using citrate over heparin as the anticoagulant during apheresis [17], possibly due to hemorrhagic side effects of heparin, which needs to be confirmed in other studies.
In conclusion, apheresis is highly efficacious in rapidly reducing serum TG levels. Due to uncontrolled nature of data, reporting bias and lack of comparison group, it is difficult to draw definitive conclusions on the efficacy of apheresis in reducing the severity of AP based on published literature. We propose patients with HTG-related AP who may be best suitable for apheresis, type of studies needed and outcome measures that should be included in these studies to provide empiric data on the role of apheresis in HTG-related AP.
Supplementary Material
Acknowledgments
Presented in part at the Digestive Diseases Week 2014 and published in abstract form in Gastroenterology 2014; 146(5):Suppl 1, Page S619. The authors report no conflicts relevant to this manuscript. Benjamin Click reports support from NIH T32 training grant 5T32DK063922–12.
Abbreviations:
- AP
acute pancreatitis
- APACHE
acute physiology and chronic health evaluation
- BMI
body mass index
- CT
computed tomography
- CTSI
computed tomography severity index
- DM
diabetes mellitus
- HTG
hypertriglyceridemia
- ICU
intensive care unit
- LDL
low density lipoprotein
- OF
organ failure
- SIRS
systemic inflammatory response syndrome
- TG
triglyceride
Footnotes
Appendix A.: Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.pan.2015.02.010.
References
- [1].Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol 2014;48:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology 2013;144:1272–81. [DOI] [PubMed] [Google Scholar]
- [3].Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med 2011;3 107ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Noel P, Patel K, Durgampudi C, Trivedi RN, de Oliveira C, Crowell MD, et al. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut 2014. 10.1136/gutjnl-2014-308043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hong S, Qiwen B, Ying J, Wei A, Chaoyang T. Body mass index and the risk and prognosis of acute pancreatitis: a meta-analysis. Eur J Gastroenterol Hepatol 2011;23:1136e43. [DOI] [PubMed] [Google Scholar]
- [6].Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and bmi to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Demerath EW, Reed D, Choh AC, Soloway L, Lee M, Czerwinski SA, et al. Rapid postnatal weight gain and visceral adiposity in adulthood: the fels longitudinal study. Obesity (Silver Spring) 2009;17:2060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sadr-Azodi O, Orsini N, Andren-Sandberg A, Wolk A. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol 2013;108:133–9. [DOI] [PubMed] [Google Scholar]
- [9].Winters JL. American society for apheresis guidelines on the use of apheresis in clinical practice: practical, concise, evidence-based recommendations for the apheresis practitioner. J Clin Apher 2014;29:191–3. [DOI] [PubMed] [Google Scholar]
- [10].Fredrickson DS. LR: a system for phenotyping hyperlipoproteinemia. Circulation 1965:321–7. [DOI] [PubMed] [Google Scholar]
- [11].Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974;139:69–81. [PubMed] [Google Scholar]
- [12].Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache ii: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [13].Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of ct in establishing prognosis. Radiology 1990;174:331–6. [DOI] [PubMed] [Google Scholar]
- [14].Ogawa M, Hirota M, Hayakawa T, Matsuno S, Watanabe S, Atomi Y, et al. Development and use of a new staging system for severe acute pancreatitis based on a nationwide survey in japan. Pancreas 2002;25:325–30. [DOI] [PubMed] [Google Scholar]
- [15].Blamey SL, Imrie CW, O’Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut 1984;25:1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gubensek J, Buturovic-Ponikvar J, Marn-Pernat A, Kovac J, Knap B, Premru V, et al. Treatment of hyperlipidemic acute pancreatitis with plasma exchange: a single-center experience. Ther Apher Dial 2009;13:314–7. [DOI] [PubMed] [Google Scholar]
- [17].Gubensek J, Buturovic-Ponikvar J, Romozi K, Ponikvar R. Factors affecting outcome in acute hypertriglyceridemic pancreatitis treated with plasma exchange: an observational cohort study. PLoS One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Acevedo-Piedra NG, Moya-Hoyo N, Rey-Riveiro M, Gil S, Sempere L, Martinez J, et al. Validation of the determinant-based classification and revision of the atlanta classification systems for acute pancreatitis. Clin Gastroenterol Hepatol 2014;12:311–6. [DOI] [PubMed] [Google Scholar]
- [19].Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg 2002;89:298–302. [DOI] [PubMed] [Google Scholar]
- [20].Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 2012;142:1476–82. quiz e1415–1476. [DOI] [PubMed] [Google Scholar]
- [21].Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitise2012: revision of the atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- [22].Leese T, Holliday M, Watkins M, Thomas WM, Neoptolemos JP, Hall C, et al. A multicentre controlled clinical trial of high-volume fresh frozen plasma therapy in prognostically severe acute pancreatitis. Ann R Coll Surg Engl 1991;73(4):207–14. [PMC free article] [PubMed] [Google Scholar]
- [23].Antonič M, Gubenšek J, Buturović-Ponikvar J, Ponikvar R. Comparison of citrate anticoagulation during plasma exchange with different replacement solutions. Ther Apher Dial 2009;13:322–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.