SUMMARY
Subjective and objective estimates of sleep are often discordant among individuals with insomnia who typically under-report sleep time and over-report wake time at night. This study examined the impact and durability of CBT for insomnia (CBTi) on improving the accuracy of sleep and wake perceptions in older adults, and tested whether changes in sleep quality were related to changes in the accuracy of sleep/wake perceptions. 159 older veterans (97% male, mean age 72.2 years) who met diagnostic criteria for insomnia disorder were randomized to: 1) CBTi (n=106), or 2) attention control (n=53). Assessments were conducted at baseline, post-treatment, 6-month, and 12-month follow-up. Sleep measures included objective (via wrist actigraphy) and subjective (via self-report diary) total sleep time and total wake time, along with Pittsburgh Sleep Quality Index (PSQI) score. Discrepancy was computed as the difference between objective and subjective estimates of wake and sleep. Minutes of discrepancy were compared between groups across time, as were the relationships between PSQI scores and subsequent changes in discrepancy. Compared to controls, participants randomized to CBTi became more accurate (i.e., minutes discrepancy was reduced) in their perceptions of sleep/wake at post-treatment, 6-month, and 12-month follow-up (p’s<.05). Improved PSQI scores at each study assessment preceded and predicted reduced discrepancy at the next study assessment (p’s<.05). CBTi reduces sleep/wake discrepancy among older adults with insomnia. The reductions may be driven by improvements in sleep quality. Improving sleep quality appears to be a viable path to improving sleep perception and may contribute to the underlying effectiveness of CBTi.
Keywords: Cognitive-Behavioral Therapy for Insomnia, Older Adults, Sleep Perception, Sleep Discrepancy, Sleep Quality
INTRODUCTION
Sleep is an important health behavior in late-life. As individuals age, sleep becomes lighter and more fragmented, and they spend more time awake and less time asleep (Floyd et al., 2000; Morgan, 2000). Although not necessarily indicative of disordered sleep, these normative changes set the stage for an increased likelihood of clinically significant sleep disturbance (Spielman et al., 1987). In fact, older adults have a high prevalence of insomnia, with estimates as high as 65% (Ohayon, 2002). The high prevalence of insomnia in late-life is clinically relevant because insomnia, and insomnia-related symptomology, are associated with a myriad of adverse consequences (Dzierzewski et al., 2018; Foley et al., 2004; Jaussent et al., 2011; Kay and Dzierzewski, 2015; Spira et al., 2014), ranging from individual (e.g., decreased quality-of-life) to societal (e.g., increased health care consumption). Effective treatment options are needed, given the high prevalence and the adverse consequences of late-life insomnia.
Various taskforces have declared cognitive-behavioral therapy for insomnia (CBTi) as an evidence-based treatment specifically recommended for late-life insomnia (Bloom et al., 2009; Morgenthaler et al., 2006; Riemann et al., 2017; Qaseem et al., 2016). Several randomized, controlled trials that provide the evidence base supporting CBTi measure sleep both objectively and subjectively (Friedman et al., 2000; Rybarczyk et al., 2002).
An interesting phenomenon has been reported in many clinical trials of CBTi for late-life insomnia – older adults with insomnia who receive CBTi typically report improvements in their sleep (i.e., subjective improvements), while objective measures of sleep demonstrate minimal or no improvements (Buysse et al., 2011; Friedman et al., 2000; Lichstein, Riedel, Wilson, Lester, & Aguillard, 2001; Okajima, Komada, & Inoue, 2011; Rybarczyk et al., 2002). Insomnia is a disorder established by subjective report of symptoms, so subjective improvement is the key component of treatment response. However, this pattern of subjective improvement without evidence of objective change has been viewed by some as a shortcoming of CBTi. To date, little empirical work has focused on the impact of CBTi on the accuracy of older adults’ perceptions of their sleep; however, there have been calls to examine the impact of sleep treatments on subjective-objective sleep discrepancy (Bastien et al., 2014; Harvey and Tang, 2012).
Kay and colleagues examined changes in discrepancy between subjective and objective estimates of sleep onset latency and wake time during the night in older adults with insomnia over the course of eight weeks of CBTi (Kay et al., 2015). They reported that treatment resulted in significant reductions in discrepancy; however, their study did not include a control condition, making it difficult to make strong conclusions related to the CBTi intervention itself (Kay et al., 2015). Lund and colleagues examined changes in sleep discrepancy (assessed by 2 nights of PSG and corresponding sleep diary) in response to 8 weekly CBTi or control sessions (Lund et al., 2013). They found that sleep efficiency and sleep onset latency discrepancy significantly improved with CBTi compared to the control condition, suggesting that sleep quality may play an important role in sleep discrepancy, but they did not observe changes in discrepancies in total sleep or wake time during the night (Lund et al., 2013). Of note, neither study investigated if self-reported sleep quality is a mechanism for reductions in sleep discrepancy or included long-term follow-up at the conclusion of CBT-I to determine whether these changes persisted over time.
The current study aimed to continue this line of research examining the impact of CBTi on sleep discrepancy in older adults, using data from a recently-published randomized controlled trial testing a novel delivery model for CBTi (Alessi et al., 2016). The parent trial demonstrated medium-large effects of CBTi for late-life insomnia when delivered by non-clinician sleep coaches, with immediate and lasting effects on self-reported sleep onset latency, wake after sleep onset, total wake time, and sleep efficiency, but no significant improvements in objective sleep metrics (assessed with wrist actigraphy).
In the current analysis, we conceptualize “sleep discrepancy” as the mismatch between an individual’s perception of their sleep and objectively measured sleep. We conceptualize “wake discrepancy” as the mismatch between subjective and objective wake time estimates. This study had two main aims. The first aim was to investigate the impact of CBTi on sleep and wake discrepancies in older adults with insomnia. Secondly, we investigated whether changes in sleep quality were related to observed changes in sleep/wake discrepancy following CBTi. We hypothesized that: (1) CBTi would significantly reduce both sleep and wake discrepancies in older adults with insomnia, and (2) changes in sleep quality would drive subsequent observed reductions in sleep and wake discrepancies resulting from CBTi. We also explored how these changes persisted for one year after the conclusion of treatment.
METHODS
This manuscript describes secondary analyses from a randomized-controlled trial (NCT00781963). In the trial, CBTi was provided to older veterans by non-clinician sleep coaches under the supervision of a sleep psychologist (Alessi et al., 2016). The current analysis utilizes data collected at baseline, posttreatment, 6-month, and 12-month follow-up as part of the larger trial. Complete methods and main outcomes have been published elsewhere (Alessi et al., 2016). The institutional review board of the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) approved the study procedures, and all participants provided written informed consent. Methods pertinent to the current analyses are described below.
Participants
Participants were 159 older adults who met diagnostic criteria for insomnia disorder lasting at least 3 months (American Academy of Sleep medicine, 2005). Inclusion criteria for the clinical trial were: age 60 years or older, an outpatient visit at one urban VA healthcare system within the prior 18 months, fulfillment of diagnostic criteria for insomnia disorder, absence of significant sleep apnea (no current positive airway pressure treatment and an apnea-hypopnea index less than 20 based on home sleep apnea testing [WatchPAT 200, Itamar Medical, Ltd, Caesarea, Israel]), Mini-Mental State Examination [MMSE; (Folstein et al., 1975)] score of 24 or higher, no severe physical or mental health issues that would interfere with study-related activities.
Design and Measures
Eligible participants were randomized via 1:1:1 allocation to either individually-delivered CBTi, group CBTi, or a sleep education attention-control condition. Both individual and group CBTi included the following techniques: psychoeducation, sleep restriction, stimulus control, and cognitive therapy, provided by master’s level non-clinicians under the supervision of licensed clinical psychologists with behavioral sleep medicine expertise, in five sessions over six weeks. The control condition was a structurally equivalent, manual-based discussion of general sleep education occurring at equal frequency and intervals as the intervention conditions (see Alessi et al., 2016 for additional details). Assessments were conducted at baseline, posttreatment, 6-month, and 12-month follow-up by staff blinded to intervention assignment.
At each assessment timepoint, participants completed self-report daily sleep diaries for seven consecutive days/nights. Subjective metrics were computed from the sleep diaries. Perceived total time awake (TWT) was computed as the total sum of the following: time from diary bedtime to sleep onset time + time awake during the night + time from final awakening to rise time. Total sleep time (TST) was computed by subtracting TWT from time in bed (i.e., elapsed time from bedtime to rise time). Both TST and TWT were expressed in minutes and averaged across all recorded nights (one week at each assessment timepoint).
Participants also wore a wrist actigraph (Actigraph Spectrum, Philips Respironics) on their dominant arm for seven consecutive days/nights simultaneous with the sleep diary completion at each assessment time point. Research staff visually inspected the raw actigraph data to identify artifacts and then scored recordings according to published guidelines (Ancoli-Israel et al., 2015), in order to compute actigraphically measured TST (objective minutes of sleep per night) and actigraphically measured TWT (objective minutes of wake time per night). As with subjective estimates, TST and TWT were expressed in minutes and averaged across all recorded nights at each timepoint.
Discrepancy scores were computed by subtracting the objective (actigraph) measures (either TST or TWT) from the subjective (sleep diary) measures (again, either TST or TWT). Negative values indicate a subjective underestimate of the amount of time spent asleep or awake. Positive values indicate a subjective overestimate of the amount of time spent asleep or awake.
Overall sleep quality was assessed at each measurement occasion via the Pittsburgh Sleep Quality Index (Buysse et al., 1989). The PSQI was modified to assess sleep quality over the past week (rather than the prior month) so that it could be used as a post-treatment assessment measure after the brief CBTi program.
Statistical Analysis
Both the individual and group CBTi conditions displayed comparable improvements in sleep (Alessi et al., 2016). As such, data were combined for the two active treatments in all analyses. The mean sleep discrepancy was estimated separately for the control group and the treatment group (including a 95% confidence interval for each group). A one sample t-test was used to test the null hypothesis that the mean sleep discrepancy, within each group, is equal to zero (which would suggest no difference between subjective estimates and objective measurements of sleep and wake). The effect size for the comparison of the control versus treatment group was estimated using Cohen’s d, along with a 95% confidence interval. These analyses were repeated for the assessments of sleep discrepancy at post-treatment, 6-months, and 12-months follow-up. These analyses were then repeated using wake discrepancy as the outcome.
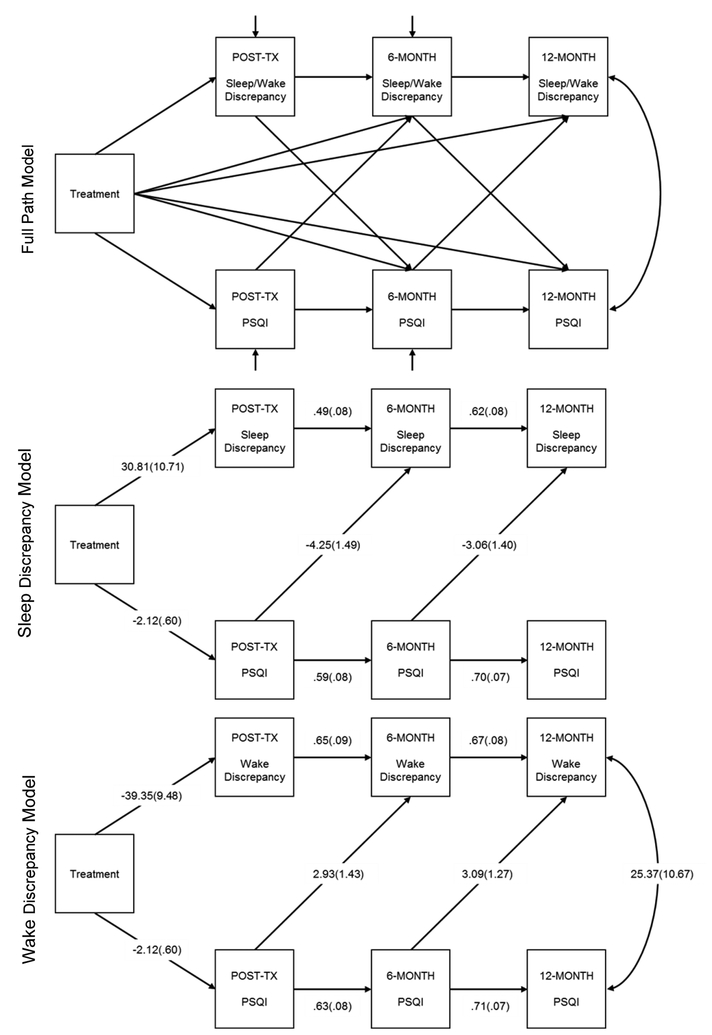
Path analysis models were used to assess the total effects, direct effects, and indirect effects of treatment group assignment on sleep and wake discrepancy at each of the three time points following treatment, the treatment group effect on sleep quality at the same three time points, and sleep quality as a mechanism for any treatment-related changes in sleep discrepancy (see top panel, Figure 3). Data preparation and t-tests were performed using Stata (StataCorp, 2013) while the path models were fit using Mplus (Muthen and Muthen, 1998). All significance tests were performed using the 5% level of significance.
Figure 3:
Full Path Diagram Modeling Sleep Quality and Sleep/Wake Discrepancy Associated with Treatment (Top Panel), along with Significant Paths from Sleep Discrepancy Model (Middle Panel) and Wake Discrepancy Model (Bottom Panel).
RESULTS
Descriptive Statistics
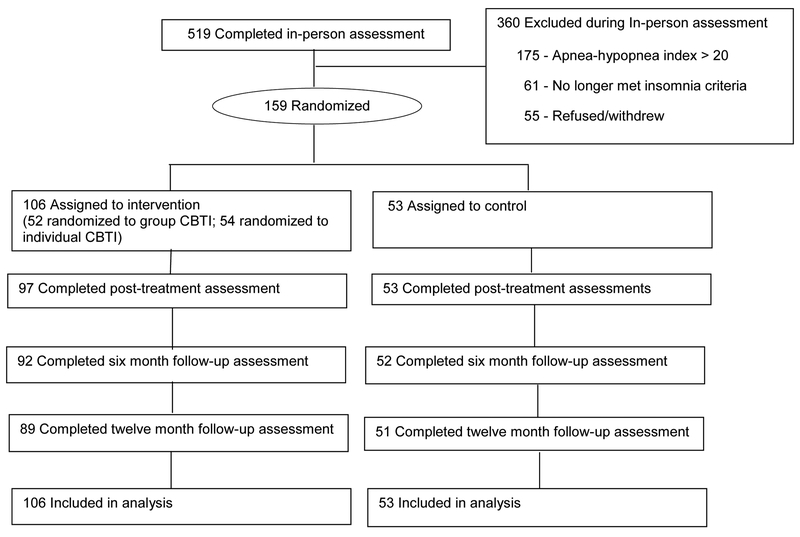
In total, 159 older adults with insomnia (mean age = 72.2, SD = 7.7) participated. One hundred six older adults with insomnia were randomized to a CBTi treatment condition (52 group and 54 individual CBTi) and 53 were randomized to the control condition. Descriptive statistics for at baseline are shown in Table 1. Participant flow is depicted in Figure 1.
Table 1.
Descriptive statistics for selected baseline characteristics of the overall sample (N=159) and for treatment (N=106) and control (N=53) groups with p-values for difference between the treatment and control groups.
| Variable | Total | Treatment | Control | |
|---|---|---|---|---|
| Age: mean, (SD) | 72.2 (7.7) | 72.1 (7.9) | 72.4 (7.3) | |
| Gender (Male) | 154 (96.9%) | 102 (96.2%) | 52 (98.1%) | |
| Race (Non-Hispanic White) | 125 (78.6%) | 83 (78.3%) | 42 (79.2%) | |
| Education | ||||
| Less than High School | 6 (3.8%) | 6 (5.7%) | 0 (0.0%) | |
| High School Graduate | 25 (15.7%) | 18 (17.0%) | 7 (13.2%) | |
| Some College | 70 (44.0%) | 44 (41.5%) | 26 (49.1%) | |
| College Graduate | 30 (18.9%) | 18 (17.0%) | 12 (22.6%) | |
| Post Baccalaureate | 28 (17.6%) | 20 (18.9%) | 8 (15.1%) | |
| Marital Status | ||||
| Married | 66 (41.5%) | 43 (40.6%) | 23 (43.4%) | |
| Living as Married | 11 (6.9%) | 8 (7.5%) | 3 (5.7%) | |
| Divorced/Separated | 48 (30.2%) | 34 (32.1%) | 14 (26.4%) | |
| Widowed | 14 (8.8%) | 10 (9.4%) | 4 (7.5%) | |
| Single/Never Married | 20 (12.6%) | 11 (10.4%) | 9 (17.0%) | |
| Employment | ||||
| Not working | 121 (76.1%) | 81 (76.4%) | 40 (75.5%) | |
| Working part time | 31 (19.5%) | 19 (17.9%) | 12 (22.6%) | |
| Working full time | 7 (4.4%) | 6 (5.7%) | 1 (1.9%) | |
| AHI | 9.4 (5.3) | 9.9 (5.4) | 8.3 (5.1) | |
| Epworth Sleepiness Scale | 5.1 (3.7) | 5.4 (4.0) | 4.6 (2.8) | |
| PHQ9 | 4.8 (4.3) | 5.1 (4.3) | 4.4 (4.3) | |
| Baseline sleep outcomes | ||||
| Total sleep time (TST), Diary | 356.2 (82.0) | 350.1 (79.0) | 368.3 (87.2) | |
| Total sleep time (TST), Actigraphy | 420.2 (67.8) | 416.7 (70.8) | 427.4 (61.4) | |
| TST discrepancy, (Diary – Actigraphy) | −64.1 (80.7) | −66.5 (78.0) | −59.1 (86.4) | |
| Total wake time (TWT), Diary | 143.2 (88.9) | 144.0 (89.5) | 141.6 (88.5) | |
| Total wake time (TWT), Actigraphy | 85.2 (39.0) | 83.0 (38.6) | 89.7 (39.7) | |
| TWT discrepancy, (Diary - Actigraphy) | 58.0 (80.3) | 61.0 (79.4) | 51.9 (82.4) | |
| PSQI | 9.1 (3.4) | 9.4 (3.5)* | 8.3 (3.2)* | |
Notes: AHI = apnea-hypopnea index; PHQ9 = Patient Health Questionnaire 9; TST = total sleep time; TWT = total wake time; PSQI = Pittsburgh Sleep Quality Index;
p < 0.05;
p < 0.01;
p < 0.001.
Figure 1:
Participant Flow in the Study. Adapted from (Alessi et al., 2016). Please refer to original source for more detailed description of participant endpoints.
Treatment Effects on Sleep Discrepancy
The upper panel of Table 2 shows the average sleep discrepancy for the control group (Column A), the treatment group (Column B), the difference in these means (Column C), the test of the significance of the difference (Column D), and the estimate of the effect size of the difference (Column E). Columns A, B, C, and E also display 95% confidence intervals around the mean values. The results from columns A, B, and C are also visualized in the left panel of Figure 2.
Table 2.
Average sleep and wake discrepancy by treatment group assignment (control vs. treatment), with the difference (control minus treatment), and standardized difference (Cohen’s d) with 95% confidence intervals and test of significance comparing treatment vs. control groups (t statistic and p-value) reported at each of four time points (baseline, 3-months, 6-months, and 12-months).
| Time | [A] Controla | [B] Treatmenta | [C] Difference | [D] t statistic and p-value | [E] Cohen’s d |
|---|---|---|---|---|---|
| Sleep Discrepancy | |||||
| Baseline | −59.1 (−81.1, −37.2)*** | −66.5 (−82.1, −51.0)*** | 7.4 (−19.5, 34.3) | t(157) = 0.55, p=0.586 | 0.09(−0.24,0.42) |
| 3-Months | −43.3 (−60.5, −26.1)*** | −12.7 (−25.4, 0.1) | −30.6 (−52.1, −9.2) | t(144) = −2.83, p=0.005 | −0.49(−0.83,−0.14) |
| 6-Months | −29.3 (−47.2, −11.5)** | −0.6 (−14.4, 13.2) | −28.7 (−51.2, −6.1) | t(129) = −2.51, p=0.013 | −0.45(−0.81,−0.09) |
| 12-Months | −26.8 (−46.0, −7.7)** | −2.2 (−17.1, 12.8) | −24.6 (−48.9, −0.4) | t(122) = −2.01, p=0.047 | −0.37(−0.74,−0.01) |
| Wake Discrepancy | |||||
| Baseline | 51.9 (30.1, 73.7)*** | 61.0 (45.6, 76.4)*** | −9.1 (−35.8, 17.6) | t(157) = −0.67, p=0.501 | −0.11(−0.44,0.22) |
| 3-Months | 32.2 (16.9, 47.4)*** | −7.3 (−18.6, 4.0) | 39.5 (20.5, 58.4) | t(144) = 4.11, p=0.000 | 0.71(0.36,1.06) |
| 6-Months | 20.9 (3.6, 38.3)* | −13.6 (−27.0, −0.1)* | 34.5 (12.6, 56.4) | t(129) = 3.11, p=0.002 | 0.56(0.20,0.92) |
| 12-Months | 25.0 (6.2, 43.7)** | −1.0 (−15.7, 13.6) | 26.0 (2.2, 49.8) | t(122) = 2.16, p=0.033 | 0.40(0.03,0.77) |
N(Baseline)=158, N(3 Months)=145, N(6 Months)=130, N(12 Months)=123.
Significance of the test that the average sleep or wake discrepancy equals 0 is indicated as:
p < 0.05,
p < 0.01,
p < 0.001.
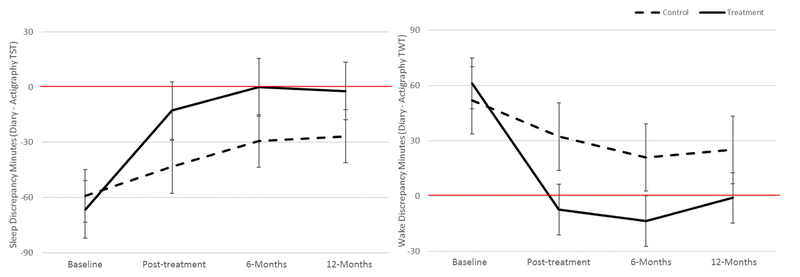
Figure 2:
Average sleep discrepancy (left panel) and wake discrepancy (right panel) with 95% confidence intervals, by treatment group (control, treatment) and time (Baseline, Post-treatment, 6-Months, and 12-Months).
Note: Sleep discrepancy values < 0 (highlighted by the solid red line) indicate an underestimation of time spent asleep. Wake discrepancy values > 0 (highlighted by the solid red line) indicate an overestimation of time spent awake.
At baseline, there was no significant difference between the treatment and control group in sleep discrepancy (p=.586). However, the difference in sleep discrepancy between the control and treatment group was significant at post-treatment (p=.005), 6 months (p=.013), and 12 months (p=.047) follow-up. The control group’s average sleep discrepancy was significantly different from zero at each of the four time points (p’s < 0.006; also see Table 2, Column A, upper panel), and was less than zero, indicating that the control group’s subjective estimate of TST was significantly less than the objective measure of TST at each measurement occasion.
The average sleep discrepancy for the treatment group at baseline was negative and significantly different from zero (p<0.001; see Table 2, Column B, upper panel). However, their sleep discrepancy was not significantly different from zero at post-treatment (p=0.053), 6-months (p=0.927), or 12-months (p=0.774). In other words, after CBTi, the treatment group became “accurate” in their perception of sleep. The left panel of Figure 2 depicts these results, illustrating mean sleep discrepancies that are significantly different from zero by confidence intervals that exclude zero and mean sleep discrepancies that are not significantly different from zero by confidence intervals that overlap zero.
Treatment Effects on Wake Discrepancy
The lower panel of Table 2 shows the average wake discrepancy for the control group (Column A), average wake discrepancy for the treatment group (Column B), difference in these means (Column C), test of the significance of the difference (Column D), and the estimated effect size of the difference (Column E). Columns A, B, C, and E also display 95% confidence intervals. The results from columns A, B, and C are also visualized in the right panel of Figure 2. As for sleep discrepancy, the findings suggest that the treatment group became “accurate” in their perceptions of wake.
Sleep Quality as a Mechanism for Reducing Sleep and Wake Discrepancies
Sleep Discrepancy
The path model depicted in Figure 3 (top panel) was fit using sleep discrepancy in the boxes labeled sleep/wake discrepancy. This model included paths from treatment to measures of sleep discrepancy at post-treatment, 6-months, and 12-months. It also includes similar paths to sleep quality at post-treatment, 6-months, and 12-months. The model posits cross lagged paths between sleep quality and sleep discrepancy (e.g., sleep quality at post-treatment to sleep discrepancy at 6-months; and from sleep discrepancy at post-treatment to sleep quality at 6-months).
A simplified version of this path diagram showing just the significant paths is shown in Figure 3 (middle panel). The path from treatment to sleep quality at post-treatment was significant (B=−2.1, SE=.6, p<0.001) and the path from treatment to sleep discrepancy at post-treatment was significant (B=30.8, SE=10.7, p=0.004). At post-treatment, the PSQI scores for the treatment group were 2.1 units lower than the control group and the sleep discrepancy scores were 30.8 minutes smaller for the treatment group vs. the control group. The paths from sleep quality to sleep discrepancy at the subsequent time were significant (post-treatment PSQI to 6-months sleep discrepancy: B= −4.3, SE=1.5, p = 0.004; 6-months PSQI to 12-months Sleep Discrepancy: B= −3.1, SE=1.4, p = 0.028). These results indicate that at 12-month follow-up, for example, a one unit increase in PSQI score was associated with a 3.1 minute decrease in the discrepancy between self-report and objectively assessed TST (see Figure 3, middle panel).
Wake Discrepancy
The path model depicted in Figure 3 (top panel) was next fit using wake discrepancy in the boxes labeled sleep/wake discrepancy. A simplified version of the path diagram showing just the significant paths is shown in Figure 3 (bottom panel). A similar pattern of results was found for wake discrepancy as those reported above for sleep discrepancy. Please refer to Figure 3 (bottom panel) for full results.
DISCUSSION
The results of the present investigation confirm previous evidence suggesting that CBTi reduces sleep discrepancy in older adults (Kay et al., 2015; Lund et al., 2013), while also extending the literature in several important ways. In addition to demonstrating that CBTi reduced the amount of discrepancy in older adults with insomnia, we also revealed that the reduction in discrepancy (after 5 sessions of CBTi) was of such a magnitude that perception of sleep and wake no longer significantly differed from objective measurement of sleep or wake. Perceptions of sleep transformed from pessimistic (i.e., significantly under reporting TST and over reporting TWT) to realistic (i.e., reporting TST and TWT that did not differ from objective measurements) in older adults with insomnia disorder. We also discovered that changes in self-reported sleep quality preceded and predicted changes in sleep and wake discrepancies, suggesting a potential mechanism for the improvements in the accuracy of sleep and wake perceptions.
Previous research has shown that good sleepers do not misperceive their sleep (Baekeland and Hoy, 1971; Manconi et al., 2010; Mendelson, 1995). We discovered that following CBTi, the average sleep and wake discrepancies of the treatment group also did not differ from zero. However, the average sleep and wake discrepancy for the control group remained significantly lower than zero, indicating they remained pessimistic in their assessment of TST and TWT. That CBTi resulted in sleep estimation patterns similar to those established in good sleeping individuals adds additional evidence to support the effectiveness of CBTi in the treatment of late-life insomnia.
Our investigation extended previous findings (Lund et al., 2013) by demonstrating that self-reported sleep quality may be a mechanism through which CBTi influences subsequent improvements in sleep and wake discrepancy. Given the multiple assessment occasions employed in the current investigation, we were able to investigate numerous potential temporal pathways for change in sleep and wake discrepancy. The direct effects of treatment on sleep and wake discrepancy were only significant at the immediate post-treatment assessment, with all other treatment effects mediated by previous changes in sleep and wake discrepancy (i.e., an autoregressive effect) and changes in self-reported sleep quality (PSQI score).
While there have been previous reports that sleep and wake discrepancies are temporally related to sleep quality (Kay et al., 2015; Lund et al., 2013), our results confirm these suspicions through a rigorous RCT with 12-month follow-up. Not only did changes in sleep quality precede changes in sleep and wake discrepancy, changes in sleep quality directly influenced long-term improvements in sleep and wake discrepancy. One potential explanation for the observed relationships between sleep quality and sleep and wake discrepancy could be that individuals with better sleep quality spend less time awake during the night and thus have less opportunity to overestimate time awake and underestimate time asleep. Importantly, our study demonstrates that CBTi for insomnia in older adults not only improves the accuracy of perceptions of sleep and wake, but that these improvements are durable for at least 1-year post treatment.
This study has several limitations that need to be acknowledged. First, our objective measure of sleep, actigraphy, is more accurately defined as a method for estimating sleep and wake based on limb movement. However, actigraphy has been validated against PSG (Marino et al., 2013; Martin and Hakim, 2011), and is commonly used in the investigation of sleep misperception (Hughes et al., 2018; Tang and Harvey, 2006; Tang and Harvey, 2004; Van Den Berg et al., 2008; Williams et al., 2013; Williams et al., 2013). However, it is important to recognize that actigraphy may not capture the full spectrum of nightly sleep and wakefulness behavior that could contribute to sleep discrepancy (e.g., sleep stages). Secondly, our older veteran sample is comprised of mostly male participants, and the results may not generalize to non-veteran women with insomnia.
To conclude, CBTi in older adults results in improved perceptual accuracy of both TST and TWT. In fact, the magnitude of improvement is so substantial that subjective estimates of sleep and wake no longer differ from objective measurement after treatment. Given that individuals with insomnia consistently demonstrate difficulty in accurately estimating their nightly sleep and wake patterns (Harvey and Tang, 2012; Kay et al., 2015; Tang and Harvey, 2004; Williams et al., 2013), and that good sleeping individuals do not show this pattern (Baekeland and Hoy, 1971; Manconi et al., 2010; Mendelson, 1995), the ability of CBTi to result in accurate estimates of nighttime sleep and wakefulness may prove to be a useful indicator of treatment success. The improved accuracy of sleep and wake perceptions observed in the current investigation were driven by previous improvement in sleep quality. As such, sleep quality may be a viable target for focused interventions intent on improving the accuracy of perceptions of disturbed sleep. Future treatment trials should include strategies designed specifically to target sleep quality and/or sleep perceptions in older adults with insomnia, and should include sleep and wake discrepancy as important outcome measures.
Acknowledgments
Support: K23AG049955, UCLA/NIA (5P30AG028748), NIH/NCATS/UCLA/CTSI (UL1TR000124), VA HSR&D (IIR:123532), K23AG045937, K24HL143055, American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies, VA Advanced Geriatrics Fellowship. The authors disclose no conflicts of interest.
ClinicalTrials.gov Identifier: NCT00781963
REFERENCES
- Alessi C, Martin JL, Fiorentino L, et al. Cognitive Behavioral Therapy for Insomnia in Older Veterans Using Nonclinician Sleep Coaches: Randomized Controlled Trial. J. Am. Geriatr. Soc, 2016, 64: 1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav. Sleep. Med, 2015, 13 Suppl 1: S4–S38. [DOI] [PubMed] [Google Scholar]
- Baekeland F, Hoy P Reported vs recorded sleep characteristics. Arch. Gen. Psychiatry, 1971, 24: 548–551. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Ceklic T, St-Hilaire P, et al. Insomnia and sleep misperception. Pathol. Biol, 2014, 62: 241–251. [DOI] [PubMed] [Google Scholar]
- Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J. Am. Geriatr. Soc, 2009, 57: 761–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch. Intern. Med, 2011, 171: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res., 1989, 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Dzierzewski JM, Dautovich N, Ravyts S Sleep and Cognition in the Older Adult. Sleep Med. Clin, 2018, 13: 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd JA, Medler SM, Ager JW, Janisse JJ Age-related changes in initiation and maintenance of sleep: A meta-analysis. Res. Nurs. Health, 2000, 23: 106–117. [DOI] [PubMed] [Google Scholar]
- Foley D, Ancoli-Israel S, Britz P, Walsh J Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J. Psychosom. Res, 2004, 56: 497–502. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res, 1975, 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J. Geriatr. Psychiatry Neurol, 2000, 13: 17–27. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Tang N (Mis)Perception of Sleep in Insomnia: A puzzle and a resolution. Psychol. Bull, 2012, 138: 77–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JM, Song Y, Fung CH, et al. Measuring Sleep in Vulnerable Older Adults: A Comparison of Subjective and Objective Sleep Measures. Clin. Gerontol, 2018, 41: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaussent I, Bouyer J, Ancelin M-L, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep, 2011, 34: 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DB, Buysse DJ, Germain A, Hall M, Monk TH Subjective-objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment. J. Sleep Res, 2015, 24: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DB, Dzierzewski JM Sleep in the Context of Healthy Aging and Psychiatric Syndromes. Sleep Med. Clin, 2015, 10: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL, Riedel BW, Wilson NM, Lester KW, Aguillard RN Relaxation and sleep compression for late-life insomnia: a placebo-controlled trial. J. Consult. Clin. Psychol, 2001, 69: 227–239. [DOI] [PubMed] [Google Scholar]
- Lund HG, Rybarczyk BD, Perrin PB, Leszczyszyn D, Stepanski E The discrepancy between subjective and objective measures of sleep in older adults receiving CBT for comorbid insomnia. J. Clin. Psychol, 2013, 69: 1108–1120. [DOI] [PubMed] [Google Scholar]
- Manconi M, Ferri R, Sagrada C, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J. Sleep Res, 2010, 19: 478–486. [DOI] [PubMed] [Google Scholar]
- Marino M, Li Y, Rueschman MN, et al. Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography. Sleep, 2013, 36: 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Hakim AD Wrist Actigraphy. Chest, 2011, 139: 1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson WB Effects of flurazepam and zolpidem on the perception of sleep in normal volunteers. Sleep, 1995, 18: 88–91. [DOI] [PubMed] [Google Scholar]
- Morgan K Sleep and Aging In: Lichstein K and Morin C (eds.) Treatment of late-life insomnia. Sage Publications, Thousand Oaks, CA: (2000). [Google Scholar]
- Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep, 2006, 29: 1415–1419. [PubMed] [Google Scholar]
- Muthen LK, Muthen BO Mplus. Muthen & Muthen, Los Angeles, CA, 1998. [Google Scholar]
- Ohayon MM Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med. Rev, 2002, 6: 97–111. [DOI] [PubMed] [Google Scholar]
- Okajima I, Komada Y, Inoue Y A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol. Rhythms, 2011, 9: 24–34. [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg T, for the Clinical Guidelines Committee of the American College of Physicians. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med, 2016, 165: 125–133. [DOI] [PubMed] [Google Scholar]
- Riemann D, Baglioni C, Bassetti C, Biorvatn B Dolenc Groseli L, et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res, 2017, 26: 675–700. [DOI] [PubMed] [Google Scholar]
- Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol. Aging, 2002, 17: 288–298. [PubMed] [Google Scholar]
- Spielman AJ, Caruso LS, Glovinsky PB A behavioral perspective on insomnia treatment. Psychiatr. Clin. North Am, 1987, 10: 541–553. [PubMed] [Google Scholar]
- Spira AP, Kaufmann CN, Kasper JD, et al. Association between insomnia symptoms and functional status in U.S. older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci, 2014, 69 Suppl 1: S35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp Stata Statistical Software. StateCoprt LP, College Station, TX, 2013. [Google Scholar]
- Tang NKY, Harvey AG Correcting distorted perception of sleep in insomnia: a novel behavioural experiment? Behav. Res. Ther, 2004, 42: 27–39. [DOI] [PubMed] [Google Scholar]
- Tang NKY, Harvey AG Altering misperception of sleep in insomnia: behavioral experiment versus verbal feedback. J. Consult. Clin. Psychol, 2006, 74: 767–776. [DOI] [PubMed] [Google Scholar]
- Van Den Berg JF, Van Rooij FJA, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J. Sleep Res, 2008, 17: 295–302. [DOI] [PubMed] [Google Scholar]
- Williams JM, Kay DB, Rowe M, McCrae CS Sleep discrepancy, sleep complaint, and poor sleep among older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci, 2013, 68: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Classification of Sleep Disorders: Diagnostic and Coding Manual. American Academy of Sleep Medicine, Westchester, IL, 2005. [Google Scholar]