Abstract
Background:
Bulimia nervosa (BN) is associated with functional abnormalities in frontostriatal and frontolimbic circuits. Although structural alterations in the frontal portions of these circuits have been observed, this is the first study of subcortical surface morphometry and the largest study of subcortical volume in BN.
Methods:
Anatomical MRI scans were acquired from 62 females with full and subthreshold BN (18.7±4.0 years) and 65 group-matched healthy controls (19.3±5.7 years). General linear models were used to compare groups and assess the significance of group-by-age interactions on the shape and total volume of 15 subcortical structures (p < 0.05, FWE-corrected). Associations with illness severity and duration were assessed in the BN group.
Results:
Subcortical volumes did not differ across groups, but vertex-wise analyses revealed inward shape deformations on the anterior surface of the pallidum in BN relative to control participants that were associated with binge eating frequency and illness duration. Inward deformations on the ventrolateral thalamus and dorsal amygdala were more pronounced with advancing age in the BN group, and inward deformations on the caudate, putamen, and amygdala were associated with self-induced vomiting frequency.
Conclusions:
Our findings point to localized deformations on the surface of subcortical structures in areas that comprise both reward and cognitive control circuits. These deformations were more pronounced among older BN participants and among those with the most severe symptoms. Such precise localization of alterations in subcortical morphometry may ultimately aid in efforts to identify markers of risk and BN persistence.
Keywords: bulimia nervosa, structural MRI, subcortical shape, vertex analysis, basal ganglia, amygdala
Introduction
Bulimia nervosa (BN) affects 1–3% of women (1–3) and is associated with significant medical complications, high rates of comorbid psychopathology, and considerable psychosocial impairment (1, 4). Only 30–50% of individuals with BN respond to first-line treatments (5, 6). Therefore, improved identification and characterization of structural and functional brain abnormalities that could maintain recurrent binge eating, defined by a sense of “loss of control over eating” (7), and compensatory behaviors (e.g., self-induced vomiting, (1)) and serve as direct targets for novel treatments are vital.
Little is known about structural alterations that may contribute to the development or persistence of BN, and it is not clear what alterations pre-date or result from BN symptoms (8). To date, the relatively few anatomical MRI studies of BN have focused on cortical surface morphology and white and gray matter volume. Adults and adolescents with BN show reduced volume and thickness in frontoparietal and temporal cortices relative to controls and in association with behavioral and cognitive symptoms of BN (9–12). Volume reductions on the surface of inferior frontal cortices are more pronounced with advancing age and associated with greater illness severity and greater deficits in cognitive control processes (9). In contrast, adult women with BN show increased volumes of ventral anterior insula and medial orbitofrontal cortex, the latter of which has been associated with sucrose pleasantness ratings (13, 14). Together, these findings point to structural abnormalities in cortical regions involved in both control and reward processes in BN.
Findings focused on subcortical structures are mixed, with voxel-based-morphometric (VBM) approaches showing increased gray matter density in ventral striatum (13), and traditional volumetric (i.e., total volume) approaches showing normal (15) nucleus accumbens volumes in BN compared to control participants. Other VBM and traditional volumetric findings suggest decreased (14–16) or normal (17) gray matter density and volumes of dorsal striatum, specifically caudate nucleus. These disparate results may be related to small sample sizes, different analytic tools and volumetric measurements, or the inherent limitations of voxel-based morphometry (VBM). Although VBM can detect more localized alterations than can total volume analyses, VBM results reflect a mix of variations in size, shape and/or position of brain structures (18). In addition, VBM findings depend on smoothing extents and accurate tissue-type classification (19).
Shape (i.e., vertex) analysis has been used to overcome these limitations in studies of subcortical morphology in healthy individuals (20) and in psychiatric and neurological disorders (e.g., 21, 22–24). This approach does not depend on tissue-type classification and includes no additional smoothing. Therefore, compared to VBM, vertex analysis permits comparison of subcortical morphology across groups of individuals with much greater anatomical precision and sensitivity to detect subtle regional differences (e.g., inward or outward deformations). To date, vertex analysis has only been used in the eating disorders field to characterize thalamic shape abnormalities associated with anorexia nervosa (25).
In the current study, we used high-resolution anatomical MRI and a validated, model-based segmentation toolbox in FSL (FIRST; 19) to examine the volume and shape of subcortical structures in a large sample of adolescent and adult females with BN compared with group-matched healthy controls. Consistent with some prior findings of reduced gray matter density and total volumes (14–16), we hypothesized that BN would be associated with localized inward deformations, or “inversions,” on the surface of caudate and putamen, along with reduced total caudate and putamen volume. As this is the first application of shape analysis in BN, we also examined whether BN was associated with localized morphometric alterations in other subcortical structures (nucleus accumbens, pallidum, thalamus, hippocampus, amygdala, and brain stem). Exploratory analyses tested whether alterations would be more pronounced in older individuals and associated with more chronic BN (i.e., longer durations of illness) and more severe acute bulimic symptoms (i.e., more frequent current bulimic symptoms). The subcortical structures previously implicated in BN by functional magnetic resonance imaging (fMRI) and volumetric studies consist of subregions with distinct projections that comprise distinct cortico-basal ganglia and corticolimbic circuits that support specific functions (26). Thus, more accurate and precise localization of subcortical alterations that are associated with BN and scale with age and bulimic symptom measures is an important next step to inform future longitudinal research. Such focal alterations may be more sensitive biomarkers for the disorder.
Method
Participants and Study Design
Structural MRI scans were acquired from healthy control (HC) females and adolescent and adult females with BN or a BN-spectrum Otherwise Specified Eating Disorder (BN with subthreshold frequency or duration of symptoms). Participants were recruited via flyers posted in the surrounding community, online advertisements, and through the Eating Disorders Clinic at the New York State Psychiatric Institute (NYSPI), where BN participants could receive no-cost treatment. Participants in the BN group were enrolled in the study if they engaged in an average of at least one loss-of control eating episode (regardless of episode size) or one compensatory behavior episode per week within the past 3 months. BN symptom severity in the past 3 months was assessed using an abbreviated version of the Eating Disorders Examination (27).
Individuals with any history of neurological illness, seizures, head trauma with loss of consciousness, mental retardation, or pervasive developmental disorder were excluded. HC participants had no lifetime history of Axis I disorders, and BN participants did not meet criteria for any comorbid Axis I disorders other than major depression and anxiety disorders. BN diagnoses and comorbidities were established (28–31) and full-scale IQs were estimated (32) using standard measures (see Supplement).
All participants over 18 years of age provided written, informed consent. Legal guardians of adolescents under 18 years provided written informed consent and adolescents provided written assent to participate. The New York State Psychiatric Institute’s Institutional Review Board approved this study.
Image Acquisition
Imaging was performed on a 3T GE Signa whole body scanner (Milwaukee, WI) using an eight-channel coil. High-resolution, T1-weighted images were acquired with a fast-spoiled gradient echo (FSPGR) three-dimensional pulse sequence using the following parameters: inversion time = 500 ms, echo time = 1.3 ms, repetition time = 4.7 ms, 1 excitation, matrix size = 256 × 256, FOV = 25 cm, flip angle = 11°, number of slices = 164, slice thickness = 1 mm, voxel dimensions = .976 × .976 × 1.0 mm.
Subcortical Shape Analyses
Image preprocessing, including intensity correction and skull stripping, was performed using the DARTEL algorithm from the VBM8 toolbox for SPM (http://www.neuro.unijena.de/vbm/download). A Bayesian model-based segmentation toolbox in FSL (FIRST; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST; 19) was used to segment each anatomical image and create vertex meshes for 15 subcortical structures (brain stem and bilateral nucleus accumbens, putamen, caudate, palladium, thalamus, hippocampus, and amygdala).
Quality control of the subcortical segmentations was performed by an experienced image analyst (Z.W.) following FSL FIRST guidelines (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST/UserGuide) and included the following procedures: 1) Each raw T1-weighted structural image was visually inspected for distortion or tissue loss; 2) Output of the DARTEL algorithm, which performed skull stripping, removed bias and noise, and corrected the global intensity of each raw T1 image, was visually inspected for artifacts or distortion; 3) For each subject, FSL FIRST output was overlaid onto the corresponding subject-specific skull-stripped T1 image and visually inspected to ensure correct subcortical structural segmentation. No participants had poor structural segmentation.
Vertex analysis was conducted using standard FIRST procedures (19) via the first_utils command. Briefly, for each participant and subcortical structure, vertex indices were calculated based on the signed perpendicular distance from the surface mesh of the corresponding structure in the MNI template. Positive indices represented outward deformations on the surface of a given structure and negative indices represented inward deformations or inversions. These values were then submitted to statistical analysis. Additionally, we computed the volume of each subcortical structure by generating a mask file in 3D volume space for each structure and multiplying the number of voxels in the mask by voxel size (mm).
Statistical Analyses
Group differences in subcortical shape were assessed with general linear model (GLM)-based group comparisons in FSL randomise that included age as a covariate. The significance of group-by-age interactions was assessed to determine the differential effect of age on subcortical shape in the BN and control groups. We report results corrected for multiple comparisons using threshold-free cluster enhancement with a family-wise error (FWE) rate of p < 0.05 (two-tailed) by running 5000 random permutations. Group differences in subcortical volumes were assessed via GLMs including age as a covariate, and group-by-age interactions tested the differential effects of age on subcortical volume across groups. These volumetric analyses were Bonferroni-corrected for multiple comparisons (two-tailed alpha = 0.003).
Given the wide range of current illness severity and duration in our sample, exploratory analyses within the BN group using FSL randomise examined associations of the shape of each subcortical structure with acute illness severity, as measured by bulimic symptoms in the past 28 days (33–41), and months with BN (p < 0.05, FWE corrected). Clinical variables were log-transformed because of significant skewness and included: (1) objectively large binge eating episodes (“objective bulimic episodes”), 2) self-induced vomiting episodes, and 3) illness duration (months).
Sensitivity analyses, presented in detail in the Supplement, assessed potential confounding effects of subthreshold BN (n = 11), comorbid major depressive disorder (MDD; n = 19), comorbid anxiety disorders (n = 8), a lifetime history of anorexia nervosa (AN; n = 11), psychotropic medications (n = 16), and treatment seeking (n = 39).
Results
Participants
Scans were acquired from 65 HC participants and 62 BN participants. Eleven of the 62 BN participants endorsed loss-of-control-eating frequency that was subthreshold for DSM-5 BN diagnosis (42). The BN group included 17 inpatients and 22 outpatients who completed study procedures up to 1 month before admission or before starting outpatient treatment; the remaining 23 were not seeking treatment in our clinic (see Supplement for further detail). Duration of illness ranged from 2–192 months. This sample included 26 HC and 29 BN participants whose data were included in prior analyses of cerebral surface morphology (9). The BN and HC groups did not differ on age, body mass index, full-scale IQ or race/ethnicity (Table 1).
Table 1.
Demographic, Clinical, and Neuropsychological Characteristics of Participants
| Characteristic | BN |
HC |
T or X2 | p | ||||
|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | |||
| Age (years) | 62 | 18.8 | 4.0 | 65 | 19.3 | 5.7 | −0.63 | 0.53 |
| BMI (adults)a | 37 | 22.4 | 2.5 | 35 | 22.6 | 2.8 | −0.34 | 0.74 |
| zBMI (adolescents) | 25 | 0.4 | 0.6 | 30 | 0.1 | 0.7 | 1.48 | 0.15 |
| WASI Full-Scale IQ | 62 | 109 | 11 | 65 | 112 | 16 | −1.22 | 0.23 |
| Eating Disorder Severity | ||||||||
| Duration of illness (months) | 62 | 43.7 | 40.5 | - | ||||
| Objective bulimic episodes (past 28 days) | 62 | 24.6 | 27.8 | - | ||||
| Self-induced vomiting episodes (past 28 days)b | 62 | 44.9 | 49.8 | - | ||||
| Comorbid Symptoms | ||||||||
| BDI-II Score | 50 | 19.0 | 11.9 | 53 | 2.6 | 4.6 | 9.11 | <0.001 |
| Prior AN, # (%) | 62 | 11 (17.7) | - | |||||
| Medication, # (%) | 62 | 16 (25.8) | - | |||||
| Comorbid MDD, # (%) | 62 | 19 (30.6) | - | |||||
| Comorbid Anxiety Disorder, # (%) | 62 | 8 (12.9) | - | |||||
| Race/Ethnicity | 5.14 | 0.399 | ||||||
| White/Caucasian, # (%) | 41 (66.1) | 35 (53.8) | ||||||
| African American/Black, # (%) | 4 (6.5) | 10 (15.4) | ||||||
| Hispanic, # (%) | 7 (11.3) | 11 (16.9%) | ||||||
| Asian, # (%) | 6 (9.7) | 7 (10.8) | ||||||
| Native American/American Indian, # (%) | 1 (1.6) | 0 (0) | ||||||
| Other/Mixed, # (%) | 3 (4.8) | 2 (3.1) | ||||||
BMI calculation in youth under age 18 is adjusted for age.
Most of the BN sample (92%) endorsed self-induced vomiting, whereas only 39% endorsed driven exercise, 20% endorsed laxative misuse, and 2% endorsed diuretic misuse.
Abbreviations: HC, healthy controls; BN, bulimia nervosa; BMI, body mass index; WASI, Wechsler Abbreviated Scale of Intelligence; BDI-II, Beck Depression Inventory-II; AN, anorexia nervosa; MDD, major depressive disorder. Values are mean (SD) unless otherwise specified.
Group Differences in Subcortical Morphology
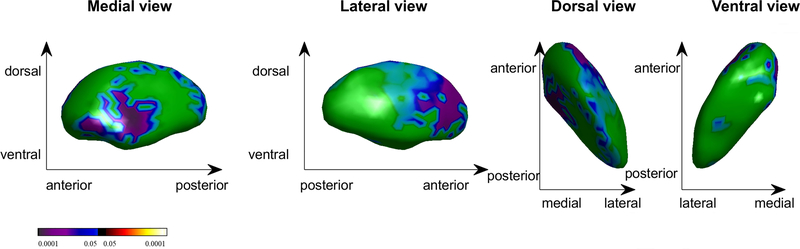
Significantly smaller vertex indices (i.e., inward deformations) were detected in the BN compared to HC group on the surface of anterior aspects of the lateral and medial right pallidum (Table 2; Figure 1; p = 0.038). Projecting this area to a 3D volume atlas (43) using a K-nearest neighbors algorithm revealed that the peak group difference and 57% of the vertices were located in the internal globus pallidus (GPi), and 43% were in the external globus pallidus (GPe). Groups did not differ in total volume of any subcortical structure (Table S1).
Table 2.
Group Differences and Interactions of Group with Age on Subcortical Shape
| Hem | Structure | Vertices | Peak MNI Coordinates |
t | p* | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Group Difference | |||||||
| R | Pallidum | 28 | 14 | −4 | −4 | −3.40 | 0.038 |
| Group x Age Interactions | |||||||
| L | Thalamus | 42 | −12 | −26 | −3 | −3.90 | 0.028 |
| R | Amygdala | 483 | 1714 | −7 | −13 | −4.05 | 0.0002 |
p < 0.05, family-wise error corrected.
Group differences were adjusted for age. Abbreviations: Hem, hemisphere; L, left; R, right; MNI, Montreal Neurological Institute
Figure 1. Group Differences in Right Pallidum Shape (p < 0.05, family-wise error corrected).
Cool colors (blues) indicate shape inversions in the bulimia nervosa compared to healthy control group. The color bar indicates t values.
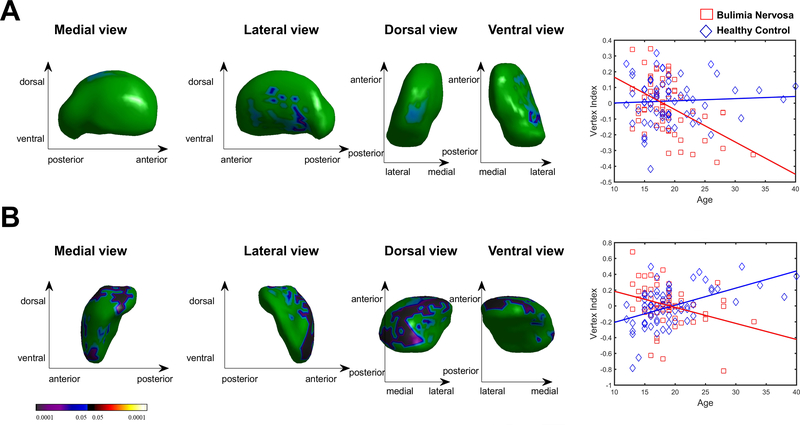
Group-by-age interactions were detected on the surface of left thalamus (p = 0.028) and right amygdala (Figure 2; p = 0.0002). Scatterplots of these interactions revealed associations of age with inward deformations on these structures in the BN group (ps < 0.05) in contrast to outward deformations on the surface of right amygdala in the HC group (p < 0.001; Figure 2B) and no association of age with thalamus shape in the HC group (p = 0.661). Similarly, a groupby-age interaction for right amygdala volume (p = 0.0009) revealed that age was associated with decreased volume in the BN group in contrast to increased volume in the HC group (Table S1).
Figure 2. Interaction of Group with Age on Subcortical Shape (p < 0.05, family-wise error corrected).
A) Shape of the left thalamus was inversely associated with age in the bulimia nervosa group (shown in red) and unassociated with age in the healthy control group (shown in blue), contributing to a group-by-age interaction. B) Shape of the right amygdala was inversely associated with age in the bulimia nervosa group and positively associated with age in the healthy control group, contributing to a group-by-age interaction. The color bar indicates t values.
Exploratory Symptom Severity Correlates within the BN Group
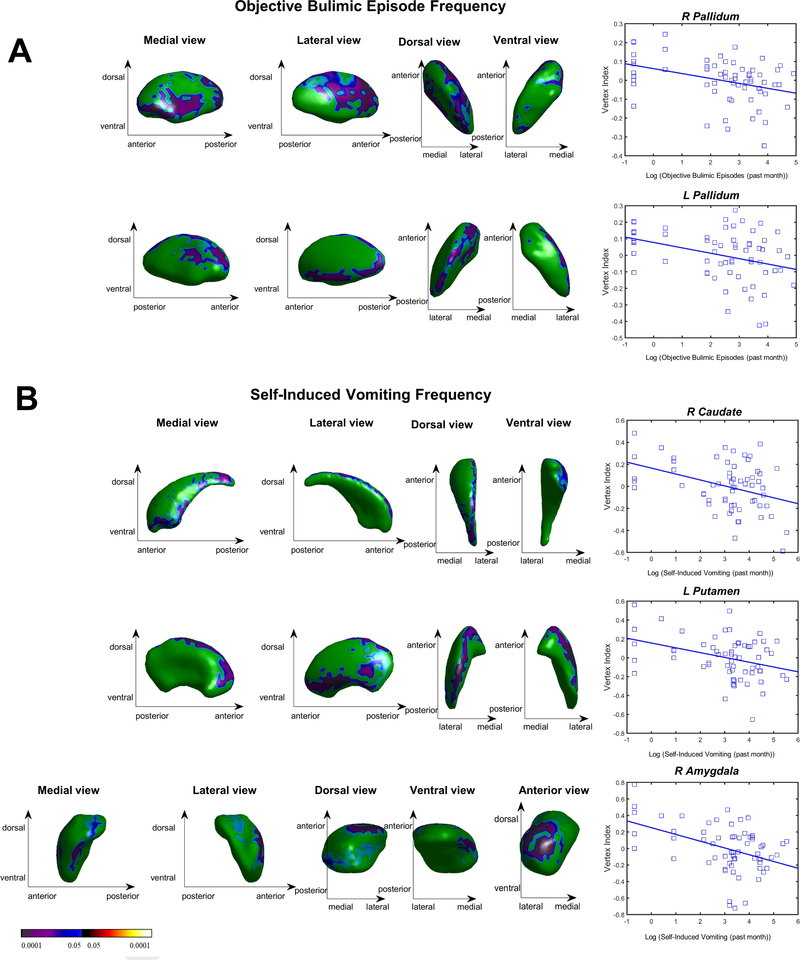
In the BN group, the frequency of objective bulimic episodes was associated with inward deformations on lateral and medial right pallidum (p = 0.013) and anterior medial and dorsal aspects of the left pallidum (p = 0.043; Table S2; Figure 3A). A total of 35.7% of the vertices of the right pallidum associated with objective bulimic episode frequency also showed inward deformations relative to HC.
Figure 3. Associations of Subcortical Shape with Binge Eating and Self-Induced Vomiting Episodes in the Bulimia Nervosa Group (p < 0.05, family-wise error corrected).
Blue colors indicate inverse associations of shape with the frequency of bulimic symptom episodes in the month prior to MRI scanning, with stronger associations in darker, more purple shades. A) Right pallidum shape, as measured by vertex index, is plotted on the y axis with binge eating (log-transformed objective bulimic episodes) plotted on the x axis. B) Right caudate, left putamen and right amygdala shape, as measured by vertex index, is plotted on the y axis with log-transformed self-induced vomiting episodes plotted on the x axis. The color bar indicates t values.
Vomiting episode frequency was associated with inward deformations on dorsolateral and ventromedial aspects of the right caudate body and tail and anterior ventromedial aspects of the right caudate head (Figure 3B; p = 0.042), predominantly lateral aspects (dorsal posterior and ventral anterior) of the left putamen (p = 0.011), and dorsal anterior aspects of the right amygdala (p = 0.008). Illness duration was associated with inward deformations on right (p = 0.005) and left (p = 0.002) pallidum, left caudate (p = 0.021), and right amygdala (p = 0.013; Table S2; Figure S1). Because age, illness duration, and objective bulimic episode frequency were themselves inter-correlated (rs = 0.42–0.74; ps ≤ 0.001), an additional exploratory model included all three variables simultaneously. None of these variables were associated with subcortical shape when controlling for the others.
Sensitivity Analyses
Excluding BN participants taking medications and those with subthreshold BN or a comorbid anxiety disorder did not appreciably impact findings of group differences in subcortical shape or interactions with age (see Supplement). After excluding those with a history of AN, group differences in right pallidum shape were no longer statistically significant (p = 0.093), but inward deformations on the surface of right nucleus accumbens emerged in the BN compared to HC group (p = 0.010). Excluding those with comorbid MDD revealed significant inward deformations on the surface of lateral, medial, and ventral aspects of left caudate head (p = 0.045). However, caudate shape was unassociated with scores on the Beck Depression Inventory-II (BDI-II; 44) in the full BN group. Excluding BN participants with comorbid MDD additionally revealed a group-by-age interaction in right pallidum, deriving from an association of age with inward deformations on the lateral surfaces of this region in the BN group (p = 0.039).
Discussion
This is the first study to examine subcortical shape abnormalities in a large sample of adolescents and adults with BN. As has been observed in studies of other disease states (21, 22, 24, 45), vertex-wise shape analyses were more sensitive than volumetric analyses, permitting detection of surface deformations in localized areas that comprise reward (46, 47) and cognitive control (48) circuits. Our findings suggest a link between morphometric alterations of basal ganglia structures and BN symptoms. Compared to group-matched controls, BN participants had significant inward deformations on the surface of the anterior right pallidum that were more pronounced among individuals with more frequent binge eating episodes and longer illness durations. Inversions on the surface of the striatum (right caudate and left putamen) were specifically associated with self-induced vomiting. In addition, our findings of group-by-age interactions on the surface of left thalamus and the surface and total volume of right amygdala may indicate altered developmental trajectories within these structures in BN (49). Inward surface deformations are thought to reflect atrophy in neurodegenerative disorders (50) and aberrant neurodevelopment (e.g., dendritic arborization) in developmental disorders (51). Although we can only speculate about the functional significance of our findings, the localization of these subcortical shape alterations is a first step toward identifying precise structural markers of the trajectory or maintenance of BN from adolescence to adulthood.
Structural and functional imaging findings from healthy individuals and primates implicate the specific anatomical locations of our findings within the pallidum, striatum, and amygdala in reward and control circuits (26, 52–54). Reward circuitry includes dopaminergic projections from midbrain to the nucleus accumbens, ventral pallidum, ventral caudate, ventral putamen, amygdala, and prefrontal cortex (46, 47). The ventral pallidum is specifically involved in “liking” palatable foods (55), the consumption of preferred foods (56, 57), eating past satiety (58), and predicting how pleasant future food consumption will be (59). Across all ages, we detected inward shape deformations in BN on anterior ventromedial aspects of the pallidum, areas that project to medial and orbitofrontal cortices involved in reward valuation (26). These deformations were associated with increased binge eating frequency in the BN group. More pronounced inward shape deformations were detected among older BN participants on subregions of the amygdala that are also involved in reward valuation, learning, and feeding regulation (60, 61). However, inward deformations on the surface of right amygdala were also associated with illness duration, suggesting that these surface abnormalities may reflect an altered developmental trajectory, a consequence of prolonged bulimic symptoms, or both.
Although we did not replicate prior findings of decreased caudate or putamen gray matter density and volume in BN (14–16), caudate shape was associated with illness duration, and caudate and putamen shape were associated with self-induced vomiting. BN participants who engaged in more frequent vomiting episodes and were ill for longer had more prominent inward shape deformations on the ventral anterior putamen, caudate head, and ventral caudate body, areas that are structurally and functionally connected to orbitofrontal cortices in limbic reward circuits (26, 53). The directionality of these associations is inconsistent with previous findings linking increased caudate gray matter volume to purging severity in BN (13); however, inward deformations in these areas have been implicated in other psychiatric disorders (62–64), and the current findings add to evidence linking ventral striatal abnormalities to BN symptoms (65, 66). Without additional behavioral and self-report measures, we can only speculate that ventral pallidum, ventral striatal, and amygdala shape alterations may relate to the reward circuit dysfunction and altered reward-based learning hypothesized to promote binge eating among individuals with BN (8, 65–70). This interpretation is consistent with findings of altered activation in ventral striatum and amygdala during the anticipation or receipt of food stimuli (8, 65, 66, 71–73) and monetary rewards (70) among BN participants relative to controls.
We detected inversions on the surface of other subcortical regions that are critical components of basal ganglia pathways that control voluntary actions. Projections from the dorsal caudate and putamen to medial and lateral aspects of the pallidum comprise the “direct” and “indirect” pathways that are thought to mediate “go” and “stop” signals via the ventral anterior and lateral thalamus (48). Mid- and anterior aspects of the pallidum project to premotor cortex and dorsolateral prefrontal cortex and are parts of the “motor” and “associative” cortico-basal ganglia circuits that control cognition and behavior (26, 54). Anterior portions of the right and left lateral pallidum (i.e., GPe) are specifically involved in action cancelation and prevention, respectively (74). Thus, inward shape deformations of the anterior GPe and age-associated deformations on the surface of the ventrolateral thalamus, which projects to motor cortex (75), could contribute to or result from altered action initiation and inhibition signaling and relate to a wide range of impulsive behaviors in BN (42, 76). Inward deformations on the surface of the GPe and on localized areas of the dorsal caudate and dorsal posterior putamen that project to lateral prefrontal and premotor cortices (26) were associated with the frequency of BN symptoms and illness duration. Of note, inward deformations on the surface of dorsal striatum are also associated with trichotillomania, a disorder characterized by poor control over compulsive behaviors (24). In BN, such striatal structural abnormalities may relate to the impaired control over eating behaviors and urges to self-induce vomiting. Consistent with this interpretation, girls and women with BN show reduced pallidum activation when engaging inhibitory control (41, 77), and binge eating frequency is associated with deficient caudate activation during the engagement of control in adults with BN (41, 78).
Results of sensitivity analyses also point to inward surface deformations of areas involved in reward and control circuits in BN. For example, inward deformations were detected on the surface of right nucleus accumbens in BN participants without a history of AN, which may relate to increased reward sensitivity in individuals with BN compared to AN (79) and the central role of the nucleus accumbens in the brain reward circuit. Excluding BN participants with comorbid MDD revealed age effects on the surface of right pallidum and inward shape deformations on the left caudate head and lateral caudate body. As BDI-II scores were unrelated to caudate shape in the full BN sample, and outward shape deformations of the caudate have not been documented in individuals with MDD (21), this result may be better explained by BN duration than depressive symptoms. Illness duration tended to be shorter in the BN participants with (compared to without) comorbid MDD (p = 0.053, d = 0.58), but these subgroups did not differ in age or in the severity of their BN symptoms (ps > 0.143). Since longer illness duration was associated with inward deformations on left caudate in the full BN sample, excluding those with MDD (and less persistent BN) may have revealed left caudate inversions relative to controls that were only apparent among those with the longest BN durations. Future transdiagnostic, longitudinal studies should assess developmental changes in caudate and accumbens structure in BN participants with and without MDD and AN to delineate their contributions to the reward and cognitive control circuit alterations characteristic of all three disorders (80–83).
This study has several limitations. First, our cross-sectional design cannot determine whether any of the subcortical shape abnormalities detected herein precede BN symptoms, precipitating their development, or instead represent sequelae of these behaviors. Relatedly, age, illness duration, and objective bulimic episode frequency were intercorrelated, and none of these variables were associated with subcortical shape when controlling for the others. Future studies of samples in which these variables are uncorrelated are required to disentangle potential effects of altered neurodevelopment from illness duration, and prospective longitudinal studies are needed to delineate causes from consequences and to distinguish state-specific from trait-like subcortical shape alterations in BN. Second, assumptions about the functional implications of structural abnormalities in certain regions of the brain are only speculative. Future studies of subcortical morphology in BN should include behavioral measures of control and reward processes to determine if the shape of the pallidum, caudate, putamen, thalamus, and amygdala is in fact associated with impairments in these functions. Third, our sample was not large enough to conclusively determine the impact of comorbidities. Given that the population comorbidity of BN is 50.1% with MDD and 80.6% with anxiety disorders, and that 25–41% of individuals with BN have a history of AN (2, 84, 85), the heterogeneity of our BN sample (30.6% with MDD, 17.7% with an anxiety disorder, 17.7% with prior AN) likely bolsters the external validity of our findings. Nevertheless, future studies should include larger samples of individuals with and without these comorbidities to better understand their impact on subcortical morphology in BN. Third, our use of an automated segmentation procedure may be viewed as a limitation to this study, but these procedures now are commonly used in studies of subcortical morphology (e.g., 21), replicable, and are less biased and time consuming than manual methods. Fourth, our results are only applicable to females with BN, thus further study is needed to examine whether inversions on the surface of the pallidum are also present in males with BN. In addition, we did not limit scanning to a specific menstrual cycle phase or time of day or systematically collect information on other factors that may impact brain structure, including acute weight change, hydration, length of treatment, hormonal contraceptive use, sleep, or among adolescents, pubertal stage (86, 87).
Despite these limitations, this is one of the largest anatomical MRI studies of BN to date and the first to employ vertex-wise subcortical shape analysis, which can reveal subtler, more precisely localized alterations than volumetric approaches. Our findings indicate that inward deformations of the basal ganglia are exaggerated in more symptomatic individuals with BN and that deformations on the thalamus and amygdala are more pronounced in older individuals with BN. Shape analysis is particularly advantageous for heterogeneous structures like the pallidum, which integrates motivational and emotional information (88) and plays a central role in processing food reward and controlling consummatory behavior (89). Our findings of inward shape deformations in BN are consistent with previously documented functional alterations in brain regions and circuits involved in control and reward processes (8, 41, 65, 66, 70–73, 77, 78, 90, 91), and may reflect synaptic loss, premorbid abnormal maturation, or both. Post-mortem tissue analyses and future longitudinal, multi-modal investigations are necessary to understand relationships among shape, volume, and functional changes in subcortical structures and their contribution to BN maintenance. Ultimately, precisely localized structural alterations may prove useful in detecting early risk for BN, predicting outcome, evaluating change over time, or evaluating mechanistic effects of interventions.
Supplementary Material
Acknowledgments
This work and preparation of this manuscript was supported in part by grants from the National Institute of Mental Health (R01MH090062 (RM), K01MH077652 (RM), and F32MH108311 (LAB)). We thank staff in the Eating Disorders Research Unit at the New York State Psychiatric Institute for facilitating recruitment and conducting assessments with participants. In addition, we thank the participants for their time.
Footnotes
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
ClinicalTrials.gov Registration: NCT00345943
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders: Fouth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association. [Google Scholar]
- 2.Hudson JI, Hiripi E, Pope HG, Kessler RC (2007): The prevalence and correlates of eating disorders in the National Comorbidity Survey replication. Biological Psychiatry 61:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keski-Rahkonen A, Hoek HW, Linna MS, Raevuori A, Sihvola E, Bulik CM, et al. (2009): Incidence and outcomes of bulimia nervosa: a nationwide population-based study. Psychological Medicine 39:823–831. [DOI] [PubMed] [Google Scholar]
- 4.Wonderlich SA, Mitchell JE (1997): Eating disorders and comorbidity: Empirical, conceptual, and clinical implications. Psychopharmacology Bulletin 33:381–390. [PubMed] [Google Scholar]
- 5.Mitchell JE, Agras S, Wonderlich S (2007): Treatment of bulimia nervosa: Where are we and where are we going? International Journal of Eating Disorders 40:95–101. [DOI] [PubMed] [Google Scholar]
- 6.Wilson GT, Grilo CM, Vitousek KM (2007): Psychological treatment of eating disorders. American Psychologist 62:199–216. [DOI] [PubMed] [Google Scholar]
- 7.Mond JM, Latner JD, Hay PH, Owen C, Rodgers B (2010): Objective and subjective bulimic episodes in the classification of bulimic-type eating disorders: Another nail in the coffin of a problematic distinction. Behaviour Research and Therapy 48:661–669. [DOI] [PubMed] [Google Scholar]
- 8.Frank GKW (2013): Altered brain reward circuits in eating disorders: chicken or egg? Current psychiatry reports 15:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh R, Stefan M, Bansal R, Hao X, Walsh BT, Peterson BS (2013): Anatomical characteristics of the cerebral surface in bulimia nervosa. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cyr M, Kopala-Sibley DC, Lee S, Chen C, Stefan M, Fontaine M, et al. (2017): Reduced Inferior and Orbital Frontal Thickness in Adolescent Bulimia Nervosa Persists Over Two-Year Follow-Up. J Am Acad Child Adolesc Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berner LA, Stefan M, Lee S, Wang Z, Terranova K, Attia E, et al. (2018): Altered cortical thickness and attentional deficits in adolescent girls and women with bulimia nervosa. J Psychiatry Neurosci 43:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westwater ML, Seidlitz J, Diederen KMJ, Fischer S, Thompson JC (2018): Associations between cortical thickness, structural connectivity and severity of dimensional bulimia nervosa symptomatology. Psychiatry Res Neuroimaging 271:118–125. [DOI] [PubMed] [Google Scholar]
- 13.Schafer A, Vaitl D, Schienle A (2010): Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. NeuroImage 50:639–643. [DOI] [PubMed] [Google Scholar]
- 14.Frank GK, Shott ME, Hagman JO, Mittal VA (2013): Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. American Journal of Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutinho J, Ramos AF, Maia L, Castro L, Conceição E, Geliebter A, et al. (2014): Volumetric alterations in the nucleus accumbens and caudate nucleus in bulimia nervosa: A structural magnetic resonance imaging study. International Journal of Eating Disorders.n/a-n/a [DOI] [PubMed] [Google Scholar]
- 16.Amianto F, Caroppo P, D’Agata F, Spalatro A, Lavagnino L, Caglio M, et al. (2013): Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a voxel-based morphometry study. Psychiatry Research: Neuroimaging 213:210–216. [DOI] [PubMed] [Google Scholar]
- 17.Joos A, Kloppel S, Hartmann A, Glauche V, Tuscher O, Perlov E, et al. (2010): Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res 182:146–151. [DOI] [PubMed] [Google Scholar]
- 18.Zatorre RJ, Fields RD, Johansen-Berg H (2012): Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgaleta M, Sanjuán A, Ventura-Campos N, Sebastian-Galles N, Ávila C (2016): Bilingualism at the core of the brain. Structural differences between bilinguals and monolinguals revealed by subcortical shape analysis. NeuroImage 125:437–445. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Liang H, Han D, Mo Y, Li Z, Cheng Y, et al. (2016): The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. NeuroImage: Clinical 11:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemmi F, Sabatini U, Rascol O, Péran P (2015): Parkinson’s disease and local atrophy in subcortical nuclei: insight from shape analysis. Neurobiol Aging 36:424–433. [DOI] [PubMed] [Google Scholar]
- 23.Mancke F, Herpertz SC, Hirjak D, Knies R, Bertsch K (2018): Amygdala structure and aggressiveness in borderline personality disorder. Eur Arch Psychiatry Clin Neurosci 268:417–427. [DOI] [PubMed] [Google Scholar]
- 24.Isobe M, Redden SA, Keuthen NJ, Stein DJ, Lochner C, Grant JE, et al. (2018): Striatal abnormalities in trichotillomania: a multi-site MRI analysis. Neuroimage Clin 17:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biezonski D, Cha J, Steinglass J, Posner J (2016): Evidence for Thalamocortical Circuit Abnormalities and Associated Cognitive Dysfunctions in Underweight Individuals with Anorexia Nervosa. Neuropsychopharmacology 41:1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, et al. (2008): Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 28:7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper Z, Fairburn CG (1987): The Eating Disorder Examination: a semistructured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord 6:1–8. [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). . New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- 30.Fairburn CG, Cooper Z, O’Connor M (2008): Eating Disorder Examination (Edition 16.0D). In: Fairburn CG, editor. Cognitive Behavior Therapy and Eating Disorders New York: Guilford Press. [Google Scholar]
- 31.Fairburn CG, Cooper Z (1993): The Eating Disorder Examination (twelfth edition). In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. New York: Guilford Press, pp 317–360. [Google Scholar]
- 32.Wechsler D (1999): Wechsler Abbreviated Scale of Intelligence Manual. San Antonio: The Psychological Corporation. [Google Scholar]
- 33.Marsh R, Stefan M, Bansal R, Hao X, Walsh BT, Peterson BS (2015): Anatomical Characteristics of the Cerebral Surface in Bulimia Nervosa. Biological Psychiatry 77:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cyr M, Kopala-Sibley DC, Lee S, Chen C, Stefan M, Fontaine M, et al. (2017): Reduced Inferior and Orbital Frontal Thickness in Adolescent Bulimia Nervosa Persists Over Two-Year Follow-Up. J Am Acad Child Adolesc Psychiatry 56:866–874.e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domakonda MJ, He X, Lee S, Cyr M, Marsh R (in press): Increased Functional Connectivity Between Ventral Attention and Default Mode Networks in Adolescents with Bulimia Nervosa. Journal of the American Academy of Child & Adolescent Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cyr M, Fontaine M, Stefan M, Terranova K, Kopala-Sibley DC, Attia E, et al. (2018): A longitudinal functional magnetic resonance imaging study of task control circuits and bulimic symptoms over adolescence. Journal of Child Psychology and Psychiatry 59:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreyfuss MFW, Riegel ML, Pedersen GA, Cohen AO, Silverman MR, Dyke JP, et al. (2017): Patients with bulimia nervosa do not show typical neurodevelopment of cognitive control under emotional influences. Psychiatry Research: Neuroimaging 266:59–65. [DOI] [PubMed] [Google Scholar]
- 38.Wonderlich JA, Breithaupt L, Thompson JC, Crosby RD, Engel SG, Fischer S (2018): The impact of neural responses to food cues following stress on trajectories of negative and positive affect and binge eating in daily life. Journal of Psychiatric Research 102:14–22. [DOI] [PubMed] [Google Scholar]
- 39.Wonderlich JA, Breithaupt LE, Crosby RD, Thompson JC, Engel SG, Fischer S (2017): The relation between craving and binge eating: Integrating neuroimaging and ecological momentary assessment. Appetite 117:294–302. [DOI] [PubMed] [Google Scholar]
- 40.Fischer S, Breithaupt L, Wonderlich J, Westwater ML, Crosby RD, Engel SG, et al. (2017): Impact of the neural correlates of stress and cue reactivity on stress related binge eating in the natural environment. Journal of Psychiatric Research 92:15–23. [DOI] [PubMed] [Google Scholar]
- 41.Marsh R, Steinglass JE, Gerber AJ, O’Leary KG, Walsh BT, Peterson BS (2009): Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Archives of General Psychiatry 66:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition (DSM-5). Washington. D.C.: American Psychiatric Association. [Google Scholar]
- 43.Pauli WM, Nili AN, Tyszka JM (2018): A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Scientific Data 5:180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck A, Steer R, Brown G (1996): Beck Depression Inventory—Second Edition. Manual The Psychological Corporation, San Antonio, TX. [Google Scholar]
- 45.Mamah D, Alpert KI, Barch DM, Csernansky JG, Wang L (2016): Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. NeuroImage: Clinical 11:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beier Kevin T, Steinberg Elizabeth E, DeLoach Katherine E, Xie S, Miyamichi K, Schwarz L, et al. (2015): Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 162:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith Y, Kieval JZ (2000): Anatomy of the dopamine system in the basal ganglia. Trends Neurosci 23, Supplement 1:S28–S33. [DOI] [PubMed] [Google Scholar]
- 48.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014): Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17:1022. [DOI] [PubMed] [Google Scholar]
- 49.Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ, et al. (2012): Regional changes in thalamic shape and volume with increasing age. NeuroImage 63:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Younes L, Ratnanather JT, Brown T, Aylward E, Nopoulos P, Johnson H, et al. (2014): Regionally selective atrophy of subcortical structures in prodromal HD as revealed by statistical shape analysis. Hum Brain Mapp 35:792–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu A, Adler M, Crocetti D, Miller MI, Mostofsky SH (2010): Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 49:539–551, 551.e531–534. [DOI] [PubMed] [Google Scholar]
- 52.Haber SN (2016): Corticostriatal Circuitry In: Pfaff DW, Volkow ND, editors. Neuroscience in the 21st Century. New York, NY: Springer New York, pp 1–21. [Google Scholar]
- 53.Choi EY, Yeo BT, Buckner RL (2012): The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saga Y, Hoshi E, Tremblay L (2017): Roles of Multiple Globus Pallidus Territories of Monkeys and Humans in Motivation, Cognition and Action: An Anatomical, Physiological and Pathophysiological Review. Frontiers in Neuroanatomy 11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berridge K, Ho C, Richard J, Deifeliceantonio A (2010): The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res 1350:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Covelo IR, Patel ZI, Luviano JA, Stratford TR, Wirtshafter D (2014): Manipulation of GABA in the ventral pallidum, but not the nucleus accumbens, induces intense, preferential, fat consumption in rats. Behav Brain Res 270:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson PI, Stellar JR, Paul AD (1993): Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology 32:1305–1314. [DOI] [PubMed] [Google Scholar]
- 58.Stratford TR, Kelley AE, Simansky KJ (1999): Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res 825:199–203. [DOI] [PubMed] [Google Scholar]
- 59.Simmons WK, Rapuano KM, Ingeholm JE, Avery J, Kallman S, Hall KD, et al. (2014): The ventral pallidum and orbitofrontal cortex support food pleasantness inferences. Brain structure & function 219:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baxter MG, Murray EA (2002): The amygdala and reward. Nat Rev Neurosci 3:563–573. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Raine A, Narr KL, Colletti P, Toga AW (2009): Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry 66:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y, Liang H, Han D, Mo Y, Li Z, Cheng Y, et al. (2016): The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. Neuroimage Clin 11:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakravarty MM, Rapoport JL, Giedd JN, Raznahan A, Shaw P, Collins DL, et al. (2015): Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: A longitudinal study. Human Brain Mapping 36:1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garza-Villarreal EA, Chakravarty MM, Hansen B, Eskildsen SF, Devenyi GA, Castillo-Padilla D, et al. (2017): The effect of crack cocaine addiction and age on the microstructure and morphology of the human striatum and thalamus using shape analysis and fast diffusion kurtosis imaging. Translational Psychiatry 7:e1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohon C, Stice E (2011): Reward abnormalities among women with full and subthreshold bulimia nervosa: A functional magnetic resonance imaging study. International Journal of Eating Disorders 44:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frank GKW, Reynolds JR, Shott ME, O’Reilly RC (2011): Altered temporal difference learning in bulimia nervosa. Biological Psychiatry 70:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broft AI, Berner LA, Martinez D, Walsh BT (2011): Bulimia nervosa and evidence for striatal dopamine dysregulation: A conceptual review. Physiol Behav 104:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mueller SV, Mihov Y, Federspiel A, Wiest R, Hasler G (2017): Neural response to catecholamine depletion in remitted bulimia nervosa: Relation to depression and relapse. Eur Neuropsychopharmacol 27:633–646. [DOI] [PubMed] [Google Scholar]
- 69.Frank GKW (2016): The Perfect Storm - A Bio-Psycho-Social Risk Model for Developing and Maintaining Eating Disorders. Front Behav Neurosci 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cyr M, Wang Z, Tau GZ, Zhao G, Friedl E, Stefan M, et al. (2016): Reward-based spatial learning in teens with bulimia nervosa. J Am Acad Child Adolesc Psychiatry 55:962–971.e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radeloff D, Willmann K, Otto L, Lindner M, Putnam K, Leeuwen SV, et al. (2012): High-fat taste challenge reveals altered striatal response in women recovered from bulimia nervosa: A pilot study. The World Journal of Biological Psychiatry 0:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon JJ, Skunde M, Walther S, Bendszus M, Herzog W, Friederich H-C (2016): Neural signature of food reward processing in bulimic-type eating disorders. Soc Cogn Affect Neurosci 11:1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ely AV, Wierenga CE, Bischoff-Grethe A, Bailer UF, Berner LA, Fudge JL, et al. (2017): Response in taste circuitry is not modulated by hunger and satiety in women remitted from bulimia nervosa. Journal of Abnormal Psychology 126:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo Y, Schmitz TW, Mur M, Ferreira CS, Anderson MC (2018): A supramodal role of the basal ganglia in memory and motor inhibition: Meta-analytic evidence. Neuropsychologia 108:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansen-Berg H, Behrens TEJ, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, et al. (2005): Functional–Anatomical Validation and Individual Variation of Diffusion Tractography-based Segmentation of the Human Thalamus. Cerebral Cortex 15:31–39. [DOI] [PubMed] [Google Scholar]
- 76.Kaltiala-Heino R, Rissanen A, Rimpela M, Rantanen P (2003): Bulimia and impulsive behaviour in middle adolescence. Psychotherapy & Psychosomatics 72:26–33. [DOI] [PubMed] [Google Scholar]
- 77.Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, et al. (2011): An fMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. American Journal of Psychiatry 168:1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skunde M, Walther S, Simon JJ, Wu M, Bendszus M, Herzog W, et al. (2016): Neural signature of behavioural inhibition in women with bulimia nervosa. Journal of psychiatry & neuroscience: JPN 41:E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harrison A, O’Brien N, Lopez C, Treasure J (2010): Sensitivity to reward and punishment in eating disorders. Psychiatry Res 177:1–11. [DOI] [PubMed] [Google Scholar]
- 80.Luking KR, Pagliaccio D, Luby JL, Barch DM (2016): Reward Processing and Risk for Depression Across Development. Trends in Cognitive Sciences 20:456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, Schachar R (2014): Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. Journal of Abnormal Psychology 123:429. [DOI] [PubMed] [Google Scholar]
- 82.O’Hara CB, Campbell IC, Schmidt U (2015): A reward-centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neurosci Biobehav Rev 52:131–152. [DOI] [PubMed] [Google Scholar]
- 83.Hirst RB, Beard CL, Colby KA, Quittner Z, Mills BM, Lavender JM (2017): Anorexia nervosa and bulimia nervosa: A meta-analysis of executive functioning. Neuroscience & Biobehavioral Reviews 83:678–690. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan PF, Bulik CM, Carter FA, Gendall KA, Joyce PR (1996): The significance of a prior history of anorexia in bulimia nervosa. Int J Eat Disord 20:253–261. [DOI] [PubMed] [Google Scholar]
- 85.Bardone-Cone AM, Maldonado CR, Crosby RD, Mitchell JE, Wonderlich SA, Joiner TE, et al. (2008): Revisiting Differences in Individuals with Bulimia Nervosa with and without a History of Anorexia Nervosa: Eating Pathology, Personality, and Maltreatment. The International journal of eating disorders 41:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frank GKW, Favaro A, Marsh R, Ehrlich S, Lawson EA (2018): Toward valid and reliable brain imaging results in eating disorders. The International journal of eating disorders 51:250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.King JA, Frank GKW, Thompson PM, Ehrlich S (2018): Structural Neuroimaging of Anorexia Nervosa: Future Directions in the Quest for Mechanisms Underlying Dynamic Alterations. Biological Psychiatry 83:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Napier TC, Mickiewicz AL (2009): The role of the ventral pallidum in psychiatric disorders. Neuropsychopharmacology 35:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Root DH, Melendez RI, Zaborszky L, Napier TC (2015): The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130:29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lock J, Garrett A, Beenhakker J, Reiss AL (2011): Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. American Journal of Psychiatry 168:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cyr M, Yang X, Horga G, Marsh R (2018): Abnormal fronto-striatal activation as a marker of threshold and subthreshold bulimia nervosa. Human Brain Mapping 39:1796–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.