Abstract
Locally advanced and metastatic invasive bladder cancer (BC) has a poor prognosis, and no advanced therapies beyond cisplatin‐based combination chemotherapy have been developed. Therefore, it is an urgent issue to elucidate the underlying mechanisms of tumor progression and metastasis of invasive BC for the development of new therapeutic strategies. Here, we clarified a novel role of exosomes containing ErbB2 and CRK in a formation of premetastatic niches and subsequent metastases. CRK adaptors were overexpressed in invasive UM‐UC‐3 BC cells. In an orthotopic xenograft model, metastases to lung, liver, and bone of UM‐UC‐3 cells were completely abolished by CRK elimination. Mass spectrometry analysis identified that ErbB2 was contained in UM‐UC‐3‐derived exosomes in a CRK‐dependent manner; the exosomes significantly increased proliferation and invasion properties of low‐grade 5637 BC cells and HUVECs through FAK and PI3K/AKT signaling pathways. In athymic mice educated with UM‐UC‐3‐derived exosomes, i.v. implanted UM‐UC‐3 cells were trapped with surrounding PKH67‐labeled exosomes in lung and led to development of lung metastasis with disordered vascular proliferation. In contrast, exosomes derived from CRK‐depleted BC cells failed to induce these malignant features. Taken together, we showed that CRK adaptors elevated the expression of ErbB2/3 in BC cells, and these tyrosine kinase/adaptor units were transferred from host BC cells to metastatic recipient cells by exosomes, leading to vascular leakiness and proliferation and contributing to the formation of distant metastasis. Thus, CRK intervention with ErbB2/3 blockade might be a potent therapeutic strategy for patients with ErbB2 overexpressing advanced and metastatic BC.
Keywords: bladder cancer, CRK, ErbB2, exosome, metastasis
1. INTRODUCTION
Bladder cancer (BC) is one of the most common malignant epithelial tumors, causing an estimated 429 800 new cases and 165 100 deaths in 2012 worldwide.1 At the initial diagnosis, approximately 30% of cases are diagnosed as muscle‐invasive bladder cancer (MIBC), which frequently leads to local invasion and distant metastasis. Patients with MIBC have a poor prognosis, and the 5‐year survival rates for locally advanced and metastatic MIBCs are less than 35% and 5%, respectively.2 The remaining 70% of cases are diagnosed as non‐muscle invasive bladder cancer (NMIBC). Although the 5‐year survival rate for NMIBC is more than 90%, 50–70% of NMIBC patients are highly relapsed, and 10–20% of these patients progress to MIBC.3, 4, 5, 6 Radical cystectomy has been considered as the standard curative treatment for patients with MIBC. However, approximately half of MIBC patients who receive radical cystectomy developed local recurrence and distant metastasis. The mainstay of treatment for these patients is cisplatin‐based combination chemotherapy, such as M‐VAC (methotrexate, vinblastine, doxorubicin, and cisplatin) or GC (gemcitabine and cisplatin) regimens. These regimens have prolonged the median overall survival of patients with locally advanced and metastatic MIBC up to 15.2 months for M‐VAC and 14.0 months for GC.7 However, these regimens frequently produce serious side‐effects in some BC patients, making it impossible for them to continue. Moreover, tumors treated with these regimens ultimately acquire platinum resistance. Therefore, in the past 30 years, there has been no standard second‐line therapy following these cisplatin‐based combination chemotherapies. Molecular targeted therapy is anticipated to become one appropriate treatment for selected BC patients. The Cancer Genome Atlas (TCGA) Research Network has suggested the presence of potential therapeutic targets in 69% of BC patients, including 42% with targets in the PI3K/AKT/mTOR pathway and 45% with targets in the receptor tyrosine kinase (RTK)/MAPK pathway.8 However, none of these molecular targeted therapies have yet been widely accepted in routine clinical practice. Therefore, the development of new molecular targeted treatment strategies is urgently required to improve outcomes for BC patients.
Recently, exosomes have attracted attention as important mediators of intercellular communication. Exosomes contain significant amounts of proteins, nucleic acids, and transcription factors from the cell of origin.9, 10, 11, 12 In the extracellular environment, these functional biomolecules are stably protected from external degrading enzymes by a lipid bilayer of exosomal membrane.13 Thus, exosomes play an important role in the horizontal transfer of this proteomic and genetic information to target cells.14 Several reports have shown that tumor‐derived exosomes can facilitate tumor progression and metastasis.10, 11, 15, 16 Melanoma‐derived exosomes induce cancer cell recruitment, ECM deposition, and vascular proliferation in lymph nodes in preparation for metastasis.17 In addition, metastatic melanoma‐derived exosomes induce vascular leakiness, inflammation, and bone marrow progenitor cell recruitment in premetastatic niches, contributing to the formation of distant metastases.18 Costa‐Silva et al reported the sequential steps for liver premetastatic niches by exosomal migration inhibitory factor derived from pancreatic cancer.19 The expression patterns of exosomal integrins might be useful to predict the metastatic propensity and organ sites of future metastasis.20 In BC, exosomal EDIL‐3 protein from invasive BC cells promotes tumor angiogenesis, cell migration, and invasiveness in vitro, and exosomal EDIL‐3 isolated from the urine can successfully distinguish BC patients from healthy controls as a biomarker.21 Furthermore, exosomes isolated from BC patient urine induce epithelial‐mesenchymal transition in recipient cells.22 These results suggest that BC‐derived exosomes can transform recipient cells to facilitate tumor progression. Thus, it is necessary to clarify the further exosomal role in the formation of distant metastases in BC to establish effective treatment strategies.
The v‐Crk (CT10 regulator of kinase) protein was originally isolated as the oncogene fusion product of the CT10 retrovirus in chicken fibrosarcomas.23 Cellular homologues of v‐Crk encode 2 alternatively spliced isoforms (CRK‐I and CRK‐II) and the related protein (CRKL).24, 25 CRK‐I contains an Src homology (SH) 2 domain and an SH3 domain, whereas CRK‐II has an additional SH3 domain at the C‐terminus and a linker region (Y221) between 2 SH3 domains. CRK has been well known as an adaptor protein that mediates the receptor of tyrosine kinase and small G‐protein interactions. CRK lacks enzymatic activity, but it plays a crucial role in cellular functions including cell growth, migration, adhesion, and invasion.26, 27 Overexpression of CRK has been identified in various human cancers.28, 29, 30, 31, 32, 33, 34
We have previously reported that CRK is overexpressed in BC and induces epithelial‐mesenchymal transition, contributing to the formation of metastasis.35 However, the relationship between CRK and exosomes has remained unknown. In this study, we describe a novel role of exosomal ErbB2 regulated by CRK in the process of tumor progression and metastasis in human invasive BC.
2. MATERIALS AND METHODS
2.1. Cell lines and establishment of BC lines stably expressing tdTomato‐Luc2 and Crk knockdown BC cells
The human BC cell lines UM‐UC‐3, J82, TCC‐SUP, T24, and 5637 cells were purchased from ATCC (Manassas, VA, USA) and stably transfected with pCSII‐CMV‐tdTomato‐Luc2 as described previously.35 UM‐UC‐3 and J82 cells were also transfected with a plasmid producing siRNAs for CRK as described previously.36 The HUVECs were from Riken Cell Bank (Tsukuba, Japan). The detailed culture conditions are described in Appendix S1.
2.2. Exosome purification and fluorescent labeling
Fetal bovine serum was ultracentrifuged at 110 000 g for 2 hours for depletion of exosomes in bovine serum, so‐called exosome‐depleted FBS. Exosomes of human BC cell lines were purified by sequential centrifugation as previously described with minor modifications.18, 20 Purified exosomes were fluorescently labeled using PKH67 green membrane dye (Sigma‐Aldrich, St. Louis, MO, USA).37 The detailed methods were described in Appendix S1.
2.3. Uptake and internalization of exosomes in vitro
For in vitro exosome uptake assays, 5637 cells or HUVECs (1 × 105) were incubated with 20 μg PKH67‐labeled exosomes derived from UM‐UC‐3 cells for 24, 48, and 72 hours. Cellular uptake and internalization of the exosomes were observed under a fluorescence microscope. As a control, the membranes of 5637 cells and HUVECs were directly labeled with PKH67 dye, according to the protocol provided by the manufacturer.
2.4. RNA extraction and gene expression analysis
Total RNAs of BC cells and exosomes were extracted using the RNeasy Mini kit (Qiagen, Valencia, CA, USA), whereas microRNA was using the mirVana miRNA Isolation Kit. cDNA was synthesized using Superscript ViLo (Invitrogen, Carlsbad, CA, USA), and quantitative RT‐PCR was undertaken using the StepOne real‐time PCR system (Applied Biosystems, Foster City, CA, USA) as described previously.38 The sequences of the primers are listed in Table S1. The relative expression levels of total RNA and microRNA to control samples were normalized to GAPDH and RNU6B levels, respectively.
2.5. Immunoblotting analysis and Abs
Immunoblot analyses were carried out as described previously.32, 39 The detailed protocols are described in Appendix S1. Antibodies against the following proteins were purchased. ErbB3 (C‐17), DOCK180 (H4), C3G (C19), Gab1, CRKL (C20), and CD63 (MX‐49.129.5) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and paxillin, p130Cas, and CRK were from BD Transduction Laboratories (Lexington, KY, USA). Epidermal growth factor receptor (EGFR), phospho‐EGFR (Y1148), c‐Met, phospho‐c‐Met (Y1234/1235), ErbB2 (29D8), focal adhesion kinase (FAK), phospho‐FAK (Y397), phospho‐Gab1 (Y307), Src, phospho‐Src (Y416), ERK1/2, phospho‐ERK1/2, Akt, and phospho‐Akt (S473) were from Cell Signaling Technology (Beverly, MA, USA). Actin was from Chemicon International (Temecula, CA, USA) and α‐tubulin was from Sigma‐Aldrich.
2.6. Mass spectrometry analysis
Coomassie brilliant blue staining and silver staining (PlusOne Silver Staining Kit, Protein; GE Healthcare, Little Chalfont, UK) of gels after SDS‐PAGE were carried out according to the protocol provided by the manufacturer. Mass spectrometry analyses of exosomes were performed at the Instrumental Analysis Division, Global Facility Center, Creative Research Institution, Hokkaido University (Sapporo, Japan). Approximately 1 μg UM‐UC‐3 cell‐derived exosomes was analyzed by reversed‐phase nanoliquid chromatography‐tandem mass spectrometry.
2.7. Proliferation assay
For the proliferation analysis of cells treated with exosomes, 5637 cells (1 × 105) and HUVECs (2 × 104) were treated with UM‐UC‐3 cell‐derived exosomes. The numbers of cells were counted using a cell counter after 48, 96, and 144 hours.
2.8. Matrigel invasion assay and chemotaxis assay
The 5637 cells were pretreated with UM‐UC‐3 cell‐derived exosomes for 48 hours, and the Matrigel invasion assay was carried out as described previously.32, 39 The HUVECs were pretreated with UM‐UC‐3 exosomes for 48 hours, and 5 × 105 cells were seeded into the upper part of a Transwell cell culture chamber (24‐well chambers) with 8‐μm pores (Costar, Tewksbury, MA, USA). The following method is similar to the Matrigel invasion assay.
2.9. Orthotopic xenograft mouse model and bioluminescent imaging
All animal studies were carried out in accordance with the protocol approved by the Institutional Animal Care and Use Committee at Hokkaido University Faculty of Medicine. We have previously reported the methods for the orthotopic xenograft models of bladder cancer cells.35 After the primary and metastatic tumors were identified by the IVIS Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA, USA),35 they were excised and isolated.
2.10. Education of mice with exosomes and in vivo tracking
Six‐week‐old BALB/cAJcl nu/nu female mice and NOD/ShiJic‐scid Jcl mice (CLEA Japan, Tokyo, Japan) were used for the exosome education studies. Ten micrograms of PKH67‐labeled exosomes was i.v. injected every other day for 2 weeks as described previously.18 The mice were divided into 3 groups: Empty‐exos group, CRKi‐exos group, and PBS injected group as control. UM‐UC‐3 parent cells (1 × 106) labeled with tdTomato‐Luc2 were injected into the tail vein. After 24 hours, the tumor cells were detected using the IVIS Spectrum system, and the targeted organs were excised. The distribution of PKH67‐labeled exosomes and tdTomato‐expressing UM‐UC‐3 cells in the targeted organs was analyzed under a fluorescence microscope. For histological analysis, the tissues were sectioned and stained with H&E.
2.11. Detection of lung metastasis in exosome‐educated mice
After the exosome‐educated mice were injected with tumor cells as described above, the metastatic formation was traced using the IVIS system.35 After the metastatic tumors were identified, the metastatic organs were excised and analyzed under a fluorescence microscope. The tissues were also utilized for histological H&E staining and MIB‐1 staining.
2.12. In vitro vascular permeability assay
The in vitro vascular permeability assay was carried out using a 24‐well in vitro vascular permeability assay kit (CultreCoat; R&D Systems). Briefly, 2 × 105 HUVECs were precultured in the insert for 24 hours and treated with or without 20 μg exosomes (parent, Emp‐1, and CRKi‐1) for a further 24 hours. FITC‐Dextran was added and the cells were incubated for 5 minutes. Fluorescence was measured at 485 nm excitation, 520 nm emission using Spectra MAX Paradigm (Molecular Devices, San Jose, CA, USA).
2.13. Statistical analysis
Graphical data are presented as the mean and SD. Student's t test or the Mann‐Whitney U test was chosen to analyze significant differences. P < .05 and P < .01 were considered as significant and highly significant, respectively. JMP version 10 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
3. RESULTS
3.1. UM‐UC‐3 BC cells with high expression of CRK promote invasiveness and metastasis in vivo
In 5 human BC cell lines (high‐grade invasive BC: UM‐UC‐3, J82, TCC‐SUP, and T24; low‐grade BC: 5637), UM‐UC‐3 and T24 cells showed higher expression of CRK‐II and CRK‐I (Figure 1A). The phosphorylated form of CRK‐II was clearly detected in UM‐UC‐3 cells (Figure 1A, arrowhead), suggesting a substantial activation of CRK‐mediated downstream signaling. UM‐UC‐3 cells showed the highest invasiveness (Figure 1B). For in vivo analysis, BC cells stably expressing tdTomato‐Luc2 were established (Figure 1C) and injected into the bladder muscle layer of nude mice as an orthotopic xenograft model (each n = 4). After 35 days, distant metastases were detected only in UM‐UC‐3‐injected mice, whereas J82 and TCC‐SUP cells failed to form either primary bladder tumor or metastases, despite the increased number of injected cells and prolonged observation period (Figure 1D). In UM‐UC‐3‐injected mice, tissues of primary bladder tumors and metastases to lung, liver, and bone were resected, and tumor cells with tdTomato fluorescence could be isolated (Figure 1E). Based on these malignant features, the UM‐UC‐3 cells were used for further investigations of the metastatic progression of BC.
Figure 1.
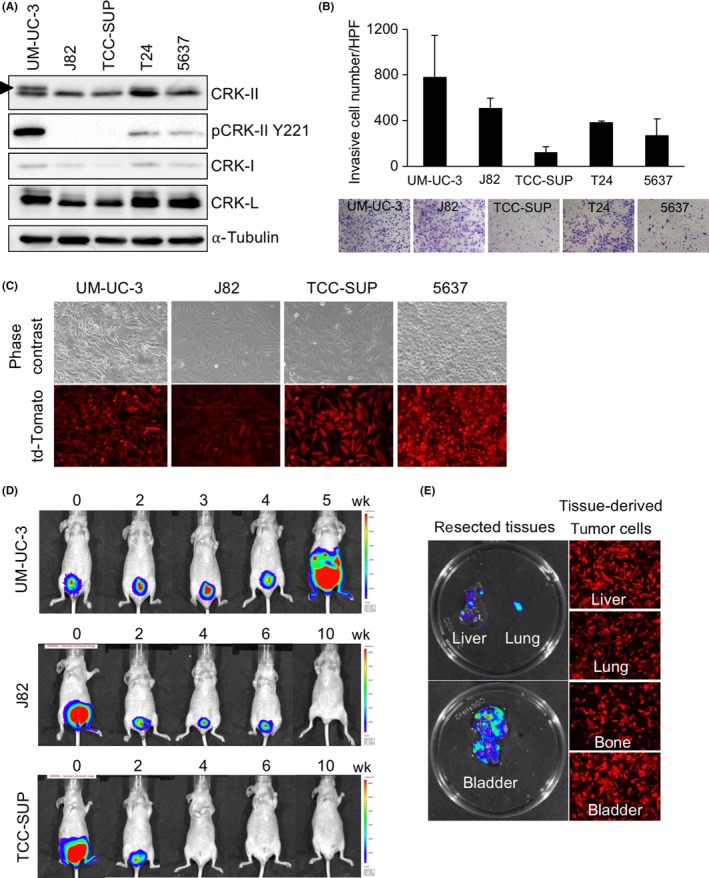
Bladder cancer cells with high expression of CRK develop metastasis in vivo. A, Expression levels of CRK family adaptor proteins were examined by immunoblotting (IB) in 5 bladder cancer (BC) cell lines. Arrowhead indicates a phosphorylated form of CRK‐II. α‐Tubulin was used as a loading control. B, Matrigel invasion assay. The BC cells were seeded in Matrigel‐coated Transwell chambers. After 20 h of incubation, the invading cells under the filter were counted and depicted as the mean ± SD. Representative photomicrographs are shown. C, Photographs of UM‐UC‐3, J82, TCC‐SUP, and 5637 cells labeled with tdTomato‐luc2 are shown. D, UM‐UC‐3, J82, and TCC‐SUP cells labeled with tdTomato‐luc2 were orthotopically injected into the bladder muscle layer in nude mice (n = 5, each group). Tumor growth was monitored weekly using the IVIS Spectrum imaging system. E, After 35 days, the UM‐UC‐3‐injected mice were killed, and bioluminescent imaging photons from primary bladder tumor and liver‐ and lung‐metastatic tumor burden were obtained ex vivo (left). Fluorescence microscopy showed tdTomato‐expressing tumor cells isolated in the culture dish (right)
3.2. CRK‐depleted BC cells suppress tumor progression and metastasis through a decrease in ErbB2 and ErbB3 expression
To clarify the role of CRK adaptors in the malignant features of UM‐UC‐3 cells, we established CRK‐I/‐II‐knockdown cells (CRKi) and control cells (Empty) (Figure 2A). We have previously reported that a depletion of CRK suppresses cell motility, invasion, and growth of invasive BC.35 Furthermore, CRK elimination in UM‐UC‐3 cells resulted in a significant suppression of primary BC progression and a complete abolishment of metastasis in an orthotopic xenograft model (Figure 2B,C). To clarify CRK‐regulated underlying molecular mechanisms, we investigated the expression levels of mRNAs of RTKs that have been shown to be highly expressed in BC cells. Of 11 RTKs, mRNAs of EGFR, cMET, ErbB2, and ErbB3 were decreased by CRK elimination (Figures 2D and S1A). No obvious differences were detected in the expression levels of their ligands and related microRNAs (Figure S1B,C). Of note, protein expressions of ErbB2 and ErbB3, but not EGFR or cMet, were clearly decreased in CRK‐depleted UM‐UC‐3 cells (Figure 2E), and a similar trend was also observed in J82 cells (Figure S1D,E). In CRK‐mediated signaling, the expression levels of paxillin, Dock180, FAK, AKT, and Src and the phosphorylation of ERK seem to be decreased by CRK depletion (Figures 2F and S1F). Thus, CRK increases the expressions of ErbB2 and ErbB3 and also probably stabilizes the proteins, leading to an activation of signaling pathways essential for tumor development and metastasis in invasive BC.
Figure 2.
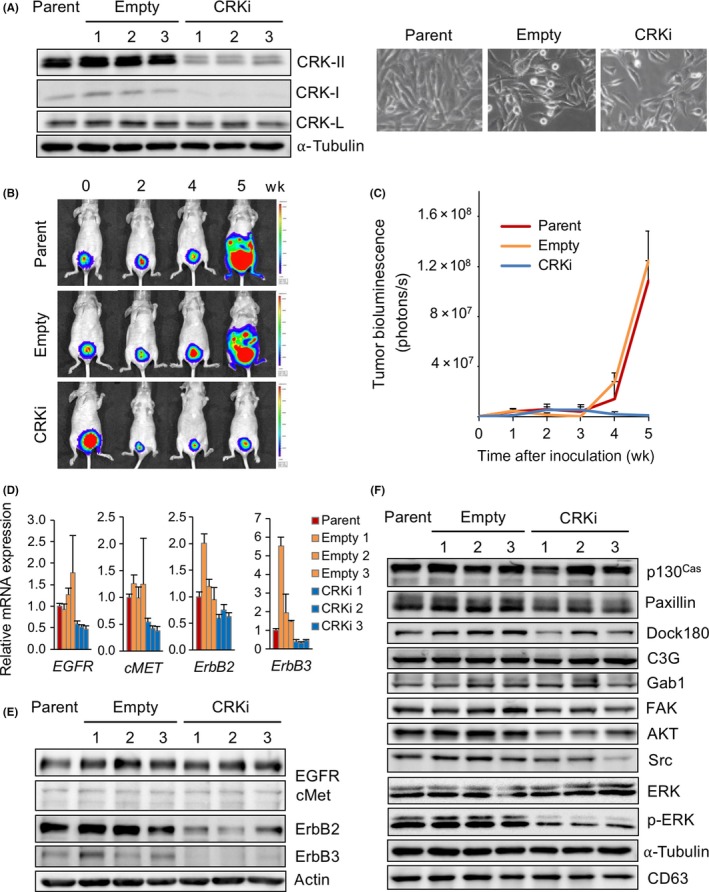
Depletion of CRK decreases expression of ErbB2 and ErbB3 in bladder cancer cells and abolishes metastasis of bladder cancer in vivo. A, Establishment of CRK knockdown bladder cancer cells. UM‐UC‐3 cells were stably transfected with expression plasmids producing shRNA targeting CRK (CRKi) or its control vector (Empty). Cell lysates of parent, control (Empty), and CRK knockdown cells (CRKi) were subjected to immunoblotting with anti‐CRK and CRK‐L Abs. α‐Tubulin was used as a loading control. Photomicrographs of the cells were obtained under bright‐field illumination (right panels). B,C, tdTomato‐luc2‐labeled UM‐UC‐3 cells (parent, empty, and CRKi) were injected into the bladder muscle layer in athymic mice (n = 4, each group). Tumor growth was measured weekly using the IVIS Spectrum imaging system (B) and graphed as the mean ± SD (C). In CRKi cell‐injected mice, metastasis to the liver and lung was absent. D, Total RNAs were isolated from UM‐UC‐3 cells (parent, empty, and CRKi), and endogenous expression levels of EGFR,cMET, ErbB2, and ErbB3 mRNAs were analyzed by quantitative RT‐PCR. E,F, Levels of expression and phosphorylation of epidermal growth factor receptor (EGFR), cMET, ErbB2, and ErbB3 (E) and CRK‐related signaling molecules (F) were examined by immunoblotting
3.3. Exosomes derived from invasive BC contain ErbB2 through CRK regulation
To investigate a participation of exosomes in CRK‐regulated malignant features of invasive BC, exosomes were isolated from conditioned medium of UM‐UC‐3 cells with or without CRK. Mass spectrometry analysis was carried out to identify proteins that were downregulated in exosomes by CRK depletion (Figure 3A, Emp‐1 vs Crki‐1), and many proteins, including EGFR, ErbB2, EPHA2, EPHB4, Src, integrin α2, and integrin α3, were nominated (Table S2). ErbB2 and integrin α2, also likely ErbB3 and FAK, seem to be declined in CRKi exosomes (CRKi‐exos) (Figures 3B and S2A). In contrast, expression levels of EPHA2, EPHA4, EPHB4, and integrin α3 remained unchanged, irrespective of CRK expression (Figure S2A). CRK has no effect on expression of mRNAs of RTKs and their ligands, and RTK‐related microRNAs in exosomes (Figure S2B‐D). It is noteworthy that we were able to detect a considerable amount of CRK‐II protein in exosomes from parental and control (empty) UM‐UC‐3 cells (Figure 3B); therefore, invasive BC‐derived exosomes might play an as yet unidentified important role in the formation of distant metastasis by transferring tyrosine kinases, such as ErbB2/B3 and CRK adaptor unit, from host cells into recipient cells.
Figure 3.
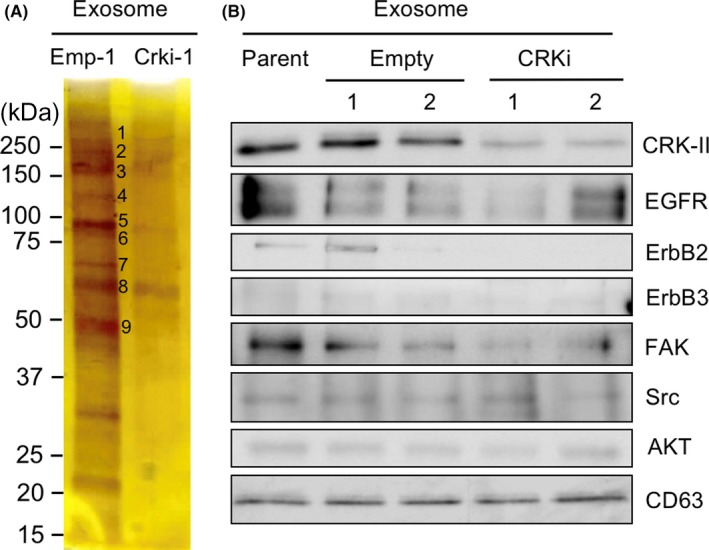
Elimination of CRK reduces expression of ErbB2 and ErbB3 in exosomes derived from bladder cancer cells. A, An equivalent amount of protein (1 μg) from exosomes derived from UM‐UC‐3 cells (empty [Emp‐1] and CRKi) were subjected to SDS‐PAGE, followed by silver staining. B, In exosomes derived from UM‐UC‐3 cells (parent, empty, and CRKi), expression levels of the indicated proteins were examined by immunoblotting. CD63 is an exosomal marker and was used as a loading control. EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase
3.4. Exosomes derived from high‐grade BC promote proliferation and invasion of low‐grade BC cells through ErbB2 signaling
Lipophilic carbocyanine dyes such as PKH67 are useful for monitoring exosome uptake into recipient cells. To investigate the uptake and internalization of BC‐derived exosomes into recipient cells, 5637 cells (low‐grade BC) were treated with PKH67 green fluorescence‐labeled exosomes derived from parental UM‐UC‐3 cells (high‐grade BC). After 24 hours, the exosomes were incorporated into the cytoplasm of more than 90% of 5637 cells (Figure 4A) and persisted at least until 72 hours (Figure S3A). By comparison, in 5637 cells directly labeled with PKH67 dye, the fluorescence signal was diffused throughout the cells (Figure S3B).
Figure 4.
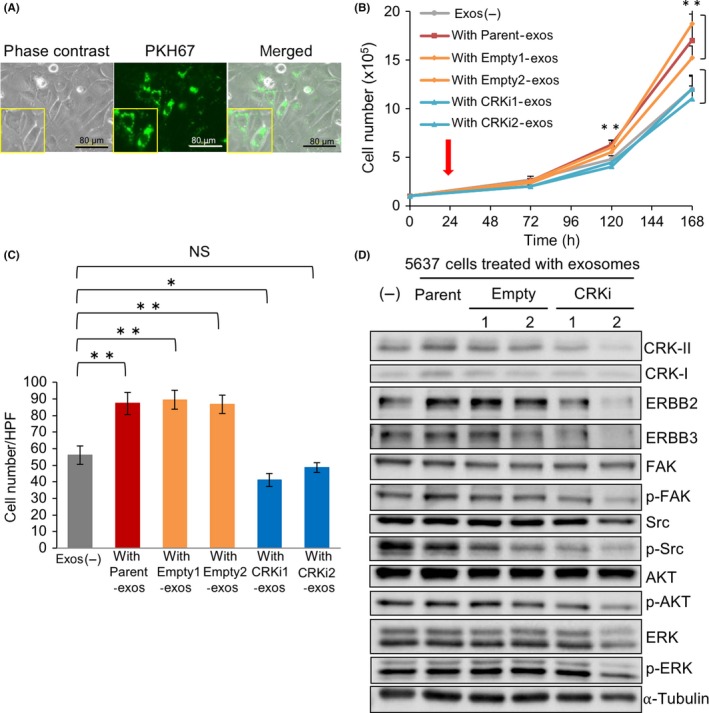
Exosomes derived from high‐grade bladder cancer cells facilitate proliferation and invasion of low‐grade bladder cancer cells through an activation of ErbB2 signaling. A, 5637 cells (low‐grade) were treated with 20 μg PKH67‐labeled exosomes (green) derived from UM‐UC3 cells (high‐grade) for 48 h. Photomicrographs of the cells were obtained under bright‐field illumination (left) and using a fluorescence microscope (middle). B, Proliferation assay. The 5637 cells treated with exosomes derived from UM‐UC‐3 cells (parent, empty, and CRKi) (red arrow) were counted under a microscope at the indicated time points and expressed as the mean ± SD of 3 independent experiments. Cells treated with PBS were used as a control (Exos (−)). **P < .01 vs exos (−). C, Matrigel invasion assay. The 5637 cells were treated with exosomes derived from UM‐UC‐3 cells with or without CRK for 24 h, and the invading cells under the filter were counted and depicted as the mean ± SD. *P < .05 and **P < .01 vs Exos (−). D, In 5637 cells treated with UM‐UC‐3‐derived exosomes, expression and phosphorylation of the indicated proteins were examined by immunoblotting. FAK, focal adhesion kinase
We examined a biological effect of invasive BC‐derived exosomes on recipient cells. The proliferation and invasiveness of 5637 cells treated with parent and control (Empty)‐exos significantly increased compared with the cells without exos (exos (−)) or with CRKi‐exos (Figure 4B,C). Treatment with parent‐ and empty‐exos increased the expression levels of ErbB2 and ErbB3 in 5637 cells, but CRKi‐exos did not (Figure 4D). Phosphorylation levels of FAK, Src, AKT, EGFR, and cMET seemed to decline followed by treatment with CRK‐depleted exosomes (Figures 4D and S3C). In contrast, there were no differences in mRNA expression levels of RTKs and their ligands, or in RTK‐related microRNAs, in 5637 cells treated with or without CRK (Figure S3D‐F).
3.5. Human umbilical vascular endothelial cells promote cell proliferation and invasion through exosomes derived from high‐grade BC
To further determine the role of BC‐derived exosomes on tumor angiogenesis and distant metastasis, HUVECs were treated with exosomes derived from UM‐UC‐3 cells (Figure 5A). The amount of uptake of exosomes in HUVECs was almost equal irrespective of the presence or absence of CRK (data not shown). Like 5637 cells (Figure 4), the proliferation and chemotactic ability of HUVECs treated with parent and control (Empty)‐exos significantly increased, whereas CRKi‐exos did not (Figure 5B,C). Furthermore, the expression levels of ErbB2, ErbB3, and CRK‐II and the phosphorylation levels of FAK and AKT increased following treatment with parent and Empty‐exos, but CRKi‐exos had no effect (Figures 5D and S4).
Figure 5.
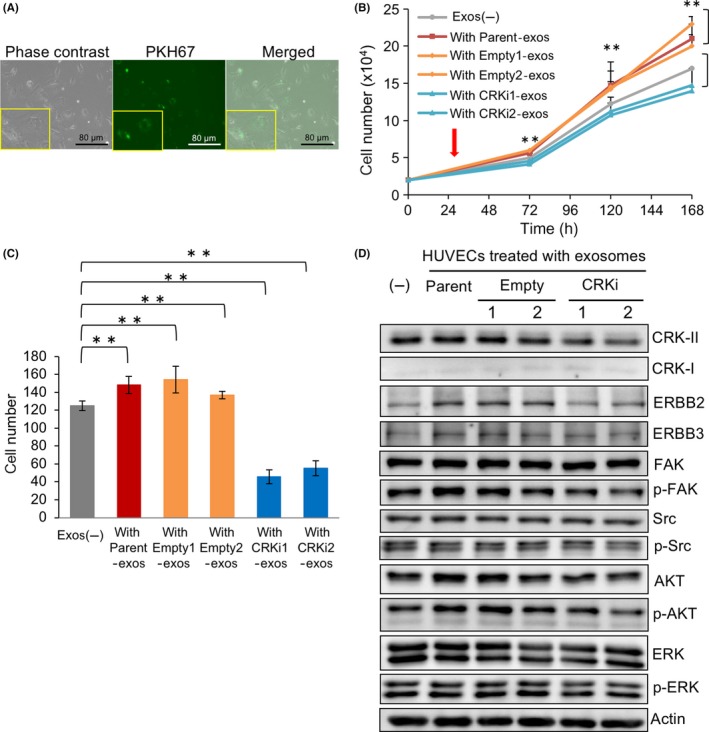
Human umbilical vascular endothelial cells promote proliferation and migration by incorporating exosomes derived from high‐grade bladder cancer cells. A, HUVECs were treated with 20 μg PKH67‐labeled exosomes derived from UM‐UC3 cells for 48 h. Photomicrographs of the cells were obtained under bright‐field illumination (left) and using a fluorescence microscope (middle). B, Proliferation of HUVECs treated with exosomes derived from UM‐UC‐3 cells (parent, empty, and CRKi) (red arrow) were counted under a microscope at the indicated time points and expressed as the mean ± SD of 3 independent experiments. Cells treated with PBS were used as a control (exos (–)). **P < .01. C, Migration of HUVECs treated with exosomes derived from UM‐UC‐3 cells with or without CRK were analyzed by chemotactic assay. The cells migrating through the filter were counted and depicted as the mean ± SD. **P < 0.01. D, In HUVECs treated without or with UM‐UC‐3‐derived exosomes (parent, empty, and CRKi), levels of expression and phosphorylation of the indicated proteins were investigated by immunoblotting
3.6. Exosomes derived from high‐grade BC educate premetastatic niches and facilitate distant metastasis of BC
To clarify the role of exosomes in the formation of distant metastasis of BC, we investigated the distribution of BC cells in target organs educated with BC‐derived exosomes. Emp‐exos or CRKi‐exos derived from UM‐UC‐3 were injected into tail veins of nude mice every other day for 2 weeks. After the last treatment, tdTomato‐Luc2‐labeled UM‐UC‐3 cells were injected i.v. After a further 24 hours, distinct bioluminescence was detected in the lung of Emp‐exos‐educated mice (Figure 6A). Indeed, under fluorescence microscopy, UM‐UC‐3 cells (red) were trapped with surrounding PKH‐labeled exosomes (green) in lung following Emp‐exos education (Figure 6B, upper panels). In contrast, few tumor cells were observed in CRKi‐exos‐educated lung (Figure 6B, lower panels), although the uptake of Emp‐exos and CRKi‐exos in lung was almost equivalent.
Figure 6.
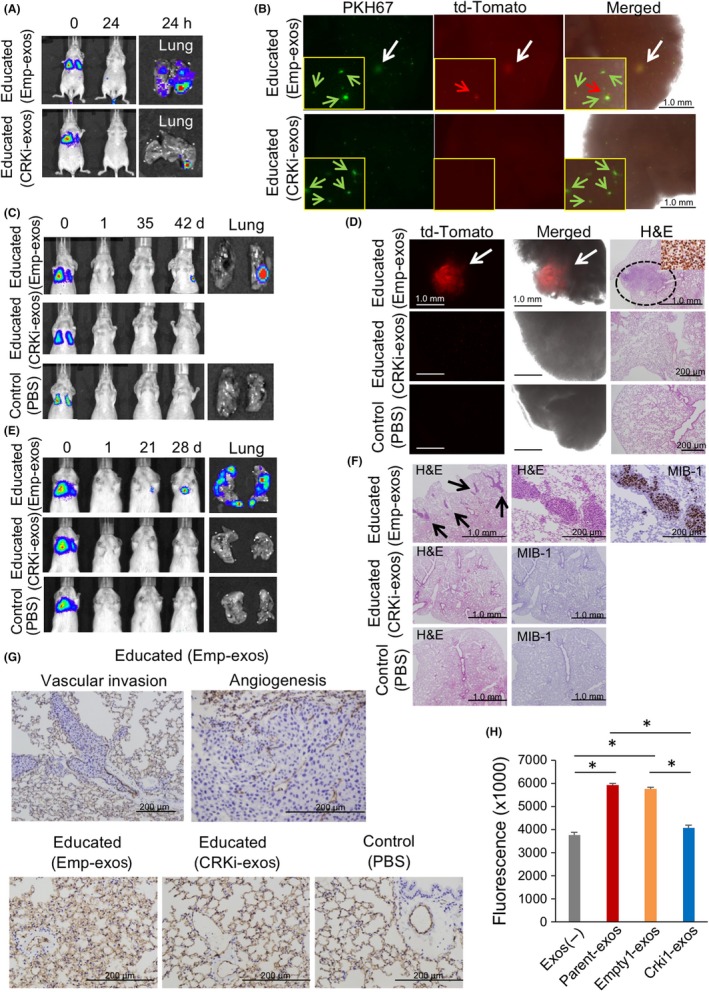
Education of the metastatic target organ with high‐grade bladder cancer‐derived exosomes (exos) facilitate metastasis in vivo. A, Mice were divided into 3 groups: Emp‐exos group, CRKi‐exos group, and PBS‐injected group as a control. PKH67‐labeled exosomes (10 μg Emp‐exos or CRKi‐exos) or PBS were injected i.v. in athymic nude mice every other day for 2 weeks. After exosome education, UM‐UC‐3 parent cells (1 × 106 cells) were injected into the tail vein. After 24 h, the tumor cells trapped in lung were detected using the IVIS Spectrum system. B, In lung tissue displayed in (A), the biodistribution of PKH67‐labeled exosomes and tdTomato‐expressing UM‐UC‐3 cells were analyzed under a fluorescence microscope. C,E, After exosome education as described in (A), UM‐UC‐3 cells (1 × 106 cells) were injected in BALB/cAJclnu/nu mice (C) and NOD/ShiJic‐scid Jcl mice (E) into the tail vein (n = 3, each group). Metastatic formations were tracked using the IVIS system. When the metastatic tumors were identified, the lung tissues were excised. D,F, Lung metastases were confirmed using a fluorescence microscope and histological examination such as H&E staining and immunostaining for MIB‐1. G, Resected lung tissues were subjected to immunostaining for CD31. H, HUVECs were treated with or without 20 μg exosomes (parent, Emp‐1, and CRKi‐1) for 24 h. After FITC‐Dextran was added, vascular permeability was measured at 485 nm excitation, 520 nm emission. *P < .0005
In another xenograft setting, we investigated the metastatic progression of BC cells after exosome education. After education for 2 weeks, tdTomato‐Luc2‐expressing UM‐UC‐3 cells were injected into nude mice as described above, and metastatic progression was evaluated using the IVIS Spectrum system every week (Figure 6C). Six weeks later, metastases to the lung and lower abdomen were detected only in the mice educated with Emp‐exos (Figure 6C) and were confirmed under a fluorescence microscope and by histological examination (Figure 6D). Similar results were obtained in NOD‐Scid mice at an earlier phase (Figure 6E,F). Of note, in lung metastatic tumors educated with Emp‐exos, vascular invasion and angiogenesis were clearly promoted with a disturbed pulmonary alveolus (Figures 6G and S5). Supporting this data, the permeability of the vascular sheet formed by HUVECs increased to 1.57‐ and 1.53‐fold following treatment with parent‐ and Empty1‐exos, respectively, relative to HUVECs without exosomes, whereas Crki1‐exos had no effect (1.08‐fold; Figure 6H).
In summary, exosomes containing CRK/ErbB2 proteins contribute to formation of distant metastases in invasive BC by educating premetastatic niches, leading to vascular leakiness and proliferation, in addition to facilitation of proliferation and invasion of BC cells (Figure 7).
Figure 7.
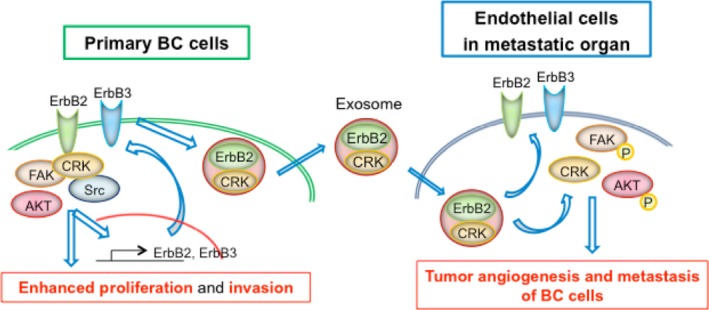
Putative mechanisms of CRK‐facilitated distant metastasis of bladder cancer (BC) by exosomes. CRK adaptors elevate the expression of ErbB2 and ErbB3 in BC cells and promote the proliferation and invasion through ErbB2/3‐mediated activation of downstream signaling pathways. In addition, CRK and ErbB2/3 proteins can be included in exosomes and transferred to recipient cells in metastatic target organs, such as endothelial cells, contributing to formation of premetastatic niches and subsequent metastasis of BC
4. DISCUSSION
Invasive BC with advanced progression and distant metastasis has shown a poor prognosis in the past 30 years. Therefore, it is an urgent issue to reveal the underlying mechanisms of tumor progression and metastasis to develop new treatment strategies for BC patients. In the present study, we unveiled a novel function of CRK/ErbB2 through exosomes; this function plays an important role in tumor progression and metastatic formation in invasive BC.
We here clarified that CRK elevates the expression of ErbB2/3 mRNAs and protein levels in human BC cells (Figures 2D,E and S1E,F). ErbB2/3 genes encode members of the EGFR family of transmembrane receptor tyrosine kinases. Particularly, ErbB2 triggers various cellular phenotypes, such as cell proliferation, differentiation, motility, and survival.40 It has been reported that ErbB2 receptor is correlated with the coupling of p130Cas to CRK to drive migration and invasion in breast cancer cells.41, 42 Therefore, it seems that CRK contributes to an activation of ErbB2 signaling in cancer cells. The expression of ErbB2 has been shown to be elevated in various cancers, and currently, ErbB2‐targeted therapy is one of the most important treatment options for patients with ErbB2‐overexpressing cancers.43, 44, 45, 46, 47 In BC, ErbB2 was overexpressed from 9% to 81% and amplified 10% to 20%,48, 49, 50, 51, 52, 53, 54 and hence, overexpression without gene amplification of ErbB2 was more common in BC.55 Based on our previous evidence,35 CRK‐mediated signaling pathways might enhance the transcriptional activity of the ErbB2 gene in BC cells.
The high expression level of ErbB2 in patients with invasive BC was correlated with metastatic progression and a poor prognosis,49, 56, 57, 58, 59, 60 raising the possibility that ErbB2 might be not only a prognostic factor but also a potential therapeutic target for BC.49, 56 Although TCGA found that ErbB2 is a potential therapeutic target in 9% of BC patients,8 no significant efficacy of molecular target therapy, including ErbB2‐targeted therapy, has been reported in metastatic BC tumors.6, 61 Recently, a clinical randomized phase II trial found that the addition of trastuzumab to platinum‐based chemotherapy could not improve progression‐free survival, overall response rate, or overall survival of patients with locally advanced or metastatic BC.62 As a reason for the failure, the blockade of ErbB2 signaling by trastuzumab might generate an alternative activation of other RTK signaling, such as ErbB3. The Cancer Genome Atlas project suggested that ErbB3 could also be a potential therapeutic target in 6% of BC,8 and both ErbB2 and ErbB3 tended to be associated with a poor prognosis in BC patients.50 In the present study, we showed that CRK is a key regulator of ErbB2/3 expression and CRK‐mediated ErbB2/3 elevation in exosomes contributed to the metastatic progression of BC. Therefore, CRK intervention could be a powerful treatment strategy to suppress metastasis of BC by complete blockage of ErbB2/3 signaling.
Tumor‐derived exosomes have protumorigenic roles, and oncogenic RTKs are present in exosomes. Peinado et al18 showed that exosomes are able to horizontally transfer MET from melanoma to bone marrow progenitor cells to educate them toward a prometastatic phenotype. Exosomes derived from glioma cells have been shown to contribute to the horizontal transfer of oncogenic EGFRvIII.15 However, the role of exosomes in tumor progression and metastasis of BC has remained unclear. Here, we found that ErbB2 proteins are contained in invasive BC‐derived exosomes in a CRK‐dependent manner (Figure 3B) and transferred to the recipient cells (Figures 4D and 5D). Wolfers et al63 showed that some tumors secreted exosome‐like microvesicles containing many proteins, including ErbB2. Ascites‐exosomes purified from patients with ovarian and breast cancer include tumor‐specific proteins such as ErbB2.64 Furthermore, human breast cancer cells cultured with tumor‐derived exosomes containing highly ErbB2 proteins increased tumor cell proliferation.65 Thus, invasive BC‐derived exosomes containing CRK and ErbB2 proteins are capable of educating target organs in preparation for metastasis, indeed, CRK and ErbB2 in exosomes are most likely to be transferred to endothelial cells of the target organ, elevating tumor angiogenesis with disrupted structures (Figures 6G and S5), which can give rise to facile formation of distant metastases. Special attention should be paid to clarify the role of CRK in biogenesis and secretion of exosomes in future studies.
In the present study, we found that UM‐UC‐3‐derived exosomes activated the phosphorylations of FAK and AKT and the subsequent downstream signaling cascades in recipient cells such as 5637 cells and HUVECs, but exosomes derived from CRK‐depleted UM‐UC‐3 cells did not. A previous study reported that gastric cancer‐derived exosomes promoted tumor cell proliferation through MAPK/ERK and PI3K/Akt activation.66 The exosomes from glioma stimulated EGFRvIII transfer and activated downstream signaling pathways such as MAPK and AKT cascades in recipient cells.15 Recently, BC‐derived exosomes were reported to activate the Akt and ERK pathways to induce cell proliferation.67 Taken together, we showed that CRK and ErbB2 in BC‐derived exosomes act as key regulators of cellular transformation within premetastatic niches containing endothelial cells and fibroblasts, through FAK and PI3K/AKT signaling activation, in addition to increases in tumor cell proliferation and invasion.
In conclusion, we herein report that CRK elevates the expression of ErbB2/3 in BC cells, and these oncoproteins are transferred to the recipient cells in target organs by exosomes, contributing to the distant metastasis of BC. Thus, we propose that CRK intervention could be a powerful treatment strategy for patients with ErbB2 overexpressing advanced and metastatic BC.
DISCLOSURE
The authors declare no competing financial interests.
Supporting information
ACKNOWLEDGMENTS
We thank Dr. Kyoko Hida (Hokkaido University, Sapporo, Japan) for providing the pCSII‐CMV‐tdTomato‐Luc2 plasmid, and Ms. Kyoko Fujii for technical assistance. This work was supported, in part, by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the Japanese Society for the Promotion of Science, and the Ministry of Health, Labor, and Welfare of Japan, as well as a grant from the Japanese Science and Technology Agency. In addition, this research was supported by the Global Station for Soft Matter, a project of the Global Institution for Collaborative Research and Education at Hokkaido University. The Institute for Chemical Reaction Design and Discovery (ICReDD) was established by the World Premier International Research Initiative (WPI), MEXT, Japan.
Yoshida K, Tsuda M, Matsumoto R, et al. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. 2019;110:2119–2132. 10.1111/cas.14080
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5‐29. [DOI] [PubMed] [Google Scholar]
- 3. Rübben H, Lutzeyer W, Fischer N, Deutz F, Lagrange W, Giani G. Natural history and treatment of low and high risk superficial bladder tumors. J Urol. 1988;139(2):283‐285. [DOI] [PubMed] [Google Scholar]
- 4. Schenk‐Braat EA, Bangma CH. Immunotherapy for superficial bladder cancer. Cancer Immunol Immunother. 2005;54(5):414‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sylvester RJ. Natural history, recurrence, and progression in superficial bladder cancer. ScientificWorldJournal. 2006;6:2617‐2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374(9685):239‐249. [DOI] [PubMed] [Google Scholar]
- 7. von der Maase H, Sengelov L, Roberts JT, et al. Long‐term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602‐4608. [DOI] [PubMed] [Google Scholar]
- 8. Network CGAR . Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 10. Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ratajczak J, Wysoczynski M, Hayek F, Janowska‐Wieczorek A, Ratajczak MZ. Membrane‐derived microvesicles: important and underappreciated mediators of cell‐to‐cell communication. Leukemia. 2006;20(9):1487‐1495. [DOI] [PubMed] [Google Scholar]
- 12. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907‐1920. [DOI] [PubMed] [Google Scholar]
- 13. El Andaloussi S, Lakhal S, Mäger I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65(3):391‐397. [DOI] [PubMed] [Google Scholar]
- 14. Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575‐581. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619‐624. [DOI] [PubMed] [Google Scholar]
- 16. Jung T, Castellana D, Klingbeil P, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11(10):1093‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792‐3801. [DOI] [PubMed] [Google Scholar]
- 18. Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro‐metastatic phenotype through MET. Nat Med. 2012;18(6):883‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costa‐Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre‐metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoshino A, Costa‐Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beckham CJ, Olsen J, Yin PN, et al. Bladder cancer exosomes contain EDIL‐3/Del1 and facilitate cancer progression. J Urol. 2014;192(2):583‐592. [DOI] [PubMed] [Google Scholar]
- 22. Franzen CA, Blackwell RH, Todorovic V, et al. Urothelial cells undergo epithelial‐to‐mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis. 2015;4:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988;332(6161):272‐275. [DOI] [PubMed] [Google Scholar]
- 24. Matsuda M, Tanaka S, Nagata S, Kojima A, Kurata T, Shibuya M. Two species of human CRK cDNA encode proteins with distinct biological activities. Mol Cell Biol. 1992;12(8):3482‐3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feller SM. Crk family adaptors‐signalling complex formation and biological roles. Oncogene. 2001;20(44):6348‐6371. [DOI] [PubMed] [Google Scholar]
- 26. Kobashigawa Y, Sakai M, Naito M, et al. Structural basis for the transforming activity of human cancer‐related signaling adaptor protein CRK. Nat Struct Mol Biol. 2007;14(6):503‐510. [DOI] [PubMed] [Google Scholar]
- 27. Birge RB, Kalodimos C, Inagaki F, Tanaka S. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun Signal. 2009;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsuda M, Tanaka S. Roles for crk in cancer metastasis and invasion. Genes Cancer. 2012;3(5–6):334‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishihara H, Tanaka S, Tsuda M, et al. Molecular and immunohistochemical analysis of signaling adaptor protein Crk in human cancers. Cancer Lett. 2002;180(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 30. Miller CT, Chen G, Gharib TG, et al. Increased C‐CRK proto‐oncogene expression is associated with an aggressive phenotype in lung adenocarcinomas. Oncogene. 2003;22(39):7950‐7957. [DOI] [PubMed] [Google Scholar]
- 31. Fathers KE, Bell ES, Rajadurai CV, et al. Crk adaptor proteins act as key signaling integrators for breast tumorigenesis. Breast Cancer Res. 2012;14(3):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linghu H, Tsuda M, Makino Y, et al. Involvement of adaptor protein Crk in malignant feature of human ovarian cancer cell line MCAS. Oncogene. 2006;25(25):3547‐3556. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Tabu K, Kimura T, et al. Signaling adaptor protein Crk is indispensable for malignant feature of glioblastoma cell line KMG4. Biochem Biophys Res Commun. 2007;362(4):976‐981. [DOI] [PubMed] [Google Scholar]
- 34. Watanabe T, Tsuda M, Makino Y, et al. Adaptor molecule Crk is required for sustained phosphorylation of Grb2‐associated binder 1 and hepatocyte growth factor‐induced cell motility of human synovial sarcoma cell lines. Mol Cancer Res. 2006;4(7):499‐510. [DOI] [PubMed] [Google Scholar]
- 35. Matsumoto R, Tsuda M, Wang L, et al. Adaptor protein CRK induces epithelial‐mesenchymal transition and metastasis of bladder cancer cells through HGF/c‐Met feedback loop. Cancer Sci. 2015;106(6):709‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagashima K, Endo A, Ogita H, et al. Adaptor protein Crk is required for ephrin‐B1‐induced membrane ruffling and focal complex assembly of human aortic endothelial cells. Mol Biol Cell. 2002;13(12):4231‐4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle‐mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahabir R, Tanino M, Elmansuri A, et al. Sustained elevation of Snail promotes glial‐mesenchymal transition after irradiation in malignant glioma. Neuro Oncol. 2014;16(5):671‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watanabe T, Tsuda M, Tanaka S, et al. Adaptor protein Crk induces Src‐dependent activation of p38 MAPK in regulation of synovial sarcoma cell proliferation. Mol Cancer Res. 2009;7(9):1582‐1592. [DOI] [PubMed] [Google Scholar]
- 40. Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19(10):2013‐2023. [DOI] [PubMed] [Google Scholar]
- 41. Spencer KS, Graus‐Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148(2):385‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cabodi S, Tinnirello A, Bisaro B, et al. p130Cas is an essential transducer element in ErbB2 transformation. FASEB J. 2010;24(10):3796‐3808. [DOI] [PubMed] [Google Scholar]
- 43. Joensuu H, Kellokumpu‐Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809‐820. [DOI] [PubMed] [Google Scholar]
- 44. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376(9742):687‐697. [DOI] [PubMed] [Google Scholar]
- 45. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med. 2005;353(16):1659‐1672. [DOI] [PubMed] [Google Scholar]
- 46. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med. 2005;353(16):1673‐1684. [DOI] [PubMed] [Google Scholar]
- 47. Bellmunt J, Hussain M, Dinney CP. Novel approaches with targeted therapies in bladder cancer. Therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit Rev Oncol Hematol. 2003;46(Suppl):S85‐S104. [DOI] [PubMed] [Google Scholar]
- 48. Zhau HE, Zhang X, von Eschenbach AC, et al. Amplification and expression of the c‐erb B‐2/neu proto‐oncogene in human bladder cancer. Mol Carcinog. 1990;3(5):254‐257. [DOI] [PubMed] [Google Scholar]
- 49. Grivas PD, Day M, Hussain M. Urothelial carcinomas: a focus on human epidermal receptors signaling. Am J Transl Res. 2011;3(4):362‐373. [PMC free article] [PubMed] [Google Scholar]
- 50. Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7(7):1957‐1962. [PubMed] [Google Scholar]
- 51. Gandour‐Edwards R, Lara PN, Folkins AK, et al. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer. 2002;95(5):1009‐1015. [DOI] [PubMed] [Google Scholar]
- 52. Sato K, Moriyama M, Mori S, et al. An immunohistologic evaluation of C‐erbB‐2 gene product in patients with urinary bladder carcinoma. Cancer. 1992;70(10):2493‐2498. [DOI] [PubMed] [Google Scholar]
- 53. Mellon JK, Lunec J, Wright C, Horne CH, Kelly P, Neal DE. C‐erbB‐2 in bladder cancer: molecular biology, correlation with epidermal growth factor receptors and prognostic value. J Urol. 1996;155(1):321‐326. [DOI] [PubMed] [Google Scholar]
- 54. Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor‐2/neu‐positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol. 2007;25(16):2218‐2224. [DOI] [PubMed] [Google Scholar]
- 55. Coombs LM, Pigott DA, Sweeney E, et al. Amplification and over‐expression of c‐erbB‐2 in transitional cell carcinoma of the urinary bladder. Br J Cancer. 1991;63(4):601‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60(2):350‐357. [DOI] [PubMed] [Google Scholar]
- 57. Jimenez RE, Hussain M, Bianco FJ, et al. Her‐2/neu overexpression in muscle‐invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res. 2001;7(8):2440‐2447. [PubMed] [Google Scholar]
- 58. Jalali Nadoushan MR, Taheri T, Jouian N, Zaeri F. Overexpression of HER‐2/neu oncogene and transitional cell carcinoma of bladder. Urol J. 2007;4(3):151‐154. [PubMed] [Google Scholar]
- 59. Kolla SB, Seth A, Singh MK, et al. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int Urol Nephrol. 2008;40(2):321‐327. [DOI] [PubMed] [Google Scholar]
- 60. Zhao J, Xu W, Zhang Z, et al. Prognostic role of HER2 expression in bladder cancer: a systematic review and meta‐analysis. Int Urol Nephrol. 2015;47(1):87‐94. [DOI] [PubMed] [Google Scholar]
- 61. Wülfing C, Machiels JP, Richel DJ, et al. A single‐arm, multicenter, open‐label phase 2 study of lapatinib as the second‐line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115(13):2881‐2890. [DOI] [PubMed] [Google Scholar]
- 62. Oudard S, Culine S, Vano Y, et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer. 2015;51(1):45‐54. [DOI] [PubMed] [Google Scholar]
- 63. Wolfers J, Lozier A, Raposo G, et al. Tumor‐derived exosomes are a source of shared tumor rejection antigens for CTL cross‐priming. Nat Med. 2001;7(3):297‐303. [DOI] [PubMed] [Google Scholar]
- 64. Andre F, Schartz NE, Movassagh M, et al. Malignant effusions and immunogenic tumour‐derived exosomes. Lancet. 2002;360(9329):295‐305. [DOI] [PubMed] [Google Scholar]
- 65. Koga K, Matsumoto K, Akiyoshi T, et al. Purification, characterization and biological significance of tumor‐derived exosomes. Anticancer Res. 2005;25(6A):3703‐3707. [PubMed] [Google Scholar]
- 66. Qu JL, Qu XJ, Zhao MF, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis. 2009;41(12):875‐880. [DOI] [PubMed] [Google Scholar]
- 67. Yang L, Wu XH, Wang D, Luo CL, Chen LX. Bladder cancer cell‐derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep. 2013;8(4):1272‐1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


